Introduction
Endometriosis is a common gynecologic condition that may be visualized on 18F-FDG PET/CT and mimic lesions of malignancy. We analyzed the interference of known or suspected endometriosis in reporting 18F-FDG PET/CT performed in another indication.
Results
The PET/CT images of 18 women with known (n = 15) or suspected (n = 3) endometriosis were analyzed. Based on clinical follow-up and results of other imaging, biopsy, and/or postsurgical histology, the presence of lesions of endometriosis at the time of 18F-FDG PET/CT was confirmed in 13 of 18 patients (72%). The per-patient positivity rate of 18F-FDG PET/CT was 8/18 (44%; 95% confidence interval, 22%–69%). The patient-based detection rate of 18F-FDG PET/CT in patients with confirmed lesions of endometriosis was 8/13 (62%; confidence interval, 32%–86%). On per-lesion/site basis, 18F-FDG PET/CT detected 11 of 20 sites (55%) of endometriosis. The SUVmax of these lesions/sites ranged between 1.8 and 5.3 (median, 3.8). In 9 of 18 patients (50%), a total of 13 non–endometriosis-related lesions/sites were detected by 18F-FDG PET/CT; their SUVmax ranged between 2.7 and 23 (median, 9.4).
Conclusion
The interference of known or suspected endometriosis in reporting 18F-FDG PET/CT performed in another indication was limited but possible and should be kept in mind, even in postmenopausal women, as the oldest patient with 18F-FDG–positive endometriosis was aged 63 years. The lesions of endometriosis showed inconstant 18F-FDG uptake with overlap of SUVmax with low-grade malignancies. In our series, the greatest SUVmax value of lesion of endometriosis was 5.3, somewhat higher than the threshold of 4 previously proposed for identification of malignant transformation of endometriosis.
Key Words: 18F-FDG PET/CT, diagnostic pitfall, endometriosis
Endometriosis is currently defined as presence of endometrial-like tissue epithelial outside the uterus with both endometrial gland and stroma. However, with advances in disease knowledge, endometrial stromal and glands have been shown to represent only a minor component of endometriotic lesions, and they are often absent in some disease forms. On the one hand, in rectovaginal nodules, the glandular epithelium is often not surrounded by stroma,1 and frequently no epithelium can be identified in the wall of ovarian endometriomas.2 On the other hand, a smooth muscle component and fibrosis represent consistent features of all disease forms.3 Based on these observations, Vigano et al3 proposed the rewording of definition of endometriosis as “A fibrotic condition in which endometrial stroma and epithelium can be identified.” The main aims for this proposed change are to foster the evaluation of fibrosis in studies on endometriosis pathogenesis, to limit potential false negative diagnoses if pathologists stick stringently to the current definition of endometriosis requiring the demonstration of endometrial stromal and glands, and to consider fibrosis as a potential target for treatment in endometriosis.4
Endometriosis is a common gynecologic condition occurring in 5% to 10% of women of reproductive age5 and may also be present in 2% to 5% of postmenopausal women.6,7 Regardless of predominant pathophysiology of endometriosis, both inflammation and active fibrosis may lead to increased glucose metabolism, which is detected on functional PET imaging with 18F-FDG. On 18F-FDG PET, lesions of endometriosis may potentially mimic lesions of newly diagnosed or recurrent malignancies,8 particularly when localized in atypical localizations, with a significant impact on therapeutic management. Therefore, it could be anticipated as a frequent cause of false-positive suspicion of malignancy on 18F-FDG PET in female patients referred for characterization of lesions, in particular adnexal masses, or for cancer staging or restaging. This actually seems not to be the case.8–10 In a series of adnexal masses proven to be benign, only a minority of lesions of endometriosis corresponded to foci of increased 18F-FDG.
Our aim was to shed some light on the likelihood of endometriosis as the cause of 18F-FDG foci on PET/CT performed for another condition, to avoid misinterpretation and improve female patients' management. We will consider, in each patient, whether endometriosis was diagnosed recently or was part of her past history or was only suspected and on 18F-FDG PET/CT the localization of the foci and the intensity of 18F-FDG uptake.
PATIENTS AND METHODS
We performed a retrospective search in our prospective databases including electronic reports of 18F-FDG PET/CTs, using the keyword “endometriosis,” appearing in the summary of patient's history and/or as a potential diagnosis matching the images. This search was performed in 2 centers and covered the years 2010 to 2020 and retrieved the 18F-FDG PET/CT scans of 21 patients. The criteria for inclusion in the study were that endometriosis was part of the past or present history of the patient or was highly suspicious at the time of 18F-FDG PET/CT, which confirmed later that endometriosis was not the (main) indication for 18F-FDG PET/CT and that follow-up data were available. Eighteen patients (9 from each center) met those criteria.
This search overall resulted in a series of 18 patients with known or suspected endometriosis subsequently confirmed referred to 18F-FDG PET/CT for search and characterization of lesions in the context of cancer staging or follow-up, active granulomatous disease, or inflammatory syndrome (Table 1).
TABLE 1.
