Abstract
Background
Acute renal failure (ARF) is associated with substantial morbidity and mortality. Some studies have reported a survival advantage among patients dialyzed with biocompatible membranes (BCM) compared to bioincompatible membranes (BICM). These findings were not consistently observed in subsequent studies.
Objectives
To ascertain whether the use of BCM confers an advantage in either survival or recovery of renal function over the use of BICM in adult patients with ARF requiring intermittent hemodialysis.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, in The Cochrane Library), MEDLINE (from 1966), EMBASE (from 1980), the Mexican Index of Latin American Biomedical Journals IMBIOMED (from 1990), the Latin American and Caribbean Health Sciences Literature Database LILACS (from 1982), and reference lists of articles. Search date: January 2007
Selection criteria
Randomized and quasi‐randomized controlled trials comparing the use of a BCM with a BICM in patients > 18 years of age with ARF requiring intermittent hemodialysis.
Data collection and analysis
Two authors extracted the data independently. Cellulose‐derived dialysis membranes were classified as BICM, and synthetic dialyzers were considered as BCM. The main outcomes were all‐cause mortality and recovery of renal function by type of dialyzer. We further explored these outcomes according to the flux properties (high‐flux or low‐flux) of each of these dialyzers. A meta‐analysis was conducted by combining data using a random‐effects model.
Main results
Ten studies were included in the primary analysis of mortality, with a total of 1100 patients. None of the pooled risk ratios (RRs) reached statistical significance. The pooled RR for mortality was 0.93 (95% confidence interval (CI) 0.81 to 1.07). The overall RR for recovery of renal function, which was inclusive of 1038 patients from nine studies, was 1.09 (95% CI 0.90 to 1.31). The pooled RR for mortality by dialyzer flux property was 1.05 (95% CI 0.81 to 1.37). The pooled RR for recovery of renal function by flux property was 1.30 (95% CI 0.83 to 2.02). A meta‐analysis of mortality among kidney transplant recipients was not possible, however the analysis of recovery of renal function in this patient population revealed an RR of 1.05 (95% CI 0.87 to 1.26). Results of sensitivity analyses did not differ significantly from the primary analyses.
Authors' conclusions
There is no demonstrable clinical advantage to the use of BCM versus BICM in patients with ARF who require intermittent hemodialysis.
Plain language summary
The use of biocompatible dialysis membranes compared with biocompatible membranes does not appear to have a different effect on mortality in patients with acute renal failure (ARF)
ARF is a common complication of critically ill patients. Death rates are high despite technological advances in kidney replacement treatments, including the use of a dialyzer or artificial kidney to remove toxins from the blood. Dialyzers are manufactured with different materials and are classified as bioincompatible (BICM) or biocompatible (BCM), as they elicit different biological responses when they come into contact with blood. An initial reported benefit of BCM over BICM, was not confirmed by subsequent studies. In this systematic review, a meta‐analysis combining results of several studies of patients with dialysis‐requiring ARF, no clinical advantage was demonstrable with the use of BCM.
Background
Acute renal failure (ARF) is a syndrome characterized by a rapid decline (hours to weeks) in glomerular filtration rate and retention of nitrogenous waste products such as blood urea nitrogen and creatinine. ARF complicates approximately 5% of hospital admissions and up to 30% of admissions to intensive care units (Brady 2004), and is associated with a substantial morbidity and mortality. Current mortality rates range from 40% to 80% when renal replacement therapy (RRT) is required (Karsou 2000; Nolan 1998; Thadhani 1996). This high mortality has not significantly decreased in the last 40 years despite advances in dialysis therapy, probably due to increasing disease complexity and patient age (Liano 1989; Pascual 1997). No conclusive evidence exists on the use of pharmacological agents to ameliorate recovery from ARF (Pascual 1997).
Modalities of RRT include hemodialysis, peritoneal dialysis, hemofiltration and hemodiafiltration. Intermittent hemodialysis is the most commonly used modality of RRT in ARF. Continuous therapies such as hemofiltration and hemodiafiltration have important international variations.
During hemodialysis, several factors may have an impact on outcomes in ARF. These factors include dialysis modality, type and performance characteristics of the dialysis membrane, and timing of initiation and adequacy of dialysis. Except for the type of dialysis membrane and the adequacy of dialysis, the link between these dialysis related factors and clinical outcomes remain largely unexplored.
Dialysis membranes are manufactured with different materials. They can be classified as biocompatible membranes (BCM) or as bioincompatible membranes (BICM), depending on the degree of complement and leukocyte activation that occurs when they come into contact with blood. However, neither all membranes considered as BCM nor all of those considered as BICM are equally biocompatible, as they elicit different degrees of biological responses.
Some clinical studies initially reported a survival advantage among patients dialyzed with BCM compared to BICM (Hakim 1994; Himmelfarb 1998; Schiffl 1994; Schiffl 1995). However, these findings have not been confirmed by subsequent studies (Albright 2000; Assouad 1996; Gastaldello 2000; Jörres 1999Kurtal 1995). Furthermore, two published meta‐analyses comparing the impact of dialysis membranes on clinical outcomes of patients with ARF yielded conflicting results (Jaber 2002; Subramanian 2002; Teehan 2003).
As a consequence, there is no consensus regarding the preferred dialysis membrane among patients with ARF requiring dialysis.
Objectives
The aim of this meta‐analysis was to ascertain whether the use of BCM confers an advantage in either survival or recovery of renal function over the use of BICM to adult patients with ARF requiring intermittent hemodialysis.
Methods
Criteria for considering studies for this review
Types of studies
Published and unpublished reports of randomised controlled trials (RCTs) and quasi‐RCTs (studies in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) of patients with ARF (as defined in the individual studies) requiring intermittent hemodialysis, comparing biocompatible to bioincompatible dialyzer membranes.
Types of participants
The studies were restricted to hospitalized patients older than 18 years of age with ARF requiring intermittent hemodialysis and presumable hemodynamic stability, otherwise patients would have required continuous RRT. Patients were drawn from either medical or surgical services (wards and intensive care units). A subgroup analysis included kidney transplant recipients with delayed allograft function.
Types of interventions
Studies comparing cellulose‐derived membranes to synthetic membranes were retained. For the purpose of this review, all synthetic dialyzers were defined as biocompatible, and all cellulose‐derived dialyzers as bioincompatible.
Types of outcome measures
The primary outcome of this analysis was mortality within the study period. Although not uniformly reported in the individual studies, we considered recovery of renal function (defined by the discontinuation of dialysis) as a secondary outcome.
Search methods for identification of studies
We searched the medical literature for clinical studies examining the effect of dialysis membranes in humans with ARF. The following data sources were searched: Cochrane Central Register of Controlled Trials (CENTRAL, in The Cochrane Library ‐ Issue 1, 2007), MEDLINE (1966 to January 2007), EMBASE (1980 to January 2007), the Mexican Index of Latin American Biomedical Journals IMBIOMED (1990 to January 2007) and the Latin American and Caribbean Health Sciences Literature Database LILACS (1982 to January 2007).
Medical subject headings and key words used for search included: acute kidney failure, acute renal failure, ARF, biomaterial, biocompatible material, artificial membrane, hollow fiber membrane, dialysis, dialysis membrane, and combinations of these terms. (See Additional Table 1 ‐ Electronic search strategies).
1. Electronic search strategies.
| Database | Search terms |
| CENTRAL | #1 KIDNEY FAILURE ACUTE #2 (acute next kidney next failure) #3 (acute next renal next failure) #4 arf #5 (#1 or #2 or #3 or #4) #6 BIOCOMPATIBLE MATERIALS #7 ((biocompatib* or bioincompatib*) and membrane*) #8 MEMBRANES ARTIFICIAL #9 biomaterial #10 (dialysis next membrane) #11 (#6 or #7 or #8 or #9 or #10) #12 (#5 and #11) |
| MEDLINE | 1) exp Kidney Failure Acute/ 2) acute kidney failure.tw. 3) acute renal failure.tw. 4) ARF.tw. 5) or/1‐4 6) Biocompatible Materials/ 7) ((biocompatib$ or bioincompatib$) and membrane$).tw. 8) Membranes Artificial/ 9) or/6‐8 (31354) 10) 5 and 9 (187) |
| EMBASE | 1) Acute Kidney Failure/ 2) acute kidney failure.tw. 3) acute renal failure.tw. 4) ARF.tw. 5) or/1‐4 6) Biomaterial/ 7) artificial membrane/ or hollow fiber membrane/ 8) ((biocompatib$ or bioincompatib$) and membrane$).tw. 9) dialysis membrane/ 10) or/6‐9 11) 5 and 10 |
We also manually searched references cited in review articles and published studies, as well as abstracts published in proceedings of meetings related to nephrology, dialysis and artificial organs.
To minimize the effect of publication bias, both full‐length published articles as well as abstracts were included in the review. If the same group of authors published more than one paper of the same study, data from the most inclusive report were retrieved to avoid duplication bias. Location and language biases were minimized by including non‐English language and Latin American databases in our searches. Finally, data from an unpublished study was also included in some of the analyses.
Data collection and analysis
Two authors (AA and BLJ) extracted the data independently, and differences were resolved by consensus.
Cellulose‐derived dialysis membranes with high complement‐inducing potential were classified as BICM. These dialyzers are made of unsubstituted cellulose (cuprophan, CU) and substituted cellulose (cellulose acetate, CA) material. Although CA has a lower complement‐activating potential compared with CU (Pascual 1997), this membrane was classified as bioincompatible, because complement levels during dialysis with this membrane are higher than with synthetic membranes. Dialysis membranes manufactured with synthetic polymers including polysulfone (PS), polyamide (PA), polyacrylonitrile (PAN), and polymethylmethacrylate (PMMA), were classified as BCM due to minimal complement activation.
The flux intervention was defined by the porosity of the dialysis membrane: the flux was classified as low if the manufacturer's mean β2‐microglobulin clearance was less than 10 mL/min and as high if the ultrafiltration coefficient was more than 14 mL/h/mm Hg and the mean β2‐microglobulin clearance was more than 20 mL/min.
