Abstract
The short-day plant Pharbitis nil is a model plant for the study of photoperiodic control of floral initiation. Flower formation can be induced at the cotyledon stage by a single long night of at least 14 h in duration. Using differential display of mRNA we identified a P. nil ortholog of the Arabidopsis CONSTANS (CO) gene, which will be referred to as PnCO. Expression of PnCO was high after a 14-h night, but low when the dark period was 12 h or less. Our results indicate that the level of the PnCO transcript is photoperiodically regulated. After transfer from continuous light to darkness, PnCO showed a circadian pattern of expression. Expression of the CAB gene, which is a molecular marker for the circadian clock, exhibited a different pattern of expression than did PnCO and was not subject to the same photoperiodic control. A major portion of the PnCO transcripts contained an unspliced intron. Only the intron-free PnCO was able to complement the co mutant of Arabidopsis by shortening the time to flower.
The classical studies on photoperiodic control of flowering have shown that the leaf perceives the inductive photoperiod, whereas the flower primordia are formed in the apical meristem. This observation implies the movement of a signal, the floral stimulus, or florigen from the leaf to the shoot apex. Grafting experiments between different photoperiodic response types have provided evidence that the floral stimulus is exchangeable, and thus very similar or identical in short-day plants (SDP) and long-day plants (LDP; Lang, 1965; Zeevaart, 1976). The nature of the inductive processes in the leaf, as well as the identity of florigen, have remained elusive so far. It is clear that elucidation of the flowering process is one of the major outstanding problems in plant biology.
In more recent work the facultative LDP Arabidopsis has been used as a model plant for molecular genetic studies of flowering (Koornneef et al., 1998; Levy and Dean, 1998), and several genes involved in flowering in Arabidopsis have been cloned. An example is the CONSTANS (CO) gene, which encodes a putative zinc finger transcription factor (Putterill et al., 1995). Consistent with its role in flowering, expression of CO was up-regulated by long days (LD), which in turn resulted in up-regulation of floral meristem identity genes, such as LEAFY (Putterill et al., 1995; Simon et al., 1996; Nilsson et al., 1998).
In some species flowering can be induced at an early stage. A well-characterized example is Pharbitis nil; (Imamura, 1967). This SDP can be induced to flower by a single dark period of at least 14 h just after the cotyledons have fully expanded. Physiological evidence indicates that during the inductive dark period a floral stimulus is produced in the cotyledons, which is subsequently exported to the apical meristem (Zeevaart, 1962). These physiological characteristics make P. nil an attractive model plant for research on flowering (Vince-Prue and Gressel, 1985). In several studies at the molecular level, attempts have been made to identify genes associated with flowering (Lay-Yee et al., 1987; Ono et al., 1993, 1996; Zheng et al., 1993, 1998; O'Neill et al., 1994; Sage-Ono et al., 1998). However, there is no conclusive evidence that any of these genes play a role in flower initiation.
We have used differential display of mRNA to identify genes in P. nil that are specifically expressed in response to a single inductive dark period and whose products may be related to the induction of flowering. In this paper we report the isolation from P. nil of an ortholog of CO of Arabidopsis and provide evidence for its role in floral induction.
RESULTS
Identification of a CO Ortholog by Differential Display of mRNA
Five-day-old, light-grown P. nil seedlings were exposed to three different photoperiodic conditions, namely to an inductive 14-h dark period yielding seven to eight flower buds per plant and, as controls, to 8 h of darkness, or to 14 h of darkness interrupted after 8 h by 5 min of red light. All control plants remained vegetative. Cotyledons were collected immediately after these treatments and RNA was isolated for differential display. A cDNA corresponding to a transcript whose level increased upon photoperiodic induction was cloned. The nucleotide sequence of this cDNA fragment showed similarity to that of the Arabidopsis CO gene (data not shown). Full-length clones of this putative CO ortholog were isolated from a cDNA library prepared from P. nil cotyledons.
Alignment of the amino acid sequences predicted from the P. nil cDNA and the CO cDNAs of Arabidopsis and Brassica napus shows a high level of amino acid identity in the amino- and carboxy-terminal regions (Fig. 1). The conserved amino-terminal region contains regularly spaced Cys residues indicative of a zinc-finger DNA-binding domain (Putterill et al., 1995). The conserved carboxy-terminal region is rich in Arg and Lys and may include a nuclear localization sequence (Robert et al., 1998). The cloned P. nil cDNA is henceforth referred to as PnCO.
Figure 1.
Comparison of the predicted amino acid sequences of CO proteins. PnCO, P. nil, (accession no. AF300700); AtCO, Arabidopsis CO1 (accession no. A56133); and BnCO, oilseed rape COa1, (accession no. AF016009). Regularly spaced Cys residues of a putative Zn finger are indicated with asterisks. A putative bipartite nuclear localization signal is indicated with plus signs. The site where the intron is inserted in the corresponding nucleotide sequence is marked with ▾. The annealing sites for primers TN178 (upstream) and TN179 (downstream) are indicated with solid lines above the corresponding amino acid sequences. The alignment was performed with the CLUSTAL W program (Thompson et al., 1994).
