Abstract
Background
Total thyroidectomy is the most common surgical treatment of thyroid diseases, and postoperative hypocalcemia is its most common complication. Hypocalcemia prolongs the patient’s hospital stay and impairs his or her quality of life. Although a low vitamin D level is a recognized risk factor, the utility of preoperative vitamin D administration to prevent postoperative hypocalcemia is unclear. In this trial, therefore, we studied the effect of giving vitamin D before total thyroidectomy.
Methods
In a multicenter, randomized, minimally interventional trial (registration number: DRKS 00005615), patients about to undergo total thyroidectomy were randomized either to an intervention group that received 0.5 µg of calcitriol per os twice daily for three days up to the day immediately before surgery, or to a control group that did not (no placebo was given). The primary endpoint was the absence of hypocalcemia (serum calcium <2.1 mmol/L) in the postoperative course.
Results
Of the 287 patients recruited in six hospitals over the period 23 July 2014 to 20 March 2017, 246 were included in the final analysis. The intervention and control groups did not differ significantly with respect to the rate of postoperative hypocalcemia (29.2% and 33.6%, respectively; p = 0.546, power 8.8%). The duration of postoperative hypocalcemia was, however, shorter in the intervention group (3.5 vs. 7 days; p = 0.016, power 68%). The rates of hypocalcemia in the individual trial locations varied widely, ranging from 13.9% to 71.4%.
Conclusion
Short-term administration of calcitriol did not affect the rate of occurrence of hypocalcemia after thyroidectomy, but did shorten its duration. The rate of postoperative hypocalcemia varied widely across hospitals, probably because of differences in surgical technique.
Thyroid operations are among the more common visceral surgical procedures in Germany: in 2017, for example, approximately 70 000 thyroid operations were documented (1). More than half of all thyroid operations involve the total removal of the gland (1). The current S2 guideline of the German Association of Endocrine Surgeons (CAEK) recommends for total thyroidectomy, in particular, for multinodular goiter and for autoimmune thyropathy of Graves type (2).
Hypocalcemia arises after approximately one in three total thyroidectomies and is thus by far the most common complication of thyroid surgery. This is due to the anatomical proximity of the parathyroid glands to the thyroid gland, which renders them vulnerable to accidental removal or to impairment of their blood supply. There is no uniform definition of postoperative hypoparathyroidism; depending on the criteria that are applied, rates of hypocalcemia from 0% to 46% have been reported (3). Hypocalcemia manifests itself clinically with symptoms ranging from hand and acral paresthesia to carpopedal spasm. Severe hypocalcemia causes cardiac arrhythmia, tetany, laryngospasm, and bronchospasm (4). The manifestations of hypocalcemia are usually transient, with recovery in a few weeks to months, but 0.8%–4.4% of the affected patients suffer from permanent effects of postoperative hypoparathyroidism, of varying severity (3). In severe cases, basal ganglionic calcification, nephrocalcinosis, cataracts, and mental changes can arise that impair the quality of life and shorten the life span (5).
Postoperative hypoparathyroidism requires evaluation with additional tests and treatment with additional medication; thus, aside from its harmful physical effects, it also places a burden on the resources of the health care system (6). Clearly, it is important to identify ways to prevent this common complication.
The administration of activated vitamin D in the form of calcitriol is now well established as an acute treatment of postoperative hypocalcemia (2, 7). Postoperative hypocalcemia is more likely in the presence of vitamin D deficiency, and the preoperative prophylactic administration of vitamin D was already recommended in the past (8, 9). We present the findings of a multicenter, prospective, randomized, minimally interventional trial that was carried out to determine whether the short-term preoperative administration of activated vitamin D can influence the rate and duration of hypocalcemia after total thyroidectomy.
Methods
A multicenter, minimally interventional randomized controlled trial (RCT) was conducted. The patients in the intervention group received calcitriol 0.5 µg p.o. b.i.d. for three days before surgery, while the patients in the control group were not given vitamin D (nor were they given a placebo). In all other respects, the patients received sandard treatment, including treatment of postoperative hypocalcemia. The trial was registered prospectively (DRKS 00005615), and the trial protocol has been published (10). Patients undergoing elective total thyroidectomy for what was preoperatively judged to be benign thyroid disease were eligible for inclusion. The primary endpoint of the trial was the rate of postoperative hypocalcemia, defined as a serum calcium concentration below 2.1 mmol/L. The secondary endpoints were: hypocalcemia as defined in the individual trial centers; the duration to recovery of biochemical normocalcemia; the length of hospital stay; and the postoperative quality of life. An intention-to-treat analysis was performed. The hypocalcemia rates of the intervention and control groups were compared with a χ2 test. Logistic regression models were calculated as well. Further information can be found in the eMethods section.
Results
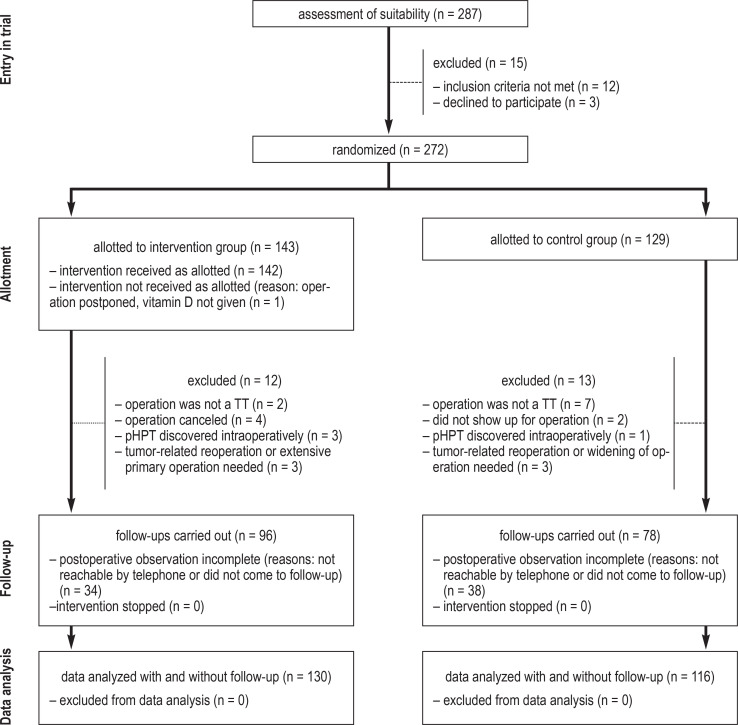
From July 23, 2014, to March 20, 2014, 287 patients were screened in six different hospitals, and 272 who fulfilled the inclusion criteria were recruited for participation in the trial. 143 patients were randomized to the intervention group and 129 to the control group. 13 subjects in each of the two groups were excluded from the final analysis, for reasons listed in the CONSORT diagram (figure 1). Randomization, stratified by center, was found to be evenly distributed (p = 0.887 by the Mantel-Haenszel test). The data sets of the 246 patients were complete up to hospital discharge and were suitable for inclusion in the modified intention-to-treat analysis (130 patients in the intervention group, 116 in the control group). The data sets from follow-up at 30 days were complete for 96 patients (64.6%) in the intervention group and 78 (51.3%) in the control group.
