Abstract
Kisspeptin (encoded by the Kiss1 gene) and its receptor, KISS1R (encoded by the Kiss1r gene), have well-established roles in stimulating reproduction via central actions on reproductive neural circuits, but recent evidence suggests that kisspeptin signaling also influences metabolism and energy balance. Indeed, both Kiss1 and Kiss1r are expressed in many metabolically-relevant peripheral tissues, including both white and brown adipose tissue, the liver, and the pancreas, suggesting possible actions on these tissues or involvement in their physiology. In addition, there may be central actions of kisspeptin signaling, or factors co-released from kisspeptin neurons, that modulate metabolic, feeding, or thermoregulatory processes. Accumulating data from animal models suggests that kisspeptin signaling regulates a wide variety of metabolic parameters, including body weight and energy expenditure, adiposity and adipose tissue function, food intake, glucose metabolism, respiratory rates, locomotor activity, and thermoregulation. Herein, the current evidence for the involvement of kisspeptin signaling in each of these physiological parameters is reviewed, gaps in knowledge identified, and future avenues of important research highlighted. Collectively, the discussed findings highlight emerging non-reproductive actions of kisspeptin signaling in metabolism and energy balance, in addition to previously documented roles in reproductive control, but also emphasize the need for more research to resolve current controversies and uncover underlying molecular and physiological mechanisms.
Keywords: Kisspeptin, KISS1, KISS1R, GPR54, Metabolism, Energy expenditure, Body weight, Obesity, Adipose, Fat, Insulin, Diabetes, Feeding, Food intake
1. Overview of kisspeptin and its role in reproduction
Over the past two decades, the peptide kisspeptin, encoded by the Kiss1 gene, and its receptor, KISS1R (previously termed GPR54), have been established as critical regulators of reproduction (Clarke, Dhillo, & Jayasena, 2015; Kauffman, 2010; Terasawa, Guerriero, & Plant, 2013). The role of kisspeptin in reproduction was first revealed in humans and mice displaying mutations in the Kiss1r (and later, Kiss1) gene, which cause infertility, impaired puberty, and very low levels of gonadotropins and sex steroids (d’Anglemont De Tassigny et al., 2007; de Roux et al., 2003; Funes et al., 2003; Seminara et al., 2003). Kisspeptin was subsequently shown to be a potent secretagogue of gonadotropin-releasing hormone (GnRH), the primary hormone controlling the secretion of luteinizing hormone (LH) and follicle stimulating hormone (FSH). The reproductive deficits observed in Kiss1 and Kiss1r mutants are due to the important role of neural kisspeptin in regulating the pulsatile and surge modes of GnRH and LH release. Accordingly, much research has focused on hypothalamic kisspeptin-expressing neurons projecting to KISS1R-expressing GnRH neurons located in the anterior hypothalamic preoptic area (POA).
In mammals, two major populations of kisspeptin neurons exist in the hypothalamus: one in the arcuate nucleus (ARC; infundibular nucleus in primates and humans) right above the median eminence and another sexually dimorphic population more rostrally in the continuum spanning the anteroventral periventricular nucleus and periventricular nucleus (AVPV/PeN; also termed the RP3V; POA in primates and humans), in which females typically have more kisspeptin neurons than males (Semaan & Kauffman, 2010). Kisspeptin neurons in the AVPV/PeN are positively regulated by estradiol (E2) and mediate E2’s positive feedback induction of the preovulatory GnRH/LH surge in females, while ARC kisspeptin neurons stimulate the tonic, pulsatile release of GnRH/LH that controls gonadal sex steroid synthesis, gametogenesis, and the menstrual cycle, and are inhibited by circulating sex steroids (negative feedback) (Smith et al., 2005; Terasawa et al., 2013). Less well studied are the functions of kisspeptin and KISS1R cells more recently identified in other non-hypothalamic brain areas, such as the amygdala, bed nucleus of the stria terminalis, and lateral septum (Cravo et al., 2011; Di Giorgio et al., 2014; Kim et al., 2011; Xu et al., 2012; Yeo et al., 2016) and a variety of peripheral tissues, including the gonads, uterus, placenta, liver, pancreas, adipose tissue, and gut (Brown et al., 2008; Hauge-Evans et al., 2006; Herbison et al., 2010; Kotani et al., 2001; Ohtaki et al., 2001; Tolson et al., 2020).
In addition to its well-characterized role in stimulating reproduction, kisspeptin has recently been shown to be involved in the regulation of metabolism and energy balance. Reproduction is energetically costly; thus, the energy status of an organism can have a significant effect on its reproductive status and ability to reproduce. It is therefore not surprising that kisspeptin neurons have been shown to be regulated by metabolic factors and conditions, and integrate multiple peripheral metabolic cues (e.g., leptin, insulin, and ghrelin) either directly or indirectly to adjust reproductive function according to energy status, as reviewed in detail elsewhere (Castellano & Tena-Sempere, 2016; Dudek, Ziarniak, & Sliwowska, 2018; Navarro, 2020; Rodríguez-Vázquez, Tena-Sempere, & Castellano, 2020; Talbi & Navarro, 2020). However, over the past few years it has also become clear that not only do metabolic cues regulate the kisspeptin system, but the inverse is also true: kisspeptin signaling affects metabolism and energy balance, via both central (hypothalamic) and peripheral sites of action. Thus, there is a bi-directional relationship between kisspeptin/KISS1R and metabolism and energy balance. As the effects of metabolic factors on the kisspeptin system are already well-reviewed, this present article will focus specifically on the emerging actions of kisspeptin signaling on metabolism and energy balance, including modulation of body weight, adipose tissue, feeding and energy expenditure, insulin secretion and glucose homeostasis, and thermoregulation. Primary discussion will be on metabolic and thermogenic effects of kisspeptin signaling likely occurring outside the brain at the level of several peripheral target tissues, including the pancreas and adipose tissue (summarized later in Fig. 5).
Fig. 5.
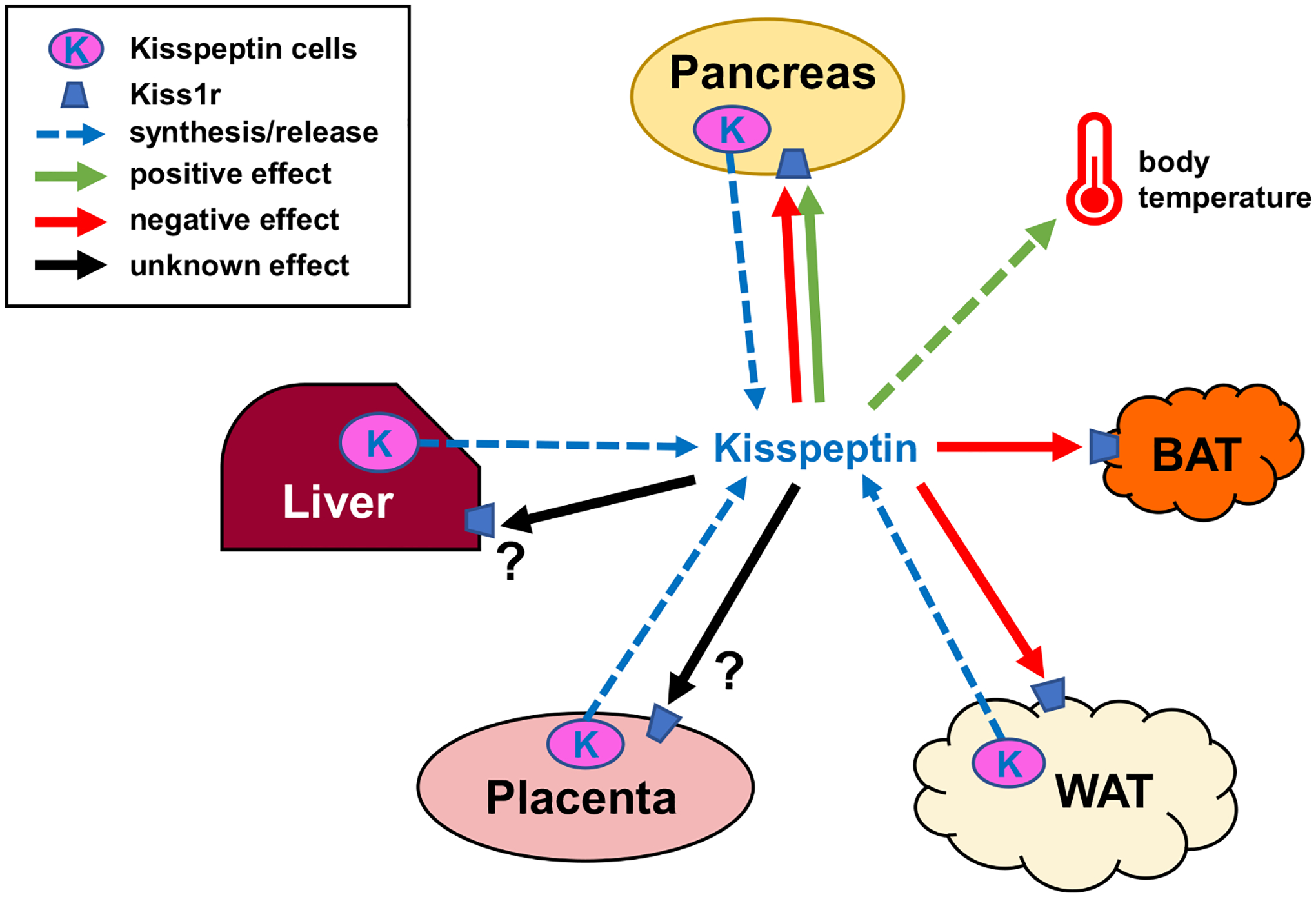
Schematic showing possible metabolic actions of peripheral kisspeptin signaling. Blue dotted arrows show potential peripheral tissue sources of circulating kisspeptin the blood. Green arrows denote positive effects of kisspeptin on target tissues or cells, red arrows denote negative effects, while black arrows denote unknown/unstudied effects. Kiss1r is expressed in a wide variety of target tissues including the brain (not shown), liver, pancreas, adipose, BAT, gonad, and placenta (Brown et al., 2008; Hauge-Evans et al., 2006; Herbison et al., 2010; Kotani et al., 2001; Tolson et al., 2020). Kisspeptin signaling has been shown to affect several of these tissues, although for most peripheral effects the endogenous physiological source (specific tissue or cell type) of kisspeptin is unknown. Moreover, it is not clear if there are also autocrine/paracrine effects of kisspeptin acting on the same peripheral tissue that synthesized it. Indeed, the role of kisspeptin synthesis and possible secretion from many peripheral tissues has not yet been studied; for example, the pancreas and white adipose tissue both express Kiss1 and presumably synthesize kisspeptin (Brown et al., 2008; Cockwell et al., 2013; Dudek et al., 2016; Ohtaki et al., 2001; Tolson et al., 2020) but it is presently unclear where such kisspeptin acts or what its specific role(s) might be.
2. Effects of kisspeptin signaling on body weight and energy expenditure
One of the earliest indications that kisspeptin might affect metabolism and energy balance was the observed effects of global Kiss1r knockout (KO) on body weight (BW) and energy expenditure in rodent models (summarized in Fig. 1). In a 2014 study by Tolson et al., adult Kiss1r KO female mice displayed a large increase in BW versus WT female littermates (Tolson et al., 2014); this overweight phenotype was replicated in a follow-up study and was shown to emerge ~10–12 weeks of age, with Kiss1r KO females weighing a notable 30% more than controls by 18 weeks of age (Tolson et al., 2016). This elevated BW in females was further confirmed in a different mouse line: Zp3-Cre/Kiss1rfl/fl (whole body Kiss1r KO) females displayed increased BW and elevated adiposity similar to the original global Kiss1r KOs (Tolson et al., 2019). In contrast, adult male Kiss1r KO mice did not have increased BW compared to WT control males in either study (Tolson et al., 2014; Tolson et al., 2019). However, for reasons still unclear, other studies have reported a reduced BW in Kiss1r KO males at younger ages up until 6 weeks of age (Velasco et al., 2019) or between 9 and 12 weeks of age (Lapatto et al., 2007). By comparison, studies of Kiss1 KO mice (knocking out the kisspeptin gene) have either found no difference in BW in young adult males and females (Lapatto et al., 2007), or slightly lower BW in younger animals ≤8 weeks old (d’Anglemont De Tassigny et al., 2007; Goto et al., 2020). While the lower BW phenotype in males matches that seen in some studies of younger Kiss1r KO males (Lapatto et al., 2007; Velasco et al., 2019), the reason for the difference in BW phenotype between Kiss1 KO (smaller or normal BW) and Kiss1r KO (heavier BW) females is not immediately apparent, although each of these studies used different KO models generated with different techniques. However, is noteworthy that the obese BW phenotype in female Kiss1r KOs first emerges at older ages (~10–12 weeks) than the ages studied for Kiss1 KO mice; it remains unknown if older (e.g., 18–20 weeks old) Kiss1 KO mice also show signs of obesity similar to adult Kiss1r KO females. It is also possible that BW differences between Kiss1 and Kiss1r KOs reflects off-target kisspeptin signaling at another RFamide receptor, such as neuropeptide FF receptor 1 (NPFFR1) (Oishi et al., 2010).
Fig. 1.
