Abstract
Sex-based differences in cardiovascular disease (CVD) presentation, diagnosis, and response to therapies are well established, but mechanistic understanding and translation to clinical applications are limited. Blood-based biomarkers have become an important tool for interrogating biologic pathways. Understanding sexual dimorphism in the relationship between biomarkers and CVD will enhance our insights into CVD pathogenesis in women, with potential to translate to improved individualized care for men and women with or at risk for CVD. In this review, we examine how biologic sex associates with differential levels of blood-based biomarkers and influences the effect of biomarkers on disease outcomes. We further summarize key differences in blood-based cardiovascular biomarkers along central biologic pathways, including myocardial stretch/injury, inflammation, adipose tissue metabolism, and fibrosis pathways in men vs women. Finally, we present recommendations for leveraging our current knowledge of sex differences in blood-based biomarkers for future research and clinical innovation.
Keywords: Biomarkers, Cardiovascular Disease, Women, Sex, Gender
INTRODUCTION
Sex-related differences in cardiovascular disease (CVD) risk, pathophysiology, disease presentation, response to therapy, and prognosis have been well characterized, but mechanistic understanding and implications for clinical practice are incompletely understood.1 This is in part due to limitations of existing tools to interrogate biological pathways in men vs women. CV biomarkers offer an opportunity to expand our insights into these sex-based differences in CVD, which may translate to improved risk prediction, prognostication, and therapeutic options uniquely tailored to men and women. In this review, we use the term sex to represent biologic sex assigned at birth and reference sex as a binary variable (male/men vs female/women).2 As most clinical studies relied on self-identified gender as a proxy for biologic sex, we use the terms male/female and men/women interchangeably. We acknowledge that this approach does not capture the complex interplay between sex and gender.
What is a biomarker?
A biomarker (biological marker), as defined by the National Institutes of Health, refers to “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.”3 While the term ‘biomarker’ has become synonymous with laboratory measured markers (blood-based biomarkers), biomarkers in fact may range from physical signs (e.g. pulse and blood pressure) to more complex readouts such as imaging findings. In this review, we focus specifically on blood-based biomarkers, recognizing the complementary insights provided by other CV biomarkers.
In cardiology clinical practice, blood-based biomarkers may be useful for disease screening, diagnosis, and risk prediction among individuals without CVD, as well as prognostication among individuals with established CVD.4 Given the significant influence of biologic sex on biomarker levels and clinical manifestations of CVD, there is a major unmet need to translate sex-specific biology to clinical implications and eventual implementation into practice. For example, sex-specific normative values and biomarker cut-points have been studied but are not yet widely adopted in practice. Beyond clinical application, blood-based biomarkers may also offer important biological insights into disease mechanisms. With the introduction of high throughput large-scale -omic platforms for biomarker discovery (e.g. proximity extension assay and modified aptamer proteomics, next-generation DNA sequencing among others) that are able to interrogate fundamental biology from genome, transcriptome, proteome, and metabolome to disease phenotype, we are poised to leverage high-dimensional biomarker readouts to better understand the underlying biologic basis of sex differences across CVD.4 Sex differences can arise at any step along the relationship of genome, transcriptome, proteome, metabolome, and disease phenotype resulting from X-chromosome and sex-specific gene expression and transcriptional regulation, sexual dimorphism in circulating biomarkers, interaction networks, and system biology, as well as sex hormones (Figure 1). In this review, we will examine how sex influences blood-based biomarkers, review sources of sex differences in biomarker biology, and discuss potential clinical implications (Figure 2). Specifically, we will highlight representative biomarkers of key pathways related to CVD and summarize established sex differences and examine potential clinical and research implications for each selected biomarker. Finally, we will identify important future areas of investigation to address current knowledge gaps around sex differences in CVD and offer opportunities for blood-based biomarkers to inform and more precisely guide clinical care for men and women with CVD.
Figure 1.
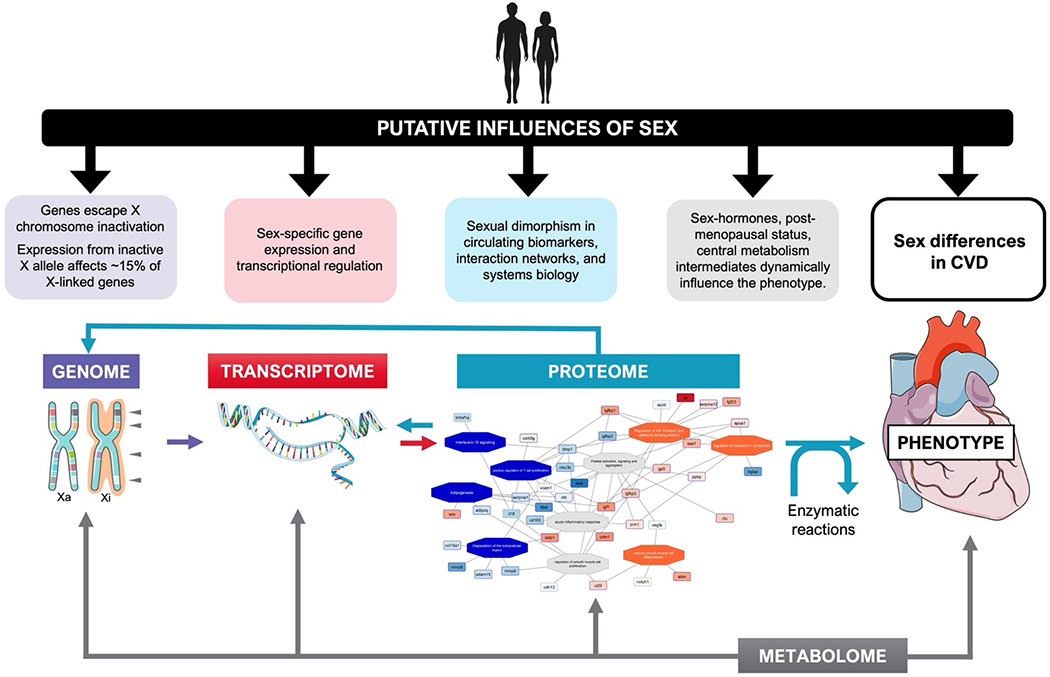
Sex differences can arise at multiple steps along the relationship of genome, transcriptome, proteome, metabolome, and disease phenotype. Biomarkers measured at each level may elucidate sex differences in disease pathogenesis that contribute to unique disease phenotypes in men vs women.
Figure 2.
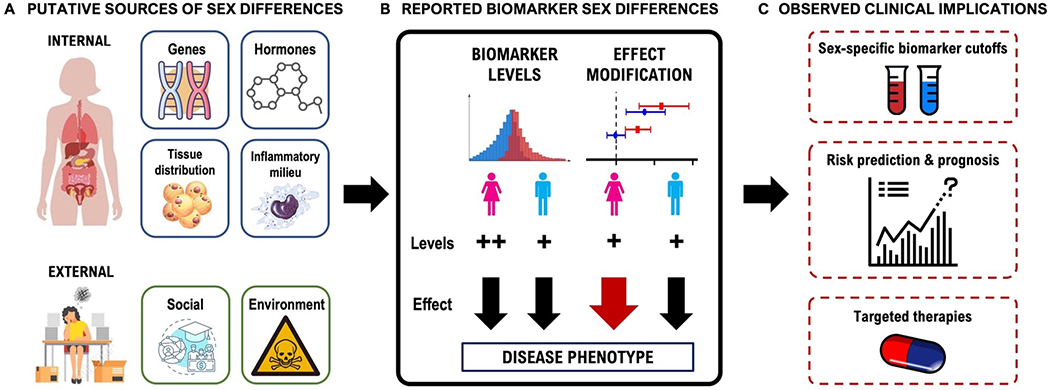
Sex differences in blood-based biomarkers and potential clinical implications. (A) Biologic determinants of sex differences in blood-based biomarkers can be broadly classified into internal and external factors. Internal factors that contribute to sex differences in biomarkers include genes and sex chromosomes, sex hormones, tissue distribution, and inflammatory milieu. External factors include social/cultural and environmental factors. (B) Sex differences in biomarkers can manifest in two ways. First, a given biomarker may portend a similar risk of CVD in men and women, yet inherent differences in circulating baseline levels of biomarkers in men vs women may contribute to sex differences in disease risk. Second, sex may modify the effect of a biomarker on a disease outcome. In other words, the same biomarker increment may portend a differential risk in men vs women, resulting in sex differences in disease. (C) The clinical applications of sex-based differences in blood-based biomarkers remain unclear, but sex differences can translate to sex-specific biomarker cutoffs for improved diagnosis of CVD, improved risk prediction and prognosis for men and women, and sex-specific targeted therapies.
Biomarkers as predictors of CVD: Why and how does sex matter?
Recognition of sex-related differences in CVD has motivated an interest in understanding sex-based differences in blood-based biomarkers. Biologic sex can influence blood-based biomarkers in two ways (Figure 2, Panel B). First, plasma concentrations of blood-based biomarkers may differ in men vs women.5 For example, cardiac natriuretic peptides (NP) are approximately two times higher in women compared with men in the general population.6, 7 Of note, these differences necessitate well-conducted studies that adjust for confounders of the relationship between sex and biomarker. Second, and less well characterized, sex can modify the effect of a given biomarker on a disease outcome. In 30,443 individuals from four community-based European studies, amino-terminal-peptide N terminal-pro BNP (NT-pro BNP) was more strongly associated with incident heart failure (HF) in men compared with women.8 Finally, both effects may be at play. Most sex-specific investigations have focused on differences in plasma concentrations of blood-based biomarkers in men vs women, but further characterization of how sex interacts with biomarkers and disease phenotype is needed to fully understand the impact of biologic sex on CVD pathogenesis.
The biological underpinnings that drive whether a biomarker demonstrates sex differences are complex. Any given biomarker has its own unique combination of sex-specific factors that dictate its regulation, which makes a global summary of driving factors for sex differences in biomarkers challenging. However, there are broad categories of physiological drivers that can be considered when gleaning a stronger understanding of sex differences in any given biomarker (Figure 2, Panel A), which include:
Differences in sex chromosome complement: Sex differences in blood-based biomarkers are influenced first and foremost by sex differences in genetic make-up and gene expression. Historically, some biologists have downplayed potential gene dose differences by leaning on the dogma that X inactivation of one allele of all X chromosome genes in females is complete and consistent across all tissues and across the lifespan.9 Mounting evidence indicates that this assumption is flawed, and we are only beginning to understand how escape from X inactivation affects female gene expression in different tissues and physiological contexts, including the CV system.10–12 For example, incomplete X-chromosome inactivation has been implicated in sex-biased expression of endothelial inflammatory genes in women with HF with preserved ejection fraction.13
Levels of and/or fluctuations in gonadal hormones: Gonadal hormones, including estrogen and progesterone in women and testosterone in men, promote pleiotropic effects across tissues distal to the reproductive system, including potent effects influencing cardiac and vascular physiology.14, 15 Furthermore, other sex related hormones such as oxytocin, luteinizing hormone (LH), and follicle stimulating hormone (FSH) may also exert sex-biased effects on cardiovascular function.16–18
Differences in body size and body composition: Differences in body size as well as adipose tissue composition and distribution between men and women influences sex-differences in blood-based biomarkers. For example, greater subcutaneous adiposity and lower visceral adiposity, and lower muscle mass in women vs men may translate to important sex differences in biomarkers as visceral and subcutaneous adipose tissue are associated with unique metabolic biomarker profiles.19
Inflammatory milieu: Existing data suggest that female sex strongly modulates immune responses.20, 21 Women incur ~80% of incident autoimmune disease in the US and mount strong innate immune responses which may influence responses to select infectious diseases.22, 23 Greater systemic inflammation in women has therefore been implicated in driving sex differences in CVD risk, pathogenesis, and disease manifestations.24, 25
Sociocultural contributions: Behaviors elicited by gendered norms manifested within different cultural contexts including family dynamics and social networks strongly contribute to biomarker biology and are of critical importance. For example, socioeconomic factors including educational attainment, socioeconomic status, and occupation have all been associated with CV biomarkers.26 Detailed discussion of these factors is beyond the scope of this review.
