Abstract
A surprising finding of recent studies in mouse is the dominance of widespread movement-related activity throughout the brain, including in early sensory areas. In awake subjects, failing to account for movement risks misattributing movement-related activity to other (e.g., sensory or cognitive) processes. In this article, we (1) review task designs for separating task-related and movement-related activity, (2) review three “case studies” in which not considering movement would have resulted in critically different interpretations of neuronal function, and (3) discuss functional couplings that may prevent us from ever fully isolating sensory, motor, and cognitive-related activity. Our main thesis is that neural signals related to movement are ubiquitous, and therefore ought to be considered first and foremost when attempting to correlate neuronal activity with task-related processes.
Keywords: behavior, cognition, movement, neural coding, sensorimotor
Introduction
A major goal of systems and behavioral neuroscience is to understand how neurons in different brain regions organize to generate behavior. Instead of tackling complex behaviors in total, this pursuit has been aided by the cognitive psychology framework of identifying specific internal processes that mediate between a stimulus and response. Cognitive psychologists have developed behavioral tasks that emphasize, for example, sensory detection (Green and Swets, 1966), attention (Posner, 1980), sensory selection (Treisman, 1964), evidence accumulation (Ratcliff and McKoon, 2008), working memory (Berg, 1948), motor planning (Rosenbaum, 1980), and impulse control (Logan et al., 1984). By pairing neuronal recordings with these behavioral tasks, researchers aim to determine the neuronal implementation of specific cognitive processes (e.g., Moran and Desimone, 1985; Funahashi et al., 1989; Roitman and Shadlen, 2002).
Within the past 10 years, there has been a surge in the use of the mouse and rat in these combined behavioral and neuronal recording studies. This development is promising in that it enables the use of a wide array of advanced genetic and physiological tools to resolve neuronal organization at unprecedented spatial, temporal, and genetic resolutions (O'Connor et al., 2009; Carandini and Churchland, 2013; Hanks and Summerfield, 2017). However, these rodent studies are revealing and re-emphasizing essential challenges in relating neuronal activity to task performance. Accounting for movement is one such challenge.
As we detail throughout this article, movements can be divided into two general categories, task-instructed and task-uninstructed (Musall et al., 2019). Task-instructed movements refer to the actions that are required for the subjects to solve a task, such as nose-poking, lever-pressing, and/or licking. Task-uninstructed movements refer to the movements that are not explicitly required to solve the task (e.g., whisking in a visual task that does not involve the whisker system). Consequently, “movement-related” neuronal activity may involve several distinct mechanisms: it may reflect overt behavioral responses required for obtaining reward; it may reflect preparatory postural shifts which may only be detected via EMG (Corneil et al., 2008; Wong et al., 2015); it may reflect the sensory consequences of self-generated movements (Flossmann and Rochefort, 2021); it may reflect changes in autonomic and behavioral arousal because of reward expectation (Hassani et al., 2001). Each of these “movement-related signals” may temporally and spatially overlap with, and indeed may contain components that are intimately intertwined with, processes considered as sensory and cognitive (see section below titled Blurred lines between sensory, cognitive, and motor processes).
Distinguishing movement-related activity has become acutely concerning because of recent findings demonstrating that, during wakefulness, movement is the dominant source of variance in neuronal activity throughout the mouse brain (Musall et al., 2019; Steinmetz et al., 2019; Stringer et al., 2019; Kauvar et al., 2020; Salkoff et al., 2020). This conclusion is based on two, interrelated sets of observations. First, mice move frequently, even under head fixation. In a revealing study, Musall et al. (2019) trained mice in a spatial discrimination task, in which head-fixed mice were required to report the locations of auditory or visual stimuli. By video monitoring during task performance, this group identified high dimensional movements of the face and body of each subject. Notably, most of these movements were considered uninstructed. Second, movements evoke widespread neural activity throughout the brain. In the same study, the authors also recorded cortex-wide neuronal activity during task performance. Using linear regression, they determined that the variance of cortical activity related to movements (both instructed and uninstructed) was several times larger than that of sensory, choice, and reward variables. Furthermore, variance related to uninstructed movements was larger than that of instructed movements. Movement-related activity dominated not only motor cortices, but primary sensory cortices as well. Concurrent studies in mice using an array of neural recording techniques also identified robust, cortex-wide and brain-wide neuronal activities related to spontaneous and task-instructed movements (Steinmetz et al., 2019; Stringer et al., 2019; Salkoff et al., 2020). Stringer et al. (2019) recorded spiking activity from mouse cortex, thalamus, basal ganglia, and midbrain and found that spontaneous behaviors were strongly encoded in each region (Stringer et al., 2019). Importantly, the encoding was multidimensional rather than just a single correlate of motor behavior that might correspond to arousal or vigilance. That is, each of these brain regions encoded details about multiple aspects of observable behavior. Additionally, movement-related signals preceded overt behavior, and were observed throughout the brain at least 50 ms before detectable movement onset (Steinmetz et al., 2019). Last, it is worth noting that movement-related signals may reflect general movement initiation that is not action-specific (Kaufman et al., 2016).
The important lesson from these recent studies is that, regardless of the brain region under investigation, movement-related signals are represented in large populations of neurons. Furthermore, while we focus this discussion on studies in rodents, isolating movement-related neuronal activity from sensory and cognitive-related activity is not unique to rodents. Studies in nonhuman primates have reported that small eye movements, “microsaccades,” strongly affect neuronal responses in visual cortices (e.g., Leopold and Logothetis, 1998; Martinez-Conde et al., 2000; Herrington et al., 2009). Furthermore, dominant, brain-wide movement-related activity has also been observed in zebrafish (Ahrens et al., 2012), Drosophila (Aimon et al., 2019), and Caenorhabditis elegans (Kato et al., 2015). Therefore, movement ought to be considered as a primary source of neuronal variance for many, if not all, animal models.
The following two sections of this article describe strategies and case studies for accounting for movement-related neuronal activity. However, we recognize that movement does not merely “contaminate” sensory and cognitive signals, but may play essential roles in sensation and cognition. In the final section of this article, we briefly describe some intrinsic couplings between sensory, cognitive, and motor processes.
