Abstract
Light-induced changes in the volume of protoplasts bathed in a medium of constant osmolarity are useful indications of light-dependent cellular osmoregulation. With this in mind, we investigated the effect of light on the volume of protoplasts isolated from the elongating stems of pea (Pisum sativum) seedlings raised under red light. The protoplasts were isolated separately from epidermal peels and the remaining peeled stems. Under continuous red light, the protoplasts of peeled stems swelled steadily, but those of epidermal peels maintained a constant volume. Experiments employing far-red light and phytochrome-deficient mutants revealed that the observed swelling is a light-induced response mediated mainly by phytochromes A and B with a little greater contribution by phytochrome A. Protoplasts of epidermal peels and peeled stems shrank transiently in response to a pulse of blue light. The blue light responsiveness in this shrinking response, which itself is probably mediated by cryptochrome, is under the strict control of phytochromes A and B with equal contributions by these phytochromes. We suggest that the swelling response participates in the maintenance of high tissue tension of elongating stems and that the shrinking response is involved in stem growth inhibition. Other findings include the following: The swelling is caused by uptake of K+ and Cl−. The presence of Ca2+ in the bathing medium is required for phytochrome signaling in the swelling response, but not in the response establishing blue light responsiveness. Phytochrome A mediates the two responses in a totally red/far-red light reversible manner, as does phytochrome B.
Red light (R) has been shown to induce swelling of the protoplasts isolated from etiolated grass leaves (Blakeley et al., 1983; Kim et al., 1986; Bossen et al., 1988; Chung et al., 1988; Zhou et al., 1990) and mung bean hypocotyls (Long et al., 1995). These swelling responses are largely far-red light (FR) reversible, indicating that they are mediated by phytochrome. More recently, protoplasts of maize coleoptiles (Wang and Iino, 1997) and Arabidopsis hypocotyls (Wang and Iino, 1998) have been shown to shrink in response to blue light. These shrinking responses, bearing many kinetic similarities, have been observed in the protoplasts isolated from R-grown seedlings. The response is absent in the hy4 mutant of Arabidopsis (Wang and Iino, 1998), indicating that it is mediated by cryptochrome 1 (Ahmad and Cashmore, 1993).
In studies investigating the effect of blue light Wang and Iino (1997, 1998) observed that the protoplasts of maize coleoptiles and Arabidopsis hypocotyls swell continuously under background R. At least in Arabidopsis, the observed swelling appears to be a phytochrome-mediated response because it is less evident in phytochrome-deficient mutants (Wang and Iino, 1998). Furthermore, it has been shown that in addition to inducing a swelling response, phytochrome controls the occurrence of the cryptochrome-dependent shrinking response. In fact, the shrinking response is absent from the mutant deficient in phytochromes A and B.
A change in the volume of the protoplasts bathed in a medium of constant osmolarity reflects a nearly parallel change in the cellular content of osmotic solutes. Therefore, the change in protoplast volume induced by light is a measure of light-dependent osmoregulation. The solute content can be modulated metabolically or by control of ion fluxes through the plasma membrane. It has not yet been clarified in which way the protoplasts of grass leaves increase the solute content for the phytochrome-mediated swelling response (Blakeley et al., 1983; Bossen et al., 1988). On the other hand, the cryptochrome-mediated shrinking and the phytochrome-mediated swelling in Arabidopsis hypocotyl protoplasts appear to be based entirely on the control of ion fluxes (Wang and Iino, 1998). Both responses are inhibited by the plasma-membrane H+-ATPase inhibitor vanadate, and the shrinking response is inhibited by the anion channel inhibitor, 5-nitro-2-(3-phenylpropylamino)-benzoic acid (NPPB). The R-dependent swelling and the volume recovery after the blue-light-induced shrinkage do not occur at neutral pH or when K+ or Cl− in the bathing medium is replaced by a membrane impermeant ion. These results together indicate that protoplasts shrink by excreting K+ and anions (mainly Cl−) and swell by taking up K+ and Cl− from the bathing medium.
It has been suggested that the phytochrome-mediated swelling of grass leaf protoplasts is causally related to leaf unrolling (Zhou et al., 1990). However, as mentioned above, the swelling response can also be induced in hypocotyl protoplasts. The physiological significance of phytochrome-mediated swelling responses, at least of those observed in hypocotyl protoplasts, is yet to be investigated. On the other hand, the blue light-sensitive shrinking response may be causally related to blue light-dependent growth inhibition. Growth measurements made on maize coleoptiles have provided evidence for this possibility (Wang and Iino, 1997). However, the suggested causality between the protoplast shrinking response and the growth inhibition in Arabidopsis hypocotyls (Wang and Iino, 1998) has to be reevaluated in view of the recent results on photoreceptor-growth relationships obtained using photoreceptor mutants of Arabidopsis (Casal and Mazzella, 1998; Neff and Chory, 1998; Parks et al., 1998; Poppe et al., 1998). This issue will be considered in “Discussion.”
In understanding the physiological roles of light-dependent osmoregulation it would be important to determine whether the responses described above are expressed or blocked in a tissue specific manner. Most of the protoplasts isolated from Arabidopsis hypocotyls expressed cryptochrome- and phytochrome-mediated osmoregulation, as well as the phytochrome control of blue light responsiveness (Wang and Iino, 1998). So far, there is no evidence that any of these responses has tissue specificity.
The present study was initiated to obtain more information about light-dependent osmoregulation. The growing stem of R-grown pea (Pisum sativum) seedlings was chosen as the material. Because the epidermal layer can easily be peeled off from the pea stem, it was possible to investigate the light-induced swelling and shrinking responses using protoplasts isolated separately from epidermal peels and the remaining, peeled stems. We have been able to confirm and extend the results obtained using Arabidopsis protoplasts and to obtain insights into the possible roles played by light-dependent osmoregulation at the tissue level.
RESULTS
As described in detail in “Materials and Methods,” protoplasts were prepared from a defined elongating zone of the third internode of R-grown pea seedlings. During this preparation, tissues and protoplasts were freely exposed to R (2–3 μmol m−2 s−1), but not to any other light. The freshly prepared protoplasts were incubated under background R (50 μmol m−2 s−1) on the sample stage of an inverted microscope.
Epidermal peels and the remaining, peeled stems were used separately to obtain protoplasts. Almost all of the protoplasts isolated from epidermal peels did not contain chloroplasts that emit red fluorescence when excited with blue light in a fluorescence microscope. This observation, confirmed on different occasions, indicates that the protoplasts derived almost entirely from epidermis. On the other hand, it is to be expected that the protoplasts from peeled stems originated from many tissues other than the epidermis. The protoplasts of epidermal peels and those of peeled stems are referred to here as the epidermal protoplasts and nonepidermal protoplasts, respectively.
Unless otherwise specified, the following experiments were conducted using seedlings of the cultivar Alaska.
Changes in Protoplast Volume under Background R and following Blue Light Stimulation
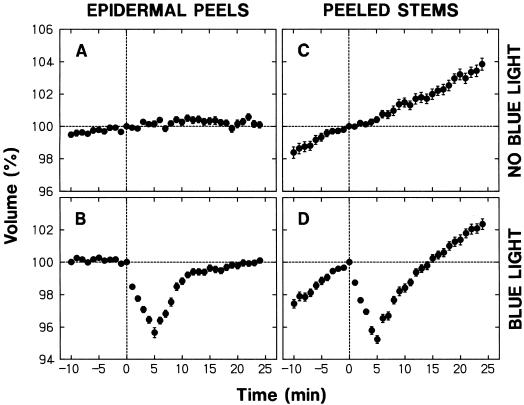
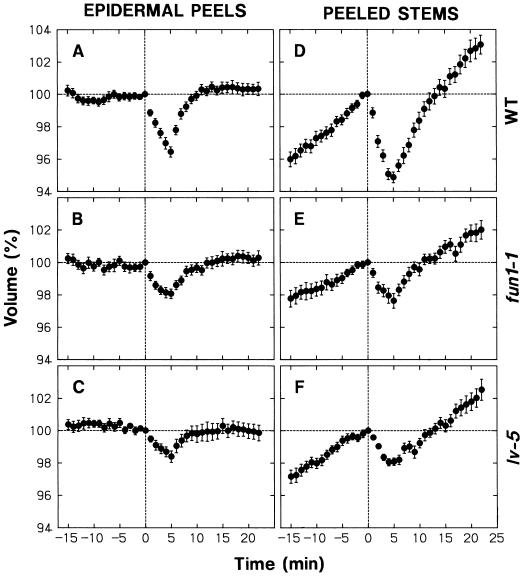
The nonepidermal protoplasts showed a steady increase in volume under background R (Fig. 1C). In sharp contrast, the epidermal protoplasts maintained a nearly constant volume (Fig. 1A). When treated with blue light for 30 s (fluence: 3,500 μmol m−2) under background R, epidermal and nonepidermal protoplasts responded with shrinkage (Fig. 1, B and D). The shrinkage could be detected 1 min after the onset of the blue light pulse, and the minimal volume (about 95%) was established at about 5 min. We also carried out similar experiments using protoplasts isolated from unpeeled stems. The time courses of the mean volume were nearly identical to those obtained with nonepidermal protoplasts (not shown), in agreement with the prediction that the epidermal protoplasts constituted only a small portion of the entire population.
