Abstract
We are using the facultative hemiparasite, Triphysaria, as a model for studying host-parasite signaling in the Scrophulariaceae. Parasitic members of this family form subterranean connections, or haustoria, on neighboring host roots to access host water and nutrients. These parasitic organs develop in response to haustorial-inducing factors contained in host root exudates. A well-characterized inducing factor, 2, 6-dimethoxy-p-benzoquinone (DMBQ), can be used to trigger in vitro haustorium formation in the roots of Triphysaria. We have assayed three species, Triphysaria eriantha (Benth.) Chuang and Heckard, Triphysaria pusilla (Benth.) Chuang and Heckard, and Triphysaria versicolor Fischer and C. Meyer, for haustorium development in response to DMBQ. There were significant differences between the species in their ability to recognize and respond to this quinone. Ninety percent of T. versicolor individuals responded, whereas only 40% of T. pusilla and less than 10% of T. eriantha formed haustoria. Within field collections of self-pollinating T. pusilla, differential responsiveness to DMBQ was seen in distinct maternal families. Assaying haustorium development in subsequent generations of self-pollinated T. pusilla showed that DMBQ responsiveness was heritable. Reciprocal crosses between T. eriantha and T. versicolor demonstrated that DMBQ responsiveness was influenced by maternal factors. These results demonstrate heritable, natural variation in the recognition of a haustorial-inducing factor by a parasitic member of the Scrophulariaceae.
An approximate 1% of flowering plant species fulfill at least some of their nutritional requirements through the parasitism of other plants (Press and Graves, 1995). The ability of plants to invade neighboring host plants and deplete them of water and other nutrients has originated multiple times during the evolution of angiosperms (Kuijt, 1969; Nickrent et al., 1998). As a consequence, parasitic plants have various forms, habits, and routes of host invasion. Plant parasitism proceeds through a series of coordinated developmental changes in response to chemicals exchanged between host and parasitic plants. Because many of these developmental responses can be readily monitored in vitro, these associations afford a remarkable opportunity to investigate genetic consequences of signal exchanges between plants.
Parasite species in the Scrophulariaceae invade host plants via subterranean connections between parasite and host plant roots. The Scrophulariaceae is an interesting family for parasitic plant studies because it includes parasitic and non-parasitic members. Most Scrophulariaceae, including the well-studied genera Antirrhinum and Mimulus, are non-parasitic autotrophs. However, a distinct clade of about 30 genera of Scrophulariaceae are heterotrophic parasites that invade the roots of neighboring plants. Molecular phylogeny places all parasitic members of the Scrophulariaceae on a common clade, suggesting that parasitism arose one time in the evolution of this family (dePamphilis et al., 1997).
The degree to which different species rely on host resources varies considerably. Some Scrophulariaceae are obligate parasites that require attachment to a host root within days of germination to survive. Within this group are the agriculturally devastating weeds, Striga and Alectra, which cause significant yield losses in monocot and dicot crops (Parker and Riches, 1993). Facultative parasites are able to grow to maturity without host connections, though they are much more vigorous when grown in the presence of other plants (Thurman, 1966; Marvier, 1998b). Facultative hemiparasites are typically generalists that invade a broad spectrum of host species (Press and Graves, 1995).
Triphysaria is a small genus of five hemiparasitic species that are common in grassland stands throughout the Pacific Coast (Chuang and Heckard, 1991; Hickman, 1993). These springtime annuals are diploids with perfect flowers that produce about 1,000 seeds per plant (Chuang and Heckard, 1982). Triphysaria versicolor Fischer and C. Meyer and Triphysaria eriantha (Benth.) Chuang and Heckard are allogamous, bee-pollinated species with conspicuous white, yellow, or purple flowers. These species readily cross hybridize and hybrids are commonly observed in the wild (Thurman, 1966). A gametophytic self-incompatibility system with multiple recognition alleles controls self-incompatibility in T. versicolor and T. eriantha (Yoder, 1998). In contrast, the autogamous species Triphysaria pusilla (Benth.) Chuang and Heckard grows decumbent with many small, inconspicuous flowers having large calyxes that hide a purple corolla. T. pusilla is highly cleistogamous and pollination occurs in the unopened flower when the anthers grow past the receptive stigma (Atsatt, 1970). Pollen from T. pusilla can be crossed manually onto T. versicolor and T. eriantha flowers, resulting in self-fertile F1 hybrids (Yoder, 1998).
Triphysaria invades host roots via haustoria, parasitic plant-specific organs that function in host attachment, tissue penetration, and as physiological bridges through which nutrients are obtained from the hosts. Roots of field grown Triphysaria can have hundreds of secondary haustoria, so named because they initiate proximal to the root tip, in contrast to primary haustoria that are terminal meristem differentiations (Kuijt, 1969). In the field and in vitro, Triphysaria will invade a broad spectrum of hosts, including maize, clover, and Arabidopsis (Estabrook and Yoder, 1998). Although hemiparasitic Scrophulariaceae can parasitize many host species, not all hosts are equally beneficial to the parasites (Gibson and Watkinson, 1989; Matthies, 1996; Marvier, 1998a). Attachment to certain hosts may even have a negative effect on parasite performance (Atsatt and Strong, 1970). Host discrimination, therefore, might be a useful feature in generalist parasites.
