Abstract
While opioids produce both analgesia and side effects by action at μ-opioid receptors (MORs), at spinal and supraspinal sites, the potency of different opioids to produce these effects varies. While it has been suggested that these differences might be because of bias for signaling via β-arrestin versus G-protein α-subunits (Gα), recent studies suggest that G-protein-biased MOR agonists still produce clinically important side effects. Since bias also exists in the role of Gα subunits, we evaluated the role of Gαi/o subunits in analgesia, hyperalgesia, and hyperalgesic priming produced by fentanyl and morphine, in male rats. We found that intrathecal treatment with oligodeoxynucleotides antisense (AS-ODN) for Gαi2, Gαi3, and Gαo markedly attenuated hyperalgesia induced by subanalgesic dose (sub-AD) fentanyl, while AS-ODN for Gαi1, as well as Gαi2 and Gαi3, but not Gαo, prevented hyperalgesia induced by sub-AD morphine. AS-ODN for Gαi1 and Gαi2 unexpectedly enhanced analgesia induced by analgesic dose (AD) fentanyl, while Gαi1 AS-ODN markedly reduced AD morphine analgesia. Hyperalgesic priming, assessed by prolongation of prostaglandin E2-induced hyperalgesia, was not produced by systemic sub-AD and AD fentanyl in Gαi3 and Gαo AS-ODN-treated rats, respectively. In contrast, none of the Gαi/o AS-ODNs tested affected priming induced by systemic sub-AD and AD morphine. We conclude that signaling by different Gαi/o subunits is necessary for the analgesia and side effects of two of the most clinically used opioid analgesics. The design of opioid analgesics that demonstrate selectivity for individual Gαi/o may produce a more limited range of side effects and enhanced analgesia.
SIGNIFICANCE STATEMENT Biased μ-opioid receptor (MOR) agonists that preferentially signal through G-protein α-subunits over β-arrestins have been developed as an approach to mitigate opioid side effects. However, we recently demonstrated that biased MOR agonists also produce hyperalgesia and priming. We show that oligodeoxynucleotide antisense to different Gαi/o subunits play a role in hyperalgesia and analgesia induced by subanalgesic and analgesic dose (respectively), of fentanyl and morphine, as well as in priming. Our findings have the potential to advance our understanding of the mechanisms involved in adverse effects of opioid analgesics that could assist in the development of novel analgesics, preferentially targeting specific G-protein α-subunits.
Keywords: analgesia, fentanyl, G-protein, hyperalgesic priming, morphine, opioid-induced hyperalgesia
Introduction
Approximately 30% of medicines in use today are G-protein-coupled receptor (GPCR) ligands, as are 20% of drugs in clinical trials (Hauser et al., 2017). GPCRs mediate their effects through the activation of guanine nucleotide binding proteins (G-proteins; Childers, 1988; Reisine and Bell, 1993; Uhl et al., 1994; Pasternak and Standifer, 1995; Standifer et al., 1996) and β-arrestins (Gainetdinov et al., 2004; Williams et al., 2013). G-proteins are composed of three distinct subunit families (α, β, and γ), which couple GPCRs to downstream second messengers and effectors. α-Subunits are pharmacologically relevant because of their intrinsic GTPase activity (Standifer and Pasternak, 1997). Different α-subunits (e.g., Gαi, Gαo, Gαs, Gαq, Gx/αz) establish the differential modulation of cAMP by GPCRs (Standifer and Pasternak, 1997). Activated Gα proteins have a variety of effects, including activation of mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (Leurs et al., 2005). Activation of the enzyme phospholipase (PL) A2 may also occur, which induces the release of arachidonic acid, as well as the inhibition of the Na+/H+ exchanger in the plasma membrane, and the lowering of intracellular Ca2+ (Leurs et al., 2005). How these various downstream signaling pathways are differentially activated by GPCRs remains to be elucidated.
Oligodeoxynucleotides (ODNs) antisense (AS) for α-subunit mRNA have been used to implicate different α-subunits in various opioid receptor-mediated functions (Pasternak and Standifer, 1995; Rossi et al., 1995; Standifer et al., 1996; Hadjimarkou et al., 2002; Silva et al., 2002; Wainford and Kapusta, 2012). For example, while intracerebroventricular administration of AS-ODN directed against either Gαi2 or Gαo reduces morphine, but not morphine-6β-glucuronide (M6G) analgesia, AS-ODN directed against Gαi1 or Gx/αz reduces M6G, but not morphine analgesia (Rossi et al., 1995; Standifer et al., 1996). In contrast, fentanyl displays high potency for activation of Gαi1 and Gαo (Saidak et al., 2006).
While opioids produce both analgesia and side effects by action at the μ-opioid receptor (MOR), their relative potencies that produce these diverse effects vary between opioids (Araldi et al., 2018a, c, 2019; Ferrari et al., 2019; Khomula et al., 2021). It has been suggested that this difference between opioid analgesics might be because of biased signaling by GPCR via β-arrestin versus Gαi (Raehal and Bohn, 2014). However, several recent studies of MOR agonists biased toward G-proteins indicate that they still produce substantial side effects, such as constipation, tolerance, and respiratory depression (Viscusi et al., 2016; Olson et al., 2017; Hill et al., 2018; Gillis et al., 2020). We recently found that two such biased MOR agonists (i.e., TRV130 and PZM21) still produce hyperalgesia [opioid-induced hyperalgesia (OIH)] and hyperalgesic priming (opioid-induced hyperalgesic priming), as well as analgesia (Araldi et al., 2018c). In this study, we tested the hypothesis that spinal opioid-induced analgesia, OIH, and hyperalgesic priming produced by two clinically important opioid analgesics, fentanyl and morphine, are mediated by different Gαi/αo proteins.
Materials and Methods
Animals.
Experiments were performed on 260–380 g adult male Sprague Dawley rats (Charles River Laboratories). Given the large number of experiments required to establish the role of multiple Gαs in the analgesic and side effects of different doses of multiple opioid analgesics, we elected to perform experiments in female rats in a subsequent study. Animals were housed three per cage, under a 12 h light/dark cycle, in a temperature- and humidity-controlled animal care facility at the University of California, San Francisco. Food and water were available ad libitum. Experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of California, San Francisco, and adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Effort was made to minimize the number of animals used and their suffering.
Nociceptive threshold testing.
Mechanical nociceptive threshold was quantified using an analgesy-meter (Randall Selitto - paw-withdrawal device, Ugo-Basile), which applies a linearly increasing mechanical force to the dorsum of the hindpaw of a rat, as previously described (Taiwo et al., 1989; Taiwo and Levine, 1989; Araldi et al., 2015, 2017, 2018a,c, 2019). Rats were placed in cylindrical acrylic restrainers designed to provide ventilation, allow hindleg extension from lateral ports in the cylinder during the assessment of nociceptive threshold, and minimize restraint stress. To acclimatize rats to the testing procedure, they were placed in restrainers for 1 h before starting each training session, for 3 consecutive daily training sessions, and for 40 min before subsequent experimental manipulations. Nociceptive threshold was defined as the force in grams at which a rat withdrew its paw. Baseline paw-pressure nociceptive threshold was defined as the mean of the three readings taken before test agents were injected. To minimize experimenter bias, individuals conducting the behavioral experiments (D.A. and I.J.M.B.) were blinded to experimental interventions.
Drugs.
The following compounds were used in this study: fentanyl citrate salt (an MOR agonist), prostaglandin-E2 (PGE2; a direct-acting hyperalgesic agent that sensitizes nociceptors), and morphine sulfate salt pentahydrate (an agonist at μ, δ, and κ opioid receptors), all purchased from Sigma-Aldrich. The stock solution of PGE2 (1 µg/μl) was prepared in 100% ethanol, and further dilutions were made with physiological saline (0.9% NaCl), yielding a final ethanol concentration of <2%. Fentanyl and morphine were dissolved in saline.
Intradermal administration of PGE2 was performed on the dorsum of the hindpaw, at the site of nociceptive testing, using a 30 gauge hypodermic needle adapted to a 50 μl syringe (Hamilton) by a segment of PE-10 polyethylene tubing (Becton Dickinson). Our in vivo control experiments have previously shown that the final concentration of ethanol (2%), used to prepare the PGE2 solution, alone had no effect on the mechanical threshold (Ferrari et al., 2016).
Systemic administration of fentanyl (sub-AD, 0.01 mg/kg, s.c.; AD, 0.03 mg/kg, s.c.) and morphine (sub-AD, 0.03 mg/kg, s.c.; AD, 3 mg/kg, s.c.) was performed at the nape of the neck (Araldi et al., 2018a, 2019). Rats received an injection of fentanyl or morphine, and mechanical nociceptive threshold was evaluated 1 h later (Araldi et al., 2018a, 2019). Fentanyl and morphine were diluted in saline and administered (100 μl/100 g body weight, s.c.).
Oligodeoxynucleotides antisense to G-protein α-subunit mRNAs.
To investigate the role of G-protein α-subunits in the analgesia, hyperalgesia, and priming, induced by systemic sub-AD and AD fentanyl and morphine, validated AS-ODNs for Gαi1, Gαi2, Gαi3, and Gαo mRNA (Rossi et al., 1995; Standifer et al., 1996; Hadjimarkou et al., 2002; Silva et al., 2002; Wainford and Kapusta, 2012) were used.
AS-ODN sequences, directed against unique regions of the rat mRNA for G-protein α-subunits, were as follows:
Gαi1 AS-ODN: 5′-AGACCACTGCTTTGTA-3′ (Gnai1; GenBank accession no. NM_013145.1)
Gαi2 AS-ODN: 5′-CTTGTCGATCATCTTAGA-3′ (Gnai2; GenBank accession no. NM_031035.3)
Gαi3 AS-ODN: 5′-AAGTTGCGGTCGATCAT-3′ (Gnai3; GenBank accession no. NM_013106.1)
Gαo AS-ODN: 5′-CGCCTTGCTCCGCTC-3′ (Gnao1; GenBank accession no. NM_017327.1)
As a control, the following sense (SE) ODN sequences were used:
Gαi1 SE-ODN: 5′-TACAAAGCAGTGGTCT-3′
Gαi2 SE-ODN: 5′-TCTAAGATGATCGACAAG-3′
Gαi3 SE-ODN: 5′-ATGATCGACCGCAACTT-3′
Gαo SE-ODN: 5′-GAGCGGAGCAAGGCG-3′
ODNs were synthesized by Thermo Fisher Scientific. A nucleotide BLAST (Basic Local Alignment Search Tool) search was performed to confirm that the mRNA sequences targeted by the AS-ODN were not homologous to any other sequences in the rat database and that SE-ODN control sequences were not homologous to any sequences in the rat database as well. Before use, lyophilized ODNs were reconstituted in nuclease-free 0.9% NaCl and then administered intrathecally at a dose of 6 µg/µl in a volume of 20 µl (120 µg/20 µl). AS-ODNs or SE-ODNs were injected daily for 4 consecutive days. On the fourth day, fentanyl (AD, 0.03 mg/kg; sub-AD, 0.01 mg/kg) or morphine (AD, 3 mg/kg; or sub-AD, 0.03 mg/kg) was then injected subcutaneously on the back of the neck. Mechanical nociceptive threshold was evaluated 1 h after systemic fentanyl and morphine; 5 d later, PGE2 (100 ng) was injected intradermally and mechanical nociceptive threshold evaluated 30 min and 4 h later. As described previously (Alessandri-Haber et al., 2003), after rats were anesthetized with isoflurane (2.5% in O2), ODN was injected using a 0.3 ml syringe (300 U/µl; Walgreens) with a 29 gauge hypodermic needle, inserted into the subarachnoid space between the L4 and L5 vertebrae. The intrathecal site of injection was confirmed by a sudden flick of the tail of the rat, a reflex that is evoked by accessing the subarachnoid space and bolus injection (Mestre et al., 1994). A total of 120 μg of ODN, in a volume of 20 μl, was then injected. Rats regained consciousness ∼2 min after anesthesia was stopped. Use of intrathecal AS-ODN to attenuate the expression of proteins, essential for their role in nociceptor sensitization, is well supported by previous studies (Song et al., 2009; Su et al., 2011; Bogen et al., 2012; Quanhong et al., 2012; Sun et al., 2013; Oliveira-Fusaro et al., 2017; Araldi et al., 2019; Ferrari et al., 2019; Pagliusi et al., 2020).