Figure 1.
Changes in protoplast volume under background R and following a pulse of blue light. Protoplasts were prepared under R (2–3 μmol m−2 s−1) from the elongating stem zone of R-grown pea cv Alaska seedlings. The protoplasts were isolated from epidermal peels or the remaining peeled stems. The isolated protoplasts were bathed in a standard medium containing 0.5 m sorbitol, 10 mm KCl, 1 mm CaCl2, 20 mm Glc, and 10 mm MES-KOH (pH 6.0). A 200-μL portion of freshly prepared protoplasts was added to an all-side clear cuvette and incubated under background R (50 μmol m−2 s−1) on the sample stage of an inverted microscope. During incubation the protoplasts were subjected to time-lapse photography to monitor their volume. The volume of each protoplast at a given time was calculated as a percentage of the volume at time 0, which corresponded to 40 min after the onset of incubation on the microscope stage. The protoplasts received no additional light treatment (A and C) or were irradiated with blue light (115 μmol m−2 s−1) for 30 s immediately after obtaining the photograph at time zero (B and D). The data shown are the means ± se obtained from more than 30 protoplasts.
The size of protoplasts used for the analysis varied considerably (see “Materials and Methods”). To investigate whether the protoplast size had any relationship to the observed changes in volume, the protoplasts used to obtain each set of the results in Figure 1 were divided into three groups (small, medium, and large) with respect to size at time zero, and time courses of the mean relative volume were obtained separately (not shown). The size division was made in such a way that equal numbers of protoplasts were distributed into the three groups. In every case the time courses obtained separately for the three groups were similar to the one obtained for the entire population. The relative rate of swelling and the extent of shrinkage tended to become smaller with protoplast size. However, the differences among the three groups were relatively small; the relative rate of swelling or the extent of shrinkage in large protoplasts was not less than 75% of that in small protoplasts.
To investigate whether the volume changes observed in Figure 1 were characteristic of the protoplasts isolated from the elongating stem zone we carried out additional experiments using protoplasts isolated from unpeeled segments of the second internode, which had almost ceased to elongate. These protoplasts retained a nearly constant volume under background R. They showed a blue light-sensitive shrinking response, but the extent of shrinkage was not more than 20% of that observed for the unpeeled segments of the rapidly elongating second internode (not shown). These results indicate that the swelling under background R and the blue light-sensitive shrinking response are positively correlated with the growth activity.
Involvement of Phytochrome
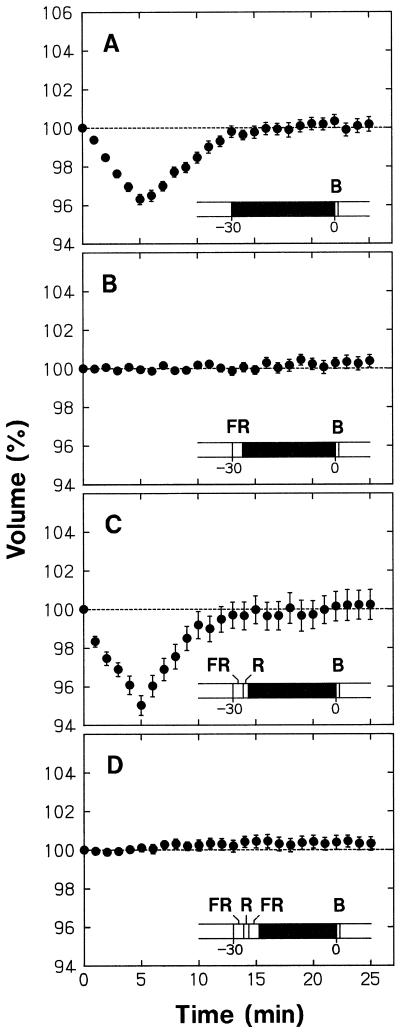
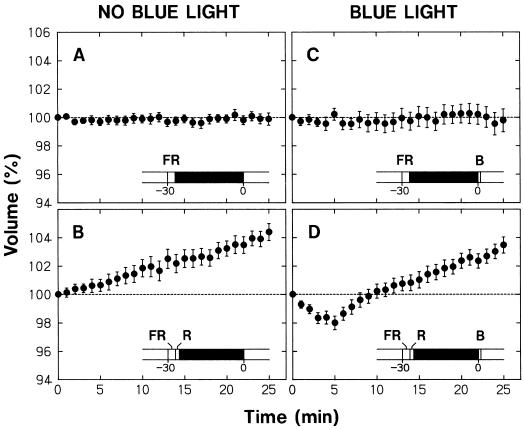
The epidermal protoplasts pretreated with 30 min of darkness showed a blue light-sensitive shrinking response (Fig. 2A) comparable with that found without dark pretreatment (Fig. 1B). However, when the protoplasts were exposed to a pulse of FR at the beginning of the 30-min pretreatment period, the shrinking response disappeared (Fig. 2B). The occurrence of shrinking response could be reversibly controlled by subsequent pulses of R and FR (Fig. 2, C and D). Therefore, the ability of protoplasts to respond to blue light depended strictly on the FR-absorbing form of phytochrome (Pfr) generated just before the dark period and probably sustained during this period.
Figure 2.
Phytochrome regulation of the blue light-sensitive shrinking response in epidermal protoplasts. Protoplasts isolated from epidermal peels of pea cv Alaska seedlings were set on the microscope stage and incubated under background R as described for Figure 1. The R was turned off after 10 min of incubation and the protoplasts were subjected to different 30-min treatments that included darkness, a 3-min pulse of FR (150 μmol m−2 s−1), and a 90-s pulse of R (50 μmol m−2 s−1). Following the 30-min treatment period, background R irradiation was resumed, time-lapse photography for volume determination was initiated, and the protoplasts were treated with a 30-s pulse of blue light (115 μmol m−2 s−1). The onset of R, the first photograph at time zero, and the onset of blue light were within a few seconds of each other. The treatment protocol is illustrated in each panel, with the black bar representing darkness. The volume of each protoplast was calculated as a percentage of the volume at time zero, the time at which the first photograph was obtained. The means ± se from 21 to 25 protoplasts are shown.
Figure 3 summarizes the results from a series of similar experiments conducted with nonepidermal protoplasts. As described above, these protoplasts swelled under background R. The results in Figure 3 (A–D), which were obtained without blue light treatment, indicated that the occurrence of swelling could be reversibly controlled by R and FR given before a dark pretreatment period; the protoplasts swelled when the dark period followed R (Fig. 3, A and C), but maintained a steady volume when the dark period followed FR (Fig. 3, B and D). Therefore, the swelling of protoplasts also depended strictly on the Pfr formed just before the dark period and probably sustained during this period.
Figure 3.
The involvement of phytochrome in the light-dependent volume changes observed in protoplasts of peeled stems. Experiments were conducted as described for Figure 2 with the treatment protocol illustrated in each panel. The protoplasts in E through H were exposed to a pulse of blue light immediately after time zero (see Fig. 2), but those in A through D were not exposed. The means ± se from 23 to 30 protoplasts are shown.
As in the case of epidermal protoplasts, the occurrence of blue light-induced shrinking was reversibly controlled by pulses of R and FR in nonepidermal protoplasts (Fig. 3, E–H). Under the conditions in which the shrinking response took place (i.e. when the dark period followed R) the protoplasts recovered to volumes exceeding the initial 100% level (Fig. 3, E and G). On the other hand, under the conditions in which the shrinking response could not take place (i.e. when the dark period followed FR) the protoplasts maintained a steady volume (Fig. 3, F and H). In view of the results in Figure 3 (A–D), it is apparent that the protoplasts recovered their volume exceeding the initial level in the former case because phytochrome-mediated swelling accompanied the shrinking response, and they maintained a steady volume in the latter case because the phytochrome-mediated swelling was also abolished by the FR treatment.
In the experiments shown in Figures 2 (A–D) and 3 (E–H), protoplasts were exposed to a blue light pulse immediately after the background irradiation with R was resumed to obtain time-lapse photographs. Therefore, the lack of blue light responsiveness in the protoplasts pretreated with FR and darkness was found even when Pfr was present during and after blue light stimulation. This result indicates that Pfr cannot immediately establish the blue light responsiveness.
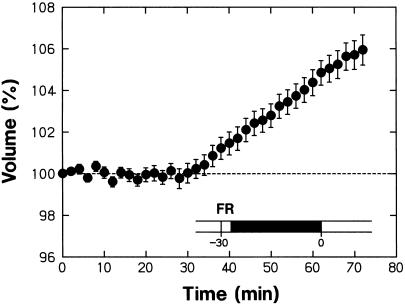
The reversible control of swelling by R and FR could also be resolved by measuring the protoplast volume after the background irradiation with R was resumed (Fig. 3, E–H). It appeared that the swelling response was induced by the Pfr present before the volume-recording period of 25 min, but not by the Pfr present during this period. As shown in Figure 4, the protoplasts pretreated with FR and darkness began to swell 30 min after the background R was turned on. This result indicates that the phytochrome-mediated swelling response was induced with a lag of 30 min after Pfr formation. It is apparent that the protoplasts pretreated with FR and darkness did not swell in response to the R given during the volume-recording period of 25 min because the Pfr produced by this R could not yet induce the swelling response.