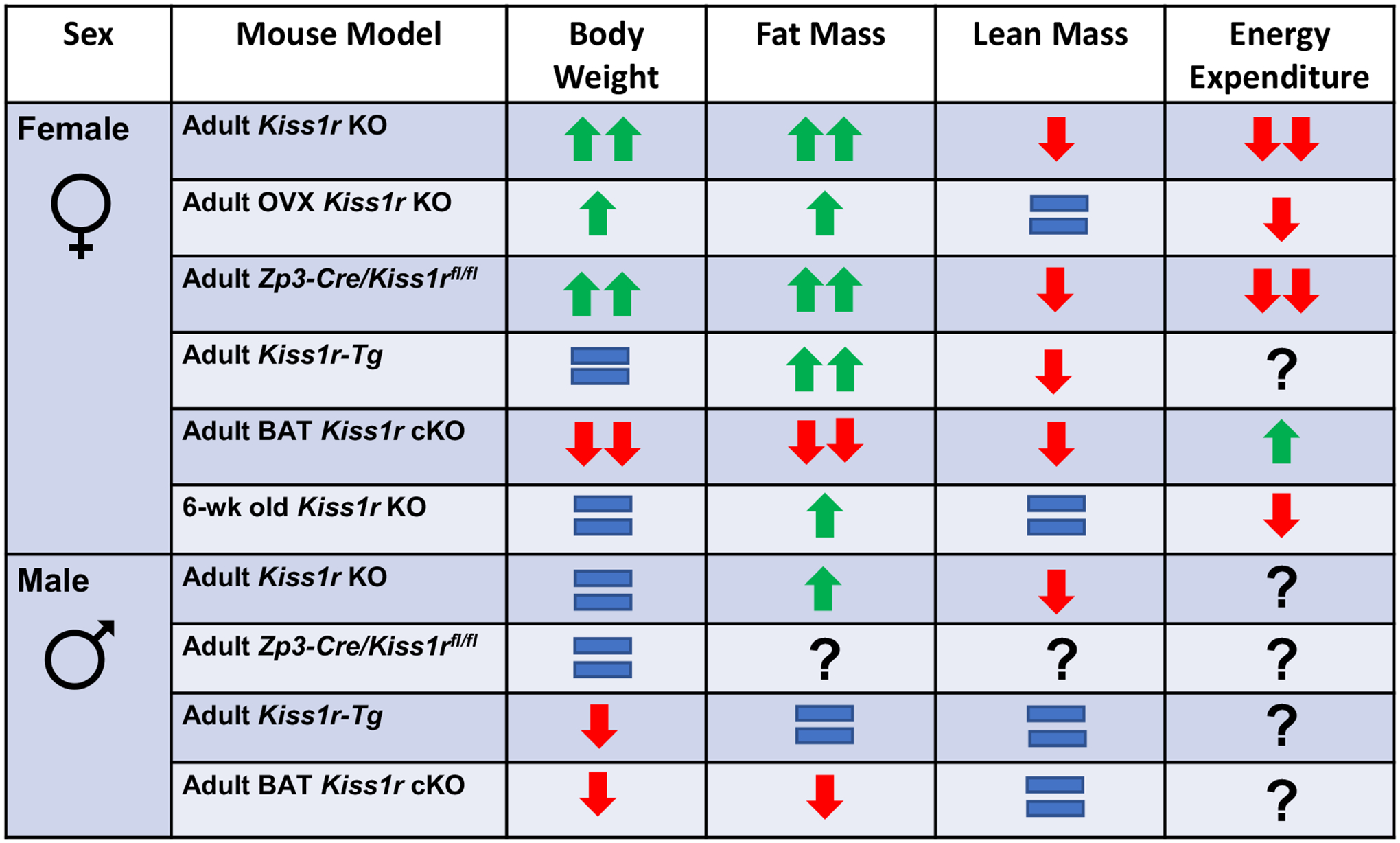
Summary of the phenotypes of various Kiss1r KO mouse models on bodyweight, fat mass, lean mass, and energy expenditure. Adult Kiss1r KOs are whole body (“global) Kiss1r KO mice examined around 4–5.5 months of age (Tolson et al., 2014; Tolson et al., 2016; Tolson et al., 2019; Velasco et al., 2019), while OVX Kiss1r KO mice were had their ovaries removed in before puberty or in young adulthood and then were studied 3–4 months later around 5 months of age (and compared to similarly OVX littermate controls). Adult Kiss1r-Tg mice (~4 months old) are whole body Kiss1r KO mice with KISS1R selectively re-expressed only in GnRH neurons, which restores normal reproductive function and gonadotropin/sex steroid levels (Velasco et al., 2019). BAT Kiss1r cKO mice (4–5.5 months old) have Kiss1r selectively knocked out from BAT (Ucp-1 cells) (Tolson et al., 2020). Zp3-Cre/Kiss1rfl/fl mice (4–5.5 months old) are whole-body Kiss1r KO mice generated by breeding mice expressing Cre under the Zp3 promoter with Kiss1rfl/fl mice, which results in global KO of the Kiss1r gene (Tolson et al., 2019).
Kiss1r KO females, in accordance with their increased BWs, also showed a 100% (2-fold) increase in fat mass and a small but significant decrease in lean mass, indicating that their BW phenotype was due entirely to increased adiposity (Tolson et al., 2014; Tolson et al., 2016; Tolson et al., 2019). Fat mass was also higher in Kiss1r KO males though to a smaller magnitude of 50% versus control males. However, Kiss1r KO males also displayed a greater decrease in lean mass compared to KO females (25% versus 6%), potentially explaining the lack of a detectable overall BW increase in the males despite their increased adiposity (Tolson et al., 2014; Tolson et al., 2016; Tolson et al., 2019). Accordingly, leptin levels were greatly increased by 450% in female Kiss1r KOs but only elevated by 300% in males, though that is still a large increase (Tolson et al., 2014). The decrease in lean mass in both sexes may be related to the role of kisspeptin signaling in the indirect stimulation of growth hormone secretion via neuropeptide Y (NPY) neurons, although this effect has only been shown in short-term fasted ewes (Foradori et al., 2017) and growth hormone was not measured in the mouse models. When fed a high fat diet (HFD), both male and female Kiss1r KOs exhibited higher BWs than HFD-fed controls, indicating that Kiss1r KO males may be more sensitive to obesogenic insults than WT males (Velasco et al., 2019).
As might be predicted from the female Kiss1r KO mouse data, two studies from Sahin and colleagues have shown that chronic i.c.v. kisspeptin treatment (50 pmol daily, at 1000–1200 h) from postnatal day (PND) 26–60 decreased BW at PND 60 in female rats (starting from PND 28) (Sahin et al., 2020). While this treatment regimen increased serum LH levels and advanced the date of vaginal opening, as expected, it did not change uterine or ovarian weight (Sahin et al., 2015). Since sex steroid levels were not directly measured in these studies, it is unclear whether they may have influenced the alterations in BW in kisspeptin-treated rats. If sex steroids were normal (unchanged), this effect of central kisspeptin administration on BW might suggest that kisspeptin signaling in the brain, in addition to the periphery, is involved in kisspeptin’s modulation of BW. Supporting this possibility, Cre-dependent silencing of kisspeptin neurons using conditional viral expression of the light chain of the tetanus toxin (TeTx) (which prevents synaptic transmission but does not kill neurons) also induced increased BW in females, indicating that kisspeptin neurons could be a key component of the overall Kiss1r KO obesity phenotype (Padilla et al., 2019). However, it is important to note that the Cre-dependent silencing method inhibits the entire kisspeptin neuron, inhibiting release of all signaling factors from that cell, including but not limited to kisspeptin. Thus, it is not clear from that study alone if the increased BW is due exclusively to diminished kisspeptin signaling or to reduced signaling by other co-transmitters (e.g., Glutamate, GABA, NKB, dynorphin, etc). Regardless, as will be discussed below, there is considerable evidence that kisspeptin action in the periphery may affect BW and adiposity. Yet, these two sites of action are not mutually exclusive, and it is likely that both central and peripheral kisspeptin signaling affect BW.
What is the underlying cause of the increased BW and adiposity in adult Kiss1r KOs? The first obvious possibility is increased food intake. However, this was surprisingly shown not to be the case; as discussed later, Kiss1r KO mice actually display decreased feeding (see “Kisspeptin regulation of feeding”), despite ultimately becoming very overweight. Thus, perhaps counterintuitively, the increased BW is not due to increased energy intake but rather occurs in spite of decreased energy intake. This suggested the possibility instead that Kiss1r KOs have decreased energy expenditure. Indeed, using CLAMS metabolic cages to assess metabolic rates and energy expenditure, we demonstrated that adult Kiss1r KO females on a normal diet have dramatically reduced locomotor activity, oxygen consumption (VO2), and carbon dioxide production (VCO2), resulting in a decreased respiratory exchange rate (RER) and energy expenditure, especially during the dark phase when mice are more active (Tolson et al., 2014). Since Kiss1r KOs did not consume more food than controls, this indicates that the female Kiss1r KO obesity phenotype stems primarily from reduced energy expenditure. The impaired metabolic phenotypes in global Kiss1r KO females were further confirmed in the Zp3-Cre/Kiss1rfl/fl mouse line (whole body Kiss1r KO engineered via Cre/lox technology) in which females similarly displayed hypogonadism, increased BW, elevated adiposity and leptin, and reduced dark phase energy expenditure (see Fig. 1 for summary) (Tolson et al., 2019). Unfortunately, because there was not an overt BW phenotype in adult Kiss1r KO males, no study has yet measured energy expenditure or metabolic rates in Kiss1r KO males. A recent study in male rats found that a single i.c.v. injection of kisspeptin (3 nmol Kp-10) reduced RER, the opposite of what might be expected from the female Kiss1r KO results (Cázarez-Márquez et al., 2021). It is not clear whether this reflects a species or sex difference in kisspeptin effects on RER, as the necessity of KISS1R for RER and energy expenditure has not yet been tested in KO male mice and these studies had different experimental designs (global Kiss1r KO in mice vs acute kisspeptin administration in rats).
The developmental trajectory of the metabolic phenotype in Kiss1r KO females has also been studied. Obesity is not apparent in Kiss1r KO females until mid-adulthood but impaired energy expenditure is already present from a young adult age. Specifically, when raised on a normal chow diet, 6-week-old Kiss1r KO females (young adults only a few weeks post-puberty) show no difference in BW compared to same age controls, but already show a moderate elevation in fat mass, mildly reduced VO2 and VCO2, and overall lower energy expenditure (Tolson et al., 2016). Increased BW begins to emerge at 10 weeks of age, along with a greater increase in underlying adiposity; BW ultimately reaches its greatest genotype difference around 18 weeks (De Bond et al., 2016; Tolson et al., 2014; Tolson et al., 2016; Tolson et al., 2019), correlating with the age of the largest reductions in metabolic rates. Another study found lower BWs in Kiss1r KO females in the first 6 weeks of age, providing more evidence that the overweight phenotype in females does not emerge until adulthood (Velasco et al., 2019). Along with increased fat mass, Kiss1r KOs show a reduction in lean mass compared to WT controls (Tolson et al., 2014; Tolson et al., 2016; Tolson et al., 2020; Velasco et al., 2019) that is not apparent at younger ages but emerges in early-to-mid adulthood (Tolson et al., 2016).
An important consideration in the metabolic consequences of kisspeptin signaling is the effect of Kiss1r KO on sex steroids (estrogen and androgen) which can regulate a variety of metabolic functions (López & Tena-Sempere, 2015; Navarro et al., 2015). Given neural kisspeptin’s stimulatory actions on reproductive brain circuits, mice and humans with mutations in the Kiss1 and Kiss1r genes display severe hypogonadism and robustly decreased sex steroid production (Kauffman, 2010). Conditions of long-term low (or absent) estrogen levels, as occurs in ovariectomized (OVX) mice or in women after menopause, are associated with increased BW and decreased energy expenditure (Carr, 2003; López & Tena-Sempere, 2015), with similar findings in aromatase KO mice which cannot produce estrogen (Jones et al., 2000). To what degree is the BW and metabolic phenotype of Kiss1r KOs due to absent estrogen versus a direct effect of kisspeptin signaling on those measures? Some studies of Kiss1r KO mice attempted to address this by using chronically OVX mice for all genotypes, including the controls, thereby removing gonadal estrogen from the equation across the board. Those studies found that OVX Kiss1r KO still displayed higher BW, lower VO2 and VCO2, and lower energy expenditure than similarly long-term OVX WT controls, even though both genotypes were lacking gonadal estrogen for many months. Thus, the absence of estrogen in the Kiss1r KO females cannot solely explain their obesity and reduced metabolism. However, in the OVX studies, the genotype difference was at a slightly lower magnitude than in ovary-intact mice, suggesting that some portion of the metabolic phenotype in intact mice was due to absent estrogen while the rest was due to effects of absent kisspeptin signaling independent of estrogen levels (Tolson et al., 2014; Tolson et al., 2016). In support of this, Kiss1r KO females have higher BWs than GnRHhpg female mice (i.e., GnRH KOs), which are also hypogonadal with greatly reduced sex steroid levels (Tolson et al., 2014), suggesting a role for kisspeptin signaling in the control of BW separate from its upstream stimulation of gonadal steroids. However, another study employed a different approach with differing results. Velasco et al. compared global Kiss1r KO to Kiss1r KO mice that had KISS1R selectively rescued just in GnRH neurons; these mice were termed Kiss1r-Tg mice and lack KISS1R in all peripheral tissues but have KISS1R in GnRH neurons, resulting in restored reproductive function with normal gonadotropin and sex steroid levels (Velasco et al., 2019). Interestingly, Kiss1r-Tg females did not develop increased BW like global Kiss1r KO females, suggesting that the effects of Kiss1r KO on BW are due primarily to reduced estrogen levels (Velasco et al., 2019). This is in contrast to the results obtained in chronically OVX Kiss1r KOs and OVX controls (Tolson et al., 2014). However, in the Kiss1r-Tg study, Kiss1r-Tg males and global Kiss1r KO males both showed lower BW than controls, indicating a metabolic effect that is not simply due to decreased androgen signaling in the global KOs (Velasco et al., 2019). Further research is required to evaluate the cause of BW sex differences in the various Kiss1r KO lines.
Adding to the complexity of the regulation of BW and energy expenditure by kisspeptin is the role of kisspeptin signaling in brown adipose tissue (BAT). Because KISS1R is absent in all cell-types in global Kiss1r KOs, it is unknown in which specific tissue(s) the absent kisspeptin signaling is causing the metabolic phenotype in those mice. Thus, our lab selectively knocked out KISS1R from just BAT to see if this targeted absence of kisspeptin signaling elicited similar increases in BW and adiposity as in the global Kiss1r KO. This was achieved with a conditional knockout of KISS1R from Ucp1-expressing cells, which are predominantly found in BAT tissue. These BAT Kiss1r cKO mice, lacking KISS1R in just BAT, surprisingly showed a reduction in BW in both males and females (Tolson et al., 2020), opposite to the increased BW in global Kiss1r KOs. Correlating with this, VO2, VCO2, and energy expenditure were all increased in BAT Kiss1r cKO mice, opposite to the decreased metabolism in global Kiss1r KOs (Tolson et al., 2014; Tolson et al., 2020). Kisspeptin signaling may have differing effects in multiple tissues that result in a net positive effect on BW in global Kiss1r KOs that is not observed when kisspeptin signaling is removed from just BAT. Another study conditionally knocked out KISS1R from just the pancreas by targeting PDX1-expressing cells (termed Panc-KISS1R mice) and found no difference in BW between Panc-KISS1R and Kiss1rfl/fl controls. However, this outcome is perhaps not surprising as only male Panc-KISS1R mice aged 6–8 weeks were examined, and global Kiss1r KO males themselves exhibit either no change in BW or small decreases in BW. Moreover, global Kiss1r KO females do not manifest signs of obesity until after 10 weeks old. Therefore, it is possible that if older Panc-KISS1R males or females were examined, an increase in BW might emerge, as occurs in global Kiss1r KO females in mid-adulthood (Song et al., 2014).
