Summary
Heart remodeling occurs as a compensation mechanism for the massive loss of tissue during initial heart failure and the consequent inflammation process. During heart remodeling fibroblasts differentiate to myofibroblasts activate their secretion functions and produce elevated amounts, of extracellular matrix (ECM) proteins, mostly collagen, that form scar tissue and alter the normal degradation of ECM. Scar formation does replace the damaged tissue structurally; however, it impedes the normal contractive function of cardiomyocytes (CMs) and results in long-lasting effects after heart failure. Besides CMs and cardiac fibroblasts, endothelial cells (ECs) and circulating endothelial progenitor cells (cEPCs) contribute to heart repair. This review summarizes the current knowledge of EC-CM crosstalk in cardiac fibrosis (CF), the role of cEPCs in heart regeneration and the contribution of Endothelial-mesenchymal transition (EndoMT).
Keywords: Cardiac fibrosis, Endothelial-mesenchymal transition, Endothelial progenitor cells, Cardiac fibroblasts, Cardiomyocytes, Endothelial cells, Agiotensin II, Tumor Growth Factor-β
Introduction
Cardiovascular disease is the main cause of death in developed countries worldwide. Cardiac dysfunction is the primary consequence of cardiac remodeling, caused by the hypoxic and inflammation response during heart ischemia. Approximately 50 % of patients diagnosed with cardiac dysfunction will die within five years. In addition, 40 % of patients die within one year after hospitalization for cardiac failure (Liu and Eisen 2014). A significant part of deaths associated with cardiac remodeling and dysfunction is caused by sudden death, which suggests that even though a patient is asymptomatic it does not guarantee of good prognosis (Pimentel et al. 2014). Despite the increased survival with modern therapies, mortality rates are still at extremely high levels (Braunwald 2013).
The tissue injured by heart ischemia and massive cell death requires a relatively rapid process to compensate for cellular loss and activates regeneration and remodeling processes. Even though these reparation processes quickly compensate for the structural damage, they are not able to replace the functionality of the tissue, which has long-term effects. The regulation of the tissue regeneration and remodeling could be beneficial for partial recovery of the function; however, any treatment intervention must be preceded by detailed knowledge of the molecular processes in place.
Most studies focus on the CMs’ response to heart ischemia as a cause of CF. The role of ECs in the process has been neglected, even though they are a major contributor to the myofibroblast pool (Pardali et al. 2017). In heart failure patients without coronary artery disease, coronary endothelial dysfunction correlates with adverse cardiac remodeling and contractile abnormalities; however, the endothelial function of peripheral arteries is preserved. This suggests that localized endothelial dysfunction and crosstalk between CMs and ECs are responsible for cardiac remodeling and hypertrophy.
EC-CM crosstalk guides CF
In the adult heart, capillaries are densely distributed throughout the myocardium, and the number of ECs is approximately 3 times higher than CMs. Together, CMs and ECs form their physiological niche by secreting of a large number of signaling molecules that affect the surrounding cells and facilitate the homeostasis of the tissue.
CMs have a significant effect on ECs’ function during cardiac remodeling. They are a major player in angiogenesis and neovascularization during heart repair. CMs are the main contributor to the vascular endothelial growth factor (VEGF) pool which is the key controller of angiogenesis (Colliva et al. 2020, Giordano et al. 2001). Despite the fact that CMs constitute less than one-third of the total number of cells in the heart, the CM-specific deletion of VEGF gene decreases the total mRNA levels by more than 85 %.
Recent data also show that CMs directly stimulate the angiogenesis of resident ECs by paracrine stimulation in response to cardiac remodeling signaling (Zhang et al. 2021b). In a report by Gladka and colleagues CMs activated the Zinc finger E-box-binding homeobox 2 (ZEB2) expression in response to remodeling stimulation, which resulted in an increase of circulating levels of Tymosin β4 (TMSB4) and Prothymosin α (PTMA). Both TMS4 and PTMA have been linked to cardioprotection by regulation of neovascularisation, angiogenesis, and apoptosis (Gladka et al. 2021, Smart et al. 2010). It was shown that TMSB4 stimulates the formation of new CMs, originating from the epicardium, and promotes neovascularization (Malinda et al. 1998, Tang et al. 2021). PTMA has also been shown to stimulate cardiac EC migration, angiogenesis, and wound healing (Halder et al. 2020). Interestingly, ZEB2 was also shown to initiate EndoMT in carcinogenesis, which suggest that regulation of ZEB2 signaling is crucial for the balance between heart regeneration and fibrosis (Comijn et al. 2001).
