Abstract
Objective
To identify subgroups likely to benefit from monoclonal antibody and antiviral therapy by evaluating the relationship between comorbidities and hospitalization among US adolescents with symptomatic coronavirus disease 2019 (COVID-19).
Study design
We analyzed the relationship between presence of comorbidities and need for hospitalization within 28 days of COVID-19 diagnosis for adolescents aged 12-17 years listed in the Pediatric COVID-19 US registry, a multicenter retrospective cohort of US pediatric patients with COVID-19. Comorbidities assessed included obesity, chronic kidney disease (CKD), diabetes, immunosuppressive disease or treatment, sickle cell disease (SCD), heart disease, neurologic disease/neurodevelopmental disorders, and pulmonary disease (excluding patients with mild asthma). We used multivariable logistic regression to determine race/ethnicity-adjusted associations between comorbidities and hospitalization.
Results
A total of 1877 patients met our inclusion criteria, of whom 284 (15%) were hospitalized within 28 days of their COVID-19 diagnosis. In a race/ethnicity-adjusted model, the following comorbidities were independently associated with increased odds of hospitalization: SCD (aOR, 6.9; 95% CI, 3.0-15.9), immunocompromising condition (aOR, 6.4; 95% CI, 3.8-10.8), obesity (aOR, 3.2; 95% CI, 2.1-4.9), diabetes (aOR, 3.0; 95% CI, 1.4-6.2), neurologic disease (aOR, 2.8; 95% CI, 1.8-4.3), and pulmonary disease (excluding mild asthma) (aOR, 1.9; 95% CI, 1.2-3.1). Heart disease and CKD were not independently associated with hospitalization.
Conclusions
SCD, immunocompromising conditions, obesity, diabetes, neurologic disease, and pulmonary disease (excluding mild asthma) were associated with hospitalization for symptomatic COVID-19. Adolescents with acute COVID-19 and these comorbidities should be prioritized for consideration of therapy to avert hospitalization.
Keywords: COVID-19, adolescent, pediatrics, monoclonal antibodies, hospitalization
As of January 2022, more than 8 million children in the US had been infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 New SARS-CoV-2 variant strains have added to the burden of coronavirus disease 2019 (COVID-19) among children and adolescents, despite the authorization of vaccinations for children aged ≥5 years.2, 3, 4 Even though most children and adolescents with COVID-19 are asymptomatic or experience mild illness, some progress to severe disease.5 Documented COVID-19-associated hospitalization rates are higher in adolescents aged 12-17 years than in younger children aged 5-11 years.6 An evaluation of SARS-CoV-2–associated deaths from February to July 2020 in patients aged <21 years identified the most common underlying conditions as obesity, asthma, and developmental disorders.7 Although chronic comorbidities have been associated with severe disease in children,5 , 8, 9, 10, 11, 12 data are limited on which conditions most strongly predict progression.
To inform the rational use of COVID-19 therapies, there is a need to understand the risk for progression to severe disease among adolescents with COVID-19. Patients aged ≥12 years weighing at least 40 kg with mild or moderate COVID-19 and ≥1 specified comorbidity are eligible to receive monoclonal antibodies (mAbs), including sotrovimab, or antivirals, including nirmaltrelvir plus ritonavir, to prevent progression to severe disease.13, 14, 15 Bamlanivimab plus etesevimab and casirivimab plus imdevimab have demonstrated reduced activity against the SARS-CoV-2 Omicron variant.16, 17, 18 The Food and Drug Administration's Emergency Use Authorizations (EUAs) for mAbs identify the following conditions as risk factors for hospitalization: elevated body mass index (for adolescents, ≥85th percentile for age and sex), pregnancy, chronic kidney disease (CKD), diabetes, immunocompromising conditions, sickle cell disease (SCD), cardiovascular disease (including congenital heart disease), chronic lung diseases, neurodevelopmental disorders, and medical-related technology dependence.13 However, multicenter expert guidance on the pediatric use of mAb therapy has underscored the paucity of evidence supporting these risks.19 In addition, given the rise in COVID-19 cases and emergence of the Omicron variant, there is a limited supply of active mAbs and preventive antiviral therapies, driving the need to identify adolescents most likely to benefit from treatment.
In the present study, we analyzed data from the Pediatric COVID-19 US Registry to identify predictors of hospitalization among adolescents diagnosed with COVID-19.
Methods
The Pediatric COVID-19 US Registry is a multicenter retrospective cohort study of US patients aged <21 years diagnosed with laboratory-confirmed COVID-19 after April 1, 2020.20 This voluntary national registry collected deidentified medical history and clinical data from medical records. Clinicians treating children were invited via national email listservs; medical centers in 36 states reported data to the registry (Appendix; available at www.jpeds.com). Centers were requested to submit data for any children with acute COVID-19 seen at inpatient or outpatient medical facilities, regardless of underlying conditions or hospitalization status. Clinicians or clinical researchers identified cases and reviewed medical records at participating institutions and entered detailed clinical data using a standardized electronic REDCap survey that encompassed events occurring within 7 days of COVID-19 diagnosis. Submitting sites received a follow-up survey for each reported case at 28 days after initial submission. St Jude Children's Research Hospital served as the data collection and coordinating center. The registry was approved as an exempt study [45 CFR 46.104(d)(4)] under US federal regulations by the St Jude Institutional Review Board. The Boston Children's Hospital Institutional Review Board also approved submission of deidentified case information and this analysis of registry data as an exempt study.
