Abstract
Abnormal lipid metabolism is common in breast cancer with the three main subtypes, hormone receptor (HR) positive, human epidermal growth factor 2 (HER2) positive, and triple negative, showing common and distinct lipid dependencies. A growing body of studies identify altered lipid metabolism as impacting breast cancer cell growth and survival, plasticity, drug resistance, and metastasis. Lipids are a class of nonpolar or polar (amphipathic) biomolecules that can be produced in cells via de novo synthesis or acquired from the microenvironment. The three main functions of cellular lipids are as essential components of membranes, signaling molecules, and nutrient storage. The use of mass spectrometry-based lipidomics to analyze the global cellular lipidome has become more prevalent in breast cancer research. In this review, we discuss current lipidomic methodologies, highlight recent breast cancer lipidomic studies and how these findings connect to disease progression and therapeutic development, and the potential use of lipidomics as a diagnostic tool in breast cancer. A better understanding of the breast cancer lipidome and how it changes during drug resistance and tumor progression will allow informed development of diagnostics and novel targeted therapies.
Keywords: Breast Cancer, Lipidomics, Lipid, Fatty Acid
1. Introduction
Metabolic reprograming is a long-standing hallmark of cancer that encompasses diverse processes involved in energy production, macromolecule biosynthesis and degradation. Abnormally regulated lipid metabolism is relatively understudied compared to other branches of cancer metabolism despite playing a critical role in tumor cell biology. Lipid metabolism is notably altered in breast cancer versus normal breast cells, and is hypothesized to contribute to tumor cell plasticity, therapeutic resistance, and metastasis [1]. However, studies find considerable variation in both lipid anabolic and catabolic pathways, potentially due to intertumoral heterogeneity and the existence of distinct disease subtypes, and thus there is no clear targetable metabolic signature at present.
Breast cancer is the most common malignancy among women, comprising 30% of newly diagnosed cancer cases in the US [2]. Breast cancer is generally stratified into three main subtypes based on the presence of estrogen receptor (ER) and progesterone receptor (PR) (or hormone receptor (HR) positive), amplification/overexpression of human epidermal growth factor receptor 2 (HER2+), or lack of all three markers [triple negative breast cancer (TNBC)] [3]. Positivity for HR or HER2 initially stratifies patients into anti-endocrine- or HER2-targeted therapies, respectively [4, 5]. Acquired resistance and recurrence occurs in 10-41% of HR+ breast cancers depending on grade/stage [6, 7]. Late-stage HR+ tumors are treated with inhibitors to CDK4/6 and PIK3CA [8-11]. Treatment of TNBC primarily relies on chemotherapy, with limited options for PARP inhibitors or immunotherapy [12]. Almost all refractory breast cancers eventually develop resistance to second line drugs [13, 14]. Targeting other processes in breast cancer including metabolism has long been thought to hold therapeutic potential. Discovery of the Warburg Effect, or the preference towards anaerobic metabolism in the presence of oxygen, originally highlighted that tumor cell metabolism could be a targetable vulnerability [13, 14]. Unfortunately, development of anti-cancer drugs targeting metabolic changes has not been successful in breast cancer to date. Lipid regulatory pathways may be effective therapeutic targets, especially in breast cancers where lipid metabolism plays a central role both normal mammary gland and tumor biology.
Lipid metabolism includes the enzymatic biosynthesis, covalent modification, and degradation of fatty acids (FA) and their lipid derivatives. Prior to the use of mass spectrometry to identify organic molecules, technical limitations restricted the study of cellular lipids [15]. The development of electron spray ionization (ESI) and matrix assisted laser desorption ionization (MALDI) techniques 30 years ago improved the ability to detect and quantify lipids within biological samples, and the field of lipidomics emerged [16, 17]. In the past decade, these techniques have been further refined and their use has steadily increased in biological research. At present, there are multiple methods for lipid extraction/separation, mass spectrometry, metabolite annotation, and data analysis/normalization, which provides challenges for data sharing and points to the importance of technical "gold standards" [18, 19]. Lipidomics has the potential to provide previously unavailable information on cellular lipidomes and lipid metabolism, which can be combined with proteomic and genomic data to identify novel therapeutic strategies in breast cancer.
In this review, we cover recent developments in lipid metabolism and the lipidome in the three main subtypes of breast cancer, and their potential meaning to cancer biologists. We include discussion of various lipidomic methods and data analysis present in the breast cancer literature. Lastly, we highlight the role of breast cancer lipid signatures and their contribution to disease detection, progression, and treatment.
2. Mammalian lipid structures and functions
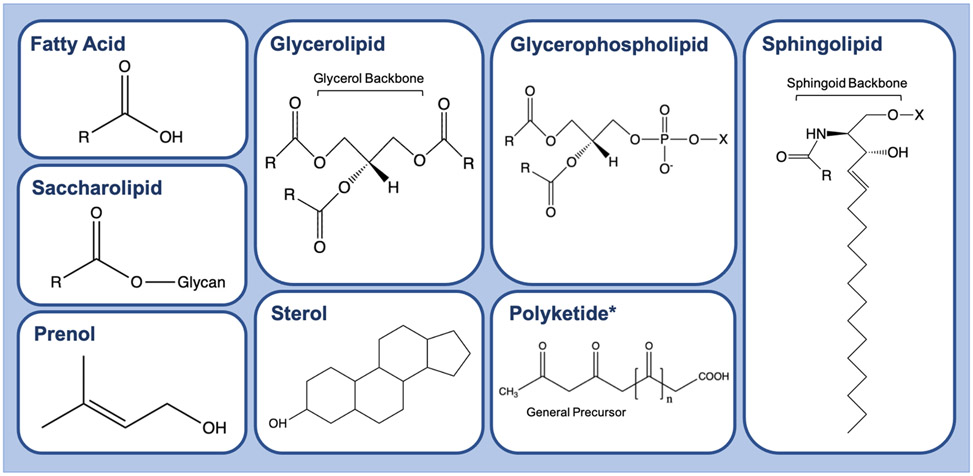
Lipids are hydrophobic or amphipathic (having both hydrophobic and hydrophilic regions) small molecules that serve essential functions as membrane components, energy storage, and signaling molecules in mammalian cells. Lipids can be broadly classified into eight categories, seven of which are found in mammals: fatty acyls, glycerolipids, glycerophospholipids, sphingolipids, saccharolipids, sterols, and prenols (Fig.1) [20]. These categories can also be subdivided into either nonpolar, neutral lipids or polar (amphipathic) lipids. Hydrophobicity is determined by one or more hydrocarbon chains that vary in chain length and degree of saturation. Some lipids contain headgroups and modifications that provide amphipathic characteristics. In mammalian cells, a family of enzymes termed elongases regulate the length of lipid hydrocarbon tails. Chain length typically ranges from 4-22 carbons; however, chains with >24 carbons are occasionally present in mammals [21]. Hydrocarbon chain saturation, or the number of double bonds, is regulated by saturase and desaturase enzymes and thus characterized as unsaturated, monounsaturated, or polyunsaturated. The essential linoleic and linolenic acids must be acquired from the diet since mammalian cells lack the desaturase enzymes necessary to produce these FA from their 18-carbon precursors [22]. The degree of lipid saturation impacts properties such as interaction with other organic molecules [23]. The LIPID MAPS consortium devised a lipid nomenclature system for researchers to effectively describe the position and degree of lipid saturation [24].
Fig. 1.
Lipid Classes. The eight lipid classes: fatty acids, glycerolipids, glycerophospholipids, sphingolipids, sterol lipids, prenol lipids, saccharolipids, and polyketides. Fatty acids and fatty acyls (activated fatty acids) are the simplest lipid category that serves as building blocks for complex lipids; includes eicosanoids, docosanoids, fatty alcohols, fatty aldehydes, fatty esters, fatty amides, fatty nitriles, fatty ethers, and hydrocarbons. Glycerolipids are neutral lipids containing a glycerol backbone; includes monoacylglycerol (MG), diacylglycerol (DG), and triacylglycerol (TG) species. Glycerophospholipids are membrane lipids that contain a phosphodiester linked to a hydroxyl group of glycerol and are differentiated by the type of moieties (X) esterified to the phosphate. Sphingolipids contain a sphingoid base backbone and vary by polar moieties (X) esterified to the backbone. Sterols contain a common steroid core of a fused four-ring structure with a hydrocarbon side chain and an alcohol group, cholesterol being the most common and functionally important for membrane integrity. Prenol lipids consist of one or more 5 carbon prenol derivatives that can link in chain or ring-like structures. Saccharolipids generally consist of fatty acids directly esterified to a sugar. (R) represents hydrocarbon chain at an arbitrary length. (n) represents repeating carbonyl components
The main lipid species in cell membranes are phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), phosphatidylinositol (PI), phosphatidylglycerol (PG), phosphatidic acid (PA) and cardiolipin (CL). Phospholipids are amphipathic in nature by containing a polar phosphate and glycerol head group and non-polar fatty acyl chains. The extracellular plasma membrane is a lipid bilayer mainly containing structural phospholipid species: PC, PE, PI, PS, and the phospholipid-precursor, phosphatidic acid. PC is the most abundant phospholipid in eukaryotic cell membranes and accounts for about 50% of total cellular phospholipid mass [25]. PC head groups have a cylindrical geometry which provides a planar shape to lipid bilayers. PE is another abundant membrane phospholipid and contributes to membrane curvature with a smaller conical headgroup geometry. The ratio of PC and PE species within the membrane can impose curvature stress onto the membrane, which is used for budding, fission and fusion [26]. PS is almost exclusively found in the inner cytoplasmic leaflet of the plasma membrane and, when flipped to the outer leaflet, is a signal for apoptosis and platelet activation [27, 28].
Sphingomyelin (SM) and sterols also comprise a large component of the membrane. SM belong to the class of sphingolipids and differ from phospholipids by a long-chain nitrogenous base backbone, termed sphingosine. The saturated (or trans-unsaturated) SM tails allow these species to form longer and narrower cylinders than PC of the same chain length. Consequently, SM can assemble tightly together, a phenomenon sometimes referred to as "lipid-packing", resulting in a more rigid membrane state. Neutral sterols such as cholesterol balance SM structural rigidity and maintain membrane fluidity [29]. Sphingolipids, cholesterol, and the degree of phospholipid hydrocarbon saturation affect overall membrane fluidity [23]. It is hypothesized that lipid bilayers do not exist as a homogenous lipid composition, but rather clusters of dense and fluid areas [30]. Denser areas, referred as lipid rafts, are comprised of packable lipids and clusters of membrane-bound proteins [31]. More fluid membrane areas contain unsaturated phospholipids as their non-linear acyl tails prevent tight "lipid-packing" interactions [32]. The plasma membrane also contains structures involved in endocytosis such as caveolae, which studies have found are rich in SM [33]. Most intracellular organelles also contain lipid bilayers, including the endoplasmic reticulum, golgi, nucleus, mitochondria, and lysosomes. Their membranes consist of PC, PE, PI, and some cholesterol which result in a dynamic flexible interface. The endoplasmic reticulum is the primary location of phospholipid, glycerolipid, and cholesterol synthesis. The golgi membrane closely resembles the endoplasmic reticulum membrane and contains similar lipid species with increased SM and PS content [34]. In mammalian cells, the golgi is the main producer of complex sphingolipids like SM, glucosylceramide (GlcCer), and lactosylceramide (LacCer), although, Cer from the endoplasmic reticulum is required for golgi-mediated sphingolipid metabolism [35, 36]. Nuclear membranes are also enriched in PC, PE, PI and cholesterol which contribute to flexible membrane dynamics. While there are fewer studies on eukaryotic nuclear and nucleolar membrane compositions, they are thought to be similar to the endoplasmic reticulum [37]. The mitochondria are unique in having two membranes separating spaces with different pH. Unlike the plasma membrane, mitochondrial membranes are composed of about 15% CL and low levels of sphingolipids and cholesterol [38]. CL plays an essential role in regulating mitochondrial transporter function and mitochondria-organelle interactions [39, 40]. Lysosomal lipid membranes are low in cholesterol and high in sphingolipid content [41]. Lysosomes are also involved in lipid trafficking, specifically cholesterol and exogenous triacylglycerol (TG), sterols, and phospholipids from endosomes [42].
