Abstract
Objective:
Extracts of Artemisia scoparia (SCO) have antidiabetic properties in mice and enhance adipogenesis in vitro, but the underlying mechanisms are unknown. Thiazolidinediones, including rosiglitazone (ROSI), are pharmacological activators of Peroxisome Proliferator Activated Receptor Gamma (PPARγ) that also promote adipogenesis. Our aim was to examine adipogenic pathways responsible for SCO-mediated adipogenesis and identify potential differences between SCO and ROSI in the ability to promote adipocyte development.
Methods:
We examined the ability of SCO or ROSI to promote adipogenesis in 3T3-L1 cells following systematic omission of the common triad of adipogenic effectors: dexamethasone, 1-methyl-3-isobutylxanthine (MIX) and insulin. Adipogenesis was assessed by both neutral lipid quantitation and adipocyte marker gene expression.
Results:
Our results demonstrate that SCO and ROSI promote adipogenesis and increase the expression of several PPARγ target genes involved in lipid accumulation in the absence of MIX. However, ROSI can enhance adipogenesis in the absence of MIX and insulin, and differentially regulates adipogenic and lipid metabolism genes as compared to SCO.
Conclusions:
These data demonstrate the adipogenic capabilities of SCO are similar, but not identical to ROSI, thereby warranting further research into SCO as a promising source of therapeutic compounds in the treatment of metabolic disease states.
Keywords: adipocyte, botanical, adipogenesis
Introduction
Obesity is a global epidemic that has contributed to increased incidence of metabolic syndrome and Type 2 diabetes (1). Currently, metformin is prescribed as the first line of pharmacological defense against Type 2 diabetes. However, metformin alone is not sufficient in achieving glucose control for many individuals and improved therapeutics are needed (2). A variety of Artemisia species have been used as homeopathic medicines in Eastern Asia (3). We and others have previously shown that extracts of Artemisia scoparia (SCO) have positive effects on systemic metabolism. Specifically, SCO supplementation in food significantly improves glucose homeostasis and hepatic steatosis in high-fat diet induced obese mice (4–6). Although the underlying mechanisms are unclear, SCO promotes the differentiation of adipocytes (6) and this may help confer the metabolic benefits of SCO diet supplementation. Adipogenesis is necessary for proper adipose tissue development and the safe storage of lipids. Impaired adipogenesis results in ectopic lipid storage and metabolic dysfunction (7). Moreover, reduced adipogenesis is associated with obesity and insulin resistance (8), suggesting that enhancing the differentiation of adipocytes may improve insulin sensitivity. These data highlight the importance of investigating pro-adipogenic compounds as a means to improve metabolic health.
In culture, adipogenesis is typically induced by three main effectors, insulin, dexamethasone and 1-methyl-3-isobutylxanthine (MIX), each activating primary drivers of adipocyte differentiation. Insulin induces a signaling cascade ultimately resulting in nuclear exclusion of the anti-adipogenic protein, Forkhead box-O1 (FOXO1), thereby preventing its activity (9). In addition, insulin induces Sterol Regulatory Element Binding Protein 1c (SREBP1c) expression, a key lipogenic protein that can indirectly activate Peroxisome Proliferator Activated Receptor Gamma (PPARγ) through the generation of lipid ligands (10,11). PPARγ is required for adipocyte differentiation and is considered the master regulator of adipogenesis (12). Dexamethasone is a synthetic agonist for the glucocorticoid receptor (GR), a transcription factor that positively regulates expression of two of the CCAAT Enhancer Binding Proteins, Cebpb and Cebpd, upstream regulators of PPARγ (13,14). In addition to activating cAMP, a second messenger in early adipogenesis (15), MIX also induces Cebpb transcription and contributes to the positive regulation of other pro-adipogenic genes, including Cluster of differentiation 36 (Cd36) and Fatty acid binding protein (Fabp4). CD36 and FABP4 which are involved in lipid accumulation and transport, and are PPARγ target genes (14,16–19). Ablation of PPARγ prevents adipogenesis in vitro (12). Conversely, expression and activation of PPARγ can rescue adipogenesis in the absence of other key adipogenic genes (20). Thiazolidinediones (TZDs), a class of drugs that activate PPARγ and can induce adipogenesis in the absence of these effectors in vitro (21) and have also been shown to promote adipocyte differentiation in vivo (22). TZD’s have been used clinically to successfully treat Type 2 Diabetes in humans (23,24), supporting the notion that activation of PPARγ and adipogenesis is a viable treatment option for individuals with metabolic disease. Unfortunately, treatment with some of these TZD agents is accompanied by harmful side effects including an increased risk of cardiac dysfunction, stroke and even death (23,24), thereby limiting their therapeutic potential. Identifying compounds that yield similar, yet not identical results in terms of PPARγ activity and adipogenesis may prove beneficial to finding a safer yet effective alternative to TZDs.
SCO extracts are comprised of numerous natural compounds (25). SCO extracts can activate the ligand binding domain of PPARγ, but the response is distinct from rosiglitazone (ROSI), a widely studied TZD (5). Here we examine the signaling pathways involved in SCO-enhanced adipogenesis. In addition, our studies have identified differences between the pro-adipogenic actions of SCO and ROSI. Our experimental approach included a systematic omission of the three adipogenic effectors commonly used to induce adipocyte differentiation (MIX, dexamethasone and insulin) in the presence of either SCO or ROSI. Our data suggests that SCO activates a similar signaling cascade as MIX and that the adipogenic capabilities of SCO are not identical to ROSI.
Methods
Preparation and Source of SCO extract –
Artemisia scoparia extracts were prepared at Rutgers University (26). The herb was greenhouse-grown from seed and periodically harvested at the flowering stage, then freeze-dried and stored at −20°C. Dried herb was extracted in 80% ethanol (1∶20 w/v) at 50°C with sonication. Solid material was removed by centrifugation and the solvent was subsequently removed by evaporation. Dried extracts were solubilized in 100% dimethyl sulfoxide (DMSO).
Cell culture treatments and Oil Red O staining –
Murine 3T3-L1 preadipocytes were passaged and grown in 10% calf serum and Dulbecco’s Modified Eagle’s Medium (DMEM) that included 1% penicillin, streptomycin, and amphoteracin D (PSA). Preadipocytes were differentiated at two days post confluence with 1-methyl-3-isobutylxanthine (MIX; 500 μM), dexamethasone (1 μM) and insulin (1.7 μM), in the presence of either vehicle (DMSO; 1 μL/mL), SCO (50 μg/mL) or ROSI (1 μM) in 10% fetal bovine serum (FBS) and 1% PSA DMEM for three days. Media was then changed to only include insulin (0.425 μM) for an additional two days. DMEM, MIX, dexamethasone, insulin, and DMSO were purchased from Sigma-Aldrich (St. Louis, MO), and FBS from Hyclone (GE Healthcare Life Sciences, Logan, UT). To visually determine lipid accumulation, cells were fixed with 10% formaldehyde and incubated for one hour with 0.3% Oil Red O (ORO) in isopropanol between days 4 and 5 post initial induction depending on differentiation status. To quantify the amount of staining, isopropanol was added to the fixed, stained cells to extract the dye. Absorbance was measured at A492 via a spectrophotometer.