How to isolate sensory and cognitive from movement-related neuronal activity
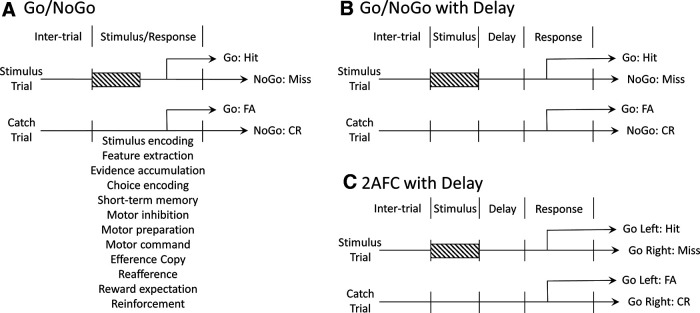
The most important consideration in attributing neuronal activity to sensory, cognitive, or motor processes is the behavioral task design. Figure 1A illustrates a common Go/NoGo sensory detection task design (e.g., Ollerenshaw et al., 2012; Pinto et al., 2013; Sachidhanandam et al., 2013; Martins and Froemke, 2015; Yang et al., 2016; Rodenkirch et al., 2019; Banerjee et al., 2020; McBurney-Lin et al., 2020). After a variable intertrial interval, the subject is presented with a stimulus trial (stimulus present) or catch trial (stimulus absent). Alternatively, for sensory discrimination, the two trial types are a target stimulus trial (target present) and distractor stimulus trial (target absent). In both designs, immediately following stimulus onset, the mouse has a specific response window in which to perform a motor action (Go) to report target stimulus detection. When recording from early sensory areas (e.g., primary sensory cortex), it has been common practice to interpret the activity within the early post-stimulus window (e.g., first 100 or 200 ms) as sensory-related. However, at least for simple detection tasks, this “sensory” window may also contain the earliest response times, with mean response times for salient stimuli approaching 200 ms (Ollerenshaw et al., 2012; Sachidhanandam et al., 2013; Yang et al., 2016; Steinmetz et al., 2019; Aruljothi et al., 2020; McBurney-Lin et al., 2020). Therefore, this “early post-stimulus” window includes not only sensory processing, but a multitude of processes, including decision formation, motor planning, motor initiation, movement, reward expectation, and reinforcement signaling (Fig. 1A). Given the widespread movement-related signals throughout the mouse brain (see above), this task design is insufficient for isolating sensory processing. In other words, even when recording in early sensory areas, interpreting this window as “sensory” may misattribute neuronal activity that relates to other processes. Identical concerns apply when attempting to attribute choice-related signals in this Go/NoGo task design.
Figure 1.
Behavioral task design and associated internal processes. A, Standard Go/NoGo task structure. After the intertrial interval, one of two trials are presented: stimulus present (stimulus trial) or stimulus absent (catch trial). Within this design, stimulus presentation and response windows overlap. Beneath this combined stimulus–response window, we indicate some of the internal processes likely to be deployed. Trial outcomes include response present (Go) and response absent (NoGo). Hit: Stimulus/Go; Miss: Stimulus/NoGo; False alarm (FA): Catch/Go; Correct rejection (CR): Catch/NoGo. B, Same Go/NoGo trial structure as in A, but with the inclusion of a delay between stimulus and response windows. This affords a separation of sensory and decision-making processes during the stimulus window from the task-instructed motor processes and reinforcement signaling during the response window. C, 2AFC trial structure. The same trial types are presented as in A and B, yet subjects are required to report both stimulus present and stimulus absent with different motor actions (e.g., Go Left, Go Right). This trial structure affords a separation between choice encoding (deciding stimulus present vs absent) and generalized motor initiation. How the processes listed in A distribute across task epochs in B and C will depend on the precise details of each task and should be considered for all task designs.
Two partial solutions to the problem are presented in Figure 1B, C. The first partial solution (Fig. 1B) is to include a delay between stimulus onset and the earliest allowable response window (Sachidhanandam et al., 2016; Aruljothi et al., 2020; Esmaeili et al., 2021). The addition of this delay makes it possible to dissociate early sensory and choice processing from task-instructed movements and reinforcement signals. A related practice, applied post hoc, is to exclude the trials with early response times from analyses of sensory and choice processing (Kwon et al., 2016; Yang et al., 2016). However, neither approach eliminates the confound of potential task-uninstructed movements, including postural movements in preparation for executing the Go response. For example, Zareian et al. (2021) included a short (200 ms) delay between stimulus onset and earliest allowable response window. Nonetheless, overt movements were observable by ∼100 ms after stimulus onset. Thus, in that task, neural activity after 100 ms is confounded by movement, despite the inclusion of a 200 ms delay. Uninstructed and preparatory movements during a post-stimulus, pre-response delay period have been reported by others as well (Esmaeili et al., 2021).
The second partial solution is to use a 2-alternative forced choice (2AFC) design instead of a Go/NoGo design (Fig. 1C) (Green and Swets, 1966; Miyashita and Feldman, 2013; O'Connor et al., 2013; Guo et al., 2014; Li et al., 2016; Burgess et al., 2017). With this design, subjects are instructed to initiate a motor response regardless of sensory detection (e.g., lick left for stimulus detection, lick right for no stimulus detection) and are rewarded every correct trial. While 2AFC does not solve the potential confound of uninstructed movements, this design likely enables the experimenter to dissociate choice activity from nonselective, instructed movements as well as from reward expectation and reinforcement signals. Steinmetz et al. (2019) recorded brain-wide neuronal activity during a modified 2AFC task. This task required mice to turn a wheel to the left or right on trials with lateralized visual stimuli (away from the side of the stronger stimulus) but withhold movement on trials without visual stimuli. This design allowed the experimenters to distinguish neuronal activity related to nonselective movement (turning the wheel in either direction vs no turning) and choice-related, direction-specific movement (turning left vs turning right). Nonselective movement-related activity was observed globally throughout the brain. In contrast, choice-related signals were much more restricted to subsets of neurons within specific brain regions. These findings emphasize the need for caution when interpreting “choice-related” signals in Go/NoGo tasks, as they may reflect general, nonselective movement initiation rather than specific action selection.
In all three task designs described above, motor planning is likely to be initiated immediately following sensory processing since the stimulus–response associations are constant throughout training. Other task designs can better isolate sensory or cognitive processes from motor planning, by requiring the motor response to be contingent on specific sequences of stimuli. In sequential sensory discrimination tasks (LaMotte and Mountcastle, 1975) or delayed matching to sample tasks (Miller et al., 1996), the motor response depends on a comparison of the first with subsequent stimulus presentations. In Pro/Anti tasks (Everling and Munoz, 2000; Duan et al., 2021), the rule for how to pair a stimulus–response varies across trials. By cueing the rule before presentation of a target stimulus, this task design can vary context without altering the expected sensory and motor content.
While task design could mitigate the overlap of instructed movements and sensory and cognitive processing, the potential confound of uninstructed movements remains unresolved. For this, movement monitoring is essential. For example, working memory is commonly tested by cueing a target location, followed by a 2-6 s delay during which the cue is absent, followed by a Go signal which instructs the subject to make a movement toward the remembered target location. In early studies of working memory in nonhuman primates, it was uncertain whether subjects were covertly remembering the target location (requiring a persistent internal representation) or making overt postural movements toward the target (which may or may not require persistent internal representation). In response to this confound, Funahashi et al. (1989) developed an oculomotor delayed-response task, for which the experimenters used a scleral search coil to precisely measure eye position. With eye position monitoring, the researchers were able to train their subjects to maintain fixation throughout the delay, thereby encouraging the use of covert working memory instead of postural movements to solve the task. However, more recent studies indicate that, during delayed saccades, neck EMG responses correlate strongly with upcoming eye movements (Corneil et al., 2008). On the one hand, the prevalence of such uninstructed movements highlights the need for rigorous movement monitoring beyond task-related outputs (e.g., monitoring only whisker movements during whisker-based tasks, or monitoring only eye movements during visual tasks). On the other hand, the prevalence of uninstructed movements suggests that overt movements may be intimately linked with cognitive processes. An important topic of ongoing and future research is to determine the extent to which these uninstructed movements are part of the decision-making process.
