Summary
There are only few studies concerning about long-term effect of growth hormone (GH) replacement therapy on bone mineral density and bone microstructure. To assess effect of GH replacement therapy on bone mineral density (BMD) and trabecular bone score (TBS) in adult GH deficient (AGHD) subjects over period of 10 years. From 2005 to 2018, a prospective study of AGHD patients was conducted in national referral center for treatment of GHD. All patients received subcutaneous recombinant human GH in an IGF-1-normalizing regimen once a day. Lumbar spine (L-spine) and total hip (TH) BMD using Hologic densitometers were measured at baseline and every two years during treatment with rhGH. TBS was derived from L1–L4 DXA using iNsight® software (Medimaps, France) at each time point. Periods of measurement were baseline, year 2; 4; 6; 8 and 10. In total, 63 patients (38 males, 25 females, mean age 25.1±16 years) were included in the study. After 10 years of GH treatment, IGF-1 significantly increased (~35 %), with greatest increase at year 2. During 10-year follow-up, L-spine BMD increased approximately of 7 % (NS). TH BMD increase of 11 % during follow-up (p=0.0003). The greatest increment of BMD was achieved at year 6 on both sites, L-spine (+6 %) and TH BMD (+13 %) (p<0.05). There was no significant change of TBS during whole follow-up. In this study, sustaining positive effect of GH replacement therapy on bone density in subjects with adult GH deficiency over 10 years of follow-up was observed. The study did not show effect on TBS, as indirect measure of trabecular bone microarchitecture.
Keywords: Growth hormone, IGF-1, Bone mineral density, Trabecular bone score
Introduction
Growth hormone (GH) affects bone metabolism directly by binding to the GH receptor (GHR) or indirectly by insulin-like growth factor-1 (IGF-1). The anabolic effect of RH and IGF-1 is manifested by stimulation of bone proliferation, wherein linear bone growth is promoted by activation of epiphyseal pre-chondrocytes. In addition, osteoclasts are activated, stimulating thus bone resorption (Appelman-Dijkstra et al. 2014). This effect of GH is necessary to achieve peak bone mass (PBM) and appropriate trabecular bone microarchitecture during adolescence and early adulthood. PBM is highest after growth and later in life, bone mass declines (Kužma et al. 2019). IGF-1 together with RH plays a key role in the regulation of bone remodeling, longitudinal bone growth in childhood and appositional growth in adulthood. IGF-1 is thought to play a greater role in regulating bone size than RH (Lindsey and Mohan 2016).
A high prevalence of fractures has been observed in adult GH deficiency (AGHD), including GH deficiency with onset in childhood and adulthood. (Kristensen et al. 2012). Patients with child onset of GHD have a lower BMD in adulthood than patients with the adult onset of GHD, explained by insufficient PBM and the risk of bone fractures in these patients is higher in individuals with BMD below 1 SD for a given age. (Saggese et al. 1996).
The effect of GH replacement therapy on BMD in patients with AGHD was not observed in short-term studies. An increase in BMD is observed only in studies longer than 12–24 months (Clanget et al. 2001a, Elbornsson et al. 2012a, Elbornsson et al. 2012c, Kuzma et al. 2014). GH replacement therapy has been found to increase bone turnover and expand the bone remodeling space, a process that results in an initial decrease or no change in BMC and BMD among patients with organic GHD, followed by a subsequent time-related increase in bone densitometric endpoints. However, studies on the long-term effect of GH replacement therapy on BMD vary in the results.
One of the longest studies concerning the effect of RH treatment on bone to date, bone mineral content (BMC) and lumbar spine (LS) BMD were significantly higher after 15 years of treatment than before treatment and the largest increase was during the first 7–10 years, which may be explained by an increase in bone formation (Elbornsson et al. 2012a). In the metanalysis of Barake et al. (2018) authors among women with GHD and concurrent osteoporosis treatment did not proved GH replacement therapy effect on BMD in comparison to placebo but suggested GH replacement therapy effect on fracture risk reduction. Thus, effect of GH on bone microstructure could play a key role in fracture risk. In the most recent study (Yang et al. 2020), GHRT in 9 AOGHD subjects for 24 weeks led to increase of vBMD, cortical area and cortical thickness, as assessed by high resolution peripheral quantitative computed tomography (HR-pQCT) - a “gold standard” method for bone quality assessment. However, trabecular parameters were not improved. In the longer studies with trabecular bone score (TBS), an indirect measure of trabecular bone, controversial results were obtained (Kuzma et al. 2014, Allo Miguel et al. 2016).