ECs, on the other hand, control CM contractility by a number of growth factors. During heart inflammation and heart remodeling, EC-CM communication guides tissue fibrosis by regulation of nitric oxide (NO) bioavailability and Angiotensin converting enzyme 2 (Ace2) production (Han et al. 2018, Heiss et al. 2015). NO is one of the most significant signaling molecules regulating vascular function and cell recruitment during injury. It is synthetized by the NO synthase class of enzymes (eNOS). Different eNOS types are either constitutively active and produce NO in response to calcium, or inducible by cytokine stimulation in response to tissue injury. In the adult heart, NO is mainly produced by ECs and controls vascular smooth muscle cells, and the onset of ventricular relaxation, thus optimizing pump function at every cardiac contraction (Heiss et al. 2015). eNOS catalytic activity requires the cofactor tetrahydrobiopterin (BH4). Reactive oxygen spieces (ROS) are one of the major regulators of eNOS activity through oxidation of BH4 to 7,8-dihydrobiopterin (BH2) (McNeill and Channon 2012). BH2 changes eNOS enzymatic activity, which produces superoxide in place of NO. This mechanism decreases NO bioavailability and significantly contributes to fibrosis by the dysregulation of vasoconstriction and NO signaling for stem cell recruitment discussed later.
Angiotensin II (AngII) is a major inducer of CF in vitro and only recently AngII inhibition was shown to have a cardioprotective effect in patients with hypertension-induced heart failure (Huang et al. 2020). The level of AngII in heart is strictly controlled by hydrolysis by Ace2 (De Mello and Danser 2000). Ace2’s analog Ace, however, does not share this functionality and Ace2-to-Ace ratio in circulation is responsible for the control of AngII levels (Fig. 1). This delicate physiological balance is disrupted during cardiac stress, when EC expression of Brahma-related gene-1 chromatin remodeler (Brg1) and forkhead box M1 (FoxM1) transcription factors cause the formation of a protein complex at the Ace/Ace2 promoter site, followed by concurrent activation of Ace and repression of Ace2. This shifts the Ace-to-Ace2 ratio balance in favor of Ace, resulting in AngII production. Subsequently, AngII stimulates fibrosis in two phases (Yang et al. 2016). In the early phase of CF, AngII stimulates direct association between the Toll-like receptor 4 (Tlr4) and Signal transducer and activator of transcription 3 (STAT3) (Han et al. 2018). Phosphorylated STAT3 activates its transcription targets including interleukin-6 (IL-6), resulting in increased IL-6 production, which induces late phase of STAT3 transcription and its targets including the transforming growth factor β (TGF-β). IL-6 and TGF-β are notorious inducers of cardiac remodeling.
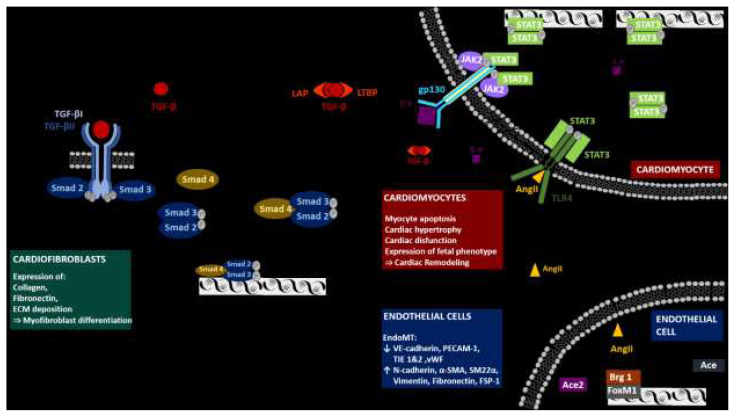
Fig. 1.
