Abstract
Background
Major depressive disorder is the most common neuropsychiatric comorbidity of human immunodeficiency virus (HIV), and women are more frequently affected in the general population and among those with HIV. The rate of depression in HIV is three times higher than the general population. Differences in biomarkers in neuroendocrine and inflammatory pathways are one possible explanation for the increased prevalence of depression in individuals with HIV, especially biological women. Therefore, we aimed to perform a systematic review identifying differences in neuroendocrine factors leading to depression in men versus women with HIV.
Methods
A comprehensive search of 8 databases was performed, followed by title and abstract screening and later full-text screening by two independent researchers. A risk of bias assessment was completed.
Results
Twenty-six full-text articles were included in the review. Significant correlations between depression and neuroendocrine marker levels were found for cortisol (both sexes), testosterone (only in men), oxytocin (only tested in women), and estradiol (only in women). No significant correlation between depression and hormone level was found for prolactin, dehydroepiandrosterone (DHEAS), or sex hormone binding globulin (SHBG). Nearly all studies included only men or women and did not directly compare neuroendocrine markers between the two sexes. One study found that the correlation between cortisol levels and depression scores was stronger in women than men.
Conclusion
Neuroendocrine systems are highly active in the brain and important in the development and persistence of mental illness. Given that HIV can, directly and indirectly, impact hormone signaling, it is likely contributing to the high rate of depression in individuals with HIV. However, few studies explore neuroactive hormones in depression and HIV, nor how this connection may differ between the sexes. More high-quality research is needed in this area to explore the link further and inform possible avenues of treatment.
Keywords: HIV, depression, hormones, Sex, psychiatry
1. Introduction
Major depressive disorder (MDD) is the most common neuropsychiatric comorbidity of individuals with human immunodeficiency virus (HIV), with the prevalence of depression in HIV-infected people being 2 to 4 times higher than in the general population.1 Depression in people with HIV also may be treatment-resistant, as a recent Cochrane review showed inconclusive evidence that the standard treatment regimens were effective in this population.2 This comorbidity is associated with low quality of life and a wide range of adverse clinical outcomes, including poor antiretroviral adherence, lower CD4 counts, and higher viral loads.1
It is well established that women experience higher levels of major depression in comparison to their male counterparts.3 This pattern continues in people with HIV, with multiple studies citing a higher rate of depression in women with HIV than men with HIV. For example, in one study of 3,863 people with HIV in Africa, 32% of women with HIV reported depressive symptoms versus 22% of men with HIV.4 Women have also been shown to have depressive symptoms of more severe intensity than men.5 Despite these differences, there are few studies exploring why depression is more common and more severe in HIV-infected women than men.
One explanation for this discrepancy lies in the biological differences between men and women. Studies have explored possible biological causes for the higher prevalence of depression in individuals with HIV, including increased inflammatory cytokines, neurobiological changes in the central nervous system (CNS), and neuroendocrine pathway disruptions.6-8 Hormonal pathways are of particular significance due to the previously established relationship between depression and neuroendocrine pathways.9,10 Some studies have begun to explore this relationship in people with HIV. In one recent study of 65 people with HIV, salivary afternoon basal cortisol levels were examined by remitted depression determined using the SCID. Higher cortisol levels were found in women with remitted MDD than women with no MDD; however, this association was not observed in men with HIV.11
Although the prevalence of depression among people with HIV has been noted, and research has begun to consider sex as a biological variable in this context, there are outstanding questions regarding the role of hormones on different rates of depression in men and women with HIV. This systematic review will explore the biological differences between men and women with HIV and depression by comparing levels of neuroendocrine markers such as cortisol, testosterone, and estrogen. These discoveries will aid in the understanding of the pathophysiology behind depression and HIV and potentially illuminate new therapeutic targets.
2. Methods
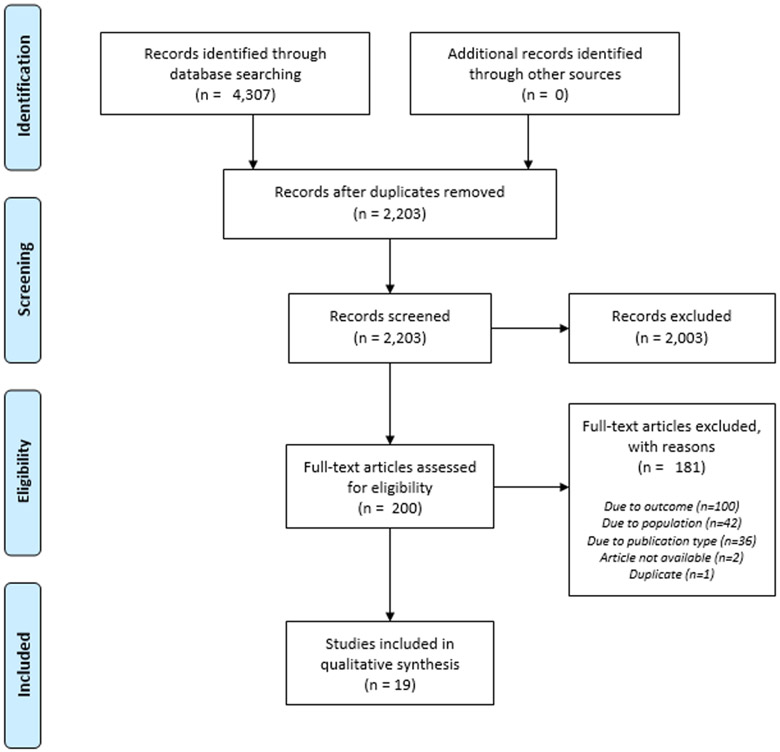
We conducted a comprehensive search of 8 databases: PubMed, Medline, Embase and PsycINFO via Ovid, Web of Science Core Collection, Global Index Medicus, ClinicalTrials.gov, and Cochrane Library via Wiley. Per best practices,12 the search combined natural language searching and controlled vocabulary to reflect the concepts of HIV, hormones, and depression. A complete search strategy is available in Appendix A. The search was conducted in September 2020. We placed no limitations on the language of publication or study design. Title and abstract screening were completed using Rayyan, a web-based systematic review screening tool.13 Two independent reviewers reviewed titles and abstracts, and any discrepancies were resolved through consensus or a third party where necessary. Full-text screening was also completed by two independent reviewers, and reasons for exclusion were recorded and reported in Figure 1.14
Figure 1.
PRISMA Flow Diagram
Inclusion and Exclusion Criteria
Inclusion criteria required HIV, Depression, and measurement of a hormone. Participants needed to be over 18 years old. Depression had to be measured using a validated method that was reported. Both randomized and non-randomized studies were included.
Exclusion criteria included articles that focused on pediatric populations and patients at risk or affected by but not personally diagnosed with human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS). Articles that included both men and women but did not report data for these populations separately were excluded. Single case reports and papers that did not report original data, such as opinion pieces, were excluded.
