Abstract
Purpose of review
New knowledge on rotavirus infection in children and well established mouse models has renewed interest in whether rotavirus could cause biliary atresia, an idiopathic, obliterative infantile disease of bile ducts that is the primary indication for liver transplant in children.
Recent findings
Studies in the rotavirus mouse model of biliary atresia indicate that infection of biliary epithelium is an inaugural event leading to biliary inflammation and obstruction, which is preceded by systemic spread of rotavirus, which also occurs during human rotavirus enteric infections. Viral factors, including rotavirus gene 4, are important for biliary infection and biliary atresia in mice. Specific host factors related to inflammatory processes (natural killer and T cells, interferon) are also critical, and a paucity of regulatory T cells in neonates may play a key role in pathogenesis in experimental biliary atresia. Rotavirus vaccination has substantially decreased rotavirus diarrheal disease worldwide and might enable demonstration of a cause–effect relationship between rotavirus infection and biliary atresia in humans.
Summary
Rotavirus can be detected in the serum of mice and children and causes biliary atresia in neonatal mice. Approaches to re-examine whether rotavirus causes biliary atresia in children are discussed based on concepts from the mouse model of biliary atresia and rotavirus vaccination programs.
Keywords: biliary atresia, impact of vaccination, rotavirus
INTRODUCTION
Biliary atresia presents exclusively in early infancy, is characterized by a progressive fibro-inflammatory obliteration of intrahepatic and extrahepatic bile ducts in infants, and is the primary indication for liver transplant in children. Biliary atresia occurs at low frequency, affecting one in 12 000 live births in the United States [1]. In the vast majority of biliary atresia cases, infants are born apparently healthy and the cause of biliary atresia is unknown. A highly reproducible mouse model of rotavirus-induced biliary atresia that resembles biliary atresia in children demonstrates that an acute viral infection of neonates (<2 days of age) can cause biliary atresia by triggering hepatobiliary inflammation and obstruction [2,3]. On the basis of the information from the mouse model of rotavirus-induced biliary atresia, other mouse models of rotavirus-induced diarrheal disease, and the implementation of rotavirus vaccination programs, re-evaluation of whether rotavirus causes biliary atresia in children is discussed.
MOUSE MODELS OF ROTAVIRUS DISEASE PROVIDE DATA LEADING TO NEW INSIGHTS INTO ROTAVIRUS INFECTIONS IN CHILDREN
Rotavirus is well established as the leading cause of severe dehydrating gastroenteritis in children less than 5 years of age throughout the world [4]. Rotavirus was initially considered to be an exclusive mucosal pathogen that infects differentiated intestinal epithelial cells and induces acute, noninflammatory diarrheal disease in young children and animals. Several mouse models of rotavirus-induced infection and disease clearly demonstrate that clinical outcomes of infection differ depending on the age of the mouse at inoculation (Fig. 1). Virus strain and mouse strain also influence clinical outcome in all models of rotavirus-induced disease.
FIGURE 1.
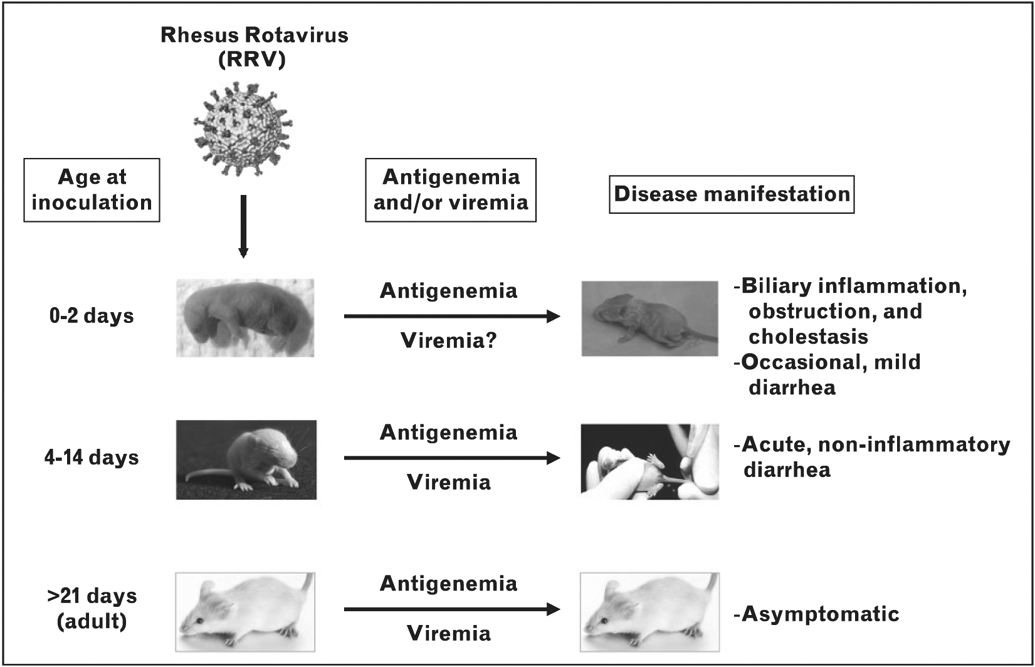
Rotavirus infection and age-dependent disease outcomes. Rotavirus spreads systemically (leading to antigenemia/viremia) in mice of all ages, but only causes disease in neonatal and suckling mice.
