Abstract
The human coronavirus disease 2019 (COVID-19) pandemic has affected overall healthcare delivery, including prenatal, antenatal and postnatal care. Hyperglycemia in pregnancy (HIP) is the most common medical condition encountered during pregnancy. There is little guidance for primary care physicians for providing delivery of optimal perinatal care while minimizing the risk of COVID-19 infection in pregnant women. This review aims to describe pragmatic modifications in the screening, detection and management of HIP during the COVID- 19 pandemic. In this review, articles published up to June 2021 were searched on multiple databases, including PubMed, Medline, EMBASE and ScienceDirect. Direct online searches were conducted to identify national and international guidelines. Search criteria included terms to extract articles describing HIP with and/or without COVID-19 between 1st March 2020 and 15th June 2021. Fasting plasma glucose, glycosylated hemoglobin (HbA1c) and random plasma glucose could be alternative screening strategies for gestational diabetes mellitus screening (at 24–28 weeks of gestation), instead of the traditional 2 h oral glucose tolerance test. The use of telemedicine for the management of HIP is recommended. Hospital visits should be scheduled to coincide with obstetric and ultrasound visits. COVID-19 infected pregnant women with HIP need enhanced maternal and fetal vigilance, optimal diabetes care and psychological support in addition to supportive measures. This article presents pragmatic options and approaches for primary care physicians, diabetes care providers and obstetricians for GDM screening, diagnosis and management during the pandemic, to be used in conjunction with routine antenatal care.
Keywords: COVID-19, gestational diabetes mellitus, hyperglycemia, pandemic, pre-gestational diabetes mellitus, pregnancy
Introduction
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) has had an immense impact on healthcare systems globally, including the continuum of care during the prenatal, antenatal and postnatal periods.[1,2] Hyperglycemia is reported to be the most frequent medical condition seen during pregnancy. According to 2019 estimates by the International Diabetes Federation (IDF), one in six live births (16.8%) occur in women with some form of hyperglycemia in pregnancy (HIP).[3] HIP could present as pre-existing or newly detected overt diabetes in pregnancy (DIP) or gestational diabetes mellitus (GDM) [Figure 1].[3,4] It is associated with adverse pregnancy outcomes and transgenerational impact on the offspring.[4,5] Prompt detection and appropriate management is, therefore, critical but frequent healthcare visits increase the risk of infection.[1] COVID-19 in pregnant women with hyperglycemia further increases the risk of complications.[6,7]
Figure 1.
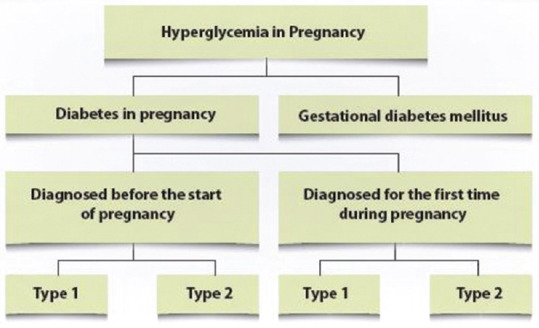
Classification of Hyperglycemia in Pregnancy (2; Adapted from WHO 2013)
Therefore, there is an urgent need to formulate strategies that balance continued delivery of optimal healthcare, most of which is provided by primary care physicians (PCPs), with the obligation to minimize the risk of infection in this vulnerable population.[8,9,10] In a rapidly evolving pandemic situation, there is a dearth of published literature to guide PCPs, obstetricians, and diabetologists engaged in the detection and management of HIP.
The main objective of this review was to assess current guidelines and alternate strategies for the detection and management of HIP during the COVID-19 pandemic and suggest a pragmatic clinical approach PCPs, diabetes care providers and obstetricians.
Methods
The PubMed, Medline, Embase, and ScienceDirect databases were searched for articles published up to 15th June 2021, using the keywords ‘SARS-CoV-2,’ ‘coronavirus,’ ‘hyperglycemia in pregnancy (HIP),’ ‘antenatal care,’ ‘gestational diabetes mellitus (GDM),’ ‘gestational diabetes clinical practices, or recommendation.’ All guidelines and recommendations that have been issued as temporary alternative strategies for the detection and management of GDM and/or DIP were evaluated.[11,12,13,14,15,16,17,18,19,20] We also reviewed the global interim guidance on COVID-19 during pregnancy and puerperium from the International Federation of Gynecologists and Obstetricians (FIGO) and allied partners.[21] All searches were limited to publications and guidelines in the English language. Since this manuscript is based on a review of existing literature and no additional human studies were conducted, ethical or institutional permission was not required.
Screening for Hyperglycemia in Pregnancy during the Pandemic
Early detection of HIP is critical because it facilitates timely and appropriate management. Most guidelines recommend assessment of glycemic status at the first antenatal visit with fasting plasma glucose (FPG) or random plasma glucose (RPG) and/or glycosylated hemoglobin (HbA1c) to detect pre-existing diabetes or early GDM. In women with normal results at early screening, repeat testing during the second trimester is recommended. The 75-g 2-h oral glucose tolerance test (OGTT) is recommended as the gold standard for the detection of GDM between 24 and 28 weeks.[3,4,11,22,23,24,25,26,27] However, there exists significant heterogeneity in the screening methods (universal versus risk-based screening, one-step versus two-step screening, use of 50-g versus 75-g glucose load and the cut-off values for plasma glucose) [Table 1]. Diabetes in Pregnancy Study Group India (DIPSI) recommends one-step universal screening test which is simple, feasible and cost-effective, especially in resource-limited settings.[12,22,28,29]
Table 1.
| Guidelines | FPG mg/dl (mmol/l) | Glucose Challenge | 1-hour plasma glucose mg/dl (mmol/l) | 2-hour plasma glucose mg/dl (mmol/l) |
|---|---|---|---|---|
| WHO 2013[4]# | ≥92 (5.1) | 75g OGTT | ≥180 (10.0) | ≥153 (8.5) |
| ACOG 2018[1]### | ≥95 (5.3) | 100g OGTT | ≥180 (10.0) | ≥155 (8.6) |
| Canadian Diabetes Association 2018[27]### | ≥95 (5.3) | 75g OGTT | ≥191 (10.6) | ≥162 (9) |
| IADPSG 2010[23]#### | ≥92 (5.1) | 75g OGTT | ≥180 (10.0) | ≥153 (8.5) |
| DIPSI 2010[12,22]# | Not required | 75g OGTT | Not required | ≥140 (7.8) |
| ADA 2015[25]# | ≥92 (5.1) | 75g OGTT | ≥180 (10.0) | ≥153 (8.5) |
| Australia 2014[11]# | ≥92 (5.1) | 75g OGTT | ≥180 (10.0) | ≥153 (8.5) |
| FIGO 2017[3]# | ≥ 92 (5.1) | 75g OGTT | ≥180 (10.0) | ≥153 (8.5) |
#1 value sufficient for diagnosis, ##≥2 values required for diagnosis; ###≥ 2 values required for diagnosis, ####1 value is sufficient for diagnosis. ADA: American Diabetes Organization; ACOG: American College of Obstetricians and Gynecologists; DIPSI: Diabetes in Pregnancy Study Group in India; FIGO: International Federation of Gynecology and Obstetrics; GCT: Glucose challenge test; IADPSG: International Association of Diabetes and Pregnancy Study Groups; OGTT: Oral glucose tolerance test
However, OGTT requires visiting and spending time at the sample collection center and multiple blood samples. This inadvertently increases the women's risk of exposure to COVID-19 infection and places greater burden on PCPs dealing with a pandemic and calls for a need to find pragmatic alternatives.[8,10]
Various countries have suggested modifications to the algorithm for GDM screening during the pandemic, but these have not been sufficiently validated.[13,14,15,16,17,18,19,20,21,30,31] Fasting or random plasma glucose and/or HbA1c have been suggested in place of the 2-hour OGTT. Table 2 provides a comparison between pre-existing guidelines and revised temporary guidance during the pandemic. Screening methods for GDM in women with risk factors during the evolving COVID-19 pandemic are depicted in Figure 2.[20]
Table 2.
