Abstract
Transient neuromodulation can have long-lasting effects on neural circuits and motivational states1-4. We examined dopaminergic mechanisms underlying mating drive and its persistence in male mice. Brief investigation of females primes a male’s interest to mate for tens of minutes, while a single successful mating triggers satiety that gradually recovers over days5. We found that both processes are controlled by specialized anteroventral and preoptic periventricular (AVPV/PVpo) dopamine neurons in the hypothalamus. During investigations of females, dopamine is transiently released in the medial preoptic area (MPOA), an area critical for mating behaviors. Optogenetic stimulation of AVPV/PVpo dopamine axons in the MPOA recapitulates the priming effect of female exposure. Using optical and molecular methods for tracking and manipulating intracellular signaling, we show that this priming effect emerges from accumulation of mating-related dopamine signals in the MPOA via accrual of cyclic adenosine monophosphate (cAMP) levels and protein kinase A (PKA) activity. Dopamine transients in the MPOA are abolished following a successful mating, likely ensuring abstinence. Consistent with this idea, inhibiting AVPV/PVpo dopamine neurons selectively demotivates mating, while stimulating these neurons restores the motivation to mate following sexual satiety. Therefore, accumulation or suppression of signals from specialized dopamine neurons regulates mating behaviors across minutes and days.
In humans, drugs that boost dopamine release are known to increase male mating drive6. Similarly, in male rats, direct infusion of dopamine agonists or antagonists into the MPOA rapidly altered mating drive7-9. These results are echoed by recent findings demonstrating a central role for dopamine in the Drosophila mating-drive circuitry10. However, the identity of the relevant dopamine neurons in the mammalian brain, their activity pattern during mating behaviors, and their roles in driving changes in downstream mating-related circuits have remained unknown for almost four decades. Here, we sought to identify such dopamine neurons and uncover the circuit- and molecular-level mechanisms by which they control mating behaviors.
Long-term satiety and short-term priming
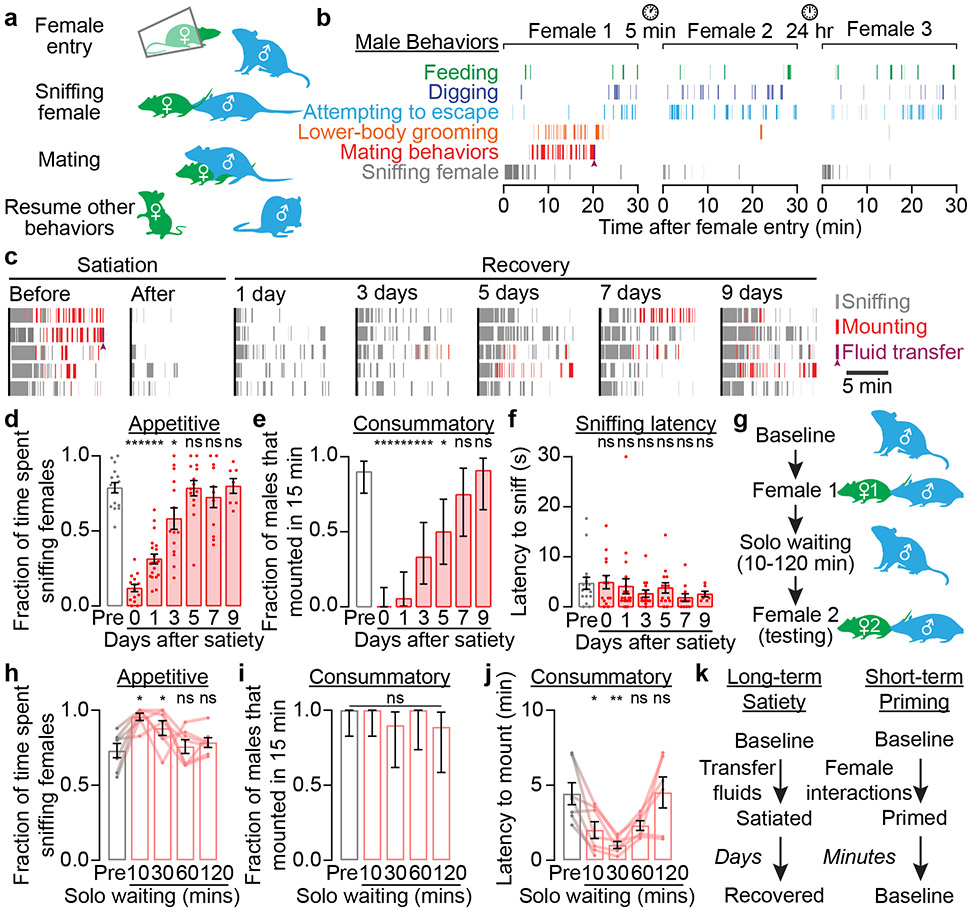
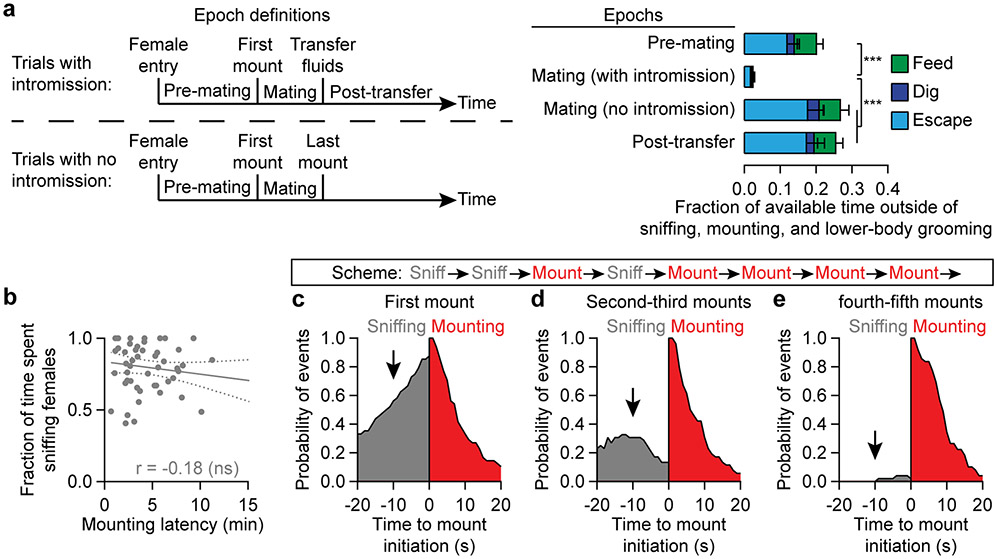
To measure male mating drive, we adapted an established method11,12 in which a female mouse in estrous is introduced into a male’s home cage. Typically, the male quickly moved to sniff the female and initiated mounting behaviors minutes later (Fig. 1a, b, Supplementary Video 1). After gaining intromission, the male vigorously and almost exclusively engaged in mating-related behaviors (Fig 1b, Extended Data Fig. 1a, Supplementary Video 2). After transferring reproductive fluids (i.e., ejaculation; Supplemental Movie 3), he reverted to non-mating behaviors (Fig 1b, Extended Data Fig. 1a), and spent little time investigating or trying to mate with newly introduced, sexually receptive females (Fig. 1b). This abstinence from both sniffing of females and mounting behaviors was consistent across mice and lasted for days (Fig. 1c).
Fig. 1 ∣. Motivational regulation of male mating behaviors across minutes and days.
a,b, A typical male C57BL/6J mouse became satiated after transferring reproductive fluids (arrowhead).
c, After the initial sexual satiety, sniffing (gray) and mounting (red) gradually returned over days (n = 5 example males).
d-f, Both the fraction of time that males were engaged in sniffing behavior during the pre-mounting window (up to 5 minutes, d: n = 16, 14, 20, 14, 14, 11, 7 males [dots]) and the fraction of males that tried to mount within the first 15 minutes after female entry (e: mean ± 95% c.i.) decreased after satiety and gradually recovered over ~5-7 days. The latency to sniff did not change (f).
g, In a priming assay, males were first primed by interacting with a female for 15 min (no transfer of fluids was allowed), and then tested with a second female.
h-j, After priming, males (n = 7) more frequently sniffed a second female that was introduced up to 30 minutes later (h), and mounted with shorter latency (j). The fraction of trials with mounting remained near ceiling (i: mean ± 95% c.i.). Gray bars: before priming.
k, Model: regulation of motivational drive across timescales of days (long-term satiety) and minutes (short-term priming).
Mean ± s.e.m. unless otherwise specified. *p<0.05, **p<0.01, ***p<0.001. See Supplementary Table 1 for statistics.
We quantified engagement in appetitive mating behaviors as the fraction of time that a male spent sniffing a female during the first 5 minutes of the pre-mounting period. This metric did not correlate with mounting latency (Extended Data Fig. 1b). Before satiety, males that had been individually housed for two weeks (referred to below as baseline) spent about 75% of the pre-mounting period sniffing females (Fig. 1d). After satiation, this percentage dropped to ~10% and gradually recovered over ~5 days of isolation from females (Fig. 1d). Likewise, consummatory mating behavior, which was quantified as the fraction of males that initiated mounting within 15 minutes of female entry, plummeted from 90% to 0% after satiation and gradually recovered over ~7 days (Fig. 1e). In contrast, the latency to initial investigation of the female intruder was unaffected by satiety (Fig. 1f).
Although mating drive is diminished after one successful mating, it is unlikely to be a binary variable. We found that mating drive could be increased above baseline by a priming protocol in which a male was initially paired with a female for 15 min but not allowed to transfer fluids (Fig. 1g). The male then spent more time sniffing a second female, and mounted with a shorter latency even when she was introduced 10-30 minutes after the removal of the first female (Fig. 1h-j). Even during sessions with the first female, the male’s mounts became progressively less dependent on preceding sniffs (Extended Data Fig. 1c-e). These results show that repeated investigations of a female serve not only to ascertain sensory information, but also to build up the male’s mating drive. Therefore, interactions with females and fluid transfer alter male mating drive on the timescales of tens of minutes (short-term priming) and days (long-term satiety), respectively (Fig. 1k).
Mating-related dopamine release in MPOA
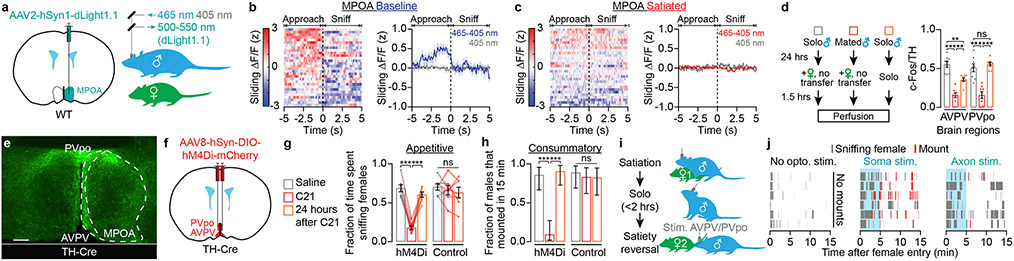
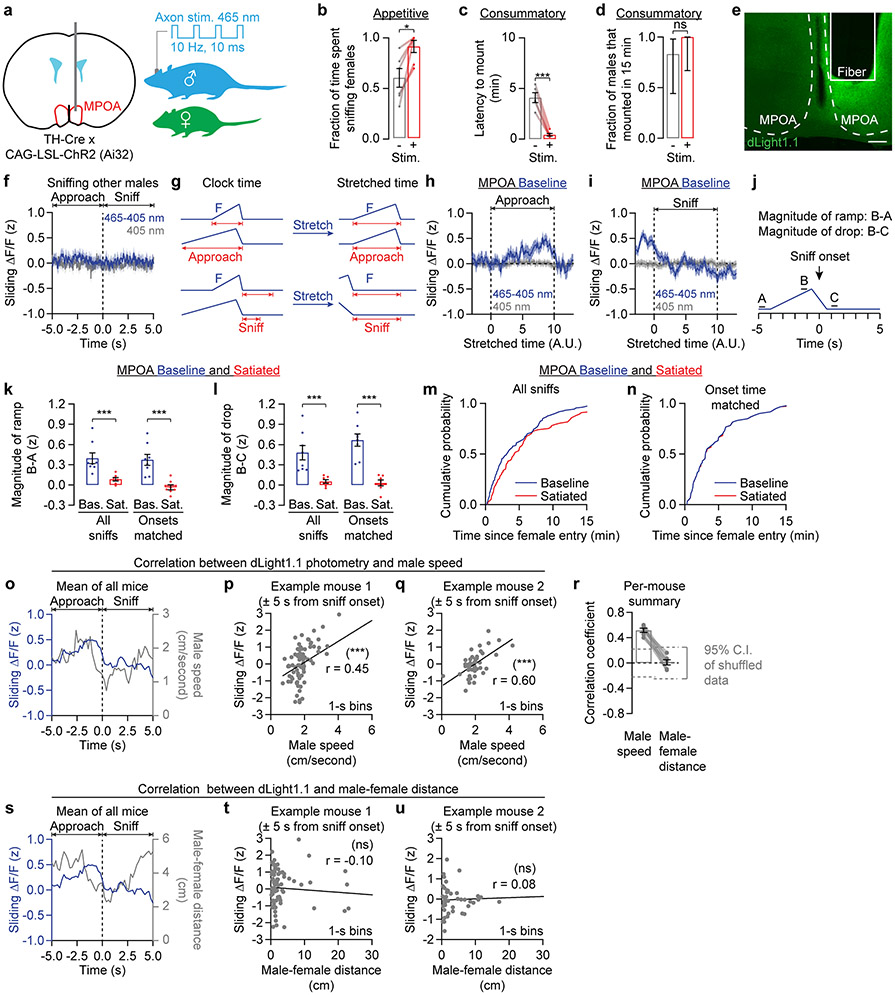
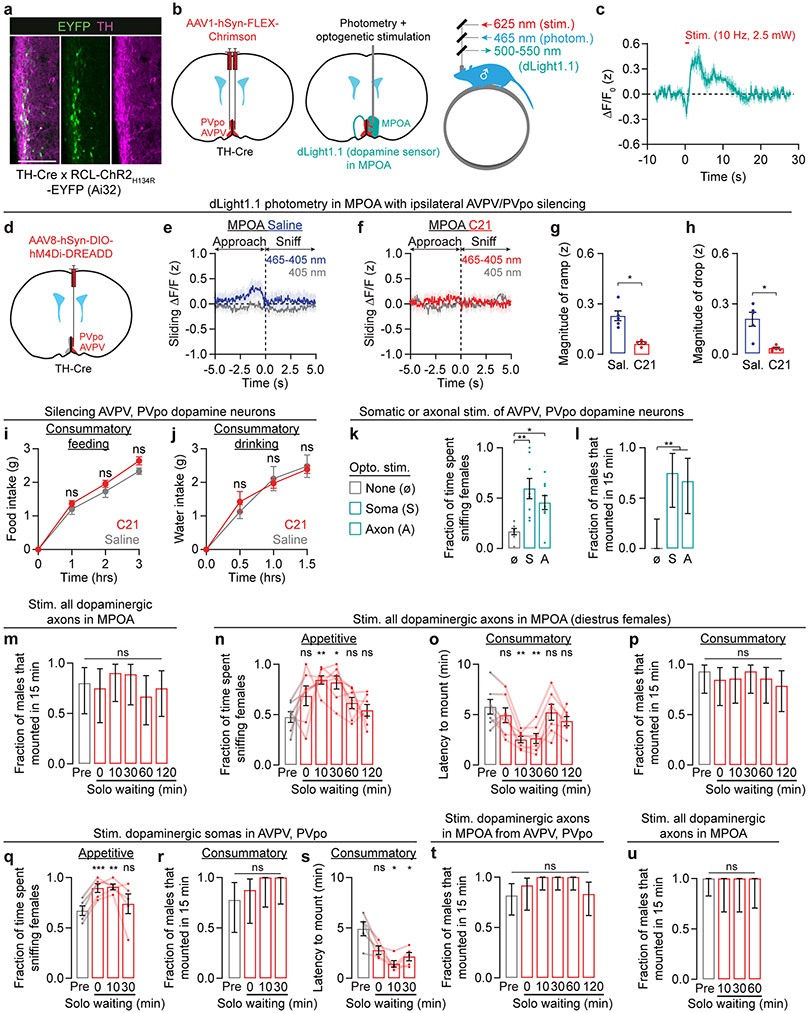
In rats, infusion of dopamine receptor agonists (e.g., apomorphine) in the MPOA increases the vigor of male mating behaviors8 – a result that we reproduced in mice by optogenetically stimulating all dopaminergic inputs to this area (Extended Data Fig. 2a-d). To assess when dopamine might be released in the MPOA during the appetitive sniffing phase, we used fiber photometry to record the fluorescence of the dopamine sensor dLight1.113 (Fig. 2a, Extended Data Fig. 2e). Dopamine release in the MPOA ramped up as the male initiated approach towards the female, and returned to the pre-approach level as the male began to sniff the female (Fig. 2b). This ramp was not present when males sniffed other males (Extended Data Fig. 2f) and was not due to averaging of step-like dopamine increases of varying latency across bouts (Extended Data Fig. 2g-i). We did not observe dopamine ramps or drops in satiated mice that had mated on the previous day (Fig. 2c, Extended Data Fig. 2j-l), even when correcting for differences in sniffing latency (Extended Data Fig. 2k-n). On a bout-by-bout basis, the ramp and drop of dLight1.1 signals roughly tracked the male’s speed (albeit at a delay) (Extended Data Fig. 2o-r) but not his distance from the female (Extended Data Fig. 2r-u).