Even with the most rigorous experimental design, neural activity that resembles choice formation may still be related to other processes. One way to determine whether a brain area causally influences choice is to perturb the neural activity in that area and observe the perturbation's influence on behavior. If perturbing a brain area influences the trial outcome, then that brain area may putatively be involved in choice formation. Recent studies have shown that optogenetic perturbations of primary sensory areas and secondary motor areas, but not other areas of dorsal cortex, influence the decision of the mouse (Guo et al., 2014; Inagaki et al., 2019; Daie et al., 2021; Zatka-Haas et al., 2021). If, however, the perturbation alters overt movement regardless of the experimental paradigm, that brain area may relate more directly to motor activation (Corbit et al., 2020; Ruder et al., 2021) or contribute to both decision formation and motor commands (Gold and Shadlen, 2000; Selen et al., 2012). Interpretations of perturbation experiments require additional considerations (e.g., Otchy et al., 2015; Li et al., 2019). Nonetheless, they are indispensable for identifying neuronal activity causally related to choice formation and motor processing.
Case studies in the importance of accounting for movement
Below, we review three studies in mice and rats in which careful accounting for movement was essential for the proper attribution of sensory and cognitive-related neuronal activity. These studies highlight three different ways in which movement may complicate the evaluation of sensory and cognitive-related signaling. First, rodents may develop an unexpected behavioral strategy to, presumably, reduce task difficulty and cognitive load; second, rodents may use a combination of overt movement and covert motor planning when preparing a choice response; third, movement during decision formation may confound sensory, choice, and reward signaling.
The first series of studies relate to an active sensing, whisker-based spatial discrimination task in head-fixed mice (Fig. 2A) (O'Connor et al., 2010a,b). In this task, on each trial, a pole was presented adjacent to the mouse's snout, at either an anterior or posterior location. Both locations were accessible within the mouse's whisking range. In this Go/NoGo task design, the posterior location was the target stimulus; anterior, the distractor. O'Connor et al. (2010a) recorded neuronal activity in primary somatosensory cortex during task performance. They observed substantially more responsive neurons on hit (target, Go) compared with correct rejection (distractor, NoGo) trials. A tempting interpretation of these data would be that distractor touch signals were attenuated or filtered out along the ascending sensory pathway before reaching primary somatosensory cortex. However, by high-speed video monitoring of the whiskers, they demonstrated that mice adapted a clever behavioral strategy to solve this task. Instead of sampling both locations equally, mice retracted their whiskers to sample only the posterior/target location (O'Connor et al., 2010b) (Fig. 2A). Because of this sampling strategy, there were much fewer touch events on anterior/distractor trials. Thus, by selective sampling, the mice converted a spatial discrimination task into an object detection task. Accordingly, the authors recognized that the reduced responsiveness on anterior/distractor trials was due, at least in part, to reduced sensory drive.
Figure 2.
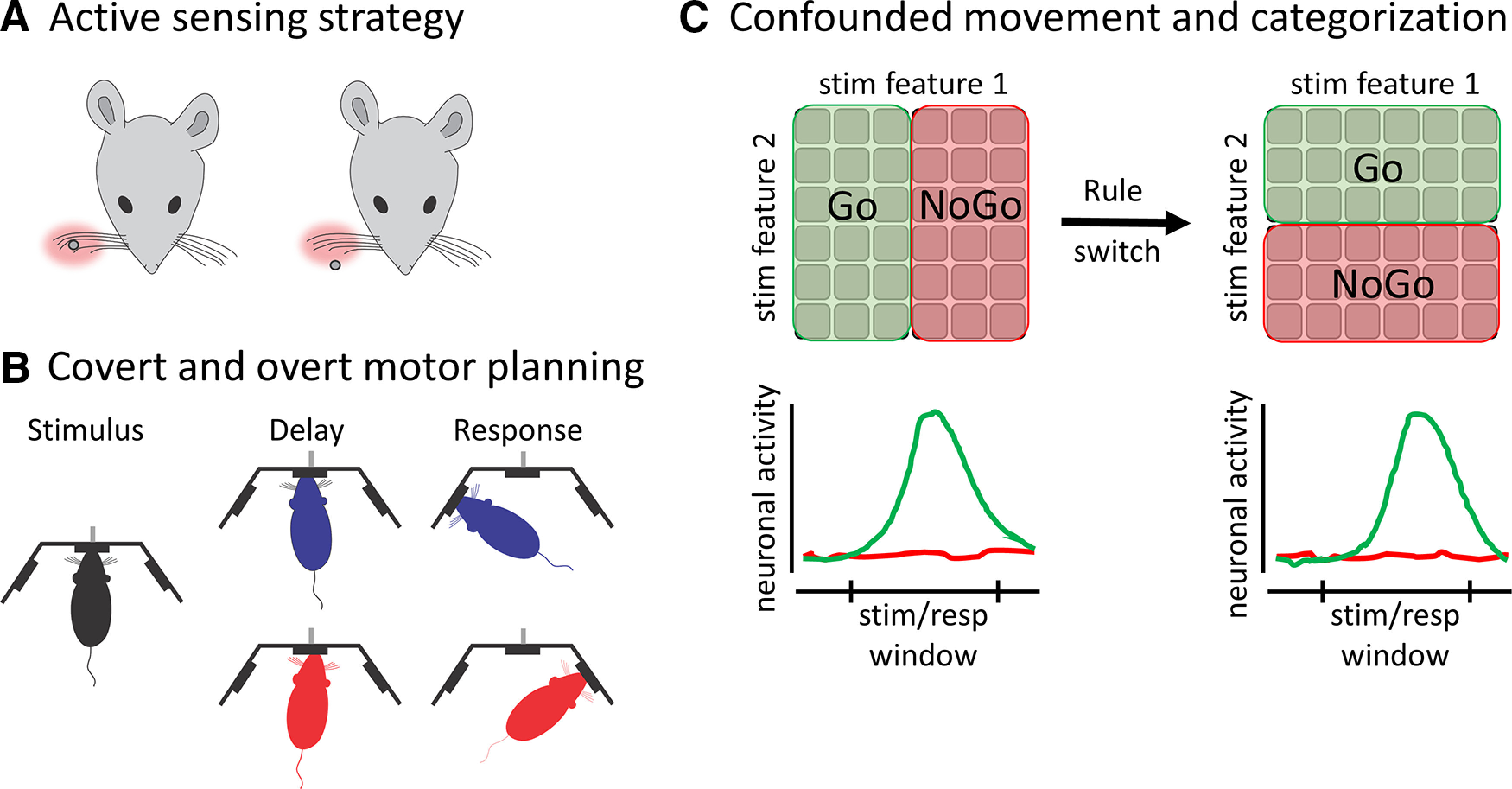
Examples of how movement may complicate the evaluation of sensory and cognitive processes. A, Schematic of the active sensing whisker-based spatial discrimination task of O'Connor et al. (2010b). Mice were trained to discriminate posterior (left) from anterior (right) pole locations. Instead of sampling both locations equally, mice developed a strategy of only sampling (pink shade) the posterior (Go) location. B, Schematic of the freely moving 2AFC memory-guided orienting task from Erlich et al. (2011). Rats were trained to report high and low stimulus frequencies at left and right nose ports. During the delay period while still in the central nose port, rats slightly oriented toward the expected reward port, thus using a combination of covert and overt strategies to remember the reward location. C, Top, Schematic of the category assignments before and after rule switch from Reinert et al. (2021). Before the rule switch, mice learned to respond to stimuli according to stimulus feature 1 and ignore stimulus feature 2; after rule switch, mice learned to respond to stimuli according to stimulus feature 2 and ignore stimulus feature 1. Bottom, Illustration of a hypothetical neuron's response to each category on successful Go (green) and NoGo (red) trials, before (left) and after (right) rule switch. Because of the Go/NoGo task design without a delay, neuronal activity following Go stimuli may reflect stimulus category representation and/or response-related movements.
