Abstract
Background
Implementation of universal antiretroviral therapy (ART) has significantly lowered vertical transmission rates but has also increased numbers of human immunodeficiency virus (HIV)–exposed uninfected children, who remain vulnerable to morbid effects. In the current study, we investigated whether T-cell alterations in the placenta contribute to altered immune status in HIV-exposed uninfected.
Methods
We analyzed T cells from term placenta decidua and villous tissue and paired cord blood from pregnant women living with HIV (PWH) who initiated ART late in pregnancy (n = 21) with pregnant women not living with HIV (PWNH) (n = 9).
Results
Placentas from PWH showed inverted CD4/CD8 ratios and higher proportions of tissue resident CD8+ T cells in villous tissue relative to control placentas. CD8+ T cells in the fetal capillaries, which were of fetal origin, were positively correlated with maternal plasma viremia before ART initiation, implying that imbalanced T cells persisted throughout pregnancy. In addition, the expanded memory differentiation of CD8+ T cells was confined to the fetal placental compartment and cord blood but was not observed in the maternal decidua.
Conclusions
T-cell homeostatic imbalance in the blood circulation of PWH is reflected in the placenta. The placenta may be a causal link between HIV-induced maternal immune changes during gestation and altered immunity in newborn infants in the absence of vertical transmission.
Keywords: CD4, CD8, T cells, placenta, HIV, HEU, HIV-exposed, placenta pathology, villous tissue
In adults, human immunodeficiency virus (HIV) causes severe immune dysregulation, characterized by systemic depletion of CD4+ T cells, increased HIV-1–specific CD8+ T cells, inflammation and a progressive failure of the immune system [1–3]. Initiation of antiretroviral therapy (ART) has been shown to augment HIV-specific CD4+ T-cell responses, but normalization of the CD4/CD8 T-cell ratio does not occur in a large proportion of people with HIV [4]. In pregnant women living with HIV (PWH), there is evidence that women who start ART before pregnancy are more likely to have adverse birth outcomes than those who start ART during pregnancy [5].
Placentas from PWH exhibit increased signs of inflammation and injury affecting maternal vasculature and circulation [6, 7]. Studies also show that using protease inhibitor–based ART during pregnancy is associated with placental injury affecting maternal vascularization and impaired decidualization [8, 9]. In addition, although maternal and fetal circulation within the placenta takes place in distinct compartments, there is evidence that maternal HIV and viral load affects the fetal immune system. HIV-exposed uninfected children (HEU) have been shown to have lower CD4+ T-cell counts and CD4/CD8 T-cell ratios at birth [10, 11], and this appears to be related to maternal viral loads >1000 RNA copies/mL [12]. HIV-unexposed uninfected children (HUU) have an almost completely naive T-cell repertoire, but at birth HEU can have increased proportions of differentiated immune cells, suggestive of antigen experience in utero [10, 13]. Indeed, a number of factors including impaired thymic output and functioning may underlie the immune alterations in HEU [14, 15]. Here, we sought to investigate how HIV exposure in utero may contribute to altered HEU immunity [16, 17].
To test the hypothesis that maternal HIV infection is associated with disruption of T-cell homeostasis in the placenta and cord blood from HEU newborns, we examined term placentas from PWH from a randomized trial in pregnant mothers starting dolutegravir (DTG) versus efavirenz (EFV)–containing therapy in the third trimester (DolPHIN-2; NCT03249181) [18], as well as pregnant women not living with HIV (PWNH), as controls. We show that placentas from PWH have inverted CD4/CD8 ratios with higher CD8+ T-cell counts in villous tissue relative to control placentas, contributing to T-cell homeostatic imbalance in the placenta at birth.
METHODS
Cohort
We included 21 placentas with 9 paired cord blood samples from PWH and HEU and 9 placentas from PWNH with 5 cord blood samples from HUU infants in this study. The PWH group was nested in the DolPHIN-2 study recruited from the Gugulethu Community Health Centre, Cape Town, South Africa [18]. PWNH were enrolled from Khayelitsha Site B Midwife Obstetric Unit, Cape Town. All placentas were from term deliveries (>37 weeks’ gestation).
Clinical Data Collection
As part of DolPHIN-2, maternal systemic CD4+ T-cell counts and plasma viral loads copies were measured at ART initiation at 28 weeks’ gestation (visit 1), at 29 (visit 2), 33 weeks (visit 3), and 36 weeks (visit 4), and at day 14 after delivery (visit 5). The level of HIV-1 RNA detection was 50 copies/mL [18].
Placenta and Cord Blood Processing
Cells were isolated from each placenta, as described elsewhere [19] and illustrated in Supplementary Figure 1. Placentas were collected in RPMI 1640 supplemented with 10% fetal calf serum and penicillin-streptomycin at room temperature, and processing was performed within 6 hours after delivery. Each placenta was dissected to obtain the decidua parietalis, basalis, and villous tissue. Enzymatic lymphocyte isolation was performed using collagenase I and DNAse I. The lymphocyte fraction was obtained after Percoll density centrifugation and incubated with violet amine reactive viability dye (VIVID; Thermofisher). The cells were then fixed using BD FACS lysing solution and cryopreserved in liquid nitrogen until analysis. Cord blood mononuclear cells were isolated on Ficoll, fixed, and cryopreserved until analysis.
Placental Pathology
Whole placentas were fixed in 10% buffered formalin before histopathology. Specimens were macroscopically examined, and samples from the umbilical cord, placental membranes and placental disk were obtained based on the Amsterdam Placental Workshop Group consensus statement [20]. Briefly, 4 blocks were prepared from each placenta, including a roll of the placental membranes, 2 cross-sections of the umbilical cord, and full-thickness sections of the placental parenchyma, and examined in detail, as described elsewhere [21].
Flow Cytometry
Placental and cord blood cells were labeled with fluorochrome-conjugated monoclonal antibodies: CD3 (clone UCHT1), CD4 (clone SK3), CD8 (clone SK1), CD45RA (clone H100), CD28 (clone CD28.2), CD14 (clone MHCD1417), and CD45 (clone MHCD4530). Samples were acquired using an LSR II flow cytometer (BD Biosciences). Total CD4+ and CD8+ T cells were expressed as proportions of CD3+ T cells (Supplementary Figure 2).
Immunohistochemistry
Formalin-fixed paraffin-embedded placenta tissue blocks were cut into 5-µm sections and stained with CD8 (clone C8/144B), with tonsillar tissue serving as a control. Briefly, the slides were baked overnight at 56°C, rehydrated in xylene followed by varying concentrations of alcohol, and then incubated in 3% hydrogen peroxide. Heat-mediated antigen retrieval was performed using an ethylenediaminetetraacetic acid buffer (pH 9). The slides were then incubated with 1% bovine serum albumin and stained with anti-CD8. The images were acquired on Zeiss Axioskop 200 upright fluorescence microscope with an AxioCam high-resolution color camera.