3. Kisspeptin regulation of adipose tissue
Adipose tissue may be a direct target of kisspeptin signaling (summarized in Fig. 2). Kiss1r and Kiss1 are both expressed in adipose tissue in rodents (Brown et al., 2008; Dudek et al., 2016; Pruszyńska-Oszmałek et al., 2017; Tolson et al., 2020) and humans (Cockwell et al., 2013; Muir et al., 2001; Ohtaki et al., 2001). As a potential source of kisspeptin in the periphery, several studies have measured changes in Kiss1 expression in adipose tissue, including in conditions of obesity. However, the results and designs of these studies have been inconsistent. One study found reduced Kiss1 expression in the subcutaneous and gonadal fat of young adult (6–7-week-old) male obese Zucker rats as well as in the gonadal fat of peripubertal (PND 40) HFD male Sprague-Dawley rats (Brown et al., 2008). However, another study in adult (17-week-old) male Wistar rats found no effect of HFD on Kiss1 expression in gonadal fat (Dudek et al., 2016). It is unclear whether the different outcome reflects differences in the age, strain, and/or duration of HFD. In the former study, 3-weeks of HFD in peripubertal rats did not increase BW but still resulted in decreased Kiss1 levels in adipose tissue. On the other hand, in the latter study, a longer duration of 6 weeks of HFD in adults increased BW but did not alter Kiss1 levels in adipose tissue. Females were unfortunately not examined in either rat study. In humans, one study of women found a positive correlation between BMI and Kiss1 expression in abdominal fat but not subcutaneous fat (gonadal fat was not studied, nor were men) (Cockwell et al., 2013). Clearly, more research is required to understand the effects of obesity and metabolic challenges on adipose Kiss1 expression and whether it differs by sex, age, species, or tissue (gonadal vs. abdominal fat). Just as importantly, the function of kisspeptin made in adipose tissue still needs to be determined, which would ultimately help understand any observed changes (or lack thereof) in adipose Kiss1 mRNA. Several studies discussed further below have begun to assess possible effects of adipose kisspeptin signaling.
Fig. 2.
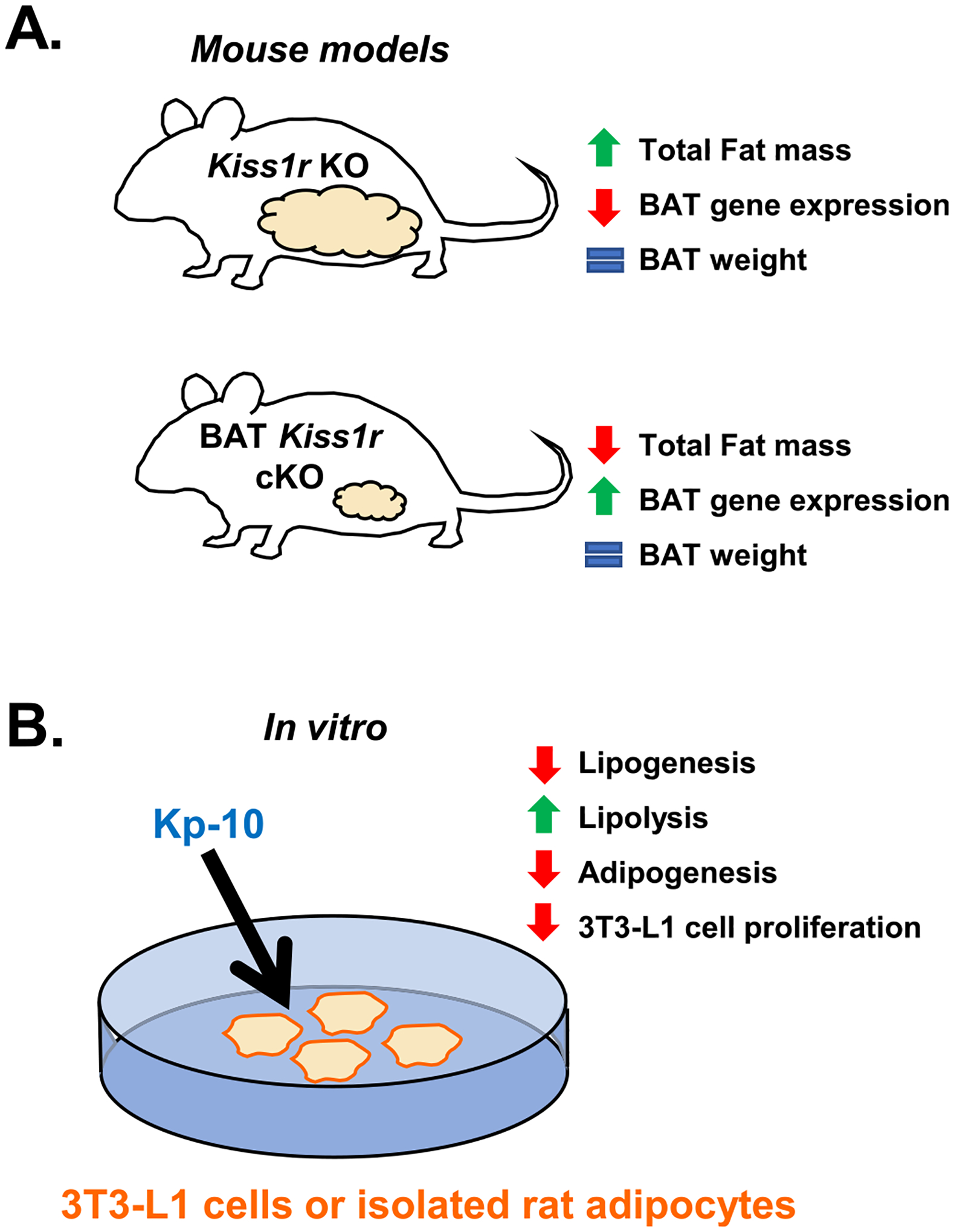
The effects of kisspeptin signaling on adipose tissue in vivo (A) and in vitro (B). (A) Kiss1r KO females lacking kisspeptin signaling throughout the body display increased fat mass (white adipose tissue) (Tolson et al., 2014; Tolson et al., 2016; Tolson et al., 2019; Velasco et al., 2019) but no difference in BAT weight (Tolson et al., 2020). However, Kiss1r KO females have decreased expression of the BAT genes Prdm16, Cox8b, and Ucp1 (Tolson et al., 2020). In contrast, BAT Kiss1r cKO females have decreased overall fat mass (white adipose tissue) and increased Cox8b expression in BAT, with normal BAT weight (Tolson et al., 2020). (B) Effects of Kp-10 administration in vitro in 3 T3-L1 cells and cultured male rat adipocytes. Kisspeptin decreased lipogenesis, adipogenesis, and 3 T3-L1 cell proliferation while increasing lipolysis, indicating that kisspeptin may inhibit lipid accumulation (Pruszyńska-Oszmałek et al., 2017), and could explain, in part, fat mass accumulation in Kiss1r KO mice lacking endogenous kisspeptin signaling.
Kiss1 regulation in adipose tissue is further complicated by the effects of sex steroids. Brown et al. found that subcutaneous injection of 10 μg estradiol in OVX female rats or 1 mg testosterone in castrated (CAST) males increased Kiss1 levels in gonadal fat by ~450% and ~ 250% respectively (Brown et al., 2008). Both androgen and estrogen receptors are expressed in adipose tissue (Mayes & Watson, 2004), suggesting that this could be direct effects of sex steroids on the Kiss1 gene. Indeed, it is well established that Kiss1 gene expression in the brain is strongly affected by sex steroids (primarily activated by sex steroids in most regions other than the arcuate nucleus where steroids inhibit Kiss1 levels) (Kauffman, 2009; Kauffman, Clifton, & Steiner, 2007; Popa, Clifton, & Steiner, 2008; Smith, 2013; Stephens & Kauffman, 2017). Thus, it is not surprising that sex steroids may also alter Kiss1 levels in peripheral tissues such as adipose. If so, this is an important caveat to interpreting studies measuring peripheral Kiss1 (or kisspeptin protein) levels and must be considered for proper conclusions, since many conditions or treatments can directly or indirectly alter sex steroid levels.
Several studies have attempted to correlate blood kisspeptin levels with various measures of obesity, especially body mass index (BMI). Results for these studies have been mixed, reporting either a positive correlation (Pita et al., 2011; Sitticharoon et al., 2021; Zhu et al., 2016), a negative correlation (Hestiantoro et al., 2019; Kołodziejski et al., 2018), or no association (Çelik, Belviranli, & Okudan, 2016; Rafique & Latif, 2015; Sithinamsuwan et al., 2020). However, since the source of circulating kisspeptin in the blood is unclear and could be from one or more tissues (fat, liver, pancreas, gonad, or other tissue), interpreting these blood kisspeptin studies is difficult, if not impossible. Thus, it is not clear to what degree the increased serum kisspeptin levels in obese or overweight conditions reflects the increased adiposity (i.e., more kisspeptin coming from adipose sources) or changes in some other peripheral tissue, such as the liver (which is a significant site of kisspeptin synthesis, especially in obese conditions). Additional studies including specific measurements of Kiss1 mRNA or kisspeptin protein levels in particular tissues, and their association with obesity, will help to further clarify the issue.
Adipose cells have KISS1R, and kisspeptin itself may have direct physiological effects on adipose tissue function (Fig. 2). Knocking out endogenous kisspeptin signaling in global Kiss1r KOs correlates with higher levels of fat mass in both males and females (Tolson et al., 2020; Velasco et al., 2019). Whether this increase in adiposity is due to diminished kisspeptin signaling directly in adipose cells or instead caused indirectly by impaired kisspeptin signaling elsewhere is not known, though in vitro studies discussed below indicate the possibility for direct adipose effects. Additionally, global Kiss1r KOs show lower expression of energy expenditure-related genes, including Ucp1 and Cox8b, in BAT (Tolson et al., 2020), though again, it is not known if this is due to altered kisspeptin signaling directly in BAT cells or due to an indirect effect of changes in other tissues. This was tested by studying mice with BAT-specific KO of Kiss1r. Interestingly, opposite to global Kiss1r KOs, conditional knockout of Kiss1r in just BAT lowered animals’ overall fat mass (primarily white adipose), and increased Cox8b levels in BAT, with no effect on Ucp1 levels (Tolson et al., 2020). This suggests that the effects of global Kiss1r KO on increased BAT Ucp1 and Cox8b gene expression are not due to kisspeptin action directly in BAT but rather to cumulative effects of absent kisspeptin signaling in other tissues. Despite that conclusion, the fact that BAT Kiss1r cKOs show lower BWs and decreased adiposity (and higher metabolic rates and body temperature, as discussed later) clearly indicates that kisspeptin signaling directly in BAT has some metabolic effects. Similar studies selectively knocking out Kiss1r from just WAT have not yet been reported but would be very informative.
Another consideration is the secondary effect of reduced sex steroids due to absent kisspeptin signaling on observed changes in WAT or BAT in Kiss1r KO mice. Several studies found that OVX Kiss1r KOs still exhibit increased overall fat mass when compared to OVX controls, indicating that kisspeptin signaling has a role in regulating adipose tissue independent of its effects on sex steroids (Tolson et al., 2014; Tolson et al., 2016). In contrast, Kiss1r-Tg mice (Kiss1r KO mice with restored KISS1R just in GnRH neurons and thus normal sex steroid levels), show no increase in overall fat mass in either sex (Velasco et al., 2019). With regards to BAT tissue, OVX Kiss1r KO females still have lower Ucp1 and Cox8b gene expression than OVX controls, but the magnitude of the effect is lower than gonad-intact animals, suggesting that part of the decrease is due to secondary effects from diminished sex steroids and part is due to kisspeptin signaling beyond the HPG axis (Tolson et al., 2020). Since adipocytes express Kiss1r (Pruszyńska-Oszmałek et al., 2017; Tolson et al., 2020; Wang et al., 2018), it is possible that kisspeptin exerts effects directly in adipocytes (Fig. 2B). To study this, Pruszyńska-Oszmalek et al. conducted a series of in vitro experiments in 3 T3-L1 cells and rat primary adipocytes (Pruszyńska-Oszmałek et al., 2017). They found that addition of kisspeptin-10 (Kp-10) into the growth media reduced the proliferation and viability of 3 T3-L1 cells and also reduced adipogenesis. Moreover, in both 3 T3-L1 cells and primary rat adipocytes, Kp-10 decreased rates of lipogenesis while increasing lipolysis (Pruszyńska-Oszmałek et al., 2017). The decrease in adipogenesis induced by Kp-10 may be due to inhibition of peroxisome proliferator-activated receptor gamma (PPAR-γ) and CCAAT/enhancer-binding protein β (CEBPβ) gene expression, which are either required for (PPAR-γ) or enhance (CEBPβ) adipocyte differentiation (Steger et al., 2010). Additionally, Kp-10 in rat adipocytes decreased secretion of adiponectin, an adipogenic hormone, but increased secretion of leptin, a lipolytic and anorexigenic hormone (Pruszyńska-Oszmałek et al., 2017; Stern, Rutkowski, & Scherer, 2016). Taken together, these results imply that kisspeptin signaling in adipose tissue is generally anti-obesogenic, which makes sense in the context of the observed increase in fat mass in global Kiss1r KO mice. However, it is important to note that many of the effects observed in the Pruszyńska-Oszmalek et al. study required in vitro Kp-10 concentrations of 100 nM-1 μM, which are supraphysiological to circulating serum kisspeptin concentrations, although whether these high concentrations are achieved locally in vivo is not known. Complicating the issue, in another study by Wang et al., Kp-10 increased lipogenesis in 3 T3-L1 cells, opposite to the decrease in the previous study (Pruszyńska-Oszmałek et al., 2017; Wang et al., 2018). The opposite effects of Kp-10 on lipogenesis in these two studies occurred at similar doses (10–100 nM Kp-10), but the Kp-10 treatment occurred at different times and for different durations. Wang et al., treated 3 T3-L1 cells with Kp-10 over the course of several days during differentiation, while Pruszyńska-Oszmalek et al. treated 3 T3-L1 cells with Kp-10 for 2 h post-differentiation during stimulation of lipogenesis (Pruszyńska-Oszmałek et al., 2017; Wang et al., 2018). Regardless, the strikingly opposing results from the two studies necessitates additional investigations to resolve the issue and more definitively determine how kisspeptin directly affects adipose cells.