Data analysis.
All data are presented as the mean ± SEM of n independent observations. Statistical comparisons were made using GraphPad Prism 8.0 statistical software (GraphPad Software). A p-value < 0.05 was considered statistically significant. In the behavioral experiments, the dependent variable was change in the mechanical paw-withdrawal threshold, expressed as the percentage change from baseline. No significant difference in mechanical nociceptive thresholds was observed between G-protein α-subunit AS-ODN- and SE-ODN-treated groups of rats measured before the administration of fentanyl or morphine (as demonstrated in the figure legends) and immediately before the injection of PGE2 (average mechanical nociceptive threshold before fentanyl- and morphine-induced priming, 141.5 ± 0.837 g; average mechanical nociceptive threshold before PGE2 injection, 140.8 ± 0.839 g; n = 192 rats; paired Student's t test: t(191) = 1.87, p = 0.062. As specified in figure legends, Student's t test or two-way repeated-measures ANOVA followed by Bonferroni's post hoc test was performed to compare the magnitude of the analgesia and hyperalgesia induced by systemic fentanyl or morphine, or to compare the effect produced by the G-protein α-subunit AS-ODNs on the prolongation of hyperalgesia (evaluated 4 h after injection of PGE2) compared with the SE-ODN control groups.
Results
OIH and priming produced by subanalgesic dose fentanyl
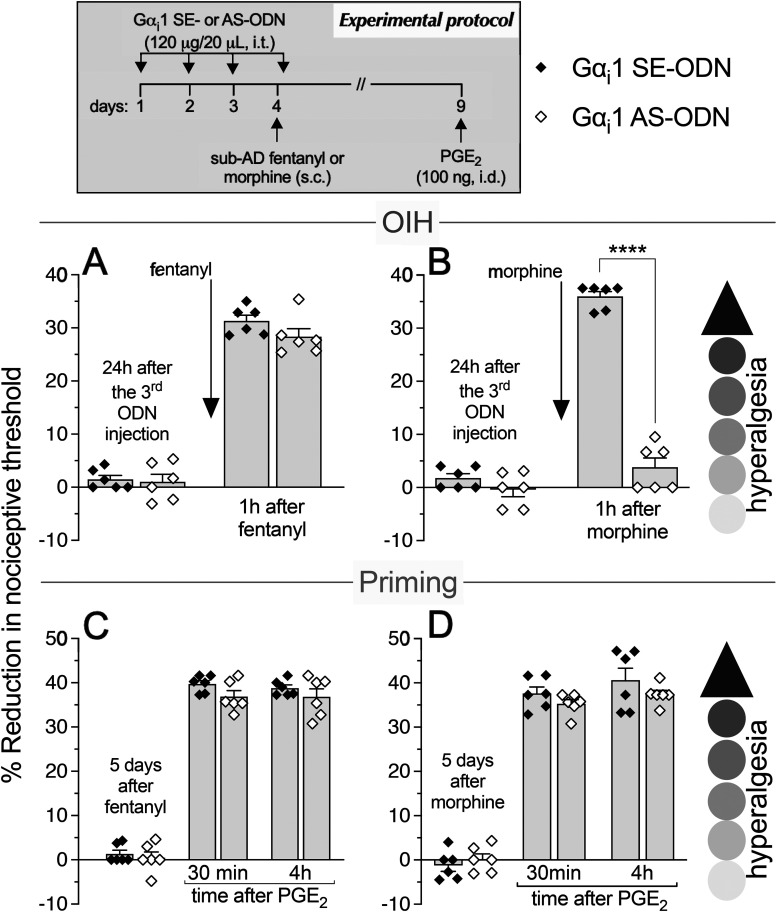
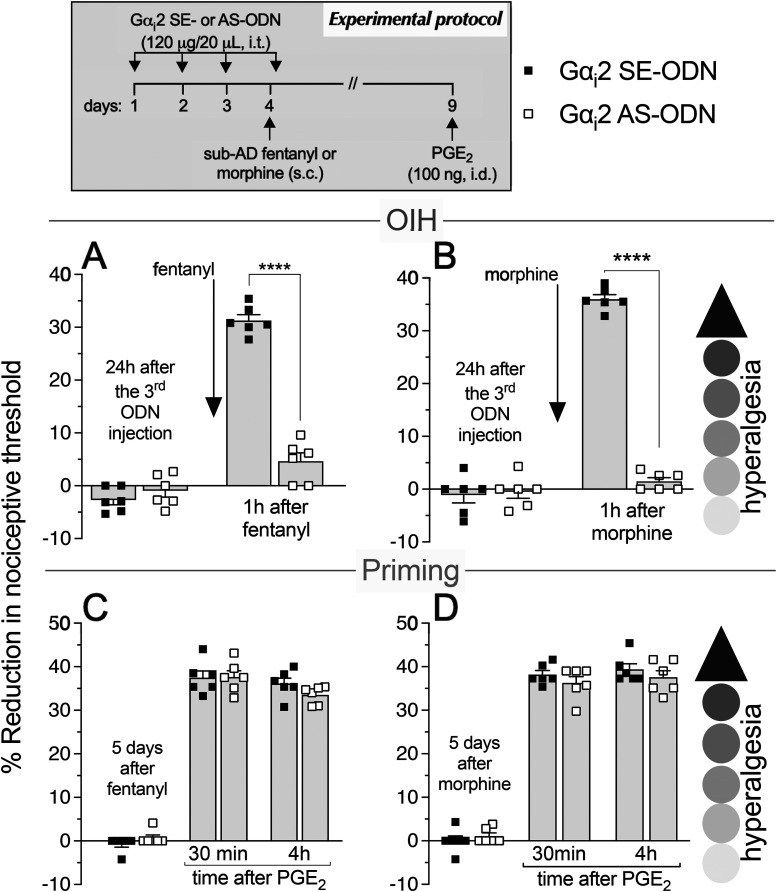
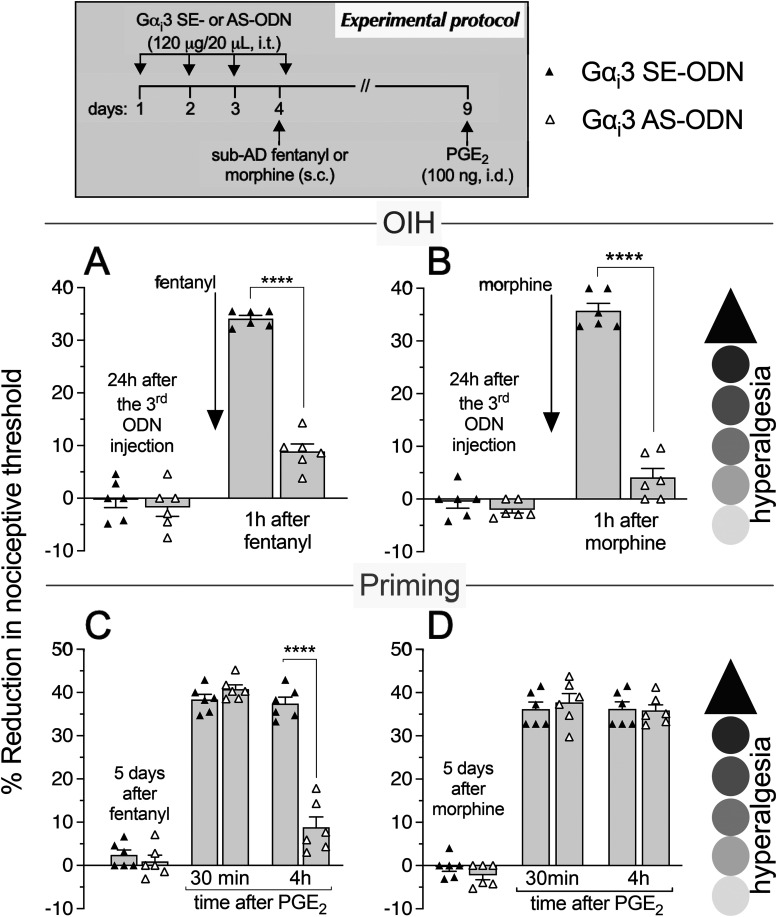
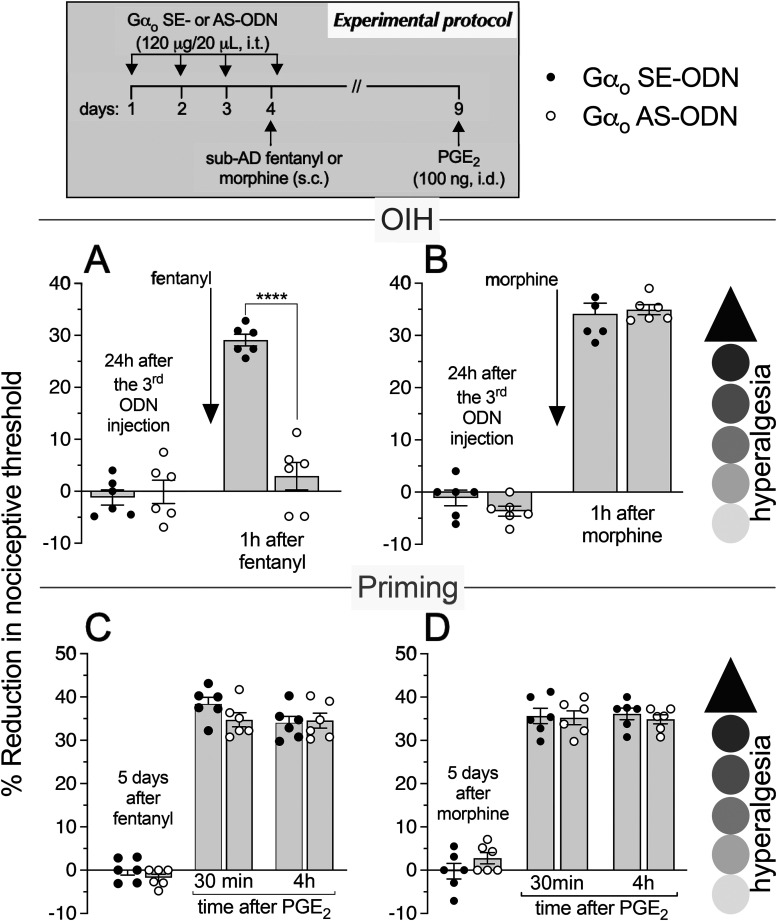
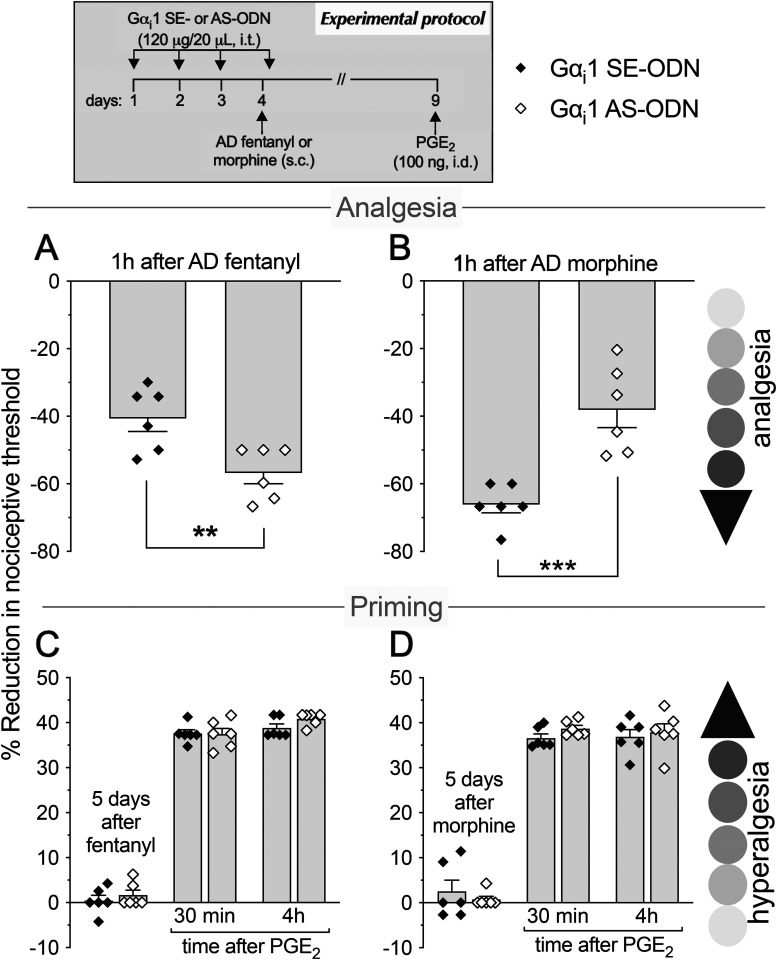
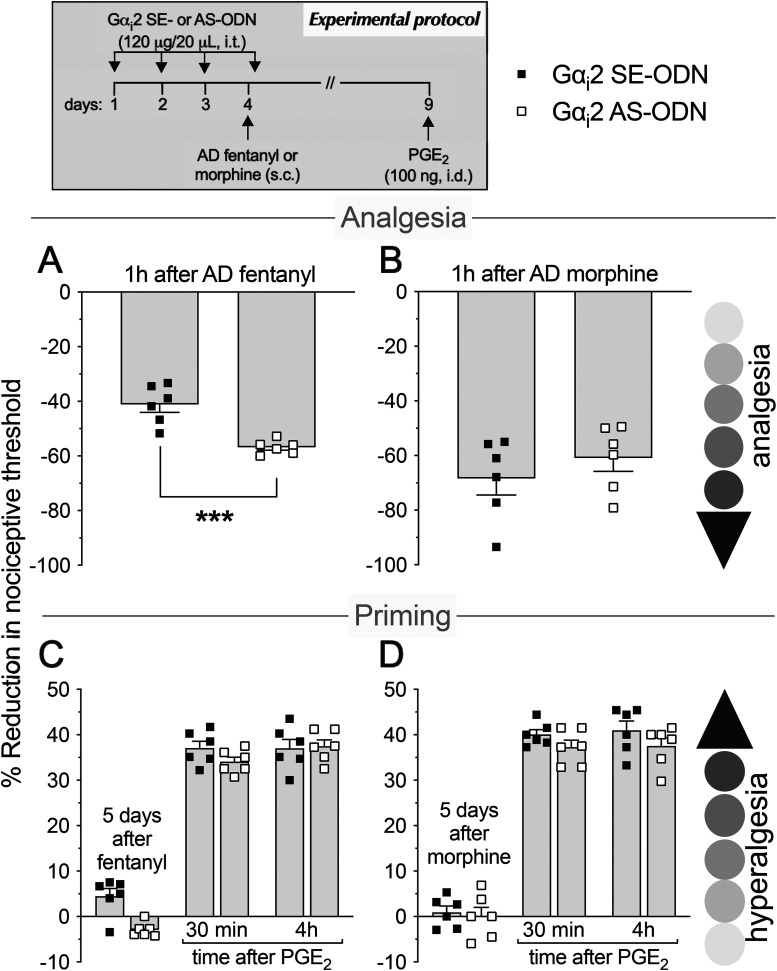
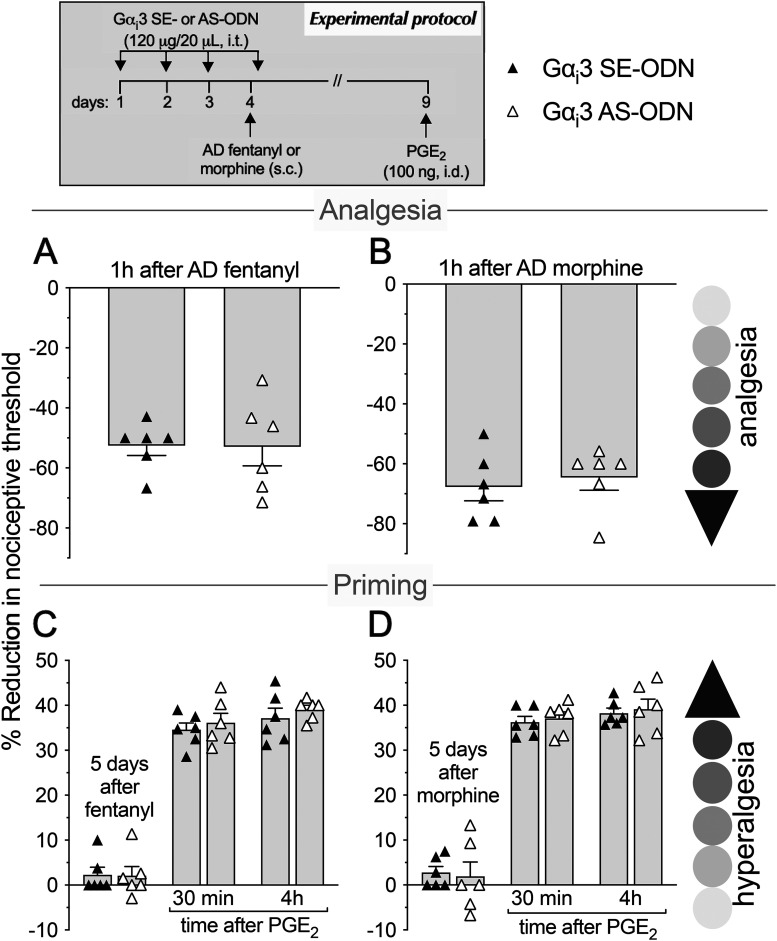
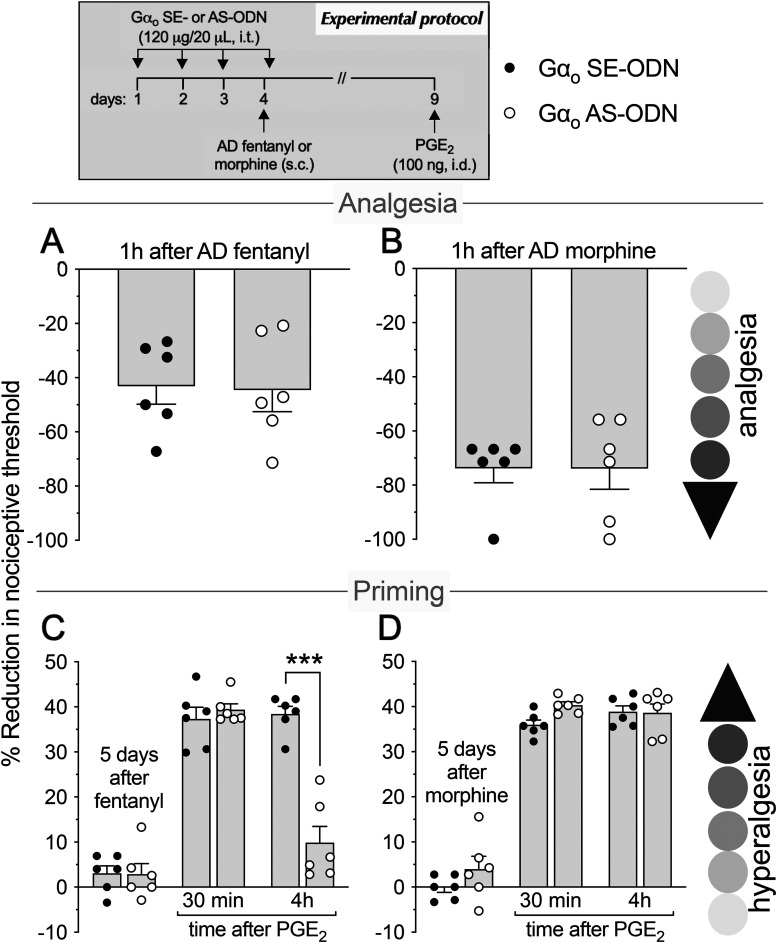