CM-EC crosstalk during heart remodeling. During heart remodeling complex Brb1/FoxM1 modifies the transcription of Ace and Ace2 in ECs, which prevents degradation of AngII. In result, AngII activates cardiomyocyte TLF4 receptor, which phosphorylates STAT3, which initiates transcription of multiple targets including IL-6. IL-6 is further secreted into extracellular space, binds to gp130 receptor and activates secondary STAT3 targets including TGF-β. TGF-β is again secreted to extracellular space and activates transcription of TGF-β targets trough Smad signaling pathway in cardiofibroblasts, CMs and ECs.
The production of TGF-β alone is however not solely responsible for the activation of the intracellular signaling cascade (Gentry and Nash 1990, Robertson and Rifkin 2016). TGF-β is secreted in a latent form that contains the TGF-β dimer, the latency-associated peptide (LAP), and a latent TGF-β-binding protein (LTBP), which sequesters the complex into the ECM (Fig. 1). The activation mechanisms of TGF-β are currently unknown. A list of possible suspects includes ROS, ECM protein interactions, and, most recently, tissue nonspecific alkaline phosphatase (Cheng et al. 2021, Hanna and Frangogiannis, 2019). TGF-β activation initiates the Smad2/3 pathway, promotes the process of CF and induces the transformation from fibroblasts into myofibroblasts (Desmoulière et al. 1993, Heldin et al. 1997, Shi and Massagué 2003). Furthermore, TGF-β increases ECM gene expression, promotes ECM deposition and inhibits the degradation of ECM by regulating the level of plasminogen activator inhibitor and tissue inhibitors of metalloproteinases (Leask 2010, Schiller et al. 2004). TGF-β also affects the CM phenotype, where TGF-β stimulation results in the hypertrophy of CMs (Lim et al. 2005). This finalizes the process of scar tissue formation.
The role of EndoMT in cardiac regeneration and CF
EndoMT is a complex biological process where ECs lose their specific cell profile, decrease vascular endothelial cadherin expression, and acquire a myofibroblast phenotype characterized by smooth muscle actin, vimentin, and type I collagen expression. Besides the acquisition of an activated pro-fibrogenic phenotype, these cells also increase their mobility and migrate to surrounding tissues.
TGF-β was shown to have more diverse effects than fibroblast transformation. Epithelial, endothelial and immune cells were shown to contribute to the total myofibroblast pool in a fibrotic heart by epithelial/endothelial-to-mesenchymal transition in response to TGF-β (Pardali et al. 2017, Xu et al. 2009, Zeisberg et al. 2007). In CF, EndoMT represents the most important contributor to the generation of fibrotic tissue (Piera-Velazquez et al. 2011). Moreover, the inhibition of TGF-β signaling prevented epithelial-to-mesenchymal transition (Meng et al. 2012). ECs themselves were also shown to contribute to TGF-β production and secretion under hypoxic conditions, which, in turn, promotes EndoMT in an autocrine loop and induces apoptosis in CMs (Sniegon et al. 2017). It appears that EndoMT and functional tissue regeneration are the opposite outcomes of a common process. Close optimization of the growth factor composition of the specific niche where regeneration and fibrosis take place may represent a novel target for the prevention of tissue fibrosis.
The role of cEPCs in heart remodeling
The contribution of bone-marrow derived stem cells to heart regeneration has been debated since the discovery of cEPC’s and mesenchymal stem cell’s differentiation abilities. EPCs are a bone marrow stem cell population, considered a remnant of developmental hemangioblast characterized by CD34, CD31, VEGF receptor 2 and CD133 expression. These progenitor cells show limited stem cell features, such as clonal expansion and angiogenic capability. A low number of EPCs is constitutively present in human blood circulation. They account for only 0.001 %-0.0001 % of peripheral blood cells in a physiological state (Asahara et al. 2011). Cardiac injury and inflammation signaling mobilizes EPC’s from blood marrow into circulating blood and homing to the injury site. The question of cEPC’s contribution to tissue repair and regeneration is controversial. It was shown that cEPCs contribute to tissue repair by paracrine signaling, and their differentiation into mature ECs and integration into the vessel wall was shown repeatedly as well. However, the extent of their contribution was repeatedly questioned.
