Abstract
In this study, the tolerance to salt stress of the photosynthetic machinery was examined in relation to the effects of the genetic enhancement of the unsaturation of fatty acids in membrane lipids in wild-type and desA+ cells of Synechococcus sp. PCC 7942. Wild-type cells synthesized saturated and mono-unsaturated fatty acids, whereas desA+ cells, which had been transformed with the desA gene for the Δ12 acyl-lipid desaturase of Synechocystis sp. PCC 6803, also synthesized di-unsaturated fatty acids. Incubation of wild-type and desA+ cells with 0.5 m NaCl resulted in the rapid loss of the activities of photosystem I, photosystem II, and the Na+/H+ antiport system both in light and in darkness. However, desA+ cells were more tolerant to salt stress and osmotic stress than the wild-type cells. The extent of the recovery of the various photosynthetic activities from the effects of 0.5 m NaCl was much greater in desA+ cells than in wild-type cells. The photosystem II activity of thylakoid membranes from desA+ cells was more resistant to 0.5 m NaCl than that of membranes from wild-type cells. These results demonstrated that the genetically engineered increase in unsaturation of fatty acids in membrane lipids significantly enhanced the tolerance of the photosynthetic machinery to salt stress. The enhanced tolerance was due both to the increased resistance of the photosynthetic machinery to the salt-induced damage and to the increased ability of desA+ cells to repair the photosynthetic and Na+/H+ antiport systems.
Salt stress is one of the main environmental factors that limit the growth and productivity of plants and micro-organisms. We have been investigating the mechanisms of the hyperosmotic stress-induced and the salt stress-induced inactivation of the photosynthetic machinery, focussing on the oxygen-evolving machinery of the photosystem II complex, which is the system that is most susceptible to such environmental stress in Synechococcus sp. PCC 7942 (hereafter Synechococcus; Allakhverdiev et al., 2000a, 2000b). Hyperosmotic stress due to 1.0 m sorbitol induces the efflux of water through water channels and reduces the volume of cells by more than 50%. This loss of water from the cytosol might be expected to increase the intracellular concentration of salts, and it leads to the rapid but reversible inactivation of the oxygen-evolving machinery (Allakhverdiev et al., 2000b).
Salt stress due to 0.5 m NaCl has both osmotic and ionic effects (Allakhverdiev et al., 2000a). The osmotic effect due to 0.5 m NaCl is not as strong as the effect of 1.0 m sorbitol and inactivates reversibly the oxygen-evolving machinery. The ionic effect of 0.5 m NaCl is caused by the influx of Na+ ions through K+(Na+) channels and the resultant increase in the intracellular concentration of Na+ ions and counterpart anions that are mostly Cl− ions (Allakhverdiev et al., 2000a). These changes result in the irreversible inactivation of the oxygen-evolving machinery. As a consequence salt stress appears to be much more damaging to the oxygen-evolving machinery than osmotic stress.
Photosynthetic organisms, including cyanobacteria, have several kinds of mechanism that allow them to acclimate to salt stress, for example, the inducible synthesis of compatible solutes. Suc is synthesized in salt-sensitive strains of cyanobacteria such as Synechococcus (Mackay et al., 1984; Reed et al., 1986; for reviews, see Joset et al., 1996; Hagemann and Erdmann, 1997, and references therein); glucosylglycerol is synthesized in strains with intermediary tolerance such as Synechocystis sp. PCC 6803 (Hagemann et al., 1987; Erdmann et al., 1992; for reviews see Joset et al., 1996; Hagemann and Erdmann, 1997, and references therein); and glycinebetaine is synthesized in salt-tolerant strains such as Synechococcus sp. PCC 7418 (Aphanothece halophytica; Mackay et al., 1984; Reed et al., 1986; for reviews, see Joset et al., 1996; Hagemann and Erdmann, 1997, and references therein). Direct evidence for the ability of these compatible solutes to protect the cyanobacterial cells has been provided from studies of transgenic systems (Deshnium et al., 1995, 1997; Ishitani et al., 1995; Nakamura et al., 1997).
Several reports have suggested that lipids might be involved in the protection against salt stress (Huflejt et al., 1990; Khamutov et al., 1990; Ritter and Yopp, 1993). When photosynthetic organisms are exposed to salt stress, the fatty acids of membrane lipids are desaturated. A hypothesis has been presented to explain the role of such desaturation, but no direct evidence for the hypothesis has been provided. We have used targeted mutagenesis to alter genes for fatty acid desaturases in Synechocystis sp. PCC 6803, and we have produced strains with decreased levels of unsaturated fatty acids in their membrane lipids (Tasaka et al., 1996) as well as decreased tolerance to salt (Allakhverdiev et al., 1999).
The aim of the present study was to examine the contribution of the unsaturation of fatty acids in membrane lipids to tolerance to salt stress using transgenic Synechococcus. This cyanobacterium normally contains only saturated and mono-unsaturated fatty acids in its membrane lipids (Murata and Wada, 1995). Transformation of this micro-organism with the desA gene from Synechocystis sp. PCC 6803 allows it to synthesize di-unsaturated fatty acids (Sakamoto et al., 1994). The results of the present study demonstrate that an increase in the unsaturation of fatty acids in membrane lipids enhances the tolerance to salt stress of the photosynthetic and Na+/H+-antiport systems of Synechococcus.
RESULTS
Fatty Acid Composition of Wild-Type and desA+ Cells
We investigated changes in the fatty acid composition of glycerolipids after transformation of Synechococcus cells with the desA+ gene for Δ12 desaturase (Table I). The most abundant fatty acids in wild-type cells were 16:0 (49% of the total fatty acids) and 16:1(9) (41%). However, we also found low levels of 18:0, 18:1(9), and 18:1(11) in wild-type cells. In desA+ cells 16:2(9, 12) appeared (15%) at the expense of 16:1(9), suggesting that some of the 16:1(9) had been desaturated to 16:2(9, 12). In desA+ cells, 18:2(9, 12) accounted for 3% of the total fatty acids. These results indicate that the wild-type cells contained only saturated and mono-unsaturated fatty acids, whereas the desA+ cells contained, in addition, di-unsaturated fatty acids such as 16:2(9, 12) and 18:2(9, 12).
Table I.
Fatty acid composition of wild-type Synechococcus sp. PCC 7942 and of desA+ cells after growth at 32°C
| Strain | Fatty Acid
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 16:0 | 16:1 (9) | 16:2 (9,12) | 18:0 | 18:1 (9) | 18:1 (11) | 18:2 (9,12) | 18:2 (?) | |
| mol % | ||||||||
| Wild type | 49 | 41 | 0 | 2 | 4 | 4 | 0 | 0 |
| desA+ | 48 | 25 | 15 | 2 | 3 | 1 | 3 | 3 |
Positions of double bonds were not determined for 18:2(?). Each value represents the average of results from four independent experiments. Experimental deviations were within 2% for 16:0, 16:1 (9), and 16:2 (9,12) and within 0.5% for the other fatty acids.