Taken together, a thorough review of each of these biological drivers will translate to a better understanding of whether a biomarker will be equally informative between men and women and under what assumptions we should evaluate its performance. While simple rubrics for risk stratification and diagnostics are attractive, biomarker biology is highly complex and oversimplification of biomarker utility and performance between sexes can contribute to misinterpretation and misapplication.27–29 Acknowledging this complex interplay, we sought to highlight important biological pathways that exhibit sexual dimorphism including myocyte injury/stretch, inflammatory, adipose tissue metabolism, and fibrosis pathways. Given the breadth of available biomarkers, we further selected specific biomarkers as case examples for discussion (Table 1).
Table 1.
Sex differences in key established cardiovascular biomarkers of myocardial injury/stretch, inflammation, adipose, and fibrosis pathways
| Biomarkers | Source of sex differences | Circulating levels | Effect of sex on biomarker-CVD association | |
|---|---|---|---|---|
| Myocardial injury/stretch | Natriuretic peptides | ● Sex hormones: E2 ↑ NP, T ↓ NP ● Gynoid fat deposits correlated with ↑ NP |
General population: women > men HF: women = men |
Prediction of incident HF: mixed Prognostic value: limited data |
| Cardiac troponins | ● Greater heart size and cardiomyocyte volume in men ● Sex hormones: T induces/E2 suppresses cardiomyocyte hypertrophy/apoptosis ● Higher prevalence of obstructive CAD in men |
General population: men > women CAD, ACS, and HF: men > women |
Prediction of CV events: mixed Prognostic value: mixed |
|
| Inflammation | C-reactive protein | ● Many immune-response related genes are X-linked ● Mixed data on sex hormones and adipose tissue |
General population: women > men MetS, stable angina, MI: women > men |
No influence of sex on predictive or prognostic abilities |
| Adipose Tissue Metabolism | Leptin | ● Greater percentage of body fat in women | General population: women > men | Leptin sensitivity may be greater in women > men |
| Adiponectin (Total and HMW) | ● More subcutaneous fat in women | General population: women > men | Unknown | |
| Fibrosis | Galectin-3 | ● Greater adipose tissue mass in women | General population: women > men HF: mixed |
Prediction of incident HF: mixed Prognostic value: unknown |
| Soluble ST2 | ● Unknown, potential role of sex hormones and obesity | General population: men > women HF: men > women |
Unknown |
Abbreviations: ACS = acute coronary syndrome, CAD = coronary artery disease, E2 = estradiol, HF = heart failure, MetS = metabolic syndrome, MI = myocardial infarction, NP = natriuretic peptide, T = testosterone.
TRADITIONAL CV BIOMARKERS
Sex differences in biomarkers are best established for traditional CV biomarkers that are routinely used in clinical practice, including NPs and cardiac troponins (cTn consisting of cardiac troponin I [cTnI] or troponin T [cTnT]).30 Both sets of biomarkers exhibit significant sex differences in circulating plasma concentrations and their interactions with disease phenotype are modified by biologic sex. Such knowledge has not yet been translated to sex-specific clinical applications in the use of NP’s and Tn to guide prediction, diagnosis, or treatment of CV disease.
Natriuretic Peptides
NPs, a group of neurohormones secreted by the myocardium in response to stretch and hypoxia stimuli to maintain volume and arterial pressure homeostasis, are widely used in contemporary clinical practice to guide diagnosis and prognosis of HF. There are two forms of NPs found in the myocardium: atrial NPs (ANP) and BNP. BNP is produced as a prohormone and selectively proteolyzed to form the biologically active BNP and pro-BNP. The available clinical assays include BNP and the amino-terminal-peptide of pro-BNP (NT-proBNP).31, 32 Sex is known to impact circulating concentrations of both BNP and NT-proBNP.33 Specifically in healthy populations, baseline levels of circulating NPs are higher in women compared with men.33 The sex differences observed in healthy populations, however, are less pronounced among patients diagnosed with HF. The differential prevalence of HF phenotype (HF with preserved ejection fraction [HFpEF] vs HF with reduced ejection fraction [HFrEF]) may explain this phenomenon.7, 30 Specifically, because NP levels are lower in patients with HFpEF vs HFrEF, similar NP concentrations between men and women may reflect greater prevalence of HFpEF among women. However, a study of 9847 outpatients (6733 men and 3114 women) with chronic HF in the Swedish HF Registry showed higher median NT-pro BNP levels across the left ventricular ejection fraction spectrum (median NT-pro BNP (inter-quartile range [IQR]), HFpEF: 1598 (709-3186) ng/L in women vs 1310 (536, 2771) ng/L in men; HFmrEF 1764 (670-3640) ng/L in women vs 1464 (640, 3173) ng/L in men; HFrEF: 2543 (1100, 5520) ng/L in women vs 2226 (1003, 4650) ng/L in men).34 Alternatively, in disease states, the overwhelming activation of NP production may effectively eliminate any contribution of sex on circulating NP levels.
Sex hormone profiles appear to be a significant driver of sex differences in circulating levels of NPs. In women, higher circulating testosterone and lower estradiol levels are associated with lower circulating proBNP levels.35 For example, women with polycystic ovarian syndrome (PCOS) with biochemical hyperandrogenism exhibit reduced NT-pro BNP levels compared with women with PCOS without hyperandrogenism (median [IQR]: 34.6 (20.0-46.7) pg/mL vs 39.8 (25.3-63.3) pg/mL).36 An independent effect of estrogen on elevated NP levels in women has also been implicated, given evidence supporting an increase in circulating BNPs following initiation of hormone replacement therapy in postmenopausal women.37 Separately, the association of body fat distribution with proBNP levels has been well established, with more female ‘gynoid’ fat deposits positively correlated with proBNP levels38 Separating influences of fat deposition from influences of sex hormones on circulating NP levels is challenging. There are minimal data indicating an overt role for sex chromosome influences on proBNP levels. However, at least one gene located on the X Chromosome, SHOX, is a known regulator of BNP expression in chondrocytes.39 Whether this or other X Chromosome factors participate in BNP regulation in the cardiomyocyte is unknown. Overall, hormone status is a major regulator of circulating proBNP levels in healthy adults and warrants careful attention in terms of reference ranges and utility of this classical biomarker for CVD applications.
NPs have been shown to aid in risk prediction of future CVD and prognostication, but data on whether sex modifies the utility of NPs for risk prediction and prognostication are conflicting. For example, in a prospective study of 78,657 healthy participants (38,001 men and 40,656 women) from the BiomarCaRE consortium, the association of NT-pro BNP with incident HF was more pronounced in men vs women (hazard ratio [HR] 1.89, 95% confidence interval [CI] [1.75-2.05] in men vs HR 1.54, 95% CI [1.37-1.74] in women, pint=0.006).8 By contrast, in a prospective study of 22,756 participants enrolled in four community-based cohorts, NPs were strongly and similarly associated with incident HF in both men and women.40 Sex-specific data on association of NPs with outcomes are limited, but one study found that NT-pro BNP levels was a predictor of long-term clinical events including mortality and HF readmissions in men but not women (HR for composite event, highest tertile vs lowest tertile: 1.74, 95% CI 1.25-2.43 in men vs HR 1.17, 95% CI 0.87-1.56 in women).41
In clinical practice, NPs are primarily used to help diagnose help diagnose HF and grade HF severity.32, 42 Recognizing differences in NP levels among the general vs HF populations, universal cutoffs have been proposed separately for ambulatory and hospitalized/decompensated patients. Despite robust evidence supporting the impact of biologic sex on cardiac NP levels in the general population, these NP cutoffs are not sex-specific.42 Many have advocated for the adoption of sex-specific NP cutoffs, particularly in the ambulatory setting, but how unique NP thresholds for men vs women will inform future clinical practice guidelines remains to be seen.
