Abstract
Glycated hemoglobin A1c (HbA1c) is considered the standard of care for the testing and monitoring of diabetes. Its ability to accurately reflect glycemia, however, is imperfect. Hemoglobin variants—mutant forms of hemoglobin caused by genetic variation present in 7% of the population—are known to adversely affect the ability of HbA1c measurement to reflect glycemic control. We report an illustrative case of a 64-year-old nondiabetic man with a steadily decreasing HbA1c and no symptoms of hypoglycemia or concerning family history. Preliminary investigative workup returned nothing of significance. Genetic sequencing, however, identified a rare benign hemoglobin variant: a heterozygous missense mutation in the gene encoding the hemoglobin β chain (c.155C > A, p.Pro51His). This variant has been reported only once previously, and the report predates genetic sequence data of the variant. Although this variant had no clinical implications for the patient, it was the cause of falsely low HbA1c levels on high-performance ion-exchange chromatography. This case highlights the importance of considering the effect of hemoglobin variants on the measurement of HbA1c. When available, family history should be carefully considered. Clinicians should suspect hemoglobin variants when HbA1c is too high or low, or discordant with the clinical picture.
Keywords: hemoglobin variant, hemoglobin A1c
Glycated hemoglobin A1c (HbA1c) is currently considered the standard of care for the testing and monitoring of diabetes and its complications [1]. HbA1c is a glycated form of normal adult hemoglobin (HbA), in which a glucose is attached to the N-terminal valine of each β chain in the hemoglobin tetramer [2, 3]. Owing to the lifespan of erythrocytes, HbA1c correlates with blood glucose over the preceding 120 days. Levels of HbA1c can be measured by many methods, and the choice of method is often center specific. Methodologies include enzyme assays and immunoassays, affinity chromatography, capillary electrophoresis, high-performance liquid chromatography, and high-performance ion-exchange chromatography (HPIEC). Regardless of methodology, measurement of HbA1c as a marker of diabetes control has distinct advantages: It is known to capture chronic hyperglycemia better than other measures, and is better associated with complications [4].
The measurement of HbA1c, however, is imperfect. Because HbA1c is based on the turnover of erythrocytes and their glycation, its accuracy can be adversely affected by conditions or states that affect these processes. Thus, comorbidities are well known to reduce the utility of using HbA1c to diagnose and monitor diabetes [4]. Iron-deficiency anemia, asplenia, and chronic alcohol consumption cause increased levels of HbA1c, whereas blood loss, transfusions, and pregnancy can cause falsely decreased levels of HbA1c [4].
Hemoglobin variants can also impede the accurate measurement of HbA1c [4-6]. HbA is composed of 4 subunits, 2 α chains, encoded by genes on chromosome 16 (HBA1 and HBA2), and 2 β chains, encoded by a gene on chromosome 11 (HBB). Genetic variants can occur in any of these genes. Most variant forms of hemoglobin, however, are the result of a missense variant leading to a single amino acid substitution in the β chain [2, 3]. Seven percent of the population has a variant form of HbA [2, 3]. The clinical presentation of these variants is heterogeneous: Variant carriers have presentations ranging from hemolytic anemia and reticulocytosis to erythrocytosis [3]. Many variants, however, are clinically silent, with carriers being asymptomatic [3]. Even clinically silent hemoglobin variants, however, can affect measurement of HbA1c, with some methodologies more affected than others [4]. Depending on the method and the hemoglobin variant, HbA1c results can be falsely low or high, potentially impeding the accurate monitoring of diabetes control, or even leading to inappropriate diagnoses or the unnecessary treatment of unaffected patients.
To illustrate the effects of measuring HbA1c in the setting of hemoglobin variants, we present a case of a clinically well individual with a measured low HbA1c due to a rare variant of hemoglobin, as well as the confirmed genetic etiology.
Case Report
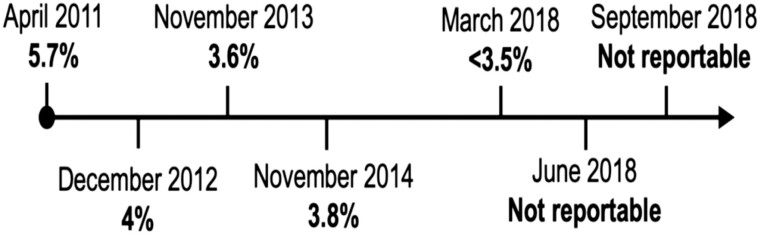
A 64-year-old man with a body mass index of 25.1 was referred to Endocrinology with low HbA1c, as measured by HPIEC, that had been decreasing over the preceding 7 years (Fig. 1). Aside from occasional dizziness, he had no classic symptoms of hypoglycemia—fasting, overnight, nor postprandial. He ate regular meals, with no eating overnight or unusual dietary patterns. He had essentially stable weight, no history of liver disease, blood dyscrasias, congenital syndromes, nor a history of bleeding or transfusions. His past medical history was significant only for well-controlled psoriasis and immunoglobulin A nephropathy with proteinuria. He took ramipril 2.5 mg daily and used a topical steroid cream as needed. Early in his presentation he reported no remarkable family history. He worked in the legal field, drank 2 to 3 beers per day, and had 4 grown healthy children. With the exception of being overweight as per his previously recorded body mass index, his exam was normal.