Inactivation of Photosystem II under Salt Stress and Osmotic Stress
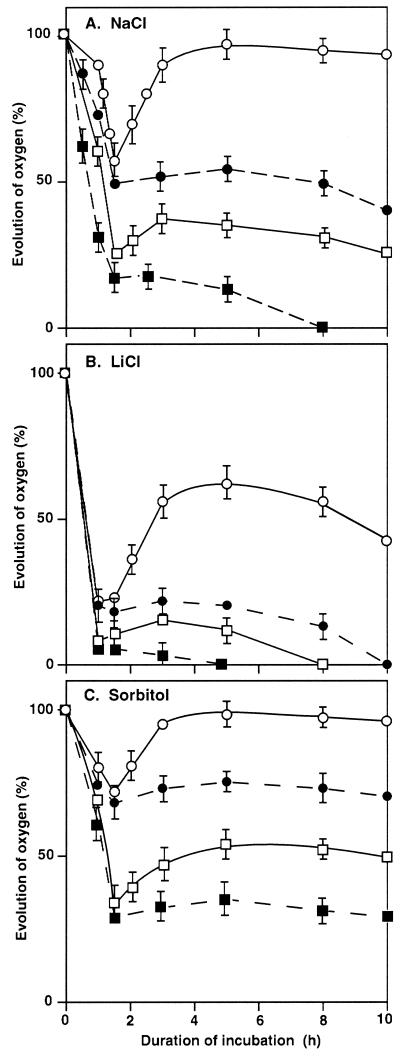
Figure 1 shows the changes in the oxygen-evolving activity of photosystem II (PSII) during incubation of wild-type and desA+ cells in the presence of NaCl, LiCl, or sorbitol. During incubation with 0.5 m NaCl in darkness (Fig. 1A), the oxygen-evolving activity of wild-type cells declined to approximately 20% of the original level within 1.5 h. Then it continued to decrease gradually until it finally disappeared at 8 h, as observed in a previous study (Allakhverdiev et al., 2000a). During the incubation of desA+ cells in darkness, the oxygen-evolving activity also declined rapidly to approximately 50% of the original level in 1.5 h. However, it remained at approximately the same level for the next several hours.
Figure 1.
Changes in the oxygen-evolving activity of PSII in wild-type and desA+ cells during incubation with NaCl, LiCl, and sorbitol. Cells were incubated in darkness or in light at 70 μE m−2 s−1 in the presence of 0.5 m NaCl (A), 0.5 m LiCl (B), or 1.0 m sorbitol (C). At designated times, a portion of the cell suspension was withdrawn. The oxygen-evolving activity was measured after addition of 1.0 mm BQ to the suspension. The activities of wild-type and desA+ cells that corresponded to 100% were 548 ± 30 and 576 ± 35 μmol O2 mg−1 Chl h−1, respectively. ▪, Wild-type cells in darkness; □, wild-type cells in light; ●, desA+ cells in darkness; ○, desA+ cells in light. Each point and bar represent the average ± se of results from four independent experiments.
When similar experiments were performed with illumination at 70 μE m−2 s−1, the oxygen-evolving activity in both wild-type and desA+ cells declined rapidly, as observed in darkness. However, light had a striking effect, namely, restoration of the oxygen-evolving activity after the initial decline. This effect was more pronounced in desA+ than in wild-type cells, and in desA+ cells the oxygen-evolving activity was fully restored within 2 h. In wild-type cells, by contrast, the extent of the restoration of activity was more limited. These observations indicated that PSII of desA+ cells was more resistant to salt stress than the PSII of wild-type cells and that the difference was especially pronounced under illumination.
We obtained similar results when cells were incubated in the presence of 0.5 m LiCl in darkness or in light (Fig. 1B). However, the PSII complex in both wild-type and desA+ cells was inactivated to a greater extent by 0.5 m LiCl than by 0.5 m NaCl. The ability of light to restore the oxygen-evolving activity was minimal in wild-type cells. By contrast, in desA+ cells, activity returned to approximately 50% of the original level within 3 h and remained at this level for the remainder of the 10-h incubation.
During incubation of cells with 1.0 m sorbitol for 1.5 h, the oxygen-evolving activity declined both in darkness and in light to approximately 30% and 70% of the original level in wild-type and desA+ cells, respectively (Fig. 1C). No restoration of activity during the subsequent 8.5-h incubation was observed in darkness in either type of cell. However, in light at 70 μE m−2 s−1, the oxygen-evolving activity returned to the original high level within 3 h in desA+ cells. In wild-type cells, only 50% of the original activity was regained. These observations suggest that desA+ cells were also more tolerant than wild-type cells to osmotic stress.
Inactivation of Photosystem I under Salt Stress and Osmotic Stress
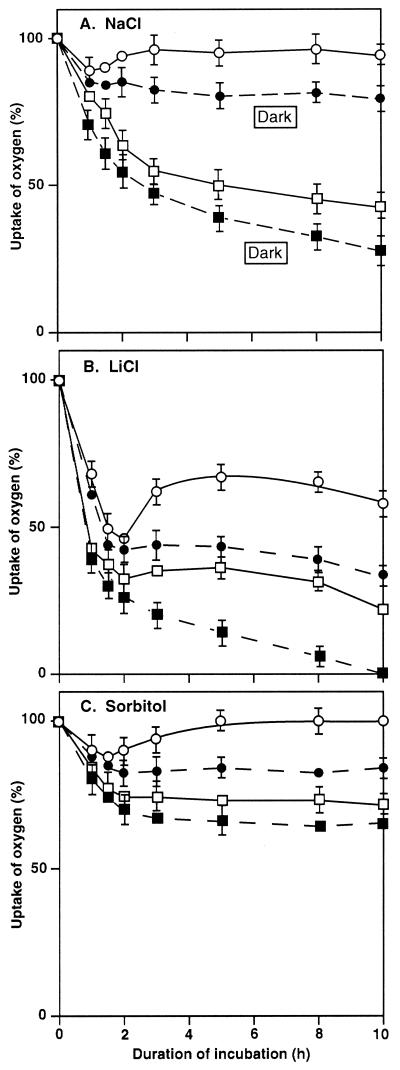
We next compared the tolerance to NaCl, LiCl, and sorbitol of wild-type and desA+ cells in terms of photosystem I (PSI) activity, which was monitored by measuring the uptake of oxygen in the presence of 3-(3′,4′-dichlorophenyl)-1,1-dimethylurea (DCMU), methyl viologen (MV), 2,6-dichlorophenolindophenol (DCIP), and ascorbate (Fig. 2). When both types of cell were incubated in the presence of 0.5 m NaCl in darkness, the PSI activity declined within 2 h to 50% and 85% of the original level, respectively (Fig. 2A). PSI activity appeared to be more tolerant to NaCl than PSII activity in both types of cell. Light alleviated the effects of NaCl, but the mitigating effect of light was more prominent in desA+ cells than in wild-type cells. Almost 100% of the original activity of PSI was restored in desA+ cells within 3 h (Fig. 2A).
Figure 2.
Changes in PSI activity in wild-type and desA+ cells during incubation with NaCl, LiCl, and sorbitol. Cells were incubated in darkness or in light at 70 μE m−2 s−1 in the presence of 0.5 m NaCl (A), 0.5 m LiCl (B), or 1.0 m sorbitol (C). At designated times, a portion of the cell suspension was withdrawn. The PSI activity was measured by monitoring the uptake of oxygen after addition of 15 μm DCMU, 0.1 mm DCIP, 5 mm sodium ascorbate, and 0.1 mm MV to the suspension. The activities of wild-type and desA+ cells that corresponded to 100% were 314 ± 27 and 332 ± 30 μmol O2 mg−1 Chl h−1, respectively. ▪, Wild-type cells in darkness; □, wild-type cells in light; ●, desA+ cells in darkness; ○, desA+ cells in light. Each point and bar represent the average ± se of results from five independent experiments.
Incubation with 0.5 m LiCl markedly inhibited the activity of PSI in both types of cell (Fig. 2B) and the activity in wild-type and desA+ cells declined within 2 h to 30% and 45% of the original level, respectively. Light at 70 μE m−2 s−1 restored some activity after the rapid decline but the effect of 0.5 m LiCl was more damaging than that of NaCl. However, it was clear that the PSI complex in desA+ cells, in darkness and in light, was much more tolerant to LiCl than that in wild-type cells.