Haustoria develop on the roots of Triphysaria and other parasitic Scrophulariaceae in response to chemical signals released by host roots into the rhizosphere (Riopel and Timko, 1995). Specific host signal molecules are called haustorial-inducing factors (HIFs). These include quinones, hydroquinones, phenolic acids, and flavonoids (Chang and Lynn, 1986; Riopel and Timko, 1995; Albrecht et al., 1998). Structurally variant quinones having similar redox potentials generally function as HIFs and the signaling of haustorium development is thought to result from the cycling of these molecules from quinone to semi-quinone forms (Smith et al., 1996). One of the most active HIFs, 2,6-dimethoxy-p-benzoquinone (DMBQ), was first isolated from sorghum roots (Chang and Lynn, 1986). DMBQ is a commonly found quinone that is released into the rhizosphere as a fungal-mediated lignin degradation product and through the enzymatic oxidation of plant-derived phenolic acids (Thomson, 1987; Siqueira et al., 1991; Kim et al., 1998).
Haustorium ontogeny in Triphysaria can be monitored in vitro by applying DMBQ to the roots of in vitro cultured seedlings. Using this assay we demonstrated that T. versicolor, T. eriantha, and T. pusilla form haustoria at different frequencies when exposed to DMBQ. Differential responsiveness was observed at all DMBQ concentrations, suggesting that there were qualitative differences in DMBQ response in the different species. Within field collections of self-pollinated T. pusilla, distinct sibling families showed differential DMBQ responsiveness. By assaying haustorium development in subsequent generations of self-pollinated T. pusilla, we showed that DMBQ responsiveness was heritable. Interspecific hybrids between responding and non-responding species suggested that maternal effects influenced DMBQ responsiveness. These results indicate that responsiveness to DMBQ is a heritable and highly polymorphic trait in natural populations of Triphysaria.
RESULTS
Haustorium Induction in Vitro by DMBQ
Haustorium development in T. versicolor root tips is characterized by localized root swelling and the emergence of haustorial hairs behind the root tip near the zone of elongation (Fig. 1). The swelling and epidermal hair proliferation was first detected about 6 h after treating the roots with DMBQ. Haustorium development continued for about 24 h at which time the haustoria were competent to attach and invade a host root. Once a mature haustorium was formed, the root tip reverted to its typical developmental program and a morphologically normal root then grew distal to the haustoria. Mock treatment with sterile water did not cause haustorium development under these conditions.
Figure 1.
DMBQ-induced haustorium in T. versicolor. A T. versicolor root tip 24 h after induction with 10 μm DMBQ. Haustorium development includes cortical cell swelling and proliferation of epidermal hairs, followed by a resumption of root tip growth.
T. versicolor radicles were competent to form haustoria within 24 h of emergence from the seed coat. Roots exposed to DMBQ 1, 3, 7, 10, 14, or 21 d after germination did not exhibit any significant differences in propensity to form haustoria (data not shown). Primary and lateral roots formed haustoria.
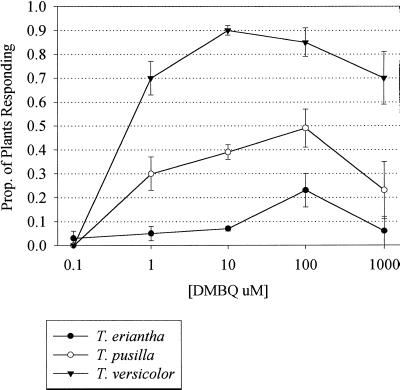
The proportion of plants that form haustoria is a function of DMBQ concentration (Fig. 2). For all species the maximal number of plants with haustoria was obtained when the roots were exposed to between 10 and 100 μm DMBQ. Induction with concentrations of DMBQ greater than 100 μm resulted in root tips becoming brown and necrotic.
Figure 2.
DMBQ concentration-dependent haustorium formation in Triphysaria species. The graph shows the proportion of plants having at least one haustorium in response to increasing concentrations of DMBQ. se bars were calculated as the square root of P(1 − P)/(N − 1), with P representing the proportion of plants forming haustoria and N representing the number of individuals screened at each concentration.
The length of exposure to DMBQ needed to induce haustorium development was determined by exposing T. versicolor roots to 10 μm DMBQ for various lengths of time, then washing the roots free of DMBQ and plating them on fresh media without DMBQ. Maximal induction of haustorium development was not observed with exposure times less than 6 h, indicating that haustorium induction during this 0- to 6-h window can be reversed. After 6 h an apparent threshold is reached that allows haustorium development to continue even when DMBQ is removed (Table I). These results are consistent with those recently reported for DMBQ-induced haustorium development in Striga (O'Malley and Lynn, 2000).
Table I.
DMBQ exposure time required to induce irreversible haustorium development
| Time of Exposure | Proportion of T. versicolor Responding |
|---|---|
| h | |
| 0 | 0.00 |
| 2 | 0.00 |
| 4 | 0.50 |
| 6 | 0.95 |
| 8 | 0.95 |
T. versicolor roots were exposed to 10 μm DMBQ for various lengths of time before being rinsed with sterile water. Twenty plants (n = 20) were screened at each time point. The zero time point represents induction by a water control. The proportion of individuals that formed at least one haustorium is shown. Data for each time point was taken once.
Triphysaria Species Are Differentially Responsive to DMBQ
Seedlings were grown in vitro from field-collected seeds of T. eriantha (TA103), T. pusilla (TA136), and T. versicolor (TA112). After 3 weeks in culture the seedlings had grown about halfway down the Petri plate and had approximately five to 10 roots per plant. We then applied 10 μm DMBQ to the roots and monitored haustorium development for up to 1 week. There were statistically significant differences between species in the proportion of plants that formed haustoria (Table II). Ninety percent of the T. versicolor seedlings, but less than 10% of T. eriantha, formed haustoria. The frequency with which T. pusilla seedlings formed haustoria was intermediate, with about 40% of the plants responding. Similar differences were observed over a range of DMBQ concentrations (Fig. 2).
Table II.