Figure 1.
CONSORT diagram for the HypoCalVID trial pHPT, primary hyperparathyroidism; TT, total thyroidectomy
All patients underwent conventional thyroidectomy as their first-ever thyroid operation. The indications for surgery were nodular goiter in 210 patients (85%) and Graves disease in 36 (15%). In 17 patients, the histopathological examination of the surgical specimen yielded a diagnosis of thyroid carcinoma, without any need for further surgery. In six patients, three each from the intervention and control groups, a thyroid carcinoma was found that was in a stage requiring more extensive surgery; these patients had to be excluded from the data analysis for the purposes of this trial. The global incidence of thyroid carcinoma in the entire patient cohort was 10.9%.
The mean age of the patients was 48.7 years (95% confidence interval: [50.2; 47.3]). 61 men (24.8%) and 185 women (75.2%) were operated upon. The patients’ demographic and clinical characteristics, including (but not limited to) their age, sex, and indication for surgery, were evenly distributed in the intervention and control groups (etable 1).
eTable 1. There were no significant differences between the intervention and control groups in the following demographic and clinical parameters, or in any others:
| Parameter | Intervention group | Control group | p value and test procedure |
| Age (years)* | 48.5 [50.45; 46.5] | 48.9 [51.2; 46.6] | p = 0.834; WMW-U test |
| Sex | 73.1% women 26.9% men |
77.6% women 22.4% men |
p = 0.503; χ² test |
| Indication for surgery | 86.9% goiter 13.1% Graves disease |
83.6% goiter 16.4% Graves disease |
p = 0.582; χ² test |
| Skin-to-skin time* | 120 [128; 111] min | 111 [119; 102] min | p = 0.247; WMW-U test |
* Mean and 95% confidence interval. WMW, Wilcoxon-Mann-Whitney
Regarding the immediate complications of surgery, 2.4% of patients overall (with rates in each hospital varying from 0 to 4.8%) underwent reoperation for a suspected postoperative hemorrhage, while 4.5% (range across hospitals, 0 to 10%) had unilateral vocal cord dysfunction (paresis or paralysis) at the time of discharge. Postoperative hypocalcemia, as defined for the purposes of this trial, was registered in 31.3% of all patients and was more commonly seen in women than in men (women, 37.8%; men, 11.5%; χ2 test, p <0.001). The median fall in serum calcium concentration was 0.290 mmol/L in women (95% confidence interval: [0.311; 0.269]) and 0.135 mmol/L in men (95% confidence interval: [0.153; 0.116], Wilcoxon-Mann-Whitney[WMW] U test; p <0.001). The mean age of patients with hypocalcemia was 44.7 years (46.0; 43.4); they were significantly younger than patients with normocalcemia (50.5 years [51.9; 49.0], t test for two sample, p <0.001). The patients with and without postoperative hypocalcemia did not differ in any other parameter, such as diagnosis, indication for surgery, duration of procedure, the presence or absence of preoperative hyperthyroidism, or the preoperative vitamin D level (in 95 patients) (table 1).
Table 1. Group characteristics of patients with postoperative hypocalcemia and those without a postoperative disturbance of calcium metabolism.
| Criterion (n = 95) |
Patients with hypocalcemia
(n = 16) |
Patients with normocalcemia
(n = 79) |
p value and test procedure |
| Age (years) | 44.7 [48.1; 41.3]* | 50.5 [54.3; 46.7]* | p <0.001; two-tailed t test |
| Sex | 37.8% of women 11.5% of men |
62.2% of women 88.5% of men |
p <0.001; χ² test |
| Indication for surgery | 81.8% goiter 18.2% Graves disease |
87% goiter 13% Graves disease |
p = 0.385; χ² test |
| Hyperthyroidism | 3.9% | 3.0% | p = 0.391; two-tailed t test |
| Skin-to-skin time | 112 [126; 94] min* | 112 [12; 97] min* | p = 0.306; WMW-U test |
|
Vitamin D preoperatively 96.3 [105;81.7] nmol/L* |
90.2 [117; 63.1] nmol/L* | 98.5 [105; 87.7] nmol/L* | p = 0.518; two-tailed t test |
*Mean and 95% confidence interval.