The data extraction form was developed by one researcher and piloted prior to further refinement. Quality assessment was completed using the Cochrane Collaboration's RoB 2 tool for randomized studies and National Heart, Lung, and Blood Institute (NHLBI) Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies for non-randomized studies. Data extraction and quality assessment were completed by an individual researcher, and a second researcher independently confirmed the findings. Quality assessment was guided by official implementation documents developed by Cochrane and NHLBI.15-17 following the previously established workflow, any discrepancies in findings or opinion between the researchers were resolved through consensus or by a third party. The protocol for the project was registered in PROSPERO (CRD42020157839).
3. Results
3.1. Flow of Studies
Our literature search produced 4,307 articles related to our search terms. Of these, 2,203 remained after duplicates were removed. The abstracts of these articles were screened by at least two reviewers independently, leaving 200 full-text articles to review for eligibility. In addition, 174 full-text articles were excluded for reasons including outcome, population, publication type, and unavailability of the article, leaving 26 full-text articles. Of these full-text articles, 7 included non-hormonal biomarkers, and we decided to include these in a separate paper. Thus, 19 papers remained in the scope of this article. Table 1 describes the characteristics of the included studies and their participants, while Table 2 describes the relevant outcomes of each included study.
Table 1.
Characteristics of included studies
| Study | Study Design (Country) |
Population | Neuroendocr ine Marker (s) (sample type, time of collection, fasting vs non-fasting) |
N | CD4 (mean (sd) cells/ mm3) |
HIV Viral Load (copies/ml) †† |
Population on ART |
|---|---|---|---|---|---|---|---|
| Antoni 2000 | RCT (USA) | Symptomatic HIV+ gay and bisexual men | Cortisol (urine, 24 hour output) | 69 | 418 (237) cells/ml | NR | 39.1% (27/69) |
| Grinspoon 2000 | RCT (USA) | HIV+ men | Testosterone (serum), estradiol (serum),SHBG (hematocrit and serum) | Total: 61 | NR | NR | NR |
| Hypogonad al: 51 | 175 (30) | 191151 (36483) | |||||
| Eugonadal: 10 | 205 (91) | 111920 (37766) | |||||
| Antoni 2005 | RCT (USA) | Symptomatic HIV+ gay and bisexual men | Cortisol (urine, 24 hour output) | Total: 25 | NR | NR | NR |
| Interventio n: 16 | 528 (273.9) | 1202 (1787) | 25% | ||||
| Control: 9 | 420.7 (165.5) | 2088 (2336) | 44% | ||||
| Kertzner 1993 | Longitudinal (USA) | Gay and bisexual men | Cortisol (urine, 24 hour output) | Total: 121* | NR | NR | NR |
| HIV+: 80 | 422 (209) | ||||||
| HIV−: 41 | 825 (266) | ||||||
| Wagner 1998a | Longitudinal (USA) | HIV+ men with sexual dysfunction, CD4<400, serum testosterone<450 | Testosterone (serum) | 54 | 109 | NR | NR |
| Wagner 1998b | Longitudinal (USA) | HIV+ men with diminished libido and comorbidity of interest** | Testosterone (serum) | 23 | 150 | NR | 65% |
| Blick 2013 | Longitudinal (USA) | Men with hypogonadism | Testosterone (serum) | 849 | NR | NR | NR |
| Seay 2014 | Longitudinal (USA) | HIV+women | Oxytocin (plasma) | 70 | 478.1 cells/uL | NR | 90.5% |
| Antoni 1991 | Cross-sectional (USA) | Gay men | Cortisol (plasma, 7:30 AM-10:30 AM, fasting) | 71 | 713 [median] | NR | NR |
| Gorman 1991 | Cross-sectional (USA) | Gay men | Cortisol (urine, 24 hour output) | Total: 187 | NR | NR | NR |
| HIV+: 112 | 401.8 (226.4) | ||||||
| HIV-: 75 | 828.2 (274.9) | ||||||
| Gorman 1992 | Cross-sectional (USA) | Gay and bisexual men | Prolactin (serum, majority collected in AM) | Total: 199 | NR | NR | NR |
| HIV+: 120 | 409 (222.6) | ||||||
| HIV−: 79 | 843.1 (263.7) | ||||||
| Goggin 1998 | Cross-sectional (USA) | HIV+ women | DHEAS (serum, 11:00 AM-5 PM) | 54 | 158 (149.9) | NR | NR |
| Ferrando 1999 | Cross-sectional (USA) | HIV+ men | DHEAS, Testosterone (serum, 11:00 AM-4:00 PM) | Total: 169 | 301 (190, 496) [median (IQR)] | NR | NR |
| HIV+ CD4 > 500: 18 | 681 (547, 748) [median (IQR)] | ||||||
| HIV+ CD4 200-500:46 | 296 (230, 361) [median (IQR)] | ||||||
| AIDS CD4 <200: 105 | 91 (21, 165) [median (IQR)] | ||||||
| Carrico 2006 | Cross-sectional (USA) | HIV+ persons | Cortisol (urine, 24 hour output) | Total: 264 | 435 (276) | 17441 (62991) | NR |
| Men: 130 | 422 (239) | 13098 (30481) | |||||
| Women: 134 | 482 (306) | 21717 (83385) | |||||
| Wisniewski 2006 | Cross-sectional (USA) | 18-50-years in high risk categories† | Cortisol (serum, 8:00 AM-10:00 AM) | 209 | NR | NR | NR |
| Lari 2012 | Cross-sectional (Iran) | HIV+ men | Prolactin (does not specify), free testosterone (serum, early morning, fasting) | 237 | NR | NR | NR |
| Sunchatawi rul 2012 | Cross-sectional (Thailand) | HIV+ men | Total testosterone, SHBG (serum, 8:00 AM-11:00 AM) | 491 | 320 | <50 copies/ml: 87.3% Undetectable; peak plasma RNA level 157 | 93.5% |
| Bekhbat 2018 | Cross-sectional study (USA) | Women | Estradiol (does not specify) | Total: 147 | NR | NR | NR |
| HIV− without depression: 37 | 1151.5 (915, 1509) [median (IQR)] | NR | N/A | ||||
| HIV− with depression: 34 | 1066.5 (831, 1349) [media (IQR)] | NR | N/A | ||||
| HIV+ without depression: 38 | 436 (309, 606) [median (IQR)] | 11500 (3850, 32000) [median (IQR)] | 65.8% | ||||
| HIV+ with depression: 38 | 499.5 (313, 631) [median (IQR)] | 5500 (340, 33000) [median (IQR)] | 50.0% | ||||
| Laan 2019 | Cross-sectional (Netherlands) | Women with or without testosterone insufficiency (TI) | Prolactin, testosterone, SHBG, FH, FSH, 17b-estradiol (plasma) | Total: 49 | NR | Undetectable in 78% | 78% |
| With TI: 18 | 653 (376) | Undetectable in 88% | 89% | ||||
| Without TI: 31 | 572 (272) | Undetectable in 71% | 72% |
ART: Antiretroviral Therapy; DHEAS: Dehydroepiandrosterone sulfate; FSH: Follicle-stimulating hormone; LH: Luteinizing hormone; SHBG: Sex hormone binding globulin
A subset of the initially enrolled 184 patients (109 HIV positive, 75 HIV negative individuals)
Comorbidities of interest: low mood, low energy, low appetite/weight loss
Risk categories included visiting HIV clinics, methadone maintenance clinics, and homeless shelters
Viral load was reported as % Undetectable with cutoff as available. If only numeric viral load was presented that was what was reported.