Fig. 2 ∣. Specialized hypothalamic dopamine neurons control mating drive.
a, Two-color photometry of the dopamine sensor dLight1.1.
b, Left: dLight1.1 fluorescence traces aligned to sniff onset, sorted by pre-sniffing dLight1.1 intensity. Right: the average dLight1.1 signal ramped up over seconds as the mouse approached a female, and dropped as the male sniffed the female (n = 6 males). Sliding ΔF/F (z): fractional change in fluorescence, filtered and z-scored.
c, After satiety, dopamine ramps before sniffing were abolished (n = 6 males).
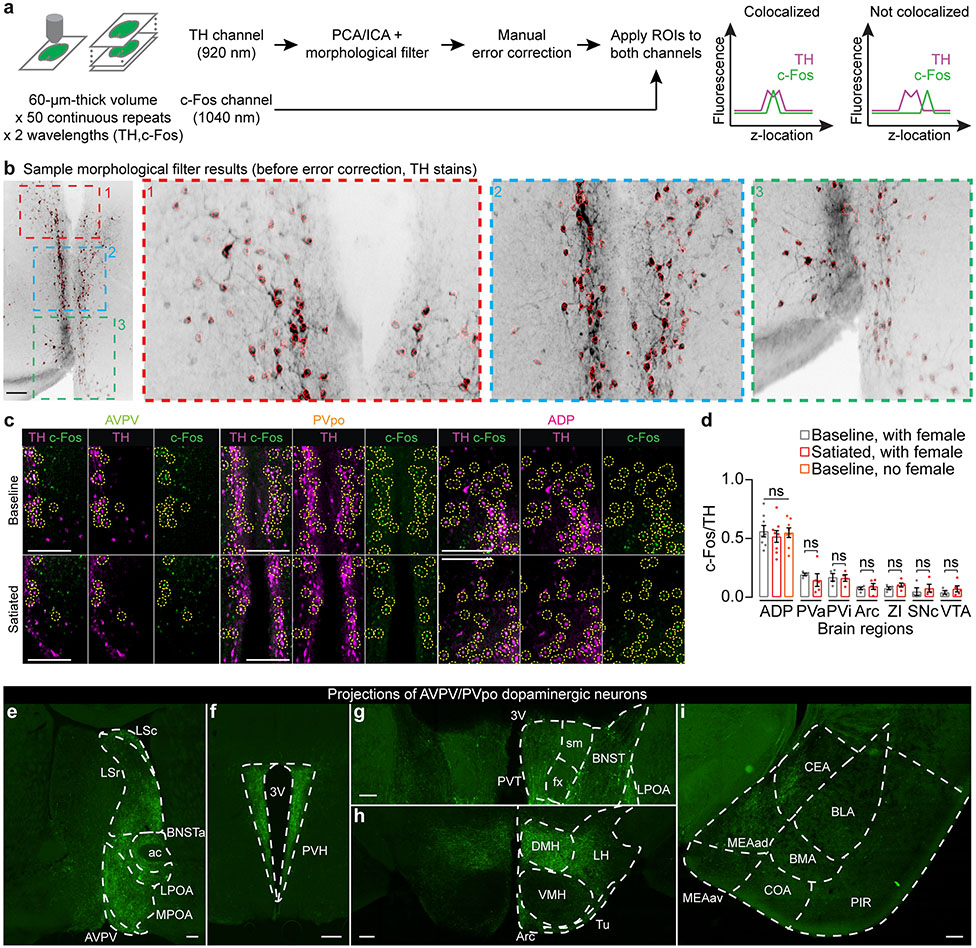
d, In the male AVPV and PVpo, c-Fos is expressed in a lower proportion of TH+ cells following a successful mating (n = 8 males).
e, Axon terminals of AVPV/PVpo dopamine neurons innervate the lateral MPOA (scale bar: 200 μm).
f-h, Bilateral chemogenetic inhibition of male AVPV/PVpo dopamine neurons decreased appetitive sniffing (g: n = 7 males) and consummatory mounting behaviors (h: n = 7, mean ± 95% c.i.).
i-j, Optogenetic stimulation of AVPV/PVpo dopamine neuron cell bodies (‘Soma’) or axons in the MPOA reinvigorates both appetitive sniffing and consummatory mounting behaviors in sexually sated mice. n = 7, 9, 9 males.
Mean ± s.e.m. unless otherwise specified. *p<0.05, **p<0.01, ***p<0.001. See Supplementary Table 1 for statistics.
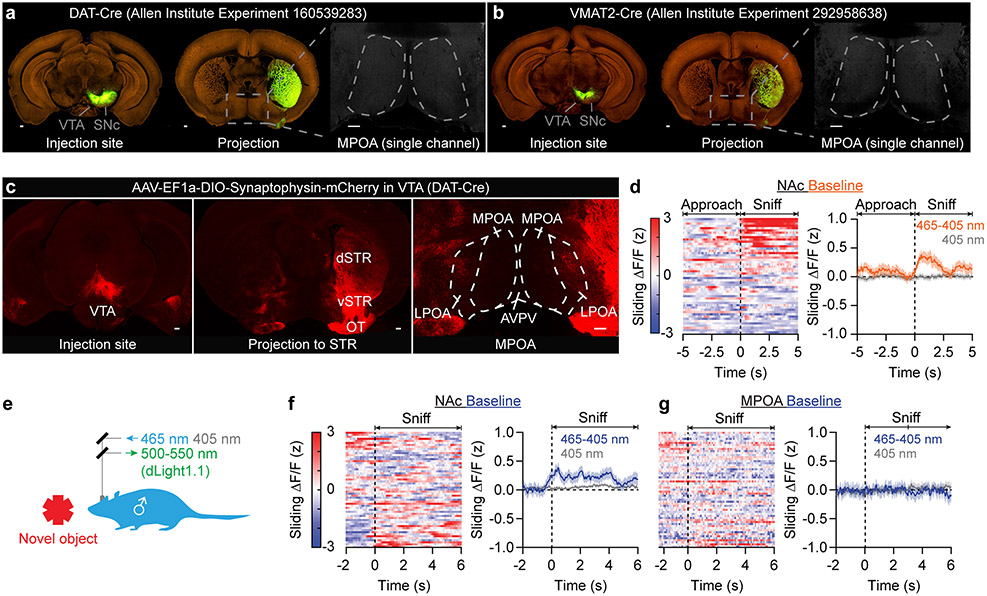
While the ramping component of MPOA dopamine release resembles previously reported ramps in midbrain dopamine activity14, the sources of dopamine are likely different. First, few midbrain dopamine neurons send axons to the MPOA15 (Extended Data Fig. 3a-c). Second, during the same female-investigation behavior, dopamine release in the nucleus accumbens (NAc) increased after sniff onsets (Extended Data Fig. 3d)16. Third, when the male investigated a novel object, dopamine was released in the NAc but not in the MPOA17 (Extended Data. Fig. 3e-g). These results suggest that functionally specialized dopamine inputs to the MPOA promote mating behaviors and are suppressed after satiation.
Dopamine neurons control mating drive
We next sought to define the source(s) of dopaminergic input to the MPOA that promote mating drive and become blunted after satiation. We hypothesized that satiety reduces the excitability of candidate dopamine neurons10, which could be reflected in the expression of the neuronal activity marker c-Fos18. We therefore mapped mating-related c-Fos activity of nine major dopamine clusters in males that either had been sexually deprived prior to exposure to a female or that had mated successfully 24 hours earlier (Fig. 2d). We observed substantially lower c-Fos expression specifically in the dopamine neurons of the anteroventral periventricular nucleus (AVPV) and the preoptic periventricular nucleus (PVpo) of satiated males (Fig. 2d, Extended Data Fig. 4a-d). We also considered a third group of males that were sexually deprived throughout the experiment (Fig. 2d). This group showed equally high c-Fos expression in PVpo dopamine neurons and intermediate expression in AVPV dopamine neurons. Together, these results suggest that the activity of AVPV and PVpo dopamine neurons reflects the male’s mating history, and the activity of AVPV neurons additionally signals the presence of a female.
To gain genetic access to AVPV and PVpo dopamine neurons, we used transgenic tyrosine hydroxylase-Cre (TH-Cre) mice. We verified that most Cre-expressing AVPV and PVpo neurons express TH (mean ± s.d. = 83 ± 9%, Extended Data Fig. 5a). Brief (1 s; 10 Hz) Chrimson19 stimulation of TH-Cre+ AVPV/PVpo neurons consistently induced dopamine release in the MPOA (Extended Data Fig. 5b,c).
AVPV/PVpo dopamine neurons send dense projections to the MPOA (Fig. 2e), but also project to other areas implicated in mating behaviors. These include the bed nucleus of the stria terminalis, the ventrolateral part of ventromedial nucleus of the hypothalamus, the lateral hypothalamus, and the medial amygdala (Extended Data Fig. 4e-i)20,21. The activity of AVPV/PVpo dopamine neurons is important for gating mating drive. In highly motivated male mice, bilateral chemogenetic inhibition22,23 of AVPV/PVpo dopamine neurons drove satiety-like decreases in sniffing and mounting behaviors (Fig. 2f-h), potentially due to the reduction of dopamine ramps in the MPOA (Extended Data Fig. 5d-h). Despite the dramatic suppression of mating behaviors, this inhibition did not decrease food or water intake in animals that were either food- or water-restricted for 24 hours (Extended Data Fig. 5i-j).
We next tested if optogenetic stimulation of AVPV/PVpo dopamine neurons can restore mating drive in recently satiated male mice (Fig. 2i). When we stimulated the somas or axons of AVPV/PVPo dopamine neurons in satiated males for the first 5 minutes after female entry (a duration that matched the typical duration of female exploration in baseline conditions, Fig. 1b-c; see also Methods), males showed significantly more sniffing and mounting behaviors (Fig. 2j, Extended Data Fig. 5k, l). Together with previous studies7,8, these results strongly suggest that AVPV/PVpo dopamine neurons motivate male mating behaviors, at least in part via projections to the MPOA.
Dopaminergic priming of mating drive
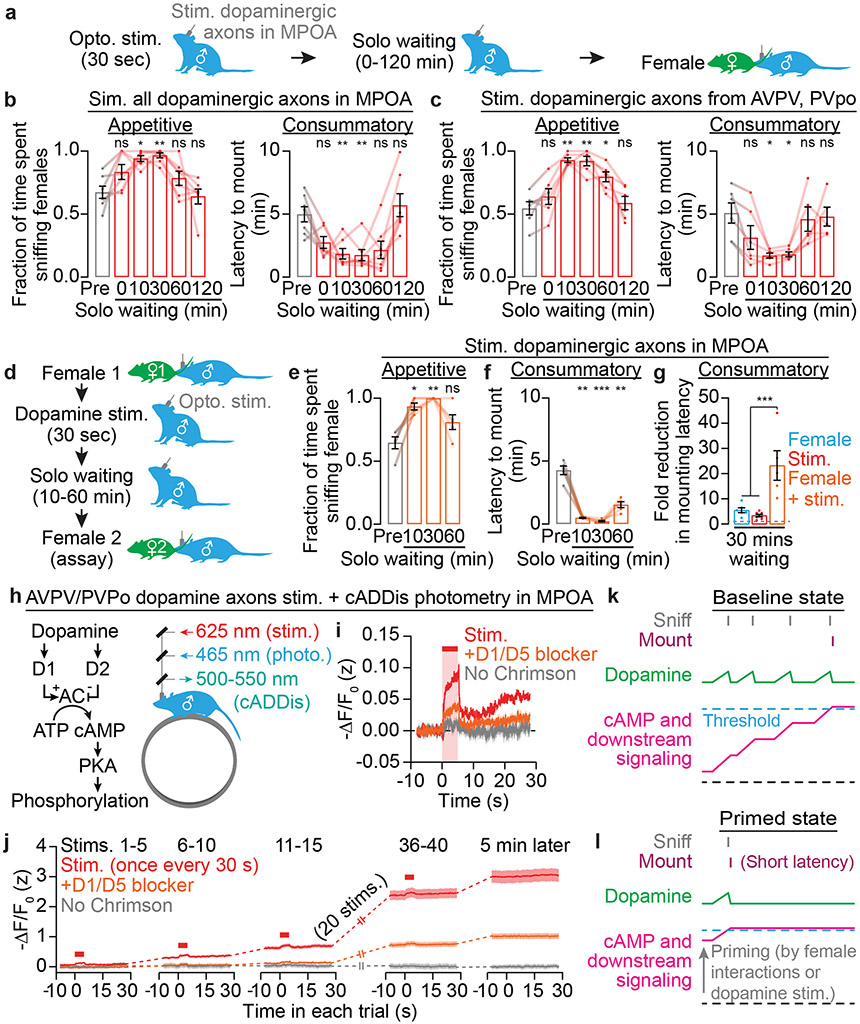
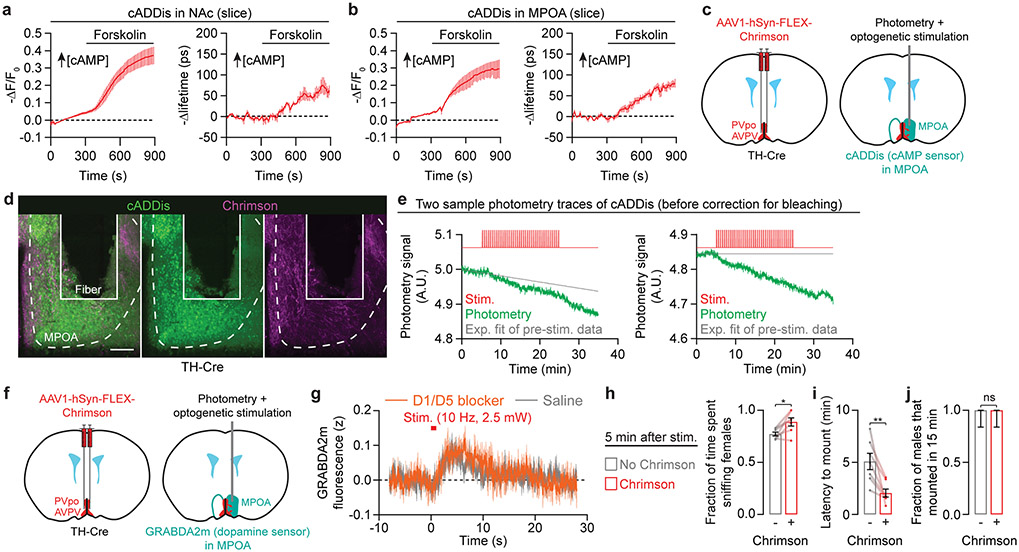
In our satiety-reversal experiments, the motivational effect persisted even following termination of AVPV/PVpo stimulation (Fig. 2j), redolent of the priming effects we observed in unmated males (Fig. 1g-k). We therefore asked whether transient dopamine release in the MPOA, which normally occurs during male-female interactions (Fig. 2b), was sufficient to prime mating drive in unsated males (Fig. 3a). We found that a 30-second pre-stimulation of all dopaminergic axons in the MPOA was sufficient to prime male mating behaviors with females (including diestrus and unreceptive females) that were introduced up to 30 minutes later (Fig. 3b, Extended Data Fig. 5m-p; the fraction of males that mounted within 15 min was already near ceiling before pre-stimulation). Similar effects were also observed when specifically stimulating AVPV/PVpo dopamine neurons (Extended Data Fig. 5q-s) or their axons in the MPOA (Fig. 3c, Extended Data Fig. 5t). Combining priming by female exposure with dopaminergic stimulation resulted in a greater elevation in mating drive than either condition alone (Fig. 3d-g, Extended Data Fig. 5u, Supplementary Video 4), consistent with a graded accumulation of both natural and artificially evoked dopaminergic signals in the MPOA.