Differential response of Triphysaria species to DMBQ
| Poisson Analysis | T. eriantha (TA103) | T. pusilla (TA136) | T. versicolor (TA112) |
|---|---|---|---|
| Total no. of plants | 330 | 329 | 335 |
| No. of plants with haustoria | 26 | 131 | 303 |
| Frequency of plants with haustoria | 0.08a | 0.40b | 0.90c |
| Total no. of root tips | 1417 | 2748 | 3204 |
| No. of root tips with haustoria | 40 | 284 | 1359 |
| Frequency of root tips with haustoria | 0.03 | 0.1 | 0.42 |
| Average no. of tips/plant | 4.3 | 8.4 | 9.6 |
| Expected frequency of plants with haustoria (μ) | 0.12 | 0.86 | 4.05 |
| Poisson value P(0) | 0.89 | 0.42 | 0.02 |
| Observed no. without haustoria | 304 | 198 | 32 |
| Expected no. without haustoria | 293 | 139 | 6 |
| Observed no. with haustoria | 26 | 131 | 303 |
| Expected no. with haustoria | 37 | 190 | 329 |
| Chi-square value | 3.68d | 43.4e | 115e |
Frequency of plants with haustoria varied significantly between the three species, using a one-way ANOVA at P < 0.001 and Tukey's multiple comparisons test at P ≤ 0.01 (Zar, 1999). Expected values represent the no. of plants without haustoria, assuming a random distribution. Poisson values were obtained using the equation P(X) = (e−μ)(μx)/X!. In the equation, P(X) represents the probability of “X” no. of haustoria occurring per plant and μ is the frequency of haustoria per plant calculated from the average proportion of roots with haustoria and the average no. of roots per plant (Zar, 1999). To determine the expected no. of plants without haustoria, X was set equal to “0.” Goodness of fit of observed and expected values were determined by chi-square at P ≤ 0.05 and P ≤ 0.10, 1df.
P ≤ 0.01.
P ≤ 0.10.
P ≤ 0.05.
The percentage of root tips that formed haustoria also varied between species (Table II). We used the Poisson distribution to determine whether haustoria were equally distributed among all the plants of a given species or whether some plants formed haustoria more readily than others. The Poisson distribution predicts the number of plants expected with and without haustoria as a function of the average number of roots per plant and the proportion without haustoria (Zar, 1999). This calculation was performed independently for each species. For T. pusilla and T. versicolor the number of plants without haustoria was significantly greater (with P ≤ 0.05) than predicted (Table II). This suggests that there are subpopulations within these species that are differentially responsive to DMBQ because the proportion of roots with haustoria were not randomly distributed among the plants. Therefore, subsequent data is represented as the proportion of plants, rather than proportion of root tips that responded to DMBQ. For T. eriantha the number of plants observed without haustoria was not significantly different from the predicted number at P ≤ 0.05, but was at P ≤ 0.10. Because T. eriantha plants rarely formed haustorium in response to DMBQ, the Poisson distribution had little power to distinguish whether they were randomly distributed.
Responsiveness to DMBQ Is Heritable in T. pusilla
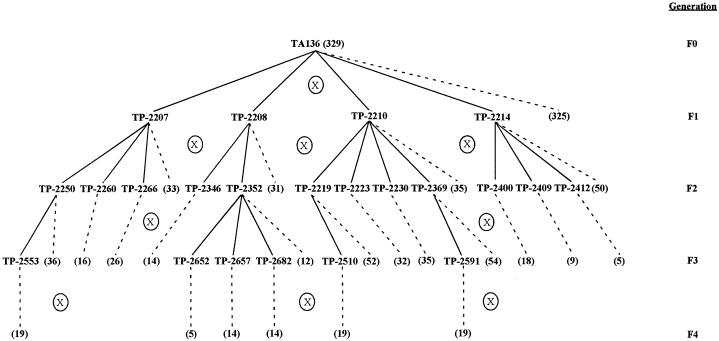
The Poisson analyses predicted that different plants within a given population had differential propensities to form haustoria in response to DMBQ. We determined that these differences were heritable by monitoring haustorium formation in sequential generations of self-pollinated T. pusilla. As described above, about 40% of T. pusilla seedlings grown from seeds pooled from many field-grown plants developed haustoria in vitro. Two haustoria-forming F1 plants (TP-2208 and TP-2214) and two non-forming F1 plants (TP-2207 and TP-2210) were rescued from the assay plates and were transplanted into soil. When the plants were mature the flowers were self-pollinated and progeny seed was harvested from individual plants. Between 33 and 53 F2 individuals from the four sibling families were then assayed for DMBQ responsiveness. Ten percent to 14% of progeny from non-responding parents formed haustoria, whereas 48% to 60% of progeny from responding parents formed haustoria (Table III). Therefore, responding and non-responding parents gave rise to distinctly responsive families, suggesting that DMBQ responsiveness was heritable.
Table III.
