Abstract
Large-conductance, Ca2+-activated, voltage-dependent K+ (BK) channel function is critical for adequate airway hydration and mucociliary function. In airway epithelia, BK function is regulated by its γ subunit leucine-rich repeat-containing protein 26 (LRRC26). Since patients with cystic fibrosis (CF)-related diabetes mellitus (CFRD) have worse lung function outcomes, this study determined the effects of hyperglycaemia on BK function in CF bronchial epithelial (CFBE) cells in vitro and evaluated the correlation between glycaemic excursions and mRNA expression of LRRC26 in the upper airways of CF and CFRD patients.
CFBE cells were re-differentiated at the air-liquid interface (ALI) in media containing either 5.5 mM or 12.5 mM glucose. BK activities were measured in Ussing chambers. Airway surface liquid (ASL) volumes were estimated by meniscus scanning and inflammatory marker expression was measured by quantitative real-time PCR (qPCR) and enzyme-linked immunosorbent assay (ELISA). CF patients were assessed by 7 days of continuous glucose monitoring. LRRC26 mRNA expression was measured by qPCR from nasal cells obtained at the end of glucose monitoring.
BK currents were significantly decreased in CFBE cells cultured under high glucose. These cells revealed significantly lower ASL volumes and increased inflammation, including RAGE, compared to cells cultured in normal glucose. In vivo, nasal cell expression of LRRC26 mRNA was inversely correlated with hyperglycaemic excursions, consistent with the in vitro results.
Our findings demonstrate that hyperglycaemia induces inflammation and impairs BK channel function in CFBE cells in vitro. These data suggest that declining lung function in CFRD patients may be related to BK channel dysfunction.
Keywords: Cystic Fibrosis, CFTR, BK channel, airway inflammation, hyperglycaemia
Summary:
In CF patients, hyperglycaemia downregulates airway epithelial BK channels that are critical for mucociliary clearance. Mechanistically, hyperglycaemia suppresses expression of the BK γ subunit LRRC26, which is required for its function in the airway.
Introduction
Cystic fibrosis (CF)-related diabetes mellitus (CFRD) is a major predictor of worse lung function and affects ~20% of adolescents and >40% of adults with CF [1–3]. However, little is known about the mechanisms by which elevated glucose levels lead to worse lung function outcomes.
Advances in care and therapeutic treatments for CF patients have led to a dramatic increase in life expectancy. However, the consequence of prolonged life is an increase in the prevalence of age- and CF-related comorbidities that can worsen pulmonary function. In fact, CFRD is the most common CF comorbidity and associated with worse lung function, increased number and severity of pulmonary exacerbations, increased risk of infection by Pseudomonas aeruginosa, and poorer prognosis compared to CF patients without diabetes [4]. Thus, it is imperative to initiate mechanistic studies that could be translated into lung function preservation trials in CFRD beyond glycaemic control.
Hyperglycaemia initiates pro-inflammatory signalling pathways [5], including the formation of advanced glycation endproducts (AGEs). These bind to the receptor for AGE (RAGE or AGER), a member of the immunoglobulin superfamily of cell-surface receptors that functions as a pattern-recognition receptor and engages pro-inflammatory signalling pathways when activated [6]. RAGE is highly expressed in the lung and is also important for cell adhesion and epithelial repair [7, 8]. RAGE signalling has been implicated to play a role in the pathogenesis of a number of respiratory disorders, including chronic obstructive pulmonary disease (COPD), asthma, and pulmonary fibrosis [9]. In CF and even more so in CFRD patients, levels of RAGE ligands in the lung inversely correlate with lung function [10, 11].
It is known that inflammation and hyperglycaemia impair airway epithelial ion channel function [12], thereby possibly contributing to respiratory decline in CFRD patients, but little is known about mechanism. Here, we specifically studied the impact of hyperglycaemia on apically expressed large-conductance, Ca2+-activated, voltage-dependent K+ (BK) channels because they are critical to maintain mucus clearance in CF bronchial epithelial (CFBE) cells [13].
Our data reveal that ASL volume reduction in hyperglycaemic conditions in CFBE cells in vitro correlated with reduced BK function and increased expression of RAGE, which can initiate pro-inflammatory signalling cascades under conditions of persistent hyperglycaemia [14]. BK dysfunction was likely due to a decrease in expression of LRRC26, the BK γ subunit needed for its function in non-excitable cells [15, 16]. Furthermore, continuous monitoring of glucose levels in CF patients with or without CFRD over a one-week period revealed that hyperglycaemic excursions inversely correlated with mRNA expression of LRRC26.
Methods
Lungs
All non-CF human bronchial epithelial (NHBE) cells were from nonsmoking donors with no known pre-existing airway or lung diseases whose lungs were not used for transplant by the Life Alliance Organ Recovery Agency at the University of Miami (Miami, FL, USA), LifeCenter Northwest (Seattle, WA, USA), and the Midwest Transplant Network (Kansas City, KS, USA). Organ donations were performed on donors appropriately consented by the organ procurement agencies. Thus, cell and tissue use are not considered human subjects research (deceased individuals). Non-CF lung donor information is presented in supplementary table S1. Most CFBE cells stemmed from explanted lungs with appropriately consented patients undergoing lung transplantation, approved by the University of Miami’s IRB. Lungs from one deceased CF donor were procured through LifeCenter Northwest. CF lung donor information is presented in supplementary table S2.
Cell culture
Culturing of NHBE and CFBE cells at the air-liquid interface (ALI) was performed as described (see also supplementary methods) [17–19]. Cells were re-differentiated at an ALI in media containing 5.5 mM or 12.5 mM glucose for a minimum of three weeks before experiments were performed. Glucose levels in media were measured with a calibrated OneTouch Verio® meter (LifeScan, Malvern, PA, USA). For HMGB1 experiments, 100 ng/mL recombinant human HMGB1 (#H4652; MilliporeSigma, Burlington, MA, USA) was added to the basolateral media 24 h before Ussing chamber and ASL volume measurements.
Quantitative PCR
NHBE and CFBE cells were lysed and total RNA isolated using the E.Z.N.A.® Total RNA Kit (Omega Bio-tek, Norcross, GA, USA). qPCR was performed as described [20, 21] using TaqMan Gene Expression Assays (ThermoFisher Scientific, Waltham, MA, USA) for AGER (Hs00153957_m1), IL-1β (Hs01555410_m1), IL-6 (Hs00985639_m1), IL-8 (Hs00174103), KCNMA1 (Hs00266938_m1), KCNN4 (Hs00158470_m1), LRRC26 (Hs02385555_g1), MMP-9 (Hs00234579_m1), COX-2 (Hs00153133_m1), and TGF-β1 (Hs00998133_m1), and normalized to reference gene GAPDH.
ELISA
Basolateral media from CFBE cells cultured in ALI media with starting glucose concentrations of 5.5 mM or 12.5 mM were collected 48 h after the previous media change. Media samples were analysed using the Ella Automated Immunoassay System and Simple Plex cartridge-based immunoassay for IL-1β, IL-6 and Il-8 (Bio-Techne Corp., Minneapolis, MN, USA).
Ussing chamber
Cystic fibrosis transmembrane conductance regulator (CFTR) and large conductance, Ca2+-activated and voltage-dependent K+ channel (BK) activities were recorded in Ussing chambers as previously described (see also supplementary methods) [16, 22]. Ussing chamber experiments were performed 24 h after apical wash and basolateral media change unless otherwise noted. CFTR and BK measurements in NHBE cells on Snapwell inserts were conducted using 1.12 cm2 aperture sliders (#P2302; Physiologic Instruments). BK measurements in CFBE cells on Transwell inserts were conducted using 0.10 cm2 aperture sliders (#P2303; Physiologic Instruments). The smaller aperture size likely allows for greater epithelial uniformity which may account for the larger BK currents observed in CFBE cells.
Airway surface liquid (ASL) volume measurements
ASL volume estimation was performed by meniscus scanning as previously published [21, 23]. Briefly, the apical surface of the cells was washed with Dulbecco’s phosphate-buffered saline (Corning, Corning, NY, USA) and ASL volumes were measured after 24 h. Basolateral media was replenished with ALI media containing either 5.5 mM or 12.5 mM glucose and ASL volumes were measured again after another 24 h. ΔASL represents the difference between these two measurements.
Human Study approval
The study was approved by the University of Kansas Medical Center IRB and informed consent was obtained from each participant.
Human subjects
Participants age 18 or older with known CF, with or without CFRD, at the University of Kansas Health System were enrolled in an observational cohort study (see supplementary material for additional enrolment criteria). CFRD was defined with fasting blood glucose >126 mg/dL or two-hour blood glucose >200 mg/dL during an oral glucose tolerance test (OGTT). Those with normal OGTT (baseline <126 mg/dL, two-hour <140 mg/dL) or impaired glucose tolerance OGTT (baseline <126 mg/dL, two-hour 140–200 mg/dL) were defined as CF only. At the initial study visit, demographics and baseline clinical variables were collected and haemoglobin A1C was measured from capillary blood using A1CNow®+ (PTS Diagnostics, Whitestown, IN, USA). Study participants wore blinded Dexcom G6® continuous glucose monitors (CGM, Dexcom, Inc., San Diego, CA, USA) for 5–10 days. Nasal cells were collected from study participants at the time of CGM removal using sterile cytology brushes (Medical Packaging Corporation, Camarillo, CA, USA) as described in supplementary methods.
Continuous glucose monitoring (CGM) analyses
CGM variables were calculated using the R statistical package cgmanalysis (R Foundation for Statistical Computing, Vienna, Austria) [24]. Imputation was used to fill missing intervals <20 min in length and for those >20 min in length, the corresponding 24 h period was censored from final analysis. CGM variables of interest were selected as being previously associated with outcomes in CFRD [mean amplitude of glycaemic excursions (MAGE), % time of glucose > 140 mg/dL] or reflective of hyperglycaemic excursions [glucose management indicator (GMI), % time of glucose > 200 mg/dL and area under the curve (AUC) of glucose > 180 mg/dL] [25, 26].