Fluorescence In Situ Hybridization
Because there were 5 male infants, we selected those placental samples to identify the origin of the infiltrating lymphocytes by looking for the Y chromosome, using XY fluorescence in situ hybridization (FISH). Briefly, the slides were baked at 90°C for 15 minutes, deparaffinized in xylene, dehydrated in 100% ethanol and then placed in 10 mmol/L citric acid (pH 6.0). The slides were then dehydrated in varying concentrations of ethanol (70%, 85%, and 100%). We then applied a working solution of DXZ1/DYZ3 (Abbott Laboratories) to the target areas, codenatured with a ThermoBrite system (Abbott Laboratories), and hybridized overnight at 37°C. The slides were then counter stained with 4’-6’-diamidino-2-phenylindole (DAPI) (Vector Laboratories). Tissue samples were scanned and the qualitative result was determined based on observed signal patterns with CytoVision (Leica Biosystems).
Statistical Analysis
All flow cytometric data were analyzed using FlowJo software (version 10; TreeStar). Statistical analyses were performed using Prism (version 8; GraphPad Software), Stata (version 12.0; StataCorp), or R [22] software. Immunohistochemistry cell counts were performed using ImageJ Fiji software version 2 (developed by W. S. Rasband, National Institutes of Health). Tests of significance were performed using Mann-Whitney U and Kruskal-Wallis tests for intergroup comparisons. The associations between cell proportions and maternal viral load or CD4+ T-cell counts were assessed using simple linear regression. All bivariate analyses including maternal and infant characteristics, and placental pathologic conditions stratified by HIV exposure or by ART regimen were compared using χ 2 or Fisher exact tests and Wilcoxon rank-sum tests.
Study Approval
The study protocol, informed consent and all data collection tools were approved by the Human Research Ethics Committee of the University of Cape Town (no. 096/2017). Written and signed informed consent was obtained from all participants, including collection of placentas before study inclusion.
RESULTS
Participant Characteristics
Maternal and newborn infant characteristics are shown in Table 1. No significant differences in maternal age were noted between PWNH and PWH at enrollment, with the median gestational ages being 30 and 28 weeks, respectively, in the groups. PWH were more likely to be multigravida (P = .003). The median gestational age at delivery was 40 weeks in the PWNH and 39 weeks in the PWH (P = .03), and there was a lower birthweight trend in HEU infants (P = .07). We collected placentas from 21 PWH: 16 (76.2%) receiving EFV (with tenofovir disoproxil fumarate and lamivudine) and 5 (23.8%) receiving DTG (with tenofovir disoproxil fumarate and lamivudine), shown in Supplementary Table 1. The median CD4+ T-cell count at ART initiation was 358/µL (interquartile range [IQR], 278–477/µL), with no difference between ART groups (Supplementary Table 2). The median viral load at ART initiation was 4.54 log10 RNA copies/mL (IQR, 3.85–4.80) in the EFV group versus 3.83 log10 RNA copies/mL (3.49–3.83) in the DTG group, with a combined viremia of 4.28 log10 RNA copies/mL (Supplementary Table 2). Both ART groups were on treatment for a median of 84 days (IQR, 44–105 days) and women in the DTG arm achieved viral suppression (cutoff ≤50 copies/mL or 1.69 log10 RNA copies/mL) at a faster rate, at 4 versus 2 weeks after delivery (Supplementary Table 2 and [18]). For the purposes of this study, we combined the placentas from 2 ART groups, because only 5 were collected from the DTG arm.
Table 1.
Maternal and Infant Characteristics
| Characteristic | PWNH (n = 9) | PWH (n = 21) | P Value |
|---|---|---|---|
| Maternal characteristics | |||
| Age group, no. (%) | >.99 | ||
| ≤24 y | 1 (11.1) | 2 (9.5) | |
| 25–29 y | 5 (55.6) | 11 (52.4) | |
| ≥30 y | 3 (33.3) | 8 (38.1) | |
| Age, median (IQR), y | 29 (25–31) | 29 (26–30) | … |
| Gestation at enrollment, median (IQR) | 30 (26–32) | 28 (28–31) | .6 |
| Gravidity, no. (%) | .003 | ||
| 1 | 5 (55.6) | 1 (4.8) | |
| 2 | 3 (33.3) | 7 (33.3) | |
| ≥3 | 1 (11.1) | 13 (61.9) | |
| Gravidity, median (IQR) | 1 (1–2) | 3 (2–3) | … |
| Infant characteristics | |||
| Sex, no. (%) | >.99 | ||
| Female | 6 (66.7) | 12 (57.1) | |
| Male | 3 (33.3) | 6 (28.6) | |
| Data missing | 0 (0) | 3 (14.3) | |
| Gestation at delivery, median (IQR), completed wk | 40 (40) | 39 (38–39) | .003 |
| Birth weight, median (IQR), g | 3420 (3420–4000) | 3305 (3010–3570) | .07 |
Abbreviations: IQR, interquartile range; PWH, pregnant women living with human immunodeficiency virus (HIV); PWNH, pregnant women not living with HIV.
Effect of HIV on Placental Weight
Table 2 shows placenta characteristics and pathologic conditions stratified by HIV status. PWNH had significantly larger placentas (median weight [IQR], 468 [426–533] g) than PWH (394 [343–469] g; P = .02), with 38% of placentas in PWH having weight below the 10th percentile for gestational age, compared with 0% in PWNH. These differences were also reflected the in the fetal-placental ratios; all fetal-placental ratios above the 90th percentile were in the PWH (P = .02). Placental histopathology identified 2 cases (9.5%) of chronic deciduitis and 6 (28.6%) of maternal vascular malperfusion (MVM), all in PWH. There were no significant differences between PWH and PWNH in the incidence of meconium exposure and chorioamnionitis, and there was no evidence of villitis of unknown cause in any of the placentas. There were no significant differences in placental weight, fetal-placental ratio, or placental pathologic conditions between the 2 ART groups (Supplementary Table 3).
Table 2.
