Abstract
Clinical, laboratory, and autopsy findings support an association between coronavirus disease-2019 (COVID-19) and thromboembolic disease. Acute COVID-19 infection is characterized by mononuclear cell reactivity and pan-endothelialitis, contributing to a high incidence of thrombosis in large and small blood vessels, both arterial and venous. Observational studies and randomized trials have investigated whether full-dose anticoagulation may improve outcomes compared with prophylactic dose heparin. Although no benefit for therapeutic heparin has been found in patients who are critically ill hospitalized with COVID-19, some studies support a possible role for therapeutic anticoagulation in patients not yet requiring intensive care unit support. We summarize the pathology, rationale, and current evidence for use of anticoagulation in patients with COVID-19 and describe the main design elements of the ongoing FREEDOM COVID-19 Anticoagulation trial, in which 3,600 hospitalized patients with COVID-19 not requiring intensive care unit level of care are being randomized to prophylactic-dose enoxaparin vs therapeutic-dose enoxaparin vs therapeutic-dose apixaban. (FREEDOM COVID-19 Anticoagulation Strategy [FREEDOM COVID]; NCT04512079)
Key Words: anticoagulation, clinical trial, coagulopathy, COVID-19
Abbreviations and Acronyms: DIC, disseminated intravascular coagulation; ICU, intensive care unit; LMWH, low–molecular weight heparin; SARS-CoV2, severe acute respiratory syndrome-coronavirus-2
Central Illustration
COVID-19, caused by the severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), has led to unprecedented morbidity and mortality in a modern global pandemic. To date, more than 200 million cases have been reported, with more than 4 million deaths worldwide.1 As the number of affected individuals continues to climb, effective strategies for treatment and prevention of the disease remains of paramount importance. Clinical and laboratory evidence have highlighted that, apart from causing acute respiratory distress syndrome, SARS-CoV-2 is also associated with thromboembolic disease, which has emerged as a common and potentially catastrophic manifestation of COVID-19.2, 3, 4, 5 More recently, it was observed that COVID-19 may also be associated with chronic physical and mental health consequences, which have been labeled as “long-COVID” or “post-COVID” conditions.6
Perturbations in laboratory markers of coagulation were noted early on among patients who were hospitalized with COVID-19, with reports demonstrating an association between elevated D-dimer and an increased risk of mortality.7 , 8 Several pathologic mechanisms have been postulated to account for the laboratory abnormalities and clinical thrombotic associations, collectively described as a “COVID-19–associated coagulopathy.”9 COVID-19 is characterized by mononuclear cell reactivity and pan-endothelialitis that contribute to a high incidence of in situ thrombosis in large and small blood vessels, both arterial and venous, including at the capillary-alveolar interface that may contribute to the high rate of respiratory failure.10, 11, 12
In this context, many have argued that there might be a role for anticoagulation therapy in patients with severe COVID-19. Early in the pandemic, observational studies suggested that anticoagulation therapy, particularly with heparins, might improve freedom from intubation and survival in hospitalized patients with COVID-19.13 , 14 As a result, prophylactic-dose heparin (at a minimum) has become accepted as the de facto standard of care.15 Case series initially from China and then from Europe and North America further suggested that therapeutic anticoagulation might be beneficial in these patients.12 , 16 Although no benefit for therapeutic heparin has been found in patients who are critically ill hospitalized with COVID-19, some studies support a possible role for therapeutic anticoagulation in patients not yet requiring intensive care unit (ICU) support, albeit with increased risk of major bleeding. In the present report, we summarize the pathology, rationale, and current evidence base for use of anticoagulation in hospitalized patients with COVID-19 and describe the main design elements of the large-scale international ongoing FREEDOM COVID-19 Anticoagulation trial.
COVID-19 Coagulopathy
SARS-CoV-2 enters host cells by binding to the transmembrane angiotensin-converting enzyme 2 receptor.17, 18, 19 This receptor is expressed in numerous tissues, including type 2 pneumocytes, macrophages, the brain, kidney, heart, and endothelium, all of which are vulnerable to viral infection. Although postulated to mitigate rates and severity of infection, no benefit of pharmacologic modulation of the renin-angiotensin-aldosterone system in patients with COVID-19 has been demonstrated.20 Viral entry through the endothelium may cause inflammation (endothelialitis) and vascular injury and, independently, mononuclear cell activation can trigger cytokine release and a procoagulant state.21 , 22 Mononuclear cell activation also independently contributes to the endothelialitis.22 Excess release of cytokines is observed in these processes, particularly tumor necrosis factor alpha and interleukins-1 and -6.23 This cytokine storm may lead to the expression of tissue factor on mononuclear cells, resulting in activation of the coagulation cascade and thrombin formation most prominently.3 These mechanisms might be involved in the diffuse in situ pulmonary microthrombosis that is seen in advanced COVID-19, as well as more traditional venous and arterial thrombotic events.24
Other mechanisms may also be associated with COVID-19 coagulopathy. Complement activation may occur as part of the innate immune response to the viral infection. The initial excess of complement particles C3a, C4a, and C5a and later deposition of C5b-9 may contribute to endothelial damage and a proinflammatory response.25 Furthermore, as part of the response to endothelial damage, there is increased release of von Willebrand factor, which promotes platelet aggregation.26 , 27 Severe COVID-19 may also be associated with positive antiphospholipid antibodies (eg, anticardiolipin, anti–β2 glycoprotein, and antiphosphatidylserine/prothrombin), which can further induce vasculitis and thrombosis.28
Laboratory and Clinical Manifestations of COVID-19 Coagulopathy
The COVID-19 coagulopathy is characterized by laboratory abnormalities including increased levels of fibrinogen and D-dimer, and mild prolongation of prothrombin time or activated partial thromboplastin time, sometimes with mild thrombocytopenia.15 , 29 Some patients hospitalized due to COVID-19 may develop a coagulopathic state that meets the International Society on Thrombosis and Haemostasis definition for disseminated intravascular coagulation (DIC). In a cohort from Wuhan, 16 of 183 hospitalized patients developed DIC, which was associated with worse prognosis (all but 1 of these patients died during the hospitalization).30 Elevated D-dimer alone at hospital admission is also associated with worse prognosis, including elevated risks for bleeding, thrombosis, critical illness, and death.31 , 32