Correlation of PnCO Expression and the Flowering Response
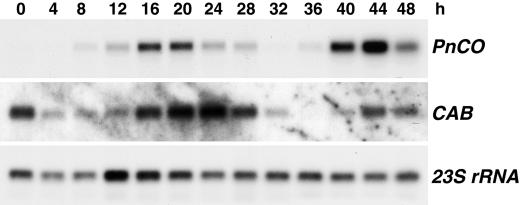
The time course of floral induction in P. nil has been established by Zeevaart (1962). After 12 h of darkness, little if any flowering was observed, whereas a dark period of 14 h elicited a full flowering response. The time course of PnCO expression in continuous darkness was studied at 4-h intervals (Fig. 2). Accumulation of PnCO mRNA increased slowly, starting at 8 h, and reached a peak between 16 and 20 h. Combining these data with those of Figure 3, one can conclude that PnCO is expressed at high levels between 14 and 20 h of darkness.
Figure 2.
Expression of PnCO in continuous darkness. Five-day-old light-grown seedlings were transferred to darkness at time 0. Cotyledons were harvested for RNA isolation at the times indicated. Poly(A)+ RNA preparations were analyzed by RNA gel blotting using a 720-bp cDNA of PnCO as probe. Residual 23S rRNA served as loading control. Plants returned to continuous light after 16 h of darkness or at later time points developed six or seven flower buds after 1 month in continuous light. Plants receiving 12 h of darkness or less remained vegetative.
Figure 3.
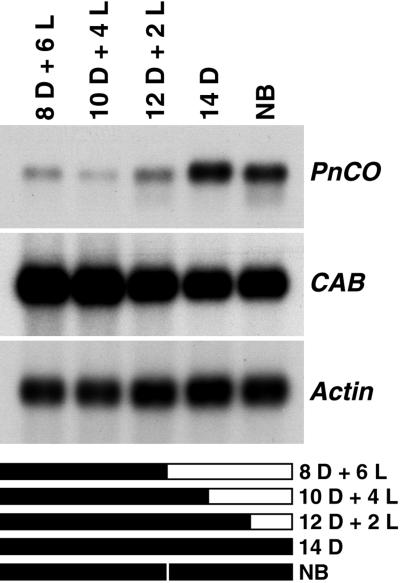
Expression of PnCO under different dark regimes. Five-day-old light-grown seedlings were transferred to darkness at time 0. Cotyledons were harvested for RNA isolation 14 h later after being exposed to the dark (D)-light (L) regime shown on the bar graphs. Poly(A)+ RNA preparations were analyzed by RNA gel blotting. The probes were as in Figure 2. Actin mRNA served as loading control. Plants kept for 14 h in the dark developed six or seven flower buds after 1 month in continuous light. Plants remained vegetative under all other photoperiodic conditions.
When transferred from continuous light to continuous darkness, the PnCO gene showed a circadian pattern of expression at the RNA level (Fig. 2). The expression pattern of the CAB gene, commonly used to monitor the circadian clock at the molecular level (Millar et al., 1992), was similar. Further experiments were designed to determine whether expression of PnCO is regulated via a mechanism shared by other clock-controlled genes such as CAB or whether expression of PnCO is controlled by photoperiod only. Light-grown P. nil seedlings were exposed to the following dark-light regimes: 8 h of darkness followed by 6 h of light; 10 h of darkness followed by 4 h of light; 12 h of darkness followed by 2 h of light; and 14 h of darkness. In all of the above treatments the level of PnCO mRNA was examined 14 h after start of the experiment (Fig. 3). A high level of PnCO expression was observed after 14 h of darkness. PnCO expression was low under all other photoperiodic conditions, which had a dark period of less than 14 h. In contrast, expression of the CAB gene reached a high level 14 h after transfer from light into dark, irrespective of the duration of the dark period (Fig. 3).
Effect of a Night Break on PnCO Expression
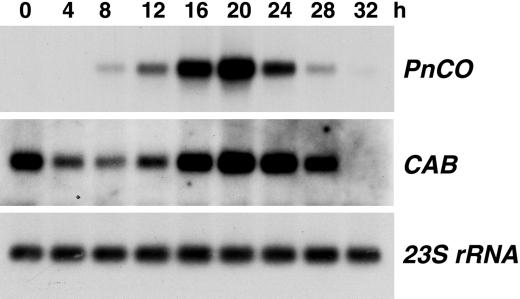
In P. nil, induction of flowering by 14 h of darkness is completely abolished by a 5-min irradiation with red light given 8 h after start of the dark period. Evidence shown in Figure 3 indicates that such a night break reduced expression of PnCO slightly when compared with the transcript level after a 14-h uninterrupted dark period. The abundance of PnCO mRNA in cotyledons with night break was significantly higher than in cotyledons that had been exposed to non-inductive dark periods of 8 to 12 h. A time course of PnCO expression following a night break after 8 h of darkness is shown in Figure 4. The time course of PnCO accumulation showed a peak similar to that without night break (Fig. 2). Again, the expression of the CAB gene did not match that of PnCO.
Figure 4.