In this study, long-term GH replacement therapy and its effect on BMD and TBS in patients with AGHD was assessed.
Patients and methods
From 2005 to 2018, a prospective study of GHD patients was conducted in national referral center for treatment of GHD. The study protocol was reviewed and approved by Comenius University and University Hospital research boards. The regional medical ethics committees in each center approved the study. All patients signed informed consent prior to the conduct of any study procedure. The diagnosis of GHD was confirmed by stimulation testing using the insulin tolerance test with hypoglycemia (ITT) according to current Endocrine Society guidelines (Molitch et al. 2006), which recommend a cutoff value for stimulated GH in ITT of 3 μg/l.
Study inclusion criteria were as follows:
Subjects with AO-GHD diagnosed according to the current guidelines of the Endocrine Society regardless of gender or etiology.
Stable replacement for other pituitary deficiencies, if present.
No present or history of treatment for osteoporosis with any antiresorptive drug.
No signs of pituitary tumor on pituitary gland MRI performed at the time of GHD diagnosis.
Growth hormone supplementation
All patients received subcutaneous recombinant human GH (rhGH) in an IGF-1-normalizing rhGH replacement regimen once a day. The initial rhGH dose was 0.2 or 0.3 mg and the dose was titrated according to the IGF-1. the average dose during follow-up was 0.35mg/day. A stable dose was reached at approximately month 6. Patients were treated for other pituitary deficiencies, and the efficacy of that treatment was monitored regularly (mostly twice yearly) by measurement of target hormone levels.
Outcome measures
Basic anthropometric measurements of height, weight, waist circumference, and body mass index (BMI) were assessed at baseline and at month 24 of treatment with rhGH. IGF-1 levels were assessed at every visit by theenzyme-labeled chemiluminescent immunometric assay in commercial laboratory. The IMMULITE® 2000 assay (interassay variability CV: 2.4–4.7 %) was used from 2005 to 2008 and the IMMULITE® 2500 assay (interassay variability CV: 2.4–4.7 %) was used from 2008. Given the two different IGF-1 standards, IGF-1 was calculated as the IGF-1 SDS score (Z-score) from a reference population, which is used by laboratory method.
Bone measures
Lumbar spine (LS), at L1–L4. and total hip (TH) BMD were measured at baseline and every two years during treatment with rhGH. BMD was measured using Hologic densitometers (Discovery and Horizon). When densitometry device was changed a cross calibration was performed to maintain continuity control of DXA scans. TBS was derived from L1–L4 DXA using iNsight® software (Medimaps, France) at each time point in retrospective manner.
All outcomes were measured at baseline, year 2; 4; 6; 8 and 10.
Statistical analysis
The statistical analysis was performed using the Analyse-It® for Microsoft Excel v5.40.2 software. Numeric data were expressed as means +/− standard deviations (SDs). The statistical significance of differences in TBS and BMD between different time points was tested by paired t-test. Between-group variances were assessed by one-way ANOVA or independent sample T-test. The BMD and TBS values were approximately normally distributed according to gender in a normality test.
Results
Baseline characteristics
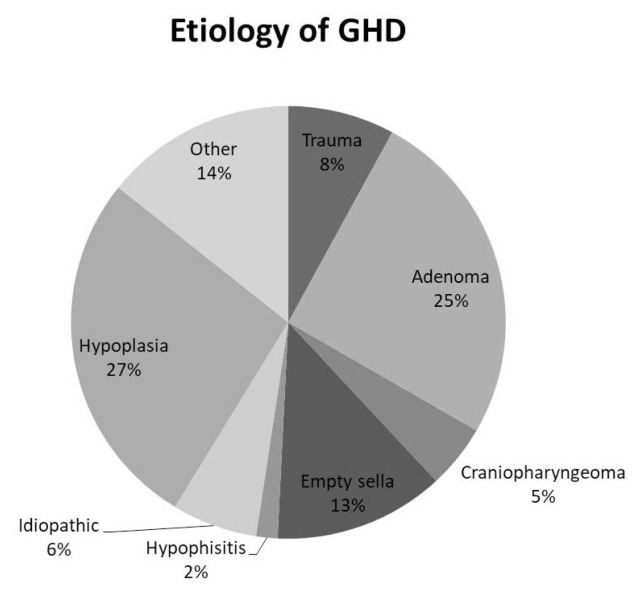
According to inclusion criteria, 63 patients (38 males, 25 females, mean age 25.1±16 years) were included in the study group. The basic demographic and baseline characteristics are shown in Table 1. Most of the subjects, 28 (44 %) had 3 other pituitary deficiencies. Average IGF-1 at baseline was 111.45±74.02 ug/l with greater values among males in comparison to females (127.49 vs 83.8. p<0.05). Males had higher values of TH BMD and TBS than females (p<0.05). The most prevalent etiology of GHD was pituitary hypoplasia and pituitary adenoma (Fig. 1).