The following data was retrieved:
-
Membrane composition
All‐cause mortality
Recovery of renal function
-
Flux property
All‐cause mortality
Recovery of renal function
-
Transplant recipients
All‐cause mortality
Recovery of renal function
-
Sensitivity analyses
All‐cause mortality
Recovery of renal function
Flux property ‐ mortality and recovery of renal function
-
Analysis of covariates at enrollment
Age
Acute Physiology and Chronic Health Evaluation II (APACHE II) score
Presence of oliguria (defined as < 400 mL/d)
(d) Presence of sepsis
The primary outcome was death within the study period, by group of membrane composition. This outcome was summarized for each treatment group in each individual study. We retrieved the total number of patients in each selected study. The proportion of patients who died was calculated in each dialyzer group. Analyses were performed to obtain the pooled risk ratio (RR) with the use of a random‐effects model. Studies were sorted by dialyzer type for subgroup analyses.
We have also attempted to assess the recovery of renal function. This secondary outcome was not uniformly reported in the individual studies, and was either not evaluated, or was defined as the duration of dialysis (in days), or as the number of dialysis sessions performed before the need for dialysis support ceased. For the purpose of this meta‐analysis, recovery of renal function was defined by discontinuation of dialysis during the study period.
The analysis on flux property was performed on studies that compared low‐flux with high‐flux dialyzers. For studies inclusive of kidney transplant recipients with delayed allograft function we assessed both mortality and recovery of renal function.
A sensitivity analysis was performed to assess all cause mortality and recovery of renal function including all the studies. In separate sensitivity analyses, we excluded a study that has not been published in neither full‐text nor abstract form, and included and excluded the studies of transplant recipients. Two other sensitivity analyses were performed by including a study comparing biocompatible membranes with different flux properties. Finally, the covariates described above were extracted and tabulated.
We subdivided outcomes into CU and CA for the analyses in the BICM group, to assess if those would have any difference.
We also performed sensitivity analyses using a fixed‐effect model and the same combinations and pooling of data described above.
The data are presented as risk ratio (RR) with 95% confidence intervals (CI).
Heterogeneity was analyzed using a Chi² test on N‐1 degrees of freedom, with an alpha of 0.1 used for statistical significance and with the I² test (Higgins 2003).
Results
Description of studies
We critically appraised 55 articles that resulted from the search strategy. Twenty five reports (23 studies) were excluded for the following reasons: three prospective studies were uncontrolled (Mehta 1996; Neveu 1996; Splendiani 1996); three were retrospective (Constentino 1994; Gasparovic 1998; Van Loo 1998); three studies compared different types of BCM only (Davenport 1993; Jones 1998; Kumar 2004); four were letters that did not provide relevant data for analysis (Brivet 1996; Shaldon 1996a; Shaldon 1996b; Shaldon 1997); two were review articles (Modi 2001; Quan 2005) six studies evaluated the impact of the dialyzers on cellular responses (Bonomini 1997; Cendoroglo 1997; Haase 2002; Jaber 2000; Morgera 2003a; Morgera 2003b); one included patients with end‐stage kidney disease (Gueler 2003); and one studied an animal model (Conger 1990).
Of the remaining 30 articles, 14 were preliminary reports, letters or duplicated reports of nine of the studies (Albright 2000; Gastaldello 2000; Himmelfarb 1998; Jörres 1999; Kes 2003; Romao (Tx) 1999; Schiffl 1994; Valeri (Tx) 1996; Woo (Tx) 2002). We excluded these 14 preliminary reports and kept the most current articles, leaving a total of 16 studies out of the 30. The characteristics of the excluded studies are described in the table ‐ Characteristics of excluded studies.
Of these 16 studies, four were conducted in kidney transplant recipients who had delayed allograft function (Michael (Tx) 1995; Romao (Tx) 1999; Valeri (Tx) 1996; Woo (Tx) 2002). These studies included a more homogeneous patient population. Therefore, a subgroup analysis comparing the outcome of these kidney transplant recipients was performed, as well as a sensitivity analysis inclusive of all the studies that qualified for analysis.
Of note, Schiffl published two articles involving overlapping patient populations. The first report (Schiffl 1994) had a total of 52 patients and was inclusive of data on recovery of renal function. The second report (Schiffl 1995) included 76 patients and had more comprehensive data on mortality. Despite a questioned validity of the data of the second report (Shaldon 1997), we used the first report in the analyses of recovery of renal function and the second report for the analyses of mortality. Hence, 10 studies described in a total of 11 articles were left for the primary analyses.
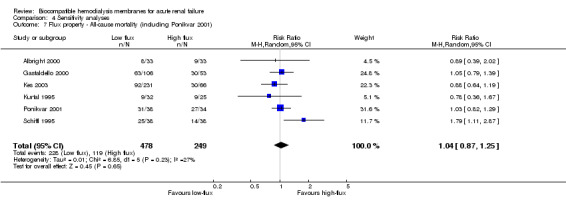
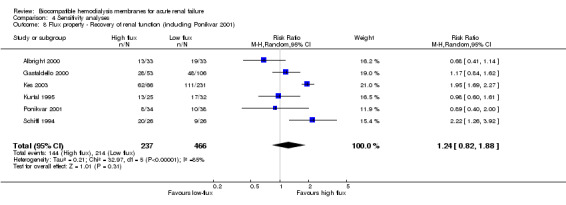
Finally, one study comparing low‐flux to high‐flux BCMs (Ponikvar 2001) was included in sensitivity analyses to further explore the influence of the flux property of the dialyzer membrane on clinical outcomes.
Studies included in primary analyses
The characteristics of the included studies are described in the table ‐ Characteristics of included studies.
The studies included in the primary analyses used a randomized or quasi‐randomized, controlled study design. Two studies were multicenter studies (Himmelfarb 1998; Jörres 1999). Seven studies used appropriate randomized allocation (Assouad 1996; Balasubramaniam 1998; Gastaldello 2000; Jaber 2004; Jörres 1999; Kes 2003; Schiffl 1994; Schiffl 1995), and two studies assigned patients to the treatment or control group by alternating order (Himmelfarb 1998; Kurtal 1995). One study initially randomized patients appropriately, but subsequently allocated dialyzers using an alternating order (Albright 2000). Only one study attempted to minimize intentional and unintentional biases by the creation of a separate randomization facility for the dialyzer allocation procedure (Jörres 1999). None of the studies were blinded, therefore, despite the elimination of the influence of confounding variables that were present at the time of randomization, all studies were confounded by variables that developed during the follow‐up period.
Most studies included both medical and surgical adult patients with ARF. Uniformly reported exclusion criteria for patients included acute glomerulonephritis, previous hemodialysis, need for continuous RRT, or pre‐existing chronic kidney disease (CKD). The definition of pre‐existing CKD was heterogeneous and was reported in only four of the studies: serum creatinine ≥ 2.5 mg/dL (Gastaldello 2000), serum creatinine ≥ 3 mg/dL (Jörres 1999; Albright 2000) or estimated creatinine clearance < 40 mL/min (Himmelfarb 1998). The study by Schiffl et al (Schiffl 1994; Schiffl 1995) excluded patients with sepsis at baseline. There was no available data on sepsis at study entry in three studies (Assouad 1996; Jaber 2004; Kes 2003). Similarly, there was no available data on oliguria at the study entry in four studies (Assouad 1996; Jaber 2004; Kes 2003; Kurtal 1995). Finally, one study included only patients with diarrhea‐induced ARF (Balasubramaniam 1998).
Five studies compared the use of a BCM versus a CA‐BICM (Albright 2000; Assouad 1996; Gastaldello 2000; Jaber 2004; Kes 2003). The remaining five studies compared BCM to CU membranes (Balasubramaniam 1998; Himmelfarb 1998; Jörres 1999; Kurtal 1995; Schiffl 1995). One study (Himmelfarb 1998) used two types of BCM dialyzers (PMMA and PS) compared to CU‐BICM. One study failed to indicate the type of BCM dialyzers that were used (Kes 2003).
The studies differed in the flux properties of the dialyzers. While three studies evaluated a high‐flux BCM with a low‐flux BICM (Albright 2000; Kurtal 1995; Schiffl 1994; Schiffl 1995), two studies compared a low‐flux BCM with a low‐flux BICM (Jaber 2004; Jörres 1999). Two studies failed to provide data on the flux property of the BCM dialyzer (Assouad 1996; Balasubramaniam 1998). In one study, there were three groups of patients randomized to a low‐flux BICM, a low‐flux BCM and a high‐flux BCM (Gastaldello 2000). Other study had four groups, two groups of either flux property of both BCM and BICM (Kes 2003). For the purpose of this meta‐analysis, the BCM groups were combined for comparison with the BICM groups for mortality and recovery of renal function by membrane composition, and the low and high‐flux groups were combined for comparison of the outcomes by flux property of the dialyzer. Therefore, for the purpose of flux property analyses, only high‐flux groups compared with low‐flux groups were pooled, leaving only five studies available for this particular type of analyses (Albright 2000; Gastaldello 2000; Kes 2003; Kurtal 1995; Schiffl 1995), whereas 10 studies were available for analyses of mortality and recovery of renal function.
In the studies included in the primary analyses, the mean age of patients ranged from 57 to 70 years. The mean follow up ranged from 21 to 90 days. The mean APACHE II score ranged from 21 to 28. Differences of these variables were not statistically significant between the two groups.
Analyses of studies inclusive of kidney transplant recipients
We analyzed the four studies inclusive of kidney transplant recipients with delayed allograft function (Michael (Tx) 1995; Romao (Tx) 1999; Valeri (Tx) 1996; Woo (Tx) 2002). All four studies were randomized appropriately. They excluded patients with acute rejection and primary non‐function. Two used a PS‐BCM dialyzer (Romao (Tx) 1999; Woo (Tx) 2002) and two used a PMMA‐BCM (Michael (Tx) 1995; Valeri (Tx) 1996). All four studies used a CU dialyzer in the BICM group. For the assessment of mortality we used data from the intention‐to‐treat analysis of one study (Valeri (Tx) 1996). The other studies did not report deaths, and were therefore excluded from the pooled analysis of mortality, as there was no estimable data available.
For the analysis of recovery of renal function, we used data from the intention‐to‐treat analysis of Valeri (Tx) 1996 and data from the initial randomization of the study by Woo (Tx) 2002. We considered patients with rejection from the study of Romao (Tx) 1999, as patients who failed to recover their renal function and included them in the pooled analysis.
Follow up ranged from 21 to 30 days. One study had a long‐term patient and allograft survival re‐assessment at two years after transplantation (Valeri (Tx) 1996). Mean age in these studies ranged from 35 to 50 years. Of interest, age was significantly different between the BCM and the BICM groups of Romao (Tx) 1999. No other baseline characteristics were different in the three studies.