Figure 4.
The changes in protoplast volume during prolonged R irradiation after pretreatment with FR and darkness. Protoplasts isolated from peeled stems were pretreated with a 3-min FR pulse and darkness as illustrated and were subsequently incubated under background R. The experimental conditions were identical to those used to obtain the data in Figure 3B except that the volume was monitored for a longer period. The means ± se from 26 protoplasts are shown.
Contribution of Phytochromes A and B
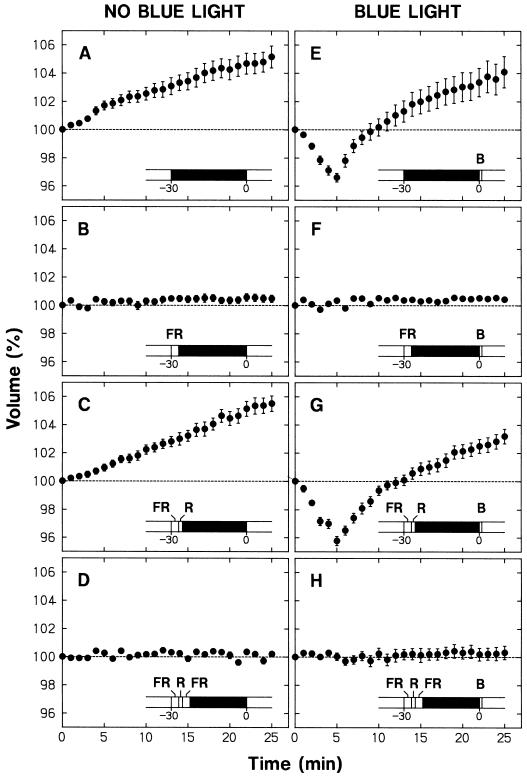
The fun1-1 and lv-5 pea mutants (Weller et al., 1995, 1997) were used to extend the study on phytochrome involvement. The fun1-1 and lv-5 mutants most probably do not contain any functional phytochrome A and B, respectively, because the mutated phytochrome genes contain stop codons within their sequences (S. Batge, N. Beauchamp, and J.B. Reid, unpublished data; J.B. Reid, personal communication). Experiments were conducted as described for Figure 1 (B and D), but with a little longer period of volume recording before blue light stimulation. The results are summarized in Figure 5 together with those obtained for the wild type (cv Torsdag).
Figure 5.
Light-dependent volume changes in protoplasts from fun1-1 and lv-5 mutants. Protoplasts were isolated from epidermal peels (left) or peeled stems (right) of R-grown seedlings of the mutants fun1-1 (B and E) and lv-5 (C and F), and the wild type, cv Torsdag (A and D) and were incubated on the microscope stage under R as described for Figure 1. In all cases, protoplasts were exposed to a 30-s pulse of blue light (115 μmol m−2 s−1) immediately after time zero (45 min after the onset of incubation). The means ± se from 21 to 25 protoplasts are shown.
Epidermal and nonepidermal protoplasts of wild-type seedlings shrank in response to a pulse of blue light (Fig. 5, A and D). The swelling under background R (see the data before time zero) was found in nonepidermal protoplasts (Fig. 5D), but not in epidermal protoplasts (Fig. 5A). These results are essentially identical to those from cv Alaska seedlings (Fig. 1).
The epidermal protoplasts (Fig. 5, B and C) and the nonepidermal protoplasts (Fig. 5, E and F) of the two mutants showed a smaller blue light-sensitive shrinking response. These results indicate that phytochromes A and B both contribute to the control of blue light responsiveness. The shrinking response was similar between the two mutants, and the response in either mutant was at most one-half of that in the wild type. It appeared that phytochromes A and B, being equally responsible for the control of blue light responsiveness, are the major phytochromes involved.
The swelling response was also smaller in fun1-1 and lv-5 mutants (Fig. 5, E and F). The sum of the swelling rates in the two mutants was a little greater than the rate in the wild type. Although the results do not rule out the possible contribution of phytochrome species other than A and B, they at least indicated that phytochromes A and B are the major phytochromes involved in the swelling response. The swelling rate was somewhat less in the fun1-1 mutant than in the lv-5 mutant, suggesting that phytochrome A made a slightly greater contribution than phytochrome B.
The results obtained here indicate that phytochrome A mediates a substantial portion of the R-induced swelling response and the R-dependent control of blue light responsiveness. Because the effects of R were totally FR reversible (Figs. 2 and 3), it appeared that phytochrome A mediates these responses in an R/FR reversible manner. To confirm this point, we next investigated whether or not the effects of R in the lv-5 mutant, which are expected to be due mainly to phytochrome A, could be reversed by FR (Fig. 6). The swelling under background R (Fig. 6A) and the blue light-sensitive shrinking response (Fig. 6C) were both abolished by the treatment with an FR pulse and darkness (compare with Fig. 5D). When an R pulse followed the FR pulse, the protoplasts showed clear swelling (Fig. 6B) and the blue light-sensitive shrinking response became inducible (Fig. 6D). These results substantiate the conclusion that phytochrome A mediates the two responses in an R/FR reversible manner. The significance of this finding will be considered in “Discussion.”
Figure 6.
Reversion by FR of the R effect in protoplasts isolated from peeled stems of lv-5 mutant seedlings. Experiments were conducted as described for Figure 2 with the treatment protocol illustrated in each panel. The protoplasts in C and D were exposed to a pulse of blue light immediately after time zero, but those in A and B were not (see Fig. 3). The means ± se from 21 to 25 protoplasts are shown.
Ion Relationships in the Swelling Response
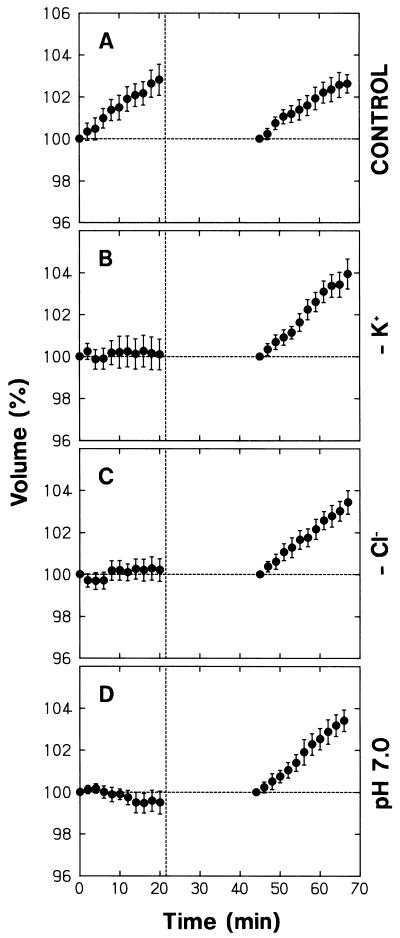
We next investigated the relationships of the protoplast swelling under background R, demonstrated to be a phytochrome-mediated response, with ion compositions of the bathing medium. The major inorganic ions of the medium were K+ and Cl−. When K+ or Cl− was replaced by an impermeant ion (tetraethylammonium ion [TEA+] or iminodiacetic acid [IDA], respectively), protoplasts underwent no swelling (Fig. 7, B and C; the data between 0 and 20 min). These protoplasts recovered swelling upon resuspension in the standard medium (Fig. 7, B and C; the data after 45 min). It is clear that the swelling response strictly depended on the presence of K+ and Cl− in the bathing medium. The results indicate that protoplasts swell by taking up K+ and Cl− and that K+ and Cl− must be present for the uptake of either ion.
Figure 7.
Dependence of R-induced protoplast swelling on K+, Cl−, and H+ of the bathing medium. Protoplasts isolated from peeled stems of pea cv Alaska seedlings were washed with and suspended in the standard medium (A; see Fig. 1 for the compositions), a medium in which K+ was replaced by TEA+ (B), a medium in which Cl− was replaced by IDA (C), and a medium adjusted to pH 7.0 (D). The protoplasts were incubated under background R as described for Figure 1 and were subjected to time-lapse photography from time zero, which was 25 min after the onset of incubation. After obtaining a photograph at 20 min, protoplasts were washed with and resuspended in the standard medium, incubated again under background R, and subjected to time-lapse photography. The dashed vertical line indicates the time at which the protoplasts were suspended in a large volume of the standard medium (the first step of protoplast washing). The volume of each protoplast was calculated as a percentage of the initial volume before or after the washing treatment. The means ± se from 19 to 23 protoplasts are shown.