The two other essential lipid functions are providing metabolic fuel and acting as signaling molecules. Lipids are stored in droplets in the cytosol, composed mainly of neutral lipid species: TG, diacylglycerol (DG), and cholesterol species. Lipid droplets are formed off the endoplasmic reticulum where newly synthesized TGs can be readily packaged by the perilipin (PLIN) family of 5 proteins [43]. This process is particularly important for alveolar cells in the lactating mammary gland where lipids obtained via de novo synthesis and diet feed into TG synthesis for milk-fat globule production [44]. In times of nutrient deprivation, cells can initiate hydrolysis of TG and DG from lipid droplets, releasing FAs from glycerol for degradation by fatty acid oxidation (FAO). Not all lipid droplet components are broken down for energy. DG serves as a lipid messenger activating protein kinase C (PKC) and intracellular Ca2+ release [45]. DGs also trigger the translocation of protein kinase D which catalyzes the formation of secretory vesicles [46, 47]. Phosphorylation of DG or hydrolysis of phospholipids results in phosphatidic acid, another multifunctional lipid second messenger. Phosphatidic acid has been shown to attenuate hippo pathway signaling through lipid-protein interference [48]. Although PI is a membrane component, its phosphorylation plays a role in PI3K signaling and AKT activation, the most frequently mutated pathway in HR+ breast cancer [49]. Additionally, Cer synthesized at high levels triggers a cellular apoptotic program through JNK and p38 signaling [50]. Prostaglandins and other eicosanoids are involved in immune cell signaling and inflammation through a variety G-protein coupled receptors. The functions described here give a brief overview of the fundamental role lipids play in most cellular processes.
3. Current analytical methods for lipidomics
The collective lipid content in a cell is termed the lipidome. Lipidomics is the study of the lipidome through identification and quantification of lipid analytes within a given sample. The term "lipidomics" emerged in the early 2000s when mass spectrometry (MS)-based methods were optimized for lipid identification studies. In the past decade, lipidomics have been conducted for the study of human diseases such as cancer. Technological advancements in MS now allow different analytic coverage (global/untargeted or targeted lipidomics) and has broadened the scope of lipid research. There are several detailed technical reviews on lipidomics [51-53]; here we give a brief overview of current analytical methods and their application towards breast cancer research.
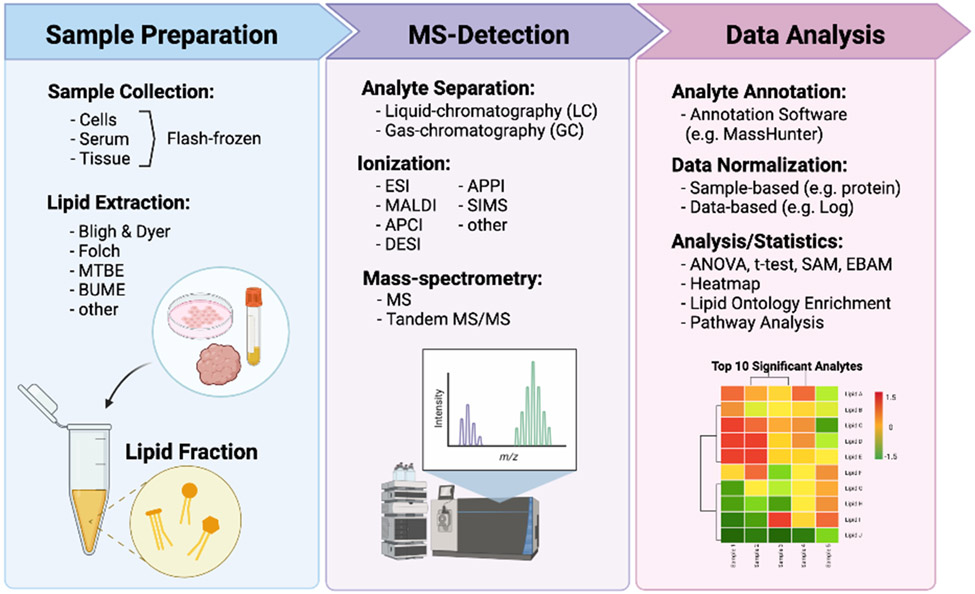
The general workflow of lipidomic experiments typically involves sample preparation, MS acquisition, and data processing (Fig. 2). Liquid chromatography (LC)-MS is the most common technique which utilizes columns for analyte separation before MS detection and can be used for targeted or untargeted approaches. Direct-infusion techniques such as shot-gun lipidomics take advantage of the chemical properties of lipids for lipid identification and allow for direct use of samples with minimal preparation. Most lipidomic techniques utilize extracts prepared from biological samples (i.e. cell lines, tissues); however, MS imaging can analyze whole tissue slices (e.g. MALDI MS-imaging). Sample extraction isolates the lipid fraction of a biological sample for MS analysis. A few common extraction methods include the Modified Bligh and Dyer, Modified Folch, Methyl tertiary-butyl ether (MTBE), and butanol-methanol (BUME) methods, each varying in solvent and solvent ratios. Unfortunately, there is not a single extraction method that captures all lipid species with high recovery percentage, yet each of the following liquid-liquid extraction methods have their advantages and disadvantages. Modified Bligh and Dyer method uses chloroform/methanol/H2O (1:1:0.9, v/v/v) for extraction of small biological samples (<50mg of tissue) and traps lipids in the chloroform phase [54]. The Modified Folch method is similar to Bligh and Dyer and uses chloroform/methanol (2:1, v/v) for biological tissue extraction (~100mg), then water or 0.9% NaCl (0.2 volume) is added to wash extracts [55]. This method was designed to improve capture from lipid-rich samples which may otherwise be excluded using the Bligh and Dyer method. The MTBE method uses MTBE/methanol/water (5:1.5:1.45, v/v/v), trapping total lipids in the top MTBE fraction [56]. The benefit of this method is that uses fewer toxic solvents and is more feasible for high throughput or automated set ups. The BUME method uses a volume of butanol/methanol (3:1, v/v) and a small aqueous phase volume. An equal volume of heptane/ethyl acetate (3:1, v/v) is then added followed by an equal volume 1% acetic acid [57]. This method is proposed to reduce water-soluble contaminants that may be found in the previously described methods. The methods described each use organic systems for "wide net" lipid analyte capture and are used for both targeted and untargeted analyses [58]. The choice of extraction method for targeted analyses is dependent on the subset of lipids in question. Neutral lipid species with higher hydrophobicity are best captured by methods with nonpolar solvents such as cyclohexane or toluene. Intermediate polar lipids such as sphingolipids or phospholipids are best extracted with polar solvents like chloroform or MTBE [58]. It is important to note that MALDI lipidomics require little to no sample preparation and was recently discussed in a detailed technical review [59]. After extraction, additional steps may be necessary depending on the type of MS being conducted. For direct-infusion based approaches, it is important to simplify sample extracts since no chromatography separation is applied prior to MS analysis [52]. This can be achieved through physical (phase separation) or chemical approaches (base hydrolysis) to enrich low abundance lipids [60, 61]. Lipid derivation is another option that chemically tags specific functional groups on lipids and can aid in MS identification [61]. The benefit of these steps is that they increase detection of target analytes (i.e. sphingolipids) in low abundance samples without the use of columns for analyte separation for direct-infusion approaches such as shot-gun lipidomics.
Fig. 2.
General Workflow of Lipidomics for Breast Cancer Research. Three main steps to lipidomic analysis include sample preparation, MS-Detection, and Data Analysis. MTBE = methyl tert-butyl ether, BUME = butanol/methanol, ESI = Electrospray ionization, MALDI = Matrix assisted laser desorption ionization, SIMS = Secondary ion mass spectrometry, APCI = Atmospheric Pressure Chemical Ionization, APPI = atmospheric pressure polarization ionization, DESI = Desorption electrospray ionization, MS = Mass spectrometry, ANOVA = Analysis of variance, SAM = Significance Analysis of Microarrays, EBAM = Empirical Bayes Analysis of Microarrays.
Ionization is the next step following lipid extract preparation. The type of ionization depends on whether direct infusion MS (shotgun lipidomics) or chromatography-based MS (LC-based lipidomics) is being performed. It is important to note that MS imaging requires ionization as well. The two most popular ionization techniques are ESI and MALDI. ESI is a soft ionization technique that uses an electrospray produced from a strong electric field applied to a liquid passing through a capillary. This results in a fine aerosol from which ions are formed by desolvation [16]. MALDI is also a soft ionization technique but allows analysis of larger and labile molecules like peptides, proteins, and lipids. This technique is useful for MS imaging of tissue and establishes a matrix for analytes that absorbs energy at the wavelength of the laser. As the pulsed laser hits analytes, this triggers ablation and desorption from the matrix which facilitates analyte ionization [17]. Other popular ionization techniques include Atmospheric Pressure Chemical Ionization (APCI), Atmospheric Pressure Polarization (APPI), Secondary Ion Mass Spectrometry (SIMS), and Desorption ESI (DESI) which have been reviewed in detail [62]. Ion mobility is an optional step that furthers ion separation according to their charge shape and size [63]. Following ionization and ion mobility, full MS or tandem mass spectrometry (MS/MS) is performed depending on whether global or targeted analysis is desired. After MS analysis, the data is represented as MS spectra, MS/MS spectra, ion chromatogram, or images (MS-imaging).
Following data acquisition, spectral MS data undergoes deisotoping to remove spectral complications from the presence of isotopic clusters. This allows for easier mass identification and analyte annotation by lipidomics software. Lipidomic software match molecular masses to lipid identifiers specific to comprehensive databases such as LIPID MAPS, SWISS LIPIDS, Chemical Entities of Biological Interest (ChEBI), KEGG compound database, or human metabolome database (HMBD) [24, 64, 65]. Once qualitative and quantitative data are acquired, the results are further processed for bioinformatic analysis. There are many free online analysis tools available to apply statistical calculations and most accept raw spectra (mzML, mzXML or mzData) or MS peak intensities (e.g. Metaboanalyst) [66]. A key aspect to consider in data analysis is method of normalization. Currently, there lacks a "gold standard" method for lipidomic data normalization; however, there are several accepted methods in the literature (discussed in [19]). Data normalization can include both sample-based (e.g. sample protein,) and data-based (e.g. Log transformation) methods. Both are easily applicable to spectral data on the mentioned online analysis platforms; however, it is important to clearly document which methods are applied and make raw data publicly available at publication. Down-stream analysis of lipidomics data include pathway or enrichment analyses. These analyses are currently better suited for genetic data; however, new online tools tailored to lipids, such as Lipid Ontology Enrichment Analysis (LION) or Lipid Pathway Enrichment Analysis (LIPEA), have been recently developed [67, 68]. With the lipidomic analysis tools available today, we can conduct statistical comparisons between samples of normal and diseased states, calculate disease specific pathway enrichment, and assess global impacts of lipid metabolic networks as a consequence of disease. There is a growing application of lipidomics to biological research.
4. Breast cancer lipid signatures in cell lines and tumors
Advances in lipidomics technologies has led to an increase in the number of lipid studies focusing on breast cancer within the past 5 years. In 2020, a PubMed search query for “lipidomics” returned 1,560 articles, and adding “breast cancer” reduced the list to 28 publications. Here we will highlight several recent research studies utilizing breast cancer research lipidomics (2012-2021) that we found insightful to the scope of this review. These fall into two general categories: studies conducted in breast cancer cell lines versus those conducted in clinical breast tumor specimens. A summary of results from the studies discussed in this section can be found in Table 1.
Table 1.