Expression of PnCO following night-break treatment. Five-day-old light-grown seedlings were transferred to darkness at time 0. Cotyledons were harvested for RNA isolation at the times indicated. The night break was given after 8 h of darkness. Poly(A)+ RNA preparations were analyzed by RNA gel blotting. The PnCO and CAB probes were as in Figure 2. 23S RNA served as loading control.
An Unspliced Intron Is Present in a Majority of PnCO Transcripts
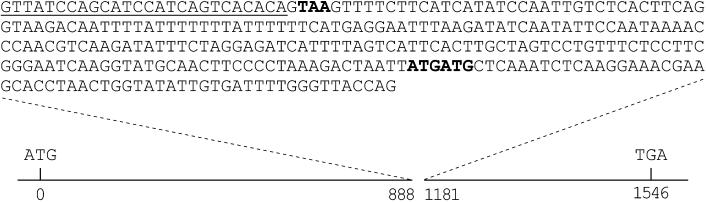
Three types of PnCO cDNA clones were isolated from the cDNA library: PnCO(ni) containing no intron, PnCO(si) containing a short intron, and PnCO(li) containing a long intron. PnCO(ni), representing a single clone, could be translated into a complete protein encompassing both conserved domains (Fig. 1). PnCO(li), representing 16 out of the 18 clones isolated, contains a 292-bp intron, which encompasses the entire corresponding intron in the genome (results not shown) and which is characterized by a stretch of 17 thymine residues (Fig. 5). This intron contains an in-frame premature stop codon. PnCO(si) represents a single clone that retained an alternatively spliced short intron of 26 bp. Presence of this short intron results in a frame shift and a premature in-frame stop codon. Protein sequences from the two intron-containing cDNAs would be truncated, lacking the conserved C-terminal domain.
Figure 5.
The unspliced long intron of the PnCO(li) transcript. ATG and TGA indicate the start and stop codons in PnCO(ni), which encodes the protein shown in Figure 1. In PnCO(li) the open reading frame is interrupted between nucleotides 888 and 1,181 by the intron sequence shown above. This introduces the premature stop codon TAA (highlighted). A second open reading frame of 429 bp starts at the highlighted ATG and ends at the same stop codon as does the uninterrupted PnCO(ni). The short intron sequence in PnCO(si) is underlined. It results in a stop codon (TGA) between nucleotides 1,228 and 1,230.
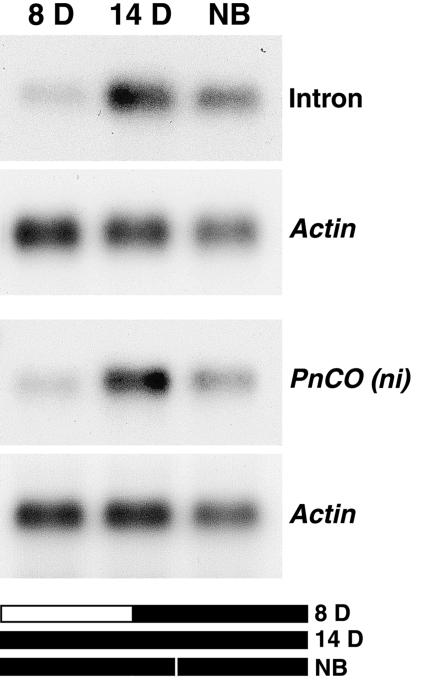
When an RNA gel blot was probed with the long intron, the same pattern of PnCO expression was detected as with the PnCO probe that did not contain an intron (Fig. 6). This result confirms that of our library screen, namely that a major fraction of PnCO transcripts contains the long intron. Using reverse transcriptase- (RT) PCR, the short-intron-containing transcript could be detected with ease, whereas the intron-free mRNA was found in an amount close to our detection limit. There was no indication for differential expression of the intron-free mRNA and the short intron-containing transcripts at various time points of floral induction and in various types of tissues such as the shoot apex, hypocotyl, cotyledons, and root (data not shown).
Figure 6.
RNA gel-blot analysis of PnCO transcripts probed with the long intron or the intron-less PnCO(ni) cDNA. Five-day-old light-grown seedlings were transferred to the light-dark regimes shown on the bar graphs. Cotyledons were harvested at the end of each dark period. Poly(A)+ RNA preparations were analyzed by RNA gel blotting. Actin mRNA served as loading control. The blot was probed with the long intron and with the intron-free, full-length cDNA clone PnCO(ni). Plants that had been kept in uninterrupted darkness for 14 h developed six or seven flower buds after 1 month in continuous light. Plants that had received 8 h of darkness or 14 h of darkness with a night break after 8 h remained vegetative.