Figure 1.
A1c timeline from 2011 to 2018. A1c deemed “not reportable” by the laboratory in June and September 2018 because of an identified abnormal hemoglobin peak affecting the appropriate measurement of A1c.
Initial investigations and imaging were largely unremarkable (Table 1). In contrast to the low HbA1c, random and fasting glucose were both normal (5.1 mmol/L). At follow-up visits the patient’s random glucose measurements remained between 5.1 and 5.2 mmol/L. The patient had an appropriately normal fasting glucose, insulin, and C-peptide level (703 pmol/L).
Table 1.
Relevant investigations and results. Investigations were undertaken June 2018 unless otherwise specified
| Investigation | Result |
|---|---|
| Hemoglobin A1c | Not reportable |
| Glucose | 5.1 mmol/L, random |
| 5.1 mmol/L, fasting | |
| Insulin | 48 pmol/L (< 120 pmol/L) |
| C-peptide | 703 pmol/L (260-1730 pmol/L) |
| CBC | HgB 148 g/L |
| Hct 0.441 | |
| RDW | |
| MCV normal | |
| WBC 5.39 × 109/L | |
| Plt 226 × 109/L | |
| Peripheral blood smear | Normal |
| Hemoglobin electrophoresis | No hemoglobin variant detected, no thalassemia |
| TSH | 4.84 mIU/L (0.35-4.3 mIU/L) |
| 8 am serum cortisol | 434 nmol/L |
| Fructosamine (calculated HbA1c) | 196 μmol/L (205-285 μmol/L), September 2018 |
| [A1c (%) = 0.017 × fructosamine + 1.61] (calculated HbA1c = 4.9%; measured HbA1c not reportable, per laboratory) | |
| AST, ALT, ALP, GGT | Normal |
| Urine protein | 0.3 g/L, March 2021 |
| Bilirubin, albumin, INR | Normal |
| Creatinine (GFR) | 91 μmol/L (77 mL/min/1.73 m2) |
| Abdominal ultrasound | Normal liver, normal pancreas, normal kidneys normal spleen (10 cm), December 2018 |
Abbreviations: ALT, alanine transaminase; ALP, alkaline phosphatase; AST, aspartate transaminase; CBC, complete blood count; GFR, glomerular filtration rate; GGT, γ-glutamyltransferase; HbA1c, glycated hemoglobin A1c; Hct, hematocrit; HgB, hemoglobin; INR, international normalized ratio; MCV, mean corpuscular volume; Plt, platelets; RDW, red blood cell distribution width; TSH, thyrotropin; WBC, white blood cell count.
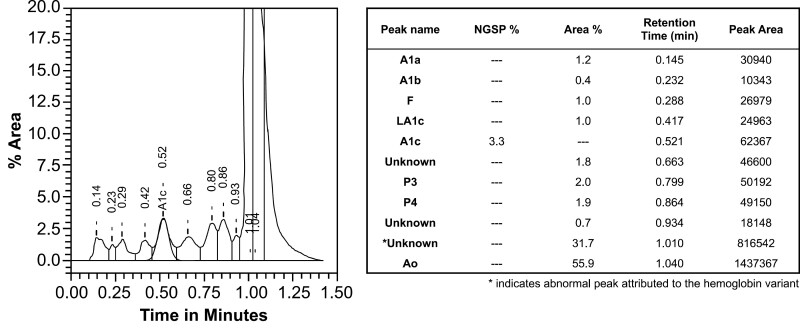
At the time of his visit, HbA1c was deemed not reportable. The HbA1c assays, including those measurements previously taken in the community, were performed locally on the Variant II Turbo (Bio-Rad Laboratories). This is based on an HPIEC that has no interferences from hemoglobin S, C, D, or E variants. The presence of β-thalassemia trait, fetal hemoglobin (up to 25%), and carbamylated hemoglobin and labile A1c also do not affect the accurate reporting of HbA1c. When calculated using a fructosamine assay, the HbA1c was 4.9% (fructosamine = 196 μmol/L; A1c (%) = 0.017 × fructosamine + 1.61 as per Cohen et al 2003 [7]), which remains discordant with HbA1c measured by HPIEC, despite a possible lower glycosylation of fructosamine secondary to our patient’s ongoing proteinuria. Thus, hemoglobin electrophoresis was undertaken to investigate explanatory hemoglobin variants. Initial hemoglobin electrophoresis was normal: HbA 97.1% and HbA2 2.9%. Ultimately, on repeat analysis of HbA1c, an abnormal hemoglobin peak on HPIEC was noted by the laboratory during manual review (Fig. 2).