Study Population
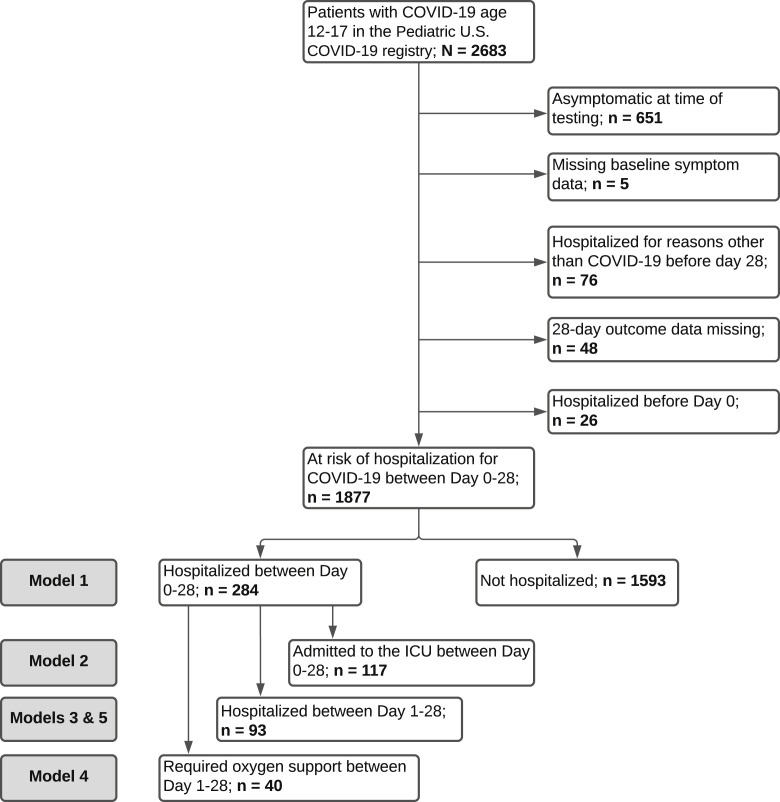
The study population comprised children aged 12-17 years diagnosed with symptomatic acute COVID-19 (defined as any symptoms that prompted COVID-19 testing up to 3 days before testing) reported to the registry between April 1, 2020, and April 30, 2021, for whom 28-day follow-up data were submitted by August 5, 2021. We excluded patients who were already hospitalized for any reason before the day of testing, hospitalized for a reason other than COVID-19 by day 28, or had missing 28-day follow-up hospitalization status.
Covariables and Outcomes
Race and ethnicity were categorized as White non-Hispanic, Hispanic, Black non-Hispanic, Asian non-Hispanic, other non-Hispanic, or “missing.” Age (in years) and sex were also assessed as covariates.
The primary exposure was preexisting comorbidities specified in the mAb EUAs.13 , 17 , 18 Obesity, CKD, diabetes (any type), SCD, heart disease, neurologic disease, and immunocompromising conditions were captured as binary survey variables in the registry; additional details about diagnoses were available for some, but not all, comorbidities. Using available survey data, we specified a variable for pulmonary disease excluding mild asthma, defined as asthma not requiring daily inhaled corticosteroids. We defined medical technology dependence as use of tracheostomy with or without mechanical ventilation, need for total parenteral nutrition, or use of a gastrostomy/gastrostomy-jejunostomy tube. Although technology dependence was evaluated in descriptive analysis, it was removed from analyses of associations owing to the small sample size. The secondary exposure was trichotomized total comorbidity burden (0 comorbidities, 1 comorbidity, or 2 or more comorbidities); the comorbidity burden did not include technology dependence.
The primary outcome was hospitalization for COVID-19 (a clinical determination adjudicated by investigators at the time of data entry) at any time between day 0 (COVID-19 diagnosis) and day 28, which served as a proxy for severe disease. The secondary outcomes were intensive care unit admission between days 0 and 28, hospitalization between days 1 and 28 among patients not hospitalized on day 0, and the need for any form of oxygen support between days 1 and 28. Secondary outcomes were selected to represent the incidence of severe disease and progression of COVID-19 disease among patients presenting with mild-to-moderate illness. When dates of hospitalization or initiation of oxygen support were not specified, they were assumed to have occurred after day 0; we made this assumption because 97% and 100% of cases with these missing data, respectively, were reported in the 28-day follow-up assessment of hospitalization, which did not include a field for date of hospitalization or date of oxygen initiation, rather than in the initial day 0-7 survey.
Because of conflicting evidence about the associations between different forms of preexisting pulmonary disease and risk for COVID-19–related hospitalization,21 , 22 we performed a sensitivity analysis using the following variables as the exposure of interest: asthma necessitating daily inhaled corticosteroids, preexisting pulmonary disease except for asthma, and preexisting pulmonary disease, including mild asthma.
Statistical Analyses
We used frequencies and percentages to describe demographic characteristics, comorbidities, and outcomes. We used the Fisher exact test and χ2 test for categorical variables and the t test for continuous variables to assess univariable associations between exposures/covariates and outcomes. We evaluated for possible collinearity between comorbidities using point-biserial pairwise correlation coefficients, with a correlation of >0.8 indicating potential for collinearity.
We performed multivariable logistic regression to model associations between exposures and outcomes. All comorbidity variables except technology dependence (owing to small sample size) were included in models because they were hypothesized a priori to independently predict outcomes and also to enable adjustment for the presence of multiple comorbidities. Models were adjusted for demographic characteristics (age, sex, and race/ethnicity) when univariable tests of association between these factors and outcomes were significant at P < .1. Significance in multivariable analysis was set at P < .05. In cases of zero cells, relevant variables were removed from the models to preserve sample size.
We constructed models to evaluate independent associations between comorbidities and the following outcomes: hospitalization between days 0 and 28 (model 1), intensive care unit (ICU) admission between days 0 and 28 (model 2), and hospitalization between days 1 and 8, excluding patients hospitalized on day 0 (model 3). We constructed 2 additional models to assess relationships between comorbidities specified using the trichotomized comorbidity variable and the outcomes of oxygen requirement between days 1 and 28, excluding patients requiring oxygen on day 0 (model 4), and hospitalization on day 0 versus hospitalization on days 1-28 (model 5). Model 5 assessed whether the odds of hospitalization associated with comorbidities were greater in the days following diagnosis of COVID-19 than at diagnosis. Models 3, 4, and 5 evaluated the risk of disease progression. We used the trichotomized comorbidity burden score rather than individual comorbidities in models 4 and 5 because of concerns for model overfitting, given the small numbers of outcomes. We performed sensitivity analyses with different definitions of pulmonary disease using model 1.
Finally, we conducted an exploratory analysis for interaction between comorbidities on risk of hospitalization, hypothesizing that the interaction of comorbidities would confer an additive risk of hospitalization. We used logistic or exact logistic regression models, including each pair of comorbidities and their interaction term to screen for potentially significant interactions. We then performed a multivariable interaction model that included interaction terms that were significant at the P < .1 level in the screening analysis, as well as all comorbidities and race/ethnicity.
Results
The Pediatric COVID-19 US registry contained 2683 patients aged 12-17 years with COVID-19, of whom 1877 were included in our primary analysis (Figure 1 ). The mean patient age was 15.3 ± 1.4 years, and 992 (53%) were female (Table I ). By day 28 after COVID-19 diagnosis, 284 patients (15%) were hospitalized, of whom 93 (5%) were hospitalized at ≥1 days after diagnosis, suggesting progression of disease. There were no documented deaths.
Figure 1.
Diagram of included patients.
Table I.