Sensitivity analyses
We decided to include kidney transplant recipients in the sensitivity analyses of both all‐cause mortality and recovery of renal function.
One study has not been published in either full‐text or abstract form (Jaber 2004). We therefore decided to perform sensitivity analyses (1) excluding this study and, (2) excluding this study but including the studies of kidney transplant recipients.
The study by Jaber 2004 and the studies of kidney transplant recipients (Michael (Tx) 1995; Romao (Tx) 1999; Valeri (Tx) 1996; Woo (Tx) 2002) compared low‐flux BCM with low‐flux BICM. Therefore, none of these five studies were included in the flux modality analyses. Sensitivity analyses of flux assessment including or removing these studies were not necessary.
Finally, sensitivity analyses of mortality and recovery of renal function were performed including a study comparing a low‐flux PS‐BCM with a high‐flux PAN‐BCM (Ponikvar 2001).
For the sensitivity analyses using the fixed‐effect model, we used the same studies and combinations and pooling of data described in the methods section.
Analyses of covariates
We attempted to evaluate the following covariates that may have influenced the results of individual studies: age at study entry, APACHE II score, number of patients with oliguric ARF (defined as urine output of less than 400 mL/d), and sepsis at time of randomization. These variables have been described above. However, we found inconsistent reporting of these covariates in independent studies, and analyses with these covariates were not possible.
Four studies did not provide data on mean age of patients (Assouad 1996; Balasubramaniam 1998; Kes 2003; Michael (Tx) 1995). One study did not provide mean age, but provided ranges (Albright 2000); the median age in this study was in the range between 60 and 70 years.
Eight studies did not provide APACHE II scores (Assouad 1996; Balasubramaniam 1998; Jaber 2004; Kes 2003; Michael (Tx) 1995; Romao (Tx) 1999; Valeri (Tx) 1996; Woo (Tx) 2002). Of note, none of the four studies in transplant recipients evaluated APACHE II scores.
Three studies did not evaluate oliguria at study entry (Assouad 1996; Jaber 2004; Kes 2003; Kurtal 1995), and one implied oliguria as an inclusion criteria, but failed to provide information on how it was defined (Balasubramaniam 1998). Schiffl 1994 included data on oliguria, but data was not available from the second report of that study (Schiffl 1995).
Three studies failed to provide data on sepsis at time of randomization (Assouad 1996; Jaber 2004; Kes 2003). None of the transplant recipients had sepsis at time of transplantation or initiation of dialysis. Two studies excluded patients with baseline sepsis (Balasubramaniam 1998; Schiffl 1994; Schiffl 1995).
The one study included in the sensitivity analyses of membrane flux (Ponikvar 2001) provided information on all of the covariates. However, these covariates were not tabulated, as this study did not compare a BCM to a BICM.
Risk of bias in included studies
All studies included in the primary analyses were RCTs or quasi‐RCTs. For studies included in the primary analyses, randomized allocation was appropriate in seven studies (Assouad 1996; Balasubramaniam 1998; Gastaldello 2000; Jaber 2004; Jörres 1999; Kes 2003; Schiffl 1994; Schiffl 1995), whereas in the remaining three studies, patients were assigned to the treatment or control group by alternating order (Himmelfarb 1998; Kurtal 1995), or through partial randomization and alternating order (Albright 2000). It is remarkable to mention that not a single study was blinded. The four studies inclusive of kidney transplant recipients with delayed allograft function had appropriate randomization (Michael (Tx) 1995; Romao (Tx) 1999; Valeri (Tx) 1996; Woo (Tx) 2002). The unpublished study had a randomized controlled design (Jaber 2004). We did not use a scoring system to evaluate the methodological quality of the individual studies. Further information regarding individual studies can be found in the table ‐ Characteristics of included studies.
Effects of interventions
Analyses of dialysis membrane composition
There were ten studies included in the primary analysis of mortality (Analysis 1.1), with a total of 1100 patients. There were 575 patients in the BCM group and 525 patients in the BICM group. The number of deaths was 241 and 228, respectively. The pooled RR for mortality was 0.93 (95% CI 0.81 to 1.07), a trend in favor of BCM. Only one study had a significant RR favoring the use of a BCM (Schiffl 1995). The test for heterogeneity among studies was not significant (Chi² = 8.31, P = 0.50; I² = 0%).
1.1. Analysis.
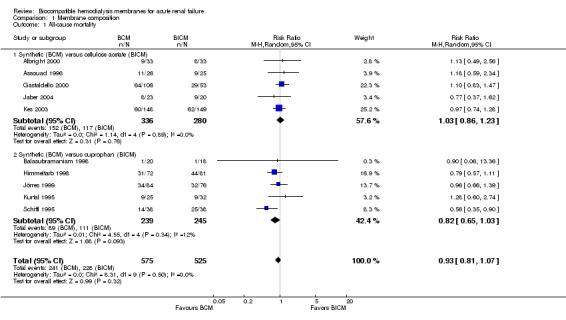
Comparison 1 Membrane composition, Outcome 1 All‐cause mortality.
In subgroup analyses, the RR for mortality of the BCM versus CA dialyzer was 1.03 (95% CI 0.86 to 1.23). The RR for mortality of the BCM versus CU was 0.82 (95% CI 0.65 to 1.03).
A total of nine studies with 1038 patients were available for analysis of recovery of renal function Analysis 1.2). There were 543 patients in the BCM group and 495 patients in the BICM group, with 299 and 254 patients in whom dialysis was discontinued, respectively. The overall RR for recovery of renal function was 1.09 (95% CI 0.90 to 1.31). Of note, only two original studies demonstrated a significant difference in favor of the use of BCM (Himmelfarb 1998; Schiffl 1995). In subgroup analyses, the RR for recovery of renal function was 0.97 (95% CI 0.83 to 1.14), for the BCM group when compared to the CA group, and 1.30 (95% CI 0.94 to 1.79) for the BCM group when compared with the CU group.
1.2. Analysis.
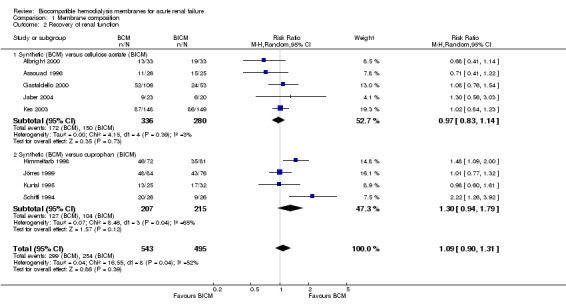
Comparison 1 Membrane composition, Outcome 2 Recovery of renal function.
Analyses of dialysis membrane flux properties
Only five studies with a total of 655 patients qualified for analysis of mortality by dialyzer flux property (Analysis 2.1). The pooled RR was 1.05 (95% CI 0.81 to 1.37). Schiffl 1995 appeared to be an outlier, as it was the only study that reached a significant difference in favor of high‐flux membranes.
2.1. Analysis.
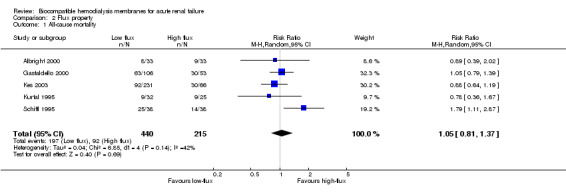
Comparison 2 Flux property, Outcome 1 All‐cause mortality.
A total of 631 patients from five studies were assessed for recovery of renal function (Analysis 2.2). The RR for recovery of renal function by flux property was 1.30 (95% CI 0.83 to 2.02), a tendency towards a better outcome in favor of high‐flux membranes.
2.2. Analysis.
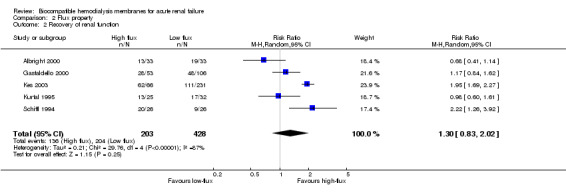
Comparison 2 Flux property, Outcome 2 Recovery of renal function.
Analyses of dialysis membrane composition in kidney transplant recipients
One study did not report mortality data (Michael (Tx) 1995) and two studies of kidney transplant recipients had no analyzable data on mortality (Romao (Tx) 1999; Woo (Tx) 2002). Therefore, a pooled analysis of mortality was not possible. Only one study reported deaths (Analysis 3.1) with a non‐significant RR of 0.22 (95% CI 0.03 to 1.79) in the direction of favoring BCM. On the other hand, all four studies, with a total of 163 patients were available for pooled analysis of recovery of renal function (Analysis 3.2). The RR was 1.05 (95% CI 0.87 to 1.26).
3.1. Analysis.
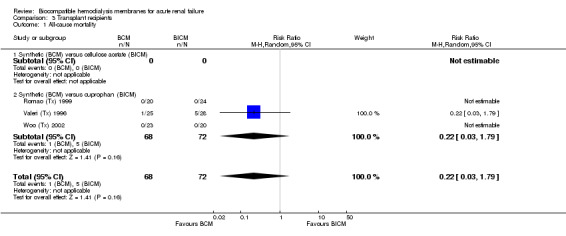
Comparison 3 Transplant recipients, Outcome 1 All‐cause mortality.
3.2. Analysis.
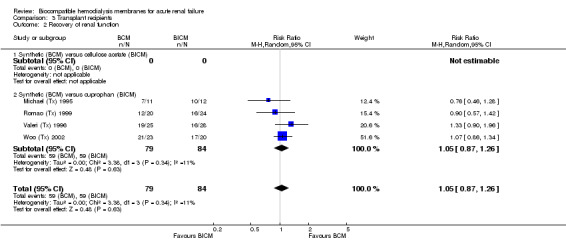
Comparison 3 Transplant recipients, Outcome 2 Recovery of renal function.
Sensitivity analyses
A sensitivity analysis for mortality (Analysis 4.1) including data from the 13 estimable studies, with a total of 1240 patients, showed a RR of 0.93 (95% CI 0.81 to 1.06).
4.1. Analysis.
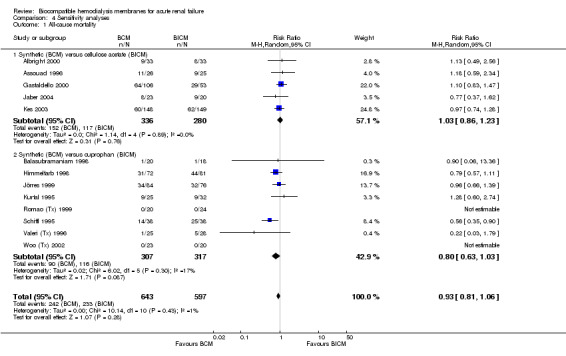
Comparison 4 Sensitivity analyses, Outcome 1 All‐cause mortality.