As most opioid analgesics produce hyperalgesia at sub-ADs, we investigated whether OIH and hyperalgesic priming produced by systemic sub-AD fentanyl (0.01 mg/kg, s.c.) are dependent on Gαi/o (i.e., Gαi1, Gαi2, Gαi3, and/or Gαo). Rats were treated intrathecally with AS-ODN or SE-ODN to Gαi1 (Fig. 1), Gαi2 (Fig. 2), Gαi3 (Fig. 3), or Gαo (Fig. 4) mRNA, daily for 4 d. On the fourth day, ∼17 h after the third injection of ODN, sub-AD fentanyl (0.01 mg/kg) was administered systemically (subcutaneous, s.c.), and mechanical nociceptive threshold measured 1 h later. When compared with SE-ODN-treated rats the hyperalgesia induced by sub-AD fentanyl was markedly attenuated in rats pretreated with AS-ODN against Gαi2 (Fig. 2A), Gαi3 (Fig. 3A), or Gαo (Fig. 4A). Treatment with Gαi1 AS-ODN did not affect sub-AD fentanyl-induced hyperalgesia (Fig. 1A). At the end of the fourth day, rats received the last intrathecal administration of G-protein α-subunit AS-ODN or SE-ODN. Five days after systemic sub-AD fentanyl administration, PGE2 (100 ng) was injected intradermally and the mechanical nociceptive threshold evaluated 30 min and 4 h later. In the rats treated with Gαi3 AS-ODN, the prolongation PGE2 hyperalgesia at the fourth hour was not present (Fig. 3C). However, prolonged PGE2-induced hyperalgesia was still present in Gαo, Gαi1, and Gαi2 AS-ODN- and SE-ODN-treated rats (Figs. 1C, 2C, and 4C). Our data support the following suggestions: (1) that some side effects of fentanyl (OIH and hyperalgesic priming) are Gαi/o dependent; (2) that a side effect (OIH) may be dependent on multiple Gαi/o subunits; and (3) that the Gαi/o subunits mediating one side effect (Gαi2, Gαi3, and Gαo mediating OIH) may differ from those mediating another side effect (only Gαi3 mediating sub-AD fentanyl-induced priming).
Figure 1.