During incubation with 1.0 m sorbitol, PSI activity declined within 2 h to 70% and 85% of the original level in wild-type and desA+ cells, respectively (Fig. 2C). During subsequent incubation in light for 4 h, the PSI activity of desA+ cells returned to the original level and remained at that level for the remainder of the 10-h incubation. In darkness, the PSI activity of desA+ cells was always higher than that of wild-type cells.
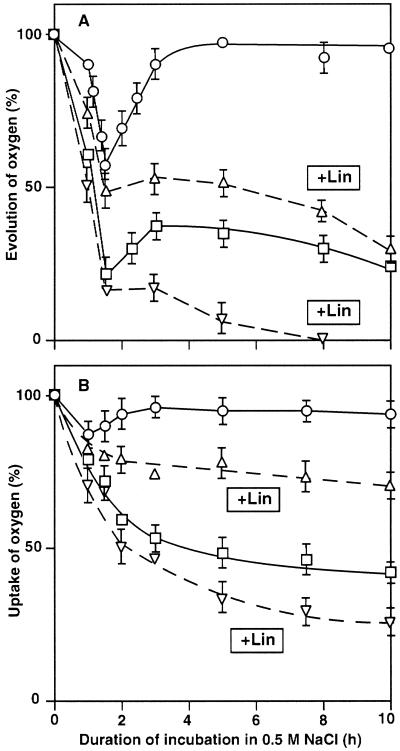
Effects of Lincomycin on the Salt-Induced Inactivation of PSII and PSI
To investigate the possible involvement of protein synthesis in the tolerance to salt stress, we examined the effects of lincomycin, an inhibitor of protein synthesis, on the NaCl-induced inactivation of PSII and PSI in light (Fig. 3). During incubation of wild-type and desA+ cells in medium that contained 0.5 m NaCl, lincomycin at 200 μg mL−1 eliminated the ability of light to restore the oxygen-evolving activity of PSII in both types of cell (Fig. 3A). Essentially the same result was obtained in the case of PSI activity (Fig. 3B). By contrast, lincomycin had no significant effect on the salt-induced inactivation of PSII and PSI in darkness in wild-type and desA+ cells (data not shown). These observations suggested that synthesis of proteins was involved in the light-induced restoration of the activities of PSII and PSI, in particular in desA+ cells.
Figure 3.
Effects of lincomycin (Lin) on the NaCl-induced inactivation of PSII and PSI in wild-type and desA+ cells. Cells were incubated with 0.5 m NaCl in light at 70 μE m−2 s−1 in the presence of lincomycin at 200 μg mL−1 (dashed lines) or in its absence (solid lines). At designated times, a portion of the cell suspension was withdrawn. A, The oxygen-evolving activity of PSII was measured after addition of 1.0 mm BQ to the suspension. The oxygen-evolving activities of wild-type and desA+ cells that corresponded to 100% were 568 ± 32 and 576 ± 39 μmol O2 mg−1 Chl h−1, respectively. B, PSI activity was measured by monitoring the uptake of oxygen after addition of 15 μm DCMU, 0.1 mm DCIP, 5 mm sodium ascorbate, and 0.1 mm MV to the suspension. The oxygen-uptake activities of wild-type and desA+ cells that corresponded to 100% were 286 ± 24 and 288 ± 27 μmol O2 mg−1 Chl h−1, respectively. Wild-type (□) and desA+ (○) cells in the absence of lincomycin. Wild-type (▿) and desA+ (▵) cells in the presence of lincomycin. Each point and bar represent the average ± se of results from five independent experiments.
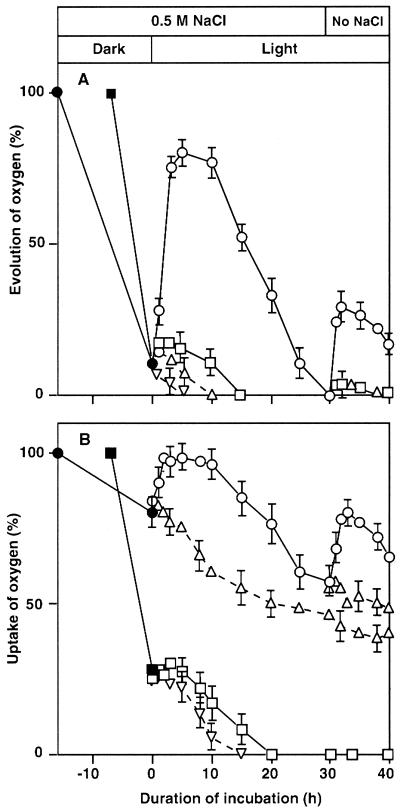
Light-Dependent Recovery of PSII and PSI from NaCl-Induced Inactivation
Figure 4A shows the effects of light at 70 μE m−2 s−1 on the recovery of oxygen-evolving activity after almost all activity had been lost during the incubation of wild-type and desA+ cells in 0.5 m NaCl in darkness. Under these conditions, more than 75% of the original oxygen-evolving activity of PSII was restored within 3 h in desA+ cells, whereas the extent of recovery was less than 10% in wild-type cells. the oxygen-evolving activity subsequently started to decrease and ceased completely at 15 h in wild-type cells and at 30 h in desA+ cells.
Figure 4.
Effects of light and the removal of NaCl on the recovery of PSII and PSI activities in wild-type and desA+ cells after NaCl-induced inactivation. Wild-type and desA+ cells were incubated for 7 and 16 h, respectively, in darkness in the presence of 0.5 m NaCl. Then cells were further incubated in light at 70 μE m−2 s−1 in the presence of lincomycin at 200 μg mL−1 or in its absence. After incubation for 30 h in light, the cells were collected by centrifugation, resuspended in fresh BG-11 medium with no added NaCl, and incubated in light for a further 10 h. At designated times, a portion of the cell suspension was withdrawn. A, The oxygen-evolving activity of PS II was measured after addition of 1.0 mm BQ to the suspension. The oxygen-evolving activities of wild-type and desA+ cells that corresponded to 100% were 527 ± 36 and 543 ± 33 μmol O2 mg−1 Chl h−1, respectively. B, PSI activity was measured by monitoring the uptake of oxygen after the addition of 15 μm DCMU, 0.1 mm DCIP, 5 mm sodium ascorbate, and 0.1 mm MV to the suspension. The oxygen-uptake activities of wild-type and desA+ cells that corresponded to 100% were 315 ± 26 and 309 ± 21 μmol O2 mg−1 Chl h−1, respectively. ▪ and □, Wild-type cells; ● and ○, desA+ cells in the absence of lincomycin. Wild-type (▿) and desA+ (▵) cells in the presence of lincomycin. Each point and bar represent the average ± se of results from four independent experiments.
When NaCl was removed at 30 h by pelleting and resuspension of cells, the PSII activity in desA+ cells recovered to a small but significant extent with restoration of approximately 25% of the original activity in 2 h and then started to decrease again. No similar recovery was observed in wild-type cells. The presence of lincomycin (200 μg mL−1) completely eliminated the recovery of the oxygen-evolving activity in both types of cell (Fig. 4A). These results clearly suggested that protein synthesis was required for the recovery of PSII activity in light and after removal of NaCl.
Figure 4B shows the recovery of PSI activity after NaCl-induced inactivation in wild-type and desA+ cells. Wild-type and desA+ cells were incubated with 0.5 m NaCl for 7 and 16 h, respectively, in darkness, which caused approximately 75% and 20% inactivation of PSI, respectively. Then they were exposed to light at 70 μE m−2 s−1. Under these conditions, the PSI activity in desA+ cells returned almost to the original level within 3 h. In wild-type cells, the extent of recovery was less than 5%, but then the PSI activity started to decrease again and disappeared completely within 20 h. By contrast, in desA+ cells 60% of the original activity was detectable at 30 h.