Revised Temporary Recommendations for Screening for GDM during COVID-19 pandemic
| Country | Recommending Body for revised recommendation | Pre-existing Guidelines | Revised Temporary Guidelines |
|---|---|---|---|
| UK | RCOG Guidance for maternal medicine services in the evolving COVID-19 pandemic | Recommend NICE practice guidelines with 2-h OGTT in high-risk pregnant women at 24-28 weeks. 2-h OGTT with FPG ≥100 mg/dl (5.6 mmol/L) or 2-hour value ≥140 mg/dl (7.8 mmol/L) is diagnostic of GDM.[26] | HbA1c and RPG should be measured along with routine blood tests at the initial visit. HbA1c ≥6.5% or RPG ≥200 mg/dl (11.1 mmol/L) - overt diabetes; HbA1c 5.9-6.4% or RPG 162-199 mg/dl (9-11 mmol/L) - GDM. Measure HbA1c and FPG or RPG at 28 weeks in all high-risk women. FPG ≥100 mg/dl (5.6 mmol/L), HbA1c ≥5.7% or RPG ≥162 mg/dl (9 mmol/L) defines GDM. Consider FPG ≥95 mg/dl (5.3 mmol/L) as diagnostic of GDM if resources allow to further improve detection rates. If a woman has clinical suspicion of diabetes at any time during pregnancy (heavy glycosuria, nocturia, polydipsia, large for gestational age or polyhydramnios), she should be tested for GDM.[20] |
| Canada | Joint Consensus Statement by the Diabetes Canada Clinical Practice Guidelines Steering Committee and the Society of Obstetricians and Gynecologists of Canada | 2018 Diabetes Canada Clinical Practice Guidelines of Diabetes and Pregnancy: High-risk women should be screened for overt diabetes in early pregnancy with HbA1c and/or FPG if HbA1c is unreliable. For all women, re-screening is recommended at 24-28 weeks with 50g glucose challenge followed by 75g OGTT if 1-hour glucose value is 140-199 mg/dL (7.8-11.0 mmol/L).[27] | Screening of high-risk women in early pregnancy with HbA1c and/or FPG for overt diabetes remains unaltered during the pandemic. At 24-28 weeks, all pregnant women should be screened with an HbA1c and RPG. If HbA1c <5.7% or RPG is <200 mg/dl (11.1 mmol/L), no further action is required but testing can be repeated if there is high clinical suspicion of diabetes. If HbA1c ≥5.7% or RPG ≥200 mg/dl (11.1 mmol/L), they are diagnosed and managed as GDM.[15] |
| Italy | Position statement of the Italian Association of Clinical Diabetologists (AMD) and the Italian Diabetes Society (SID), Diabetes, and Pregnancy Study Group | All pregnant women should be screened for the presence of overt diabetes. The criterion for the diagnosis of overt diabetes is either FPG ≥126 mg/dL (7 mmol/L) or RPG ≥200 mg/dL (11.1 mmol/L), or HbA1c ≥6.5% (13). OGTT is recommended at 16-18 weeks in those women who are at high risk (obesity with BMI >30 kg/m2, previous GDM with a FPG 100-125 mg/dl), and is repeated at 24-28 weeks if the first test was normal. An OGTT at 24-28 weeks is recommended in women at medium risk (age >35 years, overweight, previous GDM or fetal macrosomia, family history of type 2 diabetes or high-risk ethnicity).[13] | Screening for overt diabetes in early pregnancy remains same. When the OGTT cannot be safely performed, FPG ≥92 mg/dL (5.1 mmol/L) alone can be used as a surrogate marker for the diagnosis of GDM. Measurement of FPG is recommended at 16-18 weeks of gestation in those women who are at high risk. GDM is diagnosed if FPG ≥92 mg/dl (5.1 mmol/L). If FPG is <92 mg/dl (5.1 mmol/L), test is repeated at 24-28 weeks. In women at medium risk, a single measurement of FPG is recommended at 24-28 weeks. However, if the OGTT can be safely performed, compliance with social distancing precautions must be followed.[13] |
| Australia and New Zealand | ADIPS, ADS, ADEA, DA, RANZOG, Queensland | In women at high risk, OGTT should be done in first trimester. For women who have not been diagnosed with diabetes, 2-hour formal OGTT is recommended at 24-28 weeks. On OGTT, GDM is diagnosed if FPG ≥92 mg/dL (5.1 mmol/L), 1-hour value is ≥180 mg/dl (10 mmol/L) or 2- hour value is ≥153 mg/dl (8.5 mmol/L).[16,17] | First trimester HBA1c is recommended in high-risk women - GDM is diagnosed if HbA1c is ≥5.9%. If HbA1c is <5.9% and in all other women, FPG is measured at 24-28 weeks. If FPG <85 mg/dL (4.7 mmol/L), OGTT is not required; if FPG ≥92 mg/dL (5.1 mmol/L), GDM is diagnosed and if FPG 85-92 mg/dl (4.7-5.0 mmol/L), OGTT is recommended. Women with previous GDM may be assumed to have GDM. In areas of low risk and where OGTT can be safely performed with adequate precautions, it should be considered as routine.[16-18] |
| Bangladesh | Bangladesh Endocrine Society | Screening during first antenatal visit with FPG, RPG or HbA1c. 2-hour OGTT at 24-28 weeks in all women who have not been diagnosed with overt diabetes or GDM earlier. | For women at high risk of GDM, measure HbA1c or RPG: HbA1c >6.5 or RPG ≥200 mg/dL (11.1 mmol/L) - overt diabetes; HbA1c 6-6.5% or RPG 162-199mg/dL (9-11 mmol/L) - GDM; HbA1c<6% or RPG <162 mg/dL (9 mmol/L) - reassess at 28 weeks with HbA1c, FPG or RPG. FPG>100 mg/dL (5.6 mmol/L), RPG >162 mg/dL (9 mmol/L) or HbA1c >5.7% - GDM[30] |
ADEA: Australian Diabetes Educators Society; ADIPS: Australasian Diabetes in Pregnancy Society; ADS: Australian Diabetes Society; BMI: body mass index; COVID-19: coronavirus disease 2019; DA: Diabetes Australia; FPG: Fasting plasma glucose; GDM: gestational diabetes mellitus; HbA1c: glycosylated hemoglobin; NICE: National Institute of Clinical Excellence; OGTT: oral glucose tolerance test; PCOS: polycystic ovary syndrome; RANZOG: Royal Australian and New Zealand College of Obstetricians and Gynecologists: RCOG: Royal College of Obstetricians and Gynecologists; RPG: Random plasma glucose; UK: United Kingdom
Figure 2.
Screening for GDM in women with risk factors during the evolving COVID-19 pandemic. (Figure adapted from the Royal College of Obstetricians and Gynecologists’ Guidance for maternal medicine in the evolving coronavirus (COVID-19) pandemic, 20). FPG: Fasting plasma glucose; RPG: Random plasma glucose
All temporary guidelines support the use of an early pregnancy HbA1c ≥5.9% to identify GDM, although some offer other options (FPG, RPG and/or HbA1c). Due to safety concerns and burden on healthcare resources, guidelines propose alternate testing with FPG, RPG and/or HbA1c at 24-28 weeks instead of OGTT.[10,19,32,33,34,35,36,37]
These temporary recommendations are patient-centered and at the same time safety-motivated in the light of the current unprecedented health crisis.[8,10,32,33,34] Table 3 compares different strategies for screening and diagnosis of GDM. While FPG and HbA1c have high specificity (low false positive rates), they may be associated with low sensitivity (high false negative rates). Therefore, the diagnosis of GDM may be missed in a substantial proportion of women.[10,32,33,34] On the contrary, if lower cut-offs are used, while this may increase the sensitivity, false positive rates are likely to be higher, leading to significant burden on PCPs engaged in dealing with the pandemic.
Table 3.