Statistical analyses
For in vitro studies, data are shown as mean ± S.E.M. Data that followed normal distribution by Shapiro-Wilk were analysed by parametric tests. Otherwise, non-parametric statistics were performed. Multiple groups were analysed by one-way ANOVA followed by the appropriate post hoc test. Two groups were compared with a paired or unpaired t-test as appropriate. Results were considered statistically significant at p < 0.05. Clinical study data are shown as dot plots / bar graph combinations with mean ± S.E.M or trend lines with 95% confidence intervals. Differences between two groups were compared by parametric or non-parametric tests as indicated in the figure captions depending on whether the data passed Shapiro-Wilk normality testing. Pearson’s correlation coefficient was used to assess correlation between continuous variables. Results were considered statistically significant at p < 0.05. All analyses were performed using Prism (GraphPad Software, San Diego, CA, USA).
Results
Hyperglycaemia differentially affects ion channel function in normal human bronchial epithelial (NHBE) cells in vitro
NHBE cells cultured at the ALI were exposed to high levels of glucose in the basolateral media (12.5 mM or 225 mg/dL). Glucose levels decreased over time, reaching normal blood levels 48 h after media change (figure 1a). BK conductance was measured 24 h and 72 h after media change, corresponding to time points with high (24 h range: 7.2 – 12.5 mM) and normal glucose (72 h range: 3.1 – 5.7 mM) conditions, respectively. BK currents were reduced by 50% in NHBE cells under high glucose (24 h) compared to normal glucose (72 h) conditions (figure 1b,c). BK channels comprise the pore-forming α subunit, encoded by the KCNMA1 gene, and auxiliary β and γ subunits [27]. BK function in airway epithelial cells requires the γ regulatory subunit LRRC26 and is directly related to LRRC26 expression [16]. We found that NHBE cells under high glucose expressed lower levels of LRRC26 mRNA compared to cells under normal glucose (figure 1d), consistent with the functional BK data. On the other hand, KCNMA1 mRNA expression levels remained unchanged (figure 1e). NHBE cells under high glucose showed a significantly greater response to mallotoxin, a BK opener that is more effective in the absence of LRRC26 (figure 1f,g), providing further evidence that high glucose-induced decreases in BK conductance are likely due to reduced LRRC26 expression.
FIGURE 1. High glucose negatively impacts BK channel function in NHBE cells in vitro.
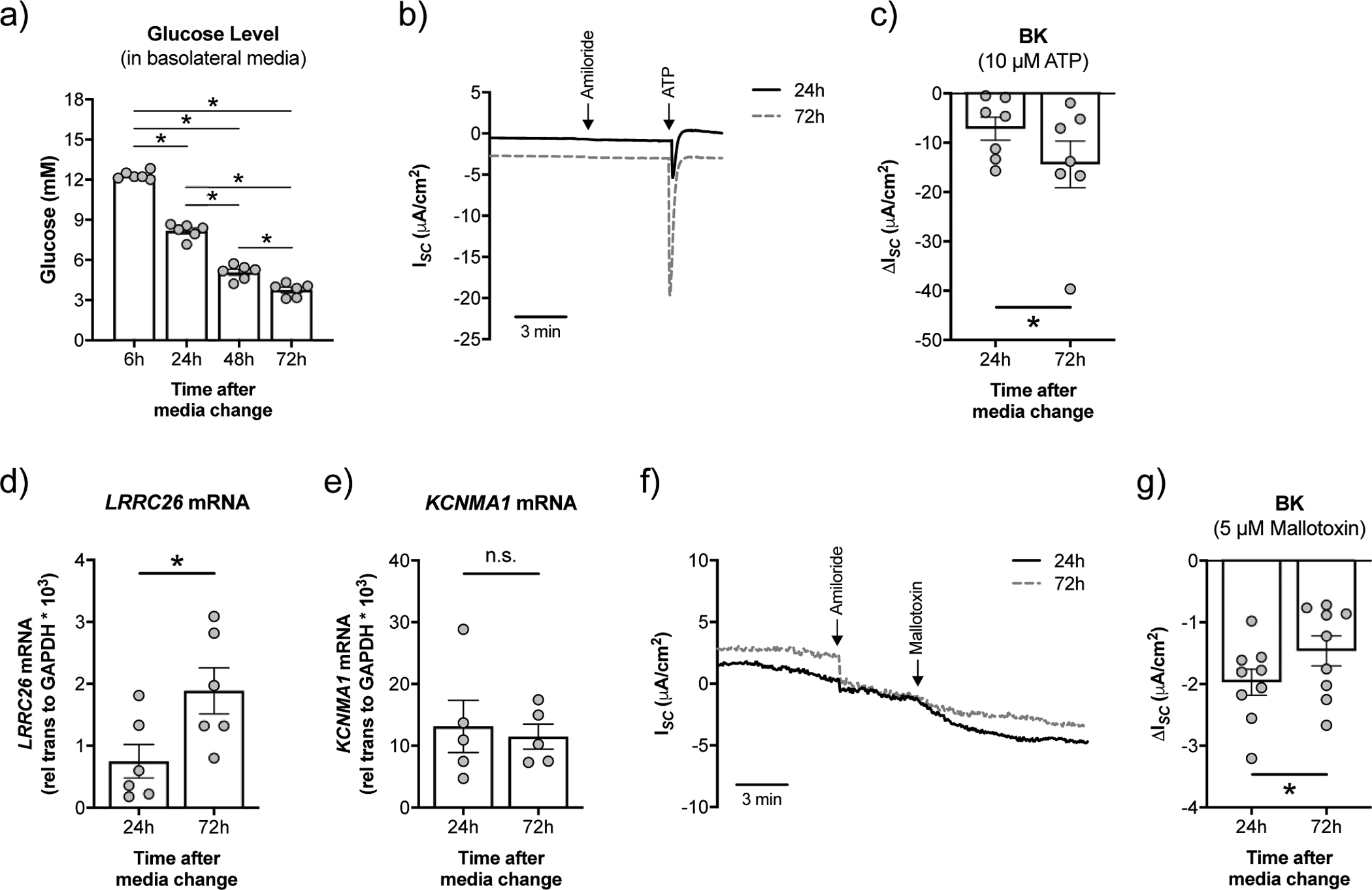
a) Fully differentiated NHBE cells were cultured in ALI media with high glucose (12.5 mM or 225 mg/dL). Measurement of glucose levels in the basolateral media over time (n=6 lungs). * p < 0.05, one-way ANOVA with Tukey’s. b) Representative tracing of ATP-stimulated short-circuit current (ISC) measurements in Ussing chambers with a basolateral to apical K+ and apical to basolateral Na+ gradient [34]. ISC is a measure of net ionic current across the epithelium and thus near zero at baseline. c) BK activity is significantly lower at 24 h than at 72 h after media change (n=7 lungs). * p < 0.05, one-tailed t-test. d) mRNA expression levels of LRRC26 correlate inversely with glucose levels (n=6 from 3 lungs). * p < 0.05, unpaired t-test. e) Expression of KCNMA1 mRNA is not significantly different in NHBE cells 24 h and 72 h after media change (n=6 lungs). n.s. = not significant. f) Representative tracing of mallotoxin-stimulated ISC measurements in Ussing chambers with a basolateral to apical K+ gradient [13]. g) Mallotoxin-activated BK is significantly greater in NHBE cells 24 h after media change compared to 72 h (n=9 from 4 lungs). These data indicate that KCNMA1 is still at the plasma membrane and opens with mallotoxin when LRRC26 associations are reduced [13]. * p < 0.05, Student’s t-test. Data are shown as mean ± S.E.M.
To determine the extent by which chronic elevations in glucose induce changes in ion channel function, we cultured NHBE cells in ALI media supplemented with either 5.5 mM (normal) or 12.5 mM (high) glucose throughout redifferentiation at the ALI. High glucose did not significantly impact transepithelial electrical resistance (TEER) of cultures (supplementary figure S1). BK channel activity was significantly reduced in NHBE cells cultured under high glucose compared to normal glucose (figure 2a,b). On the other hand, NHBE cells cultured under high glucose conditions showed a significant increase in CFTR conductance (figure 2d–f), with a concomitant decrease in the activity of calcium-activated chloride channels (CaCC) (supplementary figure S1). Expression of CFTR mRNA was correlated with elevated levels of glucose (supplementary figure S1). Despite this increase, the change in ASL volume over a 24 h period was not significantly different between NHBE cells cultured under high glucose compared to normal glucose (figure 2c), likely due to the balance of changes in ion channel activities.
FIGURE 2. High glucose differentially affects ion channel function in NHBE cells in vitro.