When NaCl was removed at 30 h, approximately 80% of the original activity was detected in desA+ cells within 2 h but no recovery was observed in wild-type cells (Fig. 4B). Lincomycin prevented the recovery of PSI activity in light and upon removal of NaCl in desA+ cells (Fig. 4B), results that suggested that protein synthesis was required for the recovery of PSI activity under these conditions.
NaCl-Induced Inactivation of the Oxygen-Evolving Machinery in Vitro
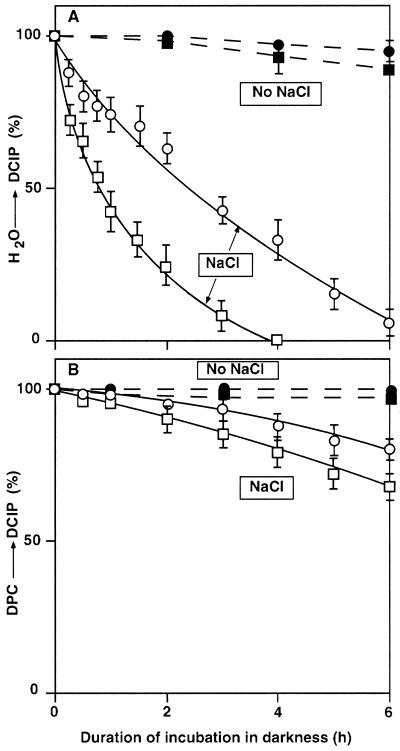
We compared the effects of NaCl on the oxygen-evolving activity of isolated thylakoid membranes from wild-type and desA+ cells. Figure 5 shows that, during incubation of thylakoid membranes in the presence of 0.5 m NaCl in darkness, the transport of electrons from water to DCIP was inhibited much more rapidly than in intact cells of both types. Moreover, the inactivation in thylakoid membranes from wild-type cells was more rapid than that in membranes from desA+ cells: The time required for a 50% inactivation was 50 and 150 min for thylakoid membranes from wild-type and desA+ cells, respectively. In the absence of NaCl, the inactivation of thylakoid membranes from both types of cell was very slow (Fig. 5A). The transport of electrons from 1,5-diphenylcarbazide (DPC) to DCIP, which bypasses the oxygen-evolving site (Yamashita and Butler, 1969), was not inactivated as rapidly during incubation with 0.5 m NaCl as transport from water to DCIP (Fig. 5B). These observations demonstrated that incubation of thylakoid membranes with NaCl resulted primarily in damage to the oxygen-evolving site in the PSII complex. Another set of experiments indicated that light at 70 μE m−2 s−1 had no effect on the NaCl-induced inactivation of the oxygen-evolving machinery in isolated thylakoid membranes (data not shown).
Figure 5.
Changes in the PSII activity of thylakoid membranes isolated from wild-type and desA+ cells. Thylakoid membranes (10 μg Chl mL−1) were incubated in darkness in the presence of 0.5 m NaCl or in its absence. At designated times, a portion of the suspension was withdrawn and the light-induced reduction of DCIP was measured after addition of 0.1 mm DCIP or 0.1 mm DCIP and 0.5 mm DPC to the suspension. A, The transport of electrons from water to DCIP. The activities that corresponded to 100% were 173 ± 15 and 180 ± 17 μmol DCIP reduced mg−1 Chl h−1 in thylakoid membranes from wild-type and desA+ cells, respectively. B, The transport of electrons from DPC to DCIP. The activities that corresponded to 100% were 344 ± 20 and 332 ± 25 μmol DCIP reduced mg−1 Chl h−1 in thylakoid membranes from wild-type and desA+ cells, respectively. □, Thylakoid membranes from wild-type cells; ○, thylakoid membranes from desA+ cells; both were incubated with 0.5 m NaCl. ▪, Thylakoid membranes from wild-type cells; ●, thylakoid membranes from desA+ cells; both were incubated in the absence of added NaCl. Each point and bar represent the average ± se of results from four independent experiments.
NaCl-Induced Inhibition of the Reduction of P700+
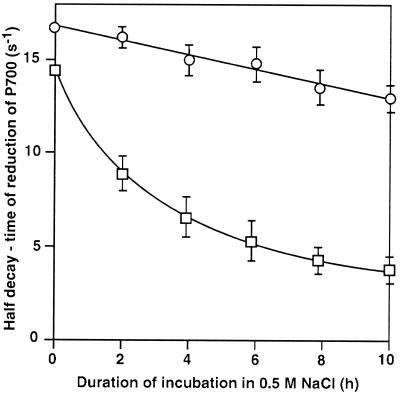
To examine whether incubation with NaCl might affect the redox reaction of P700 in the PSI complex, we determined the rate of reduction of P700+ after illumination of intact cells with a 5-ms flash of saturating light in the presence of DCMU, MV, and the reduced form of DCIP. The one-half decay time of the reduction in the absence of NaCl was approximately 67 and 62 ms in wild-type and desA+ cells, respectively. Figure 6 shows that incubation of wild-type and desA+ cells in the presence of 0.5 m NaCl in light at 70 μE m−2 s−1 retarded the reduction of P700+. The rate of reduction of P700+ decreased much more rapidly in wild-type cells than in desA+ cells. The extent of the light-induced oxidation of P700 was almost unchanged during incubation of both types of cell with 0.5 m NaCl.
Figure 6.
Changes in the rate of reduction of P700+ in wild-type and desA+ cells during incubation in light at 70 μE m−2 s−1 in the presence of 0.5 m NaCl. At designated times, a portion of the cell suspension was withdrawn, and the rate of reduction of P700+ was measured after addition of 15 μm DCMU, 0.1 mm DCIP, 5 mm sodium ascorbate, and 0.1 mm MV to the suspension. The oxidation-reduction kinetics of P700 were examined at 820 nm with 10 flashes of 5-ms duration at a saturating light intensity of 4.5 mE m−2 s−1 from a xenon discharge lamp (XMT 103; Walz, Germany) in a multiple-turnover mode with 20-s intervals. The results were averaged. □, Wild-type cells; ○, desA+ cells. Each point and bar represent the average ± se of results from five independent experiments.
We observed a similar delay in the reduction of P700+ when both types of cell were treated with 1 mm KCN or HgCl2 (data not shown), both of which inhibit the transport of electrons from plastocyanin to P700+ (Izawa, 1980; Trebst, 1980). These findings suggested that incubation with NaCl might primarily have inactivated the transport of electrons from plastocyanin to P700+. This reaction in desA+ cells was more resistant to the damaging effects of NaCl than that in wild-type cells.
NaCl-Induced Inactivation of the Na+/H+ Antiport System
In a previous study (Allakhverdiev et al., 1999), we demonstrated that the activity of Na+/H+ antiporters is important in the protection of cyanobacterial cells against salt stress. Therefore, we examined the activity of the Na+/H+ antiport system in wild-type and desA+ cells. When a small amount of a suspension of cells in 0.5 m NaCl (20 μL) was added to 2 mL of Na+-free medium that contained acridine orange, the fluorescence of acridine orange was quenched. This phenomenon was due to the efflux of Na+ ions from cells and the influx of H+ ions into cells via the activity of the Na+/H+ antiport system (Blumwald et al., 1984; Garbarino and DuPont, 1989). Addition of 100 mm NaCl to the suspension suppressed the quenching of fluorescence, perhaps because of an increase in intracellular alkalization due to the efflux of H+ ions coupled with the influx of Na+ ions as a result of the activity of Na+/H+ antiport system (Blumwald et al., 1984; Garbarino and DuPont, 1989; for review, see Padan and Schuldiner, 1994, and references therein). Further addition of Triton X-100 to a final concentration of 0.04% (v/v) rapidly increased the fluorescence to a final steady-state level.