Patient's Demographics, Clinical Context, and Result of 18F-FDG PET/CT
| Patient No. | Age, y | Indication of 18F-FDG PET/CT | History of Endometriosis at the Time of 18F-FDG PET/CT | Lesion(s) of Endometriosis Known or Suspected Confirmed During Follow-up | SUVmax of Lesions of Endometriosis Detected as 18F-FDG Foci | Localization and Type of Non–Endometriosis-Related Lesion(s) | SUVmax of Non–Endometriosis-Related Lesion(s) Detected as 18F-FDG Foci |
|---|---|---|---|---|---|---|---|
| 1 | 66 | Characterization of a left adnexal mass. Breast cancer 14 y earlier. Tamoxifen from 14 until 10 y earlier | 40 y | — | — | Left ovarian cancer | 13 |
| Uterus adjacent to left ovarian cancer | 11.5 | ||||||
| 2 | 34 | Characterization of a right pleural nodule | 15 y | Pleural mass* | 4.5 | Diffuse right pleural reaction after 2 talc pleurodeses (15 and 4 y earlier) for spontaneous pneumothorax | 9.4 |
| Pleural nodule* | 1.8 | ||||||
| Umbilicus† | 2.6 | ||||||
| 3 | 47 | Characterization of pulmonary mass | 11 y | Uterosacral* ligament | 5.3 | Sarcomatous lung cancer | 23 |
| Torus* | 4.3 | Mediastinal metastatic lymph node | 21.3 | ||||
| Right ovary* | — | ||||||
| 4 | 37 | Inflammatory syndrome | 5 y | Left ovary* | — | Uterine myoma adjacent to left ovary | 7.0 |
| Right ovary† | — | ||||||
| 5 | 54 | Surveillance 3 y after radiochemotherapy for nasopharyngeal cancer | 5 y | — | — | — | |
| 6 | 43 | Surveillance of adenocarcinoma of endometrioid origin, resected 5 and then 3 y before PET/CT. Endometriosis was also found | 5 y | Postsurgical fibrosis in the left pelvic wall | — | — | — |
| 7 | 63 | Surveillance of left breast cancer resected 3 y earlier; letrozole since then | 4 y | Uterosacral* ligament | — | — | — |
| Torus* | — | ||||||
| Rectosigmoid transition zone* | 3.8 | ||||||
| 8 | 39 | Surveillance of a triple-negative breast cancer with BRCa1 mutation, resected 3.5 y earlier | 3.5 y | — | — | Metastasis of breast cancer in left mediastinal subcentimeter lymph node | 16.8 |
| Metastasis of breast cancer in left supraclavicular subcentimeter lymph node | 10.6 | ||||||
| 9 | 45 | Staging of recently resected right breast cancer with metastases in axillary lymph nodes, BRCa1 | 2 y | Pelvic peritoneum* (multiple lesions) | 5 | — | — |
| 10 | 41 | Staging of mediastinal sarcoidosis | 2 y | Juxtauterine cyst* | 2.3 | Sarcoidosis of mediastinal lymph nodes | 7.2 |
| Uterine myoma | 4.4 | ||||||
| 11 | 38 | Systemic autoimmune disease with recurrent ascites | 2 y | — | — | Left breast fibroadenoma | 2.7 |
| 12 | 30 | Surveillance after hysterectomy and oophorectomy for endometriosis with partial malignant transformation | 1 y | Cystoid lesion on the left side of pelvis* | — | — | — |
| 13 | 26 | Abdominal pain. Oophorectomy for right borderline ovarian tumor 1 y ago. Squamous cell carcinoma of uterus cervix, 1 y after conization and reconization 4 mo ago | 1 y | Cystic lesions in the left ovary* | — | Nonspecific colitis | 6.2 |
| 14 | 35 | Etiology of tracheal stenosis. Arthralgia. Suspicion of vasculitis | 6 mo | — | — | Polychondritis | — |
| 15 | 34 | Characterization and staging of left juxtaureteral mass | 1 mo | Cystic lesion* | — | Left juxtaureteral mass: low-grade urothelial cancer | 4.6 |
| 16 | 50 | Characterization and staging of lesion in rectosigmoid junction | Suspected endometriosis | Rectosigmoid transition zone† | 3.6 | — | — |
| 17 | 47 | Characterization and staging of left ovarian lesion | Suspected endometriosis | Left ovary† | 4.5 | — | — |
| 18 | 36 | Anemia, abdominal pain | Suspected endometriosis | Right and left parametria† | 3.7 | — | — |
*Known.
†Suspected.
The indication for 18F-FDG PET/CT, the age of the patient, and the delay since the diagnosis of endometriosis were recorded.
18F-FDG PET/CT was performed during the proliferative phase of the cycle in 15 of 16 patients and during the menstrual flow in 1 of 16 nonovariectomized premenopausal women without oral contraception.
Fifteen PET/CT procedures were performed on a Gemini TF-16 machine and 3 on a Siemens Biograph mCT flow machine, starting approximately 60 minutes after intravenous injection of 2 to 3 MBq/kg of body mass of 18F-FDG. The field of view of PET covered the top of the skull to midthighs at 3-minute acquisition per bed position.
The location and SUVmax of each nonphysiologic 18F-FDG foci were recorded. For each patient, a composite standard of truth based on clinical follow-up, results of other imaging modalities, and biopsy or postsurgical histology was subsequently determined, in order to characterize the nature of those 18F-FDG foci.
RESULTS
The patients' demographics, clinical context, and the result of 18F-FDG PET/CT (lesions detected on 18F-FDG PET/CT and their SUVmax of according to their nature) are provided in Table 1.
The data of 18 women with mean age at 18F-FDG PET/CT of 42.5 years (median, 40 years; range, 26–66 years) were analyzed. Fifteen of them (83%) (patients 1–15) had a past or recent history of endometriosis. The mean time interval since the diagnosis of endometriosis was 5.4 years (median, 2 years; longest time interval of 40 years at the time of 18F-FDG PET/CT); 4 of 18 (22%) (patients 1, 8, 12, and 13) of these patients had undergone unilateral or bilateral salpingo-oophorectomy or partial oophorectomy because of endometriosis. Endometriosis was suspected as one potential diagnosis in 3 of 18 patients (17%) (patients 16–18) referred to 18F-FDG PET/CT for characterization of a pelvic lesion or staging of a pelvic tumor and was subsequently confirmed during follow-up.
According to the composite standard of truth, the presence of lesions of endometriosis at the time of 18F-FDG PET/CT was confirmed in 13 of 18 patients (72%) (patients 2–4, 6, 7, 9, 10, 12, 13, and 15–18). In contrast, in 1 patient, the left adnexal mass suspected to correspond to a late recurrence of her endometriosis was in fact an ovarian cancer with extension to the uterus. In 4 patients (5, 8, 11, and 14), no recurrence of endometriosis was detected either on other imaging modalities at the time of 18F-FDG PET/CT or during follow-up.
