Abstract
Idiopathic pulmonary fibrosis (IPF) is a chronic interstitial lung disease. The pathogenesis of IPF is not completely understood. However, numerous genes are associated with the development and progression of pulmonary fibrosis, indicating there is a significant genetic component to the pathogenesis of IPF. Epigenetic influences on the development of human disease, including pulmonary fibrosis, remain to be fully elucidated. In this paper, we identify miR-338-3p as a microRNA severely downregulated in the lungs of patients with pulmonary fibrosis and in experimental models of pulmonary fibrosis. Treatment of primary human lung fibroblasts with miR-338-3p inhibits myofibroblast differentiation and matrix protein production. Published and proposed targets of miR-338-3p such as TGFβ receptor 1, MEK/ERK 1/2, Cdk4, and Cyclin D are also not responsible for the regulation of pulmonary fibroblast behavior by miR-338-3p. miR-338-3p inhibits myofibroblast differentiation by preventing TGFβ-mediated downregulation of phosphatase and tensin homolog (PTEN), a known antifibrotic mediator.
Keywords: fibroblast, lung, miRNA, pulmonary fibrosis
INTRODUCTION
Idiopathic pulmonary fibrosis (IPF) is a progressive interstitial lung disease affecting ∼3 million people worldwide (1). Although the pathogenesis of IPF is not entirely established, the current paradigm postulates that repeated epithelial injury leads to sustained and aberrant wound healing resulting in the excess deposition of matrix proteins in the interstitial space, thickened alveolar walls and impaired gas exchange (2).
In the interstitium, the primary cell type responsible for matrix production and secretion is the fibroblast (3). Fibroblasts are active participants in the homeostatic wound-healing process. In response to injury, fibroblasts differentiate into contractile, α smooth muscle (αSMA)-expressing myofibroblasts (4). As a part of the resolution process, myofibroblasts undergo apoptosis allowing for a return to homeostasis. In fibrosis, there is an abundance of myofibroblasts, which become resistant to apoptosis, and the wound healing process fails to resolve (5–7).
Transforming growth factor β (TGFβ) is a master regulator of fibrosis and induces myofibroblast differentiation (8). TGFβ affects a variety of cellular processes including matrix protein production, proliferation, apoptosis, and glycolysis (9–12). Our previous work demonstrates TGFβ induces a glycolytic shift, resulting in the induction of lactate dehydrogenase A (LDHA) (13). LDHA is required for myofibroblast differentiation, as well as to the development of pulmonary fibrosis in animal models (14–16). Available LDHA inhibitors are highly toxic and narrow therapeutic windows limit their use beyond the bench. To effectively target LDHA and minimize toxicity, we began to explore how microRNAs (miRNAs) may be a novel mechanism for regulating LDHA.
MiRNAs are small, noncoding RNAs that utilize seed sequence of 2–8 base pairs to bind to the 3′-untranslated region (UTR) of an mRNA transcript and induce target inhibition or degradation (17). This enables microRNAs to potentially target hundreds of transcripts and regulate multiple pathways. The biological role of many miRNAs remains unclear, particularly within the context of human disease. In addition, the function of specific miRNAs varies widely between tissues and cell types. MiRNA function is also altered by aging, cellular environment, and transcript abundance (18).
Using data published by Schulz et al. (19), we screened for miRNAs that were downregulated in lung tissue of patients with IPF (20), hypothesizing that miRNAs with antifibrotic potential would be downregulated in disease state. We then cross-referenced this list with miRNAs that were predicted to negatively regulate LDHA (21, 22). miR-338-3p is downregulated in IPF and predicted to target LDHA. We hypothesized miR-338-3p would block myofibroblast differentiation via inhibition of LDHA. Our data demonstrate miR-338-3p does indeed inhibit TGFβ-induced myofibroblast differentiation and matrix protein secretion. In addition, miR-338-3p inhibits the fibroblast proliferation in response to TGFβ. However, it does not inhibit LDHA in primary human lung fibroblasts. We explored other potential mechanisms including the inhibition of other glycolytic enzymes such as PKM2, PFKFB3, and PFKFB4. We also investigated if miR-338-3p blocked canonical and noncanonical TGFβ signaling and matrix protein regulators. Published literature indicated miR-338-3p blocked cyclin-associated proteins, leading us to assess the expression profile of several cell cycle proteins. Ultimately, we identified miR-338-3p inhibits myofibroblast differentiation in part through the induction of phosphatase and tensin homolog (PTEN) and the inhibition of a negative regulator of PTEN, PREX2. Our data provide novel information about the mechanism of action of miR-338-3p within the context of human lung disease. In addition, this contribution highlights the cell- and tissue-specific functions of miR-338-3p.