Significant Identified Lipids in Breast Cancer. Increased and decreased lipid species compared to MCF10A cells, normal adjacent tissue, or healthy patient serum for breast cancer cell lines, tumor tissue, breast cancer patient serum, respectively. NR = None reported
| HR+ Breast Cancer | ||
| T47D Cell Line | Up | PE(32:2), PE(36:5), PE(38:0), DG(32:0), DG(34:0), LPC(16:0), LPC(18:0) |
| Down | NR | |
| MCF7 Cell Line | Up | TG(46:1), TG(46:2), TG(48:2), TG(50:2), TG(50:3), TG(52:2), TG(52.3), TG(54:3), PC(28:0), PC(28:1), PC(30:1), PC(40:2),PE(32:2), PE(36:5), SM(44:1), MUFAs |
| Down | NR | |
| CAMA-1 Cell Line | Up | PC(28:0), PC(28:1), PC(30:1), PE(P34:1/O-34:2), PE(P34:1/O-34:3), PE(P32:1), PE(36:3), PE(38:0), SM(44:1), SM(44:2), DG(32:0), DG(34:0) |
| Down | SM(32:1) | |
| Tumors | NR | |
| Serum | NR | |
| HER2+ Breast Cancer | ||
| SK-BR-3 Cell Line | Up | TG(40:0), TG(40:1), TG(42:0), TG(42:1), TG(44:0), TG(46:1), TG(46:2), PC(28:0), PC(28:1), PC(30:1), PE(P-32:1), PE(32:2), PE(36:5) |
| Down | PE(P-36:4), PE(O-34:2), PE(P-38:4), PE(O-38:5) | |
| Tumors | Up | PC(16:1), PC( 32:2) |
| Down | NR | |
| Serum | NR | |
| Triple Negative Breast Cancer | ||
| MDA-MB-231 Cell Line |
Up | PC(34:0), PC(O-34:0), DG(32:0), DG(34:0), LPC(18:0), PUFAs |
| Down | PE(P-34:1/O-34:2), SM(32:1) | |
| MDA-MB-436 Cell Line | Up | PC(34:0), PC(O-34:0), SM(34:2), DG(32:0), DG(34:0), LPC(16:0), LPC(18:0) |
| Down | PE(P-34:1/O-34:2) | |
| Tumors | Up | PC(32:1), PC(30:0), PC(32:0), PE(36:1) |
| Down | NR | |
| Serum | Up | PC(32:1), Cer(43:1), stearic acid |
| Down | NR | |
| General Breast Cancer versus Normal | ||
| Tumors | Up | PC(34:1), PC(32:0), PC(34:1), SM(d18:1/16:0), PE(P-16:0/22:6), PS(38:3), Free FAs |
| Down | NR | |
| Serum | Up | PC(32:1), Total TGs |
| Down | NR | |
| Recurrent versus Non-recurrent Breast Cancer | ||
| Tumors | Up | PC(32:1), PC(30:0) |
| Down | NR | |
Established cell lines models provide the most feasible models for querying lipid profiles under baseline and manipulated states. Three studies compared lipid profiles of breast cancer subtypes. Eiriksson et al. was the first study to conduct untargeted LC-MS lipidomics on a panel of seven widely used cell lines: non-tumorigenic MCF10A cells, and ER+ (MCF7, T47D, CAMA-1), TNBC (MDA-MB-231, MDA-MB-436), and HER2+ (SK-BR-3) breast cancer cells [69]. Based on their analysis, they concluded that breast cancer cells (with one exception) have lipid profiles distinct from MCF10A cells, and that each breast cancer subtype had a distinct lipidome. SK-BR-3 cells displayed the most distinct lipid profile with a relative abundance of TGs (less than C46) versus MCF10A and the other breast cancer cell lines. This could potentially be due to the high rate of de novo FA synthesis reported in HER2+ breast cancers [70]. ER+ MCF7 and T47D cells contained similar lipid signatures with a notable increase of PE(32:2) and PE(36:5) compared to MCF10A cells while CAMA-1 cells showed minimal difference. MDA-MB-231 and MDA-MB-436 TNBC cell lines exhibited an abundance of medium chain PC species (C<40) and saturated DG(32:0) and DG(34:0) species versus MCF10A cells. Therefore, TG and PC abundance may serve as a key lipid profile indicator of breast cancer subtype based on elevated de novo FA and PC synthesis observed in HER2+ and TNBC, respectively. It is important to note these studies were conducted in the absence of steroid hormone treatments in ER+ breast cancer cell lines. Future studies in the presence of hormones (i.e. estrogen and progesterone), and endocrine therapies (i.e. tamoxifen) will be important for deciphering the lipidome in ER+ breast cancer cells.
The process of epithelial-mesenchymal transition (EMT) is associated with metabolic changes yet studies on lipid metabolism in EMT are lacking. To investigate this, Giudetti et al. measured proteomic profiles via LC-MS and lipid profiles using GC/MS and NMR in MCF7 and MDA-MB-231 breast cancer cells as models of epithelial-like and mesenchymal-like cells, respectively [71]. By proteomic analysis, MDA-MD-231 cells had reduced expression of lipogenic enzymes compared to MFC7 cells (i.e. FASN, ACC, ACLY). Lipidomic analysis found that MDA-MB-231 cells exhibited increased levels of PUFAs, most notably PI(38:4), and cholesterol compared to MCF7 cells. Conversely, MCF7 cells displayed a higher percentage of monounsaturated fatty acids (MUFAs) which the authors suggested could be due to their observation that stearoyl-CoA desaturase (SCD) is overexpressed in MCF7 versus MDA-MB-231 cells. SCD has also been found to be elevated in ER+ versus TNBC clinical samples [72]. While this study identified potentially interesting changes in lipid use during EMT, a limitation is the inclusion of a single cell line of each type. These observations merit extension to additional breast cancer cell lines of various subtypes, as well as cells that have been induced to undergo EMT which would identify EMT-induced changes in the same genetic background.
One study investigated lipid changes associated with metastatic potential. Nishida-Aoki et al. compared lipidomic profiles between parental MDA-MB-231 cells and two syngeneic sublines selected for high (D3H2LN) vs low (D3H1) lymph node-metastatic potential [73]. Lipidomics were conducted on the three cell lines in addition to their secreted extracellular vesicles. D3H2LN vs parental and D3H1 cells had increased abundance of LPE, SM, PA, and hexosylceramide (HexCer) in their lipidome, and it was hypothesized the relative percent changes in these lipid species may be associated with metastatic potential. Both D3H2LN cells and EVs had significantly increased saturated DG(14:0/22:0) vs parental and D3H1 derived EVs, which could potentially activate PKD/PKC signaling in surrounding endothelial cells to promote angiogenesis. However the authors found no significant difference in the ability of EVs from either subline to activate PKC and thus the implication of saturated DG(14:0/22:0) is still undetermined [74].
The extracellular microenvironment plays a significant role in influencing tumor cell metabolism. Several studies measured the impact of specific environmental stressors on the cellular lipid landscape. Enhanced glycolysis is a common feature of cancer cells and results in an acidic tumor microenvironment from the production of lactate [75]. Urbanelli et al. determined the effect of microenvironment acidification (pH 6.5) on the lipid profiles of several cancer cell lines including MCF7 [76]. Under acidic vs baseline pH conditions, MCF7 cells decreased PC chain saturation and increased elongase and desaturase enzyme expression. These data insinuate a protective effect of longer, unsaturated phospholipid remodeling against acid pH that requires further study. Nutrient deprivation can also occur in specific tumor microenvironments. For example, methionine (Met) is an essential amino acid required for cancer cells to grow under in vitro conditions and is also important in lipid biosynthesis due to the requirement for S-adenyl-methionine [77]. Borrego et al. assessed the impact of Met stress (deprivation) on the cellular lipid composition of TNBC MDA-MB-468 cells and a Met stress insensitive derivative, MDA-MB-468res-R8, under control and Met-stress conditions [78]. There was a rapid and extensive decrease in lipid abundance, except for unsaturated TGs, in Met-dependent MDA-MB-468 cells that was not observed in Met-res cells, and there was an associated increase in cytoplasmic lipid droplets reflecting an overall increase in neutral storage lipids. Replacement of Met with its metabolic precursor, homocysteine, in cell culture media decreased total lipids and increased TGs in MDA-MB-468 sensitive compared to Met-stress resistant cells. The authors attributed these changes to stress-induced lipid oxidation and the unfolded protein response. Changes in gene expression were also observed, although they were delayed relative to the changed lipid profile and were better correlated with the unfolded protein response. Additional studies will need to determine the mechanisms by which cancer cells overcome Met dependence and its role in lipid metabolism. Numerous other microenvironmental stressors likely impact the breast cancer cell lipidome including both glucose and L-glutamine which serve as critical carbon sources in many tumor cells. The stress impacted lipidome could be a potential tumor cell vulnerability.
It is well-established that cell culture dimensionality affects cell phenotype [79, 80]. To address this issue, Vidavsky et al. compared the lipid profiles of 2D versus 3D spheroid cultures of a series of MCF10A cells. These included parental MCF10A to mimic “nonmalignant” cells, MCF10DCIS.com to mimic “pre-malignant” cells and MCF10CA1 (HRAS transformed, invasive in vivo) to mimic malignant or “invasive” cells [81]. Storage of lipids upon both conditions was examined using Oil Red O staining. In 2D culture, parental MCF10A cells were void of lipid droplets while MCF10DCIS.com and MCF10CA1 had abundant lipid storage. In 3D culture, parental MCF10A cells showed occasional lipid droplets. Interestingly, the 3D pre-malignant and invasive spheroids exhibited larger lipid droplets (vs 2D) that were concentrated near the spheroid center. To assess global lipid profiles, LC-MS was run on each of the three cell lines in 2D vs 3D cultures. Total phospholipid content differed between 2D vs 3D cells, where 2D cells displayed a higher percentage of PC and PE lipid species. Invasive 3D spheroids also showed increased SM, DG, and acylglycerols compared to pre-cancerous spheroids. Notably, the lysophosphatidylcholines (LPC) pool in MCF10CA1a (invasive cancer) spheroids had shorter chain lengths compared to MCF10DCIS (pre-cancerous) spheroids. These data suggest that growth in 3D profoundly affects lipid production and distribution. We speculate the reduction in total lipid content in 3D spheroids could be indicative of reduced lipid synthesis in a 3D state. Furthermore, the distribution of lipid droplets near the spheroid center could reflect an adaption to meet the energy demands of the surrounding cells through lipid transfer. Tumor centers are typically hypoxic and necrotic. Lipid droplets may serve as a central energy storage since access to nutrients is not evenly distributed in 3D as it is in 2D cell culture. Increased SM levels in invasive 3D spheroids could result from upregulated de novo sphingolipid synthesis. While this has been reported in multiple cancers, it remains unclear whether upregulated sphingolipid biosynthesis is connected to invasiveness or cell survival pathways and requires more study [82]. This study clearly indicates the importance of including 3D culture conditions in the experimental design for future lipid studies in breast cancer. It would also be interesting to determine the effect on including adipocytes in 3D cultures to determine whether the observed changes are intrinsic to the tumor cells, or reflect their growth in 3D in the absence of adipocytes that could serve as a source of lipids for tumor cells.
Breast cancer cell line models that allow for comparison of factors in tightly controlled systems have given a solid indication of how important lipid metabolism is to the disease. However, they must be ultimately confirmed in patient samples. To conduct lipidomics on human tissues, samples are typically flash-frozen at time of tumor-resection or biopsy before sample preparation. Two studies focused specifically on TNBC tumors. Purwaha et al. conducted LC-MS on 70 TNBC tumors looking to identify biomarkers associated with clinical outcome and identify potential therapeutic targets [83]. They found elevated SM and sphingoid bases were correlated with better patient disease-free survival. This is in contrast to the studies in TNBC and MCF10A cells that associated elevated SM with more invasive and metastatic properties and highlights the need for additional in vivo and patient studies to resolve the different observations. above. Notably, Cer levels had no correlation with disease free survival in this study. Hosokawa et al. investigated TNBC patient tumors to define lipid markers correlated with tumor recurrence [84]. Using MALDI-MS on a small set of recurrent (n=3) and non-recurrent (n=6) TNBC tumors, PC(32:1) and PC(30:0) were identified as significantly increased in the recurrent tumors. PC(30:0) was identified by two additional studies as associated with TNBC or Grade 3/ER− tumors [85, 86]. Thus, there is some potential for specific lipid species to serve as predictive markers.
Lipid signatures of clinical breast cancer specimens has also established potential subtype differences. Hilvo et al. conducted lipidomics on 267 patient tumors, the largest study of this kind thus far [86]. They found PC(14:0/16:0) and PE (18:0/18:1) lipid species were correlated with ER− tumors and PC(16:0/16:0) was associated with decreased patient survival in confirmation of the observations from Hosowaka et al. [84]. A smaller study of 34 tumors reported PC(32:1) and PC(30:0) were increased in TNBC tumors compared to normal adjacent tissue [85]. PC(18:1/16:0) was also consistently present in all breast tumors compared to normal adjacent tissue suggesting PC(34:1) may serve as a general breast cancer biomarker [85]. Notably, HER2+ tumors exhibited elevated levels of short chain PC(16:1) lipids. Collectively, lipidomic analysis on breast cancer cell lines and patient tumors has identified a subset of potential prognostic or predictive lipid markers (Table 1). However, the biological significance of these lipid species and their validation as reliable prognostic markers will require extensive additional study. Additionally, the lipid contribution of stromal or adipose cells were not discussed in many of these publications. This warrants discussion as these cells could play potential roles in lipid trafficking or signaling crosstalk with tumor cells.
5. Major players of lipid metabolism and their regulation in breast cancer
Deregulated energy metabolism is a hallmark of cancer and is often associated with aberrant glucose metabolism, or the Warburg effect, and glutamine metabolism [13, 87]. An emerging hallmark of cancer metabolism as described by Pavlova et al. is “the use of glycolysis and tricarboxylic acid (TCA) cycle intermediates for biosynthesis”, a major component of which is lipid metabolism [87]. FAs are critical components of cell membranes, energy homeostasis, and signaling. The regulation of these processes is only partially understood in breast cancer. Normal breast tissue undergoes extensive metabolic rewiring, largely resulting from transcriptional changes, to prepare for milk production during lactation [reviewed in [44, 88]]. Therefore, breast cancers originate from cells that have the machinery to undergo dynamic lipid remodeling. Whether these processes are retained during tumorigenesis is unclear. However, breast cancer cells show an exceptional ability to utilize anabolic and catabolic lipid metabolism to fulfill survival and proliferative needs. Here we discuss current knowledge on how lipid metabolism is regulated in breast cancer.
Breast cancer cells can obtain lipids through uptake from their microenvironment or through de novo synthesis, therefore the expression of genes involved in lipid transport or fatty acid biosynthesis repress two extreme phenotypes for tumor cells that require lipids, and the ability to toggle between these two states may be critical for metabolic flexibility and tumor survival. Exogenous FA uptake is mediated through specialized transporters that facilitate FA movement across the plasma membrane. FA translocase (FAT/CD36) and six FA transport proteins (FATP1-6/SLC271-6) are the best characterized molecules that mediate uptake and are over expressed in many cancers [89]. High CD36 expression is associated with poor prognosis in breast cancer and reported to enhance therapy resistance in each of the three main breast cancer subtypes [90-92]. Additionally, breast cancer cells can uptake FAs through secondary mechanisms such as endocytosis [93]. Cancer associated fibroblasts are the most abundant cell type in the tumor stroma and have been shown to transfer FAs to breast cancer cells through the extracellular matrix, lipid droplets, and microvessicles [94, 95]. Adipocytes, especially abundant in the breast stroma, can also supply FAs to breast cancer cells [96].