In an autopsy series from the Mount Sinai Health System in New York City, 11 of 26 patients who died of COVID-19 infection had evidence of thromboembolic disease, including pulmonary emboli in 4 patients.13 In 15 patients, the lungs contained an extensive amount of fibrin thrombi, in the context of diffuse alveolar damage.13 In a cohort of 400 hospitalized patients with COVID-19 in Massachusetts (144 critically ill patients), 4.8% had radiographically confirmed venous thromboembolism, including 10 patients with pulmonary embolism.32 COVID-19 may also be associated with arterial thrombotic events, which occurred in 2.8% of patients in that cohort (myocardial infarction, unstable angina, radial artery thrombosis).32 In a separate report from New York, 36% of 2,736 patients hospitalized due to COVID-19 had an elevated troponin level within 24 hours of admission, denoting a significant degree of myocardial injury.33 Troponin elevation was independently associated with death in a dose-dependent fashion33 and with various electrocardiographic or echocardiographic abnormalities, such as left systolic or diastolic dysfunction, right ventricular dysfunction, and pericardial effusions.34 Other arterial thrombotic events also have been associated with COVID-19, including stroke, acute renal injury, acute limb, and mesenteric ischemia.35, 36, 37, 38
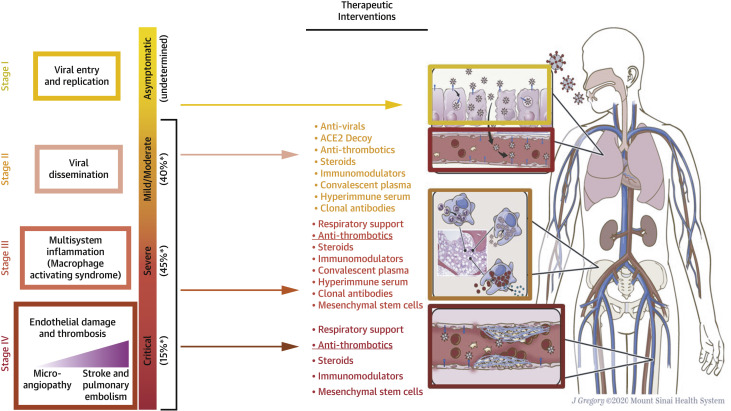
In more critically ill COVID-19 cohorts, the prevalence of thrombotic events has been reported to be as high as 35%.12 , 39 A meta-analysis of 49 studies reported the pooled incidences of venous thromboembolism and pulmonary embolism among hospitalized patients with COVID-19 to be 17% and 7.1%, respectively, whereas the incidence of major bleeding was 3.9%.40 The incidence of thromboembolic events was higher in critically ill patients (27.9% vs 7.1% in ward patients) and bleeding rates were higher among those receiving therapeutic anticoagulation (21.4%).40 Importantly, the rates of overall thrombotic events in hospitalized patients with COVID-19 seem to be higher than in other critical illness and respiratory viral infections such as influenza.41 , 42 These clinical manifestations of the distinct “COVID-19–associated coagulopathy” have led to interest in early preventive anticoagulation (Figure 1 ), explored both in observational and randomized studies.
Figure 1.
Proposed Stages of COVID-19, With Suggested Therapeutic Interventions
Antithrombotic medications might be helpful in all stages of disease. Other possible therapeutic interventions are shown for each disease stage, as well as a hypothetical pathophysiology mechanism. ∗Approximate distribution of primary staging of disease among symptomatic patients. Adapted from Cordon-Cardo et al.22 ACE2 = angiotensin-converting enzyme 2.
Observational Studies
Large-scale observational studies have suggested that prophylactic-dose enoxaparin improves freedom from intubation and survival in hospitalized patients with COVID-19,13 , 14 and, thus, despite the absence of randomized trials, most centers have adopted routine use of prophylactic anticoagulation in these patients.15 However, whether full-dose anticoagulation is more effective than prophylactic-dose anticoagulation, and to a sufficient degree to offset a potential increase in major bleeding complications, is not clear.
Early studies focused on comparing patients with COVID-19 receiving heparin (usually low doses) vs no anticoagulation. In a retrospective Chinese study, 99 of 449 consecutive patients received heparin for 7 days or longer.8 No difference in 28-day mortality was seen between patients receiving heparin but among those with elevated D-dimer or high DIC score, heparin therapy was associated with lower 28-day mortality (OR in the subgroup with D-dimer >3 μg/mL: 0.44; 95% CI: 0.23-0.87; P = 0.017; OR in the subgroup with DIC score ≥4: 0.37; 95% CI: 0.15-0.90; P = 0.029).8 In a retrospective study of 2,773 patients hospitalized due to COVID-19 from the Mount Sinai Health System, those who received full treatment–dose systemic anticoagulation (either oral, subcutaneous, or intravenous) had longer median survival time compared with those not on anticoagulants (21 vs 14 days), a promising effect that might have in part been inflated by immortal time bias, emphasizing the need for randomized trials.2 In a national cohort from the U.S. Department of Veterans Affairs, 84.4% of all 4,297 patients hospitalized with COVID-19 received prophylactic anticoagulation, mainly with subcutaneous heparin or enoxaparin.14 Prophylactic anticoagulation compared with no anticoagulation was associated with a 27% decrease in the risk of 30-day mortality (HR: 0.73; 95% CI: 0.66-0.81), without increasing severe bleeding. In a study of 538 patients with COVID-19 from 8 ICUs in France, the use of a higher-dose prophylactic anticoagulation regimen compared with standard doses was associated with a reduced risk of thrombotic complications (HR: 0.81; 95% CI: 0.66-0.99), without significantly increasing bleeding.43
An observational study of 4,389 patients admitted to Mount Sinai Hospital in New York demonstrated an association between in-hospital administration of anticoagulation and reduction in the risks of mortality and intubation.13 Further analyses suggested more pronounced benefit with therapeutic as opposed to prophylactic dosing.13 Bleeding rates were low overall, but higher among patients on therapeutic anticoagulation. Finally, although exploratory in nature, a signal for benefit was observed in patients on novel oral anticoagulant therapy (primarily apixaban) at therapeutic doses compared with low–molecular weight heparin (LMWH), both regarding prevention of thrombotic complications and fewer bleeding complications.13
As previously mentioned, COVID-19 may lead to the development of symptoms persisting for more than 4 weeks after the acute infection. “Long-COVID,” “post-COVID syndrome,” and “post-acute sequelae of SARS-CoV-2 infection” are some of the terms coined to describe this syndrome.44 The pathophysiology of the post-COVID syndrome remains unclear, and more research is needed to understand the possible role of thrombosis and endotheliopathy in this process.45 In a registry study from New York, within 90 days after discharge of 4,906 patients who were hospitalized due to COVID-19, 1.6% and 1.7% developed new venous and arterial thromboembolic events, respectively.46 Postdischarge anticoagulation was associated with a 46% reduction in the risk of thromboembolism.46 Conversely, a study from the UK reported similar rates of venous thromboembolism after a COVID-19 hospitalization compared with the postdischarge rates observed after medical hospitalizations before the pandemic (4.8 events/1,000 patients vs 3.1 events/1,000 patients at 42 days after discharge; P = 0.20).47 Routine anticoagulation is not presently recommended after the acute phase of COVID-19 in patients who did not develop a thromboembolic event.48
Randomized Clinical Trials
Although the observational studies suggested a benefit for use of anticoagulation, the lack of rigorous evidence establishing the optimal anticoagulation regimen has resulted in varying anticoagulation practices by individual physicians and institutions in patients with COVID-19. The optimal choice of anticoagulant, dosing, and duration of treatment are still not well understood more than 18 months into the pandemic. Nor have the observational studies been able to establish whether the selection of specific anticoagulation regimens should be tailored according to clinical or laboratory risk factors affecting prognosis. Several large-scale randomized trials were thus initiated to determine the relative effectiveness and safety of different anticoagulation strategies in patients with COVID-19 at varying risks (Table 1 ).49
Table 1.