Relative advantages and disadvantages of different screening methods for GDM
| Method | Pros | Cons |
|---|---|---|
| 2-hour 75g OGTT (3 samples) | High sensitivity and specificity Correlation with adverse pregnancy outcomes Well validated in clinical trials |
Fasting required Need for administration of glucose load Need for three samples at timed intervals Greater exposure and more risk of infection |
| One-step, non-fasting 2 h 75g OGTT (DIPSI) | Fasting not required One sample can be drawn at any time of the day Glucose load can be taken at home Capillary blood glucose may be used in remote areas where laboratory sampling is not feasible |
Need for administration of glucose load 12% of GDM cases may be missed If capillary blood glucose is used, the thresholds are not defined and the levels may vary due to meter variability or user error, temperature, humidity and hematocrit |
| FPG | Single sample High specificity |
Fasting required 25% of GDM cases may be missed Ethnic differences in FPG cut-off values FPG criterion in early pregnancy not well-defined Indeterminate values may need to be confirmed by OGTT |
| HbA1c | Single sample Fasting not required Can be taken at any time of the day Some studies have demonstrated association with adverse pregnancy outcomes Not affected by acute disturbances such as diet, exercise or stress Greater pre-analytical stability than plasma glucose Low biological variability and high reproducibility |
Proposed cut-off of 5.7% reflects the 99th centile of HAPO cohort - high specificity but low sensitivity One-third of GDM cases may be missed Association with adverse pregnancy outcomes not as well validated as for standard 2-hour 75g OGTT Measure of long-term glycemic control - may not reflect glycemic change over short period of time Standardized tests not widely available Biphasic changes during pregnancy with initial decline with nadir at 24 weeks, followed by slow rise as pregnancy approaches term Thresholds may vary across ethnicities Influenced by red cell life - possible false positives in renal failure, HIV, hemoglobinopathies, and anemia - iron deficiency anemia is common in pregnant women in developing countries |
| RPG | Fasting not required Single sample Can be taken at any time of the day |
Significantly impacted by recent meal and activity levels High specificity but low sensitivity |
| Fructosamine | Fasting not required Single sample Can be taken at any time of the day Measure of short-term glycemic control |
Significantly influenced by albumin turnover, especially during pregnancy Has not been correlated with adverse pregnancy outcomes in studies |
| SMBG | Home monitoring Prior GDM well acquainted with SMBG |
GDM may not recur and SMBG should be need based Thresholds are not defined and the levels may vary due to meter variability or user error, temperature, humidity and hematocrit |
DIPSI: Diabetes in Pregnancy Study Group India; FPG: fasting plasma glucose; GDM: gestational diabetes mellitus; HAPO: Hyperglycemia and Adverse Pregnancy Outcomes; HbA1c: glycosylated hemoglobin; HIV: human immunodeficiency virus: OGTT: oral glucose tolerance test; RPG: random plasma glucose; SMBG: self-monitoring of blood glucose
The role of HbA1c in GDM diagnosis remains highly debated. Rajput et al.[38] and Renz et al.[39] suggest that HbA1c may obviate the need for OGTT in one-third of women. However, others suggest that at any HbA1c cut-off with acceptable sensitivity, false-positive rates would be high.[40] The proposed cut-off for HbA1c of 5.7% in the UK and Canadian guidelines has high specificity (good rule-in test) but low sensitivity (not a good rule-out test).[40] It is also ideal to investigate the relevance and reliability of HbA1c estimates in resource-poor areas where National Glycohemoglobin Standardization Program (NGSP)-certified methods are not available.
Moreover, the association of HbA1c with adverse pregnancy outcomes has not been uniformly established. In the HAPO cohort, the association of HbA1c with birth weight, skin fold thickness plus percent body fat >90th percentile and cord blood C-peptide levels was weaker than glucose values.[41] Ho et al.[42] reported HbA1c levels were associated with the risk of gestational hypertension, preeclampsia, preterm delivery, low birth weight as well as macrosomia and the need for neonatal intensive care.
Using FPG alone is likely to miss one-third to one-half of GDM cases.[37,43] Lamain-de Ruiter reported that a combined approach of HbA1c ≥5.7% and FPG ≥92 mg/dL (5.1 mmol/L) resulted in a detection rate of 51% with false-positive rate of 12% when compared to National Institute of Clinical Excellence (NICE) criteria. However, the rates of complications including large for gestational age (LGA), small for gestational age (SGA), stillbirth, preterm birth and Cesarean section were similar.[44]
McIntyre et al.[37] suggested that the approach recommended in Australian guidelines would miss 25% of GDM cases, but their outcome would be similar to women without GDM. However, a higher threshold of FPG and RPG in Canadian or UK guidelines would result in missing a substantially high number of women with GDM at increased risk of adverse outcomes.[33,45] Meek et al.[33] evaluated the diagnostic and prognostic performance of such alternate strategies. The diagnosis of GDM according to IADPSG criteria correlated with FPG as well as HbA1c at 28 weeks.
Personalized risk calculators
The use of personalized risk calculators in first antenatal visit may help identify women at greater risk and increase the yield of testing.[46,47] Many risk calculators have been validated to predict the risk of GDM, including the Monash risk calculator. Sensitivity is 61.3% with a specificity of 71.4%.[44,48] The factors that have been found to be useful to predict the risk of GDM in several studies include maternal age and BMI, previous GDM or family history of diabetes and ethnicity.[49] The application of these risk calculators can be a pragmatic approach during the pandemic that allows for optimal resource utilization and minimizes risk of exposure.
Seshiah et al.[50] emphasized the importance of single-test, non-fasting procedure recommended by DIPSI as an evidence-based and viable option for screening during the pandemic. While this may be useful to minimize the need for frequent sampling, it still requires the administration of oral glucose load and sampling after 2 hours. While some studies[22,51] have demonstrated the feasibility and cost-effectiveness of the DIPSI criteria in resource-limited settings, others suggest that DIPSI method has less sensitivity and may miss a substantial proportion of women with GDM.[52,53]
Panel recommendations
Universal screening for HIP is recommended for all pregnant women at the first antenatal visit and then at 24-28 weeks of gestation
At the first antenatal visit, FPG or RPG and HbA1c should be measured along with other routine investigations. If FPG ≥126 mg/dL (7.0 mmol/L) or RPG ≥200 mg/dL (11.1 mmol/L) or HbA1c ≥6.5% (7.7 mmol/L), it confirms the diagnosis of overt diabetes. If FPG is 92-125 mg/dl (5.1-6.9 mmol/L) or HbA1c is ≥5.7-6.4%, it should be labeled as GDM
If OGTT is not feasible, screening for GDM at 24-28 weeks can be done using FPG and HbA1c. Women with FPG ≥92 mg/dl (5.1 mmol/L) and/or HbA1c ≥5.7% should be considered as having GDM. Regular screening protocols with 2-hour OGTT should be resumed once the pandemic is over.
Pre-Pregnancy Planning in Women with Pre-Existing Diabetes during COVID-19
Women with pre-existing diabetes are at a greater risk of preterm birth, perinatal mortality, congenital malformations and neonatal hypoglycemia and need pre-pregnancy optimization.[54,55] Pregnant women with diabetes are at a greater risk of severe COVID-19 infection with greater morbidity, need for hospitalization, intensive care unit (ICU) admission and mechanical ventilation as well as mortality.[56,57] Therefore, they should strictly adhere to social distancing, hand hygiene and use of face masks.[1,14,20]
Optimal glycemic and metabolic control at this time is not only important to reduce the risk of severe COVID-19 infection, it also ensures optimal metabolic control.[58] This can be planned through scheduled telemedicine or physical visits, as necessary.[4,14] Additionally, periodic screening to assess any complications or comorbidities is strongly recommended.[58] Folic acid supplementation should also be started.[3,4]
Panel recommendations
We recommend that all women with pre-existing diabetes should discuss pregnancy planning with their diabetes care team, keeping in mind the risk of inadvertent infection from frequent healthcare visits
Glycemic control, blood pressure and weight should be optimized prior to pregnancy. It is advisable to switch previous antidiabetic therapy to insulin along with self-monitoring of blood glucose (SMBG) and discontinue medications that are contraindicated in pregnancy
Telemedicine services for pre-pregnancy counseling and management can reduce the risk of exposure in women planning pregnancy
Management of Hyperglycemia in Pregnancy during COVID-19
Management of women with HIP should follow existing standards of care.[25] An ideal approach to the management of HIP includes dietary and lifestyle advice, frequent blood glucose self-monitoring, weight and blood pressure (BP) monitoring, and pharmacological therapy.[14,20,50] Minimizing hospital visits for diabetes care to align them with visits to the obstetrician or for ultrasonography (USG) can increase positive outcomes during COVID-19.[1,59] Women should be instructed on appropriate safe distancing precautions, hand hygiene and the use of face masks. Strict social distancing measures should be implemented and visits scheduled in a manner to avoid overcrowding.[60] The healthcare staff should also use adequate personal protective equipment (PPE) that includes triple-layered surgical or N95 facemask and gloves.
Women with pre-existing diabetes
As soon as pregnancy is confirmed, women with pre-existing diabetes should undergo detailed evaluation with HbA1c, renal and thyroid function tests, urinary albumin creatinine ratio and fundus evaluation.[20] The women should be counseled about medical nutrition therapy, physical activity, home monitoring, medications and insulin, recognition and management of hypoglycemia management as well as sick day guidelines in the first visit.[8] Continuous glucose monitoring (CGM) may be used for glycemic monitoring and allows for the detection of glycemic variability and remote transmission of data.[61] A telephonic or electronic mode of follow-up should be established.
Continued fetal monitoring should follow established standards of care, including nuchal translucency (NT) scan, fetal movement count and fetal ultrasound for monitoring of fetal growth and amniotic fluid. Royal College of Obstetricians and Gynecologists (RCOG) recommends that for women with DIP, face-to-face obstetric visits can coincide with planned ultrasound at 28 and 32 weeks. A comprehensive review is recommended at 34-36 weeks to plan for time and mode of delivery. This can be done remotely if feasible.[20] Close communication between obstetrician and diabetes care team is recommended throughout pregnancy. COVID-19 testing is advised in all pregnant women within a week prior to planned delivery.
