Abstract
Background
Combination antibiotic therapy with an antitoxin agent, such as clindamycin, is included in some guidelines for severe, toxin-mediated Staphylococcus aureus infections. The evidence to support this practice is currently limited to in vitro, animal and observational human case-series data, with no previous randomized controlled trials (RCTs).
Objectives
This pilot RCT aimed to determine the feasibility of conducting a clinical trial to examine if adjunctive clindamycin with standard therapy has greater efficacy than standard therapy alone for S. aureus infections.
Methods
We performed an investigator-initiated, open-label, multicentre, pilot RCT (ACTRN12617001416381p) in adults and children with severe S. aureus infections, randomized to standard antibiotic therapy with or without clindamycin for 7 days.
Results
Over 28 months, across nine sites, 127 individuals were screened and 34 randomized, including 11 children (32%). The primary outcome—number of days alive and free of systemic inflammatory response syndrome ≤14 days—was similar between groups: clindamycin (3 days [IQR 1–6]) versus standard therapy (4 days [IQR 0–8]). The 90 day mortality was 0% (0/17) in the clindamycin group versus 24% (4/17) in the standard therapy group. Secondary outcomes—microbiological relapse, treatment failure or diarrhoea—were similar between groups.
Conclusions
As the first clinical trial assessing adjunctive clindamycin for S. aureus infections, this study indicates feasibility and that adults and children can be incorporated into one trial using harmonized endpoints, and there were no safety concerns. The CASSETTE trial will inform the definitive S. aureus Network Adaptive Platform (SNAP) trial, which includes an adjunctive clindamycin domain and participants with non-severe disease.
Introduction
Invasive Staphylococcus aureus infections are common and cause considerable mortality, particularly at the margins of the age continuum.1 They are the most common infective reason for admission to an ICU in both adults2 and children,3 with high mortality identified amongst these cohorts: 23%–33% in adults4 and 9% in children.5 Contributing to this severe phenotype is the ability of S. aureus to express multiple extracellular toxins that cause additional tissue damage.6
Despite this disease burden, fewer than 3000 adults7 and 300 children8 have been randomly assigned into clinical trials to assess the efficacy of treatments for S. aureus bacteraemia. Hence, opinions on best management vary widely,9,10 with an adjunctive protein synthesis inhibitor antibiotic suggested by some experts in severe, toxin-mediated S. aureus infections.11–13 The most frequently used protein synthesis inhibitor in this setting, clindamycin,10 decreases exotoxin production by binding to the 50S bacterial ribosomal subunit.6 The evidence to support adjunctive protein synthesis inhibitor use is currently limited to in vitro, animal and observational human case-series data, with no previous randomized controlled trials (RCTs).14
In this multicentre pilot RCT, we aimed to determine feasibility and to inform the design of a definitive RCT, the S. aureus network adaptive platform (SNAP) trial (https://www.snaptrial.com.au/). One of the aims of the SNAP trial will be to examine if the addition of clindamycin to standard antibiotic therapy (β-lactam, vancomycin or daptomycin) has greater clinical efficacy than standard therapy alone in adults and children with S. aureus infections. In addition, we aimed to provide proof of concept of including adults and children in the same RCT.
Methods
Study design and setting
We performed an investigator-initiated, open-label, multicentre, parallel-group, pilot RCT across four states in Australia, at six adult and three paediatric tertiary referral hospitals in adults and children with severe S. aureus infections from July 2018 to October 2020. This trial was endorsed by the Australasian Society of Infectious Diseases Clinical Research Network (ASID-CRN) and registered with the Australian and New Zealand Clinical Trials Registry (ACTRN12617001416381p). The published protocol14 was prospectively approved at each participating site with ethical approval (HREC/17/HNE/411). Written legal guardian (<18 years) or participant/surrogate decision-maker (≥18 years) informed consent was required for all participants before enrolment by the site principal investigator (PI).
Participants
Eligibility criteria were: hospital inpatients ≥28 days old expected to remain an inpatient at the study site for ≥7 days post-randomization; S. aureus cultured from ≥1 clinically relevant site (defined as any specimen where the site investigator determines the organism to be contributing to the patient’s clinical syndrome); and index tissue (including blood) culture drawn within ≤48 h of hospital admission (to exclude nosocomial infections) and ≤72 h pre-randomization. Evidence of severe S. aureus disease was defined as at least one of the following: (i) septic shock (Appendix S1, available as Supplementary data at JAC-AMR Online);14 (ii) severe lung (necrotizing or multifocal pneumonia) or pleural space infection (Appendix S2); or (iii) multifocal disease (>1 non-contiguous site or >1 contiguous anatomical site involved).