We evaluated 1201 patients of the 13 studies available for analysis of recovery of renal function (Analysis 4.2). The RR was 1.07 (95% CI 0.95 to 1.38).
4.2. Analysis.
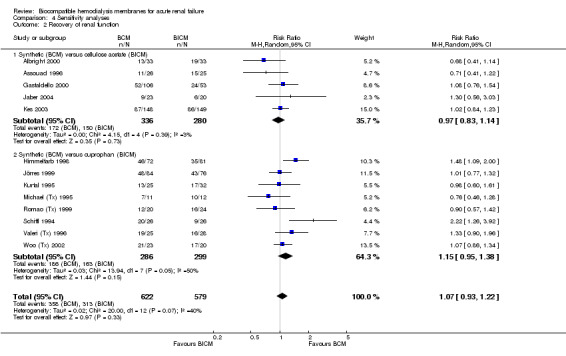
Comparison 4 Sensitivity analyses, Outcome 2 Recovery of renal function.
Further analyses excluding Jaber 2004, with or without including studies of kidney transplant recipients did not differ significantly from the former analyses of mortality and recovery of renal function.
The sensitivity analyses of flux property with the inclusion of the study comparing two BCMs (Ponikvar 2001) did not reveal differences. The RR for mortality was 1.04 (95% CI 0.87 to 1.25) and the RR for recovery of renal function was 1.24 (95% CI 0.82 to 1.88).
We also included analyses of the same groups of data using a fixed‐effect model. There was no difference in the analyses of all‐cause mortality by either membrane composition or flux property. Interestingly, for the outcome of recovery of renal function by membrane composition, in the subgroup of studies comparing a synthetic (BCM) versus CU (BICM), there was a statistically significant advantage favoring BCM membranes over CU membranes, with a RR of 1.26 (95% CI 1.06 to 1.51). The pooled analysis including the CA studies was not significant, however. Similarly, there appeared to be an advantage of high‐flux membranes over low‐flux membranes for the outcome of recovery of renal function, with a RR of 1.46 (95% CI 1.28 to 1.67). When we incorporated the studies of kidney transplant recipients, the CU versus synthetic subgroup became statistically significant for the outcome of all‐cause mortality, favoring BCM, with a RR of 0.80 (95% CI 0.65 to 0.99). Regardless, the pooled analysis inclusive of the CA studies remained without statistical significance. Those differences were also seen for recovery of renal function when we included kidney transplant recipients, with a RR of 1.19 (95% CI 1.04 to 1.35), favoring BCM over CU membranes. Again, when all the studies were included, there was no statistically significant difference between BCM and BICM. No difference was seen in the analyses using a fixed‐effect model with the inclusion or the exclusion of Jaber 2004.
Analyses of covariates
We extracted data on age, APACHE II score, oliguria and sepsis at entry. We could not use any of these covariates for regression analyses because of insufficient data and lack of variation in the aggregate mean levels.
Discussion
During hemodialysis, exposure of blood to the dialysis membrane can elicit biological responses of circulating cells and plasma components. The magnitude of leukocyte activation has been used as an index of biocompatibility (Pereira 1999). These blood‐membrane interactions have been linked to adverse clinical outcomes (Bloembergen 1999; Hakim 1996). Unlike patients with end‐stage renal disease (ESRD), in whom these interactions have been primarily studied (Pereira 1999), patients with ARF who require dialysis are often critically ill and have sepsis (Breen 1998). Consequently, the biological responses to membrane biocompatibility among patients with ARF are more difficult to decipher, compared to patients with ESRD.
In 1994, Hakim 1994 and Schiffl 1994 published the initial results of two clinical studies examining the effect of BCM on clinical outcomes among patients with ARF. Both studies observed a significant recovery of renal function and a trend towards superior survival among patients dialyzed with BCM compared to BICM. The publication of these studies was followed by a major shift toward the use of synthetic membranes for patients with dialysis‐requiring ARF. However, over the past decade, the benefit of synthetic membranes has not been validated in subsequent studies.
The contradictory conclusions drawn from the individual studies examined in this meta‐analysis might result from an insufficient sample size, lack of standardization of protocols, and absence of blinding. Possible sources of variability include the definition of BCM and BICM, age, co‐morbid conditions, severity of acute illness, etiology of ARF, presence of oliguria, dialysis membrane characteristics (e.g. surface area, efficiency and flux property), timing of dialysis initiation, intensity and adequacy of dialysis, follow‐up duration, sample size and allocation concealment.
In addition, the purity of the dialysate might be another potential confounding factor influencing the lack of difference between the different dialyzer membranes. Studies in vitro demonstrate that the composition and flux property of the membrane can affect the permeability to bacterial endotoxin and other cytokine‐inducing bacterial substances when a standard dialysate is used. This is particularly true with high‐flux BCMs. By contrast, low‐flux BICMs are less permeable to endotoxin and might be associated with less activation of pro‐inflammatory cytokines. In vivo studies suggest that higher dialysate purity is required if dialyzer membranes are more permeable to endotoxin. Nevertheless, while reduced targets for bacterial and endotoxin contamination improve the quality of dialysis, large randomized clinical studies are still needed to determine the true value of ultrapure dialysate and its impact on clinical outcomes (Bommer 2006). Meta‐analyses of the published literature allow similar clinical studies to be combined quantitatively, thereby, increasing the power and precision of the estimation of effect (Giatras 1997; Lau 1992). Hence, we performed a meta‐analysis to examine the effect of biocompatibility of dialysis membranes on mortality among patients with ARF.
Our meta‐analysis failed to demonstrate a mortality or recovery of renal function difference between the two types of dialysis membranes. Schiffl 1994 and Schiffl 1995 appeared to be an outlier in most analyses. In fact, this study included a large number of surgical patients that may have skewed the results. The validity of this study has been a matter of debate (Vanholder 1999). In Himmelfarb 1998, only after adjusting the analysis for APACHE II score were the original authors able to demonstrate a significantly lower mortality rate among patients dialyzed with BCM. Although such finding warrants the need for meta‐regression analyses to explore for interactions between the outcome and covariates, no such analyses were performed due to inconsistent reporting of the covariates, and insufficient variation in the reported values.
The choice of a random‐effects model versus a fixed‐effect model was based on our prior belief that heterogeneity was present. We included, however, some exploration of the data using a fixed‐effect model as part of our sensitivity analyses. The fact that there were some discrepant results, mainly in the subgroups of studies comparing CU versus BCM, further strengthens the belief that the data comes from heterogeneous populations, and that the possibility of subgroup effects cannot be excluded. The random‐effects model provides wider confidence intervals when heterogeneity is present. More future data, however might change that estimate or, if future studies of specific sub‐populations are published (e.g. comparing CU versus BCM), meta‐analyses of subgroups should be the focus. In summary, for the current meta‐analysis, adding fixed‐effect analyses and the data found with this model, enforces the idea that heterogeneity is present. As heterogeneity is present, the use of a random‐effects model is more appropriate for the main analyses of the current meta‐analysis. Given that limited current data exists, future studies would need to focus on subpopulations to confirm or refute the observations seen with the use of a fixed‐effect model.
Publication bias may affect the results of pooled analyses. We attempted to partially account for this limitation by including abstracts, because negative studies may be more likely to remain unpublished after being presented in abstract form (Krzyzanowska 2003).
There are several limitations to our meta‐analysis that are worthy of mention. First, the definition of BCM and BICM adopted in this analysis assumes that all cellulose‐derived and synthetic membranes behave similarly. Second, we included data derived from abstracts that had not undergone a peer review process, as well as an unpublished report. However, by doing so, we tried to minimize publication bias by including data derived from more studies. Nevertheless, we acknowledge that our results may have been affected by differences in the quality of studies, the inconsistent randomization process, the absence of a dialyzer blinding process, and the incomplete documentation of statistical procedures in the case of the abstracts. Finally, this meta‐analysis is limited to summary measures of unadjusted mortality rates and to the duration of follow‐up presented in each published report. Indeed, it does not allow for the use of survival analyses, which can consider the incomplete follow‐up of individual persons. Pooled analyses of individual patient data would provide the opportunity to obtain information on potential confounding factors and adjust mortality analyses for the variables that are potentially important predictors of outcome such as age, APACHE II score, presence of sepsis or co‐morbid conditions such as diabetes mellitus, pulmonary or cardiac disease.
Although two studies suggested that the use of CU ‐ considered the most bioincompatible membrane in terms of complement activation ‐ was associated with higher mortality rates when compared with BCM (Himmelfarb 1998; Schiffl 1995), the biological mechanisms behind these potential benefits have not yet been elucidated. Many investigators have questioned the clinical relevance of blood‐membrane interactions in the setting of ARF. In fact, the acuity of the illness that accompanies ARF is often associated with the activation of various pro‐ and anti‐inflammatory cascades, which may obscure the consequences of these interactions between blood components and the dialysis membrane. In an attempt to address this concern, a study comparing the effect of a PS‐BCM with a CA‐BICM on leukocyte functions in patients with ARF failed to demonstrate an impact of these dialysis membranes on several cellular responses (Jaber 2000).
The results of this meta‐analysis call into question the preferential use of BCM over BICM dialysis membranes for patients with ARF who require dialysis. However, this meta‐analysis does not exclude the hypothesis that CU may indeed be the most bioincompatible membrane, and may be associated with worse outcomes in ARF.
A national survey of practicing nephrologists in the United States demonstrated that there was a trend towards the use of BCM for patients with ARF who require dialysis (Eid 1996). In addition, these authors observed that high cost and unavailability of BCM were associated with their restricted, in‐hospital use.
In summary, this meta‐analysis did not demonstrate a survival advantage conferred by the use of BCM versus BICM dialyzers in patients with ARF who require hemodialysis. However, pooled analysis of individual patient‐level data will be required to assess sources of variability among studies and to examine fatal and non‐fatal outcomes of ARF, in particular, adjusted all‐cause mortality rates, septic deaths and recovery of renal function. In the interim, there is no evidence of a survival disadvantage associated with the use of BICM. Consequently, if the cost between BCM and BICM does not require consideration, the use of either a BCM or a CA BICM would appear appropriate.