DMBQ responsiveness in genetically defined T. pusilla families
| Generation | Parent | Parental Response | N | Proportion of Plants Responding |
|---|---|---|---|---|
| F0 | TA136 | −/+ | 329 | 0.40 |
| F1 | TP-2207 | − | 36 | 0.14 |
| TP-2210 | − | 39 | 0.10 | |
| TP-2208 | + | 33 | 0.48 | |
| TP-2214 | + | 53 | 0.60 | |
| F2 | TP-2219 | − | 53 | 0.00 |
| TP-2223 | − | 32 | 0.13 | |
| TP-2230 | − | 35 | 0.00 | |
| TP-2250 | − | 37 | 0.00 | |
| TP-2260 | − | 16 | 0.13 | |
| TP-2266 | − | 26 | 0.00 | |
| TP-2369 | − | 55 | 0.00 | |
| TP-2346 | + | 14 | 0.50 | |
| TP-2352 | + | 15 | 0.20 | |
| TP-2400 | + | 18 | 1.00 | |
| TP-2409 | + | 9 | 0.44 | |
| TP-2412 | + | 5 | 0.80 |
Generation F0 refers to the progeny of parents that were screened, numbered, and propagated from the pooled, field-collected seed in TA136. Generation F2 refers to the plants grown from self-seed collected from pedigreed parents in generation F1. The parent column lists the pedigree no., prefixed with a “TP,” belonging to the individual plant from which the family is descended (see Fig. 3). N represents the no. of individuals comprising each family. A parental response score of (−) indicates that the parent did not form any haustoria in response to induction with 10 μm DMBQ; a (+) score indicates that the parent formed at least one haustorium in response to induction with 10 μm DMBQ. Responders and non-responders varied significantly in haustorium development according to a one-way ANOVA at P < 0.001 and Tukey's multiple comparisons test at P ≤ 0.01.
Responsive and non-responsive F2 individuals were rescued by transplanting them to soil and they self-pollinated (Fig. 3). Seeds obtained from selfing seven non-responsive and five responsive F2 individuals were assayed with DMBQ and again, responsive and non-responsive parents gave rise to responsive and non-responsive progeny. Only six out of 254 progeny (2%) obtained from non-responsive parents formed haustoria. These six haustoria (+) lines were derived from only two parents. In contrast, almost 60% (36/61) of progeny obtained from responsive parents formed haustoria. All responsive families had at least 20% responding siblings. In the case of one family (TP-2400), all 18 of the examined F3 progeny formed haustoria.
Figure 3.
Pedigree of T. pusilla families. The parental generation (F0) comprised the field-grown plants from which TA136 seeds were collected. Generation F1 were plants germinated from TA136 seed and assayed in vitro for DMBQ responsiveness. After screening, selected plants were rescued from the assay plates and grown to maturity in soil; these individuals were given the pedigree numbers TP-2207 to TP-2214. Seeds were harvested from individual, self-pollinated plants. This second generation (F2) was similarly screened for responsiveness to DMBQ, transplanted to soil, and self-pollinated. The F3 generation was assayed in the same way. Pedigree numbers that follow a solid line represent individual plants. The numbers in parentheses that follow a dashed line represent the number of siblings that were analyzed, but not subsequently rescued.
T. pusilla That Do Not Respond to DMBQ Form Haustoria When Treated with Arabidopsis Exudates
Six families derived from F3 individuals were screened with DMBQ and Arabidopsis exudates (Table IV). The three DMBQ responsive families screened in this generation were all descended from F2 parent TP-2352 (Fig. 3). When treated with DMBQ, 40% to 64% of the progeny in different families formed haustoria. Similar levels of haustoria formation were obtained when treated with Arabidopsis exudates. Overall, 52% (17/33) of the progeny in responding lines formed haustoria with DMBQ, whereas 76% (25/33) formed haustoria with exudates.
Table IV.
Non-responding T. pusilla families form haustoria with Arabidopsis exudates
| Generation | Parent | Parental Line | N | Proportion of Plants Responding
|
|
|---|---|---|---|---|---|
| DMBQ | Exudates | ||||
| Treatment 1 | Treatment 2a | ||||
| F3 | TP-2510 | − | 19 | 0.16 | 0.68 |
| TP-2553 | − | 19 | 0.00 | 0.53 | |
| TP-2591 | − | 19 | 0.00 | 0.37 | |
| Treatment 3a | Treatment 4a | ||||
| TP-2652 | + | 5 | 0.40 | 0.60 | |
| TP-2657 | + | 14 | 0.64 | 0.86 | |
| TP-2682 | + | 14 | 0.43 | 0.71 | |
F3 families were induced with 100 μm DMBQ and Arabidopsis exudates. The parent column lists the pedigree no., prefixed with a “TP,” belonging to the individual plant from which the family is descended (see Fig. 3). N represents the no. of individuals comprising each family. Parental line (−) indicates that the family descends from a DMBQ non-responsive line. Parental line (+) indicates that the family descends from a DMBQ-responsive line. One-way ANOVA of the four treatment groups (non-responders with DMBQ, non-responders with exudates, responders with DMBQ, and responders with exudates) indicated a significant difference among the groups at P < 0.001. Further analysis with Tukey's multiple comparisons test (Zar, 1999) showed the non-responding families induced with DMBQ to be significantly different from the other three treatment groups at P ≤ 0.01. Non-responding families induced with Arabidopsis exudates and responding families induced with DMBQ and exudates were not significantly different at P ≤ 0.01.
P ≤ 0.01.
Three non-responsive families were also screened with DMBQ and Arabidopsis exudates (Table IV). Consistent with the observations from previous generations, progeny from non-responding lines made few haustoria when treated with DMBQ (3/57, or 5%). In contrast, these plants did form haustoria when treated with exudates (Table IV). Twenty-nine of 57 individuals, or 51% of the non-responding family members, developed haustoria in response to Arabidopsis exudates. Using Tukey's multiple comparisons test (Zar, 1999), there were no significant differences in the number of haustoria formed between non-responding plants induced with exudates and responding plants induced with exudates or DMBQ. The non-responding lines treated with DMBQ were significantly different. Therefore the DMBQ non-responsive plants are not defective in haustoria formation, but are unresponsive to treatment with a specific haustorial-inducing factor, DMBQ.