To facilitate recruitment, after 12 months, the eligibility criteria for multifocal infection was broadened (initially it only included >1 non-contiguous site) to also include >1 contiguous anatomical site. The rationale for this change was supported in the literature, with an association of Panton–Valentine leucocidin (PVL)-positive S. aureus disease and complicated osteomyelitis (thrombosis and pyomyositis).15,16
Exclusion criteria related to patients who: had significant immunosuppression (defined as prednisolone >0.5 mg/kg/day for ≥14 days in the last 30 days, other immunosuppressive medication, known HIV with CD4 cell count <200 cells/mm3 or congenital immunodeficiency); were expected to die within 24 h with or without treatment; had current severe diarrhoea (defined as >6 stools per day or clinician-determined severe diarrhoea in children) or onset of Clostridioides difficile-associated diarrhoea within 48 h prior to enrolment; had necrotizing fasciitis; were known to be pregnant; had a history of severe allergy to β-lactams, glycopeptides, lincosamides or daptomycin; had previously participated in the trial or were currently receiving a protein synthesis inhibitor antibiotic that could not be ceased or substituted. Other exclusions were the presence of polymicrobial culture containing other clinically significant isolates and where the primary clinician was unwilling to enrol the patient.
Randomization
Participants were randomized by a computerized central randomization schedule generated by a statistician in a 1:1 ratio to standard therapy (control group) or standard therapy plus clindamycin (adjunctive clindamycin group), stratified by age (<18 years versus ≥18 years), in permuted blocks of variable size. We pre-specified inclusion of one-third of children in our target sample.
Procedures
Standard therapy comprised IV flucloxacillin, 4–6 hourly or cefazolin, 6–8 hourly (both 50 mg/kg/dose for MSSA) or IV vancomycin [with dosing adjustments to maintain trough levels at 15–20 mg/L], daptomycin or ceftaroline (Appendix S3) for MRSA. Clindamycin was given IV 10 mg/kg/dose up to 600 mg four times daily or oral 450 mg three times daily in adults (10 mg/kg/dose in children) for 7 days. The choice and duration of standard antibiotic therapy and the decision to switch to oral clindamycin were clinician determined.
Outcomes and measurements
The primary outcome was number of days alive and free of systemic inflammatory response syndrome (SIRS) (defined as meeting <2 simultaneous SIRS criteria on a calendar day) ≤14 days post-randomization (Appendix S2). The secondary outcomes were: (i) all-cause mortality at 14, 42 and 90 days; (ii) time to first resolution of SIRS (number of days until the patient meets <2 simultaneous SIRS criteria); (iii) proportion with microbiological relapse by Day 90 (positive blood culture for S. aureus ≥72 h after a preceding negative culture); (iv) proportion with microbiological treatment failure by Day 90 (positive sterile site culture for S. aureus ≥14 days after randomization); (v) number of surgical procedures needed to achieve source control; (vi) duration of IV antibiotic treatment; (vii) C. difficile-associated diarrhoea (three or more loose stools per day with a positive laboratory test for C. difficile toxin); (viii) all cause diarrhoea (three or more loose stools per day); and (ix) time to a ≥50% decrease in C-reactive protein (CRP) in the first 14 days post-randomization (excluding participants missing a baseline CRP level).
Routine haematological parameters, repeat cultures, surgery for source control for S. aureus, patient progress and treatment were gathered from medical records, pathology results and treating teams onto an electronic case report form on Days 1, 3, 7, 10 and 14 and then weekly thereafter if an inpatient, and out to 90 days post-randomization (REDCap database).17 All-cause mortality data were collected through participant contact at 90 days or retrieved from medical records. Adverse events (AEs) were captured daily whilst an inpatient and up until 90 days post-randomization. All serious adverse events (SAEs) as defined in the protocol14 to any of the study drugs reported.
Laboratory methods
Identification of S. aureus was based on routine phenotypic and/or genotypic methods used in clinical laboratories of participating hospitals.
Statistical methods
The principal analysis of both primary and secondary endpoints was according to intention-to-treat principles. All participants with data available for the endpoint were analysed according to the treatment allocation, regardless of what treatment they received. Analyses of aggregated outcomes was initially performed by a statistician blinded to treatment groups, who was subsequently unblinded. Summary statistics were calculated for each outcome by trial arm and differences and 95% CIs were calculated using appropriate regression models. Given that this is a pilot study which is not powered for formal hypothesis testing, P values were not reported. All regression analyses were adjusted by the randomization covariate of age group (<18 years versus ≥18 years), and both unadjusted and adjusted estimates are reported.
For the primary outcome, days alive and free of SIRS, the difference between treatment arms was estimated using a zero-inflated negative binomial regression model, adjusted by days observed to account for participants with missing data. Binary secondary outcomes were assessed using logistic regression to estimate differences in proportions. All-cause mortality in both treatment arms was illustrated in a Kaplan–Meier survival curve. Competing risks regression was used to estimate HR for time-to-event outcomes. Difference in number of surgical procedures was estimated with negative binomial regression adjusted for days observed. All analyses were performed using Stata v16.0 (StataCorp LLC, College Station, TX, USA).