Discussion of other meta‐analyses
Two meta‐analyses on this specific topic have previously been published with discordant results (Jaber 2002; Subramanian 2002). In the first meta‐analysis, Jaber 2002 examined seven studies, comprising two randomized controlled trials and five non‐randomized controlled trials. Unsubstituted and substituted cellulose membranes were grouped together as BICM and compared to synthetic membranes, which were categorized as BCM. A total of 722 patients were examined, after selecting the most inclusive and updated studies in order to maximize the sample size. Overall death rate was not different between BCM and BICM (45% versus 46%). Using a random‐effects model, the RR of death was not significantly lower among patients dialyzed with BCM (RR = 0.92, 95% CI 0.76 to 1.13; P = 0.44). The difference in the meta‐analysis by Jaber 2002 and the ensuing meta‐analysis by Subramanian 2002 is the addition of Neveu 1996, which markedly affected the overall result. Indeed, this publication was a prospective cohort study of patients with ARF, where dialysis modality was not limited to intermittent hemodialysis, and where dialysis membrane use reflected on the practice pattern of the participating centers. Of note, this observational study had more patients examined than any clinical study (N = 169). The inclusion of this study carried significant weight in the compiled meta‐analysis, resulting in a statistically significant overall lower relative risk of death among patients dialyzed with BCM compared with BICM (RR = 0.73, 95% CI 0.55 to 0.98; P = 0.03). Neither meta‐analysis demonstrated an overall impact of dialysis membranes on recovery of renal function.
The results of these two meta‐analyses highlight the issue of study selection in systematic reviews. The rationale for selecting RCTs in a meta‐analysis of treatments is to limit confounding factors and to insure more reliable estimates of treatment effects. RCTs provide the highest level of evidence, minimize bias, and help establish causality. Both meta‐analyses are limited by the use of quasi‐RCTs and non‐RCTs. Although they may help reveal associations, non‐RCTs are unable to overcome hidden confounders.
Authors' conclusions
Implications for practice.
There is no demonstrable advantage to the use of BCM versus BICM in patients with ARF that require intermittent hemodialysis.
Implications for research.
Current evidence does not permit a recommendation for or against the use of BCM in ARF.
Large studies and pooled analyses of individual patient‐level data will be required to asses sources of variability among studies and non‐fatal outcomes in ARF.
What's new
| Date | Event | Description |
|---|---|---|
| 13 May 2009 | Amended | Contact details updated. |
History
Protocol first published: Issue 2, 2005 Review first published: Issue 2, 2005
| Date | Event | Description |
|---|---|---|
| 27 August 2008 | Amended | Converted to new review format. |
| 10 November 2007 | New citation required and conclusions have changed | Substantive amendment |
| 31 January 2007 | Amended | A subsequent article search on January 2007 yielded 15 new articles that had not been previously identified. Two of these studies, although not more recent to the 2005 publication of this Systematic Review, met our inclusion criteria. This version incorporates these two studies in the analyses. A study that was excluded in the first version of this review, comparing low‐flux vs high‐flux biocompatible membranes, was included in sensitivity analyses of flux property. |
Acknowledgements
The authors would like to thank;
Narelle Willis, from the Cochrane Renal Group, for her valuable advice that made this review possible;
Drs Alison Macleod, Robert Richardson, Marcello Tonelli and Raymond Vanholder for their editorial advice during the preparation of this review and its update; and
the patients who participated in the original studies and the investigators who conducted these clinical studies.
Data and analyses
Comparison 1. Membrane composition.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 10 | 1100 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.81, 1.07] |
| 1.1 Synthetic (BCM) versus cellulose acetate (BICM) | 5 | 616 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.86, 1.23] |
| 1.2 Synthetic (BCM) versus cuprophan (BICM) | 5 | 484 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.65, 1.03] |
| 2 Recovery of renal function | 9 | 1038 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.90, 1.31] |
| 2.1 Synthetic (BCM) versus cellulose acetate (BICM) | 5 | 616 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.83, 1.14] |
| 2.2 Synthetic (BCM) versus cuprophan (BICM) | 4 | 422 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.94, 1.79] |
Comparison 2. Flux property.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 5 | 655 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.81, 1.37] |
| 2 Recovery of renal function | 5 | 631 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.83, 2.02] |
Comparison 3. Transplant recipients.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 3 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.03, 1.79] |
| 1.1 Synthetic (BCM) versus cellulose acetate (BICM) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Synthetic (BCM) versus cuprophan (BICM) | 3 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.03, 1.79] |
| 2 Recovery of renal function | 4 | 163 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.87, 1.26] |
| 2.1 Synthetic (BCM) versus cellulose acetate (BICM) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Synthetic (BCM) versus cuprophan (BICM) | 4 | 163 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.87, 1.26] |
Comparison 4. Sensitivity analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 13 | 1240 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.81, 1.06] |
| 1.1 Synthetic (BCM) versus cellulose acetate (BICM) | 5 | 616 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.86, 1.23] |
| 1.2 Synthetic (BCM) versus cuprophan (BICM) | 8 | 624 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.63, 1.03] |
| 2 Recovery of renal function | 13 | 1201 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.93, 1.22] |
| 2.1 Synthetic (BCM) versus cellulose acetate (BICM) | 5 | 616 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.83, 1.14] |
| 2.2 Synthetic (BCM) versus cuprophan (BICM) | 8 | 585 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.95, 1.38] |
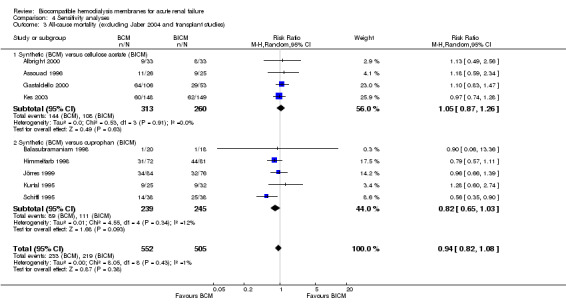
| 3 All‐cause mortality (excluding Jaber 2004 and transplant studies) | 9 | 1057 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.82, 1.08] |
| 3.1 Synthetic (BCM) versus cellulose acetate (BICM) | 4 | 573 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.87, 1.26] |
| 3.2 Synthetic (BCM) versus cuprophan (BICM) | 5 | 484 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.65, 1.03] |
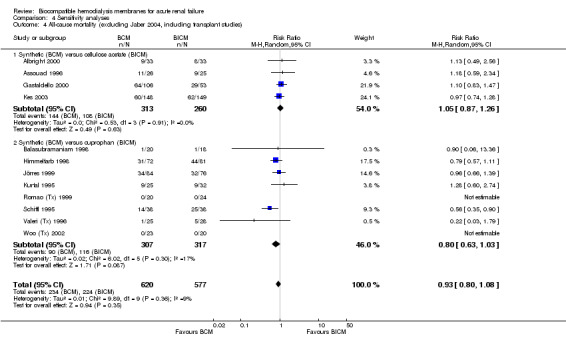
| 4 All‐cause mortality (excluding Jaber 2004, including transplant studies) | 12 | 1197 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.80, 1.08] |
| 4.1 Synthetic (BCM) versus cellulose acetate (BICM) | 4 | 573 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.87, 1.26] |
| 4.2 Synthetic (BCM) versus cuprophan (BICM) | 8 | 624 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.63, 1.03] |
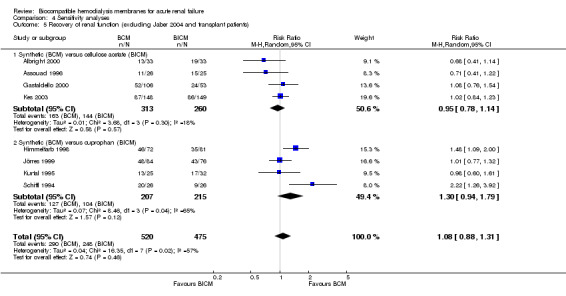
| 5 Recovery of renal function (excluding Jaber 2004 and transplant patients) | 8 | 995 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.88, 1.31] |
| 5.1 Synthetic (BCM) versus cellulose acetate (BICM) | 4 | 573 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.78, 1.14] |
| 5.2 Synthetic (BCM) versus cuprophan (BICM) | 4 | 422 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.94, 1.79] |
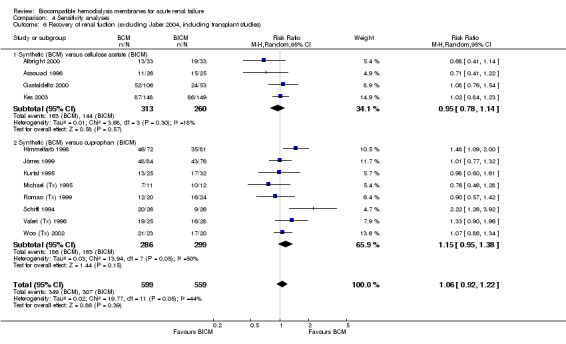
| 6 Recovery of renal fuction (excluding Jaber 2004, including transplant studies) | 12 | 1158 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.92, 1.22] |
| 6.1 Synthetic (BCM) versus cellulose acetate (BICM) | 4 | 573 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.78, 1.14] |
| 6.2 Synthetic (BCM) versus cuprophan (BICM) | 8 | 585 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.95, 1.38] |
| 7 Flux property ‐ All‐cause mortality (including Ponikvar 2001) | 6 | 727 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.87, 1.25] |
| 8 Flux property ‐ Recovery of renal function (including Ponikvar 2001) | 6 | 703 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.82, 1.88] |
4.3. Analysis.
Comparison 4 Sensitivity analyses, Outcome 3 All‐cause mortality (excluding Jaber 2004 and transplant studies).
4.4. Analysis.
Comparison 4 Sensitivity analyses, Outcome 4 All‐cause mortality (excluding Jaber 2004, including transplant studies).
4.5. Analysis.
Comparison 4 Sensitivity analyses, Outcome 5 Recovery of renal function (excluding Jaber 2004 and transplant patients).
4.6. Analysis.
Comparison 4 Sensitivity analyses, Outcome 6 Recovery of renal fuction (excluding Jaber 2004, including transplant studies).
4.7. Analysis.
Comparison 4 Sensitivity analyses, Outcome 7 Flux property ‐ All‐cause mortality (including Ponikvar 2001).
4.8. Analysis.
Comparison 4 Sensitivity analyses, Outcome 8 Flux property ‐ Recovery of renal function (including Ponikvar 2001).