Table 2.
Hormone levels by sex and correlation with depression scales
| Study | Average Hormone Level | Depression scale (Cut-off) |
Correlation between hormones and depression |
|
|---|---|---|---|---|
| Men | Women | |||
| Cortisol | ||||
| Antoni 1991 | 170 ng/ml | N/A | POMS depression subscale (continuous) | Yes (p =NR) |
| Antoni 2000 | Control: 11 ug/24hrs Intervention: 13 ug/24 hrs | N/A | POMS depression subscale (continuous) | Yes (r = 0.32, p < 0.05) |
| Antoni 2005 | Control: 9 ug/24 hrs Intervention: 14 ug/24 hrs | N/A | POMS depression subscale (continuous) | NR |
| Carrico 2006 | 6.1/24h (with square root correction) | N/A | BDI affective subscale (continuous) | No correlation |
| Gorman 1991 | 62 ug/24 hours | N/A | HAM-D (continuous) | Yes (r = 25, p = 0.01) |
| Kertzner 1993 | 70 ug/24 hours | N/A | HAM-D (continuous) | Yes (r = 0.25, p < 0.01) |
| Wisniewski 2006 | 17.5 μg/dL | 16.46 μg/dL | CES-D (>23, continuous) | Yes (2% increase in odds of depressive symptoms per unit increase) |
| DHEAS | ||||
| Ferrando 1999 | 158 ug/dL | N/A | BDI (>10 significant depressive symptoms) | No correlation |
| Goggin 1998 | N/A | 96.5 ug/dL | HAM-D (continuous) | No correlation |
| Estradiol | ||||
| Bekhbat 2018 | N/A | No depression: 43.0pg/mL Depression: 37.5pg/mL |
CES-D (>16) | NR |
| Grinspoon 2000 | hypogonodal: 12.0 (1.1) pg/m Leugonadal: 14.2 (2.6) pg/mL. |
N/A | BDI (>18) | No correlation |
| Oxytocin | ||||
| Seay 2014 | 21.3 pg/ml | N/A | BDI (no cutoff) | Yes (p = 0.05) |
| Prolactin | ||||
| Gorman 1992 | CDC Stage II: 9.2 (3.2) ng/ml CDC Stage III: 10.2 (3.4) ng/ml CDC Stage IV: 9.9 (3.9) ng/ml |
N/A | HAM-D (no cutoff) | No correlation |
| SHBG | ||||
| Grinspoon 2000 | hypogonodal: 34.0 nmol/L eugonadal: 44.0 nmol/L |
N/A | BDI (18) | No correlation |
| Sunchatawi rul 2012 | 42.0 nmol/L | N/A | Thai HADS (>10 on the even numbered questions) | No correlation |
| Free Testosterone | ||||
| Blick 2013 | 50 pg/ml | N/A | PHQ-9 (continuous) | NR |
| Ferrando 1999 | 87 pg/ml | N/A | BDI (continuous) | No correlation |
| Grinspoon 2000 | hypogonodal: 13 pg/ml eugonadal: 22 pg/ml |
N/A | BDI (18) | Yes (p = NR) |
| Lari 2012 | 4.8 vs. 6.6 pg/ml | N/A | BDI (18) | Yes (p = NR) |
| Total Testosterone | ||||
| Blick 2013 | 375 ng/dL | N/A | PHQ-9 (continuous) | Yes (p = NR) |
| Goggin 1998 | 25.6 dg/sl | N/A | HAM-D (cutoff?) | No correlation |
| Grinspoon 2000 | hypogonodal: 427 ng/d leugonadal: 738 ng/dl |
N/A | BDI (18) | Yes (p = NR) |
| Laan 2018 | With insufficiency: 14 ng/dl Without insufficiency: 35ng/dl |
N/A | BDI (20) | No correlation |
| Sunchatawi rul 2012 | Median 19 nmol/L | Median 19 nmol/L | Thai HADS (>10 on even numbered questions) | No correlation |
| Wagner 1998 | 344 ng/dl | N/A | HAM-D (continuous) | Yes (p = NR) |
| Wagner 1998 | 550 ng/dl; week 11:1287 ng/dl | N/A | HAM-D (continuous) | Yes (t = 6.0, p = .000) |
BDI: Beck Depression Inventory; CES-D: Center for Epidemiologic Studies Depression Scale; HADS: Hospital Anxiety and Depression Scale; HAM-D: Hamilton Depression Rating Scale; PHQ-9: Patient Health Questionnaire-9; POMS: Profile of Mood States
3.2. Overview of Included Studies
The majority of the studies were conducted in the USA (n=16), with others conducted in Iran (n=1), Thailand (n=1), and the Netherlands (n=1). The studies used a wide variety of screening tools for depression, including the Profile of Mood States (POMS), Patient Health Questionnaire 9 (PHQ-9), Beck Depression Inventory I and II (BDI I/II), Hamilton Depression Rating Scale (HAM-D), Structured Clinical Interview for DSM-IV (SCID), and one used the Thai Hospital Anxiety and Depression Scale (Thai HADS). The number of study participants ranged from 23 - 849 participants. Inclusion criteria varied by study, with some including any people with HIV, many including only biological men or women, and some with other specific criteria (men who have sex with men, men with hypogonadism, etc.). The studies measured various hormones; cortisol (n=7) and testosterone (n=8) were the most frequently measured hormones. In addition, one study measured Prolactin, Testosterone, SHBG, LH, FSH, and 17b-estradiol.18 The table also includes average CD4 counts, HIV viral load, and % of participants on ART when available in the studies.
3.3. Outcomes
3.3.1. Cortisol
Of the seven papers that measured cortisol, five found some correlation between cortisol levels and measures of depression. 19-23 One study found no correlation,24 while one did not directly correlate the POMS score with cortisol levels.25 Interestingly, one paper reported a negative correlation between plasma cortisol levels and physiological distress using the POMS depression subscale.23 The remaining papers reported a positive correlation between depression and plasma cortisol, with r values ranging from 0.25-0.35.19-22 A study by Wisnieweski found a 2% increase in the odds of experiencing depressive symptoms for every unit (μg/d) increase in cortisol. They also found the correlation stronger in female participants than male participants.22
3.3.2. Dehydroepiandrosterone Sulfate (DHEAS)
Two studies measured DHEAS.26,27 Ferrando included only male participants, while Goggin included only female participants. Neither study found any correlation between plasma DHEAS levels and depression score (utilizing the BDI and HAM-D scales, respectively).