We planned to enrol 60 patients, at least 20 of whom were aged <18 years. As a pilot study, the sample size was based on the number achievable and the number needed to determine feasibility and to refine assumptions and study design. No assumptions were made about the rate of occurrence of the primary outcome in the control group nor for anticipated treatment effects of the intervention.
Results
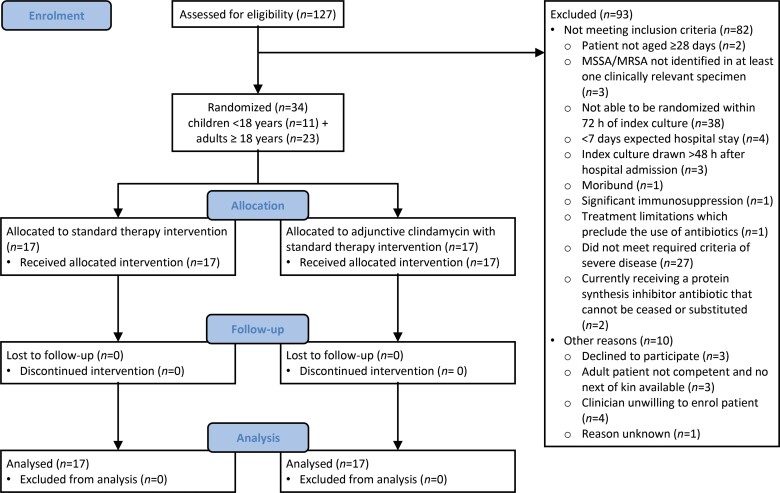
Over 28 months (July 2018–October 2020), across nine sites, 127 individuals were screened and 34 randomized from 40 eligible participants (85% consent rate). The recruitment rate was 1.2 participants/per month. Key reasons for exclusion included: inability to enrol participants within 72 h of the index culture (38/127, 30%) and not meeting the severe S. aureus disease definition (27/127, 21%). Consented participants were randomized to receive either standard therapy (n = 17) or standard therapy plus adjunctive clindamycin (n = 17) (Figure 1).
Figure 1.
Study flow diagram.
The trial was stopped prior to the anticipated sample size of 60 patients due to the slow recruitment rate and because the SNAP trial was planned to commence in 2021 and includes a domain for adjunctive treatment with clindamycin.
Baseline characteristics were similar between groups (Table 1). One third of the cohort were children <18 years (11/34, 32%) and female (11/34, 32%). All S. aureus infections (34/34, 100%) were community acquired and 24% (8/34) were caused by MRSA.
Table 1.
Baseline demographic, infection and treatment characteristics by study arm (overall and by age group [<18 years and ≥18 years])
| Demographics | Overall n = 34 | <18 years n = 11 | ≥18 years n = 23 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| standard therapy | adjunctive clindamycin therapy | total | standard therapy | adjunctive clindamycin therapy | total | standard therapy | adjunctive clindamycin therapy | total | |
| Age, years, median (IQR) | 44 (11–63) | 33 (12–60) | 41 (11–63) | 10 (10–11) | 8 (0–12) | 10 (0–11) | 45 (41–68) | 59 (33–75) | 54 (37–70) |
| Gender, female | 4/17 (24) | 7/17 (41) | 11/34 (32) | 3/5 (60) | 2/6 (33) | 5/11 (45) | 1/12 (8) | 5/11 (45) | 6/23 (26) |
| Weight, kg, median (IQR) | 87 (48–90) | 78 (44–87) | 78 (44–90) | 32 (28–36) | 30 (7–44) | 32 (7–44) | 89 (84–98) | 85 (77–102) | 87 (78–102) |
| Comorbid conditions | |||||||||
| Diabetes mellitus | 3/17 (18) | 2/17 (12) | 5/34 (15) | 0/5 (0) | 0/6 (0) | 0/11 (0) | 3/12 (25) | 2/11 (18) | 5/23 (22) |
| Chronic renal impairment (eGFR <50) | 0/17 (0) | 2/17 (12) | 2/34 (6) | 0/5 (0) | 0/6 (0) | 0/11 (0) | 0/12 (0) | 2/11 (18) | 2/23 (9) |
| Laboratory confirmed influenza ≤14 days prior to randomization | 1/17 (6) | 2/17 (12) | 3/34 (9) | 0/5 (0) | 1/6 (17) | 1/11 (9) | 1/12 (8) | 1/11 (9) | 2/23 (9) |
| Laboratory characteristics | |||||||||
| MRSA | 5/17 (29) | 3/17 (18) | 8/34 (24) | 2/5 (40) | 2/6 (33) | 4/11 (36) | 3/12 (25) | 1/11 (9) | 4/23 (17) |
| MSSA | 12/17 (71) | 14/17 (82) | 26/34 (76) | 3/5 (60) | 4/6 (67) | 7/11 (64) | 9/12 (75) | 10/11 (91) | 19/23 (83) |
| Infection site | |||||||||