Cardiac Troponins
cTns including cTnI and cTnT are regulatory proteins involved in the calcium-mediated interaction between actin and myosin that is integral to myocardial contraction. Elevations in cTn, even mild elevations, indicate myocardial damage. Sex differences in plasma concentrations of cTn are well described but incompletely understood. In the general population, circulating levels of cTn are consistently higher in men compared with women across all age groups (Roche Diagnostics standard (fourth generation) cTnT assay [pooled median value ± standard deviation (SD)]: 5.5 ± 2.2 ng/L in men vs 3.6 ± 1.3 ng/L in women, limit of detection [LoD]: 0.01 ng/L; Abbott cTnI assay: 2.6 ± 1.1 ng/L in men vs 1.8 ± 1.0 ng/L in women, LOD: 1.7 ng/L).30, 43–46 This is also true for disease states including stable coronary artery disease (CAD)47, acute coronary syndromes (ACS), and acute and chronic HF. For example, in 1865 patients presenting with unstable angina and non-ST elevation myocardial infarction (NSTEMI) from the TACTICS-TIMI 18 study, men were more likely to have elevated cTns compared with women even after adjustment for baseline comorbidities.48
Numerous mechanisms have been proposed to explain these sex-related differences in cTns. The majority of studies point to differences in heart size and cardiomyocyte volume to explain higher cTn levels in men.49 However, hormonal milieu is believed to play a role as well, as testosterone has been shown to induce myocardial hypertrophy and cardiomyocyte apoptosis, while estrogen suppresses cardiomyocyte damage.49–52 Furthermore, among patients presenting with ACS, men are more likely to have evidence of obstructive CAD or more severe atherosclerosis at time of coronary angiography, while women have a higher prevalence of coronary microvascular dysfunction and nonobstructive coronary disease.32, 53 Tendency toward macroscopic coronary plaque translates to higher cTn in men because myocardial injury in the setting of epicardial coronary disease is often more severe. Finally, sex differences in myocardial response to ischemia and reperfusion have also been implicated; in a small study of 17 age-matched men and women undergoing cardiac surgery, cTn release was markedly higher in men vs women despite similar duration of cardiopulmonary bypass and aortic cross-clamp times.54
cTn has important value in prediction and prognostication of CV events, including MI and HF. Whether the predictive value of cTns is influenced by sex is less evident as the available data are conflicting. For example, in 19,501 healthy participants enrolled in the Generation Scotland Scottish Family Health Study, cTn concentrations were more strongly predictive of cardiovascular events in women vs men (HR cTnI of 10 ng/L relative to limit of blank [LoB], the highest apparent analyte concentration expected to be detected when a blank sample without actual analyte is assayed: HR 9.7 in women vs HR 5.6 in men; HR high sensitivity cTnT (hs-cTnT) of 10 ng/L relative to LoB: HR 3.7 in women vs HR 2.2 in men).55 Other studies have found that the predictive value of cTns for incident CV events was comparable in men vs women. The data for the prognostic value of cTns is similarly mixed. In 5626 patients with stable CAD, preprocedural hs-cTnT was a strong predictor of mortality in both men and women, but the effect was particularly pronounced in men (HR 6.45, 95% CI 4.68-8.87 in men vs HR 4.29, 95% CI 2.36-9.03 in women).56 This was seen again in a prospective study of patients with HFpEF, where cTnI was more strongly associated with adverse events in men vs women.57 These findings were in contrast to other studies that demonstrated similar associations of cTnT concentration with adverse outcomes in men vs women.58
Acknowledgement of the role of sex in influencing reference levels of cTns has led to the adoption of sex-specific reference levels and cutoffs in clinical practice. Sex-specific 99th percentiles for the 5th generation high sensitivity-cTnT assay (hs-cTnT) assay have been reported (20 ng/L for men and 13 ng/L for women).59 Moreover, the 4th Universal Definition of Myocardial Infarction (MI) incorporated sex-specific thresholds for the diagnosis of MI in clinical practice.60 This guideline update comes after consistent evidence supporting improved risk stratification in patients with ACS using sex-specific thresholds,49, 61 and is particularly important in the context of ACS guidelines that recommend a conservative treatment strategy for low-risk women presenting with chest pain in the absence of positive biomarkers of myocardial injury.62 Despite the potential benefit of adopting sex-specific thresholds for MI diagnosis, the real-world benefits have been less clear.63 While several analyses have found better reclassification of MI (with increase in MI diagnoses in women), reclassification did not translate to improved outcomes for women perhaps owing to persistent disparities in the management of men and women presenting with MI.63
INFLAMMATORY PATHWAYS
The contribution of inflammation to promoting CVD has been firmly established; concomitantly, sex-specific activation of immune and inflammatory pathways has been implicated as an important driver of sex differences in CVD. Female sex strongly modulates immune responses as reflected by higher incidence of autoimmunity among women vs men and lower susceptibility to most infectious diseases.22, 64 Immune pathway biomarkers reflect this sexual dimorphism as demonstrated by overexpression of select inflammatory biomarkers in women as compared with men in several large-scale proteomic studies. For example, in an analysis of 71 circulating CVD biomarkers in 7184 healthy participants from the Framingham Heart Study (FHS), CVD biomarkers preferentially expressed in woman were enriched for those involved in inflammation-related pathways including C-reactive protein (CRP), hemopexin, and C2.5 A complementary analysis of 3439 healthy individuals from the Dallas Heart Study (DHS) found significant sex-based differences in biomarkers of inflammation including higher levels of high sensitivity CRP (hsCRP), D-dimer, and osteoprotegerin and lower levels of interleukin-18 and lipoprotein phospholipase A2 among women.65
Genetic differences, specifically X-linked genes, are thought to play a significant role in sexual dimorphism observed in expression of inflammatory biomarkers. The human X chromosome includes a significant number of immune response related genes, such as interleukin 2 (IL-2) receptor-γ chain, IL-3 receptor-α chain, IL-9 receptor, IL-13 receptor-α chains, Toll-like receptor 7 (TLR7), TLR8, IL-1 receptor-associated kinase 1, as well as multiple transcriptional and translational effectors.20, 21 While X-chromosome inactivation provides dosage compensation for X-linked genes between XX females and XY males, approximately 15% of X genes in humans escape or have skewed X inactivation and are found in higher copy number in women compared with men.66 The role of sex hormones is less clear. Estrogen affects functional activity of innate immune cells that influence downstream adaptive immune responses. For example, low endogenous E2 levels have been shown to enhance the production of markers of innate immune activation and inflammation, including IL-1, IL-6, and tumor necrosis factor. However, the effect of treatment with estrogen on inflammatory markers is less clear. Previous studies have demonstrated an increase in circulating neutrophils after treatment with estrogen (17β-estradiol, E2), but others have found that high concentrations of estrogen reduce the production of pro-inflammatory cytokines.67–69 In a proteomic analysis from the FHS, menopause or hormone status did not appear to significantly influence levels of inflammatory markers.5 Finally, adipose tissue contributes to overexpression of select inflammatory markers in women. For example, adipose tissue stimulates hepatic production of CRP and has been strongly correlated with CRP levels, particularly among women.70 Cross-sectional analyses from the DHS found that the association between female sex and hsCRP was completely attenuated after adjustment for body mass index (BMI).65 This was further corroborated by a study of 353 healthy men and pre-menopausal women that found that adjusting for subcutaneous adipose tissue abolished sex differences in baseline CRP concentrations.71 Other studies, however, have found significant sex differences in CRP levels independent of BMI and obesity.5
To demonstrate how biologic sex can modulate inflammatory pathway biomarkers, we highlight sex-based differences in CRP, an acute phase reactant released from the liver in response to cytokine stimulation.72 CRP and its high-sensitivity assay (hsCRP) are the best studied markers of inflammation and their associations with CVD have been well characterized. Studies from the general population have shown that hsCRP levels are 30-50% higher in women compared with men across all ethnic subgroups even after adjustment for traditional cardiovascular risk factors BMI.73–75 While most of the literature examining hsCRP in men vs women has been limited to the general population, higher levels of hsCRP in women have also been demonstrated in patients with metabolic syndrome,76 stable angina,77 and following acute MI.78, 79
The clinical application of hsCRP has been limited to prediction and prognostication in select populations. Large population-based studies have shown that baseline levels of CRP predict long term CVD risk,80, 81 but the predictive value of CRP beyond clinical risk factors is small, particularly in healthy populations. As such, professional guidelines do not recommend routine screening of hsCRP in low atherosclerotic CVD risk individuals. Whether to screen individuals at intermediate risk for CVD is actively debated, and has been left to the discretion of the clinician to help guide further diagnostics and therapeutic options in the most recent Centers for Disease Control/American Heart Association statement on markers of inflammation and CVD.82 While there does not appear to be a sex difference in the predictive ability of CRP, hsCRP was incorporated into the Reynolds Risk Score, a risk assessment tool that was developed specifically for use in women acknowledging that previous existing tools underestimated risk in women.83 Finally, CRP has been shown to perform well as a marker of prognosis, particularly after ACS, but the prognostic value does not appear to be influenced by sex. For example, elevated levels of hsCRP 1-month post-acute MI were associated with poor health status including symptoms, functional capacity, and quality of life at 12 months follow-up in both men and women enrolled in the VIRGO study.78 Other trials including PROVE-IT TIMI 22 and GUSTO IV ACS found that both male and female participants with elevated CRP levels had significantly greater risk of an adverse outcome including new or worsening HF or mortality.84, 85
ADIPOSE PATHWAYS
Adipose tissue secretes many signaling factors that play important roles in systemic metabolic regulation. There is significant enthusiasm in the scientific community for examining adipose-derived biomarkers, or adipokines, as a biologic link between obesity, metabolic dysregulation, and CVD risk. Sex differences in these adipokines have been described in large-scale proteomic studies from the DHS and FHS including higher levels of leptin, adiponectin, and resistin in women vs men.5, 65 We will here focus on sex-differences in leptin and adiponectin, two of the main adipokines that have been linked to cardiometabolic diseases.
Leptin is produced and secreted from adipocytes and is regulated by various hormones including estrogen and insulin.86 The main action of leptin appears to be regulation of energy balance and metabolism, although it also has a role in fertility and bone function.87 Paradoxically, while leptin appears to promote insulin sensitivity and anorexigenic behavior, levels are increased with obesity despite limited physiological effects leading to a condition of ‘leptin resistance’ similar to insulin resistance.86 The specific role of leptin in cardiovascular physiology is controversial, with some research supporting an association with HF and CAD risk in contrast to other work demonstrating no relationship or even beneficial effects on cardiac metabolism and function.86
There are clear sex-differences in circulating leptin levels, with women evidencing 3-4x greater levels relative to men.88 Of note, estrogen is a potent stimulus for leptin secretion.89, 90 88 Higher body fat in women also likely contributes to sexual dimorphism of leptin.91 Beyond higher levels of secreted leptin in women vs men, sex differences in sensitivity and resistance to leptin action have not been fully elucidated but may also be of critical significance.86, 92 Whether sex hormones and other sex-dependent biological factors (e.g. sex chromosome dependent gene expression, body composition differences) contributes to leptin sensitivity warrants additional study.
Adiponectin, another adipose tissue derived factor that has been linked to CVD risk, also displays sexual dimorphism. Adiponectin is a cardioprotective adipokine that is abundantly produced and secreted by adipose tissue in response to pro-inflammatory factors, reactive oxygen species, and hypoxia. It modulates glucose regulation and has been shown to improve insulin sensitivity and suppress both inflammation and atherogenesis.93 Adiponectin circulates in three forms, low molecular weight (LMW), moderate molecular weight (MMW), and high molecular weight (HMW) oligomers, with the HMW form considered the most biologically active form. Low plasma levels of total adiponectin and HMW adiponectin have been associated with increased risk of cardiometabolic disease including obesity, insulin resistance, diabetes, and CAD in healthy individuals.94, 95 Paradoxically, in patients with established CVD, higher levels of adiponectin have been associated with poor prognosis.96 In particular, HMW adiponectin is associated with increased risk of CAD.97 This conundrum, dubbed the ‘adiponectin paradox’, limits the application of adiponectin as a biomarker for clinical use.98
Women have significantly higher levels of circulating total and HMW adiponectin levels compared with men in healthy populations.65, 99–101 Sex differences in adiponectin levels in disease states are not well characterized, but differences in regional distribution of fat (subcutaneous vs. visceral) in men vs women may contribute to sex differences in levels of circulating total and HMW adiponectin.102–104 Sex hormones are not known to play a significant role in adiponectin regulation.105, 106 While baseline levels of adiponectin are higher in women vs men, it is not known whether sex modifies the predictive and prognostic value of adiponectin in CVD.
As highlighted by the case examples of leptin and adiponectin, adipose-derived biomarkers display significant sexual dimorphism, likely related to inherent differences in adiposity between men and women. While their clinical utility remains limited, adipokines can provide important biologic insights into the drivers of sex differences in cardiometabolic disease.