Comparison 5. Covariates.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Age at entry | Other data | No numeric data | ||
| 2 APACHE II score | Other data | No numeric data | ||
| 3 Oliguria (< 400 mL/d) | Other data | No numeric data | ||
| 4 Sespis at entry | Other data | No numeric data |
5.1. Analysis.
Comparison 5 Covariates, Outcome 1 Age at entry.
| Age at entry | ||
|---|---|---|
| Study | BCM | BICM |
| Albright 2000 | 60‐70 | 60‐70 |
| Assouad 1996 | ND | ND |
| Balasubramaniam 1998 | ND | ND |
| Gastaldello 2000 | 60 | 60 |
| Himmelfarb 1998 | 57 | 58 |
| Jaber 2004 | 63 | 58 |
| Jörres 1999 | 62 | 56 |
| Kes 2003 | ND | ND |
| Kurtal 1995 | 65 | 64 |
| Michael (Tx) 1995 | ND | ND |
| Romao (Tx) 1999 | 35 | 45 |
| Schiffl 1994 | 64 | 65 |
| Schiffl 1995 | 60 | 63 |
| Valeri (Tx) 1996 | 42 | 43 |
| Woo (Tx) 2002 | 39 | 50 |
5.2. Analysis.
Comparison 5 Covariates, Outcome 2 APACHE II score.
| APACHE II score | ||
|---|---|---|
| Study | BCM | BICM |
| Albright 2000 | median 17‐25 | median 17‐25 |
| Assouad 1996 | ND | ND |
| Balasubramaniam 1998 | ND | ND |
| Gastaldello 2000 | 24 | 23 |
| Himmelfarb 1998 | 28 | 26 |
| Jaber 2004 | ND | ND |
| Jörres 1999 | 24 | 23 |
| Kes 2003 | ND | ND |
| Kurtal 1995 | 21 | 23 |
| Michael (Tx) 1995 | ND | ND |
| Romao (Tx) 1999 | ND | ND |
| Schiffl 1994 | 24 | 24 |
| Schiffl 1995 | 23 | 24 |
| Valeri (Tx) 1996 | ND | ND |
| Woo (Tx) 2002 | ND | ND |
5.3. Analysis.
Comparison 5 Covariates, Outcome 3 Oliguria (< 400 mL/d).
| Oliguria (< 400 mL/d) | ||
|---|---|---|
| Study | BCM | BICM |
| Albright 2000 | 24/33 | 26/33 |
| Assouad 1996 | ND | ND |
| Balasubramaniam 1998 | 20/20 | 18/18 |
| Gastaldello 2000 | 64/106 | 26/53 |
| Himmelfarb 1998 | 33/72 | 35/81 |
| Jaber 2004 | ND | ND |
| Jörres 1999 | 28/84 | 30/76 |
| Kes 2003 | ND | ND |
| Kurtal 1995 | ND | ND |
| Michael (Tx) 1995 | ND | ND |
| Romao (Tx) 1999 | 14/20 | 19/14 |
| Schiffl 1994 | 18/26 | 18/26 |
| Schiffl 1995 | ND | ND |
| Valeri (Tx) 1996 | 16/18 | 16/18 |
| Woo (Tx) 2002 | ND | ND |
5.4. Analysis.
Comparison 5 Covariates, Outcome 4 Sespis at entry.
| Sespis at entry | ||
|---|---|---|
| Study | BCM | BICM |
| Albright 2000 | 6/33 | 6/33 |
| Assouad 1996 | ND | ND |
| Balasubramaniam 1998 | 0 | 0 |
| Gastaldello 2000 | 66/106 | 24/53 |
| Himmelfarb 1998 | 14/72 | 15/81 |
| Jaber 2004 | ND | ND |
| Jörres 1999 | 22/84 | 14/76 |
| Kes 2003 | ND | ND |
| Kurtal 1995 | 5/25 | 10/32 |
| Michael (Tx) 1995 | ND | ND |
| Romao (Tx) 1999 | ND | ND |
| Schiffl 1994 | 0 | 0 |
| Schiffl 1995 | 0 | 0 |
| Valeri (Tx) 1996 | ND | ND |
| Woo (Tx) 2002 | ND | ND |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Albright 2000.
| Methods | Partly randomized, partly alternating order. Country: USA Follow‐up: 30 days Intention‐to‐treat: NA | |
| Participants | Patients > 18 years requiring hemodialysis for ARF. Exclusions: CKD, CRRT. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Assouad 1996.
| Methods | Randomized Country: USA Follow‐up: NA Intention‐to‐treat: NA | |
| Participants | Patients >18 years requiring hemodialysis for ARF. | |
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Balasubramaniam 1998.
| Methods | Randomized Country: India Follow‐up: NA Intention‐to‐treat: NA | |
| Participants | Patients unknown age requiring hemodialysis for diarrhea‐induced ARF. | |
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Gastaldello 2000.
| Methods | Randomized Country: Belgium Follow‐up: 80 days Intention‐to‐treat: NA | |
| Participants | Patients > 18 years requiring hemodialysis for ARF. Exclusions: Acute GN, transplant, CKD. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Himmelfarb 1998.
| Methods | Alternating order Country: USA Follow‐up: NA Intention‐to‐treat: No Multicenter | |
| Participants | Patients > 18 years requiring hemodialysis for ARF. Exclusions: Transplant, CKD. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Jaber 2004.
| Methods | Randomized Country: USA Follow‐up: 60 days Intention‐to‐treat: Yes | |
| Participants | Patients > 18 years requiring hemodialysis for ARF. | |
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Jörres 1999.
| Methods | Randomized Country: Germany Follow‐up: 90 days Intention‐to‐treat: NA Multicenter | |
| Participants | Patients > 18 years requiring hemodialysis for ARF. Exclusions: Malignancy, immunodeficiency, transplant, CKD, previous CRRT. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Kes 2003.
| Methods | Randomized Country: Croatia Follow‐up: NA Intention‐to‐treat: NA | |
| Participants | Patients > 18 years requiring hemodialysis for ARF; medical and surgical patients. | |
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Kurtal 1995.
| Methods | Alternating order Country: Germany Follow‐up: 30 days Intention‐to‐treat: NA | |
| Participants | Patients > 18 years requiring hemodialysis for ARF. | |
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Michael (Tx) 1995.
| Methods | Randomized Country: USA Follow‐up: NA Intention‐to‐treat: NA | |
| Participants | Renal transplant recipients with ATN after transplant. | |
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Ponikvar 2001.
| Methods | Randomized Country: Slovenia Follow‐up: NA Intention‐to‐treat: NA | |
| Participants | Patients > 18 years requiring hemodialysis for ARF and failure of at least two organ systems. Exclusions: CKD |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Romao (Tx) 1999.
| Methods | Randomized Country: Brazil Follow‐up: ˜30 days Intention‐to‐treat: NA | |
| Participants | Renal transplant recipients with delayed allograft function. Exclusions: Acute rejection, vascular thrombosis, primary nonfunction. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Schiffl 1994.
| Methods | Randomized Country: Germany Follow‐up: 21 days Intention‐to‐treat: NA | |
| Participants | Patients >18 year requiring hemodialysis for ARF after cardiovascular surgery. Exclusions: CKD |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Schiffl 1995.
| Methods | Randomized Country: Germany Follow‐up: NA Intention‐to‐treat: NA | |
| Participants | Patients > 18 ears requiring hemodialysis for ARF; medical and surgical patients. Exclusions: Sepsis before ARF, acute GN |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Valeri (Tx) 1996.
| Methods | Randomized Country: USA Follow‐up: 15 days Intention‐to‐treat: Yes | |
| Participants | Renal transplant recipients with delayed allograft function. Exclusions: Acute rejection, primary nonfunction, obstruction. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Woo (Tx) 2002.
| Methods | Randomized Country: UK Follow‐up: 30 days Intention‐to‐treat: Yes | |
| Participants | Renal transplant recipients with delayed allograft function. | |
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
ARF: acute renal failure; BCM: biocompatible membrane; BICM: bioincompatible membrane; CA: Cellulose acetate; CKD: chronic kidney disease; CRRT: continuous renal replacement therapy; CU: Cuprophan; GN: glomerulonephritis; HF: high flux; LF: low flux; MC: modified cellulose; NA: data not available; PA: Polyamide; PAN: Polyacrylonitrile; PMMA: Polymethylmethacrylate; PS: Polysulfone; S: Synthetic
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bonomini 1997 | Impact of dialyzer on neutrophil activation. |
| Brivet 1996 | Did not contain relevant data for analysis. |
| Cendoroglo 1997 | Impact of dialyzer on cellular response. |
| Conger 1990 | Animal model. |
| Constentino 1994 | Retrospective study. |
| Davenport 1993 | Hemofiltration, two BCM dialyzers. |
| Gasparovic 1998 | Retrospective study. |
| Gueler 2003 | Patients with end‐stage kidney disease. |
| Haase 2002 | Hemofiltration; measurement of T‐lymphocyte function. |
| Jaber 2000 | Impact of dialyzer on cellular response. |
| Jones 1998 | Comparison between two high‐flux BCM dialyzers. |
| Kumar 2004 | Comparison between two BCM dialyzers; two methods of frequency of hemodialysis. |
| Mehta 1996 | Prospective uncontrolled design. |
| Modi 2001 | Review article. |
| Morgera 2003a | Leukocyte activation; continuous veno‐venous hemofiltration. |
| Morgera 2003b | Leukocyte activation; continuous veno‐venous hemofiltration. |
| Neveu 1996 | Prospective uncontrolled design. |
| Quan 2005 | Review article. |
| Shaldon 1996a | Did not contain relevant data for analysis. |
| Shaldon 1996b | Did not contain relevant data for analysis. |
| Shaldon 1997 | Did not contain relevant data for analysis. |
| Splendiani 1996 | Prospective uncontrolled design. |
| Van Loo 1998 | Retrospective cohort. |
Contributions of authors
Conceiving the review: BLJ, JL
Designing the review: AA, BLJ
Coordinating the review: AA, BLJ, JL
Data collection for the review: AA, BLJ
Undertaking searches: AA, Cochrane Renal Group
Screening search results: AA, BLJ
Organising retrieval of papers: AA, BLJ, Cochrane Renal Group
Screening retrieved papers against inclusion criteria: AA, BLJ, Cochrane Renal Group
Appraising quality of papers: AA, BLJ
Abstracting data from papers: AA, BLJ
Providing additional data about papers: BLJ
Obtaining and screening data on unpublished studies: BLJ
Data management for the review: AA
Entering data into RevMan: AA
Analysis of data: AA, BLJ, JL
Interpretation of data: AA, BLJ, JL
Providing a methodological perspective: JL
Providing a clinical perspective: BLJ
Writing the review: AA, BLJ, JL
Providing general advice on the review: BLJ, JL
Performing previous work that was the foundation of current study: BLJ, JL
Sources of support
Internal sources
No sources of support supplied
External sources
Agency for Healthcare Research and Quality grant R25 HS09796 (Dr. Lau), USA.