Characteristics of included patients and primary and secondary outcomes
| Characteristics | Total (N = 1877) | Not hospitalized (n/N = 1593/1877) | Hospitalized on days 0-28 (n/N = 284/1877) | ICU admission on days 0-28 (n/N = 117/1877) | Hospitalized on days 1-28 (n/N = 93/1686)∗ | Oxygen requirement on days 1-28 (n/N = 40/1771)† |
|---|---|---|---|---|---|---|
| Age, y, mean (SD) | 15.3 (1.4) | 15.3 (1.4) | 15.3 (1.3) | 15.5 (1.3) | 15.4 (1.3) | 15.7 (1.3) |
| Sex, n (%)‡ | ||||||
| Female | 992 (53) | 848 (85) | 144 (15) | 55 (6) | 55 (6) | 23 (2) |
| Male | 882 (47) | 742 (84) | 140 (16) | 62 (7) | 38 (5) | 17 (2) |
| Other/unknown | 3 (0.2) | 3 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Race/ethnicity, n (%)‡ | ||||||
| White, non-Hispanic | 694 (37) | 643 (93) | 51 (7) | 25 (4) | 25 (4) | 10 (1) |
| Hispanic | 400 (21) | 301 (75) | 99 (25) | 45 (11) | 26 (8) | 12 (3) |
| Black, non-Hispanic | 398 (21) | 323 (81) | 75 (19) | 27 (7) | 26 (7) | 15 (4) |
| Asian, non-Hispanic | 36 (2) | 25 (69) | 11 (31) | 5 (14) | 3 (11) | 0 (0) |
| Other, non-Hispanic | 33 (2) | 23 (70) | 10 (30) | 4 (12) | 2 (8) | 0 (0) |
| Missing | 316 (17) | 278 (88) | 38 (12) | 11 (3) | 11 (4) | 3 (1) |
| Comorbidities, n (%)‡ | ||||||
| SCD | 27 (1.4) | 12 (44) | 15 (56) | 0 (0) | 6 (33) | 1 (4) |
| Immunocompromising condition | 78 (3.7) | 39 (50) | 39 (50) | 10 (13) | 21 (35) | 10 (14) |
| Obesity | 131 (7.0) | 81 (62) | 50 (38) | 31 (24) | 17 (17) | 8 (8) |
| Diabetes | 39 (2.1) | 23 (59) | 16 (41) | 7 (18) | 7 (23) | 5 (14) |
| Neurologic disease | 123 (6.6) | 84 (68) | 39 (32%) | 15 (12) | 17 (17) | 4 (4) |
| CKD | 13 (0.7) | 5 (38) | 8 (62) | 1 (8) | 6 (55) | 2 (15) |
| Pulmonary disease (excluding mild asthma) | 106 (5.6) | 74 (70) | 32 (30) | 18 (17) | 13 (15) | 12 (13) |
| Heart disease | 53 (2.8) | 34 (64) | 19 (36) | 10 (19) | 9 (21) | 7 (15) |
| Technology dependence | 6 (0.3) | 2 (33) | 4 (67) | 2 (33) | 0 (0) | 1 (25) |
| Any prespecified comorbidity§, n (%)‡ | 448 (24) | 294 (66) | 154 (34) | 61 (14) | 58 (16) | 29 (7) |
| 1 comorbidity | 349 (19) | 241 (69) | 108 (31) | 41 (12) | 33 (12) | 17 (6) |
| 2 or more comorbidities | 99 (5.3) | 53 (54) | 46 (46) | 20 (20) | 25 (32) | 12 (14) |
For analysis of patients hospitalized on days 1-28, the total number was 1686 after excluding 191 patients hospitalized on day 0.
For analysis of patients initiating oxygen on days 1-28, the total number was 1771 after excluding 106 patients requiring oxygen on day 0.
The percentage in the first column refers to the percentage of the total cohort with the characteristic (ie, the column percentage), and the percentage in the second through sixth columns refers to the percentage of patients with the specified characteristic who experienced the specified outcome (ie, the row percentage).
Excludes patients with technology dependence, for consistency with regression analyses.
Among the included patients, 448 (24%) had ≥1 comorbidity (Table I and Table II; available at www.jpeds.com). The most common conditions were obesity (7.0%), neurologic disease (6.6%), pulmonary disease excluding mild asthma (5.6%), and immunocompromising conditions (3.7%). In univariable analysis, all comorbidities were significantly associated with hospitalization between day 0 and 28 (Table III; available at www.jpeds.com). All pairwise point biserial correlations were <0.25.
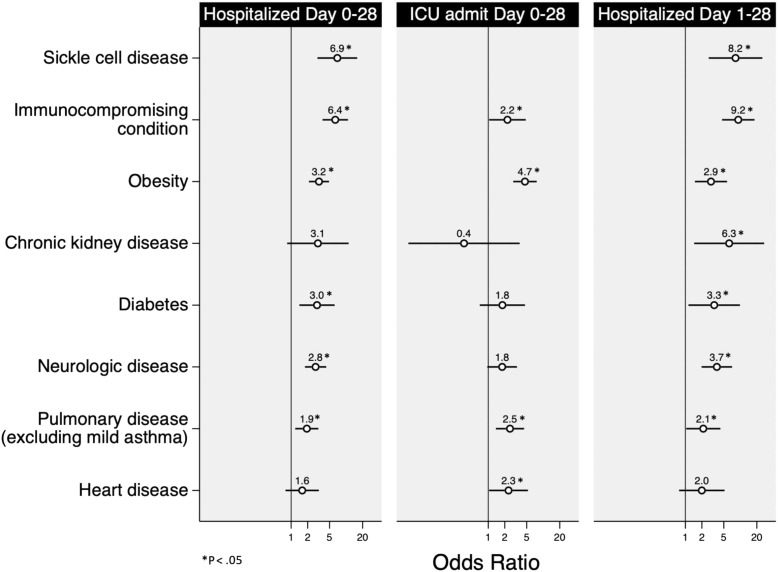
In the model of hospitalization between days 0 and 28 (model 1), SCD (aOR, 6.9; 95% CI, 3.0-15.9), immunocompromising condition (aOR, 6.4; 95%CI 3.8–10.8), obesity (aOR, 3.2; 95%CI 2.1–4.9), diabetes (aOR, 3.0; 95%CI 1.4–6.2), neurologic disease (aOR, 2.8; 95% CI, 1.8-4.3), and pulmonary disease (excluding mild asthma) (aOR, 1.9; 95% CI, 1.2-3.1) were significantly associated with hospitalization (Figure 2 and Table IV ). CKD and heart disease were not significant predictors.