The per-patient positivity rate of 18F-FDG PET/CT showing foci evocative of metabolically active lesions of endometriosis was 8 of 18 (44%; 95% confidence interval, 22–69%) (patients 2, 3, 7, 9, 10, and 16–18). The 18F-FDG positivity of endometriosis was influenced neither by the menopausal nor oophorectomy status of the patient (Fisher test P = 0.3) nor by the time interval since the diagnosis of endometriosis (mean, 6.3 years if 18F-FDG–negative vs. 4.3 years if 18F-FDG–positive P = 0.5). The oldest patient with 18F-FDG–positive endometriosis was aged 63 years; the longest time interval between the diagnosis of endometriosis and the detection of 18F-FDG–positive foci of endometriosis was 15 years. The presence of pelvic implants of endometrium was detected in 4 patients on MRI (patients 4, 12, 13, and 15) and in 1 patient (patient 6) during surgery, but in those patients, no foci were visible on 18F-FDG PET/CT. Thus, the patient-based detection rate of 18F-FDG PET/CT limited to patients with confirmed lesions of endometriosis was 8 of 13 (62%; confidence interval, 32%–86%).
On per-lesion/site basis, 18F-FDG PET/CT detected 11 of 20 sites (55%) of endometriosis. The SUVmax of these lesions/sites ranged between 1.8 and 5.3 (median, 3.8).
In 9 of 18 patients (50%) (patients 1, 3, 4, 8, 10, 11, 13, and 5), a total of 13 non–endometriosis-related lesions/sites were detected by 18F-FDG PET/CT; their SUVmax ranged between 2.7 and 23 (median, 9.4). All 7 malignant lesions/sites were 18F-FDG–positive (ovarian cancer, urothelial cancer, sarcomatous lung cancer with lymph node metastases, lymph node recurrence of breast cancer); their SUVmax ranged between 4.6 and 23 (median, 13). Three benign tumors took up 18F-FDG: 2 myomas (SUVmax 4.4 and 7.0) and 1 breast fibroadenoma (SUVmax 2.7). Three inflammatory lesions also took up 18F-FDG: colitis, sarcoidosis, and pleural inflammation after talc pleurodesis (SUVmax 6.2, 7.2, and 9.4, respectively). In 1 patient subsequently diagnosed with polychondritis, no 18F-FDG focus was visible (Fig. 1).
FIGURE 1.
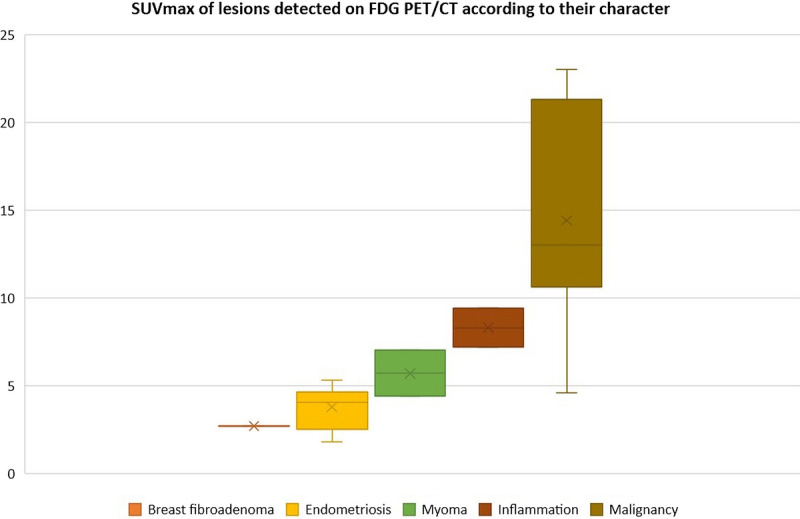
SUVmax of lesions detected on 18F-FDG PET/CT according to their location and origin.
The illustrative cases of 18F-FDG–avid endometriosis in a patient with active (patient 3) and remittent malignancy (patient 7) are provided in Figure 2 and Figure 3. The illustrative case of mildly 18F-FDG–avid ovarian endometrioma in a patient with suspected ovarian cancer is provided in Figure 4.
FIGURE 2.
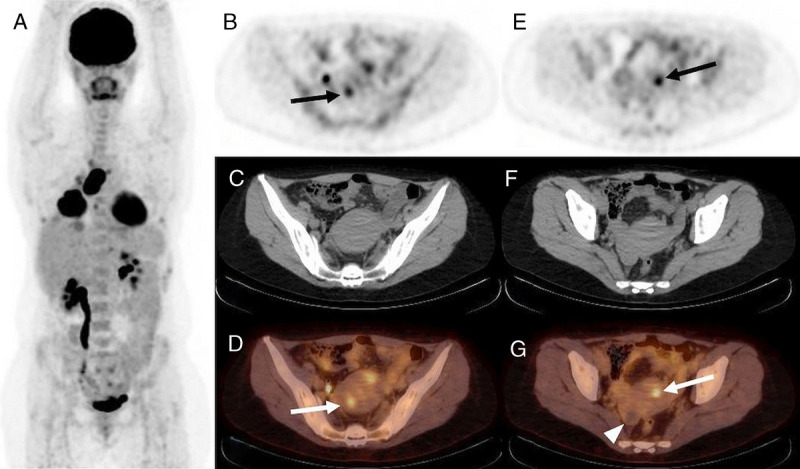
18F-FDG PET/CT, A: MIP, B and E: PET, C and F: CT, and D and G: PET/CT, axial slice. Staging of sarcomatous lung cancer in a 47-year-old woman (patient 3) with 11-year history of endometriosis at the time of 18F-FDG PET/CT. Intense 18F-FDG uptake by primary lung cancer (SUVmax 23) and its lymph node and pleural metastases. Two foci of increased 18F-FDG uptake in lesions of endometriosis in the uterine wall (SUVmax 4.3 and 5.3, B, D, and E, G, arrow). No 18F-FDG uptake by known right ovarian endometrioma (G, arrowhead).