Role of Gi-protein α1 subunit (Gαi1) in OIH and hyperalgesic priming produced by systemic sub-AD fentanyl and morphine. Rats received intrathecal (i.t.) injection of AS-ODN (120 μg/20 μl/d, i.t.) or SE-ODN (120 μg/20 μl/d, i.t.) to Gαi1 mRNA for 3 consecutive days. A, B, On the fourth day, at which time mechanical nociceptive threshold was not different from the pre-ODN baseline (A: SE-ODN-treated group: t(5) = 0.5; p = 0.64; AS-ODN-treated group: t(5) = 0.82; p = 0.45; B: SE-ODN-treated group: t(5) = 2.17; p = 0.08; AS-ODN-treated group: t(5) = 0.62; p = 0.56 when the mechanical nociceptive threshold is compared before and ∼17 h after the third ODN injection; paired Student's t test), sub-AD fentanyl (A: 0.01 mg/kg, s.c.) or morphine (B: 0.03 mg/kg, s.c.) was injected and the mechanical nociceptive threshold was evaluated 1 h later. Gαi1 AS-ODN did not affect sub-AD fentanyl-induced hyperalgesia (F(1,10) = 1.94, p = 0.19, when hyperalgesia was compared between the Gαi1 SE-ODN- and AS-ODN-treated groups 1 h after systemic sub-AD fentanyl; two-way repeated-measures ANOVA followed by Bonferroni post hoc test; A). However, in the Gαi1 AS-ODN-treated group, OIH produced by sub-AD morphine was markedly attenuated (F(1,10) = 189.4, ****p < 0.0001, when the hyperalgesia in the Gαi1 SE-ODN- and the AS-ODN-treated groups is compared at 1 h after systemic sub-AD morphine; two-way repeated-measures ANOVA followed by Bonferroni post hoc test; B). At the end of the fourth day, rats again received intrathecal Gαi1 AS- or SE-ODN. C, D, Five days after systemic sub-AD fentanyl and morphine, at which time mechanical nociceptive threshold was not different from preopioid baselines (C: SE-ODN-treated group: t(5) = 1.58; p = 0.18; AS-ODN-treated group that received sub-AD fentanyl: t(5) = 0.39; p = 0.71; D: SE-ODN-treated group: t(5) = 0.88; p = 0.42; AS-ODN-treated group that received sub-AD morphine: t(5) = 0.2; p = 0.85 when the mechanical nociceptive threshold is compared before and 5 d after systemic sub-AD opioid administration; paired Student's t test), PGE2 (100 ng/5 μl, i.d.) was injected and the mechanical nociceptive threshold was evaluated 30 min and 4 h later. Treatment with Gαi1 AS-ODN did not prevent PGE2-induced prolonged hyperalgesia in either fentanyl-treated (C) or morphine-treated (D) groups (C: F(1,10) = 2.12, p = 0.17; D: F(1,10) = 1.02, p = 0.34, when the hyperalgesia in the Gαi1 SE-ODN- and the AS-ODN-treated groups is compared at the fourth hour after intradermal PGE2 administration; two-way repeated-measures ANOVA followed by Bonferroni post hoc test). These findings support the suggestion that Gαi1 plays a role in OIH produced by systemic sub-AD morphine, but not fentanyl, and is not involved in hyperalgesic priming produced by sub-AD fentanyl or morphine (n = 6 paws/6 rats/group).
Figure 2.
Role of Gαi2 in hyperalgesia and hyperalgesic priming produced by systemic sub-AD fentanyl and morphine. Rats received injections of AS-ODN (120 μg/20 μl/d, i.t.) or SE-ODN (120 μg/20 μl/d, i.t.) against Gαi2 mRNA, for 3 consecutive days. A, B, Approximately 17 h after the third injection, at which time the mechanical nociceptive threshold was not different from pre-ODN baseline levels (A: SE-ODN-treated group: t(5) = 0.41, p = 0.70; AS-ODN-treated group: t(5) = 0.56; p = 0.57; B: SE-ODN-treated group: t(5) = 0.15, p = 0.89; AS-ODN-treated group: t(5) = 0.13, p = 0.90, when the mechanical nociceptive threshold is compared before and after the third ODN injection; paired Student's t test), sub-AD fentanyl (A: 0.01 mg/kg, s.c.) or morphine (B: 0.03 mg/kg, s.c.) was administered and mechanical nociceptive threshold was evaluated 1 h later. Treatment with Gαi2 AS-ODN prevented hyperalgesia induced by both sub-AD fentanyl (A) and morphine (B), when it was compared with the SE-ODN-treated group (A: F(1,10) = 75.5, ****p < 0.0001; B: F(1,10) = 298.3, ****p < 0.0001, when the hyperalgesia in the Gαi2 SE-ODN-treated and the AS-ODN-treated groups is compared at 1 h after systemic sub-AD fentanyl and morphine; two-way repeated-measures ANOVA followed by Bonferroni's post hoc test). Rats again received intrathecal Gαi2 AS- or SE-ODN on the fourth day. C, D, Five days after sub-AD opioids, at which time the mechanical nociceptive threshold was not different from preopioid baseline (C: SE-ODN-treated group: t(5) = 1.0, p = 0.36; AS-ODN-treated group that received sub-AD fentanyl: t(5) = 0.54, p = 0.61; D: SE-ODN-treated group: t(5) = 0.73, p = 0.49; AS-ODN-treated group that received sub-AD morphine: t(5) = 0.59, p = 0.57, when the mechanical nociceptive threshold is compared before and 5 d after systemic sub-AD opioids; paired Student's t test), PGE2 (100 ng/5 μl, i.d.) was injected and the mechanical nociceptive threshold was evaluated 30 min and 4 h later. In both the Gαi2 AS-ODN-treated and SE-ODN-treated groups, the prolongation of PGE2 hyperalgesia was present 5 d after systemic sub-AD fentanyl and morphine (C and D, respectively; C: F(1,10) = 0.11, p = 0.74; D: F(1,10) = 0.50, p = 0.49, when the hyperalgesia in the Gαi2 AS-ODN-treated and the SE-ODN-treated groups is compared at the fourth hour after intradermal PGE2; two-way repeated-measures ANOVA followed by Bonferroni's post hoc test). Our data indicate that OIH, but not priming, produced by systemic sub-AD fentanyl and morphine is Gαi2 dependent (n = 6 paws/6 rats/group).
Figure 3.
Role of Gαi3 in hyperalgesia and hyperalgesic priming produced by systemic sub-AD fentanyl and morphine. Rats received injection of AS-ODN (120 μg in 20 μl/d, i.t.) or SE-ODN (120 μg in 20 μl/d, i.t.) against Gαi3 mRNA, daily for 3 consecutive days. A, B, On the fourth day, ∼17 h after the third intrathecal administration of ODNs, at which time mechanical nociceptive threshold was not significantly different from pre-ODN baselines (A: SE-ODN-treated group: t(5) = 0.67, p = 0.53; AS-ODN-treated group: t(5) = 0.18, p = 0.86; B: SE-ODN-treated group: t(5) = 0.39, p = 0.71; AS-ODN-treated group: t(5) = 0.15, p = 0.89, when the mechanical nociceptive threshold is compared before and after the third Gαi3 ODN injection; paired Student's t test), sub-AD fentanyl (A: 0.01 mg/kg, s.c.) or morphine (B: 0.03 mg/kg, s.c.) was administered and the mechanical nociceptive threshold was evaluated 1 h later. In the Gαi3 AS-ODN-treated group, systemic sub-AD of neither fentanyl (A) nor morphine (B) produced hyperalgesia, measured 1 h after its administration, as is observed in the Gαi3 SE-ODN-treated group (A: F(1,10) = 186.6, ****p < 0.0001; B: F(1,10) = 193.9, ****p < 0.0001, when the hyperalgesia in the Gαi3 SE-ODN-treated and the AS-ODN-treated groups was compared at 1 h after systemic sub-AD opioids; two-way repeated-measures ANOVA followed by Bonferroni post hoc test). At the end of the fourth day, rats again received intrathecal Gαi3 AS-ODN or SE-ODN. C, D, Five days after systemic sub-AD fentanyl and morphine, at which time mechanical nociceptive threshold was not different from preopioid baselines (C: SE-ODN-treated group: t(5) = 1.98, p = 0.11; AS-ODN-treated group that received sub-AD fentanyl: t(5) = 1.07, p = 0.33; D: SE-ODN-treated group: t(5) = 0.22; p = 0.83; AS-ODN-treated group that received sub-AD morphine: t(5) = 2.23, p = 0.07, when the mechanical nociceptive threshold is compared before and 5 d after systemic sub-AD opioid; paired Student's t test), PGE2 (100 ng/5 μl, i.d.) was injected and mechanical nociceptive threshold was evaluated 30 min and 4 h later. In the Gαi3 AS-ODN-treated group, which received systemic sub-AD fentanyl, PGE2-induced hyperalgesia was not present at the fourth hour (C: F(1,10) = 42.9, ****p < 0.0001, when the hyperalgesia in the Gαi3 AS-ODN-treated and the SE-ODN-treated groups is compared at the fourth hour after intradermal PGE2; two-way repeated-measures ANOVA followed by Bonferroni's post hoc test). However, prolongation of PGE2-induced hyperalgesia was not affected by the treatment with Gαi3 AS-ODN in the systemic sub-AD morphine-treated group (D: F(1,10) = 0.04, p = 0.85, when hyperalgesia was compared between the Gαi3 SE-ODN-treated and AS-ODN-treated groups at the fourth hour after intradermal PGE2; two-way repeated-measures ANOVA followed by Bonferroni's post hoc test). These findings indicate that Gαi3 plays a role in hyperalgesia induced by both sub-AD fentanyl and morphine. However, priming induced by sub-AD fentanyl, but not sub-AD morphine, is dependent on Gαi3 (n = 6 paws/6 rats/group).
Figure 4.
Role of Gαo in hyperalgesia and hyperalgesic priming induced by systemic sub-AD fentanyl and morphine. Rats received injection of AS-ODN (120 μg in 20 μl/d, i.t.) or SE-ODN (120 μg in 20 μl/d, i.t.) against Gαo mRNA daily for 3 consecutive days. On the fourth day, at which time the mechanical nociceptive threshold was not different from the pre-ODN baselines (A: SE-ODN-treated group: t(5) = 0.67; p = 0.53; AS-ODN-treated group: t(5) = 0.65; p = 0.54; B: SE-ODN-treated group: t(5) = 0.74; p = 0.49; AS-ODN-treated group: t(5) = 1.66; p = 0.15, when the mechanical nociceptive threshold is compared before and ∼17 h after the third ODN injection; paired Student's t test), sub-AD fentanyl (A, 0.01 mg/kg, s.c.) or morphine (B, 0.03 mg/kg, s.c.) was administered and the mechanical nociceptive threshold was evaluated 1 h later. In the group of rats treated with Gαo AS-ODN, systemic sub-AD fentanyl-induced hyperalgesia was prevented (A, F(1,10) = 53.3, ****p < 0.0001, when the hyperalgesia in the Gαo SE-ODN-treated and the AS-ODN-treated groups was compared at 1 h after systemic sub-AD fentanyl; two-way repeated-measures ANOVA followed by Bonferroni's post hoc test). However, systemic sub-AD morphine-induced hyperalgesia was not affected by the treatment with Gαo AS-ODN (B, F(1,10) = 0.24, p = 0.63, when the hyperalgesia in the Gαo SE-ODN-treated and the AS-ODN-treated groups was compared at 1 h after systemic sub-AD morphine; two-way repeated-measures ANOVA followed by Bonferroni's post hoc test). At the end of the fourth day, rats again received intrathecal Gαo AS-ODN or SE-ODN. Five days after systemic sub-AD fentanyl and morphine, when the mechanical nociceptive threshold was not different from preopioid baselines (C: SE-ODN-treated group: t(5) = 0.75; p = 0.48; AS-ODN-treated group that received sub-AD fentanyl: t(5) = 2.15; p = 0.08; D: SE-ODN-treated group: t(5) = 0.68; p = 0.53; AS-ODN-treated group that received sub-AD morphine: t(5) = 1.90; p = 0.11, when the mechanical nociceptive threshold is compared before and 5 d after systemic sub-AD opioids; paired Student's t test), PGE2 (100 ng/5 μl, i.d.) was administered and the mechanical nociceptive threshold was evaluated 30 min and 4 h later. Treatment with Gαo AS-ODN did not prevent the prolongation of PGE2-induced hyperalgesia in both fentanyl-treated (C) and morphine-treated (D) groups of rats (C: F(1,10) = 2.15, p = 0.17; D: F(1,10) = 0.18, p = 0.68, when the hyperalgesia in the Gαo SE-ODN-treated and the AS-ODN-treated groups is compared at the fourth hour after intradermal PGE2; two-way repeated-measures ANOVA followed by Bonferroni's post hoc test). These findings support the suggestion that the Gαo subunit plays a role in OIH produced by systemic sub-AD fentanyl, but not morphine, and is not involved in hyperalgesic priming produced by sub-AD fentanyl and morphine. (n = 6 paws/6 rats/group).