FIBROSIS PATHWAYS
Sexual dimorphism in fibrosis and fibrosis-related pathways has been previously described. For example, in a proteomic analysis from FHS, circulating levels of fibrosis biomarkers including tetranectin and TIMP1 were higher in men vs women. Despite the important contribution of fibrosis in the pathogenesis of CVD and HF, the majority of identified fibrosis biomarkers have not been adopted for clinical use. We will here focus on sex differences galectin-3 (Gal-3) and soluble ST2 (sST2), two fibrosis biomarkers that are currently recommended for risk stratification in HF patients.107, 108
Gal-3 is a β-galactoside-binding lectin that is thought to play an important role in cardiac fibrosis and heart failure.109 Gal-3 is secreted by activated macrophages and is highly expressed in adipose tissue, respiratory tract, and hematopoietic tissue. In population-based studies, Gal-3 concentrations are consistently higher in women compared with men (pooled median value [IQR]: 12.8 [10.9-15.1] ng/L in men vs. 14.2 [11.9-16.7] ng/L in women).110–113 Data on sex differences in Gal-3 concentrations in the HF population are less consistent. While Gal-3 levels are higher HF compared with healthy individuals for both men and women,114 whether levels are higher in women vs men in HF is actively debated. Some studies have reported higher levels in women with HF vs men,115 while others have shown the opposite.116, 117 In a sub-analysis of the Valsartan Heart Failure Trial, for example, female sex was a predictor of both baseline and changes in Gal-3 levels in symptomatic HF patients.115 The exact mechanisms driving Gal-3 ‘excess’ in women, particularly in the healthy population, are not precisely known, but differences in distribution of adipose tissue have been implicated. Direct associations with adipose measures (including BMI, total body fat, abdominal fat, and body fat distribution) and Gal-3 levels have been observed in both children and adults.111, 118–120 Other comorbidities including diabetes and chronic kidney disease are also strongly associated with Gal-3 levels, and differences in comorbidity profile may explain observed sex-based differences. In a randomized trial of modified citrus pectin, a Gal-3 inhibitor vs. placebo, administered to human subjects with hypertension and elevated Gal-3 levels, the association between female sex and Gal-3 levels was attenuated after multivariable adjustment for age, BMI, DM, systolic blood pressure, and estimated glomerular filtration rate.121
Gal-3 has been shown to predict incident HF, CV death, and all-cause mortality in the general population.110, 113, 122 Whether sex modifies the association of Gal-3 with incident CV outcomes is unclear. An analysis of 8444 healthy participants from the FINRISK 1997 cohort found similar associations between Gal-3 levels and CV outcomes in men and women (HR for HF: 1.16, 95% CI 1.03-1.31 in men vs 1.22, 95% CI 1.07-1.40 in women).113 Longitudinal changes in Gal-3 have also been shown to predict incident HF,123 but there are no sex-specific data on the predictive value of longitudinal Gal-3 changes. Finally, baseline Gal-3 concentrations can be used for risk stratification and prognostication in patients with acute and chronic HF.107, 112, 113, 124–126 Clinically, measurement of Gal-3 alone or in a multi-marker strategy is recommended for risk stratification in patients with HF, but sex-specific cutoffs have not been advised.32
sST2 is a member of member of the interleukin-1 receptor family and is a marker of cardiomyocyte stress and fibrosis. It acts as a decoy receptor of IL-33 by binding to IL-33 and blocks the cardioprotective effects derived from the interaction between IL-33 and transmembrane ST2 ligand. Biologic sex is an important modulator of sST2 concentrations, with significantly higher levels in healthy men compared with women that become apparent in adolescence (sST2 [median ± SD]: 24.0 ± 0.78 ug/L in men vs 17.2 ± 1.18 ug/L in women).65, 127–131 Among patients with HF, sST2 levels are also higher in men vs women.116, 132 The source of sex related differences in sST2 levels is not clearly known, although limited data support the influence of sex hormones on circulating levels. For example, in 3109 individuals from the FHS, exogeneous estrogen therapy was associated with lower sST2 levels.131 By contrast, sST2 was not independently associated with sex hormones including testosterone, estradiol, FSH, and LH in 528 healthy blood donors.130 Small animal and human studies have also supported an association between obesity and sST2 levels.133, 134 In a study of 80 morbidly obese individuals pre- and post- bariatric surgery, sST2 levels decreased significantly following bariatric surgery, highlighting the potential role of obesity on modulating sST2 concentrations.134
sST2 has been shown to predict incident HF in community-based studies,135 but sex-specific data on predictive value of sST2 are lacking. The primary clinical application of sST2 is for risk stratification in patients with acute or chronic HF given its strong association with hospitalization and death in patients with HF beyond traditional biomarkers such as NPs.136–143 Higher levels of sST2 are considered a strong independent marker of poor prognosis in patients with HF, and a universal prognostic cut-point of 35 ug/L has been proposed.32 Despite consistent evidence of higher levels of sST2 in men, sex-specific more outcomes-based data are needed to clarify the need for sex-specific cutoffs.
FUTURE DIRECTIONS
These examples of CV biomarkers representative of myocardial injury/stretch, inflammation, adipose tissue metabolism, and fibrosis pathways illustrate widespread sex differences in their associations with CVD. While significant knowledge gaps remain, CV biomarkers have enormous potential to expand biologic insights into disease mechanism and to improve contemporary clinical practice by refining our diagnostic, risk prediction, and even therapeutic capabilities specific to men vs women. We are beginning to understand that sex modulates CV biomarker biology, but the biologic underpinnings are poorly characterized and translation to clinical practice has been limited. This is in large part due to paucity of sex-specific studies and uncertainty regarding how to more precisely tailor clinical care for men and women separately.
In this review, we have summarized the effect of sex on biomarker biology and highlighted sex-specific aspects of key CV biomarkers along important established biologic pathways. Evolving molecular technologies such as metabolomics and epigenetics will no doubt identify additional sex-based biomarkers. Looking ahead, we offer specific recommendations for future research on sex-based differences in CV biomarkers and explore opportunities for improved targeted clinical application of CV biomarkers in men vs women (Table 2). Broadly, we would like to highlight the following areas for future study:
Table 2.
Future directions: proposed focus areas for future CV biomarker research and future clinical applications
| Proposed Focus Areas of Future CV Biomarker Research | |
|---|---|
| Recruitment & Enrollment | ● Increase recruitment and retention of female subjects in research studies including animal studies, human observational studies, and human clinical trials.144, 145 ● Consider sex-specific biomarker thresholds when biomarker cut-points are used as eligibility criteria for inclusion into research studies. ● Enroll specialized populations historically excluded from research studies, such as pregnant women.147 |
| Study Design | ● Adopt sex-specific thresholds when biomarkers are used as disease or endpoint surrogates in research studies. ● Consider single sex research studies and trials when further investigating a sex-specific signal.148 ● Interrogate sex-based differences using deep phenotyping approaches (e.g. large scale multi -omic studies, clonal hematopoiesis of indeterminate potential [CHIP], and deep sequencing). ● Investigate the role of X-linked genes and X chromosome inactivation in cardiovascular disease pathogenesis.149 ● Incorporate ascertainment of female-specific factors (e.g. menopause, adverse pregnancy outcomes, parity, menses, hormone therapy, oral contraceptive use, etc.) in research study design. Female-specific factors, however, should not be conflated with biologic sex differences.29 ● Identify and adjust for relevant confounders that influence the association between sex and biomarker. |
| Reporting | ● For all biomarker research studies, report sex-stratified analyses when possible.146 ● When reporting sex-specific results of biomarker analyses, include sex-specific plasma concentrations, cut-points, risk ratios, and prediction models.7, 146 |
| Future Clinical Applications | |
| Diagnosis | ● For biomarkers that display clinically relevant sex differences, consider sex-specific thresholds.146 |
| Therapy | ● Do not use underpowered sex-specific biomarker analyses as support for withholding evidence-based therapies for women.150 |
First, women are consistently underrepresented in biomedical research, and strategies to recruit, enroll, and retain women in CV biomarker research have been inadequate.144, 145 We highlight an opportunity to leverage sex-specific biomarker thresholds to more precisely target women for enrollment into clinical trials and also endorse the inclusion of specialized populations of women who have been historically excluded from clinical studies.
Second, biologic sex has not been systematically incorporated in research study design.146 We enumerate potential opportunities to integrate biologic sex and female-specific factors into research study design and emphasize the importance of performing well-conducted studies that adjust for confounders that influence the association between sex and biomarker. These recommendations will enable more rigorous investigation into the mechanisms that drive sex-based differences in CVD.
Third, sex-stratified analyses are not routinely reported. We propose that whenever possible, biomarker research studies should report sex-stratified analyses. Suggested metrics for reporting sex-stratified results include sex-specific plasma concentrations, cut-points, risk ratios, and prediction models.7
Finally, the clinical application of biomarkers has yet to integrate the role of biologic sex. We suggest considering the incorporation of sex-specific thresholds only when clinically relevant sex differences exist for a given biomarker. Importantly, because sex-specific biomarker analyses are often underpowered owing to insufficient recruitment of women, absence of significant findings should not be used as evidence to support withholding evidence-based therapies for women.
Taken together, these approaches may ultimately enhance prevention strategies and clinical care for both men and women with CVD.
Source of Funding
E.S.L is supported by the American Heart Association (853922). M.V.Z. is supported by NIH- R01-HL137562, NIH R01-HL146267, and K24-AI157882. J.E.H. is supported by NIH R01-HL134893, R01-HL140224, and K24-HL153669. S.H.S. is supported by NIH R01-HL146145, R01- HL127009, and R21-AI158786.
Disclosures
M.V.Z. is Principal Investigator of an Investigator-initiated research grant from Gilead to her institution (MGH). J.E.H. has received research grant support from Bayer, AG. S.H.S. receives research support through a sponsored research project to Duke University from Verily Inc., Lilly Inc., and Astra-Zeneca.