| Multifocal | 10/17 (59) | 13/17 (76) | 23/34 (68) | 5/5 (100) | 3/6 (50) | 8/11 (73) | 5/12 (42) | 10/11 (91) | 15/23 (65) |
| Pleuropulmonary | 7/17 (41) | 6/17 (35) | 13/34 (38) | 3/5 (60) | 3/6 (50) | 6/11 (55) | 4/12 (33) | 3/11 (27) | 7/23 (30) |
| CNS | 3/17 (18) | 4/17 (24) | 7/34 (21) | 0/5 (0) | 0/6 (0) | 0/11 (0) | 3/12 (25) | 4/11 (36) | 7/23 (30) |
| Osteoarticular (native) | 5/17 (29) | 6/17 (35) | 11/34 (32) | 4/5 (80) | 3/6 (50) | 7/11 (64) | 1/12 (8) | 3/11 (27) | 4/23 (17) |
| Infective endocarditis | 2/17 (12) | 5/17 (29) | 7/34 (21) | 0/5 (0) | 0/6 (0) | 0/11 (0) | 2/12 (17) | 5/11 (45) | 7/23 (30) |
| Skin and soft tissue | 6/17 (35) | 3/17 (18) | 9/34 (26) | 1/5 (20) | 1/6 (17) | 2/11 (18) | 5/12 (42) | 2/11 (18) | 7/23 (30) |
| Bloodstream infection | 8/17 (47) | 12/17 (71) | 20/34 (59) | 3/5 (60) | 3/6 (50) | 6/11 (55) | 5/12 (42) | 9/11 (82) | 14/23 (61) |
| Infection severity | |||||||||
| ICU admission | 12/17 (71) | 10/17 (59) | 22/34 (65) | 2/5 (40) | 2/6 (33) | 4/11 (36) | 10/12 (83) | 8/11 (73) | 18/23 (78) |
| Septic shock | 8/17 (47) | 10/17 (59) | 18/34 (53) | 1/5 (20) | 2/6 (33) | 3/11 (27) | 7/12 (58) | 8/11 (73) | 15/23 (65) |
| SIRS on Day 1 | 16/17 (94) | 16/17 (94) | 32/34 (94) | 4/5 (80) | 5/6 (83) | 9/11 (82) | 12/12 (100) | 11/11 (100) | 23/23 (100) |
| SOFA scorea, median (IQR) | 6 (3–12) | 7 (5–11) | 7 (5–11) | N/A | N/A | N/A | 6 (3–12) | 7 (5–11) | 7 (5–11) |
| Baseline CRPb mg/L, median (IQR) | 245 (153–280) | 206 (164–349) | 235 (160–327) | 146 (140–270) | 249 (86–349) | 170 (139–327) | 261 (235–395) | 206 (164–374) | 243 (164–374) |
| Surgical source control | 6/17 (35) | 9/17 (53) | 15/34 (44) | 3/5 (60) | 2/6 (33) | 5/11 (45) | 3/12 (25) | 7/11 (64) | 10/23 (43) |
| Antibiot8ic use ≤72 h prior to randomization | |||||||||
| Any antibiotics | 16/17 (94) | 16/17 (94) | 32/34 (94) | 4/5 (80) | 5/6 (83) | 9/11 (82) | 12/12 (100) | 11/11 (100) | 23/23 (100) |
| Any vancomycin | 13/17 (76) | 15/17 (88) | 28/34 (82) | 1/5 (20) | 5/6 (83) | 6/11 (55) | 12/12 (100) | 10/11 (91) | 22/23 (96) |
| Any beta lactam | 16/17 (94) | 16/17 (94) | 32/34 (94) | 4/5 (80) | 5/6 (83) | 9/11 (82) | 12/12 (100) | 11/11 (100) | 23/23 (100) |
| Any protein synthesis inhibitor antibiotic | 8/17 (47) | 8/17 (47) | 16/34 (47) | 1/5 (20) | 1/6 (17) | 2/11 (18) | 7/12 (58) | 7/11 (64) | 14/23 (61) |
| Predominant therapy Day 1–14 | |||||||||
| MSSA: cefazolin | 1/12 (8) | 4/14 (29) | 5/26 (19) | 1/3 (33) | 1/4 (25) | 2/7 (29) | 0/9 (0) | 3/10 (30) | 3/19 (16) |
| MSSA: flucloxacillin | 11/12 (92) | 10/14 (71) | 21/26 (81) | 2/3 (67) | 3/4 (75) | 5/7 (71) | 9/9 (100) | 7/10 (70) | 16/19 (84) |
| MRSA: vancomycin | 4/5 (80) | 2/3 (67) | 6/8 (75) | 1/2 (50) | 1/2 (50) | 2/4 (50) | 3/3 (100) | 1/1 (100) | 4/4 (100) |
| MRSA: ceftaroline | 1/5 (20) | 1/3 (33) | 2/8 (25) | 1/2 (50) | 1/2 (50) | 2/4 (50) | 0/3 (0) | 0/1 (100) | 0/4 (0) |
| Non-study antibiotic use Day 1–90 | |||||||||
| Any non-study protein synthesis inhibitor in standard therapy arm | 8/17 (47) | N/A | N/A | 2/5 (40) | N/A | N/A | 6/12 (50) | N/A | N/A |
Values are shown as n (%) unless otherwise specified.