Fig. 3 ∣. Dopaminergic input to the MPOA builds up a persistent motivation to mate.
a-c, Brief optogenetic pre-stimulation (a) of all dopamine axons in the MPOA (b: n = 7 males) or of AVPV/PVpo dopamine axons in MPOA (c: n = 6) led to sustained increases in sniffing behavior and sustained decreases in mounting latency.
d-g, Priming by exposure to a female followed by pre-stimulation of all dopaminergic inputs to the MPOA (d) resulted in increases in appetitive sniffing (e: n = 5 males) and decreases in mounting latency (f: n = 5) that were greater than for either manipulation alone (g: n = 7, 7, 5).
h-j, Dopamine signals through D1- and D2-family receptors to alter cAMP levels and PKA activity (h). Brief Chrimson photostimulation of AVPV/PVpo dopamine axons in the MPOA in vivo (h) triggered local cAMP production (measured by cADDis photometry) that can be attenuated by D1/D5 antagonist SCH23390 (0.6 mg/kg, i.p.) (i: n = 6-7 males). Strikingly, cAMP levels did not completely return to baseline after stimulation but instead accumulated across repeated stimulations and persisted for minutes after the last stimulation (j: n = 6-7). The sign of the Y-axis is flipped, as cADDis fluorescence decreases with increasing cAMP. AC, adenylyl cyclase.
k,l, A model of motivational accumulation. Mean ± s.e.m. *p<0.05, **p<0.01, ***p<0.001. See Supplementary Table 1 for statistics.
Dopamine signals to downstream neurons via D1- or D2-type receptors that alter cAMP production and PKA activity24 (Fig. 3h). To detect persistence at the level of cAMP, we used a fluorescent sensor of cAMP, cADDis25 (Extended Data Fig. 6a, b). We used fiber photometry to record cADDis signals in the MPOA of awake, head-fixed males in response to local photostimulation of AVPV/PVpo dopamine axons (Fig. 3h, Extended Data Fig. 6c, d). Evoked dopamine release triggered cAMP production via D1-type dopamine receptors (Fig. 3i). More importantly, the residual cAMP at the end of each stimulation gradually accumulated and persisted for many minutes (Fig. 3j, Extended Data Fig. 6e). This persistence in cAMP levels was neither due to sustained dopamine release (Extended Data Fig. 5b, c, 6f, g) nor to autocrine effects of the stimulation (Extended Data Fig. 6f, g). Following this stimulation protocol, males showed elevated appetitive and consummatory mating behaviors (Extended Data Fig. 6h-j).
These findings led us to propose a model of motivational accumulation: dopamine ramps during female investigations are accumulated via accrual of cAMP and downstream signals in MPOA neurons, ultimately exceeding a threshold for a male to initiate mounts (Fig. 3k; see also Supplementary Discussion). Priming preemptively brings the accumulation to a near-threshold level, reducing mounting latency (Fig. 3l). Satiety abolishes dopamine ramps and cAMP accumulation, ensuring abstinence. Since accumulation requires multiple episodes of close investigation, this mechanism ensures that a male has thoroughly investigated the female before deciding to mate.
A persistent mode of cAMP-PKA signaling
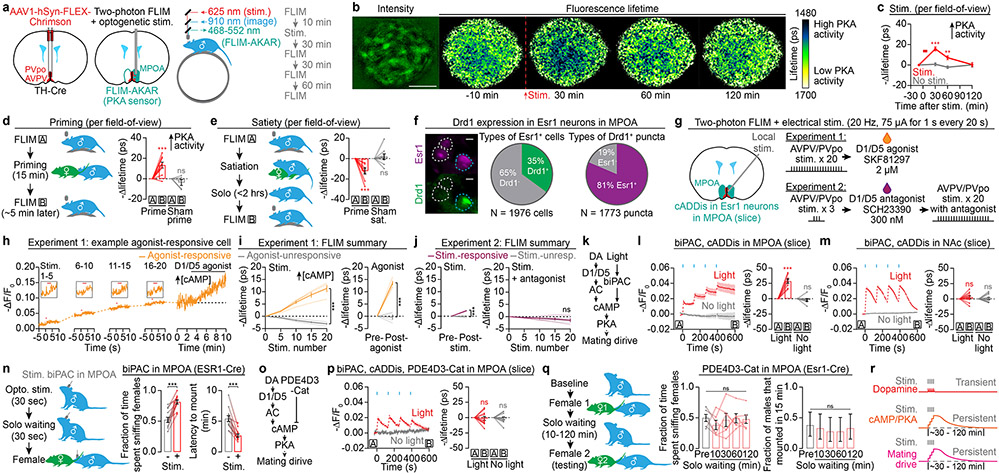
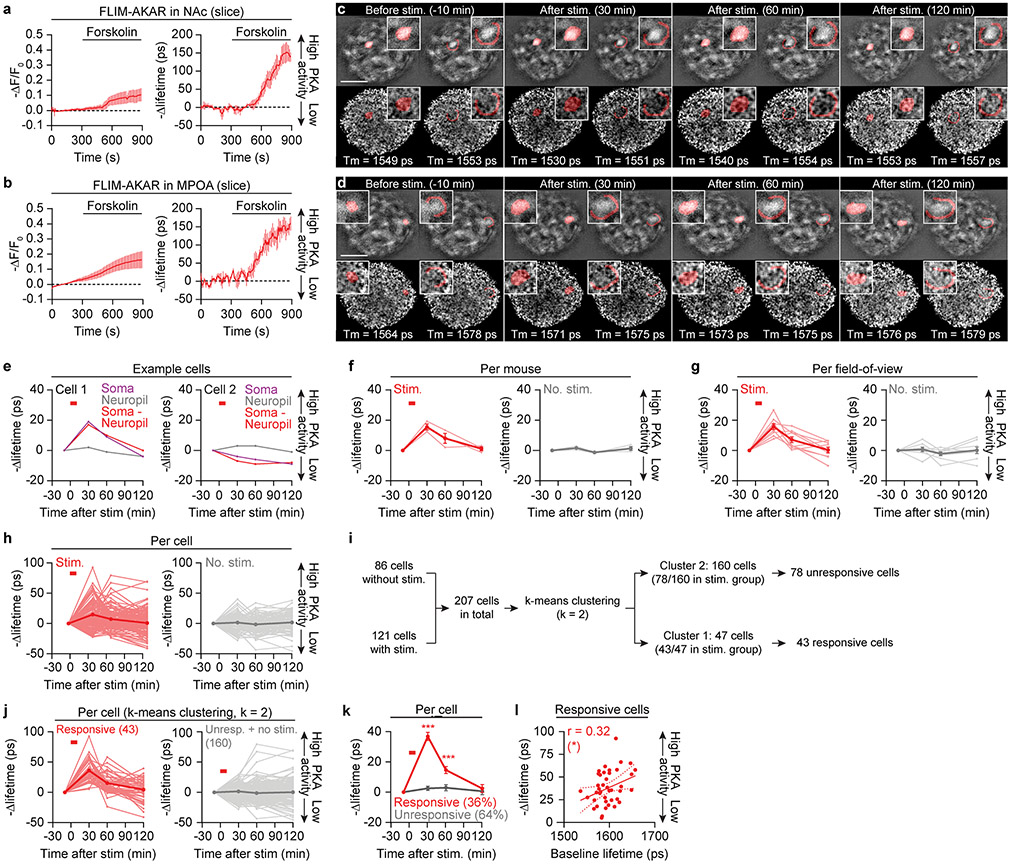
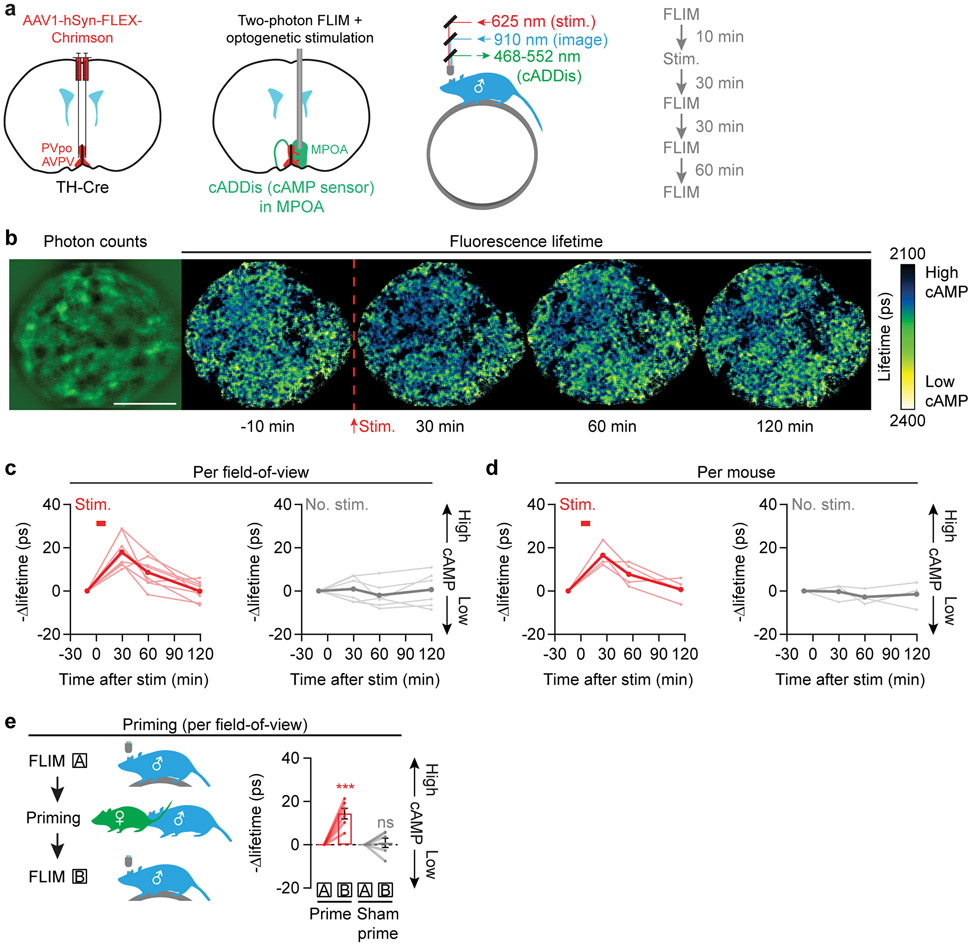
If this accrual of cAMP indeed underlies the accumulation of mating drive, these two phenomena should decay to baseline at similar rates (Fig. 1h, j). To quantitatively estimate the dynamics of cAMP/PKA signaling without the confound of bleaching, we performed two-photon fluorescence-lifetime imaging microscopy (FLIM) of the PKA sensor FLIM-AKAR26 (Extended Data Fig. 7a, b). We imaged FLIM-AKAR signals in MPOA neurons of awake, head-fixed mice through a gradient refractive index (GRIN) lens27, while optogenetically stimulating local AVPV/PVpo dopamine axons (Fig. 4a). We observed an increase in PKA activity that took ~1-2 hours to return to baseline (Fig. 4b, c, Extended Data Fig. 7c-h), matching the decay rates of both natural and optogenetic priming of mating drive (Fig. 1h, j, 3b, c). No changes in PKA activity were observed in no-stimulation controls (Fig. 4c, Extended Data Fig. 7f-h). 95% of the increase in PKA activity (averaged across all neurons) was accounted for by 36% of the neurons, commonly those with lower initial PKA activity (Extended Data Fig. 7i-l). We observed similar decay dynamics when directly estimating changes in cAMP levels using FLIM measurements of the cADDis sensor (Extended Data Fig. 6a, b, 8a-d).
Fig 4. ∣. Elevated cAMP levels and PKA activity in the MPOA underlie mating drive persistence.
a, In vivo 2p-FLIM imaging of PKA activity in MPOA neurons expressing FLIM-AKAR, during Chrimson photostimulation of dopaminergic inputs from AVPV/PVpo.
b,c, Dopamine stimulation induced a persistent decrease in FLIM-AKAR lifetime (increase in PKA activity) that gradually returned to baseline over tens of minutes (n = 8-9 fields of view). Scale bar: 200 μm.
d,e, FLIM imaging revealed a sustained increase in PKA activity in the MPOA from before (‘A’) to after (‘B’) priming of males (d). Satiety had the opposite effect (e). n = 9 fields of view.