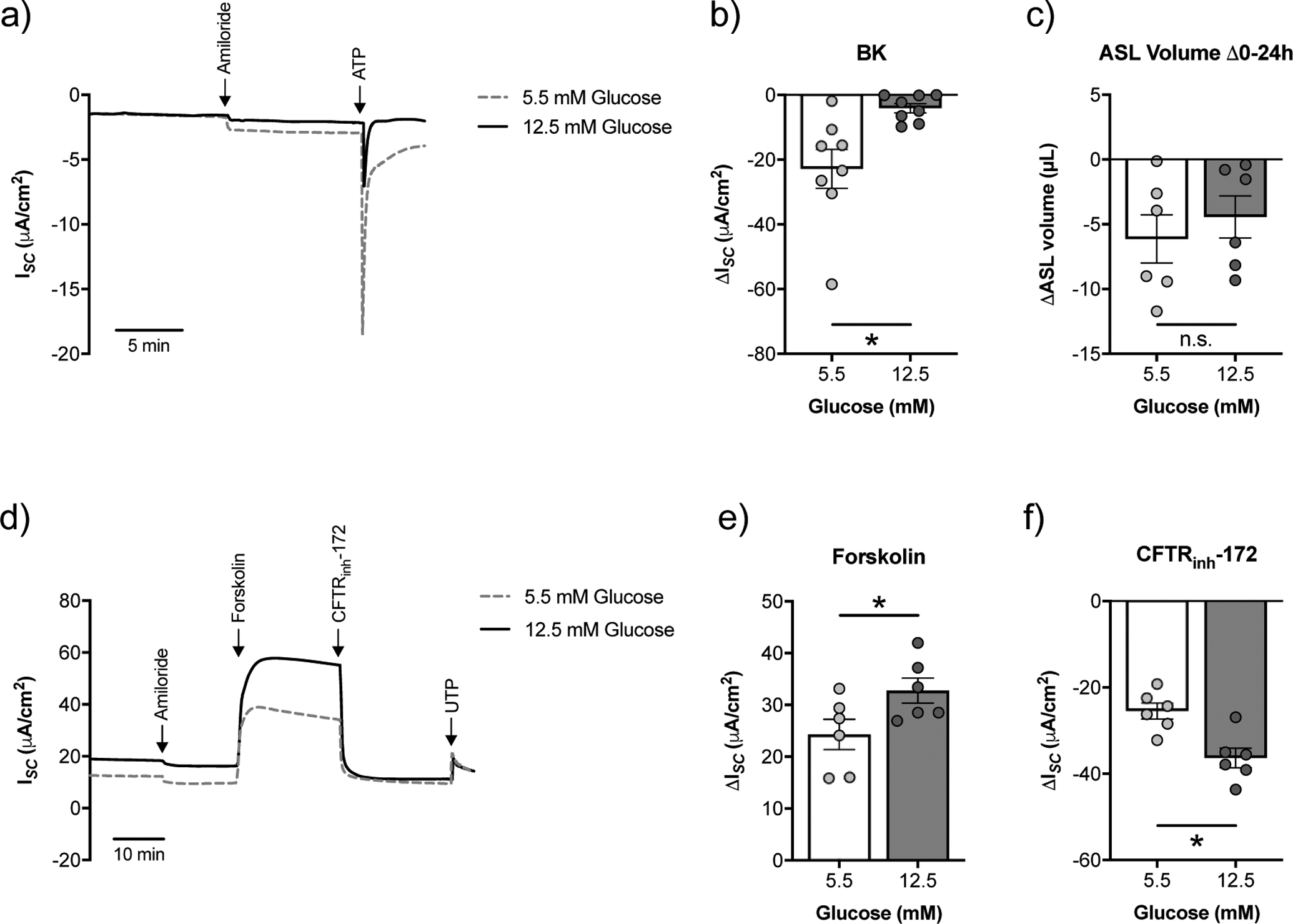
a) Representative tracing of ATP-stimulated ISC with a basolateral to apical K+ gradient [34] from fully differentiated NHBE cells cultured in ALI media with starting glucose concentrations of 5.5 mM or 12.5 mM. Ussing chamber experiments were performed on NHBE cells 20–24 h after basolateral media change. b) BK activity is significantly decreased in NHBE cells cultured under high glucose (n=8 from 6 lungs). c) Changes in ASL volume (ΔASL) over a 24 h period did not significantly differ between NHBE cells cultured under 5.5 mM or 12.5 mM glucose (n=6 from 3 lungs). d) Representative tracing of ISC following forskolin stimulation and CFTR inhibition by CFTRinh-172 from fully differentiated NHBE cells cultured in ALI media with 5.5 mM or 12.5 mM glucose. Ussing chamber experiments were performed on NHBE cells 20–24 h after basolateral media change. e) CFTR currents as measured by forskolin are significantly increased in NHBE cells cultured under high glucose (n=6 lungs). f) CFTR currents as measured by CFTRinh-172 inhibition are significantly increased in NHBE cells cultured under high glucose (n=6 lungs). * p < 0.05, Student’s t-test. Data are shown as mean ± S.E.M.
Hyperglycaemia induces the expression of inflammatory markers in NHBE cells in vitro
Hyperglycaemia can activate a pronounced inflammatory response [5], but whether it increases expression of inflammatory markers in the airway epithelium remains largely unknown. Compared to NHBE cells cultured under normal glucose, NHBE cells cultured under high glucose conditions showed significant increases in the mRNA expression levels of a number of proinflammatory cytokines, including interleukin-1 beta (IL-1β), interleukin-6 (IL-6), interleukin-8 (IL-8) (figure 3a–c), as well as the inflammatory markers matrix metalloproteinase-9 (MMP-9) and transforming growth factor-β1 (TGF-β1) (figure 3d,e).
FIGURE 3. High glucose induces expression of inflammatory markers in NHBE cells in vitro.
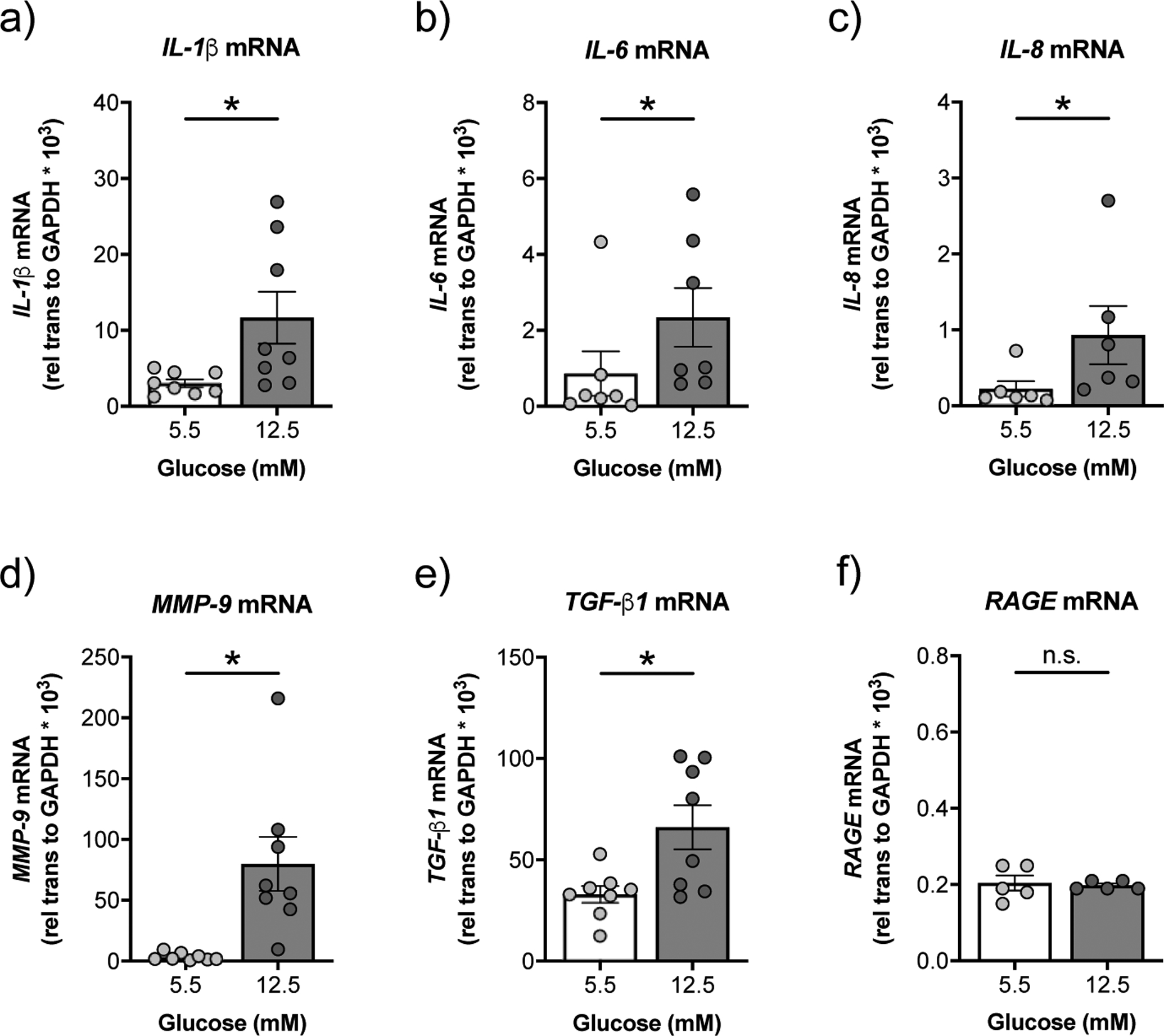
a-e) NHBE cells cultured under high glucose show significant increases in mRNA expression levels of IL-1β (a), IL-6 (b), IL-8 (c), MMP-9 (d), and TGF-β1 (e) compared to NHBE cells cultured under normal glucose (n≥6 from 6 lungs). f) High glucose does not affect the expression of RAGE mRNA in NHBE cells (n=5 from 4 lungs). * p < 0.05, Wilcoxon or Student’s t-test. Data are shown as mean ± S.E.M.
Hyperglycaemia induces BK channel dysfunction, loss of ASL volume, and inflammation in CFBE cells in vitro
We next sought to determine the impact of high glucose on BK channel function and ASL volumes in primary CFBE cells in vitro. CFTR mutations of donor lungs are listed in supplementary table S2. CFBE cells cultured under high glucose (24 h range: 10.1 – 12.5 mM) showed a significant reduction in BK channel function compared to CFBE cells cultured under normal glucose (24 h range: 2.5 – 5.5 mM) conditions (figure 4a,b and supplementary figure S2). The decrease in BK activity in CFBE cells also correlated with reduced LRRC26 mRNA expression (figure 4c), while KCNMA1 mRNA expression was not affected by glucose levels (figure 4e). Furthermore, CFBE cells under high glucose showed significantly greater rates of ASL absorption over a 24 h period (figure 4d). ATP-stimulated K+ currents in CFBE cells cultured under both normal and high glucose were completely inhibited by the BK blocker paxilline (figure 4g,h). KCa3.1 potassium channels, which are encoded by the KCNN4 gene, are also expressed at the apical membrane of bronchial epithelial cells and can be blocked by TRAM-34 [28]. However, glucose levels did not impact KCNN4 mRNA expression in CFBE cells (figure 4f). Furthermore, TRAM-34 had no effect on ATP-stimulated K+ currents in CFBE cells cultured in normal glucose (figure 4g). Although ATP-stimulated K+ currents were reduced by TRAM-34 in CFBE cells cultured in high glucose, the decrease was not significant (figure 4h).