Table 4 provides a checklist for the first antenatal visit[14,20] and Figure 3 provides an algorithm for the management of diabetes in women with pre-gestational diabetes.[14,20]
Table 4.
Checklist for the first antenatal visit in women with pre-existing diabetes during the COVID-19 pandemic
| What to do | |
|---|---|
| Assess and evaluate | Assess glycemic and metabolic status - weight, body mass index, blood pressure, blood glucose records, HbA1c Assess for complications and comorbidities - hemogram, renal and liver functions, thyroid function, urinary albumin creatinine ratio, fundus examination |
| Educate and empower | Nutrition advice, exercise recommendations Home monitoring: SMBG and record keeping or CGM, discuss glycemic targets (fasting and premeal values <95 mg/dl, 1-h post- meal value <140 mg/dl and 2-h post-meal value <120 mg/dl), BP and weight, urinalysis (ketones) Insulin technique, targets and titration Hypoglycemia recognition, prevention and management Advise on social distancing precautions Sick day guidelines |
| Treat and optimize | Review SMBG or CGM records Review of insulin regimen (preferably initiate basal bolus insulin regimen); for those on insulin pump, review insulin dose and pump settings If not on insulin, initiate insulin therapy Readjustment of therapies such as antihypertensives and antidiabetic medications - discontinue oral antidiabetics, ACE inhibitors, ARBs and statin Folic acid supplementation |
| Formulate a follow-up plan | Establishing a mode of remote monitoring (teleconsultation or video consultation or email for periodic review of glycemic status) For physical in-person visits, coordinate simultaneous visits for ultrasound scans, antenatal check-up and review with healthcare team |
ACE: angiotensin converting enzyme: ARB: angiotensin receptor blocker; BMI: body mass index; CGM: continuous glucose monitoring: HbA1c: glycosylated hemoglobin; SMBG - self-monitoring of blood glucose
Figure 3.
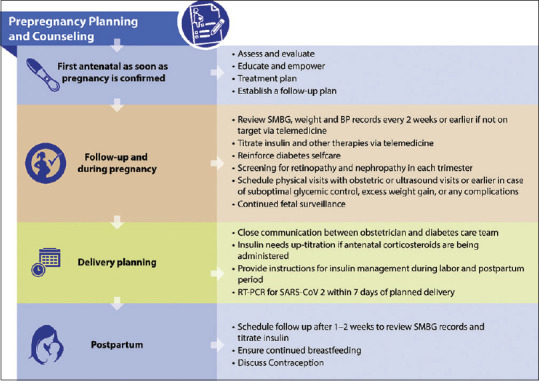
Algorithm for the management of diabetes in pregnant women with pre-existing diabetes during the COVID-19 pandemic
Women with gestational diabetes mellitus
Following a diagnosis of GDM, the first consultation should include advice on medical nutrition therapy, SMBG and glycemic targets. The usual protocols for dietary advice, physical activity, blood glucose monitoring and insulin initiation should be followed.[16] Regular follow-up of these women every 2 weeks can be done via telemedicine to review SMBG records. For women who need pharmacological treatment, diabetes care can be followed up remotely. Obstetric visits are needed at 28 and at 32 weeks concomitant with ultrasound. Obstetric review at 36 weeks is recommended to plan time and mode of delivery. Timing of delivery should be planned in accordance with maternal risk factors for perinatal morbidity.[16]
Table 5 provides a checklist for the first consultation and Figure 4 provides an algorithm for the management of GDM during the COVID-19 pandemic.[14,20]
Table 5.
Checklist for the first consultation for gestational diabetes mellitus during the COVID-19 pandemic
| What to do | |
|---|---|
| Assess and evaluate | Assess maternal weight, BMI and blood pressure Assess glycemic and metabolic status including blood glucose and thyroid functions |
| Educate and empower | Advice on nutrition and physical activity Home monitoring: SMBG and record keeping, discuss glycemic targets (fasting and premeal values <95 mg/dl, 1-hour post-meal value <140 mg/dl and 2-h post-meal value <120 mg/dl), BP and weight monitoring Advice on ketone testing if blood glucose persistently elevated Advice on targets for glycemic control (premeal blood glucose <95 mg/dl and 2-hour post-meal <120 mg/dl) Insulin technique, targets and titration if insulin initiation needed Hypoglycemia recognition, prevention and management Sick day guidelines Advise on social distancing precautions |
| Treat and optimize | Medical nutrition therapy and exercise Pharmacological treatment if there is significant hyperglycemia - insulin forms the mainstay of treatment. Metformin may be considered in women refusing insulin If only on lifestyle modification, discuss/sensitize about possible need for insulin initiation if targets are not attained |
| Formulate a follow-up plan | Establishing a mode of remote monitoring (teleconsultation or video consultation or email for periodic review of glycemic status) Schedule in-person visits if there is a need to initiate insulin or with obstetric or ultrasound visits |
BMI: body mass index; HbA1c: glycosylated hemoglobin; SMBG - self-monitoring of blood glucose
Figure 4.
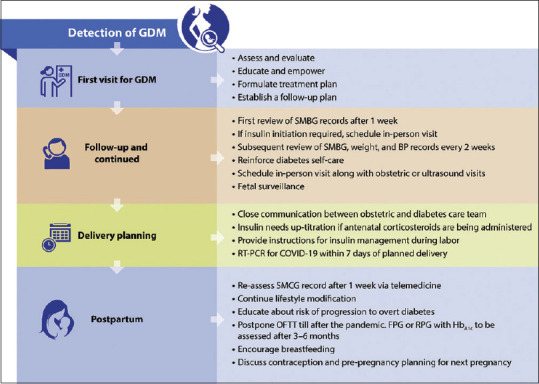
Algorithm for the management of gestational diabetes mellitus during the COVID-19 pandemic
Role of telemedicine in care delivery during the pandemic
Currently, majority of antenatal diabetes and obstetric visits are being provided remotely.[62] Owing to the pandemic, maternity support has transitioned from in-person hospital visits to virtual home-based training for self-assessment and management/monitoring of blood glucose and blood pressure.[63,64] Remote monitoring of glucose and other records through phone or other online platforms can be used for follow-up.[1,10,14,65,66] Video conference platforms, smart phones and internet apps can be used to host virtual GDM classes and teach insulin technique.[9,15,66]
The use of telemedicine to evaluate the effect of mobile health (mHealth) interventions on pregnancy weight management, blood glucose control, and pregnancy outcomes has been evaluated in numerous studies, suggesting a reduced incidence of adverse pregnancy outcomes.[63,65] Dodesini et al.[67] reported that a combination of telemedicine and lockdown measures was associated with very few women with pregestational diabetes testing positive for COVID-19, highlighting the importance of these measures.
Antenatal corticosteroids
For women who are at risk of imminent preterm birth, antenatal corticosteroid treatment is prescribed to promote fetal lung maturity.[3,4] However, even a short course of antenatal corticosteroids can have a deleterious impact on glycemic control. In women already on insulin, this requires an appropriate increment in insulin doses, in accordance with blood glucose monitoring while in women on lifestyle modification alone, short-term use of insulin may be required.[68,69]
Postpartum Care and Screening for Diabetes in Women with GDM
Women with GDM are at increased risk of overt diabetes; therefore, guidelines recommend OGTT to assess glycemic status at 4-12 weeks postpartum.[1,4] However, temporary guidelines issued during the pandemic agree that postpartum screening with a formal OGTT at 4-12 weeks should be deferred to prevent undue exposure to the mother and her child.[15]
Women may be advised to monitor capillary blood glucose at home if they have persistent hyperglycemia after delivery in the immediate postpartum period or had high insulin requirements during pregnancy.[13,70] These women can be monitored via telemedicine services for continued care. RCOG and Australian guidelines recommend HbA1c for screening instead of OGTT at 3-6 months.[17,18,20]
Panel recommendations
Women with GDM should be advised to continue adherence to lifestyle modification, optimization of body weight, blood glucose and blood pressure and contraception after delivery. It is also advisable to discuss future pregnancy planning with the diabetes care team
We recommend that glycemic status should be determined in the immediate postpartum period in women with GDM by frequent capillary glucose monitoring
Women with persistent hyperglycemia or those with high insulin dose requirements during pregnancy should be advised to continue SMBG at home and contact diabetes care team via telemedicine services
For women who become normoglycemic after delivery, HbA1c and FPG or RPG can be measured at 3-6 months. Routine OGTT should be deferred till after the pandemic.