WMW, Wilcoxon-Mann-Whitney
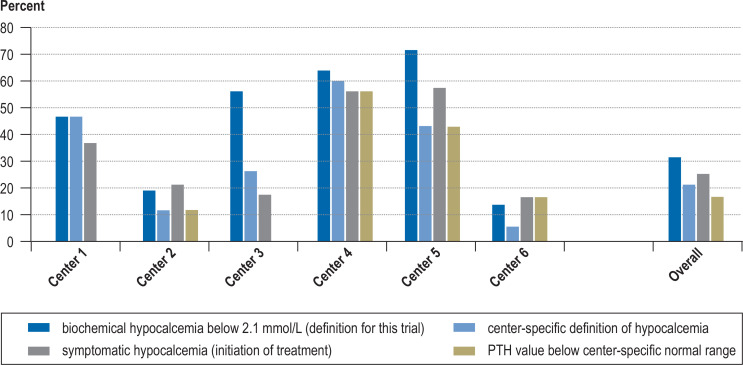
The rates of postoperative hypocalcemia in the individual hospitals ranged from 13.9% to 71.4% (p <0.001; Mantel-Haenszel-Zimmermann test). These differences remained when hypocalcemia was defined on the basis of other serum calcium levels (e.g., <2.0 mmol/L: mean hypocalcemia rate 19.5%, range 5.6–71.4%) or other discriminatory variables, such as the need for calcium supplementation in symptomatic patients (mean 25.2%, range 17.6–57.1%) or a low parathormone level the day after surgery (mean 16.7%, range 11.9–56%) (figure 2).
Figure 2.
Differences in the rates of postoperative hypocalcemia across centers: these differences remained even when analyzed according to alternative definitions, e.g., according to center-specific definitions of hypocalcemia, or the percentage of patients who had symptoms or required treatment. PTH, parathormone.
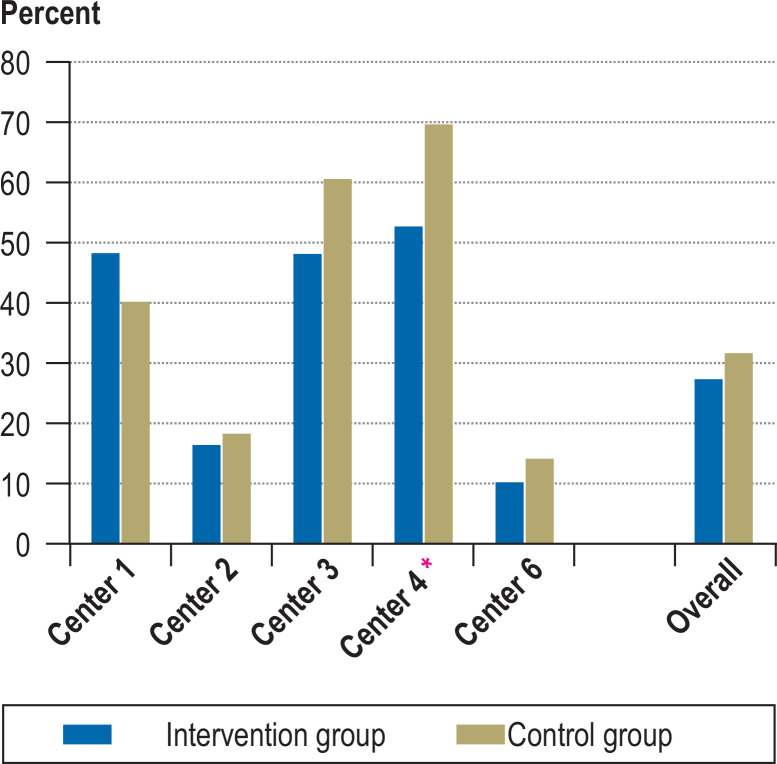
The primary endpoint “postoperative hypocalcemia” was defined as a decline in the serum calcium concentration to less than 2.1 mmol/L. According to this definition, the overall frequency of postoperative hypocalcemia in the intervention group was 29.2% (38 of 130 patients), and in the control group 33.6% (39 of 116 patients). Using a serum calcium concentration of less than 2.0 mmol/L as the criterion would have lowered these figures to 16.9% and 22.4%, respectively. The difference between the two groups was not significant for either criterion (χ2 test; p = 0.546 for calcium concentration <2.1 mmol/L; p = 0.356 for calcium concentration <2.0 mmol/L). When the analysis was stratified by the participating hospitals, a trend emerged, but the effect of the preoperative brief intervention with vitamin D on the rate of postoperative hypocalcemia was still not statistically significant (efigure). The heterogeneous distribution of the primary endpoint was unexpected. Case numbers were recalculated as part of the planned interim analysis, and it was determined that, because of this heterogeneity, a clinically relevant significance level would not be reached even with the inclusion of more patients in the trial. This analysis was therefore the final evaluation, and the trial was ended.
eFigure.
Percentage distribution of hypocalcemia rates in the individual centers and overall (intervention group = with preoperative short-term calcitriol administraiton, control group = without any intervention). χ² test for serum calcium < 2.1 mmol/L, p = 0.546; for serum calcium <2.0 mmol/L, p = 0.35.
Logistical regression analysis revealed that the two predictors “treatment” and “center” were not collinear and did not interact. The single influential factor that emerged in the univariate model was the center. Patients in centers 1–5 were 4.7 times more likely to develop postoperative hypocalcemia within 48 hours than patients in center 6 (table 2).
Table 2. Logistic regression analysis for predictors of postoperative hypocalcemia within 48 hours (n = 246).
| Parameter (the risk group is indicated) | Univariate analysis | Multivariate analysis (n = 246) | ||||
| Odds ratio | 95% CI | p value | Odds ratio | 95% CI | p value | |
| No intervention | 1.128 | [0.650; 1.960] | 0.668 | 1.117 | [0.625; 1.996] | 0.709 |
| Hospitals 1 - 5 | 4.725 | [2.452; 9.103] | <0.001 | 4.721 | [2.450; 9.097] | <0.001 |
CI, confidence interval
The occurrence of postoperative hypocalcemia had no effect on either the results of the postoperative 36-Item Short Form Survey (SF-36) summary scores (WMW-U test, mental health summary score): p = 0.479; physical health summary score: p = 0.813) or the disease-specific stress scores (p = 0.599; WMW-U test). These data are presented more extensively in the eResults section and in eTable 2.
eTable 2. Data from the SF-36 questionnaire on the patients’ preoperative quality of life.