The protoplasts did not swell when bathed in the medium adjusted to pH 7 (Fig. 7D; the data between 0 and 20 min). After resuspension in the standard medium (pH 6) the protoplasts recovered swelling (Fig. 7D; the data after 45 min), indicating that the lack of swelling was not due to unspecific cellular damage that might be caused by incubation at pH 7. Thus, the high H+ concentration of the standard medium was necessary for the phytochrome-mediated swelling response.
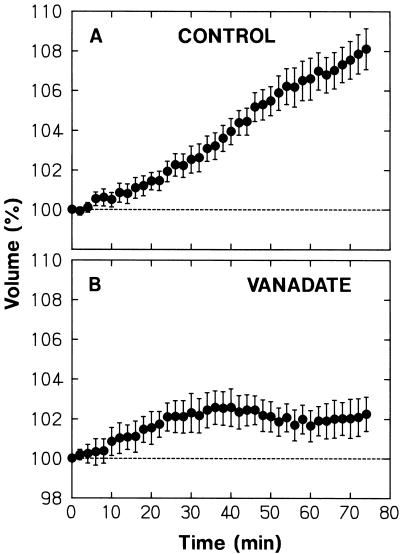
The swelling was inhibited by vanadate added to the bathing medium (Fig. 8). Complete inhibition occurred after about 70 min of incubation in the vanadate-containing medium (see the legend to Fig. 8). The result indicated that the plasma membrane H+-ATPase participates directly or indirectly to the phytochrome-mediated swelling response. Once the swelling was completely inhibited, the protoplasts retained a constant volume for at least 90 min (see the legend to Fig. 8).
Figure 8.
The effect of vanadate on R-induced protoplast swelling. Protoplasts isolated from peeled stems of R-grown pea cv Alaska seedlings were washed with and suspended in the standard medium (A) or the medium to which vanadate was added at a concentration of 500 μm (B). The protoplasts were incubated under R as described for Figure 1. Time zero corresponded to 15 min after the onset of incubation (about 40 min after the protoplasts were put in contact with vanadate). The vanadate-treated protoplasts (A) retained a nearly constant volume during the additional 46 min. The means ± se from 20 (A) or 22 (B) protoplasts are shown.
Dependence on Ca2+ of Shrinking and Swelling Responses
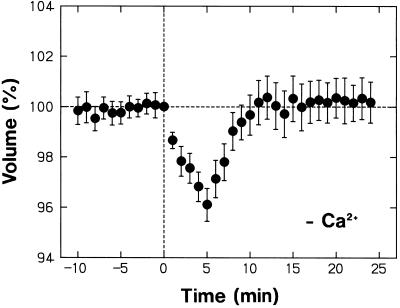
Possible roles played by Ca2+ in the bathing medium were investigated using nonepidermal protoplasts. The protoplasts bathed in the Ca2+-free medium showed a normal shrinking response to a pulse of blue light, including the volume recovery phase (Fig. 9; compare with Fig. 1D). Therefore, the shrinking response did not depend on the Ca2+ in the bathing medium. In the experiment of Figure 9 the protoplasts were incubated in the Ca2+-free medium for more than 40 min before time zero. As shown by the results in Figure 3, the protoplasts lose the capacity to respond to blue light when phytochrome cannot function for 30 min. Therefore, the result in Figure 9 also indicates that the phytochrome-mediated control of blue light responsiveness does not require the presence of Ca2+ in the bathing medium.
Figure 9.
Dependence of the blue light-induced protoplast shrinking on Ca2+ in the bathing medium. Protoplasts isolated from peeled stems of R-grown pea cv Alaska seedlings were washed with and suspended in a Ca2+-free medium (no CaCl2 and 1 mm EGTA; otherwise as the standard medium) and incubated under R on the microscope stage as described for Figure 1. The protoplasts were exposed to a 30-s pulse of blue light (115 μmol m−2 s−1) immediately after time zero. The means ± se from 20 protoplasts are shown. The data should be compared with those in Figure 1D, which were obtained using identical conditions except for the medium compositions.
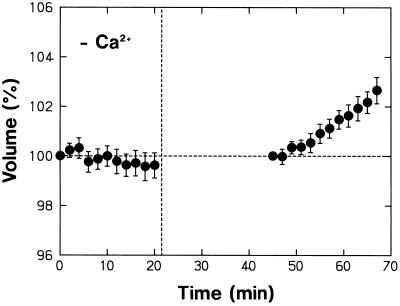
Although the protoplasts showed a normal shrinking response in the Ca2+-free medium, the swelling response that takes place under background R was not evident in this medium (compare Figs. 1D and 9). The lack of swelling in the Ca2+-free medium could be confirmed in the result shown in Figure 10 (the data between 0 and 20 min). In this experimen, it was also ascertained that the protoplasts could recover the swelling response upon resuspension in the standard medium (Fig. 10; the data after 45 min). Therefore, the lack of swelling in the Ca2+-free medium was clearly not caused by any unspecific cellular damage. These results demonstrate that the phytochrome-mediated swelling response requires the presence of Ca2+ in the bathing medium, in contrast to the blue light-sensitive shrinking response and the phytochrome-mediated control of blue light responsiveness.
Figure 10.
Dependence of R-induced protoplast swelling on Ca2+ in the bathing medium. The experiment was conducted as described for Figure 7. Protoplasts isolated from peeled stems of R-grown pea cv Alaska seedlings were washed with and suspended in a Ca2+-free medium (see Fig. 9). The protoplasts were incubated under R on the microscope stage and the data from 0 to 20 min were obtained. Protoplasts were washed with and resuspended in the standard medium and the data after 45 min were obtained. The means ± se from 20 or 21 protoplasts are shown.
DISCUSSION
Light-Dependent Control of Protoplast Volume and Tissue Specificity
The protoplasts of pea stems have been shown to shrink in response to a pulse of blue light (Fig. 1). The time course of this shrinking response is comparable with that shown previously in protoplasts of maize coleoptiles (Wang and Iino, 1997) or Arabidopsis hypocotyls (Wang and Iino, 1998) under comparable conditions. In Arabidopsis, cryptochrome 1 is the nearly sole photoreceptor of the shrinking response (Wang and Iino, 1998). A cryptochrome 1 homolog is probably responsible for the shrinking response of pea protoplasts. The shrinking response was induced in most of the protoplasts isolated from peeled stems. Furthermore, the protoplasts of epidermal peels, identified to be almost entirely of epidermal origin, could also show a clear shrinking response (Fig. 1B). It is concluded that the protoplast-shrinking response has little tissue specificity.
In Arabidopsis the cryptochrome-mediated shrinking response is controlled by phytochrome in such a way that the ability of the protoplasts to respond to blue light depends strictly on Pfr (Wang and Iino, 1998). The results obtained using phytochrome-deficient mutants indicate that phytochromes A and B contribute to the control of blue light responsiveness and are the nearly sole photoreceptors involved in this control. Herein we have further shown that the blue light-sensitive shrinking response of pea stem protoplasts is also under the strict control of phytochrome (Figs. 2 and 3) and that phytochromes A and B are the major phytochrome species involved (Fig. 5). The results also indicate that the shrinking responses of epidermal and nonepidermal protoplasts are similarly controlled by phytochromes A and B.
The protoplasts of maize coleoptiles (Wang and Iino, 1997) and Arabidopsis hypocotyls (Wang and Iino, 1998) were found to swell steadily under background R. At least in the Arabidopsis protoplasts the observed swelling appeared to be a phytochrome-mediated response because the rate of swelling was significantly reduced in phytochrome-deficient mutants. The similar swelling of pea stem protoplasts observed under background R (Fig. 1C) is clearly a phytochrome-mediated response; this was demonstrated by R/FR reversibility (Fig. 3, A–D), as well as by reduced swelling rates in fun1-1 and lv-5 mutants (Fig. 5).
We found that the phytochrome-mediated swelling observed in nonepidermal protoplasts does not take place in epidermal protoplasts (Figs. 1, 2, and 5). Therefore, the swelling response is not expressed in epidermal protoplasts in a tissue-specific manner. Because phytochromes A and B control the blue light responsiveness in epidermal protoplasts, it is clear that these phytochromes found to mediate the swelling response are present in epidermal protoplasts. It can be concluded that the epidermal protoplast cannot transduce the Pfr signal to cause the swelling response.
Kinetic Features of Phytochrome Responses in Protoplasts
With regard to the phytochrome control of the cryptochrome-mediated shrinking response in Arabidopsis protoplasts, Wang and Iino (1998) have shown that Pfr does not directly interact with cryptochrome or any signal transduction component involved in the shrinking response. This conclusion is based on the result that once the protoplasts have lost blue light responsiveness after treatment with FR and darkness, R cannot immediately bring about blue light responsiveness. In fact, a period longer than 10 min was required before any blue light responsiveness begins to be detectable after Pfr formation. Although we have not investigated the temporal relationship between phytochrome and blue light responsiveness in detail, it could at least be demonstrated that R does not immediately establish blue light responsiveness in pea protoplasts.
The phytochrome-mediated swelling response disappears within 30 min after treatment with an FR pulse (Fig. 3, A–D) and is induced with a lag of about 30 min after the onset of R irradiation (Fig. 4). In view of such a long lag time in this cellular response it appears probable that the underlying signal transduction pathway involves gene expression, as also suggested for the control of blue light responsiveness (Wang and Iino, 1998).