ATSI; Aboriginal and Torres Strait Islander; eGFR; estimated glomerular filtration rate; LOS; length of stay; ID; infectious diseases; CRP; C-reactive protein.
SOFA scores are only calculated for adult participants. Two missing baseline platelet measurements, four missing baseline bilirubin measurements, one missing baseline creatinine and urinary output measurements, four missing baseline CRP measurements. Where values are missing, a score of zero was assumed.
Four participants missing baseline CRP measurements.
The majority of participants (32/34, 94%) met SIRS criteria on Day 1 of randomization (Table 1). The median SOFA score in adults was 7 (IQR 5–11) and underlying comorbidities were present in 35% (8/23) of adults and 9% (1/11) of children. Half (18/34, 53%) developed septic shock and 65% (22/34) required admission to the ICU, which was less frequent in children (4/11, 36%) compared with adults (18/23, 78%). Receipt of antibiotics prior to randomization was frequent overall (32/34, 94%), including with a β-lactam (32/34, 94%), vancomycin (28/34, 82%) or protein synthesis inhibitor antibiotic (16/34, 47%).
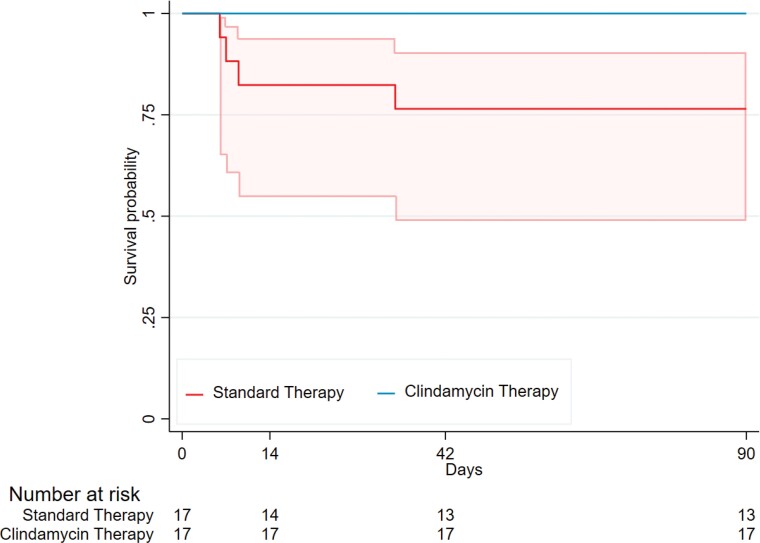
The primary endpoint, number of days alive and free of SIRS, was similar between the clindamycin group (median 3 days [IQR 1–6]) and standard therapy group (4 days [IQR 0–8]; adjusted difference [aD] −0.31 days [CI −3.20, 2.57]) (Table 2). For the secondary outcome of all-cause mortality, there were no deaths (0/17, 0%) up to 90 days in the clindamycin group. In the standard therapy group, 14 day mortality was 18% (3/17) and 42 and 90 day mortality (all in adults) were 24% (4/17) (Figure 2). There were no differences in microbiological relapse (3/17, 18% versus 1/17, 6%; aD 12% [CI −10%, 33%]) or microbiological treatment failure (1/17, 6% in both; aD 0% [CI −16%, 16%]) in the clindamycin compared with standard therapy group.
Table 2.