Oral administration of rotavirus to infant mice (4–15 days of age) induces diarrhea due to absorptive enterocyte destruction and changes in cellular calcium homeostasis and other signaling pathways that alter absorption and secretion. Rotavirus infects adult mice without observed disease; virus replicates for approximately 7 days and is detected based on fecal shedding or an antibody response [5].
Results from studies in suckling and adult mice have changed concepts of rotavirus pathogenesis. A key unexpected finding is that rotavirus infection is not limited to the intestinal mucosa but, instead, the virus escapes into the circulation where both viral antigen (antigenemia) and infectious virus (viremia) are detectable [6-14]. Subsequent confirmation of these data in other animals and in immunocompetent children ultimately resulted in a paradigm shift, with the acceptance that rotavirus routinely spreads beyond the intestine, and antigenemia frequently can be detected in a patient’s serum. Recognition that rotaviruses spread beyond the gastrointestinal mucosa is an important advancement in addressing the question of whether rotavirus might cause biliary atresia in children because a viral etiologic agent must be able to reach the biliary epithelium. If rotavirus causes biliary atresia in children, this would be the first proven clinical manifestation of extra-intestinal rotavirus spread.
VIRAL AND HOST FACTORS INFLUENCE DEVELOPMENT OF BILIARY ATRESIA IN NEONATAL MICE
Studies using the rotavirus-induced biliary atresia mouse model show that both viral and host factors are important for disease outcome.
Viral factors
First, only live virus induces biliary atresia [15■, 16] and a relatively high viral dose is required. The difference in the dose required to cause biliary atresia in 50% (BA50 dose) of 24–48-h-old animals injected intraperitoneally with purified rhesus rotavirus (RRV) is approximately 104 higher than the viral infectious dose (ID50) [15■]. The necessity for such high doses of virus remains unknown, but may reflect a need to infect a large number of cholangiocytes to trigger the immune-mediated events that lead to biliary atresia. Cholangiocytes are relatively resistant to infection; at least 100-fold more RRV is required to infect immortalized BALB/c mouse cholangiocytes than monkey kidney cells that are routinely used to cultivate rotavirus [3]. This relative resistance could be a factor in the age dependence of biliary atresia, with cholangiocytes in neonatal animals potentially being more susceptible to rotavirus infection than cells in older mice.
The route of infection and virus strain are other critical factors for the induction of biliary atresia. The initial biliary atresia mouse model showed disease in animals inoculated orally at 2 days of age with two different rotavirus strains. Hepatobiliary disease developed in approximately 42% of pups with complete bile duct obstruction present in a subset of symptomatic animals [17]. Intraperitoneal injection of virus into pups has been adopted because it more reproducibly results in biliary atresia than oral inoculation [17,18]. Although use of nonoral routes of inoculation is artificial, this simply bypasses host or viral factors that may affect extra-intestinal spread of virus and allows the consequences of rotavirus infection of the biliary tract to be studied reproducibly and at reasonable frequency.
The molecular basis of rotavirus tropism for cells of hepatobiliary origin is beginning to be defined by identifying viral virulence factors important for virus replication in the biliary epithelium [19,20■]. Only some rotavirus strains cause biliary atresia and strain-specific characteristics dictate tropism for cells of hepatobiliary origin, which in turn impacts induction of biliary atresia [17-19,20■,21-25].
The rotavirus genome consists of 11 segments of double-stranded RNA [4]. Identification of individual rotavirus genes critical for pathogenesis can be determined once two parental strains of rotavirus are characterized to have distinct biliary atresia-inducing abilities. Single-gene reassortant virus strains in which all the genes but one are derived from one parent strain are then made and tested. Testing of two independent sets of reassortants from different sets of parental rotaviruses (simian RRV and simian TUCH or RRV and bovine UK), has identified gene 4 that codes for the viral spike protein, VP4, as a primary determinant for biliary epithelial tropism [19,20■]. In-vitro studies show that VP4 governs virus attachment and infection of cholangiocytes. Analyses of gene sequence differences between RRV and TUCH or UK reveal differences that might affect binding of these viruses to sialic acid, or integrin-a2pi, which are proposed receptors for rotaviruses [26,27]. Cholangiocytes, but not hepatocyes, express α2β1 in vitro and in vivo, and cell surface expression of the integrin-α2β1 is proposed to play a role in cholangiocyte susceptibility to RRV [28].