Complementation of the Arabidopsis co Mutant with PnCO
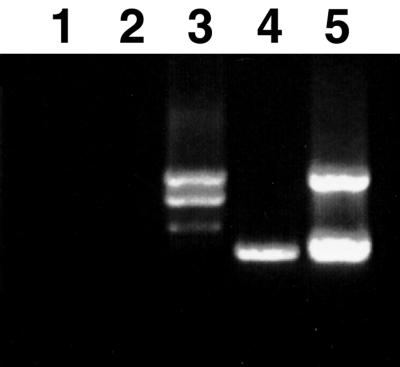
To study the function of PnCO, cDNAs of PnCO(ni), PnCO(si), and PnCO(li) were fused to the cauliflower mosaic virus 35S promoter and were introduced into the co-1 mutant of Arabidopsis using Agrobacterium-mediated transformation. A number of primary transformants were recovered and allowed to self-pollinate. Single insertion lines were identified in the T2 generation. Their homozygous progenies were identified in the T3 generation and were scored for flowering-time phenotype. Expression of the intron-free PnCO cDNA restored early flowering in LD-grown co mutants to the flowering time of wild-type plants (Fig. 7; Table I). The Arabidopsis co mutant transformed with the intron-less PnCO cDNA flowered also much earlier under SD than did the wild type and co mutants (Fig. 7; Table I). Flowering time was virtually unchanged in transgenic co plants expressing PnCO containing the long intron (Fig. 7; Table I). Transgenic plants expressing the PnCO cDNA with the short intron had a phenotype similar to that of plants transformed with PnCO(li) (data not shown). Examination by RT-PCR showed that the large intron was not spliced to yield intron-less PnCO (Fig. 8).
Figure 7.
Phenotypes of transgenic Arabidopsis plants. Plants were grown in SD (9 h of white fluorescent light supplemented with incandescent light) or in LD (9 h of the same light conditions as in SD and 15 h of continued incandescent light). The plants in LD were 18 d old, those in SD were 35 d old. The transgenic plants were all in the Columbia co background: 35S::PnCO(ni) did not contain an intron; 35S::PnCO(li) contained the long intron.
Table I.
Flowering time and segregation of kanamycin resistance in the progeny of Arabidopsis co-1 mutant plants transformed with the Pharbitis nil CO ortholog PnCO
| Transformant Lines | Ratio of Kanamycin-Resistant Seedlings in T2a | Ratio of Kanamycin-Resistant Seedlings in T3b | Average Leaf No.
at Flowering of T3 Plantsc
|
|
|---|---|---|---|---|
| LD | SD | |||
| PnCO(ni) | ||||
| 1-4 | 2.8 /1 | 1 /0 | 5.5 ± 0.5 | 10.8 ± 1.1 |
| 2-1 | 2.5 /1 | 1 /0 | 5.6 ± 0.5 | 11.5 ± 1.4 |
| 3-2 | 3.1 /1 | 1 /0 | 5.4 ± 0.7 | 10.9 ± 1.1 |
| 4-4 | 3.3 /1 | 1 /0 | 5.5 ± 0.6 | 12.3 ± 1.5 |
| PnCO(li) | ||||
| 2-8 | 3.2 /1 | 1 /0 | 8.7 ± 1.0 | 23.0 ± 3.2 |
| 3-1 | 3.2 /1 | 1 /0 | 8.9 ± 0.8 | 19.2 ± 3.8 |
| Columbia | – | – | 5.0 ± 0.4 | 37.5 ± 7.1 |
| co1 | – | – | 9.7 ± 0.9 | 20.6 ± 3.6 |
Over 80 plants were tested.
Over 40 plants were tested.
No. of rosette leaves at the time when the first flower bud appeared in the rosette. Sixteen plants from each transformant line were tested. The values given are ±se.
Figure 8.
RT-PCR analysis of PnCO expression in transgenic Arabidopsis plants. Poly(A)+ RNA preparations used for reverse transcription were isolated from aerial parts of plants harvested at the time when the first flower bud appeared in the rosette. Primers from the regions flanking the intron (TN178 and TN179, see Fig. 1) were used for the RT-PCR reaction. The PCR products were separated on a 2% (w/v) agarose gel. Lanes 1 and 2 are control reactions (without added reverse transcriptase) for the reactions in lanes 3 and 4, respectively. Lane 3, RT-PCR products from plants transformed with 35S::PnCO(li). Lane 4, RT-PCR product from plants transformed with 35S::PnCO(ni). Lane 5, Reference DNA amplified with the same primer set from the plasmids PnCO(ni), PnCO(si), and PnCO(li). The upper band 1 kb in length corresponds to DNA derived from PnCO(li); the lower band is about 700 bp in length and corresponds to PnCO(si) and PnCO(ni), which are 26 bp apart.
DISCUSSION
PnCO Is an Ortholog of the Arabidopsis CO and Its Expression Is Photoperiodically Controlled
The following evidence indicates that PnCO is a functional ortholog of the Arabidopsis CO gene: First, the PnCO gene encodes two highly conserved protein domains found in the CO gene of Arabidopsis (Putterill et al., 1995) and Brassica (Robert et al., 1998; Fig. 1), and, second, the PnCO cDNA complements the co mutation in Arabidopsis (Fig. 7; Table I).