DMBQ Responsiveness in Intra- and Interspecific Hybrids of T. eriantha and T. versicolor
We evaluated DMBQ responsiveness in maternal families harvested from individual field-grown plants of T. versicolor and T. eriantha. Because these species are self-incompatible and were growing allopatricly in the areas we collected, these were typically intraspecific hybrids. T. versicolor families had a higher proportion of progeny responding to DMBQ (80%–95%) than T. eriantha families (11%–48%) (Table V). The proportion of responding progeny averaged over the three T. eriantha families (27%) was higher than obtained when seeds were pooled (TA103) from hundreds of plants (Table II). This is primarily due to one plant (TE146–3) having an aberrantly high number of responding progeny (48%). As with T. pusilla, there appears to be differential responsiveness to DMBQ between different individuals within a single field population.
Table V.
DMBQ responsiveness of F1 families between T. eriantha and T. versicolor
| Species/Hybrid | N | Proportion Responding |
|---|---|---|
| Intraspecific hybrids | ||
| T. versicolora | ||
| TV145-1 | 79 | 0.80 |
| TV145-2 | 92 | 0.86 |
| TV145-3 | 81 | 0.95 |
| T. erianthab | ||
| TE146-1 | 46 | 0.22 |
| TE146-2 | 45 | 0.11 |
| TE146-3 | 93 | 0.48 |
| Interspecific hybrids | ||
| T. versicolor × T. eriantha F1sc | ||
| TV2191 × TE2198 | 9 | 0.89 |
| TV105-1 × TE102-1 | 85 | 0.80 |
| TV145-1 × TE146-1 | 50 | 0.52 |
| TV145-3 × TE146-2 | 53 | 0.53 |
| TV2450 × TE2475 | 45 | 0.76 |
| T. eriantha × T. versicolor F1sb | ||
| TE2198 × TV2191 | 48 | 0.44 |
| TE102-1 × TV105-1 | 28 | 0.43 |
| TE146-1 × TV145-1 | 41 | 0.24 |
| TE146-3 × TV145-2 | 39 | 0.49 |
| TE2431 × TV2424 | 33 | 0.24 |
| TE2434 × TV2427 | 45 | 0.24 |
Individuals prefixed with “TE” represent T. eriantha plants and individuals prefixed with “TV” represent T. versicolor plants. Those individuals with a three-digit label followed by a dashed no. represent plants that were collected in the field after open pollination was allowed to occur, generating the intraspecific families. They were then brought back to the greenhouse where cross-pollinations were performed to generate the interspecific families. Individuals numbered with a “TE” or “TV” prefix, followed by a four-digit numeral represent those that were propagated from seed in a laboratory setting and grown in a controlled environment facility where the crosses were performed. Crosses are written as female × male. ANOVA indicated that there were significant differences among the groups of hybrid families at P < 0.001.
Tukey's multiple comparisons test confirmed that there were significant differences between the groups at P ≤ 0.01 (Zar, 1999). The T. eriantha intraspecific hybrids and the T. eriantha × T. versicolor F1s were not significantly different at P ≤ 0.01.
Reciprocal crosses were successfully achieved with two parent pairs (TE2191 × TV2198 and TV145–1 × TE146–1). The direction of the cross significantly influenced the expression of hybrid responsiveness in each case (Table V). When T. versicolor was maternal parent, 58% of progeny (34/59) made haustoria, whereas in reciprocal crosses only 34% of progeny (30/89) responded.
Seven additional F1 populations, three from a maternal T. versicolor and four from a maternal T. eriantha, were examined. When T. versicolor was the mother, 80%, 76%, and 53% of the progeny responded, in contrast to crosses with T. eriantha as the mother where 24%, 24%, 43%, and 49% of progeny responded (Table V). Therefore, there is a strong maternal component to the transmission of DMBQ responsiveness.
DISCUSSION
Triphysaria is an annual hemiparasite common to grassland stands along coastal bluffs, hot inland valleys, and mountain foothills. Ecological studies of T. pusilla report host associations with up to 33 different species residing in 16 families. In the field a single Triphysaria plant can invade multiple host plants of the same or different species (Marvier, 1998b). As an annual, Triphysaria must have the ability to recognize and associate with new host plants on a yearly basis. Dominant species in these communities vary greatly from year to year, and to survive over time the hemiparasite population needs to maintain the ability to parasitize a broad spectrum of host plants (McNaughton, 1968).
Although hemiparasitic plants have the ability to photosynthesize and can survive without a host, they are unlikely to be competitive and reproductively fit in a natural setting without the benefit of host nutrients (Thurman, 1966; Marvier, 1998b). Attachment of a single parasite to multiple host species enhanced growth of the hemiparasitic plant, Castilleja wightii, due to increased variety of nutrients available to the parasite and enhanced resistance to herbivory (Marvier, 1998a). However, not all host plants afford equal advantages to the hemiparasite. Legumes have frequently been described as preferred hosts, presumably for their rich reserve of nitrogenous resources (Gibson and Watkinson, 1989; Matthies, 1996). When the hemiparasite Castilleja exerta was grown with different host plants in a “no choice” pot experiment, there was marked increase in fecundity with some, but not all, hosts. In some cases, associations with a host decreased fecundity compared with autotrophic growth (Atsatt and Strong, 1970). It seems reasonable that secondary metabolites produced by some species will be inhibitory to growth. Therefore, in the long term, hemiparasites must compromise between invading all available roots for nutrient uptake and selectively avoiding detrimental host associations.
It is unclear from field studies whether all Triphysaria individuals parasitize a broad range of hosts or whether different individuals in a highly polymorphic seed bank encompass multiple host recognition determinants. A single Triphysaria plant will produce hundreds of seeds, most of which fall to the ground for lack of animal vectors. Because only a small fraction of these produce plants in subsequent years, it is possible that different host recognition races of the hemiparasite are successful each year. The obligate parasites Striga and Orobanche need host-specific factors to germinate, a mechanism that ensures the presence of appropriate host roots before these parasites commit to growth (Parker and Riches, 1993). Triphysaria and other generalist hemiparasites do not need host-derived germination factors. If host discriminatory mechanisms exist in these hemiparasites, they must function post-germination. The first stage in host recognition and invasion for the hemiparasite is initiation of haustorium development.