4. Kisspeptin regulation of food intake and hypothalamic feeding circuits
Mixed evidence exists for the role of kisspeptin in directly regulating food intake (summarized in Fig. 3). Several early studies in male rats found no effect of i.c.v. Kp-10 administration on food intake in either ad libitum fed or 12 h fasted rats given repeated Kp-10 injections in the early light phase (0900 h, 1 nmol Kp-10 daily for 7 days) (Castellano et al., 2005) or a single Kp-10 injection in either the early light phase (0830–0930 h, 0.3–3 nmol Kp-10) [66]or onset of the dark phase (1900 h, 3 nmol Kp-10) (Thompson et al., 2004). A similar lack of feeding effect was also observed in ad libitum fed OVX ewes, with no effect on food intake even after several hours of central kisspeptin infusion (Clarke et al., 2012). However, a study in male mice found that a single i.c.v Kp-10 injection (0900 h, ~0.8–2.3 nmol Kp-10) decreased food intake in the first 4 h of the light phase, but only after overnight fasting (Stengel et al., 2011). Subsequent studies similarly showed that i.c.v. Kp-10 reduces food consumption in the first 4–5 h of return to ad libitum feeding after 24 h of fasting in male rats (1000 h, ~4.6 nmol Kp-10) or 48 h fasting in female (but not male) desert jerboas (0800–1100 h, ~3 nmol Kp-10) (Saito et al., 2019; Talbi et al., 2016). In each of these studies, cumulative food intake at 24 h after kisspeptin treatment was not different from controls (Saito et al., 2019; Stengel et al., 2011; Talbi et al., 2016), which is not surprising since a single injection is unlikely to have maintained physiological action for more than a couple hours. However, a recent study in ad-libitum fed male rats found that a single i.c.v. injection of Kp-10 (1130–1230 h, 3 nmol Kp-10) decreased cumulative food intake 24 h post-injection (Cázarez-Márquez et al., 2021) and another study of male and female mice (combined into a single group) reported that a single i.p. injection of Kp-10 (1800 h, 2 nmol Kp-10) reduced cumulative food intake 24 h post-injection (Dong et al., 2020). These are especially long time courses for an effect of a single kisspeptin treatment given that the half-life of Kp-10 in the blood (in humans) is ~4 min (Jayasena et al., 2011) and that kisspeptin-stimulated increases in LH level return to baseline in ~2 h (Navarro et al., 2005). It is also interesting that neither study required extended fasting to observe an anorexigenic effect of acute Kp-10 treatment, unlike the previous studies (Saito et al., 2019; Stengel et al., 2011; Talbi et al., 2016). In all but one of these studies, the Kp-10 doses given were shown to be effective at increasing either LH or testosterone (Castellano et al., 2005; Cázarez-Márquez et al., 2021; Saito et al., 2019; Stengel et al., 2011; Talbi et al., 2016; Thompson et al., 2004), regardless of whether food intake was altered, indicating that the dose of Kp-10 was not too low in those studies. Taken together, the majority of the evidence indicates that acute kisspeptin can induce anorexigenic effects by acting within the brain (Fig. 3B), but future studies taking into account the timing and specific brain location of kisspeptin administration will be informative.
Fig. 3.
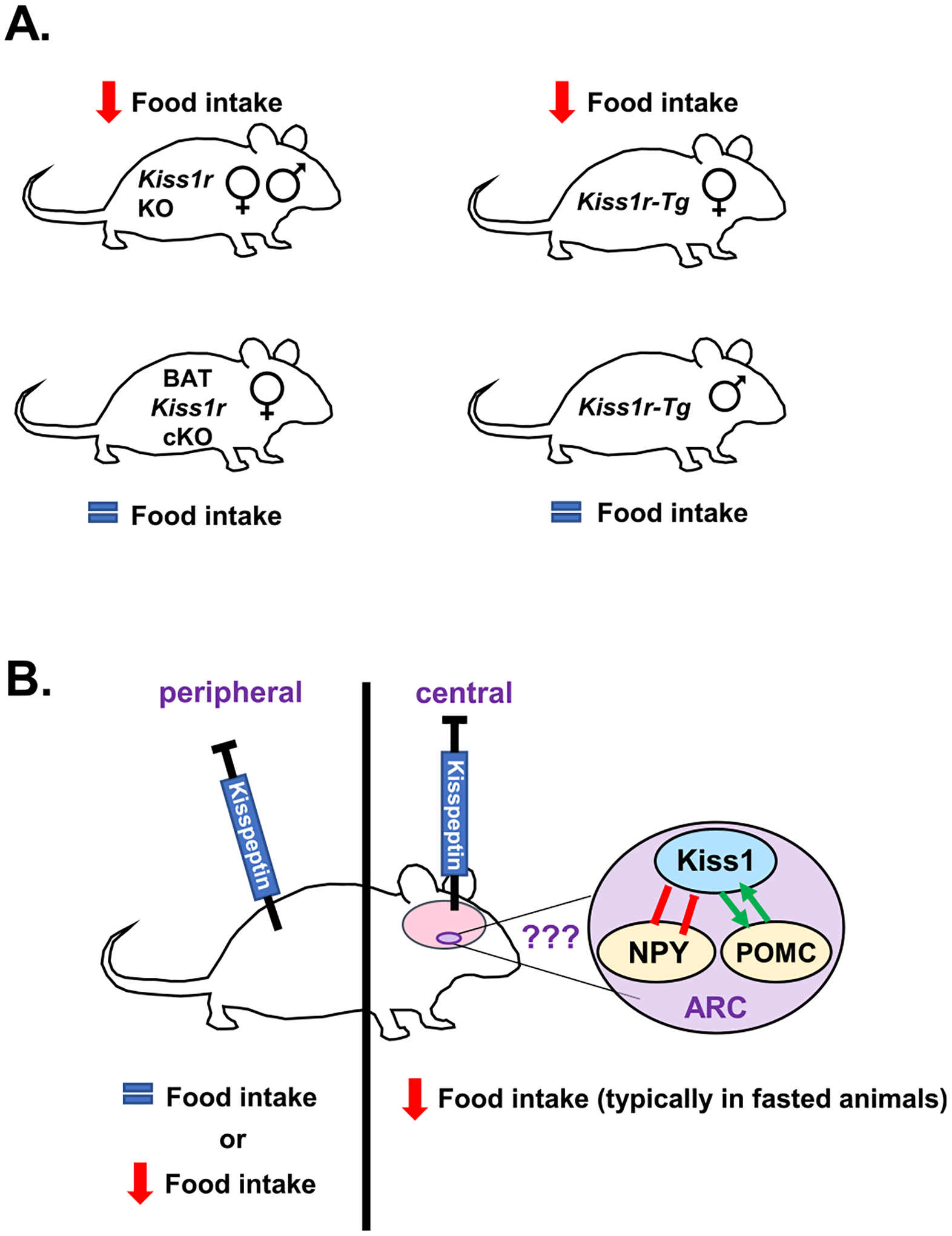
The effects of kisspeptin signaling on food intake. (A) Global Kiss1r KO male and female mice show reduced food intake, especially during the dark phase when mice consume most of their food (Tolson et al., 2014; Velasco et al., 2019). Similarly, female Kiss1r-Tg mice (global Kiss1r KOs that have KISS1R rescued back into GnRH neurons), show decreased food intake (Velasco et al., 2019), though males do not. In contrast, selective knockout of Kiss1r from just BAT (BAT Kiss1r cKO) had no effect on daily food intake (Tolson et al., 2020). (B) Rodent studies of kisspeptin administration in vivo have found either no effect on feeding or an inhibition of food intake. More specifically, several studies administering kisspeptin peripherally found no effect on food intake (Izzi-Engbeaya et al., 2018; Stengel et al., 2011) whereas one study reporting a decrease in 24 h cumulative food intake after peripheral kisspeptin treatment (Dong et al., 2020). In contrast, most studies infusing kisspeptin centrally (i.c.v) report anorexigenic effects (decreased feeding) (Sahin et al., 2015; Saito et al., 2019; Stengel et al., 2011; Talbi et al., 2016); the anorexigenic effects in rodents generally required extended fasting before the refeeding period, except for one study reporting an anorexigenic effect in ad libitum fed male rats (Cázarez-Márquez et al., 2021). The anorexigenic effects of i.c.v. kisspeptin may be due to effects on hypothalamic hunger/satiety circuits (NPY and POMC neurons which are known to be regulated by arcuate kisspeptin neurons), although other brain areas may also be involved, and this still needs more investigation.
A study by Sahin et al. examined the effects of chronic i.c.v. kisspeptin treatment on food intake in young female rats. From PND 26–60, female rats received daily infusions of kisspeptin (50 pmol) between 1000 and 1200 h, which were effective at increasing serum LH levels and advancing vaginal opening, as expected (Sahin et al., 2015). Interestingly, chronic kisspeptin caused a decrease in food intake in unfasted, ad libitum fed rats specifically from PND 30–45, after which food intake was lower than controls but not significantly different. This anorexigenic effect suggests a possible role of neural kisspeptin signaling in the control of food intake and was eliminated by coinfusion of the KISS1R antagonist p234. However, these results are in contrast to a previous study of chronic kisspeptin exposure which found no effect of kisspeptin on feeding in adult male rats receiving daily i.c.v. injections of kisspeptin (1 nmol at 0900 h) for 7 days (Castellano et al., 2005). Sahin et al. observed an anorexigenic effect of chronic kisspeptin only during a specific developmental period (PND30–45), which was not examined in the latter study, and also studied female rather than male rats. Further studies of the effects of chronic kisspeptin in both sexes at the same ages will clarify whether this is an age, sex, or technical difference.
Thus far, only two studies have examined the effect of kisspeptin on food intake in humans. In overnight-fasted human males, kisspeptin-54 was reported to have no effect on either food intake (Izzi-Engbeaya et al., 2018), brain activity in response to food images (Yang et al., 2020), or psychometric measures of appetite (Yang et al., 2020). No human studies have yet measured the effect of kisspeptin on food intake in females or after extended fasting, which resulted in an anorexigenic effect in several rodent studies (Saito et al., 2019; Stengel et al., 2011; Talbi et al., 2016). Therefore, more work is required to ascertain whether kisspeptin affects food intake in humans.
Given the multiple reports of inhibitory effects of kisspeptin treatment on food intake in rodents (usually after fasting), it might be predicted that removal of kisspeptin signaling would have the opposite effect of increasing feeding. However, chronically absent kisspeptin signaling, as occurs in global Kiss1r KO mice, was surprisingly associated with reduced food intake during both the light and dark phases in both sexes (Fig. 3A) (Tolson et al., 2014; Tolson et al., 2016; Tolson et al., 2019; Velasco et al., 2019). Moreover, female Kiss1r-Tg mice, who have rescued KISS1R signaling in GnRH neurons but absent KISS1R in all other cells, also exhibited reduced food intake (Fig. 3A), suggesting that the decrease in feeding in global Kiss1r KOs is not primarily due to absent sex steroids, at least in females (Velasco et al., 2019). The reason for similar diminished feeding with either exogenous kisspeptin treatment or absent kisspeptin receptor is not immediately clear. Further clouding the issue, unlike the females, Kiss1r-Tg males did not show decreased food intake. While the reason for this sex difference is unknown, it is noteworthy that in desert jerboas, the inhibitory effect of kisspeptin treatment on food intake was similarly sexually dimorphic, also occurring in females but not males (Talbi et al., 2016). This further underlines the importance of studying both sexes in kisspeptin biology, especially since many of the early kisspeptin feeding studies were performed only in males.
The contrasting effects of central kisspeptin administration on food intake (anorexigenic) vs. the putative effects of endogenous kisspeptin signaling on food intake inferred from global Kiss1r KOs (orexigenic) are not entirely conflicting since they ultimately have very different experimental designs. Indeed, studies showing an anorexigenic effect of kisspeptin have observed this effect only after central (Sahin et al., 2015; Saito et al., 2019; Stengel et al., 2011; Talbi et al., 2016) but not peripheral (Izzi-Engbeaya et al., 2018; Stengel et al., 2011; Yang et al., 2020) administration of kisspeptin, except for one study by Dong et al. (Dong et al., 2020), and often enhanced after fasting (Fig. 3B). Kiss1r KO studies showing an anorexigenic effect have typically eliminated kisspeptin signaling from all tissues and cells, both brain and periphery, in ad libitum fed animals (Tolson et al., 2014; Tolson et al., 2016; Tolson et al., 2019). Thus, the KO models have altered kisspeptin signaling in multiple tissues, which could ultimately directly or indirectly affect feeding and energy balance. For example, Kiss1r KOs have increased adiposity and therefore greatly increased circulating levels of leptin, an anorexigenic hormone (Klok, Jakobsdottir, & Drent, 2007) which may contribute to the reduced food intake in these mice (Tolson et al., 2014; Tolson et al., 2016; Tolson et al., 2019; Velasco et al., 2019).
The inhibitory effects of centrally administered kisspeptin on feeding may be mediated by hunger/satiety circuits in the hypothalamus. The hypothalamic arcuate nucleus is home to key populations of orexigenic neurons expressing neuropeptide Y/Agouti-related peptide (NPY/AgRP) and anorexigenic neurons expressing proopiomelanocortin/ cocaine- and amphetamine-regulated transcript (POMC/CART), along with kisspeptin neurons (De Jonghe, Hayes, & Bence, 2011; Kauffman, 2010; Timper & Brüning, 2017). Arcuate kisspeptin neurons exhibit reciprocal connectivity with both NPY/AgRP and POMC/CART neurons (Backholer et al., 2010; Nestor et al., 2016; Padilla et al., 2017), have been shown via electrophysiological experiments to activate POMC/CART neurons and inhibit NPY/AgRP neurons (Fu & van den Pol, 2010; Nestor et al., 2016; Qiu et al., 2011; Qiu et al., 2018) and are themselves inhibited by optogenetic activation of AgRP neurons (Padilla et al., 2017). In some of these studies, optogenetic activation of kisspeptin neurons stimulates POMC/CART neurons and inhibits NPY/AgRP neurons via the co-release of other neurotransmitters such as glutamate (Nestor et al., 2016; Qiu et al., 2018). However, kisspeptin itself may also directly regulate those neurons. Both NPY/AgRP and POMC/CART neurons express Kiss1r (Fu & van den Pol, 2010; Qiu et al., 2018) and kisspeptin directly excites POMC/CART neurons, potentially through activation of the sodium‑calcium exchanger (NCX) and non-selective cation channels (such as TRPC channels) (Fu & van den Pol, 2010; Qiu et al., 2018). In contrast, kisspeptin inhibits NPY/AgRP neuron firing, either directly through non-canonical activation of the RFRP-3 receptor GPR147 (Qiu et al., 2018), or indirectly through the activation of intermediary GABAergic neurons projecting to NPY/AgRP neurons (Fu & van den Pol, 2010). Either inhibition of NPY neurons or excitation of POMC/CART neurons by kisspeptin could explain, in part, the anorexigenic effects of exogenous kisspeptin treatments, but this has yet to be directly tested.