Gene and protein expression –
3T3-L1 preadipocytes were differentiated at two days post confluence with 1-methyl-3-isobutylxanthine (MIX; 250 μM), dexamethasone (500 nM) and insulin (0.85 μM), in the presence of either vehicle (DMSO; 1 μl/mL), SCO (50 μg/mL) or ROSI (1 μM) in 10% fetal bovine serum (FBS) and 1% PSA DMEM for up to three days. RNA lysates were prepared using RNA lysis buffer, and RNA was extracted and purified using the RNeasy mini-kit (Qiagen, Hilden, Germany). RNA was quantified using NanoDrop ND-1000 UV-Vis Spectrophotometer. Reverse-transcription was performed using the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA) plus RNasin (Promega, Madison, WI) according to manufacturers’ guidelines. Quantitative PCR (qPCR) gene expression analyses were performed using primers from Integrated DNA Technologies (Skokie, IL; primer sequences shown in Table 1) and SYBR Premix (Takara Bio USA, Mountain View, CA). Assays were run on the Applied Biosystems 7900HT system and data were analyzed with SDS 2.3 software. Cycling conditions were as follows: 2 min, 50 °C; 10 min, 95°C; 40 cycles of 15 s at 95 °C and 1 min at 60°C; dissociation stage: 15 s, 95 °C; 15 s, 60 °C; 15 s, 95 °C. Quantification was measured using the standard curve method. Relative quantities of target genes were normalized to those of the reference gene, non-POU-domain-containing, octamer binding protein (Nono).
Table 1.
Sequences for primers used in qPCR experiments.
| Gene symbol | Forward | Reverse |
|---|---|---|
| Nono | CATCATCAGCATCACCACCA | TCTTCAGGTCAATAGTCAAGCC |
| Pparg | TCACAAGAAATTACCATGGTTGACA | CGAGTGGTCTTCCATCACGG |
| Cebpa | TCATTGTCACTGGTCAACTCC | ACAAGAACAGCAACGAGTACC |
| Cebpb | CCGCAGGAACATCTTTAAGTGA | GTTTCGGGACTTGATGCAATC |
| Cebpd | TTTCTGTACCTTACTCCACTGC | GTAATTCAAATCCCTGCCCAAA |
| Klf5 | TGGAGAAGCGACGTATCCAC | AGGTGCACTTGTAGGGCTTC |
| Stat5a | ** see below | |
| Fabp4 | CCCTCCTGTGCTGCAGCCTTT | GTGGCAAAGCCCACTCCCACTT |
| Cd36 | GAACACAGCGTAGATAGACCTG | GCCAAGCTATTGCGACATGA |
| Srebf1 | CATGCCCTCCATAGACACATC | AGAACCTGACCCTACGAAGT |
| Glut4 | GAGAATACAGCTAGGACCAGTG | TCTTATTGCAGCGCCTGAG |
| Fasn | GGCATCATTGGGCACTCCTT | ACCAACAGCTGCCATGGATC |
| Lpl | CCCACGCCGCGTAGTTCCAG | AATCTCTTCCCGCGTCTGCTGC |
Stat5a primer assay was ordered from Qiagen (catalog no: 330001_PPM04026C)
For protein lysates, cell monolayers were harvested in a non-denaturing extraction buffer containing 10 mM Tris, pH 7.4, 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 1% Triton X-100, 0.5% Igepal CA-630, 1 mM PMSF, 1 μM pepstatin, 50 trypsin inhibitory milliunits of aprotinin, 10 μM leupeptin, 1 mM 1, 10-phenanthroline, and 0.2 mM sodium vanadate. Lysates were subjected to one freeze-thaw cycle, and passed 3 times through a 20-gauge needle. The homogenates were then centrifuged at 17,500 x g for 10 minutes at 4 °C. After removing the floating lipid layer, the protein concentrations of the supernatants were determined by a BCA kit (Thermo Scientific, Rockford, IL) according to the manufacturer’s instructions. Reduced and heat-denatured samples were separated on 10–15% SDS-polyacrylamide gels (acrylamide from National Diagnostics). Following gel electrophoresis, proteins were transferred to nitrocellulose membranes in transfer buffer containing 25 mM Tris, 192 mM glycine, and 20% methanol. Membranes were blocked in 4% milk and probed with primary antibodies for 1.5 hours at room temperature or overnight at 4 °C on a rotating platform, then subjected to secondary antibodies for 1.5 hours at room temperature. Primary antibodies included: CEBPβ (abcam; ab32358), PPARγ (Santa Cruz Biotechnology; sc-7273), FABP4 (abcam; ab76659), Vinculin (Cell Signaling Technology; 4650) and α- Tubulin (ABclonal; AC012), with α-Tubulin and Vinculin serving as loading controls. Results were visualized with horseradish peroxidase-conjugated anti-rabbit and anti-mouse IgG secondary antibodies Jackson ImmunoResearch (West Grove, PA) and enhanced chemiluminescence (Thermo Scientific, Rockford, IL). Optical densities of all protein bands were analyzed using Image Studio Lite software (Licor Biosciences, Lincoln, NE).
Statistics –
Statistical analyses were performed by one-way ANOVA with Tukey’s multiple comparisons test of the means on GraphPad Prism, version 8.4.2. Experiments were performed three times unless otherwise noted and values are reported as mean ± standard deviation (SD). Significance was set to p<0.05, where asterisks indicate significance in comparison to the DMSO condition of the corresponding adipogenic cocktail treatment and dollar signs indicate significant differences between SCO and ROSI.
Results
SCO promotes lipid accumulation in the absence of 1-methyl-3-isobutylxanthine
Previous studies from our lab have shown that SCO enhances terminal adipogenesis in the 3T3-L1 cell line (6,26); however, the underlying pathways that contribute to this effect have not been assessed. In vitro adipogenesis of white adipocytes is typically achieved with the use of three primary adipogenic effectors, dexamethasone, insulin and isobutyl-methylxanthine (MIX) (27). Rosiglitazone (ROSI), a well characterized PPARγ agonist, also enhances terminal adipogenesis (22). To assess the ability of SCO to replace the need for the known adipogenic effectors, ROSI was included in our experiments as a positive control. This also allowed us the ability to examine potential differences between the adipogenic capabilities of SCO and ROSI. To assess the degree of adipogenesis achieved among the various treatment conditions, lipid accumulation was measured via Oil Red O (ORO). As shown in Figure 1A, confluent preadipocytes were induced to differentiate with MIX (M), dexamethasone (D) and insulin (I), or in the absence of one or more of these effectors. The cells were also treated with DMSO (vehicle control), SCO or ROSI for three days that was added at the same time as the induction cocktail. To allow for complete adipogenesis, after three days the media was changed to include only insulin for the following two days. ORO staining (Figure 1B) and quantification (Figure 1C) was assessed. We did not detect significant differences among any of the conditions in the absence of MDI (vehicle in Figure 1C and data not shown). Also, there were no differences between vehicle, SCO, or ROSI in the presence of the full MDI cocktail as the cells were terminally differentiated. To observe the adipogenic promoting capabilities of SCO or ROSI, the cells have to be harvested prior to complete adipogenesis as they reach the same level of adipogenesis. Using a systematic omission approach with components of the adipogenic cocktail, we observed less lipid was accrued when insulin was removed from the induction cocktail (MD) in the DMSO condition (0.54 ± 0.02). However, in the absence of insulin, there was a noted ability of both SCO (0.76 ± 0.06; p=0.019) and ROSI (0.84 ± 0.01; p=0.009) to increase lipid accumulation as compared to vehicle conditions.