FIGURE 3.
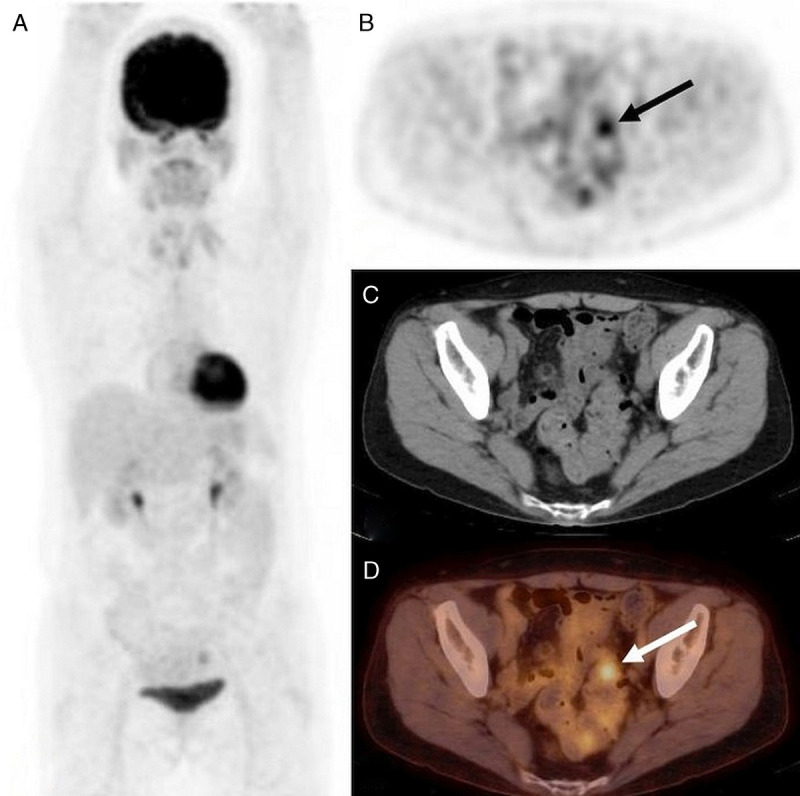
18F-FDG PET/CT, A: MIP, B: PET, C: CT, and D: PET/CT, axial slice. Surveillance of left breast cancer resected 3 years earlier in a 63-year-old woman (patient 7); letrozole since then. The endometriosis was known for 4 years at the time of 18F-FDG PET/CT. Focally increased 18F-FDG uptake in rectosigmoid junction (SUVmax 3.8; B and D, arrow) confirmed by biopsy as endometriosis.
FIGURE 4.
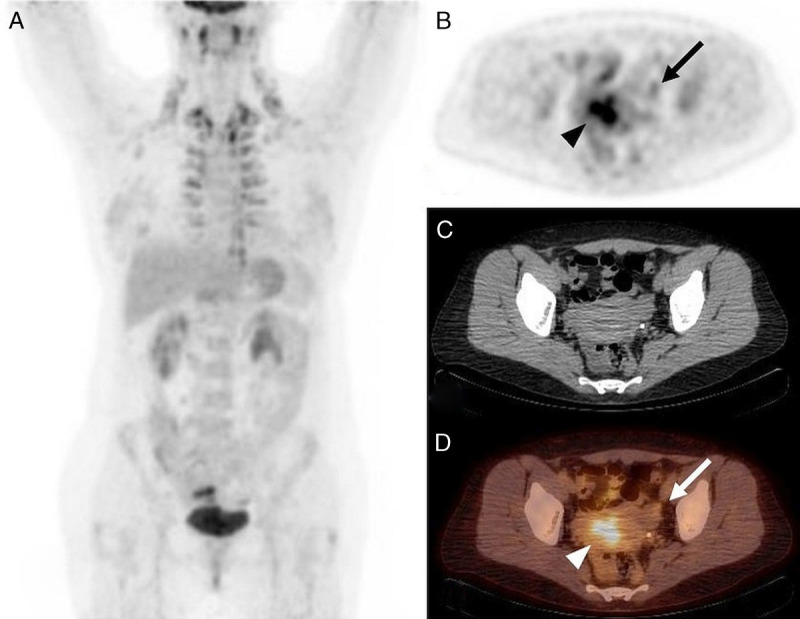
18F-FDG PET/CT, A: MIP, B: PET, C: CT, and D: PET/CT, axial slice. Characterization and staging of newly diagnosed left ovarian mass complicated by constriction of left ureter and hydronephrosis in a 47-year-old woman (patient 17) with no history of endometriosis. Mildly increased, isolated 18F-FDG uptake in the peripheral part of cystoid left ovarian lesion (SUVmax 4.5, B and D: arrow) and increased 18F-FDG uptake in uterine cavity during menstrual flow (SUVmax 7.45, B and D: arrowhead). Left ovarian endometrioma was confirmed by histology.
DISCUSSION
Frequency of 18F-FDG Uptake by Confirmed Lesions of Endometriosis
In endometriosis, fibrosis and inflammation are likely to be the nonspecific diagnostic targets on FDG PET/CT, with a variable intensity of uptake (Table 2). Cases have been reported of 18F-FDG uptake by progressive deep invasive pelvic endometriosis11 or by endometriosis lesions in the lung,12 the canal of Nuck,20 or lymph nodes,14 but in general, a significantly increased glucose metabolism does not seem to be a part of typical and consistent features of endometrioma.21,22 In the study by Rieber et al,8 18F-FDG PET, performed for characterization of asymptomatic ovarian mass suspicious for carcinoma, showed positive foci in 4 of 22 patients (18%) with ovarian endometrioma. On the same year, the same team10 reported increased 18F-FDG uptake corresponding to the area of adnexal endometriosis in 5 of 23 cases (22%) only. Furthermore, when compared with MRI, the 18F-FDG foci could be clearly attributed to endometrioma in 1 of 5 cases; in the remaining cases, the 18F-FDG uptake was caused by gastrointestinal activity. In the subsequent study by Fastrez et al,9 the preoperative 18F-FDG PET/CT brought negative result in all 9 consecutive patients aged 31 ± 10 years with confirmed ovarian and/or pelvic endometriosis (6/9 had at least stage III endometriosis, according to the American Society of Reproductive Medicine). In the series by Fastrez et al9 including patients with suspected endometriosis, 18F-FDG uptake was observed in none of the lesions of the 9 patients with confirmed endometriosis. In the study by Setubal et al13 including 9 patients with suspected endometriosis, 18F-FDG foci were visible in 4 of 8 patients with confirmed endometriosis, allowing only a partial visualization of the disease spread in those 18F-FDG–positive cases.