Figure 2.
Associations among comorbidities, hospitalization, and ICU admission.
Table IV.
Associations between comorbidities and hospitalization on days 0-28, ICU admission on days 0-28, and hospitalization on days 1-28
| Comorbidities∗ | Adjusted odds of hospitalization |
||
|---|---|---|---|
| Hospitalized on days 0-28 (n/N = 284/1877), OR (95% CI) | ICU admission on days 0-28 (n/N = 117/1877), OR (95% CI) | Hospitalized on days 1-28 (n/N = 93/1686), OR (95% CI)† | |
| SCD | 6.9 (3.0-15.9) | ---‡ | 8.2 (2.7-25.2) |
| Immunocompromising condition | 6.4 (3.8-10.8) | 2.2 (1.0-4.8)§ | 9.2 (4.7-18.1) |
| Obesity | 3.2 (2.1-4.9) | 4.7 (2.8-7.6) | 2.9 (1.5-5.7) |
| CKD | 3.1 (0.8-11.1) | 0.4 (0.1-3.7) | 6.3 (1.4-27.2) |
| Diabetes | 3.0 (1.4-6.2) | 1.7 (0.7-4.7) | 3.3 (1.1-9.9) |
| Neurologic disease | 2.8 (1.8-4.3) | 1.8 (0.9-3.3) | 3.7 (2.0-7.1) |
| Pulmonary disease (excluding mild asthma) | 1.9 (1.2-3.1) | 2.5 (1.4-4.5) | 2.1 (1.0-4.3)§ |
| Heart disease | 1.6 (0.8-3.2) | 2.3 (1.0-5.3)§ | 2.0 (0.8-5.2) |
| Race/ethnicity (reference: white, non-Hispanic) | |||
| Hispanic | 4.0 (2.7-5.9) | 2.8 (1.7-4.7) | 2.1 (1.2-3.9) |
| Black, non-Hispanic | 2.4 (1.6-3.7) | 1.5 (0.8-2.7) | 1.6 (0.9-3.1) |
| Asian, non-Hispanic | 5.9 (2.6-13.1) | 3.5 (1.2-10.2) | 3.8 (1.0-14.4)§ |
| Other, non-Hispanic | 3.9 (1.6-9.5) | 2.9 (0.9-9.4) | 1.7 (0.3-8.4) |
| Missing | 1.6 (1.0-2.6)§ | 0.9 (0.4-1.8) | 0.9 (0.4-2.0) |
The models are adjusted for race/ethnicity.
For each comorbidity variable, the reference is absence of that comorbidity. Models are adjusted for all comorbidities and race/ethnicity.
Patients who were hospitalized on day 0 have been removed from the analysis of patients hospitalized between days 1 and 28.
SCD was removed from the model because no patients with SCD were admitted to the ICU, and thus OR could not be computed.
P < .05.
Notably, compared with patients identified as White non-Hispanic, those who were Asian non-Hispanic (aOR, 5.9; 95% CI, 2.6-13.1), Hispanic (aOR, 4.0; 95% CI, 2.7-5.9), other non-Hispanic (aOR, 3.9; 95% CI, 1.6-9.5), or Black non-Hispanic (aOR, 2.4; 95% CI, 1.6-3.7), or who were missing race/ethnicity data (aOR, 1.6; 95% CI, 1.0-2.6) had increased odds of hospitalization, adjusting for comorbidities.
Immunocompromising conditions, obesity, pulmonary disease (excluding mild asthma), and heart disease were associated with ICU admission between days 0 and 28 (Figure 2 and Table IV). Immunocompromising conditions, SCD, CKD, diabetes, neurologic disease, obesity, and pulmonary disease (excluding mild asthma) were associated with hospitalization between days 1 and 28 (Figure 2 and Table IV). The presence of 1 (aOR, 6.7; 95% CI, 3.1-14.7) or ≥2 comorbidities (aOR, 17.1; 95% CI, 7.2-40.7) was associated with the need for oxygen support between days 1 and 28, after adjusting for age and race/ethnicity (model 4). Adjusting for race/ethnicity, the presence of ≥2 comorbidities (vs no comorbidities) was associated with increased odds of hospitalization on days 1-28 compared with day 0 (aOR, 3.1; 95% CI, 1.5-6.4), although the presence of only 1 comorbidity was not (aOR, 1.3; 95% CI, 0.7-2.3) (model 5).
In sensitivity analyses examining different definitions of pulmonary disease, patients with any pulmonary disease including mild asthma (aOR, 1.7; 95% CI, 1.2-2.4) or excluding any asthma (regardless of inhaled corticosteroid use) (aOR, 3.4; 95% CI, 1.6-6.9) had increased odds of hospitalization (Table V; available at www.jpeds.com). There was no increase in the odds of hospitalization when pulmonary disease was specified as the presence of asthma on inhaled corticosteroids (Table V).
In the interaction analysis, the interaction term for obesity and immunocompromising condition was significant in screening analysis (P = .042). This interaction term remained significant in the multivariable interaction model, although the effect was negative (aOR, 0.04; 95% CI, 0.005-0.35), indicating that the risk conferred by these 2 comorbidities was less than additive for the 5 patients who had both comorbidities. The observed hospitalization rate in this group was 40%, which was similar to the observed hospitalization rate in immunocompromised patients overall (50%) and in patients with obesity overall (38%). Because the interaction finding was not intuitive, we examined the 5 patients with obesity and immunocompromising conditions in more detail and noted that 4 of 5 patients had other prespecified comorbidities. Therefore, we interpret the interaction finding as suggesting that these patients’ risk of hospitalization was likely accounted for by the contributions of other comorbidities, rather than just the combination of obesity and immunocompromising conditions.
Discussion
We used a national registry of 1877 adolescents with symptomatic acute COVID-19 to investigate risk factors for COVID-19–related hospitalization within 28 days of diagnosis. Adjusting for race/ethnicity, we found that SCD, immunocompromising conditions, obesity, diabetes, neurologic disease, and pulmonary disease (excluding mild asthma) were associated with hospitalization at or following COVID-19 diagnosis. The same conditions, with the addition of CKD, were associated with progression to hospitalization between days 1 and 28. Adolescents with these comorbidities should be prioritized to receive interventions to avert disease progression.