P. nil is an SDP with a well-established daylength requirement (Imamura, 1967). A single 14-h dark period given to seedlings at the cotyledon stage is sufficient for full floral induction. Excision experiments have shown that complete induction is only obtained if the cotyledons remain attached to the plant in the dark or light for an additional 4 h, indicating that a saturating amount of the floral stimulus has moved out of the cotyledons by that time (Zeevaart, 1962). If differential expression of one or more genes is associated with photoperiodic induction in P. nil cotyledons, it must occur within the 14-h dark period. It is evident from our results that the expression pattern of PnCO is correlated with the daylength requirement of floral induction in P. nil. PnCO mRNA accumulated to a high level after a long night of 14 to 20 h (Figs. 2, 3, and 6). Under noninductive conditions of 12 h of darkness or less, the expression of PnCO transcript was substantially lower than under inductive conditions. The simplest explanation is that this was due to the different durations of darkness, but it is also possible that re-entrainment of the circadian rhythm by the shift from darkness to light in the 8 h of darkness + 6 h of light, 10 h of darkness + 4 h of light, and 12 h of darkness + 2 h of light treatments prevented mRNA from reaching the same level as in the 14-h-of-darkness treatment.
In the facultative LDP Arabidopsis, expression of CO is promoted by LD (Putterill et al., 1995). Therefore, the CO gene appears to be the target of photoperiodic regulation in LDP and SDP. Although the correlation between photoperiodic induction and expression of PnCO is consistent, a night break during an inductive dark period did not have the expected results. Under such conditions flowering is completely inhibited. However, the level of PnCO mRNA exhibited little, if any, reduction (Figs. 3, 4, and 6) following a night break. Therefore, the night break does not appear to abolish flowering by reducing expression of PnCO.
PnCO exhibited a circadian pattern of expression upon transfer of P. nil from continuous light to continuous darkness (Fig. 2). CO in Arabidopsis is also subject to circadian control with a peak in mRNA abundance during the night (Valverde et al., 2000). Other flowering-time genes show circadian expression patterns as well (Hicks et al., 1996; Schaffer et al., 1998; Wang and Tobin, 1998; Fowler et al., 1999; Park et al., 1999). However, the 14-h peak in the accumulation of PnCO mRNA, which would have occurred in continuous darkness, was not observed following a noninductive dark period of 12 h or less (Fig. 3). Such a change in rhythmicity is inconsistent with PnCO being a true circadian-clock gene. Instead, it suggests the existence of a timing mechanism that monitors the night length in P. nil and regulates, accordingly, transcription of photoperiodically controlled genes such as PnCO. This timing mechanism is not the same as that controlling the expression of the CAB gene because CAB responds differently to the daylength conditions used to examine expression of PnCO (Fig. 3).
Some PnCO Transcripts Contain an Unspliced Intron
Eighteen PnCO cDNAs of three distinct lengths were isolated from our cDNA library. Of these, 16 contained a complete, unspliced intron, one contained a truncated sequence of the same intron, and one was properly spliced with an open reading frame of the expected length. RT-PCR analysis confirmed that RNA preparations from P. nil cotyledons contained transcripts that corresponded to all three types of cDNAs. Both introns introduced an in-frame stop codon that would prematurely terminate translation of the respective mRNA. The resulting protein would lack the conserved C-terminal domain that contains a putative nuclear localization signal (Robert et al., 1998) and would not be targeted to the nucleus. The truncated protein derived from the intron-containing transcripts could, therefore, not act as a transcription factor, which is the proposed role of CO in Arabidopsis (Putterill et al., 1995).
PnCO Complements the co Mutant of Arabidopsis
We transformed the co mutant of Arabidopsis with the three types of PnCO cDNAs to determine the function of the proteins that they may encode. Whereas the two intron-containing cDNAs did not complement the co mutation, the intron-less cDNA did (Fig. 7; Table I). In LD, co mutants transformed with the intron-less cDNA flowered at the same time as did wild-type plants. In SD, expression of the intron-less PnCO cDNA also advanced flowering of co mutants (Fig. 7; Table I). The intron-less transcript encodes, therefore, a protein that is involved in determining flowering time. Our results also support the previous conclusion that delayed flowering of wild-type Arabidopsis plants in SD is, at least in part, due to low levels of CO expression (Putterill et al., 1995; Simon et al., 1996).
The introns of Arabidopsis CO and PnCO are similar in length and are located at similar positions in the genes (Putterill et al., 1995; Fig. 5). Whereas the intron of CO is efficiently spliced, the intron of PnCO is not, in P. nil or in transgenic Arabidopsis plants (Fig. 8). This evidence points to the existence of a unique sequence structure in the intron of PnCO that attenuates splicing, rather than different splicing mechanisms in the two plants. It is not known whether the intron-containing transcripts are translated and whether their protein products fulfill any function. The second longest open reading frame of the long-intron-containing RNA begins within the intron and codes for the highly conserved C-terminal domain of PnCO (Fig. 5). This protein could conceivably have some regulatory function. The presence of unspliced introns in mRNAs is not unusual. FCA, another gene whose product is also involved in controlling flowering time in Arabidopsis, also has variant transcripts with all or parts of intron 3 included (Macknight et al., 1997).