| Mental heath summary scale | p value and test procedure | Physical health summary scale | p value and test procedure | |||
| preoperative | postoperative | preoperative | postoperative | |||
| All patients | 47.1 [48.7; 45.44] | 52.6 [54.3; 50.9] | 0.02 (MWP) | 52.9 [54.3; 51.5] | 50.1 [51.5; 48.5] | 0.05 (MWP) |
| All patients with hypocalcemia | 46.1 [49.4; 42.8] | 51.2 [53.6; 46.8] | 0.479 (WMW) | 53.9 [56.75; 51.2] | 51.9 [54.3; 49.6] | 0.813 (WMW) |
| All patients without vitamin D | 47.9 [50.2; 45;7] | 51.8 [54.4; 49.3] | 0.002 (MWP) | 52.3 [54.5; 50.2] | 49.2 [51.6; 46.9] | 0.001 (MWP) |
| All patients with vitamin D | 46.3 [48.7; 44.0] | 53.3 [55.3; 51.2] | 0.001 (MWP) | 53.4 [55.1; 46.9] | 50.7 [52.8; 48.6] | 0.02 (MWP) |
| Women without vitamin D | 47;6 [44.9; 50.2] | 52.6 [55.3; 49.9] | 0.001 (MWP) | 52.2 [54.8; 49.5] | 48.7 [45.9; 51.4] | 0.001 (MWP) |
| Women with vitamin D | 45.5 [48.2; 42.7] | 52.2 [54.9; 49.6] | 0.001 (WMW) | 53.0 [55.2; 50.9] | 49.6 [52.1; 47.1] | 0.001 (WMW) |
| Men without vitamin D | 49;1 [53.7; 44.6] | 49.6 [56.4; 42.7] | 0.458 (MWP) | 52.9 [56.9; 48.8] | 50.9 [55.4; 46.3] | 0.585 (MWP) |
| Men with vitamin D | 48.3 [52.9; 43.8] | 56.4 [58.3; 54.4] | 0.058 (WMW) | 54.3 [57.0; 51.6] | 54.2 [57.1; 51.3] | 0.572 (WMW) |
Comparisons between the pre- and postoperative summary scales within each group were performed with the Wilcoxon matched pairs test (WMP); comparisons of summary scales across groups (e.g., the overall patient group compared to patients with hypocalcemia) were performed with the Wilcoxon-Mann-Whitney U test (WMW). Means and 95% confidence intervals are given. SF, short form.
The biochemical signs of postoperative hypocalcemia regressed more rapidly in patients who had received activated vitamin D in the intervention group. The patients in the intervention group had a low serum calcium concentration for a median of 3.5 days (95% confidence interval [3.7; 3.26], range: 1–21 days). Biochemical hypocalcemia persisted twice as long in control group patients, with a median duration of 7 days (95% CI: [7.18; 6.77], range: 1–35 days; WMW-U test; p = 0.016). The mean length of hospital stay among all patients who underwent surgery was 2.52 days (95% CI: [2.65; 2.4]). Patients with postoperative hypocalcemia stayed in the hospital for a mean duration of 2.84 days (95% CI: [3.07; 2.61]); nearly one in two patients with hypocalcemia stayed a day longer in the hospital than patients without hypocalcemia did. The duration of inability to work was not affected: patients in both groups went back to work after a mean of 19.8 days (95% CI: [21.7; 17.9]).
Stable normocalcemia is a prerequisite for safe discharge from the hospital. Therefore, the rates of serum calcium concentrations ≥ 2.1 mmol/L on each postoperative day were studied, and the corresponding numbers needed to treat were calculated. For example, on postoperative day 3, the normocalcemia rate was 89% in the entire intervention group and 81% in the entire control group (global NNT: 12.5). Calculations of this type for each of the participating hospitals yielded NNTs ranging from 3 to 16. Centers with a higher rate of hypocalcemia can lower it by briefly administering vitamin D preoperatively even to a small number of patients (table 3).
Table 3. Number needed to treat (NNT) among patients with postoperative hypocalcemia to achieve a normal serum calcium concentration on the day of hospital discharge*.
| Rate of hypocalcemia | Normocalcemic patientsin the control group on postoperative day 3 | Normocalcemic patientsin the vitamin D group on postoperative day 3 | Absolute risk reduction | NNT | |
| Center 1 | 41.5% | 90% | 70.6% | −19.4% | −5.2 |
| Center 2 | 20% | 84% | 90% | 6.3% | 16 |
| Center 3 | 62.5% | 60% | 93.3% | 33.3% | 3 |
| Center 4 | 71.4% | 40% | 66.7% | 26.7% | 3.8 |
| Center 6 | 16% | 91% | 98.1% | 6.7% | 15 |
| All centers | 33.6% | 81% | 89% | 8% | 12.5 |
*Depending on the rates of postoperative hypocalcemia in the individual centers, the NNT was between 3 and 16.
Discussion
Despite the steady decline in the number of thyroid operations performed each year, total thyroidectomy is still a common procedure. Specialization and technical progress have led to shorter skin incisions, a lower risk of postoperative bleeding, and, above all, a lower frequency of permanent vocal cord motility disturbances. This was also demonstrated by the prospectively acquired data for patients who underwent thyroidectomy in the present trial. The rates of vocal cord dysfunction and postoperative bleeding that were found in this trial are representative of the typical clinical outcomes in thyroid surgery centers certified by the endocrine surgery working group of the German Society for General and Visceral Surgery (Deutsche Gesellschaft für Allgemein- und Viszeralchirurgie [DGAVC], Chirurgische Arbeitsgruppe Endokrinologie [CAEK]), and they accord with the findings of comparable studies from other experienced centers (15, 16).