ABBREVIATIONS
- ACS
acute coronary syndrome
- ANP
atrial natriuretic peptide
- BMI
body mass index
- CAD
coronary artery disease
- CI
confidence interval
- CRP
C-reactive protein
- cTn
cardiac troponin
- CVD
cardiovascular disease
- DHS
Dallas Heart Study
- FHS
Framingham Heart Study
- FSH
follicle stimulating hormone
- Gal-3
galectin-3
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- HR
hazard ratio
- hs-cTnT
high sensitivity cardiac troponin T
- hsCRP
high sensitivity C-reactive protein
- IL
interleukin
- IQR
inter-quartile range
- LH
luteinizing hormone
- MI
myocardial infarction
- NP
natriuretic peptide
- NSTEMI
non-ST elevation myocardial infarction
- NT-pro BNP
amino-terminal-peptide N terminal-pro BNP
- PCOS
polycystic ovarian syndrome
- sST2
soluble ST2
- TLR
toll-like receptor
REFERENCES
- 1.Bairey Merz CN, Shaw LJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Pepine CJ, Mankad S, Sharaf BL, Rogers WJ, Pohost GM, Lerman A, Quyyumi AA and Sopko G. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol. 2006;47:S21–9. [DOI] [PubMed] [Google Scholar]
- 2.Madsen TE, Bourjeily G, Hasnain M, Jenkins M, Morrison MF, Sandberg K, Tong IL, Trott J, Werbinski JL and McGregor AJ. Sex- and Gender-Based Medicine: The Need for Precise Terminology. Gender and the Genome. 2017;1:122–128. [Google Scholar]
- 3.Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clinical pharmacology and therapeutics. 2001;69:89–95. [DOI] [PubMed] [Google Scholar]
- 4.Gerszten RE and Wang TJ. The search for new cardiovascular biomarkers. Nature. 2008;451:949–52. [DOI] [PubMed] [Google Scholar]
- 5.Lau ES, Paniagua SM, Guseh JS, Bhambhani V, Zanni MV, Courchesne P, Lyass A, Larson MG, Levy D and Ho JE. Sex Differences in Circulating Biomarkers of Cardiovascular Disease. J Am Coll Cardiol. 2019;74:1543–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ndumele CE, Matsushita K, Sang Y, Lazo M, Agarwal SK, Nambi V, Deswal A, Blumenthal RS, Ballantyne CM, Coresh J and Selvin E. N-Terminal Pro-Brain Natriuretic Peptide and Heart Failure Risk Among Individuals With and Without Obesity: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2016;133:631–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suthahar N, Meems LMG, Ho JE and de Boer RA. Sex-related differences in contemporary biomarkers for heart failure: a review. Eur J Heart Fail. 2020;22:775–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magnussen C, Niiranen TJ, Ojeda FM, Gianfagna F, Blankenberg S, Vartiainen E, Sans S, Pasterkamp G, Hughes M, Costanzo S, Donati MB, Jousilahti P, Linneberg A, Palosaari T, de Gaetano G, Bobak M, den Ruijter HM, Jørgensen T, Söderberg S, Kuulasmaa K, Zeller T, Iacoviello L, Salomaa V and Schnabel RB. Sex-Specific Epidemiology of Heart Failure Risk and Mortality in Europe: Results From the BiomarCaRE Consortium. JACC Heart Fail. 2019;7:204–213. [DOI] [PubMed] [Google Scholar]
- 9.Berletch JB, Yang F, Xu J, Carrel L and Disteche CM. Genes that escape from X inactivation. Hum Genet. 2011;130:237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi W, Sheng X, Dorr KM, Hutton JE, Emerson JI, Davies HA, Andrade TD, Wasson LK, Greco TM, Hashimoto Y, Federspiel JD, Robbe ZL, Chen X, Arnold AP, Cristea IM and Conlon FL. Cardiac proteomics reveals sex chromosome-dependent differences between males and females that arise prior to gonad formation. Developmental cell. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tukiainen T, Villani AC, Yen A, Rivas MA, Marshall JL, Satija R, Aguirre M, Gauthier L, Fleharty M, Kirby A, Cummings BB, Castel SE, Karczewski KJ, Aguet F, Byrnes A, Lappalainen T, Regev A, Ardlie KG, Hacohen N and MacArthur DG. Landscape of X chromosome inactivation across human tissues. Nature. 2017;550:244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma W, Bonora G, Berletch JB, Deng X, Noble WS and Disteche CM. X-Chromosome Inactivation and Escape from X Inactivation in Mouse. Methods in molecular biology (Clifton, NJ). 2018;1861:205–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Florijn BW, Bijkerk R, van der Veer EP and van Zonneveld AJ. Gender and cardiovascular disease: are sex-biased microRNA networks a driving force behind heart failure with preserved ejection fraction in women? Cardiovascular Research. 2017;114:210–225. [DOI] [PubMed] [Google Scholar]
- 14.Moreau KL. Modulatory influence of sex hormones on vascular aging. Am J Physiol Heart Circ Physiol. 2019;316:H522–h526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baños G, Guarner V and Pérez-Torres I. Sex steroid hormones, cardiovascular diseases and the metabolic syndrome. Cardiovascular & hematological agents in medicinal chemistry. 2011;9:137–46. [DOI] [PubMed] [Google Scholar]
- 16.Lizneva D, Rahimova A, Kim SM, Atabiekov I, Javaid S, Alamoush B, Taneja C, Khan A, Sun L, Azziz R, Yuen T and Zaidi M. FSH Beyond Fertility. Frontiers in endocrinology. 2019;10:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Habashi JP, MacFarlane EG, Bagirzadeh R, Bowen C, Huso N, Chen Y, Bedja D, Creamer TJ, Rykiel G, Manning M, Huso D and Dietz HC. Oxytocin antagonism prevents pregnancy-associated aortic dissection in a mouse model of Marfan syndrome. Science translational medicine. 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hyde Z, Norman PE, Flicker L, Hankey GJ, McCaul KA, Almeida OP, Chubb SA and Yeap BB. Elevated LH predicts ischaemic heart disease events in older men: the Health in Men Study. European journal of endocrinology. 2011;164:569–77. [DOI] [PubMed] [Google Scholar]
- 19.Neeland IJ, Ayers CR, Rohatgi AK, Turer AT, Berry JD, Das SR, Vega GL, Khera A, McGuire DK, Grundy SM and de Lemos JA. Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity. 2013;21:E439–E447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein SL and Flanagan KL. Sex differences in immune responses. Nature reviews Immunology. 2016;16:626–38. [DOI] [PubMed] [Google Scholar]
- 21.Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nature reviews Immunology. 2008;8:737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobson DL, Gange SJ, Rose NR and Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clinical immunology and immunopathology. 1997;84:223–43. [DOI] [PubMed] [Google Scholar]
- 23.Addo MM and Altfeld M. Sex-based differences in HIV type 1 pathogenesis. The Journal of infectious diseases. 2014;209 Suppl 3:S86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiechter M, Haider A, Bengs S, Marȩdziak M, Burger IA, Roggo A, Portmann A, Warnock GI, Schade K, Treyer V, Becker AS, Messerli M, Felten EV, Benz DC, Fuchs TA, Gräni C, Pazhenkottil AP, Buechel RR, Kaufmann PA and Gebhard C. Sex Differences in the Association between Inflammation and Ischemic Heart Disease. Thrombosis and haemostasis. 2019;119:1471–1480. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan S, Young A, Hammadah M, Lima BB, Levantsevych O, Ko YA, Pearce BD, Shah AJ, Kim JH, Moazzami K, Driggers EG, Haffar A, Ward L, Herring I, Hankus A, Lewis TT, Mehta PK, Bremner JD, Raggi P, Quyyumi A and Vaccarino V. Sex differences in the inflammatory response to stress and risk of adverse cardiovascular outcomes among patients with coronary heart disease. Brain, behavior, and immunity. 2020;90:294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aiello AE and Kaplan GA. Socioeconomic position and inflammatory and immune biomarkers of cardiovascular disease: applications to the Panel Study of Income Dynamics. Biodemography Soc Biol. 2009;55:178–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sobhani K, Nieves Castro DK, Fu Q, Gottlieb RA, Van Eyk JE and Noel Bairey Merz C. Sex differences in ischemic heart disease and heart failure biomarkers. Biol Sex Differ. 2018;9:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.AlBadri A, Wei J, Mehta PK, Shah R, Herscovici R, Gulati M, Shufelt C and Bairey Merz N. Sex differences in coronary heart disease risk factors: rename it ischaemic heart disease! Heart (British Cardiac Society). 2017;103:1567–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ouyang P, Wenger NK, Taylor D, Rich-Edwards JW, Steiner M, Shaw LJ, Berga SL, Miller VM and Merz NB. Strategies and methods to study female-specific cardiovascular health and disease: a guide for clinical scientists. Biol Sex Differ. 2016;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Motiwala SR, Sarma A, Januzzi JL and O’Donoghue ML. Biomarkers in ACS and heart failure: should men and women be interpreted differently? Clin Chem. 2014;60:35–43. [DOI] [PubMed] [Google Scholar]
- 31.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F and Kathrine Skibelund A. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. European heart journal. 2021;42:3599–3726. [DOI] [PubMed] [Google Scholar]
- 32.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW and Westlake C. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776–803. [DOI] [PubMed] [Google Scholar]
- 33.Cediel G, Codina P, Spitaleri G, Domingo M, Santiago-Vacas E, Lupón J and Bayes-Genis A. Gender-Related Differences in Heart Failure Biomarkers. Front Cardiovasc Med. 2020;7:617705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faxén UL, Lund LH, Orsini N, Strömberg A, Andersson DC, Linde C, Dahlström U and Savarese G. N-terminal pro-B-type natriuretic peptide in chronic heart failure: The impact of sex across the ejection fraction spectrum. Int J Cardiol. 2019;287:66–72. [DOI] [PubMed] [Google Scholar]
- 35.Ying W, Zhao D, Ouyang P, Subramanya V, Vaidya D, Ndumele CE, Sharma K, Shah SJ, Heckbert SR, Lima JA, deFilippi CR, Budoff MJ, Post WS and Michos ED. Sex Hormones and Change in N-Terminal Pro-B-Type Natriuretic Peptide Levels: The Multi-Ethnic Study of Atherosclerosis. The Journal of clinical endocrinology and metabolism. 2018;103:4304–4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kałużna M, Krauze T, Ziemnicka K, Wachowiak-Ochmańska K, Kaczmarek J, Janicki A, Wykrętowicz A, Ruchała M and Guzik P. Cardiovascular, anthropometric, metabolic and hormonal profiling of normotensive women with polycystic ovary syndrome with and without biochemical hyperandrogenism. Endocrine. 2021;72:882–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maffei S, Del Ry S, Prontera C and Clerico A. Increase in circulating levels of cardiac natriuretic peptides after hormone replacement therapy in postmenopausal women. Clinical science (London, England : 1979). 2001;101:447–53. [PubMed] [Google Scholar]