Figure 1: SCO promotes adipogenesis in the absence of MIX, 1-methyl-3-isobutylxanthine.
(A) Schematic of study design. 3T3-L1 preadipocytes were treated with full differentiation cocktail (MIX (M), DEX (D) and insulin (I); MDI), MD, DI or D with either vehicle (DMSO), SCO or ROSI for three days. Following the third day, media was changed to include only insulin for all conditions for up to two days. (B) Lipid was detected using Oil Red O staining and (C) lipid quantification was performed by measuring the absorbance of staining following an isoproterenol extraction method. This experiment is a representative of an experiment independently performed four times on different batches of cells. Data are normalized to standard differentiation (DMSO MDI condition) and presented as average ± SD. Data are normalized to standard differentiation (DMSO MDI condition) and presented as mean ± SD. Statistical analyses were performed using a one-way ANOVA with multiple comparisons, significance was set at p<0.05. * indicates significance compared to DMSO; $ indicates significance between SCO and ROSI.
As shown Figures 1B and C, there is visibly less lipid accumulation when MIX is removed from the induction cocktail (DI) in the control condition (DMSO; 0.18 ± 0.02), but lipid content was significantly elevated in SCO (0.63 ± 0.03; p=0.002) and ROSI (0.93 ± 0.04; p=0.0003) conditions, with ROSI resulting in greater lipid accumulation than SCO (p=0.005). When both MIX and insulin were removed from the induction cocktail (D), lipid accumulation was only observed in ROSI treated cells (DMSO 0.17 ± 0.0002; SCO 0.19 ± 0.01; ROSI 0.51 ± 0.002; p<0.0001). Other treatment combinations were assessed, but were not significantly different compared to control DMSO conditions (data not shown). Overall, we observed that SCO could promote adipogenesis in the absence of MIX and insulin, but not without dexamethasone.
SCO and ROSI enhance expression of late stage adipogenic markers
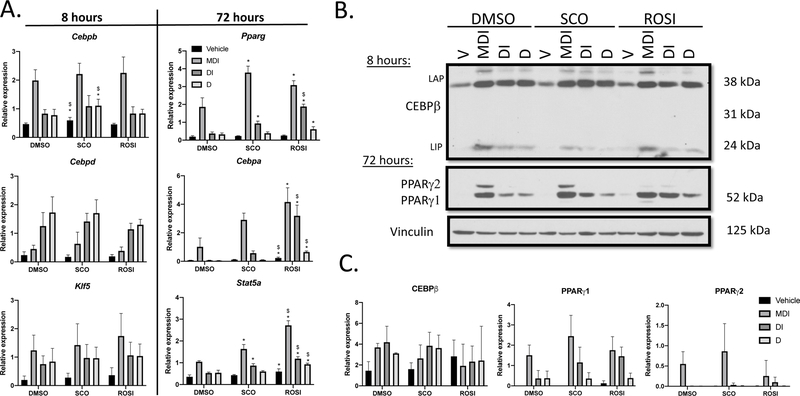
Utilizing the same treatment paradigm, we collected RNA and protein at various time points after the induction of adipogenesis in the systematic omission experiments. CCAAT enhancer binding protein genes (Cebpb and Cebpd) and Kruppel-like factor 5 (Klf5), are induced early in adipocyte differentiation (13). We also examined later markers of adipogenesis (28) including Pparg, Cebpa and Signal transducer and activator of transcription 5A (Stat5a). Based on preliminary studies not shown, we focused on an 8-hour (early) and 72-hour (late) time points. There were no differences that would explain the promotion of lipid accumulation in the absence of MIX in any of the early-response adipogenic targets assessed (Figure 2A; Table 2). However, at 72 hours post-induction Pparg, Cebpa, and Stat5a transcripts were significantly elevated across several cocktail treatments in the SCO and ROSI conditions when compared to DMSO (Table 2). Additionally, differential expression was detected between SCO and ROSI in response to V (Cebpa), MDI (Stat5a), DI (Pparg, Cebpa and Stat5a) and D cocktail treatments (Cebpa and Stat5a; Table 2).
Figure 2: SCO and ROSI enhance expression of late stage adipogenic markers.
3T3-L1 preadipocytes were induced to differentiate and treated as described in Figure 1A. (A) Cells were lysed at 8 hours (n=3) and 72 hours (n=3) to assess mRNA expression of adipogenic genes. Quantification was determined by qPCR using the standard curve method and transcript expression is presented relative to Nono. (B) Whole cell extracts were collected at 8 hours and 72 hours and subjected to western blots analysis. (C) Bands were imaged on film using the ECL method and quantified and normalized to vinculin using the Image Studio Lite software. The results shown are representative of three independent experiments. Data are presented as mean ± SD. Statistical analyses were performed using a one-way ANOVA with multiple comparisons, significance was set at p<0.05. * indicates significance compared to DMSO; $ indicates significance between SCO and ROSI.
Table 2: Differentiation transcript analysis.
Values are reported as mean ± SD. Statistics were generated using a one-way ANOVA with Tukey’s test for multiple comparisons.