3.3.3. Estradiol
Estradiol was numerically lower in women with HIV with CES-D ≤ 15 than in women without both depression and HIV or who had one or the other.28 One study compared estradiol levels in men but did not significantly correlate with depression scores via the BDI (r= 0.15, P=0.24).29
3.3.4. Oxytocin
Only one study measured Oxytocin.30 It only included female participants. They found a U-shaped curve with baseline oxytocin levels and depressive symptoms at ten weeks.
3.3.5. Prolactin
One study by Gormin et al. measured plasma prolactin levels.31 They had only male participants, separated into three groups according to the CDC stages of HIV/AIDS infections (utilizing stages II, III, and IV). They did not find any significant correlation between plasma prolactin levels and HAM-D scores in any group.
3.3.6. Sex Hormone Binding Globulin (SHBG)
Two studies measured SHBG, and both included only male participants.29,32 Neither found a significant correlation between plasma SHBG levels and depression scores.
3.3.7. Total Testosterone (TT)
Seven studies measured total testosterone levels.18,27,29,32-35 Most of the studies used the BDI or the HAM-D scales to measure depressive symptoms. Of these, four reported a statistically significant negative correlation between total testosterone levels and depression scores.29,33-35 Several of these studies were intervention-based and measured depression levels before and after a trial of testosterone supplementation therapy.34,35
3.3.8. Free Testosterone (FT)
Four studies measured free testosterone, two of which also measured total testosterone and are discussed above.29,33 Two of these four, both using the BDI scale for depression, found a statistically significant correlation between depression scores and FT levels.29,36 While Blick did not directly correlate depression and FT; they showed that as FT levels significantly increased, PHQ-9 scores also significantly improved.33 The remaining study found no correlation between FT and depression.26
3.3.9. Non-hormonal outcomes
While this review focuses on depression and hormone levels as a primary outcome, the included studies sometimes focused on additional outcomes. For example, several studies addressed sexual dysfunction. Others explored nutritional status, exercise, drug use, and neuropsychiatric outcomes. We will not comment on these outcomes here.
3.4. Risk of Bias Assessment
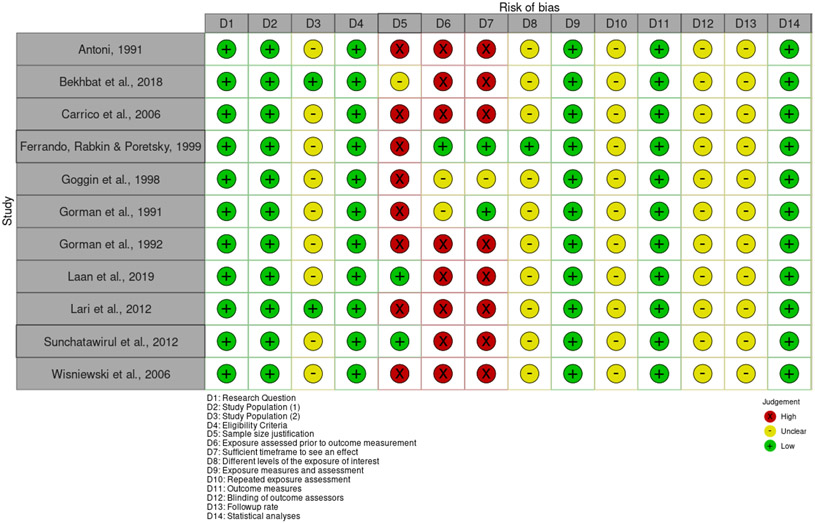
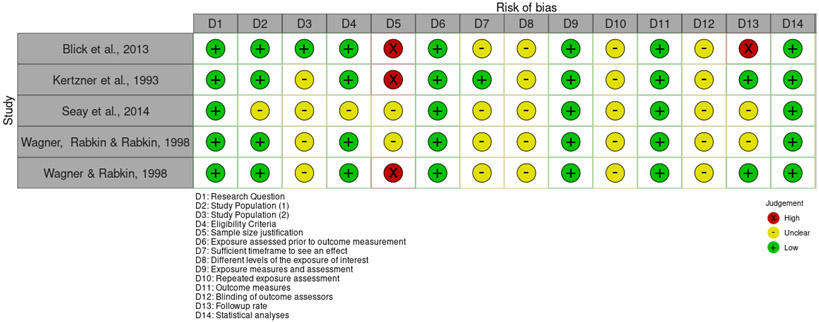
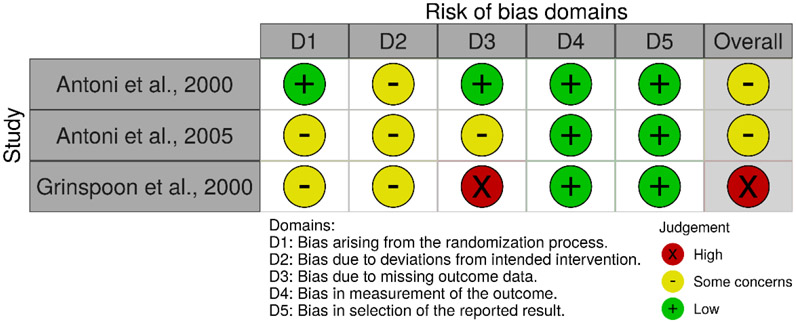
No studies were found to be entirely free of potential risk of bias, as represented in Figures 2, 3 and 4. Within the set of nonrandomized studies, only two studies justified their sample sizes. However, the lack of sample size justification may not be a significant concern in the case of more exploratory research. There was an unclear risk of bias for the majority of studies when considering participation rates. As the studies generally relied on convenience samples, and the discrepancy between those approached and those who agreed to participate is not always clearly articulated, there may be some concern that the study participants may not represent the larger population of interest. Some of the other domains of potential bias, such as the risk of bias associated with the timing of exposure assessment and outcome measurement, are due to the prevalence of cross-sectional studies. Within the three randomized studies, unclear risk of bias was most associated with a lack of details describing the randomization process and adherence to intervention.
Figure 2.
Risk of bias in cross-sectional studies
Figure 3.
Risk of bias in longitudinal studies
Figure 4.
Risk of bias in randomized studies
4. Discussion
Unsurprisingly, cortisol, long linked with stress, was correlated with depression in nearly all studies. DHEAS was measured in men and women in one separate study each and was not significantly associated with depression in either study. Both total and free testosterone were linked to depression in men, although not in women (women tested in only one study). Oxytocin had a complex U-shaped relationship with depression, although only tested in women. Prolactin was only measured in men in one study and was not significant. Finally, we only found two studies that evaluated estradiol. In women, estradiol was numerically lower in those depressed with HIV than controls. In men, estradiol and depression were not significantly correlated.
4.1. Cortisol
Correlations between cortisol and depression in the general population are complex. Most studies show altered cortisol responses in depression.37-39 Treatment with electroconvulsive therapy for major depression was shown to produce a long-term reduction in cortisol.40 Cortisol has been shown to have different effects in men and women, with men having more reactivity and women having a blunted response.41 HIV is known to alter adrenal function with higher basal cortisol levels and risk for adrenal insufficiency.42 HIV medications improve outcomes, but increased inflammation from increased cortisol remains.43 As such, we think our findings are consistent with the literature.