Table 1.
Baseline study group characteristics. Differences between males and females.
| Baseline characteristics | All (N=63) | Males (n=38) | Females (n=25) |
|---|---|---|---|
| Age (years) | 25.1 ± 16 | 23.76 ± 14.86 | 27.04 ± 17.72 |
| weight (kg) | 79.81 ± 15.99 | 81.9 ± 14.18 | 75.91 ± 18.83 |
| height (cm) | 169.1 ± 11.2 | 175.71 ± 7.18 | 159.06 ± 8.70 |
| waist circum. (cm) | 96.45 ± 11.03 | 97.75 ± 9.12 | 95 ± 13.25 |
| BMI (kg/m2) | 27.34 ± 5.30 | 26.44 ± 4.55 | 29.04 ± 6.29 |
| IGF1 (μg/l) | 111.45 ± 74.02 | 127.49 ± 79.38* | 83.84 ± 55.43* |
| TSH (mIU/l) | 3.83 ± 19.80 | 5.66 ± 25.21 | 0.93 ± 1.47 |
| fT4 (IU/l) | 14.68 ± 5.58 | 14.43 ± 4.92 | 15.07 ± 6.57 |
| ACTH (ng/l) | 21.08 ± 15.81 | 22.24 ± 15.28 | 19.57 ± 16.97 |
| LH (IU/l) | 3.83 ± 6.54 | 2.48 ± 3.98 | 5.80 ± 8.88 |
| FSH (IU/l) | 5.77 ± 12.56 | 2.75 ± 4.11 | 10.21 ± 18.54 |
| PRL (μg/l) | 45.98 ± 198.51 | 15.32 ± 17.62 | 90.28 ± 309.36 |
| N of AO / CO - GHD | 35/28 | 21/17 | 14/11 |
| Isolated GHD (number) | 2 | 2 | 0 |
| N of pts with 1 other (than GHD) pituitary deficiency | 11 | 6 | 5 |
| N of pts with 2 other (than GHD) pituitary deficiencies | 15 | 5 | 10 |
| N of pts with 3 other (than GHD) pituitary deficiencies | 28 | 21 | 7 |
| N of pts with panhypopituitarism | 7 | 4 | 3 |
| BMD LS (g/cm 2 ) | 0.9660 ± 0.1774 | 0.9584 ± 0.1090 | 0.9789 ± 0.2637 |
| BMD TH (g/cm 2 ) | 0.8906 ± 0.1341 | 0.9324 ± 0.1117 * | 0.8071 ± 0.1439* |
| TBS | 1.38 ± 0.09 | 1.43 ± 0.05 * | 1.35 ± 0.10* |
| vitamin D (μg/l) | 23.92 ± 11.13 | 24.98 ± 9.01 | 21.64 ± 15.57 |
| Ca (mmol/l) | 2.38 ± 0.11 | 2.39 ± 0.13 | 2.37 ± 0.06 |
| P (mmol/l) | 1.26 ± 0.18 | 1.26 ± 0.18 | 1.25 ± 0.17 |
| PTH (pmol/l) | 5.42 ± 4.81 | 3.87 ± 1.63 | 7.55 ± 6.84 |
p ≤ 0.05 males vs. Females. Continous data are expressed as value ± SD.
Fig. 1.