As is known from the literature, postoperative hypocalcemia was the most common side effect of total thyroidectomy. While the 31.3% overall hypocalcemia rate that we found seems to be in the same range as previously reported, we also found a marked divergence of hypocalcemia rates across established thyroid surgery centers, which had not previously reported elsewhere to any comparable extent. Publications to date, however, have been limited to single-center interventional trials or pooled data (3– 11, 16– 23). This prospective trial is thus the first that enables a direct comparison with respect to this particular endpoint across certified institutions for endocrine surgery. One may plausibly assume that it is mainly the details of operative technique used to protect the parathyroid glands that affect the rate of hypocalcemia in each center. This assumption is supported by the finding that “center” is a predictor for the outcome of thyroid surgery, even independently of the definition of hypocalcemia that is used.
This trial did not detect a protective effect of the preoperative prophylactic administration of activated vitamin D on the rate of postoperative hypocalcemia. The numerical differences between the intervention and control groups did not reach statistical significance. Because calcitriol exerts its effects within 1–2 days of its administration, any real effect on postoperative hypocalcemia, ought to be detectable after the administration of vitamin D during a brief preoperative interval, as in this trial (4).
There have been only a few comparable studies of the utility of preoperative calcitriol administration. In studies in which calcitriol was given preoperatively to patients in an intervention group, but postoperatively to all patients as part of standard postoperative care, the event rates in both groups were low and no relevant difference was found between groups with respect to the course of the postoperative calcium concentration or the rate of symptomatic hypocalcemia (19, 20). Two further single-center studies are comparable with the present trial, in that the patients in the control groups were only given calcitriol in response to biochemical or clinical evidence of hypocalcemia, while the patients in the intervention groups continued to take calcitriol postoperatively. In these two RCTs, the patients who had been treated with calcitriol preoperatively for 5 or 7 days had lower rates of symptomatic hypocalcemia. The absolute risk reduction in these two studies was 0.3 and 0.4 (21, 22).
This effect was not achieved in the present multicenter trial, but preoperative calcitriol was indeed found to shorten the duration of postoperative hypocalcemia. The duration till the calcium concentration rises back to normal, in relation to the administration or non-administration of calcitriol preoperatively, was not investigated in any of the studies mentioned above. The present trial shows that, on the third day after surgery, a significantly higher number of patients who had undergone the intervention before surgery already had normal calcium levels again. The higher the rate of postoperative hypocalcemia in each center, the more marked the clinical effect of calcitriol. The number of patients who need to be treated preoperatively in this way so that one patient will have a clinically relevant benefit (NNT) ranged from 3 to 16 across the hospitals participating in this trial.
One limitation of this trial is its multicentric, minimally interventional design, which did not enable the use of blinding, a placebo control, or strict standardization with respect to potential cofactors promoting hypocalcemia or with respect to the details of surgical technique. At the same time, however, it was only the use of real-world data that enabled us to demonstrate the major disparities in event rates across centers. A systematic assessment of preoperative vitamin D levels might have been useful as well, as one may surmise from the subgroup analysis of patients whose preoperative vitamin D values were available: of the 27 patients with very low values (i. e. ≤ 25 nmol/L), 11 were in the control group and 6 of these patients had hypocalcemia postoperatively, compared to only one of the 16 patients in the control group. As has already been suggested elsewhere, perioperative interventions in patients with a documented vitamin D deficit can be investigated in future studies (8, 23). Furthermore, the findings of the present RCT support the speculative hypothesis that the best way to lower the rate of symptomatic postoperative hypocalcemia is to start the patient on calcitriol before surgery and to continue doing so afterward (23).
Overview
Independently of the vitamin D level, short-term prophylaxis with the administration of activated vitamin D for three days does not lower the rate of postoperative hypocalcemia, but it does significantly shorten the time until the renormalization of the serum calcium level. In hospitals were postoperative hypocalcemia is common, this can be a simple and rapid way to improve the standard of treatment, by enabling patients to leave the hospital earlier, on average, than they would have done without such treatment.
Supplementary Material
eMethods
Trial design and patients
The trial was planned as a multicenter, prospective, randomized, minimally interventional trial with an intervention group and an active control group. The patients in the intervention group were given 0.5 µg of calcitriol twice a day for three days before surgery; the patients in the control group were not given vitamin D, nor were they given a placebo. All other aspects of treatment, including the treatment of postoperative hypocalcemia, accorded with the standards of the participating hospitals and thus varied across study centers but were identical for the two patient groups within each study center. The trial was approved by the Ethics Committee of the Hesse State Medical Association (Landesärztekammer Hessen), and participating clinics were enrolled in the trial after local attestation by the criteria of good clinical practice (GCP). All patients gave written informed consent to their participation. The trial was entered into the German Clinical Trial Registry (DRKS 00005615), and its protocol was published (10).
Patients undergoing elective total thyroidectomy for what was preoperatively judged to be benign thyroid disease were eligible for inclusion. Patients were recruited at six hospitals with specialized experience in endocrine surgery. All of the participating surgeons were members of a competence or reference center of the endocrine surgery working group (CAEK) of the German Society for General and Visceral Surgery (DGAVC) and thus had special expertise in thyroid surgery. All of the operations were conventional thyroid resections.
The exclusion criteria included any diseases or drugs that alter calcium metabolism (e.g., musculoskeletal disease, hyperparathyroidism, and drugs containing vitamin D or hydrochlorothiazide). Patients who had undergone thyroid or parathyroid surgery were likewise excluded, as were those who required further surgery beyond total thyroidectomy because of an intra- or postoperatively established diagnosis of thyroid carcinoma.
The primary endpoint of the trial was the rate of postoperative hypocalcemia, defined as the number of patients on any particular day of treatment up to discharge whose serum calcium concentration was below 2.1 mmol/L (10, 11). The secondary endpoints included, among others: hypocalcemia as defined in the individual trial centers; the duration to recovery of biochemical normocalcemia; the length of hospital stay; and the postoperative quality of life.
The quality of life was assessed by the Short Form (SF-36) questionnaire, as per Bullinger et al. (12), according to the guidelines of the International Quality of Life Assessment Association (IQOLA). The normal values used for comparison were those reported by Ellert and Mellbach (13). The HypoPara questionnaire, which was used in this trial as well, was developed for chronic hypoparathyroidism and has been validated as a disease-specific instrument of assessment (14). Through the subjects’ responses, the symptoms and sequelae of hypoparathyroidism are rated on a scale and then represented as a summary score and a stress score that reflects the intensity of symptom-related stress, on a scale from 0 to 160.