MATERIALS AND METHODS
Cell Culture and Reagents
Primary human lung fibroblasts were isolated from biopsy as previously described (23). The diagnosis of IPF was made on the basis of the American Thoracic Society (ATS) consensus statement including the identification of definite or probable usual interstitial pneumonia (UIP) on biopsy. All donors gave written informed consent. Cells were cultured with 10% FBS (Sigma-Aldrich), 1% l-glutamine and 1% antibiotic-antimycotic in Eagle’s minimum essential media (Gibco). RNA was harvested with QIAzol (Qiagen), and protein was harvested in a 2% SDS-Tris solution. Smartpool nontargeting control siRNA and PTEN siRNA were purchased from Dharmacon and utilized at a dose of 100 nM. Transfections were performed with X-treme Gene siRNA transfection reagent (Millipore-Sigma) in basal media without added antibiotics. TGFβ was purchased from R&D Systems and was used at a dose of 1 ng/mL. Nontargeting microRNA constructs and miR-338-3p were purchased from Thermo Fisher Scientific and MyBiosource, respectively.
Single Transfections
For microRNA transfections, fibroblasts were pretreated with 1 ng/mL TGFβ 6 h before transfection. Transfections were performed in basal MEM without added antibiotics and X-treme Gene siRNA Transfection Reagent. The transfection reagent and miRNA construct were incubated for 20 min in basal media before the mixture was applied to cells. MicroRNA was used at a final volume of 200 nM in each well. Single siRNA transfections were performed with the same methods, however, using a final concentration of 100 nM per well.
Dual Transfections
When microRNAs and siRNAs were used concurrently, both were used at a final dose of 100 nM, such that the total amount of genetic material used never exceeded 200 nM, which is the same total concentration of microRNA utilized in previous experiments. We assessed cytotoxicity (Fig. 4, C and D) and there was no cytotoxicity associated with a dose of 200 nM microRNA. To perform the double transfections, the Dharmacon DharmaFECT Duo Transfection Reagent was used, which is a reagent specifically designed for the delivery of multiple constructs concurrently. Manufacturer’s instructions were followed. Cells were harvested 72 h after transfection.
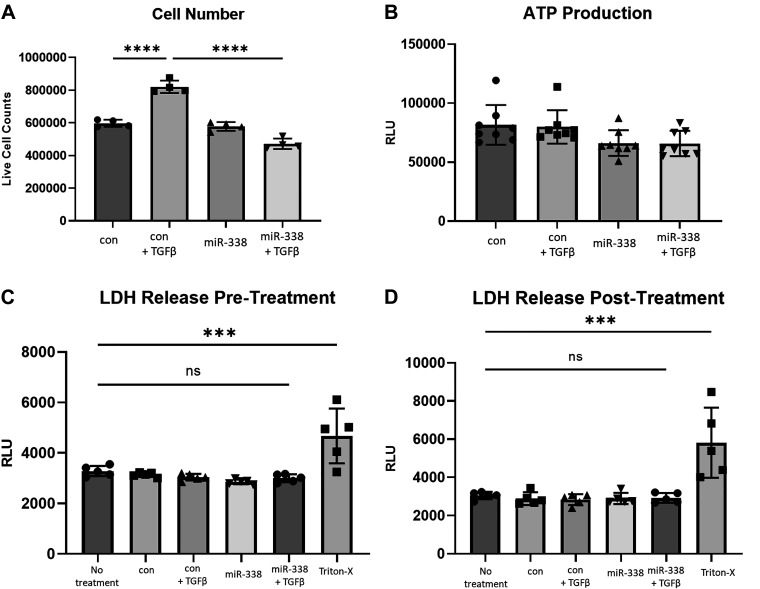
Figure 4.
miR-338-3p blocks TGFβ-induced matrix proliferation. Primary human lung fibroblasts from three different donors were treated with 200 nM nontargeting control microRNA or miR-338-3p ±1 ng/mL TGFβ. A: 72 h after treatment cells were counted utilizing trypan blue, only live cells were counted, n = 4/group. B: ATP production was assessed by cleavage of a luminescent substrate 72 h after treatment, n = 8/group. Cell death was assessed via LDH release prior to treatment (C) and 72 h after treatment (D), n = 5/group (***P < 0.005, ****P < 0.0001 by ANOVA).