| Pathway | Gene | Cocktail | DMSO | SCO | ROSI |
|---|---|---|---|---|---|
| Early Differentiation | Cebpb | Vehicle | 0.47 ± 0.04 | 0.60 ± 0.10 *p=0.01; $p=0.004 |
0.46 ± 0.04 |
| MDI | 1.99 ± 0.37 | 2.21 ± 0.37 | 2.25 ± 0.56 | ||
| DI | 0.83 ± 0.14 | 1.09 ± 0.37 | 0.83 ± 0.24 | ||
| D | 0.78 ± 0.20 | 1.11 ± 0.23 *p=0.01; $p=0.04 |
0.84± 0.14 | ||
| Cebpd | Vehicle | 0.24 ± 0.12 | 0.17 ± 0.07 | 0.19 ±0.07 | |
| MDI | 0.45 ± 0.13 | 0.63 ± 0.41 | 0.39 ± 0.14 | ||
| DI | 1.25 ± 0.47 | 1.41 ± 0.29 | 1.14 ± 0.21 | ||
| D | 1.73 ± 0.55 | 1.71 ± 0.47 | 1.30 ± 0.19 | ||
| Klf5 | Vehicle | 0.20 ± 0.14 | 0.28 ± 0.16 | 0.37 ± 0.26 | |
| MDI | 1.24 ± 0.53 | 1.43 ± 0.75 | 1.74 ± 0.79 | ||
| DI | 0.75 ± 0.29 | 0.97 ± 0.47 | 1.06± 0.46 | ||
| D | 0.84 ± 0.47 | 0.96 ± 0.39 | 1.04 ± 0.42 | ||
| Late Differentiation | Pparg | Vehicle | 0.19 ± 0.05 | 0.23 ± 0.02 | 0.26 ± 0.03 |
| MDI | 1.87 ± 0.51 | 3.79 ± 0.36 *p=0.005 |
3.09 ± 0.25 *p=0.03 |
||
| DI | 0.37 ± 0.08 | 0.93 ± 0.13 *p=0.002 |
1.89 ± 0.13 *p<0.0001; $p=0.0001 |
||
| D | 0.32 ± 0.08 | 0.39 ± 0.07 | 0.61 ± 0.15 *p=0.035 |
||
| Cebpa | Vehicle | 0.07 ± 0.01 | 0.12 ± 0.02 | 0.23 ± 0.06 *p=0.004; $p=0.021 |
|
| MDI | 1.02 ± 0.62 | 2.91 ± 0.48 NS, p=0.089 |
4.16 ± 0.99 *p=0.013 |
||
| DI | 0.06 ± 0.02 | 0.57 ± 0.16 | 3.19 ± 0.75 *p=0.003; $p=0.0009 |
||
| D | 0.05 ± 0.01 | 0.10 ± 0.01 | 0.65 ± 0.07 *$p<0.0001 |
||
| Stat5a | Vehicle | 0.36 ± 0.09 | 0.43 ± 0.03 | 0.59 ± 0.13 *p=0.041 |
|
| MDI | 1.05 ± 0.05 | 1.64 ± 0.20 *p=0.041 | 2.72 ± 0.21 *p=0.0004; $p=0.002 |
||
| DI | 0.53 ± 0.04 | 0.87 ± 0.09 *p=0.005 | 1.19 ± 0.09 *p=0.0001; $p=0.007 |
||
| D | 0.54 ± 0.12 | 0.60 ± 0.04 | 0.93 ± 0.06 *p=0.003; $p=0.006 |
indicates significance compared to DMSO and $ indicates significance when comparing SCO and ROSI.
Western blots were also performed to examine protein expression (Figure 2B–C). CEBPβ expression was clearly elevated in the MDI treatment when compared to control, undifferentiated cells; however, the removal of effectors had little to no effect on expression and this was consistent across all conditions. PPARγ1 expression was not different among the various conditions. Interestingly, PPARγ2 expression was considerably lower in the ROSI full cocktail MDI condition when compared to DMSO or SCO treatments, and though this finding was consistent, it not statistically significant due to variability between experiments. Collectively, these data show SCO enhances late stage adipogenic gene transcription, but does not significantly alter the expression of PPARγ when compared to DMSO.
Expression of PPARγ targets is elevated in SCO and ROSI in the absence of MIX
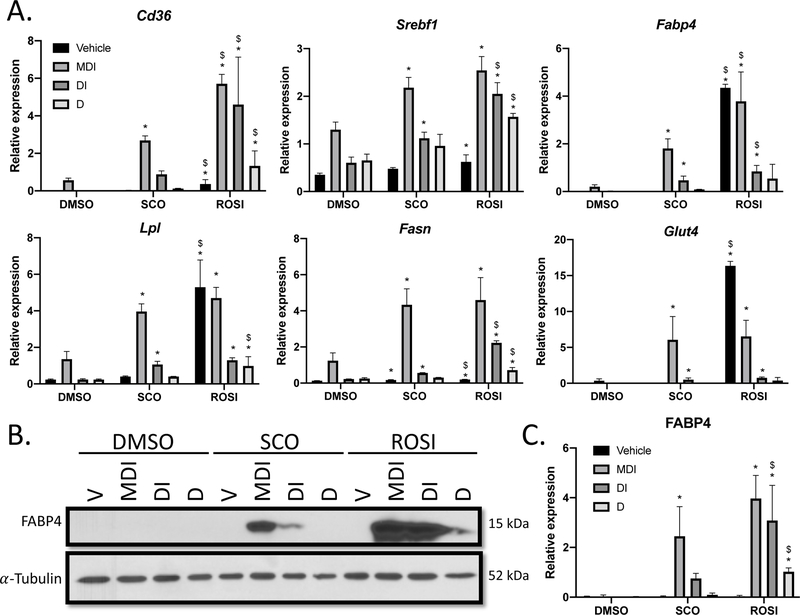
Lipid synthesis and transport are also important in the late stage differentiation, and MIX and PPARγ agonists are known to activate several genes in these processes (17,19,29–31). Therefore, we examined the mRNA expression of a subset of genes involved in lipid metabolism and accumulation that are upregulated during adipogenesis, including Fatty acid binding protein 4 (Fabp4), Cluster of differentiation 36 (Cd36), Lipoprotein lipase (Lpl), Sterol regulatory element binding transcription factor 1 (Srebf1) and Fatty acid synthase (Fasn), as well as the primary glucose transporter in adipocytes Glucose transporter type 4 (Glut4; Figure 3A). The mRNA expression of several genes was significantly elevated across the differentiation treatments in SCO and ROSI conditions when compared to DMSO (Table 3). Particularly, in the absence of MIX, SCO increased Srebf1, Fabp4, Lpl, Fasn and Glut4 expression and ROSI increased expression of all transcripts, when compared to DMSO. ROSI was also able to enhance expression of Cd36, Srebf1, Lpl and Fasn in the absence of insulin and MIX, as well as all transcripts in the lack of treatment (Vehicle), when compared to DMSO. Furthermore, significant differences in gene expression were observed when comparing the effects of SCO and ROSI, with ROSI resulting in greater mRNA induction across all genes for several cocktail treatments.
Figure 3: SCO promotes expression of PPARγ target genes in the absence of MIX.
3T3-L1 preadipocytes were induced and treated as described in Figure 1A. (A) Cell monolayers were harvested at 72 hours (n=3) examine mRNA expression of genes involved in lipid accumulation. Quantification was determined by qPCR using the standard curve method and transcript expression is presented relative to Nono. (B) Whole cell extracts were collected at 72 hours and subject to western blot analysis. (C) FABP4 expression was quantified and normalized using a-Tubulin expression with use of Image Studio Lite software. This experiment is a representative of an experiment independently performed three times on different batches of cells. Data are presented as mean ± SD. Statistical analyses were performed using a one-way ANOVA with multiple comparisons, significance was set at p<0.05. * indicates significance compared to DMSO; $ indicates significance between SCO and ROSI.