4.2. DHEAS
DHEAS has broad neurologic properties and is known to have both anxiolytic and antidepressant properties.44 It acts directly on the gamma-aminobutyric acid (GABA) and N-methyl-d-aspartate (NMDA) pathways, important in mental illness.45 A recent meta-analysis showed that DHEAS levels are associated with developing depression, with DHEAS decreasing with age.46 Higher levels of DHEAS are protective against depression in the elderly.47 HIV is associated with low DHEAS, and low DHEAS is a poor prognostic indicator.26 As such, we would expect DHEAS to be associated with depression in people with HIV, but likely our sample size is too small.
4.3. Testosterone
Testosterone is strongly associated with depression in men.48 Testosterone is also strongly associated with depression in women with polycystic ovarian syndrome (PCOS)49 In women without PCOS, there is also a correlation between depression and testosterone, although with a complex relationship and potentially J-shaped curve to the testosterone level.50 Early studies showed decreased testosterone associated with HIV,51 but more recent ones with individuals on HIV therapy showed some alterations but less so.52 In women, AIDS wasting is associated with androgen deficiency.53 Our study from Laan showed that testosterone was frequently low in women with HIV.18 Overall, the literature suggests that our findings in men with HIV are to be expected; more data is needed in women with HIV for depression and testosterone.
4.4. Oxytocin
Oxytocin is closely aligned with perinatal depression, which was excluded in this work, with higher oxytocin being protective.54 However, oxytocin is known to have antidepressant effects in animal models and post-mortem studies of individuals who had been depressed.55 Sanwald et al. evaluated epigenetic regulation of the gene coding for oxytocin; they found significant differences between men and women.56 Specifically in depression, oxytocin coding is less activated in women than men. However, oxytocin was not correlated with depression severity overall.56 In addition, oxytocin immunoreactivity was found to be decreased in hypothalamic neurons in people with HIV.7 Thus, we would expect differences between men and women in depression and oxytocin’s effects. We also would expect complex relationships with oxytocin which our data show, although more testing should be done, especially in men.
4.5. Prolactin
Like oxytocin, prolactin has been studied in depression among peripartum women, although the link between depression and prolactin is somewhat equivocal.57 In mice, prolactin levels in the hypothalamus are increased due to stress, which is thought to regulate depression.57 Electroconvulsive therapy, an effective treatment for depression, increases prolactin in people with depression.58 In men with HIV, bioactive prolactin was elevated, while the response of immunoreactive and bioactive prolactin suggested a decrease in dopaminergic tone.59 In a population of Nigerian women, they found that prolactin levels did not vary significantly between those with HIV and controls at coordinated phases of their menstrual cycle.60 We did not see an association between depression and prolactin, but we only found one study in men.
4.6. Estradiol
One study of estradiol in men found men under 60 years old with depression had higher estradiol levels.61 Estradiol has been shown to regulate the expression of monoamine oxidases, the serotonin transporter, and the serotonin-1A receptor in women.62 Given the known role of serotonin and monoamine oxidases in depression, estradiol likely has profound impacts on depression in women. In men, estradiol has not been associated with depression.63 In addition, there is data showing that estradiol can regulate infections, including HIV, and that estradiol may be protective against HIV, although it depends on the phase of the infection.64 Overall, estradiol needs more study in depression and HIV but is more relevant for women, with our data being consistent with that finding.
4.7. Progesterone
Progesterone and its metabolite allopregnanolone are important in both men and women and are synthesized in the nervous system, the adrenal gland, and, in women, the ovaries.65 Allopregnanolone acts directly on the GABA receptor, and progesterone is known to have a key role in neuroprotection. Low progesterone has been associated with depression and anxiety.66 Similar to estradiol, there is data showing that progesterone can regulate infections, including HIV, and that progesterone may protect against HIV early in the disease course. However, later in the disease course, women progress to AIDS faster than men.64 We did not find papers with progesterone in our search for hormones and depression in HIV, which the literature suggests is an area that should be further studied.
4.8. Limitations
This systematic review has several important limitations. First, despite the large body of literature screened, relatively few studies met inclusion criteria. Second, these studies were heterogeneous, generally including different measures of depression and considering different biomarkers. Third, few studies included both male and female participants, limiting our ability to draw conclusions regarding the role of sex in this context. Finally, methodologically, most of the studies featured incomplete reporting. Lack of details regarding selection, randomization, timing, and adherence creates difficulties when assessing research and applying findings. However, it is possible that reporting standards changed since the time of the articles’ initial publications, as several studies were published in the early 1990s. Overall, the lack of statistical conclusions and consistency between the studies left us unable to perform a meta-analysis of this data. The prevalence of depression in people with HIV and its importance in treatment outcomes is a large and important gap in existing research. More meaningful work should be done in this area to better understand the pathophysiology of depression in people with HIV and how gender may affect this comorbidity. We have intentionally avoided describing these results as evidence bioreductionism, and acknowledge that depression in people with HIV is a complex disease process that undoubtedly includes psychological and social influences such as stigma, living with a chronic illness, and other social determinants of health experienced by people with HIV. However, we maintain that neuroendocrine markers and other biological factors are worth studying as one aspect of the pathophysiology.
Of note, we do not see transgender individuals in our results. Transgender individuals were not excluded; however, they did not come up in our search. It could be because of a lack of biomarker testing or understudy of a marginalized population. Indeed, we know depression is very high among transgender individuals, with a recent Canadian study of transgender women with HIV who had a rate of depression of 45%.67 Further work to evaluate hormone therapy in this population is warranted.
Also, we did not see reports of women on hormonal contraceptives. These were, again, not excluded but not found in our search. A review looking at hormonal contraceptives showed improving mental health in women with premenstrual dysphoric disorder.68 They report there are not consistent findings of mood disorders with hormonal contraceptives, although there are individual groups with susceptibilities. Further study in women with HIV would be helpful.
Finally, given our narrow inclusion criteria the majority of studies were from the USA. We did not limit countries and would have preferred more representation but were limited by the literature. Specifically, while we found papers from Iran, Thailand, and the Netherlands, we did not find papers from Africa or South America. We suspect the range of countries will expand with more interest in depression in people with HIV.
5. Conclusion
This systematic review of hormones in people with HIV using sex as a biological variable found substantial differences in relevant hormones in their importance for depression by sex. However, these were nearly all studies done in one sex, not comparisons between both. Given the importance of hormones in depression and the impact of HIV on these pathways, better understanding the pathophysiology of the neuropsychiatry in HIV is crucial. Since hormone levels vary significantly by sex yet are important for both biological sexes, further work in individual biological sexes and comparison between groups is needed. Additionally, there was a paucity of data overall to understand this pathophysiology. We call for further research in this area to improve the lives of people with HIV.
Highlights.
-Significant correlations between HIV-associated depression and hormone levels were found for cortisol (both sexes), testosterone (only in men), oxytocin (only tested in women), and estradiol (only in women). -No significant correlation between depression and hormone level was found for prolactin, dehydroepiandrosterone (DHEAS), or sex hormone binding globulin (SHBG) and no studies measured progesterone.