SCD was associated with hospitalization and illness progression, although no patients with SCD were admitted to the ICU. Assessing the risk of COVID-19–related hospitalization in patients with SCD is complicated by admissions for acute chest syndrome and vaso-occlusive crises.23 It is plausible that these SCD complications could predispose to more severe COVID-19. A review of published series from the Surveillance Epidemiology of Coronavirus (COVID-19) Under Research Exclusion-SCD registry found infrequent severe COVID-19 outcomes among pediatric patients with SCD overall, although the source literature may have been biased toward hospitalized patients.24 , 25 Similar to the approach for patients with immunocompromising conditions, centers may have protocols to admit patients with SCD presenting with acute respiratory symptoms and fever; specific factors leading to admission were not collected in the Pediatric COVID-19 US registry. Our analysis also was limited by the small number of patients with SCD in our cohort.
Immunocompromising conditions in our cohort included various primary and secondary immunodeficiencies. Immunocompromised patients may be unable to mount effective adaptive immune responses to infection or vaccination26 and thus may especially benefit from such therapies as mAbs. Conversely, COVID-19 outcomes may be more favorable in immunocompromised patients than in patients with other comorbidities, owing to a weaker inflammatory response and thus a milder disease presentation.27 , 28 Protocols requiring hospitalization for fever in stable patients with mild symptoms may increase admissions among immunocompromised patients with COVID-19, limiting the utility of hospitalization as a proxy for severe disease. Nonetheless, we also found that immunocompromising conditions increased the risk for ICU admission in our cohort. Prior pediatric studies have reported heterogeneous effects of immunocompromising conditions, particularly relating to cancer/chemotherapy,29 transplantation,28 , 30 other immunosuppressive medications,31 and primary immunodeficiencies.32 However, our findings add to the existing literature that supports an overall association between immunocompromising conditions and severe COVID-19.
A number of hypotheses linking obesity and the risk of severe COVID-19 have been advanced, including impaired chest wall compliance, increased inflammation and risk of thrombosis, overexpression of angiotensin0converting enzyme-2 in adipocytes, and baseline organ dysfunction.33, 34, 35, 36 Our findings are consistent with previous studies, including a meta-analysis of controlled studies that found an association between childhood obesity and increased risk of severe COVID-19.35 A study using billing codes to identify comorbidities similarly found an increased risk of hospitalization for obese children, as well as an increased risk of severe disease among those hospitalized with COVID-19.10 Notably, in this study, as in ours, obesity likely was underreported, which is a limitation of our findings. In our study, only 5.6% of subjects were reported to have obesity, compared with an estimated prevalence of 21.2% among US adolescents aged 12-19 years.37
Diabetes and CKD were each associated with progression to hospitalization after day 0, but our analyses were limited by the small numbers of patients in each category. Diabetes also was associated with hospitalization, but CKD was not. Despite sample limitations, our results contribute to accumulating evidence identifying both type 1 and type 2 diabetes as risk factors for severe COVID-19 in children and adults.12 , 38 One pediatric study found that subjects with either type 1 or type 2 diabetes and COVID-19 were at elevated risk for hospitalization, although the magnitude of risk differed between the types.10 In addition, COVID-19 has led to increased rates of diabetic ketoacidosis and disruptions to long-term diabetes care.39 Diabetes was reported to the Pediatric COVID-19 US registry without differentiation between types 1 and 2, which may confer risk for severe disease through different pathways. Large registries have identified CKD as a risk factor for COVID-19 severity and mortality, although no clear mechanism has been determined.40 , 41 Challenges in studying CKD-associated risk include the rarity of this condition among children and its association with a variety of comorbidities that also may predispose to severe illness. In our cohort, only 13 patients had CKD.
Our results concur with previous research showing that neurodevelopmental disorders are prevalent among US adolescents hospitalized with COVID-196 and represent a risk factor for hospitalization and progression.10 , 42 The mechanisms underlying this association are unknown. As in our study, Kompaniyets et al identified neurodevelopmental disorders as risk factors for hospitalization, but these disorders were not associated with severe illness among hospitalized adolescents.10 In that study, neurodevelopmental disorders consisted mostly of mental health disorders, and the identified association may reflect the COVID-19 and mental health syndemics, rather than a physiologic predisposition to severe COVID-19.10 Neurologic disease also is often a marker of medical complexity, and our findings may reflect an increased need for hospital-based supportive care for adolescents with complex conditions.9 , 43
We found that pulmonary disease (excluding mild asthma) was associated with hospitalization, ICU admission, and hospitalization after day 0. Previous studies have reported conflicting findings regarding the risk of severe COVID-19 in patients with asthma and non-asthma pulmonary disease. Large observational studies have found an association between asthma and the risk of hospitalization,6 , 10 although smaller retrospective studies have identified asthma as protective against hospitalization.21 Little data exist to guide risk assessment of adolescents with non-asthma pulmonary disease.
We found no association between heart disease and hospitalization, although patients with heart disease were at increased risk of ICU admission. Consistent with the current EUAs, our study included adolescents with hypertension as well as congenital cardiac structural and muscular diseases.17 , 18 Hypertension has been identified as a risk factor for severe disease among adults with COVID-19,44 whereas congenital heart disease has been associated with increased risk among young children.10 Adolescents with cardiovascular comorbidities may be more tolerant of acute COVID-19 compared with adults, given differences in epidemiology and comorbidities associated with heart disease, or young children, given the timing of corrective surgeries and circulatory mechanics. Our analysis leaves open the possibility that subgroups of patients with heart disease may be at high risk; for example, no adolescents with heart failure were included in our cohort, and other specific conditions were infrequent.
Race and ethnicity have emerged as important risk factors for COVID-19–related hospitalization among US adolescents, and nonwhite children and adolescents are overrepresented among US COVID-19 cases.6 , 45 Our analysis reaffirms the results of previous studies showing that race/ethnicity and specific comorbidities are independently associated with hospitalization.10 , 45
In our interaction model, we found that the interaction between obesity and immunocompromising conditions was statistically significant, although our results did not show additive risk. Four of 5 patients with obesity and immunocompromising conditions had at least 1 other prespecified comorbidity, and their hospitalization risk likely was accounted for by the other condition(s). Interpretation of the interaction analysis also was limited by small sample sizes. We found that a higher total number of comorbidities was associated with increased odds of oxygen need between days 1 and 28, as well as odds of hospitalization between day 1-28 compared with on day 0. Additional investigation into the combined effects of specific comorbidities is needed to better risk-stratify adolescents with medical complexity.