Main Results of Randomized Clinical Trials Investigating Anticoagulation Regimens in Patients With COVID-19
| Trial (Country) | N | Population | Intervention | Comparator | Main Outcome | Main Results |
|---|---|---|---|---|---|---|
| ACTION (Brazil)56 | 615 | Hospitalized patients with COVID-19 and elevated D-Dimer | Therapeutic anticoagulation: rivaroxaban 20 or 15 mg daily for stable patients (94%), LMWH or UFH | Prophylactic anticoagulation: enoxaparin or UFH | Composite of time to death, duration of hospitalization or duration of supplemental oxygen to day 30 (win ratio method) | 34.8% wins (therapeutic) vs 41.3% wins Win ratio: 0.86; 95% CI: 0.59-1.22; P = 0.40 Major or clinically relevant bleeding: 8% (therapeutic) vs 2% (P = 0.001) |
| ACTIV-4B (USA)52 | 657 | Symptomatic clinically stable outpatients with COVID-19 | Therapeutic-dose apixaban (5 mg orally twice daily)a | Prophylactic-dose apixaban (2.5 mg orally twice daily); placebo | Composite of all-cause death, symptomatic venous or arterial thromboembolism, myocardial infarction, stroke, or hospitalization for a cardiovascular or pulmonary cause at 45 d | The trial was terminated early because event rates were lower than anticipated (1.4% in the 5-mg apixaban arm and 0.7% in the other arms) Risk difference: 5-mg apixaban vs placebo: 1.4% (95% CI: -1.5%-5.0%); 2.5-mg apixaban vs placebo: 0.7% (95% CI: -2.1%-4.1%) Bleeding events: 9.1% (5-mg apixaban); 6.7% (2.5-mg apixaban); 2.2% (placebo) |
| HEP-COVID (USA)55 | 257 | Hospitalized COVID-19 patients with need of oxygen supply and either elevated D-dimer or high SIC score | Therapeutic-dose enoxaparin (1 mg/kg SC twice daily) | Standard of care pharmacologic thromboprophylaxis (intermediate doses were allowed) | Composite of VTE, arterial thromboembolism (myocardial infarction, stroke, systemic embolism), all-cause death at 30 d | 28.7% (therapeutic dose) vs 41.9% Relative risk: 0.68; 95% CI: 0.49-0.96; P = 0.03 Major bleeding: 4.7% (therapeutic) vs 1.6% - NS |
| INSPIRATION (Iran)53 | 562 | Critically ill COVID-19 patients | Intermediate-dose heparin (enoxaparin, 1 mg/kg SC daily) | Standard prophylactic anticoagulation (enoxaparin, 40 mg daily) | Composite of venous or arterial thrombosis, treatment with extracorporeal membrane oxygenation, or mortality within 30 d | 45.7% (intermediate dose) vs 44.1% OR: 1.06; 95% CI: 0.76-1.48; P = 0.70 Major bleeding: 2.5% (intermediate dose) vs 1.4%, P for noninferiority >0.99 |
| Multiplatform trial (ATTACC, ACTIV-4a, and REMAP-CAP) in non–critically ill patients (International)58 | 2,219 | Hospitalized patients with COVID-19 not in need of critical care–level organ support at enrollment | Therapeutic-dose anticoagulation with heparin | Usual-care pharmacologic thromboprophylaxis | Organ support–free days, ie, an ordinal scale combining in-hospital death and the number of days free of CV or respiratory organ support up to day 21 (Bayesian statistical model) | Probability that therapeutic anticoagulation increases organ support–free days: 98.6% OR: 1.27; 95% CrI: 1.03-1.58 Major bleeding: 1.9% (therapeutic) vs 0.9% (probability that therapeutic anticoagulation is inferior: 95.5%) |
| Multiplatform trial (REMAP-CAP, ACTIV-4a, and ATTACC) in critically ill patients (International)57 | 1,098 | Critically ill COVID-19 patients | Therapeutic-dose anticoagulation with heparin | Usual-care pharmacologic thromboprophylaxis | Organ support–free days – same definition as above (Bayesian statistical model) | Probability of futility: 99.9% OR: 0.83; 95% CrI: 0.67-1.03 Major bleeding: 3.8% (therapeutic) vs 2.3% (probability that therapeutic anticoagulation is inferior: 87.2%) |
| Perepu US et al (USA)60 | 176 | COVID-19 patients admitted to an ICU or with laboratory evidence of coagulopathy | Intermediate-dose enoxaparin (1 mg/kg SC daily) | Standard prophylactic enoxaparin (40 mg SC daily) | All-cause mortality at 30 d | 15% (intermediate dose) vs 21% HR: 0.67; 95% CI: 0.33-1.37; P = 0.28 Major bleeding: 2% vs 2% |
| RAPID (International)54 | 465 | Moderately ill COVID-19 ward patients with elevated D-dimer | Therapeutic-dose heparin (LMWH or UFH) | Prophylactic heparin (LMWH or UFH) | Composite of death, ICU admission, noninvasive or invasive mechanical ventilation at 28 days | 16.2% (therapeutic heparin) vs 21.9% Odd ratio: 0.69; 95% CI: 0.43-1.10; P = 0.12 Major bleeding: 0.9% (therapeutic) vs 1.7% - NS |
| Phase 2 trial | ||||||
| HESACOVID (Brazil)61 | 20 | COVID-19 patients requiring mechanical ventilation and with laboratory evidence of coagulopathy | Therapeutic enoxaparin (1 mg/kg SC BID) | Standard anticoagulant thromboprophylaxis with SC UFH | Change in the ratio of PaO2 over the FiO2 from baseline to 14 d | Significant increase in the PaO2/FiO2 ratio was observed in the therapeutic group but not in the control group |
| Trial not yet peer-reviewed | ||||||
| MICHELLE (Brazil)51 | 320 | Patients discharged from a COVID-19 hospitalization and at increased risk for VTE, as assessed using the IMPROVE score. | Rivaroxaban 10 mg/daily | No anticoagulation | Composite of VTE, either symptomatic or detected by image tests, and arterial thrombotic events (myocardial infarction, nonhemorrhagic stroke, major adverse limb events, and CV death) at 35 d | 3.14% (rivaroxaban) vs 9.43% Relative risk: 0.33; 95% CI: 0.13-0.90; P = 0.03 No major bleedings detected |
The trials listed have reported their results either in a full publication or congress presentation by the time this manuscript was written. A list of ongoing trials is presented in Talasaz et al.49 All trials had an open-label design, except for HEP-COVID (where patients and most but not all trial personnel were blinded), and ACTIV-4B (double-blinded).