-Nearly all studies included only men or women and did not directly compare biomarkers between the two sexes.
-More research is needed to compare hormone signaling among those with HIV and depression by sex.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Dr. Lofgren is supported by the National Institute of Mental Health (K23MH121220).
Appendix A: Search Strategy for Embase via Ovid
exp Human immunodeficiency virus/
("human immunodeficiency virus" or "human immunodeficiency viruses" or "HIV-1" or "HIV-2" or HIV or "LAV-HTLV-III" or "HTLV-III" or "Lymphadenopathy-Associated Virus" or "Lymphadenopathy Associated Virus" or "Lymphadenopathy-Associated Viruses" or "Lymphadenopathy Associated Viruses" or "AIDS virus" or "AIDS viruses" or "Acquired Immune Deficiency Syndrome Virus" or "Acquired Immune Deficiency Syndrome Viruses").tw,kw.
(human adj2 ("t cell" or "t-cell") adj2 ("leukemia virus*" or "lymphotropic virus")).tw,kw.
or/1-3
exp progesterone derivative/
exp sex hormone/
(progesterone or estrogen* or oestrogen* or testosterone or estradiol or dihydrotestosterone or androgen* or progestrogen* or endocrinopath* or algestone or hydroxyprogesterone* or pregnenedione or epitestosterone or hydroxytestosterone* or methenolone or mehtyltestosterone or estrane* or anaprotin or andractim or dihydroepitestosterone or androstanolone or mesterolone).tw,kw.
exp biological factor/
(cytokine* or interferon* or interleukin* or chemokine* or intercrine* or chemotactic or "growth differentiation factor* ").tw,kw.
or/5-9
4 and 10
exp mood disorder/
(depressive or depression or depressed or depressions or MDD or mood or (mental adj1 (health or illness* or disorder*)) or psychiatr* or psycholog*).tw,kw.
or/12-13
11 and 14
exp Adults/
exp child/
exp infant/
exp adolescent/
or/17-19
20 not 16
15 not 21
exp human/
exp animal/
24 not 23
22 not 25
..dedup 26
Footnotes
Declarations of interest: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nanni MG, Caruso R, Mitchell AJ, Meggiolaro E, Grassi L. Depression in HIV Infected Patients: a Review. Current Psychiatry Reports. 2014/November/21 2014;17(1):530. doi: 10.1007/s11920-014-0530-4 [DOI] [PubMed] [Google Scholar]
- 2.Eshun-Wilson I, Siegfried N, Akena DH, Stein DJ, Obuku EA, Joska JA. Antidepressants for depression in adults with HIV infection. Cochrane Database of Systematic Reviews. 2018;(1)doi: 10.1002/14651858.CD008525.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bromet E, Andrade LH, Hwang I, et al. Cross-national epidemiology of DSM-IV major depressive episode. BMC medicine. 2011;9:90–90. doi: 10.1186/1741-7015-9-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seth P, Kidder D, Pals S, et al. Psychosocial functioning and depressive symptoms among HIV-positive persons receiving care and treatment in Kenya, Namibia, and Tanzania. Prev Sci. Jun 2014;15(3):318–28. doi: 10.1007/s11121-013-0420-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reis RK, Haas VJ, Santos CB, Teles SA, Galvão MT, Gir E. Symptoms of depression and quality of life of people living with HIV/AIDS. Rev Lat Am Enfermagem. Jul-Aug 2011;19(4):874–81. doi: 10.1590/s0104-11692011000400004 [DOI] [PubMed] [Google Scholar]
- 6.Del Guerra FB, Fonseca JL, Figueiredo VM, Ziff EB, Konkiewitz EC. Human immunodeficiency virus-associated depression: contributions of immuno-inflammatory, monoaminergic, neurodegenerative, and neurotrophic pathways. J Neurovirol. Aug 2013;19(4):314–27. doi: 10.1007/s13365-013-0177-7 [DOI] [PubMed] [Google Scholar]
- 7.Langford D, Baron D, Joy J, Del Valle L, Shack J. Contributions of HIV infection in the hypothalamus and substance abuse/use to HPT dysregulation. Psychoneuroendocrinology. Jun 2011;36(5):710–9. doi: 10.1016/j.psyneuen.2010.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawson MA, Kelley KW, Dantzer R. Intracerebroventricular administration of HIV-1 Tat induces brain cytokine and indoleamine 2,3-dioxygenase expression: a possible mechanism for AIDS comorbid depression. Brain Behav Immun. Nov 2011;25(8):1569–75. doi: 10.1016/j.bbi.2011.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tichomirowa MA, Keck ME, Schneider HJ, et al. Endocrine disturbances in depression. J Endocrinol Invest. Jan 2005;28(1):89–99. doi: 10.1007/bf03345535 [DOI] [PubMed] [Google Scholar]
- 10.Dwyer JB, Aftab A, Radhakrishnan R, et al. Hormonal Treatments for Major Depressive Disorder: State of the Art. Am J Psychiatry. Aug 1 2020;177(8):686–705.doi: 10.1176/appi.ajp.2020.19080848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubin LH, Langenecker SA, Phan KL, et al. Remitted depression and cognition in HIV: The role of cortisol and inflammation. Psychoneuroendocrinology. Apr 2020;114:104609. doi: 10.1016/j.psyneuen.2020.104609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane. 2021. www.training.cochrane.org/handbook [Google Scholar]
- 13.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. December 2016;5(1):210. doi: 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. March 2021;18(3):e1003583. doi: 10.1371/journal.pmed.1003583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. Oct 18 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JPT SJ, Page MJ, Sterne JAC. Revised Cochrane risk-of-bias tool for randomized trials (RoB 2). Updated 2021.2021. https://drive.google.com/open?id=19R9savfPdCHC8XLz2iiMvL_71lPJERWK [Google Scholar]
- 17.National Heart L, and Blood Institute. Development and use of Study Quality Assessment Tools. . NHLBI. Updated 2021. 2021. https://www.nhlbi.nih.gov/node/80102 [Google Scholar]
- 18.Laan ETM, Prins JM, van Lunsen RHW, Nieuwkerk PT, Nievaard-Boon MAF. Testosterone Insufficiency in Human Immunodeficiency Virus-Infected Women: A Cross-Sectional Study. Sexual Medicine. 2019 2019;7(1):72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kertzner RM, Goetz R, Todak G, et al. Cortisol levels, immune status, and mood in homosexual men with and without HIV infection. The American journal of psychiatry. 1993 1993;150(11):1674–8. [DOI] [PubMed] [Google Scholar]