Primary, secondary and post-hoc outcomes in the standard therapy versus the adjunctive clindamycin therapy group, including summary statistics and estimates for the difference in outcome between groups
| Standard therapy, n = 17 | Adjunctive clindamycin therapy, n = 17 | Unadjusted | Adjusted | |||
|---|---|---|---|---|---|---|
| estimate | 95% CI | estimate | 95% CI | |||
| Estimated differences in count, including the primary outcome of days alive and free of SIRS | ||||||
| Days alive and free of SIRSa, mean (SD)/median (IQR) | 4.59 (4.11)/4 (0–8) | 4.76 (4.87)/3 (1–6) | 0.05 | −2.99, 3.09 | −0.31 | −3.20, 2.57 |
| Number of surgical procedures, mean (SD)/median (IQR) | 0.65 (1.06)/0 (0–1) | 1.18 (1.63)/1 (0–2) | 0.35 | −0.53, 1.23 | 0.35 | −0.53, 1.23 |
| Length of stay, daysb, median (IQR) | 15 (10–31) | 29 (15–46) | — | — | — | — |
| Days of bacteraemiab, median (IQR) | 2 (1–5) | 3 (1–8) | — | — | — | — |
| Days febrileb, median (IQR) | 2 (0–7) | 3 (1–6) | — | — | — | — |
| Estimated differences in probability | ||||||
| All-cause mortalityc, n (%) | ||||||
| Day 14 | 3/17 (18) | 0/17 (0) | — | — | — | — |
| Day 42 | 4/17 (24) | 0/17 (0) | — | — | — | — |
| Day 90 | 4/17 (24) | 0/17 (0) | — | — | — | — |
| Microbiological relapsed, n (%) | 1/17 (6) | 3/17 (18) | 12% | −10%, 33% | 12% | −10%, 33% |
| Microbiological treatment failuree, n (%) | 1/17 (6) | 1/17 (6) | 0% | −16%, 16% | 0% | −16%, 16% |
| C. difficile-associated diarrhoeac,f, n (%) | 1/17 (6) | 0/17 (0) | — | — | — | — |
| All cause diarrhoeag, n (%) | 6/17 (35) | 8/17 (47) | 12% | −21%, 45% | 12% | −21%, 45% |
| Estimated hazard ratios, where standard therapy is considered the reference | ||||||
| Days to first resolution of SIRSh, median survival (95% CI) | 9 (5, 12) | 9 (2, 10) | 1.53 | 0.72, 3.27 | 1.57 | 0.75, 3.31 |
| Days till CRP >50% decreasei, median survival (95% CI) | 5 (3, 7) | 7 (3, 14) | 0.55 | 0.30, 0.99 | 0.59 | 0.30, 1.13 |
Number of days patient is alive and meets <2 simultaneous SIRS criteria on a calendar day within ≤14 days post-randomization.
Post-hoc outcome: summary statistics presented only.
Difference in probability unable to be estimated due to zero events in one group.
Positive blood culture for MSSA or MRSA at least 72 h after a preceding negative culture within ≤14 days post-randomization.
Positive sterile site culture for MSSA or MRSA >14 days after randomization.
Three or more loose stools per day along with a positive laboratory test for C. difficile toxin.
Three or more loose stools per day.
Number of days until the patient first meets <2 simultaneous SIRS criteria on a calendar day.
Number of days till CRP decreases by >50% ≤14 days post randomization.
Figure 2.
Kaplan–Meier survival curves and 95% CI for all-cause mortality in the standard therapy (n = 17) and clindamycin therapy (n = 17) treatment groups. We were unable to estimate 95% CIs for clindamycin therapy due to zero events in that group.
All-cause diarrhoea occurred in 47% (8/17) of the clindamycin group compared with 35% (6/17) of the standard therapy group (aD 12% [CI −21%, 45%]), with one case (1/17, 6%) of C. difficile-associated diarrhoea occurring in the standard therapy group. The median days to first resolution of SIRS was equivalent between groups; 9 days (CI 2–10) in the clindamycin and 9 days (CI 5–12) in the standard therapy group; (adjusted hazard ratio [aHR] 1.57 [CI 0.75, 3.31]). The median days until CRP decreased by over 50% were similar between groups: 7 days (CI 3–14) in the clindamycin group and 5 days (CI 3–7) in the standard therapy group (aHR 0.59 [CI 0.30, 1.13]) (Table 2).
AEs were more common in the clindamycin group (10/17, 59%) compared with standard therapy (5/17, 29%) (Appendix S4). Study investigators determined most events to be unrelated to the study treatment and to be mild in severity.
Discussion
This is the first clinical trial to assess adjunctive clindamycin for severe S. aureus infections. Key findings from the CASSETTE trial include that it is feasible to conduct an RCT examining adjunctive clindamycin therapy, adults and children can be incorporated into one trial using harmonized endpoints, and there were no serious safety concerns evident from this preliminary cohort.
This study took almost 2.5 years to recruit a relatively small number of participants despite a large cohort being screened (34/127, 27%). This was due, in part, to difficulty identifying patients that met the severe S. aureus infection definition within 72 h of the index culture (Figure 1), with trial recruitment performed during the working week by clinician study investigators. This information has provided future direction for the planned SNAP trial, which will expand inclusion criteria to all individuals with S. aureus bacteraemia, regardless of illness severity and contain an adjunctive clindamycin domain. Importantly, clinician equipoise and patient acceptability were high, with 85% of eligible participants providing consent and no participant withdrawals or discontinued interventions noted (Figure 1). This trial experience confirms feasibility and has progressed the partnerships needed for a robust adult and paediatric infectious diseases trial network.
This pilot RCT has also provided insights into the utility of trial endpoints. The primary endpoint (number of days alive and free of SIRS), was pragmatically selected, given the availability of a validated scoring system that aligns in both adults18 and children.19 It was reasoned it would reflect decreased systemic inflammation due to decreased exotoxin production. Despite the rationale, the primary outcome, days alive and free of SIRS, were comparable, yet a difference in mortality was demonstrated (0% [0/17] 90 day mortality: clindamycin group versus 24% [4/17]: standard therapy group), although small numbers limit any definitive conclusions. Given these hypothesis-generating findings, and the much larger planned sample size for the SNAP trial, the more clinically relevant endpoint of all-cause mortality20 has been selected as the primary outcome for this planned RCT.