The second study involved freely moving rats in a 2AFC memory-guided orienting task (Fig. 2B) (Erlich et al., 2011). Rats were placed in a behavioral box consisting of a central nose port to trigger stimulus delivery consisting of auditory clicks, and two lateral nose ports to report high-frequency (left) or low-frequency (right) stimulus content. The authors used a delay period between stimulus presentation and a Go cue to measure response preparatory signals in secondary motor cortex. Rats were trained to remain in the central nose port throughout the delay period. As described above (Funahashi et al., 1989), activities during the delay period of memory-guided tasks have been commonly used in primates to measure working memory and preparatory signals in frontal cortex. And yet, a previous study in rats showed that neuronal activity in frontal cortex is highly sensitive to subtle differences in overt behavior (Euston and McNaughton, 2006). By video monitoring, Erlich et al. (2011) demonstrated that rats did not solely encode motor preparation internally, but also slightly orientated their snouts toward the expected reward port (Fig. 2B). By accounting for this overt motor strategy, the authors were able to attribute neural activity in secondary motor cortex during the delay to a combination of head direction and preparatory signaling. Using receiver operator characteristic analyses, the authors demonstrated that head direction accounted for only a small proportion of the preparatory signals recorded in secondary motor cortex. This suggests the combined use of both overt and covert processes contributing to response preparation. Whether the overt movements were necessary for performance of the task (and for the signals observed in secondary motor cortex) remains an open question.
The third study set out to explore representations of learned categories in mouse PFC (Fig. 2C) (Reinert et al., 2021). The authors monitored the activities of the same PFC neurons throughout category learning, generalization, and rule switching. Notably, rule switching required mice to categorize and generalize based on an orthogonal feature of the same stimulus set. The task structure was Go/NoGo, without a post-stimulus delay. A substantial percentage of PFC neurons were active following the Go stimuli, before and after the rule switch (Fig. 2C). One possible interpretation of this finding is that these neurons represented the Go stimulus categories before and after the rule switch. This would require a remapping of their inputs since half of the previous Go/NoGo stimulus assignments were inverted after the rule switch. An alternative interpretation, however, is that these PFC neurons stably represented the Go action (licking) instead of the stimulus category. Due to a lack of a post-stimulus delay, the sensory, choice, Go movement, and reward signals all overlapped in the response window. To dissect the contributions of category, choice, movement, and reward, the authors used a linear regression model to determine the extent to which these task features (variables) accounted for neuronal activity. Since these variables were likely to be highly correlated in this task design, the authors took the additional step of measuring each variable's unique contribution by shuffling the variable and quantifying the loss in performance of the model. By this method, the authors concluded that the PFC neurons were likely representing a combination of category, choice, movement, and reward (Reinert et al., 2021). Future studies, using tasks designed to temporally dissociate these representations, are needed to test their conclusions.
Computational modeling enabled the previous studies to begin to disentangle behavioral and cognitive signals: the investigators created predictive models of neural activity from behavior and cognitive variables and found that models which included cognitive variables outperformed behavior-only models. It is important to note that current methods of behavioral monitoring and modeling are very likely to underestimate the neuronal correlates of movement and motor planning. First, even the most rigorous monitoring approaches do not sample all movements and muscle activity. Second, experimenters can only infer the onset of motor planning and the neuronal coordination of motor sequences. Behavior-only models of neural activity will likely continue to improve as behavioral tracking and computational models improve (Hausmann et al., 2021). Therefore, future studies ideally should record as many behavioral features as possible to create a “null” model, which relates neuronal activity to behavioral representations in the absence of a goal-directed task (Stringer et al., 2019). Creating this “null” model using neural activity recorded before learning may additionally enable the study of changes in neural representations of behavior by task demands (e.g., Driscoll et al., 2017; Henschke et al., 2020).
Blurred lines between sensory, cognitive, and motor processes
Above, we have discussed various strategies to aid proper attribution of neuronal activity. Different strategies are useful at different stages of a project, from planning (behavioral task design) and data gathering (movement monitoring) to data analyses (modeling of the multiple potential factors driving neuronal activity). These strategies ought to be considered regardless of the brain region under study, given the brain-wide movement-related signals observed in mice (Steinmetz et al., 2019; Stringer et al., 2019). However, even with these strategies in place, is it ever possible to fully dissociate the neuronal activities related to motor, sensory, and cognitive processes? Below, we discuss why confounds among these functions may not merely reflect experimental limitation, but may be embedded within the sensory-motor couplings of neuronal systems.