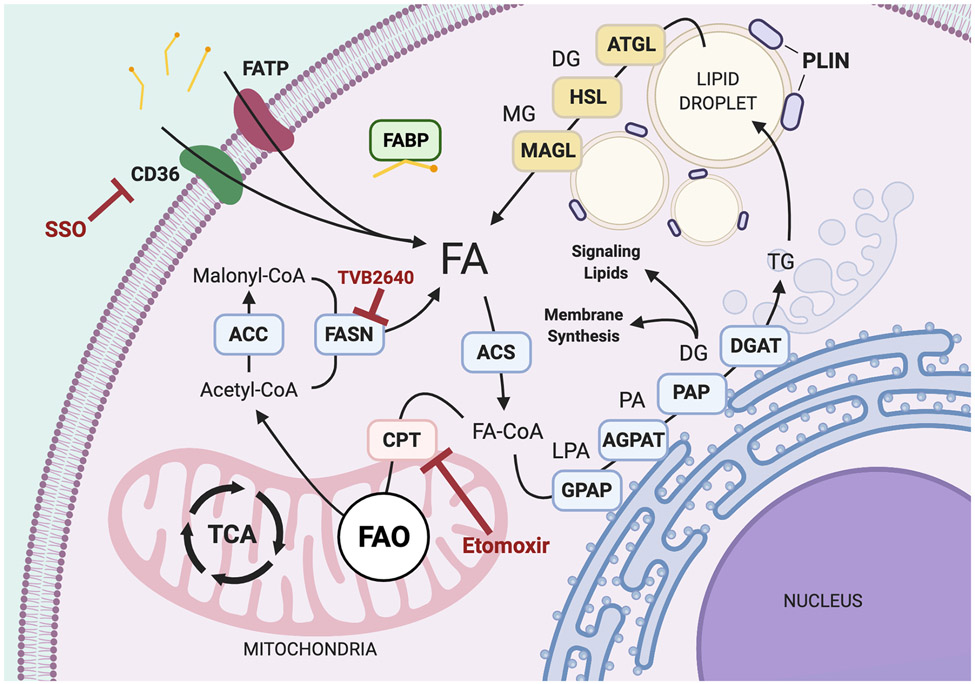
FA de novo synthesis is the anabolic process of building intracellular FAs. Most metabolic processes depend on central pools of acetyl-CoA, a fundamental metabolite building block (Fig. 3). Acetyl-CoA is derived from citrate or acetate – which are either imported or broken down from larger carbohydrates. Acetyl-CoA and malonyl-CoA are the necessary substrates for de novo FA synthesis. Acetyl-CoA is converted to Malonyl-CoA by acetyl-CoA carboxylase (ACC), a rate limiting enzyme in de novo FA synthesis. FA synthase (FASN) is the master enzyme that assembles acetyl-CoA and malonyl-CoA into palmitate (C16), or other FAs. FASN is overexpressed in breast cancer compared to normal, nonlactating tissue, with the highest expression in HER2+ followed by HR+ and TNBC [97]. Increased FASN activity has been linked with increased Pentose Phosphate Pathway (PPP) activity in non-Hodgkin lymphoma [98]. The PPP generates NADPH and 5-carbon sugars needed for nucleotide synthesis. FASN consumption of NADPH relieves feedback inhibition of Phosphogluconate Dehydrogenase (PGDH) and resupplies NADP+ for PDGH to synthesis ribulose-5-phosphate. These two interdependent biosynthetic pathways are likely essential for lipogenic breast cancer growth and their cooperation warrants further research. In addition, upregulated de novo synthesis may be a metabolic adaptation to breast cancer tumor microenvironment or specific metastatic sites. Ferraro et al. recently showed that FASN activity was required for growth of tumor cells in the brain but not in the mammary gland using their HER2-enriched breast cancer models [99]. This represents the first example of a tissue-specific requirement for FASN and fatty acid biosynthesis and suggests this metabolic change could be required for brain metastasis in this model.
Fig. 3.
Overview of Lipid Metabolism. General anabolic and catabolic pathways for intracellular fatty acids. Acetyl-CoA carboxylase (ACC), acetyl-CoA synthase (ACS), 1-acyl glycerol-3-phosphate acyltransferase (AGPAT), adipose triglyceride ligase (ATGL), cluster of differentiation 36 (CD36), carnitine palmitoyl transferase 1/2 (CPT1/2), diacylglycerol (DA), diacylglycerol acyltransferase (DGAT), fatty acids (FA), fatty acid binding protein (FABP), fatty acid oxidation (FAO), fatty acid synthase (FASN), fatty acid transport protein (FATP), glycerol-3-phosphate acyltransferase (GPAT), hormone sensitive lipase (HSL), lysophosphatidic acid (LPA), monoacylglycerol (MG), monoacylglycerol lipase (MGL), phosphatidic acid (PA), phosphatidic acid phosphatase (PAP), perilipin (PLIN), triacylglycerol (TG), tricarboxylic acid cycle (TCA)
Upstream regulation of de novo synthesis occurs largely through sterol regulatory element-binding proteins (SREBPs). There are two SREBP genes in mammals that encode three isoforms (SREBP1a, SREBP1c, and SREBP2) [100]. SREBPs reside in the endoplasmic reticulum or golgi depending on high or low cholesterol levels, respectively [101, 102], and must be cleaved in order to translocate the nucleus where they activate transcription of lipogenic genes including FASN, ACC, and ATP citrate lyase (ACLY) [103, 104]. PPARγ, NR1H2/3, and CEBPs are additional transcription factors that regulate lipid enzyme transcription. SREBP regulation of lipid biosynthetic pathways was largely defined in the lactating mammary gland where SREBP1c plays a critical role in initiating milk-globule production [105]. HER2 is also reported to induce FASN expression, however it is unclear if this is in an SREBP-dependent manner [70]. In HR+ breast cancers, both estrogen and progesterone are reported to increase lipogenic gene expression [106]. Thus, HR+ and HER2+ breast cancers have highly activated lipid biosynthetic pathways, and we speculate that this may be retained from cells that originally required these processes for lactation, that will require further study.
Palmitate, the most abundant and fundamental saturated FA, can be further processed into glycerolipids by enzymes in the golgi (GPAT, LPAT, PAP) and shuttled into lipid storage, membrane synthesis, or signaling lipids. Palmitate itself serves as important protein lipid modification in cancer cells. The Wnt signaling pathway is an important driver of several cancers and is frequently activated in breast cancer cells. Wnt ligands undergo palmitoylation and depalmitoylation for trafficking between the plasma membrane and cytosol. Palmitoylation occurs when a protein forms an enzyme-mediated thioester bond with a palmitoyl group and this process is responsible for tethering a number of proteins to the plasma membrane such as Ras, and CD36. Palmitoylation and myristoylation are just a few of the key protein lipid modifications prevalent in cancer which are being explored as therapeutic targets [107]. Glycerolipids destined for storage are processed into MG, DG, and TGs. The PLIN family of proteins package these species into lipid droplets with cholesterol. Lipid droplets visible by microscope are associated with a lipogenic cellular phenotype and commonly reported in HR+ and HER2+ breast cancer [108]. Lipid droplets mainly serve as energy storage but may have other consequential effects. For example, lipid droplets in breast cancer cells have been shown to provide cytotoxic protection by sequestering chemotherapeutic agents [109]. Under times of nutrient deficiency, lipases associated with lipid droplets (ATGL, HSL, MGL) can release FAs from their glycerol backbone through hydrolysis reactions. Intracellular FAs can recycle to other anabolic synthetic pathways or be shuttled for oxidation.
FAO is a catabolic process that breaks down FAs into acetyl-CoA. This process begins in the mitochondria with transport proteins bound to the mitochondrial membrane that participate in the carnitine shuttle. Mitochondria consist of two membranes, the inner and outer membranes. Carnitine palmitoyltransferase I (CPT1) is incorporated in the outer mitochondrial membrane and facilitates transfer of FAs across this membrane, while CPT2 coordinates FA transport across the inner membrane. Through a series of reactions, FAs are broken down to yield acetyl-CoA, NADH, and FADH2. This mechanism serves as an alternative to drive TCA cycle movement during insufficient glucose or glutamine availability. Breakdown of branched and very long chain FAs require α- and β-oxidation by peroxisomes [110]. Once these FAs are converted to shorter chain FAs, they can be imported into the mitochondria via the CPT-mediated carnitine shuttle to complete further oxidation steps. FAO is emerging as an important metabolic process that contributes to deregulated breast cancer metabolism, especially in TNBC [111]. The MYC oncogene is frequently amplified in TNBC and has been shown to drive FAO in addition to glycolysis [112, 113]. TNBCs with MYC overexpression have been shown to upregulate PGC1α, CPT1B, and CDCP1 while downregulating FASN and ACC [113]. In addition, FAO gene signatures have been associated with poor clinical outcome in MYC-expressing TNBCs, suggesting that this process is a contributing factor to TNBC pathogenesis [113].
Oncogenes play a critical role in regulating lipid metabolism. PIK3CA is one of the most commonly mutated genes in carcinomas with up to 40% of breast cancers exhibiting gain-of-function mutations [114, 115]. The protein generated by PIK3CA, phosphoinositide 3-kinase (PI3K), participates in the PI3K/AKT/mTOR signaling axis which regulates cell growth and proliferation as well as sensing availability of nutrients, hormones, and growth factor stimulation [116]. Loss of the tumor suppressor PTEN also occurs frequently in breast cancer; PTEN acts as a lipid phosphatase, converting phosphatidylinositol (3,4,5)-trisphosphate (PIP3), to phosphatidylinositol (4,5)-bisphosphate (PIP2), thereby depressing PI3K and AKT activation. 80% of HER2+ breast cancer tumors display increased phosphorylation AKT, an indicator of active PI3K signaling [117]. The connection between active PI3K signaling and a lipogenic phenotype in HER2+ breast cancer is not fully understood but is suggested to be a consequence of AKT downstream targets [118, 119]. As discussed, MYC is associated with activating glycolysis and lipogenesis/FAO in TNBC [113, 120]. MYC is also frequently mutated or amplified in breast cancer and likely contributes to breast cancer aggressiveness through its regulation of multiple branches of metabolism [115]. Thus, the various genetic changes present in the different breast cancer subtypes can drive changes in lipid metabolism. There are two excellent recent revies on breast cancer oncogenes and metabolism [121, 122].
6. Serum lipidomics: clinical diagnostic potential?
Preclinical and clinical studies to date support that lipid metabolism is aberrantly altered in breast cancer compared to benign breast tissue. A key question is whether breast tumor lipid metabolites in patient serum have diagnostic potential. Mammograms and magnetic resonance imaging (MRI) are the current standard for breast cancer screening, yet mammograms alone display a rate of over-diagnosis between 0-30% [123]. Follow up MRIs and tissue sampling can be costly and inconclusive. In addition, some subtypes such as TNBC can be difficult to detect by mammogram until tumors are of a size that negatively impacts treatment and outcome [124]. Thus, there remains a need for cost-effective breast cancer screening alternatives. Serum tumor markers provide an alternative, noninvasive and less costly methods for breast cancer diagnostic screening. For example, advances in capturing circulating tumor cells and circulating tumor DNA provides prognostic and disease-state information [125]. Current advancements in mass spectrometry detection of lipids may offer an additional serum screening option.
Several recent studies conducted lipidomic analysis of serum from breast cancer patients and non-cancer controls to determine if tumor-associated lipids could be detected. Three independent studies identified increased levels of PC(32:1) in serum from women with breast cancer compared to non-affected women [126-128]. PC(32:1) was also an increased lipid analyte detected in studies of TNBC tumors described previously [84, 85]. In addition, total TGs were increased in breast cancer patient serum compared to control [129]. Total serum TGs also distinguished menopausal and HR status in breast cancer patients as well as pathological complete response rate to neoadjuvant chemotherapy [126, 127]. Notably, TGs containing mainly oleic acid (C18:1) were associated with decreased disease-free survival in breast cancer patients [129]. Additionally, serum LPC and cholesterol esters were elevated in breast cancer patients compared to healthy control [129].