Table 3: Lipid accumulation transcript analysis.
Values are reported as mean ± SD. Statistics were generated using a one-way ANOVA with Tukey’s test for multiple comparisons.
| Gene | Cocktail | DMSO | SCO | ROSI |
|---|---|---|---|---|
| Cd36 | Vehicle | 0.0002±0.0003 | 0.02±0.007 | 0.36±0.24 p=0.013; $p=0.017 |
| MDI | 0.56 ±0.12 | 2.69 ± 0.24 *p<0.0001 |
5.71 ± 0.50 *$p<0.0001 |
|
| DI | 0.01 ± 0.004 | 0.88 ± 0.18 | 4.61 ± 2.53 *p=0.004; $p=0.014 |
|
| D | 0.002 ± 0.0002 | 0.11 ± 0.02 | 1.33 ± 0.80 *p=0.007; $p=0.012 |
|
| Srebf1 | Vehicle | 0.35 ± 0.03 | 0.48 ± 0.03 | 0.62 ± 0.15 *p=0.005 |
| MDI | 1.30 ± 0.16 | 2.18 ± 0.21 *p=0.001 |
2.54 ± 0.29 *p<0.0001 |
|
| DI | 0.60 ± 0.12 | 1.12 ± 0.13 *p=0.005 |
2.05 ± 0.23 *$p<0.0001 |
|
| D | 0.65 ± 0.13 | D 0.96 ± 0.24 | 1.57 ± 0.07 *p<0.0001; $p=0.001 |
|
| Fabp4 | Vehicle | 0.0001 ± 0.00003 | 0.004 ± 0.001 | 4.35 ± 0.15 *$p<0.0001 |
| MDI | 0.20 ± 0.08 | 1.81 ± 0.40 *p=0.035 |
3.78 ± 1.23 *p=0.0002; $p=0.012 |
|
| DI | 0.02 ± 0.005 | 0.47 ± 0.18 *p=0.016 |
0.84 ± 0.25 *p=0.0003; $p=0.037 |
|
| D | 0.02 ± 0.003 | 0.09 ± 0.003 | 0.54 ± 0.60 | |
| Lpl | Vehicle | 0.24 ± 0.03 | 0.40 ± 0.03 | 5.30 ± 1.49 *$p<0.0001 |
| MDI | 1.36 ± 0.42 | 3.96 ± 0.42 *p<0.0001 |
4.71 ± 0.58 *p<0.0001 |
|
| DI | 0.24 ± 0.04 | 1.06 ± 0.18 *p<0.0001 |
1.30 ± 0.13 *p<0.0001 |
|
| D | 0.24 ± 0.03 | 0.40 ± 0.01 | 0.98 ± 0.51 *p=0.014; $p=0.048 |
|
| Fasn | Vehicle | 0.12 ± 0.01 | 0.16 ± 0.02 *p=0.008 |
0.19 ± 0.01 *p=0.0002; $p=0.043 |
| MDI | 1.25 ± 0.42 | 4.34 ± 0.88 *p=0.003 |
4.60 ± 1.25 *p=0.002 |
|
| DI | 0.22 ± 0.01 | 0.56 ± 0.01 *p<0.0001 |
2.23 ± 0.11 *$p<0.0001 |
|
| D | 0.25 ± 0.05 | 0.30 ± 0.01 | 0.72 ± 0.15 *p=0.0001; $p=0.0003 |
|
| Glut4 | Vehicle | 0.002 ± 0.002 | 0.01 ± 0.01 | 16.35 ± 0.62 *$p<0.0001 |
| MDI | 0.36 ± 0.26 | 6.06 ± 3.23 *p=0.016 |
6.53 ± 2.25 *p=0.01 |
|
| DI | 0.01 ± 0.01 | 0.51 ± 0.23 *p=0.004 |
0.75 ±0.13 *p=0.0002 |
|
| D | 0.01 ± 0.01 | 0.04 ± 0.01 | 0.37 ± 0.44 |
indicates significance compared to DMSO and $ indicates significance when comparing SCO and ROSI.
To determine if protein expression was also elevated, we assessed FABP4 by western blot (Figure 3B–C). FABP4 is responsible for lipid transport and is a marker of terminal differentiation (32,33). Additionally, Fabp4 is a direct target of PPARγ (18). In our exposure in the linear range, there were no visible bands for FAPB4 in vehicle (DMSO treatments), but its expression was evident with a longer exposure (data not shown). We observed robust expression of FABP4 when SCO was added to MDI (2.45 ± 1.19, p=0.034), but was not significantly different from DMSO in the absence of the full cocktail. Although not statistically significant, FABP4 was consistently detected across experiments in the absence of MIX in the SCO condition (DI 0.75 ± 0.20, NS). ROSI induced FAPB4 expression under the following cocktail treatments (MDI 3.97 ± 0.93, p=0.004; DI 3.08 ± 1.41, p=0.02; D 1.02 ± 0.16, p=0.0004) when compared to DMSO (MDI 0.03 ± 0.06; DI 0.01 ± 0.004; D 0.01 ± 0.01). FABP4 expression was statistically different in SCO (DI p=0.031; D 0.09 ± 0.08, p<0.0001) and ROSI. These data show that SCO is positively regulating several genes involved in lipid accumulation and terminal differentiation and this is reflected at the protein level for FABP4. Taken together, the results reported suggest that increased lipid accumulation in the SCO and ROSI conditions is due to enhanced lipid metabolic and transport processes, and that SCO and ROSI promote PPARγ activity in the absence of MIX (summarized in Figure 4).
Figure 4: Model for SCO and ROSI induced adipogenesis.
Dexamethasone (DEX), 1-methyl-3-isobutylxanthine (MIX) and insulin are common components of the classic adipogenic cocktail which lead to the activation of the master regulators of adipogenesis, including PPARγ. MIX and PPARγ agonists positively regulate a select subset of genes involved in lipid accumulation, and in the absence of MIX the processes of adipocyte differentiation and lipid accumulation are non-existent. SCO leads to the induction of several of these PPARγ target genes and is able to promote adipogenesis in the absence of MIX, similar to ROSI. However, the ability to stimulate differentiation in the absence of MIX and insulin is unique to ROSI.