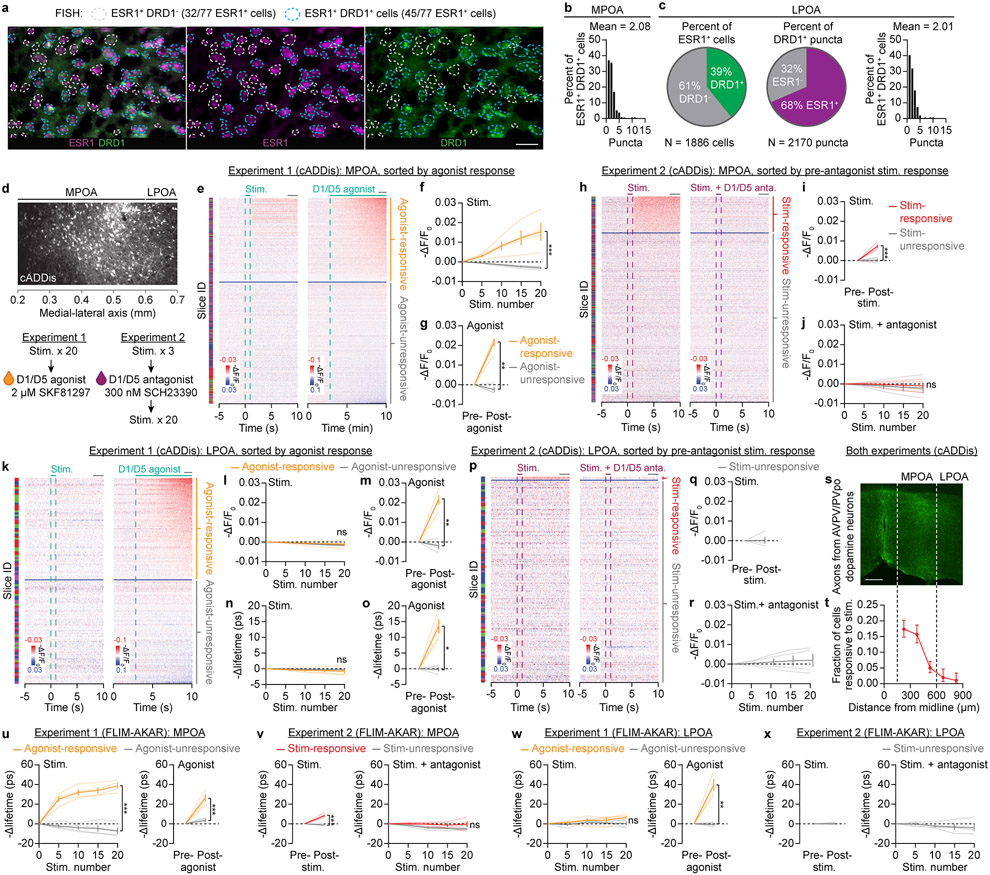
f, Two-color FISH experiment. 35% of MPOA Esr1 cells express Drd1, and Esr1 cells contain 81% of Drd1 puncta. Left panel: three example Esr1 cells. Scale bar: 20 μm.
g, Experimental design for mapping the circuitry downstream of AVPV/PVpo dopamine neurons in acute coronal slices.
h, Example D1/D5-agonist-responsive Esr1 neuron in the MPOA. cAMP was persistently elevated following AVPV/PVpo stimulation.
i, Using FLIM imaging of cADDis, MPOA Esr1 neurons were classified into two groups: D1/D5-agonist-responsive and agonist-unresponsive (right). Only neurons within the agonist-responsive group responded to AVPV/PVpo stimulation (left). n = 4 slices.
j, In MPOA Esr1 neurons that responded to AVPV/PVpo stimulation (left), responses were blocked by a D1/D5 antagonist (right). n = 4 slices.
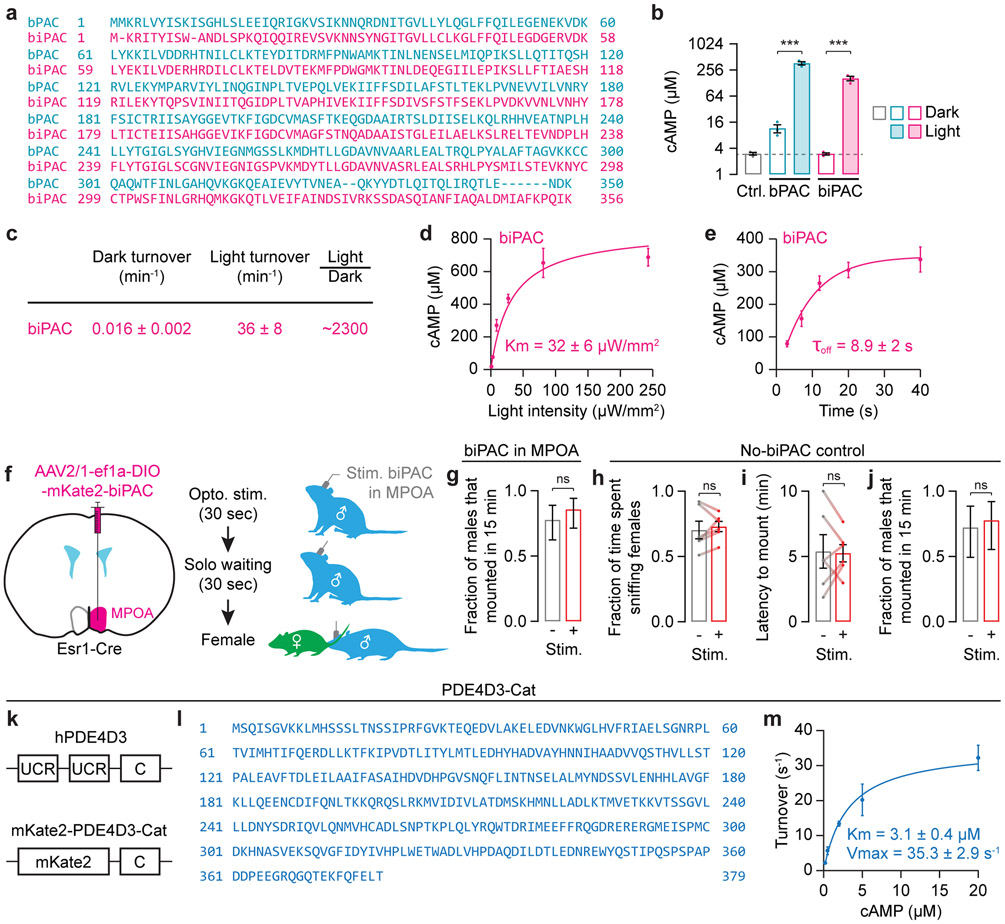
k, Optogenetic stimulation of biPAC produces cAMP.
l,m, in vitro optogenetic stimulation of biPAC in the MPOA induced persistent increases in cAMP that accumulate across trials (l: n = 8 slices). Such persistence was not observed in the NAc (m: n = 12 slices).
n, Optogenetic pre-stimulation of biPAC in Esr1 neurons in the MPOA caused subsequent increases in appetitive sniffing and decreases in mounting latency (n = 12 males).
o, Constitutively active PDE4D3-Cat degrades cAMP.
p, Same as l but in cells that also express PDE4D3-Cat. No cAMP persistence was observed (n = 11 slices).
q, AAV expression of PDE4D3-Cat in MPOA Esr1 neurons abolished the priming effect of female interactions (n = 6 males, right: mean ± c.i.).
r, Model: transient dopamine release during female investigation induces a persistent increase in mating drive by accruing cAMP levels and PKA activity in the MPOA. Mean ± s.e.m. unless otherwise specified. **p<0.01, ***p<0.001. See Supplementary Table 1 for statistics.
In addition to optogenetic stimulation, natural dopamine release during brief interaction with a female also induced persistent increases in both cAMP levels and PKA activity in the MPOA (Figs. 2b, 4d, Extended Data Fig. 8e). In contrast, after sexual satiation (which suppresses dopamine release from AVPV/PVpo dopamine neurons, Fig. 2c), we observed a large decrease in PKA activity (Fig. 4e). These results suggest that both cAMP levels and PKA activity in MPOA neurons track levels of mating drive.
One potential target of the dopaminergic mating-drive signal is the set of MPOA neurons that express the estrogen receptor Esr128,29, 35% of which express Drd1 (the gene that encodes the D1 receptor) (Fig. 4f, Extended Data Fig. 9a, b). When we applied focal electrical stimulation to AVPV/PVpo (Fig. 4g), we observed persistent cAMP elevations that accumulated across trials (Fig. 4h, i), but only in neurons that also responded to a D1/D5 agonist (Fig. 4i, Extended Data Fig. 9d-g). Separate experiments suggested that these elevations are mediated by direct dopamine transmission from AVPV/PVpo neurons, as they were blocked by a D1/D5 antagonist (Fig. 4g, j, Extended Data Fig. 9h-j). In the neighboring lateral preoptic area (LPOA), Esr1 neurons also express Drd1 (Extended Data Fig. 9c). However, almost none responded to AVPV/PVpo stimulation (Extended Data Fig. 9k-r), consistent with a gradual decrease in the fraction of responsive neurons with increasing distance from the midline (Extended Data Fig. 9s, t). Similar findings were observed using the PKA sensor FLIM-AKAR (Extended Data Fig. 9u-x). Together, these results strongly suggest that AVPV/PVpo dopamine neurons drive cAMP signaling in MPOA Esr1 neurons via activation of D1/D5 receptors.
We next asked whether cAMP persistence is caused by the prolonged activation of dopamine signal transduction or the slow clearance of cAMP. To distinguish between the two scenarios, we directly stimulated cAMP production using biPAC, a light-activated bacterial adenylyl cyclase that we engineered to exhibit minimal dark activity (Fig. 4k, Extended Data Fig. 10a-d). As with dopamine stimulation (Fig. 3j, 4h), optogenetically induced cAMP in MPOA neurons lingered for minutes after each light pulse, and accumulated across pulses (Fig. 4l). This phenomenon was not due to persistent biPAC activation (Toff = ~9 s) (Extended Data Fig. 10e), and was not observed in identical experiments in the NAc (Fig. 4m). These findings suggest that cAMP persistence in the MPOA is due to slow clearance. Strikingly, this direct elevation of cAMP in MPOA Esr1 neurons was also sufficient to increase male mating behaviors (Fig. 4n, Extended Data Fig. 10f-j).
To test the necessity of cAMP persistence for priming, we engineered PDE4D3-Cat, a high-affinity, constitutively active, and cAMP-degrading phosphodiesterase (Fig. 4o, Extended Data Fig. 10k-m). AAV expression of PDE4D3-Cat eliminated cAMP persistence in MPOA neurons (Fig. 4p). Behaviorally, PDE4D3-Cat expression in MPOA Esr1 neurons blocked priming by female interactions (Fig. 4q) and reduced the probability of male mounting from ~90% (Fig. 1i) to ~30% (Fig. 4q). Together, these findings indicate that persistence of cAMP in MPOA Esr1 neurons underlies the priming of mating drive (Fig. 4r).
Discussion
In this study, we have identified AVPV/PVpo dopamine neurons that project to the MPOA as a key regulator of mating drive in male mice. Our findings suggest that neurons in different brain areas (e.g. MPOA and NAc) exhibit different kinetics of cAMP clearance that enable persistent or episodic30-32 modes of neuromodulation (see also Supplementary Discussion). AVPV dopamine neurons are regulated by sex hormones33,34 and are sexually dimorphic in their anatomy34,35. In female mice, these neurons have been implicated in parental behaviors35, which also exhibit persistence4.
Our findings may help explain how various drugs affect male libido. Drugs that boost or inhibit dopamine signaling have hyper- or hypo-sexuality side effects, respectively6,36, possibly via actions on the MPOA. Non-dopamine-related medications such as selective serotonin reuptake inhibitors (SSRIs) also decrease libido37. Elevated serotonin caused by SSRIs could activate Gi-coupled serotonin receptors in the MPOA38 to prevent motivational cAMP accumulation.
Methods
Data reporting
No statistical methods were used to predetermine sample size. Sample sizes were chosen to reliably measure experimental parameters and keep with standards in the relevant fields16,39-44, while remaining in compliance with ethical guidelines to minimize the number of experimental animals. Experiments did not involve experimenter-blinding. Randomization was used to determine experimental order and group assignments.
Animals
All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee at Beth Israel Deaconess Medical Center. Animals were housed in a 12-hour-light/12-hour-darkness environment (temperature 20-22 °C, humidity 30-70%) with standard mouse chow and water provided ad libitum, unless specified otherwise. Male and female mice older than 8 weeks were used in experiments, but due to the scope of this study, recordings, perturbations, and behavioral analyses were focused on the males. We used the following genotypes: male and female C57BL/6J (WT; 000664, The Jackson Laboratory), male B6.Cg-7630403G23RikTg(Th-cre)1Tmd/J (TH-Cre; 008601, The Jackson Laboratory), male F1 progeny of TH-Cre and B6;129S-Gt(ROSA)26Sortm32(CAG-COP4*H134R/EYFP)Hze/J (Ai32(RCL-ChR2(H134R)/EYFP); 012569, The Jackson Laboratory), and male C57BL/6-Esr1tm2.1(cre)And/J (Esr1-Cre; 017913, The Jackson Laboratory). We used TH-Cre mice to label AVPV/PVpo dopamine neurons because these cells only sparsely express DAT (Slc6a3)45 and therefore cannot be targeted using DAT-Cre mice. Because males’ mating behaviors and ability to process sensory cues from conspecifics are strongly improved by having at least some social experience46-48, we paired males individually with hormonally primed females (see Supplementary Methods), two weeks before any experiment (except for slice experiments). The two-week time window was chosen to be longer than the recovery time from satiety (Fig. 1d, e). After the female was removed, the male was housed solo in his home cage and remained so throughout the experiments. Females were housed in groups.
Stereotaxic surgeries
Viral injections, fiber implantations, and GRIN lens implantations were generally performed as described in Lutas et al.41 with the following specifications and modifications. We note that for experiments using 400-μm diameter fibers or 500-μm diameter GRIN lens, in order to ensure a snug fit for the fiber or lens, to reduce brain motion, and to accelerate recovery, we pre-set the insertion tracks by lowering needles with matching diameters (27 gauge or 25 gauge, respectively) to a depth of 0.1 mm above the final depth of the fiber or lens. All AAVs were injected at a titer of 3-15 1013 gc/ml. In experiments where unmixed cADDis and PDE4D3 viruses were used, both AAVs were pre-diluted 1:3 to a final titer of 3-4 1013 gc/ml before injections to minimize potential effects on cell heath (which we verified in post hoc histological analyses). We also note that, due to the close proximity of the AVPV and PVpo nuclei, they were co-infected by the viral-injection steps, which we verified with histology after each experiment. In the minority of cases where viral expression was absent (<10% of surgeries), we excluded the data from subsequent analyses. All animals were allowed to recover for at least 3 weeks prior to the onset of experiments. No obvious capsid competition was seen in histology. Please see Supplementary Methods for surgical details of each experiment.
Assays of male mating behaviors
All mating behavior experiments were conducted during the circadian dark hours inside the home cages of the males. Food bowls, huts, running wheels, and any other materials that could obstruct the view of the inside of the cage were temporarily removed for the duration of the experiments. The cages were then placed in a dark environment illuminated only with IR lamps. Videos of the cage were taken with Flea3 cameras and Flycapture 2 (FLIR systems) at 30 Hz. Hormonally primed females (see Supplementary Methods) were gently lowered into the cage in a transparent cup (~10 cm in diameter) under dim red light, which was subsequently turned off.
To measure a male’s mating behaviors, we used a 15-min assay that starts at female entry. To measure mating behaviors without satiating the male’s mating drive (i.e., transfer of reproductive fluids), we typically either stopped the recording and separated the animals after the male gained intromission (i.e. last step in the sequence of mating behaviors before transfer of fluids), or gently shook the cage to separate them in the cases for which we could not stop the video (e.g., for recovery ethograms in Fig. 1c). The same procedure was also used for priming experiments where the male was prevented from successfully mating with the first female, which would have otherwise resulted in satiety prior to the assay.
To satiate a male’s mating drive during subsequent imaging, histology, and behavioral experiments, we let the video recording proceed for as long as needed, and spot-checked for mating plugs afterward. However, if a male had failed to gain intromission in 30 min, we switched the female and restarted the process. In experiments where we measured a male’s mating behaviors more than once (e.g., during recovery from satiety), we ensured that the male was never paired with the same female more than once. In some experiments, we tested the males every day with different females, and no cross-day priming was observed in those experiments (e.g., Fig. 1h-j). All males tested in this paper were in the C57BL/6J background, a strain that has a recovery time of several days from sexual satiety (Fig. 1d, e)12,49. However, the exact recovery time is likely strain-dependent49.