ACTION = AntiCoagulaTlon cOroNavirus; ACTIV = Accelerating Covid-19 Therapeutic Interventions and Vaccines-4; ATTACC = Antithrombotic Therapy to Ameliorate Complications of Covid-19; CrI = Credible interval; CV = cardiovascular; FiO2 = fraction of inspired oxygen; HEP-COVID = Systemic Anticoagulation With Full Dose Low Molecular Weight Heparin (LMWH) Vs Prophylactic or Intermediate Dose LMWH in High Risk COVID-19 Patients; HESACOVID = Therapeutic versus prophylactic anticoagulation for severe COVID-19: A randomized phase II clinical trial; ICU = intensive care unit; INSPIRATION = Intermediate vs Standard-Dose Prophylactic Anticoagulation in Critically-ill Patients With COVID-19: An Open Label Randomized Controlled Trial; LMWH = low–molecular weight heparin; MICHELLE = Medically Ill hospitalized Patients for Covid - THrombosis Extended ProphyLaxis with rivaroxaban ThErapy; NS = nonsignificant; PaO2 = partial pressure of arterial oxygen; RAPID = Therapeutic Anticoagulation versus Standard Care as a Rapid Response to the COVID-19 Pandemic; REMAP-CAP = Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia; SC = subcutaneous; SIC = sepsis-induced coagulopathy; UFH = unfractionated heparin; VTE = venous thromboembolism.
ACTIV-4B also tested aspirin 81 mg once daily with equal patient allocation in the 4 arms (placebo, aspirin, 2.5-mg apixaban, 5-mg apixaban).
The MICHELLE (Medically Ill hospitalized Patients for Covid - THrombosis Extended ProphyLaxis with rivaroxaban ThErapy) trial randomized 320 adult patients discharged from a COVID-19 hospitalization and at increased risk for a thromboembolic event to receive rivaroxaban 10 mg daily for 35 days vs no anticoagulation.50 The trial was conducted in Brazil and the primary endpoint was a composite of venous thromboembolic events, either symptomatic or detected using routine image tests, and arterial thrombotic events. Results were not yet peer-reviewed, but the investigators have reported that rivaroxaban reduced the primary endpoint by 67% at 35 days after discharge (relative risk: 0.33; 95% CI: 0.13-0.90; P = 0.03), without any major bleedings reported.51 Recently, the National Institutes of Health–sponsored ACTIV-4B (Accelerating COVID-19 Therapeutic Interventions and Vaccines) Outpatient Thrombosis Prevention Trial, testing apixaban in mildly symptomatic COVID-19 outpatients, was halted prematurely after enrollment of 9% of the planned total number of participants due to the low incidence of thrombotic events in this population.52 The primary composite endpoint of all-cause death, symptomatic venous or arterial thromboembolism, myocardial infarction, stroke, or hospitalization for cardiovascular or pulmonary cause at 45 days occurred in 0.7% (1 patient) of those in the placebo group and 1.4% (2 patients) of those randomized to apixaban 5 mg daily.52 Other clinical trials focused on nonhospitalized patients with COVID-19 are still underway, testing rivaroxaban (NCT04508023) and LMWH (NCT04492254; NCT04400799).