Two additional genes associated with biliary atresia induction in mice are RRV gene 3, which may affect virus replication rates [20■]. and non-structural protein 1, which can enhance virus replication due to its ability to block type I interferon (IFN) production [19]. Studies of more sets of rotavirus reassortants from different parental viruses are required for definitive conclusions about whether other virus genes are important for biliary atresia induction. Some rotavirus strains replicate only in the periportal area within the liver, and not in the biliary epithelium, and induce minimal biliary epithelial injury and mortality [21]. Overall, these results show that the ability of a rotavirus to infect and replicate in the biliary epithelium is critical to trigger the subsequent development of biliary atresia. They also highlight that detection of virus in liver tissue might not correlate with the induction of biliary atresia, as the virus may be replicating in nonbiliary cells, which is unlikely to cause biliary atresia.
Host factors
Age of infection and mouse strain (i.e., host genetics) are both critical in determining development of biliary atresia. Biliary atresia incidence is highest in newborn BALB/c pups administered RRV within 12 h after birth, and biliary atresia incidence and lethality decrease with increasing age. Postnatal infection is also a key factor; RRV infection of pregnant dams does not cause biliary atresia in pups, although these mice have infectious virus in their livers and brains [29]. Thus, there is a critical time window for inducing biliary atresia in neonates.
New data support a role of regulatory T cells (Tregs) in experimental biliary atresia. Tregs, which represent a small percentage of the CD4+ T cells that are indispensable for the maintenance of peripheral tolerance and prevention of autoimmune disease, are not generated in the thymus before 3 days of age. A recent study has found a postnatal paucity of Tregs in the liver may allow hepatic dendritic cells to act unopposed to activate naïve natural killer (NK) cells, key effectors in experimental biliary atresia [30]. Infection with RRV at birth failed to induce prompt Treg responses, but Tregs were activated following RRV infection of mice at 7 days of age. Importantly, adoptive transfer of CD4+ cells (including Tregs) into neonatal mice injected with RRV attenuated the biliary atresia phenotype [31■■]. These studies support the attractive hypothesis that the absence of Tregs in the first 3 days of life renders the newborn biliary tract susceptible to an unrestrained pro-inflammatory response to a viral challenge.
Studies in the rotavirus-induced biliary atresia mouse model have extensively dissected the pathogenic cascade in the liver and bile ducts at the early stages of the disease that leads to inflammation and biliary atresia. Studies of infections of several knockout mouse strains as well as in mice treated with immune cell-depleting antibodies are summarized in Table 1 [3,15■,20■,22,25,28,30,32-35] and details have been reviewed elsewhere [1,36,37]. Inactivation of IFNγ and targeted depletion of lymphocyte subpopulations reveal key roles for IFNγ, CD8+ T cells, and NK cells in the initiation of neonatal bile duct obstruction [3,30,33].
Table 1.
Rotavirus-induced biliary atresia in mice with engineered immune deficitsa
| Molecule or receptor inactivated |
Genetic or antibody- mediated inactivation (and genetic background) |
Effects on disease (vs. WT/ unmanipulated mouse on same genetic background) |
Interpretation |
|---|---|---|---|
| CB-17 mouse (SCID) [25] | Gene mutation (PRKDCSCID) | Increased rates of cholestasis and mortality More frequent hepatic virus and higher hepatic viral loads; presence of viremia |
Loss of cellular and humoral immunity leads to lack of viral clearance and worsens cholestasis |
| IFNγ [3] | Gene KO (BALB/c) | Spontaneous resolution of cholestasis Lower mortality rates No effect on viral clearance from liver |
IFNγ-mediated cellular immune response is required for irreversible biliary obstruction, but not for development of cholestasis. Viral clearance is not necessary for resolution of cholestasis |
| IL-12 [35] | Gene KO (BALB/c) | No effect on cholestasis or mortality rates No effect on viral clearance from liver |
IL-12 is not required for cholestasis |
| TNFα receptor I [34] | Gene KO (BALB/c) | Decreased severity of cholestasis (lower bilirubin levels), but increased mortality Impaired viral clearance from liver |
TNFα receptor I may contribute to severity of cholestasis, but is not required. Lessens mortality in this model, possibly related to enhanced viral clearance from the liver |
| Osteopontin [15■] | Gene KO (BALB/c) | No effect on rates of cholestasis Increased mortality | Osteopontin’s Th1 cytokine (or other) activity is not required for cholestasis |
| CD4+ lymphocytes [33] | Anti-CD4 (GK1.5) antibody (BALB/c) | Mildly delayed onset of cholestasis but no effect on rates of cholestasis No effect on viral clearance from liver |
CD4+ lymphocytes may contribute to cholestasis, but are not required |