FIGURE 4. High glucose reduces BK channel function and leads to a loss of ASL volume in CFBE cells in vitro.
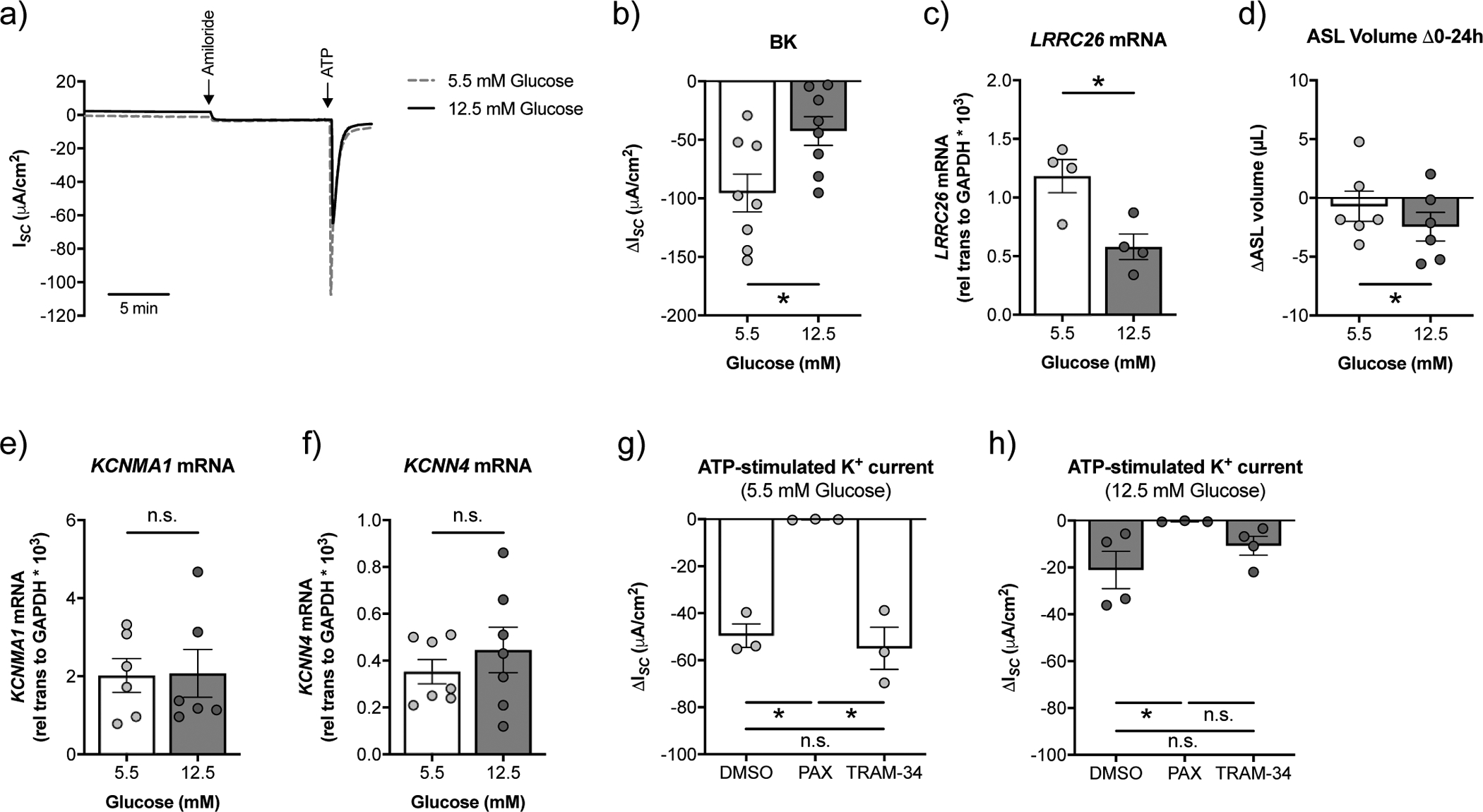
a) Representative tracing of ATP-stimulated ISC with a basolateral to apical K+ and apical to basolateral Na+ gradient [34] from fully differentiated CFBE cells cultured in ALI media with starting glucose concentrations of 5.5 mM or 12.5 mM. Ussing chamber experiments were performed on CFBE cells 20–24 h after basolateral media change. b) BK activity is significantly decreased in CFBE cells cultured under high glucose (24 h range: 10.1 – 12.5 mM) compared to normal glucose (24 range: 2.5 – 5.5 mM) (n=8 from 7 CF lungs). * p < 0.05, Student’s t-test. c) mRNA expression levels of LRRC26 inversely correlate with glucose levels (n=4 CF lungs). * p < 0.05, Student’s t-test. d) CFBE cells cultured under high glucose show increased ASL absorption (as indicated by a more negative ΔASL volume) over a 24 h period compared to CFBE cells under normal glucose (n=6 CF lungs). * p < 0.05, Student’s t-test. e) Expression of KCNMA1 mRNA is not significantly different in CFBE cells cultured in ALI media with starting glucose concentrations of 5.5 mM and 12.5 mM (n=6 from 6 CF lungs). n.s.=not significant, Wilcoxon test. f) The KCa3.1 potassium channel is encoded by KCNN4. High glucose does not affect the expression of KCNN4 mRNA in CFBE cells (n=7 from 7 CF lungs). n.s.=not significant, Wilcoxon test. g) ATP-stimulated K+ currents are significantly reduced by 10 μM paxilline (PAX), but not 1 μM TRAM-34, in CFBE cells cultured in ALI media with a starting glucose concentration of 5 mM (n=3 CF lungs). * p < 0.05, one-way ANOVA with Tukey’s. h) ATP-stimulated K+ currents are significantly reduced by 10 μM paxilline (PAX) in CFBE cells cultured in ALI media with a starting glucose concentration of 12.5 mM. The reduction in ATP-stimulated K+ currents by TRAM-34 was not significant (n ≥ 3 CF lungs). * p < 0.05, Kruskal-Wallis test with Dunn’s. n.s.=not significant. Data are shown as mean ± S.E.M.
High glucose did not lead to a significant increase in the expression of IL-1β, IL-8, or TGF-β1 mRNAs in CFBE cells (supplementary figure S3). On the other hand, CFBE cells cultured in high glucose showed significant increases in the mRNA expressions of MMP-9 and cyclooxygenase-2 (COX-2) compared to CFBE cells cultured in normal glucose media (figure 5a,b). Advanced glycation endproducts (AGEs) are formed during hyperglycaemia and bind to the pro-inflammatory receptor for AGE (RAGE) [14]. Interestingly, CFBE cells in high glucose demonstrated a significant increase in the expression of RAGE mRNA compared to CFBE cells in normal glucose (figure 5c). A similar increase in RAGE mRNA expression was not observed in NHBE cells in response to high glucose (figure 3F). We also detected a significant increase in secreted IL-1β and IL-8 protein in the basolateral media of CFBE cells cultured in high glucose compared to CFBE cells cultured in normal glucose (figure 5d,e). Levels of IL-6 in the basolateral media remained unchanged (figure 5e).
FIGURE 5. High glucose induces expression of inflammation markers and RAGE in CFBE cells in vitro.