Etiology of GHD
Follow-up
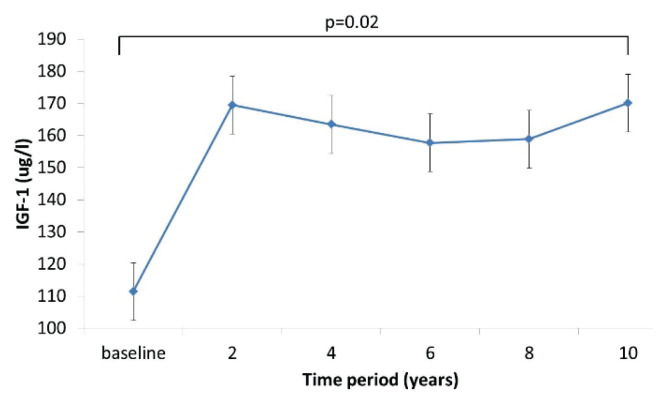
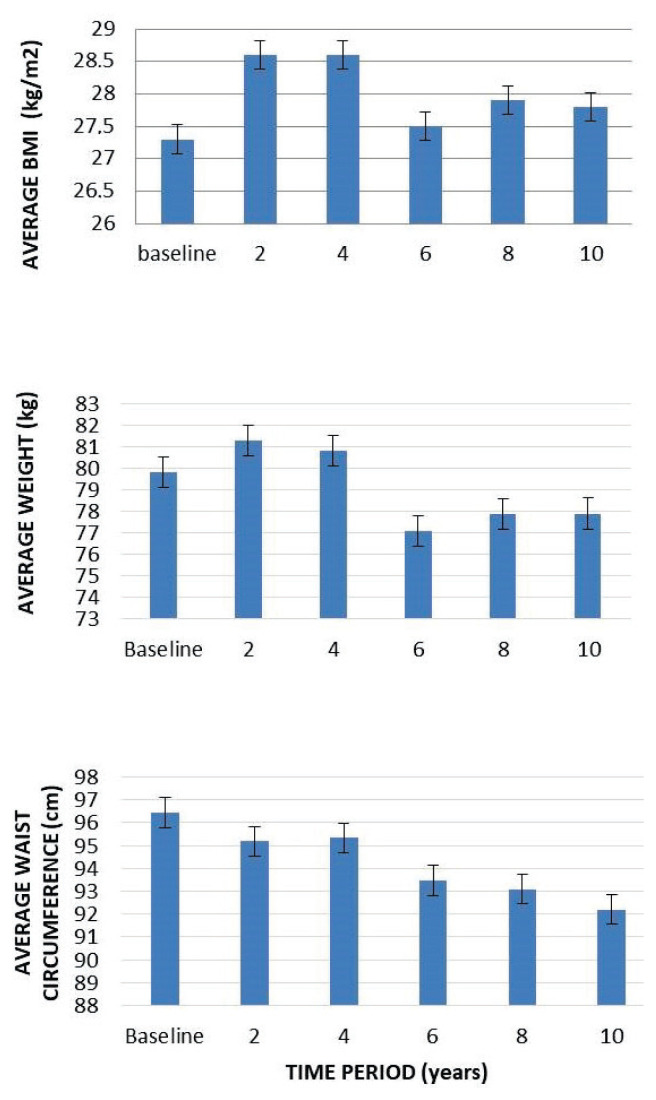
After 10 years of rhGH treatment, IGF-1 significantly increased (~35 %), with greatest increase at year 2 (Fig. 2). During follow up, up to 4th year initial trend to increase in body weight and BMI was observed. Later, body weight and BMI decreased and remained stable for whole follow-up. Waist circumference gradually decreased during whole follow-up (Fig. 3).
Fig. 2.
IGF-1 levels during follow-up. Initial higher increase of IGF-1 is associated with rhGH replacement start.
Fig. 3.
Change in anthropomoetric measures during follow-up.
Change in bone measures
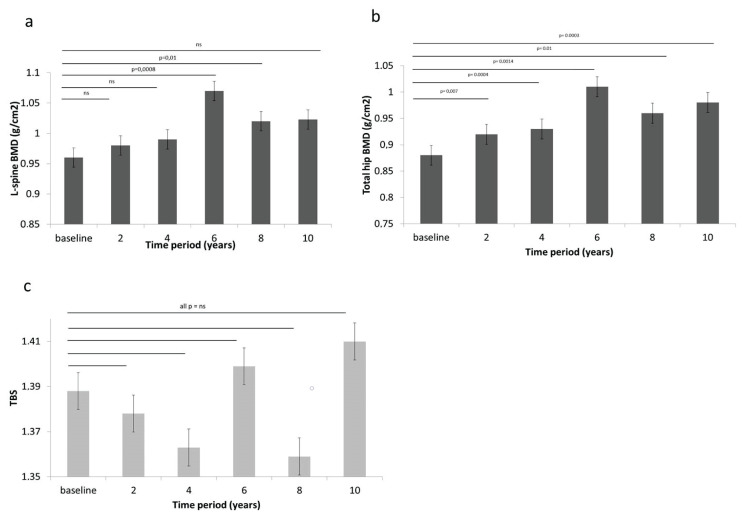
During 10-year follow-up L-spine BMD increased approximately of 7 % (NS). The greatest increment of L-spine BMD was observed during baseline and year 6 (+11 %; p<0.001), followed by decline in next 4 years (Fig. 4a). TH BMD increase of 11 % during follow-up (p<0.001 with greatest increment at year 6 (13 %; p=0.001) was observed. Increment of TH BMD was significant during whole follow-up (Fig. 4b). TBS increase of 1.5 % was observed. There was no significant change of TBS during whole follow-up (Fig. 4c).