One study investigated specific lipid signatures as a diagnostic test. Using 166 plasma samples, Eghlimi et al. established a 19-lipid biomarker panel capable of distinguishing early stage TNBC from controls (AUROC=0.93, sensitivity = 0.89, specificity = 0.76), as well as a 5-lipid biomarker panel differentiating ES-TNBC from non-ES-TNBC serum samples (AUROC=0.95, sensitivity = 0.95, specificity = 0.87) [128]. Of the 19-lipid panel, stearic acid and Cer(43:1) are elevated (fold change (FC) >1.3) in TNBC while DGs and LPCs were generally decreased compared to serum from non-affected women (FC<0.7). The smaller 5-lipid panel was sufficient to detect DG(34:2) as significantly decreased (FC>0.2) in TNBC. In this study, a statically tested lipid biomarker panel was established, however, clinical use of this panel still requires further validation with larger patient serum cohorts. Despite these challenges there is sufficient promise in the utility of serum-based biomarkers for breast cancer detection that merit further study.
7. Therapeutic targets in tumor lipid biology and metabolism
As discussed in this review, potential lipid metabolism targets have been identified in each breast cancer subtype. Cancer metabolism therapies have largely been unsuccessful in clinical trials, with the exception of isocitrate dehydrogenase 1 inhibitors. However, our understanding of cancer metabolism continues to improve. Here, we give a brief overview of several lipid metabolic and transport inhibitors at preclinical or clinical trial stages for breast cancer.
FASN is perhaps the most widely targeted lipogenic enzyme in breast cancer due to its consistent overexpression. Genetic or pharmacological inhibition of FASN in preclinical studies has shown efficacy in decreasing cell proliferation in vitro and tumor growth in vivo in all subtypes of breast cancer, and there are excellent reviews on the topic [130]. Unfortunately, clinical translation has not been successful. Existing selective inhibitors of FASN have limited solubility and adverse side effects that have prohibited their clinical use. Cerulenin, C75, and C93 are inhibitors that target the β-ketoacyl synthase domain of FASN induce anorexia and weight loss in murine models [131, 132]. EGCG, G28UCM, GSK2194069, and GSK837149A also target the β-ketoacyl synthase domain but are ineffective in vivo due to low solubility [133-135]. To date, TVB-2640 is the only FASN inhibitor that is has reached a phase II clinical trial for breast cancer. Trial NCT03179904 is testing the efficacy of TVB-2640 in combination with paclitaxel and Trastuzumab in breast cancer patients with metastatic HER2+ disease. While the trial is still ongoing, additional inhibitors, such as Fasnall, that block FASN co-factor binding, are under preclinical study [136]. Furthermore, FASN inhibitors have shown promise against some forms of endocrine resistant HR+ breast cancers in vivo [137]. Some preclinical studies have also tested inhibiting alternative de novo synthesis targets such as ACC [138].
Elevated cholesterol has long been associated with increased risk of breast cancer and specific metabolites, such as 27-hydroxychoelesterol, have been shown to facilitate metastasis and evasion of immune cells in breast cancer [139]. Farnesyltransferase or HMG-CoA reductase inhibitors (statins) that target the mevalonate pathway and are commonly used to treat hypercholesterolemia and meta-analyses have shown statin users have reduced in breast cancer specific mortality [140]. Statin drugs are an appealing therapeutic target especially in advanced HER2+ and TNBC that exhibit enhanced cholesterol dependency. Preclinical studies have shown efficacy of statins in therapy-resistant HER2+ breast cancer models [141]. Simvastatin has reached phase II clinical trial (NCT03324425) in combination with targeted HER2 therapies for advanced HER2+ breast cancer. For TNBC, Atorvastatin is being evaluated in conjunction with the bisphosphonate zoledronate and adjuvant chemotherapy in phase II clinical trial NCT03358017. Some studies show adverse effects of statins such as increased expression of cholesterol synthesis enzymes through heightened feedback regulation from SREBP2 [142]. To circumvent this resistance mechanism, alternative targets of cholesterol synthesis are being investigated. For example, RORγ was recently identified as new upstream target for mevalonate pathway inhibition in TNBC [143, 144].
There is also interest in targeting lipid transporters, which has mostly centered on the best described FA transporters CD36 and FATP. However, there are likely many other promiscuous FA transporters of the SLC family. In low nutrient conditions, breast cancer cells can bypass dependency on de novo synthesis by increasing FA uptake. For example, studies show that drug resistant HER2+ breast cancer cells compensate for FASN inhibition by increasing extracellular FA uptake [91, 145]. Feng et al. showed that lapatinib-resistant breast cancer cells upregulate CD36 and regain drug sensitivity under CD36 inhibition [91]. Unfortunately, there are few available CD36 inhibitors, likely due in part to incomplete understanding of CD36 mechanisms and functions. Sulfo-N-succinimidyl esters of long-chain FAs, such as Sulfosuccinimidyl Oleate (SSO), efficiently inhibit CD36 and have been used in multiple in vitro studies. Large chemical screens have identified additional potential inhibitors; however, further development is needed for these compounds to advance into preclinical studies [146]. It may be important to use lipid transport inhibitors in conjunction with inhibitors of fatty acid synthesis to prevent resistance to the latter by increased lipid transport.
FAO or beta-oxidation is the mitochondrial break down of FAs to provide metabolic fuel. FAO has emerged as an attractive target in breast cancer. TNBC cells in particular have been reported to utilize FAO [92]. Since FAO occurs within the mitochondria, the primary target for this pathway is the outer mitochondrial membrane transporter and rate-limiting enzyme CPT1. The best known CPT1 inhibitor is Etomoxir which continues to be widely used in preclinical studies. Etomoxir failed in clinical trials due to cardiotoxicity. There is a continued effort to develop tolerable anti-CPT1 analogs to target FAO-dependent breast cancers.
Diabetic drugs may have efficacy in breast cancer treatment. Metformin, the most common drug taken for diabetes, mediates hepatic glucose production and insulin sensitivity through inhibition of mitochondrial complex 1 and AMPK pathway activation [147]. Women with Type 2 diabetes taking Metformin have decreased risk of post-menopausal breast cancer [148]. Metformin is currently involved in 18 clinical trials for breast cancer, several of which are investigating its efficacy as a neoadjuvant treatment (NCT04387630, NCT04170465, NCT03238495). While the effects of Metformin on breast cancer lipid metabolism remain unclear, current studies suggest Metformin may be a promising therapeutic for FAO-dependent breast cancers [149].
In addition to enzymes and transporters, lipids themselves are believed to hold therapeutic potential. Omega3 or PUFA supplementation is currently under investigation for use in neoadjuvant breast cancer therapy (NCT02831582). Joint pain or discomfort is a common side effect of endocrine therapies and PUFAs have been shown to reduce joint inflammation by competing with pro-inflammatory prostaglandin signals [150]. Three clinical trials are investigating the benefits of PUFA supplementation with standard endocrine and chemotherapies. PUFAs could potentially be advantageous for breast cancers exhibiting enhanced lipid uptake and warrants further study.
Another novel therapeutic approach is to target tumor cell lipidomes. There is increased interest in lipidome-based therapies as we learn more about tumor specific lipid dependencies [reviewed in [151]]. For example, Toric et al. successfully screened a library of layer-by-layer nanoparticles to determine the surface layer that best interacts with STAT3 expressing TNBC cell membranes [152]. The NP coating identified from the screen allowed selective cisplatin NP delivery to STAT3-active TNBC cells, avoiding non-STAT3 activated cells. This study demonstrates the potential of exploiting distinct lipidomes for targeted drug delivery. Other novel methods of lipidome targeting are certain to emerge.
8. Summary and future perspectives
Advancements in mass spectrometry-based lipidomics have increased our understanding of breast cancer lipidomes, yet there remain several obstacles to accelerating the field. The first obstacle resides within the technique itself (see Fig. 2). Numerous forms of mass spectrometry have been utilized (i.e., shotgun MS, LC-MS, LC-MS/MS, GC-MS), each using various methods of sample separation, detection, and identification, which is also dependent on whether the desired approach is global or targeted. The lack of a technical "gold standard" makes data sharing and comparison difficult. However, the two main data repositories, Metabolomics Workbench (https://www.metabolomicsworkbench.org/) and Metabolites (https://www.ebi.ac.uk/metabolights/), accept lipidomics data in different formats but require thorough methodological detail to aid in interpretation of shared data. In addition to methodological discrepancies, global lipidomics conducted on any biological sample can detect over 1800 validated lipid species which adds to the difficulty of connecting lipidome alterations to biological consequence or changes in cellular phenotype [153, 154]. Despite these attempts to achieve methodological transparency, it remains difficult to compare different datasets unless similar MS methods and data handling were used.
The second obstacle is the types of breast cancer models used in previous published work. The majority of lipidomic studies discussed in this review used either breast cancer cell lines [69, 71, 73, 76, 78, 81] or primary tumor samples [83-86]. As highlighted in Table 1, there is a clear disparity between lipid analytes detected in 2D cell line monoculture versus patient tumor samples, with only several key overlapping lipid species. While primary tumor samples may be the most directly relevant, they have several variables that can complicate interpretations including tumor heterogeneity and inclusion of multiple cell types in the microenvironment. In addition, factors such as diet, body mass index, and tumor stage/burden may impact results. While breast cancer cell lines eliminate many of these variables, whether they reflect the more complex in vivo situation is uncertain. As discovered by Vidavsky et al. in comparing lipidomics in MCF10A 2D monoculture vs 3D spheroids, spatial orientation within a 3D cell structure significantly impacts lipid content [81]. In addition, the lipid content of cell culture medium directly impacts cellular morphology and behavior [155]. The FA content of fetal bovine serum can vary between commercial source and lot number. Many companies do not provide detailed FA information since it is difficult to measure and not a concern for all consumers. Charcoal stripped serum is frequently used to remove hormones, particularly estrogens, and likely also removes a subset of lipids. To our knowledge, additional lipidome studies on breast cancer 3D cultures, tumor xenografts, or syngeneic mammary tumor models have not been reported. There is therefore a need for future pre-clinical lipidomic studies to utilize 3D cultures, organoids or cell line or patient-derived xenograft models to better incorporate spatial influences.
A third variable lies in technological limitations in studying specific lipid analytes. Unlike genetic or pharmacological manipulation of an individual gene product, lipid metabolites are not as easily modified. Studying the relevance of individual lipid species or classes requires an understanding of the proteins that regulate their synthesis, uptake, degradation, an intracellular location. However, there are tools that are routinely used. These include fluorescent tagged lipid species (i.e. BODIPY) for tracking cell uptake and location and the long standing method of stable isotope tracing which allows us to depict FA usage in cells over time [156]. Stable isotope lipids containing C14 within its structure are routinely used in lipidomics and aid in absolute quantification of analytes in interest [157].
Analyzing lipids in patient serum samples has diagnostic potential. The studies discussed herein defined preliminary panels of tumor-associated lipids in patient serum that could aid in breast cancer diagnoses [126-129]. This method could be particularly useful for early detection of breast cancer subtypes that are difficult to detect in mammograms [158]. Patient-specific factors such a dietary lipids, lifestyle, and menopausal status may complicate the efficacy of these panels. For example, overweight or individuals with obesity have increased levels of total serum lipids and lipoproteins compared to normal individuals [159]. Diets high in palmitic acid (palmitate) have also been shown to increase an individual’s serum cholesterols levels [160]. Once protocols are established to account for these factors, serum lipid panels may serve as an alternative or complementary diagnostic test to the mammogram. Serum lipidomics could also be used to predict patient response to specific therapies. For example, Hilvo et al. identified lipid analytes associated with positive response to chemotherapy [129]. A lipid panel could prospectively be developed for endocrine therapies. In time, we predict serum lipidomics will indicate useful clinical information such as tumor burden, therapeutic response, and development of metastases.
In summary, the breast is a dynamic organ which responds to hormonal and environmental cues to undergo drastic remodeling and lipid production. Breast cancer has well documented reliance on lipid metabolism – however a link between processes in normal and malignant breast tissues has been difficult to define – as have consistent targetable lipid dependencies. This is underscored by a general paucity of understanding of the lipidome in breast cancer cells and the complication of different breast cancer subtypes and significant intra- and inter- tumoral heterogeneity. The studies highlighted have made significant progress in understanding the global lipidome and its impact on breast cancer cell phenotype. However, there remain multiple gaps in our knowledge, including how lipids are impacted by spatial location of the cell withing the tumor, tumor microenvironment, metastasis, and resistance to drug treatment etc. With emerging models such as tumor-derived organoids and patient-derived xenografts, coupled with continuous improvements to lipidomics and analysis tools, these gaps will be hopefully become filled and lipids a regular measurement of breast cancer cell state and therapeutic vulnerability.