Consider you are recording from a primary sensory area (e.g., V1 or A1), a brain region that presumably does not have direct roles in sending out motor commands. During a sensory discrimination task, would you be able to ascribe all activity in this region to externally generated, task-related stimuli? Emphatically, no! First, in addition to encoding sensory stimuli, primary sensory cortices, like many other brain areas, also encode the sensory consequences of self-generated movements, called “reafference” (Fee et al., 1997; Crapse and Sommer, 2008; Russ et al., 2016). For example, while walking, we generate sequences of foot contacts, stepping sounds, and visual streaming. Reafference may be due to spontaneous and uninstructed movements, instructed movements, and/or reflexive movements triggered in response to task-related stimuli. Second, sensory circuits in the brain also receive internal copies of motor commands, referred to as “efference copy” or “corollary discharge” (Sommer and Wurtz, 2002; Eliades and Wang, 2003; Poulet and Hedwig, 2006; Schneider et al., 2014). Efference copy signals, presumably, help to discriminate between self-generated (reafferent) and externally generated (afferent) sensory inputs (Wurtz and Sommer, 2004; Crapse and Sommer, 2008; Sun and Goldberg, 2016; Straka et al., 2018). Sensory regions receive additional cognitive signals pertaining to attention (Moran and Desimone, 1985; Fries et al., 2001; McAdams and Reid, 2005; Chalk et al., 2010), expectation (Summerfield et al., 2008; Vinken et al., 2017), timing (Shuler and Bear, 2006; Namboodiri et al., 2015), and arousal (Niell and Stryker, 2010; Fu et al., 2014; Vinck et al., 2015). Thus, because of bottom-up inputs (afference, reafference) and top-down inputs (efference copy, attention, expectation, timing), neuronal activity even in primary sensory regions may reflect combinations of externally generated sensory stimuli, self-generated sensory stimuli, motor plans, and cognitive processes. How movement-related and other contextual signals integrate with afferent sensory signals to influence goal-directed behavior is an active topic of current and future research (Parker et al., 2020).
Further complicating this analysis, primary sensory cortices may perform both sensory and motor functions. This was demonstrated elegantly in the mouse cortical whisker system, in which primary somatosensory cortex was shown to drive whisker retraction directly (rather than indirectly through motor cortex) (Matyas et al., 2010). Motor functions of sensory cortices may facilitate sensorimotor coordination during active sensing, in this case, possibly, quickly retracting following detection of an object. However, it is currently unknown whether this direct sensory-motor coupling is unique to the whisker somatosensory system, unique to rodents, or a general feature of all sensory cortices.
Last, processes we consider as “cognitive” are not independent from sensory and motor systems. One example is covert spatial attention: the ability to shift one's attention without physically re-orienting toward the attended location. Cognitive psychology studies demonstrated functional linkages between covert attention and motor planning/execution (Rizzolatti et al., 1987; Hoffman and Subramaniam, 1995; Deubel and Schneider, 1996). This led to the premotor theory of attention (Rizzolatti et al., 1987), which proposed that there is no supramodal attentional brain structure distinct from sensory and motor circuits. Indeed, truly “covert” attention may be illusory, as features of covert visual attention are highly correlated with small, transient eye movements (microsaccades) toward the attended region (Lowet et al., 2018). Studies spanning multiple decades, mainly in nonhuman primates, have demonstrated how spatial attention, motor preparation, and movement are different functions of the same neural structures (Tanji and Evarts, 1976; Moore and Fallah, 2001; Moore et al., 2003; Churchland et al., 2010; Mazer, 2011; Kaufman et al., 2014). More broadly, perception, cognition, and action may be implemented in a shared and distributed manner rather than subserved each by distinguishable subsystems (Cisek and Kalaska, 2010).
Conclusion
Recent findings have demonstrated that movement-related signals are dominant and ubiquitous in the mouse brain. In light of these new findings, we suspect that previous reports of sensory and cognitive-related neuronal activity in rodents may have been confounded by movement, leading to inaccurate conclusions about the contributions of neuronal populations to behavior. We conclude with four general strategies that we believe will help curtail potential misattributions: (1) Behavioral task design. When designing a task, consider all the processes that are likely to be deployed within each task period, and not just the intended focus of the study. (2) Movement monitoring. Given the prevalence of spontaneous and uninstructed movements, some of which may be time-locked to the trial structure, monitoring beyond task-related outputs is essential. (3) Causality testing. Determining whether neuronal activity contributes to a specific process, such as sensory encoding, choice formation, or working memory, requires perturbation. (4) Movement first. When evaluating our data or reviewing the work of others, consider the hypothesis that all reported signals can be accounted for by movement (including spontaneous, uninstructed, and instructed movements, reafferent and efferent signals). Once this hypothesis has been addressed, then consider alternative explanations. Experimental limitations and biological realities may prevent us from fully dissociating neuronal circuits and signals related to sensory, motor, and cognitive processes. However, recognizing and explicitly reporting potential confounds is a crucial step in linking neurons to behavior.
Footnotes
This work was supported by National Institutes of Health Grants R01NS107599 to E.Z., R01MH123686 to G.L., R01NS104834 to D.H.O., and R01NS107355 and R01NS112200 to H.Y.; Intramural Research Program of National Institute of Mental Health, National Institutes of Health ZIAMH002959 to S.L.; NYU-ECNU Institute of Brain and Cognitive Science at NYU Shanghai and the 111 Project, Base B16018 to J.C.E.; Klingenstein-Simons Foundation to N.A.S. and H.Y.; Pew Biomedical Scholars program to N.A.S.; and Howard Hughes Medical Institute to C.S. We thank members of the E.Z. laboratory for helpful comments on the manuscript.
The authors declare no competing financial interests.
References
- Ahrens MB, Li JM, Orger MB, Robson DN, Schier AF, Engert F, Portugues R (2012) Brain-wide neuronal dynamics during motor adaptation in zebrafish. Nature 485:471–477. 10.1038/nature11057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimon S, Katsuki T, Jia T, Grosenick L, Broxton M, Deisseroth K, Sejnowski TJ, Greenspan RJ (2019) Fast near-whole-brain imaging in adult Drosophila during responses to stimuli and behavior. PLoS Biol 17:e2006732. 10.1371/journal.pbio.2006732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruljothi K, Marrero K, Zhang Z, Zareian B, Zagha E (2020) Functional localization of an attenuating filter within cortex for a selective detection task in mice. J Neurosci 40:5443–5454. 10.1523/JNEUROSCI.2993-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Parente G, Teutsch J, Lewis C, Voigt FF, Helmchen F (2020) Value-guided remapping of sensory cortex by lateral orbitofrontal cortex. Nature 585:245–250. 10.1038/s41586-020-2704-z [DOI] [PubMed] [Google Scholar]