Randomization and blinding
The included patients were randomly allotted to the intervention and control groups, in a ratio of 1:1, as soon as the date of the upcoming operation was determined. Randomization was performed centrally and was stratified by center with Random Allocation Software V 1.1.0. There was no intent to blind either the patients or the treating personnel to the intervention, or to use a placebo control.
The course of the trial
The trial procedure is described in detail in the published protocol (10). The patients in the intervention group were given six tablets containing 0.5 µg of calcitriol and were instructed to take two tablets per day up to and including the day before surgery. The details of surgery and individual operative technique were not influenced by participation in the trial or by allotment to the intervention or control group. The perioperative checks and treatment routines were identical for the two groups and were in accordance with the local standards of the participating centers, as well as with the recommendations of the endocrine surgery working group of the German Society for General and Visceral Surgery in its guidelines. The relevant parameters for the trial were assessed preoperatively, on postoperative days 1 and 2, on the day of discharge, and at 30 ± 3 days (figure 1).
Statistical evaluation
The case-number calculation was based on a retrospective evaluation of published data (10). There is no uniform definition of postoperative hypocalcemia, and the published rates range from 0 to 46% (6). For the purposes of this investigation, it was assumed that patients in the participating specialized clinics would have a 25% rate of hypocalcemia in the control group and a rate that was 10% lower in the intervention group. 500 patients per group would give the trial 80% power with a χ2 test and a two-tailed significance level of 0.05. Assuming that 10% of the enrolled patients would be lost to analysis, it was concluded that 540 patients should be randomized. Because of marked variation in the existing data on the rate of postoperative hypocalcemia that could be expected, the adaptive trial protocol included an interim analysis after the recruitment of 270 patients (half of the originally intended total). The results of the planned interim analysis are presented here. Because of these results, recruitment was terminated earlier than originally planned.
The primary analysis was carried out in an intention-to-treat population. Individual missing values of variables were replaced with the last observation carried on forward (LOCF). The primary endpoint – the rates of hypocalcemia in the two groups – was subjected to a a χ2 test, as were all nominal values. Tests for associations among multiple groups were performed with the Mantel-Haenszel test (MHT). Comparisons of two independent samples were performed with the two-sample t test. The Wilcoxon-Mann-Whitney U test was used for variables that were not normally distributed. These analyses were carried out by the Institute of Biostatistics and Mathematical Modeling of the Universitätsklinik Frankfurt am Main.
Uni- and multivariate logistical regression models were also created, with the intervention and center as predictors, and with postoperative hypocalcemia within 48 hours as the outcome to be explained. A preliminary check was performed for the possible collinearity or interaction of the two predictive factors.
eResults
Assessment of the quality of life with the SF-36 and HypoPara questionnaires
191 patients (77.6% of all patients included in the trial) received quality-of-lfe questionnaires preoperatively, and 154 (62.6%) postoperatively. 145 patients (58.9%, of whom 78 were in the intervention group and 67 in the control group) completed the questionnaires both pre- and postoperatively.
In the overall patient group, changes were seen after surgery in the SF-36 summary scores for both physical and mental health. Relevant differences between the intervention and control groups were found among women, who experienced a more marked improvement on the physical health summary scale than men did, as well as a more marked worsening on the mental health summary scale. The occurrence of postoperative hypocalcemia did not affect the postoperative SF-36 summary scores (WMW-U test for mental health: p = 0.479; for physical health: p = 0.813). These data are summarized in eTable 2.
The mean stress score derived from the HypoPara questionnaire rose in the overall patient group from 10.8 (95% confidence interval: [13.3; 8.4]) before surgery to 12.9 ([15.1; 10.8]) afterward (p = 0.0002, WMP test). The postoperative stress score was lower in the intervention group of patients who had received vitamin D preoperatively than in the control group (11.3; [14.2; 8.3] vs. 14.75; [19.5; 9.9], Wilcoxon matched pairs test; p <0.001). Postoperative hypocalcemia was not in itself associated with any difference in stress scores (p = 0.599; WMW-U test).
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Trial sponsor
Klinikum Offenbach GmbH
Statement on data sharing
The trial protocol has been published elsewhere (10). The anonymized patient data on which the findings of this trial are based will be made available to scientists whose project proposals are approved by a committee that has been established for this purpose. Project proposals can be submitted via the senior author’s correspondence address (see below) up to 36 months after the publication of this article.
Acknowledgement
We express our sincere thanks to the patients who participated in this trial, and to the staff of the participating hospitals who carried out additional work for this trial beyond their existing clinical duties.
Registration
Trial registration number DRKS 00005615.
Footnotes
Conflict of interest statement
The authors declare that they have no conflict of interest.