Some trials targeted at hospitalized patients with COVID-19 have already reported their main results. The INSPIRATION (Intermediate vs Standard-Dose Prophylactic Anticoagulation in Critically-ill Patients With COVID-19: An Open Label Randomized Controlled) trial compared intermediate-dose vs standard-dose prophylactic anticoagulation among 562 patients with COVID-19 receiving ICU support in Iran.53 Enoxaparin 1 mg/kg daily was used as intermediate-dose anticoagulation and enoxaparin 40 mg daily was used as the standard dose. The primary outcome was a composite of venous or arterial thrombosis, treatment with extracorporeal membrane oxygenation, or mortality within 30 days. No significant difference was observed between groups (OR: 1.06; 95% CI: 0.76-1.48; P = 0.70).53 Bleeding events, although rare, were more frequent in the intermediate-dose group and included intracranial and fatal bleeding.53
Focusing particularly on patients who were moderately ill hospitalized due to COVID-19 with an elevated D-dimer, the RAPID (Therapeutic Anticoagulation versus Standard Care as a Rapid Response to the COVID-19 Pandemic) trial compared therapeutic vs prophylactic anticoagulation with heparin.54 Among 465 randomized patients, a nonsignificant trend toward a reduction in the 28-day composite of death, invasive mechanical ventilation, noninvasive mechanical ventilation, or ICU admission was observed in the therapeutic anticoagulation group (OR: 0.69; 95% CI: 0.43-1.10; P = 0.12).54 The incidence of all-cause death was reduced in the therapeutic anticoagulation group (OR: 0.22; 95% CI: 0.07-0.65), a finding that should be regarded as hypothesis generating.54 The HEP-COVID (Systemic Anticoagulation with Full-Dose LMWH versus Prophylactic or Intermediate-Dose LMWH/Unfractionated Heparin in High-Risk COVID-19 Patients) trial also used elevated D-dimer levels or a high sepsis-induced coagulopathy score as part of the eligibility criteria.55 A total of 257 patients who were hospitalized due to COVID-19 were randomized, most of them (67.2%) not requiring ICU level of care.55 Therapeutic-dose enoxaparin, compared with institutional standard prophylactic or intermediate-dose heparin, led to a 32% reduction in the primary outcome of venous or arterial thromboembolism or death from any cause (relative risk: 0.68; 95% CI: 0.49-0.96; P = 0.03), mainly driven by a reduction in thromboembolism (relative risk: 0.37; 95% CI: 0.21-0.66; P < 0.001).55
Using a win ratio design, the Brazilian Consortium recently reported the results of the ACTION (Full Anticoagulation Versus Prophylaxis in COVID-19: COALIZAO ACTION Trial), an open-label study assessing whether therapeutic anticoagulation improves outcomes in patients hospitalized with COVID-19.56 A total of 615 patients hospitalized due to COVID-19 with elevated D-dimer were randomized to either prophylactic or therapeutic anticoagulation. Prophylactic anticoagulation included enoxaparin or unfractionated heparin. In the therapeutic anticoagulation group, 90% of patients received in-hospital oral rivaroxaban (20 mg or 15 mg daily).56 The primary efficacy outcome (time to death, duration of hospitalization, or duration of supplemental oxygen to day 30) was not different between the therapeutic and prophylactic anticoagulation arms, with 28,899 (34.8%) and 34,288 (41.3%) wins, respectively (win ratio: 0.86; 95% CI: 0.59-1.22; P = 0.40).56 The investigators concluded that rivaroxaban and other direct oral anticoagulants should be avoided in hospitalized patients with COVID-19 in the absence of an evidence-based indication for oral anticoagulation.
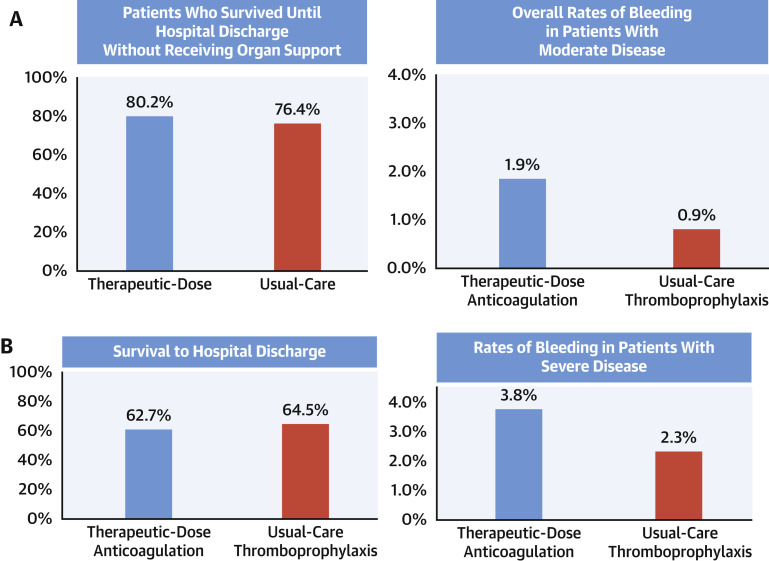
The largest study reported to date was a multiplatform investigation from a prospective federation of 3 adaptive randomized trials in which hospitalized patients with COVID-19 were randomized to therapeutic anticoagulation with LMWH or unfractionated heparin vs usual care pharmacologic prophylaxis.57 , 58 The participating trials included the ATTACC (Antithrombotic Therapy to Ameliorate Complications of Covid-19), REMAP-CAP (Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia), and the National Institutes of Health–sponsored ACTIV-4a. Study outcomes were reported separately for critically ill and not critically ill patient strata. The study stratum of severely ill patients receiving ICU-level support at baseline was stopped prematurely for futility after randomization of 1,098 patients and demonstrated no improvements in the primary outcome of survival free of organ support, whereas major bleeding tended to be increased with the use of full-dose compared with prophylactic-dose heparin (Figure 2B ).57 In contrast, among 2,219 moderately ill patients not initially receiving ICU-level support, surviving through day 21 without requiring organ support was more likely to be achieved with use of therapeutic-dose compared with prophylactic-dose anticoagulation (80.2% vs 76.4%; adjusted absolute between-group difference: 4.0%; 95% CI: 0.5%-7.2%) (Figure 2A).58 This positive finding also resulted in early termination of this stratum of the trial. However, only small differences between groups were noted in in-hospital major thrombotic events (1.1% vs 2.1%, respectively) and in-hospital mortality (7.3% vs 8.2%, respectively). The median number of organ support–free days was 22 in both groups. Major bleeding occurred in 1.9% in the therapeutic-dose anticoagulation group and 0.9% in the prophylactic-dose anticoagulation group. Although the reasons underlying the differences in outcomes between the 2 strata are unknown, critically ill patients may have had disease that was too advanced to benefit from therapeutic heparin, with sequestered or organized thrombi resistant to the action of antithrombin, the endogenous anticoagulant potentialized by heparin.59 In addition, other modifying differences in the patient populations or geographies of recruitment, specific heparin agents and doses used, or concomitant therapies may have been present. In an accompanying editorial, ten Cate wrote: “first, the available evidence does not support use of therapeutic-dose heparin or LMWH for thrombosis prevention in critically ill patients. Second, whether intermediate or therapeutic doses of thromboprophylactic drugs are effective and safe in moderately ill patients with Covid-19 remains an important question.”59 To further inform this latter issue, the Icahn School of Medicine at Mount Sinai is sponsoring a large international trial in hospitalized patients with COVID-19 not requiring ICU level of care, the FREEDOM COVID Anticoagulation trial, discussed in the next section.