Animal Exposures and Tissue Processing
Six- to eight-week-old male C57BL/6 mice were treated with vehicle or bleomycin by oropharyngeal aspiration. After 21 days, the animals were euthanized and lung tissue was collected (16). Another cohort of C57BL/6 mice was exposed to a single dose of 5 Gy total body and 10 Gy thoracic radiation to induce pulmonary fibrosis. After 26 wk, the animals were euthanized and lung tissue was harvested (24). All animal work was performed under the proper review and approval of the IACUC.
Reverse Transcription-Quantitative Real-Time PCR
RNA was isolated from human lung fibroblasts and mouse lung tissue as previously described (25). Reverse transcription and RT-PCR amplification was conducted with iScript cDNA synthesis kit (Bio-Rad) and SYBR Green (Bio-Rad), respectively. PCR for detection of miR-338-3p and U6 was performed by utilization of the TaqMan Reverse Transcription and Taqman microRNA PCR assay kits combined with TaqMan universal PCR mix, according to manufacturer’s instructions (Applied Biosciences). Pre- and postamplification primers for miR-338-3p and U6 were purchased from Applied Biosciences. All other primers were designed using Integrated DNA Technologies (IDT) and sequences are listed in Supplemental Table S1 (all Supplemental Material is available at https://doi.org/10.6084/m9.figshare.16867312).
Western Blotting and Slot Blotting
Cell lysates were run on an SDS-PAGE gel and transferred using the Trans-Blot Turbo Transfer System (Bio-Rad). Western blots were blocked using EveryBlot Blocking Buffer (Bio-Rad) and imaged using the Chemidoc MP fluorescent imager (Bio-Rad). Western blots were quantified utilizing Image Lab Software 6.1 (Bio-Rad). Western blots were not stripped and reprobed; however, a strategic use of antibodies produced in different species and multiple fluorophores allowed for multiplexed analysis of singular western blots. For slot blots, supernatants were collected 72 h after treatment. For each sample, 5-µL supernatant was diluted into 195-µL PBS. These samples were run through a slot blot apparatus (Bio-Rad) with a PVDF membrane and two pieces of filter paper for 5 min, followed by three washes with 1× PBS. The membrane was then blocked with 5% milk in TBST for 1 h and probed like a Western blot. All primary antibodies used are listed in Supplemental Table S2. Mouse and rabbit starbright antibodies (with wavelengths of 520 and 700, respectively) were used at a dilution of 1:10,000 (Bio-Rad). All raw Western blot images are provided in the supplement and are paired with the corresponding quantification.
Cell Counts
Primary human lung fibroblasts were seeded on 24-well plates (n = 4/group) and given 24 h to adhere before treatment. Fibroblasts were transfected with a nontargeting microRNA or miR-338-3p as described and treated with 1 ng/mL TGFβ where indicated. Seventy-two hours after transfection, the cells were harvested with trypsin, incubated with trypan blue, and counted using the Biorad TC20 Automated Cell Counter, utilizing the same gating settings across all samples. Only live cell counts were used in data analysis; viability was over 90% across all samples.
ATP Production Assay
ATP production was assessed using the Cell Titer-Glo Kit from Promega. Primary human lung fibroblasts were plated in 96-well plates and were transfected with a nontargeting microRNA or miR-338-3p as described and/or treated with 1 ng/mL TGFβ. After 72 h, a cell lysis solution containing a proprietary oxyluciferase substrate was introduced. Cells are incubated at room temperature with gentle mixing to ensure complete lysis. Luminescence was then measured with a Tecan Infinite F-Plex plate reader. The luminescent signal produced is proportional to the amount of ATP produced in each well.