| CD8+ lymphocytes [33] | Anti-CD8 (GK2.43) antibody (BALB/c) | Spontaneous resolution of cholestasis Impaired clearance of virus from liver | CD8+ lymphocytes are required for irreversible biliary obstruction, but not for development of cholestasis. Viral clearance is not necessary for resolution of cholestasis |
| α2-Integrin subunit [28] | Anti-α2 antibody (BALB/c) | Reduced rates of cholestasis and mortality Decreased virus in extrahepatic bile ducts |
α2-Integrin subunit is important for biliary infection with RV, which is required for development of BA |
| NK cells or NK receptor [30] | Anti-asialo GM1 antibody (depletes NK cells) or anti-Nkg2d (NK receptor on cholangiocytes) (BALB/c) | Reduced rates (asialo GM1) or spontaneous resolution (Nkg2d) of cholestasis Decreased mortality Increased hepatic viral load (asialo GM1) |
NK function is required for irreversible biliary obstruction. Viral clearance is not necessary for resolution of cholestasis |
| IFNα/β receptor [22,32] | Gene KO (129SV) | Increased rates cholestasis Increased mortality vs. WT [32] or no mortality in KO or WT mice [22] |
IFNα/β protect against development of cholestasis and, in neonatal mice, decrease mortality. Not clear whether this is due to antiviral effects |
| IFNγ receptor [22,32] | Gene KO (129SV) | Same rates of cholestasis [32] or no cholestasis in either WT or KO mice [22] Increased mortality vs. WT [32] or no disease in either WT or KO mice [22] |
IFNγ receptor (examined in this study) is not required for irreversible cholestasis/biliary obstruction (as with IFNγ in study above); different mouse strain and age of inoculation could contribute to different outcomes |
| IFNα/β and IFNγ receptors [22,32] | Gene KO (129SV) | Same [32] or increased [22] rates of cholestasis Increased mortality Delayed viral clearance from liver and extrahepatic bile ducts [22] |
IFNα/β and IFNγ receptors, together, may reduce rates of cholestasis and mortality, possibly due to enhanced clearance of virus from the liver and bile ducts |
| STAT1 [22] | Gene KO (129SV) | Increased rates of cholestasis and mortality Delayed viral clearance from liver and extrahepatic bile ducts |
Loss of this downstream effector of IFNα/β and IFNγ worsens cholestasis similarly to loss of IFNα/β and IFNγ receptors and is, thus, critical for their protective effect in this model |
BA, biliary atresia; IFN, interferon; IL, interleukin; KO, knockout; NK, natural killer; PRKDC, protein kinase, DNA-activated, catalytic (protein); RV, rotavirus; SCID, severe combined immune deficiency; Th1, helper T-cell 1; TNF, tumor necrosis factor; WT, wild-type
Results in this table reflect effects on cholestasis, defined as jaundice, oily fur, and pale stools, following inoculation with rhesus rotavirus at 0–2 days of age or at 5 days [20■] of age.
Other studies provide support for an autoimmune-mediated cause for biliary atresia, which is based on identification of autoreactive cells specific to bile duct epithelia [2,33]. Adoptive transfer of hepatic T cells from biliary atresia mice into naïve immunodeficient recipients results in bile duct-specific inflammation and injury. Recent studies also have focused on the potential role of humoral autoimmunity in biliary atresia based on the observation that immunoglobulin deposits can be detected colocalizing with and surrounding bile duct epithelia [2]. Screening of serum immunoglobulin G (IgG) antibodies from mice with rotavirus-induced biliary atresia for their reactivity with proteins in a mouse cholangiocyte cell line detected α-enolase as a reactive cytosolic bile duct epithelial antigen [38■]. Further analysis showed that antirotavirus antibody and anti-enolase antibody cross-react with α-enolase. Several noncontiguous regions of sequence homology exist between RRV VP4 and enolase, suggesting that molecular mimicry might activate humoral autoimmunity and contribute to the pathogenesis of biliary atresia [38■]. This is interesting data that should be further analyzed based on the sequences of VP4s from rotaviruses that do and do not induce biliary atresia (discussed above).
NEW APPROACHES TO DETERMINE WHETHER ROTAVIRUS CAUSES BILIARY ATRESIA IN CHILDREN
Numerous studies have sought a viral cause for biliary atresia by testing liver or biliary tissues for virus or serum for antibody to viruses. Some evidence for rotavirus, reovirus, cytomegalovirus (CMV), human papillomavirus, and Epstein–Barr virus as possible etiologic agents exists, but findings have not been highly reproducible [1,37]. On the basis of clear data that rotavirus causes biliary atresia in neonatal mice that we find compelling, we hypothesize that rotaviruses cause biliary atresia in at least a subset of children. This idea does not exclude other possible viral causes of biliary atresia. Criteria for rotavirus or any viral etiologic agent for biliary atresia should include the virus being able to infect neonates, cause viremia, replicate in cholangiocytes, and trigger a host inflammatory immune response. As these criteria seem straightforward, why has it been so difficult to prove that rotavirus, or another virus, can cause biliary atresia? Several possibilities exist.