Management of Hyperglycemia in Pregnant Women Infected with COVID-19
Immunological changes in pregnancy make women more susceptible to severe respiratory infections.[21] However, the effect of COVID-19 infection on the outcomes of pregnancy is not well-documented. Studies have reported that the rates of preterm birth, pre-eclampsia and stillbirth are increased in pregnant women infected with COVID-19 infection.[71,72,73] They were more likely to be hospitalized, need ICU admission or mechanical ventilation, but mortality was not higher.[57,74] Women who had pre-existing diabetes, gestational hypertension, pre-eclampsia and obesity were more likely to have severe COVID-19 infection.[75]
Hyperglycemia can lead to impaired immune response to infections and increased inflammation, both of which have been linked to worse outcomes of COVID-19 infection.[58] This not only increases the susceptibility of pregnant women with diabetes to severe infection, but also can lead to worsening of glycemic control and diabetic ketoacidosis (DKA). In addition, medications such as glucocorticoids that are used in the management of severe disease may also cause/worsen hyperglycemia.[58] Several cases of new onset hyperglycemia due to COVID-19 infection have been reported.[76,77,78] DKA has been reported during pregnancy with COVID-19 infection and this may occur at relatively lower blood glucose concentrations.[77,78] DKA can be associated with risk of fetal mortality.[79] Therefore, close gluco-vigilance is imperative in COVID-19 infected pregnant women with HIP. In addition, evaluation for ketonuria and ketonemia is needed even if the blood glucose levels are not significantly elevated.
Since COVID-19 infection may lead to stress hyperglycemia even in individuals without prior history of diabetes mellitus, it would be prudent to monitor blood glucose for 2-3 days in all COVID-19 infected pregnant women even if they do not have previously diagnosed HIP, especially if there are receiving glucocorticoids.
Suspected, probable or confirmed cases of COVID-19 infection should be advised home or institute quarantine, based on the severity of infection.[21] Table 6 summarizes the management of pregnant women with HIP who are suspected or have COVID-19 infection. Pregnant women with HIP who are asymptomatic or have mild infection can be managed via regular teleconsultation at home. They should be advised on nutrition, adequate hydration, increased capillary glucose monitoring (6-7 times per day), self-titration of insulin doses, hypoglycemia care and sick-day guidelines.[14,20,76] Proper fluid and caloric intake must be ensured to prevent dehydration. In case of any deterioration in clinical state, occurrence of significant hyperglycemia or ketosis or in moderate to severe infection, they should be managed as in-patients.
Table 6.
| Asymptomatic or Mild COVID-19 infection | Confirmed Moderate to Severe COVID-19 infection | |
|---|---|---|
| General supportive care | Asymptomatic or mild case can be advised to self-isolate at home Monitoring of vitals - temperature, HR, BP, oxygen saturation Maintain hydration, fluid and electrolyte balance Antipyretics, antibiotics for superadded bacterial infection as per local guidelines |
Moderate to severe infection requires admission to COVID-19 specific units Multidisciplinary care including obstetrician and diabetes care provider in addition to the COVID-19 response team Monitoring of vitals - temperature, HR, BP, oxygen saturation High flow nasal oxygen or mechanical ventilation as required Antipyretics, antibiotics for superadded bacterial infection as per local guidelines Antiviral therapy may be considered as per local guidelines and informed consent with discussion of potential adverse effects Glucocorticoids for severe disease or critically ill patients |
| Diabetes Care | Blood glucose monitoring - pre-meals and post-meals or CGM and urine ketones Insulin - basal bolus regimen with administration of correction bolus as required Regular teleconsultation with diabetes care team to adjust insulin doses |
Critically ill patients - 1 hourly blood glucose monitoring or CGM, with intravenous insulin infusion Non-critically patients - 6-7-point capillary blood glucose monitoring (pre-meals and post-meals) or CGM with administration of subcutaneous insulin as a basal bolus regimen; correction bolus as required in case of blood glucose remaining above targets Remote consultation with diabetes care team for insulin dose titration |
| Maternal surveillance | Self-monitoring of temperature, HR, BP and oxygen saturation Regular review of clinical status via telemedicine Low threshold for admission in case of any clinical deterioration or worsening hyperglycemia |
Close and vigilant monitoring of vital signs and oxygen saturation to minimize maternal hypoxia Arterial blood-gas analysis if any signs of hypoxia or respiratory distress Chest imaging (X-ray or CT chest) may be done if indicated with informed consent Regular evaluation of complete blood count, renal and liver function, coagulation profile and inflammatory markers |
| Fetal surveillance | Fetal movement count Follow-up scan for fetal well-being and amniotic fluid after 2 weeks | Cardiotocography for fetal heart rate if gestational age is beyond the limit of viability (23-28 weeks) Fetal movement count Follow-up scan for fetal well-being and amniotic fluid after 2 weeks |
The management of in-patient hyperglycemia in pregnancy should be with insulin therapy.[80] If the woman is taking metformin or glyburide, these should be discontinued, and patient started on insulin.[76] Critically ill women will require continuous intravenous insulin infusion with hourly blood glucose monitoring. In non-critically ill women, use of basal-bolus approach with 6- 7-point blood glucose profile is advisable.[80] In women on continuous subcutaneous insulin pump, it can be continued with frequent monitoring and administration of correction boluses. CGM may help facilitate glycemic control, can minimize the burden on nursing staff and allow for remote in-patient monitoring of glucose levels.[81] In women who receive glucocorticoids, insulin dose needs to be appropriately titrated to ensure glycemic control.
Antenatal evaluation for GDM or DIP should follow standard protocols in pregnancies affected by COVID-19 and at present, there is no evidence to suggest more frequent antenatal testing.[76,82,83,84,85,86,87,88] In-person antenatal follow-up visit can be postponed by 14 days to minimize exposure to other antenatal women clinic staff.[21] In the meantime, telemedicine services should continue to be utilized for delivery of diabetes care.
Though no reports of fetal malformations following SARS-CoV-2 infection have been reported, FIGO recommends a fetal morphology scan at 18-23 weeks of gestation if the woman was infected with COVID-19 in early pregnancy.[21] The Federation of Obstetric and Gynecological Societies of India (FOGSI) recommends fetal ultrasound 2 weeks after the infection for fetal well-being and amniotic fluid.[89] Timing and the mode of delivery are individualized, depending on maternal and fetal status and gestational age.[89] COVID-19 infected pregnant women may develop significant anxiety and require continued psychological support.
If the mother has been infected within 14 days prior to delivery, COVID-19 testing of the newborn should be considered. It is not known if SARS-CoV-2 can be transmitted via breastmilk, but there is a risk of contact transmission from mother to the child. Guidelines recommend that the benefits of breastfeeding outweigh the risk of infection to the neonate.[21,89] The mother should be advised to wash her hands and breast and wear a facemask when lactating. Alternatively, breastmilk may be expressed with a breast pump for feeding.
Panel recommendations
We recommend that blood glucose monitoring should be intensified in pregnant women with hyperglycemia who are infected with COVID-19
Asymptomatic women or those with mild infection can be managed at home with advice on maintaining hydration, nutrition, frequent SMBG and urinary ketone testing, insulin dose self-titration, hypoglycemia care and sick day guidelines. Insulin remains the mainstay of treatment for glycemic control and insulin doses can be adjusted via telemedicine or remote services. They should promptly contact the diabetes care team in case of any worsening of symptoms or glycemic control or appearance of ketonuria. A low index of suspicion for hospitalization should be maintained.
Pregnant women with pre-existing of gestational diabetes who develop moderate to severe COVID-19 infection need hospitalization. Insulin administered as a basal bolus approach should be titrated as per severity of infection, nutritional status and glycemic control. Insulin dose may be significantly higher in women requiring glucocorticoids for the management of COVID-19.
Conclusion
Primary care physicians are often engaged in the care of pregnant women with HIP. The pandemic has compromised routine care and there is lack of guidance for a pragmatic approach to the detection and management of HIP during the pandemic. Reducing the number of clinic visits in pregnant women is important to reduce the risk of COVID-19. Use of alternate strategies using FPG and HbA1c instead of the 2-hour OGTT has been suggested for the screening of HIP. Virtual healthcare services with remote monitoring and delivery of care in women with GDM or DIP via the use of telemedicine platforms can improve outcomes while minimizing the risk of infection. Pregnant women with diabetes who develop COVID-19 infection should be closely monitored and hospitalized if there is any deterioration in clinical condition.
Most of the recommendations for the management of HIP during the pandemic need to be assessed further in terms of diagnostic and therapeutic efficacy along with the long-term implications of these decisions.
Disclosures
All authors had full access to the articles reviewed in this manuscript, have read and reviewed the final draft of this manuscript and take complete responsibility for the integrity and accuracy of this manuscript. The details published herein are intended for informational, educational, academic and/or research purposes and are not intended to substitute for professional medical advice, diagnosis or treatment.
Compliance with ethical guidance
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We would like to thank Dr Rajshri Mallabadi and Dr Chinappa A B from BioQuest Solutions Pvt. Ltd, Bangalore, for providing medical writing assistance and editorial support in the preparation of this manuscript. We also thank Dr S Amarnath for editorial and scientific support.