CONCLUSIONS
Our results show that expression of the CO gene in P. nil is promoted by SD as it is by LD in Arabidopsis. Therefore, it can be assumed that the product of the CO gene mediates induction of flowering in plants with different photoperiodic responses. Our complementation experiments confirmed that the product of the PnCO gene fulfills the same function as that of CO in Arabidopsis. The intron in PnCO mRNA is not removed by splicing, and any proteins that may be derived from such transcripts are inactive in advancing flowering time in transgenic Arabidopsis plants. A number of questions arise from our work. What is the sequence of the promoter of the CO gene in P. nil? How does it compare with that of CO in Arabidopsis? Can one derive any conclusions from such a comparison with respect to the signal that mediates photoperiodic induction in both plants? What would be the effect of promoter swapping on the expression of the CO gene and on photoperiodic sensitivity in Arabidopsis? Supplementing ongoing work on the control of flowering in Arabidopsis with work using a SDP such as P. nil may shed light on processes of photoperiodic induction that are shared by LDPs and SDPs.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Seeds of Pharbitis nil Choisy cv Violet were purchased from the Marutane Co. (Kyoto). The seeds were scarified in concentrated sulfuric acid for 1 h and were then rinsed in running tap water for 1 h. They were imbibed in aerated distilled water at 30°C overnight for approximately 17 h. Seeds with emerging radicles were selected for uniformity, planted in wet vermiculite, covered with a mixture of gravel and vermiculite, and kept in the dark at 32°C for 2 d. The seedlings were then transferred to a growth chamber and kept at 30°C under continuous cool-white fluorescent light (125 μmol m−2 s−1) for 2 d after which they were grown at 23°C until photoperiodic treatments started. The photoperiodic conditions were as specified for each experiment. In some experiments the 14-h inductive dark period was interrupted after 8 h by 5 min of red light (15 μmol m−2 s−1). This night break eliminated induction of flowering. Plants were scored approximately 1 month after the photoperiodic treatment for the presence of terminal and axillary flowers or vegetative buds.
The co-1 mutant of Arabidopsis, accession Columbia, was obtained from the Arabidopsis Biological Resources Center (Ohio State University) and has been described previously (Rédei, 1962; Koornneef et al., 1991). Arabidopsis seeds were surface sterilized with 50% (v/v) bleach containing 0.02% (v/v) Triton X-100 for 7 min and after rinses in sterilized, distilled water, they were spread onto plates containing one-half-strength Murashige and Skoog salts (2.16 g/L; Gibco-BRL, Rockville, MD), 1% (w/v) Suc, and 0.8% (w/v) agar. After cold treatment at 4°C in the dark for 3 d, the seeds were allowed to germinate for a week at 23°C in a SD or LD growth chamber. Seedlings were then selected for uniformity and transferred to soil before being returned to the respective growth chambers. The SD photoperiod was 9 h of a combination of white fluorescent and weak incandescent lights at 140 μmol m−2 s−1 and 15 h of darkness; LD conditions were 9 h of the same light regime as in the SD chamber, followed by 15 h of incandescent light (15 μmol m−2 s−1) at 20°C.
Isolation of Nucleic Acids
Genomic DNA was isolated from P. nil and Arabidopsis according to Doyle and Doyle (1990) and total RNA was isolated according to Verwoerd et al. (1989). The PolyATtract kit (Promega, Madison, WI) was used to enrich poly(A)+ RNA.
Isolation of PnCO
Total RNA was isolated from cotyledons of P. nil after various photoperiodic treatments. Following reverse transcription, gene expression was analyzed by differential display (Liang and Pardee, 1992) using the RNAimage kit (GenHunter, Nashville, TN). Two differentially displayed cDNA bands, 178 and 179, were identified using the primers H-T11-G and H-AP62 (AAGCTTGCAAGTT). The cDNA fragments were cloned into a TA vector (Promega). The 178 and 179 clones were later determined to derive from the same gene, PnCO.
A cDNA library was constructed using poly(A)+ RNA isolated from the same tissue samples as used for the differential display. cDNAs were unidirectionally inserted between the EcoRI and XhoI sites of the Uni-ZAP XR phage vector (Stratagene, La Jolla, CA). Full-length cDNA clones were isolated from the library using the cloned differential display product as a probe. The nucleotide sequences were determined at the Michigan State University DNA sequencing facility.
RNA Gel-Blot Analysis
One microgram of poly(A)+ RNA was subjected to electrophoresis on formaldehyde-agarose gels and transferred to Hybond N+ nylon membrane (Amersham, Piscataway, NJ). RNA was bound to the membrane by cross-linking with UV. The PnCO probe was, unless otherwise stated, a 715-bp cDNA fragment from the divergent region located between the two highly conserved domains (Fig. 1). This fragment (between nucleotides 343 and 1,057) was amplified by PCR from the intron-free cDNA clone PnCO(ni) with TN178 (5′-CACCGTGTCCCGATTCTG) and TN179 (5′-GAACTCTGGCCTCCCTGTCC) as primers. The intron probe was amplified by PCR from PnCO(li), the cDNA clone containing the long intron, with TN182 (5′-GAGAATTTTAACACGGGATA) and JZ203 (5′-CCTGGTAACCCAAAATCACA) as primers. The CAB probe was generated from an Arabidopsis expressed sequence tag clone (GenBank accession no. TI3309). Labeling of the probes, hybridization, and washing were as described by Liu et al. (1999). Probes were used at similar specific radioactivities. X-ray film (Amersham) was exposed to the blot hybridized with PnCO for about 20 h, and to the same blot hybridized with CAB, 23S rRNA, or an actin probe for 0.5 to 2 h.