At 4°C, the swelling response of wheat protoplasts was already apparent 10 min after the onset of a 5-min R irradiation (Blakeley et al., 1983). At 15°C, the wheat leaf protoplasts began to swell within 1 min after the onset of a 3-min R irradiation and reached a maximal volume in about 15 min (Bossen et al., 1988). These results indicate that wheat leaf protoplasts swell much more rapidly than pea stem protoplasts. This kinetic difference might represent contributions of distinct mechanisms. Because we used R-grown seedlings and R-adapted protoplasts in contrast to the experiments with wheat protoplasts in which etiolated seedlings were used, it is also possible that distinct mechanisms operate under the different light conditions. Although the different temperature conditions used preclude direct comparisons, the other studies conducted with etiolated seedlings indicate that the swelling response is induced with a lag longer than in wheat protoplasts, but shorter than in pea protoplasts (Chung et al., 1988; Zhou et al., 1990; Long et al., 1995). At present it cannot be decided whether the kinetic difference represents different materials or light conditions.
Contribution of Phytochromes A and B, and R/FR Reversibility
In Arabidopsis protoplasts it was noted that phytochrome A contributes to the swelling response more than phytochrome B does (Wang and Iino, 1998). This relationship was also found for pea protoplasts, although the difference was less pronounced (Fig. 5). In Arabidopsis protoplasts phytochrome B participated more than phytochrome A in the establishment of blue light responsiveness (Wang and Iino, 1998). In pea protoplasts the two phytochromes appeared to participate to similar extents in the corresponding response (Fig. 5).
These results were obtained using seedlings raised under continuous R and preparing protoplasts under R. Under such conditions most of the light-labile phytochrome A, which is present in large amount in etiolated seedlings, would have been degraded in the seedlings and protoplasts used for the experiments (see Furuya, 1993). Therefore, the results described above indicate that the contribution of phytochrome A is not minor and can even be greater than that of phytochrome B in the material in which most light-labile phytochrome A is expected to be absent.
It is generally believed that phytochrome A is solely responsible for the very-low-fluence response and the FR-sensitive high-irradiance response and that phytochrome B is the major phytochrome species responsible for the R/FR reversible, low-fluence response (see Smith, 1995; Casal et al., 1997). As demonstrated using phytochrome A-deficient Arabidopsis mutants, phytochrome A functions in white light-grown green plants for photoperiod perception (Johnson et al., 1994; Reed et al., 1994) and stem-growth control (Yanovsky et al., 1995). Phytochrome A can control the inhibition of hypocotyl growth in an R/FR reversible manner in the transgenic tobacco and Arabidopsis overexpressing phytochrome A (Boylan and Quail, 1991; Nagatani et al., 1991). To date, however, there is no clear evidence that phytochrome A mediates these responses in an R/FR reversible manner in wild-type plants.
As shown previously, the phytochrome-mediated control of blue light responsiveness in Arabidopsis protoplasts is almost entirely R/FR reversible and yet the phytochrome A clearly participates in the response (Wang and Iino, 1998). The present study has indicated further that not only the control of blue light responsiveness, but also the swelling response is almost entirely R/FR reversible in pea protoplasts (Fig. 3). Because the two responses are impaired in the phytochrome A-deficient mutant, it is apparent that phytochrome A contributes to and limits these responses in the presence of phytochrome B (Fig. 5E). Furthermore, it has been resolved that the portion of these responses remaining in the phytochrome B-deficient mutant is entirely FR reversible (Fig. 6). These results demonstrate that phytochrome A can function in an R/FR reversible manner in the material lacking the majority of light-labile phytochrome A.
Osmoregulation Underlying Light-Induced Protoplast Volume Changes
Blue light has been shown to transiently depolarize the plasma membrane in cucumber hypocotyls (Spalding and Cosgrove, 1989) and to activate plasma membrane anion channels in Arabidopsis hypocotyls (Cho and Spalding, 1996). The depolarization response (Cho and Spalding, 1996) and the protoplast-shrinking response (Wang and Iino, 1997, 1998) are inhibited by the anion channel inhibitor NPPB. In light of these results and the kinetics of individual responses, Wang and Iino (1998) presented a model in which protoplasts are thought to shrink by extruding anions (mainly Cl−) and K+ through activated ion channels, thereby reducing the content of osmotic solutes. This model can also account for the shrinking response of pea stems, which has been shown to share basic properties with that of Arabidopsis protoplasts.
Blakeley et al. (1983) reported that full expression of the phytochrome-mediated swelling of wheat leaves requires the presence of K+ in the medium. However, the swelling response partially took place in the absence of K+. Bossen et al. (1988) showed that phytochrome-mediated swelling of wheat leaf protoplasts does not depend on the presence of K+ in the medium. The maximal swelling occurred in a medium containing only 1 mm CaCl2, 0.5 m sorbitol, and 5 mm MES [2-(N-morpholino)-ethanesulfonic acid]-Tris. It is unlikely that protoplasts take up Ca2+ (and Cl−) to an extent that allows the observed swelling (Bossen et al., 1988). Wheat leaf protoplasts can most probably swell by metabolically enhancing their content of osmotic solutes, and the swelling by this mechanism seems to dominate under certain experimental conditions.
The phytochrome-mediated swelling of Arabidopsis hypocotyl protoplasts depended on the presence of K+ and Cl− in the bathing medium (Wang and Iino, 1998). The strict and specific dependence on either K+ or Cl− of the swelling response could be clearly demonstrated in pea stem protoplasts (Fig. 7, B and C). We conclude that the protoplasts of Arabidopsis hypocotyls and pea stems swell by taking up K+ and Cl− from the medium. As also suggested above in view of the difference in lag periods, the mechanism of the swelling response is probably distinct between protoplasts of wheat leaves and those of Arabidopsis hypocotyls and pea stems.
Nonepidermal pea protoplasts retained a nearly constant volume (i.e. did not undergo any obvious shrinkage) when they were not able to swell in the K+- or Cl−-free medium and in the medium of neutral pH (Fig. 7). Vanadate-treated protoplasts retained a nearly constant volume for quite a long period after complete inhibition of swelling (Fig. 8). Similar observations have been made with Arabidopsis protoplasts (Wang and Iino, 1998). Treatment with NPPB, which totally inhibited the blue light-sensitive shrinking response, did not result in any enhancement of swelling in Arabidopsis protoplasts (Wang and Iino, 1998). Together, these results suggest that the protoplasts bear very low ion efflux activities. Based on this interpretation and on the fact that protoplasts retain a constant volume when they cannot undergo swelling in the absence of Pfr (Fig. 3, B and D), we conclude that Pfr induces swelling by enhancing K+ and Cl− influxes from nearly inactive states.
The sustained influxes of K+ and Cl− underlying the phytochrome-mediated protoplast swelling are interdependent processes, as indicated by the fact that protoplasts cannot swell when either K+ or Cl− is absent from the medium. Although the exact mechanism that allows such coordinated and charge-balancing ion influxes are not known, the following plasma membrane components are expected to play central roles: the H+-ATPase that generates the proton motive force used in the process of ion uptake (Serrano, 1990) and the two ion transporters, inward-rectifying K+ channel (Maathuis et al., 1997) and Cl−/H+ symporter (Beilby and Walker, 1981; Felle, 1994). The Cl−/H+ symporter has been least characterized. In the protoplast system the H+ gradient required for Cl− uptake through Cl−/H+ symporters is largely determined by the pH of the bathing medium. In fact, the swelling response cannot take place at neutral pH (i.e. at pH comparable with the cytosolic pH), supporting the possible participation of a Cl−/H+ symporter (Wang and Iino, 1998; Fig. 7D). The inside negative membrane potential can also fuel the symporter-mediated Cl− uptake if one Cl− is taken up together with more than one H+. However, we have obtained no evidence for a contribution of the membrane potential (note that the protoplast swelling was totally inhibited at the neutral pH). As far as the requirement for K+ and Cl− and the sensitivity to medium pH are concerned the ion uptake mechanism for phytochrome-mediated swelling is similar to that for the volume recovery after blue light-induced shrinkage (Wang and Iino, 1998).
It is expected that phytochrome controls the activity of one or more of the above mentioned membrane components. Because Pfr induces protoplast swelling with a lag time as long as 30 min, it is unlikely that Pfr acts directly on the H+-ATPase or ion transporters. Rather, the long lag suggests that the phytochrome signaling involves gene expression for the limiting membrane component itself or the transduction component acting on the latter. At the moment it is not clear which membrane component limits the ion uptake activity and is more directly controlled by phytochrome. The swelling response is totally inhibited by vanadate (Fig. 8). Although this result alone does not suggest that phytochrome acts on the H+-ATPase, the possibility that Pfr enhances the net H+-ATPase activity is supported by the result that R stimulates in an R/FR reversible manner the H+ excretion by excised segments of coleoptiles and hypocotyls (Pike and Richardson, 1977; Brownlee and Kendrick, 1979; Roth-Bejerano and Hall, 1986). In Mougeotia cells, inward-rectifying K+ channels are indirectly activated by Pfr (Serlin et al., 1996). Such activation could also account for the protoplast-swelling response. The sustained protoplast swelling probably involves an exocytotic increase of the plasma-membrane surface area (see Sutter et al., 2000). It is another basic question whether or not any such control of the plasma membrane area precedes the net ion uptake underlying the protoplast-swelling response.