Fig. 4.
Change in bone measures during treatment with rhGH. a) L-spine BMD b) TH BMD c) TBS
Discussion
GH replacement has demonstrated its efficacy in reducing cardiovascular morbidity and mortality (Rosén et al. 1993), improving lipid profile (de Boer et al. 1994), changing body composition (Colson et al. 2006) and increasing quality of life (Arwert et al. 2005b). Prior studies document that GH treatment improves BMD (Biermasz et al. 2001, Clanget et al. 2001b, Davidson et al. 2004, Arwert et al. 2005a, Rota et al. 2008, Conway et al. 2009, Jorgensen et al. 2011, Elbornsson et al. 2012b, Elbornsson et al. 2012d) and reduces fracture risk (Finkelstein et al. 1992, Rosen et al. 1997). However, only few studies are longer than 5 years of follow-up.
In this study, during ten years of GH replacement therapy in IGF-1 normalizing regimen, significant effect on BMD was observed. BMD increase of 7 % and 11 % at L-spine and TH was observed, respectively. The greatest increase in BMD on both sites at 6 year was achieved. However, no effect on TBS during follow-up was proven. Interestingly, significant positive effect on body composition, as represented by decrease in BMI, weight and waist circumference was observed.
Although, significant improvement in BMD was observed in studies up to 24 months of duration, (Clanget et al. 2001a, Elbornsson et al. 2012a, Elbornsson et al. 2012c, Kuzma et al. 2014) longer studies vary in results on different measured sites. However, a similar pattern with greatest increase after 5–7 years of GH replacement therapy followed by plateau was observed (Gotherstrom et al. 2007, Elbornsson et al. 2012a, Appelman-Dijkstra et al. 2014) in accordance to results presented in this study.
This can be explained by the fact that the GH dose was gradually adjusted during the studies and it can be assumed that the GH dose at the end of the study was not sufficient to maintain the increase in BMD. In a study of the 15 years GH replacement therapy effect they reached BMD values as at the beginning of treatment (Elbornsson et al. 2012a) supporting the fact that patients with the best response to treatment, i.e. with the most pronounced increase in BMC and BMD, also had the greatest increase in serum IGF-1 and received the highest doses of rhGH.
The positive effect of GH on BMD in the lumbar spine may not be associated with the same increase in bone microarchitecture (Allo Miguel et al. 2016). At present, the prevailing opinion is that bone quality is more important in the development of fractures than BMD, and microarchitecture, geometry and mineral composition of the bone matrix are considered to be the primary determinants of bone strength (Lindsey and Mohan 2016, Kuzma et al. 2017).
The gold standard in the evaluation of bone quality is currently bone biopsy, which is subsequently evaluated by histomorphometry and high-resolution peripheral quantitative CT (HR-pQCT) (McCloskey et al. 2016). The disadvantages of these tests are invasiveness (in the case of a bone biopsy) and availability. However, there are also methods that use a commonly available bone densitometer (DXA) and can provide information on bone microarchitecture (McCloskey et al. 2016). One of the most commonly used is the trabecular bone score (TBS) method. More recently, DXA scans can also be used to assess bone microarchitecture in the proximal femur, the method is called 3D-Shaper (formerly 3D-DXA). TBS provides us with information on trabecular bone microarchitecture, and in some forms of secondary osteoporosis, TBS appears to be a more sensitive marker of fracture than BMD.
GH and IGF-1 are considered positive predictors of bone microarchitecture, while GH is a positive determinant of trabecular thickness and IGF-1 is a positive predictor of cortical bone structure (Bredella et al. 2012). On the contrary, the results of biopsies from the study from 2005 suggest that RH affects cortical bone more. In another study of men with AO-GHD treated with GH, there was a significant increase in BMD over five years, but the increase in TBS was only negligible. Qualitative evaluation by histomorphometry showed endosteal and periosteal formation, from which it can be concluded that RH had a greater effect on cortical bone than on trabecular bone (Bravenboer et al. 2005) (Bravenboer et al. 1996).