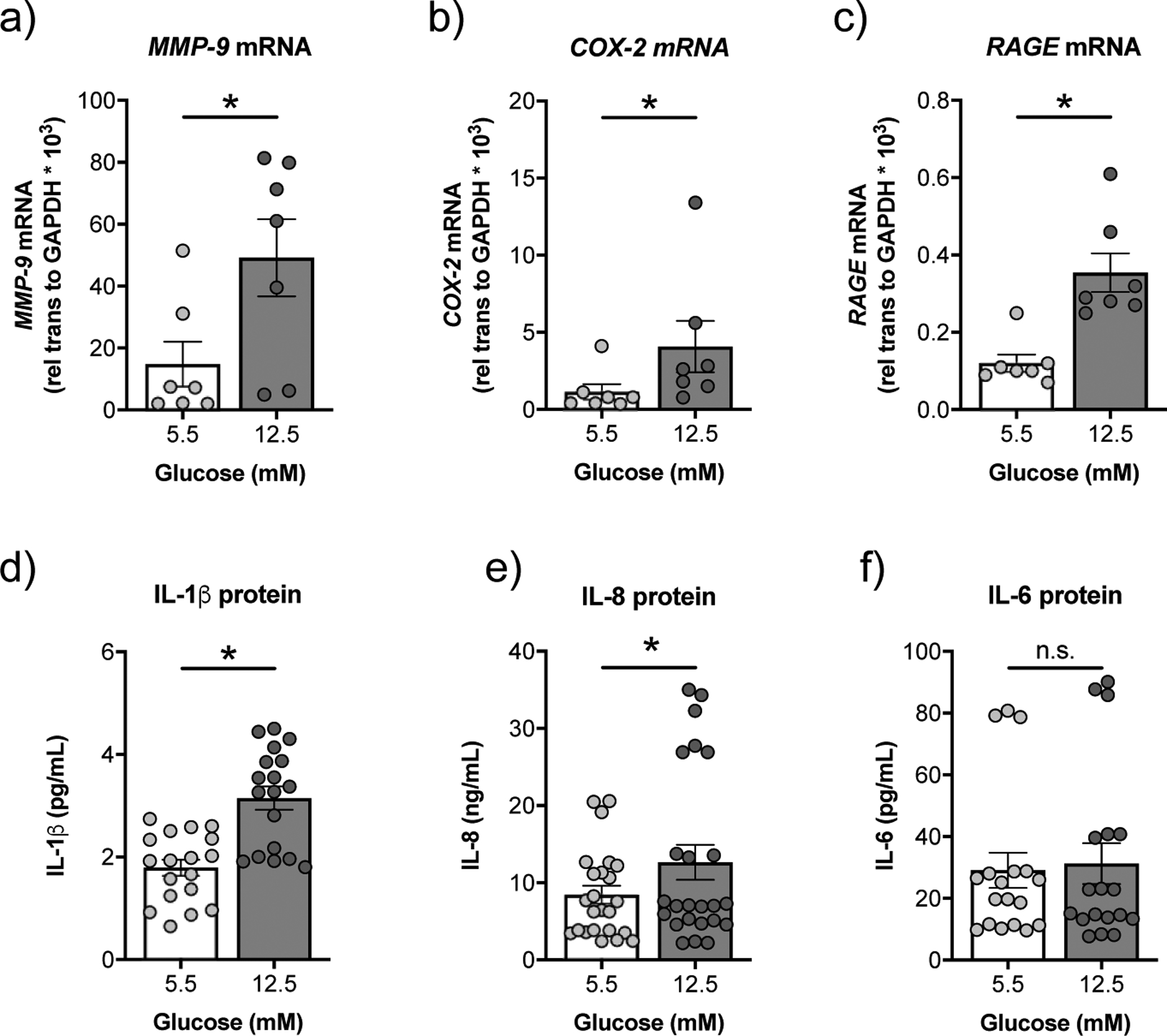
a-c) CFBE cells cultured under high glucose show significant increases in mRNA expression levels of MMP-9 (a), COX-2 (b), and RAGE (c) compared to CFBE cells cultured under normal glucose conditions (n=7 CF lungs). d) CFBE cells cultured under high glucose show a significant increase in expression levels of IL-1β protein in the basolateral media as determined by ELISA (n=18, triplicates from 6 CF lungs) and so was (e) IL-8 (n=24, triplicates from 6 CF lungs). Expression levels of IL-6 (f) are not significantly different in the basolateral media of CFBE cells cultured under normal vs. high glucose (n=18, triplicates from 6 CF lungs). * p < 0.05, n.s.=not significant, Wilcoxon test. Data are shown as mean ± S.E.M.
The RAGE agonist HMGB1 induces BK dysfunction and ASL volume loss in CFBE cells in vitro
It is possible that the increase in RAGE expression in CFBE cells is caused by BK dysfunction induced by hyperglycaemia. To address this possibility, we treated CFBE cells cultured under normal (5.5 mM) glucose conditions with recombinant high mobility group box 1 (HMGB1) protein and measured BK function. HMGB1 is a RAGE ligand and marker of airway epithelial injury that induces a strong pro-inflammatory response [29–31]. CFBE cells treated with HMGB1 (100 ng/mL) in the basolateral media for 24 h showed a significant reduction in BK channel function compared to controls (figure 6a,b). The reduction in BK activity correlated with increased ASL absorption (Figure 6c). These data suggest that RAGE activation is upstream of BK channel dysfunction.
FIGURE 6. HMGB1 reduces BK channel function and leads to a loss of ASL volume in CFBE cells in vitro.
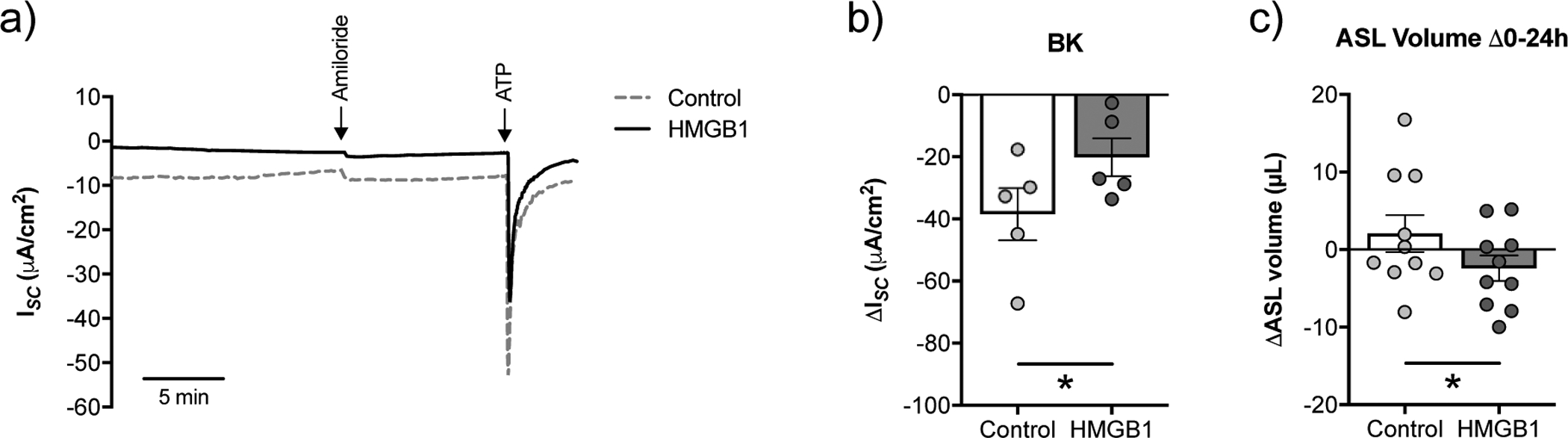
a) Representative tracing of ATP-stimulated ISC with a basolateral to apical K+ gradient and apical to basolateral Na+ gradient [34] from fully differentiated CFBE cells cultured in ALI media with starting glucose concentrations of 5.5 mM treated for 24 h with recombinant HMGB1 (100 ng/mL) or vehicle (water) control. b) BK activity is significantly decreased in CFBE cells treated with recombinant HMGB1 (n=5 from 4 CF lungs). c) CFBE cells treated with recombinant HMGB1 show increased ASL absorption (as indicated by a more negative ΔASL volume) over 24 h. * p < 0.05, Student’s t-test. Data are shown as mean ± S.E.M.
CGM variables of hyperglycaemia correlate with decreased LRRC26 expression in those with CF and CFRD
Based on our in vitro data, we initiated an observational study to determine whether there was a correlation between glycaemic excursions, as measured by continuous glucose monitoring (CGM), and LRRC26 expression in the upper airways of CF patients. A total of 21 participants (CF = 6, CFRD = 15) were enrolled in the study (see supplementary table S3 for baseline participant characteristics). Four subjects (CF = 1, CFRD = 3) were excluded from analysis due to CGM malfunction, CGM use <5 days, or inadequate mRNA in nasal brush samples. The CFRD group was older (mean age: 32 versus 22; 95% CI, 5.3–14.7) and had lower baseline lung function (mean ppFEV1: 64% versus 98%; 95% CI, 50.77–17.23) as compared to the CF group without diabetes. There were no differences in CFTR mutation group (F508del homozygous or heterozygote / minimum function) or CFTR modulator use between groups. CGM variables of interest were uniformly higher in the CFRD group as compared to the CF group (supplementary table S4).
A significant, inverse correlation was found between LRRC26 expression in nasal cells and % time >140 mg/dL (r = −0.51, p = 0.03), % time > 200 mg/dL (r = −0.54, p = 0.02), and AUC >180 mg/dL (r = −0.48, p = 0.04) (figure 7a–c). Other CGM variables of interest, including GMI (−0.46, p = 0.06) and MAGE (−0.35, p = 0.17) demonstrated a negative trend with LRRC26 expression but did not achieve significance (figure 7d,e). There was no significant association between age and LRRC26 expression or a difference in LRRC26 expression between female and male sex or CF and CFRD (supplementary figure S4). A significant correlation was also found in the CFRD group between RAGE expression and CGM variables including % time >140 mg/dL and MAGE (p<0.05) (figure 8a,b) and other CGM variables of interest demonstrated positive trends but failed to reach significance. As this small sample size is sensitive to outliers, excluding the participant with the greatest RAGE expression resulted in a significant association between RAGE expression and AUC glucose >180 mg/dL, % time >200 mg/dL and GMI (p <0.05; figure7c–e).
FIGURE 7. Hyperglycaemic excursions correlate with decreased LRRC26 mRNA expression in participants with CF and CFRD.