Placental Characteristics and Pathologic Conditions at Delivery
| Characteristics and Pathologic Conditions | Placentas, No. (%)a | P Value | |
|---|---|---|---|
| From PWNH (n = 9) | From PWH (n = 21) | ||
| Placental basal plate weight (g), median (IQR) | 468 (426–533) | 394 (343–469) | .02 |
| Placental weight | |||
| Small (<10th percentile) | 0 (0) | 8 (38.1) | .03 |
| Appropriate (10th–90th percentile) | 9 (100.0) | 12 (57.1) | |
| Large (>90th percentile) | 0 (0) | 0 (0) | |
| Data missing | 0 (0) | 1 (4.8) | |
| Fetal-placental weight ratio | |||
| Small (<10th percentile) | 1 (11.1) | 0 (0) | .02 |
| Appropriate (10th–90th percentile) | 8 (88.9) | 11 (52.4) | |
| Large (>90th percentile) | 0 (0) | 7 (33.3) | |
| Data missing | 0 (0) | 3 (14.9) | |
| Placenta dimension, median (IQR) | |||
| Greatest diameter, mm | 180 (172–210) | 180 (170–199) | .6 |
| Thickness, mm | 20 (15–20) | 25 (16.5–27.5) | .2 |
| Cord insertion | |||
| Central | 3 (33.3) | 3 (33.3) | .7 |
| Off center | 5 (55.6) | 12 (57.1) | |
| Marginal | 1 (11.1) | 5 (23.8) | |
| Data missing | 0 (0) | 1 (4.76) | |
| Pathologic conditions at delivery | |||
| Meconium exposure | |||
| Yes | 3 (33.3) | 2 (9.5) | .1 |
| No | 6 (66.7) | 19 (90.5) | |
| Chorioamnionitis | |||
| Maternal inflammatory response | |||
| Yes | 2 (22.2) | 2 (9.5) | .6 |
| No | 7 (77.8) | 19 (90.5) | |
| Fetal inflammatory response | |||
| Yes | 2 (22.2) | 3 (14.3) | .6 |
| No | 7 (77.8) | 18 (85.7) | |
| Chronic deciduitis | |||
| Yes | 0 (0) | 2 (9.5) | .5 |
| No | 9 (100) | 19 (90.5) | |
| Maternal vascular malperfusion | |||
| Yes | 0 (0) | 6 (28.6) | .09 |
| No | 9 (100) | 15 (71.4) | |
| Villitis of unknown cause | |||
| Yes | 0 (0) | 0 (0) | >.99 |
| No | 9 (100) | 21 (100) |
Abbreviations: IQR, interquartile range; PWH, pregnant women living with human immunodeficiency virus (HIV); PWNH, pregnant women not living with HIV.
aData represent no. (%) of placentas unless other specified.
Effects of HIV Infection During Pregnancy on Placental CD4+ and CD8+ T-Cell Proportions
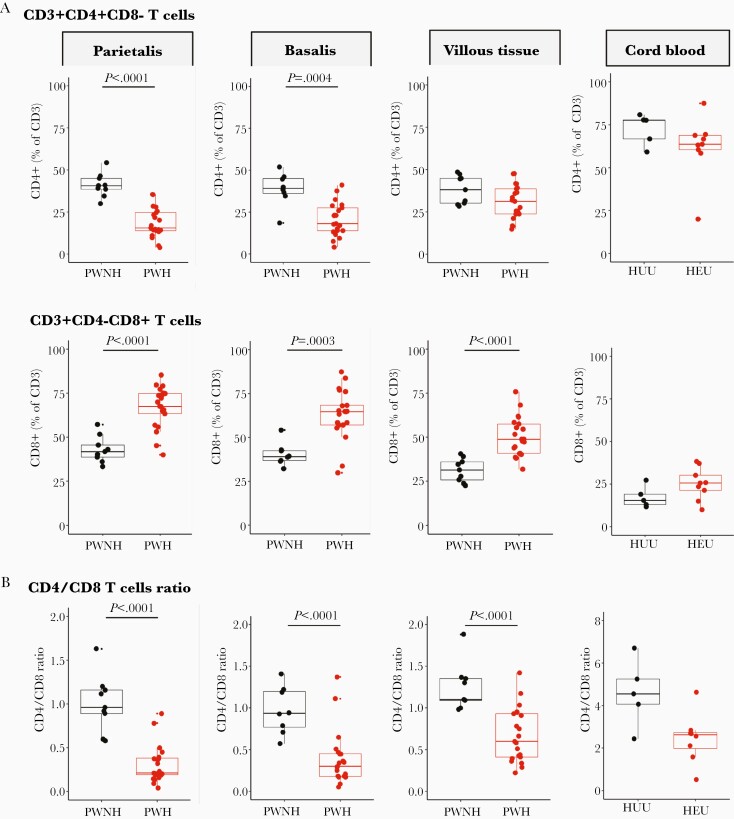
Figure 1A shows significantly lower proportions of CD4+ T cells in decidua parietalis and basalis, but not in the villous tissue, comparing placentas from PWH with those from PWNH. The proportion of CD8+ T cells was significantly increased (Figure 1A) in all 3 placental compartments, resulting in significantly lower CD4/CD8 T-cell ratios in the 3 placental tissues from PWH (Figure 1B). Notably, the inverted CD4/CD8 ratio in villous tissue was due to the increased CD8+ T cells in the villous tissue. The inverted CD4/CD8 ratio was partially reflected in cord blood from HEU infants (Figure 1B). No differences were identified in T-cell proportions when stratified by the different ART regimens (Supplementary Figure 3). The proportions of CD4+ and CD8+ T cells in the placenta were not associated with placental weight, gravidity, or gestational age at delivery (Supplementary Figures 4, 5, and 6).
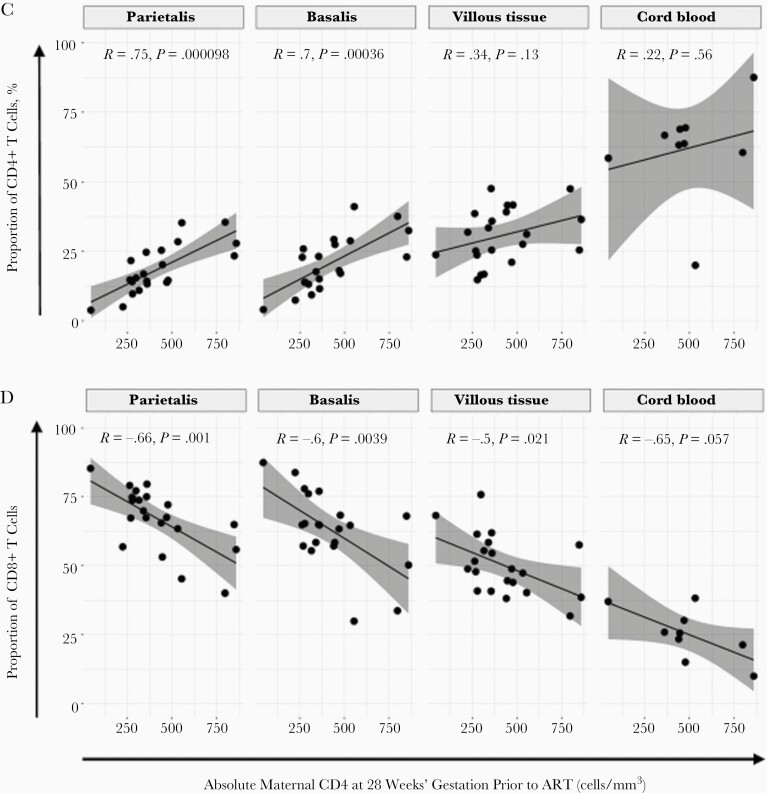
Figure 1.