Our study has several limitations. The Pediatric US COVID-19 registry is a voluntary surveillance study using secondary data abstracted from medical records. Although the registry captured the need for hospitalization due to COVID-19, the specific reasons for hospitalization were not available. Some hospitalizations may have reflected policies for treatment of high-risk patients rather than disease severity. In addition, the infrequency of hypoxemia precluded an analysis of associations between this outcome and specific comorbidities. Furthermore, because the registry did not capture granular data on comorbidity characteristics, we were unable to evaluate subgroups of patients with specified comorbidities (eg, type 1 vs type 2 diabetes). Some comorbidities may have been underreported, including obesity. Additionally, race and ethnicity were based on information available in medical records, which has substantial limitations, including missing data, discrepant categories across electronic record systems, and lack of self-reports.46 Furthermore, we were unable to measure markers of socioeconomic status, which affect the association between race/ethnicity and health outcomes. Because pediatric hospitals were more likely than nonpediatric centers to contribute cases, registry data may be enriched for more severe cases, as reflected by the 15% rate of hospitalization in our cohort.11 Finally, case submission was stopped in April 2021, before the surges associated with the Delta and Omicron variants and before the widespread vaccination of adolescents. The risk of hospitalization for adolescents with COVID-19 will continue to change as new variants emerge and as vaccine policy and access evolve. Nonetheless, as underscored by the recent shortages in mAbs and antivirals effective against the Omicron variant, it remains important to understand which adolescents are most likely to experience severe illness and disease progression.
Our study also has some key strengths. The data are derived from a national multicenter pediatric-focused registry gleaned from a detailed chart review that captured information on comorbidities. The database size enabled evaluation of adolescents specifically and assessment of risk conferred by rare conditions, such as CKD, that have not been reported in observational or surveillance studies. The registry prospectively captured COVID-19–related hospitalizations within 28 days of diagnosis, enabling analysis of progression to hospitalization (models 3 and 5), which is the outcome that mAbs have been shown to prevent. Finally, we specifically selected conditions included in current mAb guidance, making our results directly relevant to decisions that clinicians currently face.
Acknowledgments
We thank Dr Robert Husson of Boston Children's Hospital; Dr Gabriela Maron, Dr Ronald Dallas, Dr Jose Ferrolino, Ms Hailey Skonhovd Ross, and Ms Sapna Ashokkumar Pardasani of the Pediatric COVID-19 US Registry Coordinating Center Team; and all collaborators and contributors to the Pediatric COVID-19 US Registry (Appendix).
Footnotes
The Pediatric COVID-19 US Registry has received support from the American Lebanese Syrian Associated Charities (ALSAC). J.C. was supported by Agency for Healthcare Research and Quality Grant T32 HS000063 as part of the Harvard-wide Pediatric Health Services Research Fellowship Program. M.D. was supported by Grant T32 AI007433 from the National Institute of Allergy and Infectious Diseases. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or Agency for Healthcare Research and Quality. The authors declare no conflicts of interest.
Portions of this study were presented as a poster during the IDWeek meeting, September 29-October 3, 2021 (virtual).
Supplementary Data
Appendix
Table II.
Comorbidities (N = 1877)
| Comorbidities | n (%) |
|---|---|
| SCD | 27 (1.4) |
| Immunocompromising condition | 78 (3.7) |
| Hematopoietic stem cell transplant or cellular therapy recipient | 5 (0.2) |
| Solid organ transplant recipient | 16 (0.9) |
| Other immunocompromising condition (not transplanted) | 57 (3.0) |
| Malignancy (solid or liquid) | 26 (1.4) |
| Primary immunodeficiency | 3 (0.2) |
| Rheumatologic conditions | 10 (0.5) |
| Other/not specified | 18 (1.0) |
| Technology dependence | 6 (0.3) |
| Tracheostomy dependence | 1 (0.1) |
| Enteral feeding tube dependence | 5 (0.2) |
| Obesity | 131 (7.0) |
| Diabetes | 39 (2.1) |
| Neurologic disease | 123 (6.6) |
| Hypoxic-ischemic encephalopathy | 3 (0.2) |
| Neurodegenerative disease | 3 (0.2) |
| Seizure disorder | 39 (2.1) |
| Other/not specified | 79 (4.2) |
| CKD | 13 (0.7) |
| Pulmonary disease (excluding mild asthma) | 106 (5.6) |
| Moderate/severe asthma | 65 (3.5) |
| Bronchopulmonary dysplasia | 1 (0.1) |
| Cystic fibrosis | 3 (0.2) |
| Pulmonary hypertension | 1 (0.1) |
| Other/not specified | 36 (1.9) |
| Heart disease | 53 (2.8) |
| Congenital cardiac disease | 12 (0.6) |
| Cardiomyopathy | 3 (0.2) |
| Cardiovascular or coronary artery disease | 2 (0.1) |
| Hypertension | 13 (6.9) |
| Other/not specified | 25 (1.3) |
| Any prespecified comorbidity | 449 (24) |
| No prespecified comorbidity | 1428 (76) |
Table III.
P values for univariable tests of association between predictors/covariates and primary and secondary outcomes
| Variables | Hospitalized on days 0-28 (n/N = 284/1877) | ICU admission on days 0-28 (n/N = 117/1877) | Hospitalized on days 1-28 (n/N = 93/1686)∗ | Oxygen requirement on days 1-28 (n/N = 40/1771)† |
|---|---|---|---|---|
| Age, y | .579 | .233 | .700 | .072 |
| Female sex | .657 | .352 | .398 | .656 |
| Race/ethnicity | <.001 | <.001 | .018 | .042 |
| Comorbidities | ||||
| SCD | <.001 | .408 | <.001 | .411 |
| Immunocompromising condition | <.001 | .026 | <.001 | <.001 |
| Obesity | <.001 | <.001 | <.001 | .002 |
| Diabetes | <.001 | .009 | .001 | .001 |
| Neurologic disease | <.001 | .010 | <.001 | .303 |
| CKD | <.001 | .568 | <.001 | .033 |
| Pulmonary disease (excluding mild asthma) | <.001 | <.001 | .001 | <.001 |
| Heart disease | <.001 | .001 | <.001 | <.001 |
| Any prespecified comorbidity | <.001 | <.001 | <.001 | <.001 |
| Number of prespecified comorbidities (0 vs 1 vs 2+) | <.001 | <.001 | <.001 | <.001 |
For analysis of patients hospitalized on days 1-28, the total number was 1686 after excluding 191 patients hospitalized on day 0.
For analysis of patients initiating oxygen on days 1-28, the total number was 1771 after excluding 106 patients requiring oxygen on day 0.
Table V.