The correlation between the number of cEPC and the state of the cardiovascular system is an appealing argument in favor of cEPCs’ role in cardiac regeneration. The enumeration of cEPCs is a diagnostic parameter that reflects the condition of the vascular network, and their number decreases in multiple diseases that affect the cardiovascular network like diabetes and artherosclerosis (Chironi et al. 2007, Fadini 2014). Hill’s assay evaluates patients with different cardiovascular risk factors dependent on the number of cEPCs. A recent study showed a correlation between the cEPC count, severity of coronary artery disease and cardiovascular risk (Hill et al. 2003, Zhang et al. 2021a). It was shown that cEPCs contribute to endothelial repair at the moment of endothelial injury, and their depletion or decrease has a detrimental effect on injury response and tissue regeneration (Kocher et al. 2001, Zhang et al. 2021a). It was demonstrated that neoangiogenesis by cEPCs inhibits the apoptosis of hypertrophied myocardium, minimizes abnormal collagen deposition and scar formation, and optimizes ventricular function (Kocher et al. 2001, Wang et al. 2021). A recent study found that cardiac resynchronization therapy increases cEPC count and improves cardiovascular outcome (Cristóvão et al. 2020, Yang et al. 2020). As a previous study showed IL-6, as a major biomarker of inflammation response, is reduced after 3 months of biventricular pacing (Theodorakis et al. 2006). Therefore, increased cEPC mobilization can be directly linked to a decrease in inflation signaling.
EPC mobilization during heart remodeling is, however, obstructed. Endothelium-derived NO is a major regulator of both EPC mobilization from bone marrow and its function. (Aicher et al. 2003, Sandri et al. 2016). Due to the low bioavailability of NO during heart remodeling EPC mobilization is limited (Chironi et al. 2007, Samman Tahhan Ayman et al. 2017). A recent study showed the recovery of EPC mobilization through the activation of eNOS with fenofibrate (Huang et al. 2021). Furthermore, the half-life of EPCs is also reduced in heart failure, which further drives heart remodeling and adverse outcomes (Samman Tahhan Ayman et al. 2017).
Although the differentiation of cEPCs into CMs was repeatedly demonstrated in vitro, it still remains controversial (Bachelier et al. 2020, Badorff et al. 2003, Koyanagi et al. 2005, Murasawa et al. 2005). The reason is that this process was never proven to contribute to heart regeneration in vivo. Resident cardiac ECs were recently shown to express CM myofibril (CMF) genes and have open chromatin at CMF gene promoters. However, these open chromatin signitures disappeared at EC cultivation in vitro (Yucel et al. 2020). These data demonstrate that ECs maintain fully open chromatin at CM-specific genes, which suggests certain phenotype plasticity. The fact that cEPCs and resident cardiac ECs contribute to fibrosis by EMT and were also shown to transdifferentiate into functional CMs raises the possibility that the specific CM-EC niche can be modulated to favor of differentiation into partially functional heart tissue.
Conclusion
ECs significantly contribute to cardiac remodeling and fibrosis. It appears that the remodeling program that takes place in the environment of inflammation signaling favors the highly proliferating fibroblast population that creates scar tissue and reduces the functionality of the cardiac tissue. Moreover, cells with regenerative capabilities and cardiac trans-differentiation potential are affected by the paracrine signaling and go through EndoMT transition. Shifting the delicate balance of CM-EC crosstalk to favor angiogenesis and trans-differentiation of ECs and EPCs into CMs instead of EndoMT could significantly improve the tissue function. The modulation of the CM-EC specific cardiac niche is an interesting new target for CF therapy.
Abbreviations
- Brg1
Brahma-related gene-1 chromatin remodeler
- FoxM1
forkhead box M1
- TIMPS
tissue inhibitors of metalloproteinases
- Ace
Angiotensin converting enzyme Ace2, Angiotensin converting enzyme 2
- AngII
angiotensin II
- BH2
7,8-dihydrobiopterin
- BH4
tetrahydrobiopterin
- cEPC
circulating endothelial progenitor cells
- CF
cardiac fibrosis
- CMF
cardiomyocyte myofibril
- CMs
cardiomyocytes
- ECM
extracellular matrix
- ECs
endothelial cells
- EndoMT
endothelial- mesenchymal transition
- eNOS
NO synthase class of enzymes
- IL-6
interleukin 6
- NO
nitric oxide
- PTMA
Prothymosin α
- ROS
reactive oxidative species
- STAT 3
Signal transducer and activator of transcription 3
- TGF-β
transforming growth factor β
- Tlr4
Toll-like receptor 4
- TMSB4
Tymosin β4
- TNF-α
tumor necrosis factor α
- ZEB2
Zinc finger E-box-binding homeobox 2
Footnotes
Conflict of Interest
There is no conflict of interest.