Proportions of T cells in the placenta and cord blood. A, Box plots (showing medians and interquartile ranges) of CD3+CD4+CD8− and CD3+CD4−CD8+ T-cell proportions isolated from the decidua parietalis, decidua basalis, villous tissue, and cord blood from pregnant women not living with human immunodeficiency virus (HIV) (PWNH) and pregnant women living with HIV (PWH) and cord blood from HIV-unexposed uninfected (HUU) and HIV exposed uninfected (HEU) infants. B, Box plots (showing medians and interquartile ranges) of CD4/CD8 T-cell ratios in the decidua parietalis, decidua basalis, villous tissue, and cord blood from PWNH and PWH and cord blood from HUU and HEU infants. Tests of significance were performed using the Mann-Whitney U test. C, Correlation plots between the absolute maternal CD4+ T-cell count at 28 weeks’ gestation before antiretroviral therapy (ART) initiation and the proportions of CD4+ T cells isolated from the decidua parietalis, basalis, villous tissue, and cord blood. Statistical analysis was performed using the Spearman rank test; gray-shaded areas represent 95% confidence intervals. D, Correlation plots between the absolute maternal CD4+ T-cell count at 28 weeks’ gestation (before ART initiation) and the proportion of CD8+ T cells isolated from the decidua parietalis, basalis, villous tissue. and cord blood. Statistical analysis was performed using the Spearman rank test, and gray-shaded areas represent 95% confidence intervals.
Maternal absolute peripheral blood CD4+ T-cell counts, measured before ART at a median of 28 weeks’ gestation, were positively correlated with the proportion of CD4+ T cells in the decidua and negatively correlated with the proportion of CD8+ T cells in decidua and villous tissue (Figure 1C and 1D). A similar trend was observed in cord blood. Thus, the inverted placental tissue CD4/CD8 T-cell ratios appeared to reflect the maternal peripheral immune status but were mirrored to a lesser extent in the cord blood of HEU infants. This correlation was temporally dissociated, where correlations were made between blood measured at 28 weeks and placentas measured at 38–40 weeks of gestation, suggesting that inverted T-cell ratios persisted throughout pregnancy.
Pre-ART Maternal Viral Load and Placental and Cord Blood CD4+ and CD8+ T-Cell Proportions
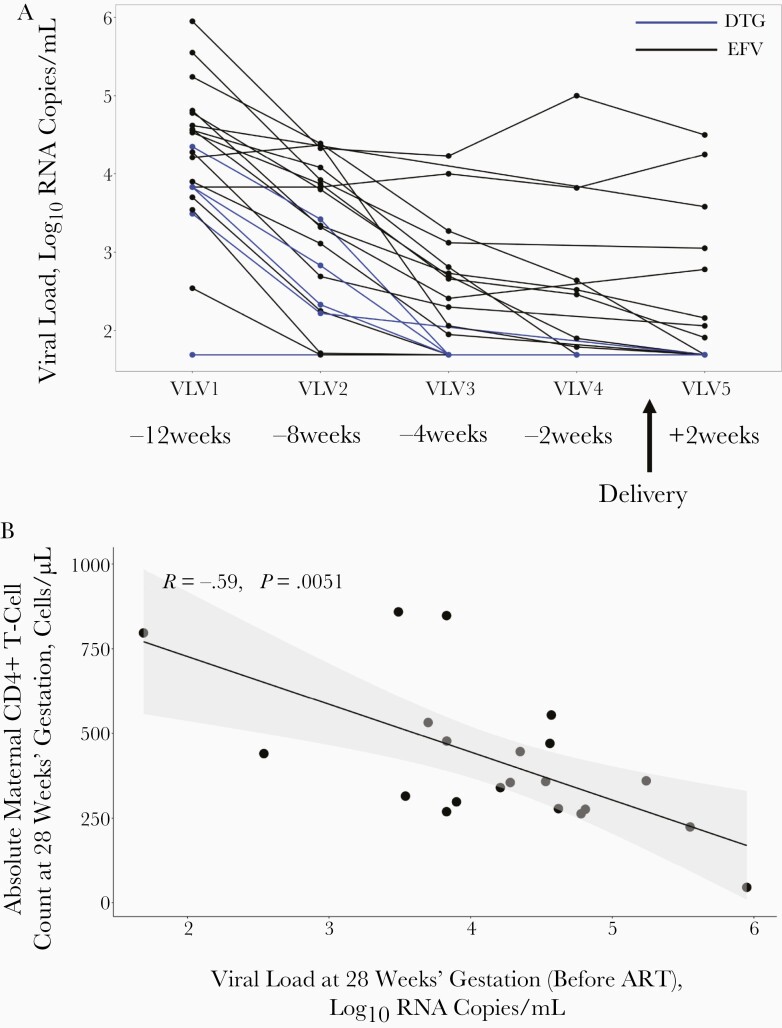
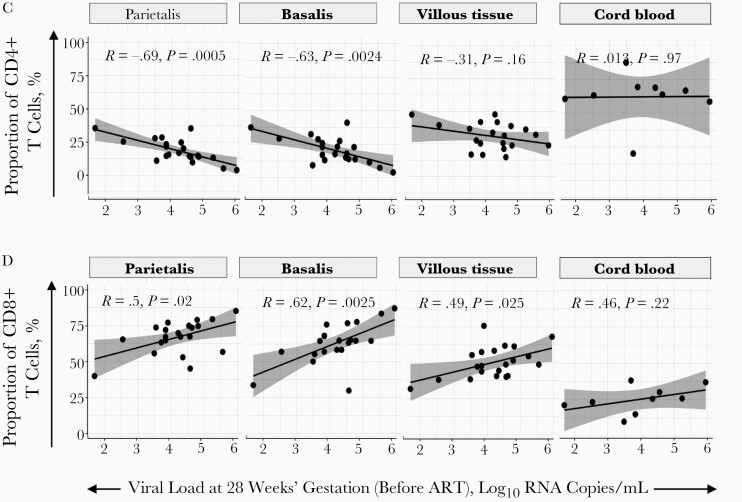
Maternal viremia dropped over time from enrollment to delivery (12 weeks beforehand), where PWH receiving DTG decreased at a faster rate (Figure 2A) [18]. As expected, the enrollment plasma viremia, ranging from 1.69–6.0 log10 RNA copies/mL, was significantly inversely correlated with the absolute maternal peripheral blood CD4+ T-cell count determined before ART, at enrollment (Figure 2B). Pre-ART viremia also showed a significant negative correlation with proportions of CD4+ T cells in the decidua parietalis and basalis (Figure 2C) and a positive correlation with CD8+ T cells in the decidua parietalis, basalis and villous tissue at delivery (Figure 2D). The association between maternal viral load over time and the proportions of decidual CD4+ and CD8+ T cells was maintained at 8 and 4 weeks before delivery for CD4+ T cells (Supplementary Figure 7) and up to 8 weeks before delivery for CD8+ T cells (Supplementary Figure 8). Maternal viremia was not correlated with the proportion of T cells in the cord blood (Figure 2C and 2D), suggesting that maternal viremia (before and after ART initiation) can influence the homeostatic balance of T cells in the placenta, but not in the “newborn” immune compartment.