- Berg EA (1948) A simple objective technique for measuring flexibility in thinking. J Gen Psychol 39:15–22. 10.1080/00221309.1948.9918159 [DOI] [PubMed] [Google Scholar]
- Burgess CP, Lak A, Steinmetz NA, Zatka-Haas P, Bai Reddy C, Jacobs EA, Linden JF, Paton JJ, Ranson A, Schroder S, Soares S, Wells MJ, Wool LE, Harris KD, Carandini M (2017) High-yield methods for accurate two-alternative visual psychophysics in head-fixed mice. Cell Rep 20:2513–2524. 10.1016/j.celrep.2017.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carandini M, Churchland AK (2013) Probing perceptual decisions in rodents. Nat Neurosci 16:824–831. 10.1038/nn.3410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalk M, Herrero JL, Gieselmann MA, Delicato LS, Gotthardt S, Thiele A (2010) Attention reduces stimulus-driven gamma frequency oscillations and spike field coherence in V1. Neuron 66:114–125. 10.1016/j.neuron.2010.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Cunningham JP, Kaufman MT, Ryu SI, Shenoy KV (2010) Cortical preparatory activity: representation of movement or first cog in a dynamical machine? Neuron 68:387–400. 10.1016/j.neuron.2010.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF (2010) Neural mechanisms for interacting with a world full of action choices. Annu Rev Neurosci 33:269–298. 10.1146/annurev.neuro.051508.135409 [DOI] [PubMed] [Google Scholar]
- Corbit VL, Piantadosi SC, Wood J, Liu G, Choi CJ, Witten IB, Gittis AH, Ahmari SE (2020) Dissociable roles of central striatum and anterior lateral motor area in initiating and sustaining naturalistic behavior. bioRxiv 899070. doi: 10.1101/2020.01.08.899070 [DOI] [Google Scholar]
- Corneil BD, Munoz DP, Chapman BB, Admans T, Cushing SL (2008) Neuromuscular consequences of reflexive covert orienting. Nat Neurosci 11:13–15. 10.1038/nn2023 [DOI] [PubMed] [Google Scholar]
- Crapse TB, Sommer MA (2008) Corollary discharge across the animal kingdom. Nat Rev Neurosci 9:587–600. 10.1038/nrn2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daie K, Svoboda K, Druckmann S (2021) Targeted photostimulation uncovers circuit motifs supporting short-term memory. Nat Neurosci 24:259–265. 10.1038/s41593-020-00776-3 [DOI] [PubMed] [Google Scholar]
- Deubel H, Schneider WX (1996) Saccade target selection and object recognition: evidence for a common attentional mechanism. Vision Res 36:1827–1837. 10.1016/0042-6989(95)00294-4 [DOI] [PubMed] [Google Scholar]
- Driscoll LN, Pettit NL, Minderer M, Chettih SN, Harvey CD (2017) Dynamic reorganization of neuronal activity patterns in parietal cortex. Cell 170:986–999.e916. 10.1016/j.cell.2017.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan CA, Pagan M, Piet AT, Kopec CD, Akrami A, Riordan AJ, Erlich JC, Brody CD (2021) Collicular circuits for flexible sensorimotor routing. Nat Neurosci 24:1110–1120. 10.1038/s41593-021-00865-x [DOI] [PubMed] [Google Scholar]
- Eliades SJ, Wang X (2003) Sensory-motor interaction in the primate auditory cortex during self-initiated vocalizations. J Neurophysiol 89:2194–2207. 10.1152/jn.00627.2002 [DOI] [PubMed] [Google Scholar]
- Erlich JC, Bialek M, Brody CD (2011) A cortical substrate for memory-guided orienting in the rat. Neuron 72:330–343. 10.1016/j.neuron.2011.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmaeili V, Tamura K, Muscinelli SP, Modirshanechi A, Boscaglia M, Lee AB, Oryshchuk A, Foustoukos G, Liu Y, Crochet S, Gerstner W, Petersen CC (2021) Rapid suppression and sustained activation of distinct cortical regions for a delayed sensory-triggered motor response. Neuron 109:2183–2201.e2189. 10.1016/j.neuron.2021.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euston DR, McNaughton BL (2006) Apparent encoding of sequential context in rat medial prefrontal cortex is accounted for by behavioral variability. J Neurosci 26:13143–13155. 10.1523/JNEUROSCI.3803-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everling S, Munoz DP (2000) Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. J Neurosci 20:387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fee MS, Mitra PP, Kleinfeld D (1997) Central versus peripheral determinants of patterned spike activity in rat vibrissa cortex during whisking. J Neurophysiol 78:1144–1149. 10.1152/jn.1997.78.2.1144 [DOI] [PubMed] [Google Scholar]
- Flossmann T, Rochefort NL (2021) Spatial navigation signals in rodent visual cortex. Curr Opin Neurobiol 67:163–173. 10.1016/j.conb.2020.11.004 [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R (2001) Modulation of oscillatory neuronal synchronization by selective visual attention. Science 291:1560–1563. 10.1126/science.1055465 [DOI] [PubMed] [Google Scholar]
- Fu Y, Tucciarone JM, Espinosa JS, Sheng N, Darcy DP, Nicoll RA, Huang ZJ, Stryker MP (2014) A cortical circuit for gain control by behavioral state. Cell 156:1139–1152. 10.1016/j.cell.2014.01.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS (1989) Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol 61:331–349. 10.1152/jn.1989.61.2.331 [DOI] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN (2000) Representation of a perceptual decision in developing oculomotor commands. Nature 404:390–394. 10.1038/35006062 [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA (1966) Signal detection theory and psychophysics. New York: Wiley. [Google Scholar]
- Guo ZV, Li N, Huber D, Ophir E, Gutnisky D, Ting JT, Feng G, Svoboda K (2014) Flow of cortical activity underlying a tactile decision in mice. Neuron 81:179–194. 10.1016/j.neuron.2013.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks TD, Summerfield C (2017) Perceptual decision making in rodents, monkeys, and humans. Neuron 93:15–31. 10.1016/j.neuron.2016.12.003 [DOI] [PubMed] [Google Scholar]
- Hassani OK, Cromwell HC, Schultz W (2001) Influence of expectation of different rewards on behavior-related neuronal activity in the striatum. J Neurophysiol 85:2477–2489. 10.1152/jn.2001.85.6.2477 [DOI] [PubMed] [Google Scholar]
- Hausmann SB, Vargas AM, Mathis A, Mathis MW (2021) Measuring and modeling the motor system with machine learning. Curr Opin Neurobiol 70:11–23. 10.1016/j.conb.2021.04.004 [DOI] [PubMed] [Google Scholar]
- Henschke JU, Dylda E, Katsanevaki D, Dupuy N, Currie SP, Amvrosiadis T, Pakan JM, Rochefort NL (2020) Reward association enhances stimulus-specific representations in primary visual cortex. Curr Biol 30:1866–1880.e1865. 10.1016/j.cub.2020.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington TM, Masse NY, Hachmeh KJ, Smith JE, Assad JA, Cook EP (2009) The effect of microsaccades on the correlation between neural activity and behavior in middle temporal, ventral intraparietal, and lateral intraparietal areas. J Neurosci 29:5793–5805. 10.1523/JNEUROSCI.4412-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman JE, Subramaniam B (1995) The role of visual attention in saccadic eye movements. Percept Psychophys 57:787–795. 10.3758/bf03206794 [DOI] [PubMed] [Google Scholar]