Home-cage optogenetic experiments
Optogenetic stimulation50 experiments were carried out using a 465-nm LED (Plexon LED and driver). Please see Supplementary Methods for the design of the LED controller and the experimental setup. For optogenetic stimulation of ChR2, blue light was pulsed at 10 Hz (10 ms square waves) and a power of 2.5 mW. In pilot experiments stimulating all dopamine axons in the MPOA (Extended Data Fig. 2a-d), the LED pulses were turned on when the female entered the cage and turned off when the experiment ended. In the satiety-reversal experiments (Fig. 2i, j), the LED pulses were turned on 1 min before female entry and turned off 5 min after female entry. The reason we chose this protocol is that, in the pilot experiments in which we stimulated AVPV/PVpo dopamine axons in the MPOA, we were never able to observe male mounting if we left the pulses on, and the mounting happened only after we turned off the pulses. We are currently investigating the biological reasons behind this observation. In optogenetic priming experiments (e.g., Fig. 3a-c), the duration of the pulse train was 30 seconds, and the stimulation was performed 10-120 min before female entry while the male was alone in the cage. The exception was the 0-min solo waiting condition, where the stimulation (still 30 seconds in duration) started when the female entered. For optogenetic stimulation of biPAC, a single 30-sec square wave of 465-nm light was delivered from 60 seconds to 30 seconds prior to female entry. We used this protocol because our pilot experiments showed that biPAC required relatively long light pulses to produce cAMP. Such long light pulses may increase the temperature in the MPOA and may agitate the male51. We therefore turned off the activation of biPAC before female entry. Based on our MPOA slice recordings (Fig. 4l) we expect that this protocol should nevertheless result in a long-lasting elevation in cAMP that persists after female entry.
Home-cage two-color photometry experiments
Two-color photometry was carried out using a 465-nm LED and a 405-nm LED (Plexon LED and driver). The 405-nm light was used to excite dLight1.1 at its isosbestic wavelength, thereby measuring dopamine-independent signals13. Please see Supplementary Methods for the design of the LED controller and the experimental setup.
For two weeks before photometry experiments, each male was habituated to handling and to attachment of a patch cord to his fiber implant. Before each recording, a male was allowed to explore his cage in darkness for ~1 min after attaching the patch cord and placing the heat shrink. During this time, we verified the signal intensity and quality. Each recording session started when a hormonally primed female was lowered into the cage as described above, and lasted for 15 minutes. Simultaneous video recording (using IR illumination) of the behaviors was conducted as in the behavioral experiments, with the exception that the cameras were externally triggered at 30 Hz using an Arduino. The video trigger signals were also collected together with the photometry-related signals to allow synchronization of behavioral and photometry analyses. No transfer of fluids was allowed during the recording to prevent satiety. For experiments that involve the investigation of a novel object, we lowered a ball of red tape into the male’s cage instead of a female, and the experiments were conducted in a dimly lit environment.
Head-fixed photometry experiments
Head-fixed photometry experiments were used to test the effect of optogenetically stimulating AVPV/PVpo dopamine axons on dopamine release or cAMP production in the MPOA. The experiments were carried out using head-fixed mice that could run freely on a circular treadmill52 in order to reduce potential motion artifacts. Please see Supplementary Methods for the design of the LED controller and the experimental setup.
To estimate the effect of photobleaching, in every trial, we first recorded photometry signals for 5 min before starting optogenetic stimulation. For dLight1.1 and GRABDA2m recordings, the stimulation was turned on for 1 second every 30 seconds (for 10 rounds spanning 300 seconds). This repeated stimulation followed by a long inter-stimulation interval was used to mimic the phasic increase in dopamine release seen in Fig. 2b. For cADDis recordings, the stimulation was turned on for 5 seconds every 30 seconds (for 40 rounds spanning 1200 seconds), followed by an additional 20 minutes of recording after the last stimulation to estimate persistent changes in the cADDis signal. For two weeks prior to photometry experiments, each male was habituated to handling and head-fixation.
In experiments that used the D1/D5 antagonist SCH23390, we diluted the antagonist to 300 μM in saline and injected 150 μl intraperitoneally (~0.6 mg/kg dosage) 15 min before the recordings. The animal’s movement was typically noticeably reduced after injection.
Home-cage feeding and drinking experiments
Home-cage feeding experiments were carried out as described in Krashes et al.40, and the drinking experiments were performed using a similar method. Please see Supplementary Methods for details.
Chemogenetic silencing
Chemogenetic silencing experiments were performed as in Garfield et al.53. Please see Supplementary Methods for details.
Histology
Perfusion and histology were performed as described in Garfield et al.53. Brain slices (60 μm thick) were collected and one of every three consecutive slices was scanned. The primary antibodies used in this paper are chicken anti-GFP (1:1000, Invitrogen, A10262, Lot 2035172 and 2089131; used for GFP and GCaMP6s), rat anti-mCherry (1:1000, ThermoFisher, M11217, Lot UJ287711; used for mCherry and tdTomato), rabbit anti-TH (1:500, Millipore, AB152, Lot 2115918), mouse anti-TH (1:1000, ImmunoStar, 22941), and rabbit anti-c-Fos (1:1000, Millipore, ABE457, Lot 3142408). The secondary antibodies are donkey anti-rabbit 594 (1:1000, Invitrogen, A21207, Lot 21450222), goat anti-mouse 488 (1:1000, Invitrogen, A11001, Lot 1664729), donkey anti-chicken 488 (1:1000, Jackson, 703-545-155, Lot 138498), and donkey anti-rat 594 (1:1000, Jackson, 712-585-153, Lot 134903).
Fluorescence in situ hybridization
Fluorescence in situ hybridization experiments were performed using the RNAscope kit (ACD). Please see Supplementary Methods for details.
Two-photon imaging
Two-photon imaging was performed using a two-photon resonant-galvo scanning microscope (NeuroLabWare) as described previously52. An InSight X3 laser (Spectra-Physics) was used to excite the fluorophores (910-1050 nm), and the emission light was filtered (green: 510/84 nm; red: 607/70 nm; Semrock) before collection with photomultiplier tubes (H10770B-40, Hamamatsu). The XY scanning was performed using resonant/galvo mirrors and the Z scanning was achieved with an electrically tunable lens (Optotune).
Two-photon FLIM microscopy
Two-photon fluorescence-lifetime imaging microscopy (FLIM) was performed as described previously54. Data were collected using SPCM (Berker and Hickl). Please see Supplementary Methods for microscope details.
Two-photon fluorescence intensity and FLIM imaging of acute brain slices
Acute brain slices were prepared as described in Lutas et al.41 and transferred to a recording chamber perfused with ACSF (oxygenated with 95% O2 and 5% CO2; flow rate: 2-5 mL/min) at room temperature. Two-photon slice imaging was performed using a 16x water-immersion objective (NA 0.8, CFI75 LWD, Nikon) at 910-920 nm.
In pharmacological experiments involving the cAMP sensor cADDis or the PKA sensor FLIM-AKAR, each slice was imaged for 15 minutes (one frame every 10 seconds) and 50 μM forskolin was applied via perfusion starting at 5 minutes.
In optogenetic experiments involving biPAC, each slice was imaged for 10.5 minutes (15.5 frames per second). At 0.5 min, 2.5 min, 4.5 min, and 6.5 min, the PMT was powered off, a 470-nm LED (1 mW/mm2, Luxeon Star LEDs) driven by an Arduino-controlled driver (Luxeon Star LEDs) was then turned on for 2 seconds, and the PMT power was then powered on again. The PMT was turned off and on to protect it from the LED light. Control experiments were conducted as above except for LED was not turned on.
In experiments involving electrical stimulation of AVPV/PVpo, a concentric bipolar electrode was positioned with a motorized manipulator (Scientifica) such that the tip of the electrode was in contact with AVPV/PVpo (but not too deep to avoid tissue warping). Local stimulation current was controlled by a custom-programmed microcontroller and delivered as 1-s trains of 20 Hz, 5 ms, 75 μA pulses that occurred once every 20 s. These parameters were chosen based on pilot experiments using GCaMP6s-expressing mice, and we found the radius of direct stimulation to be ~100 μm. Two-photon images were taken from the MPOA (defined as the subregion of the preoptic area within 600 μm from the midline) and LPOA (defined as the subregion beyond 600 μm from the midline). In experiments that involved the D1/D5 agonist SKF81297, we did 20 rounds of stimulation first, and then washed on 2 μM SKF81297 afterward to distinguish Esr1 neurons that could respond to the agonist from those that could not. In experiments that involved the D1/D5 antagonist SCH23390, we first did 3 rounds of stimulation (the minimal number needed to assess whether neurons responded to the stimulation), then washed on the antagonist (300 nM) for 10 min, and then delivered 20 more rounds of stimulation in the presence of the antagonist. We kept pre-antagonist stimulation to a minimum to prevent cAMP/PKA responses from saturating prior to application of the antagonist.
In vivo two-photon FLIM experiments
In vivo two-photon imaging of the MPOA via a GRIN lens in head-fixed males was generally performed as described in Lutas et al.41. The excitation wavelength is 910-920 nm. The experiments were carried out using head-fixed mice that could run freely on a circular treadmill52. Imaging was performed with a 4x 0.2 NA air objective (Nikon) on mice implanted with a doublet GRIN lens (NEM-050-25-10-860-DM, Grintech; NA 0.19 on the air side). Light shield material was used to protect the lens and the objective from external light. Imaging fields-of-view were at depths of 100–300 μm below the face of the GRIN lens. For each time point (e.g., −10 min before dopamine stimulation), we collected five 5-s FLIM frames of the same field of view to be averaged later (10 seconds between frames).
For the optogenetic experiments, between initial and subsequent FLIM imaging of the same MPOA neurons, we performed an intervening dopamine axon photostimulation in the MPOA via the GRIN lens on a different microscope (the FLIM microscope did not have full-field red optogenetic stimulation capability). For optogenetic excitation, a 617-nm LED (ThorLabs, M617L3) was focused onto the GRIN lens using the same 4x objective (power was measured to be 1 mW below the objective). The stimulation protocol was a 5-second train of 15.5 Hz, 8 ms pulses that were repeated every 30 seconds for 20 rounds spanning 600 s. After the stimulation, the male was head-fixed again under the FLIM microscope to collect three post-stimulation time points at +30 min, +60 min, and +120 min. Fields-of-view were matched manually before each time point to ensure stable tracking of the same cell bodies.
For imaging experiments involving priming by female interactions, each male was allowed to interact with (but not to transfer fluids to) a receptive female in his home cage for 15 min following the baseline session of head-fixed imaging of cADDis or FLIM-AKAR. After this interaction, the male was head-fixed again for post-priming imaging of cADDis or FLIM-AKAR. In control experiments, males went through the same protocol but no female was present.
For imaging experiments to assess PKA activity prior to and following sexual satiety, each male was allowed to mate with a female in his home cage after the head-fixed recording of the baseline FLIM-AKAR signal. About 1.5-2 hours after mating, the males were head-fixed again to image post-satiety FLIM-AKAR measurements of PKA activity. In control experiments, males went through the same protocol but no female was present
Two-photon imaging of fixed brain slices
Please see Supplementary Methods for experimental details of imaging fixed brain slices.
In vitro cAMP assays
Please see Supplementary Methods for in vitro cAMP assays.
Data analysis
All data analyses were performed using custom scripts in MATLAB (MathWorks), Python, ImageJ (NIH), SPCImage (Becker and Hickl), and Prism (GraphPad).
Analyses of male mating behaviors
The onset of a bout of sniffing was defined as the moment when a male initiated a bout of movement toward the female that culminated in the male beginning to sniff the female (usually either around the face or anus). Since the individual sniffs were fast, we inferred that the sniffing bout was terminated when the male made one of the following actions: turning his head away from the female, turning his body away from the female, moving forward but in a different direction from the female, remaining still when the female moved forward, or beginning other non-sniffing behaviors. The onset of a bout of mounting was defined as the moment when the male climbed over a female and began rapid, shallow lower body thrusting, and terminated when the male was no longer over the female. During a bout of mounting, the male could gain intromission, which was evident from the male’s thrusting movements becoming deeper and slower (usually about once per 1-2 sec). The transfer of fluids (i.e., ejaculation) was marked by a specific series of events where an intromitted male began to shake for a few seconds, then fell on his side, then became temporarily immobilized even when the female moved away, and then stood up again to clean himself. When we spot-checked mating plugs after the assays, the results consistently matched the behaviors of the male. Lower-body grooming bouts were defined as those moments when the male bent his body forward to lick his lower-body, usually around the genital area. Digging bouts were defined as those moments when the male used his forelimbs to dig in the bedding. Feeding bouts were defined as those moments when the male ate chow in the cage. Attempts to escape were defined as those moments the male attempted to jump and climb up on the side of the cage.
To measure a male’s appetitive mating behavior (which is commonly defined as the sniffing of females55), we calculated the fraction of time that a male spent in sniffing females in the time period before the first mount, or up to five minutes from the moment that the female is introduced, whichever came first. We used this 5-minute maximum time window because most sniffing occurred in the minutes immediately after female entry and, by focusing on the pre-mounting time, we avoided the situation where a male who began mounting early (or at all) or spent more time mounting may paradoxically spend less time sniffing. We chose five minutes as the maximum time window, because it roughly matched the mean latency to the first mounts by wild-type males (5.46 min). We note that, on average, animals with a fiber in the MPOA show ~10% lower values in both the appetitive sniffing metric and the consummatory mounting metric than animals without a fiber in the MPOA, possibly due to minor damage to this region. Note, however, that the experiments involving optic fibers mostly involved within-animal comparisons.
To measure a male’s consummatory mating behavior, we calculated either 1) the fraction of trials in which a male began mounting in the first 15 minutes after female entry, or 2) the latency of the male’s first mount, depending on the experiment. In experiments where we expected a decrease in mounting behaviors (e.g., Fig. 2h), we used the first metric because estimating mounting latency is difficult when there were few mounts. In experiments where we expected an increase in mating drive (e.g., Extended Data Fig. 2c), we used the second metric because the fraction of trials with mounting was typically near the ceiling (~0.85) in the baseline state. In Fig. 4q, the low mounting rate precludes the estimations of changes in mounting latencies.
Analyses of home-cage photometry experiments
Two-color photometry data were analyzed with custom MATLAB scripts, and the analysis code is available online (https://github.com/xzhang03/Photometry_analysis). Pulses that trigger the 465-nm and 405-nm LEDs (50 Hz each) were used to determine when the two LEDs were on. For each pulse (6 ms), a median function was applied to the corresponding points (the same 6 ms) of the photodetector traces to determine the photometry signal. This resulted in two 50 Hz traces: one for the 465-nm channel and another for the 405-nm channel. Because the power of the two LEDs was matched, they photo-bleached at similar rates. Since the biological signals reported by dLight1.1 do not contain very high frequency content13, we applied a 10 Hz low-pass filter to both traces.
To calculate the [465 nm – 405 nm] trace, we first fitted the 405-nm trace to the 465-nm trace linearly, and then subtracted the resulting scaled and shifted 405-nm trace from the 465-nm trace. This subtraction was done for each pair of 465-nm and 405-nm traces. After subtraction, we added back the Y-offset such that the mean of the [465 nm – 405 nm] trace equals the mean of the raw 465-nm trace (and roughly equals the mean of the raw 405-nm trace). This allowed for a ΔF/F calculation of the [465 nm – 405 nm] trace using the equation ΔF/F = (F – F0)/F0, where F0 is a running estimate of baseline fluorescence calculated as the 10th percentile of F(t) in the previous 32-second sliding window56. We then applied the same sliding-window ΔF/F estimation method to the 405-nm trace. To calculate the z-scored version of the traces, we pooled the ΔF/F traces of all the [465 nm – 405 nm] and 405-nm signals across days from the same animal and calculated a single standard deviation value that was then applied to all the traces for that animal. These z-scored ΔF/F traces are shown in Figures 2b-c and Extended Data Fig. 3d, f, g, 5e, f.
The sniffing-triggered heat maps show traces that are aligned to the onsets of close sniffing of a female. In Extended Data Fig. 2g-i, stretched the photometry traces linearly in time so that the corresponding actions (approach or sniff) all span the same duration of time (10 seconds).