TABLE 2.
18F-FDG Uptake by Lesions of Endometriosis
| Reference | No. Patients With Suspected or Confirmed EndometriosisPatient-Based 18F-FDG PET/CT DR | Localization of 18F-FDG–Positive Lesions of Endometriosis | SUVmax of Lesions of Endometriosis Median (Range) |
|---|---|---|---|
| Rieber et al8 | 22 DR: 4/22 = 18% |
Ovary | NA |
| Fenchel et al10 | 23 DR: 5/23 = 22% |
Adnexal tumors | NA |
| Jeffry et al11 | 1 DR: 1/1 |
Ovary | 4.5 |
| Derman et al12 | 1 DR: 1/1 |
Lung | NA |
| Fastrez et al9 | 10 DR: 0/9 = 0% |
- | - |
| Setubal et al13 | 9 DR: 4/8 = 50% * |
Ovary/intestine, adnexal area, abdominal muscle, rectovaginal septum | 5.16 (3.52–5.56) |
| Akiyama et al14 | 1 DR: 1/1 |
left ureter, ovary, and internal iliac lymph node | NA |
| Ge et al15 | 1 DR: 1/1 |
Right ovary, liver capsule, perihepatic nodules, greater omentum, mesentery | 1.7–2.6 |
| Maffione et al16 | 1 DR: 1/1 |
Retroperitoneum | 4.8 |
| Agarwal Sharma et al17 | 1 DR: 1/1 |
Both ovaries, diffuse peritoneal dissemination | NA |
| Kusunoki et al18 | 11 DR: 5/11 = 45% |
Ovary | 2.7 (1–4) |
| Li et al19 | 1 DR: 1/1 |
Ovaries, vaginal and bladder walls | NA |
| Present series | 18 DR: 8/13 = 62%* |
Pleura, umbilicus, pelvic peritoneum, juxtauterine cyst, uterosacral ligament, torus, ovary, rectosigmoid junction | 4.3 (1.8–5.3) |
*Endometriosis finally confirmed in a lesser number of patients.
DR, detection rate; NA, not available.
This inconstant 18F-FDG uptake by lesions makes 18F-FDG PET/CT not suited for routine localization of lesions of endometriosis.3,4 In our series of patients referred to 18F-FDG PET/CT for another indication but with past or recent diagnosis or suspected endometriosis, the patient-based detection rate of endometriosis with 18F-FDG was 8 of 13 (62%), nonsignificantly greater than 32 of 73 (44%) obtained by pooling the results of 5 previous studies (Fisher exact test, P = 0.4).8–10,13,18 It should be noted that the detection rate was highly variable between those series, from none of 9 (0%)9 to 18 of 22 (82%)8 in case of ovarian masses.
On 18F-FDG PET/CT requested in another indication, an incidental 18F-FDG uptake by lesions of endometriosis, even if infrequent,23,24 may be a source of misinterpretation. We will consider criteria that may help to circumvent this pitfall.
Are 18F-FDG–Positive Lesions of Endometriosis Highly Unlikely in a Postmenopausal Patient?
Active endometriosis also occurs in 2% to 5% postmenopausal women6,7 and commonly represents an adverse effect of hormone replacement therapy or tamoxifen treatment25–27; in a few cases, postmenopausal endometriosis has been described in women who had no history of endometriosis on imaging or surgery prior to menopause.26 Those results and a case report17 justify considering endometriosis as a potential diagnosis and source of 18F-FDG foci even in postmenopausal patients.
In this cohort, we observed 18F-FDG active lesions of endometriosis in 1 of 4 postmenopausal or postoophorectomy patients. Two postmenopausal patients with history of endometriosis were referred for localization of recurrent breast cancer. One patient was being treated with letrozole, an aromatase inhibitor that has an inhibitory effect on endometriosis, but that did not impede 18F-FDG uptake in this patient. The other patient had been treated with tamoxifen more than 10 years before and no lesion of endometriosis was found. In a 66-year-old patient, the suspected ovarian endometriomas corresponded to an ovarian cancer infiltrating the uterus, and in a 63-year-old patient with confirmed endometriosis in uterosacral ligament, torus, and rectosigmoid junction, the 18F-FDG PET/CT localized 1 lesion of endometriosis in the rectosigmoid junction.
May Endometriosis Be Ruled Out in Some 18F-FDG–Positive Lesions According to Their Location?
The common sites of endometriosis include ovaries, fallopian tubes, pelvic peritoneum, and pelvic ligaments, whereas atypical sites include the colon, urinary bladder, ureter, abdominal wall, and pleura.28,29
For pelvic sites, endometriosis shares the typical localization with ovarian cancer, the abdominal cavity being the typical localization of metastases.30
In the present series, the lesions of confirmed endometriosis were located not only in the pelvic peritoneum, adnexal area, and rectosigmoid junction, but also in the abdominal wall and in the thorax in 1 patient (patient 2).
Non–endometriosis-related malignant, inflammatory, or benign 18F-FDG–positive lesions were detected in 5 patients in the abdomen or the pelvis and also in 5 patients in the thorax (patients 2, 3, 8, 10, and 11).
May the Intensity of 18F-FDG Uptake Help Differentiating Endometriosis From Lesions of Another Origin?