References
- AICHER A, HEESCHEN C, MILDNER-RIHM C, URBICH C, IHLING C, TECHNAU-IHLING K, ZEIHER AM, DIMMELER S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- ASAHARA T, KAWAMOTO A, MASUDA H. Concise review: circulating endothelial progenitor cells for vascular medicine. STEM CELLS. 2011;29:1650–1655. doi: 10.1002/stem.745. [DOI] [PubMed] [Google Scholar]
- BACHELIER K, BERGHOLZ C, FRIEDRICH EB. Differentiation potential and functional properties of a CD34-CD133+ subpopulation of endothelial progenitor cells. Mol Med Rep. 2020;21:501–507. doi: 10.3892/mmr.2019.10831. [DOI] [PubMed] [Google Scholar]
- BADORFF C, BRANDES RP, POPP R, RUPP S, URBICH C, AICHER A, FLEMING I, BUSSE R, ZEIHER AM, DIMMELER S. Transdifferentiation of blood-derived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation. 2003;107:1024–1032. doi: 10.1161/01.CIR.0000051460.85800.BB. [DOI] [PubMed] [Google Scholar]
- BRAUNWALD E. Heart failure. JACC: Heart failure. 2013;1:1–20. doi: 10.1016/j.jchf.2012.10.002. [DOI] [PubMed] [Google Scholar]
- CHENG X, WANG L, WEN X, GAO L, LI G, CHANG G, QIN S, ZHANG D. TNAP is a novel regulator of cardiac fibrosis after myocardial infarction by mediating TGF-β/Smads and ERK1/2 signaling pathways. EBioMedicine. 67:2021. doi: 10.1016/j.ebiom.2021.103370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIRONI G, WALCH L, PERNOLLET M-G, GARIEPY J, LEVENSON J, RENDU F, SIMON A. Decreased number of circulating CD34+KDR+ cells in asymptomatic subjects with preclinical atherosclerosis. Atherosclerosis. 2007;191:115–120. doi: 10.1016/j.atherosclerosis.2006.02.041. [DOI] [PubMed] [Google Scholar]
- COLLIVA A, BRAGA L, GIACCA M, ZACCHIGNA S. Endothelial cell-cardiomyocyte crosstalk in heart development and disease. J Physiol. 2020;598:2923–2939. doi: 10.1113/JP276758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMIJN J, BERX G, VERMASSEN P, VERSCHUEREN K, VAN GRUNSVEN L, BRUYNEEL E, MAREEL M, HUYLEBROECK D, van ROY F. The two-handed e box binding zinc finger protein SIP1 downregulates E-Cadherin and induces invasion. Mol Cell. 2001;7:1267–1278. doi: 10.1016/S1097-2765(01)00260-X. [DOI] [PubMed] [Google Scholar]
- CRISTÓVÃO G, MILNER J, SOUSA P, VENTURA M, CRISTÓVÃO J, ELVAS L, PAIVA A, GONÇALVES L, RIBEIRO CF, ANTÓNIO N. Improvement in circulating endothelial progenitor cells pool after cardiac resynchronization therapy: increasing the list of benefits. Stem Cell Res Ther. 2020;11:194. doi: 10.1186/s13287-020-01713-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De MELLO WC, DANSER AHJ. Angiotensin II and the heart. Hypertension. 2000;2020;35:1183–1188. doi: 10.1161/01.HYP.35.6.1183. [DOI] [PubMed] [Google Scholar]
- DESMOULIÈRE A, GEINOZ A, GABBIANI F, GABBIANI G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FADINI GP. A reappraisal of the role of circulating (progenitor) cells in the pathobiology of diabetic complications. Diabetologia. 2014;57:4–15. doi: 10.1007/s00125-013-3087-6. [DOI] [PubMed] [Google Scholar]
- GENTRY LE, NASH BW. The pro domain of pre-pro-transforming growth factor beta 1 when independently expressed is a functional binding protein for the mature growth factor. Biochemistry. 1990;29:6851–6857. doi: 10.1021/bi00481a014. [DOI] [PubMed] [Google Scholar]