Despite the reported effects of kisspeptin on short-term electrical recordings of POMC/CART and NPY/AgRP neurons (i.e., neuron firing), whether kisspeptin also regulates the expression of Npy or Pomc mRNA is less clear. In vitro 24 h Kp-10 treatment (10 nM) increased Npy expression in mHypoE-38 cells (Kim, Dhillon, & Belsham, 2010), while in OVX ewes, 20 h of i.c.v. kisspeptin infusion (5 μg/h) increased Npy but decreased Pomc expression (Backholer et al., 2010). However, in fasted female jerboas, a single i.c.v. Kp-10 infusion (4 μg) increased Pomc expression and had no effect on Npy 1.5 h after infusion (Talbi et al., 2016). It should be noted that the effects of Kp-10 on Npy expression were seen only after prolonged (20–24 h) continuous treatment (Backholer et al., 2010; Kim et al., 2010). Likewise, the increase in Pomc expression induced by acute Kp-10 treatment in female desert jerboas (Talbi et al., 2016) may have been missed in the studies that examined the effects of much longer Kp-10 administration (Backholer et al., 2010; Kim et al., 2010), but this still requires testing.
In contrast to exogenous kisspeptin administration, studies of Kiss1r KO mice have shown virtually no effect of absent kisspeptin signaling on Npy or Pomc gene expression independent of effects on gonadal hormones (De Bond et al., 2016; Velasco et al., 2019). Specifically, Pomc, but not Npy, expression was increased in male and female Kiss1r KO mice, but this effect was eliminated when compared to gonadectomized control mice that similarly lacked gonadal sex steroids (De Bond et al., 2016; Velasco et al., 2019). Likewise, Velasco et al. found increased Pomc expression in Kiss1r KOs of both sexes, but no effect on Pomc expression in Kiss1r-Tg mice in which gonads developed normally (Npy expression was not studied), implying that the elevated Pomc expression was due to the secondary effects of absent sex steroids (Velasco et al., 2019). These findings raise the caveat that some of the previously discussed effects of kisspeptin on Pomc and Npy expression in non-transgenic animals, as well as i.c.v effects of kisspeptin on feeding, may be due in part to altered sex steroid secretion caused by kisspeptin activation of the reproductive axis. Future studies assessing effects of kisspeptin on feeding or hypothalamic energy balance physiology would benefit from controlling sex steroid conditions.
5. Effects of kisspeptin on insulin secretion and diabetic phenotypes
Kisspeptin signaling may have an important role in glucose homeostasis (summarized in Fig. 4). Kiss1r is expressed in the pancreas and Kiss1 is expressed in both the liver and pancreas (Kotani et al., 2001; Ohtaki et al., 2001; Song et al., 2014), both of which are critical to the regulation of glucose metabolism (Tengholm & Gylfe, 2017). Glucagon is secreted by pancreatic α islet cells in response to low blood glucose levels and binds to glucagon receptors on hepatocytes to stimulate glucose production. Conversely, when blood glucose is high, β islet cells secrete insulin which reduces glucose production (Tengholm & Gylfe, 2017). The first in vitro study of the role of kisspeptin in glucose-stimulated insulin secretion (GSIS) found that 1 μM kisspeptin increased GSIS in a pancreatic β cell line (Hauge-Evans et al., 2006). Subsequent studies had mixed results, with some reporting inhibition of GSIS by kisspeptin in pancreases dissected from rats (Silvestre et al., 2008) and isolated mouse islet cells (Vikman & Ahrén, 2009), while others reported that kisspeptin stimulated GSIS in isolated mouse islet cells (Bowe et al., 2009; Bowe et al., 2012; Schwetz, Reissaus, & Piston, 2014) and rhesus monkeys (Wahab, Riaz, & Shahab, 2011). Most in vitro studies showing an enhanced GSIS after kisspeptin treatment used a supraphysiological dose (1 μM) (Bowe et al., 2012; Hauge-Evans et al., 2006; Schwetz et al., 2014), while other studies using lower kisspeptin doses (e.g., nanomolar range) have shown inhibition of GSIS (Silvestre et al., 2008; Vikman & Ahrén, 2009) (Fig. 4). One exception is a report showing a dose-dependent enhancement of GSIS at doses from 60 nM to 1 μM of kisspeptin (Bowe et al., 2009). These discrepancies led Song et al. to hypothesize that kisspeptin may have different effects on GSIS depending on the dose. In cultured mouse islet cells, they found that 10 nM kisspeptin (physiological dose) inhibited GSIS while 1 μM kisspeptin (supra-physiological dose) increased GSIS. Moreover, conditional knockout of Kiss1r selectively from the pancreas eliminated suppression of GSIS by 10 nM kisspeptin but not the enhancement elicited by 1 μM kisspeptin, suggesting that the physiological role of kisspeptin signaling in rodents may be to suppress GSIS (Song et al., 2014). However, the discrepancy between studies may also depend on the concentration of glucose used for GSIS measurements. Inhibition of GSIS by kisspeptin was often found at lower glucose concentrations (~2.8–11 mM glucose) (Silvestre et al., 2008; Vikman & Ahrén, 2009), while enhancement of GSIS tended to use higher glucose concentrations (10–20 mM glucose with most using 20 mM glucose) (Bowe et al., 2009; Bowe et al., 2012; Hauge-Evans et al., 2006; Izzi-Engbeaya et al., 2018; Schwetz et al., 2014).
Fig. 4.
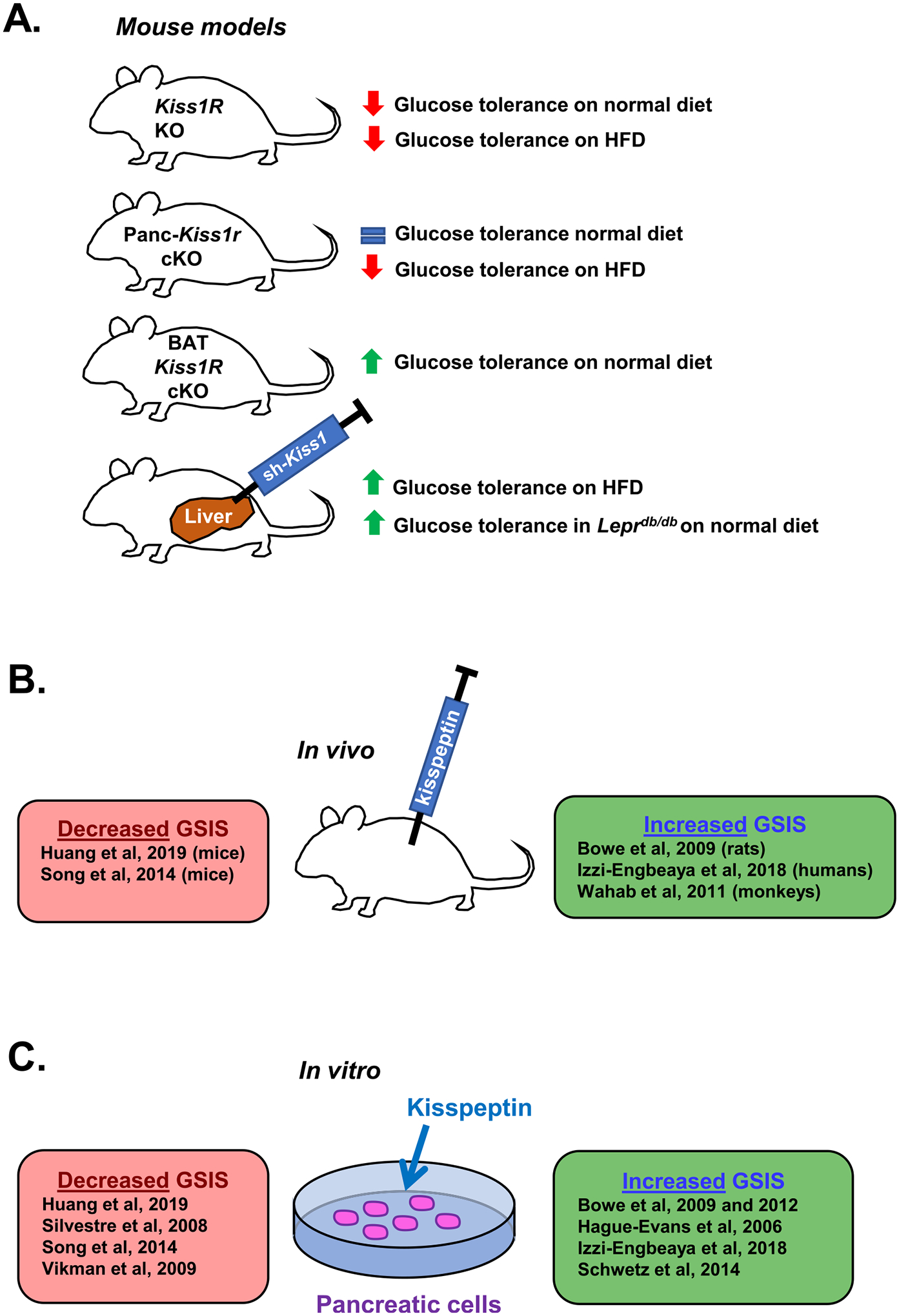
The effects of kisspeptin on glucose metabolism. (A) Effects of kisspeptin signaling on glucose tolerance. Adult global Kiss1r KO female mice, including gene trap Kiss1r KOs and Zp3-Cre/Kiss1rfl/fl Kiss1r KOs, exhibit impaired glucose tolerance (Tolson et al., 2014; Tolson et al., 2019). Conditional knockout of Kiss1r from just the pancreas (Panc-Kiss1r cKOs) in male mice had no effect on glucose tolerance on a normal diet (consistent with Kiss1r KO males (Tolson et al., 2014)), but ameliorated the impaired glucose tolerance observed on HFD (Song et al., 2014). Conditional knockout of Kiss1r from just BAT (BAT Kiss1r cKOs) in female mice improved glucose tolerance (Tolson et al., 2020). Viral knockdown of Kiss1 in the liver (correlating with reduced circulating kisspeptin levels in the blood) of Leprdb/db and HFD mice improves glucose tolerance with no effect on glucagon levels (Song et al., 2014). (B and C) The effect of exogenous kisspeptin administration on GSIS in vivo and in vitro. Multiple studies have come to different conclusions on the effect of kisspeptin on GSIS. Some studies report that kisspeptin decreases GSIS in vivo in mice (Huang et al., 2019; Song et al., 2014) and in vitro in NIT-1 cells (Huang et al., 2019), mouse pancreata (Silvestre et al., 2008), and isolated mouse islet cells (Song et al., 2014; Vikman & Ahrén, 2009). In contrast, other studies report kisspeptin increases GSIS in vivo in rats (Bowe et al., 2009), rhesus monkeys (Wahab et al., 2011), and men (Izzi-Engbeaya et al., 2018), and in vitro in mouse islet cells (Bowe et al., 2009; Bowe et al., 2012; Hauge-Evans et al., 2006; Schwetz et al., 2014), human islet cells (Bowe et al., 2012; Hauge-Evans et al., 2006; Izzi-Engbeaya et al., 2018), and porcine islet cells (Bowe et al., 2012). The discrepancy between these studies may be due in part to differences in kisspeptin or glucose concentrations used in the experiments, both of which tended to be lower in studies observing decreased GSIS after kisspeptin treatment.
The studies discussed above tested acute kisspeptin treatment on GSIS, but a more recent study examined the effects of long-term kisspeptin exposure on insulin secretion (Huang et al., 2019). NIT-1 cells (a mouse pancreatic β cell line) were transfected with a plasmid overexpressing the Kiss1 gene. Cells overexpressing Kiss1 had decreases in both GSIS (11 mM glucose) and non-glucose stimulated insulin secretion. Moreover, continuous in vivo delivery of Kp-10 (0.4 nmol/h) in male mice via osmotic minipump decreased GSIS after 6 or 11 days of treatment (Huang et al., 2019). Serum kisspeptin levels during the in vivo Kp-10 infusion were not measured in that study so it is not known if they were physiological or higher.
Regarding the effects of kisspeptin on fasting insulin levels, a recent study of male and female mice (grouped together) found an in vivo increase in serum insulin levels 30 min after a single i.p. kisspeptin injection (2 nmol) (Dong et al., 2020). However, another study in human men (1 nmol/kg/h kisspeptin-54, discussed below) found no effect of i.v. kisspeptin treatment on fasting insulin (Izzi-Engbeaya et al., 2018). Whether this represents a species difference, a sex difference, or a difference in the form of kisspeptin administered (Kp-10 in mice and Kp-54 in humans), is not yet clear and will require further investigation.
In humans, a study in men found that kisspeptin-54 infusion (1 nmol/kg/h, resulting in ~2–3 nM serum kisspeptin) enhanced GSIS both in vivo and in isolated human islet cells (at both 2.7 nM and 1 μM doses), with no effect on fasting insulin secretion prior to glucose infusion (Izzi-Engbeaya et al., 2018). In human islet cells, stimulation of GSIS by kisspeptin was observed at 17 mM glucose, but no effect was observed at 3 mM glucose (Izzi-Engbeaya et al., 2018). Another study with both men and women found that serum kisspeptin levels were inversely related to GSIS (Andreozzi et al., 2017), although the tissue source(s) of circulating kisspeptin and other factors that may have influenced either GSIS or kisspeptin levels are unclear. Future studies in rodents and humans carefully controlling the dose of both kisspeptin and glucose will be required to better understand the effects of kisspeptin on GSIS.
Kiss1r KO mouse models of absent kisspeptin signaling have generally reported a sex-dependent impairment in glucose tolerance. Kiss1r KO females on a normal diet show impaired glucose tolerance (Fig. 4A), with no effect observed in males; only KO females are obese, which may explain this glucose tolerance sex difference (Tolson et al., 2014; Tolson et al., 2016; Tolson et al., 2019; Velasco et al., 2019). To this point, Kiss1r KO males exposed to a HFD become overweight and also show glucose intolerance like females, suggesting that the glucose intolerance is secondary to being overweight/obese and not a direct effect of kisspeptin, at least in males (Velasco et al., 2019). Indeed, the link between HFD, obesity, and glucose intolerance is well-established (Winzell & Ahrén, 2004). Importantly, the impaired glucose tolerance in Kiss1r KO females fed a normal diet appears to be largely independent of any indirect effects owing to kisspeptin stimulation of the reproductive axis (i.e., absent ovarian estrogen secretion), as glucose tolerance was impaired in OVX Kiss1r KO females relative to OVX controls (Tolson et al., 2014; Tolson et al., 2016) and also in Kiss1r KO females with kisspeptin signaling rescued only in GnRH neurons (Kiss1r-Tg; not hypogonadal), albeit with a smaller effect size (Velasco et al., 2019). Taken together, the studies of absent kisspeptin signaling in Kiss1r KOs (Fig. 4A) might suggest that, when present, endogenous kisspeptin signaling normally acts to improve glucose tolerance and therefore could be an anti-diabetic factor. However, the direct effects of kisspeptin signaling on glucose metabolism vs. the secondary effects of increased BW on glucose metabolism are difficult to disentangle. Some studies have investigated the link between kisspeptin signaling and diabetes by examining changes in Kiss1 and Kiss1r expression in rodent models of diabetes. Studies of streptozotocin-induced diabetes in male rats, a model of type-1 diabetes, (termed “DM1 rats”) have shown increased levels of Kiss1 expression in both the pancreas and gonadal fat and increased Kiss1r levels in both the pancreas and liver (Dudek et al., 2016). Since these DM1 rats did not have higher BW than controls, the effects on Kiss1 gene expression are not due to altered BW.