- 38.Chlabicz M, Jamiołkowski J, Paniczko M, Sowa P, Łapińska M, Szpakowicz M, Jurczuk N, Kondraciuk M, Raczkowski A, Sawicka E and Kamiński KA. Independent Impact of Gynoid Fat Distribution and Free Testosterone on Circulating Levels of N-Terminal Pro-Brain Natriuretic Peptide (NT-proBNP) in Humans. Journal of clinical medicine. 2019;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marchini A, Häcker B, Marttila T, Hesse V, Emons J, Weiss B, Karperien M and Rappold G. BNP is a transcriptional target of the short stature homeobox gene SHOX. Human molecular genetics. 2007;16:3081–7. [DOI] [PubMed] [Google Scholar]
- 40.Suthahar N, Lau ES, Blaha MJ, Paniagua SM, Larson MG, Psaty BM, Benjamin EJ, Allison MA, Bartz TM, Januzzi JL Jr., Levy D, Meems LMG, Bakker SJL, Lima JAC, Cushman M, Lee DS, Wang TJ, deFilippi CR, Herrington DM, Nayor M, Vasan RS, Gardin JM, Kizer JR, Bertoni AG, Allen NB, Gansevoort RT, Shah SJ, Gottdiener JS, Ho JE and de Boer RA. Sex-Specific Associations of Cardiovascular Risk Factors and Biomarkers With Incident Heart Failure. J Am Coll Cardiol. 2020;76:1455–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim HL, Kim MA, Choi DJ, Han S, Jeon ES, Cho MC, Kim JJ, Yoo BS, Shin MS, Seong IW, Ahn Y, Kang SM, Kim YJ, Kim HS, Chae SC, Oh BH, Lee MM and Ryu KH. Gender Difference in the Prognostic Value of N-Terminal Pro-B Type Natriuretic Peptide in Patients With Heart Failure - A Report From the Korean Heart Failure Registry (KorHF). Circulation journal : official journal of the Japanese Circulation Society. 2017;81:1329–1336. [DOI] [PubMed] [Google Scholar]
- 42.Bozkurt B, Coats AJ, Tsutsui H, Abdelhamid M, Adamopoulos S, Albert N, Anker SD, Atherton J, Böhm M, Butler J, Drazner MH, Felker GM, Filippatos G, Fonarow GC, Fiuzat M, Gomez-Mesa JE, Heidenreich P, Imamura T, Januzzi J, Jankowska EA, Khazanie P, Kinugawa K, Lam CSP, Matsue Y, Metra M, Ohtani T, Francesco Piepoli M, Ponikowski P, Rosano GMC, Sakata Y, SeferoviĆ P, Starling RC, Teerlink JR, Vardeny O, Yamamoto K, Yancy C, Zhang J and Zieroth S. Universal Definition and Classification of Heart Failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. Journal of cardiac failure. 2021. [DOI] [PubMed] [Google Scholar]
- 43.Jia X, Sun W, Hoogeveen RC, Nambi V, Matsushita K, Folsom AR, Heiss G, Couper DJ, Solomon SD, Boerwinkle E, Shah A, Selvin E, de Lemos JA and Ballantyne CM. High-Sensitivity Troponin I and Incident Coronary Events, Stroke, Heart Failure Hospitalization, and Mortality in the ARIC Study. Circulation. 2019;139:2642–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Osibogun O, Ogunmoroti O, Tibuakuu M, Benson EM and Michos ED. Sex differences in the association between ideal cardiovascular health and biomarkers of cardiovascular disease among adults in the United States: a cross-sectional analysis from the multiethnic study of atherosclerosis. BMJ Open. 2019;9:e031414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu JY, Jia QW, Zang XL, Wang RH, Li CJ, Wang LS, Ma WZ, Yang ZJ and Jia EZ. Age-sex distribution of patients with high-sensitivity troponin T levels below the 99th percentile. Oncotarget. 2017;8:75638–75645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS and Katus HA. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem. 2010;56:254–61. [DOI] [PubMed] [Google Scholar]
- 47.Omland T, de Lemos JA, Sabatine MS, Christophi CA, Rice MM, Jablonski KA, Tjora S, Domanski MJ, Gersh BJ, Rouleau JL, Pfeffer MA and Braunwald E. A sensitive cardiac troponin T assay in stable coronary artery disease. The New England journal of medicine. 2009;361:2538–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wiviott SD, Cannon CP, Morrow DA, Murphy SA, Gibson CM, McCabe CH, Sabatine MS, Rifai N, Giugliano RP, DiBattiste PM, Demopoulos LA, Antman EM and Braunwald E. Differential Expression of Cardiac Biomarkers by Gender in Patients With Unstable Angina/Non–ST-Elevation Myocardial Infarction. Circulation. 2004;109:580–586. [DOI] [PubMed] [Google Scholar]
- 49.Bhatia PM and Daniels LB. Highly Sensitive Cardiac Troponins: The Evidence Behind Sex-Specific Cutoffs. J Am Heart Assoc. 2020;9:e015272–e015272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rubio-Gayosso I, Ramirez-Sanchez I, Ita-Islas I, Ortiz-Vilchis P, Gutierrez-Salmean G, Meaney A, Palma I, Olivares I, Garcia R, Meaney E and Ceballos G. Testosterone metabolites mediate its effects on myocardial damage induced by ischemia/reperfusion in male Wistar rats. Steroids. 2013;78:362–9. [DOI] [PubMed] [Google Scholar]
- 51.Deschamps AM, Murphy E and Sun J. Estrogen receptor activation and cardioprotection in ischemia reperfusion injury. Trends Cardiovasc Med. 2010;20:73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iorga A, Cunningham CM, Moazeni S, Ruffenach G, Umar S and Eghbali M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol Sex Differ. 2017;8:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Oosterhout REM, de Boer AR, Maas AHEM, Rutten FH, Bots ML and Peters SAE. Sex Differences in Symptom Presentation in Acute Coronary Syndromes: A Systematic Review and Meta-analysis. J Am Heart Assoc. 2020;9:e014733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwarzenberger JC, Sun LS, Pesce MA, Heyer EJ, Delphin E, Almeida GM and Wood M. Sex-based differences in serum cardiac troponin I, a specific marker for myocardial injury, after cardiac surgery. Critical care medicine. 2003;31:689–93. [DOI] [PubMed] [Google Scholar]
- 55.Kimenai DM, Shah ASV, McAllister DA, Lee KK, Tsanas A, Meex SJR, Porteous DJ, Hayward C, Campbell A, Sattar N, Mills NL and Welsh P. Sex Differences in Cardiac Troponin I and T and the Prediction of Cardiovascular Events in the General Population. Clinical Chemistry. 2021;67:1351–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harada Y, Michel J, Koenig W, Rheude T, Colleran R, Giacoppo D, Kastrati A and Byrne RA. Prognostic Value of Cardiac Troponin T and Sex in Patients Undergoing Elective Percutaneous Coronary Intervention. J Am Heart Assoc. 2016;5:e004464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gohar A, Chong JPC, Liew OW, den Ruijter H, de Kleijn DPV, Sim D, Yeo DPS, Ong HY, Jaufeerally F, Leong GKT, Ling LH, Lam CSP and Richards AM. The prognostic value of highly sensitive cardiac troponin assays for adverse events in men and women with stable heart failure and a preserved vs. reduced ejection fraction. Eur J Heart Fail. 2017;19:1638–1647. [DOI] [PubMed] [Google Scholar]
- 58.Aimo A, Januzzi JL Jr., Vergaro G, Ripoli A, Latini R, Masson S, Magnoli M, Anand IS, Cohn JN, Tavazzi L, Tognoni G, Gravning J, Ueland T, Nymo SH, Brunner-La Rocca HP, Bayes-Genis A, Lupón J, de Boer RA, Yoshihisa A, Takeishi Y, Egstrup M, Gustafsson I, Gaggin HK, Eggers KM, Huber K, Tentzeris I, Tang WHW, Grodin J, Passino C and Emdin M. Prognostic Value of High-Sensitivity Troponin T in Chronic Heart Failure: An Individual Patient Data Meta-Analysis. Circulation. 2018;137:286–297. [DOI] [PubMed] [Google Scholar]
- 59.Apple FS, Ler R and Murakami MM. Determination of 19 cardiac troponin I and T assay 99th percentile values from a common presumably healthy population. Clin Chem. 2012;58:1574–81. [DOI] [PubMed] [Google Scholar]
- 60.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA and White HD. Fourth Universal Definition of Myocardial Infarction (2018). Circulation. 2018;138:e618–e651. [DOI] [PubMed] [Google Scholar]
- 61.Cullen L, Greenslade JH, Carlton EW, Than M, Pickering JW, Ho A, Greaves K, Berndt SL, Body R, Ryan K and Parsonage WA. Sex-specific versus overall cut points for a high sensitivity troponin I assay in predicting 1-year outcomes in emergency patients presenting with chest pain. Heart. 2016;102:120–6. [DOI] [PubMed] [Google Scholar]
- 62.O’Donoghue M, Boden WE, Braunwald E, Cannon CP, Clayton TC, de Winter RJ, Fox KA, Lagerqvist B, McCullough PA, Murphy SA, Spacek R, Swahn E, Wallentin L, Windhausen F and Sabatine MS. Early invasive vs conservative treatment strategies in women and men with unstable angina and non-ST-segment elevation myocardial infarction: a meta-analysis. Jama. 2008;300:71–80. [DOI] [PubMed] [Google Scholar]
- 63.Romiti GF, Cangemi R, Toriello F, Ruscio E, Sciomer S, Moscucci F, Vincenti M, Crescioli C, Proietti M, Basili S and Raparelli V. Sex-Specific Cut-Offs for High-Sensitivity Cardiac Troponin: Is Less More? Cardiovasc Ther. 2019;2019:9546931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fischer J, Jung N, Robinson N and Lehmann C. Sex differences in immune responses to infectious diseases. Infection. 2015;43:399–403. [DOI] [PubMed] [Google Scholar]
- 65.Lew J, Sanghavi M, Ayers CR, McGuire DK, Omland T, Atzler D, Gore MO, Neeland I, Berry JD, Khera A, Rohatgi A and de Lemos JA. Sex-Based Differences in Cardiometabolic Biomarkers. Circulation. 2017;135:544–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carrel L and Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–4. [DOI] [PubMed] [Google Scholar]
- 67.Jilma B, Eichler HG, Breiteneder H, Wolzt M, Aringer M, Graninger W, Röhrer C, Veitl M and Wagner OF. Effects of 17 beta-estradiol on circulating adhesion molecules. The Journal of clinical endocrinology and metabolism. 1994;79:1619–24. [DOI] [PubMed] [Google Scholar]
- 68.Robinson DP, Hall OJ, Nilles TL, Bream JH and Klein SL. 17β-estradiol protects females against influenza by recruiting neutrophils and increasing virus-specific CD8 T cell responses in the lungs. Journal of virology. 2014;88:4711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bouman A, Heineman MJ and Faas MM. Sex hormones and the immune response in humans. Human reproduction update. 2005;11:411–23. [DOI] [PubMed] [Google Scholar]
- 70.Moshage HJ, Roelofs HM, van Pelt JF, Hazenberg BP, van Leeuwen MA, Limburg PC, Aarden LA and Yap SH. The effect of interleukin-1, interleukin-6 and its interrelationship on the synthesis of serum amyloid A and C-reactive protein in primary cultures of adult human hepatocytes. Biochemical and biophysical research communications. 1988;155:112–7. [DOI] [PubMed] [Google Scholar]
- 71.Cartier A, Côté M, Lemieux I, Pérusse L, Tremblay A, Bouchard C and Després J-P. Sex differences in inflammatory markers: what is the contribution of visceral adiposity? The American Journal of Clinical Nutrition. 2009;89:1307–1314. [DOI] [PubMed] [Google Scholar]
- 72.Baumann H and Gauldie J. Regulation of hepatic acute phase plasma protein genes by hepatocyte stimulating factors and other mediators of inflammation. Molecular biology & medicine. 1990;7:147–59. [PubMed] [Google Scholar]
- 73.Lakoski SG, Cushman M, Criqui M, Rundek T, Blumenthal RS, D’Agostino RB Jr. and Herrington DM. Gender and C-reactive protein: data from the Multiethnic Study of Atherosclerosis (MESA) cohort. American heart journal. 2006;152:593–8. [DOI] [PubMed] [Google Scholar]
- 74.Khera A, McGuire DK, Murphy SA, Stanek HG, Das SR, Vongpatanasin W, Wians FH Jr., Grundy SM and de Lemos JA. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol. 2005;46:464–9. [DOI] [PubMed] [Google Scholar]