Multiple chemicals have been isolated that induce haustorium development in Triphysaria and other Scrophulariaceae (Riopel and Timko, 1995). We have used a well-characterized inducing factor, DMBQ, to assay Triphysaria populations for heritable differences in recognition of a host signal (Chang and Lynn, 1986). In studies with Striga asiatica, six or more hours of exposure to micromolar concentrations of DMBQ were required to irreversibly initiate haustorium development (Smith et al., 1990). We see a similar pattern of haustorium development in Triphysaria, where 6 h of exposure to 10 μm DMBQ is needed to complete the developmental program (Table I). In both systems haustorial swelling is visible in newly emergent radicles approximately 6 to 8 h post-induction, and a mature haustorium is formed within 24 h. Similarities in DMBQ concentration requirement and temporal development of haustoria suggest that fundamentally equivalent host recognition mechanisms exist in these species.
We have found that different populations of Triphysaria respond differentially to DMBQ as a haustorial-inducing factor. When examining the progeny of individual field-collected plants of each species, there were significant differences in DMBQ responsiveness between maternal families, indicating a high level of polymorphism for this trait within these populations. These differences were not dependent on DMBQ concentration or exposure time, suggesting a qualitative nature to the discrimination. DMBQ responsiveness was a heritable trait, as seen by breeding for differential responsiveness in subsequent generations of self-pollinated T. pusilla. A previous study demonstrated that the related hemiparasite, C. exerta, could be bred for increased autotrophic growth (Atsatt and Strong, 1970). It has also been observed that genetically isolated populations of the obligate parasite Striga develop host preferences through selection for host germination signals in the field (Parker and Riches, 1993). However, our research is the first account of heritability in responsiveness to a specific haustorial-inducing factor.
Responsive and non-responsive T. pusilla families formed haustoria when induced with Arabidopsis root exudate, indicating that DMBQ non-responsive plants were capable of forming haustoria. This result suggests that the Arabidopsis exudates contained inducers other than DMBQ and that non-responding plants were capable of recognizing one or more of these to initiate haustorium development. Therefore, there is heritable variation in the recognition of host factors in natural populations of Triphysaria. The distribution of host recognition variants in hemiparasite populations will be influenced by annual changes in dominant host species.
There was a significant difference in DMBQ responsiveness between T. versicolor and T. eriantha. F1 progeny generated by cross-hybridizing individuals from these two populations responded to DMBQ in a manner that was strongly influenced by the choice of maternal parent. F1 progeny generated using T. eriantha as maternal parent were less responsive to DMBQ than those having T. versicolor as mothers. Of the 11 hybrid families evaluated, not one exhibited a level of DMBQ responsiveness that was similar to the paternal parent. In fact statistical analysis showed that the T. eriantha × T. versicolor hybrids were not significantly different from the T. eriantha hybrids in their response. The maternal effect operating on DMBQ responsiveness was particularly evident in reciprocal crosses (Table V). These results show that there are maternal factors influencing DMBQ responsiveness in Triphysaria.
Variation due to a maternal effect has been reported in other members of the Scrophulariaceae as well. The non-parasitic annual Collinsia verna has a number of size-related traits that are significantly impacted by maternal inheritance (Thiede, 1998). Also, a high level of variation in the number of mtDNA haplotypes has been reported for isolated populations of Penstemon haydenii (Caha et al., 1998). From an evolutionary standpoint, traits most influenced by maternal effects tend to be those important in the early life cycle of a plant (Thiede, 1998). Characters influenced by maternal effects often play a significant role in survival and fecundity, as well as potentially changing the rate at which a population will evolve in response to environmental selection (Rossiter, 1996). Therefore, responsiveness to specific host signals, in this case DMBQ, may play an important role early in the life cycle of Triphysaria. Host recognition determinants may also function in facilitating hemiparasite fitness in a host environment that fluctuates year to year.
MATERIALS AND METHODS
Seeds and Plants
All seed collections labeled with the prefix “TA” were harvested directly from grassland stands around northern California. Collections TA103 (T. eriantha subsp. eriantha [Benth.] Chuang and Heckard), TA112 (T. versicolor subsp. faucibarbata [A. Gray] Fischer and C. Meyer), and TA136 (T. pusilla [Benth.] Chuang and Heckard) represent pooled populations of seed harvested from hundreds of individual plants within about a 1 ha area. Individual plants were also removed from the ground, transplanted into 5-gallon containers, and transported to the greenhouse where they were used for hybridizations. The seeds obtained from these individuals include the collections TE102, TE144, and TE146 (T. eriantha subsp. eriantha), and collections TV105 and TV145 (T. versicolor subsp. faucibarbata).
Assay for Haustorium Development
Triphysaria seeds were sterilized using a solution of 50% (v/v) bleach (sodium hypochlorite 2.13% [w/v] final) and 0.1% (v/v) Triton X-100 (Sigma, St. Louis), and were then thoroughly rinsed in 4 to 6 volumes of sterile de-ionized water. The seeds were then placed in round Petri dishes (100 × 25 mm) containing 0.25× Hoagland nutrient media [1.25 mm Ca(NO3)2, 1.25 mm KNO3, 0.25 mm KH2PO4, 0.50 mm MgSO4, and micronutrients (50 μm H3BO3, 9.0 μm MnCl2·4H2O, 70 nm ZnSO4·7H2O, 30 nm CuSO4·5H2O, and 10 nm Na2MoO4·2H2O)] (Johnson, 1977), 1% (w/v) Suc, and 0.5% (w/v) Phytagar (Gibco-BRL Life Technologies, Rockville, MD). The plates were sealed with parafilm and placed in a 16°C growth chamber under a 12-h light regimen. The Triphysaria seeds germinated after 7 to 10 d under these conditions.