Discussion
The classic MDI treatment is a widely used method to differentiate 3T3-L1 preadipocytes and other precursor cells into mature adipocytes. Here we tested the potential mechanisms contributing to SCO’s ability enhance adipogenesis and observed that SCO is able to promote adipogenesis in the absence of MIX and that SCO actions are similar, but not identical to ROSI. Similar to our findings, others have shown that MIX is a necessary component in adipocyte differentiation in vitro. In the absence of MIX, 3T3-L1 cells do not undergo adipogenesis and are unable to produce lipids (15). Collectively, our data suggests that SCO is able to enhance lipid accumulation through upregulation of lipid synthesis and transport. It is important to note that MIX is a well-known phosphodiesterase (PDE) inhibitor, allowing for enhanced cAMP activity and increased adipogenesis (15). PDE inhibitory activity has been characterized in some Artemisia species (34,35). One of the compounds responsible, sesquiterpene lactone, has been identified in SCO fractions that have pro-adipogenic activity (26). However, although we did not test cAMP activity in these experiments, our lab has previously reported that SCO does not affect isoproterenol-induced lipolysis in mature adipocytes (36). Therefore, it is possible, but unlikely that the increased lipid accumulation observed here is attributable to changes in cAMP activity and more work will be needed to confirm these speculations. CEBPβ is a key player in the induction of adipogenesis (37) and is a well-known target of MIX (16). Surprisingly, after assessing gene and protein expression at several time points, we did not detect significant decreases in CEBPβ expression in the absence of MIX in any of the conditions tested. These data indicate that induction of CEBPβ is not the primary mechanism by which MIX is contributing to the adipogenic process. Furthermore, there were no meaningful differences of SCO and ROSI across variations of the MDI treatment in the genes regulating early differentiation. Cebpb, Cebpd and Klf5 are all involved in the induction of Pparg (13,38), indicating that SCO is likely acting downstream of these genes. We did observe elevations in Pparg and Cebpa at the transcript level in response to SCO and ROSI when compared to vehicle conditions. Since there was no indication that SCO was affecting the upstream regulators of Pparg and given the evidence that PPARγ and CEBPα positively regulate the expression of one another (20), these findings support the notion that SCO activates PPARγ.
Lipid metabolism and transport genes are also important in differentiation and several are regulated by PPARγ, with many of these being induced by MIX as well. The regulation of PPARγ and MIX across a common subset of lipid accumulation genes such as Cd36, Fabp4 and others is likely due to the fact that MIX activates CBP, a coactivator of PPAR that is necessary for PPARγ-induced adipocyte differentiation (39,40). We found significant upregulation of an array of genes involved in lipid synthesis and transport in the presence and absence of MIX in SCO and ROSI conditions, including Fabp4, where the protein level was elevated with SCO and ROSI with and without the full adipogenic cocktail. Fabp4 is a direct target of PPARγ (18) and has previously been shown to be strongly induced by MIX (17,29). The increase in FABP4 gene and protein expression, as well as enhanced transcription of other PPARγ targets such as Cebpa, Cd36 and Lpl (19,30,41) further supports the idea that SCO, similar to ROSI, is increasing PPARγ activity. As previously indicated, there are several compounds in SCO, making it difficult to determine exact mechanisms resulting in enhanced adipogenesis, which is a notable limitation of this study. Although we did not assess PPARγ activity directly, these data indicate that SCO treatment is promoting adipogenesis by enhancing lipid storage genes, including the PPARγ target genes involved in adipogenesis and lipid accumulation. It is important to note that elevated FABP4 has recently been associated with negative outcomes of metabolic health such as obesity and heart disease (29,42). While the majority of these findings are correlative, they could suggest FABP4 as a negative regulator of metabolic health. Indeed, recent evidence indicates FABP4 propagates lipolytic activity (43), and lipolysis is elevated in obesity and is strongly associated with metabolic disease, see review (44). However, the aforementioned studies were performed in mature adipocytes or whole adipose tissue, while our data are from cells undergoing adipogenesis. Though mature adipocytes originate from preadipocytes, there are a variety of types and sources of preadipocytes that they have vastly different expression profiles and perform different functions, therefore direct comparisons cannot be made. Although we do not know if SCO directly upregulates FABP4 in mature adipocytes, our lab has previously shown that SCO significantly reduces lipolysis (36). Therefore, we do not think that the increase in FABP4 in differentiating adipocytes contradicts the hypothesis that SCO-induced adipogenesis may be mediating the attenuation of metabolic disease outcomes observed in our mouse models.
Our lab has previously shown that SCO is able to improve glucose homeostasis and enhance adipogenesis (5,6), similar to TZD’s. However, it is important to note that we observed several differences among SCO and ROSI conditions across the adipogenic treatments. Specifically, ROSI was able to promote the greater lipid accumulation in nearly all treatment conditions, with the exception of vehicle and MDI; whereas SCO was similar to DMSO when MIX and insulin were removed (D). In line with this, we observed the induction of FABP4 expression by ROSI when MIX and insulin were removed (D). Although not significant, we also observed differences in PPARγ2 expression between SCO and ROSI in the MDI treatment, where SCO was again similar to DMSO. TZD’s have been shown to decrease PPARγ protein expression due to rapid proteasomal degradation following ligand activation, therefore the reduced expression of PPARγ2 in our study indicates greater activation when compared to SCO (45). However, the discrepancy between PPARγ expression and activation becomes less evident in conditions where there is reduced adipogenesis compared to MDI, as this could also indicate that expression of PPARγ was lower in general. Nevertheless, this finding provides more evidence to suggest that SCO-induced PPARγ activation is not occurring through the same mechanism as ROSI. Lastly, ROSI resulted in the greatest gene expression across treatments for several adipogenic targets, most notably in the vehicle treatment there were elevations in Cebpa, Cd36, Fabp4, Lpl and Glut4, all PPARγ target genes; whereas, SCO resulted in much lower expression of these genes, and was not different than DMSO vehicle treatment. These data indicate that although SCO is enhancing adipogenesis and PPARγ activity, as assessed by increased PPARγ target genes, and is similar to ROSI, there are distinct differences in their outcomes. This is a promising finding since TZD treatment is effective at improving glucose homeostasis in Type 2 diabetes, but is associated with deleterious side effects and improved therapeutics are needed. SCO extract includes many compounds and much additional research is needed to identify the compounds that promote adipogenesis. The importance of botanical research in biomedical research comes from the use of Metformin, the first line of pharmacological treatment for Type 2 diabetes, and many other pharmaceuticals that have been derived or developed from plant extracts. In summary, our data provide evidence that the effects of SCO on adipocyte development are not identical to ROSI, and as such may not result in the same undesirable clinical manifestations. Therefore, continued study regarding the clinical safety and efficacy is warranted.
Conclusion
The findings reported here reveal the ability of SCO to induce adipogenesis in the absence of MIX and our data suggest this is likely associated with enhanced PPARγ activity. Our results demonstrate unique characteristics of SCO when compared to ROSI, highlighting the need for further investigation of SCO as a potential therapeutic in metabolic disease treatment.
What is already known about this subject?
Artemisia scoparia (SCO) extracts attenuate insulin resistance and hepatic steatosis in vivo and promote adipogenesis in vitro. SCO treatment is associated with PPARγ activation.
Agonists of PPARγ, such as rosiglitazone (ROSI) have been used to treat type 2 diabetes and are known to promote adipogenesis in vivo and in vitro, but have limited medicinal use due to a variety of negative side effects.
Unpublished data from our lab suggests that SCO and ROSI are not enhancing PPARγ activity by the same mechanism, and therefore SCO may promote beneficial effects of PPARγ agonism without the harmful side effects.
What are the new findings in your manuscript?