SUVmax values of endometriosis lesions reported in literature are summarized in Table 2. In the published case reports, the SUVmax of lesions of endometriosis located in the pelvic or abdominal muscle ranged from 1.7 to 4.8.11,15,16 In ovarian endometriosis, the SUVmax ranged from 1 to 4 in a series of 5 cases.18 Among 8 patients with endometriosis, Setubal et al13 observed 18F-FDG uptake in 3 of 5 patients with deep endometriosis (SUVmax 4.88, 5.44, and 5.56). SUVmax of abdominal wall endometriosis was 3.52, whereas ovarian endometrioma and superficial endometriosis were misdiagnosed on 18F-FDG PET/CT in 1 case each. A trend for a higher 18F-FDG uptake by deep endometriosis, as measured by SUVmax, compared with superficial peritoneal lesions may be explained by a greater lesion size of deep endometriosis, whereas peritoneal lesions are often of small size. Another explanation is related to the type of peritoneal lesions red, black, or Wright stain–positive. Indeed, red lesions are very active lesions with a high angiogenesis, whereas Wright stain–positive lesions are mainly composed of fibrosis, and black lesions correspond to intermediate state, but also by chronic inflammatory and/or fibrotic character of the pathology.3,23 In the present series, the SUVmax values of endometriosis lesions fell into the same range. The lesions of endometriosis with the highest 18F-FDG uptake were located in pelvic peritoneum and adnexal area (SUVmax 2.3–5.3). In contrast, the lesions of endometriosis in the rectosigmoid junction (SUVmax 3.6–3.8), the abdominal wall (2.6), and the thorax (1.8–4.5) were less metabolically active. This finding may be explained by a larger fibrotic component and a lesser glandular component of endometriosis in those latter organs and structures.
The SUVmax values reported in case of malignant transformation of endometriosis (MTOE) are provided in Table 3. Malignant transformation of endometriosis is a particular clinical situation that occurs in fewer than 0.1% of patients. A significant difference in 18F-FDG uptake (P < 0.01) was observed between 11 patients with nonmalignant endometrioma (median SUVmax, 2.7; range, 1–4) and 21 patients with MTOE (median SUVmax, 8.4; range, 2–18).18 A cutoff value of SUVmax >4.0 was capable of excluding endometriosis, with 75% sensitivity, 100% specificity, 100% positive predictive value, and 68.8% negative predictive value (area under the curve 90%).18 Accordingly, in a case of rectal MTOE reported by Li et al,19 the SUVmax was 15.7. In another patient with MTOE in the abdominal wall, the SUVmax on 18F-FDG PET/MRI was 9.61 in the abdominal wall and 4.25 in metastatic retroperitoneal and inguinal lymph nodes.32 In the case reported by Yoshida et al,31 18F-FDG was taken up by MTOE in the groin, as well as by ipsilateral metastatic pelvic lymph nodes in a postmenopausal patient, but the 18F-FDG uptake values were not provided.
TABLE 3.
18F-FDG Uptake in Case of Malignant Transformation of Endometriosis
| Reference | No. Patients (Patient-Based 18F-FDG PET/CT Positivity Rate) | Location of Malignant Transformation of Endometriosis | SUVmax in Case of Malignant Transformation of Endometriosis Median or Mean (Range) |
|---|---|---|---|
| Kusunoki et al18 | 11 (5/11) | Ovary | 8.4 (2–18) |
| Li et al19 | 1 (1/1) | Rectosigmoid | 15.7 |
| Yoshida et al31 | 1 (1/1) | Groin, right pelvic lymph nodes | NA |
| Wang et al32 | 1 (1/1) PET/MRI, delay between 18F-FDG administration and acquisition of images not provided |
Abdominal wall and lymph nodes | 9.61 and 4.25 |
Other types of malignancies usually result in a high value of SUVmax, but there is an overlap with some lesions of endometriosis. Just focusing on gynecological malignancies, Takagi et al33 reported a mean SUVmax value of 11.0 (range, 2.6–22.4) in 41 ovarian cancers, 13.7 (2.0–35.2) in 63 endometrial cancers, and 15.9 (5.8–29.3) in 15 patients with uterine sarcomas. In this study, SUVmax values less than 5.6 were found only in ovarian cancers at International Federation of Gynecology and Obstetrics stage 1 (n = 19; mean, 9.8; range, 2.6–22.4) and stage 2 (n = 5; mean, 10.1; range, 4.0–18.6). Recently, Park et al34 reported the SUVmax according to the dualistic model of ovarian carcinogenesis; SUVmax values less than 5.6 may be observed with type I (n = 90; mean, 9.5; range, 2.5–23.8) and type II (n = 80; mean, 13.0; range, 3.2–41.2). In the present series, the SUVmax of ovarian cancer lesions was 13 in the pelvis and 11.5 in the thorax; the SUVmax of sarcomatous lung cancer and its metastatic lymphadenopathy was 23 and 21.3, respectively. However, the SUVmax of a retroperitoneal low-grade urothelial tumor was 4.6, similar to the value observed by Maffione et al16 in an infiltrative retroperitoneal endometriosis, overlapping with the range of SUVmax of endometriosis lesions. In the 3 patients who had 18F-FDG–positive lesions originating from a malignancy and others from endometriosis, all the malignant lesions had a greater SUVmax than all the lesions of endometriosis.
Apart from endometriosis, other benign gynecological tumors can take up 18F-FDG. In the series of 47 patients with uterine myomas by Ma et al,35 18F-FDG positivity rate was 19/47 (40.4%). The SUVmax of 18F-FDG–positive myomas was highly variable (4.38 ± 2.57; range, 1.4–10.81) and was higher in younger and premenopausal patients.35 Takagi et al33 reported 18F-FDG uptake by 12 uterine myomas with SUVmax of 6.0 ± 5.4 (range, 2.7–22.4). Therefore, 18F-FDG SUVmax of myomas seems to be similar to or higher than that of lesions of endometriosis. This inconstant and variable 18F-FDG uptake by myomas may be explained by various levels of inflammation in the microenvironment suitable for myoma onset and development.36 According to the results of the retrospective study by Nezhat et al37 performed in 244 patients, 181 of 208 (87%) patients with a chief concern of symptomatic myomas also had histology-proven endometriosis. In the present study, 18F-FDG–positive uterine myoma was found in 2 patients (patients 4 and 10), associated with lesions of endometriosis, which were 18F-FDG–positive in 1 case. Therefore, in patients with uterine myoma, the presence of foci on 18F-FDG PET/CT corresponding to lesions of endometriosis must be considered.