Where might kisspeptin be coming from to act on pancreatic cells? Song et al. found that liver Kiss1 mRNA levels were increased in humans with diabetes mellitus type 2 (DM2), as well as in mouse model of DM2 (HFD mice and Leprdb/db mice) (Song et al., 2014). Whether this elevated liver Kiss1 connects to the pathology of diabetes is an interesting question. In the study by Song et al., liver Kiss1 expression was stimulated by glucagon but inhibited by insulin, both in vitro in mouse hepatocytes and in vivo in male mice (Song et al., 2014). Since hyperglucagonemia is often observed in diabetes (Baron et al., 1987; Reaven et al., 1987), this may explain the higher liver Kiss1 levels in humans with DM2 and in Leprdb/db and HFD mice (which also exhibited hyperglucagonemia) (Song et al., 2014). Supporting this possibility, viral Cre-mediated knockdown of glucagon receptor (Gcgr) expression in the liver of Gcgrfl/fl mice eliminated glucagon-induced increases in both liver Kiss1 expression and circulating blood kisspeptin levels, and also improved glucose tolerance (Song et al., 2014). Moreover, treatment with a glucagon receptor antagonist lowered liver kisspeptin levels in HFD mice and LepRdb/db mice, while also improving glucose tolerance. These findings indicate that glucagon stimulates Kiss1 expression in the liver (and hence, kisspeptin levels in the blood), correlating with impaired glucose tolerance. How might elevated kisspeptin, made by the liver, affect glucose homeostasis? Song et al. demonstrated that cultured mouse islet cells secrete decreased insulin when conditioned with plasma containing high levels of kisspeptin (from DM2 patients), and that this effect was eliminated in mouse islet cells lacking KISS1R (tissues obtained from Panc-Kiss1r KO mice) (Song et al., 2014). Furthermore, viral-mediated knockdown of Kiss1 expression in the liver in HFD and LepRdb/db mice (which normally have high Kiss1 levels) also improved glucose tolerance without affecting plasma glucagon levels (Song et al., 2014). These pieces of evidence suggest that the improvements in glucose tolerance were due to direct effects of kisspeptin signaling in the pancreas. Indeed, HFD Panc-Kiss1r KO male mice (lacking KISS1R only in the pancreas) had improved glucose tolerance compared to HFD controls. Collectively, these results suggest that dysfunctional secretion of liver kisspeptin due to hyperglucagonemia in diabetes could contribute to hyperglycemia by kisspeptin-mediated inhibition of insulin secretion. This is an exciting hypothesis, though these experiments have yet to be repeated and were mostly performed in young adult (6 to 8-week-old) male mice, which is not representative of the typical age of onset of DM2 in humans (Koopman et al., 2005). Moreover, recent evidence suggests that knockout of KISS1R from pancreatic β cells in male and female mice impairs glucose tolerance in older mice, beginning ~6 months of age; this suggests that at older ages, kisspeptin signaling in β cells may normally act to improve hyperglycemia (Smith et al., 2021). Although there seems to be strong effects of kisspeptin on pancreas function, more research in models of diabetes considering the developmental timing of insulin resistance and changes over aging are required to determine the specific role of peripheral kisspeptin in diabetes pathology.
Kisspeptin may also play a significant role in altered glucose metabolism during pregnancy. Blood serum kisspeptin levels rise dramatically during pregnancy, reaching several thousand-fold of normal levels by the third trimester (Dhillo et al., 2006; Horikoshi et al., 2003), and elevated kisspeptin can also be detected in saliva of pregnant women (Jayasena et al., 2015). In addition to the liver and adipose tissue, kisspeptin is also expressed in both the uterus and placenta, where it may serve multiple functions including embryo implantation and placental development (reviewed in (Babwah, 2015)); placental kisspeptin is believed to be the primary source of the greatly increased serum kisspeptin during pregnancy (Horikoshi et al., 2003). During pregnancy, many adaptations occur in glucose homeostasis, including greater insulin secretion and decreased insulin sensitivity (Bowe et al., 2019; Butte, 2000; Xue et al., 2010), for which circulating kisspeptin (from the placenta or other tissues) may play a role. For example, KISS1R antagonist treatment impaired glucose tolerance and GSIS in pregnant mice (Bowe et al., 2019). Moreover, knockout of Kiss1r from β islet cells also reduced glucose tolerance and GSIS in pregnant but not non-pregnant female mice, and reduced β cell proliferation during late gestation, suggesting that kisspeptin plays a role in β cell function during pregnancy (Bowe et al., 2019). Furthermore, in human pregnancy, circulating kisspeptin levels are positively correlated with GSIS and negatively correlated with fasting glucose levels (albeit with a small effect size) (Bowe et al., 2019) and are also lower in patients with gestational diabetes (Bowe et al., 2019; Cetković et al., 2012). Thus, high levels of circulating kisspeptin during pregnancy may help compensate for insulin resistance by enhancing insulin secretion from β islet cells, whereas low levels of kisspeptin during pregnancy may be a risk factor for gestational diabetes.
6. Role of kisspeptin in thermoregulation
In mammals and other endotherms, physiological maintenance of core body temperature within a narrow range is critical to metabolic and cellular processes and is a significant source of energy expenditure (Chondronikola et al., 2016; Mekjavic & Eiken, 2006). Several lines of evidence suggest that kisspeptin signaling may contribute to thermoregulation. Infusion of 2 μg of kisspeptin-13 i.c.v. (0830 h) in intact male rats elevated core body temperature for several hours during the light phase (the inactive phase for rats), causing a sharp peak immediately after infusion and an elevated plateau that persisted until the onset of the dark phase (Csabafi et al., 2013). Since kisspeptin-13 was given i.c.v, this body temperature change is likely due to kisspeptin signaling in the brain rather than peripherally. However, where in the brain and how such thermoregulatory effects would be elicited by neural kisspeptin action remain unknown. Silencing of arcuate kisspeptin neurons by injection of a conditional viral vector expressing TeTx in female mice reduced the amplitude of circadian body temperature oscillations (Padilla et al., 2019) but whether this result is due specifically to kisspeptin signaling is unknown, as part or perhaps all of this effect is likely due to reduced co-transmission of neurokinin B (NKB) from these neurons (Padilla et al., 2018). Indeed, NKB released from actuate kisspeptin neurons projecting to thermoregulatory regions of the preoptic area is known to regulate body temperature and produces hot flushes during menopause (Dacks, Krajewski, & Rance, 2011; Krull et al., 2017; Mittelman-Smith et al., 2012; Rance et al., 2013).
In the absence of kisspeptin signaling, gonad-intact and OVX female Kiss1r KO mice both display lower body temperatures than intact or OVX controls during the dark phase when mice are more active, while body temperature is normal in Kiss1r KO males (which also do not present obesity or glucose intolerance) (Tolson et al., 2020). Kiss1r KO females are also much less active than control females and, likely relatedly, have decreased energy expenditure during the dark phase (Tolson et al., 2014), suggesting that the reduced body temperature may be linked, in part, to general decreases in activity. Yet, OVX Kiss1r KO mice exhibit impairments in the ability to maintain body temperature during acute cold exposure, indicating a decreased ability to thermoregulate via shivering and/or non-shivering thermogenesis (though this was not tested) (Tolson et al., 2020).
How the body temperature effect in Kiss1r KO mice is occurring is not immediately clear since these global KO mice lack KISS1R in all tissues and cell types. One possibility is BAT, a tissue that is critical for non-shivering thermogenesis and maintaining normal body temperatures (Cannon & Nedergaard, 2004). Kiss1r KO females exhibit reduced expression of the thermogenic genes Ucp1 (a mitochondrial protein involved in heat generation), Cox8b (an oxidase involved in the mitochondrial electron transport (Wikstrom, 1977)), and Prdm16 (a regulator of thermogenic gene transcription) in BAT tissue (Tolson et al., 2020). To probe this issue further, our group selectively knocked out Kiss1r from just BAT, while leaving the receptor present in other tissues. In this conditional Kiss1r KO model (BAT Kiss1r cKO), females surprisingly had increased body temperature along with increased expression of Cox8b in BAT tissue (males were not studied), suggesting that endogenous kisspeptin signaling directly in BAT normally restrains BAT-mediated thermogenesis (Tolson et al., 2020). Therefore, peripheral kisspeptin signaling may play an important role in temperature control by acting directly in Ucp1-expressing cells in BAT. Interestingly, although Ucp1 gene expression in BAT was increased in global Kiss1r KOs, BAT Ucp1 levels were normal in BAT Kiss1r cKOs (Tolson et al., 2020). Moreover, BAT weight was not affected by global Kiss1r KO or selective knockout of Kiss1r from BAT (Tolson et al., 2020), suggesting that endogenous kisspeptin’s effects on body temperature or energy expenditure do not involve alterations in the “browning” of fat tissue, but rather BAT function itself (Fenzl & Kiefer, 2014).
The increase in both body temperature and BAT Cox8b expression in BAT Kiss1r cKO mice suggests that endogenous kisspeptin signaling in BAT normally activates signaling pathways which restrain BAT cell energy production. However, the decrease in body temperature and Cox8b expression in global Kiss1r KOs indicates that the cumulative effects of impaired kisspeptin signaling in multiple other tissues, both in the periphery and brain, may ultimately culminate in inhibited BAT activity. This could be explained in part by the increased adiposity in global Kiss1r KO mice, as increased pro-inflammatory signaling and non-esterified fatty acid accumulation in obesity can inhibit BAT activity and induce mitochondrial dysfunction (Alcalá et al., 2019; Bournat & Brown, 2010). Potential effects of central kisspeptin signaling on body temperature (still awaiting further validation studies) might also possibly override the peripheral kisspeptin effects directly in BAT. Future research into the specific role of central kisspeptin, independent of NKB signaling arising from kisspeptin neurons, on temperature regulation and/or BAT function is required to understand how kisspeptin actions in different tissues regulate body temperature. Importantly, the tissue source(s) of peripherally circulating kisspeptin that might be acting directly on BAT is unknown, as BAT itself does not express Kiss1; thus, peripheral kisspeptin acting on BAT must originate in other tissues such as white adipose tissue, the liver, or pancreas (Dudek et al., 2016).
Increased energy expenditure due to heightened thermoregulation may also be an influential component of the sexually dimorphic Kiss1r KO BW phenotype. Typically, housing mice below thermoneutral conditions (29–31 °C (Cannon & Nedergaard, 2011)) increases metabolic demands and food intake due to the need to generate more body heat (Cannon & Nedergaard, 2011). Halvorson et al. hypothesized that the obese phenotype in Kiss1r KO females was exacerbated when housed at sub-thermoneutral conditions (room temperature; 22 °C) because impaired thermoregulation in Kiss1r KOs would fail to offset their increased energy intake at lower temperatures compared with at higher room temperatures (30 °C) (Halvorson et al., 2020). Supporting this hypothesis, housing Kiss1r KO females at warmer, thermoneutral conditions (30 °C) ameliorated their obesity and (Halvorson et al., 2020) reduced adiposity compared to mice housed at lower room temperatures (Halvorson et al., 2020). Surprisingly, thermoneutral conditions increased BW in male Kiss1r KO mice, which do not exhibit increased BW at room temperature (22 °C). The reason for this unexpected increase in BW in males is unclear. One possibility that requires further investigation may be sex differences in the normal regulation of BAT (Harshaw, Culligan, & Alberts, 2014; Rodrıguez-Cuenca et al., 2002) (Cypess et al., 2009), with greater BAT activity and mitochondrial activation reported in females than males, suggesting that males may use different mechanisms of thermogenesis than females.
7. Perspectives and future directions
Novel functions of kisspeptin beyond its role in reproduction continue to be discovered. As the epidemic of obesity and associated metabolic diseases continues to grow, understanding the role of kisspeptin signaling in metabolism will be increasingly important. Our understanding of kisspeptin’s metabolic effects is very much in its infancy, and more investigation is still required to fully understand its actions, especially in the periphery. Clearly, from studies of different global and conditional Kiss1r KO animal models, the effects of kisspeptin are complex and tissue specific (Fig. 5). For example, global Kiss1r KO increases BW in females, but selective Kiss1r KO from just BAT decreases BW, suggesting an even greater countereffect of kisspeptin signaling on BW in one or more other non-BAT tissues. Likewise, selective KO of the Kiss1r gene from just the pancreas improves glucose tolerance in males, but similar pancreas-specific KO of Kiss1r worsens glucose tolerance in pregnant females and has no effect in nonpregnant females. In contrast, global Kiss1r deletion impairs glucose tolerance in nonpregnant females, indicating that the effects of Kiss1r KO on glucose tolerance may depend on the cumulative effects from multiple Kiss1r-expressing tissues. Moreover, the function(s) of kisspeptin made in several different tissues that are critically important to energy homeostasis and metabolism remain unstudied, most notably endogenous kisspeptin in the pancreas and white adipose tissue (Fig. 5), and their potential contribution to the global Kiss1r KO metabolic phenotype remains unknown. Additionally, although the results of Song et al. suggest a dose-dependence of the effect of kisspeptin on insulin secretion in mice (with inhibition at nM concentrations) (Song et al., 2014), recent in vivo evidence in humans suggest doses of kisspeptin resulting in nM concentrations in blood serum actually enhance GSIS (Izzi-Engbeaya et al., 2018). However, normal kisspeptin serum concentrations have yet to be properly established in mice, and varying results in the picomolar to nanomolar (but not micromolar) range have been reported in humans (Andreozzi et al., 2017; Çelik et al., 2016; Dhillo, Murphy, & Bloom, 2007; Horikoshi et al., 2003; Izzi-Engbeaya et al., 2018; Kołodziejski et al., 2018; Pita et al., 2011; Sitticharoon et al., 2021; Song et al., 2014), which complicates the translation of these results in rodents to humans. Moreover, thus far only one study has examined the effect of kisspeptin administration on GSIS in humans, in men only (Izzi-Engbeaya et al., 2018), and found that kisspeptin enhanced GSIS but had no effect on fasting insulin levels. Since most of kisspeptin effects in animal models are on GSIS, rather than fasting insulin as measured in most human studies, more clinical investigation of the effects of kisspeptin administration on GSIS in both men and women are required. Similarly, only two studies have examined kisspeptin effects on feeding or appetite in humans (Izzi-Engbeaya et al., 2018; Yang et al., 2020), both including only men and neither using fasting paradigms similar to those that elicited the strongest feeding effects in rodents. Further adding to the complexity are the contrasting results of human studies examining correlations between circulating serum kisspeptin and insulin levels. These studies have come to a variety of different conclusions and the mechanisms are not easily interpretable because the anatomical source(s) of circulating kisspeptin in each case is unclear.