Ca2+ Uptake as a Step of Phytochrome Signal Transduction
The phytochrome-mediated protoplast swelling in protoplasts of wheat leaves (Bossen et al., 1988) and mung bean hypocotyls (Long et al., 1998) has been shown to depend strictly on the presence of Ca2+ in the bathing medium. Such a dependence on Ca2+ was not clear in protoplasts of Arabidopsis hypocotyls (Wang and Iino, 1998). However, we could clearly demonstrate here that the swelling response of pea stem protoplasts requires the presence of Ca2+ (Figs. 9 and 10). It has been shown that R induces Ca2+ uptake in Mougeotia cells (Dreyer and Weisenseel, 1979) and in protoplasts of maize leaves (Das and Sopory, 1985), oat leaves (Chae et al., 1990), and mung bean hypocotyls (Long et al., 1998). These results suggest that the phytochrome signal transduction involves Ca2+ uptake and that Ca2+ functions as a second messenger (Roux, 1994). Although the mechanism of osmoregulation underlying the phytochrome-mediated protoplast swelling may be distinct between leaves (at least of etiolated wheat seedlings) and stems (at least of R-grown pea seedlings) as discussed above, Ca2+ uptake appears to occur as a common step of the signal transduction for protoplast swelling responses.
The result shown in Figure 9 has indicated that the phytochrome-mediated control of blue light responsiveness does not require external Ca2+. Therefore, unlike the swelling response, the control of blue light responsiveness does not involve Ca2+ uptake as a step of the phytochrome signal transduction. Using a microinjection technique and investigating phytochrome-dependent expression of genes for photosynthesis and anthocyanin biosynthesis, Chua and coworkers have shown that Ca2+-dependent and -independent pathways can operate in a single cell (Neuhaus et al., 1993; Bowler et al., 1994). Our results suggest that such different pathways may also be present within a single cell for the expression of genes that are involved in the two phytochrome-mediated responses investigated in the present study.
The volume recovery after the blue light-induced shrinkage, which probably share ion uptake mechanisms with the phytochrome-mediated swelling (see above), does not require the presence of Ca2+ in the medium (Wang and Iino, 1998; Fig. 9). This fact favors the idea that Ca2+ is involved more specifically in the transduction of the phytochrome signal.
Physiological Significance of Blue Light-Induced Protoplast Shrinking
Wang and Iino (1997) observed that the growth of R-grown maize coleoptiles is inhibited rapidly and transiently by a blue light pulse and also following the onset of continuous blue light with good kinetic correlations to the blue light-induced protoplast shrinking response. In light of such results they hypothesized that the mechanism allowing the protoplast shrinking response mediates the blue light-induced growth inhibition. The growth of pea stems is inhibited by blue light (Laskowski and Briggs, 1989; Warpeha and Kaufman, 1990). Laskowski and Briggs (1989) could resolve a rapid blue light-induced growth inhibition in R-grown pea seedlings. The occurrence of such a growth inhibition in the material used in the present study agrees with the above-mentioned hypothesis. Our observation that the blue light-sensitive shrinking response was expressed only weakly in the protoplasts isolated from stems that had almost ceased to elongate (see “Results”) is also in agreement with the hypothesis.
The cryptochrome 1-deficient hy4 mutant of Arabidopsis is impaired in the blue light-induced inhibition of hypocotyl growth (Koornneef et al., 1980). Therefore, the suggested relationship between the protoplast-shrinking response and the growth inhibition is supported by the fact that the former response does not occur in hy4 seedlings (Wang and Iino, 1998). Furthermore, the cryptochrome 1-mediated shrinking response in Arabidopsis protoplasts depends on the preceding presence of the FR-absorbing form of phytochromes A and B in agreement with the result that the full expression of cryptochrome 1-mediated growth inhibition requires the co-action of phytochromes (Casal and Baccalandro, 1995; Ahmad and Cashmore, 1997).
More recent studies with photoreceptor mutants of Arabidopsis have provided results that call for further careful considerations. Casal and Mazzella (1998) confirmed that cryptochrome 1-mediated growth inhibition depends on phytochrome B by stimulating Arabidopsis seedlings with 3 h of blue light each day while they were exposed to continuous R. However, when blue light was given continuously together with R, no evidence for phytochrome dependence could be obtained. Neff and Chory (1998) and Poppe et al. (1998) could not obtain any evidence that the expression of cryptochrome 1-mediated growth inhibition during continuous blue light depends on phytochrome A or B. It seems likely that the cryptochrome 1-mediated growth inhibition is composed of phytochrome-dependent and -independent responses and that a major part of the phytochrome-dependent one is expressed transiently.
The idea that the cryptochrome 1-mediated protoplast shrinking response participates in the rapid blue light-induced growth inhibition is challenged by the results of Parks et al. (1998). They found that the rapid growth inhibition observed within 30 min after the onset of blue light irradiation in etiolated Arabidopsis hypocotyls is not impaired in the cryptochrome 1-deficient mutant. The difference in growth rate between wild-type and mutant seedlings became apparent only after exceeding this period. Although the results indicate that a cryptochrome 1-independent growth inhibition constitutes a major part of the rapid growth inhibition in etiolated seedlings, it is possible that a cryptochrome 1-mediated rapid growth inhibition occurs in R-grown seedlings. This possibility follows the fact that the expression of the cryptochrome 1-mediated protoplast shrinking response requires the preceding presence of Pfr (see above).
Even if the protoplast-shrinking response is causally related to the growth inhibition, the mechanism by which growth inhibition is achieved is not clear. It is predicted that the osmoregulation underlying the protoplast shrinking response results in a drop in turgor pressure in the cells from which the protoplasts have been obtained. Wang and Iino (1998) suggested that such a turgor drop may somehow lead to a growth inhibition (compare with Cosgrove, 1988). Protoplasts of Arabidopsis hypocotyls swell in response to R (Wang and Iino, 1998) and yet the growth of these organs is inhibited by R (Koornneef et al., 1980). This negative relationship appeared to conflict with the above idea, but we found that the phytochrome-mediated swelling response does not take place in pea epidermal protoplasts. Because the growth of stems is probably limited by the extensibility of the epidermis, it is possible that only the turgor in the epidermal cells is linked to growth control.
Physiological Significance of Phytochrome-Mediated Protoplast Swelling
Zhou et al. (1990) detected a phytochrome-mediated (R/FR reversible) swelling response in protoplasts of etiolated, rolled leaves of maize, but not in protoplasts of R-pretreated, unrolled leaves. Furthermore, they did not find any phytochrome-mediated swelling in the protoplasts isolated from etiolated maize coleoptiles and pea leaves. Based on these results, they concluded that the phytochrome-mediated swelling of leaf protoplasts is causally related to the R-induced leaf unrolling, a response typical to grass leaves, rather than to the R-induced growth stimulation that can be observed in coleoptiles and leaves.
As described above, the phytochrome-mediated swelling response occurs in protoplasts of Arabidopsis hypocotyls and pea stems. This fact does not disprove the possibility that the swelling response of grass leaf protoplasts is involved in the leaf-unrolling process. However, the swelling response found in protoplasts of hypocotyls and stems must be attributed to a physiological process other than leaf unrolling. The growth of hypocotyls and stems is generally inhibited by R. In Arabidopsis hypocotyls (e.g. Neff and Chory, 1998) and pea stems (Weller et al., 1995), R-induced growth inhibition is clearly a phytochrome-mediated response. Therefore, the alternative possibility that the phytochrome-mediated protoplast swelling is causally related to growth stimulation is also unlikely.
What could then be the physiological role for the phytochrome-mediated swelling of hypocotyl and stem protoplasts? It is expected that the osmoregulation underlying the swelling response causes a rise in turgor pressure when expressed in the cells. Our results suggest that such a rise in turgor occurs in stem tissues other than the epidermis. Furthermore, the swelling response was undetectable in the protoplasts isolated from the stem that had nearly ceased to elongate (see “Results”). The phytochrome-mediated osmoregulation may play a role in maintaining a high tissue tension, which probably represents an important physical and physiological condition in growing stems (see Kutschera, 1987). This interesting possibility warrants further investigation.
MATERIALS AND METHODS
Plant Materials
Seeds of Alaska peas (Pisum sativum) were purchased from Watanabe Inc. (Miyagi, Japan) and those of fun1-1 and lv-5 mutants and the corresponding wild type, cv Torsdag, were provided by Dr. J. B. Reid (University of Tasmania, Australia). The seeds were surface sterilized with an NaOCl solution, soaked in running tap water for 9 h, and sown on moist paper towels in trays (Haga and Iino, 1997). They were incubated at 25°C under continuous R (2–3 μmol m−2 s−1) in a light-tight growth room (see Wang and Iino, 1997 for the R sources). The seedlings were used for the experiments 5 d after sowing. At this stage of development the third internode constituted the top elongating internode in all materials used.