References
- 1.Gesundheitsberichterstattung des Bundes (GBE) „Operationen und Prozeduren der vollstationären Patientinnen und Patienten in Krankenhäusern“. www.gbe-bund.de/gbe10/I?I=662:36258844D (last accessed on 1 September 2021) [Google Scholar]
- 2.AWMF. Leitlinie: Benigne Schilddrüsenerkrankungen, operative Therapie. www.awmf.org/leitlinien/detail/ll/088-007.html (last accessed on 1 September 2021) [Google Scholar]
- 3.Mehanna HM, Jain A, Randeva H, et al. Postoperative hypocalcaemia—the difference a definition makes. Head Neck. 2010;32:279–283. doi: 10.1002/hed.21175. [DOI] [PubMed] [Google Scholar]
- 4.Shoback D. Hypoparathyroidism. N Engl J Med. 2008;359:391–403. doi: 10.1056/NEJMcp0803050. [DOI] [PubMed] [Google Scholar]
- 5.Almquist M, Ivarsson K, Nordenström E, et al. Mortality in patients with permanent hypoparathyroidism after total thyroidectomy. Br J Surg. 2018;105:1313–1318. doi: 10.1002/bjs.10843. [DOI] [PubMed] [Google Scholar]
- 6.Kara M, Tellioglu G, Krand O, et al. Predictors of hypocalcaemia ocurring after a total/near total thyroidectomy. Surg Today. 2009;39:752–757. doi: 10.1007/s00595-009-3957-1. [DOI] [PubMed] [Google Scholar]
- 7.Grzegory A, Pomoski L. Perioperative calcium and vitamin D supplementation in patients undergoing thyroidectomy—literature review. Pol Przgel Chir. 2018;90:47–51. doi: 10.5604/01.3001.0012.0975. [DOI] [PubMed] [Google Scholar]
- 8.Kirkby-Bott J, Haridimos M, Skandarhaja A, et al. Preoperative vitamin D deficiency predicts postoperative hypocalcemia after total thyroidectomy. World J Surg. 2011;35:324–330. doi: 10.1007/s00268-010-0872-y. [DOI] [PubMed] [Google Scholar]
- 9.Erbil Y, Barbaros U, Temel B, et al. The impact of age, vitamin D3 level, and incidental parathyroidectomy on postoperative hypocalcemia after total or near total thyroidectomy. Am J Surg. 2009;197:439–446. doi: 10.1016/j.amjsurg.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 10.Wolak S, Scheunchen M, Holzer K, Busch M, Trumpf E, Zielke A. Impact of preoperative Vitamin D3 administration on postoperative hypocalcaemia in patients undergoing total thyroidectomy: study protocol for a randomized controlled trial. Trials. 2016;17 doi: 10.1186/s13063-016-1216-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quinn EM, Neary PM, O`Connor OJ, Shafiq A, Kelly J, Redmond HP. Routine calcium measurement is not necessary after most thyroid surgeries: a prospective clinical study. Clin Otolaryngol. 2010;35:4368–4373. doi: 10.1111/j.1749-4486.2010.02222.x. [DOI] [PubMed] [Google Scholar]
- 12.Bullinger M, Kirchberger I. Der SF-36-Fragebogen zum Gesundheitszustand: Handbuch für die deutschsprachige Fragebogenversion. Hoegrefe-Verlag, Göttingen. 1997 [Google Scholar]
- 13.Ellert U, Mellbach MB. Der SF-36-Bundesgesundheitssurvey - Beschreibung einer aktuellen Normstichprobe. Gesundheitswesen. 1999;61(Suppl 2):184–190. [PubMed] [Google Scholar]
- 14.Boher T, Fleischmann P, Tersteegen A, Hasse C. Das weitgehend unbekannte Krankheitsbild des postoperativen Hypoparathyreoidismus: Konzeption und Validierung eines Fragebogeninstrumentes. Zentralbl Chir. 2005;130:440–448. doi: 10.1055/s-2005-836820. [DOI] [PubMed] [Google Scholar]
- 15.Bures C, Klatte T, Friedrich G, Kober F, Hermann M. Guidelines for complications after thyroid surgery: pitfalls in diagnosis and advices for continuous quality improvement. Eur Surg. 2014;46:38–47. [Google Scholar]
- 16.Bartsch DK, Dotzenrath C, Vorländer C, et al. Current practice of surgery for benign goitre An analysis of the prospective DGAV StuDoQ thyroid registry. J Clin Med. 2019;8:477–483. doi: 10.3390/jcm8040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Testa A, Fant V, De Rosa A, et al. Calcitriol plus hydrochlorothiazide prevents transient post-thyroidectomy hypocalcemia. Horm Metab Res. 2006;38:821–826. doi: 10.1055/s-2006-956504. [DOI] [PubMed] [Google Scholar]
- 18.De Pasquale L, Sartori PV, Vicentini L, et al. Necessity of therapy for post-thyroidectomy hypocalcaemia: a multicenter experience. Langb Arch Surg. 2015;400:319–324. doi: 10.1007/s00423-015-1292-0. [DOI] [PubMed] [Google Scholar]
- 19.Donahue C, Pantel HJ, Yarlagadda BB, Brams D. Does preoperative calcium and calcitriol decrease rates of post-thyroidectomy hypocalcemia? A randomized clinical trial. J Am Coll Surg. 2021;232:848–854. doi: 10.1016/j.jamcollsurg.2021.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Shonka DC Jr, Maxwell AK, Petroni GR, Jameson MJ. Phase II randomized study of preoperative calcitriol to prevent hypocalcemia following thyroidectomy. Head Neck. 2021;43:2935–2945. doi: 10.1002/hed.26775. [DOI] [PubMed] [Google Scholar]
- 21.Jaan S, Shegal A, Wani RA, et al. Usefulness of pre- and post-operative calcium and vitamin D supplementation in prevention of hypocalcaemia after total thyroidectomy: a randomized controlled trial. Ind J Endocrinol Metab. 2017;21:51–55. doi: 10.4103/2230-8210.195997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai CK, Zainira WZ, Imsiairi AH, et al. Randomized control study using vitamin D in preventing post total thyroidectomy transient hypocalcaemia. Surg Chron. 2018;23:26–30. [Google Scholar]
- 23.Khatiwada AS, Harris AS. Use of pre-operative calcium and vitamin D supplementation to prevent post-operative hypocalcaemia in patients undergoing thyroidectomy: a systematic review. J Laryngol Otol. 2021;135:568–573. doi: 10.1017/S0022215121001523. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
Trial design and patients
The trial was planned as a multicenter, prospective, randomized, minimally interventional trial with an intervention group and an active control group. The patients in the intervention group were given 0.5 µg of calcitriol twice a day for three days before surgery; the patients in the control group were not given vitamin D, nor were they given a placebo. All other aspects of treatment, including the treatment of postoperative hypocalcemia, accorded with the standards of the participating hospitals and thus varied across study centers but were identical for the two patient groups within each study center. The trial was approved by the Ethics Committee of the Hesse State Medical Association (Landesärztekammer Hessen), and participating clinics were enrolled in the trial after local attestation by the criteria of good clinical practice (GCP). All patients gave written informed consent to their participation. The trial was entered into the German Clinical Trial Registry (DRKS 00005615), and its protocol was published (10).