OIH and priming produced by subanalgesic dose morphine
We next investigated whether OIH and hyperalgesic priming induced by sub-AD of another clinical opioid analgesic, morphine (0.03 mg/kg, s.c.; Araldi et al., 2019) are also Gαi/o dependent and whether they are dependent on the same Gαi/o subunits as these effects of sub-AD fentanyl. Rats received intrathecal AS-ODN or SE-ODN to Gαi1 (Fig. 1), Gαi2 (Fig. 2), Gαi3 (Fig. 3), or Gαo (Fig. 4) mRNA, daily for 4 d. On the fourth day, sub-AD morphine (0.03 mg/kg, s.c.) was administered and the mechanical nociceptive threshold was measured 1 h later. In rats treated with AS-ODN against Gαi1 (Fig. 1B), Gαi2 (Fig. 2B), and Gαi3 (Fig. 3B) mRNA, sub-AD morphine did not induce hyperalgesia. However, in rats treated with Gαo AS-ODN, systemic sub-AD morphine still induced hyperalgesia (Fig. 4B). At the end of the fourth day, rats again received AS-ODN or SE-ODN. Five days after systemic sub-AD morphine administration, PGE2 (100 ng) was injected intradermally and the mechanical nociceptive threshold was evaluated 30 min and 4 h later. The prolongation of PGE2-induced hyperalgesia was still present in all AS-ODN-treated rats (Figs. 1D, 2D, 3D, and 4D). These findings support the following suggestions: (1) that the Gαi/o subunits mediating side effects (OIH and hyperalgesic priming) differ between the sub-AD of clinical opioid analgesics (fentanyl and morphine); and (2) that the same side effect (OIH) produced by two opioid analgesics may be dependent only partially on overlapping Gαi/o subunits (while OIH produced by sub-AD morphine is dependent on Gαi1, Gαi2, and Gαi3, OIH produced by sub-AD fentanyl is Gαi2, Gαi3, and Gαo dependent). Whether opioid-induced hyperalgesic priming produced by sub-AD morphine is G-protein independent or dependent on one of the Gα subunits not evaluated in the present experiments remains to be established.
Analgesia and hyperalgesic priming produced by analgesic dose (AD) fentanyl
Since AD opioids produce hyperalgesic priming as well as analgesia, we next determined whether analgesia and priming induced by AD fentanyl (0.03 mg/kg, s.c.; Khomula et al., 2019, 2021) are Gαi/o dependent and whether the same G-proteins mediate opioid-induced hyperalgesic priming produced by sub-AD and AD fentanyl. Groups of rats were treated with AS-ODN or SE-ODN for Gαi1 (Fig. 5), Gαi2 (Fig. 6), Gαi3 (Fig. 7), or Gαo (Fig. 8) mRNA, daily for 4 consecutive days. On the fourth day, ∼17 h after the third dose of ODN, AD fentanyl was administered (0.03 mg/kg, s.c.), and the mechanical nociceptive threshold was evaluated 1 h later. Unexpectedly, analgesia induced by AD fentanyl was increased in the Gαi1 (Fig. 5A) and Gαi2 (Fig. 6A) AS-ODN-treated groups of rats; while in the groups treated with either Gαi3 (Fig. 7A) or Gαo AS-ODN (Fig. 8A) fentanyl-induced analgesia was not affected. At the end of the fourth day, rats again received AS-ODN or SE-ODN. Five days after AD fentanyl, PGE2 (100 ng, i.d.) was injected and mechanical nociceptive threshold evaluated 30 min and 4 h later. In rats treated with Gαo (Fig. 8C), but not Gαi1, Gαi2, and Gαi3 (Figs. 5C, 6C, and 7C) AS-ODN the prolongation of PGE2-induced hyperalgesia was eliminated.
Figure 5.
Role of Gαi1 in systemic AD fentanyl- and morphine-induced analgesia and priming. Rats received intrathecal injections of AS-ODN (120 μg in 20 μl/d, i.t.) or SE-ODN (120 μg in 20 μl/d, i.t.) against Gαi1 mRNA, daily for 3 consecutive days. On the fourth day, AD fentanyl (A, 0.03 mg/kg, s.c.) or morphine (B, 3 mg/kg, s.c.) was administered and the mechanical nociceptive threshold was evaluated 1 h later. Treatment with Gαi1 AS-ODN increased analgesia induced by systemic AD fentanyl (A), while it decreased AD morphine-induced analgesia (B), compared with their respective Gαi1 SE-ODN-treated groups (A: t(10) = 3.24, ** p = 0.0088; B: t(10) = 4.81, *** p = 0.0007, when the analgesia in the Gαi1 AS-ODN-treated and the SE-ODN-treated groups is compared at 1 h after systemic AD fentanyl and morphine; unpaired Student's t test). At the end of the fourth day, rats again received intrathecal Gαi1 AS-ODN or SE-ODN. Five days after systemic AD fentanyl and morphine administration, at which time the mechanical nociceptive threshold was not different from preopioid baselines (C: SE-ODN-treated group: t(5) = 0.39, p = 0.71; AS-ODN-treated group that received systemic AD fentanyl: t(5) = 1.4, p = 0.22; D: SE-ODN-treated group: t(5) = 1.0, p = 0.36; AS-ODN-treated group that received systemic AD morphine: t(5) = 0.58, p = 0.59, when the mechanical nociceptive threshold is compared before and 5 d after systemic AD opioids; paired Student's t test), PGE2 (100 ng/5 μl, i.d.) was administered and the mechanical nociceptive threshold was evaluated 30 min and 4 h later. Prolongation of PGE2-induced hyperalgesia was not affected by treatment with Gαi1 AS-ODN in both AD fentanyl-treated (C) and morphine-treated (D) groups of rats (C: F(1,10) = 1.28, p = 0.28; D: F(1,10) = 0.13, p = 0.72, when the hyperalgesia in the Gαi1 SE-ODN-treated and the AS-ODN-treated groups is compared at the fourth hour after intradermal PGE2 administration; two-way repeated-measures ANOVA followed by Bonferroni's post hoc test). These findings support the suggestion that Gαi1 plays a role in analgesia, but not in hyperalgesic priming, produced by AD fentanyl and morphine (n = 6 paws/6 rats/group).
Figure 6.
Role of Gαi2 in analgesia and hyperalgesic priming induced by systemic analgesic dose fentanyl and morphine. Rats received injections of AS-ODN (120 μg/20 μl/d, i.t.) or SE-ODN (120 μg/20 μl/d, i.t.) against Gαi2 mRNA, daily for 3 consecutive days. Approximately 17 h after the third injection of ODNs, AD fentanyl (A: 0.03 mg/kg, s.c.) or morphine (B: 3 mg/kg, s.c.) was administered and the mechanical nociceptive threshold was evaluated 1 h later. Treatment with Gαi2 AS-ODN increased analgesia induced by AD fentanyl (A); however, it did not affect analgesia induced by AD morphine (B), when compared with the SE-ODN-treated group (A: t(10) = 5.07, ***p = 0.0005; B: t(10) = 0.96, p = 0.36, when the analgesia in the Gαi2 AS-ODN-treated and the SE-ODN-treated groups is compared at 1 h after systemic AD fentanyl and morphine, respectively; unpaired Student's t test). Rats again received Gαi2 AS-ODN or SE-ODN later on the fourth day. Five days after AD opioids, at which time mechanical nociceptive threshold was not different from preopioid baselines (C: SE-ODN-treated group: t(5) = 2.5, p = 0.06; AS-ODN-treated group that received AD fentanyl: t(5) = 0.67; p = 0.53; D: SE-ODN-treated group: t(5) = 1.0, p = 0.36; AS-ODN-treated group that received AD morphine: t(5) = 0.12, p = 0.91, when the mechanical nociceptive threshold is compared before and 5 d after systemic AD opioids; paired Student's t test), PGE2 (100 ng/5 μl, i.d.) was administered and the mechanical nociceptive threshold was evaluated 30 min and 4 h later. In both the Gαi2 AS-treated and SE-ODN-treated groups, the prolongation of PGE2-induced hyperalgesia was present 5 d after systemic AD fentanyl and morphine (C and D, respectively; C: F(1,10) = 0.68, p = 0.43; D: F(1,10) = 2.84, p = 0.12, when the hyperalgesia in the Gαi2 AS-ODN-treated and the SE-ODN-treated groups is compared at the fourth hour after intradermal PGE2 administration; two-way repeated-measures ANOVA followed by Bonferroni's post hoc test). Our data support the suggestion that the Gαi2 subunit plays a role in analgesia produced by AD fentanyl, but not morphine; also, hyperalgesic priming produced by AD fentanyl and morphine is not Gαi2 dependent (n = 6 paws/6 rats/group).
Figure 7.
Role of Gαi3 in analgesia and hyperalgesic priming induced by systemic AD fentanyl and morphine. Rats received intrathecal injections of AS-ODN (120 μg in 20 μl/d, i.t.) or SE-ODN (120 μg in 20 μl/day, i.t.) against Gαi3 mRNA, daily for 3 consecutive days. On the fourth day, ∼17 h after the third injection of ODNs, systemic AD fentanyl (A: 0.03 mg/kg, s.c.) or morphine (B: 3 mg/kg, s.c.) was injected and mechanical nociceptive threshold evaluated 1 h later. Treatment with Gαi3 AS-ODN did not affect the analgesia produced by either AD fentanyl (A) or morphine (B), measured 1 h after their administration, as observed in the Gαi3 SE-ODN-treated group (A: t(10) = 0.054; ***p = 0.96; B: t(10) = 0.51, p = 0.62, when the analgesia in the Gαi3 AS-ODN-treated and the SE-ODN-treated groups is compared at 1 h after systemic AD fentanyl and morphine, respectively; unpaired Student's t test). At the end of the fourth day, rats again received intrathecal Gαi3 AS-ODN or SE-ODN. Five days after systemic AD fentanyl and morphine, at which time the mechanical nociceptive threshold was not different from preopioid baseline levels (C: SE-ODN-treated group: t(5) = 1.38, p = 0.23; AS-ODN-treated group that received systemic AD fentanyl: t(5) = 1.06, p = 0.34; D: SE-ODN-treated group: t(5) = 2.0, p = 0.1; AS-ODN-treated group that received systemic AD morphine: t(5) = 0.72, p = 0.5, when the mechanical nociceptive threshold is compared before and 5 d after systemic AD opioids; paired Student's t test), PGE2 (100 ng/5 μl, i.d.) was injected and the mechanical nociceptive threshold was evaluated 30 min and 4 h later. In both the Gαi3 AS-ODN-treated and SE-ODN-treated groups, the prolongation of PGE2-induced hyperalgesia was present 5 d after systemic AD fentanyl and morphine (C and D, respectively; C: F(1,10) = 0.58, p = 0.46; D: F(1,10) = 0.1, p = 0.75, when the hyperalgesia in the Gαi3 AS-ODN-treated and the SE-ODN-treated groups is compared at the fourth hour after intradermal PGE2; two-way repeated-measures ANOVA followed by Bonferroni's post hoc test). These findings support the suggestion that Gαi3 did not play a role in analgesia and hyperalgesic priming induced by AD fentanyl or morphine (n = 6 paws/6 rats/group).
Figure 8.