In the CASSETTE trial, mortality in the standard treatment arm occurred only in adults and was consistent with mortality in other adult S. aureus bacteraemia trials.1,2 To have no deaths in the clindamycin arm despite balanced groups with severe disease suggests a possible benefit of clindamycin, but this could have occurred by chance given the small numbers. In the literature the human clinical studies previously examining adjunctive clindamycin include only four case series in the setting of necrotizing pneumonia (n = 92 adults/adolescents),21 skin and soft tissue infections (SSTIs) (n = 269 adults),22 severe influenza-MRSA pneumonia (n = 29 children)23 and PVL-positive invasive S. aureus infections (n = 141 adults).24 Two case series in the severe pneumonia setting demonstrated lower mortality with adjunctive protein synthesis inhibitor antibiotics compared with standard therapy alone,21,23 but all have major study design limitations. A definitive trial is needed to objectively determine if this observed mortality difference reflects a true benefit or a chance finding.
The burden of daily data collection for the primary endpoint was evident. This has since been refined to a single study timepoint (Day 5) for the equivalent secondary outcome in SNAP. In addition, the statistical model for SNAP acknowledges the infrequent occurrence of trial endpoints in children (particularly death, 0/11, 0%) and will incorporate Bayesian hierarchical models that specify borrowing of data from adult patients to children.25,26 This pilot RCT has importantly enabled significant refinement of trial design in preparation for the definitive SNAP trial.
Limitations of this study include a small sample size, limiting conclusions regarding efficacy and mortality between treatment groups. However, this was a pilot RCT designed primarily to assess feasibility. There were some missing data points, however primary and key secondary outcomes including death and relapse were complete. We were unable to assess the total duration of IV antibiotic therapy or timing of IV to oral switch due to incomplete data. Some of the standard therapy group received adjunctive clindamycin prior to enrolment, which may have impacted results. There was no placebo available in the standard therapy group.
Lessons from the CASSETTE trial will inform the SNAP trial, which includes an adjunctive clindamycin domain and will be powered to determine whether clindamycin reduces the likelihood of death. Incorporating both adults and children with S. aureus infections in an RCT is feasible, despite challenges in analysis and data interpretation.
Supplementary Material
Acknowledgements
Preliminary results of this study were presented virtually as an oral presentation (number 131) at the Australian Society of Infectious Diseases Annual Scientific Virtual Meeting on 25 March 2021.
We would like to acknowledge the participants and their families involved in this study, as well as the study-site clinical and microbiology laboratory staff.
Funding
This work was supported by a National Health and Medical Research Council HOT NORTH grant (grant number 1131932), a Telethon Kids Institute, Wesfarmers Centre for Vaccines and Infectious Diseases Seed Fund grant, a National Health and Medical Research Council PhD scholarship to A.J.C (grant number APP1133670), National Health and Medical Research Council fellowship to A.C.B (grant number APP1175509) and a National Health and Medical Research Council fellowship to S.Y.C.T. (grant number 1145033).
Transparency declarations
S.Y.C.T has received consultancy fees from Roivant Sciences for advising on a clinical trial design. All other authors: none to declare.
Author contributions
J. S. Davis, S. Y. C. Tong and A. C. Bowen were involved in the conceptualization, methodology and supervision of this clinical trial. J. S. Davis, S. Y. C. Tong, A. C. Bowen, R. Dotel and A. J. Campbell were involved in the trial methodology. Statisticians N. Meagher and D. J. Price were involved in trial methodology, formal analysis and data visualization. Study coordinators J. Nelson and A. Whelan contributed to trial resources, data curation and project administration. A. J. Campbell, J. S. Davis, S. Y. C. Tong and A. C. Bowen wrote the original draft manuscript. All authors contributed to methodology, trial investigation and reviewing and editing of the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work. A. C. Bowen was involved in funding acquisition for the project.
Supplementary data
Appendices S1 to S4 are available as Supplementary data at JAC-AMR Online.