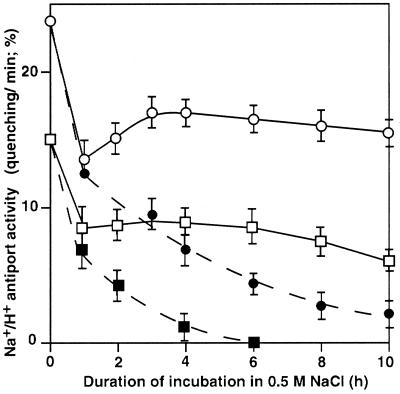
As shown in Figure 7, the Na+/H+ antiport activity of desA+ cells was higher than that of wild-type cells. Figure 7 also reveals that incubation for 1 h of wild-type and desA+ cells with 0.5 m NaCl in darkness or in light reduced the Na+/H+ antiport activity to approximately 55% and 45% of the original level, respectively. In desA+ cells the activity returned to 75% of the original level at 2 h and remained stable for the next 8 h in light. The salt-induced inactivation of the Na+/H+ antiport system in darkness was more rapid than in light, and inactivation was complete within 6 h in wild-type cells. In desA+ cells, approximately 10% of the original Na+/H+ antiport activity remained at 10 h under these conditions.
Figure 7.
Changes in the activity of the Na+/H+ antiport system in wild-type and desA+ cells during incubation in the presence of 0.5 m NaCl. Cells were incubated in the presence of 0.5 m NaCl in darkness or in light at 70 μE m−2 s−1. At designated times, 20 μL of the suspension of cells was withdrawn and diluted 100-fold with Na+-free medium that contained 5 μm acridine orange. Then the fluorescence of acridine orange was monitored as described in “Materials and Methods.” The activity of the Na+/H+ antiport system was calculated from the initial rate of recovery of fluorescence quenching upon addition of NaCl, divided by the difference between the fluorescence before the addition of NaCl and the steady-state level of fluorescence 1 min after the addition of Triton X-100 at a final concentration of 0.04% (v/v). ▪, Wild-type cells in darkness; □, wild-type cells in light; ●, desA+ cells in darkness; ○, desA+ cells in light. Each point and bar represent the average ± se of results from five independent experiments.
DISCUSSION
Possible Sites of NaCl-Induced Inactivation of PSII and PSI
In a previous study of the effects of NaCl on chlorophyll (Chl) fluorescence in Synechococcus cells, we showed that incubation of cells in the presence of NaCl did not damage QA, pheophytin, and P680 but blocked the transport of electrons from water to P680 (Allakhverdiev et al., 2000a). This earlier result is strongly supported by the results of the incubation of isolated thylakoid membranes with NaCl (Fig. 5). In the isolated membranes, the transport of electrons from water to DCIP but not from DPC to DCIP was suppressed by NaCl. Since DPC donates electrons to P680 (Yamashita and Butler, 1969), it seems likely that the oxygen-evolving machinery (Kuwabara and Murata, 1983; Miyao and Murata, 1983; Murata and Miyao, 1985) was inactivated in the presence of 0.5 m NaCl.
In the present study we also examined the effects of salt stress on PSI activity (Figs. 2–4), which was monitored by quantitating the uptake of oxygen by intact cells in the presence of DCIP, sodium ascorbate, MV, and DCMU. In this system, electrons are transported from the reduced form of DCIP to MV through plastocyanin, P700, phylloquinone (vitamin K1), and iron sulfur centers (Izawa, 1980; Trebst, 1980; Golbeck, 1994). Incubation of cells with 0.5 m NaCl suppressed the reduction of P700+ in particular in wild-type cells (Fig. 6). Since P700+ is reduced by plastocyanin (Izawa, 1980; Trebst, 1980; Golbeck, 1994), it seems likely that the association of plastocyanin with the PSI complex was distorted by the presence of NaCl.
In a previous study we also demonstrated that incubation of cells with NaCl inactivated the Na+/H+ antiport system (Allakhverdiev et al., 2000a). This inactivation might be due to inhibition of the synthesis of proteins that are involved in the Na+/H+ antiport system. Because the Na+/H+ antiport system is responsible for maintaining the intracellular concentration of Na+ ions at a certain low level in the cytosol, inactivation of the system would be expected to accelerate damage due to Na+ ions.
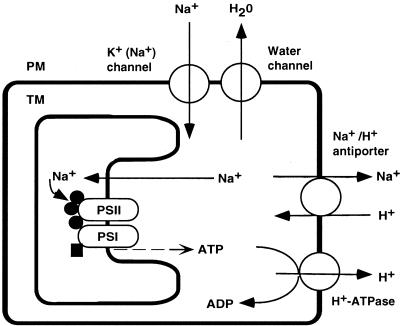
Our various results can be explained by the hypothetical scheme shown in Figure 8. Previous studies demonstrated that water channels are predominantly responsible for the hyperosmotic stress-induced inactivation of PSII and PSI by sorbitol (Allakhverdiev et al., 2000b), as well as for part of the rapid phase of the NaCl-induced inactivation of PSII and PSI (Allakh-verdiev et al., 2000a). These kinds of inactivation are reversible, and protein synthesis is not required for recovery. Such rapid and reversible inactivation might be caused by the dissociation of the three kinds of extrinsic protein of the oxygen-evolving complex that is due to an increase in the concentration of NaCl in the thylakoid lumen.
Figure 8.
A schematic explanation of the effects of Na+ ions and the unsaturation of fatty acids in membrane lipids on the activities of PSI and PSII in cyanobacterial cells. ●, Three extrinsic proteins, namely the 33-kD protein, Cyt c550, and PsbU, of the oxygen-evolving machinery of PSII complex; ▪, plastocyanin associated with the PSI complex; PM, plasma membrane; TM, thylakoid membrane.
When NaCl is supplied to the medium, K+(Na+) channels mediate an influx of Na+ ions into the cytosol, which induces the slow and irreversible inactivation of PSII and PSI (Allakhverdiev et al., 2000a). This slow and irreversible inactivation might be due to the destruction of the Mn cluster, the catalytic center of the oxygen-evolving complex. The Mn cluster might be destroyed while the three extrinsic proteins are dissociated from the PSII complex during long-term incubation of Synechococcus cells with a high concentration of NaCl (Stewart et al., 1985; Shen et al., 1992; Allakhverdiev et al., 2000a).
Protective Effects of Light on the NaCl-Induced Inactivation and Recovery of PSI and PSII
We demonstrated previously that inhibition of the PSII and PSI activities of cyanobacterial cells by incubation of the cells with NaCl is composed of two phases: rapid and slow (Allakhverdiev et al., 2000a). The rapid phase of 2-h duration is reversible and is induced partly by osmotic effects that reduce the amount of water in the cytosol via the efflux of water through water channels. The slow phase, which occurs over the course of about 8 h, is irreversible and is induced by ionic effects due to the influx of Na+ ions through K+(Na+) channels.
In the present study, we demonstrated that light restored the activities of PSII and PSI during the slow phase of the NaCl-induced inactivation, in particular in desA+ cells (Figs. 1 and 2). Moreover, light was effective in the recovery of the activities of PSII and PSI after incubation of cells with 0.5 m NaCl, which reduced these activities to low levels (Fig. 4).