- 75.Wong ND, Pio J, Valencia R and Thakal G. Distribution of C-reactive protein and its relation to risk factors and coronary heart disease risk estimation in the National Health and Nutrition Examination Survey (NHANES) III. Preventive cardiology. 2001;4:109–114. [DOI] [PubMed] [Google Scholar]
- 76.Garcia VP, Rocha HN, Sales AR, Rocha NG and da Nóbrega AC. Sex Differences in High Sensitivity C-Reactive Protein in Subjects with Risk Factors of Metabolic Syndrome. Arquivos brasileiros de cardiologia. 2016;106:182–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garcia-Moll X, Zouridakis E, Cole D and Kaski JC. C-reactive protein in patients with chronic stable angina: differences in baseline serum concentration between women and men. European heart journal. 2000;21:1598–606. [DOI] [PubMed] [Google Scholar]
- 78.Lu Y, Zhou S, Dreyer RP, Spatz ES, Geda M, Lorenze NP, D’Onofrio G, Lichtman JH, Spertus JA, Ridker PM and Krumholz HM. Sex Differences in Inflammatory Markers and Health Status Among Young Adults With Acute Myocardial Infarction: Results From the VIRGO (Variation in Recovery: Role of Gender on Outcomes of Young Acute Myocardial Infarction Patients) Study. Circulation Cardiovascular quality and outcomes. 2017;10:e003470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eggers KM, Lindhagen L, Baron T, Erlinge D, Hjort M, Jernberg T, Johnston N, Marko-Varga G, Rezeli M, Spaak J and Lindahl B. Sex-differences in circulating biomarkers during acute myocardial infarction: An analysis from the SWEDEHEART registry. PloS one. 2021;16:e0249830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, Curhan GC, Rifai N, Cannuscio CC, Stampfer MJ and Rimm EB. Inflammatory Markers and the Risk of Coronary Heart Disease in Men and Women. New England Journal of Medicine. 2004;351:2599–2610. [DOI] [PubMed] [Google Scholar]
- 81.Ridker PM, Cushman M, Stampfer MJ, Tracy RP and Hennekens CH. Inflammation, Aspirin, and the Risk of Cardiovascular Disease in Apparently Healthy Men. New England Journal of Medicine. 1997;336:973–979. [DOI] [PubMed] [Google Scholar]
- 82.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC Jr., Taubert K, Tracy RP and Vinicor F. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. [DOI] [PubMed] [Google Scholar]
- 83.Michos ED, Vasamreddy CR, Becker DM, Yanek LR, Moy TF, Fishman EK, Becker LC and Blumenthal RS. Women with a low Framingham risk score and a family history of premature coronary heart disease have a high prevalence of subclinical coronary atherosclerosis. American heart journal. 2005;150:1276–81. [DOI] [PubMed] [Google Scholar]
- 84.James SK, Armstrong P, Barnathan E, Califf R, Lindahl B, Siegbahn A, Simoons ML, Topol EJ, Venge P and Wallentin L. Troponin and C-reactive protein have different relations to subsequent mortality and myocardial infarction after acute coronary syndrome: a GUSTO-IV substudy. J Am Coll Cardiol. 2003;41:916–24. [DOI] [PubMed] [Google Scholar]
- 85.Scirica BM, Cannon CP, Sabatine MS, Jarolim P, Sloane S, Rifai N, Braunwald E and Morrow DA. Concentrations of C-reactive protein and B-type natriuretic peptide 30 days after acute coronary syndromes independently predict hospitalization for heart failure and cardiovascular death. Clin Chem. 2009;55:265–73. [DOI] [PubMed] [Google Scholar]
- 86.Poetsch MS, Strano A and Guan K. Role of Leptin in Cardiovascular Diseases. Frontiers in endocrinology. 2020;11:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Margetic S, Gazzola C, Pegg GG and Hill RA. Leptin: a review of its peripheral actions and interactions. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2002;26:1407–33. [DOI] [PubMed] [Google Scholar]
- 88.Belin de Chantemèle EJ. Sex Differences in Leptin Control of Cardiovascular Function in Health and Metabolic Diseases. Adv Exp Med Biol. 2017;1043:87–111. [DOI] [PubMed] [Google Scholar]
- 89.Machinal-Quélin F, Dieudonné MN, Pecquery R, Leneveu MC and Giudicelli Y. Direct in vitro effects of androgens and estrogens on ob gene expression and leptin secretion in human adipose tissue. Endocrine. 2002;18(12):179–84. [DOI] [PubMed] [Google Scholar]
- 90.Brann DW, De Sevilla L, Zamorano PL and Mahesh VB. Regulation of leptin gene expression and secretion by steroid hormones. Steroids. 1999;64:659–63. [DOI] [PubMed] [Google Scholar]
- 91.Hellström L, Wahrenberg H, Hruska K, Reynisdottir S and Arner P. Mechanisms behind gender differences in circulating leptin levels. Journal of internal medicine. 2000;247:457–62. [DOI] [PubMed] [Google Scholar]
- 92.Côté I, Green SM, Toklu HZ, Morgan D, Carter CS, Tümer N and Scarpace PJ. Differential physiological responses to central leptin overexpression in male and female rats. J Neuroendocrinol. 2017;29: 10.1111/jne.12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fisman EZ and Tenenbaum A. Adiponectin: a manifold therapeutic target for metabolic syndrome, diabetes, and coronary disease? Cardiovascular diabetology. 2014;13:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yoo HJ and Choi KM. Adipokines as a novel link between obesity and atherosclerosis. World journal of diabetes. 2014;5:357–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu N, Pankow JS, Ballantyne CM, Couper D, Hoogeveen RC, Pereira M, Duncan BB and Schmidt MI. High-molecular-weight adiponectin and the risk of type 2 diabetes in the ARIC study. The Journal of clinical endocrinology and metabolism. 2010;95:5097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sook Lee E, Park SS, Kim E, Sook Yoon Y, Ahn HY, Park CY, Ho Yun Y and Woo Oh S. Association between adiponectin levels and coronary heart disease and mortality: a systematic review and meta-analysis. International journal of epidemiology. 2013;42:1029–39. [DOI] [PubMed] [Google Scholar]
- 97.Rizza S, Gigli F, Galli A, Micchelini B, Lauro D, Lauro R and Federici M. Adiponectin Isoforms in Elderly Patients with or without Coronary Artery Disease. Journal of the American Geriatrics Society. 2010;58:702–706. [DOI] [PubMed] [Google Scholar]
- 98.Kim-Mitsuyama S, Soejima H, Yasuda O, Node K, Jinnouchi H, Yamamoto E, Sekigami T, Ogawa H and Matsui K. Total adiponectin is associated with incident cardiovascular and renal events in treated hypertensive patients: subanalysis of the ATTEMPT-CVD randomized trial. Scientific reports. 2019;9:16589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nishizawa H, Shimomura I, Kishida K, Maeda N, Kuriyama H, Nagaretani H, Matsuda M, Kondo H, Furuyama N, Kihara S, Nakamura T, Tochino Y, Funahashi T and Matsuzawa Y. Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes. 2002;51:2734–41. [DOI] [PubMed] [Google Scholar]
- 100.Degawa-Yamauchi M, Dilts JR, Bovenkerk JE, Saha C, Pratt JH and Considine RV. Lower serum adiponectin levels in African-American boys. Obesity research. 2003;11:1384–90. [DOI] [PubMed] [Google Scholar]
- 101.Hanley AJ, Bowden D, Wagenknecht LE, Balasubramanyam A, Langfeld C, Saad MF, Rotter JI, Guo X, Chen YD, Bryer-Ash M, Norris JM and Haffner SM. Associations of adiponectin with body fat distribution and insulin sensitivity in nondiabetic Hispanics and African-Americans. The Journal of clinical endocrinology and metabolism. 2007;92:2665–71. [DOI] [PubMed] [Google Scholar]
- 102.Yang Y, Xie M, Yuan S, Zeng Y, Dong Y, Wang Z, Xiao Q, Dong B, Ma J and Hu J. Sex differences in the associations between adiposity distribution and cardiometabolic risk factors in overweight or obese individuals: a cross-sectional study. BMC public health. 2021;21:1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reneau J, Goldblatt M, Gould J, Kindel T, Kastenmeier A, Higgins R, Rengel LR, Schoyer K, James R, Obi B, Moosreiner A, Nicholson K, Sahoo D and Kidambi S. Effect of adiposity on tissue-specific adiponectin secretion. PloS one. 2018;13:e0198889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maimaituxun G, Fukuda D, Izaki H, Hirata Y, Kanayama HO, Masuzaki H, Sata M and Shimabukuro M. Levels of Adiponectin Expression in Peri-Renal and Subcutaneous Adipose Tissue and Its Determinants in Human Biopsied Samples. Frontiers in endocrinology. 2019;10:897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hall N, White C and O’Sullivan AJ. The relationship between adiponectin, progesterone, and temperature across the menstrual cycle. Journal of endocrinological investigation. 2009;32:279–83. [DOI] [PubMed] [Google Scholar]
- 106.Sieminska L, Wojciechowska C, Niedziolka D, Marek B, Kos-Kudla B, Kajdaniuk D and Nowak M. Effect of postmenopause and hormone replacement therapy on serum adiponectin levels. Metabolism: clinical and experimental. 2005;54:1610–4. [DOI] [PubMed] [Google Scholar]
- 107.Chow SL, Maisel AS, Anand I, Bozkurt B, de Boer RA, Felker GM, Fonarow GC, Greenberg B, Januzzi JL Jr., Kiernan MS, Liu PP, Wang TJ, Yancy CW and Zile MR. Role of Biomarkers for the Prevention, Assessment, and Management of Heart Failure: A Scientific Statement From the American Heart Association. Circulation. 2017;135:e1054–e1091. [DOI] [PubMed] [Google Scholar]
- 108.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW and Westlake C. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. [DOI] [PubMed] [Google Scholar]
- 109.Hara A, Niwa M, Noguchi K, Kanayama T, Niwa A, Matsuo M, Hatano Y and Tomita H. Galectin-3 as a Next-Generation Biomarker for Detecting Early Stage of Various Diseases. Biomolecules. 2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ho JE, Liu C, Lyass A, Courchesne P, Pencina MJ, Vasan RS, Larson MG and Levy D. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol. 2012;60:1249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.de Boer RA, van Veldhuisen DJ, Gansevoort RT, Muller Kobold AC, van Gilst WH, Hillege HL, Bakker SJ and van der Harst P. The fibrosis marker galectin-3 and outcome in the general population. Journal of internal medicine. 2012;272:55–64. [DOI] [PubMed] [Google Scholar]
- 112.Daniels LB, Clopton P, Laughlin GA, Maisel AS and Barrett-Connor E. Galectin-3 is independently associated with cardiovascular mortality in community-dwelling older adults without known cardiovascular disease: The Rancho Bernardo Study. American heart journal. 2014;167:674–82.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jagodzinski A, Havulinna AS, Appelbaum S, Zeller T, Jousilahti P, Skytte-Johanssen S, Hughes MF, Blankenberg S and Salomaa V. Predictive value of galectin-3 for incident cardiovascular disease and heart failure in the population-based FINRISK 1997 cohort. Int J Cardiol. 2015;192:33–9. [DOI] [PubMed] [Google Scholar]