References
- 1. van Hal SJ, Jensen SO, Vaska VLet al. . Predictors of mortality in Staphylococcus aureus bacteremia. Clin Microbiol Rev 2012; 25: 362–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kaukonen KM, Bailey M, Suzuki Set al. . Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA 2014; 311: 1308–16. [DOI] [PubMed] [Google Scholar]
- 3. Schlapbach LJ, Straney L, Alexander Jet al. . Mortality related to invasive infections, sepsis, and septic shock in critically ill children in Australia and New Zealand, 2002-13: a multicentre retrospective cohort study. Lancet Infect Dis 2015; 15: 46–54. [DOI] [PubMed] [Google Scholar]
- 4. Lambert M-L, Suetens C, Savey Aet al. . Clinical outcomes of health-care-associated infections and antimicrobial resistance in patients admitted to European intensive-care units: a cohort study. Lancet Infect Dis 2011; 11: 30–8. [DOI] [PubMed] [Google Scholar]
- 5. Miles F, Voss L, Segedin Eet al. . Review of Staphylococcus aureus infections requiring admission to a paediatric intensive care unit. Arch Dis Child 2005; 90: 1274–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Poehlsgaard J, Douthwaite S. The bacterial ribosome as a target for antibiotics. Nat Rev Microbiol 2005; 3: 870–81. [DOI] [PubMed] [Google Scholar]
- 7. Holland TL, Chambers HF, Boucher HWet al. . Considerations for clinical trials of Staphylococcus aureus bloodstream infection in adults. Clin Infect Dis 2019; 68: 865–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McMullan BJ, Campbell AJ, Blyth CCet al. . Clinical management of Staphylococcus aureus bacteremia in neonates, children, and adolescents. Pediatrics 2020; 146: e20200134. [DOI] [PubMed] [Google Scholar]
- 9. Liu C, Strnad L, Beekmann SEet al. . Clinical practice variation among adult infectious disease physicians in the management of Staphylococcus aureus bacteremia. Clin Infect Dis 2019; 69: 530–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Campbell AJ, Tong SYC, Davis JSet al. . Infectious diseases clinician's variation in the management of pediatric Staphylococcus aureus bacteraemia and equipoise for clinical trials. Front Pediatr 2019; 7: 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu C, Bayer A, Cosgrove SEet al. . Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 2011; 52: 285–92. [DOI] [PubMed] [Google Scholar]
- 12. Health Protection Agency UK . Guidance on the Diagnosis and Management of PVL-Associated Staphylococcus aureus Infections (PVL-SA) in England, 2nd edn. Health Protection Agency UK, 2008. [Google Scholar]
- 13. Gillet Y, Dumitrescu O, Tristan Aet al. . Pragmatic management of Panton-Valentine leukocidin-associated staphylococcal diseases. Int J Antimicrob Agents 2011; 38: 457–64. [DOI] [PubMed] [Google Scholar]
- 14. Dotel R, Tong SYC, Bowen Aet al. . CASSETTE—clindamycin adjunctive therapy for severe Staphylococcus aureus treatment evaluation: study protocol for a randomised controlled trial. Trials 2019; 20: 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gonzalez BE, Teruya J, MahoneyDH, Jr.et al. . Venous thrombosis associated with staphylococcal osteomyelitis in children. Pediatrics 2006; 117: 1673–9. [DOI] [PubMed] [Google Scholar]
- 16. Bocchini CE, Hulten KG, Jr MEet al. . Panton-Valentine leukocidin genes are associated with enhanced inflammatory response and local disease in acute hematogenous Staphylococcus aureus osteomyelitis in children. Pediatrics 2006; 117: 433–40. [DOI] [PubMed] [Google Scholar]
- 17. Harris PA, Taylor R, Thielke Ret al. . Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bone RC, Balk RA, Cerra FBet al. . Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992; 101: 1644–55. [DOI] [PubMed] [Google Scholar]
- 19. Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005; 6: 2–8. [DOI] [PubMed] [Google Scholar]
- 20. Harris PNA, McNamara JF, Lye DCet al. . Proposed primary endpoints for use in clinical trials that compare treatment options for bloodstream infection in adults: a consensus definition. Clin Microbiol Infect 2017; 23: 533–41. [DOI] [PubMed] [Google Scholar]
- 21. Li H-T, Zhang T-T, Huang Jet al. . Factors associated with the outcome of life-threatening necrotizing pneumonia due to community-acquired Staphylococcus aureus in adult and adolescent patients. Respiration 2011; 81: 448–60. [DOI] [PubMed] [Google Scholar]
- 22. Wargo KA, McCreary EK, English TM. Vancomycin combined with clindamycin for the treatment of acute bacterial skin and skin-structure infections. Clin Infect Dis 2015; 61: 1148–54. [DOI] [PubMed] [Google Scholar]
- 23. Randolph AG, Xu R, Novak Tet al. . Vancomycin monotherapy may be insufficient to treat methicillin-resistant Staphylococcus aureus coinfection in children with influenza-related critical illness. Clin Infect Dis 2018; 68: 365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boan P, Tan H-L, Pearson Jet al. . Epidemiological, clinical, outcome and antibiotic susceptibility differences between PVL positive and PVL negative Staphylococcus aureus infections in Western Australia: a case control study. BMC Infect Dis 2015; 15: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schoenfeld DA, Hui Z, Finkelstein DM. Bayesian design using adult data to augment pediatric trials. Clin Trials 2009; 6: 297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huff RA, Maca JD, Puri Met al. . Enhancing pediatric clinical trial feasibility through the use of Bayesian statistics. Pediatr Res 2017; 82: 814–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.