SCO and ROSI can promote adipogenesis in the absence of one or more of the classic differentiation cocktail components, 1-methyl-3-isobutylxanthine (MIX), insulin and dexamethasone.
The preservation of lipid accumulation by SCO and ROSI in the absence of MIX is accompanied by enhanced expression of genes and proteins involved in lipid accumulation, many of which are PPARγ target genes.
Differential regulation of gene expression and adipogenic capabilities are observed when comparing SCO and ROSI.
These data indicate that SCO is upregulating PPARγ activity, but is not identical to the effects of ROSI.
How might your results change the direction of research or the focus of clinical practice?
These findings support previous evidence that SCO extracts include compounds that have significant adipogenic capabilities and provide insight into the underlying pathways involved in this process.
Adipogenesis can be associated with improved metabolic health and future research into specific SCO compounds that confer these positive effects may provide potential therapeutic targets that would mimic the beneficial effects of ROSI and other PPARγ agonists without the detrimental outcomes.
Funding:
I.H. was supported by T32AT00409 and the research was supported by P50AT002776 from NCCIH.
Footnotes
Disclosure: The authors declared no conflicts of interest
References
- 1.Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep [Internet]. 2018. Feb 26 [cited 2018 May 1];20(2):12. Available from: http://link.springer.com/10.1007/s11906-018-0812-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lingvay I, Catarig A-M, Frias JP, Kumar H, Lausvig NL, le Roux CW, et al. Efficacy and safety of once-weekly semaglutide versus daily canagliflozin as add-on to metformin in patients with type 2 diabetes (SUSTAIN 8): a double-blind, phase 3b, randomised controlled trial. Lancet Diabetes Endocrinol [Internet]. 2019;7(11):834–44. Available from: http://www.sciencedirect.com/science/article/pii/S2213858719303110 [DOI] [PubMed] [Google Scholar]
- 3.Willcox M Artemisia Species: From Traditional Medicines to Modern Antimalarials—and Back Again. J Altern Complement Med [Internet]. 2009;15(2):101–9. Available from: 10.1089/acm.2008.0327 [DOI] [PubMed] [Google Scholar]
- 4.Wang ZQ, Zhang XH, Yu Y, Tipton RC, Raskin I, Ribnicky D, et al. Artemisia scoparia extract attenuates non-alcoholic fatty liver disease in diet-induced obesity mice by enhancing hepatic insulin and AMPK signaling independently of FGF21 pathway. Metabolism [Internet]. 2013. [cited 2018 Jul 11];62:1239–49. Available from: 10.1016/j.metabol.2013.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richard AJ, Burris TP, Sanchez-Infantes D, Wang Y, Ribnicky DM, Stephens JM. Artemisia extracts activate PPARγ, promote adipogenesis, and enhance insulin sensitivity in adipose tissue of obese mice. Nutrition [Internet]. 2014. Jul [cited 2018 May 2];30(7–8 SUPPL.):S31–536. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24985103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richard AJ, Fuller S, Fedorcenco V, Beyl R, Burris TP, Mynatt R, et al. Artemisia scoparia enhances adipocyte development and endocrine function in vitro and enhances insulin action in vivo. PLoS One. 2014;9(6):e98897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gavrilova O, Marcus-Samuels B, Graham D, Kim JK, Shulman GI, Castle AL, et al. Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. J Clin Invest [Internet]. 2000. Feb [cited 2019 Mar 1];105(3):271–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10675352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith U, Kahn BB. Adipose tissue regulates insulin sensitivity: role of adipogenesis, de novo lipogenesis and novel lipids. J Intern Med [Internet]. 2016. Nov 1 [cited 2019 Feb 27];280(5):465–75. Available from: http://doi.wiley.com/10.1111/joim.12540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakae J, Kitamura T, Kitamura Y, Biggs WH, Arden KC, Accili D. The forkhead transcription factor Fox01 regulates adipocyte differentiation. Dev Cell. 2003;4(1):119–29. [DOI] [PubMed] [Google Scholar]
- 10.Kim JB, Wright HM, Wright M, Spiegelman BM. ADD1/SREBP1 activates PPARγ through the production of endogenous ligand. Proc Natl Acad Sci U S A. 1998;95(8):4333–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dif N, Euthine V, Gonnet E, Laville M, Vidal H, Lefai E. Insulin activates human sterol-regulatory-element-binding protein-1c (SREBP-1c) promoter through SRE motifs. Biochem J. 2006;400(1):179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosen ED, Hsu CH, Wang X, Sakai S, Freeman MW, Gonzalez FJ, et al. C/EBPα induces adipogenesis through PPARγ: A unified pathway. Genes Dev. 2002;16(1):22–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oishi Y, Manabe I, Tobe K, Tsushima K, Shindo T, Fujiu K, et al. Krüppel-like transcription factor KLF5 is a key regulator of adipocyte differentiation. Cell Metab [Internet]. 2005. Jan 1 [cited 2019 Mar 7];1(1):27–39. Available from: https://www.sciencedirect.com/science/article/pii/S1550413104000063?via%3Dihub [DOI] [PubMed] [Google Scholar]
- 14.Merrett JE, Bo T, Psaltis PJ, Proud CG. Identification of DNA response elements regulating expression of CCAAT/enhancer-binding protein (C/EBP) β and δ and MAP kinase-interacting kinases during early adipogenesis. Adipocyte. 2020;9(1):427–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersen RK, Madsen L, Pedersen LM, Hallenborg P, Hagland H, Viste K, et al. Cyclic AMP (cAMP)-Mediated Stimulation of Adipocyte Differentiation Requires the Synergistic Action of Epac- and cAMP-Dependent Protein Kinase-Dependent Processes. Mol Cell Biol. 2008;28(11):3804–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao Z, Umek RM, McKnight SL. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991;5(9):1538–52. [DOI] [PubMed] [Google Scholar]
- 17.Sun L, Nicholson AC, Hajjar DP, Gotto AM, Han J. Adipogenic differentiating agents regulate expression of fatty acid binding protein and CD36 in the J744 macrophage cell line. J Lipid Res. 2003;44(10):1877–86. [DOI] [PubMed] [Google Scholar]
- 18.Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev [Internet]. 1994. May 15 [cited 2019 Mar 7];8(10):1224–34. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7926726 [DOI] [PubMed] [Google Scholar]
- 19.Lefterova MI, Zhang Y, Steger DJ, Schupp M, Schug J, Cristancho A, et al. PPARγ and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 2008;22(21):2941–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Z, Rosen ED, Brun R, Hauser S, Adelmant G, Troy AE, et al. Cross-regulation of C/EBPα and PPARγ controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol Cell. 1999;3(2):151–8. [DOI] [PubMed] [Google Scholar]