The ramp and drop sizes were calculated as shown in Extended Data Fig. 2j. The magnitude of the ramp in fluorescence prior to the onset of sniffing was calculated as the mean of the ΔF/F trace from 1.5 seconds before sniffing onset to 1 second before sniffing onset (‘B’), minus the mean of the ΔF/F trace from 5 seconds before sniffing onset to 4.5 seconds before sniffing onset (‘A’). The magnitude of the drop in fluorescence at the onset of sniffing was calculated as ‘B’ minus the mean of the ΔF/F trace from 1 second after sniffing onset to 1.5 seconds after sniffing onset. Ramp magnitudes and drop magnitudes were plotted on a per-mouse basis.
To test the hypotheses that differences in ramp and drop magnitudes may be due to differences in the time of sniffs after female entry, we re-calculated the mean ramp and drop magnitude by subsampling without replacement from pairs of sniffs, one in each of the two conditions, that were within 2 seconds of each other. This procedure matched ~80% of the sniffs (Extended Data Fig. 2m, n).
We used Deeplabcut57 for automated behavioral tracking. Please see Supplementary Methods.
Analyses of head-fixed photometry experiments
Head-fixed photometry data were analyzed with custom MATLAB scripts (https://github.com/xzhang03/Photometry_analysis). Pulses that trigger the 465-nm LED (50 Hz) were used to determine when the LED was on. For each 6-ms pulse, the photometry signal was defined as the median of the corresponding data points in the photodetector trace. We then applied a 10 Hz low-pass filtered to the resulting 50 Hz trace.
To estimate bleaching, we fitted the pre-stimulation data (first 5 min of each trial) with a mono-exponential function (see Extended Data Fig. 6e for examples) and subtracted the values from the photometry trace. Because the cADDis experiments focus on persistent cAMP activity, we did not use the sliding-window method to estimate ΔF/F, but instead used either the average photometry signal in the 5 seconds before each stimulation as the baseline (Fig. 3i) or the average photometry signal in the 5 seconds before the first stimulation (for Fig. 3j, where we highlight the accumulation of cAMP). We also did not use the sliding-window method for the head-fixed dLight1.1 experiments to avoid overlooking any potential accumulation of extracellular dopamine. The ΔF/F traces were then pooled across days and z-scored as above.
Quantification of c-Fos/TH colocalization
Quantification of c-Fos/TH colocalization was performed using a script in MATLAB (https://github.com/xzhang03/sbxSlide). The pipeline is shown in Extended Data Fig. 4a. Please see Supplementary Methods for additional details.
FLIM analysis procedures
FLIM data were preprocessed using SPCImage (Becker and Hickl) to generate intensity and lifetime images (see Supplementary Methods for details). The remainder of the data processing for FLIM experiments was performed in MATLAB (https://github.com/xzhang03/SPC_analysis). Both intensity and lifetime images were downsampled spatially by a factor of four. Image registration was performed using the intensity images, and the shifts were applied to the lifetime images. For in vivo imaging experiments, frames that we were unable to register (due to brain motion) were removed. For slice imaging experiments, all frames were registered and no downsampling was performed. Somas were then segmented from the intensity images by first locally enhancing contrast58 and then applying the morphological filter (see Supplementary Methods). The soma ROIs were then applied to the lifetime images to estimate the average lifetime per cell. A typical cell had a mean pixel intensity of 10-15 photons/pixel and the cell’s lifetime was estimated from ~20000-26000 photons.
For in vivo FLIM experiments, we also calculated the lifetimes of the neuropil rings surrounding each cell. The purpose of this procedure was to subtract lifetime changes in the neuropil from those in the soma, thereby estimating the contribution of changes in lifetime at the soma, above and beyond changes that may be due to contamination from nearby neuropil. Please see Supplementary Methods for details of neuropil determination.
Because cADDis and FLIM-AKAR show decreased fluorescence lifetime and intensity when cAMP concentration and PKA activity increase, we plot their lifetime and intensity changes on a flipped Y-axis (−ΔF/F0 and −Δlifetime, respectively). Decreases in FLIM-AKAR lifetime could also be caused by a decrease in phosphatase activity. However, since we almost always observed concerted changes in cAMP levels and PKA activity, it is more likely that FLIM-AKAR reports PKA activity changes in our experiments.
For experiments that used FLIM to test the cAMP sensor cADDis and the PKA sensor FLIM-AKAR in slices, intensity and lifetime changes were calculated relative to the first 5 min (pre-forskolin) as a baseline (e.g., Extended Data Fig. 6a).
For in vivo FLIM experiments, we averaged the lifetime estimates from the last 4 of the 5 FLIM frames per time point to avoid the imaging aberration in the first frame (when the resonant mirror just started scanning). Soma ROIs were segmented from each time point and manually matched between time points.
Analyses of two-photon slice experiments involving biPAC and electrical stimulation
Image registration was performed using a previously published method in MATLAB59. To extract cell bodies, we down-sampled images spatially by a factor of four. Then, we used the same morphological filter to extract soma ROIs (see Supplementary Methods).
For experiments involving electrical stimulation of AVPV/PVpo, we subtracted the responses of the surrounding neuropil rings from the soma responses. This was done to remove global influences from the estimation of proportions of neurons that responded to electrical stimulation and to D1/D5 agonists. Estimates of ΔF/F0 for each component of the experiment (e.g., stimulation and agonist delivery) were calculated separately. For the purpose of heat maps (e.g., Extended Data Fig. 9e), the response of each cell was re-zeroed to the pre-stimulation level. For the agonist experiments (e.g., Extended Data Fig. 9e), we classified each neuron based on its response to the D1/D5 agonist. To do so, we used a classifier52 that is based on the area under receiver operating characteristic curve (auROC) applied to ΔF/F0 data from the pre-agonist baseline (defined as the last minute before wash on) and the post-agonist period (defined as the last minute of the session). Statistical significance was determined using two-tailed bootstrapping and Bonferroni corrected for multiple comparisons. Within each group, the cells were sorted by their agonist response. The classification results and sorting order were also applied to the stimulation data to determine the correspondence between a neuron’s stimulation response and its agonist response. The same ROIs and neuropil rings were also applied to the lifetime data to estimate lifetime changes. For the antagonist experiments (e.g., Extended Data Fig. 9h), the auROC classifier was used on the pre-antagonist stimulation data (comparing the last 2 seconds before each stimulation and the 2-s window 8 seconds after each stimulation onset) to determine which neurons were stimulation-responsive. The classifications and sorting orders were then applied to the post-antagonist stimulation responses. For FLIM-AKAR experiments, all analyses were performed on the fluorescence lifetime data, since the sensor has a weak ΔF/F0 response (Extended Data Fig. 7a, b).
Statistics and reproducibility
Two-tailed t-test and ANOVA were performed using Prism. For ANOVA, multiple comparisons were done using the Sidak host hoc correction. Also for ANOVA, F values (F) and degrees of freedom (DF) are indicated in Supplementary Table 1. For these tests, each mouse was treated as an independent sample. We typically averaged data from 2-3 tests per mouse, except for the early experiments in which most animals were tested only once. For two-photon FLIM experiments, each field of view was treated as an independent sample (2-3 fields of view per mouse). Hypothesis testing of fractions was done with two-tailed Fisher’s exact tests in MATLAB, and multiple comparisons were adjusted using the Bonferroni correction. The fractions are based on the numbers of trials. We also note the numbers of animals in figure legends (typically 2-3 trials per animal). For all tests, non-significant (ns) outcomes indicate that the null hypothesis cannot be rejected (i.e., p ≥ 0.05). Asterisks indicate *p<0.05, **p<0.01, ***p<0.001. Exact p-values are given whenever appropriate. For plots of fractions, error bars represent 95% Jeffrey’s confidence interval (95% c.i.). For all other plots, error bars represent the standard error of the mean (s.e.m.) or standard deviation (s.d.). For each panel where a representative image is shown (with the exceptions of Extended Data Fig. 3a, b), similar independent images from different mice were reproduced at least 3 times.
Extended Data
Extended Data Fig. 1 ∣. Males suppress non-mating behaviors and gradually increase their sexual arousal during mating epochs.
a, In the pre-mating epoch (after female entry and before the first mount), males engaged in feeding, digging, or trying to escape during ~25% of the “available” time (defined as the time not spent in sniffing the female, mounting the female, or lower-body grooming). In trials where males gained intromission at least once, they became hyper-engaged in mating. In the subsequent mating epoch (i.e. in the time between the first mount and the transfer of reproductive fluids), males spent almost no available time feeding, digging, or trying to escape. After transferring fluids, these males re-engaged in these non-mating behaviors. In trials where males never gained intromission (defined as the male’s thrusting movements becoming deeper and slower), they did not demonstrate a similarly hyper-engaged mode restricted to mating-related behaviors (n = 7, 6, 5, 6 males).
b, We defined the appetitive sniffing metric as the fraction of pre-mounting time (i.e. the time from introduction of the female to up to 5 minutes later or to the first mount, whichever came first) spent in sniffing females. This metric was not significantly correlated with mounting latency (n = 47 first mounts from 20 males).
c, About 80% of the first mounts were immediately preceded by a bout of sniffing (black arrows). This fraction gradually dropped across subsequent mounts, to about 5% for the fourth and fifth mounts (1st mounts: n = 48; 2nd and 3rd mounts: n = 52; 4th and 5th mounts: n = 49 mounts; 20 males).
***p<0.001. See Supplementary Table 1 for statistics.
Extended Data Fig. 2 ∣. Specialized dopamine release in the MPOA during appetitive mating behaviors.
a-d, Optogenetically stimulating all dopaminergic inputs to the MPOA (in TH-Cre males, a) increased appetitive sniffing behavior (b: n = 6 males) and decreased mounting latency (c: n = 5 males). The same stimulation did not further increase the fraction of males that mount in the first 15 minutes after introduction of the female (d), as this fraction was already near ceiling in the absence of stimulation (n = 6 trials from 6 males, mean ± 95% c.i.).
e, dLight1.1 expression and fiber location in the MPOA (scale bar: 200 μm).
f, The average dLight1.1 signal remained largely flat when a male mouse approached another male (n = 7 males).
g-i, Normalizing the durations of different bouts of approach to the female to a fixed “stretched" time across bouts (g) still results in a ramp-and-drop profile in the dLight1.1 signal (h). This suggests that ramps are not the result of averaging of square-shaped signals of varying durations. For illustration purposes, we set this fixed stretched time to 10 seconds, the approximate median length of a sniffing bout. Normalizing the bout lengths of sniffs in a similar manner did not reveal any increase in dopamine release either during or after the sniffs (i) (n = 6 males). dLight1.1 signal is calculated as the difference between the signal at 465-nm excitation and the signal at the isosbestic excitation wavelength of 405 nm, after the 405-nm trace has been linearly scaled to fit the 465-nm trace. See also Fig. 2b.
j-n, Quantification of the magnitude of the ramp and of the drop in the dLight1.1 signal (j) shows greatly diminished dopamine ramps (k) and drops (l) after satiety. The differences between baseline and post-satiety magnitudes of the ramp and of the drop persisted even after we sub-selected all pairs of sniffs (one per condition) with matching latencies to sniff onset (i.e. with latencies that differed by <2 seconds; 81% of the sniffs were matched) (m,n). Latency to sniff onset was defined as the number of minutes since female entry (n = 8 baseline and 6 satiated males).
o, Overlay of changes in MPOA dLight1.1 signal and male speed surrounding each sniff.
p,q, Scatter plots of dLight1.1 signal and male speed (both in 1-s bins) in the 5-s window surrounding the sniffs in two example males (Pearson correlation; p: n = 80 trials; q = 50 trials).
r, Moment-to-moment dLight1.1 signal in the MPOA is more correlated with male speed than with male-female distance, which also depends on female movements (n = 6 males). Correlation coefficients are calculated as in p and q.
s-u, Same as o-q but for correlation between dLight1.1 signal and male-female distance (Pearson correlation; t: n = 80 trials; u = 50 trials).
Mean ± s.e.m. unless otherwise specified. ***p<0.001. See Supplementary Table 1 for statistics.
Extended Data Fig. 3 ∣. Anatomically and functionally separable sources of dopamine in MPOA and NAc.
a,b, VTA and SNc dopamine neurons (which can be labeled by either DAT-Cre or VMAT2-Cre following injection of a Cre-dependent reporter virus) send few or no axons to the MPOA. The right panels of a and b only show the GFP (green) channel. Scale bar: 200 μm. Data from Allen Institute15.
c, Cre-dependent expression of AAV8-EF1a-DIO-synaptophysin-mCherry in VTA of a DAT-Cre (Slc6a3-Cre) mouse shows dopamine terminals in the dorsal striatum (dSTR), the ventral striatum (vSTR, NAc), and the olfactory tubercle (OT). Some axons terminate in lateral preoptic area (LPOA). Almost no axons terminate in the MPOA. Scale bar: 200 μm.
d, In the NAc, dLight1.1 photometry shows dopamine release after sniff onsets (n = 7 males).
e-g, When males sniffed a novel, inanimate object (d), photometry recordings of dLight1.1 showed dopamine transients in the NAc (e) but not in the MPOA (f) (e: n = 7 males; f: n = 6). Mean ± s.e.m. for this figure.
Extended Data Fig. 4 ∣. Dopamine neurons in the AVPV and PVpo nuclei of the hypothalamus.
a,b, Data acquisition and analysis steps for quantifying TH and c-Fos co-localization. Brief description (see Supplementary Methods for details): high-redundancy volumes of 60-μm thick brain slices (50 volumes of 15-30 steps, 2-4 μm/step) that have been stained for TH and c-Fos were collected using a two-photon microscope. To segment dopaminergic somas, we first identified independent components of the TH volume along the z-axis through standard PCA/ICA analysis60. We then applied a morphological filter to the independent components, each usually containing a few closely located cells, to separate the individual somas. The preliminary segmentation results (see b for a sample; scale bar: 200 μm) were then manually corrected and validated before the regions-of-interest (ROIs) were applied to the c-Fos channel. An ROI was considered positive for both TH and c-Fos only if the two intensity profiles (along the z-axis) contained co-localized peaks.
c, Representative images of co-localization of TH and c-Fos in AVPV, PVpo, and ADP. Cells positive for both TH and c-Fos are highlighted with dashed circles (Scale bars: 200 μm).
d, Brain regions that did not show differences in c-fos expression in dopamine neurons (n = 8 males for ADP; 4-5 for all other brain regions; mean ± s.e.m). ADP, anterior dorsal preoptic area; PVa, anterior periventricular nucleus; PVi, intermediate periventricular nucleus; Arc, arcuate nucleus; ZI, zona incerta; SNc, substantia nigra pars compacta; VTA, ventral tegmental area.
e-i, Projections of AVPV/PVpo dopamine neurons (identified by injecting Cre-dependent axon-GCaMP6s in unilateral AVPV/PVpo of a TH-Cre male). Note that no axons were observed in the basolateral amygdala, which receives strong mesolimbic dopamine inputs. 3V, third ventricle; ac, anterior commissure; Arc, arcuate hypothalamic nucleus; BLA, basolateral amygdala nucleus; BMA, basomedial amygdala nucleus; BNST, bed nucleus of stria terminalis; BNSTa, anterior bed nucleus of the stria terminalis; CEA, central amygdala nucleus; COA, cortical amygdala area; DMH, dorsomedial nucleus of the hypothalamus; fx, column of fornix; LPOA, lateral preoptic area; LSc, caudal lateral septum; LSr, rostral lateral septum; MEAad, anterodorsal medial amygdala nucleus; MEAav, anteroventral medial amygdala; PIR, piriform area; PVH, paraventricular nucleus of the hypothalamus; PVT, paraventricular nucleus of the thalamus; sm, stria medullaris; Tu, tuberal nucleus; VMH, ventromedial nucleus of the hypothalamus. Scale bars: 200 μm.