RT-PCR Analysis
For first-strand cDNA synthesis, 20 ng of poly(A)+ RNA was copied using an oligo dT primer and reverse transcriptase (Gibco-BRL) according to the manufacturer's instructions. A 2-μL aliquot of the 20-μL reaction mixture was used in a 50-μL PCR mixture. To detect the RNA species with the long and short intron, the TN178 and TN179 primers, which flank the intron site, were used. PCR was terminated after 20 cycles while the amplification was still in the exponential phase. To specifically detect the intron-free RNA, the TN178 primer and a primer that spans the intron (JZ399, 5′-AAATGGAGAC-CATATCCCGT, with the hyphen indicating the intron position) were used.
Analysis of Transgenic Arabidopsis Plants
A plant transformation vector, pBI121 (CLONTECH, Palo Alto, CA), was chosen for the transformation experiments. All three types of PnCO cDNA clones isolated from the P. nil cDNA library were subcloned as follows. First, the entire cDNA sequences were amplified using Pfu polymerase (Promega) with the reverse primer and T7 primer located on the vector (pBluescript) arms of the clones. The sequences were cloned into a TA cloning vector (Invitrogen, Carlsbad, CA) and released by XbaI and SacI. The released sequences were then inserted into the XbaI/SacI site of pBI121. After confirmation of the sequences, Agrobacterium tumefaciens C58C1 (pGV3101; Koncz and Schell, 1986) was transformed with the sense constructs pPnCO(ni), pPnCO(si), and pPnCO(li). The T-DNA with the PnCO insert or the vector alone was introduced into the co mutant of Arabidopsis as described by Clough and Bent (1998).
Kanamycin resistance was used to identify primary transformants, single-insertion lines, and homozygous families. Early flowering was an additional trait seen in a majority of primary transformants carrying pPnCO(ni) and was also used to trace genetic segregation.
Flowering time was measured as described by Koornneef et al. (1991), counting the number of rosette leaves at the time when the first flower bud appeared and the number of days from sowing to the appearance of the first flower bud.
Footnotes
This work was supported by the U.S. Department of Energy (grant no. De–FG02–91ER20021).
LITERATURE CITED
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL. Isolation of plant DNA from fresh tissue. Focus. 1990;12:13–15. [Google Scholar]
- Fowler S, Lee K, Onouchi H, Samach A, Richardson K, Morris B, Coupland G, Putterill J. GIGANTEA: a circadian clock-controlled gene that regulates photoperiodic flowering in Arabidopsis and encodes a protein with several membrane-spanning domains. EMBO J. 1999;18:4679–4688. doi: 10.1093/emboj/18.17.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks KA, Millar AJ, Carre IA, Somers DE, Straume M, Meeks-Wagner DR, Kay SA. Conditional circadian dysfunction of the Arabidopsis early-flowering 3 mutant. Science. 1996;274:790–792. doi: 10.1126/science.274.5288.790. [DOI] [PubMed] [Google Scholar]
- Imamura S. Photoperiodic induction and the floral stimulus. In: Imamura S, editor. Physiology of Flowering in Pharbitis nil. Tokyo: Japanese Society of Plant Physiologists; 1967. pp. 15–28. [Google Scholar]
- Koncz C, Schell J. ) The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genet. 1986;204:383–396. [Google Scholar]
- Koornneef M, Alonso-Blanco C, Peeters AJM, Soppe W. Genetic control of flowering in Arabidopsis. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:345–370. doi: 10.1146/annurev.arplant.49.1.345. [DOI] [PubMed] [Google Scholar]
- Koornneef M, Hanhart CJ, van der Veen JH. A genetic and physiological analysis of late-flowering mutants in Arabidopsis thaliana. Mol Gen Genet. 1991;229:57–66. doi: 10.1007/BF00264213. [DOI] [PubMed] [Google Scholar]
- Lang A. Physiology of flower initiation. In: Ruhland W, editor. Handbuch der Pflanzenphysiologie. XV/1. Berlin: Springer-Verlag; 1965. pp. 1380–1536. [Google Scholar]
- Lay-Yee M, Sachs RM, Reid MS. Changes in cotyledon mRNA during floral induction of Pharbitis nil cv. Violet Planta. 1987;171:104–109. doi: 10.1007/BF00395073. [DOI] [PubMed] [Google Scholar]
- Levy YY, Dean C. The transition of flowering. Plant Cell. 1998;10:1973–1989. doi: 10.1105/tpc.10.12.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P, Pardee AB. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992;257:967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- Liu JY, Huang YH, Ding B, Tauer CG. cDNA cloning and expression of a sweetgum gene that shows homology with Arabidopsis AGAMOUS. Plant Sci. 1999;142:73–82. [Google Scholar]