Figure 2.
High-performance ion exchange chromatography results for the determination of hemoglobin A1c. This graph was generated by the analyzer and is shown as is, with the table transcribed verbatim. % Area is represented by the y-axis, Time in minutes by the x-axis, and the abnormal peak (subsequently identified as the Hemoglobin North Manchester peak) is identified by the asterisk.
In light of the abnormal heterozygotic peak on his HbA1c assay and normal hematologic workup including electrophoresis, genetic sequencing was undertaken. Sequencing identified a heterozygotic variant in HBB, the gene encoding the hemoglobin β chain. The single-nucleotide variant (NM_000518.4[HBB]:c.155C > A, NC_ 000011.10:g.5226737G > T) is a missense variant at coding position 155 (cysteine to adenosine), resulting in a single amino acid substitution, from a proline to a histidine residue. This substitution results in a β chain with increased mass, creating an abnormal additional peak (and smaller normal β-chain peak) when analyzed by mass spectrometry [8]. It has been reported once previously as the Hb North Manchester Mutation [8]; however, this was by amino acid sequence only. In our patient, the laboratory reported the hemoglobin variant resulting from the detected nucleotide variant as benign. Carriers of benign variants are expected to have a normal phenotype, as the variant is not predicted to have any clinical effects. Of note, however, the variant is exceedingly rare: The genome aggregation database (https://gnomad.broadinstitute.org) reports only one instance of the variant, giving an allele frequency of 7 × 10–6. Among other pieces of evidence, allele frequency is used to determine the pathogenicity of genetic variants, with higher-frequency variants being less likely to cause penetrant disease [9].
After receiving the genetic diagnosis, our patient continued to be asymptomatic, including normal point-of-care glucose monitoring. He was counseled on alternative methods of screening for diabetes including fasting glucose levels and oral glucose tolerance tests, which are not affected by the genetic variant. It was further determined that he had a sibling with a similar abnormal hemoglobin peak on a recent HbA1c, although further genetic testing could not be pursued in this individual.
Discussion
This case is unique because it is one of the first to demonstrate the nucleotide variant underlying the Hb North Manchester in a patient. Furthermore, it is the first report of the variant in North America, as well as the first in a patient without diabetes: To date, only the original report of the Hb North Manchester variant in 2 related patients in the United Kingdom and a 42-year-old Chinese man with the variant have been published [8, 10]. Both of these previously reported cases involved individuals with diabetes with discordant HbA1c and glucose measurements affected by the heterozygotic presence of the variant. Of note, a different variant at the same site, where an arginine replaces the proline, has been described as Hemoglobin Willamette [8]. Although most patients described with the Hemoglobin Willamette variant are asymptomatic, Barbosa et al report [11] on a patient who presented with a symptomatic refractory microcytic anemia. Their patient’s laboratory tests showed a hemoglobin of 84 g/L, hematocrit of 23.4%, mean corpuscular volume of 75.0 fL, reticulocytes count of 4.8%, normal iron studies, and a peripheral smear featuring anisochromia, microcytosis, and polychromasia. Importantly, to date, no homozygous carriers of the Hb North Manchester variant have been reported. Taken together with the possibility of a clinical phenotype in a heterozygous carrier of a variant at this site, it is certainly possible that this sequence is conserved for functional reasons, and that a homozygous variant at this site could be pathogenic.
Hemoglobinopathies can affect HbA1c values in 3 ways: by influencing the binding of glucose to hemoglobin, by decreasing the lifespan of red blood cells, or by affecting chromatography peak measurements [12]. In the case of chromatography, coelution of the hemoglobin variant with HbA1c will cause gross overestimation of HbA1c, while coelution of the hemoglobin variant with HbA, with resolution of the glycated hemoglobin variant from HbA1c, will underestimate the HbA1c results, as seen in this case. Wiener et al [8] demonstrated that the Hb North Manchester amino acid substitution was present in approximately half the total hemoglobin β chains. In turn, HbA1c measured by HPIEC was half that of the value as measured by affinity chromatography. In our patient, the cause of the gradual decrease in HbA1c over time, however, remains unknown. It could be attributed to factors known to lower HbA1c, [4] but it could be due to mosaicism that led to greater proportions of total hemoglobin represented by mutated chains over time. This could be further tested through molecular hemoglobin analysis of the patient’s sibling with reported low HbA1c. Unfortunately, the sibling did not consent to further investigations or genetic confirmation of the variant.