- Inagaki HK, Fontolan L, Romani S, Svoboda K (2019) Discrete attractor dynamics underlies persistent activity in the frontal cortex. Nature 566:212–217. 10.1038/s41586-019-0919-7 [DOI] [PubMed] [Google Scholar]
- Kato S, Kaplan HS, Schrodel T, Skora S, Lindsay TH, Yemini E, Lockery S, Zimmer M (2015) Global brain dynamics embed the motor command sequence of Caenorhabditis elegans. Cell 163:656–669. 10.1016/j.cell.2015.09.034 [DOI] [PubMed] [Google Scholar]
- Kaufman MT, Churchland MM, Ryu SI, Shenoy KV (2014) Cortical activity in the null space: permitting preparation without movement. Nat Neurosci 17:440–448. 10.1038/nn.3643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MT, Seely JS, Sussillo D, Ryu SI, Shenoy KV, Churchland MM (2016) The largest response component in the motor cortex reflects movement timing but not movement type. eNeuro 3:ENEURO.0085-16.2016. 10.1523/ENEURO.0085-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauvar IV, Machado TA, Yuen E, Kochalka J, Choi M, Allen WE, Wetzstein G, Deisseroth K (2020) Cortical observation by synchronous multifocal optical sampling reveals widespread population encoding of actions. Neuron 107:351–367.e319. 10.1016/j.neuron.2020.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon SE, Yang H, Minamisawa G, O'Connor DH (2016) Sensory and decision-related activity propagate in a cortical feedback loop during touch perception. Nat Neurosci 19:1243–1249. 10.1038/nn.4356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte RH, Mountcastle VB (1975) Capacities of humans and monkeys to discriminate vibratory stimuli of different frequency and amplitude: a correlation between neural events and psychological measurements. J Neurophysiol 38:539–559. 10.1152/jn.1975.38.3.539 [DOI] [PubMed] [Google Scholar]
- Leopold DA, Logothetis NK (1998) Microsaccades differentially modulate neural activity in the striate and extrastriate visual cortex. Exp Brain Res 123:341–345. 10.1007/s002210050577 [DOI] [PubMed] [Google Scholar]
- Li N, Daie K, Svoboda K, Druckmann S (2016) Robust neuronal dynamics in premotor cortex during motor planning. Nature 532:459–464. 10.1038/nature17643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Chen S, Guo ZV, Chen H, Huo Y, Inagaki HK, Chen G, Davis C, Hansel D, Guo C, Svoboda K (2019) Spatiotemporal constraints on optogenetic inactivation in cortical circuits. Elife 8:e48622. 10.7554/eLife.48622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, Davis KA (1984) On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform 10:276–291. 10.1037/0096-1523.10.2.276 [DOI] [PubMed] [Google Scholar]
- Lowet E, Gomes B, Srinivasan K, Zhou H, Schafer RJ, Desimone R (2018) Enhanced neural processing by covert attention only during microsaccades directed toward the attended stimulus. Neuron 99:207–214.e203. 10.1016/j.neuron.2018.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Conde S, Macknik SL, Hubel DH (2000) Microsaccadic eye movements and firing of single cells in the striate cortex of macaque monkeys. Nat Neurosci 3:251–258. 10.1038/72961 [DOI] [PubMed] [Google Scholar]
- Martins AR, Froemke RC (2015) Coordinated forms of noradrenergic plasticity in the locus coeruleus and primary auditory cortex. Nat Neurosci 18:1483–1492. 10.1038/nn.4090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyas F, Sreenivasan V, Marbach F, Wacongne C, Barsy B, Mateo C, Aronoff R, Petersen CC (2010) Motor control by sensory cortex. Science 330:1240–1243. 10.1126/science.1195797 [DOI] [PubMed] [Google Scholar]
- Mazer JA (2011) Spatial attention, feature-based attention, and saccades: three sides of one coin? Biol Psychiatry 69:1147–1152. 10.1016/j.biopsych.2011.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdams CJ, Reid RC (2005) Attention modulates the responses of simple cells in monkey primary visual cortex. J Neurosci 25:11023–11033. 10.1523/JNEUROSCI.2904-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBurney-Lin J, Sun Y, Tortorelli LS, Nguyen QA, Haga-Yamanaka S, Yang H (2020) Bidirectional pharmacological perturbations of the noradrenergic system differentially affect tactile detection. Neuropharmacology 174:108151. 10.1016/j.neuropharm.2020.108151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Erickson CA, Desimone R (1996) Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J Neurosci 16:5154–5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita T, Feldman DE (2013) Behavioral detection of passive whisker stimuli requires somatosensory cortex. Cereb Cortex 23:1655–1662. 10.1093/cercor/bhs155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T, Armstrong KM, Fallah M (2003) Visuomotor origins of covert spatial attention. Neuron 40:671–683. 10.1016/s0896-6273(03)00716-5 [DOI] [PubMed] [Google Scholar]
- Moore T, Fallah M (2001) Control of eye movements and spatial attention. Proc Natl Acad Sci USA 98:1273–1276. 10.1073/pnas.021549498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran J, Desimone R (1985) Selective attention gates visual processing in the extrastriate cortex. Science 229:782–784. 10.1126/science.4023713 [DOI] [PubMed] [Google Scholar]
- Musall S, Kaufman MT, Juavinett AL, Gluf S, Churchland AK (2019) Single-trial neural dynamics are dominated by richly varied movements. Nat Neurosci 22:1677–1686. 10.1038/s41593-019-0502-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namboodiri VM, Huertas MA, Monk KJ, Shouval HZ, Hussain Shuler MG (2015) Visually cued action timing in the primary visual cortex. Neuron 86:319–330. 10.1016/j.neuron.2015.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niell CM, Stryker MP (2010) Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65:472–479. 10.1016/j.neuron.2010.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor DH, Huber D, Svoboda K (2009) Reverse engineering the mouse brain. Nature 461:923–929. 10.1038/nature08539 [DOI] [PubMed] [Google Scholar]
- O'Connor DH, Peron SP, Huber D, Svoboda K (2010a) Neural activity in barrel cortex underlying vibrissa-based object localization in mice. Neuron 67:1048–1061. 10.1016/j.neuron.2010.08.026 [DOI] [PubMed] [Google Scholar]
- O'Connor DH, Clack NG, Huber D, Komiyama T, Myers EW, Svoboda K (2010b) Vibrissa-based object localization in head-fixed mice. J Neurosci 30:1947–1967. 10.1523/JNEUROSCI.3762-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor DH, Hires SA, Guo ZV, Li N, Yu J, Sun QQ, Huber D, Svoboda K (2013) Neural coding during active somatosensation revealed using illusory touch. Nat Neurosci 16:958–965. 10.1038/nn.3419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollerenshaw DR, Bari BA, Millard DC, Orr LE, Wang Q, Stanley GB (2012) Detection of tactile inputs in the rat vibrissa pathway. J Neurophysiol 108:479–490. 10.1152/jn.00004.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otchy TM, Wolff SB, Rhee JY, Pehlevan C, Kawai R, Kempf A, Gobes SM, Olveczky BP (2015) Acute off-target effects of neural circuit manipulations. Nature 528:358–363. 10.1038/nature16442 [DOI] [PubMed] [Google Scholar]