Figure 2.
Proportions of CD4+ and CD8+ T cells in the placenta and maternal viral load. A, Line plot depicting participant viral load trajectories over time at antiretroviral therapy (ART) initiation, was at 28 weeks’ gestation (VLV1; 12 weeks before delivery); at 29, 33, and 36 weeks’ gestation (VLV2, VLV3, and VLV4, respectively; 8, 4, and 2 weeks before delivery); and at day 14 after delivery (VLV5; approximately 2 weeks after delivery). The women received efavirenz (EFV; with tenofovir disoproxil fumarate [TDF] and lamivudine [3TC]) (black lines) or dolutegravir (with TDF+3TC) (blue lines). B, Correlation plot between maternal systemic absolute CD4+ T-cell counts at enrollment, before ART initiation, with maternal viral load at enrollment and ART initiation (at 28 weeks’ gestation). Statistical analysis was performed using the Spearman rank test. C, Correlation plots between CD4+ T-cell proportions in the placenta and maternal viral load at enrollment and ART initiation (28 weeks’ gestation) in the decidua parietalis, basalis, villous tissue and cord blood. Statistical analysis was performed using the Spearman rank test, and gray-shaded areas represent 95% confidence intervals. D, Correlation plots between CD8+ T-cell proportions in the placenta and maternal viral load at enrollment and ART initiation (28 weeks’ gestation) in the decidua parietalis, basalis, villous tissue and cord blood. Statistical analysis was performed using the Spearman rank test, and gray-shaded area represents 95% confidence intervals.
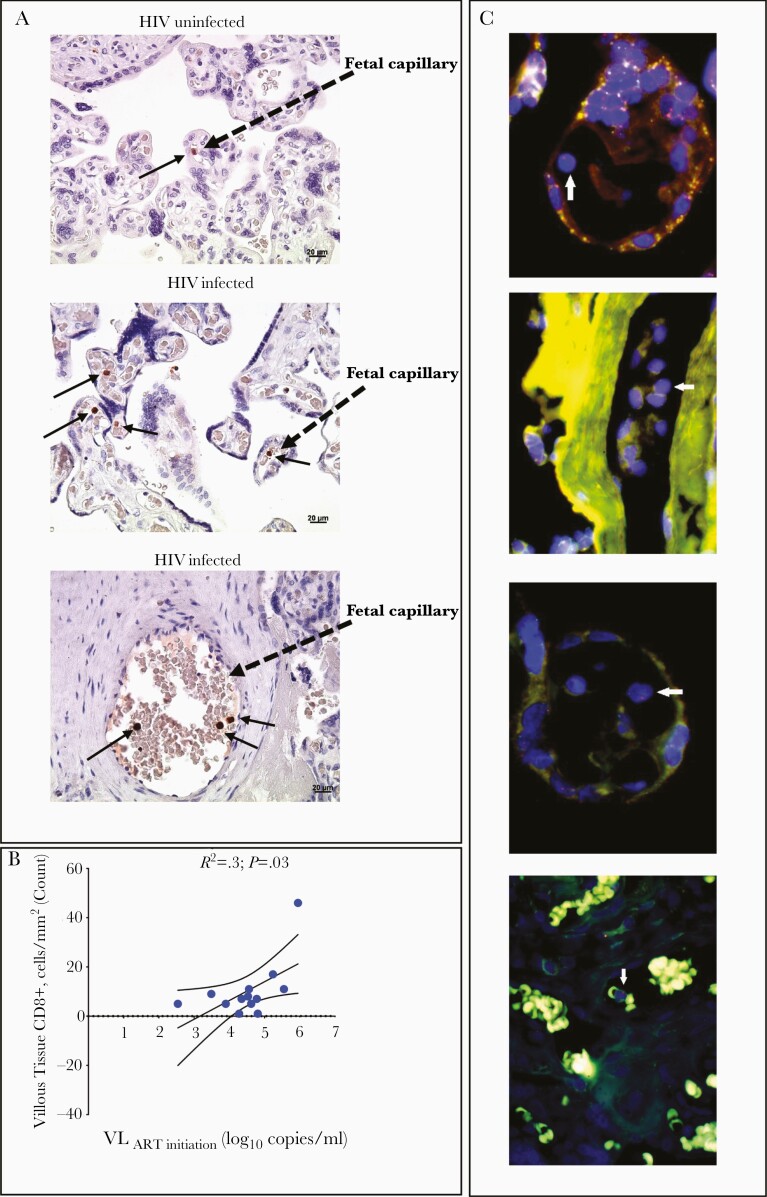
Immunohistochemical Confirmation of Anatomic Location and Fetal Origin of CD8+ T Cells in Villous Tissue
Analysis of placental villi from 13 PWH and 3 PWNH controls showed the presence of CD8+ T cells in the fetal capillaries (Figure 3A). The numbers of CD8+ T cells were positively correlated with pre-ART maternal viremia (Figure 3B), confirming the flow cytometric analysis (Figure 1A and 1D). Using fluorescence in situ hybridization to detect X and Y chromosomes in cells located within the villi from 5 placentas with male births, we confirmed the presence of male (fetal) cells in fetal capillaries (Figure 3C). Thus, the immunohistochemistry analysis confirms the anatomic location and fetal origin of the increased proportions of CD8+ T cells in villous tissue. The fetal (male) origin of the CD8+ T cells in placental villi is consistent with the absence of villitis of unknown cause, a lesion characterized by maternal immune infiltrates [23, 24].
Figure 3.
Anatomic location of CD8+ T cells in the villous tissue. A, Representative immunohistochemical stained images of CD8+ T cells in villous tissue sections denoted by brown dots and black arrows in the villi of placentas from human immunodeficiency virus (HIV)–infected and HIV-uninfected mothers (×40 magnification). B, Correlation plot between the density of tissue-bound CD8+ T cells in the villi and maternal viral load (VL) at antiretroviral therapy (ART) initiation. Statistical analysis was performed using the Spearman rank test; curved lines represent the 95% confidence intervals. C, Representative fluorescence in situ hybridization images of lymphocytes (arrows) in the villous tissue from placentas from male infants. The X chromosome is denoted in green, and the Y chromosome in red (digitally scanned slides).