The NaCl-induced inactivation of Na+/H+ antiporters in Synechococcus cells also consisted of rapid and slow phases. Light restored the activity of the Na+/H+ antiporters during the slow phase (Fig. 7). The recovery of the Na+/H+-antiport activity after incubation of cells with 0.5 m NaCl was also supported by light (A.I. Allakhverdiev, M. Hagemann, and N. Murata, unpublished data).
The intensity of light, 70 μE m−2 s−1, that we used for restoration and recovery of the activities of PSII, PSI, and Na+/H+ antiporters, was the same as that used for growth of Synechococcus cells. It is likely that the effects of light were mediated by photosynthesis, which might have generated energy such as ATP in cells (Fig. 8). The energization of cells by light might have allowed the recovery of the activities of PSII and PSI, and the Na+/H+ antiporters. The uncoupler carbonylcyanide m-chlorophenylhydrazone and carbonylcyanide p-trifluoro-methoxyphenylhydrazone, each of which induces the de-energization of cells, prevented the restoration and the recovery of the activities of PSII and PSI in light (A.I. Allakhverdiev, M. Hagemann, and N. Murata, unpublished data). These observations confirm that the energization of cells is important for the tolerance of the photosynthetic machinery to salt stress.
The effects of light were completely eliminated by lincomycin, an inhibitor of protein synthesis (Figs. 3 and 4). Thus, it is clear that when cyanobacterial cells are exposed to salt stress protein synthesis is important for the restoration and recovery of the photosynthetic machinery and the Na+/H+ antiporters.
A close correlation between the synthesis of proteins and the salt stress in Synechocystis sp. PCC 6803 cells has been shown by Hagemann et al. (1991). They demonstrated that salt stress by NaCl reduced the synthesis of most proteins to approximately 30% to 35% of the control level but specifically increased the synthesis of several proteins that might be related to salt stress. Our results in the present study are consistent with these observations.
Effects of the Unsaturation of Fatty Acids in Membrane Lipids on the NaCl-Induced Inactivation and Recovery of PSI and PSII
In the present study, we investigated the role of the unsaturation of fatty acids in membrane lipids in the tolerance of the photosynthetic machinery to salt stress using wild-type and desA+ cells of Synechococcus, in which the extent of unsaturation of the fatty acids in membrane lipids differed (Table I). Wild-type cells contained saturated and monounsaturated fatty acids but no di-unsaturated fatty acids. By contrast, desA+ cells also synthesized di-unsaturated fatty acids, namely 16:2 and 18:2.
As we demonstrated in previous studies, the NaCl-induced inactivation of PSII and PSI consists of the rapid and slow phases (Allakhverdiev et al., 2000a). In the present study, we demonstrated that the unsaturation of fatty acids in membrane lipids protected PSII and PSI against both the rapid and the slow phase of NaCl-induced inactivation. The combination of light and the unsaturation of fatty acids was the most effective in protecting the photosynthetic machinery during the slow phase.
It is likely that the extent of NaCl-induced inactivation is a result of a balance between NaCl-induced damage and recovery from such damage. Lincomycin inhibited protein synthesis and, thus, blocked recovery. Therefore, in the presence of this drug only the NaCl-induced damage was apparent. Recovery could be examined after removal of NaCl from the medium or by exposure of cells to light. The results in Figures 3 and 4 demonstrate that when repair was inhibited by lincomycin, the NaCl-induced damage to PSII and PSI was alleviated by the unsaturation of fatty acids. This result was consistent with the results in Figure 5, which shows that the NaCl-induced inactivation in isolated thylakoid membranes from desA+ cells occurred more slowly than that in membranes from wild-type cells, since no repair mechanism was operative in the isolated membranes. By contrast, the recovery of PSII and PSI activities, which was assessed directly in illuminated intact cells, was much more pronounced in desA+ cells than in wild-type cells (Fig. 4). These observations suggested that the unsaturation of fatty acids had two effects: it alleviated the NaCl-induced damage to PSI and PSII complexes and enhanced the repair of PSI and PSII from the damage. These possibilities are consistent with our previous finding in Synechocystis sp. PCC 6803 that increased saturation of fatty acids in membrane lipids as a consequence of targeted mutagenesis of genes for fatty acid desaturases increased the extent of NaCl-induced damage and suppressed recovery (Allakhverdiev et al., 1999).
We demonstrated previously that the unsaturation of fatty acids in membrane lipids protected PSII against inactivation in strong light (Tasaka et al., 1996; Gombos et al., 1997). In this earlier study, however, the unsaturation of fatty acids in membrane lipids did not affect the extent of light-induced damage but accelerated the recovery from damage. We suggested previously that translation of the psbAII/III gene for the precursor to the D1 protein was accelerated by the unsaturation of fatty acids that resulted from transformation of Synechococcus with the desA gene (Sippola et al., 1998) as in the present study.
Possible Sites Affected by the Unsaturation of Fatty Acids in Membrane Lipids
Wild-type cells were more sensitive to NaCl and less able to recover from its effects than desA+ cells. There are at least four possible explanations for these observations, as follows. (a) Water channels, the activity of which is responsible for the sorbitol-induced inactivation (Allakhverdiev et al., 2000b) and the rapid phase of the NaCl-induced inactivation (Allakh-verdiev et al., 2000a), are located on the plasma membrane. Therefore, it is quite possible that their activity might be affected by the unsaturation of membrane lipids or by changes in the fluidity of the membrane. Such effects might explain why the unsaturation of fatty acids minimized the sorbitol-induced inactivation and the rapid phase of the NaCl-induced inactivation. (b) K+(Na+) channels are also located on the plasma membrane, and their activities might be depressed by the unsaturation of fatty acids of membrane lipids. Such effects might explain why the unsaturation of fatty acids counteracted the slow phase of NaCl-induced inactivation. (c) The Na+/H+ antiport system, consisting of Na+/H+ antiporter(s) and H+-ATPase(s), is located in the plasma membrane. The unsaturation of fatty acids in membrane lipids might activate the Na+/H+ antiport system via enhanced fluidity of the membrane with resultant protection of PSII and PSI activities. The activities of several membrane-bound enzymes are known to be affected by changes in membrane fluidity (Kates et al., 1984; Kamada et al., 1995). (d) The unsaturation of fatty acids might stimulate the synthesis of the Na+/H+ antiporter(s) and/or H+-ATPase(s). The increased density in the membrane of these components of the antiport system might result in a decrease in the concentration of Na+ ions in the cytosol, which would tend to protect PSII and PSI against NaCl-induced inactivation and to accelerate the recovery of PSII and PSI activities.
Efforts to elucidate the underlying mechanisms of salt tolerance at the level of intact cells have met with little success to date. The data of the present study provide direct evidence that the unsaturation of fatty acids in membrane lipids is important for the maintenance of the photosynthetic machinery under salt stress.
MATERIALS AND METHODS
Cells, Growth Conditions, and Exposure of Cells to Salt Stress
Wild-type Synechococcus sp. PCC 7942 (Anacystis nidulans strain R2) and desA+ cells (Sakamoto et al., 1994) were grown photo-autotrophically in glass tubes (2.5 cm, i.d., × 20 cm; 120 mL) at 32°C, under constant illumination from incandescent lamps at 70 μE m−2 s−1, in BG-11 medium (Stanier et al., 1971) supplemented with 20 mm 2-[4-(2-hydroxyethyl)-1-piperazinyl]-ethanesulfonic acid-NaOH (HEPES-NaOH; pH 7.5), which contained 20 mm Na+ ions with aeration by sterile air that contained 1% (v/v) CO2 (Ono and Murata, 1981). After 4 d, cells were harvested by centrifugation at 9,000g for 10 min at 32°C and resuspended in fresh BG-11 medium. They were then incubated at 32°C with gentle stirring every 15 min in BG-11 medium or in BG-11 medium suplemented with 0.5 m NaCl, 0.5 m LiCl, or 1.0 m sorbitol at a density of 10 μg Chl mL−1 in glass tubes (1.5 cm, i.d., × 17.5 cm; 35 mL), in darkness or in light at 70 μE m−2 s−1.