First, biliary atresia is a rare disease, so large studies are necessary and controls are essential. Additionally, if more than one virus causes biliary atresia, then smaller studies focused exclusively on identifying rotavirus (or another single virus) as a trigger for biliary atresia may not have sufficient power to demonstrate an association between that virus and biliary atresia, but testing for more than one cholangiotropic virus, such as reovirus or CMV or others, may be useful. Study design must consider that rotavirus is a common infection in children (although most acute infections are in children >2 months of age), so other factors must be important for a child to get biliary atresia. There are sufficient other examples of common enteric viruses causing disease in a subset of children such as central nervous system disease caused by poliovirus or enterovirus 71, and liver disease caused by hepatitis A.
Second, detection of virus in tissue may not be possible because liver disease is usually quite advanced when biliary atresia is diagnosed in children, usually 4–6 weeks of age. By the time diagnosis is made, the virus likely has been cleared. In addition, detection of virus in liver tissue must be viewed with caution, as it could reflect virus replicating in cells other than biliary epithelium, which would not be associated with hepatobiliary injury and induction of biliary atresia.
Third, attempts to detect an antibody response are complicated by transplacentally acquired maternal antibody so that evaluating rotavirus-specific IgG in children less than 4–6 months of age may not detect a primary infection in children. IgM has been sought but may be too transient.
Thus, it is not surprising that previous studies have failed to unequivocally show an association between rotavirus and biliary atresia. Together with all that is now known from the mouse model, however, it might be worthwhile to consider two new approaches to search for evidence of a rotavirus cause. One might consider looking for rotavirus-specific IgA in serum samples from biliary atresia cases less than 2 months of age (prior to the first rotavirus vaccine dose). IgA testing can detect primary infections in children, can persist for months, is not affected by existing maternal antibody, and has been used successfully to monitor rotavirus vaccine takes in children. Therefore, testing for rotavirus-specific IgA might be useful in screening for evidence of neonatal infection.
A second approach to consider is to look for antigenemia, or the viral genome, using meta-genomic sequencing, in early serum samples from biliary atresia cases and controls. Metagenomic sequencing is an unbiased, high-throughput approach that can detect the presence of any viral nucleic acid, so it can detect molecular footprints of more than a single virus that might be associated with biliary atresia. Metagenomic sequencing has recently discovered many new viruses, including at least three novel astroviruses, in stools of children with gastroenteritis [39,40], so it is possible that a previously unknown virus might be discovered to cause biliary atresia. A final approach that may demonstrate that rotavirus causes biliary atresia could come from postlicensure evaluations of the new rotavirus vaccines, as discussed below.
IF ROTAVIRUS CAUSES BILIARY ATRESIA IN CHILDREN, WILL ROTAVIRUS VACCINATION REDUCE BILIARY ATRESIA?
Rotavirus-induced diarrhea is now a vaccine preventable childhood disease and two effective rotavirus vaccines, a single-strain attenuated human rotavirus vaccine (Rotarix, GlaxoSmithKline Biologicals, Wavre, Belgium) and a multistrain bovine-reassortant vaccine (RotaTeq, Merck and Company, Whitehouse Station, New Jersey, USA), are now available and recommended for routine immunization of all infants by the WHO.
Possible indirect effects of rotavirus vaccination programs
Rotavirus vaccines are administered orally to infants at 2, 4, and 6 months of age, so they would not be expected to have a direct effect on preventing biliary atresia in children, as a viral infection that leads to biliary atresia likely occurs at an earlier age. However, initial assessments of rotavirus vaccination on the burden of severe childhood diarrhea demonstrate a rapid, easily measured, and substantial direct and indirect protective effect [41,42■■,43]. In addition, large declines in diarrhea hospitalizations have been seen in children less than 5 years of age who were not vaccinated or who were too young or too old to be vaccinated. Thus, the decline in disease is not solely in vaccine-eligible children, which suggests substantial indirect protection among unvaccinated populations through herd immunity.
Recognition that herd immunity develops after rotavirus vaccination is potentially important to be able to test the hypothesis that rotavirus causes biliary atresia in children. If overall levels of circulating rotavirus and virus transmission are reduced with a sustained vaccination program, one can predict that fewer neonates will become infected and, thus, the numbers of cases of biliary atresia will decrease. Epidemiologic studies that demonstrate and prove a hypothesis of rotavirus cause of a biliary atresia would be reminiscent of the landmark studies that followed implementation of at-birth hepatitis B virus (HBV) vaccination and ultimately ended many years of debate about whether HBV could cause liver cancer in humans [44]. As in the case of the mouse model of rotavirus-induced biliary atresia, several animal models supported the claim that HBV caused liver cancer, but proving this in humans was more challenging.