- 20.Antoni MH, Cruess S, Cruess DG, et al. Cognitive-behavioral stress management reduces distress and 24-hour urinary free cortisol output among symptomatic HIV-infected gay men. Annals of behavioral medicine. 2000 2000;22(1):29–37. [DOI] [PubMed] [Google Scholar]
- 21.Gorman JM, Kertzner R, Cooper T, et al. Glucocorticoid level and neuropsychiatric symptoms in homosexual men with HIV infection. The American journal of psychiatry. 1991 1991;148(1):41–5. [DOI] [PubMed] [Google Scholar]
- 22.Wisniewski AB, Brown TT, John M, et al. Cortisol levels and depression in men and women using heroin and cocaine. Psychoneuroendocrinology. 2006 2006;31(2):250–5. [DOI] [PubMed] [Google Scholar]
- 23.Antoni MH, Schneiderman N, Klimas N, LaPerriere A, Ironson G, Fletcher MA. Disparities in psychological, neuroendocrine, and immunologic patterns in asymptomatic HIV-1 seropositive and seronegative gay men. Biological Psychiatry. 1991 1991;29(10):1023–1041. [DOI] [PubMed] [Google Scholar]
- 24.Carrico AW, Ironson G, Antoni MH, et al. A path model of the effects of spirituality on depressive symptoms and 24-h urinary-free cortisol in HIV-positive persons. Journal of psychosomatic research. 2006 2006;61(1):51–8. [DOI] [PubMed] [Google Scholar]
- 25.Antoni MH, Cruess DG, Klimas N, et al. Increases in a marker of immune system reconstitution are predated by decreases in 24-h urinary cortisol output and depressed mood during a 10-week stress management intervention in symptomatic HIV-infected men. Journal of psychosomatic research. 2005 2005;58(1):3–13. [DOI] [PubMed] [Google Scholar]
- 26.Ferrando SJ, Rabkin JG, Poretsky L. Dehydroepiandrosterone sulfate (DHEAS) and testosterone: Relation to HIV illness stage and progression over one year. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology. 1999 1999;22(2):146–154. [DOI] [PubMed] [Google Scholar]
- 27.Goggin K, Engelson ES, Rabkin JG, Kotler DP. The relationship of mood, endocrine, and sexual disorders in human immunodeficiency virus positive (HIV+) women: An exploratory study. Psychosomatic Medicine. 1998 1998;60(1):11–16. [DOI] [PubMed] [Google Scholar]
- 28.Bekhbat M, Mehta CC, Kelly SD, et al. HIV and symptoms of depression are independently associated with impaired glucocorticoid signaling. Psychoneuroendocrinology. Oct 2018;96:118–125. doi: 10.1016/j.psyneuen.2018.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grinspoon S, Corcoran C, Stanley T, Baaj A, Basgoz N, Klibanski A. Effects of hypogonadism and testosterone administration on depression indices in HIV-infected men. Journal of clinical endocrinology and metabolism. 2000 2000;85(1):60–65. [DOI] [PubMed] [Google Scholar]
- 30.Seay JS, Lattie E, Schneiderman N, et al. Linear and quadratic associations of plasma oxytocin with depressive symptoms in ethnic minority women living with HIV. Journal of Applied Biobehavioral Research. 2014 2014;19(1):70–78. [Google Scholar]
- 31.Gorman JM, Warne PA, Begg MD, et al. Serum prolactin levels in homosexual and bisexual men with HIV infection. The American journal of psychiatry. 1992 1992;149(3):367–70. [DOI] [PubMed] [Google Scholar]
- 32.Sunchatawirul K, Tantiwongse K, Chathaisong P, Thongyen S, Chumpathat N, Manosuthi W. Hypogonadism among HIV-infected men in Thailand. International Journal of STD and AIDS. 2012 2012;23(12):876–881. [DOI] [PubMed] [Google Scholar]
- 33.Blick G, Khera M, Bhattacharya RK, Kushner H, Miner MM. Testosterone replacement therapy in men with Hypogonadism and HIV/AIDs: results from the TRiUS registry. Postgraduate medicine. 2013 2013;125(2):19–29. [DOI] [PubMed] [Google Scholar]
- 34.Wagner GJ, Rabkin JG. Testosterone therapy for clinical symptoms of hypogonadism in eugonadal men with AIDS. International Journal of STD and AIDS. 1998 1998;9(1):41–44. [DOI] [PubMed] [Google Scholar]
- 35.Wagner G, Rabkin J, Rabkin R. Exercise as a mediator of psychological and nutritional effects of testosterone therapy in HIV+ men. Medicine and Science in Sports and Exercise. 1998 1998;30(6):811–817. [DOI] [PubMed] [Google Scholar]
- 36.Lari MA, Faramarz H, Shams M, Marzbana M, Parsa N. Depression, testosterone concentration, sexual dysfunction and methadone use among men with hypogonadism and HIV infection. Retrovirology. 2012 2012;9 [DOI] [PubMed] [Google Scholar]
- 37.Fiksdal A, Hanlin L, Kuras Y, et al. Associations between symptoms of depression and anxiety and cortisol responses to and recovery from acute stress. Psychoneuroendocrinology. Apr 2019;102:44–52. doi: 10.1016/j.psyneuen.2018.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jia Y, Liu L, Sheng C, et al. Increased Serum Levels of Cortisol and Inflammatory Cytokines in People With Depression. J Nerv Ment Dis. Apr 2019;207(4):271–276. doi: 10.1097/nmd.0000000000000957 [DOI] [PubMed] [Google Scholar]
- 39.Mayer SE, Lopez-Duran NL, Sen S, Abelson JL. Chronic stress, hair cortisol and depression: A prospective and longitudinal study of medical internship. Psychoneuroendocrinology. Jun 2018;92:57–65. doi: 10.1016/j.psyneuen.2018.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yrondi A, Sporer M, Péran P, Schmitt L, Arbus C, Sauvaget A. Electroconvulsive therapy, depression, the immune system and inflammation: A systematic review. Brain Stimul. Jan-Feb 2018;11(1):29–51. doi: 10.1016/j.brs.2017.10.013 [DOI] [PubMed] [Google Scholar]
- 41.Zorn JV, Schür RR, Boks MP, Kahn RS, Joëls M, Vinkers CH. Cortisol stress reactivity across psychiatric disorders: A systematic review and meta-analysis. Psychoneuroendocrinology. Mar 2017;77:25–36. doi: 10.1016/j.psyneuen.2016.11.036 [DOI] [PubMed] [Google Scholar]
- 42.Mayo J, Collazos J, Martínez E, Ibarra S. Adrenal function in the human immunodeficiency virus-infected patient. Arch Intern Med. May 27 2002; 162(10):1095–8. doi: 10.1001/archinte.162.10.1095 [DOI] [PubMed] [Google Scholar]
- 43.Endocrine Norbiato G., metabolic, and immunologic components of HIV infection. Ann N Y Acad Sci. Jul 2012;1262:51–5. doi: 10.1111/j.1749-6632.2012.06620.x [DOI] [PubMed] [Google Scholar]