Analysis of Lipids and Fatty Acids
Lipids and fatty acids were analyzed as described by Sato and Murata (1988). Extracted lipids were subjected to methanolysis in the presence of a mixture of HCl and methanol (5:95, w/w) at 85°C for 150 min. The esterified fatty acids were analyzed with a gas-liquid chromatograph (GC-7A; Shimadzu, Kyoto) equipped with a hydrogen flame-ionization detector.
Measurement of Photosynthetic Activities
Activities of PSI and PSII in intact cells were measured at 32°C by monitoring the concentration of evolved oxygen with a Clark-type oxygen electrode (Hansatech, King's Lynn, UK). The sample, in a 3-mL cuvette, was illuminated with incandescent light that had been passed through a red optical filter (R-62; Hoya Glass, Tokyo) and an infrared-absorbing filter (HA-50; Hoya Glass). The intensity of light at the surface of the cuvette was 2 mE m−2 s−1. For measurements of the oxygen-evolving activity of PSII, 1.0 mm 1,4-benzoquinone (BQ) was added as an artificial electron acceptor. The activity of PSI in intact cells was measured by monitoring the uptake of oxygen in the presence of 15 μm DCMU, 5 mm sodium ascorbate, 0.1 mm DCIP, and 0.1 mm MV.
The redox state of P700 in intact cells was determined at 25°C as the change in A820 with a flash of 5-ms duration at a saturating light intensity with a fluorometer (PAM-101; Walz, Effeltrich, Germany) equipped with an emitter-detector system (ED-800T; Walz) as described previously (Schreiber et al., 1988; Asada et al., 1992).
Measurement of Na+/H+ Antiport Activity
The Na+/H+ antiport activity of intact cells was determined by monitoring the quenching and recovery of the fluorescence of acridine orange as described previously (Blumwald et al., 1984; Garbarino and DuPont, 1989) with minor modifications (Allakhverdiev et al., 1999, 2000a). Fluorescence was measured at 25°C with a spectrofluorometer RF-500; Shimadzu) with excitation and emission wavelengths of 495 and 540 nm, respectively. The reaction mixture contained 35 mm N-methylglucamine-gluconate (pH 7.8), 0.6 m mannitol, and 5 μm acridine orange.
Quantitation of PSII Activity of Isolated Thylakoid Membranes
Thylakoid membranes were isolated from wild-type and desA+ cells as described previously (Allakhverdiev et al., 2000b). They were incubated at 32°C in darkness in 50 mm HEPES-NaOH, pH 7.5, that contained 400 mm Suc and 5 mm CaCl2. The transport of electrons from water to DCIP in isolated thylakoid membranes was monitored in the presence of 0.1 mm DCIP, while that from DPC to DCIP was monitored in the presence of 0.5 mm DPC and 0.1 mm DCIP. The light-induced reduction of DCIP was measured at 25°C by monitoring changes in A580 with a reference beam of light at 500 nm in a dual-wavelength spectrophotometer (UV-300; Shimadzu). The change in the concentration of DCIP was calculated from the change in absorbance during the 30 s that followed the start of illumination. We calculated the differential absorption coefficient of DCIP at pH 7.5 (Δε580−500 = 15.78 mm−1 cm−1) for the optical parameters of our assay system using the absorption coefficient of DCIP at pH 6.5 that was reported by Armstrong (1963). Red actinic light at 1.2 mE m−2 s−1 was obtained by passage of light from an incandescent lamp through two optical filters, as mentioned above. Concentrations of Chl were determined as described by Arnon et al. (1974).
ACKNOWLEDGMENT
The authors are grateful to Ms. U. Makino (Center for Analytical Instruments, National Institute for Basic Biology) for measurements of Na+/H+ antiport activity.
Footnotes
This work was supported by a Grant-in-Aid for Specially Promoted Research from the Ministry of Education, Science and Culture, Japan (grant no. 08102011 to N.M.), and in part by the National Institute for Basic Biology Cooperative Research Program on the Stress Tolerance of Plants. S.I.A. was the recipient of an Invitation Fellowship for Research in Japan from the Japan Society for the Promotion of Science.
LITERATURE CITED
- Allakhverdiev SI, Nishiyama Y, Suzuki I, Tasaka Y, Murata N. Genetic engineering of the unsaturation of fatty acids in membrane lipids alters the tolerance of Synechocystis to salt stress. Proc Natl Acad Sci USA. 1999;96:5862–5867. doi: 10.1073/pnas.96.10.5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allakhverdiev SI, Sakamoto A, Nishiyama Y, Inaba M, Murata N. Ionic and osmotic effects of NaCl-induced inactivation of photosystems I and II in Synechococcus sp. Plant Physiol. 2000a;123:1047–1056. doi: 10.1104/pp.123.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allakhverdiev SI, Sakamoto A, Nishiyama Y, Murata N. Inactivation of photosystems I and II in response to osmotic stress in Synechococcus: contribution of water channels. Plant Physiol. 2000b;122:1201–1208. doi: 10.1104/pp.122.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong JM. The molar extinction coefficient of 2,6-dichlorophenol indophenol. Biochim Biophys Acta. 1963;86:194–197. doi: 10.1016/0304-4165(64)90180-1. [DOI] [PubMed] [Google Scholar]
- Arnon DI, McSwain BD, Tsujimoto HY, Wada K. Photochemical activity and components of membrane preparations from blue-green algae: I. Coexistence of two photosystems in relation to chlorophyll a and removal of phycocyanin. Biochim Biophys Acta. 1974;357:231–245. doi: 10.1016/0005-2728(74)90063-2. [DOI] [PubMed] [Google Scholar]
- Asada K, Heber U, Schreiber U. Pool size of electrons that can be donated to P700+ as determined in intact leaves: donation to P700+ from stromal components via the intersystem chain. Plant Cell Physiol. 1992;33:927–932. [Google Scholar]
- Blumwald E, Wolosin JM, Packer L. Na+/H+ exchange in the cyanobacterium Synechococcus 6311. Biochem Biophys Res Commun. 1984;122:452–459. doi: 10.1016/0006-291x(84)90497-2. [DOI] [PubMed] [Google Scholar]
- Deshnium P, Gombos Z, Nishiyama Y, Murata N. The action in vivo of glycinebetaine in enhancement of tolerance of Synechococcus sp. PCC 7942 to low temperature. J Bacteriol. 1997;179:339–344. doi: 10.1128/jb.179.2.339-344.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshnium P, Los DA, Hayashi H, Mustardy L, Murata N. Transformation of Synechococcus with a gene for choline oxidase enhances tolerance to salt stress. Plant Mol Biol. 1995;29:897–907. doi: 10.1007/BF00014964. [DOI] [PubMed] [Google Scholar]
- Erdmann N, Fulda S, Hagemann M. Glucosylglycerol accumulation during salt acclimation of two unicellular cyanobacteria. J Gen Microbiol. 1992;138:363–368. [Google Scholar]
- Garbarino J, DuPont FM. Rapid induction of Na+/H+ exchange activity in barley root tonoplast. Plant Physiol. 1989;89:1–4. doi: 10.1104/pp.89.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golbeck JH. Photosystem I in cyanobacteria. In: Bryant DA, editor. The Molecular Biology of Cyanobacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 319–360. [Google Scholar]