Funding:
This work was funded in part by the National Institute of Health [R01CA140985 and R01CA229697(CAS)] and Breast Cancer Research Foundation [19-144 (CAS)]. AVW was supported by the National Institutes of Health (F31 CA261053, T32 CA190216, and linked award TL1 TR002533). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- ACC
Acetyl-CoA carboxylase
- APCI
Atmospheric pressure chemical ionization
- APPI
Atmospheric pressure polarization ionization
- BmP
Bis(monoacylglycero)phosphate
- BUME
Butanol/methanol
- Cer
Ceramide
- ChEBI
Chemical entities of biological interest
- CL
Cardiolipin
- CPT1
Carnitine palmitoyltransferase I
- DESI
Desorption electrospray ionization
- DG
Diacylglycerol
- ER
Estrogen receptor
- ESI
Electron spray ionization
- FA
Fatty acid
- FAO
Fatty acid oxidation
- FASN
Fatty acid synthase
- GC-MS
Gas chromatography mass spectrometry
- GlcCer
glucosylceramide
- HER2+
Human epidermal growth factor receptor 2-positive
- HexCer
Hexosylceramide
- HMBD
Human metabolome data base
- HR
Hormone receptor
- LacCer
lactosylceramide
- LION
Lipid Ontology Enrichment Analysis
- LIPEA
Lipid Pathway Enrichment Analysis
- LPC
Lysophosphatidylcholine
- MALDI
Matrix assisted laser desorption/ionization
- Met
Methionine
- MRI
Magnetic resonance imaging
- MS
Mass spectrometry
- MS/MS
Tandem mass spectrometry
- MTBE
Methyl tert-butyl ether
- MUFA
Monounsaturated fatty acid
- NMR
Nuclear magnetic resonance
- PC
Phosphatidylcholine
- PE
Phosphatidylethanolamine
- PI
Phosphatidylinositol
- PI3K
Phosphoinositide 3-kinase
- PIP2
Phosphatidylinositol (4,5)-trisphosphate
- PIP3
Phosphatidylinositol (3,4,5)-trisphosphate
- PKC
Protein kinase C
- PLIN
Perilipin
- PR
Progesterone receptor
- PS
Phosphatidylserine
- PUFA
Poly unsaturated fatty acid
- SCD
Stearoyl-CoA desaturase
- SIMS
Secondary ion mass spectrometry
- SM
Sphingomyelin
- SREBP
Sterol regulatory element-binding protein
- SSO
Sulfosuccinimidyl oleate
- TCA
Tricarboxylic acid
- TG
Triacylglycerol
- TNBC
Triple negative breast cancer
Footnotes
Conflicts of interest/Competing interests: The authors declare no conflict of interest.
Consent for publication: The authors give their consent for publication.
References
- 1.Koundouros N and Poulogiannis G, Reprogramming of fatty acid metabolism in cancer. British Journal of Cancer, 2020. 122(1): p. 4–22. doi: 10.1038/s41416-019-0650-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, et al. , Cancer Statistics, 2021. CA Cancer J Clin, 2021. 71(1): p. 7–33. doi: 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 3.Malhotra GK, et al. , Histological, molecular and functional subtypes of breast cancers. Cancer Biol Ther, 2010. 10(10): p. 955–60. doi: 10.4161/cbt.10.10.13879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Come SE, et al. , Second International Conference on Recent Advances and Future Directions in Endocrine Manipulation of Breast Cancer: summary consensus statement. Clin Cancer Res, 2003. 9(1 Pt 2): p. 443s–6s. doi: [PubMed] [Google Scholar]
- 5.Slamon D, et al. , Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med, 2011. 365(14): p. 1273–83. doi: 10.1056/NEJMoa0910383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liedtke C, et al. , Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol, 2008. 26(8): p. 1275–81. doi: 10.1200/jco.2007.14.4147 [DOI] [PubMed] [Google Scholar]
- 7.Messina C, et al. , CDK4/6 inhibitors in advanced hormone receptor-positive/HER2-negative breast cancer: a systematic review and meta-analysis of randomized trials. Breast Cancer Res Treat, 2018. 172(1): p. 9–21. doi: 10.1007/s10549-018-4901-0 [DOI] [PubMed] [Google Scholar]
- 8.Pan H, et al. , 20-Year Risks of Breast-Cancer Recurrence after Stopping Endocrine Therapy at 5 Years. N Engl J Med, 2017. 377(19): p. 1836–1846. doi: 10.1056/NEJMoa1701830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dent R, et al. , Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res, 2007. 13(15 Pt 1): p. 4429–34. doi: 10.1158/1078-0432.Ccr-06-3045 [DOI] [PubMed] [Google Scholar]
- 10.Reddy SM, et al. , Long-term survival outcomes of triple-receptor negative breast cancer survivors who are disease free at 5 years and relationship with low hormone receptor positivity. Br J Cancer, 2018. 118(1): p. 17–23. doi: 10.1038/bjc.2017.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chumsri S, et al. , Incidence of Late Relapses in Patients With HER2-Positive Breast Cancer Receiving Adjuvant Trastuzumab: Combined Analysis of NCCTG N9831 (Alliance) and NRG Oncology/NSABP B-31. J Clin Oncol, 2019. 37(35): p. 3425–3435. doi: 10.1200/jco.19.00443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cortesi L, Rugo HS, and Jackisch C, An Overview of PARP Inhibitors for the Treatment of Breast Cancer. Target Oncol, 2021. 16(3): p. 255–282. doi: 10.1007/s11523-021-00796-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warburg O, Wind F, and Negelein E, THE METABOLISM OF TUMORS IN THE BODY. J Gen Physiol, 1927. 8(6): p. 519–30. doi: 10.1085/jgp.8.6.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanahan D and Weinberg RA, Hallmarks of cancer: the next generation. Cell, 2011. 144(5): p. 646–74. doi: S0092-8674(11)00127-9 [pii] 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 15.Griffiths J, A brief history of mass spectrometry. Anal Chem, 2008. 80(15): p. 5678–83. doi: 10.1021/ac8013065 [DOI] [PubMed] [Google Scholar]
- 16.Fenn JB, et al. , Electrospray ionization for mass spectrometry of large biomolecules. Science, 1989. 246(4926): p. 64–71. doi: 10.1126/science.2675315 [DOI] [PubMed] [Google Scholar]
- 17.Hillenkamp F, et al. , Matrix-assisted laser desorption/ionization mass spectrometry of biopolymers. Anal Chem, 1991. 63(24): p. 1193a–1203a. doi: 10.1021/ac00024a002 [DOI] [PubMed] [Google Scholar]
- 18.Murphy RC, Challenges in Mass Spectrometry-based Lipidomics of Neutral Lipids. Trends Analyt Chem, 2018. 107: p. 91–98. doi: 10.1016/j.trac.2018.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Misra BB, Data normalization strategies in metabolomics: Current challenges, approaches, and tools. Eur J Mass Spectrom (Chichester), 2020. 26(3): p. 165–174. doi: 10.1177/1469066720918446 [DOI] [PubMed] [Google Scholar]
- 20.Fahy E, et al. , A comprehensive classification system for lipids. J Lipid Res, 2005. 46(5): p. 839–61. doi: 10.1194/jlr.E400004-JLR200 [DOI] [PubMed] [Google Scholar]
- 21.Agbaga MP, Mandal MN, and Anderson RE, Retinal very long-chain PUFAs: new insights from studies on ELOVL4 protein. J Lipid Res, 2010. 51(7): p. 1624–42. doi: 10.1194/jlr.R005025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spector AA and Kim HY, Discovery of essential fatty acids. J Lipid Res, 2015. 56(1): p. 11–21. doi: 10.1194/jlr.R055095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ballweg S, et al. , Regulation of lipid saturation without sensing membrane fluidity. Nat Commun, 2020. 11(1): p. 756. doi: 10.1038/s41467-020-14528-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fahy E, et al. , Update of the LIPID MAPS comprehensive classification system for lipids. J Lipid Res, 2009. 50 Suppl(Suppl): p. S9–14. doi: 10.1194/jlr.R800095-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Testerink N, et al. , Depletion of phosphatidylcholine affects endoplasmic reticulum morphology and protein traffic at the Golgi complex. J Lipid Res, 2009. 50(11): p. 2182–92. doi: 10.1194/jlr.M800660-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marsh D, Lateral pressure profile, spontaneous curvature frustration, and the incorporation and conformation of proteins in membranes. Biophys J, 2007. 93(11): p. 3884–99. doi: 10.1529/biophysj.107.107938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki J, et al. , Calcium-dependent phospholipid scrambling by TMEM16F. Nature, 2010. 468(7325): p. 834–8. doi: 10.1038/nature09583 [DOI] [PubMed] [Google Scholar]
- 28.Suzuki J, et al. , Xk-related protein 8 and CED-8 promote phosphatidylserine exposure in apoptotic cells. Science, 2013. 341(6144): p. 403–6. doi: 10.1126/science.1236758 [DOI] [PubMed] [Google Scholar]
- 29.Ali MR, Cheng KH, and Huang J, Ceramide drives cholesterol out of the ordered lipid bilayer phase into the crystal phase in 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine/cholesterol/ceramide ternary mixtures. Biochemistry, 2006. 45(41): p. 12629–38. doi: 10.1021/bi060610x [DOI] [PubMed] [Google Scholar]
- 30.Sezgin E, et al. , The mystery of membrane organization: composition, regulation and roles of lipid rafts. Nat Rev Mol Cell Biol, 2017. 18(6): p. 361–374. doi: 10.1038/nrm.2017.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajendran L and Simons K, Lipid rafts and membrane dynamics. J Cell Sci, 2005. 118(Pt 6): p. 1099–102. doi: 10.1242/jcs.01681 [DOI] [PubMed] [Google Scholar]
- 32.Sezgin E, et al. , Adaptive lipid packing and bioactivity in membrane domains. PLoS One, 2015. 10(4): p. e0123930. doi: 10.1371/journal.pone.0123930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parton RG and Simons K, Digging into caveolae. Science, 1995. 269(5229): p. 1398–9. doi: 10.1126/science.7660120 [DOI] [PubMed] [Google Scholar]
- 34.Simons K and van Meer G, Lipid sorting in epithelial cells. Biochemistry, 1988. 27(17): p. 6197–202. doi: 10.1021/bi00417a001 [DOI] [PubMed] [Google Scholar]
- 35.Casares D, Escribá PV, and Rosselló CA, Membrane Lipid Composition: Effect on Membrane and Organelle Structure, Function and Compartmentalization and Therapeutic Avenues. Int J Mol Sci, 2019. 20(9). doi: 10.3390/ijms20092167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holthuis JC, et al. , The organizing potential of sphingolipids in intracellular membrane transport. Physiol Rev, 2001. 81(4): p. 1689–723. doi: 10.1152/physrev.2001.81.4.1689 [DOI] [PubMed] [Google Scholar]
- 37.Dazzoni R, et al. , The unprecedented membrane deformation of the human nuclear envelope, in a magnetic field, indicates formation of nuclear membrane invaginations. Sci Rep, 2020. 10(1): p. 5147. doi: 10.1038/s41598-020-61746-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Houtkooper RH and Vaz FM, Cardiolipin, the heart of mitochondrial metabolism. Cell Mol Life Sci, 2008. 65(16): p. 2493–506. doi: 10.1007/s00018-008-8030-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghosh S, et al. , An essential role for cardiolipin in the stability and function of the mitochondrial calcium uniporter. Proc Natl Acad Sci U S A, 2020. 117(28): p. 16383–16390. doi: 10.1073/pnas.2000640117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee RG, et al. , Cardiolipin is required for membrane docking of mitochondrial ribosomes and protein synthesis. J Cell Sci, 2020. 133(14). doi: 10.1242/jcs.240374 [DOI] [PubMed] [Google Scholar]
- 41.Escribá PV, et al. , Membrane lipid therapy: Modulation of the cell membrane composition and structure as a molecular base for drug discovery and new disease treatment. Prog Lipid Res, 2015. 59: p. 38–53. doi: 10.1016/j.plipres.2015.04.003 [DOI] [PubMed] [Google Scholar]
- 42.Thelen AM and Zoncu R, Emerging Roles for the Lysosome in Lipid Metabolism. Trends Cell Biol, 2017. 27(11): p. 833–850. doi: 10.1016/j.tcb.2017.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sztalryd C and Brasaemle DL, The perilipin family of lipid droplet proteins: Gatekeepers of intracellular lipolysis. Biochim Biophys Acta Mol Cell Biol Lipids, 2017. 1862(10 Pt B): p. 1221–1232. doi: 10.1016/j.bbalip.2017.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rudolph MC, et al. , Metabolic regulation in the lactating mammary gland: a lipid synthesizing machine. Physiol Genomics, 2007. 28(3): p. 323–36. doi: 10.1152/physiolgenomics.00020.2006 [DOI] [PubMed] [Google Scholar]
- 45.Fukami K, et al. , Phospholipase C is a key enzyme regulating intracellular calcium and modulating the phosphoinositide balance. Prog Lipid Res, 2010. 49(4): p. 429–37. doi: 10.1016/j.plipres.2010.06.001 [DOI] [PubMed] [Google Scholar]
- 46.Baron CL and Malhotra V, Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science, 2002. 295(5553): p. 325–8. doi: 10.1126/science.1066759 [DOI] [PubMed] [Google Scholar]