In the subset of 32 AGHD subjects in our previous study after 2 years of GH replacement therapy a significant increase in TBS was proven (Kuzma et al. 2014). In contrast, in a study by Allo Miguel et al. after seven years of RH treatment, there was no increase in TBS, but with increase of L-spine BMD (Allo Miguel et al. 2016). Although, study is significantly limited in the number of patients, including only 18 patients with AO-GHD (Allo Miguel et al. 2016), the results are very similar to our study. Some information, albeit only short-term, brings the most recent study with HR-pQCT in 9 AOGHD treated with rhGH for 24 weeks. An improvement in cortical area, but not trabecular bone was observed. Clearly, the effect on bone structure is not only the result of RH treatment, but also of other factors such as the treatment of hypogonadism, secondary adrenal insufficiency and central hypothyroidism, which are often associated with RH deficiency. Vitamin D status is also considered an important factor influencing bone metabolism in GHD (Kužma et al. 2016), although the results of studies evaluating the relationship between vitamin D and bone metabolism vary.
This study has several limitations, such as sample size and absent control group. However, the follow-up is exceptionally long and patients were regularly closely monitored in referral center, which allowed us to keep the group homogenous.
In conclusion, this study showed sustaining positive effect of GH replacement therapy on BMD in AGHD subjects over 10 years of follow-up. The study did not show effect on TBS, as indirect measure of trabecular bone microarchitecture. However, controlled studies with greater sample size are needed to prove effect of GH on bone structure. Overall, it is likely that GH replacement therapy led to decrease of fracture risk in AGHD.
Acknowledgement
Grant support: OPVaI ITMS2014+: 313012Q751
Footnotes
Conflict of Interest
There is no conflict of interest.
References
- ALLO MIGUEL G, SERRACLARA PLA A, PARTIDA MUNOZ ML, MARTINEZ DIAZ-GUERRA G, HAWKINS F. Seven years of follow up of trabecular bone score, bone mineral density, body composition and quality of life in adults with growth hormone deficiency treated with rhGH replacement in a single center. Ther Adv Endocrinol Metab. 2016;7:93–100. doi: 10.1177/2042018816643908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APPELMAN-DIJKSTRA NM, CLAESSEN KM, HAMDY NA, PEREIRA AM, BIERMASZ NR. Effects of up to 15 years of recombinant human GH (rhGH) replacement on bone metabolism in adults with growth hormone deficiency (GHD): the Leiden Cohort Study. Clin Endocrinol (Oxf) 2014;81:727–735. doi: 10.1111/cen.12493. [DOI] [PubMed] [Google Scholar]
- ARWERT LI, DEIJEN JB, MULLER M, DRENT ML. Long-term growth hormone treatment preserves GH-induced memory and mood improvements: a 10-year follow-up study in GH-deficient adult men. Horm Behav. 2005a;47:343–349. doi: 10.1016/j.yhbeh.2004.11.015. [DOI] [PubMed] [Google Scholar]
- BARAKE M, ARABI A, NAKHOUL N, EL-HAJJ FULEIHAN G, EL GHANDOUR S, KLIBANSKI A, TRITOS NA. Effects of growth hormone therapy on bone density and fracture risk in age-related osteoporosis in the absence of growth hormone deficiency: a systematic review and meta-analysis. Endocrine. 2018;59:39–49. doi: 10.1007/s12020-017-1440-0. [DOI] [PubMed] [Google Scholar]
- BIERMASZ NR, HAMDY NA, JANSSEN YJ, ROELFSEMA F. Additional beneficial effects of alendronate in growth hormone (GH)-deficient adults with osteoporosis receiving long-term recombinant human GH replacement therapy: a randomized controlled trial. J Clin Endocrinol Metab. 2001;86:3079–3085. doi: 10.1210/jcem.86.7.7669. [DOI] [PubMed] [Google Scholar]