18F-FDG PET provides a high accuracy in the differentiation of fibroadenomas from malignant tumors because fibroadenomas usually show no or mild 18F-FDG uptake.38 Accordingly, in our series, only mild 18F-FDG uptake (SUVmax 2.7) was observed in 1 case of breast fibroadenoma.
Concerning the differentiation between noninfected endometriosis and infectious or inflammatory lesions, the reported uptake values for endometriosis were lower than values reported in a patient with bilateral ovarian abscess (SUVmax 8.4), but similar to uptake values in reactive lymphadenopathy (SUVmax 2.6–5.7, mean SUVmax 4) in the same patient.39 In the present series, the chronic inflammatory pleural reaction after talc pleurodesis, lesions of sarcoidosis in mediastinal lymph nodes, and nonspecific colitis showed higher SUVmax (9.4, 7.2, and 6.2, respectively) than any lesion of confirmed endometriosis. Finally, in a patient who was not treated with corticosteroids, evolutive polychondritis did not result in foci on 18F-FDG PET/CT, in contrast with previously published results.40
CONCLUSION
According to our results derived from 18 patients, the interference of known or suspected endometriosis in reporting 18F-FDG PET/CT performed in another indication seems to be limited, but possible and should be kept in mind.
Our results confirm that the lesions of endometriosis show inconstant 18F-FDG uptake, which, in the majority of cases, is lower than that of malignant lesions, with partial overlap of SUVmax with well-differentiated, low-grade malignancies. According to the history of the patient, endometriosis should be considered as one potential origin of 18F-FDG foci, even in postmenopausal women, as the oldest patient with 18F-FDG–positive endometriosis was aged 63 years. In case endometriosis has been diagnosed several years ago, incidental 18F-FDG–positive lesion may still correspond to endometriosis, the longest time interval being 15 years in our series.
In our series, the greatest SUVmax value of lesion of endometriosis was 5.3, which is somewhat higher than the SUVmax threshold of 4 previously proposed for identification of MTOE.
Footnotes
Conflicts of interest and sources of funding: Supported by grant: KEGA 058UK-4/2020.
Contributor Information
Emile Daraï, Email: sona.balogova@aphp.fr.
Lucia Noskovicova, Email: noskovicova10@uniba.sk.
Ludovit Lukac, Email: lukac2@uniba.sk.
Jean-Noël Talbot, Email: jean-noel.talbot@aphp.fr.
Françoise Montravers, Email: francoise.montravers@aphp.fr.
REFERENCES
- 1.Donnez J Nisolle M Casanas-Roux F, et al. Stereometric evaluation of peritoneal endometriosis and endometriotic nodules of the rectovaginal septum. Hum Reprod. 1996;11:224–228. [DOI] [PubMed] [Google Scholar]
- 2.Muzii L Bianchi A Bellati F, et al. Histologic analysis of endometriomas: what the surgeon needs to know. Fertil Steril. 2007;87:362–366. [DOI] [PubMed] [Google Scholar]
- 3.Vigano P Candiani M Monno A, et al. Time to redefine endometriosis including its pro-fibrotic nature. Hum Reprod. 2018;33:347–352. [DOI] [PubMed] [Google Scholar]
- 4.Yoshino O Ono Y Honda M, et al. Relaxin-2 may suppress endometriosis by reducing fibrosis, scar formation, and inflammation. Biomedicine. 2020;8:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dmowski WP Lesniewicz R Rana N, et al. Changing trends in the diagnosis of endometriosis: a comparative study of women with pelvic endometriosis presenting with chronic pelvic pain or infertility. Fertil Steril. 1997;67:238–243. [DOI] [PubMed] [Google Scholar]
- 6.Secosan C Balulescu L Brasoveanu S, et al. Endometriosis in menopause-renewed attention on a controversial disease. Diagnostics (Basel). 2020;10:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haas D Chvatal R Reichert B, et al. Endometriosis: a premenopausal disease? Age pattern in 42,079 patients with endometriosis. Arch Gynecol Obstet. 2012;286:667–670. [DOI] [PubMed] [Google Scholar]
- 8.Rieber A Nüssle K Stöhr I, et al. Preoperative diagnosis of ovarian tumors with MR imaging: comparison with transvaginal sonography, positron emission tomography, and histologic findings. AJR Am J Roentgenol. 2001;177:123–129. [DOI] [PubMed] [Google Scholar]
- 9.Fastrez M Nogarède C Tondeur M, et al. Evaluation of 18FDG PET-CT in the diagnosis of endometriosis: a prospective study. Reprod Sci. 2011;18:540–544. [DOI] [PubMed] [Google Scholar]
- 10.Fenchel S Grab D Nuessle K, et al. Asymptomatic adnexal masses: correlation of FDG PET and histopathologic findings. Radiology. 2002;223:780–788. [DOI] [PubMed] [Google Scholar]
- 11.Jeffry L Kerrou K Camatte S, et al. Endometriosis with FDG uptake on PET. Eur J Obstet Gynecol Reprod Biol. 2004;117:236–239. [DOI] [PubMed] [Google Scholar]
- 12.Derman AY Sperling D Merav A, et al. Endometrioma presenting as a cavitary lung mass with intense 18F-FDG uptake on PET-CT. J Thorac Imaging. 2007;22:172–175. [DOI] [PubMed] [Google Scholar]
- 13.Setubal A Maia S Lowenthal C, et al. FDG-PET value in deep endometriosis. Gynecol Surg. 2011;8:305–309. [Google Scholar]
- 14.Akiyama M Suganuma I Mori T, et al. (18)F-fluorodeoxyglucose positron emission tomography/computed tomography–positive lymph node endometriosis masquerading as lymph node metastasis of a malignant tumor. Case Rep Obstet Gynecol. 2014;2014:648485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ge J Zuo C Guan Y, et al. Increased 18F-FDG uptake of widespread endometriosis mimicking ovarian malignancy. Clin Nucl Med. 2015;40:186–188. [DOI] [PubMed] [Google Scholar]