- 47.Yeaman C, et al. , Protein kinase D regulates basolateral membrane protein exit from trans-Golgi network. Nat Cell Biol, 2004. 6(2): p. 106–12. doi: 10.1038/ncb1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han H, et al. , Regulation of the Hippo Pathway by Phosphatidic Acid-Mediated Lipid-Protein Interaction. Mol Cell, 2018. 72(2): p. 328–340.e8. doi: 10.1016/j.molcel.2018.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santarpia L, et al. , Deciphering and Targeting Oncogenic Mutations and Pathways in Breast Cancer. Oncologist, 2016. 21(9): p. 1063–78. doi: 10.1634/theoncologist.2015-0369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siskind LJ, Mitochondrial ceramide and the induction of apoptosis. J Bioenerg Biomembr, 2005. 37(3): p. 143–53. doi: 10.1007/s10863-005-6567-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Züllig T, Trötzmüller M, and Köfeler HC, Lipidomics from sample preparation to data analysis: a primer. Anal Bioanal Chem, 2020. 412(10): p. 2191–2209. doi: 10.1007/s00216-019-02241-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hsu FF, Mass spectrometry-based shotgun lipidomics - a critical review from the technical point of view. Anal Bioanal Chem, 2018. 410(25): p. 6387–6409. doi: 10.1007/s00216-018-1252-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang K and Han X, Lipidomics: Techniques, Applications, and Outcomes Related to Biomedical Sciences. Trends Biochem Sci, 2016. 41(11): p. 954–969. doi: 10.1016/j.tibs.2016.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bligh EG and Dyer WJ, A rapid method of total lipid extraction and purification. Can J Biochem Physiol, 1959. 37(8): p. 911–7. doi: 10.1139/o59-099 [DOI] [PubMed] [Google Scholar]
- 55.Folch J, Lees M, and Sloane Stanley GH, A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem, 1957. 226(1): p. 497–509. doi: [PubMed] [Google Scholar]
- 56.Matyash V, et al. , Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J Lipid Res, 2008. 49(5): p. 1137–46. doi: 10.1194/jlr.D700041-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Löfgren L, Forsberg GB, and Ståhlman M, The BUME method: a new rapid and simple chloroform-free method for total lipid extraction of animal tissue. Sci Rep, 2016. 6: p. 27688. doi: 10.1038/srep27688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aldana J, Romero-Otero A, and Cala MP, Exploring the Lipidome: Current Lipid Extraction Techniques for Mass Spectrometry Analysis. Metabolites, 2020. 10(6). doi: 10.3390/metabo10060231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leopold J, et al. , Recent Developments of Useful MALDI Matrices for the Mass Spectrometric Characterization of Lipids. Biomolecules, 2018. 8(4). doi: 10.3390/biom8040173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han X, et al. , Metabolomics in early Alzheimer's disease: identification of altered plasma sphingolipidome using shotgun lipidomics. PLoS One, 2011. 6(7): p. e21643. doi: 10.1371/journal.pone.0021643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang X, et al. , Alkaline methanolysis of lipid extracts extends shotgun lipidomics analyses to the low-abundance regime of cellular sphingolipids. Anal Biochem, 2007. 371(2): p. 135–45. doi: 10.1016/j.ab.2007.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hommerson P, et al. , Ionization techniques in capillary electrophoresis-mass spectrometry: principles, design, and application. Mass Spectrom Rev, 2011. 30(6): p. 1096–120. doi: 10.1002/mas.20313 [DOI] [PubMed] [Google Scholar]
- 63.Paglia G, et al. , Applications of ion-mobility mass spectrometry for lipid analysis. Anal Bioanal Chem, 2015. 407(17): p. 4995–5007. doi: 10.1007/s00216-015-8664-8 [DOI] [PubMed] [Google Scholar]
- 64.Kanehisa M, et al. , KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res, 2017. 45(D1): p. D353–d361. doi: 10.1093/nar/gkw1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wishart DS, et al. , HMDB: the Human Metabolome Database. Nucleic Acids Res, 2007. 35(Database issue): p. D521–6. doi: 10.1093/nar/gkl923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xia J, et al. , MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucleic Acids Res, 2009. 37(Web Server issue): p. W652–60. doi: 10.1093/nar/gkp356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Molenaar MR, et al. , LION/web: a web-based ontology enrichment tool for lipidomic data analysis. Gigascience, 2019. 8(6). doi: 10.1093/gigascience/giz061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Acevedo A, et al. , LIPEA: Lipid Pathway Enrichment Analysis. bioRxiv, 2018: p. 274969. doi: 10.1101/274969 [DOI] [Google Scholar]
- 69.Eiriksson FF, et al. , Lipidomic study of cell lines reveals differences between breast cancer subtypes. PLoS One, 2020. 15(4): p. e0231289. doi: 10.1371/journal.pone.0231289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kumar-Sinha C, et al. , Transcriptome analysis of HER2 reveals a molecular connection to fatty acid synthesis. Cancer Res, 2003. 63(1): p. 132–9. doi: [PubMed] [Google Scholar]
- 71.Giudetti AM, et al. , A specific lipid metabolic profile is associated with the epithelial mesenchymal transition program. Biochim Biophys Acta Mol Cell Biol Lipids, 2019. 1864(3): p. 344–357. doi: 10.1016/j.bbalip.2018.12.011 [DOI] [PubMed] [Google Scholar]
- 72.Holder AM, et al. , High stearoyl-CoA desaturase 1 expression is associated with shorter survival in breast cancer patients. Breast Cancer Res Treat, 2013. 137(1): p. 319–27. doi: 10.1007/s10549-012-2354-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nishida-Aoki N, et al. , Lipidomic Analysis of Cells and Extracellular Vesicles from High- and Low-Metastatic Triple-Negative Breast Cancer. Metabolites, 2020. 10(2). doi: 10.3390/metabo10020067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Almena M and Mérida I, Shaping up the membrane: diacylglycerol coordinates spatial orientation of signaling. Trends Biochem Sci, 2011. 36(11): p. 593–603. doi: 10.1016/j.tibs.2011.06.005 [DOI] [PubMed] [Google Scholar]
- 75.Gatenby RA and Gillies RJ, A microenvironmental model of carcinogenesis. Nat Rev Cancer, 2008. 8(1): p. 56–61. doi: 10.1038/nrc2255 [DOI] [PubMed] [Google Scholar]
- 76.Urbanelli L, et al. , Lipidomic analysis of cancer cells cultivated at acidic pH reveals phospholipid fatty acids remodelling associated with transcriptional reprogramming. J Enzyme Inhib Med Chem, 2020. 35(1): p. 963–973. doi: 10.1080/14756366.2020.1748025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaiser P, Methionine Dependence of Cancer. Biomolecules, 2020. 10(4). doi: 10.3390/biom10040568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Borrego SL, et al. , Lipid remodeling in response to methionine stress in MDA-MBA-468 triple-negative breast cancer cells. J Lipid Res, 2021. 62: p. 100056. doi: 10.1016/j.jlr.2021.100056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kenny PA, et al. , The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol Oncol, 2007. 1(1): p. 84–96. doi: 10.1016/j.molonc.2007.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nath S and Devi GR, Three-dimensional culture systems in cancer research: Focus on tumor spheroid model. Pharmacol Ther, 2016. 163: p. 94–108. doi: 10.1016/j.pharmthera.2016.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vidavsky N, et al. , Mapping and Profiling Lipid Distribution in a 3D Model of Breast Cancer Progression. ACS Cent Sci, 2019. 5(5): p. 768–780. doi: 10.1021/acscentsci.8b00932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ogretmen B, Sphingolipid metabolism in cancer signalling and therapy. Nat Rev Cancer, 2018. 18(1): p. 33–50. doi: 10.1038/nrc.2017.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Purwaha P, et al. , Unbiased Lipidomic Profiling of Triple-Negative Breast Cancer Tissues Reveals the Association of Sphingomyelin Levels with Patient Disease-Free Survival. Metabolites, 2018. 8(3). doi: 10.3390/metabo8030041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hosokawa Y, et al. , Recurrent triple-negative breast cancer (TNBC) tissues contain a higher amount of phosphatidylcholine (32:1) than non-recurrent TNBC tissues. PLoS One, 2017. 12(8): p. e0183724. doi: 10.1371/journal.pone.0183724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kang HS, et al. , Protein and lipid MALDI profiles classify breast cancers according to the intrinsic subtype. BMC Cancer, 2011. 11: p. 465. doi: 10.1186/1471-2407-11-465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hilvo M, et al. , Novel theranostic opportunities offered by characterization of altered membrane lipid metabolism in breast cancer progression. Cancer Res, 2011. 71(9): p. 3236–45. doi: 10.1158/0008-5472.Can-10-3894 [DOI] [PubMed] [Google Scholar]
- 87.Pavlova NN and Thompson CB, The Emerging Hallmarks of Cancer Metabolism. Cell Metab, 2016. 23(1): p. 27–47. doi: 10.1016/j.cmet.2015.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smoczyński M, Role of Phospholipid Flux during Milk Secretion in the Mammary Gland. J Mammary Gland Biol Neoplasia, 2017. 22(2): p. 117–129. doi: 10.1007/s10911-017-9376-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Glatz JF, Luiken JJ, and Bonen A, Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol Rev, 2010. 90(1): p. 367–417. doi: 10.1152/physrev.00003.2009 [DOI] [PubMed] [Google Scholar]
- 90.Liang Y, et al. , CD36 plays a critical role in proliferation, migration and tamoxifen-inhibited growth of ER-positive breast cancer cells. Oncogenesis, 2018. 7(12): p. 98. doi: 10.1038/s41389-018-0107-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Feng WW, et al. , CD36-Mediated Metabolic Rewiring of Breast Cancer Cells Promotes Resistance to HER2-Targeted Therapies. Cell Rep, 2019. 29(11): p. 3405–3420.e5. doi: 10.1016/j.celrep.2019.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Casciano JC, et al. , MYC regulates fatty acid metabolism through a multigenic program in claudin-low triple negative breast cancer. Br J Cancer, 2020. 122(6): p. 868–884. doi: 10.1038/s41416-019-0711-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lupien LE, et al. , Endocytosis of very low-density lipoproteins: an unexpected mechanism for lipid acquisition by breast cancer cells. J Lipid Res, 2020. 61(2): p. 205–218. doi: 10.1194/jlr.RA119000327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lopes-Coelho F, et al. , Breast cancer metabolic cross-talk: Fibroblasts are hubs and breast cancer cells are gatherers of lipids. Mol Cell Endocrinol, 2018. 462(Pt B): p. 93–106. doi: 10.1016/j.mce.2017.01.031 [DOI] [PubMed] [Google Scholar]
- 95.Santi A, et al. , Cancer associated fibroblasts transfer lipids and proteins to cancer cells through cargo vesicles supporting tumor growth. Biochim Biophys Acta, 2015. 1853(12): p. 3211–23. doi: 10.1016/j.bbamcr.2015.09.013 [DOI] [PubMed] [Google Scholar]
- 96.Rybinska I, et al. , Adipocytes in Breast Cancer, the Thick and the Thin. Cells, 2020. 9(3). doi: 10.3390/cells9030560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Menendez JA and Lupu R, Fatty acid synthase regulates estrogen receptor-α signaling in breast cancer cells. Oncogenesis, 2017. 6(2): p. e299. doi: 10.1038/oncsis.2017.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ravi D, et al. , Oncogenic Integration of Nucleotide Metabolism via Fatty Acid Synthase in Non-Hodgkin Lymphoma. Frontiers in Oncology, 2021. 11(4373). doi: 10.3389/fonc.2021.725137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ferraro GB, et al. , FATTY ACID SYNTHESIS IS REQUIRED FOR BREAST CANCER BRAIN METASTASIS. Nat Cancer, 2021. 2(4): p. 414–428. doi: 10.1038/s43018-021-00183-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Horton JD, Goldstein JL, and Brown MS, SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest, 2002. 109(9): p. 1125–31. doi: 10.1172/jci15593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Adams CM, et al. , Cholesterol and 25-hydroxycholesterol inhibit activation of SREBPs by different mechanisms, both involving SCAP and Insigs. J Biol Chem, 2004. 279(50): p. 52772–80. doi: 10.1074/jbc.M410302200 [DOI] [PubMed] [Google Scholar]
- 102.Brown MS and Goldstein JL, The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell, 1997. 89(3): p. 331–40. doi: 10.1016/s0092-8674(00)80213-5 [DOI] [PubMed] [Google Scholar]
- 103.Yokoyama C, et al. , SREBP-1, a basic-helix-loop-helix-leucine zipper protein that controls transcription of the low density lipoprotein receptor gene. Cell, 1993. 75(1): p. 187–97. doi: [PubMed] [Google Scholar]
- 104.Bennett MK, et al. , Sterol regulation of fatty acid synthase promoter. Coordinate feedback regulation of two major lipid pathways. J Biol Chem, 1995. 270(43): p. 25578–83. doi: 10.1074/jbc.270.43.25578 [DOI] [PubMed] [Google Scholar]