Agency for Healthcare Research and Quality grant R01 HS10064 (Dr. Lau), USA.
National Institutes of Health Grant K23 DK065102‐01 (Dr Jaber), USA.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Albright 2000 {published data only}
- Albright RC, Smelser JM, McCarthy JT, Bergstahl EJ, Larson TS. Patient survival and renal recovery in acute renal failure: comparison of cellulose acetate vs. polysulfone dialysis membranes [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):197. [CENTRAL: CN‐00400036] [Google Scholar]
- Albright RC, Smelser JM, McCarthy JT, Homburger HA, Bergstrahl EJ, Larson TS. Patient survival and renal recovery in acute renal failure: randomized comparison of cellulose acetate and polysulfone membrane dialyzers. Mayo Clinic Proceedings 2000;75(11):1141‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Assouad 1996 {published data only}
- Assouad M, Tseng S, Dunn K, Gonzalez J, Brennan S, Suki W. Biocompatibility of dialyzer membranes is important in the outcome of acute renal failure [abstract]. Journal of the American Society of Nephrology 1996;7(9):1437. [CENTRAL: CN‐00444252] [Google Scholar]
Balasubramaniam 1998 {published data only}
- Balasubramaniam J, Sampathkumar K, Balasubramaniam S, Gopal R. Role of biocompatibility of dialysis membranes on the outcome of acute renal failure (arf) ‐ a study [abstract]. XXXV Congress of the European Renal Association European Dialysis & Transplant Association; 1998 Jun 6‐9; Rimini (Italy). 1998:300. [CENTRAL: CN‐00483113]
Gastaldello 2000 {published data only}
- Gastaldello K, Melot C, Kahn RJ, Tielemans C. Cellulose diacetate does as well as polysulfone for the treatment of acute renal failure in the intensive care unit [abstract]. Journal of the American Society of Nephrology 1996;7(9):1447. [CENTRAL: CN‐00583422] [Google Scholar]
- Gastaldello K, Melot C, Kahn RJ, Vanherweghem JL, Vincent JL, Tielemans C. Comparison of cellulose diacetate and polysulfone membranes in the outcome of acute renal failure: a prospective randomized study. Nephrology Dialysis Transplantation 2000;15(2):224‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Himmelfarb 1998 {published data only}
- Hakim RM, Held PJ, Stannard DC, Wolfe RA, Post FK, Daugindas JT, et al. Effect of the dialysis membrane on mortality of chronic hemodialysis patients. Kidney International 1996;50(2):566‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hakim RM, Tolkoff‐Rubin N, Himmelfarb J, Wingard RL, Parker RA. A multicenter comparison of bioincompatible (bicm) and biocompatible (bcm) membranes in the treatment of acute renal failure (arf) [abstract]. Journal of the American Society of Nephrology 1994;5(3):394. [CENTRAL: CN‐00583872] [DOI] [PubMed] [Google Scholar]
- Hakim RM, Wingard RL, Lawrence P, Parker RA, Schulman G. Use of biocompatible membranes (bcm) improves outcome and recovery from acute renal failure (arf) [abstract]. Journal of the American Society of Nephrology 1992;3(3):367. [CENTRAL: CN‐00460887] [Google Scholar]
- Hakim RM, Wingard RL, Parker RA. Effect of the dialysis membrane in the treatment of patients with acute renal failure. New England Journal of Medicine 1994;331(20):1338‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Himmelfarb J, Tolkoff Rubin NT, Chandran P, Parker RA, Wingard RL, Hakim R. A multicenter comparison of dialysis membranes in the treatment of acute renal failure requiring dialysis. Journal of the American Society of Nephrology 1998;9(2):257‐66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Parker RA, Himmelfarb J, Tolkoff‐Rubin N, Chandran P, Wingard RL, Hakim RM. Prognosis of patients with acute renal failure requiring dialysis: Results of a multicenter study. American Journal of Kidney Diseases 1998;32(3):432‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jaber 2004 {unpublished data only}
- Jaber BL. A randomized trial comparing biocompatible versus bioincompatible dialysis membranes for acute renal failure. Personal communication 2004.
Jörres 1999 {published data only}
- Jorres A, Dobis C, Gahl G, Polenakovic B, Cakalaroski K, Rutkowski B, et al. Haemodialysis membrane biocompatibility does not impact on the mortality of patients with acute renal failure: a prospective randomized international multicenter trial [abstract]. Nephrology Dialysis Transplantation 1999;14(9):A198. [CENTRAL: CN‐00583455] [DOI] [PubMed] [Google Scholar]
- Jörres J, Dobis C, Gahl CM, Polenakovic MH, Cakalaroski K, Rutkowski B, et al. Hemodialysis membrane biocompatibility does not impact on the mortality of patients with acute renal failure: a prospective randomized international multicenter trial [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):212A‐3A. [CENTRAL: CN‐00401436] [Google Scholar]
- Jörres J, Gahl GM, Dobis C, Polenakovic MH, Cakalaroski K, Rutowski B, et al. Haemodialysis‐membrane biocompatibility and mortality of patients with dialysis‐dependent acute renal failure: a prospective randomised multicentre trial. International Multicentre Study Group. Lancet 1999;354(9187):1337‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kes 2003 {published data only}
- Kes P, Ljutic D, Basic‐Jukie N. The possible effect of the dialyzer membrane on outcome of acute renal failure patients [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):198. [CENTRAL: CN‐00446064] [Google Scholar]
- Kes P, Sefer S, Ratkovic‐Gusic I. The possible effect of the dialyzer membranes on the outcome of acute renal failure patients. International Journal of Artificial Organs 1998;21(10):618. [CENTRAL: CN‐00321996] [Google Scholar]
Kurtal 1995 {published data only}
- Kurtal H, Herrath D, Schaefer K. Is the choice of membrane important for patients with acute renal failure requiring hemodialysis?. Artifical Organs 1995;19(5):391‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Michael (Tx) 1995 {published data only}
- Michael B, Francos GC, Burke JF, Jr. The use of a biocompatible dialysis membrane (bcm) in acute tubular necrosis (atn) after cadaver renal transplant (crt) does not improve outcome [abstract]. ISN XIII International Congress of Nephrology; 1995 Jul 2‐6; Madrid (Spain). 1995:402. [CENTRAL: CN‐00509353]
Ponikvar 2001 {published data only}
- Buturovic J, Rus R, Ponikvar B, Kremzar M, Horvat I, Muzlovic T, et al. Low flux vs. high flux synthetic membrane in patients with acute renal failure and multiorgan failure treated with hemodialysis: prospective randomized study [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):128A. [CENTRAL: CN‐00400429] [Google Scholar]
- Buturovic J, Rus R, Ponikvar R, Kremzar B, Horvat M, Muzlovic I, et al. Low flux vs. high flux synthetic membrane in the patients with acute renal failure and multiorgan failure treated with hemodialysis: Prospective randomized study [abstract]. International Journal of Artificial Organs 1998;21(10):625. [CENTRAL: CN‐00322003] [Google Scholar]
- Ponikvar JB, Rus RR, Kenda RB, Bren AF, Ponikvar RR. Low‐flux versus high‐flux synthetic dialysis membrane in acute renal failure: prospective randomized study. Artificial Organs 2001;25(12):946‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Romao (Tx) 1999 {published data only}
- Romao JE Jr, Abensur H, Castro MCM, Ianhez LE, Massola VC. Effect of biocompatibility on recovery from acute renal failure after cadaveric renal transplantation [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):181A. [CENTRAL: CN‐00402402] [Google Scholar]
- Romao JE Jr, Abensur H, Castro MCM, Ianhez LE, Massola VC, Sabbaga E. Effect of dialser biocompatibility on recovery from acute renal failure after cadaver renal transplantation. Nephrology Dialysis Transplantation 1999;14(3):709‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schiffl 1994 {published data only}
- Schiffl H. Biocompatible membranes in acute renal failure. Lancet 1996;347(8995):205‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schiffl H, Lang SM, Konig A, Strasser T, Haider MC, Held E. Biocompatible membranes in acute renal failure: prospective case‐controlled study. Lancet 1994;344(8922):570‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schiffl 1995 {published data only}
- Schiffl H, Sitter T, Lang S, Konig A, Haider M, Held E. Bioincompatible membranes place patients with acute renal failure at increased risk of infection. ASAIO Journal 1995;41(3):709‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Valeri (Tx) 1996 {published data only}
- Valeri A. Biocompatible membranes in acute renal failure: a study in post‐cadaveric renal transplant acute tubular necrosis [abstract]. Journal of the American Society of Nephrology 1994;5(3):481. [CENTRAL: CN‐00550476] [Google Scholar]
- Valeri A, Radhakrishnan J, Ryan R, Powell D. Biocompatible dialysis membranes and acute renal failure: a study in post‐operative acute tubular necrosis in cadaveric renal transplant recipients. Clinical Nephrology 1996;46(6):402‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Woo (Tx) 2002 {published data only}
- Woo Y, King B, Junor B, McMillan M, Briggs J, Rodger R. Dialysis with biocompatible membranes may delay recovery of graft function following renal transplantation [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):719A. [CENTRAL: CN‐00403135] [Google Scholar]
- Woo YM, Craig A‐M, King BB, Junor BJR, McMillan MA, Briggs JD, et al. Biocompatible membranes do not promote graft recovery following cadaveric renal transplantation. Clinical Nephrology 2002;57(1):38‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bonomini 1997 {published data only}
- Bonomini M, Stuard S, Settefrati N, Santarelli P, Alertazzi A. Biocompatible and bioincompatible haemodialysis membranes: a simple division into synthetic and cellulosic membranes? [abstract]. Nephrology Dialysis Transplantation 1997;12(9):A172. [CENTRAL: CN‐00509493] [Google Scholar]
Brivet 1996 {published data only}
- Brivet F, Loirat P, Kleinknecht D, Landais P. Biocompatible dialysis membrane in acute renal failure: the best choice. French Study Group on Acute Renal Failure. Intensive Care Medicine 1996;22(8):833‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cendoroglo 1997 {published data only}
- Cendoroglo M, Jaber BL, Sundaram S, King AJ, Pereira BJG. Effect of biocompatiblilty of hemodialysis membranes on polymorphonuclear cells and peripheral blood mononuclear cells functions in acute renal failure patients: a prospective crossover study [abstract]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):229A. [CENTRAL: CN‐00444723] [Google Scholar]