- BRAVENBOER N, HOLZMANN P, De BOER H, BLOK GJ, LIPS P. Histomorphometric analysis of bone mass and bone metabolism in growth hormone deficient adult men. Bone. 1996;18:551–557. doi: 10.1016/8756-3282(96)00069-5. [DOI] [PubMed] [Google Scholar]
- BRAVENBOER N, HOLZMANN PJ, TER MAATEN JC, STUURMAN LM, ROOS JC, LIPS P. Effect of long-term growth hormone treatment on bone mass and bone metabolism in growth hormone-deficient men. J Bone Miner Res. 2005;20:1778–1784. doi: 10.1359/JBMR.050613. [DOI] [PubMed] [Google Scholar]
- BREDELLA MA, LIN E, GERWECK AV, LANDA MG, THOMAS BJ, TORRIANI M, BOUXSEIN ML, MILLER KK. Determinants of bone microarchitecture and mechanical properties in obese men. J Clin Endocrinol Metab. 2012;97:4115–4122. doi: 10.1210/jc.2012-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLANGET C, SECK T, HINKE V, WUSTER C, ZIEGLER R, PFEILSCHIFTER J. Effects of 6 years of growth hormone (GH) treatment on bone mineral density in GH-deficient adults. Clin Endocrinol (Oxf) 2001a;55:93–99. doi: 10.1046/j.1365-2265.2001.01284.x. [DOI] [PubMed] [Google Scholar]
- CLANGET C, SECK T, HINKE V, WUSTER C, ZIEGLER R, PFEILSCHIFTER J. Effects of 6 years of growth hormone (GH) treatment on bone mineral density in GH-deficient adults. Clin Endocrinol (Oxf) 2001b;55:93–99. doi: 10.1046/j.1365-2265.2001.01284.x. [DOI] [PubMed] [Google Scholar]
- COLSON A, BROOKE AM, WALKER D, BESSER GM, CHEW SL, GROSSMAN AB, JENKINS PJ, DRAKE WM, MONSON JP. Growth hormone deficiency and replacement in patients with treated Cushing’s Disease, prolactinomas and non-functioning pituitary adenomas: effects on body composition, glucose metabolism, lipid status and bone mineral density. Horm Res. 2006;66:257–267. doi: 10.1159/000095168. [DOI] [PubMed] [Google Scholar]
- CONWAY GS, SZARRAS-CZAPNIK M, RACZ K, KELLER A, CHANSON P, TAUBER M, ZACHARIN M. Treatment for 24 months with recombinant human GH has a beneficial effect on bone mineral density in young adults with childhood-onset GH deficiency. Eur J Endocrinol. 2009;160:899–907. doi: 10.1530/EJE-08-0436. [DOI] [PubMed] [Google Scholar]
- DAVIDSON P, MILNE R, CHASE D, COOPER C. Growth hormone replacement in adults and bone mineral density: a systematic review and meta-analysis. Clin Endocrinol (Oxf) 2004;60:92–98. doi: 10.1111/j.1365-2265.2004.01935.x. [DOI] [PubMed] [Google Scholar]
- De BOER H, BLOK GJ, VOERMAN HJ, PHILLIPS M, SCHOUTEN JA. Serum lipid levels in growth hormone-deficient men. Metabolism. 1994;43:199–203. doi: 10.1016/0026-0495(94)90245-3. [DOI] [PubMed] [Google Scholar]
- ELBORNSSON M, GOTHERSTROM G, BOSAEUS I, BENGTSSON BA, JOHANNSSON G, SVENSSON J. Fifteen years of GH replacement increases bone mineral density in hypopituitary patients with adult-onset GH deficiency. Eur J Endocrinol. 2012;166:787–795. doi: 10.1530/EJE-11-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINKELSTEIN JS, NEER RM, BILLER BM, CRAWFORD JD, KLIBANSKI A. Osteopenia in men with a history of delayed puberty. N Engl J Med. 1992;326:600–604. doi: 10.1056/NEJM199202273260904. [DOI] [PubMed] [Google Scholar]
- GOTHERSTROM G, BENGTSSON BA, BOSAEUS I, JOHANNSSON G, SVENSSON J. Ten-year GH replacement increases bone mineral density in hypopituitary patients with adult onset GH deficiency. Eur J Endocrinol. 2007;156:55–64. doi: 10.1530/eje.1.02317. [DOI] [PubMed] [Google Scholar]
- JORGENSEN AP, FOUGNER KJ, UELAND T, GUDMUNDSEN O, BURMAN P, SCHREINER T, BOLLERSLEV J. Favorable long-term effects of growth hormone replacement therapy on quality of life, bone metabolism, body composition and lipid levels in patients with adult-onset growth hormone deficiency. Growth Horm IGF Res. 2011;21:69–75. doi: 10.1016/j.ghir.2011.01.001. [DOI] [PubMed] [Google Scholar]