Figure 2.
Principal Results From the Multiplatform REMAP-CAP, ACTIV-4a, and ATTACC Trial
(A) Clinical outcomes in the strata of noncritically ill hospitalized patients with COVID-19 not requiring intensive care unit (ICU)–level of care. (Left) Survival until hospital discharge without receiving organ support. (Right) Major bleeding tended to be increased with therapeutic-dose anticoagulation (posterior probability that therapeutic-dose anticoagulation is inferior to usual-care thromboprophylaxis, leading to more bleedings: 95.5%). (B) Clinical outcomes in the strata of critically ill hospitalized patients with COVID-19 requiring ICU-level respiratory or cardiovascular organ support. (Left) The secondary outcome of survival to hospital discharge (posterior probability of inferiority: 89.2%). (Right) Major bleeding tended to be increased with therapeutic-dose anticoagulation.
The FREEDOM COVID Anticoagulation Trial
Overview
The FREEDOM COVID-19 Anticoagulation trial (NCT04512079) is a large-scale, prospective, multicenter, open-label, randomized controlled comparative safety and effectiveness study currently enrolling patients at centers in the United States, Central and South America, Europe, and Asia (Supplemental Figure 1). Patients hospitalized with confirmed or suspected COVID-19 are randomly assigned to 1 of 3 anticoagulation regimens: prophylactic enoxaparin, therapeutic-dose enoxaparin, or therapeutic-dose apixaban. The primary objective of the study is to determine whether therapeutic doses of enoxaparin or apixaban are superior to a prophylactic dose of enoxaparin in reducing the composite incidence of all-cause mortality, need for ICU level of care, systemic thromboembolism, or ischemic stroke at 30 days after randomization. If so, noninferiority of apixaban vs enoxaparin at therapeutic doses is tested.
Study population
All noncritically ill adult patients hospitalized with either suspected or confirmed COVID-19 who are able to provide consent and who do not have any of the exclusion criteria outlined in Supplemental Table 1 are eligible for participation in the study.
Anticoagulation regimens
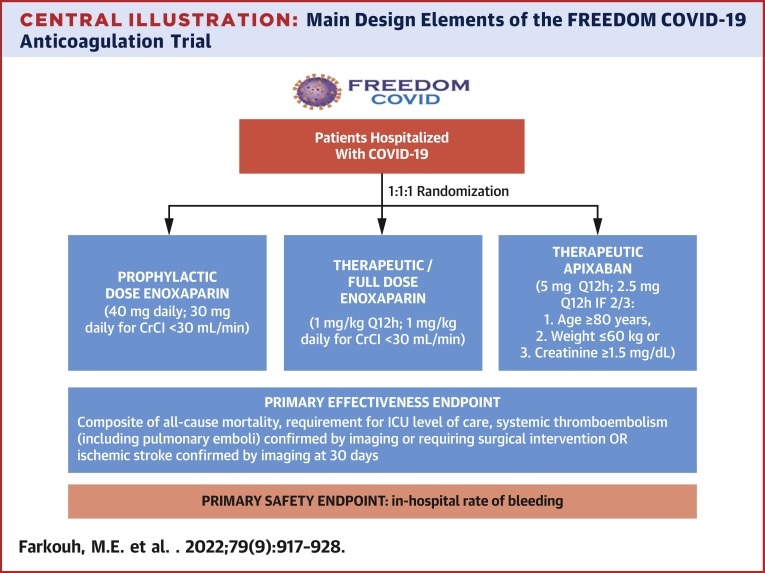
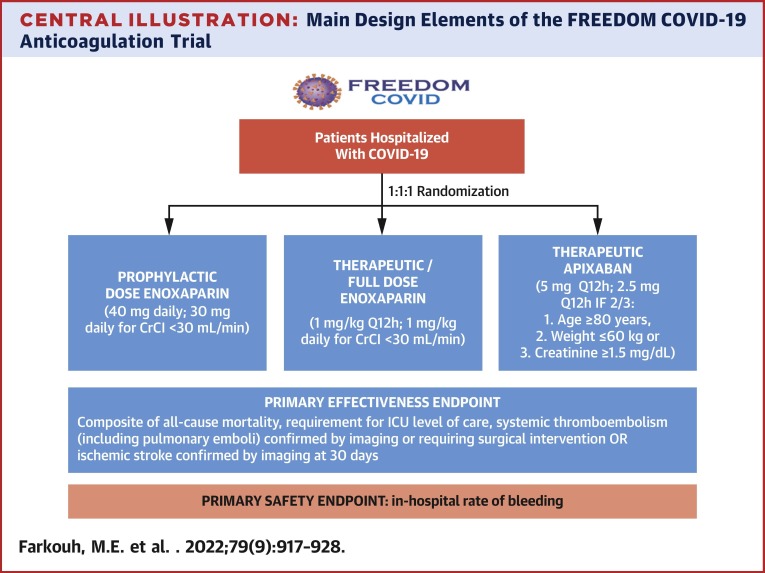
Eligible patients are randomized in a 1:1:1 fashion to 1 of 3 arms: prophylactic enoxaparin, therapeutic enoxaparin, or therapeutic apixaban (Central Illustration ). The U.S. Food and Drug Administration–approved regimens for prophylactic- and full-diose anticoagulation were chosen. Anticoagulation study drug is administered as soon as possible, but no later than 12 hours after randomization and until hospital discharge.
Central Illustration.
Main Design Elements of the FREEDOM COVID-19 Anticoagulation Trial
The FREEDOM COVID-19 Anticoagulation trial (NCT04512079) is a prospective, multicenter, open-label, randomized controlled comparative safety and effectiveness study that will enroll up to 3,600 patients. Enoxaparin is administered subcutaneously and apixaban is administered orally. CrCl = creatinine clearance; ICU = intensive care unit; Q12h = every 12 hours.
Outcome measures
The primary effectiveness outcome endpoint is the time-to-first event within 30 days after randomization of the composite of all-cause mortality, respiratory or circulatory deterioration requiring ICU level of care, systemic thromboembolism (including pulmonary emboli) confirmed using imaging or requiring surgical intervention, or ischemic stroke confirmed using imaging (Supplemental Table 2). All endpoint events are those that occurred clinically; screening for thromboembolism without symptoms is not routinely performed. All events will be adjudicated by an independent Clinical Event Committee. The primary safety outcome is the in-hospital rate of Bleeding Academic Research Consortium type 3 or 5 bleeding. Secondary outcomes are listed in Supplemental Table 3.