Sensitivity analyses using different measures of pulmonary disease
| Comorbidities∗ | Adjusted odds of hospitalization |
||
|---|---|---|---|
| Model A (n/N = 284/1877) | Model B (n/N = 284/1877) | Model C (n/N = 284/1877) | |
| SCD | 7.0 (3.0-16.3) | 6.8 (2.9-15.5) | 6.5 (2.8-15.1) |
| Immunocompromising condition | 6.7 (3.9-11.4) | 6.4 (3.8-10.9) | 6.1 (3.6-10.5) |
| Obesity | 3.2 (2.1-4.9) | 3.3 (2.2-5.0) | 3.3 (2.2-5.0) |
| CKD | 3.1 (0.9-11.5) | 3.1 (0.9-11.2) | 3.0 (0.8-10.8) |
| Diabetes | 3.1 (1.5-6.5) | 3.1 (1.5-6.5) | 2.7 (1.3-5.8) |
| Neurologic disease | 2.8 (1.8-4.4) | 2.9 (1.9-4.5) | 2.7 (1.7-4.3) |
| Pulmonary disease | |||
| Model A: any preexisting pulmonary disease | 1.7 (1.3-2.4) | --- | --- |
| Model B: asthma on inhaled corticosteroids | --- | 1.2 (0.6-2.3) | --- |
| Model C: preexisting pulmonary disease, excluding asthma | --- | --- | 3.4 (1.6-6.9) |
| Heart disease | 1.5 (0.8-3.1) | 1.7 (0.8-3.4) | 1.5 (0.7-3.0) |
All sensitivity models are adjusted for race/ethnicity.
For each comorbidity variable, the reference is absence of that comorbidity.
References
- 1.American Academy of Pediatrics, Children’s Hospital Association Children and COVID-19: state-level data report. https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report/
- 2.Delahoy M.J., Ujamaa D., Whitaker M., O’Halloran A., Anglin O., Burnset E., et al. Hospitalizations associated with COVID-19 among children and adolescents—COVID-NET, 14 states, March 1, 2020-August 14, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1255–1260. doi: 10.15585/mmwr.mm7036e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US Food and Drug Administration FDA authorizes Pfizer-BioNTech COVID-19 vaccine for emergency use in children 5 through 11 years of age. 2021. https://www.fda.gov/news-events/press-announcements/fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use-children-5-through-11-years-age
- 4.US Food and Drug Administration Coronavirus (COVID-19) update: FDA authorizes Pfizer-BioNTech COVID-19 vaccine for emergency use in adolescents in another important action in fight against pandemic. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use
- 5.Centers for Disease Control and Prevention CDC COVID-19 Response Team. Coronavirus disease 2019 in children — United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:422–426. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Havers F.P., Whitaker M., Self J.L., Chai S.J., Kirley P.D., Alden N.B., et al. Hospitalization of adolescents aged 12-17 years with laboratory-confirmed COVID-19—Covid-Net, 14 states, March 1, 2020-April 24, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:851–857. doi: 10.15585/mmwr.mm7023e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCormick D.W., Richardson L.C., Young P.R., Viens L.J., Gould C.V., Kimball A., et al. Deaths in children and adolescents associated with COVID-19 and MIS-C in the United States. Pediatrics. 2021;148 doi: 10.1542/peds.2021-052273. e2021052273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellino S., Punzo O., Rota M.C., Del Manso M., Urdiales A.M., Andrianou X., et al. COVID-19 disease severity risk factors for pediatric patients in Italy. Pediatrics. 2020;146 doi: 10.1542/peds.2020-009399. e2020009399. [DOI] [PubMed] [Google Scholar]
- 9.Shekerdemian L.S., Mahmood N.R., Wolfe K.K., Riggs B.J., Ross C.E., McKiernan C.A., et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020;174:868–873. doi: 10.1001/jamapediatrics.2020.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kompaniyets L., Agathis N.T., Nelson J.M., Preston L.E., Ko J.Y., Belay B., et al. Underlying medical conditions associated with severe COVID-19 illness among children. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.11182. e2111182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antoon J.W., Grijalva C.G., Thurm C., Richardson T., Spaulding A.B., Teufel R.J., 2nd, et al. Factors associated with COVID-19 disease severity in US children and adolescents. J Hosp Med. 2021;16:603–610. doi: 10.12788/jhm.3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woodruff R.C., Campbell A.P., Taylor C.A., Chai S.J., Kawasaki B., Meek J., et al. Risk factors for severe COVID-19 in children. Pediatrics. 2021 doi: 10.1542/peds.2021-053418. e2021053418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.US Food and Drug Administration Fact sheet for healthcare providers emergency use authorization (EUA) of sotrovimab. https://www.fda.gov/media/149534/download
- 14.Gupta A., Gonzalez-Rojas Y., Juarez E., Crespo Casa M., Moya J., Falci D.R., et al. Early treatment for covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. New Engl J Med. 2021;385:194–250. doi: 10.1056/NEJMoa2107934. [DOI] [PubMed] [Google Scholar]
- 15.US Food and Drug Administration Fact sheet for healthcare providers: emergency use authorization for paxlovid. https://www.fda.gov/media/155050/download
- 16.National Institutes of Health COVID-19 treatment guidelines panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/ [PubMed]
- 17.US Food and Drug Administration Fact sheet for health care providers emergency use authorization (EUA) of bamlanivimab and etesevimab. https://www.fda.gov/media/145802/download
- 18.US Food and Drug Administration Fact sheet for health care providers: emergency use authorization (EUA) of Regen-Cov® (casirivimab and imdevimab) https://www.fda.gov/media/145611/download
- 19.Wolf J., Abzug M.J., Wattier R.L., Sue P.K., Vora S.B., Zachariah P., et al. Initial guidance on use of monoclonal antibody therapy for treatment of coronavirus disease 2019 in children and adolescents. J Pediatric Infect Dis Soc. 2021;10:629–634. doi: 10.1093/jpids/piaa175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pediatric COVID-19 Case Registry Pediatric COVID-19 US Registry. https://www.pedscovid19registry.com/
- 21.Floyd G.C., Dudley J.W., Xiao R., Feudtner C., Taquechel K., Miller K., et al. Prevalence of asthma in hospitalized and non-hospitalized children with COVID-19. J Allergy Clin Immunol Pract. 2021;9:2077–2079.e2. doi: 10.1016/j.jaip.2021.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scorpo M.L., Ferrante G., La Grutta S. An overview of asthma and COVID-19: protective factors against SARS-COV-2 in pediatric patients. Front Pediatr. 2021;9:661206. doi: 10.3389/fped.2021.661206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrone K.A., Strumph K., Liszewski M.C., Jackson J., Rinke M.L., Silver E.J., et al. Acute chest syndrome in the setting of SARS-COV-2 infections—A case series at an urban medical center in the Bronx. Pediatr Blood Cancer. 2020;67:e28579. doi: 10.1002/pbc.28579. [DOI] [PubMed] [Google Scholar]