- GIORDANO FJ, GERBER H-P, WILLIAMS S-P, VANBRUGGEN N, BUNTING S, RUIZ-LOZANO P, GU Y, NATH AK, HUANG Y, HICKEY R, DALTON N, PETERSON KL, ROSS J, CHIEN KR, FERRARA N. A cardiac myocyte vascular endothelial growth factor paracrine pathway is required to maintain cardiac function. Proceedings Nat Acad Sci USA. 2001;98:5780–5785. doi: 10.1073/pnas.091415198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLADKA MM, KOHELA A, MOLENAAR B, VERSTEEG D, KOOIJMAN L, MONSHOUWER-KLOOTS J, KREMER V, VOS HR, HUIBERS MMH, HAIGH JJ, HUYLEBROECK D, BOON RA, GIACCA M, VAN ROOIJ E. Cardiomyocytes stimulate angiogenesis after ischemic injury in a ZEB2-dependent manner. Nat Com. 2021;12:84. doi: 10.1038/s41467-020-20361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALDER SK, MATSUNAGA H, UEDA H. Experimental evidence for the involvement of F0/F1 ATPase and subsequent P2Y12 receptor activation in prothymosin alpha-induced protection of retinal ischemic damage. J Pharmacol Sci. 2020;143:127–131. doi: 10.1016/j.jphs.2020.01.008. [DOI] [PubMed] [Google Scholar]
- HAN J, YE S, ZOU C, CHEN T, WANG J, LI J, JIANG L, XU J, HUANG W, WANG Y, LIANG G. Angiotensin II causes biphasic STAT3 activation through TLR4 to initiate cardiac remodeling. Hypertension. 2018;72:1301–1311. doi: 10.1161/HYPERTENSIONAHA.118.11860. [DOI] [PubMed] [Google Scholar]
- HANNA A, FRANGOGIANNIS NG. The role of the TGF-β superfamily in myocardial infarction. Front Cardiovasc Med. 2019;6 doi: 10.3389/fcvm.2019.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEISS C, RODRIGUEZ-MATEOS A, KELM M. Central role of eNOS in the maintenance of endothelial homeostasis. Antioxid Redox Signal. 2015;22:1230–1242. doi: 10.1089/ars.2014.6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELDIN CH, MIYAZONO K, TEN DIJKE P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- HILL JM, ZALOS G, HALCOX JPJ, SCHENKE WH, WACLAWIW MA, QUYYUMI AA, FINKEL T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. New England J Med. 2003;34:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- HUANG A, LI H, ZENG C, CHEN W, WEI L, LIU Y, QI X. Endogenous CCN5 participates in angiotensin II/TGF-β1 networking of cardiac fibrosis in high angiotensin ii-induced hypertensive heart failure. Front Pharmacol. 2020;11:1235. doi: 10.3389/fphar.2020.01235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG W-P, YIN W-H, CHEN J-S, HUANG P-H, CHEN J-W, LIN S-J. Fenofibrate attenuates doxorubicin-induced cardiac dysfunction in mice via activating the eNOS/EPC pathway. Sci Rep. 2021;11:1159. doi: 10.1038/s41598-021-80984-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOCHER AA, SCHUSTER MD, SZABOLCS MJ, TAKUMA S, BURKHOFF D, WANG J, HOMMA S, EDWARDS NM, ITESCU S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- KOYANAGI M, URBICH C, CHAVAKIS E, HOFFMANN J, RUPP S, BADORFF C, ZEIHER AM, STARZINSKI-POWITZ A, HAENDELER J, DIMMELER S. Differentiation of circulating endothelial progenitor cells to a cardiomyogenic phenotype depends on E-cadherin. FEBS letters. 2005;579:6060–6066. doi: 10.1016/j.febslet.2005.09.071. [DOI] [PubMed] [Google Scholar]
- LEASK A. Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ Res. 2010;106:1675–1680. doi: 10.1161/CIRCRESAHA.110.217737. [DOI] [PubMed] [Google Scholar]
- LIM J-Y, PARK SJ, HWANG H-Y, PARK EJ, NAM JH, KIM J, PARK SI. TGF-beta1 induces cardiac hypertrophic responses via PKC-dependent ATF-2 activation. J Mol Cell Cardiol. 2005;39:627–636. doi: 10.1016/j.yjmcc.2005.06.016. [DOI] [PubMed] [Google Scholar]