Role of Gαo in analgesia and hyperalgesic priming induced by systemic AD fentanyl and morphine. Rats received intrathecal injections of AS-ODN (120 μg in 20 μl/d, i.t.) or SE-ODN (120 μg in 20 μl/d, i.t.) against Gαo mRNA, daily for 3 consecutive days. On the fourth day, AD fentanyl (A: 0.03 mg/kg, s.c.) or morphine (B: 3 mg/kg, s.c.) was administered and the mechanical nociceptive threshold was evaluated 1 h later. In the groups of rats treated with Gαo AS-ODN, systemic AD fentanyl (A), and morphine (B) induced analgesia that was not different when compared with their respective Gαo SE-ODN-treated groups (A: t(10) = 0.13, p = 0.90; B: t(10) = 0.005, p = 0.99, when the analgesia in the Gαo AS-ODN-treated and the SE-ODN-treated groups is compared at 1 h after systemic AD fentanyl and morphine, respectively; unpaired Student's t test). At the end of the fourth day, rats again received Gαo AS-ODN or SE-ODN. Five days after systemic AD fentanyl and morphine, when the mechanical nociceptive threshold was not different from the preopioids baselines (C: SE-ODN-treated group: t(5) = 2.03; p = 0.09; AS-ODN-treated group that received AD fentanyl: t(5) = 1.26; p = 0.26; D: SE-ODN-treated group: t(5) = 1.1, p = 0.32; AS-ODN-treated group that received AD morphine: t(5) = 1.52, p = 0.19, when the mechanical nociceptive threshold is compared before and 5 d after systemic AD opioids; paired Student's t test), PGE2 (100 ng/5 μl, i.d.) was injected and the mechanical nociceptive threshold was evaluated 30 min and 4 h later. Treatment with Gαo AS-ODN markedly attenuates PGE2-induced prolonged hyperalgesia in rats that received AD fentanyl (C: F(1,10) = 23.6, *** p = 0.0007, when the hyperalgesia in the Gαo AS-ODN-treated and the SE-ODN-treated groups is compared at the fourth hour after intradermal PGE2; two-way repeated-measures ANOVA followed by Bonferroni's post hoc test). However, the prolongation of PGE2-induced hyperalgesia was not affected by treatment with Gαo AS-ODN in the AD morphine-treated group (D: F(1,10) = 2.89, p = 0.12, when hyperalgesia was compared between the Gαo SE-ODN-treated and AS-ODN-treated groups at the fourth hour after intradermal PGE2; two-way repeated-measures ANOVA followed by Bonferroni's post hoc test). These findings indicate that analgesia produced by systemic AD fentanyl and morphine is not Gαo subunit dependent; however, the Gαo subunit does play a role in hyperalgesic priming induced by AD fentanyl, but not morphine (n = 6 paws/6 rats/group).
To the best of our knowledge, our data demonstrating that Gαi1 and Gαi2 AS-ODN enhances a Gαi/o–GPCR signaling provide the first evidence that Gαi/o subunits can inhibit Gαi/o GPCR signaling. Our results in which Gαi1 and Gαi2 subunits increased analgesia induced by AD fentanyl contrasts with a fentanyl saturation-binding analysis for MOR, using urea-washed MOR membranes, where fentanyl displayed high potency in activating both Gαi1 and Gαo (Saidak et al., 2006).
Furthermore, our data support the suggestion that different Gαi/o subunits mediate priming induced by AD (Gαo) and sub-AD (Gαi3) fentanyl. While the basis for this difference is currently unknown, we have observed that sub-AD fentanyl-induced priming is attenuated by ODN antisense to Toll-like receptor 4 (TLR4) mRNA (unpublished data, Araldi D), while hyperalgesic priming induced by repeated AD fentanyl is attenuated by MOR antisense (Araldi et al., 2018a).
Analgesia and hyperalgesic priming produced by AD morphine
Finally, we investigated whether the analgesia and hyperalgesic priming induced by AD morphine (3 mg/kg, s.c.; Araldi et al., 2019) are also Gαi/o dependent. Rats received AS-ODN or SE-ODN to Gαi1 (Fig. 5), Gαi2 (Fig. 6), Gαi3 (Fig. 7), or Gαo (Fig. 8) mRNA, intrathecally daily for 4 d. On the fourth day, AD morphine was administered systemically (3 mg/kg, s.c.), and the mechanical nociceptive threshold was measured 1 h later. AD morphine-induced analgesia was markedly reduced in the Gαi1 AS-ODN-treated group, compared with its SE-ODN-treated group (Fig. 5B). On the other hand, intrathecal treatment with Gαi2 (Fig. 6B), Gαi3 (Fig. 7B), and Gαo (Fig. 8B) AS-ODNs did not affect morphine analgesia. Rats again received AS-ODN or SE-ODN at the end of the fourth day. Five days after AD morphine, PGE2 (100 ng, i.d.) was injected, and the mechanical nociceptive threshold was evaluated 30 min and 4 h later. PGE2-induced prolonged hyperalgesia was present in all four groups of AS-ODN-treated rats (Figs. 5D, 6D, 7D, and 8D). Our findings support the suggestion that, in marked contrast to the role of Gαi1 and Gαi2, which increased analgesia induced by AD fentanyl, AD morphine analgesia is reduced by Gαi1 AS-ODN. Thus, the role of Gαi1 in morphine-induced analgesia is opposite to its role in analgesia induced by AD fentanyl. Also, unexpectedly, different Gαi/o subunits mediate hyperalgesic priming induced by sub-AD and AD fentanyl, Gαi3 versus Gαo dependence, respectively; and, priming induced by both sub-AD and AD morphine are Gαi1, Gαi2, Gαi3, and Gαo independent.
Together, the experiments presented here support the following suggestions: (1) that OIH, opioid-induced hyperalgesia priming and analgesia induced by different doses of different opioid analgesics are mediated by different Gαi/o proteins; (2) that different Gαi/o subunits mediate OIH, opioid-induced hyperalgesic priming, and analgesia induced by fentanyl and morphine; (3) that OIH, opioid-induced hyperalgesic priming, and/or analgesia may be mediated by multiple Gαi/o proteins, differing for different doses and opioid analgesics; and, (4) that Gαi/o proteins may inhibit as well as mediate Gαi/o–GPCR signaling.
Discussion
Although opioids remain the most effective treatment for many forms of moderate to severe pain, they produce several treatment-limiting side effects, including analgesic tolerance, addiction, OIH, induction of the transition from acute to chronic pain (modeled in the present experiments by opioid-induced hyperalgesic priming), respiratory depression, and constipation (Chu et al., 2008; Joseph et al., 2010; Lee et al., 2011; Trang et al., 2015; Roeckel et al., 2016; Araldi et al., 2015, 2017, 2018a, b, 2019; Khomula et al., 2019, 2021). Opioid analgesics, acting at MORs, produce their effects through the activation of G-proteins (Childers, 1988; Reisine and Bell, 1993; Uhl et al., 1994; Pasternak and Standifer, 1995; Standifer et al., 1996) and β-arrestins (Gainetdinov et al., 2004; Williams et al., 2013). While opioid agonists stimulate MOR phosphorylation, β-arrestin recruitment and receptor internalization, the signaling pathways involved can vary between MOR ligands. β-Arrestin-2-dependent signaling has been implicated in clinically important dose-limiting side effects of opioid analgesics (e.g., respiratory depression, constipation, and dependence), and biased MOR agonists that preferentially activate signaling via G-proteins over β-arrestins are currently being developed (DeWire et al., 2013; Manglik et al., 2016); and one biased MOR agonist, olinvyk (oliceridine), has recently been approved for use by the US Food and Drug Administration. While such biased opioids would be expected to extend the therapeutic window, increasing the safety of opioid analgesics (DeWire et al., 2013; Manglik et al., 2016), recent studies have raised the concern that they still produce substantial side effects (Kliewer et al., 2019; Ding et al., 2020; Kliewer et al., 2020), and we recently demonstrated that the biased MOR agonist PZM21 can induce OIH and hyperalgesic priming, mediated by a unique mechanism (Araldi et al., 2018c). Previous studies have suggested a potential role of Gαi/o proteins in diverse effects of opioids (Pasternak and Standifer, 1995; Rossi et al., 1995; Standifer et al., 1996; Hadjimarkou et al., 2002; Silva et al., 2002; Wainford and Kapusta, 2012). In the present experiments, we tested the hypothesis that two side effects of clinically used opioid analgesics, OIH and hyperalgesic priming, induced by systemically administered fentanyl and morphine, acting at the level of the spinal cord and dorsal root ganglion (DRG) neurons, are Gαi/o dependent.
While hyperalgesia induced by systemic sub-AD fentanyl was prevented by AS-ODN directed against three G-proteins, Gαi2, Gαi3, and Gαo, antisense to only one of these G-proteins, Gαi3, prevented hyperalgesic priming. In contrast, when sub-AD morphine was administered, a different set of Gαi/o proteins, Gαi1, Gαi2 and Gαi3, mediated hyperalgesia, while hyperalgesic priming was not affected by AS-ODN to any of the tested α-subunits. Thus, while OIH produced by sub-AD fentanyl and morphine share Gαi2 and Gαi3 subunit dependence, Gαo plays a role in OIH produced only by sub-AD fentanyl, and Gαi1 plays a role only in OIH produced by sub-AD morphine. In contrast, hyperalgesic priming produced by sub-AD fentanyl was prevented by Gαi3 AS-ODN, while sub-AD morphine-induced priming was not affected by any of the G-protein subunit AS-ODNs. The basis of this differential contribution of G-proteins in OIH and hyperalgesic priming induced by different opioid analgesics remains to be elucidated.
We also investigated the role of Gαi/o subunits in analgesia and hyperalgesic priming induced by ADs of fentanyl and morphine. While analgesia induced by AD morphine was attenuated by Gαi1 AS-ODN, paradoxically knockdown of Gαi1 and Gαi2 enhanced fentanyl analgesia. To the best of our knowledge, this is the first demonstration that G-protein α-subunits can have a negative impact on opioid-induced analgesia, and that the same αi/o subunit can inhibit analgesia produced by one opioid analgesic (morphine) while enhancing it to another (fentanyl).
While AD fentanyl-induced priming was prevented in Gαo AS-ODN-treated rats, none of the Gα subunits tested contributed to AD morphine-induced priming. While we do not currently have an explanation for the difference in the requirement of Gαi3 versus Gαo for priming induced by sub-AD versus AD fentanyl, respectively, or for the lack of a role of any of the tested Gα subunits in priming induced by sub-AD or AD morphine, it has been suggested that some effects of sub-AD doses of opioid analgesics may be mediated by their action at a different receptor, TLR4, on nociceptors (Araldi et al., 2019). The role of Gαi/o in TLR4 signaling is currently being examined.
Given the lack of effect of Gαi/o AS-ODNs on hyperalgesic priming induced by both sub-AD and AD morphine, it may be of interest to evaluate whether other Gα proteins contribute, such as the closely related subunit Gαz that is thought to mediate the desensitization of MORs at supraspinal sites (Garzon et al., 2005). Otherwise, with respect to the differences between the Gα subunits involved in hyperalgesia and analgesia induced by sub-AD and AD morphine, respectively, it has been suggested that the difference in the effect of sub-AD and AD morphine may be a result of a bimodal dose-dependent effect of opioid receptor agonists. Electrophysiology studies of the effects of opioids on mouse DRG neurons demonstrated that opioid receptors activate an excitatory signaling pathway when exposed to very low concentrations of opioid agonists, and an inhibitory pathway when exposed to higher concentrations (Shen and Crain, 1994). Alternatively, since none of the Gαi/o AS-ODNs prevented sub-AD morphine-induced priming, it is possible that it could be because of an effect of sub-AD morphine on nociceptor TLR4 (Araldi et al., 2019).
Adenylyl cyclase (AC; Sharma et al., 1977), as well as Ca2+ channels (Hescheler et al., 1987), G-protein-coupled inwardly rectifying K+ channel (GIRK) activation (North et al., 1987; Henry et al., 1995), PLC stimulation (Spencer et al., 1997), and MAPK activation (Fukuda et al., 1996) are inhibited by MOR agonists. Low-dose morphine, administered intrathecally, can also couple to Gαs to activate protein kinase C and L-type Ca2+ channels to induce hyperalgesia (Esmaeili-Mahani et al., 2008); however, at higher opioid concentrations, Gαi/o-coupled pathways are activated (Shen and Crain, 1990). In a recent study, we found that the application of fentanyl (0.5 nm) to DRG neurons cultured from rats pretreated with AD fentanyl induced a MOR-dependent increase in [Ca2+]i and significantly decreased action potential rheobase in weakly IB4+ and IB4– small-diameter DRG neurons (Khomula et al., 2019). Since MOR can also couple to Gαo, which links to attenuation of action potential duration (Groer et al., 2007; McPherson et al., 2010), these data corroborate our findings that hyperalgesic priming induced by systemic AD fentanyl is Gαo dependent.