- 24.Sayad B., Karimi M., Rahimi Z. Sickle cell disease and COVID-19: susceptibility and severity. Pediatr Blood Cancer. 2021;68:e29075. doi: 10.1002/pbc.29075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Secure-SCD Registry Surveillance epidemiology of coronavirus (COVID-19) under research exclusion. Updates and data. https://covidsicklecell.org/updates-data/
- 26.Kennedy N.A., Goodhand J.R., Bewshea C., Nice R., Chee D., Lin S., et al. Anti-SARS-CoV-2 antibody responses are attenuated in patients with IBD treated with infliximab. Gut. 2021;70:865–875. doi: 10.1136/gutjnl-2021-324388. [DOI] [PubMed] [Google Scholar]
- 27.Minotti C., Tirelli F., Barbieri E., Giaquinto C., Donà D. How is immunosuppressive status affecting children and adults in SARS-CoV-2 infection? A systematic review. J Infect. 2020;81:e61–e66. doi: 10.1016/j.jinf.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goss M.B., Galvan N.T., Ruan W., Munoz F.M., Brewer E.D., O'Mahony C.A., et al. The pediatric solid organ transplant experience with COVID-19: an initial multi-center, multi-organ case series. Pediatr Transplant. 2021;25:e13868. doi: 10.1111/petr.13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boulad F., Kamboj M., Bouvier N., Mauguen A., Kung A.L. COVID-19 in children with cancer in New York City. JAMA Oncol. 2020;6:1459–1460. doi: 10.1001/jamaoncol.2020.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vicent M.V., Martinez A.P., Del Castillo M.T., Molina B., Sisini L., Morón-Cazalilla G., et al. COVID-19 in pediatric hematopoietic stem cell transplantation: the experience of Spanish Group of Transplant (GETMON/GETH) Pediatr Blood Cancer. 2020;67:e28514. doi: 10.1002/pbc.28514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marlais M., Wlodkowski T., Vivarelli M., Pape L., Tönshoff B., Schaefer F., et al. The severity of COVID-19 in children on immunosuppressive medication. Lancet Child Adolesc Health. 2020;4:e17–e18. doi: 10.1016/S2352-4642(20)30145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delavari S., Abolhassani H., Abolnezhadian F., Babaha F., Iranparast S., Ahanchian H., et al. Impact of SARS-CoV-2 pandemic on patients with primary immunodeficiency. J Clin Immunol. 2021;41:345–355. doi: 10.1007/s10875-020-00928-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sattar N., McInnes I.B., McMurray J.J.V. Obesity is a risk factor for severe COVID-19 infection: multiple potential mechanisms. Circulation. 2020;142:4–6. doi: 10.1161/CIRCULATIONAHA.120.047659. [DOI] [PubMed] [Google Scholar]
- 34.Kruglikov I.L., Scherer P.E. The role of adipocytes and adipocyte-like cells in the severity of COVID-19 infections. Obesity (Silver Spring) 2020;28:1187–1190. doi: 10.1002/oby.22856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsankov B.K., Allaire J.M., Irvine M.A., Lopez A.A., Sauvé L.J., Vallance B.A., et al. Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int J Infect Dis. 2021;103:246–256. doi: 10.1016/j.ijid.2020.11.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nogueira-de-Almeida C.A., Del Ciampo L.A., Ferraz I.S., Del Ciampo I.R., Contini A.A., 3 Ued F.D.V. COVID-19 and obesity in childhood and adolescence: a clinical review. J Pediatr (Rio J) 2020;96:546–558. doi: 10.1016/j.jped.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fryar C.D., Carroll M.D., Afful J. Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2-19 years: United States, 1963-1965 through 2017-2018. https://www.cdc.gov/nchs/data/hestat/obesity-child-17-18/overweight-obesity-child-H.pdf
- 38.Kim L., Garg S., O’Halloran A., Whitaker M., Pham H., Anderson E.J., et al. Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-NET) Clin Infect Dis. 2021;72:e206–e214. doi: 10.1093/cid/ciaa1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kamrath C., Rosenbauer J., Eckert A.J., Pappa A., Reschke F., Rohrer T.R., et al. Incidence of COVID-19 and risk of diabetic ketoacidosis in new-onset type 1 diabetes. Pediatrics. 2021;148 doi: 10.1542/peds.2021-050856. e2021050856. [DOI] [PubMed] [Google Scholar]
- 40.ERA-EDTA Council; ERACODA Working Group Chronic kidney disease is a key risk factor for severe COVID-19: a call to action by the ERA-EDTA. Nephrol Dial Transplant. 2021;36:87–94. doi: 10.1093/ndt/gfaa314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pakhchanian H., Raiker R., Mukherjee A., Khan A., Singh S., Chatterjee A. Outcomes of COVID-19 in CKD patients: a multicenter electronic medical record cohort study. Clin J Am Soc Nephrol. 2021;16:785–786. doi: 10.2215/CJN.13820820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeBiasi R.L., Song X., Delaney M., Bell M., Smith K., Pershad J., et al. Severe coronavirus disease-2019 in children and young adults in the Washington, DC, metropolitan region. J Pediatr. 2020;223:199–203.e1. doi: 10.1016/j.jpeds.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herman C., Mayer K., Sarwal A. Scoping review of prevalence of neurologic comorbidities in patients hospitalized for COVID-19. Neurology. 2020;95:77–84. doi: 10.1212/WNL.0000000000009673. [DOI] [PubMed] [Google Scholar]
- 44.Clark C.E., McDonagh S.T., McManus R.J., Martin U. COVID-19 and hypertension: risks and management. A scientific statement on behalf of the British and Irish Hypertension Society. J Hum Hypertens. 2021;35:304–307. doi: 10.1038/s41371-020-00451-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moreira A., Chorath K., Rajasekaran K., Burmeister F., Ahmed M., Moreira A. Demographic predictors of hospitalization and mortality in US children with COVID-19. Eur J Pediatr. 2021;180:1659–1663. doi: 10.1007/s00431-021-03955-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaplan J.B., Bennett T. Use of race and ethnicity in biomedical publication. JAMA. 2003;289:2709–2716. doi: 10.1001/jama.289.20.2709. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.