Extended Data Fig. 5 ∣. AVPV/PVpo TH-Cre+ neurons release dopamine in the MPOA and promote mating behaviors.
a, Co-localization of TH-Cre+ cells (i.e., expressing EYFP) in the AVPV/PVpo with TH staining. Both EYFP and TH proteins were visualized with antibody staining. Scale bar: 200 μm.
b,c, Optogenetic stimulation of TH-Cre+ AVPV/PVpo axonal projections to the MPOA in awake, head-fixed males triggers robust local dopamine release that can be measured with the dopamine sensor dLight1.1 (n = 5 males). Note that the mean amplitude of the optogenetically evoked dLight1.1 transient is similar to the magnitude of transients evoked by female approach behaviors in Fig. 2b-c.
d-h, Unilateral chemogenetic inhibition of AVPV/PVpo dopamine (d) neurons largely suppressed the ramp and drop of dopamine (surrounding sniffs) in the MPOA after injecting the agonist C21 (f) but not saline (e). Quantifications are shown in panels g and h, which use the same definitions of ramping and dropping as Extended Data Fig. 2j (n = 5 males).
i,j, Bilateral chemogenetic inhibition of male AVPV/PVpo dopamine neurons did not suppress ingestive behaviors (n = 10 males).
k,l, Optogenetic stimulation of AVPV/PVpo dopamine neuron cell bodies (‘Soma’) or axons in the MPOA reinvigorates both appetitive sniffing (k: n = 7, 9, 9 males) and consummatory mounting behaviors (l: n = 7, 9, 9, mean ± 95% c.i.) in sexually sated mice.
m, Optogenetic pre-stimulation of all dopamine axons in the MPOA (in TH-Cre males) did not further increase the fraction of males that mount in the first 15 minutes after introduction of the female, as this fraction was already near ceiling in the absence of stimulation (n = 10, 8, 10, 9, 12, 12 trials from 7 males, mean ± 95% c.i.).
n-p, Optogenetic pre-stimulation of all dopamine axons in the MPOA led to a sustained increase in male appetitive sniffing behavior towards diestrus females (n: n = 6 males) and a decrease in the latency to onset of the consummatory mounting behavior (o: n = 6 males) at 10 min and 30 min after the stimulation. This stimulation did not further increase the fraction of males that mount in the first 15 minutes after introduction of the female, as this fraction was already near ceiling in the absence of stimulation (p: n = 13, 14, 14, 14, 14, 14 trials from 6 males, mean ± 95% c.i.).
q-s, Brief optogenetic pre-stimulation of dopaminergic somas in the AVPV/PVpo led to a sustained increase in male appetitive sniffing behavior (q: n = 6 males) and a decrease in the latency to onset of the consummatory mounting behavior (r: n = 6 males). This stimulation did not further increase the fraction of males that mount in the first 15 minutes after introduction of the female, as this fraction was already near ceiling in the absence of stimulation (s: n = 9, 8, 7, 8 trials from 6 males, mean ± 95% c.i.).
t, Brief optogenetic pre-stimulation of AVPV/PVpo dopamine axons in the MPOA did not further increase the fraction of males that mount in the first 15 minutes after introduction of the female, as this fraction was already near ceiling in the absence of stimulation (n = 22, 12, 18, 18, 18, 18 trials from 6 males, mean ± 95% c.i.).
u, Female priming followed by pre-stimulation of all dopamine axons in the MPOA did not further increase the fraction of males that mount in the first 15 minutes after introduction of the female, as this fraction was already near ceiling in the absence of stimulation (n = 10, 7, 7, 8 trials from 5 males, mean ± 95% c.i.). Mean ± s.e.m. unless otherwise specified. *p<0.05, **p<0.01, ***p<0.001. See Supplementary Table 1 for statistics.
Extended Data Fig. 6 ∣. Measuring cAMP production with the sensor cADDis in brain slices and in vivo.
a,b, Application of forskolin (an activator of adenylyl cyclase) to brain slices of NAc (a) and MPOA (b) induces cAMP production, as measured using the cAMP sensor cADDis. cAMP production can be measured from changes either in fluorescence intensity or in fluorescence lifetime of cADDis (a: n = 6 slices from 3 mice; b: n = 2 slices from 2 mice). Photobleaching is only observed in the intensity traces (e.g., prior to application of forskolin). The y-axes are flipped to make the plots more intuitive, as cADDis fluorescence intensity and fluorescence lifetime both decrease with increasing cAMP.
c, Experimental design for cADDis photometry in the MPOA with Chrimson stimulation of AVPV/PVpo dopamine neurons.
d, Histology of cADDis expression, dopamine axons, and fiber location in the MPOA (scale bar: 200 μm).
e, Raw photometry measurements of cADDis fluorescence intensity in the MPOA of awake, head-fixed males (during two example trials) show a persistent, downward deflection (increase in cAMP) after stimulating dopamine axons in the MPOA (red). Gray traces are mono-exponential fits of the pre-stimulation photometry data (fitted using the first 5 min of the traces), in order to estimate and account for the effects of photobleaching. A.U., arbitrary units.
f, Experimental design for GRABDA2m61 (a D2-based dopamine sensor) photometry in the MPOA, together with Chrimson stimulation of AVPV/PVpo dopamine neurons.
g, Blocking D1/D5 transmission with SCH23390 (0.6 mg/kg, i.p.) did not affect optogenetically evoked dopamine release (n = 5 males).
h-j, Repeated optogenetic stimulation of AVPV/PVpo dopamine axons in the MPOA (from data collected from the same experiments as in c-e) increased male appetitive sniffing behavior (h: n = 7 males) and decreased mounting latency (i: n = 7 males). But this stimulation did not further increase the fraction of males that mount in the first 15 minutes after introduction of the female, as this fraction was already at ceiling in the absence of stimulation (j: n = 14 trials from 7 males each, mean ± 95% c.i.).
Mean ± s.e.m. unless otherwise specified. See Supplementary Table 1 for statistics.
Extended Data Fig. 7 ∣. Persistent PKA activity in the MPOA.
a,b, Application of forskolin to brain slices containing the NAc (a) or the MPOA (b) induces PKA activity, which can be measured with the PKA sensor FLIM-AKAR. PKA activity can be measured from changes either in fluorescence intensity or in fluorescence lifetime of FLIM-AKAR (a: n = 3 slices from 2 mice; b: n = 4 slices from 2 mice). Photobleaching is only seen in the fluorescence intensity traces. The y-axes are flipped to make the plots more intuitive, as FLIM-AKAR fluorescence intensity and fluorescence lifetime decrease with increasing PKA activity.
c-e, cell body ROIs and corresponding neuropil rings for two representative cells (c,d), segmented from the intensity frames (top) and applied to the lifetime frames (bottom) during in vivo two-photon FLIM imaging via a GRIN lens inserted in the MPOA. The ROIs and corresponding rings are shaded red in left and right panels, respectively, and also displayed in insets at higher magnification. The purpose of calculating lifetime changes in both ROIs and in surrounding neuropil rings was to subtract lifetime changes in the neuropil rings from those in the ROIs, thereby isolating changes in cell body PKA activity above and beyond contributions from nearby neuropil. Samples traces of the ROI, neuropil ring, and ROI after neuropil ring subtraction are shown in e (same plotting format as in Fig. 4c; red horizontal bar: Chrimson stimulation). The cell highlighted in c (Cell 1) showed increased PKA activity following stimulation in the ROI but not in the surrounding neuropil. See Methods for detailed descriptions of ROI segmentation and neuropil ring calculation. Scalebar: 200 μm.
f-h, Average lifetime traces per mouse (f: n = 3 mice), per field-of-view (g: n = 8-9 fields of view, with fields of view from the same mouse spaced a minimum of 80 μm apart along the Z-axis), and per cell (h: n = 121, 86 cells) show persistent increases in PKA activity in the MPOA after optogenetically stimulating AVPV/PVpo dopamine axons in the MPOA. Thin lines: individual traces. Thick lines: means across traces. No change in PKA activity is seen in the no-stimulation controls. Neuropil changes were subtracted for all traces. Individual traces in f and g were obtained by averaging changes in fluorescence lifetime across all cells in each mouse (f) or in each field of view (g).
i,j, K-means clustering (i, k = 2) reveals a sub-population of 36% of the MPOA neurons that show strong responses to dopamine stimulation (j, left). The other cluster includes cells that did not respond to the stimulation and cells in the no-stimulation control experiments (j, right; n = 43, 164 cells). We used this clustering method to identify responsive cells because each field of view was only imaged once per experimental condition. Neuropil changes were subtracted for all traces.
k, Mean traces of cells analyzed in j (n = 43 responsive and 78 unresponsive cells from 3 males).
l, Across responsive cells, the magnitude of change in FLIM-AKAR lifetime with dopamine stimulation was positively correlated with baseline lifetime, indicating that cells that show the strongest stimulation-evoked increases in PKA activity also exhibited lower initial PKA activity (Pearson correlation; n = 43 cells from 3 males). Mean ± s.e.m. unless otherwise specified. *p<0.05, ***p<0.001. See Supplementary Table 1 for statistics.
Extended Data Fig. 8 ∣. Persistent cAMP elevation in the MPOA.
a, Setup and protocol for optogenetic Chrimson stimulation of dopamine axons in the MPOA in awake, head-fixed males while measuring PKA activity using two-photon fluorescence lifetime imaging (FLIM) of the cAMP sensor, cADDis, via a GRIN lens. Stimulation protocol: 10-ms pulses at 10 Hz for 5 s, repeated every 30 seconds. FLIM frames were collected 10 min before stimulation as well as 30, 60, and 120 min after stimulation.
b, Left: mean cADDis fluorescence intensity image. Brighter regions: cell bodies. Scale bar: 200 μm. Right: dopamine stimulation (red dashed line) induced a persistent decrease in cADDis lifetime (increase in cAMP concentration) that gradually returned to baseline over tens of minutes. Scale bar: 200 μm.
c,d, Average lifetime changes across fields-of-view (c: n = 8, 6 fields of view) and across mice (d: n = 4 mice) demonstrated a persistent decrease in lifetime following stimulation (segmentation and neuropil ring subtraction were performed as in FLIM-AKAR imaging, cf. Extended Data Fig. 7c, d).
e, Left: experimental design. Right: head-fixed 2p-FLIM imaging sessions prior to priming (‘A’) and after priming (‘B’) show increased baseline cAMP concentration in the MPOA following priming (n = 6 fields of view from 3 mice, each value is the average of all cells in a field of view). Mean ± s.e.m. ***p<0.001. See Supplementary Table 1 for statistics.
Extended Data Fig. 9 ∣. AVPV/PVpo dopamine neurons signal to Esr1 neurons in the MPOA through D1/D5 transmission.
a,b, Fluorescence in situ hybridization (using the RNAscope kit) demonstrates high co-localization of Drd1 and Esr1 expression in the MPOA (a), with an average of ~2 Drd1 puncta per Esr1 cell (n = 3 mice). The masks were selected based on Esr1 puncta. A cropped subregion of these images is shown in Fig. 4f. Scale bars: 100 μm.
c, In the LPOA, 39% of the Esr1 neurons also express Drd1 (average ~2 Drd1 puncta per Esr1 cell), and 68% of the Drd1 puncta are found in the Esr1 cells.
d, Sample field of view of cADDis expression in the Esr1 neurons in the MPOA (defined as the subregion of the preoptic area within 600 μm from the midline) and the LPOA (defined as the subregion beyond 600 μm from the midline). The definition of the boundary at 600 μm is derived from a standard mouse atlas62. Experimental designs are replotted here. In Experiment 1, we first used a concentric bipolar electrode to locally stimulate the AVPV/PVpo area for 20 rounds (20 Hz trains, 75 μA pulses for 1 s every 20 s) and then washed on the D1/D5 agonist SKF81297 (2 μM; diluted from 100 mM stock solution in dimethyl sulfoxide). In Experiment 2, we first used a minimal stimulation protocol to identify the MPOA Esr1 neurons that responded to AVPV/PVpo stimulations, washed on the D1/D5 antagonist SCH23390 (300 nM; diluted from 50 mM stock solution in saline), and then performed 20 more rounds of AVPV/PVpo stimulation in the presence of the antagonist.
e, Heatmaps showing the cell-by-cell cADDis response in the MPOA to both AVPV/PVpo stimulation (left) and D1/D5 agonist wash-on (right). Stimulation responses are averages of 20 trials, following baseline subtraction of the pre-stimulation means, while the agonist response is a single-trial response following baseline subtraction of the pre-agonist value. Esr1 neurons are divided into those that responded to the agonist (above the horizontal blue line) and those that did not (below the horizontal blue line) using a classifier (see Methods). Cells are sorted based on their agonist response and the same order is used for both panels. Gray lines on the top denote the windows that are used for quantification and classification. The color of the ‘Slide ID’ indicates the identity of the slice (n = 983 neurons from 4 slices, 3 mice) from which the cell was recorded.
f,g, In the agonist-responsive group (orange), neurons show persistent cAMP elevations (decreases in cADDis intensity) that accumulate after each AVPV/PVpo stimulation (f). The cAMP elevations are not seen in the agonist-unresponsive group (gray in f; n = 4 slices from 3 males). Panel g summarizes agonist responses (n = 4 slices from 3 males).
h, Heatmaps showing the cell-by-cell cADDis response of MPOA Esr1 neurons to AVPV/PVpo stimulation both before (left) and after washing on the D1/D5 antagonist (right). Stimulation responses are averages of 3 and 20 trials, following baseline subtraction of the pre-stimulation means. MPOA Esr1 neurons are divided into those that responded to the pre-antagonist stimulation (above the horizontal blue line) and those that did not (below the horizontal blue line) using a classifier (see Methods). Cells are sorted based on their stimulation response, and the same order is used for both panels. Gray lines on the top denote the windows that are used for quantification and classification. The color of the ‘Slide ID’ indicates the identity of the slice (n = 947 cells from 4 slices, 3 mice) from which the cell was recorded.
i,j, In the stimulation-responsive group (red), neurons show persistent cAMP elevations that are accumulated after each AVPV/PVpo stimulation (i: n = 4 slices from 3 males). The cAMP elevations are not seen after the antagonist wash-on (j: n = 4 slices from 3 males).
k, Same as e but for LPOA Esr1 neurons (n = 192 cells from 4 slices, 3 mice).
l,m, Same as f-g but for LPOA neurons. Note that LPOA Esr1 neurons (including those in the agonist-responsive group) did not respond to AVPV/PVpo stimulation (n = 4 slices from 3 males).
n,o, Same as l-m but for fluorescence lifetime measurements (a bleaching-insensitive way of analyzing fluorescence data; see Methods). Note that LPOA Esr1 neurons did not respond to AVPV/PVpo stimulation, including those in the agonist-responsive group (n = 4 slices from 3 males).
p-r, In Experiment 2, we again observed only very few AVPV/PVpo stimulation-responsive neurons in the LPOA, before or after antagonist application (n = 198 cells from 4 slices, 3 mice). Plotting conventions are the same as above. Note that we did not plot average responses of the stimulation-responsive group due to its small size.
s,t, In combined data from Experiments 1 and 2, the fraction of cells that responded to AVPV/PVpo stimulation gradually dropped off over distance from the midline (t; n = 2320 cells from 8 slices, 6 mice). Axon fields of AVPV/PVpo dopamine neurons shown here for comparison (s). Scale bars: 200 μm.
u, In the agonist-responsive group (orange), MPOA Esr1 neurons show persistent elevations in PKA activity (decreases in FLIM-AKAR lifetime) that accumulate across repeated trials of AVPV/PVpo stimulation. The cAMP elevations are not seen in the agonist-unresponsive group (gray; n = 4 slices from 3 males). The right panel summarizes agonist responses (n = 4 slices from 3 males).
v, In the stimulation-responsive group (red), MPOA Esr1 neurons show persistent elevations in PKA activity that accumulate after each AVPV/PVpo stimulation (left: n = 4 slices from 3 males). The elevations in PKA activity are not seen after antagonist wash-on (right: n = 4 slices from 3 males).
w, Same as u but for LPOA Esr1 neurons. Note that LPOA Esr1 neurons did not respond to AVPV/PVpo stimulation, including those in the agonist-responsive group (n = 4 slices from 3 males).
x, In Experiment 2, we again only recorded very few numbers of AVPV/PVpo stimulation-responsive Esr1 neurons in the LPOA, before or after antagonist. See above for plotting conventions. Note that we did not plot average responses of the stimulation-responsive group due to its small size.