Approximately 2 weeks post-germination, Triphysaria seedlings were aseptically transferred to square Petri plates containing 0.25× Hoagland nutrient media, 1% (w/v) Suc, and 1% (w/v) Phytagar. In each plate, five seedlings were placed parallel to one another on the surface of the agar media. The plates were then wrapped with micropore tape (3 M, Health Care, St. Paul) and placed in racks, nearly vertical, to facilitate the growth of the root tips down the surface of the media. The seedlings were incubated in a 22°C growth chamber under a 16-h light regimen for 1 week prior to induction.
Induction of Triphysaria root tips was achieved by adding 2 mL of 10 μm DMBQ (Pfaltz and Bauer, Inc., Waterbury, CT) or hydroponically isolated Arabidopsis root exudates directly to the root tips. Control seedlings were mock-treated with sterile de-ionized water. Plates were kept horizontal for at least 30 min to allow absorption of the liquid inducer into the agar media.
To determine the minimum DMBQ exposure time needed to induce haustoria, plants were exposed to DMBQ for 0, 2, 4, 6, or 8 h. They were then removed from the induction plate, rinsed three times in sterile, de-ionized water, and placed on the surface of fresh media without DMBQ. Plates were re-wrapped with micropore tape and returned to the 22°C growth room for 1 to 7 d.
The number of haustoria and the number of root tips per plant was determined using a dissecting microscope at a magnification of 0.6 to 4×.
Preparation of Arabidopsis Exudates
Arabidopsis seeds were surface sterilized in 70% (v/v) ethanol for 5 min, followed by 10 min in a solution of 30% (v/v) bleach (sodium hypochlorite 1.58% [w/v] final) and 0.15% (v/v) Triton X-100 (Sigma), were then rinsed in 4 to 6 volumes of sterile de-ionized water. Using a sterile 1-mL Pasteur pipette, approximately 30 mg of seed was placed into 250-mL flasks containing 50 mL 0.5× Murashige and Skoog salts (Gibco-BRL, Grand Island, NY), 1× Nitsch and Nitsch vitamin solution (Sigma), and 0.075% (w/v) Suc at pH 5.8. The flasks were placed on a shaker at 50 rpm in 22°C with 16 h of light. After 3 weeks the plants were removed from the media, which was filter sterilized through a 0.2-μm filter (Nalge Nunc International, Rochester, NY) and stored at −20°C. The final concentration correlated to 14 mg of dry plant weight per 1 mL of exudate.
Triphysaria Growth and Genetic Hybridizations
After evaluating haustoria development in vitro, selected Triphysaria were transplanted to a mixture of 50% (w/w) sand and 50% (w/w) Sunshine Mix No. 1 soil (Sun Gro Horticulture, Bellevue, WA) in 2-inch square pots. The plants were grown at 16°C in a controlled environment chamber with 80% relative humidity and 12 h of light. Plants were subirrigated daily with 0.25× Hoagland solution.
After several weeks the Triphysaria began to flower and crosses were made using a surgical scalpel. Pollen was collected from donor anthers on the tip of a scalpel and transferred to the recipient's stigma. Between pollinations the scalpel was cleaned with 70% (v/v) ethanol. Because T. eriantha and T. versicolor are strongly self-incompatible, emasculation of the recipient flower was unnecessary (Yoder, 1998). T. pusilla flowers are strongly cleistogamous and crossing attempts with T. pusilla were unsuccessful.
Seed capsules were visible within 2 weeks of pollination and were collected over a period of 6 to 8 weeks. Capsules were stored in no. 1 coin envelopes for several weeks to dry and seeds were recovered using U.S.A. standard testing sieve No. 20 and a collection pan (Fisher Scientific Company, Pittsburgh). The seeds were subsequently stored at room temperature in 1.5-mL microfuge tubes.
ACKNOWLEDGMENTS
We thankfully acknowledge the helpful comments and insight of all members of the Yoder Lab, past and present, especially Drs. Huguette Albrecht, Elizabeth Estabrook, Marta Matvienko, and Russell Wrobel.
Footnotes
This work was funded by the U.S. Department of Agriculture National Research Initiative Competitive Grants Program (grant no. 97–01934). D.S.J. has been supported by fellowships from the University of California Systemwide Biotechnology and Education Program in Rhizosphere Biology and by the University of California Davis Biotechnology Training Program.