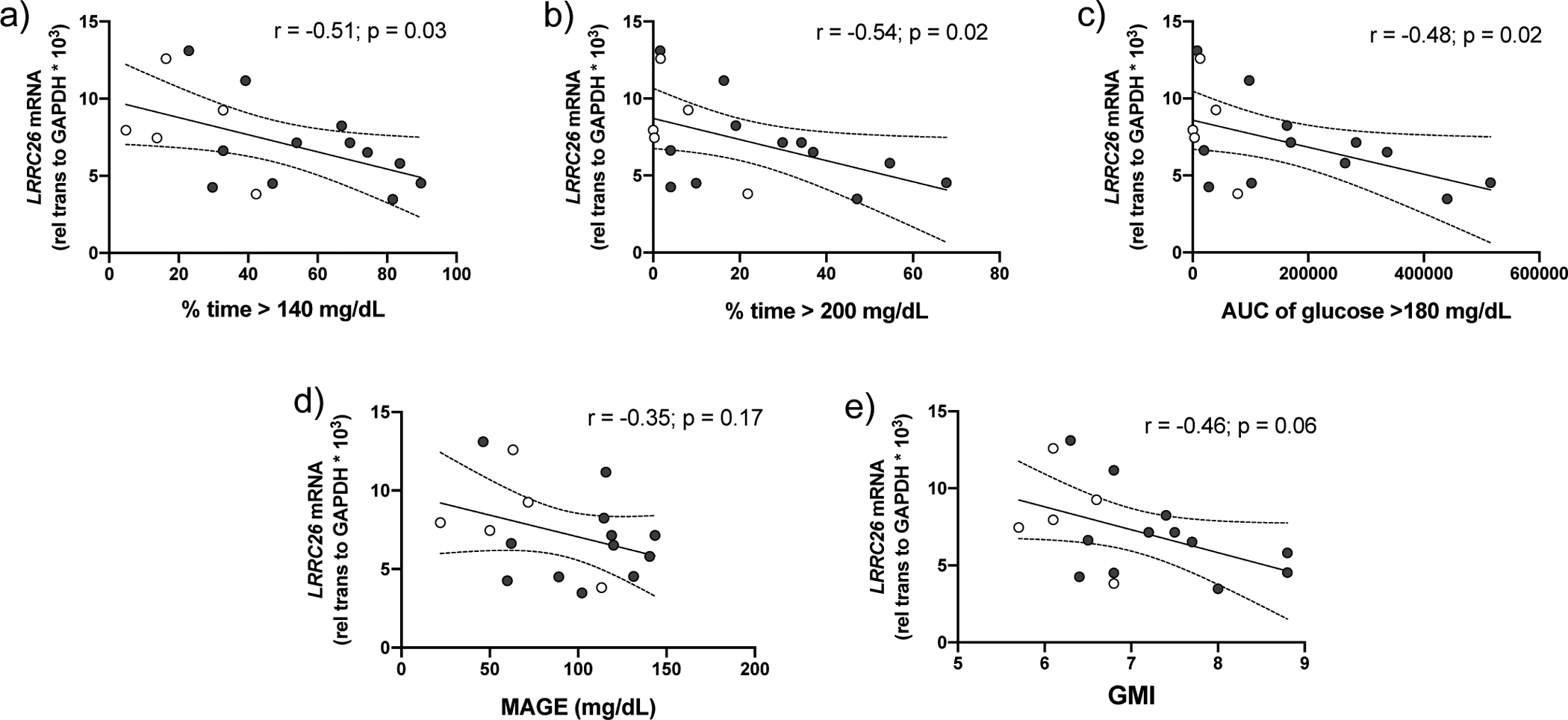
Inverse correlation between nasal cell LRRC26 expression in CF (n=5, open circles) and CFRD (n=12, shaded circles) with the CGM variables (a) percent time glucose > 140 mg/dL, (b) percent time glucose > 200 mg/dL and (c) average area under the curve of glucose >180 mg/dL. p<0.05, Pearson’s correlation coefficient. There was an inverse trend between LRRC26 expression and the CGM variables (d) mean amplitude of glycaemic excursions and (e) GMI; however, it did not reach statistical significance. p>0.05, Pearson’s correlation coefficient. Graphs include trend line (solid line) and 95% confidence intervals (dashed lines).
FIGURE 8. RAGE expression is correlated with hyperglycaemic excursions in CFRD.
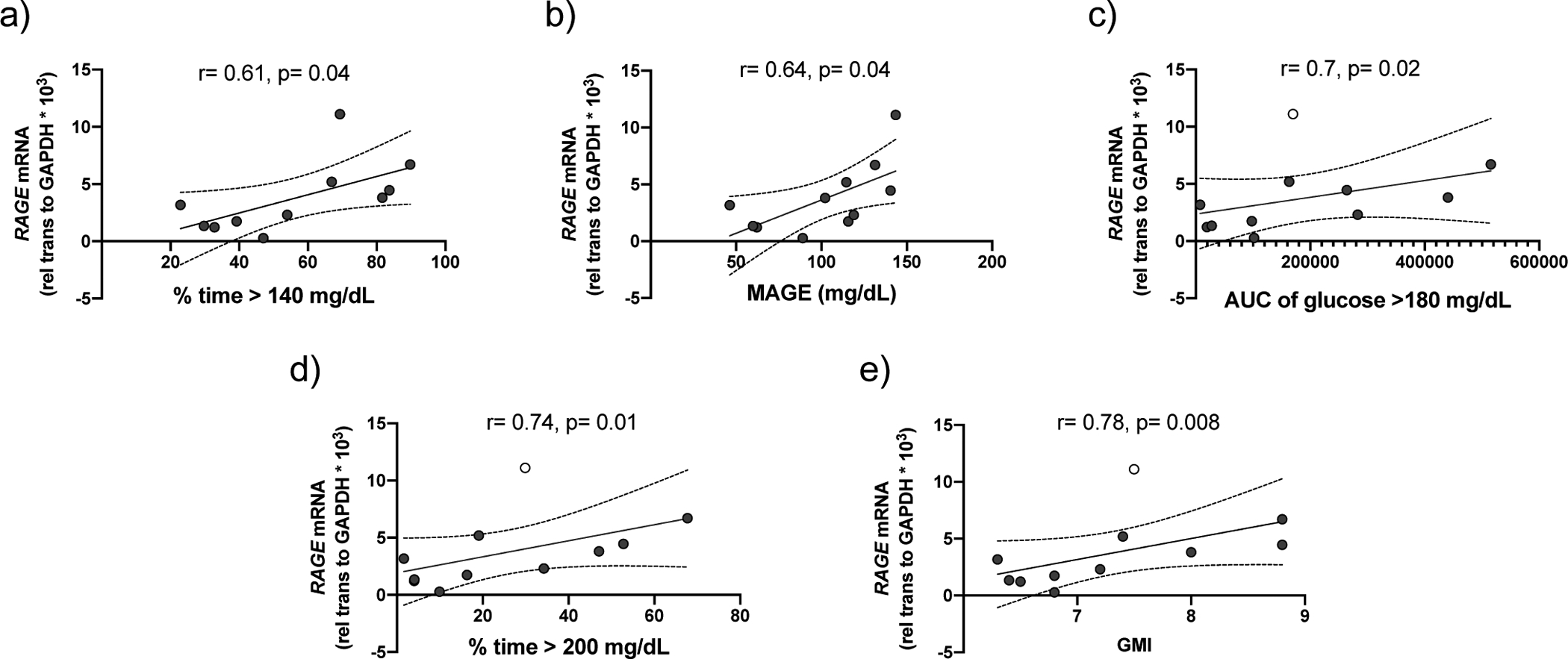
Correlation between nasal cell RAGE expression in participants with CFRD and with the CGM variables: (a) percent time glucose > 140 mg/dL and (b) mean amplitude of glycaemic excursions. p<0.05, Pearson’s correlation coefficient. After exclusion of an outlier (open circle), there is also a significant correlation between RAGE expression and the CGM variables: (c) area under the curve of glucose >180 mg/dL, (d) % time glucose >140 mg/dL and (e) GMI. P<0.05, Pearson’s correlation coefficient. Graphs include trend line (solid line) and 95% confidence intervals (dashed lines).
Discussion
CFRD is a common extrapulmonary comorbidity in adults with CF and associated with increased lung function decline [1, 2]. However, the mechanisms underlying this association are unknown. In this study, we demonstrated in an in vitro model of the CF airway epithelia that hyperglycaemia leads to impaired BK channel function, decreased ASL volume, and elevations in the expression of inflammatory markers. This finding was confirmed in vivo where LRRC26 expression, which predicts BK channel function in airway epithelia [13, 32–34], was inversely correlated with CGM variables of hyperglycaemia in study participants with CF and CFRD.
BK channels are likely to be the primary apically expressed K+ channels in the airway epithelium [13, 16]. BK function is critical for ASL hydration since inhibition of BK, either by knockdown of the pore-forming α-subunit KCNMA1 or LRRC26, causes a significant reduction in ASL volume, even in the presence of fully functional CFTR [16]. Interestingly, although high glucose correlated with reduced BK function in NHBE cells, ASL volumes were largely unaffected. This was likely due to the unexpected increase in CFTR conductance in these cells, suggesting that the airway epithelium employs mechanisms that differentially affect ion channel function to maintain ASL homeostasis during hyperglycaemic excursions. Although a previous study found that hyperglycaemia leads to a reduction in CFTR conductance, these studies were conducted at higher glucose concentrations (25 mM) in CFBE cell monolayers transduced with wild-type CFTR and may not necessarily reflect endogenous CFTR function in NHBE cells [12]. When CFTR function is limited or absent, as in CF, the airway epithelium is unlikely to maintain ASL homeostasis when glucose levels become elevated. Indeed, we found increased rates of ASL absorption in CFBE cells challenged with high glucose that correlated with reduced BK function and LRRC26 expression.
It is likely that hyperglycaemia also affects other ion channels that are important for ASL hydration. For example, activation of KCa3.1 channels, which are also expressed at the apical membrane, is thought to increase chloride secretion and improve airway hydration [28, 35]. Although high glucose did not have a significant impact on the expression or function of KCa3.1 channels in our experiments, we cannot completely rule out the possibility that KCa3.1 channels contribute to hyperglycaemia-induced changes in mucociliary clearance. Furthermore, our data in NHBE cells show that UTP-stimulated Cl− currents are impacted by high glucose. However, these studies provide some of the first in vitro evidence that high glucose can directly impact the expression and function of an ion channel known to be critical for mucociliary clearance in the CF airways.
The underlying link between BK channel dysfunction and hyperglycaemia is unclear, but we hypothesize that it is related to inflammation. In hyperglycaemia, the AGE-RAGE signalling axis is upregulated, which contributes to excess inflammation [14]. We found that CFBE cells under high glucose have significantly elevated levels of RAGE mRNA expression. Interestingly, we did not see a similar increase in RAGE expression in NHBE cells under high glucose. This is consistent with previous reports that showed significantly higher levels of RAGE expression in sputum of CF and CFRD subjects compared to non-CF diabetic subjects [11]. Indeed, levels of RAGE and RAGE ligands have been found to be increased in CF airways and levels of AGEs have been shown to negatively correlate with ppFEV1 [10, 11]. Furthermore, levels of HMGB1 are significantly elevated in sputum and serum of CF patients and particularly at the onset of CFRD [32]. The increase in IL-1β secretion in CFBE cells in response to high glucose is also particularly interesting in light of a recent study demonstrating the importance of IL-1β in promoting mucus hypersecretion and increasing the expression of proinflammatory mediators in CF airways in the absence of infection [36]. Levels of LRRC26 expression are sensitive to inflammation in CFBE cells [32]. Thus, future studies will focus on how RAGE activation, and possibly Il-1β, may potentially regulate BK function.