- 16.Maffione AM Panzavolta R Lisato LC, et al. Retroperitoneal endometriosis: a possible cause of false positive finding at (18)F-fluorodeoxyglucose positron emission tomography/computed tomography. World J Nucl Med. 2015;14:131–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwal Sharma R Lee EY Vardhanabhuti V, et al. Unusual case of postmenopausal diffuse endometriosis mimicking metastatic ovarian malignancy. Clin Nucl Med. 2016;41:e120–e122. [DOI] [PubMed] [Google Scholar]
- 18.Kusunoki S Ota T Kaneda H, et al. Analysis of positron emission tomography/computed tomography in patients to differentiate between malignant transformation of endometrioma and endometrioma. Int J Clin Oncol. 2016;21:1136–1141. [DOI] [PubMed] [Google Scholar]
- 19.Li N Zhou W Zhao L, et al. Endometriosis-associated recto-sigmoid cancer: a case report. BMC Cancer. 2018;18:905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirkpatrick A Reed CM Bui-Mansfield LT, et al. Radiologic-pathologic conference of Brooke Army Medical Center: endometriosis of the canal of Nuck. AJR Am J Roentgenol. 2006;186:56–57. [DOI] [PubMed] [Google Scholar]
- 21.Lapela M Leskinen-Kallio S Varpula M, et al. Metabolic imaging of ovarian tumors with carbon-11-methionine: a PET study. J Nucl Med. 1995;36:2196–2200. [PubMed] [Google Scholar]
- 22.Nishizawa S, Inubushi M, Okada H. Physiological 18F-FDG uptake in the ovaries and uterus of healthy female volunteers. Eur J Nucl Med Mol Imaging. 2005;32:549–556. [DOI] [PubMed] [Google Scholar]
- 23.Agostinis C Balduit A Mangogna A, et al. Immunological basis of the endometriosis: the complement system as a potential therapeutic target. Front Immunol. 2020;11:599117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holder WD Jr. White RL Jr. Zuger JH, et al. Effectiveness of positron emission tomography for the detection of melanoma metastases. Ann Surg. 1998;227:764–769; discussion 769–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeon DS Kim TH Lee HH, et al. Endometriosis in a postmenopausal woman on hormonal replacement therapy. J Menopausal Med. 2013;19:151–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snyder BM Beets JW Lessey BA, et al. Postmenopausal deep infiltrating endometriosis of the colon: rare location and novel medical therapy. Case Rep Gastrointest Med. 2018;2018:9587536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Habuchi T, Okagaki T, Miyakawa M. Endometriosis of bladder after menopause. J Urol. 1991;145:361–363. [DOI] [PubMed] [Google Scholar]
- 28.Sonavane SK, Kantawala KP, Menias CO. Beyond the boundaries—endometriosis: typical and atypical locations. Curr Probl Diagn Radiol. 2011;40:219–232. [DOI] [PubMed] [Google Scholar]
- 29.Lee HJ Park YM Jee BC, et al. Various anatomic locations of surgically proven endometriosis: a single-center experience. Obstet Gynecol Sci. 2015;58:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hess KR Varadhachary GR Taylor SH, et al. Metastatic patterns in adenocarcinoma. Cancer. 2006;106:1624–1633. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida S Onogi A Kuwahara M, et al. Clear cell adenocarcinoma arising from endometriosis in the groin: wide resection and reconstruction with a fascia Lata tensor muscle skin flap. Case Rep Obstet Gynecol. 2018;2018:2139595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H Xue Q Shou Y, et al. 18F-FDG simultaneous PET/MR findings of a malignant transformation and metastases of abdominal wall endometriosis. Eur J Nucl Med Mol Imaging. 2020;47:3190–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takagi H Sakamoto J Osaka Y, et al. Utility of 18F-fluorodeoxyglucose-positron emission tomography in the differential diagnosis of benign and malignant gynaecological tumours. J Med Imaging Radiat Oncol. 2018. [DOI] [PubMed] [Google Scholar]
- 34.Park S Kim TS Lim MC, et al. Glycolytic phenotypes in an evaluation of ovarian carcinoma based on carcinogenesis and BRCA mutation. Eur J Radiol. 2020;133:109391. [DOI] [PubMed] [Google Scholar]
- 35.Ma Y Shao X Shao X, et al. High metabolic characteristics of uterine fibroids in 18F-FDG PET/CT imaging and the underlying mechanisms. Nucl Med Commun. 2016;37:1206–1211. [DOI] [PubMed] [Google Scholar]
- 36.Orciani M Caffarini M Biagini A, et al. Chronic inflammation may enhance leiomyoma development by the involvement of progenitor cells. Stem Cells Int. 2018;2018:1716246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nezhat C Li A Abed S, et al. Strong association between endometriosis and symptomatic leiomyomas. Jsls. 2016;20:e2016.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong A Wang Y Lu J, et al. Spectrum of the breast lesions with increased 18F-FDG uptake on PET/CT. Clin Nucl Med. 2016;41:543–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim YI Kim SK Lee JW, et al. Ovarian mass mimicking malignancy: a case report. Nucl Med Mol Imaging. 2010;44:290–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lei W Zeng H Zeng DX, et al. (18)F-FDG PET-CT: a powerful tool for the diagnosis and treatment of relapsing polychondritis. Br J Radiol. 2016;89:20150695. [DOI] [PMC free article] [PubMed] [Google Scholar]