Possible direct effects of rotavirus vaccination
Another approach to learning whether rotavirus causes biliary atresia would be to develop and implement maternal immunization. Maternal immunization is effective in reducing influenza and tetanus in young children [45], and preconception oral vaccination of dams [46] or postconception intraperitoneal vaccination of dams [29,47] with live rotavirus vaccine or with a recombinant rotavirus VP6 vaccine results in transmission of rotavirus IgG elicited in dams to pups, reducing disease symptoms and improving survival in the neonatal rotavirus-induced biliary atresia mouse model. It is likely that such antibody reduces rotavirus viremia [48] and, therefore, rotavirus load and infection of the biliary epithelium, which would prevent biliary atresia. Maternal immunization with nonreplicating rotavirus vaccine would be preferable because rotavirus can be found in the liver and brains of fetuses born to dams administered live rotavirus [29].
Current rotavirus vaccination programs that are highly effective in industrialized countries show lower efficacy rates (39–77%) in developing countries, such as Africa and Asia [42■■]. Although the exact reasons for lower efficacy in these settings remains to be fully understood, earlier age of disease is one factor [49]. These findings have led to a suggestion for neonatal immunization. Such discussions need to carefully consider the safety of this approach using the currently available live vaccines, keeping in mind that rotavirus can cause biliary atresia in neonatal mice. At a minimum, any rotavirus vaccine should be tested in human cholangiocytes to ensure it cannot replicate in these cells before it is considered as a candidate for neonatal vaccination.
CONCLUSION
Findings in the murine model of rotavirus-induced biliary atresia provide tremendous insight into how a specific viral infection of biliary epithelium in a vulnerable host can cause progressive obliteration of bile ducts as is seen in human biliary atresia. On the basis of this model, biliary atresia can be triggered by a biliary viral infection, which is preceded by systemic spread of virus. Two critical events in disease development appear to be the initial biliary viral infection, which is then followed by an immune response targeted at biliary epithelium. Numerous factors may help explain why it has not been possible to demonstrate a role for viral infection in human biliary atresia, but new approaches in attempting to confirm such an association are promising.
KEY POINTS.
Biliary atresia is a progressive, obliterative biliary disease of otherwise healthy infants that may be triggered by a viral infection, although this has been difficult to demonstrate.
Studies in a well established mouse model of rotavirus-induced biliary atresia demonstrate that infection of biliary epithelium is necessary to trigger the immune-mediated destruction of bile ducts that follows inoculation with rotavirus. Extra-intestinal spread of rotavirus occurs in children and mice infected with rotavirus and is likely critical for biliary infection and biliary atresia.
Numerous host and viral factors are required for development of biliary atresia in the mouse model, and these include factors that mediate viral infection of biliary epithelium as well as those that mediate immune responses to infection.
New approaches for demonstrating an association between rotavirus (or another virus) and biliary atresia in humans are required. Methods including immunization programs and serum testing for immunoglobulin A or for systemic viral infection are worth consideration.
Acknowledgements
The authors thank Margaret Conner, Sue Crawford, Joseph Hyser, Sashi Ramani, and Sundararajah Thevananther (Baylor College of Medicine) for their helpful input.
Conflicts of interest
M.K.E. is an inventor on a rotavirus virus-like particle vaccine patent that is licensed to GlobalVaccines. Research in the authors’ laboratories is supported in part by Public Health Service grants RO1 AI080656 (to M.K.E.), P30 DK56338 that funds the Texas Medical Center Digestive Diseases Center, and by NASPGHAN/CDHNF Young Investigator Development Award (to P.M.H.).
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 83).
- 1.Mack CL. The pathogenesis of biliary atresia: evidence for a virus-induced autoimmune disease. Semin Liver Dis 2007; 27:233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mack CL, Tucker RM, Lu BR, et al. Cellular and humoral autoimmunity directed at bile duct epithelia in murine biliary atresia. Hepatology 2006; 44:1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shivakumar P, Campbell KM, Sabla GE, et al. Obstruction of extrahepatic bile ducts by lymphocytes is regulated by IFN-gamma in experimental biliary atresia. J Clin Invest 2004; 114:322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenberg HB, Estes MK. Rotaviruses: from pathogenesis to vaccination. Gastroenterology 2009; 136:1939–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McNeal MM, Belli J, Basu M, et al. Discovery of a new strain of murine rotavirus that is consistently shed in large quantities after oral inoculation of adult mice. Virology 2004; 320:1–11. [DOI] [PubMed] [Google Scholar]
- 6.Blutt SE, Kirkwood CD, Parreno V, et al. Rotavirus antigenaemia and viraemia: a common event? Lancet 2003; 362:1445–1449. [DOI] [PubMed] [Google Scholar]
- 7.Blutt SE, Matson DO, Crawford SE, et al. Rotavirus antigenemia in children is associated with viremia. PLoS Med 2007; 4:e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blutt SE, Conner ME. Rotavirus: to the gut and beyond! Curr Opin Gastroenterol 2007; 23:39–43. [DOI] [PubMed] [Google Scholar]