- Parker PR, Brown MA, Smear MC, Niell CM (2020) Movement-related signals in sensory areas: roles in natural behavior. Trends Neurosci 43:581–595. 10.1016/j.tins.2020.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto L, Goard MJ, Estandian D, Xu M, Kwan AC, Lee SH, Harrison TC, Feng G, Dan Y (2013) Fast modulation of visual perception by basal forebrain cholinergic neurons. Nat Neurosci 16:1857–1863. 10.1038/nn.3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI (1980) Orienting of attention. Q J Exp Psychol 32:3–25. 10.1080/00335558008248231 [DOI] [PubMed] [Google Scholar]
- Poulet JF, Hedwig B (2006) The cellular basis of a corollary discharge. Science 311:518–522. 10.1126/science.1120847 [DOI] [PubMed] [Google Scholar]
- Ratcliff R, McKoon G (2008) The diffusion decision model: theory and data for two-choice decision tasks. Neural Comput 20:873–922. 10.1162/neco.2008.12-06-420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert S, Hubener M, Bonhoeffer T, Goltstein PM (2021) Mouse prefrontal cortex represents learned rules for categorization. Nature 593:411–417. 10.1038/s41586-021-03452-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Riggio L, Dascola I, Umilta C (1987) Reorienting attention across the horizontal and vertical meridians: evidence in favor of a premotor theory of attention. Neuropsychologia 25:31–40. 10.1016/0028-3932(87)90041-8 [DOI] [PubMed] [Google Scholar]
- Rodenkirch C, Liu Y, Schriver BJ, Wang Q (2019) Locus coeruleus activation enhances thalamic feature selectivity via norepinephrine regulation of intrathalamic circuit dynamics. Nat Neurosci 22:120–133. 10.1038/s41593-018-0283-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman JD, Shadlen MN (2002) Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. J Neurosci 22:9475–9489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum DA (1980) Human movement initiation: specification of arm, direction, and extent. J Exp Psychol Gen 109:444–474. 10.1037/0096-3445.109.4.444 [DOI] [PubMed] [Google Scholar]
- Ruder L, Schina R, Kanodia H, Valencia-Garcia S, Pivetta C, Arber S (2021) A functional map for diverse forelimb actions within brainstem circuitry. Nature 590:445–450. 10.1038/s41586-020-03080-z [DOI] [PubMed] [Google Scholar]
- Russ BE, Kaneko T, Saleem KS, Berman RA, Leopold DA (2016) Distinct fMRI responses to self-induced versus stimulus motion during free viewing in the macaque. J Neurosci 36:9580–9589. 10.1523/JNEUROSCI.1152-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachidhanandam S, Sreenivasan V, Kyriakatos A, Kremer Y, Petersen CC (2013) Membrane potential correlates of sensory perception in mouse barrel cortex. Nat Neurosci 16:1671–1677. 10.1038/nn.3532 [DOI] [PubMed] [Google Scholar]
- Sachidhanandam S, Sermet BS, Petersen CC (2016) Parvalbumin-expressing GABAergic neurons in mouse barrel cortex contribute to gating a goal-directed sensorimotor transformation. Cell Rep 15:700–706. 10.1016/j.celrep.2016.03.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkoff DB, Zagha E, McCarthy E, McCormick DA (2020) Movement and performance explain widespread cortical activity in a visual detection task. Cereb Cortex 30:421–437. 10.1093/cercor/bhz206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider DM, Nelson A, Mooney R (2014) A synaptic and circuit basis for corollary discharge in the auditory cortex. Nature 513:189–194. 10.1038/nature13724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selen LP, Shadlen MN, Wolpert DM (2012) Deliberation in the motor system: reflex gains track evolving evidence leading to a decision. J Neurosci 32:2276–2286. 10.1523/JNEUROSCI.5273-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuler MG, Bear MF (2006) Reward timing in the primary visual cortex. Science 311:1606–1609. 10.1126/science.1123513 [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH (2002) A pathway in primate brain for internal monitoring of movements. Science 296:1480–1482. 10.1126/science.1069590 [DOI] [PubMed] [Google Scholar]
- Steinmetz NA, Zatka-Haas P, Carandini M, Harris KD (2019) Distributed coding of choice, action and engagement across the mouse brain. Nature 576:266–273. 10.1038/s41586-019-1787-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straka H, Simmers J, Chagnaud BP (2018) A new perspective on predictive motor signaling. Curr Biol 28:R232–R243. 10.1016/j.cub.2018.01.033 [DOI] [PubMed] [Google Scholar]
- Stringer C, Pachitariu M, Steinmetz N, Reddy CB, Carandini M, Harris KD (2019) Spontaneous behaviors drive multidimensional, brainwide activity. Science 364:255. 10.1126/science.aav7893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield C, Trittschuh EH, Monti JM, Mesulam MM, Egner T (2008) Neural repetition suppression reflects fulfilled perceptual expectations. Nat Neurosci 11:1004–1006. 10.1038/nn.2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun LD, Goldberg ME (2016) Corollary discharge and oculomotor proprioception: cortical mechanisms for spatially accurate vision. Annu Rev Vis Sci 2:61–84. 10.1146/annurev-vision-082114-035407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji J, Evarts EV (1976) Anticipatory activity of motor cortex neurons in relation to direction of an intended movement. J Neurophysiol 39:1062–1068. 10.1152/jn.1976.39.5.1062 [DOI] [PubMed] [Google Scholar]
- Treisman AM (1964) Selective attention in man. Br Med Bull 20:12–16. 10.1093/oxfordjournals.bmb.a070274 [DOI] [PubMed] [Google Scholar]
- Vinck M, Batista-Brito R, Knoblich U, Cardin JA (2015) Arousal and locomotion make distinct contributions to cortical activity patterns and visual encoding. Neuron 86:740–754. 10.1016/j.neuron.2015.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinken K, Vogels R, Op de Beeck H (2017) Recent visual experience shapes visual processing in rats through stimulus-specific adaptation and response enhancement. Curr Biol 27:914–919. 10.1016/j.cub.2017.02.024 [DOI] [PubMed] [Google Scholar]
- Wong AL, Haith AM, Krakauer JW (2015) Motor planning. Neuroscientist 21:385–398. 10.1177/1073858414541484 [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Sommer MA (2004) Identifying corollary discharges for movement in the primate brain. Prog Brain Res 144:47–60. 10.1016/S0079-6123(03)14403-2 [DOI] [PubMed] [Google Scholar]
- Yang H, Kwon SE, Severson KS, O'Connor DH (2016) Origins of choice-related activity in mouse somatosensory cortex. Nat Neurosci 19:127–134. 10.1038/nn.4183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zareian B, Zhang Z, Zagha E (2021) Cortical localization of the sensory-motor transformation in a whisker detection task in mice. eNeuro 8:ENEURO.0004-21.2021. 10.1523/ENEURO.0004-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatka-Haas P, Steinmetz NA, Carandini M, Harris KD (2021) Sensory coding and the causal impact of mouse cortex in a visual decision. Elife 10:e63163. 10.7554/eLife.63163 [DOI] [PMC free article] [PubMed] [Google Scholar]