While widely accepted, the classical model of G-protein signaling, cyclical GDP–GTP exchange in response to ligand binding to seven transmembrane receptors followed by dissociation of the G-protein subunit and activation of intracellular signaling cascades, has been challenged. For example, studies have shown that Gαi2 and Gαi3 can display a complex interplay in GPCR signaling. Gαi2 and Gαi3 can be activated simultaneously by a single ligand, resulting in adenylyl cyclase-mediated effects that are dependent only on Gαi2 (McClue et al., 1992), and Gαi3 can have a regulatory role, its presence inhibiting Gαi2-induced migration and GTPγS incorporation by hindering Gαi2 binding to the receptor (Thompson et al., 2007). These studies provide evidence to support the existence of a regulatory role for Gαi, independent of its effect on AC, and an interplay of Gαi proteins in transmitting G-protein-coupled receptor signals (Thompson et al., 2007). In a study of the role of T cells in “graft-versus-host” (GVH) disease, the GVH response is abolished in mice adoptively transferred with Gαi2−/− T cells but exacerbated in mice with Gαi3-deficient T cells (Jin et al., 2008). Many GPCRs are able to couple to multiple G-proteins, and different GPCR agonists can lead to signaling via different G-proteins, a phenomenon referred to as “agonist-directed trafficking” (Kenakin, 1995). For example, the dopamine D2 receptor can initiate signals via Gαi1, Gαi2, Gαi3, and Gαo1 (Gazi et al., 2003); however, ligand pharmacology can be influenced greatly by the GPCR/G-protein expression ratio (Lane et al., 2007). The D2 receptor ligand S-(—)−3-PPP shows partial agonism mediated by Gαo1 and antagonism/inverse agonism mediated by Gαi1, Gαi2, and Gαi3 in physiologically relevant end points (Arnt et al., 1983; Hjorth et al., 1983). Thus, an emerging signaling paradigm includes the capacity of one receptor to couple to and initiate signaling through multiple G-proteins and the capacity of one G-protein to activate multiple effectors (Woehler and Ponimaskin, 2009). Limitations of the use of AS-ODN to regulate protein levels in nociceptors include the following: (1) they do not completely eliminate their target protein; (2) their intrathecal administration affects cells in the spinal cord, so that additional approaches are required to specify the cells involved; and (3) their short half-life requires repeated administration.
In conclusion, the present data support the hypothesis that Gαi/Gαo signaling contributes to the side effects of clinically used opioid analgesics, providing a starting point for the design of analgesics with selectivity for individual Gαi/αo proteins, producing a more limited range of side effects. Our current findings are summarized in Table 1.
Table 1.
Gαi/o subunits mediating analgesia, hyperalgesia, and priming induced by sub-ADs and ADs of systemic fentanyl and morphine
| Fentanyl |
Morphine |
|||
|---|---|---|---|---|
| Sub-AD | AD | Sub-AD | AD | |
| OIH | ↓ Gαi2, Gαi3 and Gαo | NS | ↓ Gαi1, Gαi2 and Gαi3 | NS |
| Analgesia | NS | ↑ Gαi1 and Gαi2 | NS | ↓ Gαi1 |
| Priming | ↓ Gαi3 | ↓ Gαo | None | None |
NS, Not studied; ↑, enhances; ↓, attenuates.
Footnotes
This study was funded by the UCSF Resource Allocation Program (RAP) Pilot Award for Prevention and Treatment of Opioid Use Disorders to D.A., and the National Institutes of Health Grant NS-084545 to J.D.L. We thank Dr. Oliver Bogen for oligodeoxynucleotides, and Niloufar Mansooralavi for excellent technical assistance.
The authors declare no competing financial interests.
References
- Alessandri-Haber N, Yeh JJ, Boyd AE, Parada CA, Chen X, Reichling DB, Levine JD (2003) Hypotonicity induces TRPV4-mediated nociception in rat. Neuron 39:497–511. 10.1016/s0896-6273(03)00462-8 [DOI] [PubMed] [Google Scholar]
- Araldi D, Ferrari LF, Levine JD (2015) Repeated mu-opioid exposure induces a novel form of the hyperalgesic priming model for transition to chronic pain. J Neurosci 35:12502–12517. 10.1523/JNEUROSCI.1673-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araldi D, Ferrari LF, Levine JD (2017) Hyperalgesic priming (type II) induced by repeated opioid exposure: maintenance mechanisms. Pain 158:1204–1216. 10.1097/j.pain.0000000000000898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araldi D, Khomula EV, Ferrari LF, Levine JD (2018a) Fentanyl induces rapid onset hyperalgesic priming: type I at peripheral and type II at central nociceptor terminals. J Neurosci 38:2226–2245. 10.1523/JNEUROSCI.3476-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araldi D, Ferrari LF, Levine JD (2018b) Role of GPCR (mu-opioid)-receptor tyrosine kinase (epidermal growth factor) crosstalk in opioid-induced hyperalgesic priming (type II). Pain 159:864–875. 10.1097/j.pain.0000000000001155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araldi D, Ferrari LF, Levine JD (2018c) Mu-opioid receptor (MOR) biased agonists induce biphasic dose-dependent hyperalgesia and analgesia, and hyperalgesic priming in the rat. Neuroscience 394:60–71. 10.1016/j.neuroscience.2018.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araldi D, Bogen O, Green PG, Levine JD (2019) Role of nociceptor toll-like receptor 4 (TLR4) in opioid-induced hyperalgesia and hyperalgesic priming. J Neurosci 39:6414–6424. 10.1523/JNEUROSCI.0966-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnt J, Bøgesø KP, Christensen AV, Hyttel J, Larsen JJ, Svendsen O (1983) Dopamine receptor agonistic and antagonistic effects of 3-PPP enantiomers. Psychopharmacology (Berl) 81:199–207. 10.1007/BF00427262 [DOI] [PubMed] [Google Scholar]
- Bogen O, Alessandri-Haber N, Chu C, Gear RW, Levine JD (2012) Generation of a pain memory in the primary afferent nociceptor triggered by PKCε activation of CPEB. J Neurosci 32:2018–2026. 10.1523/JNEUROSCI.5138-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childers SR (1988) Opiate-inhibited adenylate cyclase in rat brain membranes depleted of Gs-stimulated adenylate cyclase. J Neurochem 50:543–553. 10.1111/j.1471-4159.1988.tb02945.x [DOI] [PubMed] [Google Scholar]
- Chu LF, Angst MS, Clark D (2008) Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clin J Pain 24:479–496. 10.1097/AJP.0b013e31816b2f43 [DOI] [PubMed] [Google Scholar]
- DeWire SM, Yamashita DS, Rominger DH, Liu G, Cowan CL, Graczyk TM, Chen XT, Pitis PM, Gotchev D, Yuan C, Koblish M, Lark MW, Violin JD (2013) A G protein-biased ligand at the μ-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharmacol Exp Ther 344:708–717. 10.1124/jpet.112.201616 [DOI] [PubMed] [Google Scholar]
- Ding H, Kiguchi N, Perrey DA, Nguyen T, Czoty PW, Hsu FC, Zhang Y, Ko MC (2020) Antinociceptive, reinforcing, and pruritic effects of the G-protein signalling-biased mu opioid receptor agonist PZM21 in non-human primates. Br J Anaesth 125:596–604. 10.1016/j.bja.2020.06.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmaeili-Mahani S, Shimokawa N, Javan M, Maghsoudi N, Motamedi F, Koibuchi N, Ahmadiani A (2008) Low-dose morphine induces hyperalgesia through activation of G alphas, protein kinase C, and L-type Ca 2+ channels in rats. J Neurosci Res 86:471–479. 10.1002/jnr.21489 [DOI] [PubMed] [Google Scholar]
- Ferrari LF, Khomula EV, Araldi D, Levine JD (2016) Marked sexual dimorphism in the role of the ryanodine receptor in a model of pain chronification in the rat. Sci Rep 6:31221. 10.1038/srep31221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari LF, Araldi D, Bogen O, Green PG, Levine JD (2019) Systemic morphine produces dose-dependent nociceptor-mediated biphasic changes in nociceptive threshold and neuroplasticity. Neuroscience 398:64–75. 10.1016/j.neuroscience.2018.11.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K, Kato S, Morikawa H, Shoda T, Mori K (1996) Functional coupling of the delta-, mu-, and kappa-opioid receptors to mitogen-activated protein kinase and arachidonate release in Chinese hamster ovary cells. J Neurochem 67:1309–1316. 10.1046/j.1471-4159.1996.67031309.x [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG (2004) Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci 27:107–144. 10.1146/annurev.neuro.27.070203.144206 [DOI] [PubMed] [Google Scholar]
- Garzon J, Rodriguez-Munoz M, Lopez-Fando A, Sanchez-Blazquez P (2005) The RGSZ2 protein exists in a complex with mu-opioid receptors and regulates the desensitizing capacity of Gz proteins. Neuropsychopharmacology 30:1632–1648. [DOI] [PubMed] [Google Scholar]
- Gazi L, Nickolls SA, Strange PG (2003) Functional coupling of the human dopamine D2 receptor with G alpha i1, G alpha i2, G alpha i3 and G alpha o G proteins: evidence for agonist regulation of G protein selectivity. Br J Pharmacol 138:775–786. 10.1038/sj.bjp.0705116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis A, Kliewer A, Kelly E, Henderson G, Christie MJ, Schulz S, Canals M (2020) Critical assessment of G protein-biased agonism at the μ-opioid receptor. Trends Pharmacol Sci 41:947–959. 10.1016/j.tips.2020.09.009 [DOI] [PubMed] [Google Scholar]
- Groer CE, Tidgewell K, Moyer RA, Harding WW, Rothman RB, Prisinzano TE, Bohn LM (2007) An opioid agonist that does not induce mu-opioid receptor–arrestin interactions or receptor internalization. Mol Pharmacol 71:549–557. 10.1124/mol.106.028258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjimarkou MM, Silva RM, Rossi GC, Pasternak GW, Bodnar RJ (2002) Feeding induced by food deprivation is differentially reduced by G-protein alpha-subunit antisense probes in rats. Brain Res 955:45–54. 10.1016/s0006-8993(02)03361-9 [DOI] [PubMed] [Google Scholar]
- Hauser AS, Attwood MM, Rask-Andersen M, Schiöth HB, Gloriam DE (2017) Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov 16:829–842. 10.1038/nrd.2017.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry DJ, Grandy DK, Lester HA, Davidson N, Chavkin C (1995) Kappa-opioid receptors couple to inwardly rectifying potassium channels when coexpressed by Xenopus oocytes. Mol Pharmacol 47:551–557. [PubMed] [Google Scholar]
- Hescheler J, Rosenthal W, Trautwein W, Schultz G (1987) The GTP-binding protein, Go, regulates neuronal calcium channels. Nature 325:445–447. 10.1038/325445a0 [DOI] [PubMed] [Google Scholar]
- Hill R, Disney A, Conibear A, Sutcliffe K, Dewey W, Husbands S, Bailey C, Kelly E, Henderson G (2018) The novel μ-opioid receptor agonist PZM21 depresses respiration and induces tolerance to antinociception. Br J Pharmacol 175:2653–2661. 10.1111/bph.14224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjorth S, Carlsson A, Clark D, Svensson K, Wikström H, Sanchez D, Lindberg P, Hacksell U, Arvidsson LE, Johansson A (1983) Central dopamine receptor agonist and antagonist actions of the enantiomers of 3-PPP. Psychopharmacology (Berl) 81:89–99. 10.1007/BF00428999 [DOI] [PubMed] [Google Scholar]