Analytic plan and sample size
Assuming a 25% event rate for the primary composite outcome in the prophylactic heparin arm (a conservative estimate based on prior registry-based studies)2 , 13 , 14 and a 20% event rate in both the full-dose enoxaparin and apixaban arms, randomizing 1,200 patients in each of the 3 arms (3,600 patients total) provides >95% power to demonstrate that full-dose anticoagulation (pooled across the 2 arms) is superior to the prophylactic dose regimen at a 2-sided α = 0.05. Further details on the study procedures, data collection, analytic plans, and study governance are provided in the Supplemental Appendix.
Perspective
The FREEDOM COVID-19 Anticoagulation trial is a well-powered, multicenter, open-label, global, randomized comparative safety and effectiveness study evaluating different anticoagulation regimens in noncritically ill hospitalized patients with COVID-19. To date, nearly 3,000 patients have been randomized. A major strength of FREEDOM COVID-19 Anticoagulation is that it is adequately powered to detect meaningful differences in clinical events with a frequentist design and no reliance on an ordinal scale of symptom/event severity. Completion of the FREEDOM COVID-19 Anticoagulation trial and other ongoing studies is essential to determine whether therapeutic-dose anticoagulation provides an incremental effectiveness benefit in reducing thrombotic events, preventing intubation, or improving survival compared with prophylactic-dose anticoagulation in noncritically ill hospitalized patients with COVID-19, and to assess the magnitude of the inevitable incremental increase in bleeding from full-dose anticoagulation. In addition, the FREEDOM COVID-19 Anticoagulation trial will provide insights into whether apixaban is superior to either enoxaparin regimen. The FREEDOM COVID-19 Anticoagulation trial has significant representation from North and South America as well as Europe and Asia. Thus, the findings should be informative to a diverse representation of practices across varied health-care delivery systems.
Funding Support and Author Disclosures
Dr Farkouh has received research grants from Amgen, Novo Nordisk, and Novartis. Dr Stone has received speaker honoraria from Infraredx; has served as a consultant to Valfix, TherOx, Robocath, HeartFlow, Ablative Solutions, Miracor, Neovasc, Abiomed, Ancora, Vectorious, Elucid Bio, Occlutech, CorFlow, Apollo Therapeutics, Impulse Dynamics, Cardiomech, Gore, and Amgen; and has equity/options from Ancora, Cagent, Applied Therapeutics, Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, Valfix, and Xenter. Dr Godoy is supported by the Frederick Banting and Charles Best Canada Graduate Scholarship (Doctoral Research Award) from the Canadian Institutes of Health Research. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Donald Clark, III, MD, MPH, served as Guest Associate Editor for this paper. Javed Butler, MD, MPH, MBA, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables and a figure, please see the online version of this paper.
Appendix
References
- 1.COVID-19 Map - Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html
- 2.Paranjpe I., Fuster V., Lala A., et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76:122–124. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bikdeli B., Madhavan M.V., Jimenez D., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Godoy L.C., Goligher E.C., Lawler P.R., Slutsky A.S., Zarychanski R. Anticipating and managing coagulopathy and thrombotic manifestations of severe COVID-19. CMAJ. 2020;192:E1156–E1161. doi: 10.1503/cmaj.201240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang L., Yao Q., Gu X., et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;398:747–758. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iba T., Warkentin T.E., Thachil J., Levi M., Levy J.H. Proposal of the definition for COVID-19-associated coagulopathy. J Clin Med. 2021;10:191. doi: 10.3390/jcm10020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wichmann D., Sperhake J.P., Lutgehetmann M., et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piazza G., Campia U., Hurwitz S., et al. Registry of arterial and venous thromboembolic complications in patients with COVID-19. J Am Coll Cardiol. 2020;76:2060–2072. doi: 10.1016/j.jacc.2020.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nadkarni G.N., Lala A., Bagiella E., et al. Anticoagulation, bleeding, mortality, and pathology in hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76:1815–1826. doi: 10.1016/j.jacc.2020.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rentsch C.T., Beckman J.A., Tomlinson L., et al. Early initiation of prophylactic anticoagulation for prevention of coronavirus disease 2019 mortality in patients admitted to hospital in the United States: cohort study. BMJ. 2021;372:n311. doi: 10.1136/bmj.n311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuker A., Tseng E.K., Nieuwlaat R., et al. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Advances. 2021;5:872–888. doi: 10.1182/bloodadvances.2020003763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bikdeli B., Madhavan M.V., Gupta A., et al. Pharmacological agents targeting thromboinflammation in COVID-19: review and implications for future research. Thromb Haemost. 2020;120:1004–1024. doi: 10.1055/s-0040-1713152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271. doi: 10.1016/j.cell.2020.02.052. 280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monteil V., Kwon H., Prado P., et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181:905. doi: 10.1016/j.cell.2020.04.004. 913.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopes R.D., Macedo A.V.S., de Barros E Silva P.G.M., et al. Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial. JAMA. 2021;325:254–264. doi: 10.1001/jama.2020.25864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Libby P., Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020;41:3038–3044. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cordon-Cardo C., Pujadas E., Wajnberg A., et al. COVID-19: staging of a new disease. Cancer Cell. 2020;38:594–597. doi: 10.1016/j.ccell.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levi M. Pathophysiology of coagulopathy in hematological malignancies and in COVID-19. HemaSphere. 2021;5:e571. doi: 10.1097/HS9.0000000000000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGonagle D., O'Donnell J.S., Sharif K., Emery P., Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020;2:e437–e445. doi: 10.1016/S2665-9913(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fletcher-Sandersjöö A., Bellander B.-M. Is COVID-19 associated thrombosis caused by overactivation of the complement cascade? A literature review. Thromb Res. 2020;194:36–41. doi: 10.1016/j.thromres.2020.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levi M., Thachil J., Iba T., Levy J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinelli N., Montagnana M., Pizzolo F., et al. A relative ADAMTS13 deficiency supports the presence of a secondary microangiopathy in COVID 19. Thromb Res. 2020;193:170–172. doi: 10.1016/j.thromres.2020.