References
- 1.Murphy HR. Managing diabetes in pregnancy before, during, and after COVID-19. Diabetes Technol Ther. 2020;22:454–61. doi: 10.1089/dia.2020.0223. [DOI] [PubMed] [Google Scholar]
- 2.Ghesquière L, Garabedian C, Drumez E, Lemaître M, Cazaubiel M, Bengler C, et al. Effects of COVID-19 pandemic lockdown on gestational diabetes mellitus: A retrospective study. Diabetes Metab. 2021;47:101201. doi: 10.1016/j.diabet.2020.09.008. doi:10.1016/j.diabet. 2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Federation of Gynecology and Obstetrics. Global Declaration on Hyperglycemia in Pregnancy. [Last accessed on 2020 Aug 12]. Available from: https://www.figo.org/FIGO-globaldeclaration-hip .
- 4.Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: A World Health Organization guideline. Diabetes Res Clin Pract. 2014;103:341–63. doi: 10.1016/j.diabres.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 5.HAPO Study Cooperative Research Group. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 6.Breslin N, Baptiste C, Gyamfi-Bannerman C, Miller R, Martinez R, Bernstein K, et al. Coronavirus disease 2019 infection among asymptomatic and symptomatic pregnant women: Two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM. 2020;2:100118. doi: 10.1016/j.ajogmf.2020.100118. doi:10.1016/j.ajogmf. 2020.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martínez-Perez O, Vouga M, Cruz Melguizo S, Forcen Acebal L, Panchaud A, Muñoz-Chápuli M, et al. Association between mode of delivery among pregnant women with COVID-19 and maternal and neonatal outcomes in Spain. JAMA. 2020;324:296–9. doi: 10.1001/jama.2020.10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nouhjah S, Jahanfar S, Shahbazian H. Temporary changes in clinical guidelines of gestational diabetes screening and management during COVID-19 outbreak: A narrative review. Diabetes Metab Syndr. 2020;14:939–42. doi: 10.1016/j.dsx.2020.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aziz A, Zork N, Aubey JJ, Baptiste CD, D’Alton ME, Emeruwa UN, et al. Telehealth for high-risk pregnancies in the setting of the COVID-19 pandemic. Am J Perinatol. 2020;37:800–8. doi: 10.1055/s-0040-1712121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McIntyre HD, Moses RG. The diagnosis and management of gestational diabetes mellitus in the context of the COVID-19 pandemic. Diabetes Care. 2020;43:1433–4. doi: 10.2337/dci20-0026. [DOI] [PubMed] [Google Scholar]
- 11.Rani PR, Begum J. Screening and diagnosis of gestational diabetes mellitus, where do we stand. J Clin Diagn Res. 2016;10:QE01–4. doi: 10.7860/JCDR/2016/17588.7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Junnnare KK, Adhau SR, Hegde MV, Naphade PR. Screening of gestational diabetes mellitus in antenatal women using DIPSI guidelines. Int J Res Med Sci. 2016;4:446–9. [Google Scholar]
- 13.Torlone E, Festa C, Formoso G, Scavini M, Sculli MA, Succurro E, et al. Italian recommendations for the diagnosis of gestational diabetes during COVID-19 pandemic: Position statement AMD-SID, Diabetes and Pregnancy Study Group. Nutr Metab Cardiovasc Dis. 2020;30:1418–22. doi: 10.1016/j.numecd.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torlone E, Sculli MA, Bonomo M, Di Benedetto A, Di Cianni G, Festa C, et al. Recommendations and management of hyperglycaemia in pregnancy during COVID-19 pandemic in Italy. Diabetes Res Clin Pract. 2020;166:108345. doi: 10.1016/j.diabres.2020.108345. doi:10.1016/j.diabres. 2020.108345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto JM, Donovan LE, Feig DS, Berger H. Urgent Update – Temporary Alternative Screening Strategy for Gestational Diabetes Screening during the COVID-19 Pandemic. A Joint Consensus Statement from the Diabetes Canada Clinical Practice Guidelines Steering Committee and the Society of Obstetricians and Gynecologists of Canada. [Last accessed on 2020 Sep 15]. Available from: https://sogc.org/common/Uploaded%20files/GDM-COVID-19%20temporary%20screening%20guidelines%20-%2020200402%20Agreed%20Final.pdf .
- 16.The Royal Australian and New Zealand College of Obstetricians and Gynaecologists. COVID-19 and gestational diabetes screening, diagnosis and management. [Last accessed 2020 Sep 15]. 6 April 2020, Updated on 20 Aug 2020. Available from: https://ranzcog.edu.au/news/covid-19-and-gestational-diabetes-screening,-diagn .
- 17.The Australasian Diabetes in Pregnancy Society (ADIPS), the Australian Diabetes Society (ADS), the Australian Diabetes Educators Association (ADEA), and Diabetes Australia (DA) [Last accessed on 2020 Sep 15];Diagnostic testing for gestational diabetes mellitus (GDM) during the COVID 19 pandemic: Antenatal and postnatal testing advice. Available from: https://static.diabetesaustralia.com.au/s/fileassets/diabetes-australia/e4f409b9-b9bf-4c74-96df-8a3cc424367b.pdf . [Google Scholar]
- 18.Queensland Clinical Guidelines. Notice of update during COVID-19 pandemic. Recommendations for GDM screening and oral glucose tolerance test (OGTT) during pregnancy and postpartum. 29 Jun, 2020. [Last accessed on 2020 Sep 15]. Available from: https://www.health.qld.gov.au/__data/assets/pdf_file/0022/950503/g-gdm.pdf .
- 19.Kasuga Y, Saisho Y, Ikenoue S, Ochiai D, Tanaka M. A new diagnostic strategy for gestational diabetes during the COVID-19 pandemic for the Japanese population. Diabetes Metab Res Rev. 2020;36:e3351. doi: 10.1002/dmrr.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Royal College of Obstetricians and Gynaecologists. Guidance for maternal medicine services in the evolving coronavirus (COVID-19) pandemic. Information for healthcare professionals. Version 2.4: Published Friday 10 July 2020. [Last accessed on 2020 Sep 14]. Available from: https://www.rcog.org.uk/globalassets/documents/guidelines/2020-07-10-guidance-for-maternal-medicine.pdf .
- 21.Poon LC, Yang H, Kapur A, Melamed N, Dao B, Divakar H, et al. Global interim guidance on coronavirus disease 2019 (COVID-19) during pregnancy and puerperium from FIGO and allied partners: Information for healthcare professionals. Int J Gynaecol Obstet. 2020;149:273–86. doi: 10.1002/ijgo.13156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balaji V, Balaji M, Anjalakshi C, Cynthia A, Arthi T, Seshiah V. Diagnosis of gestational diabetes mellitus in Asian-Indian women. Indian J Endocrinol Metab. 2011;15:187–90. doi: 10.4103/2230-8210.83403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.International Association of Diabetes and Pregnancy Study Groups consensus panel (IADPSG) International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycaemia in pregnancy. Diabetes Care. 2010;33:676–82. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.HAPO Study Cooperative Research Group. Hyperglycaemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 25.American Diabetes Association. Management of diabetes in pregnancy: Standards of medical care in diabetes. Diabetes Care. 2020;43:S183–92. doi: 10.2337/dc20-S014. [DOI] [PubMed] [Google Scholar]
- 26.National Institute for Health and Care Excellence. Diabetes in pregnancy: Management from preconception to the postnatal period 2015. [Last accessed on 2020 Sep 14]. Available from: www.nice.org.uk/guidance/ng3 . [PubMed]
- 27.Diabetes Canada Clinical Practice Guidelines Expert Committee. Feig DS, Berger H, Donovan L, Godbout A, Kader T, et al., editors. Diabetes and pregnancy. Can J Diabetes. 2018;42(Suppl 1):S255–82. doi: 10.1016/j.jcjd.2017.10.038. [DOI] [PubMed] [Google Scholar]
- 28.Saxena P, Verma P, Goswami B. Comparison of diagnostic accuracy of non-fasting DIPSI and HbA1c with fasting WHO criteria for diagnosis of gestational diabetes mellitus. J Obstet Gynaecol India. 2017;67:337–42. doi: 10.1007/s13224-017-0962-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vij P, Jha S, Gupta SK, Aneja A, Mathur R, Waghare S, et al. Comparison of DIPSI and IADPSG criteria for diagnosis of GDM: A study in a north Indian tertiary care center. Int J Diabetes Dev Ctries. 2015;35:285–8. [Google Scholar]
- 30.Bangladesh Endocrine Society (BES) Practical Recommendations for Management of Diabetes and Other Endocrine Diseases in Patients with COVID-19. [Last accessed on 2020 Sep 15]. Available from: http://bes-org.net/wp-content/uploads/2020/06/BES-COVID-Pract-Recomnd-06-June-Final-Copy.pdf .