- LIU L, EISEN HJ. Epidemiology of heart failure and scope of the problem. Cardiol Clin. 2014;32:1–8. doi: 10.1016/j.ccl.2013.09.009. [DOI] [PubMed] [Google Scholar]
- MALINDA KM, SIDHU GS, BANAUDHA KK, GADDIPATI JP, MAHESHWARI RK, GOLDSTEIN AL, KLEINMAN HK. Thymosin alpha 1 stimulates endothelial cell migration, angiogenesis, and wound healing. J Immunol. 1998;160:1001–1006. [PubMed] [Google Scholar]
- McNEILL E, CHANNON KM. The role of tetrahydrobiopterin in inflammation and cardiovascular disease. Thromb Haemostasis. 2012;108:832–839. doi: 10.1160/TH12-06-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENG X-M, HUANG XR, XIAO J, CHEN H, ZHONG X, CHUNG ACK, LAN HY. Diverse roles of TGF-β receptor II in renal fibrosis and inflammation in vivo and in vitro. J Pathol. 2012;227:175–188. doi: 10.1002/path.3976. [DOI] [PubMed] [Google Scholar]
- MURASAWA S, KAWAMOTO A, HORII M, NAKAMORI S, ASAHARA T. Niche-dependent translineage commitment of endothelial progenitor cells, not cell fusion in general, into myocardial lineage cells. Arterioscler Thromb Vasc Biol. 2005;25:1388–1394. doi: 10.1161/01.ATV.0000168409.69960.e9. [DOI] [PubMed] [Google Scholar]
- PARDALI E, SANCHEZ-DUFFHUES G, GOMEZ-PUERTO MC, TEN DIJKE P. TGF-β-induced endothelial-mesenchymal transition in fibrotic diseases. Int J Mol Sci. 18:2017. doi: 10.3390/ijms18102157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIERA-VELAZQUEZ S, LI Z, JIMENEZ SA. Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. American J Pathol. 2011;179:1074–1080. doi: 10.1016/j.ajpath.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIMENTEL M, ZIMERMAN LI, ROHDE LE. Stratification of the risk of sudden death in nonischemic heart failure. Arquivos Brasileiros De Cardiologia. 2014;103:348–357. doi: 10.5935/abc.20140125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTSON IB, RIFKIN DB. Regulation of the bioavailability of TGF-β and TGF-β-related proteins. Cold Spring Harb Perspect Biol. 2016;8 doi: 10.1101/cshperspect.a021907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMMAN TAHHAN A, HAMMADAH M, SANDESARA PB, HAYEK SS, KALOGEROPOULOS AP, ALKHODER A, MOHAMED KH, TOPEL M, GHASEMZADEH N, CHIVUKULA K, KO YA, AIDA H, HESAROIEH I, MAHAR ER, KIM JH, WILSON P, SHAW L, VACCARINO V, WALLER EK, QUYYUMI AA. Progenitor cells and clinical outcomes in patients with heart failure. Circ Heart Fail. 2017;10:e004106. doi: 10.1161/CIRCHEARTFAILURE.117.004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANDRI M, VIEHMANN M, ADAMS V, RABALD K, MANGNER N, HÖLLRIEGEL R, LURZ P, ERBS S, LINKE A, KIRSCH K, MÖBIUS-WINKLER S, THIERY J, TEUPSER D, HAMBRECHT R, SCHULER G, GIELEN S. Chronic heart failure and aging - effects of exercise training on endothelial function and mechanisms of endothelial regeneration: Results from the Leipzig Exercise Intervention in Chronic heart failure and Aging (LEICA) study. Eur J Prevent Cardiol. 2016;23:349–358. doi: 10.1177/2047487315588391. [DOI] [PubMed] [Google Scholar]
- SCHILLER M, JAVELAUD D, MAUVIEL A. TGF-beta-induced SMAD signaling and gene regulation: consequences for extracellular matrix remodeling and wound healing. J Dermatol Sci. 2004;35:83–92. doi: 10.1016/j.jdermsci.2003.12.006. [DOI] [PubMed] [Google Scholar]
- SHI Y, MASSAGUÉ J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/S0092-8674(03)00432-X. [DOI] [PubMed] [Google Scholar]