- 105.Rudolph MC, et al. , Sterol regulatory element binding protein and dietary lipid regulation of fatty acid synthesis in the mammary epithelium. Am J Physiol Endocrinol Metab, 2010. 299(6): p. E918–27. doi: 10.1152/ajpendo.00376.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Belkaid A, et al. , 17β-estradiol induces stearoyl-CoA desaturase-1 expression in estrogen receptor-positive breast cancer cells. BMC Cancer, 2015. 15: p. 440. doi: 10.1186/s12885-015-1452-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fhu CW and Ali A, Protein Lipidation by Palmitoylation and Myristoylation in Cancer. Frontiers in Cell and Developmental Biology, 2021. 9(1054). doi: 10.3389/fcell.2021.673647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Menendez JA and Lupu R, Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer, 2007. 7(10): p. 763–77. doi: 10.1038/nrc2222 [DOI] [PubMed] [Google Scholar]
- 109.Schlaepfer IR, et al. , Progestin modulates the lipid profile and sensitivity of breast cancer cells to docetaxel. Mol Cell Endocrinol, 2012. 363(1-2): p. 111–21. doi: 10.1016/j.mce.2012.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mast FD, et al. , Peroxisome biogenesis: something old, something new, something borrowed. Physiology (Bethesda), 2010. 25(6): p. 347–56. doi: 10.1152/physiol.00025.2010 [DOI] [PubMed] [Google Scholar]
- 111.McCleland ML, et al. , An integrated genomic screen identifies LDHB as an essential gene for triple-negative breast cancer. Cancer Res, 2012. 72(22): p. 5812–23. doi: 10.1158/0008-5472.Can-12-1098 [DOI] [PubMed] [Google Scholar]
- 112.Shen L, et al. , Metabolic reprogramming in triple-negative breast cancer through Myc suppression of TXNIP. Proc Natl Acad Sci U S A, 2015. 112(17): p. 5425–30. doi: 10.1073/pnas.1501555112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Camarda R, et al. , Inhibition of fatty acid oxidation as a therapy for MYC-overexpressing triple-negative breast cancer. Nat Med, 2016. 22(4): p. 427–32. doi: 10.1038/nm.4055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Campbell IG, et al. , Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res, 2004. 64(21): p. 7678–81. doi: 10.1158/0008-5472.Can-04-2933 [DOI] [PubMed] [Google Scholar]
- 115.Nik-Zainal S, et al. , Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature, 2016. 534(7605): p. 47–54. doi: 10.1038/nature17676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fruman DA, et al. , The PI3K Pathway in Human Disease. Cell, 2017. 170(4): p. 605–635. doi: 10.1016/j.cell.2017.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kruger DT, et al. , Hierarchical clustering of activated proteins in the PI3K and MAPK pathways in ER-positive, HER2-negative breast cancer with potential therapeutic consequences. Br J Cancer, 2018. 119(7): p. 832–839. doi: 10.1038/s41416-018-0221-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hoxhaj G, et al. , Direct stimulation of NADP(+) synthesis through Akt-mediated phosphorylation of NAD kinase. Science, 2019. 363(6431): p. 1088–1092. doi: 10.1126/science.aau3903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ward PS and Thompson CB, Signaling in control of cell growth and metabolism. Cold Spring Harb Perspect Biol, 2012. 4(7): p. a006783. doi: 10.1101/cshperspect.a006783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gouw AM, et al. , The MYC Oncogene Cooperates with Sterol-Regulated Element-Binding Protein to Regulate Lipogenesis Essential for Neoplastic Growth. Cell Metab, 2019. 30(3): p. 556–572.e5. doi: 10.1016/j.cmet.2019.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang L, Zhang S, and Wang X, The Metabolic Mechanisms of Breast Cancer Metastasis. Frontiers in Oncology, 2021. 10(2942). doi: 10.3389/fonc.2020.602416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wei Q, et al. , Metabolic rewiring in the promotion of cancer metastasis: mechanisms and therapeutic implications. Oncogene, 2020. 39(39): p. 6139–6156. doi: 10.1038/s41388-020-01432-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Morris E, et al. , Implications of Overdiagnosis: Impact on Screening Mammography Practices. Popul Health Manag, 2015. 18 Suppl 1(Suppl 1): p. S3–11. doi: 10.1089/pop.2015.29023.mor [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Krizmanich-Conniff KM, et al. , Triple receptor-negative breast cancer: imaging and clinical characteristics. AJR Am J Roentgenol, 2012. 199(2): p. 458–64. doi: 10.2214/ajr.10.6096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Krol I, et al. , Detection of clustered circulating tumour cells in early breast cancer. British Journal of Cancer, 2021. 125(1): p. 23–27. doi: 10.1038/s41416-021-01327-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Silva AAR, et al. , Multiplatform Investigation of Plasma and Tissue Lipid Signatures of Breast Cancer Using Mass Spectrometry Tools. Int J Mol Sci, 2020. 21(10). doi: 10.3390/ijms21103611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen X, et al. , Plasma lipidomics profiling identified lipid biomarkers in distinguishing early-stage breast cancer from benign lesions. Oncotarget, 2016. 7(24): p. 36622–36631. doi: 10.18632/oncotarget.9124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Eghlimi R, et al. , Triple Negative Breast Cancer Detection Using LC-MS/MS Lipidomic Profiling. J Proteome Res, 2020. 19(6): p. 2367–2378. doi: 10.1021/acs.jproteome.0c00038 [DOI] [PubMed] [Google Scholar]
- 129.Hilvo M, et al. , Monounsaturated fatty acids in serum triacylglycerols are associated with response to neoadjuvant chemotherapy in breast cancer patients. Int J Cancer, 2014. 134(7): p. 1725–33. doi: 10.1002/ijc.28491 [DOI] [PubMed] [Google Scholar]
- 130.Fhu CW and Ali A, Fatty Acid Synthase: An Emerging Target in Cancer. Molecules (Basel, Switzerland), 2020. 25(17): p. 3935. doi: 10.3390/molecules25173935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kuhajda FP, et al. , Synthesis and antitumor activity of an inhibitor of fatty acid synthase. Proc Natl Acad Sci U S A, 2000. 97(7): p. 3450–4. doi: 10.1073/pnas.050582897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ueda SM, et al. , Trophoblastic neoplasms express fatty acid synthase, which may be a therapeutic target via its inhibitor C93. Am J Pathol, 2009. 175(6): p. 2618–24. doi: 10.2353/ajpath.2009.081162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Oliveras G, et al. , Novel anti-fatty acid synthase compounds with anti-cancer activity in HER2+ breast cancer. Ann N Y Acad Sci, 2010. 1210: p. 86–92. doi: 10.1111/j.1749-6632.2010.05777.x [DOI] [PubMed] [Google Scholar]
- 134.Hardwicke MA, et al. , A human fatty acid synthase inhibitor binds β-ketoacyl reductase in the keto-substrate site. Nat Chem Biol, 2014. 10(9): p. 774–9. doi: 10.1038/nchembio.1603 [DOI] [PubMed] [Google Scholar]
- 135.Vázquez MJ, et al. , Discovery of GSK837149A, an inhibitor of human fatty acid synthase targeting the beta-ketoacyl reductase reaction. Febs j, 2008. 275(7): p. 1556–67. doi: 10.1111/j.1742-4658.2008.06314.x [DOI] [PubMed] [Google Scholar]
- 136.Alwarawrah Y, et al. , Fasnall, a Selective FASN Inhibitor, Shows Potent Anti-tumor Activity in the MMTV-Neu Model of HER2(+) Breast Cancer. Cell Chem Biol, 2016. 23(6): p. 678–88. doi: 10.1016/j.chembiol.2016.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gruslova A, et al. , FASN inhibition as a potential treatment for endocrine-resistant breast cancer. Breast Cancer Res Treat, 2021. 187(2): p. 375–386. doi: 10.1007/s10549-021-06231-6 [DOI] [PubMed] [Google Scholar]
- 138.Corominas-Faja B, et al. , Chemical inhibition of acetyl-CoA carboxylase suppresses self-renewal growth of cancer stem cells. Oncotarget, 2014. 5(18): p. 8306–16. doi: 10.18632/oncotarget.2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Baek AE, et al. , The cholesterol metabolite 27 hydroxycholesterol facilitates breast cancer metastasis through its actions on immune cells. Nature Communications, 2017. 8(1): p. 864. doi: 10.1038/s41467-017-00910-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Van Wyhe RD, Rahal OM, and Woodward WA, Effect of statins on breast cancer recurrence and mortality: a review. Breast Cancer (Dove Med Press), 2017. 9: p. 559–565. doi: 10.2147/bctt.S148080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sethunath V, et al. , Targeting the Mevalonate Pathway to Overcome Acquired Anti-HER2 Treatment Resistance in Breast Cancer. Mol Cancer Res, 2019. 17(11): p. 2318–2330. doi: 10.1158/1541-7786.Mcr-19-0756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lettiero B, et al. , Insensitivity to atorvastatin is associated with increased accumulation of intracellular lipid droplets and fatty acid metabolism in breast cancer cells. Sci Rep, 2018. 8(1): p. 5462. doi: 10.1038/s41598-018-23726-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cai D, et al. , RORγ is a targetable master regulator of cholesterol biosynthesis in a cancer subtype. Nat Commun, 2019. 10(1): p. 4621. doi: 10.1038/s41467-019-12529-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cai D, Zhang X, and Chen HW, A master regulator of cholesterol biosynthesis constitutes a therapeutic liability of triple negative breast cancer. Mol Cell Oncol, 2020. 7(2): p. 1701362. doi: 10.1080/23723556.2019.1701362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.van Weverwijk A, et al. , Metabolic adaptability in metastatic breast cancer by AKR1B10-dependent balancing of glycolysis and fatty acid oxidation. Nat Commun, 2019. 10(1): p. 2698. doi: 10.1038/s41467-019-10592-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yang J, Park KW, and Cho S, Inhibition of the CD36 receptor reduces visceral fat accumulation and improves insulin resistance in obese mice carrying the BDNF-Val66Met variant. J Biol Chem, 2018. 293(34): p. 13338–13348. doi: 10.1074/jbc.RA118.002405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rena G, Hardie DG, and Pearson ER, The mechanisms of action of metformin. Diabetologia, 2017. 60(9): p. 1577–1585. doi: 10.1007/s00125-017-4342-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bodmer M, et al. , Long-Term Metformin Use Is Associated With Decreased Risk of Breast Cancer. Diabetes Care, 2010. 33(6): p. 1304. doi: 10.2337/dc09-1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lord SR, et al. , Transcriptomic analysis of human primary breast cancer identifies fatty acid oxidation as a target for metformin. British Journal of Cancer, 2020. 122(2): p. 258–265. doi: 10.1038/s41416-019-0665-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gammone MA, et al. , Omega-3 Polyunsaturated Fatty Acids: Benefits and Endpoints in Sport. Nutrients, 2018. 11(1). doi: 10.3390/nu11010046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Preta G, New Insights Into Targeting Membrane Lipids for Cancer Therapy. Frontiers in Cell and Developmental Biology, 2020. 8(876). doi: 10.3389/fcell.2020.571237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tošić I, et al. , Lipidome-based Targeting of STAT3-driven Breast Cancer Cells Using Poly-l-glutamic Acid–coated Layer-by-Layer Nanoparticles. Molecular Cancer Therapeutics, 2021. 20(4): p. 726. doi: 10.1158/1535-7163.MCT-20-0505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yamada T, et al. , Development of a lipid profiling system using reverse-phase liquid chromatography coupled to high-resolution mass spectrometry with rapid polarity switching and an automated lipid identification software. J Chromatogr A, 2013. 1292: p. 211–8. doi: 10.1016/j.chroma.2013.01.078 [DOI] [PubMed] [Google Scholar]
- 154.Breitkopf SB, et al. , A relative quantitative positive/negative ion switching method for untargeted lipidomics via high resolution LC-MS/MS from any biological source. Metabolomics, 2017. 13(3). doi: 10.1007/s11306-016-1157-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Brovkovych V, et al. , Removal of Serum Lipids and Lipid-Derived Metabolites to Investigate Breast Cancer Cell Biology. Proteomics, 2019. 19(18): p. e1800370. doi: 10.1002/pmic.201800370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Thiele C, Wunderling K, and Leyendecker P, Multiplexed and single cell tracing of lipid metabolism. Nature Methods, 2019. 16(11): p. 1123–1130. doi: 10.1038/s41592-019-0593-6 [DOI] [PubMed] [Google Scholar]
- 157.Triebl A and Wenk MR, Analytical Considerations of Stable Isotope Labelling in Lipidomics. Biomolecules, 2018. 8(4). doi: 10.3390/biom8040151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wilson N, et al. , Lobular Breast Cancer: A Review. Frontiers in Oncology, 2021. 10(3091). doi: 10.3389/fonc.2020.591399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wattigney WA, et al. , Increasing impact of obesity on serum lipids and lipoproteins in young adults. The Bogalusa Heart Study. Arch Intern Med, 1991. 151(10): p. 2017–22. doi: [PubMed] [Google Scholar]
- 160.Grundy SM and Denke MA, Dietary influences on serum lipids and lipoproteins. J Lipid Res, 1990. 31(7): p. 1149–72. doi: [PubMed] [Google Scholar]