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuo Y., Estes S.K., Ali R.A., et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci Translational Med. 2020;12 doi: 10.1126/scitranslmed.abd3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goswami J., MacArthur T.A., Sridharan M., et al. A review of pathophysiology, clinical features, and management options of COVID-19 associated coagulopathy. Shock. 2021;55:700–716. doi: 10.1097/SHK.0000000000001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Samkari H., Karp Leaf R.S., Dzik W.H., et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136:489–500. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lala A., Johnson K.W., Januzzi J.L., et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76:533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giustino G., Croft L.B., Stefanini G.G., et al. Characterization of myocardial injury in patients with COVID-19. J Am Coll Cardiol. 2020;76:2043–2055. doi: 10.1016/j.jacc.2020.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lushina N., Kuo J.S., Shaikh H.A. Pulmonary, cerebral, and renal thromboembolic disease in a patient with COVID-19. Radiology. 2020;296:E181–E183. doi: 10.1148/radiol.2020201623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bellosta R., Luzzani L., Natalini G., et al. Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg. 2020;72:1864–1872. doi: 10.1016/j.jvs.2020.04.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhayana R., Som A., Li M.D., et al. Abdominal imaging findings in COVID-19: preliminary observations. Radiology. 2020;297:E207–E215. doi: 10.1148/radiol.2020201908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oxley T.J., Mocco J., Majidi S., et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382:e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malas M.B., Naazie I.N., Elsayed N., Mathlouthi A., Marmor R., Clary B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: a systematic review and meta-analysis. EClinicalMedicine. 2020;29 doi: 10.1016/j.eclinm.2020.100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiménez D., García-Sanchez A., Rali P., et al. Incidence of VTE and bleeding among hospitalized patients with Coronavirus Disease 2019: a systematic review and meta-analysis. Chest. 2021;159:1182–1196. doi: 10.1016/j.chest.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poissy J., Goutay J., Caplan M., et al. Pulmonary embolism in patients with COVID-19: awareness of an increased prevalence. Circulation. 2020;142:184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 42.Yu B., Li X., Chen J., et al. Evaluation of variation in D-dimer levels among COVID-19 and bacterial pneumonia: a retrospective analysis. J Thromb Thrombolysis. 2020;50:548–557. doi: 10.1007/s11239-020-02171-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tacquard C., Mansour A., Godon A., et al. Impact of high-dose prophylactic anticoagulation in critically ill patients with COVID-19 pneumonia. Chest. 2021;159:2417–2427. doi: 10.1016/j.chest.2021.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Evaluating and Caring for Patients with Post-COVID Conditions: Interim Guidance. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-index.html
- 45.Fogarty H., Townsend L., Morrin H., et al. Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J Thromb Haemost. 2021;19:2546–2553. doi: 10.1111/jth.15490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giannis D., Allen S.L., Tsang J., et al. Postdischarge thromboembolic outcomes and mortality of hospitalized patients with COVID-19: the CORE-19 registry. Blood. 2021;137:2838–2847. doi: 10.1182/blood.2020010529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberts L.N., Whyte M.B., Georgiou L., et al. Postdischarge venous thromboembolism following hospital admission with COVID-19. Blood. 2020;136:1347–1350. doi: 10.1182/blood.2020008086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anticoagulation for patients with COVID-19 being discharged from hospital – recommendation 3. https://www.hematology.org/education/clinicians/guidelines-and-quality-care/clinical-practice-guidelines/venous-thromboembolism-guidelines/ash-guidelines-on-use-of-anticoagulation-in-patients-with-covid-19#rec3
- 49.Talasaz A.H., Sadeghipour P., Kakavand H., et al. Recent randomized trials of antithrombotic therapy for patients with COVID-19: JACC State-of-the-Art Review. J Am Coll Cardiol. 2021;77:1903–1921. doi: 10.1016/j.jacc.2021.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramacciotti E., Agati L.B., Calderaro D., et al. Medically ill hospitalized patients for COVID-19 thrombosis extended prophylaxis with rivaroxaban therapy: rationale and design of the MICHELLE Trial. Am Heart J. 2021;242:115–122. doi: 10.1016/j.ahj.2021.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Medically ill hospitalized patients for COVID –19 thrombosis extended prophylaxis with rivaroxaban therapy: THE MICHELLE TRIAL. Presented by Dr. Eduardo Ramacciotti at the European Society of Cardiology Virtual Congress. August 29, 2021 doi: 10.1016/j.ahj.2021.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Connors J.M., Brooks M.M., Sciurba F.C., et al. Effect of antithrombotic therapy on clinical outcomes in outpatients with clinically stable symptomatic COVID-19: the ACTIV-4B randomized clinical trial. JAMA. 2021;326:1703–1712. doi: 10.1001/jama.2021.17272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.INSPIRATION Investigators Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA. 2021;325:1620–1630. doi: 10.1001/jama.2021.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sholzberg M., Tang G.H., Rahhal H., et al. Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with covid-19 admitted to hospital: RAPID randomised clinical trial. BMJ. 2021;375:n2400. doi: 10.1136/bmj.n2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spyropoulos A.C., Goldin M., Giannis D., et al. Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: the HEP-COVID randomized clinical trial. JAMA Intern Med. 2021;181:1612–1620. doi: 10.1001/jamainternmed.2021.6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lopes R.D., de Barros e Silva P.G.M., Furtado R.H.M., et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet. 2021;397:2253–2263. doi: 10.1016/S0140-6736(21)01203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.The REMAP-CAP, ACTIV-4a, and ATTACC Investigators Therapeutic anticoagulation with heparin in critically ill patients with Covid-19. N Engl J Med. 2021;385:777–789. doi: 10.1056/NEJMoa2103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.The ATTACC, ACTIV-4a, and REMAP-CAP Investigators Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19. N Engl J Med. 2021;385:790–802. doi: 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.ten Cate H. Surviving Covid-19 with heparin? N Engl J Med. 2021;385(9):845–846. doi: 10.1056/NEJMe2111151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perepu U.S., Chambers I., Wahab A., et al. Standard prophylactic versus intermediate dose enoxaparin in adults with severe COVID-19: a multi-center, open-label, randomized controlled trial. J Thromb Haemost. 2021;19:2225–2234. doi: 10.1111/jth.15450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lemos A.C.B., do Espírito Santo D.A., Miranda C.H. Therapeutic anticoagulation in COVID-19 patients. Thromb Res. 2021;203:72–73. doi: 10.1016/j.thromres.2021.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.