The approximately 15-mm apical zone located below the hook of the third internode was used to obtain protoplasts. Only those seedlings that had a third internode 20 to 25 mm in length were selected for use. In cv Alaska seedlings grown similarly under R, maximal and uniform elongation took place along the apical 15-mm zone (Haga and Iino, 1997). The third internode of cv Torsdag seedlings elongated similarly as that of cv Alaska seedlings until they reach the required length and also in the subsequent 2-d period. This observation suggests that the stem zone chosen for use is also the active elongation zone in cv Torsdag seedlings. The third internode of mutant seedlings also elongated similarly. The final internode length in lv-5 seedlings reached after 9 d of incubation (on average about 100 mm) was, however, longer than in cv Torsdag and fun1-1 seedlings (about 75 mm).
Preparation of Protoplasts
All steps of protoplast preparation were carried out at 25°C under R (2–3 μmol m−2 s−1) in the same growth room. Protoplasts were prepared from the epidermal layers peeled from the stem zone described above (epidermal peels) or the zone segments from which epidermal layers were peeled off (peeled stems). In a given experiment, epidermal peels were obtained from 50 to 60 seedlings. Each epidermal peel was immediately placed into 10 mL of an enzyme solution containing 1.7% (w/v) Cellulase-RS (Yakult, Tokyo), 0.1% (w/v) pectolyase Y-23 (Seishin Pharmaceutical, Tokyo), 0.5 m sorbitol, 10 mm KCl, 1 mm CaCl2, 20 mm Glc, and 10 mm MES-KOH, pH 5.5. The necessary amount of peel was collected over a period of about 30 min. Peeled stem segments were obtained from five seedlings. The segments were placed into 5 mL of an enzyme solution (as above except the concentrations of cellulase and pectolyase, which were 2.0% and 0.2% [w/v], respectively) and sliced with a razor blade. The required amount of stem slices was obtained over a period of about 6 min.
The tissues collected in an enzyme solution were vacuum infiltrated (730–750 mm Hg for 10 min) and incubated for 1.5 h on a rotating shaker (60 rpm). The mixture was next filtered through a nylon mesh to remove tissue debris and was centrifuged at 110g for 10 min. The pellet was suspended in 2 mL of a standard bathing medium that contained 0.5 m sorbitol, 10 mm KCl, 1 mm CaCl2, 20 mm Glc, and 10 mm MES-KOH, pH 6.0. The suspension was loaded, in a glass tube (10 mm in diameter), on 3 mL of an 18% (v/v) Percoll (Sigma, St. Louis) solution containing other components identical to the bathing medium. After a 5-min centrifugation at 110g, the protoplasts, located at the interface between the Percoll solution and the loaded medium, were collected using a Pasteur pipette. The collected protoplasts were washed by suspending them in 5 mL of the bathing medium and centrifuging them at 110g for 5 min. This washing procedure was repeated once again. The protoplast pellet was suspended in a small amount of the bathing medium to obtain the final preparation (1 × 105–5 × 105 protoplasts mL−1).
Measurement of Protoplast Volumes and Light Treatment of Protoplasts
Protoplasts in a microscopic field were monitored for volume using time-lapse photographs as described in Wang and Iino (1997, 1998). In brief, a 200-μL portion of the freshly prepared protoplast suspension was added to an all-side clear quartz cuvette (base area 10 × 10 mm) and was incubated on the sample stage of an inverted microscope at 25°C ± 1°C. Unless otherwise specified, protoplasts were continuously irradiated with R (50 μmol m−2 s−1) from the beginning of incubation, and time-lapse photography was initiated at 15 to 30 min of incubation. (The cuvette had to be allowed to stand for about 10 min before all protoplasts settled to the bottom and could be focused for photographing.) The volume of each protoplast was calculated from its diameter and was expressed as a percentage of the volume at a defined time point. Round protoplasts having clear margins were selected for the analysis. No selection was made for protoplast size. The initial volume of the protoplasts of epidermal peels or peeled stems varied considerably and typically ranged between 10 × 103 and 200 × 103 μm3 (for examples of size distribution, see Wang and Iino, 1997, 1998). The protoplasts bathed in the modified media described below also showed a similar size distribution.
To investigate the effect of blue light, protoplasts were treated with a pulse of blue light while being irradiated with the background R. The experiments investigating the involvement of phytochrome included pretreatments with a period of darkness and a pulse of FR. The R and other light sources were as described in Wang and Iino (1997).
Chemical Treatments of Protoplasts
The standard bathing medium (see above) was modified to investigate the roles for ions. To replace K+ in the medium with TEA+, 10 mm TEA-Cl was used in place of 10 mm KCl, and the pH was adjusted with Tris at 10 mm MES. In addition to its membrane impermeant nature, TEA+ binds to a specific locus of K+ channels to block K+ fluxes (see Brown, 1993). To replace Cl− with membrane impermeant IDA, 12 mm IDA, 10 mm KOH, and 1 mm Ca(OH)2 were used in place of 10 mm KCl and 1 mm CaCl2. To prepare a Ca2+-free medium, CaCl2 was omitted from the medium composition, and 1 mm EGTA was added. The modified medium was used from the first step of protoplast suspension (i.e. from the step of suspending the protoplast pellet obtained from the enzyme solution). When protoplasts were treated with vanadate, it was added to the standard medium at a concentration of 500 μm. The vanadate-containing medium was also used from the first step of protoplast suspension. Other details were as described in Wang and Iino (1998).
In the experiments on protoplast swelling the protoplasts treated with a modified medium were washed with and resuspended in the standard medium and subjected to further volume recording. This protocol was adopted to evaluate whether the protoplasts could recover from the inhibitory effect of the modified medium. The washing procedure was conducted under R as for the initial protoplast preparation. Because some protoplasts were lost during washing treatment, three cuvettes, each containing a 200-μL suspension of protoplasts, were prepared; one cuvette was used for volume recording, and the other two cuvettes were allowed to stand under comparable conditions. After the volume recording, the protoplasts in all cuvettes were combined and washed twice by suspending them in 15 mL of the standard medium and centrifuging them for 5 min at 110g. The final protoplast pellet was suspended in a small amount of the standard medium, and a 200-μL portion was used for volume recording.
ACKNOWLEDGMENTS
We thank Dr. James B. Reid for providing the seeds of fun1-1 and lv-5 pea mutants. We also thank Drs. Xiaojing Wang and Ken Haga for discussion and technical advice.