The surprising sex differences observed in the metabolic phenotypes of global Kiss1r KO mice are still unexplained. Sex differences in BW and glucose tolerance phenotypes of adult Kiss1r KO are still present after gonadectomy (Tolson et al., 2014), in Kiss1r KO mice with rescued kisspeptin signaling in GnRH neurons (Velasco et al., 2019), and when KISS1R is deleted from specific tissues (Tolson et al., 2020). Most of these data show a sex steroid-independent sex difference in kisspeptin signaling effects on metabolism or BW, but the underlying mechanisms for such sex differences are unknown. It is perhaps not surprising that evolution might co-opt a peptide involved in reproduction and sexual development to regulate the different metabolic needs of males and females (which have different energetic demands with respect to fertility), but the mechanism by which this occurs, the tissue source(s) of kisspeptin, and the specific target cells of kisspeptin action responsible for the phenotypes are still unknown. Understanding these sex differences and the potential off-target effects of exogenous kisspeptin treatment on metabolism (e.g., insulin secretion, changes fat accumulation, appetite, body temperature; summarized in Fig. 5) will be important when considering clinical therapies using kisspeptin administration for reproductive treatments.
Lastly, given the plethora of metabolic effects of kisspeptin reviewed here, it is surprising that no metabolic or BW phenotypes have yet been reported in humans carrying mutations in the KISS1R or KISS1 genes (Brioude et al., 2013; de Roux et al., 2003; Nalbantoğlu et al., 2019; Nimri et al., 2011; Pallais et al., 2006; Seminara et al., 2003; Topaloglu et al., 2012) or in activating mutations of KISS1 resulting in central precocious puberty (Silveira et al., 2010). The reason for the lack of metabolic phenotype in these human cases is unknown. However, it is possible that such metabolic impairments are masked by the clinical use of steroid hormones, gonadotropins, GnRH, or other medications in treating their reproductive phenotypes, as sex steroids themselves have strong metabolic effects (López & Tena-Sempere, 2015; Navarro et al., 2015). Moreover, many of the metabolic phenotypes observed thus far in rodents seem to become more obvious and of greater magnitude later in adulthood, and clinical reports of humans with KISS1 or KISS1R mutations have tended to focus on pubertal or young adult ages when the reproductive impairments are first detected or treated; following these individuals later in adulthood for possible metabolic phenotypes would be useful. Regardless, these intriguing gaps in knowledge further underscore the need for more human studies of the effects of kisspeptin on metabolism, energy balance, insulin action, and thermoregulation.
Grant support
The authors’ research is supported by National Institutes of Health (NIH) grants R01 HD090161, R01 HD100580, P50 HD012303, and T32 HD007203.
Footnotes
Declaration of Competing Interest
Both ADH and ASK declare no conflicts of interest.
References
- Alcalá M, et al. (2019). Mechanisms of impaired brown adipose tissue recruitment in obesity. Frontiers in Physiology, 10(94). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreozzi F, et al. (2017). Plasma kisspeptin levels are associated with insulin secretion in nondiabetic individuals. PLoS One 12(6), Article e0179834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babwah AV (2015). Uterine and placental KISS1 regulate pregnancy: What we know and the challenges that lie ahead. REPRODUCTION 150(4), R121. [DOI] [PubMed] [Google Scholar]
- Backholer K, et al. (2010). Kisspeptin cells in the ewe brain respond to leptin and communicate with neuropeptide Y and proopiomelanocortin cells. Endocrinology 151 (5), 2233–2243. [DOI] [PubMed] [Google Scholar]
- Baron AD, et al. (1987). Role of hyperglucagonemia in maintenance of increased rates of hepatic glucose output in type II diabetics. Diabetes 36(3), 274–283. [DOI] [PubMed] [Google Scholar]
- Bournat JC, & Brown CW (2010). Mitochondrial dysfunction in obesity. Current Opinion in Endocrinology, Diabetes, and Obesity 17(5), 446–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowe JE, et al. (2009). Kisspeptin stimulation of insulin secretion: Mechanisms of action in mouse islets and rats. Diabetologia 52(5), 855. [DOI] [PubMed] [Google Scholar]
- Bowe JE, et al. (2012). GPR54 peptide agonists stimulate insulin secretion from murine, porcine and human islets. Islets 4(1), 20–23. [DOI] [PubMed] [Google Scholar]
- Bowe JE, et al. (2019). A role for placental kisspeptin in β cell adaptation to pregnancy. JCI Insight 4(20), Article e124540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brioude F, et al. (2013). Two families with normosmic congenital hypogonadotropic hypogonadism and biallelic mutations in KISS1R (KISS1 receptor): Clinical evaluation and molecular characterization of a novel mutation. PLoS One 8(1), e53896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, et al. (2008). KiSS-1 mRNA in adipose tissue is regulated by sex hormones and food intake. Molecular and Cellular Endocrinology 281(1–2), 64–72. [DOI] [PubMed] [Google Scholar]
- Butte NF (2000). Carbohydrate and lipid metabolism in pregnancy: Normal compared with gestational diabetes mellitus. The American Journal of Clinical Nutrition 71(5), 1256S–1261S. [DOI] [PubMed] [Google Scholar]
- Cannon B, & Nedergaard J (2004). Brown adipose tissue: Function and physiological significance. Physiological Reviews 84(1), 277–359. [DOI] [PubMed] [Google Scholar]
- Cannon B, & Nedergaard J (2011). Nonshivering thermogenesis and its adequate measurement in metabolic studies. Journal of Experimental Biology 214(2), 242–253. [DOI] [PubMed] [Google Scholar]
- Carr MC (2003). The emergence of the metabolic syndrome with menopause. The Journal of Clinical Endocrinology and Metabolism 88(6), 2404–2411. [DOI] [PubMed] [Google Scholar]
- Castellano JM, & Tena-Sempere M (2016). Metabolic control of female puberty: Potential therapeutic targets. Expert Opinion on Therapeutic Targets 20(10), 1181–1193. [DOI] [PubMed] [Google Scholar]
- Castellano JM, et al. (2005). Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology 146(9), 3917–3925. [DOI] [PubMed] [Google Scholar]
- Cázarez-Márquez F, et al. (2021). Role of central kisspeptin and RFRP-3 in energy metabolism in the male Wistar rat. Journal of Neuroendocrinology, e12973 n/a(n/a). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çelik F, Belviranli M, & Okudan N (2016). Circulating levels of leptin, nesfatin-1 and kisspeptin in postmenopausal obese women. Archives of Physiology and Biochemistry 122(4), 195–199. [DOI] [PubMed] [Google Scholar]
- Cetković A, et al. (2012). Plasma kisspeptin levels in pregnancies with diabetes and hypertensive disease as a potential marker of placental dysfunction and adverse perinatal outcome. Endocrine Research 37(2), 78–88. [DOI] [PubMed] [Google Scholar]
- Chondronikola M, et al. (2016). Brown adipose tissue is linked to a distinct thermoregulatory response to mild cold in people. Frontiers in Physiology, 7(129). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke H, Dhillo WS, & Jayasena CN (2015). Comprehensive review on kisspeptin and its role in reproductive disorders. Endocrinology and Metabolism (Seoul, Korea) 30(2), 124–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke IJ, et al. (2012). Gonadotropin-inhibitory hormone is a hypothalamic peptide that provides a molecular switch between reproduction and feeding. Neuroendocrinology 95(4), 305–316. [DOI] [PubMed] [Google Scholar]
- Cockwell H, et al. (2013). KISS1 expression in human female adipose tissue. Archives of Gynecology and Obstetrics 287(1), 143–147. [DOI] [PubMed] [Google Scholar]
- Cravo RM, et al. (2011). Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience 173, 37–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csabafi K, et al. (2013). Effects of kisspeptin-13 on the hypothalamic-pituitary-adrenal axis, thermoregulation, anxiety and locomotor activity in rats. Behavioural Brain Research 241, 56–61. [DOI] [PubMed] [Google Scholar]
- Cypess AM, et al. (2009). Identification and importance of brown adipose tissue in adult humans. New England Journal of Medicine 360(15), 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacks PA, Krajewski SJ, & Rance NE (2011). Activation of neurokinin 3 receptors in the median preoptic nucleus decreases core temperature in the rat. Endocrinology 152 (12), 4894–4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Anglemont De Tassigny X, et al. (2007). Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proceedings of the National Academy of Sciences of the United States of America 104(25), 10714–10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bond JAP, et al. (2016). Unaltered hypothalamic metabolic gene expression in Kiss1r knockout mice despite obesity and reduced energy expenditure. Journal of Neuroendocrinology, 28(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jonghe BC, Hayes MR, & Bence KK (2011). Melanocortin control of energy balance: Evidence from rodent models. Cellular and Molecular Life Sciences: CMLS 68 (15), 2569–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillo WS, Murphy KG, & Bloom SR (2007). The neuroendocrine physiology of kisspeptin in the human. Reviews in Endocrine & Metabolic Disorders 8(1), 41–46. [DOI] [PubMed] [Google Scholar]
- Dhillo WS, et al. (2006). Plasma kisspeptin is raised in patients with gestational trophoblastic neoplasia and falls during treatment. American Journal of Physiology. Endocrinology and Metabolism 291(5), E878–E884. [DOI] [PubMed] [Google Scholar]
- Di Giorgio NP, et al. (2014). Impaired GABAB receptor signaling dramatically upregulates Kiss1 expression selectively in nonhypothalamic brain regions of adult but not prepubertal mice. Endocrinology 155(3), 1033–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong TS, et al. (2020). Intraperitoneal treatment of kisspeptin suppresses appetite and energy expenditure and alters gastrointestinal hormones in mice. Digestive Diseases and Sciences 65(8), 2254–2263. [DOI] [PubMed] [Google Scholar]
- Dudek M, Ziarniak K, & Sliwowska JH (2018). Kisspeptin and metabolism: The brain and beyond. Frontiers in Endocrinology, 9(145). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek M, et al. (2016). Effects of high-fat diet-induced obesity and diabetes on Kiss1 and GPR54 expression in the hypothalamic–pituitary–gonadal (HPG) axis and peripheral organs (fat, pancreas and liver) in male rats. Neuropeptides 56, 41–49. [DOI] [PubMed] [Google Scholar]
- Fenzl A, & Kiefer FW (2014). Brown adipose tissue and thermogenesis. Hormone Molecular Biology and Clinical Investigation 19(1), 25–37. [DOI] [PubMed] [Google Scholar]
- Foradori CD, et al. (2017). Kisspeptin stimulates growth hormone release by utilizing neuropeptide Y pathways and is dependent on the presence of ghrelin in the ewe. Endocrinology 158(10), 3526–3539. [DOI] [PubMed] [Google Scholar]
- Fu L-Y, & van den Pol AN (2010). Kisspeptin directly excites anorexigenic proopiomelanocortin neurons but inhibits orexigenic neuropeptide Y cells by an indirect synaptic mechanism. The Journal of Neuroscience 30(30), 10205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funes S, et al. (2003). The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochemical and Biophysical Research Communications 312(4), 1357–1363. [DOI] [PubMed] [Google Scholar]
- Goto T, et al. (2020). Testosterone supplementation rescues spermatogenesis and in vitro fertilizing ability of sperm in Kiss1 knockout mice. Endocrinology, 161(9). [DOI] [PubMed] [Google Scholar]
- Halvorson CL, et al. (2020). Thermoneutral conditions correct the obese phenotype in female, but not male, Kiss1r knockout mice. Journal of Thermal Biology 90, 102592. [DOI] [PubMed] [Google Scholar]
- Harshaw C, Culligan JJ, & Alberts JR (2014). Sex differences in thermogenesis structure behavior and contact within huddles of infant mice. PLoS One 9(1), Article e87405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauge-Evans AC, et al. (2006). A role for kisspeptin in islet function. Diabetologia 49(9), 2131–2135. [DOI] [PubMed] [Google Scholar]
- Herbison AE, et al. (2010). Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology 151(1), 312–321. [DOI] [PubMed] [Google Scholar]
- Hestiantoro A, et al. (2019). Dysregulation of kisspeptin and leptin, as anorexigenic agents, plays role in the development of obesity in postmenopausal women. International Journal of Endocrinology 2019, 1347208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikoshi Y, et al. (2003). Dramatic elevation of plasma metastin concentrations in human pregnancy: Metastin as a novel placenta-derived hormone in humans. The Journal of Clinical Endocrinology and Metabolism 88(2), 914–919. [DOI] [PubMed] [Google Scholar]
- Huang C, et al. (2019). Kisspeptin-activated autophagy independently suppresses non-glucose-stimulated insulin secretion from pancreatic β-cells. Scientific Reports 9(1), 17451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzi-Engbeaya C, et al. (2018). The effects of kisspeptin on β-cell function, serum metabolites and appetite in humans. Diabetes, Obesity and Metabolism 20(12), 2800–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasena CN, et al. (2011). The effects of kisspeptin-10 on reproductive hormone release show sexual dimorphism in humans. The Journal of Clinical Endocrinology & Metabolism 96(12), E1963–E1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasena CN, et al. (2015). The identification of elevated urinary kisspeptinimmunoreactivity during pregnancy. Annals of Clinical Biochemistry 52(Pt 3), 395–398. [DOI] [PubMed] [Google Scholar]
- Jones MEE, et al. (2000). Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proceedings of the National Academy of Sciences 97(23), 12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman AS (2009). Sexual differentiation and the Kiss1 system: Hormonal and developmental considerations. Peptides 30(1), 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman AS (2010). Coming of age in the kisspeptin era: Sex differences, development, and puberty. Molecular and Cellular Endocrinology 324(1–2), 51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman AS, Clifton DK, & Steiner RA (2007). Emerging ideas about kisspeptin–GPR54 signaling in the neuroendocrine regulation of reproduction. Trends in Neurosciences 30(10), 504–511. [DOI] [PubMed] [Google Scholar]
- Kim GL, Dhillon SS, & Belsham DD (2010). Kisspeptin directly regulates neuropeptide Y synthesis and secretion via the ERK1/2 and p38 mitogen-activated protein kinase signaling pathways in NPY-secreting hypothalamic neurons. Endocrinology 151 (10), 5038–5047. [DOI] [PubMed] [Google Scholar]
- Kim J, et al. (2011). Regulation of Kiss1 expression by sex steroids in the amygdala of the rat and mouse. Endocrinology 152(5), 2020–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok MD, Jakobsdottir S, & Drent ML (2007). The role of leptin and ghrelin in the regulation of food intake and body weight in humans: A review. Obesity Reviews 8(1), 21–34. [DOI] [PubMed] [Google Scholar]