Based on these in vitro data, we initiated a clinical study to determine whether LRRC26 expression is impacted by variations in glucose in CF patients. Interestingly, there was no significant difference in LRRC26 expression between CF and CFRD patients. Instead, the inverse correlation with LRRC26 expression occurred across a spectrum of glycaemic control in both groups. This suggests glycaemic control is an important factor in both CF and CFRD. Also mirroring the in vitro results, the correlation between RAGE expression and hyperglycaemia was observed in participants with CFRD. However, there were several limitations to our study. First, we relied on historical OGTT to define CF and CFRD which could have led to incorrect categorization of participants. Yet, as our results suggest that level of glycaemic control was more strongly associated with LRRC26 expression than CFRD status, this did not likely affect our findings. Second, a non-significant but noticeable discrepancy in CGM sampling periods between the two groups was observed. The CGM variables are means and proportions which are less likely to be affected by differences in sampling length, but this difference could lead to misestimation of true glycaemic control. Third, while CFTR modulator use was not found to be associated with LRRC26 expression, we were not able to compare differences between specific CFTR modulators given our relatively small number of subjects. It should be noted that our aims were exploratory, and no corrections were made for multiple comparisons given the small sample size.
Importantly, we believe these findings remain relevant in the context of highly effective CFTR modulator therapy. Previous observational studies have demonstrated that there are improvements in insulin secretion after initiation of highly effective CFTR modulator therapy. However, their potential effects on hyperglycaemic excursions are less well known [37, 38]. A study of subjects on ivacaftor showed that, while there was an increase in insulin after 16 weeks of ivacaftor use, there was no significant improvement in peak glucose or AUC glucose during a mixed meal tolerance test [39]. While this study was small in size and subjects had mostly normal OGTTs prior to ivacaftor use, it highlights that glycaemic excursions may persist in those receiving highly effective modulators. Furthermore, impaired pancreatic function and glycaemic abnormalities are present at birth in the CF ferret model and are prevalent in infants and young children with CF, suggesting earlier interventions are needed to preserve lung function in CFRD [40, 41]. Thus, there is a need for continued investigation into the effects of hyperglycaemia on pulmonary function decline in CF.
In conclusion and summarized in figure 9, we have demonstrated that hyperglycaemia increases inflammation with a detrimental effect on LRRC26 expression and therefore impairment in BK channel function. This is the first study to our knowledge to provide a connection between hyperglycaemic excursions and worse parameters important for mucociliary function in CF. Further mechanistic and interventional studies are needed to define therapies that will improve the disparity in outcomes between CF and CFRD.
FIGURE 9. Schematic of the effects of hyperglycaemia on airway cells in CF.
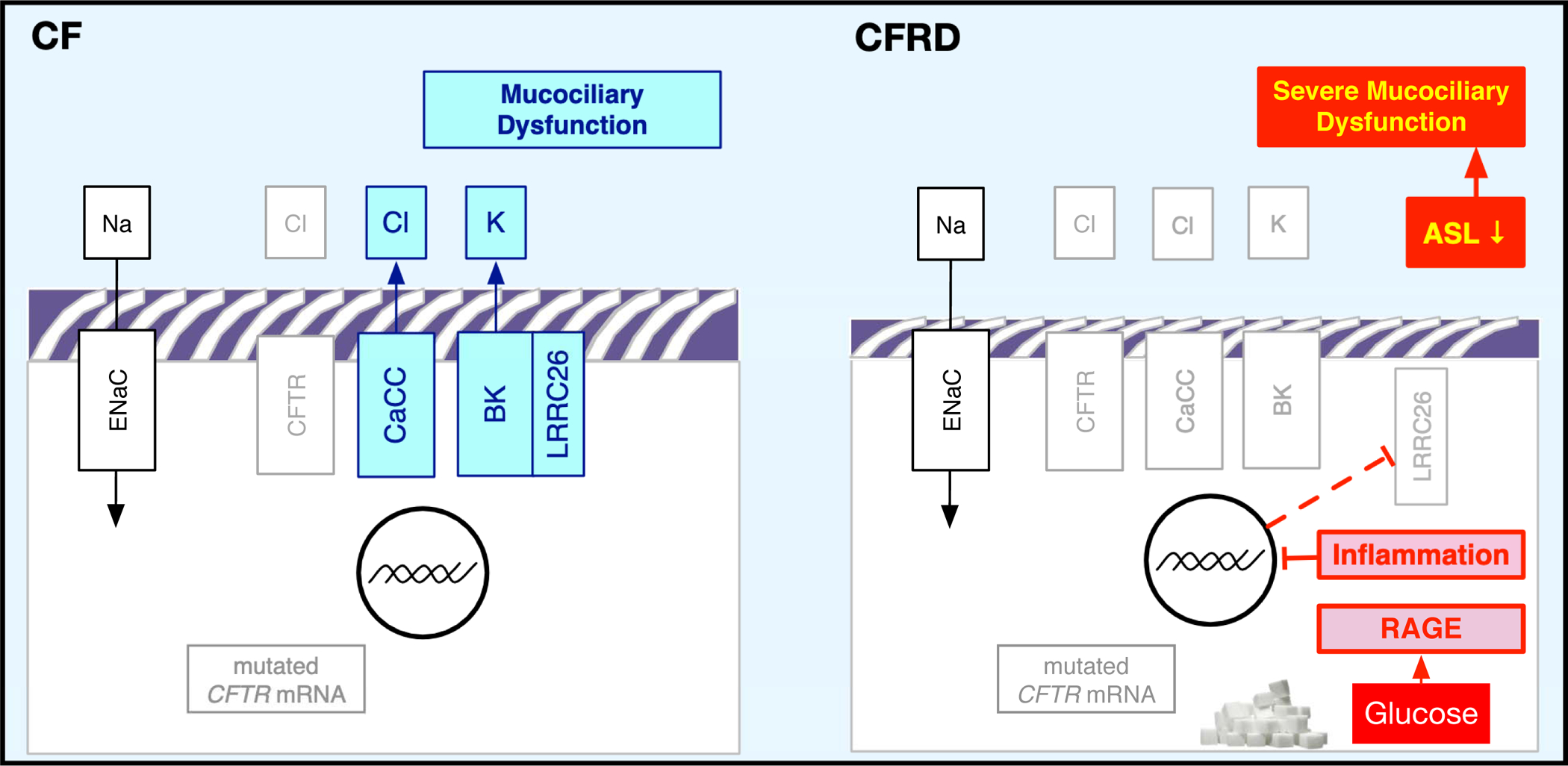
Increased glucose (sugar) leads to RAGE activation with inflammation and a decrease in LRRC26 expression, which decreases BK function (and concomitant CaCC function).
Supplementary Material
Acknowledgments:
We would like to acknowledge Dr. Michael Myerburg for providing the ASL meniscus scanning software. We also thank the Life Alliance Organ Recovery Agency at the University of Miami, LifeCenter Northwest in Seattle, WA, and Midwest Transplant Network in Kansas City, KS for providing the lungs. Finally, we owe a great deal of thanks to all the CF patients at the University of Kansas Health System who participated in the study.
Support statement:
This study was funded in part by CF Foundation (SALATH18I0, BENGTS19AC0), the NHLBI (R01 HL-133240) and a CTSA grant from NCATS awarded to the University of Kansas for Frontiers: University of Kansas Clinical and Translational Science Institute (TL1TR002368). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NCATS.
Footnotes
Publisher's Disclaimer: This is an author-submitted, peer-reviewed version of a manuscript that has been accepted for publication in the European Respiratory Journal, prior to copy-editing, formatting and typesetting. This version of the manuscript may not be duplicated or reproduced without prior permission from the European Respiratory Society. The publisher is not responsible or liable for any errors or omissions in this version of the manuscript or in any version derived from it by any other parties. The final, copy-edited, published article, which is the version of record, is available without a subscription 18 months after the date of issue publication.
Conflict of interest: None declared.