- KRISTENSEN E, HALLGRIMSSON B, MORCK DW, BOYD SK. Microarchitecture, but not bone mechanical properties, is rescued with growth hormone treatment in a mouse model of growth hormone deficiency. Int J Endocrinol. 2012;2012:294965. doi: 10.1155/2012/294965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUZMA M, JACKULIAK P, KILLINGER Z, VANUGA P, PAYER J. Issues related to secondary osteoporosis associated with growth hormone deficiency in adulthood (in Slovak) Vnitr Lek. 2017;63:658–661. doi: 10.36290/vnl.2017.128. [DOI] [PubMed] [Google Scholar]
- KUZMA M, KUZMOVA Z, ZELINKOVA Z, KILLINGER Z, VANUGA P, LAZUROVA I, TOMKOVA S, PAYER J. Impact of the growth hormone replacement on bone status in growth hormone deficient adults. Growth Horm IGF Res. 2014;24:22–28. doi: 10.1016/j.ghir.2013.12.001. [DOI] [PubMed] [Google Scholar]
- KUŽMA M, BINKLEY N, BEDNÁROVÁ A, KILLINGER Z, VAŇUGA P, PAYER J. TRABECULAR BONE SCORE CHANGE DIFFERS WITH REGARD TO 25(OH)D LEVELS IN PATIENTS TREATED FOR ADULT-ONSET GROWTH HORMONE DEFICIENCY. Endocr Pract. 2016;22:951–958. doi: 10.4158/EP151183.OR. [DOI] [PubMed] [Google Scholar]
- KUŽMA M, KILLINGER Z, JACKULIAK P, VAŇUGA P, HANS D, BINKLEY N, PAYER J. Pathophysiology of growth hormone secretion disorders and their impact on bone microstructure as measured by trabecular bone score. Physiol Res. 2019;68:S121–S129. doi: 10.33549/physiolres.934303. [DOI] [PubMed] [Google Scholar]
- LINDSEY RC, MOHAN S. Skeletal effects of growth hormone and insulin-like growth factor-I therapy. Mol Cell Endocrinol. 2016;432:44–55. doi: 10.1016/j.mce.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCLOSKEY EV, ODEN A, HARVEY NC, LESLIE WD, HANS D, JOHANSSON H, BARKMANN R, BOUTROY S, BROWN J, CHAPURLAT R, ELDERS PJM, FUJITA Y, GLUER CC, GOLTZMAN D, IKI M, KARLSSON M, KINDMARK A, KOTOWICZ M, KURUMATANI N, KWOK T, LAMY O, LEUNG J, LIPPUNER K, LJUNGGREN O, LORENTZON M, MELLSTROM D, MERLIJN T, OEI L, OHLSSON C, PASCO JA, RIVADENEIRA F, ROSENGREN B, SORNAY-RENDU E, SZULC P, TAMAKI J, KANIS JA. A Meta-Analysis of Trabecular Bone Score in Fracture Risk Prediction and Its Relationship to FRAX. J Bone Miner Res. 2016;31:940–948. doi: 10.1002/jbmr.2734. [DOI] [PubMed] [Google Scholar]
- MOLITCH ME, CLEMMONS DR, MALOZOWSKI S, MERRIAM GR, SHALET SM, VANCE ML, STEPHENS PA. Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2006;91:1621–1634. doi: 10.1210/jc.2005-2227. [DOI] [PubMed] [Google Scholar]
- ROSÉN T, EDÉN S, LARSON G, WILHELMSEN L, BENGTSSON BA. Cardiovascular risk factors in adult patients with growth hormone deficiency. Acta Endocrinol (Copenh) 1993;129:195–200. doi: 10.1530/acta.0.1290195. [DOI] [PubMed] [Google Scholar]
- ROSEN T, WILHELMSEN L, LANDIN-WILHELMSEN K, LAPPAS G, BENGTSSON BA. Increased fracture frequency in adult patients with hypopituitarism and GH deficiency. Eur J Endocrinol. 1997;137:240–245. doi: 10.1530/eje.0.1370240. [DOI] [PubMed] [Google Scholar]
- ROTA F, SAVANELLI MC, TAUCHMANOVA L, SAVASTANO S, LOMBARDI G, COLAO A, DI SC. Bone density and turnover in young adult patients with growth hormone deficiency after 2-year growth hormone replacement according with gender. J Endocrinol Invest. 2008;31:94–102. doi: 10.1007/BF03345574. [DOI] [PubMed] [Google Scholar]
- SAGGESE G, BARONCELLI GI, BERTELLONI S, BARSANTI S. The effect of long-term growth hormone (GH) treatment on bone mineral density in children with GH deficiency. Role of GH in the attainment of peak bone mass. J Clin Endocrinol Metab. 1996;81:3077–3083. doi: 10.1210/jcem.81.8.8768878. [DOI] [PubMed] [Google Scholar]
- YANG H, YAN K, XU Y, WANG L. Effects of 24 weeks of growth hormone treatment on bone microstructure and volumetric bone density in patients with childhood-onset adult GH deficiency. Int J Endocrinol. 2020:9201979. doi: 10.1155/2020/9201979. [DOI] [PMC free article] [PubMed] [Google Scholar]