Patients undergoing elective total thyroidectomy for what was preoperatively judged to be benign thyroid disease were eligible for inclusion. Patients were recruited at six hospitals with specialized experience in endocrine surgery. All of the participating surgeons were members of a competence or reference center of the endocrine surgery working group (CAEK) of the German Society for General and Visceral Surgery (DGAVC) and thus had special expertise in thyroid surgery. All of the operations were conventional thyroid resections.
The exclusion criteria included any diseases or drugs that alter calcium metabolism (e.g., musculoskeletal disease, hyperparathyroidism, and drugs containing vitamin D or hydrochlorothiazide). Patients who had undergone thyroid or parathyroid surgery were likewise excluded, as were those who required further surgery beyond total thyroidectomy because of an intra- or postoperatively established diagnosis of thyroid carcinoma.
The primary endpoint of the trial was the rate of postoperative hypocalcemia, defined as the number of patients on any particular day of treatment up to discharge whose serum calcium concentration was below 2.1 mmol/L (10, 11). The secondary endpoints included, among others: hypocalcemia as defined in the individual trial centers; the duration to recovery of biochemical normocalcemia; the length of hospital stay; and the postoperative quality of life.
The quality of life was assessed by the Short Form (SF-36) questionnaire, as per Bullinger et al. (12), according to the guidelines of the International Quality of Life Assessment Association (IQOLA). The normal values used for comparison were those reported by Ellert and Mellbach (13). The HypoPara questionnaire, which was used in this trial as well, was developed for chronic hypoparathyroidism and has been validated as a disease-specific instrument of assessment (14). Through the subjects’ responses, the symptoms and sequelae of hypoparathyroidism are rated on a scale and then represented as a summary score and a stress score that reflects the intensity of symptom-related stress, on a scale from 0 to 160.
Randomization and blinding
The included patients were randomly allotted to the intervention and control groups, in a ratio of 1:1, as soon as the date of the upcoming operation was determined. Randomization was performed centrally and was stratified by center with Random Allocation Software V 1.1.0. There was no intent to blind either the patients or the treating personnel to the intervention, or to use a placebo control.
The course of the trial
The trial procedure is described in detail in the published protocol (10). The patients in the intervention group were given six tablets containing 0.5 µg of calcitriol and were instructed to take two tablets per day up to and including the day before surgery. The details of surgery and individual operative technique were not influenced by participation in the trial or by allotment to the intervention or control group. The perioperative checks and treatment routines were identical for the two groups and were in accordance with the local standards of the participating centers, as well as with the recommendations of the endocrine surgery working group of the German Society for General and Visceral Surgery in its guidelines. The relevant parameters for the trial were assessed preoperatively, on postoperative days 1 and 2, on the day of discharge, and at 30 ± 3 days (figure 1).
Statistical evaluation
The case-number calculation was based on a retrospective evaluation of published data (10). There is no uniform definition of postoperative hypocalcemia, and the published rates range from 0 to 46% (6). For the purposes of this investigation, it was assumed that patients in the participating specialized clinics would have a 25% rate of hypocalcemia in the control group and a rate that was 10% lower in the intervention group. 500 patients per group would give the trial 80% power with a χ2 test and a two-tailed significance level of 0.05. Assuming that 10% of the enrolled patients would be lost to analysis, it was concluded that 540 patients should be randomized. Because of marked variation in the existing data on the rate of postoperative hypocalcemia that could be expected, the adaptive trial protocol included an interim analysis after the recruitment of 270 patients (half of the originally intended total). The results of the planned interim analysis are presented here. Because of these results, recruitment was terminated earlier than originally planned.
The primary analysis was carried out in an intention-to-treat population. Individual missing values of variables were replaced with the last observation carried on forward (LOCF). The primary endpoint – the rates of hypocalcemia in the two groups – was subjected to a a χ2 test, as were all nominal values. Tests for associations among multiple groups were performed with the Mantel-Haenszel test (MHT). Comparisons of two independent samples were performed with the two-sample t test. The Wilcoxon-Mann-Whitney U test was used for variables that were not normally distributed. These analyses were carried out by the Institute of Biostatistics and Mathematical Modeling of the Universitätsklinik Frankfurt am Main.
Uni- and multivariate logistical regression models were also created, with the intervention and center as predictors, and with postoperative hypocalcemia within 48 hours as the outcome to be explained. A preliminary check was performed for the possible collinearity or interaction of the two predictive factors.
eResults
Assessment of the quality of life with the SF-36 and HypoPara questionnaires
191 patients (77.6% of all patients included in the trial) received quality-of-lfe questionnaires preoperatively, and 154 (62.6%) postoperatively. 145 patients (58.9%, of whom 78 were in the intervention group and 67 in the control group) completed the questionnaires both pre- and postoperatively.
In the overall patient group, changes were seen after surgery in the SF-36 summary scores for both physical and mental health. Relevant differences between the intervention and control groups were found among women, who experienced a more marked improvement on the physical health summary scale than men did, as well as a more marked worsening on the mental health summary scale. The occurrence of postoperative hypocalcemia did not affect the postoperative SF-36 summary scores (WMW-U test for mental health: p = 0.479; for physical health: p = 0.813). These data are summarized in eTable 2.
The mean stress score derived from the HypoPara questionnaire rose in the overall patient group from 10.8 (95% confidence interval: [13.3; 8.4]) before surgery to 12.9 ([15.1; 10.8]) afterward (p = 0.0002, WMP test). The postoperative stress score was lower in the intervention group of patients who had received vitamin D preoperatively than in the control group (11.3; [14.2; 8.3] vs. 14.75; [19.5; 9.9], Wilcoxon matched pairs test; p <0.001). Postoperative hypocalcemia was not in itself associated with any difference in stress scores (p = 0.599; WMW-U test).