This case is important in that it demonstrates that variant hemoglobins, even forms that do not cause clinical disease, can cause erroneous measures of HbA1c. In this case, the patient was a carrier of a benign variant hemoglobin, Hb North Manchester, that led to falsely low HbA1c levels when measured by HPIEC. This highlights an important flaw in using HbA1c as a marker for diabetes, and should prompt clinicians to consider hemoglobin variants when HbA1c is outside the normal range and not in keeping with the clinical picture. Furthermore, it is plausible that a high HbA1c level could be masked by such a hemoglobin variant, depending on the method of measurement, leading to a false assessment of well-controlled diabetes and a missed opportunity for subsequent management.
This case also demonstrates that just because a variant is “benign”—whether that be a genetic variant or a hemoglobin variant—does not mean it will have no clinical effect. In this case, although the hemoglobin variant was not disease-causing, it did cause erroneous HbA1c levels. In turn, this led to many subsequent investigations—which, in itself, is not entirely benign.
Conclusions
This case highlights the pitfalls of measuring HbA1c, which can be erroneous in the setting of a variant hemoglobin. Even if benign, these variants can have a significant clinical effect that warrants careful consideration. Where HbA1c is used, clinicians should be mindful of the effect of hemoglobin variants on the accuracy of the measure. Variants should be suspected when HbA1c is higher than expected relative to ambient glycemia, below the nondiabetes reference range, or discordant with the clinical picture [13]. Furthermore, chromatography traces should be examined closely for anomalies if a hemoglobin variant is being considered [6]. Family history should be carefully examined because of the potential for hemoglobin variants to cause falsely low or even falsely normal HbA1c readings. In light of these pitfalls, we caution against the exclusive use of HbA1c for screening and monitoring of diabetes. Where indicated, fasting glucose or oral glucose tolerance tests can be used as alternative screening methods for diabetes and assessment of its control.
Acknowledgments
The authors would like to thank Dr Shirl Gee for her support with this case, and the patient for providing his permission to prepare this case report.
Glossary
Abbreviations
- HbA
adult hemoglobin
- HbA1c
glycated hemoglobin A1c
- HPIEC
high-performance ion-exchange chromatography
Disclosures
The authors have nothing to disclose.
Data Availability
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.
References
- 1. World Health Organization (WHO). Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus Abbreviated Report of a WHO Consultation. WHO; 2011. [PubMed] [Google Scholar]
- 2. Welsh KJ, Kirkman MS, Sacks DB. Role of glycated proteins in the diagnosis and management of diabetes: research gaps and future directions. Diabetes Care. 2016;39(8):1299-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thom CS, Dickson CF, Gell DA, Weiss MJ. Hemoglobin variants: biochemical properties and clinical correlates. Cold Spring Harb Perspect Med. 2013;3(3):a011858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Radin MS. Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med. 2014;29(2):388-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bonora E, Tuomilehto J. The pros and cons of diagnosing diabetes with A1C. Diabetes Care. 2011;34(Suppl 2):S184-S190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Little RR, Roberts WL. A review of variant hemoglobins interfering with hemoglobin A1c measurement. J Diabetes Sci Technol. 2009;3(3):446-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cohen RM, Holmes YR, Chenier TC, Joiner CH. Discordance between HbA1c and fructosamine: evidence for a glycosylation gap and its relation to diabetic nephropathy. Diabetes Care. 2003;26(1):163-167. [DOI] [PubMed] [Google Scholar]
- 8. Wiener K, Roberts NB, Green BN. The effect of an unusual haemoglobin variant (beta 51Pro –> His) on haemoglobin A1c measurement. Ann Clin Biochem. 1998;35(Pt 2):321-323. [DOI] [PubMed] [Google Scholar]
- 9. Richards S, Aziz N, Bale S, et al. ACMG Laboratory Quality Assurance Committee . Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5): 405-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yuan Y, Zhou X, Gao L, Ren Q, Linong J. Silent hemoglobin variant during capillary electrophoresis: a case report. J Diabetes Invest. 2020;11(4):1014-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barbosa OA, de Sousa Dias MM, Távora SM, de Galiza Neto GC, de Souza JH, da Silva HF. Hemoglobin Willamette (β51Pro → Arg): case report and literature review. Hematol Rep. 2017;9(1):6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lorenzo-Medina M, De-La-Iglesia S, Ropero P, Nogueira-Salgueiro P, Santana-Benitez J. Effects of hemoglobin variants on hemoglobin A1c values measured using a high-performance liquid chromatography method. J Diabetes Sci Technol. 2014;8(6): 1168-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schnedl WJ, Lahousen T, Lang T, et al. Determination of glycated hemoglobin in clinically silent hemoglobin variants. Diabetes Metab Res Rev. 2004;20(6):460-465. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.