- 9.Ramani S, Paul A, Saravanabavan A, et al. Rotavirus antigenemia in Indian children with rotavirus gastroenteritis and asymptomatic infections. Clin Infect Dis 2010; 51:1284–1289. [DOI] [PubMed] [Google Scholar]
- 10.Ramig RF. Systemic rotavirus infection. Expert Rev Anti Infect Ther 2007; 5:591–612. [DOI] [PubMed] [Google Scholar]
- 11.Patel M, Rench MA, Boom JA, et al. Detection of rotavirus antigenemia in routinely obtained serum specimens to augment surveillance and vaccine effectiveness evaluations. Pediatr Infect Dis J 2010; 29:836–839. [DOI] [PubMed] [Google Scholar]
- 12.Sugata K, Taniguchi K, Yui A, et al. Analysis of rotavirus antigenemia and extraintestinal manifestations in children with rotavirus gastroenteritis. Pediatrics 2008; 122:392–397. [DOI] [PubMed] [Google Scholar]
- 13.Chitambar SD, Tatte VS, Dhongde R, et al. High frequency of rotavirus viremia in children with acute gastroenteritis: discordance of strains detected in stool and sera. J Med Virol 2008; 80:2169–2176. [DOI] [PubMed] [Google Scholar]
- 14.Fischer TK, Ashley D, Kerin T, et al. Rotavirus antigenemia in patients with acute gastroenteritis. J Infect Dis 2005; 192:913–919. [DOI] [PubMed] [Google Scholar]
- 15.■. Hertel PM, Crawford SE, Finegold MJ, et al. Osteopontin upregulation in rotavirus-induced murine biliary atresia requires replicating virus but is not necessary for development of biliary atresia. Virology 2011; 417:281–292. This study demonstrates rotavirus antigenemia in the biliary atresia mouse model and shows that osteopontin, a molecule with cytokine properties previously suggested to play a role in biliary atresia pathogenesis, is not required for disease.
- 16.Mack CL, Tucker RM, Sokol RJ, et al. Armed CD4+ Th1 effector cells and activated macrophages participate in bile duct injury in murine biliary atresia. Clin Immunol 2005; 115:200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riepenhoff-Talty M, Schaekel K, Clark HF, et al. Group A rotaviruses produce extrahepatic biliary obstruction in orally inoculated newborn mice. Pediatr Res 1993; 33 (4 Pt 1):394–399. [DOI] [PubMed] [Google Scholar]
- 18.Petersen C, Kuske M, Bruns E, et al. Progress in developing animal models for biliary atresia. Eur J Pediatr Surg 1998; 8:137–141. [DOI] [PubMed] [Google Scholar]
- 19.Feng N, Sen A, Wolf M, et al. Roles of VP4 and NSP1 in determining the distinctive replication capacities of simian rotavirus RRV and bovine rotavirus UK in the mouse biliary tract. J Virol 2011; 85:2686–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.■. Wang W, Donnelly B, Bondoc A, et al. The rhesus rotavirus gene encoding VP4 is a major determinant in the pathogenesis of biliary atresia in newborn mice. J Virol 2011; 85:9069–9077. This study utilizes reassortant rotaviruses to determine that rotavirus gene 4 is required for cholangiocyte infection and induction of biliary atresia in the mouse model.
- 21.Allen SR, Jafri M, Donnelly B, et al. Effect of rotavirus strain on the murine model of biliary atresia. J Virol 2007; 81:1671–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng N, Kim B, Fenaux M, et al. Role of interferon in homologous and heterologous rotavirus infection in the intestines and extraintestinal organs of suckling mice. J Virol 2008; 82:7578–7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riepenhoff-Talty M, Gouvea V, Evans MJ, et al. Detection of group C rotavirus in infants with extrahepatic biliary atresia. J Infect Dis 1996; 174:8–15. [DOI] [PubMed] [Google Scholar]
- 24.Qiao H, Clark HF, DiVietro M, et al. A comparison of the effects of oral inoculation with Rotashield and pentavalent reassortant rotavirus vaccine (WC3-PV) on suckling CB17scid mice. J Gen Virol 2004; 85 (Pt 8):2245–2253. [DOI] [PubMed] [Google Scholar]
- 25.Uhnoo I, Riepenhoff-Talty M, Dharakul T, et al. Extramucosal spread and development of hepatitis in immunodeficient and normal mice infected with rhesus rotavirus. J Virol 1990; 64:361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker M, Prasad BV. Rotavirus cell entry. Curr Top Microbiol Immunol 2010; 343:121–148. [DOI] [PubMed] [Google Scholar]
- 27.Fleming FE, Graham KL, Takada Y, et al. Determinants of the specificity of rotavirus interactions with the alpha2beta1 integrin. J Biol Chem 2011; 286:6165–6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jafri M, Donnelly B, Allen S, et al. Cholangiocyte expression of alpha2beta1-integrin confers susceptibility to rotavirus-induced experimental biliary atresia. Am J Physiol Gastrointest Liver Physiol 2008; 295:G16–G26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Czech-Schmidt G, Verhagen W, Szavay P, et al. Immunological gap in the infectious animal model for biliary atresia. J Surg Res 2001; 101:62–67. [DOI] [PubMed] [Google Scholar]
- 30.Shivakumar P, Sabla GE, Whitington P, et al. Neonatal NK cells target the mouse duct epithelium via Nkg2d and drive tissue-specific injury in experimental biliary atresia. J Clin Invest 2009; 119:2281–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.■■. Miethke AG, Saxena V, Shivakumar P, et al. Postnatal paucity of regulatory T cells and control of NK cell activation in experimental biliary atresia. J Hepatol 2010; 52:718–726. This study determines that adoptive transfer of CD4+ lymphocytes including Tregs leads to improved survival in the biliary atresia mouse model and hypothesizes that a relative deficiency of Tregs in neonatal mice may contribute to the neonate’s susceptibility to biliary atresia.