- 114.Gehlken C, Suthahar N, Meijers WC and de Boer RA. Galectin-3 in Heart Failure: An Update of the Last 3 Years. Heart failure clinics. 2018;14:75–92. [DOI] [PubMed] [Google Scholar]
- 115.Anand IS, Rector TS, Kuskowski M, Adourian A, Muntendam P and Cohn JN. Baseline and serial measurements of galectin-3 in patients with heart failure: relationship to prognosis and effect of treatment with valsartan in the Val-HeFT. Eur J Heart Fail. 2013;15:511–8. [DOI] [PubMed] [Google Scholar]
- 116.Meyer S, van der Meer P, van Deursen VM, Jaarsma T, van Veldhuisen DJ, van der Wal MH, Hillege HL and Voors AA. Neurohormonal and clinical sex differences in heart failure. European heart journal. 2013;34:2538–47. [DOI] [PubMed] [Google Scholar]
- 117.Schindler EI, Szymanski JJ, Hock KG, Geltman EM and Scott MG. Short- and Long-term Biologic Variability of Galectin-3 and Other Cardiac Biomarkers in Patients with Stable Heart Failure and Healthy Adults. Clin Chem. 2016;62:360–6. [DOI] [PubMed] [Google Scholar]
- 118.Dencker M, Arvidsson D, Karlsson MK, Wollmer P, Andersen LB and Thorsson O. Galectin-3 levels relate in children to total body fat, abdominal fat, body fat distribution, and cardiac size. European journal of pediatrics. 2018;177:461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nayor M, Wang N, Larson MG, Vasan RS, Levy D and Ho JE. Circulating Galectin-3 Is Associated With Cardiometabolic Disease in the Community. J Am Heart Assoc. 2015;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pang J, Nguyen VT, Rhodes DH, Sullivan ME, Braunschweig C and Fantuzzi G. Relationship of galectin-3 with obesity, IL-6, and CRP in women. Journal of endocrinological investigation. 2016;39:1435–1443. [DOI] [PubMed] [Google Scholar]
- 121.Lau ES, Liu E, Paniagua SM, Sarma AA, Zampierollo G, López B, Díez J, Wang TJ and Ho JE. Galectin-3 Inhibition With Modified Citrus Pectin in Hypertension. JACC Basic to translational science. 2021;6:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Imran TF, Shin HJ, Mathenge N, Wang F, Kim B, Joseph J, Gaziano JM and Djoussé L. Meta-Analysis of the Usefulness of Plasma Galectin-3 to Predict the Risk of Mortality in Patients With Heart Failure and in the General Population. The American journal of cardiology. 2017;119:57–64. [DOI] [PubMed] [Google Scholar]
- 123.Ghorbani A, Bhambhani V, Christenson RH, Meijers WC, de Boer RA, Levy D, Larson MG and Ho JE. Longitudinal Change in Galectin-3 and Incident Cardiovascular Outcomes. J Am Coll Cardiol. 2018;72:3246–3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lopez-Andrès N, Rossignol P, Iraqi W, Fay R, Nuée J, Ghio S, Cleland JG, Zannad F and Lacolley P. Association of galectin-3 and fibrosis markers with long-term cardiovascular outcomes in patients with heart failure, left ventricular dysfunction, and dyssynchrony: insights from the CARE-HF (Cardiac Resynchronization in Heart Failure) trial. Eur J Heart Fail. 2012;14:74–81. [DOI] [PubMed] [Google Scholar]
- 125.van Kimmenade RR, Januzzi JL Jr., Ellinor PT, Sharma UC, Bakker JA, Low AF, Martinez A, Crijns HJ, MacRae CA, Menheere PP and Pinto YM. Utility of amino-terminal pro-brain natriuretic peptide, galectin-3, and apelin for the evaluation of patients with acute heart failure. J Am Coll Cardiol. 2006;48:1217–24. [DOI] [PubMed] [Google Scholar]
- 126.Hara A, Niwa M, Kanayama T, Noguchi K, Niwa A, Matsuo M, Kuroda T, Hatano Y, Okada H and Tomita H. Galectin-3: A Potential Prognostic and Diagnostic Marker for Heart Disease and Detection of Early Stage Pathology. Biomolecules. 2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Meeusen JW, Johnson JN, Gray A, Wendt P, Jefferies JL, Jaffe AS, Donato LJ and Saenger AK. Soluble ST2 and galectin-3 in pediatric patients without heart failure. Clinical biochemistry. 2015;48:1337–40. [DOI] [PubMed] [Google Scholar]
- 128.Dieplinger B, Januzzi JL Jr., Steinmair M, Gabriel C, Poelz W, Haltmayer M and Mueller T Analytical and clinical evaluation of a novel high-sensitivity assay for measurement of soluble ST2 in human plasma--the Presage ST2 assay. Clinica chimica acta; international journal of clinical chemistry. 2009;409:33–40. [DOI] [PubMed] [Google Scholar]
- 129.Lu J, Snider JV and Grenache DG. Establishment of reference intervals for soluble ST2 from a United States population. Clinica chimica acta; international journal of clinical chemistry. 2010;411:1825–6. [DOI] [PubMed] [Google Scholar]
- 130.Dieplinger B, Egger M, Poelz W, Gabriel C, Haltmayer M and Mueller T. Soluble ST2 is not independently associated with androgen and estrogen status in healthy males and females. Clinical chemistry and laboratory medicine. 2011;49:1515–8. [DOI] [PubMed] [Google Scholar]
- 131.Coglianese EE, Larson MG, Vasan RS, Ho JE, Ghorbani A, McCabe EL, Cheng S, Fradley MG, Kretschman D, Gao W, O’Connor G, Wang TJ and Januzzi JL. Distribution and clinical correlates of the interleukin receptor family member soluble ST2 in the Framingham Heart Study. Clin Chem. 2012;58:1673–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Anand IS, Rector TS, Kuskowski M, Snider J and Cohn JN. Prognostic value of soluble ST2 in the Valsartan Heart Failure Trial. Circ Heart Fail. 2014;7:418–26. [DOI] [PubMed] [Google Scholar]
- 133.Zhao XY, Zhou L, Chen Z, Ji Y, Peng X, Qi L, Li S and Lin JD. The obesity-induced adipokine sST2 exacerbates adipose T(reg) and ILC2 depletion and promotes insulin resistance. Science advances. 2020;6:eaay6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Demyanets S, Kaun C, Kaider A, Speidl W, Prager M, Oravec S, Hohensinner P, Wojta J and Rega-Kaun G. The pro-inflammatory marker soluble suppression of tumorigenicity-2 (ST2) is reduced especially in diabetic morbidly obese patients undergoing bariatric surgery. Cardiovascular diabetology. 2020;19:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.de Boer RA, Nayor M, deFilippi CR, Enserro D, Bhambhani V, Kizer JR, Blaha MJ, Brouwers FP, Cushman M, Lima JAC, Bahrami H, van der Harst P, Wang TJ, Gansevoort RT, Fox CS, Gaggin HK, Kop WJ, Liu K, Vasan RS, Psaty BM, Lee DS, Hillege HL, Bartz TM, Benjamin EJ, Chan C, Allison M, Gardin JM, Januzzi JL Jr., Shah SJ, Levy D, Herrington DM, Larson MG, van Gilst WH, Gottdiener JS, Bertoni AG and Ho JE. Association of Cardiovascular Biomarkers With Incident Heart Failure With Preserved and Reduced Ejection Fraction. JAMA Cardiol. 2018;3:215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dieplinger B, Gegenhuber A, Kaar G, Poelz W, Haltmayer M and Mueller T. Prognostic value of established and novel biomarkers in patients with shortness of breath attending an emergency department. Clinical biochemistry. 2010;43:714–9. [DOI] [PubMed] [Google Scholar]
- 137.Januzzi JL Jr., Peacock WF, Maisel AS, Chae CU, Jesse RL, Baggish AL, O’Donoghue M, Sakhuja R, Chen AA, van Kimmenade RR, Lewandrowski KB, Lloyd-Jones DM and Wu AH. Measurement of the interleukin family member ST2 in patients with acute dyspnea: results from the PRIDE (Pro-Brain Natriuretic Peptide Investigation of Dyspnea in the Emergency Department) study. J Am Coll Cardiol. 2007;50:607–13. [DOI] [PubMed] [Google Scholar]
- 138.Manzano-Fernández S, Mueller T, Pascual-Figal D, Truong QA and Januzzi JL. Usefulness of soluble concentrations of interleukin family member ST2 as predictor of mortality in patients with acutely decompensated heart failure relative to left ventricular ejection fraction. The American journal of cardiology. 2011;107:259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rehman SU, Mueller T and Januzzi JL Jr. Characteristics of the novel interleukin family biomarker ST2 in patients with acute heart failure. J Am Coll Cardiol. 2008;52:1458–65. [DOI] [PubMed] [Google Scholar]
- 140.Shah RV, Chen-Tournoux AA, Picard MH, van Kimmenade RR and Januzzi JL. Galectin-3, cardiac structure and function, and long-term mortality in patients with acutely decompensated heart failure. Eur J Heart Fail. 2010;12:826–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tang WH, Shrestha K, Shao Z, Borowski AG, Troughton RW, Thomas JD and Klein AL. Usefulness of plasma galectin-3 levels in systolic heart failure to predict renal insufficiency and survival. The American journal of cardiology. 2011;108:385–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.de Boer RA, Lok DJ, Jaarsma T, van der Meer P, Voors AA, Hillege HL and van Veldhuisen DJ. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Annals of medicine. 2011;43:60–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Emdin M, Aimo A, Vergaro G, Bayes-Genis A, Lupón J, Latini R, Meessen J, Anand IS, Cohn JN, Gravning J, Gullestad L, Broch K, Ueland T, Nymo SH, Brunner-La Rocca HP, de Boer RA, Gaggin HK, Ripoli A, Passino C and Januzzi JL Jr. sST2 Predicts Outcome in Chronic Heart Failure Beyond NT-proBNP and High-Sensitivity Troponin T. J Am Coll Cardiol. 2018;72:2309–2320. [DOI] [PubMed] [Google Scholar]
- 144.Lau ES, Braunwald E, Morrow DA, Giugliano RP, Antman EM, Gibson CM, Scirica BM, Bohula EA, Wiviott SD, Bhatt DL, Bonaca MP, Cannon CP, Im K, Guo J, Sabatine MS and O’Donoghue ML. Sex, Permanent Drug Discontinuation, and Study Retention in Clinical Trials: Insights From the TIMI trials. Circulation. 2021;143:685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kim Esther SH, Carrigan Thomas P and Menon V. Enrollment of Women in National Heart, Lung, and Blood Institute-Funded Cardiovascular Randomized Controlled Trials Fails to Meet Current Federal Mandates for Inclusion. Journal of the American College of Cardiology. 2008;52:672–673. [DOI] [PubMed] [Google Scholar]
- 146.Woodward M. Rationale and tutorial for analysing and reporting sex differences in cardiovascular associations. Heart. 2019;105:1701–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Committee Opinion No. 646: Ethical Considerations for Including Women as Research Participants. Obstet Gynecol. 2016;127:e100–7.doi: 10.1097/AOG.0000000000001150 [DOI] [PubMed] [Google Scholar]
- 148.Julinda M Do we still need single-sex clinical trials in cardiology? EuroIntervention. 2018;14:e261–e263. [DOI] [PubMed] [Google Scholar]
- 149.Winham SJ, de Andrade M and Miller VM. Genetics of cardiovascular disease: Importance of sex and ethnicity. Atherosclerosis. 2015;241:219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mosca L, Appel LJ, Benjamin EJ, Berra K, Chandra-Strobos N, Fabunmi RP, Grady D, Haan CK, Hayes SN, Judelson DR, Keenan NL, McBride P, Oparil S, Ouyang P, Oz MC, Mendelsohn ME, Pasternak RC, Pinn VW, Robertson RM, Schenck-Gustafsson K, Sila CA, Smith SC, Sopko G, Taylor AL, Walsh BW, Wenger NK and Williams CL. Evidence-Based Guidelines for Cardiovascular Disease Prevention in Women. Circulation. 2004;109:672–693. [DOI] [PubMed] [Google Scholar]