- 44.Sripada RK, Marx CE, King AP, et al. DHEA enhances emotion regulation neurocircuits and modulates memory for emotional stimuli. Neuropsychopharmacology. Aug 2013;38(9):1798–807. doi: 10.1038/npp.2013.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front Neuroendocrinol. Jan 2009;30(1):65–91. doi: 10.1016/j.yfrne.2008.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu G, Yin Y, Xiao CL, et al. Serum DHEAS levels are associated with the development of depression. Psychiatry Res. Sep 30 2015;229(1-2):447–53. doi: 10.1016/j.psychres.2015.05.093 [DOI] [PubMed] [Google Scholar]
- 47.Souza-Teodoro LH, de Oliveira C, Walters K, Carvalho LA. Higher serum dehydroepiandrosterone sulfate protects against the onset of depression in the elderly: Findings from the English Longitudinal Study of Aging (ELSA). Psychoneuroendocrinology. Feb 2016;64:40–6. doi: 10.1016/j.psyneuen.2015.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elliott J, Kelly SE, Millar AC, et al. Testosterone therapy in hypogonadal men: a systematic review and network meta-analysis. BMJ Open. Nov 16 2017;7(11):e015284. doi: 10.1136/bmjopen-2016-015284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cooney LG, Lee I, Sammel MD, Dokras A. High prevalence of moderate and severe depressive and anxiety symptoms in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. May 1 2017;32(5):1075–1091. doi: 10.1093/humrep/dex044 [DOI] [PubMed] [Google Scholar]
- 50.Rohr UD. The impact of testosterone imbalance on depression and women's health. Maturitas. Apr 15 2002;41 Suppl 1:S25–46. doi: 10.1016/s0378-5122(02)00013-0 [DOI] [PubMed] [Google Scholar]
- 51.Wagner G, Rabkin JG, Rabkin R. Illness stage, concurrent medications, and other correlates of low testosterone in men with HIV illness. J Acquir Immune Defic Syndr Hum Retrovirol. Feb 1 1995;8(2):204–7. [PubMed] [Google Scholar]
- 52.Slama L, Jacobson LP, Li X, et al. Longitudinal Changes Over 10 Years in Free Testosterone Among HIV-Infected and HIV-Uninfected Men. J Acquir Immune Defic Syndr. Jan 1 2016;71(1):57–64. doi: 10.1097/qai.0000000000000821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dolan SE, Grinspoon S. Androgen deficiency and the role of testosterone administration in HIV-infected women. J Acquir Immune Defic Syndr. Mar 2005;38 Suppl 1:S48–9. doi: 10.1097/01.qai.0000167050.19171.6f [DOI] [PubMed] [Google Scholar]
- 54.Moura D, Canavarro MC, Figueiredo-Braga M. Oxytocin and depression in the perinatal period-a systematic review. Arch Womens Ment Health. Aug 2016;19(4):561–70. doi: 10.1007/s00737-016-0643-3 [DOI] [PubMed] [Google Scholar]
- 55.Matsushita H, Latt HM, Koga Y, Nishiki T, Matsui H. Oxytocin and Stress: Neural Mechanisms, Stress-Related Disorders, and Therapeutic Approaches. Neuroscience. Oct 1 2019;417:1–10. doi: 10.1016/j.neuroscience.2019.07.046 [DOI] [PubMed] [Google Scholar]
- 56.Sanwald S, Gahr M, Widenhorn-Müller K, et al. Relation of Promoter Methylation of the Oxytocin Gene to Stressful Life Events and Depression Severity. J Mol Neurosci. Feb 2020;70(2):201–211. doi: 10.1007/s12031-019-01446-1 [DOI] [PubMed] [Google Scholar]
- 57.Szpunar MJ, Parry BL. A systematic review of cortisol, thyroid-stimulating hormone, and prolactin in peripartum women with major depression. Arch Womens Ment Health. Apr 2018;21(2):149–161. doi: 10.1007/s00737-017-0787-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Papakostas Y, Markianos M, Papadimitriou G, Lykouras L, Stefanis C. Thyrotropin and prolactin responses to ECT in schizophrenia and depression. Psychiatry Res. Apr 1991;37(1):5–10. doi: 10.1016/0165-1781(91)90101-t [DOI] [PubMed] [Google Scholar]
- 59.Parra A, Ramirez-Peredo J, Larrea F, et al. Decreased dopaminergic tone and increased basal bioactive prolactin in men with human immunodeficiency virus infection. Clin Endocrinol (Oxf). Jun 2001;54(6):731–8. doi: 10.1046/j.1365-2265.2001.01262.x [DOI] [PubMed] [Google Scholar]
- 60.Ukibe NR, Ukibe SN, Emelumadu OF, et al. Impact of thyroid function abnormalities on reproductive hormones during menstrual cycle in premenopausal HIV infected females at NAUTH, Nnewi, Nigeria. PLoS One. 2017;12(7):e0176361. doi: 10.1371/journal.pone.0176361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stanikova D, Luck T, Bae YJ, et al. Increased estrogen level can be associated with depression in males. Psychoneuroendocrinology. Jan 2018;87:196–203. doi: 10.1016/j.psyneuen.2017.10.025 [DOI] [PubMed] [Google Scholar]
- 62.Hernández-Hernández OT, Martínez-Mota L, Herrera-Pérez JJ, Jiménez-Rubio G. Role of Estradiol in the Expression of Genes Involved in Serotonin Neurotransmission: Implications for Female Depression. Curr Neuropharmacol. 2019;17(5):459–471. doi: 10.2174/1570159x16666180628165107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pastuszak AW, Badhiwala N, Lipshultz LI, Khera M. Depression is correlated with the psychological and physical aspects of sexual dysfunction in men. Int J Impot Res. Sep 2013;25(5):194–9. doi: 10.1038/ijir.2013.4 [DOI] [PubMed] [Google Scholar]
- 64.Cabrera-Muñoz E, Hernández-Hernández OT, Camacho-Arroyo I. Role of estradiol and progesterone in HIV susceptibility and disease progression. Mini Rev Med Chem. Oct 2012;12(11):1049–54. doi: 10.2174/138955712802762185 [DOI] [PubMed] [Google Scholar]
- 65.Schumacher M, Mattern C, Ghoumari A, et al. Revisiting the roles of progesterone and allopregnanolone in the nervous system: resurgence of the progesterone receptors. Prog Neurobiol. Feb 2014;113:6–39. doi: 10.1016/j.pneurobio.2013.09.004 [DOI] [PubMed] [Google Scholar]
- 66.Schule C, Nothdurfter C, Rupprecht R. The role of allopregnanolone in depression and anxiety. Prog Neurobiol. Feb 2014;113:79–87. doi: 10.1016/j.pneurobio.2013.09.003 [DOI] [PubMed] [Google Scholar]
- 67.Lacombe-Duncan A, Warren L, Kay ES, et al. Mental health among transgender women living with HIV in Canada: findings from a national community-based research study. AIDS Care. February 2021;33(2):192–200. doi: 10.1080/09540121.2020.1737640 [DOI] [PubMed] [Google Scholar]
- 68.Robakis T, Williams KE, Nutkiewicz L, Rasgon NL. Hormonal Contraceptives and Mood: Review of the Literature and Implications for Future Research. Curr Psychiatry Rep. June 2019;21(7):57. doi: 10.1007/s11920-019-1034-z [DOI] [PubMed] [Google Scholar]