References
- 1.Moran A, Dunitz J, Nathan B, Saeed A, Holme B, Thomas W. Cystic fibrosis-related diabetes: current trends in prevalence, incidence, and mortality. Diabetes Care 2009: 32(9): 1626–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis C, Blackman SM, Nelson A, Oberdorfer E, Wells D, Dunitz J, Thomas W, Moran A. Diabetes-related mortality in adults with cystic fibrosis. Role of genotype and sex. Am J Respir Crit Care Med 2015: 191(2): 194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kerem E, Viviani L, Zolin A, MacNeill S, Hatziagorou E, Ellemunter H, Drevinek P, Gulmans V, Krivec U, Olesen H, Group EPRS. Factors associated with FEV1 decline in cystic fibrosis: analysis of the ECFS patient registry. Eur Respir J 2014: 43(1): 125–133. [DOI] [PubMed] [Google Scholar]
- 4.Moran A, Becker D, Casella SJ, Gottlieb PA, Kirkman MS, Marshall BC, Slovis B, Committee CCC. Epidemiology, pathophysiology, and prognostic implications of cystic fibrosis-related diabetes: a technical review. Diabetes Care 2010: 33(12): 2677–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation 2002: 106(16): 2067–2072. [DOI] [PubMed] [Google Scholar]
- 6.Chuah YK, Basir R, Talib H, Tie TH, Nordin N. Receptor for advanced glycation end products and its involvement in inflammatory diseases. Int J Inflam 2013: 2013: 403460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Robaiy S, Weber B, Simm A, Diez C, Rolewska P, Silber RE, Bartling B. The receptor for advanced glycation end-products supports lung tissue biomechanics. Am J Physiol Lung Cell Mol Physiol 2013: 305(7): L491–500. [DOI] [PubMed] [Google Scholar]
- 8.Queisser MA, Kouri FM, Konigshoff M, Wygrecka M, Schubert U, Eickelberg O, Preissner KT. Loss of RAGE in pulmonary fibrosis: molecular relations to functional changes in pulmonary cell types. Am J Respir Cell Mol Biol 2008: 39(3): 337–345. [DOI] [PubMed] [Google Scholar]
- 9.Oczypok EA, Perkins TN, Oury TD. All the “RAGE” in lung disease: The receptor for advanced glycation endproducts (RAGE) is a major mediator of pulmonary inflammatory responses. Paediatr Respir Rev 2017: 23: 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunt WR, Helfman BR, McCarty NA, Hansen JM. Advanced glycation end products are elevated in cystic fibrosis-related diabetes and correlate with worse lung function. J Cyst Fibros 2016: 15(5): 681–688. [DOI] [PubMed] [Google Scholar]
- 11.Mulrennan S, Baltic S, Aggarwal S, Wood J, Miranda A, Frost F, Kaye J, Thompson PJ. The role of receptor for advanced glycation end products in airway inflammation in CF and CF related diabetes. Sci Rep 2015: 5: 8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bilodeau C, Bardou O, Maille E, Berthiaume Y, Brochiero E. Deleterious impact of hyperglycemia on cystic fibrosis airway ion transport and epithelial repair. J Cyst Fibros 2016: 15(1): 43–51. [DOI] [PubMed] [Google Scholar]
- 13.Manzanares D, Gonzalez C, Ivonnet P, Chen RS, Valencia-Gattas M, Conner GE, Larsson HP, Salathe M. Functional apical large conductance, Ca2+-activated, and voltage-dependent K+ channels are required for maintenance of airway surface liquid volume. J Biol Chem 2011: 286(22): 19830–19839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramasamy R, Vannucci SJ, Yan SS, Herold K, Yan SF, Schmidt AM. Advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology 2005: 15(7): 16R–28R. [DOI] [PubMed] [Google Scholar]
- 15.Yan J, Aldrich RW. LRRC26 auxiliary protein allows BK channel activation at resting voltage without calcium. Nature 2010: 466(7305): 513–516. [DOI] [PubMed] [Google Scholar]
- 16.Manzanares D, Krick S, Baumlin N, Dennis JS, Tyrrell J, Tarran R, Salathe M. Airway Surface Dehydration by Transforming Growth Factor beta (TGF-beta) in Cystic Fibrosis Is Due to Decreased Function of a Voltage-dependent Potassium Channel and Can Be Rescued by the Drug Pirfenidone. J Biol Chem 2015: 290(42): 25710–25716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fulcher ML, Randell SH. Human nasal and tracheo-bronchial respiratory epithelial cell culture. Methods Mol Biol 2013: 945: 109–121. [DOI] [PubMed] [Google Scholar]
- 18.Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-differentiated human airway epithelial cell cultures. Methods Mol Med 2005: 107: 183–206. [DOI] [PubMed] [Google Scholar]
- 19.Schmid A, Sutto Z, Schmid N, Novak L, Ivonnet P, Horvath G, Conner G, Fregien N, Salathe M. Decreased soluble adenylyl cyclase activity in cystic fibrosis is related to defective apical bicarbonate exchange and affects ciliary beat frequency regulation. J Biol Chem 2010: 285(39): 29998–30007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmid A, Sailland J, Novak L, Baumlin N, Fregien N, Salathe M. Modulation of Wnt signaling is essential for the differentiation of ciliated epithelial cells in human airways. FEBS Lett 2017: 591(21): 3493–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sailland J, Grosche A, Baumlin N, Dennis JS, Schmid A, Krick S, Salathe M. Role of Smad3 and p38 Signalling in Cigarette Smoke-induced CFTR and BK dysfunction in Primary Human Bronchial Airway Epithelial Cells. Sci Rep 2017: 7(1): 10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmid A, Baumlin N, Ivonnet P, Dennis JS, Campos M, Krick S, Salathe M. Roflumilast partially reverses smoke-induced mucociliary dysfunction. Respir Res 2015: 16: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harvey PR, Tarran R, Garoff S, Myerburg MM. Measurement of the airway surface liquid volume with simple light refraction microscopy. Am J Respir Cell Mol Biol 2011: 45(3): 592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vigers T, Chan CL, Snell-Bergeon J, Bjornstad P, Zeitler PS, Forlenza G, Pyle L. cgmanalysis: An R package for descriptive analysis of continuous glucose monitor data. PloS one 2019: 14(10): e0216851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan CL, Vigers T, Pyle L, Zeitler PS, Sagel SD, Nadeau KJ. Continuous glucose monitoring abnormalities in cystic fibrosis youth correlate with pulmonary function decline. J Cyst Fibros 2018: 17(6): 783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leclercq A, Gauthier B, Rosner V, Weiss L, Moreau F, Constantinescu AA, Kessler R, Kessler L. Early assessment of glucose abnormalities during continuous glucose monitoring associated with lung function impairment in cystic fibrosis patients. J Cyst Fibros 2014: 13(4): 478–484. [DOI] [PubMed] [Google Scholar]
- 27.Latorre R, Castillo K, Carrasquel-Ursulaez W, Sepulveda RV, Gonzalez-Nilo F, Gonzalez C, Alvarez O. Molecular Determinants of BK Channel Functional Diversity and Functioning. Physiol Rev 2017: 97(1): 39–87. [DOI] [PubMed] [Google Scholar]
- 28.Bernard K, Bogliolo S, Soriani O, Ehrenfeld J. Modulation of calcium-dependent chloride secretion by basolateral SK4-like channels in a human bronchial cell line. J Membr Biol 2003: 196(1): 15–31. [DOI] [PubMed] [Google Scholar]
- 29.Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol 2010: 28: 367–388. [DOI] [PubMed] [Google Scholar]
- 30.Kummarapurugu AB, Zheng S, Ledford J, Karandashova S, Voynow JA. High-Mobility Group Box 1 Upregulates MUC5AC and MUC5B Expression in Primary Airway Epithelial Cells. Am J Respir Cell Mol Biol 2018: 58(1): 126–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang W, Zhao H, Dong H, Wu Y, Yao L, Zou F, Cai S. High-mobility group box 1 impairs airway epithelial barrier function through the activation of the RAGE/ERK pathway. Int J Mol Med 2016: 37(5): 1189–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim MD, Baumlin N, Yoshida M, Polineni D, Salathe SF, David JK, Peloquin CA, Wanner A, Dennis JS, Sailland J, Whitney P, Horrigan FT, Sabater JR, Abraham WM, Salathe M. Losartan Rescues Inflammation-Related Mucociliary Dysfunction in Relevant Models of Cystic Fibrosis. Am J Respir Crit Care Med 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manzanares D, Krick S, Baumlin N, Dennis JS, Tyrrell J, Tarran R, Salathe M. Airway Surface Dehydration by Growth Factor TGF-beta in Cystic Fibrosis is Due to Decreased Function of a Voltage-dependent Potassium Channel and Can Be Rescued by the Drug Pirfenidone. J Biol Chem 2015: 290(42): 25710–25716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manzanares D, Srinivasan M, Salathe ST, Ivonnet P, Baumlin N, Dennis JS, Conner GE, Salathe M. IFN-gamma-mediated reduction of large-conductance, Ca2+-activated, voltage-dependent K+ (BK) channel activity in airway epithelial cells leads to mucociliary dysfunction. Am J Physiol Lung Cell Mol Physiol 2014: 306(5): L453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Devor DC, Singh AK, Lambert LC, DeLuca A, Frizzell RA, Bridges RJ. Bicarbonate and chloride secretion in Calu-3 human airway epithelial cells. J Gen Physiol 1999: 113(5): 743–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen G, Sun L, Kato T, Okuda K, Martino MB, Abzhanova A, Lin JM, Gilmore RC, Batson BD, O’Neal YK, Volmer AS, Dang H, Deng Y, Randell SH, Button B, Livraghi-Butrico A, Kesimer M, Ribeiro CM, O’Neal WK, Boucher RC. IL-1beta dominates the promucin secretory cytokine profile in cystic fibrosis. J Clin Invest 2019: 129(10): 4433–4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bellin MD, Laguna T, Leschyshyn J, Regelmann W, Dunitz J, Billings J, Moran A. Insulin secretion improves in cystic fibrosis following ivacaftor correction of CFTR: a small pilot study. Pediatr Diabetes 2013: 14(6): 417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsabari R, Elyashar HI, Cymberknowh MC, Breuer O, Armoni S, Livnat G, Kerem E, Zangen DH. CFTR potentiator therapy ameliorates impaired insulin secretion in CF patients with a gating mutation. J Cyst Fibros 2016: 15(3): e25–27. [DOI] [PubMed] [Google Scholar]
- 39.Kelly A, De Leon DD, Sheikh S, Camburn D, Kubrak C, Peleckis AJ, Stefanovski D, Hadjiliadis D, Rickels MR, Rubenstein RC. Islet Hormone and Incretin Secretion in Cystic Fibrosis after Four Months of Ivacaftor Therapy. Am J Respir Crit Care Med 2019: 199(3): 342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olivier AK, Yi Y, Sun X, Sui H, Liang B, Hu S, Xie W, Fisher JT, Keiser NW, Lei D, Zhou W, Yan Z, Li G, Evans TI, Meyerholz DK, Wang K, Stewart ZA, Norris AW, Engelhardt JF. Abnormal endocrine pancreas function at birth in cystic fibrosis ferrets. J Clin Invest 2012: 122(10): 3755–3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yi Y, Norris AW, Wang K, Sun X, Uc A, Moran A, Engelhardt JF, Ode KL. Abnormal Glucose Tolerance in Infants and Young Children with Cystic Fibrosis. Am J Respir Crit Care Med 2016: 194(8): 974–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