- 21.Park Y, Freedman BD, Lee EJ, Park S, Jameson JL. A dominant negative PPARγ mutant shows altered cofactor recruitment and inhibits adipogenesis in 3T3-L1 cells. Diabetologia. 2003;46(3):365–77. [DOI] [PubMed] [Google Scholar]
- 22.Tang W, Zeve D, Seo J, Jo AY, Graff JM. Thiazolidinediones regulate adipose lineage dynamics. Cell Metab. 2011. Jul 6;14(1):116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosen CJ. The Rosiglitazone story: Lessons from an FDA Advisory Committee Meeting. ACC Cardiosource Rev J. 2007;16(9):27–9. [DOI] [PubMed] [Google Scholar]
- 24.Nissen SE, Wolski K. Effect of Rosiglitazone on the Risk of Myocardial Infarction and Death from Cardiovascular Causes. N Engl J Med. 2007;356(24):2457–71. [DOI] [PubMed] [Google Scholar]
- 25.Boudreau A, Poulev A, Ribnicky DM, Raskin I. Distinct Fractions of an Artemisia scoparia Extract Contain Compounds With Novel Adipogenic Bioactivity. 2019;6(March):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boudreau A, Poulev A, Ribnicky DM, Raskin I, Rathinasabapathy T, Richard AJ, et al. Distinct fractions of an artemisia scoparia extract contain compounds with novel adipogenic bioactivity. Front Nutr [Internet]. 2019. Mar 8 [cited 2019 Mar 26];6:18. Available from: https://www.frontiersin.org/article/10.3389/fnut.2019.00018/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruiz-Ojeda FJ, Rupérez AI, Gomez-Llorente C, Gil A, Aguilera CM. Cell models and their application for studying adipogenic differentiation in relation to obesity: A review. Int J Mol Sci. 2016;17(7):1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4(4):263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu LE, Samocha-Bonet D, Whitworth PT, Fazakerley DJ, Turner N, Biden TJ, et al. Identification of fatty acid binding protein 4 as an adipokine that regulates insulin secretion during obesity. Mol Metab [Internet]. 2014;3(4):465–73. Available from: 10.1016/j.molmet.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schoonjans K, Peinado-Onsurbe J, Lefebvre AM, Heyman RA, Briggs M, Deeb S, et al. PPARα and PPARγ activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. EMBO J. 1996;15(19):5336–48. [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Z, Xie Y, Morrison RF, Bucher NLR, Farmer SR. PPARγ induces the insulin-dependent glucose transporter GLUT4 in the absence of C/EBPα during the conversion of 3T3 fibroblasts into adipocytes. J Clin Invest. 1998;101(1):22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spiegelman BM, Frank M, Green H. Molecular Cloning of mRNA from 3T3 Adipocytes. J Biol Chem. 1983;258(16):10083–9. [PubMed] [Google Scholar]
- 33.McArthur MJ, Atshaves BP, Frolov A, Foxworth WD, Kier AB, Schroeder F. Cellular uptake and intracellular trafficking of long chain fatty acids. J Lipid Res. 1999;40(8):1371–83. [PubMed] [Google Scholar]
- 34.Noori S, Hassan ZM, Rezaei B, Rustaiyan A, Habibi Z, Fallahian F. Artemisinin can inhibit the calmodulin-mediated activation of phosphodiesterase in comparison with Cyclosporin A. Int Immunopharmacol [Internet]. 2008;8(13–14):1744–7. Available from: 10.1016/j.intimp.2008.08.012 [DOI] [PubMed] [Google Scholar]
- 35.Noori S, Hassan ZM. Tehranolide inhibits cell proliferation via calmodulin inhibition, PDE, and PKA activation. Tumor Biol. 2014;35(1):257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boudreau A, Richard AJ, Burrell JA, King WT, Dunn R, Schwarz J-M, et al. An ethanolic extract of Artemisia scoparia inhibits lipolysis in vivo and has antilipolytic effects on murine adipocytes in vitro. Am J Physiol Metab [Internet]. 2018. Nov [cited 2019 Mar 26];315(5):E1053–61. Available from: https://www.physiology.org/doi/10.1152/ajpendo.00177.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka T, Yoshida N, Kishimoto T, Akira S. Defective adipocyte differentiation in mice lacking the C/EBPβ and/or C/EBPδ gene. EMBO J. 1997;16(24):7432–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu Z, Bucher NL, Farmer SR. Induction of peroxisome proliferator-activated receptor gamma during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPbeta, C/EBPdelta, and glucocorticoids. Mol Cell Biol. 1996;16(8):4128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi N, Kawada T, Yamamoto T, Goto T, Taimatsu A, Aoki N, et al. Overexpression and ribozyme-mediated targeting of transcriptional coactivators CREB-binding protein and p300 revealed their indispensable roles in adipocyte differentiation through the regulation of peroxisome proliferator-activated receptor γ. J Biol Chem. 2002;277(19):16906–12. [DOI] [PubMed] [Google Scholar]
- 40.Liu H, Jing RT, Young HC, Napolitano M, Hockman S, Taira M, et al. Importance of cAMP-response element-binding protein in regulation of expression of the murine cyclic nucleotide phosphodiesterase 3B (Pde3b) gene in differentiating 3T3-L1 preadipocytes. J Biol Chem. 2006;281(30):21096–113. [DOI] [PubMed] [Google Scholar]
- 41.Zuo Y, Qiang L, Farmer SR. Activation of CCAAT/enhancer-binding protein (C/EBP) α expression by C/EBPβ during adipogenesis requires a peroxisome proliferator-activated receptor-γ-associated repression of HDAC1 at the C/ebpα gene promoter. J Biol Chem. 2006;281(12):7960–7. [DOI] [PubMed] [Google Scholar]
- 42.Gormez S, Erdim R, Akan G, Caynak B, Duran C, Gunay D, et al. Relationships between visceral/subcutaneous adipose tissue FABP4 expression and coronary atherosclerosis in patients with metabolic syndrome. Cardiovasc Pathol [Internet]. 2020;46:107192. Available from: http://www.sciencedirect.com/science/article/pii/S1054880719303576 [DOI] [PubMed] [Google Scholar]
- 43.Ertunc ME, Sikkeland J, Fenaroli F, Griffiths G, Daniels MP, Cao H, et al. Secretion of fatty acid binding protein aP2 from adipocytes through a nonclassical pathway in response to adipocyte lipase activity. J Lipid Res. 2015. Feb 1;56(2):423–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nielsen TS, Jessen N, Jorgensen JOL, Moller N, Lund S. Dissecting adipose tissue lipolysis: molecular regulation and implications for metabolic disease. J Mol Endocrinol [Internet]. 2014. May 21 [cited 2018 Apr 30];52(3):R199–222. Available from: http://jme.endocrinology-journals.org/cgi/doi/10.1530/JME-13-0277 [DOI] [PubMed] [Google Scholar]
- 45.Hauser S, Adelmant G, Sarraf P, Wright HM, Mueller E, Spiegelman BM. Degradation of the peroxisome proliferator-activated receptor γ is linked to ligand-dependent activation. J Biol Chem. 2000. Jun 16;275(24):18527–33. [DOI] [PubMed] [Google Scholar]