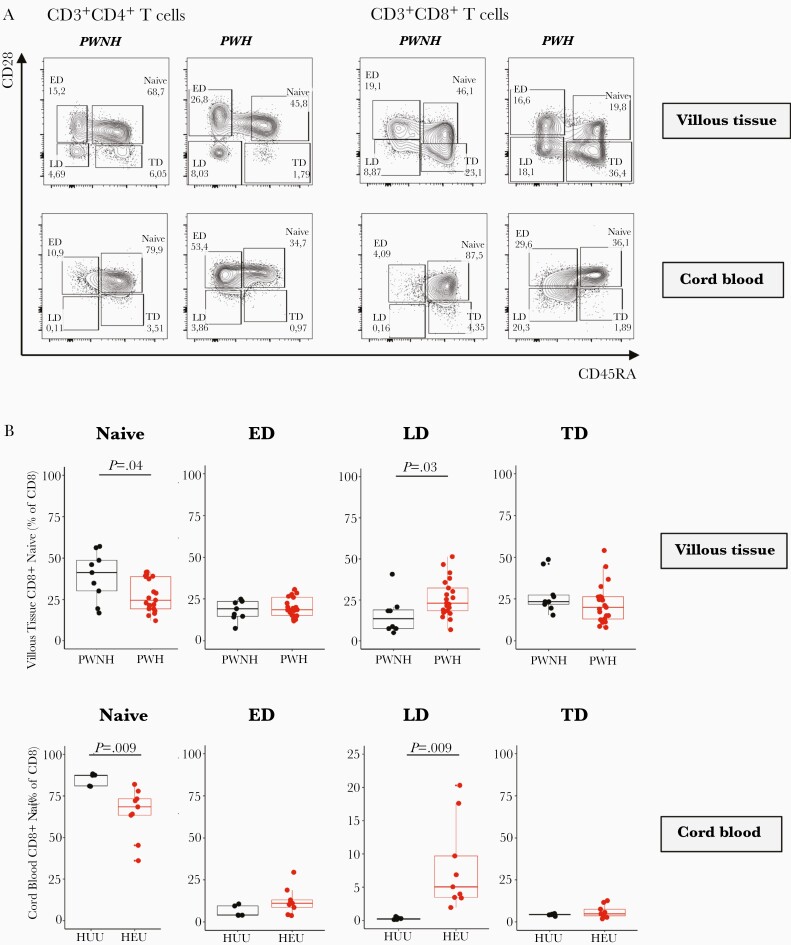
HIV Exposure and Differentiation of CD8+ T Cells in Placental Villi, Fetal Cord Blood, and Maternal Placental Compartments
CD45RA and CD28 were used to identify the proportions of naive (CD45RA+CD28+), early-differentiated (CD45RA−CD28+), late-differentiated (CD45RA−CD28−) and terminally differentiated (CD45RA+CD28−) memory CD4+ and CD8+ T cells from villous tissue and matching cord blood (Figure 4A). We observed significantly lower proportions of naive CD8+ T cells and significantly higher proportions of late-differentiated CD8+ T cells in villous tissue and cord blood of HEU compared with HUU infants (Figure 4B). The CD4+ T cells in the villous tissue were predominantly of a naive and early-differentiated phenotype, while the cord blood cells were predominantly naive. There were no significant differences in CD4+ T-cell differentiation state based on HIV exposure (Supplementary Figure 9). In addition, no significant differences in the stage of CD4+ and CD8+ T-cell differentiation in the decidua were observed between the HIV groups (Supplementary Figure 10). Thus, the increased differentiation state of CD8+ T cells is confined to the fetal placental and cord blood compartments and not observed in the maternal placental compartments. There was no correlation between maternal pre-ART viremia and memory stage of CD8+ T cells in placental villous tissue and cord blood (data not shown). We also showed that there was no association between villous tissue CD8+ T-cell memory differentiation and reported adverse events in the infant during the first 12 weeks of life (Supplementary Figure 11).
Figure 4.
Memory phenotype of CD4+ and CD8+ T cells in the villous tissue. A, Representative flow cytometric contour plots of naive, early-differentiated (ED), late-differentiated (LD), and terminally differentiated (TD) CD4+ and CD8+ T cells in the villous tissue (of placentas from pregnant women not living with human immunodeficiency virus (HIV) (PWNH) and pregnant women living with HIV (PWH) and cord blood from HIV-unexposed uninfected (HUU) and HIV-exposed uninfected (HEU) infants. B, Box plots (showing medians and interquartile ranges) of CD8+-naive, ED, LD, and TD T cells in the villous tissue and cord blood from HUU and HEU infants. Tests of significance were performed using the Mann-Whitney U test.
Discussion
We present data from a unique cohort of PWH who initiated ART late in pregnancy and show that maternal HIV infection has a clear impact on T-cell subsets in the decidua, villous tissue and cord blood. As the decidua and decidual immune cells are of maternal origin, it may not be surprising to find such a footprint [25]. Maternal HIV infection likely affects and kills maternal decidual CD4+ T cells and fewer peripheral blood T cells may traffic to decidual tissue; chemokine gradients have been shown to play a key role in the trafficking of maternal T cells into the decidua during pregnancy [26]. We show that, in contrast to the decidua, the inverted CD4/CD8 ratio in the villous tissue was largely due to an increased proportion of CD8+ T cells and not, as observed in the other tissues, due to a decrease in CD4+ T cells. These CD8+ T cells had an early to late memory differentiated phenotype, suggestive of previous antigen experience [27, 28].
The human placenta has 2 circulatory compartments: the uteroplacental unit for the trafficking of maternal blood and the fetoplacental unit for the fetal blood circulation [29, 30]. Therefore, cells in the villous tissue are likely to be predominantly from the placental reservoir. A key question is whether the increased CD8+ T-cell differentiation in villi and cord blood is due to direct exposure to HIV antigens, presence of other pathogens (eg, cytomegalovirus) or increased levels of other noninfectious inflammatory cues in placentas of PWH. Viral particles and structural and core HIV proteins have been shown to cross the placental barrier in the absence of fetal infection, leading to altered immune profiles in HEU infants [31, 32]. A number of studies also describe HIV-specific T-cell responses in HEU infants, possibly primed by exposure to HIV antigens in utero [16, 33, 34].
The lower proportions of naive cells and increased memory T cells reflected in villous tissue and cord blood mirror findings of previous studies. HEU infants have been shown to have reduced CD4+ and increased CD8+ T-cell counts compared with HUU infants at birth [35, 36]; they have also been shown to have lower numbers of naive cells, a finding thought to be due to thymic involution and frequent stimulation and expansion of the antigen-specific T cells in an effort to regenerate the T-cell pool [14, 15]. Whether the same findings would be recapitulated in PWH with suppressed viral loads before conception is unknown. Nevertheless, this study afforded us a unique opportunity to investigate the impact of starting ART late in pregnancy and how this affected placental immunity. Comparing these findings with those in women who are consistently receiving ART throughout pregnancy would reveal whether long-term viral suppression creates a more normalized immune balance in the placenta.