Conger 1990 {published data only}
- Conger JD. Does hemodialysis delay recovery from acute renal failure?. Seminars in Dialysis 1990;3:146‐8. [Google Scholar]
Constentino 1994 {published data only}
- Constentino F, Chaff C, Piedmonte M. Risk factors influencing survival in ICU acute renal failure. Nephrology Dialysis Transplantation 1994;9 Suppl 4:179‐82. [MEDLINE: ] [PubMed] [Google Scholar]
Davenport 1993 {published data only}
- Davenport A, Davison AM, Will EJ. Membrane biocompatibility: effects on cardiovascular stability in patients on hemofiltration. Kidney International ‐ Supplement 1993;41:230‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Gasparovic 1998 {published data only}
- Gasparovic V, Dukovic K, Gasparovic H, Merkler M, Radonic R, Ivanovic D, et al. Do biocompatible membranes make a difference in the treatment of acute renal failure?. Dialysis & Transplantation 1998;27(10):621‐7. [EMBASE: 1998338596] [Google Scholar]
Gueler 2003 {published data only}
- Gueler F, Schiborr C, Gwinner W, Kirsch T, Fiebeler A, Haller H, et al. Does biocompatible dialyzer membranes attenuate systemic inflammation? [abstract no: M610]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):194‐5. [CENTRAL: CN‐00445583] [Google Scholar]
Haase 2002 {published data only}
- Haase M, Morgera S, Rocktaschel J, Bohler T, Neumayer H. Increase of t‐lymphocyte proliferation in septic patients achieved with high permeability hemofiltration [abstract]. Nephrology Dialysis Transplantation 2002;17(Suppl 1):37. [CENTRAL: CN‐00583466] [Google Scholar]
Jaber 2000 {published data only}
- Jaber BL, Cendoroglo M, Balakrishnan VS, Perianayagam MC, Karsou SA, Ruthazer R, et al. Impact of dialyzer membrane selection on cellular responses in acute renal failure: A crossover study. Kidney International 2000;57(5):2107‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jones 1998 {published data only}
- Jones CH, Goutcher E, Newstead CG, Will EJ, Dean SG, Davison AM. Hemodynamics and survival of patients with acute renal failure treated by continuous dialysis and two synthetic membranes. Artificial Organs 1998;22(8):638‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kumar 2004 {published data only}
- Kumar VA, Yeun JY, Depner TA, Don BR. Extended daily dialysis vs. continuous hemodialysis for ICU patients with acute renal failure: a two‐year single center report. International Journal of Artificial Organs 2004;27(5):371‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mehta 1996 {published data only}
- Mehta R, McDonald B, Gabbai F, Pahl M, Farkas A, Pascual M, et al. Effect of biocompatible membranes on outcomes from acute renal failure in the ICU [abstract]. Journal of the American Society of Nephrology 1996;7(Program & Abstracts):1457. [Google Scholar]
Modi 2001 {published data only}
- Modi GK, Pereira BJ, Jaber BL. Hemodialysis in acute renal failure: does the membrane matter?. Seminars in Dialysis 2001;14(5):318‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Morgera 2003a {published data only}
- Morgera S, Haase M, Rocktaschel J, Bohler T, von HC, Vargas‐Hein O, et al. High permeability haemofiltration improves peripheral blood mononuclear cell proliferation in septic patients with acute renal failure. Nephrology Dialysis Transplantation 2003;18(12):2570‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Morgera 2003b {published data only}
- Morgera S, Haase M, Rocktaschel J, Bohler T, Vargas‐Hein O, Melzer C, et al. Intermittent high‐permeability hemofiltration modulates inflammatory response in septic patients with multiorgan failure. Nephron 2003;94(3):c75‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Neveu 1996 {published data only}
- Neveu G, Kleinknecht D, Brivet F, Loirat P, Landais P. Prognostic factors in acute renal failure due to sepsis: results of a prospective multicentre study. The French Study Group on Acute Renal Failure. Nephrology Dialysis Transplantation 1996;11(2):293‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Quan 2005 {published data only}
- Quan A, Quigley R. Renal replacement therapy and acute renal failure. Current Opinion in Pediatrics 2005;17(2):205‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shaldon 1996a {published data only}
- Shaldon S. Biocompatible membranes in acute renal failure. Lancet 1996;347(8995):205‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shaldon 1996b {published data only}
- Shaldon S. Bioincompatible membranes place patients with acute renal failure at increased risk of infection. ASAIO Journal 1996;42(6):1032‐3. [MEDLINE: ] [PubMed] [Google Scholar]
Shaldon 1997 {published data only}
- Shaldon S. Biocompatible membranes in acute renal failure: prospective case‐controlled study. Nephrology Dialysis Transplantation 1997;12(1):235‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Splendiani 1996 {published data only}
- Splendiani G, Mazzarella V. Is the choice of membrane important for patients with acute renal failure requiring hemodialysis?. Artifical Organs 1996;20(3):281. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Van Loo 1998 {published data only}
- Loo AA, Vanholder RC, Bernaert PR, Vermassen FE, Vennet M, Lameire NH. Pretransplantation hemodialysis strategy influences early renal graft function. Journal of the American Society of Nephrology 1998;9(3):473‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
Bloembergen 1999
- Bloembergen WE, Hakim RM, Stannard DC, Held PJ, Wolfe RA, Agodoa LY, et al. Relationship of dialysis membrane and cause‐specific mortality. American Journal of Kidney Diseases 1999;33(1):1‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bommer 2006
- Bommer J, Jaber BL. Ultrapure dialysate: facts and myths. Seminars in Dialysis 2006;19(2):115‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brady 2004
- Brady HR, Clarkson MR, Lieberthal W. Acute renal failure. In: Brenner BM editor(s). The kidney. Seventh. Elsevier, 2004:1215. [Google Scholar]
Breen 1998
- Breen D, Bihari D. Acute renal failure as a part of multiple organ failure: the slippery slope of critical illness. Kidney International ‐ Supplement 1998;66:25‐33. [MEDLINE: ] [PubMed] [Google Scholar]
Eid 1996
- Eid J, Levy EM. Variations in usage of synthetic membranes in the treatment of ARF [abstract]. Journal of the American Society of Nephrology 1996;7(Program & Abstracts):1445A. [Google Scholar]
Giatras 1997
- Giatras I, Lau J, Levey AS. Effect of angiotensin‐converting enzyme inhibitors on the progression of nondiabetic renal disease: a meta‐analysis of randomized trials. Angiotensin‐Converting‐Enzyme Inhibition and Progressive Renal Disease Study Group. Annals of Internal Medicine 1997;127(5):337‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hakim 1994
- Hakim RM, Wingard RL, Parker RA. Effect of the dialysis membrane in the treatment of patients with acute renal failure. New England Journal of Medicine 1994;331(20):1338‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hakim 1996
- Hakim RM, Held PJ, Stannard DC, Wolfe RA, Port FK, Daugirdas JT, et al. Effect of the dialysis membrane on mortality of chronic hemodialysis patients. Kidney International 1996;50(2):566‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karsou 2000
- Karsou SA, Jaber BL, Pereira BJ. Impact of intermittent hemodialysis variables on clinical outcomes in acute renal failure. American Journal of Kidney Diseases 2000;35(5):980‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Krzyzanowska 2003
- Krzyzanowska MK, Pintilie M, Tannock IF. Factors associated with failure to publish large randomized trials presented at an oncology meeting. JAMA 2003;290(4):495‐501. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lau 1992
- Lau J, Antman EM, Jimenez‐Silva J, Kupelnick B, Mosteller F, Chalmers TC. Cumulative meta‐analysis of therapeutic trials for myocardial infarction. New England Journal of Medicine 1992;327(4):248‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Liano 1989
- Liano F, Garcia‐Martin F, Gallego A, Orte L, Teruel JL, Marcen R, et al. Easy and early prognosis in acute tubular necrosis: a forward analysis of 228 cases. Nephron 1989;51(3):307‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nolan 1998
- Nolan CR, Anderson RJ. Hospital‐acquired acute renal failure. Journal of the American Society of Nephrology 1998;9(4):710‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pascual 1997
- Pascual M, Swinford RD, Tolkoff‐Rubin N. Acute renal failure: role of dialysis membrane biocompatibility. Annual Review in Medicine 1997;48:467‐76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pereira 1999
- Pereira BJG, Cheung AK. Biocompatibility of hemodialysis membranes. In: Owen WF, Pereira BJG, Sayegh MH editor(s). Dialysis and transplantation: A companion to Brenner and Rector's the kidney. Philadelphia: W.B. Saunders Company, 1999:32‐56. [Google Scholar]
Subramanian 2002
- Subramanian S, Venkataraman R, Kellum JA. Influence of dialysis membranes on outcomes in acute renal failure: a meta‐analysis. Kidney International 2002;62(5):1819‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Teehan 2003
- Teehan GS, Liangos O, Lau J, Levey S, Pereira BJ, Jaber BL. Dialysis membrane and modality in Acute Renal Failure: Understanding discordant meta‐analyses. Seminars in Dialysis 2003;16(5):356‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thadhani 1996
- Thadhani R, Pascual M, Bonventre JV. Acute renal failure. New England Journal of Medicine 1996;334(22):1448‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vanholder 1999
- Vanholder R, Lameire N. Does biocompatibility of dialysis membranes affect recovery of renal function and survival?. Lancet 1999;354(9187):1316‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Alonso 2005
- Alonso A, Lau J, Jaber BL. Biocompatible hemodialysis membranes for acute renal failure. Cochrane Database of Systematic Reviews 2005, Issue 2. [Art. No.: CD005283. DOI: 10.1002/14651858.CD005283] [DOI] [PubMed] [Google Scholar]
Jaber 2002
- Jaber BL, Lau J, Schmid CH, Karsou SA, Levey AS, Pereira BJ. Effect of biocompatibility of hemodialysis membranes on mortality in acute renal failure: a meta‐analysis. Clinical Nephrology 2002;57(4):274‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]