- 32.Kuebler JF, Czech-Schmidt G, Leonhardt J, et al. Type-I but not type-ll interferon receptor knockout mice are susceptible to biliary atresia. Pediatr Res 2006; 59:790–794. [DOI] [PubMed] [Google Scholar]
- 33.Shivakumar P, Sabla G, Mohanty S, et al. Effector role of neonatal hepatic CD8+ lymphocytes in epithelial injury and autoimmunity in experimental biliary atresia. Gastroenterology 2007; 133:268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tucker RM, Hendrickson RJ, Mukaida N, et al. Progressive biliary destruction is independent of a functional tumor necrosis factor-alpha pathway in a rhesus rotavirus-induced murine model of biliary atresia. Viral Immunol 2007; 20:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohanty SK, Shivakumar P, Sabla G, et al. Loss of interleukin-12 modifies the pro-inflammatory response but does not prevent duct obstruction in experimental biliary atresia. BMC Gastroenterol 2006; 6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bessho K, Bezerra JA. Biliary atresia: will blocking inflammation tame the disease? Annu Rev Med 2011; 62:171–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petersen C Pathogenesis and treatment opportunities for biliary atresia. Clin Liver Dis 2006; 10:73–88; vi. [DOI] [PubMed] [Google Scholar]
- 38.■. Lu BR, Brindley SM, Tucker RM, et al. Alpha-enolase autoantibodies cross-reactive to viral proteins in a mouse model of biliary atresia. Gastroenterology 2010; 139:1753–1761. This study uses the biliary atresia mouse model to identify autoantibodies to the cholangiocyte protein enolase that cross-react with rotavirus VP4 and hypothesizes a mechanism of molecular mimicry in immune-mediated biliary damage in biliary atresia.
- 39.Finkbeiner SR, Holtz LR, Jiang Y, et al. Human stool contains a previously unrecognized diversity of novel astroviruses. Virol J 2009; 6:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finkbeiner SR, Allred AF, Tarr PI, et al. Metagenomic analysis of human diarrhea: viral detection and discovery. PLoS Pathog 2008; 4:e1000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paulke-Korinek M, Kundi M, Rendi-Wagner P, et al. Herd immunity after two years of the universal mass vaccination program against rotavirus gastroenteritis in Austria. Vaccine 2011; 29:2791–2796. [DOI] [PubMed] [Google Scholar]
- 42.■■. Patel MM, Steele D, Gentsch JR, et al. Real-world impact of rotavirus vaccination. Pediatr Infect Dis J 2011; 30 (1 Suppl):S1–S5. ■■ This review summarizes the effects of rotavirus vaccination programs worldwide to date and discusses the importance of continuing evaluation of these programs.
- 43.Tate JE, Cortese MM, Payne DC, et al. Uptake, impact, and effectiveness of rotavirus vaccination in the United States: review of the first 3 years of postlicensure data. Pediatr Infect Dis J 2011; 30 (1 Suppl):S56–S60. [DOI] [PubMed] [Google Scholar]
- 44.Beasley RP. Rocks along the road to the control of HBV and HCC. Ann Epidemiol 2009; 19:231–234. [DOI] [PubMed] [Google Scholar]
- 45.Englund JA, Mbawuike IN, Hammill H, et al. Maternal immunization with influenza or tetanus toxoid vaccine for passive antibody protection in young infants. J Infect Dis 1993; 168:647–656. [DOI] [PubMed] [Google Scholar]
- 46.Turowski C, Leonhardt J, Teichmann B, et al. Preconceptional oral vaccination prevents experimental biliary atresia in newborn mice. Eur J Pediatr Surg 2010; 20:158–163. [DOI] [PubMed] [Google Scholar]
- 47.Bondoc AJ, Jafri MA, Donnelly B, et al. Prevention of the murine model of biliary atresia after live rotavirus vaccination of dams. J Pediatr Surg 2009; 44:1479–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marcelin G, Miller AD, Blutt SE, et al. Immune mediators of rotavirus antigenemia clearance in mice. J Virol 2011; 85:7937–7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gladstone BP, Ramani S, Mukhopadhya I, et al. Protective effect of natural rotavirus infection in an Indian birth cohort. N Engl J Med 2011; 365:337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]