- Jin YZ, Thompson BD, Zhou ZY, Fu Y, Birnbaumer L, Wu MX (2008) Reciprocal function of Galphai2 and Galphai3 in graft-versus-host disease. Eur J Immunol 38:1988–1998. 10.1002/eji.200737738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EK, Reichling DB, Levine JD (2010) Shared mechanisms for opioid tolerance and a transition to chronic pain. J Neurosci 30:4660–4666. 10.1523/JNEUROSCI.5530-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T (1995) Agonist-receptor efficacy. II. Agonist trafficking of receptor signals. Trends Pharmacol Sci 16:232–238. 10.1016/s0165-6147(00)89032-x [DOI] [PubMed] [Google Scholar]
- Khomula EV, Araldi D, Levine JD (2019) In vitro nociceptor neuroplasticity associated with in vivo opioid-induced hyperalgesia. J Neurosci 39:7061–7073. 10.1523/JNEUROSCI.1191-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khomula EV, Araldi D, Bonet IJM, Levine JD (2021) Opioid-induced hyperalgesic priming in single nociceptors. J Neurosci 41:31–46. 10.1523/JNEUROSCI.2160-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer A, Schmiedel F, Sianati S, Bailey A, Bateman JT, Levitt ES, Williams JT, Christie MJ, Schulz S (2019) Phosphorylation-deficient G-protein-biased μ-opioid receptors improve analgesia and diminish tolerance but worsen opioid side effects. Nat Commun 10:367. 10.1038/s41467-018-08162-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer A, Gillis A, Hill R, Schmiedel F, Bailey C, Kelly E, Henderson G, Christie MJ, Schulz S (2020) Morphine-induced respiratory depression is independent of β-arrestin2 signalling. Br J Pharmacol 177:2923–2931. 10.1111/bph.15004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane JR, Powney B, Wise A, Rees S, Milligan G (2007) Protean agonism at the dopamine D2 receptor: (S)-3-(3-hydroxyphenyl)-N-propylpiperidine is an agonist for activation of Go1 but an antagonist/inverse agonist for Gi1, Gi2, and Gi3. Mol Pharmacol 71:1349–1359. 10.1124/mol.106.032722 [DOI] [PubMed] [Google Scholar]
- Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L (2011) A comprehensive review of opioid-induced hyperalgesia. Pain Physician 14:145–161. [PubMed] [Google Scholar]
- Leurs R, Bakker RA, Timmerman H, de Esch IJ (2005) The histamine H3 receptor: from gene cloning to H3 receptor drugs. Nat Rev Drug Discov 4:107–120. 10.1038/nrd1631 [DOI] [PubMed] [Google Scholar]
- Manglik A, Lin H, Aryal DK, McCorvy JD, Dengler D, Corder G, Levit A, Kling RC, Bernat V, Hübner H, Huang XP, Sassano MF, Giguère PM, Löber S, Da D, Scherrer G, Kobilka BK, Gmeiner P, Roth BL, Shoichet BK (2016) Structure-based discovery of opioid analgesics with reduced side effects. Nature 537:185–190. 10.1038/nature19112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClue SJ, Selzer E, Freissmuth M, Milligan G (1992) Gi3 does not contribute to the inhibition of adenylate cyclase when stimulation of an α2-adrenergic receptor causes activation of both Gi2 and Gi3. Biochem J 284:565–568. 10.1042/bj2840565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson J, Rivero G, Baptist M, Llorente J, Al-Sabah S, Krasel C, Dewey WL, Bailey CP, Rosethorne EM, Charlton SJ, Henderson G, Kelly E (2010) μ-opioid receptors: correlation of agonist efficacy for signalling with ability to activate internalization. Mol Pharmacol 78:756–766. 10.1124/mol.110.066613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestre C, Pélissier T, Fialip J, Wilcox G, Eschalier A (1994) A method to perform direct transcutaneous intrathecal injection in rats. J Pharmacol Toxicol Methods 32:197–200. 10.1016/1056-8719(94)90087-6 [DOI] [PubMed] [Google Scholar]
- North RA, Williams JT, Surprenant A, Christie MJ (1987) Mu and delta receptors belong to a family of receptors that are coupled to potassium channels. Proc Natl Acad Sci U S A 84:5487–5491. 10.1073/pnas.84.15.5487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira-Fusaro MCG, Zanoni CIS, Dos Santos GG, Manzo LP, Araldi D, Bonet IJM, Tambeli CH, Dias EV, Parada CA (2017) Antihyperalgesic effect of CB1 receptor activation involves the modulation of P2X3 receptor in the primary afferent neuron. Eur J Pharmacol 798:113–121. 10.1016/j.ejphar.2017.01.030 [DOI] [PubMed] [Google Scholar]
- Olson KM, Lei W, Keresztes A, LaVigne J, Streicher JM (2017) Novel molecular strategies and targets for opioid drug discovery for the treatment of chronic pain. Yale J Biol Med 90:97–110. [PMC free article] [PubMed] [Google Scholar]
- Pagliusi M Jr, Bonet IJM, Lemes JBP, Oliveira ALL, Carvalho NS, Tambeli CH, Parada CA, Sartori CR (2020) Social defeat stress-induced hyperalgesia is mediated by nav 1.8(+) nociceptive fibers. Neurosci Lett 729:135006. 10.1016/j.neulet.2020.135006 [DOI] [PubMed] [Google Scholar]
- Pasternak GW, Standifer KM (1995) Mapping of opioid receptors using antisense oligodeoxynucleotides: correlating their molecular biology and pharmacology. Trends Pharmacol Sci 16:344–350. 10.1016/s0165-6147(00)89068-9 [DOI] [PubMed] [Google Scholar]
- Quanhong Z, Ying X, Moxi C, Tao X, Jing W, Xin Z, Li W, Derong C, Xiaoli Z, Wei J (2012) Intrathecal PLC(β3) oligodeoxynucleotides antisense potentiates acute morphine efficacy and attenuates chronic morphine tolerance. Brain Res 1472:38–44. 10.1016/j.brainres.2012.06.030 [DOI] [PubMed] [Google Scholar]
- Raehal KM, Bohn LM (2014) β-arrestins: regulatory role and therapeutic potential in opioid and cannabinoid receptor-mediated analgesia. Handb Exp Pharmacol 219:427–443. 10.1007/978-3-642-41199-1_22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisine T, Bell GI (1993) Molecular biology of opioid receptors. Trends Neurosci 16:506–510. 10.1016/0166-2236(93)90194-q [DOI] [PubMed] [Google Scholar]
- Roeckel LA, Le Coz GM, Gavériaux-Ruff C, Simonin F (2016) Opioid-induced hyperalgesia: cellular and molecular mechanisms. Neuroscience 338:160–182. 10.1016/j.neuroscience.2016.06.029 [DOI] [PubMed] [Google Scholar]
- Rossi GC, Standifer KM, Pasternak GW (1995) Differential blockade of morphine and morphine-6 beta-glucuronide analgesia by antisense oligodeoxynucleotides directed against MOR-1 and G-protein alpha subunits in rats. Neurosci Lett 198:99–102. 10.1016/0304-3940(95)11977-5 [DOI] [PubMed] [Google Scholar]
- Saidak Z, Blake-Palmer K, Hay DL, Northup JK, Glass M (2006) Differential activation of G-proteins by mu-opioid receptor agonists. Br J Pharmacol 147:671–680. 10.1038/sj.bjp.0706661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SK, Klee WA, Nirenberg M (1977) Opiate-dependent modulation of adenylate cyclase. Proc Natl Acad Sci U S A 74:3365–3369. 10.1073/pnas.74.8.3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen KF, Crain SM (1990) Cholera toxin-B subunit blocks excitatory effects of opioids on sensory neuron action potentials indicating that GM1 ganglioside may regulate Gs-linked opioid receptor functions. Brain Res 531:1–7. 10.1016/0006-8993(90)90751-v [DOI] [PubMed] [Google Scholar]
- Shen KF, Crain SM (1994) Antagonists at excitatory opioid receptors on sensory neurons in culture increase potency and specificity of opiate analgesics and attenuate development of tolerance/dependence. Brain Res 636:286–297. 10.1016/0006-8993(94)91028-6 [DOI] [PubMed] [Google Scholar]
- Silva RM, Grossman HC, Rossi GC, Pasternak GW, Bodnar RJ (2002) Pharmacological characterization of β-endorphin- and dynorphin A(1-17)-induced feeding using G-protein α-subunit antisense probes in rats. Peptides 23:1101–1106. 10.1016/S0196-9781(02)00036-0 [DOI] [PubMed] [Google Scholar]
- Song MJ, Wang YQ, Wu GC (2009) Additive anti-hyperalgesia of electroacupuncture and intrathecal antisense oligodeoxynucleotide to interleukin-1 receptor type I on carrageenan-induced inflammatory pain in rats. Brain Res Bull 78:335–341. 10.1016/j.brainresbull.2008.10.009 [DOI] [PubMed] [Google Scholar]
- Spencer RJ, Jin W, Thayer SA, Chakrabarti S, Law PY, Loh HH (1997) Mobilization of Ca2+ from intracellular stores in transfected neuro2a cells by activation of multiple opioid receptor subtypes. Biochem Pharmacol 54:809–818. 10.1016/s0006-2952(97)00243-8 [DOI] [PubMed] [Google Scholar]
- Standifer KM, Pasternak GW (1997) G proteins and opioid receptor-mediated signalling. Cell Signal 9:237–248. 10.1016/s0898-6568(96)00174-x [DOI] [PubMed] [Google Scholar]
- Standifer KM, Rossi GC, Pasternak GW (1996) Differential blockade of opioid analgesia by antisense oligodeoxynucleotides directed against various G protein alpha subunits. Mol Pharmacol 50:293–298. [PubMed] [Google Scholar]
- Su L, Wang C, Yu YH, Ren YY, Xie KL, Wang GL (2011) Role of TRPM8 in dorsal root ganglion in nerve injury-induced chronic pain. BMC Neurosci 12:120. 10.1186/1471-2202-12-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JL, Xiao C, Lu B, Zhang J, Yuan XZ, Chen W, Yu LN, Zhang FJ, Chen G, Yan M (2013) CX3CL1/CX3CR1 regulates nerve injury-induced pain hypersensitivity through the ERK5 signaling pathway. J Neurosci Res 91:545–553. 10.1002/jnr.23168 [DOI] [PubMed] [Google Scholar]
- Taiwo YO, Levine JD (1989) Prostaglandin effects after elimination of indirect hyperalgesic mechanisms in the skin of the rat. Brain Res 492:397–399. 10.1016/0006-8993(89)90928-1 [DOI] [PubMed] [Google Scholar]
- Taiwo YO, Bjerknes LK, Goetzl EJ, Levine JD (1989) Mediation of primary afferent peripheral hyperalgesia by the cAMP second messenger system. Neuroscience 32:577–580. 10.1016/0306-4522(89)90280-7 [DOI] [PubMed] [Google Scholar]
- Thompson BD, Jin Y, Wu KH, Colvin RA, Luster AD, Birnbaumer L, Wu MX (2007) Inhibition of G alpha i2 activation by G alpha i3 in CXCR3-mediated signaling. J Biol Chem 282:9547–9555. 10.1074/jbc.M610931200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trang T, Al-Hasani R, Salvemini D, Salter MW, Gutstein H, Cahill CM (2015) Pain and poppies: the good, the bad, and the ugly of opioid analgesics. J Neurosci 35:13879–13888. 10.1523/JNEUROSCI.2711-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl GR, Childers S, Pasternak G (1994) An opiate-receptor gene family reunion. Trends Neurosci 17:89–93. 10.1016/0166-2236(94)90110-4 [DOI] [PubMed] [Google Scholar]
- Viscusi ER, Webster L, Kuss M, Daniels S, Bolognese JA, Zuckerman S, Soergel DG, Subach RA, Cook E, Skobieranda F (2016) A randomized, phase 2 study investigating TRV130, a biased ligand of the μ-opioid receptor, for the intravenous treatment of acute pain. Pain 157:264–272. 10.1097/j.pain.0000000000000363 [DOI] [PubMed] [Google Scholar]
- Wainford RD, Kapusta DR (2012) Functional selectivity of central Gα-subunit proteins in mediating the cardiovascular and renal excretory responses evoked by central α(2) -adrenoceptor activation in vivo. Br J Pharmacol 166:210–220. 10.1111/j.1476-5381.2011.01662.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JT, Ingram SL, Henderson G, Chavkin C, von Zastrow M, Schulz S, Koch T, Evans CJ, Christie MJ (2013) Regulation of μ-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev 65:223–254. 10.1124/pr.112.005942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woehler A, Ponimaskin EG (2009) G protein–mediated signaling: same receptor, multiple effectors. Curr Mol Pharmacol 2:237–248. 10.2174/1874467210902030237 [DOI] [PubMed] [Google Scholar]