- Gombos Z, Kanervo E, Tsvetkova N, Sakamoto T, Aro E, Murata N. Genetic enhancement of the ability to tolerate photoinhibition by introduction of unsaturated bonds into membrane glycerolipids. Plant Physiol. 1997;115:551–559. doi: 10.1104/pp.115.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann M, Erdmann N. Environmental stresses. In: Rai AK, editor. Cyanobacterial Nitrogen Metabolism and Environmental Biotechnology. New Delhi: Narosa Publishing House; 1997. pp. 156–221. [Google Scholar]
- Hagemann M, Erdmann N, Wittenburg E. Synthesis of glucosylglycerol in salt-stressed cells of the cyanobacterium Microcystis firma. Arch Microbiol. 1987;148:275–279. [Google Scholar]
- Hagemann M, Techel D, Rensing L. Comparison of salt- and heat-induced alterations of protein synthesis in the cyanobacterium Synechocystis sp. PCC 6803. Arch Microbiol. 1991;155:587–592. [Google Scholar]
- Huflejt M, Tremolieres A, Pineau B, Lang J, Hatheway J, Packer L. Changes in membrane lipid composition during saline growth of the freshwater cyanobacterium Synechococcus 6311. Plant Physiol. 1990;94:1512–1521. doi: 10.1104/pp.94.4.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani M, Nakamura T, Han SY, Takabe T. Expression of the betaine aldehyde dehydrogenase gene in barley in response to osmotic stress and abscisic acid. Plant Mol Biol. 1995;27:307–315. doi: 10.1007/BF00020185. [DOI] [PubMed] [Google Scholar]
- Izawa S. Acceptors and donors for chloroplast electron transport. Methods Enzymol. 1980;69:413–435. [Google Scholar]
- Joset F, Jeanjean R, Hagemann M. Dynamics of the response of cyanobacteria to salt stress: deciphering the molecular events. Physiol Plant. 1996;96:738–744. [Google Scholar]
- Kamada Y, Jung US, Piotrowski J, Levin DE. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 1995;9:1559–1571. doi: 10.1101/gad.9.13.1559. [DOI] [PubMed] [Google Scholar]
- Kates M, Pugh EL, Ferrante G. Regulation of membrane fluidity by lipid desaturases. Biomembranes. 1984;12:379–395. [Google Scholar]
- Khamutov G, Fry IV, Huflejt ME, Packer L. Membrane lipid composition, fluidity, and surface charge changes in response to growth of the freshwater cyanobacterium Synechococcus 6311 under high salinity. Arch Biochem Biophys. 1990;277:263–267. doi: 10.1016/0003-9861(90)90577-l. [DOI] [PubMed] [Google Scholar]
- Kuwabara T, Murata N. Quantitative analysis of the inactivation of photosynthetic oxygen evolution and the release of polypeptides and manganese in the photosystem II particles of spinach chloroplasts. Plant Cell Physiol. 1983;24:741–747. [Google Scholar]
- Mackay MA, Horton RS, Borowitzka LJ. Organic osmoregulatory solutes in cyanobacteria. J Gen Microbiol. 1984;130:2177–2191. [Google Scholar]
- Miyao M, Murata N. Partial disintegration and reconstitution of the photosynthetic oxygen-evolution system: binding of 24 kDa and 18 kDa polypeptides. Biochim Biophys Acta. 1983;725:87–93. [Google Scholar]
- Murata N, Miyao M. Extrinsic membrane proteins in the photosynthetic oxygen-evolving complex. Trends Biochem Sci. 1985;10:122–124. [Google Scholar]
- Murata N, Wada H. Acyl-lipid desaturases and their importance in the tolerance and acclimatization to cold of cyanobacteria. Biochem J. 1995;308:1–8. doi: 10.1042/bj3080001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Yokota S, Muramoto Y, Tsutsui K, Oguri Y, Fukui K, Takabe T. Expression of a betaine aldehyde dehydrogenase gene in rice, a glycinebetaine nonaccumulator, and possible localization of its protein in peroxisomes. Plant J. 1997;11:1115–1120. doi: 10.1046/j.1365-313x.1997.11051115.x. [DOI] [PubMed] [Google Scholar]
- Ono T, Murata N. Chilling susceptibility of the blue-green alga Anacystis nidulans: I. Effect of growth temperature. Plant Physiol. 1981;67:176–181. doi: 10.1104/pp.67.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padan E, Schuldiner S. Molecular physiology of Na+/H+ antiporters, key transporters in circulation of Na+ and H+ in cells. Biochim Biophys Acta. 1994;1185:129–151. doi: 10.1016/0005-2728(94)90204-6. [DOI] [PubMed] [Google Scholar]
- Reed RH, Borowitzka LJ, Mackay MA, Chudek JA, Foster R, Warr SRC, Moore DJ, Stewart WDP. Organic solute accumulation in osmotically stressed cyanobacteria. FEMS Microbiol Rev. 1986;39:51–56. [Google Scholar]
- Ritter D, Yopp JH. Plasma membrane lipid composition of the halophilic cyanobacterium Aphanothece halophytica. Arch Microbiol. 1993;159:435–439. [Google Scholar]
- Sakamoto T, Wada H, Nishida I, Ohmori M, Murata N. Δ9 Acyl-lipid desaturases of cyanobacteria: molecular cloning and substrate specificities in terms of fatty acids, sn-positions, and polar head groups. J Biol Chem. 1994;269:25576–25580. [PubMed] [Google Scholar]
- Sato N, Murata N. Membrane lipids. Methods Enzymol. 1988;167:251–259. [Google Scholar]
- Schreiber U, Klughammer C, Neubauer C. Measuring P700 absorbance changes around 830 nm with a new type of pulse modulation system. Z Naturforsch. 1988;43c:686–698. [Google Scholar]
- Shen J-R, Ikeuchi M, Inoue Y. Stoichiometric association of extrinsic cytochrome c550 and 12 kDa protein with a highly purified oxygen-evolving photosystem II core complex from Synechococcus vulcanus. FEBS Lett. 1992;301:145–149. doi: 10.1016/0014-5793(92)81235-e. [DOI] [PubMed] [Google Scholar]
- Sippola K, Kanervo E, Murata N, Aro E-M. A genetically engineered increase in fatty acid unsaturation in Synechococcus sp. PCC 7942 allows exchange of D1 protein forms and sustenance of photosystem II activity at low temperature. Eur J Biochem. 1998;251:641–648. doi: 10.1046/j.1432-1327.1998.2510641.x. [DOI] [PubMed] [Google Scholar]
- Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales) Bacteriol Rev. 1971;35:171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AC, Siczkowski M, Ljungberg U. Glycerol stabilizes oxygen evolution and maintains binding of a 9 kDa polypeptide in photosystem II particles from the cyanobacterium Phormidium laminosum. FEBS Lett. 1985;193:175–179. [Google Scholar]
- Tasaka Y, Gombos Z, Nishiyama Y, Mohanty P, Ohba T, Ohki K, Murata N. Targeted mutagenesis of acyl-lipid desaturases in Synechocystis: evidence for the important roles of polyunsaturated membrane lipids in growth, respiration and photosynthesis. EMBO J. 1996;15:6416–6425. [PMC free article] [PubMed] [Google Scholar]
- Trebst A. Inhibitors in electron flow: tools for the functional and structural localization of carriers and energy conservation sites. Methods Enzymol. 1980;69:675–715. [Google Scholar]
- Yamashita T, Butler WL. Inhibition of the Hill reaction by Tris and restoration by electron donation to photosystem II. Plant Physiol. 1969;44:435–438. doi: 10.1104/pp.44.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]