It was not possible to tease out and distinguish the effects of HIV and ART on the placenta and fetus. There is evidence that some ART can cross the placenta [37, 38]. Additional studies show that perinatal drug exposure is associated with lower T-cell counts in the first 2 years of life in HEU infants [39]. Although we cannot discount the effect of ART alone on the placenta, the parent study did report minimal effects of ART on newborns [18]. One limitation of our study was that we had maternal CD4+ and CD8+ T-cell counts at 28 weeks’ gestation only in PWH, with no equivalent measures in PWNH. We therefore could not directly compare the effects of HIV/ART exposure on systemic T cells with those to placental T cells at delivery, or with findings in healthy mothers.
The elevated proportions of CD8+ T cells found embedded in the villi from PWH were shown to be sequestered within the fetal capillaries. These CD8+ T cells within villi were proportional to maternal viremia. The absence of overt villitis of unknown cause corroborates the finding that expanded CD8+ T-cell fractions are of fetal and not maternal origin. Previous studies have suggested that the presence of T cells in the villi during normal pregnancy reflects villitis of unknown cause [24]. However, there is emerging evidence, using immunohistochemical staining of villi sections from early elective termination placentas, of the presence of CD45+ αβ T cells, although these cells were undetectable at term [40]. In a separate study, in which there were no reported maternal infections, the villous tissue was shown to contain a mixture of fetal and maternal immune cells [41]. We cannot discount the possibility that there was also a mix of maternal and fetal CD8+ T cells in placental villi in our study.
The current study findings demonstrate that, within the fetoplacental unit, there are differences between the T-cell profiles in the villi and cord blood. We postulate that the cells we characterized in the villi may be a combination of cells within the villi and cells attached to the fetal capillaries. Contaminating circulating cord blood cells in this fraction cannot be completely ruled out, but this possibility is minimized by extensive washing of the tissues prior to isolation. Moreover, the phenotypic differences between villous and cord blood T cells as presented here suggest that the villous T cells do not include large proportions of circulating T cells. The increased differentiation state of CD8+ T cells in the villi may be due to exposure to antigens within the villous microenvironment, as well as the mechanisms of immune activation leading to T-cell adhesion and extravasation. No association was revealed by attempts to make a direct connection between the elevated numbers and proportions of CD8+ T cells found in the villous tissue with adverse health events in the first few weeks after birth; most events were resolving rashes.
Of particular note, women were ART naive during the first and second trimesters, and it is likely that prolonged HIV exposure may have contributed to altered placental development and the significantly lower placental weight observed. Interestingly, all cases of MVM were reported in placentas from PWH and possibly reflect placental injury, affecting maternal vasculature and perfusion and increasing the risk of an adverse birth outcome[42]. We have previously reported on MVM in placentas from PWH on long-term ART [21], an incidence of about 27% overall, similar to that reported by Kalk et al [6]. It is likely that HIV and/or ART exposure alters factors involved in vascular development, resulting in placental insufficiency and increased risk of adverse birth outcomes [8, 9, 43].
In conclusion, we provide evidence that in utero exposure to HIV results in an altered immune balance in both the uteroplacental and fetoplacental compartments. Despite the initiation of ART in the third trimester, resulting in either full or partial maternal viral suppression by the time of delivery, there was a significant imbalance in term placental T-cell homeostasis, and to a lesser degree in the cord blood. Overall, our results suggest that maternal immunity leaves a footprint in the placenta that may shape the neonatal/infant immune landscape.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We wish to thank all the study participants in this study, including all members of the DOLPHIN-2 clinical trial and the INFANT placenta study. We also thank Nonzwakazi Bangani, Goitseone Thamae, Michelle Barboure, Berenice Alinde, and Lizette Fick for their expert assistance with sample processing and Amsha Ramburan, PhD, for capturing fluorescence in situ hybridization images.
Author contributions. Conceptualization and design of study: N. M. I., M. L., L. M., S. K., H .B. J., and C. M. G. Panel design, sample preparation, and development of methods: N. M. I, and T. T. Histopathology scoring and interpretation: K. P. Statistical analysis: N. M. I., T. R. M., and S. D. Writing of manuscript: N. M. I., K. P., T. T. T. R. M., E. A. L. E., R. C., L. M., H. B. J., and C. M. G.
Supplement sponsorship. This supplement is sponsored by the Harvard University Center for AIDS Research.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of those of the AXA Research Fund.
Financial support. This work was supported by the AXA Research Fund (fellowship to N. M. I.), and in part by the National Institutes of Health (grants R01HD102050 to HBJ and CMG and R21HD103498 to RC and CMG), and UNITAID (sponsorship of DOLPHIN-2 clinical trial; ClinicalTrials.gov NCT03249181).
Potential conflict of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Okoye AA, Picker LJ. CD4+ T-cell depletion in HIV infection: mechanisms of immunological failure. Immunol Rev 2013; 254:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Demers KR, Makedonas G, Buggert M, et al. Temporal dynamics of CD8+ T cell effector responses during primary HIV infection. PLoS Pathog 2016; 12:e1005805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hileman CO, Funderburg NT. Inflammation, immune activation, and antiretroviral therapy in HIV. Curr HIV/AIDS Rep 2017; 14:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Okhai H, Vivancos-Gallego MJ, Hill T, Sabin CA. CD4+:CD8+ T cell ratio normalization and the development of AIDS events in people with HIV starting antiretroviral therapy. AIDS Res Hum Retroviruses 2020; 36:808–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Uthman OA, Nachega JB, Anderson J, et al. Timing of initiation of antiretroviral therapy and adverse pregnancy outcomes: a systematic review and meta-analysis. Lancet HIV 2017; 4:e21–30. [DOI] [PubMed] [Google Scholar]
- 6. Kalk E, Schubert P, Bettinger JA, et al. Placental pathology in HIV infection at term: a comparison with HIV-uninfected women. Trop Med Int Health 2017; 22:604–13. [DOI] [PubMed] [Google Scholar]
- 7. Mwanyumba F, Gaillard P, Inion I, et al. Placental inflammation and perinatal transmission of HIV-1. J Acquir Immune Defic Syndr 2002; 29:262–9. [DOI] [PubMed] [Google Scholar]