LITERATURE CITED
- Ahmad M, Cashmore AR. HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature. 1993;366:162–166. doi: 10.1038/366162a0. [DOI] [PubMed] [Google Scholar]
- Ahmad M, Cashmore AR. The blue-light receptor cryptochrome 1 shows functional dependence on phytochrome A or phytochrome B in Arabidopsis thaliana. Plant J. 1997;11:421–427. doi: 10.1046/j.1365-313x.1997.11030421.x. [DOI] [PubMed] [Google Scholar]
- Beilby MJ, Walker NA. Chloride transport in Chara: I. Kinetics and current-voltage curves for a probable proton symport. J Exp Bot. 1981;32:43–54. [Google Scholar]
- Blakeley SD, Thomas B, Hall JL, Vince-Prue D. Regulation of swelling of etiolated-wheat-leaf protoplats by phytochrome and gibberellic acid. Planta. 1983;158:416–421. doi: 10.1007/BF00397734. [DOI] [PubMed] [Google Scholar]
- Bossen ME, Dassen HHA, Kendrick RE, Vredenberg WJ. The role of calcium ions in phytochrome-controlled swelling of etiolated wheat (Triticum aestivum L.) protoplasts. Planta. 1988;174:94–100. doi: 10.1007/BF00394879. [DOI] [PubMed] [Google Scholar]
- Bowler C, Neuhaus G, Yamagata H, Chua N-H. Cyclic GMP and calcium mediate phytochrome phototransduction. Cell. 1994;77:73–81. doi: 10.1016/0092-8674(94)90236-4. [DOI] [PubMed] [Google Scholar]
- Boylan MT, Quail PH. Phytochrome A overexpression inhibits hypocotyl elongation in transgenic Arabidopsis. Proc Natl Acad Sci USA. 1991;88:10806–10810. doi: 10.1073/pnas.88.23.10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AM. Functional bases for interpreting amino acid sequences of voltage-dependent K+ channels. Annu Rev Biophys Biomol Struct. 1993;22:173–198. doi: 10.1146/annurev.bb.22.060193.001133. [DOI] [PubMed] [Google Scholar]
- Brownlee DP, Kendrick RE. Ion fluxes and phytochrome in mung bean hypocotyl segments: II. Fluxes of chloride, proton, and orthophosphate in apical and sub-hook segments. Plant Physiol. 1979;64:211–213. doi: 10.1104/pp.64.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal JJ, Boccalandro H. Co-action between phytochrome B and HY4 in Arabidopsis thaliana. Planta. 1995;197:213–218. doi: 10.1007/BF00202639. [DOI] [PubMed] [Google Scholar]
- Casal JJ, Mazzella MA. Conditional synergism between cryptochrome 1 and phytochrome B is shown by the analysis of phyA, phyB, and hy4 simple, double, and triple mutants in Arabidopsis. Plant Physiol. 1998;118:19–25. doi: 10.1104/pp.118.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal JJ, Sanchez RA, Yanovsky MJ. The function of phytochrome A. Plant Cell Environ. 1997;20:813–819. [Google Scholar]
- Chae Q, Park HJ, Hong SD. Loading of quin2 into the oat protoplast and measurement of cytosolic calcium ion concentration changes by phytochrome action. Biochim Biophys Acta. 1990;1051:115–122. doi: 10.1016/0167-4889(90)90182-d. [DOI] [PubMed] [Google Scholar]
- Cho MH, Spalding EP. An anion channel in Arabidopsis hypocotyls activated by blue light. Proc Natl Acad Sci USA. 1996;93:8134–8138. doi: 10.1073/pnas.93.15.8134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CH, Lim S-K, Song P-S, Berlin JD, Goodin JR. Swelling of etiolated oat protoplasts induced by phytochrome, cAMP and gibberellic acid: a kinetic study. Plant Cell Physiol. 1988;29:855–860. [Google Scholar]
- Cosgrove DJ. Mechanism of rapid suppression of cell expansion in cucumber hypocotyls after blue-light irradiation. Planta. 1988;176:109–116. [PubMed] [Google Scholar]
- Das R, Sopory SK. Evidence of regulation of calcium uptake by phytochrome in maize protoplasts. Biochem Biophys Res Commun. 1985;128:1455–1460. doi: 10.1016/0006-291x(85)91103-9. [DOI] [PubMed] [Google Scholar]
- Dreyer EM, Weisenseel MH. Phytochrome-mediated uptake of calcium in Mougeotia cells. Planta. 1979;146:31–39. doi: 10.1007/BF00381252. [DOI] [PubMed] [Google Scholar]
- Felle HH. The H+/Cl− symporter in root-hair cells of Sinapis alba: an electrophysiological study using ion-selective microelectrodes. Plant Physiol. 1994;106:1131–1136. doi: 10.1104/pp.106.3.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya M. Phytochromes: their molecular species, gene family, and functions. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:617–645. [Google Scholar]
- Haga K, Iino M. The short-term growth stimulation induced by external supply of IAA in internodes of intact pea seedlings. Aust J Plant Physiol. 1997;24:215–226. [Google Scholar]
- Johnson E, Bradley M, Harberd P, Whitelam GC. Photoresponses of light-grown phyA mutants of Arabidopsis: phytochrome A is required for the perception of daylength extensions. Plant Physiol. 1994;105:141–149. doi: 10.1104/pp.105.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-S, Moon D-K, Goodin JR, Song P-S. Swelling of etiolated oat protoplasts induced by cAMP and red light. Plant Cell Physiol. 1986;27:193–197. [Google Scholar]
- Koornneef M, Rolff E, Spruit JP. Genetic control of light-inhibited hypocotyl elongation in Arabidopsis thaliana (L.) Heynh. Z Pflanzenphysiol. 1980;100:147–160. [Google Scholar]
- Kutschera U. Cooperation between outer and inner tissues in auxin-mediated plant organ growth. In: Cosgrove DJ, Knievel DP, editors. Physiology of Cell Expansion During Plant Growth. Rockville, MD: The American Society of Plant Physiologists; 1987. pp. 215–226. [Google Scholar]
- Laskowski MJ, Briggs WR. Regulation of pea epicotyl elongation by blue light. Plant Physiol. 1989;89:293–298. doi: 10.1104/pp.89.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long C, Wang X, Pan R. The role of calcium ions in red light-induced swelling of mung bean protoplasts. Chinese Sci Bull. 1995;40:248–251. [Google Scholar]
- Long C, Wang X, Pan R. The effect of external Ca2+ and Ca2+-channel modulators on red-light-induced swelling of protoplasts of Phaseolus radiatus L. Cell Res. 1998;8:41–50. doi: 10.1038/cr.1998.5. [DOI] [PubMed] [Google Scholar]
- Maathuis FJM, Ichida AM, Sanders D, Schroeder JI. Roles of higher plant K+ channels. Plant Physiol. 1997;114:1141–1149. doi: 10.1104/pp.114.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatani A, Kay SA, Deak M, Chua N-H, Furuya M. Rice type I phytochrome regulates hypocotyl elongation in transgenic tobacco seedlings. Proc Natl Acad Sci USA. 1991;88:5207–5211. doi: 10.1073/pnas.88.12.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff MM, Chory J. Genetic interactions between phytochrome A, phytochrome B, and cryptochrome 1 during Arabidopsis development. Plant Physiol. 1998;118:27–36. doi: 10.1104/pp.118.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus G, Bowler C, Kern R, Chua N-H. Calcium/calmodulin-dependent and -independent phytochrome signal transduction pathways. Cell. 1993;73:937–952. doi: 10.1016/0092-8674(93)90272-r. [DOI] [PubMed] [Google Scholar]
- Parks BM, Cho MH, Spalding EP. Two genetically separable phases of growth inhibition induced by blue light in Arabidopsis seedlings. Plant Physiol. 1998;118:609–615. doi: 10.1104/pp.118.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike CS, Richardson AE. Phytochrome-controlled hydrogen ion excretion by Avena coleoptiles. Plant Physiol. 1977;59:615–617. doi: 10.1104/pp.59.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppe C, Sweere U, Drumm-Herrel H, Schäfer E. The blue light receptor cryptochrome 1 can act independently of phytochrome A and B in Arabidopsis thaliana. Plant J. 1998;16:465–471. doi: 10.1046/j.1365-313x.1998.00322.x. [DOI] [PubMed] [Google Scholar]
- Reed JW, Nagatani A, Elich TD, Fagan M, Chory J. Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol. 1994;104:1139–1149. doi: 10.1104/pp.104.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth-Bejerano N, Hall JL. Photoregulation of proton extrusion by cucumber hypocotyls and the role of ATPase activity. J Plant Physiol. 1986;122:329–336. [Google Scholar]
- Roux S. Signal transduction in phytochrome responses. In: Kendrick RE, Kronenberg GHM, editors. Photomorphogenesis in Plants. Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 187–209. [Google Scholar]
- Serlin BS, Lew RR, Krasnoshtein, Krol J, Sumida KD. Phytochrome activation of K+ channels and chloroplast rotation in Mougeotia: the escape times. Plant Cell Physiol. 1996;37:175–179. doi: 10.1104/pp.98.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano R. Plasma membrane ATPase. In: Larsson C, Møller IM, editors. The Plant Plasma Membrane. Berlin: Springer-Verlag; 1990. pp. 127–153. [Google Scholar]
- Smith H. Physiological and ecological function within the phytochrome family. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:289–315. [Google Scholar]
- Spalding EP, Cosgrove DJ. Large plasma-membrane depolarization precedes rapid blue-light-induced growth inhibition in cucumber. Planta. 1989;178:407–410. [PubMed] [Google Scholar]
- Sutter J-U, Homann U, Thiel G. Ca2+-stimulated exocytosis in maize coleoptile cells. Plant Cell. 2000;12:1127–1136. doi: 10.1105/tpc.12.7.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Iino M. Blue-light-induced shrinking of protoplasts from maize coleoptiles and its relationship to coleoptile growth. Plant Physiol. 1997;114:1009–1020. doi: 10.1104/pp.114.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Iino M. Interaction of cryptochrome 1, phytochrome, and ion fluxes in blue-light-induced shrinking of Arabidopsis hypocotyl protoplasts. Plant Physiol. 1998;117:1265–1279. doi: 10.1104/pp.117.4.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warpeha KMF, Kaufman LS. Two distinct blue-light responses regulate epicotyl elongation in pea. Plant Physiol. 1990;92:495–499. doi: 10.1104/pp.92.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller JL, Murfet IC, Reid JB. Pea mutants with reduced sensitivity to far-red light define an important role for phytochrome A in day-length detection. Plant Physiol. 1997;114:1225–1236. doi: 10.1104/pp.114.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller JL, Nagatani A, Kendrick RE, Murfet IC, Reid JB. New lv mutants of pea are deficient in phytochrome B. Plant Physiol. 1995;108:525–532. doi: 10.1104/pp.108.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovsky MJ, Casal JJ, Whitelam GC. Phytochrome A, phytochrome B and HY4 are involved in hypocotyls growth responses to natural radiation in Arabidopsis: weak de-etiolation of the phyA mutant under dense canopies. Plant Cell Environ. 1995;18:788–794. [Google Scholar]
- Zhou S, Jones AM, Scott TK. Phytochrome-mediated swelling of etiolated leaf protoplasts and its possible biological significance. Plant Cell Reports. 1990;9:435–438. doi: 10.1007/BF00232267. [DOI] [PubMed] [Google Scholar]