- SMART N, RISEBRO CA, CLARK JE, EHLER E, MIQUEROL L, ROSSDEUTSCH A, MARBER MS, RILEY PR. Thymosin β4 facilitates epicardial neovascularization of the injured adult heart. Ann NY Acad Sci. 2010;1194:97–104. doi: 10.1111/j.1749-6632.2010.05478.x. [DOI] [PubMed] [Google Scholar]
- SNIEGON I, PRIEß M, HEGER J, SCHULZ R, EULER G. Endothelial mesenchymal transition in hypoxic microvascular endothelial cells and paracrine induction of cardiomyocyte apoptosis are mediated via TGFβ1/SMAD signaling. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18112290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANG S, FAN C, IROEGBU CD, ZHOU W, ZHANG Z, WU M, CHEN W, WU X, PENG J, LI Z, YANG J. TMSB4 Overexpression enhances the potency of marrow mesenchymal stromal cells for myocardial repair. Front Cell Devel Biol. 2021;9:1381. doi: 10.3389/fcell.2021.670913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THEODORAKIS GN, FLEVARI P, KROUPIS C, ADAMOPOULOS S, LIVANIS EG, KOSTOPOULOU A, KOLOKATHIS F, PARASKEVAIDIS IA, LEFTHERIOTIS D, KREMASTINOS DT. Antiinflammatory effects of cardiac resynchronization therapy in patients with chronic heart failure. Pacing and clinical electrophysiology: PACE. 2006;29:255–261. doi: 10.1111/j.1540-8159.2006.00331.x. [DOI] [PubMed] [Google Scholar]
- WANG W, ZHANG Y, HUI H, TONG W, WEI Z, LI Z, ZHANG S, YANG X, TIAN J, CHEN Y. The effect of endothelial progenitor cell transplantation on neointimal hyperplasia and reendothelialisation after balloon catheter injury in rat carotid arteries. Stem Cell Res Ther. 2021;12:99. doi: 10.1186/s13287-021-02135-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XU J, LAMOUILLE S, DERYNCK R. TGF-β-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG J, FENG X, ZHOU Q, CHENG W, SHANG C, HAN P, LIN C-H, CHEN H-SV, QUERTERMOUS T, CHANG C-P. Pathological Ace2-to-Ace enzyme switch in the stressed heart is transcriptionally controlled by the endothelial Brg1-FoxM1 complex. Proc Natl Acad Sci U S A. 2016;113:E5628–E5635. doi: 10.1073/pnas.1525078113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG M, LI X, MORRIS JC, LIANG J, DESHMUKH AJ, HODGE D, LI Y, CHA Y-M. Outcomes of cardiac resynchronization therapy in patients with hypothyroidism and heart failure. BMC Cardiovasc Disord. 2020;20:424. doi: 10.1186/s12872-020-01693-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUCEL N, AXSOM J, YANG Y, LI L, RHOADES JH, ARANY Z. Cardiac endothelial cells maintain open chromatin and expression of cardiomyocyte myofibrillar genes. eLife. 2020;9:e55730. doi: 10.7554/eLife.55730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZEISBERG EM, TARNAVSKI O, ZEISBERG M, DORFMAN AL, MCMULLEN JR, GUSTAFSSON E, CHANDRAKER A, YUAN X, PU WT, ROBERTS AB, NEILSON EG, SAYEGH MH, IZUMO S, KALLURI R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- ZHANG B, LI D, LIU G, TAN W, ZHANG G, LIAO J. Impaired activity of circulating EPCs and endothelial function are associated with increased Syntax score in patients with coronary artery disease. Mol Med Rep. 2021a;23:1–12. doi: 10.3892/mmr.2021.11960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG W, LIANG J, HAN P. Cardiac cell type-specific responses to injury and contributions to heart regeneration. Cell Regen. 2021b;10:4. doi: 10.1186/s13619-020-00065-1. https://doi:10.1186/s13619-020-00065-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]