Mean ± s.e.m. *p<0.05, **p<0.01, ***p<0.001. See Supplementary Table 1 for statistics.
Extended Data Fig. 10 ∣. Novel optogenetic and molecular tools to manipulate intracellular cAMP in vivo.
a, Peptide sequences of bPAC63 and an improved variant, biPAC.
b, cAMP production by bPAC and biPAC in Xenopus laevis oocytes. Measurement was performed 3 days after injection of 30 ng cRNA of Venus-bPAC or Venus-biPAC in the dark (‘Dark’, non-shaded bars) or after 1 min illumination with 473 nm, 0.3 mW/mm2 light (‘Light’, shaded bars). The control bar shows uninjected oocytes (n = 3 groups of 5 oocytes). For comparison, the mean value of the uninjected oocytes is also noted with a horizontal dashed line. The Y-axis is on the log scale. Note that, in contrast to bPAC, biPAC does not exhibit increased enzymatic activity in darkness, above levels observed in baseline control conditions.
c, Enzymatic activity of Venus-biPAC in dark and light conditions (n = 4, mean ± s.d.).
d, Light intensity-dependent cAMP production of Venus-biPAC at 473 nm. After fitting with the Michaelis-Menten function, the Km value was determined to be 32 μW/mm2 (n = 4, mean ± s.d.). Venus tag was used here for protein-quantification purpose (same below).
e, cAMP concentrations at different time points in the dark after 500 ms of light stimulation. Fitting a mono-exponential function yielded an off time constant τoff = 8.9 ± 2.2 s (n = 4, mean ± s.d.).
f,g, Brief optogenetic stimulation of biPAC expressed in Esr1-Cre cells in the MPOA did not further increase the fraction of males that mount in the first 15 minutes after subsequent introduction of the female, as this fraction was already near ceiling in the absence of stimulation (ns, non-significant; Fisher’s exact test, n = 35, 36 trials from 12 males, mean ± 95% c.i.).
h-j, In mice that did not express biPAC, the same stimulation protocol failed to increase appetitive sniffing (h: n = 6 males), failed to decrease the latency to consummatory mounting behaviors (i: n = 6 males), and failed to increase the fraction of males that mounted (j: n = 18 trial from 6 males, mean ± 95% c.i.).
k,l, Design (k) and sequence (l) of PDE4D3-Cat. Upstream conserved regions (UCRs) are inhibitory, regulatory domains in the endogenous hPDE4D364. Replacement of these UCRs with mKate2 substantially increased the enzymatic activity of PDE4D3-Cat over hPDE4D3.
m, Enzymatic activity of Venus-PDE4D3-Cat (n = 3, mean ± s.d.). After fitting with the Michaelis-Menten function, the Km value was determined to be 3.1 ± 0.4 μM and the Vmax value was 35.3 ± 2.9 s−1. This enzyme is much faster than the endogenous protein (Vmax = 5-9 s−1)64.
***p<0.001. See Supplementary Table 1 for statistics.
Supplementary Material
Acknowledgements
We thank M. Baum, B. Lowell, M. Crickmore, D. Rogulja, Y. Livneh, Y. Chen, O. Yizhar, V. Ruta, R. Froemke, L. Stowers, D. Lin, M. Frank, O. Amsalem, D. Tingley, and members of the Andermann lab for useful feedback. H. Lauterwasser, C. McHugh, D. Fleharty, H. Kaul, and K. Fernando assisted with animal care, behavioral experiments, and histology. J. Madara and B. Lowell assisted with brain slice imaging and A. Verstegen shared Esr1-Cre mice. B. Lowell and G. Yellen provided the hybrid PMT and TCSPC board, respectively. S. Thornquist informed us of the lifetime properties of cADDis. S. Gupta, M. Lehtinen, Boston Children’s Hospital Viral Core, BIDMC Histology Core, and HMS Research Instrumentation Core provided technical services. Authors were supported by a Lefler Fellowship (S.X.Z.), an NIH F32 DK112589 and a Davis Family Foundation Award (A.L.), a Bertarelli Fellowship (H.F.), NIH R01 DK109930, DP1 AT010971, DP1 AT010971-02S1, the McKnight Foundation, the Klarman Family Foundation, and the Harvard Brain Science Initiative Bipolar Disorder Seed Grant, supported by Kent and Liz Dauten (M.L.A), and by the Deutsche Forschungsgemeinschaft Projektnummer 374031971 TRR 240/A04 and Projektnummer 417451587 (G.N.).
Footnotes
Competing Interests Statement
The authors declare no competing interests.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this paper.
Code availability
The custom analysis codes and designs in this paper are publicly available on Github (https://github.com/xzhang03/Code-for-Zhang-et-al-2021).
Data availability
Plasmids and sequences related to biPAC and PDE4D3-Cat have been deposited to Addgene. The data that support the findings in this study are available from the corresponding author upon request.
References
- 1.Chen Y et al. Sustained NPY signaling enables AgRP neurons to drive feeding. Elife 8, e46348 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lahiri AK & Bevan MD Dopaminergic Transmission Rapidly and Persistently Enhances Excitability of D1 Receptor-Expressing Striatal Projection Neurons. Neuron 106, 277–290 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohn R, Morantte I & Ruta V Coordinated and Compartmentalized Neuromodulation Shapes Sensory Processing in Drosophila. Cell 163, 1742–1755 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marlin BJ, Mitre M, James a D., Chao MV & Froemke RC Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature (2015) doi: 10.1038/nature14402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGill TE Sexual behavior of the mouse after long-term and short-term postejaculatory recovery periods. J. Genet. Psychol 103, 53–57 (1963). [DOI] [PubMed] [Google Scholar]
- 6.Bowers MB, Van Woert M & Davis L Sexual behavior during L-dopa treatment for Parkinsonism. Am. J. Psychiatry 127, 1691–1693 (1971). [DOI] [PubMed] [Google Scholar]
- 7.Bitran D & Hull EM Pharmacological analysis of male rat sexual behavior. Neurosci. Biobehav. Rev 11, 365–389 (1987). [DOI] [PubMed] [Google Scholar]
- 8.Hull EM et al. Dopaminergic Control of Male Sex Behavior in Rats : Effects of an Intracerebrally-Infused Agonist. Brain Res. 370, 73–81 (1986). [DOI] [PubMed] [Google Scholar]
- 9.McHenry JA, Bell GA, Parrish BP & Hull EM Dopamine D1 receptor signaling in the medial preoptic area facilitates experience-induced enhancement of mating behavior in male rats. Behav. Neurosci 126, 523–529 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang SX, Rogulja D & Crickmore MA Dopaminergic Circuitry Underlying Mating Drive. Neuron 91, 168–181 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Beach FA & Jordan L Sexual exhaustion and recovery in the male rat. Q. J. Exp. Psychol 8, 121–133 (1956). [Google Scholar]
- 12.McGill TE & Blight WC Effects of genotype on the recovery of sex drive in the male mouse. J. Comp. Physiol. Psychol 56, 887–888 (1963). [DOI] [PubMed] [Google Scholar]
- 13.Patriarchi T et al. Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science 360, eaat4422 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howe MW, Tierney PL, Sandberg SG, Phillips PEM & Graybiel AM Prolonged dopamine signalling in striatum signals proximity and value of distant rewards. Nature 500, 575–9 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeung MY et al. A mesoscale connectome of the mouse brain. Nature 15, 942–953 (2016). [Google Scholar]
- 16.Gunaydin LA et al. Natural Neural Projection Dynamics Underlying Social Behavior. Cell 157, 1535–1551 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menegas W, Babayan BM, Uchida N & Watabe-Uchida M Opposite initialization to novel cues in dopamine signaling in ventral and posterior striatum in mice. Elife 6, e21886 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheng M & Greenberg ME The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron 4, 477–485 (1990). [DOI] [PubMed] [Google Scholar]
- 19.Klapoetke NC et al. Independent optical excitation of distinct neural populations. Nat. Methods 11, 338–346 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei D, Talwar V & Lin D Neural circuits of social behaviors: innate yet flexible. Neuron 109, 1600–1620 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu X et al. Modular genetic control of sexually dimorphic behaviors. Cell 148, 596–607 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armbruster BN, Li X, Pausch MH, Herlitze S & Roth BL Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl. Acad. Sci. U. S. A 104, 5163–5168 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson KJ et al. DREADD Agonist 21 Is an Effective Agonist for Muscarinic-Based DREADDs in Vitro and in Vivo. ACS Pharmacol. Transl. Sci 72, 61–72 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beaulieu J-M & Gainetdinov RR The Physiology, Signaling, and Pharmacology of Dopamine Receptors. Pharmacol. Rev 63, 182–217 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Tewson PH, Martinka S, Shaner NC, Hughes TE & Quinn AM New DAG and cAMP Sensors Optimized for Live-Cell Assays in Automated Laboratories. J. Biomol. Screen 21, 298–305 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Saulnier JL, Yellen G & Sabatini BL A PKA activity sensor for quantitative analysis of endogenous GPCR signaling via 2-photon FRET-FLIM imaging. Front. Pharmacol 5, 56 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McHenry JA et al. Hormonal gain control of a medial preoptic area social reward circuit. Nat. Neurosci 20, 449–458 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei Y-C et al. Medial preoptic area in mice is capable of mediating sexually dimorphic behaviors regardless of gender. Nat. Commun 9, 279 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karigo T et al. Distinct hypothalamic control of same- and opposite-sex mounting behaviour in mice. Nature 589, 258–263 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goto A et al. Circuit-dependent striatal PKA and ERK signaling underlies rapid behavioral shift in mating reaction of male mice. Proc. Natl. Acad. Sci. U. S. A 112, 6718–6723 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee SJ et al. Cell-type-specific asynchronous modulation of PKA by dopamine in learning. Nature 590, 451–456 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yagishita S et al. A critical time window for dopamine actions on the structural plasticity of dendritic spines. Science 345, 1616–1620 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sar M Estradiol is Concentrated in Tyrosine Hydroxylase-Containing Neurons of the Hypothalamus. Science 223, 938–940 (1984). [DOI] [PubMed] [Google Scholar]
- 34.Simerly RB, Zee MC, Pendleton JW, Lubahn DB & Korach KS Estrogen receptor-dependent sexual differentiation of dopaminergic neurons in the preoptic region of the mouse. Neurobiology 94, 14077–14082 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scott N, Prigge M, Yizhar O & Kimchi T A sexually dimorphic hypothalamic circuit controls maternal care and oxytocin secretion. Nature 525, 519–22 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Montejo AL, Montejo L & Navarro-Cremades F Sexual side-effects of antidepressant and antipsychotic drugs. Curr. Opin. Psychiatry 28, 418–423 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Bala A, Nguyen HMT & Hellstrom WJG Post-SSRI Sexual Dysfunction: A Literature Review. Sex. Med. Rev 6, 29–34 (2018). [DOI] [PubMed] [Google Scholar]
- 38.Moffitt JR et al. Molecular, spatial, and functional single-cell profiling of the hypothalamic preoptic region. Science 362, eaau5324 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Methods References
- 39.Lin D et al. Functional identification of an aggression locus in the mouse hypothalamus. Nature 470, 221–226 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krashes MJ et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest 121, 1424–28 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lutas A et al. State-specific gating of salient cues by midbrain dopaminergic input to basal amygdala. Nat. Neurosci 22, 1820–1833 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bayless DW et al. Limbic Neurons Shape Sex Recognition and Social Behavior in Sexually Naive Males. Cell 176, 1190–1205 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inoue S et al. Periodic Remodeling in a Neural Circuit Governs Timing of Female Sexual Behavior. Cell 179, 1393–1408.e16 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen J et al. Flexible scaling and persistence of social vocal communication. Nature (2021) doi: 10.1038/s41586-021-03403-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lein ES et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176 (2007). [DOI] [PubMed] [Google Scholar]
- 46.Remedios R et al. Social behaviour shapes hypothalamic neural ensemble representations of conspecific sex. Nature 550, 388–392 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swaney WT, Dubose BN, Curley JP & Champagne FA Sexual experience affects reproductive behavior and preoptic androgen receptors in male mice. Horm. Behav 61, 472–478 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y et al. Neuronal Representation of Social Information in the Medial Amygdala of Awake Behaving Mice. Cell 1176–1190 (2017) doi: 10.1016/j.cell.2017.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valente S, Marques T & Lima SQ No evidence for prolactin’s involvement in the post-ejaculatory refractory period. Commun. Biol 4, 10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boyden ES, Zhang F, Bamberg E, Nagel G & Deisseroth K Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci 8, 1263–8 (2005). [DOI] [PubMed] [Google Scholar]
- 51.Tan CL & Knight ZA Regulation of Body Temperature by the Nervous System. Neuron 98, 31–48 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burgess CR et al. Hunger-Dependent Enhancement of Food Cue Responses in Mouse Postrhinal Cortex and Lateral Amygdala. Neuron 91, 1154–1169 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garfield AS et al. Dynamic GABAergic afferent modulation of AgRP neurons. Nat. Neurosci 19, 1628–1635 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diaz-Carcia CM et al. Quantitative in vivo imaging of neuronal glucose concentrations with a genetically encoded fluorescence lifetime sensor. J. Neurosci. Res 97, 946–960 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ball GF & Balthazart J How useful is the appetitive and consummatory distinction for our understanding of the neuroendocrine control of sexual behavior? Horm. Behav 53, 307–318 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Petreanu L et al. Activity in motor-sensory projections reveals distributed coding in somatosensation. Nature 489, 299–303 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mathis A et al. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci 21, 1281–1289 (2018). [DOI] [PubMed] [Google Scholar]
- 58.Liang L et al. A Fine-Scale Functional Logic to Convergence from Retina to Thalamus. Cell 173, 1343–1355.e24 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bonin V, Histed MH, Yurgenson S & Clay Reid R Local diversity and fine-scale organization of receptive fields in mouse visual cortex. J. Neurosci 31, 18506–18521 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mukamel EA, Nimmerjahn A & Schnitzer MJ Automated analysis of cellular signals from large-scale calcium imaging data. Neuron 63, 747–60 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun F et al. Next-generation GRAB sensors for monitoring dopaminergic activity in vivo. Nat. Methods 17, 1156–1166 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Franklin KBJ & Paxinos G The mouse brain in stereotaxic coordinates. (2007). [Google Scholar]
- 63.Stierl M et al. Light Modulation of Cellular cAMP by a Small Bacterial Photoactivated Adenylyl Cyclase, bPAC, of the Soil Bacterium Beggiatoa. J. Biol. Chem 286, 1181–1188 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lim J, Pahlke G & Conti M Activation of the cAMP-specific phosphodiesterase PDE4D3 by phosphorylation: Identification and function of an inhibitory domain. J. Biol. Chem 274, 19677–19685 (1999). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Plasmids and sequences related to biPAC and PDE4D3-Cat have been deposited to Addgene. The data that support the findings in this study are available from the corresponding author upon request.