- 31.Takkar S, Jyotsna M, Goyal P, Chaudhary A, Vipperla S, Hemalatha Y, et al. Consensus scientific statement on advisory working guidelines and recommendations for female population in COVID era by WINCARS. Ind J CVD in Women - WINCARS. 2020;5:175–94. [Google Scholar]
- 32.Vambergue A, Jacqueminet S, Lamotte MF, Lamiche-Lorenzini F, Brunet C, Deruelle P, et al. Three alternative ways to screen for hyperglycemia in pregnancy during the COVID-19 pandemic. Diabetes Metab. 2020 doi: 10.1016/j.diabet.2020.04.003. doi:10.1016/j.diabet. 2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meek CL, Lindsay RS, Scott EM, Aiken CE, Myers J, Reynolds RM, et al. Approaches to screening for hyperglycaemia in pregnant women during and after the COVID-19 pandemic. Diabet Med. 2021;38:e14380. doi: 10.1111/dme.14380. doi:10.1111/dme. 14380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.d’Emden M, McLeod D, Ungerer J, Appleton C, Kanowski D. Development of a fasting blood glucose-based strategy to diagnose women with gestational diabetes mellitus at increased risk of adverse outcomes in a COVID-19 environment. PLoS One. 2020;15:e0243192. doi: 10.1371/journal.pone.0243192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panaitescu AM, Ciobanu AM, Popa M, Duta I, Gica N, Peltecu G, et al. Screening for gestational diabetes during the COVID-19 pandemic-current recommendations and their consequences. Medicina (Kaunas) 2021;57:381. doi: 10.3390/medicina57040381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nachtergaele C, Vicaut E, Tatulashvili S, Pinto S, Bihan H, Sal M, et al. Limiting the use of oral glucose tolerance tests to screen for hyperglycemia in pregnancy during pandemics. J Clin Med. 2021;10:397. doi: 10.3390/jcm10030397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McIntyre HD, Gibbons KS, Ma RCW, Tam WH, Sacks DA, Lowe J, et al. Testing for gestational diabetes during the COVID-19 pandemic. An evaluation of proposed protocols for the United Kingdom, Canada and Australia. Diabetes Res Clin Pract. 2020;167:108353. doi: 10.1016/j.diabres.2020.108353. doi:10.1016/j.diabres. 2020.108353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rajput R, Yogesh Yadav, Rajput M, Nanda Sl. Utility of HbA1c for diagnosis of gestational diabetes mellitus. Diabetes Res Clin Pract. 2012;98:104–7. doi: 10.1016/j.diabres.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 39.Renz PB, Cavagnolli G, Weinert LS, Silveiro SP, Camargo JL. HbA1c test as a tool in the diagnosis of gestational diabetes mellitus. PLoS One. 2015;10:e0135989. doi: 10.1371/journal.pone.0135989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ye M, Liu Y, Cao X, Yao F, Liu B, Li Y, et al. The utility of HbA1c for screening gestational diabetes mellitus and its relationship with adverse pregnancy outcomes. Diabetes Res Clin Pract. 2016;114:43–9. doi: 10.1016/j.diabres.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 41.Lowe LP, Metzger BE, Dyer AR, Lowe J, McCance DR, Lappin TR, et al. Hyperglycemia and adverse pregnancy outcome (HAPO) study: Associations of maternal A1C and glucose with pregnancy outcomes. Diabetes Care. 2012;35:574–80. doi: 10.2337/dc11-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ho YR, Wang P, Lu MC, Tseng ST, Yang CP, Yan YH. Associations of mid-pregnancy HbA1c with gestational diabetes and risk of adverse pregnancy outcomes in high-risk Taiwanese women. PLoS One. 2017;12:e0177563. doi: 10.1371/journal.pone.0177563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Gemert TE, Moses RG, Pape AV, Morris GJ. Gestational diabetes mellitus testing in the COVID-19 pandemic: The problems with simplifying the diagnostic process. Aust N Z J Obstet Gynaecol. 2020;60:671–4. doi: 10.1111/ajo.13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lamain-de Ruiter M, Kwee A, Naaktgeboren CA, de Groot I, Evers IM, Groenendaal F, et al. External validation of prognostic models to predict risk of gestational diabetes mellitus in one Dutch cohort: Prospective multicentre cohort study. BMJ. 2016;354:i4338. doi: 10.1136/bmj.i4338. doi:10.1136/bmj.i4338. [DOI] [PubMed] [Google Scholar]
- 45.van-de-l’Isle Y, Steer PJ, Watt Coote I, Cauldwell M. Impact of changes to national UK Guidance on testing for gestational diabetes screening during a pandemic: A single-centre observational study. BJOG. 2021;128:917–20. doi: 10.1111/1471-0528.16482. [DOI] [PubMed] [Google Scholar]
- 46.Nombo AP, Mwanri AW, Brouwer-Brolsma EM, Ramaiya KL, Feskens EJM. Gestational diabetes mellitus risk score: A practical tool to predict gestational diabetes mellitus risk in Tanzania. Diabetes Res Clin Pract. 2018;145:130–7. doi: 10.1016/j.diabres.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 47.Artzi NS, Shilo S, Hadar E, Rossman H, Barbash-Hazan S, Ben-Haroush A, et al. Prediction of gestational diabetes based on nationwide electronic health records. Nat Med. 2020;26:71–6. doi: 10.1038/s41591-019-0724-8. [DOI] [PubMed] [Google Scholar]
- 48.Teede HJ, Harrison CL, Teh WT, Paul E, Allan CA. Gestational diabetes: Development of an early risk prediction tool to facilitate opportunities for prevention. Aust N Z J Obstet Gynaecol. 2011;51:499–504. doi: 10.1111/j.1479-828X.2011.01356.x. [DOI] [PubMed] [Google Scholar]
- 49.Cosson E, Vicaut E, Sandre-Banon D, Gary F, Pharisien I, Portal JJ, et al. Performance of a selective screening strategy for diagnosis of hyperglycaemia in pregnancy as defined by IADPSG/WHO criteria. Diabetes Metab. 2020;46:311–8. doi: 10.1016/j.diabet.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 50.Seshiah V, Balaji V, Banerjee S, Sahay R, Divakar H, Jain R, et al. Diagnosis and principles of management of gestational diabetes mellitus in the prevailing COVID-19 pandemic. Int J Diabetes Dev Ctries. 2020:1–6. doi: 10.1007/s13410-020-00860-1. doi:10.1007/s13410-020-00860-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khan S, Bal H, Khan ID, Paul D. Evaluation of diabetes in pregnancy study group of India criteria and Carpenter-Coustan criteria in the diagnosis of gestational diabetes mellitus. Turk J Obstet Gynecol. 2018;15:75–9. doi: 10.4274/tjod.57255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mohan V, Mahalakshmi MM, Bhavadharini B, Maheswari K, Kalaiyarasi G, Anjana RM, et al. Comparison of screening for gestational diabetes mellitus by oral glucose tolerance tests done in the non-fasting (random) and fasting states. Acta Diabetol. 2014;51:1007–13. doi: 10.1007/s00592-014-0660-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tripathi R, Verma D, Gupta VK, Tyagi S, Kalaivani M, Ramji S, et al. Evaluation of 75g glucose load in non-fasting state (Diabetes in Pregnancy Study group of India (DIPSI) criteria) as a diagnostic test for gestational diabetes mellitus. Indian J Med Res. 2017;145:209–14. doi: 10.4103/ijmr.IJMR_1716_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee D, Booth GL, Ray JG, Ling V, Feig DS. Undiagnosed type 2 diabetes during pregnancy is associated with increased perinatal mortality: A large population-based cohort study in Ontario, Canada. Diabet Med. 2020 doi: 10.1111/dme.14250. doi:10.1111/dme. 14250. [DOI] [PubMed] [Google Scholar]
- 55.FSRH CEU: Information to support management of individuals requesting to discontinue contraception to plan a pregnancy during the Covid-19 outbreak. 2020. Mar 26, [Last Accessed on 2020 Sep 15]. Available from: https://www.fsrh.org/documents/fsrh-ceu-information-to-support-management-of-individuals/
- 56.Priya G, Bajaj S, Grewal E, Maisnam I, Chandrasekharan S, Selvan C. Challenges in women with diabetes during the COVID-19 pandemic. Eur Endocrinol. 2020;16:100–8. doi: 10.17925/EE.2020.16.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Knight M, Bunch K, Vousden N, Morris E, Simpson N, Gale C, et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: National population based cohort study. BMJ. 2020;369:m2107. doi: 10.1136/bmj.m2107. doi:10.1136/bmj.m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Priya G, Kalra B, Grewal E, Dardi IK. Premarriage counseling in type 1 diabetes. Indian J Endocrinol Metab. 2018;22:126–31. doi: 10.4103/ijem.IJEM_550_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang H, Wang C, Poon LC. Novel coronavirus infection and pregnancy. Ultrasound Obstet Gynecol. 2020;55:435–7. doi: 10.1002/uog.22006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Royal College of Obstetricians and Gynaecologists. Coronavirus (COVID-19) Infection in Pregnancy. Information for healthcare professionals Version 12: Published Wednesday 14 October 2020. [Last accessed on Oct 15]. Available from: https://www.rcog.org.uk/globalassets/documents/guidelines/2020-10-14-coronavirus-covid-19-infection-in-pregnancy-v12.pdf .