- Kołodziejski PA, et al. (2018). Serum levels of spexin and kisspeptin negatively correlate with obesity and insulin resistance in women. Physiological Research 67(1), 45–56. [DOI] [PubMed] [Google Scholar]
- Koopman RJ, et al. (2005). Changes in age at diagnosis of type 2 diabetes mellitus in the United States, 1988 to 2000. Annals of Family Medicine 3(1), 60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani M, et al. (2001). The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. Journal of Biological Chemistry 276, 34631–34636. [DOI] [PubMed] [Google Scholar]
- Krull AA, et al. (2017). A comprehensive method to quantify adaptations by male and female mice with hot flashes induced by the neurokinin B receptor agonist senktide. Endocrinology 158(10), 3259–3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapatto R, et al. (2007). Kiss1−/− mice exhibit more variable hypogonadism than Gpr54 −/− mice. Endocrinology 148, 4927–4936. [DOI] [PubMed] [Google Scholar]
- López M, & Tena-Sempere M (2015). Estrogens and the control of energy homeostasis: A brain perspective. Trends in Endocrinology and Metabolism 26(8), 411–421. [DOI] [PubMed] [Google Scholar]
- Mayes JS, & Watson GH (2004). Direct effects of sex steroid hormones on adipose tissues and obesity. Obesity Reviews 5(4), 197–216. [DOI] [PubMed] [Google Scholar]
- Mekjavic IB, & Eiken O (2006). Contribution of thermal and nonthermal factors to the regulation of body temperature in humans. Journal of Applied Physiology 100(6), 2065–2072. [DOI] [PubMed] [Google Scholar]
- Mittelman-Smith MA, et al. (2012). Role for kisspeptin/neurokinin B/dynorphin (KNDy) neurons in cutaneous vasodilatation and the estrogen modulation of body temperature. Proceedings of the National Academy of Sciences of the United States of America 109(48), 19846–19851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir AI, et al. (2001). AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1*. Journal of Biological Chemistry 276(31), 28969–28975. [DOI] [PubMed] [Google Scholar]
- Nalbantoğlu Ö, et al. (2019). Three siblings with idiopathic hypogonadotropic hypogonadism in a nonconsanguineous family: A novel KISS1R/GPR54 loss-of-function mutation. Journal of Clinical Research in Pediatric Endocrinology 11(4), 444–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro G, et al. (2015). The role of androgens in metabolism, obesity, and diabetes in males and females. Obesity (Silver Spring, Md.) 23(4), 713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM (2020). Metabolic regulation of kisspeptin — The link between energy balance and reproduction. Nature Reviews Endocrinology 16(8), 407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM, et al. (2005). Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinology 146(1), 156–163. [DOI] [PubMed] [Google Scholar]
- Nestor CC, et al. (2016). Optogenetic stimulation of arcuate nucleus Kiss1 neurons reveals a steroid-dependent glutamatergic input to POMC and AgRP neurons in male mice. Molecular Endocrinology 30(6), 630–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimri R, et al. (2011). A novel loss-of-function mutation in GPR54/KISS1R leads to hypogonadotropic hypogonadism in a highly consanguineous family. The Journal of Clinical Endocrinology and Metabolism 96(3), E536–E545. [DOI] [PubMed] [Google Scholar]
- Ohtaki T, et al. (2001). Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 411(6837), 613–617. [DOI] [PubMed] [Google Scholar]
- Oishi S, et al. (2010). Activation of neuropeptide FF receptors by kisspeptin receptor ligands. ACS Medicinal Chemistry Letters 2(1), 53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla SL, et al. (2017). AgRP to Kiss1 neuron signaling links nutritional state and fertility. Proceedings of the National Academy of Sciences of the United States of America 114(9), 2413–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla SL, et al. (2018). A neural circuit underlying the generation of hot flushes. Cell Reports 24(2), 271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla SL, et al. (2019). Kisspeptin neurons in the arcuate nucleus of the hypothalamus orchestrate circadian rhythms and metabolism. Current Biology: CB 29(4), 592–604 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallais JC, et al. (2006). Neuroendocrine, gonadal, placental, and obstetric phenotypes in patients with IHH and mutations in the G-protein coupled receptor, GPR54. Molecular and Cellular Endocrinology 254–255, 70–77. [DOI] [PubMed] [Google Scholar]
- Pita J, et al. (2011). Circulating kisspeptin levels exhibit sexual dimorphism in adults, are increased in obese prepubertal girls and do not suffer modifications in girls with idiopathic central precocious puberty. Peptides 32(9), 1781–1786. [DOI] [PubMed] [Google Scholar]
- Popa SM, Clifton DK, & Steiner RA (2008). The role of kisspeptins and GPR54 in the neuroendocrine regulation of reproduction. Annual Review of Physiology 70(1), 213–238. [DOI] [PubMed] [Google Scholar]
- Pruszyńska-Oszmałek E, et al. (2017). Kisspeptin-10 inhibits proliferation and regulates lipolysis and lipogenesis processes in 3T3-L1 cells and isolated rat adipocytes. Endocrine 56(1), 54–64. [DOI] [PubMed] [Google Scholar]
- Qiu J, et al. (2011). Guinea pig kisspeptin neurons are depolarized by leptin via activation of TRPC channels. Endocrinology 152(4), 1503–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, et al. (2018). Estrogenic-dependent glutamatergic neurotransmission from kisspeptin neurons governs feeding circuits in females. eLife 7, Article e35656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafique N, & Latif R (2015). Serum kisspeptin levels in normal and overweight Saudi females and its relation with anthropometric indices. Annals of Saudi Medicine 35 (2), 157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rance NE, et al. (2013). Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: A novel hypothesis on the mechanism of hot flushes. Frontiers in Neuroendocrinology 34(3), 211–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven GM, et al. (1987). Documentation of hyperglucagonemia throughout the day in nonobese and obese patients with noninsulin-dependent diabetes mellitus. The Journal of Clinical Endocrinology and Metabolism 64(1), 106–110. [DOI] [PubMed] [Google Scholar]
- Rodrıguez-Cuenca S, et al. (2002). Sex-dependent thermogenesis, differences in mitochondrial morphology and function, and adrenergic response in brown adipose tissue *. Journal of Biological Chemistry 277(45), 42958–42963. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Vázquez E, Tena-Sempere M, & Castellano JM (2020). Mechanisms for the metabolic control of puberty. Current Opinion in Endocrine and Metabolic Research 14, 78–84. [Google Scholar]
- de Roux N, et al. (2003). Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proceedings of the National Academy of Sciences of the United States of America 100(19), 10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin Z, et al. (2015). Kisspeptin antagonist prevents RF9-induced reproductive changes in female rats. REPRODUCTION 149(5), 465–473. [DOI] [PubMed] [Google Scholar]
- Sahin Z, et al. (2020). Percentages of serum, liver and adipose tissue fatty acids and body weight are affected in female rats by long-term Central kisspeptin treatments. Archives of Physiology and Biochemistry, 1–9. [DOI] [PubMed] [Google Scholar]
- Saito R, et al. (2019). Centrally administered kisspeptin suppresses feeding via nesfatin-1 and oxytocin in male rats. Peptides 112, 114–124. [DOI] [PubMed] [Google Scholar]
- Schwetz TA, Reissaus CA, & Piston DW (2014). Differential stimulation of insulin secretion by GLP-1 and Kisspeptin-10. PLoS One 9(11), e113020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semaan SJ, & Kauffman AS (2010). Sexual differentiation and development of forebrain reproductive circuits. Current Opinion in Neurobiology 20(4), 424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminara SB, et al. (2003). The GPR54 gene as a regulator of puberty. The New England Journal of Medicine 349(17), 1614–1627. [DOI] [PubMed] [Google Scholar]
- Silveira LG, et al. (2010). Mutations of the KISS1 gene in disorders of puberty. The Journal of Clinical Endocrinology and Metabolism 95(5), 2276–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestre RA, et al. (2008). Kisspeptin-13 inhibits insulin secretion without affecting glucagon or somatostatin release: Study in the perfused rat pancreas. Journal of Endocrinology 196(2), 283–290. [DOI] [PubMed] [Google Scholar]
- Sithinamsuwan K, et al. (2020). Serum kisspeptin and its relation to metabolic parameters and glucose metabolism in prepubertal and pubertal obese children. International Journal of Endocrinology 2020, 8826401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitticharoon C, et al. (2021). Associations of serum kisspeptin levels with metabolic and reproductive parameters in men. Peptides 135, 170433. [DOI] [PubMed] [Google Scholar]
- Smith JT (2013). Sex steroid regulation of kisspeptin circuits. In Kauffman AS, & Smith JT (Eds.), Kisspeptin signaling in reproductive biology (pp. 275–295). New York, NY: Springer New York. [DOI] [PubMed] [Google Scholar]
- Smith JT, et al. (2005). Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146(9), 3686–3692. [DOI] [PubMed] [Google Scholar]
- Smith L, et al. (2021). A long-term role for kisspeptin in pancreatic beta-cell function. Kisspeptin virtual conference. [Google Scholar]
- Song WJ, et al. (2014). Glucagon regulates hepatic kisspeptin to impair insulin secretion. Cell Metabolism 19(4), 667–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger DJ, et al. (2010). Propagation of adipogenic signals through an epigenomic transition state. Genes & Development 24(10), 1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel A, et al. (2011). Centrally injected kisspeptin reduces food intake by increasing meal intervals in mice. Neuroreport 22(5), 253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens SBZ, & Kauffman AS (2017). Regulation and possible functions of kisspeptin in the medial amygdala. Frontiers in Endocrinology 8, 191. 10.3389/fendo.2017.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JH, Rutkowski JM, & Scherer PE (2016). Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metabolism 23(5), 770–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbi R, & Navarro VM (2020). Novel insights into the metabolic action of Kiss1 neurons. Endocrine Connections 9(5), R124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbi R, et al. (2016). Kisspeptin and RFRP-3 differentially regulate food intake and metabolic neuropeptides in the female desert jerboa. Scientific Reports 6, 36057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tengholm A, & Gylfe E (2017). cAMP signalling in insulin and glucagon secretion. Diabetes, Obesity and Metabolism 19(S1), 42–53. [DOI] [PubMed] [Google Scholar]
- Terasawa E, Guerriero KA, & Plant TM (2013). Kisspeptin and puberty in mammals. Advances in Experimental Medicine and Biology 784, 253–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson EL, et al. (2004). Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. Journal of Neuroendocrinology 16(10), 850–858. [DOI] [PubMed] [Google Scholar]
- Timper K, & Brüning JC (2017). Hypothalamic circuits regulating appetite and energy homeostasis: Pathways to obesity. Disease Models & Mechanisms 10(6), 679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolson K, et al. (2014). Impaired kisspeptin signaling decreases metabolism and promotes glucose intolerance and obesity. Journal of Clinical Investigation 124, 3075–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolson KP, et al. (2016). Metabolism and energy expenditure, but not feeding or glucose tolerance, are impaired in young Kiss1r KO female mice. Endocrinology 157(11), 4192–4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolson KP, et al. (2019). Cre/lox generation of a novel whole-body Kiss1r KO mouse line recapitulates a hypogonadal, obese, and metabolically-impaired phenotype. Molecular and Cellular Endocrinology 498, 110559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolson KP, et al. (2020). Conditional knockout of kisspeptin signaling in brown adipose tissue increases metabolic rate and body temperature and lowers body weight. The FASEB Journal 34(1), 107–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topaloglu AK, et al. (2012). Inactivating KISS1 mutation and hypogonadotropic hypogonadism. The New England Journal of Medicine 366(7), 629–635. [DOI] [PubMed] [Google Scholar]
- Velasco I, et al. (2019). Gonadal hormone-dependent vs. -independent effects of kisspeptin signaling in the control of body weight and metabolic homeostasis. Metabolism 98, 84–94. [DOI] [PubMed] [Google Scholar]
- Vikman J, & Ahrén B (2009). Inhibitory effect of kisspeptins on insulin secretion from isolated mouse islets. Diabetes, Obesity and Metabolism 11, 197–201. [DOI] [PubMed] [Google Scholar]
- Wahab F, Riaz T, & Shahab M (2011). Study on the effect of peripheral Kisspeptin administration on basal and glucose-induced insulin secretion under fed and fasting conditions in the adult male Rhesus monkey (Macaca mulatta). Hormone and Metabolic Research 43(01), 37–42. [DOI] [PubMed] [Google Scholar]
- Wang T, et al. (2018). Kisspeptin receptor GPR54 promotes adipocyte differentiation and fat accumulation in mice. Frontiers in Physiology 9, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikstrom MK (1977). Proton pump coupled to cytochrome c oxidase in mitochondria. Nature 266(5599), 271–273. [DOI] [PubMed] [Google Scholar]
- Winzell MS, & Ahrén B (2004). The high-fat diet–fed mouse: A model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 53(Suppl. 3), S215. [DOI] [PubMed] [Google Scholar]
- Xu Z, et al. (2012). Immunocytochemical localization of kisspeptin neurons in the rat forebrain with special reference to sexual dimorphism and interaction with GnRH neurons. Endocrine Journal 59(2), 161–171. [DOI] [PubMed] [Google Scholar]
- Xue Y, et al. (2010). Study on pancreatic islet adaptation and gene expression during pregnancy in rats. Endocrine 37(1), 83–97. [DOI] [PubMed] [Google Scholar]
- Yang L, et al. (2020). The effects of kisspeptin on brain response to food images and psychometric parameters of appetite in healthy men. The Journal of Clinical Endocrinology & Metabolism 106(4), e1837–e1848. 10.1210/clinem/dgaa746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo SH, et al. (2016). Visualisation of Kiss1 neurone distribution using a Kiss1-CRE transgenic mouse. Journal of Neuroendocrinology, 28(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu HJ, et al. (2016). The changes of serum leptin and kisspeptin levels in Chinese children and adolescents in different pubertal stages. International Journal of Endocrinology 2016, 6790794. [DOI] [PMC free article] [PubMed] [Google Scholar]