- Macknight R, Bancroft I, Page T, Lister C, Schmidt R, Love K, Westphal L, Murphy G, Sherson S, Cobbett C. FCA, a gene controlling flowering time in Arabidopsis, encodes a protein containing RNA-binding domains. Cell. 1997;89:737–745. doi: 10.1016/s0092-8674(00)80256-1. [DOI] [PubMed] [Google Scholar]
- Millar AJ, Short SR, Hiratsuka K, Chua N-H, Kay SA. Firefly luciferase as a reporter of regulated gene expression in higher plants. Plant Mol Biol Rep. 1992;10:324–337. [Google Scholar]
- Nilsson O, Lee I, Blázquez MA, Weigel D. Flowering-time genes modulate the response to LEAFY activity. Genetics. 1998;150:403–410. doi: 10.1093/genetics/150.1.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill SD, Zhang XS, Zheng CC. Dark and circadian regulation of mRNA accumulation in the short-day plant Pharbitis nil. Plant Physiol. 1994;104:569–580. doi: 10.1104/pp.104.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M, Sage-Ono K, Inoue M, Kamada H, Harada H. Transient increase in the level of mRNA for a germin-like protein in leaves of the short-day plant Pharbitis nil during the photoperiodic induction of flowering. Plant Cell Physiol. 1996;37:855–861. doi: 10.1093/oxfordjournals.pcp.a029022. [DOI] [PubMed] [Google Scholar]
- Ono M, Sage-Ono K, Yasui M, Okazaki M, Harada H. Changes in polypeptides in Pharbitis cotyledons during the first flower-inductive photoperiod. Plant Sci. 1993;89:135–145. [Google Scholar]
- Park DH, Somers DE, Kim YS, Choy YH, Lim HK, Soh MS, Kim HJ, Kay SA, Nam HG. Control of circadian rhythms and photoperiodic flowering by the Arabidopsis GIGANTEA gene. Science. 1999;285:1579–1582. doi: 10.1126/science.285.5433.1579. [DOI] [PubMed] [Google Scholar]
- Putterill J, Robson F, Lee K, Simon R, Coupland G. The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell. 1995;80:847–857. doi: 10.1016/0092-8674(95)90288-0. [DOI] [PubMed] [Google Scholar]
- Rédei GP. Supervital mutants of Arabidopsis. Genetics. 1962;47:443–460. doi: 10.1093/genetics/47.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert LS, Robson F, Sharpe A, Lydiate D, Coupland G. Conserved structure and function of the Arabidopsis flowering time gene CONSTANS in Brassica napus. Plant Mol Biol. 1998;37:763–772. doi: 10.1023/a:1006064514311. [DOI] [PubMed] [Google Scholar]
- Sage-Ono K, Ono M, Harada H, Kamada H. Accumulation of a clock-regulated transcript during flower-inductive darkness in Pharbitis nil. Plant Physiol. 1998;116:1479–1485. doi: 10.1104/pp.116.4.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer R, Ramsay N, Samach A, Corden S, Putterill J, Carré IA, Coupland G. The late elongated hypocotyl mutation of Arabidopsis disrupts circadian rhythms and the photoperiodic control of flowering. Cell. 1998;93:1219–1229. doi: 10.1016/s0092-8674(00)81465-8. [DOI] [PubMed] [Google Scholar]
- Simon R, Igeno MI, Coupland G. Activation of floral meristem identity genes in Arabidopsis. Nature. 1996;384:59–62. doi: 10.1038/384059a0. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde F, Suarez-Lopez P, Costa M, Coupland (2000) Pattern of expression of CONSTANS protein. Presented at the 11th International Conference on Arabidopsis Research, June 24–28, 2000, Madison, WI. Abstract no. 327
- Verwoerd TC, Dekker BMM, Hoekema A. A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res. 1989;17:2362. doi: 10.1093/nar/17.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vince-Prue D, Gressel J. Pharbitis nil. In: Halevy AH, editor. Handbook of Flowering. IV. Boca Raton, FL: CRC Press; 1985. pp. 47–81. [Google Scholar]
- Wang Z-Y, Tobin EM. Constitutive expression of the CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) gene disrupts circadian rhythms and suppresses its own expression. Cell. 1998;93:1207–1217. doi: 10.1016/s0092-8674(00)81464-6. [DOI] [PubMed] [Google Scholar]
- Zeevaart JAD. Physiology of flowering. Science. 1962;137:723–731. doi: 10.1126/science.137.3532.723. [DOI] [PubMed] [Google Scholar]
- Zeevaart JAD. Physiology of flower formation. Annu Rev Plant Physiol. 1976;27:321–348. [Google Scholar]
- Zheng CC, Bui AQ, O'Neill SD. Abundance of an mRNA encoding a high-mobility group DNA-binding protein is regulated by light and endogenous rhythm. Plant Mol Biol. 1993;23:813–823. doi: 10.1007/BF00021536. [DOI] [PubMed] [Google Scholar]
- Zheng CC, Porat R, Lu PZ, O'Neill SD. PNZIP is a novel mesophyll-specific cDNA that is regulated by phytochrome and a circadian rhythm and encodes a protein with a leucine zipper motif. Plant Physiol. 1998;116:27–35. doi: 10.1104/pp.116.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]