- 8. Mohammadi H, Papp E, Cahill L, et al. HIV antiretroviral exposure in pregnancy induces detrimental placenta vascular changes that are rescued by progesterone supplementation. Sci Rep 2018; 8:6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kala S, Dunk C, Acosta S, Serghides L. Periconceptional exposure to lopinavir, but not darunavir, impairs decidualization: a potential mechanism leading to poor birth outcomes in HIV-positive pregnancies. Hum Reprod 2020; 35:1781–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clerici M, Saresella M, Colombo F, et al. T-lymphocyte maturation abnormalities in uninfected newborns and children with vertical exposure to HIV. Blood 2000; 96:3866–71. [PubMed] [Google Scholar]
- 11. Abu-Raya B, Kollmann TR, Marchant A, MacGillivray DM. The immune system of HIV-exposed uninfected infants. Front Immunol 2016; 7:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kakkar F, Lamarre V, Ducruet T, et al. Impact of maternal HIV-1 viremia on lymphocyte subsets among HIV-exposed uninfected infants: protective mechanism or immunodeficiency. BMC Infect Dis 2014; 14:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ono E, Nunes dos Santos AM, de Menezes Succi RC, et al. Imbalance of naive and memory T lymphocytes with sustained high cellular activation during the first year of life from uninfected children born to HIV-1-infected mothers on HAART. Braz J Med Biol Res 2008; 41:700–8. [DOI] [PubMed] [Google Scholar]
- 14. Mansoor N, Abel B, Scriba TJ, et al. Significantly skewed memory CD8+ T cell subsets in HIV-1 infected infants during the first year of life. Clin Immunol 2009; 130:280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Akbar AN, Fletcher JM. Memory T cell homeostasis and senescence during aging. Curr Opin Immunol 2005; 17:480–5. [DOI] [PubMed] [Google Scholar]
- 16. Afran L, Garcia Knight M, Nduati E, Urban BC, Heyderman RS, Rowland-Jones SL. HIV-exposed uninfected children: a growing population with a vulnerable immune system? Clin Exp Immunol 2014; 176:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Slogrove AL, Powis KM, Johnson LF, Stover J, Mahy M. Estimates of the global population of children who are HIV-exposed and uninfected, 2000–18: a modelling study. Lancet Glob Heal 2020;8:e67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kintu K, Malaba TR, Nakibuka J, et al. ; DolPHIN-2 Study Group . Dolutegravir versus efavirenz in women starting HIV therapy in late pregnancy (DolPHIN-2): an open-label, randomised controlled trial. Lancet HIV 2020; 7:e332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tilburgs T, Crespo ÂC, van der Zwan A, et al. Human HLA-G+ extravillous trophoblasts: immune-activating cells that interact with decidual leukocytes. Proc Natl Acad Sci 2015; 112:7219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khong TY, Mooney EE, Ariel I, et al. Sampling and definitions of placental lesions: Amsterdam placental workshop group consensus statement. Arch Pathol Lab Med 2016; 140:698–713. [DOI] [PubMed] [Google Scholar]
- 21. Ikumi NM, Malaba TR, Pillay K, et al. ; PIMS Study Group . Differential impact of antiretroviral therapy initiated before or during pregnancy on placenta pathology in HIV-positive women. AIDS 2021; 35:717–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2020. [Google Scholar]
- 23. Enninga EAL, Raber P, Quinton RA, et al. Maternal T cells in the human placental villi support an allograft response during noninfectious villitis. J Immunol 2020; 204:2931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tamblyn JA, Lissauer DM, Powell R, Cox P, Kilby MD. The immunological basis of villitis of unknown etiology—review. Placenta 2013; 34:846–55. [DOI] [PubMed] [Google Scholar]
- 25. Ander SE, Diamond MS, Coyne CB. Immune responses at the maternal-fetal interface. Sci Immunol 2019; 4:eaat6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang Y, Zhu XY, Du MR, Li DJ. Human trophoblasts recruited T lymphocytes and monocytes into decidua by secretion of chemokine CXCL16 and interaction with CXCR6 in the first-trimester pregnancy. J Immunol 2008; 180:2367–75. [DOI] [PubMed] [Google Scholar]
- 27. Tilburgs T, Strominger JL. CD8+ effector T cells at the fetal-maternal interface, balancing fetal tolerance and antiviral immunity. Am J Reprod Immunol 2013; 69:395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. White JT, Cross EW, Kedl RM. Antigen-inexperienced memory CD8+ T cells: where they come from and why we need them. Nat Rev Immunol 2017; 17:391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dawe GS, Tan XW, Xiao ZC. Cell migration from baby to mother. Cell Adh Migr 2007; 1:19–27. [PMC free article] [PubMed] [Google Scholar]
- 30. Acharya G, Sonesson SE, Flo K, Räsänen J, Odibo A. Hemodynamic aspects of normal human feto-placental (umbilical) circulation. Acta Obstet Gynecol Scand 2016; 95:672–82. [DOI] [PubMed] [Google Scholar]
- 31. Nielsen SD, Jeppesen DL, Kolte L, et al. Impaired progenitor cell function in HIV-negative infants of HIV-positive mothers results in decreased thymic output and low CD4 counts. Blood 2001; 98:398–404. [DOI] [PubMed] [Google Scholar]
- 32. Evans C, Jones CE, Prendergast AJ. HIV-exposed, uninfected infants: new global challenges in the era of paediatric HIV elimination. Lancet Infect Dis 2016; 16:e92–e107. [DOI] [PubMed] [Google Scholar]
- 33. Kuhn L, Coutsoudis A, Moodley D, et al. T-helper cell responses to HIV envelope peptides in cord blood: protection against intrapartum and breast-feeding transmission. AIDS 2001; 15:1–9. [DOI] [PubMed] [Google Scholar]
- 34. Holditch SJ, Eriksson EM, Tarosso LF, et al. Decay kinetics of HIV-1 specific T cell responses in vertically HIV-1 exposed seronegative infants. Front Immunol 2011; 2:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huo Y, Patel K, Scott GB, et al. Lymphocyte subsets in HIV-exposed uninfected infants and HIV-unexposed uninfected infants. J Allergy Clin Immunol 2017; 140:605–608.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Borges-Almeida E, Milanez HM, Vilela MMS, et al. The impact of maternal HIV infection on cord blood lymphocyte subsets and cytokine profile in exposed non-infected newborns. BMC Infect Dis 2011; 11:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Waitt C, Orrell C, Walimbwa S, et al. Safety and pharmacokinetics of dolutegravir in pregnant mothers with HIV infection and their neonates: a randomised trial (DolPHIN-1 study). PLoS Med 2019; 16:e1002895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kreitchmann R, Schalkwijk S, Best B, et al. Efavirenz pharmacokinetics during pregnancy and infant washout. Antivir Ther 2018; 24:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pacheco SE, McIntosh K, Lu M, et al. ; Women and Infants Transmission Study . Effect of perinatal antiretroviral drug exposure on hematologic values in HIV-uninfected children: an analysis of the women and infants transmission study. J Infect Dis 2006; 194:1089–97. [DOI] [PubMed] [Google Scholar]
- 40. Bonney EA, Pudney J, Anderson DJ, Hill JA. Gamma-delta T cells in midgestation human placental villi. Gynecol Obstet Invest 2000; 50:153–7. [DOI] [PubMed] [Google Scholar]
- 41. Pique-Regi R, Romero R, Tarca AL, et al. Single cell transcriptional signatures of the human placenta in term and preterm parturition. Elife 2019; 8:e52004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ernst LM. Maternal vascular malperfusion of the placental bed. APMIS 2018; 126:551–60. [DOI] [PubMed] [Google Scholar]
- 43. Weckman AM, Ngai M, Wright J, McDonald CR, Kain KC. The impact of infection in pregnancy on placental vascular development and adverse birth outcomes. Front Microbiol 2019; 10:1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.