LITERATURE CITED
- Albrecht H, Yoder JI, Phillips DA. Flavonoids promote haustoria formation in the root parasite Triphysaria. Plant Physiol. 1998;119:585–591. doi: 10.1104/pp.119.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atsatt PR. The population biology of annual grassland hemiparasites: II. Reproductive patterns in Orthocarpus. Evolution. 1970;24:598–612. doi: 10.1111/j.1558-5646.1970.tb01794.x. [DOI] [PubMed] [Google Scholar]
- Atsatt PR, Strong DR. The population biology of annual grassland hemiparasites: I. The host environment. Evolution. 1970;24:278–291. doi: 10.1111/j.1558-5646.1970.tb01761.x. [DOI] [PubMed] [Google Scholar]
- Caha CA, Lee DJ, Stubbendieck J. Organeller genetic diversity in Penstemon haydenii (Scrophulariaceae): an endangered plant species. Am J Bot. 1998;85:1704–1709. [PubMed] [Google Scholar]
- Chang M, Lynn DG. The haustorium and the chemistry of host recognition in parasitic angiosperms. J Chem Ecol. 1986;12:561–579. doi: 10.1007/BF01020572. [DOI] [PubMed] [Google Scholar]
- Chuang TI, Heckard LR. Chromosomal numbers of Orthocarpus and related monotypic genera (Scrophulariaceae: subtribe Castillejinae) Brittonia. 1982;34:89–101. [Google Scholar]
- Chuang TI, Heckard LR. Generic realignment and synopsis of subtribe Castillejinae (Scrophulariaceae-tribe Pediculareae) Syst Bot. 1991;16:644–666. [Google Scholar]
- dePamphilis CW, Young NN, Wolfe AD. Evolution of plastid gene rps2 in a lineage of hemiparasitic and holoparasitic plants: many losses of photosynthesis and complex patterns of rate variation. Proc Natl Acad Sci USA. 1997;93:7367–7372. doi: 10.1073/pnas.94.14.7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estabrook EM, Yoder JI. Plant-plant communications: rhizosphere signaling between parasitic angiosperms and their hosts. Plant Physiol. 1998;116:1–7. [Google Scholar]
- Gibson CC, Watkinson AR. The host range and selectivity of a parasitic plant: Rhinanthus minor L. Oecologia. 1989;78:401–406. doi: 10.1007/BF00379116. [DOI] [PubMed] [Google Scholar]
- Hickman JC. The Jepson Manual: Higher Plants of California. Berkeley: University of California Press; 1993. [Google Scholar]
- Johnson H. Hydroponics: A Guide to Soil-Less Culture Systems. Science leaflet 2947. Davis: Division of Agriculture, University of California; 1977. [Google Scholar]
- Kim D, Kocz R, Boone L, Keyes WJ, Lynn DG. On becoming a parasite: evaluating the role of wall oxidases in parasitic plant development. Chem Biol. 1998;5:103–117. doi: 10.1016/s1074-5521(98)90144-2. [DOI] [PubMed] [Google Scholar]
- Kuijt J. The Biology of Parasitic Flowering Plants. Berkeley: University of California Press; 1969. [Google Scholar]
- Matthies D. Interactions between the root hemiparasite Melampyrum arvense and mixtures of host plants: heterotrophic benefit and parasite-mediated competition. Oikos. 1996;75:118–124. [Google Scholar]
- Marvier MA. A mixed diet improves performance and herbivore resistance of a parasitic plant. Ecology. 1998a;79:2616–2623. [Google Scholar]
- Marvier MA. Parasite impacts on host communities: plant parasitism in a California coastal prairie. Ecology. 1998b;79:82616–2623. [Google Scholar]
- McNaughton SJ. Structure and function in California grasslands. Ecology. 1968;49:962–973. [Google Scholar]
- Nickrent DL, Duff RJ, Colwell AE, Wolfe AD, Young ND, Steiner KE, dePamphilis CW. Molecular phylogenetic and evolutionary studies of parasitic plants. In: Soltis DE, Soltis PS, Doyle JJ, editors. Molecular Systematics ofPlants II. DNA Sequencing. Boston, MA: Kluwer Academic Publishers; 1998. [Google Scholar]
- O'Malley RC, Lynn DG. Expansin message regulation in parasitic angiosperms: marking time in development. Plant Cell. 2000;12:1445–1465. doi: 10.1105/tpc.12.8.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C, Riches CR. Parasitic Weeds of the World: Biology and Control. Wallingford, Oxon, UK: CAB International; 1993. [Google Scholar]
- Press MC, Graves JD. Parasitic Plants. New York: Chapman & Hall; 1995. [Google Scholar]
- Rossiter MC. Incidence and consequences of inherited environmental effects. Annu Rev Ecol Syst. 1996;27:451–476. [Google Scholar]
- Siqueira JO, Nair MG, Hammerschmidt R, Safir GR. Significance of Phenolic Compounds in Plant-Soil-Microbial Systems. Boca Raton, FL: CRC Press; 1991. pp. 63–121. [Google Scholar]
- Smith CE, Dudley MW, Lynn DG. Vegetative/parasitic transition: control and plasticity in Striga development. Plant Physiol. 1990;93:208–215. doi: 10.1104/pp.93.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CE, Ruttledge T, Zeng Z, O'Malley RC, Lynn DG. A mechanism for inducing plant development-the genesis of a specific inhibitor. Proc Natl Acad Sci USA. 1996;93:6986–6991. doi: 10.1073/pnas.93.14.6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiede DA. Maternal inheritance and its effect on adaptive evolution: a quantitative genetic analysis of maternal effects in a natural plant population. Evolution. 1998;52:998–1015. doi: 10.1111/j.1558-5646.1998.tb01829.x. [DOI] [PubMed] [Google Scholar]
- Thomson RH. Naturally Occurring Quinones III: Recent Advances. Ed 3. New York: Chapman & Hall; 1987. [Google Scholar]
- Thurman LD. Genecological studies in Orthocarpus subgenus Triphysaria (Scrophulariaceae). PhD Thesis. Berkeley: University of California; 1966. [Google Scholar]
- Riopel JL, Timko MP. Haustorial initiation and differentiation. In: Press MC, Graves JD, editors. Parasitic Plants. New York: Chapman & Hall; 1995. pp. 39–79. [Google Scholar]
- Yoder JI. Self and cross-compatibility in three species of the hemiparasite Triphysaria. Environ Exp Bot. 1998;39:77–83. [Google Scholar]
- Zar JH. Biostatistical Analysis. Ed 4. Upper Saddle River, NJ: Prentice Hall; 1999. pp. 571–589. [Google Scholar]