- 61.Yu Q, Aris IM, Tan KH, Li LJ. Application and utility of continuous glucose monitoring in pregnancy: A systematic review. Front Endocrinol (Lausanne) 2019;10:697. doi: 10.3389/fendo.2019.00697. doi:10.3389/fendo. 2019.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moradi F, Ghadiri-Anari A, Enjezab B. COVID-19 and self-care strategies for women with gestational diabetes mellitus. Diabetes Metab Syndr. 2020;14:1535–9. doi: 10.1016/j.dsx.2020.08.004. doi:10.1016/j.dsx. 2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guo H, Zhang Y, Li P, Zhou P, Chen LM, Li SY. Evaluating the effects of mobile health intervention on weight management, glycemic control and pregnancy outcomes in patients with gestational diabetes mellitus. J Endocrinol Invest. 2019;42:709–14. doi: 10.1007/s40618-018-0975-0. [DOI] [PubMed] [Google Scholar]
- 64.Chilelli NC, Dalfrà MG, Lapolla A. The emerging role of telemedicine in managing glycemic control and psychobehavioral aspects of pregnancy complicated by diabetes. Int J Telemed Appl 2014. 2014 doi: 10.1155/2014/621384. 621384. doi:10.1155/2014/621384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xie W, Dai P, Qin Y, Wu M, Yang B, Yu X. Effectiveness of telemedicine for pregnant women with gestational diabetes mellitus: An updated meta-analysis of 32 randomized controlled trials with trial sequential analysis. BMC Pregnancy Childbirth. 2020;20:198. doi: 10.1186/s12884-020-02892-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Albert L, Capel I, García-Sáez G, Martín-Redondo P, Hernando ME, et al. Managing gestational diabetes mellitus using a smartphone application with artificial intelligence (SineDie) during the COVID-19 pandemic: Much more than just telemedicine. Diabetes Res Clin Pract. 2020;169:108396. doi: 10.1016/j.diabres.2020.108396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dodesini AR, Galliani S, Ciriello E, Bellante R, Trevisan R. Pre-gestational diabetes during the COVID-19 pandemic in Bergamo, Italy. Int J Gynaecol Obstet. 2020 doi: 10.1002/ijgo.13306. doi:10.1002/ijgo. 13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kalra S, Kalra B, Gupta Y. Glycemic management after antenatal corticosteroid therapy. N Am J Med Sci. 2014;6:71–6. doi: 10.4103/1947-2714.127744. doi:10.4103/1947-2714.127744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jewel R, Jacob JJ. Intra-partum management of women with diabetes. J Pak Med Assoc. 2018;68:490–3. [PubMed] [Google Scholar]
- 70.Coetzee A, Mason D, Hall DR, Hoffmann M, Conradie M. Evidence for the utility of antenatal HbA1c to predict early postpartum diabetes after gestational diabetes in South Africa. Diabetes Res Clin Pract. 2018;143:50–5. doi: 10.1016/j.diabres.2018.06.021. [DOI] [PubMed] [Google Scholar]
- 71.Di Mascio D, Khalil A, Saccone G, Rizzo G, Buca D, Liberati M, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID 1–19) during pregnancy: A systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020;2:100107. doi: 10.1016/j.ajogmf.2020.100107. https://doi.org/10.1016/j.ajogmf. 20200.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Della Gatta AN, Rizzo R, Pilu G, Simonazzi G. Coronavirus disease 2019 during pregnancy: A systematic review of reported cases. Am J Obstet Gynecol. 2020;223:36–41. doi: 10.1016/j.ajog.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wei SQ, Bilodeau-Bertrand M, Liu S, Auger N. The impact of COVID-19 on pregnancy outcomes: A systematic review and meta-analysis. CMAJ. 2021;193:E540–8. doi: 10.1503/cmaj.202604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ellington S, Strid P, Tong VT, Woodworth K, Galang RR, Zambrano LD, et al. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-June 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:769–75. doi: 10.15585/mmwr.mm6925a1. doi:10.15585/mmwr.mm6925a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kayem G, Lecarpentier E, Deruelle P, Bretelle F, Azria E, Blanc J, et al. A snapshot of the Covid-19 pandemic among pregnant women in France. J Gynecol Obstet Hum Reprod. 2020;49:101826. doi: 10.1016/j.jogoh.2020.101826. doi:10.1016/j.jogoh. 2020.101826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boyles GP, Thung S, Gabbe SG, Landon MB, Costantine MM. Practical considerations for pregnant women with diabetes and severe acute respiratory syndrome coronavirus 2 infection. Am J Obstet Gynecol MFM. 2020;2:100210. doi: 10.1016/j.ajogmf.2020.100210. doi:10.1016/j.ajogmf. 2020.100210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smati S, Mahot P, Bourdiol A, Ploteau S, Hadjadj S, Cariou B, et al. Euglycaemic ketoacidosis during gestational diabetes with concomitant COVID-19 infection. Diabetes Metab. 2021;47:101181. doi: 10.1016/j.diabet.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pikovsky M, Tan MY, Ahmed A, Sykes L, Agha-Jaffar R, Yu CKH. Euglycaemic ketoacidosis in pregnant women with COVID-19: Two case reports. BMC Pregnancy Childbirth. 2021;21:427. doi: 10.1186/s12884-021-03928-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li J, Wang X, Chen J, Zuo X, Zhang H, Deng A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metab. 2020;22:1935–41. doi: 10.1111/dom.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Umpierrez GE, Hellman R, Korytkowski MT, Kosiborod M, Maynard GA, Montori VM, et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:16–38. doi: 10.1210/jc.2011-2098. [DOI] [PubMed] [Google Scholar]
- 81.U.S. Food and Drug Administration. Enforcement policy for noninvasive remote monitoring devices used to support patient monitoring during the coronavirus disease 2019 (COVID-19) public health emergency (revised) Guidance for Industry and Food and Drug Administration staff. [Last accessed on 2020 Sep 16]. Available from: https://www.fda.gov/regulatory-information/searchfda-guidance-documents/enforcement-policy-non-invasive-remotemonitoring-devices-used-support-patient-monitoring-during .
- 82.Patanè L, Morotti D, Giunta MR, Sigismondi C, Piccoli MG, Frigerio L, et al. Vertical transmission of coronavirus disease 2019: Severe acute respiratory syndrome coronavirus 2 RNA on the fetal side of the placenta in pregnancies with coronavirus disease 2019-positive mothers and neonates at birth. Am J Obstet Gynecol MFM. 2020;2:100145. doi: 10.1016/j.ajogmf.2020.100145. doi:10.1016/j.ajogmf. 2020.100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–4. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Penfield CA, Brubaker SG, Limaye MA, Lighter J, Ratner AJ, Thomas KM, et al. Detection of SARS-COV-2 in placental and fetal membrane samples. Am J Obstet Gynecol MFM. 2020:100133. doi: 10.1016/j.ajogmf.2020.100133. doi:10.1016/j.ajogmf. 2020.100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dong L, Tian J, He S, Zhu C, Wang J, Liu C, et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020;323:1846–8. doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Egloff C, Vauloup-Fellous C, Picone O, Mandelbrot L, Roques P. Evidence and possible mechanisms of rare maternal-fetal transmission of SARS-CoV-2. J Clin Virol. 2020;128:104447. doi: 10.1016/j.jcv.2020.104447. doi:10.1016/j.jcv. 2020.104447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet. 2020;395:809–15. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qiu L, Liu X, Xiao M, Xie J, Cao W, Liu Z, et al. SARS-CoV-2 is not detectable in the vaginal fluid of women with severe COVID-19 infection. Clin Infect Dis. 2020;71:813–7. doi: 10.1093/cid/ciaa375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brown A, Shenker N. Experiences of breastfeeding during COVID-19: Lessons for future practical and emotional support. Matern Child Nutr. 2021;17:e13088. doi: 10.1111/mcn.13088. doi:10.1111/mcn. 13088. [DOI] [PMC free article] [PubMed] [Google Scholar]