Cytotoxicity Assay
Cytotoxicity was measured by LDH release using the LDH-Glo Cytotoxicity Kit (Promega, J2380). Supernatants were harvested from cells and stored in an assay compatible storage buffer (200 mM Tris-HCl, 10% glycerol, 1% BSA, pH = 7.3) until the time course was complete. As a positive control, cells were treated with 10% Triton-X for 5–10 min to induce plasma membrane rupture. Complete cell death was confirmed visually. The samples were incubated with a luciferase reagent mixture for 1 h. On reduction of luciferase, a luminescent signal is released, and this signal is proportional to the concentration of LDH released. Luminescence was then measured with a Tecan Infinite F-Plex plate reader. This assay was conducted before and 72 h after transfection to ensure there were no differences in cell death before transfection that would affect the results posttransfection.
Statistical Analyses
All data are presented as means ± SD. All experiments were performed in triplicate. Data were analyzed by unpaired Student’s t test or one-way analysis of variance (ANOVA) where indicated. GraphPad Prism was used for all data analysis. Values of P < 0.05 were considered statistically significant.
RESULTS
miR-338-3p Is Reduced in Pulmonary Fibrosis
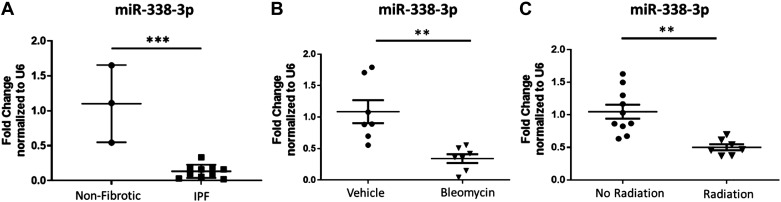
The role of microRNAs (miRNAs) in human health and disease is an area of heightened interest (17, 18, 26–28). Over the last decade, hundreds of microRNAs have been studied in human disease. Several candidates have advanced to clinical trials (29). To identify microRNAs relevant to pulmonary fibrosis, we used a database of miRNA profiles from patients with IPF and healthy controls (19, 20). We verified these findings by isolating RNA from human lung tissue obtained on biopsy, including 3 donors without fibrotic disease and 10 donors with a diagnosis of IPF. Patient demographics for these samples are listed in Table 1. One specific miRNA, miR-338-3p, was significantly downregulated in the lung tissue of patients diagnosed with pulmonary fibrosis, with expression down nearly 90% compared with control lung tissue (Fig. 1A). We also evaluated expression of miR-338-3p in animal models of fibrosis where lung tissue was obtained 21 days after oropharyngeal aspiration of bleomycin (Fig. 1B) or 26 wk after thoracic radiation exposure (Fig. 1C). In both models, miR-338-3p was significantly downregulated in animals with fibrosis compared with control animals.
Table 1.
Donor characteristics for lung tissue specimens used to assess gene expression
| Deidentified Subject No. | Condition | Sex | Age, yr | % DLCO |
|---|---|---|---|---|
| 1 | Healthy | Male | 60 | 127 |
| 2 | Healthy | Female | 72 | 86 |
| 3 | Healthy | Female | 56 | 99 |
| 4 | UIP | Male | 71 | 41 |
| 5 | UIP | Male | 55 | 29 |
| 6 | UIP | Male | 62 | 19 |
| 7 | UIP | Male | 46 | 21 |
| 8 | UIP | Male | 52 | 23 |
| 9 | UIP | Female | 52 | 29 |
| 10 | UIP | Male | 65 | 33 |
| 11 | UIP | Male | 61 | 27 |
| 12 | UIP | Male | 58 | 22 |
| 13 | UIP | Male | 72 | 53 |
DLCO, diffusing capacity of the lungs for carbon monoxide.
Figure 1.
miR-338-3p is downregulated in pulmonary fibrosis. A: human lung tissue from nonfibrotic donors (n = 3 subjects) and donors with IPF (n = 10 subjects; patient characteristics listed in Table 1) was analyzed for miR338-3p expression. B: mice (n = 7 mice/group) were treated with vehicle or 1.5 U/kg bleomycin by oropharyngeal aspiration and lung tissue was harvested after 21 days. C: lung tissue from mice exposed to a single dose of 5 Gy total body and 10 Gy thoracic radiation (n = 7–9 mice/group) were harvested 26 wk after exposure and tissue was analyzed for miR-338-3p. All data was analyzed by t test (**P < 0.01, ***P < 0.005).
miR-338-3p Blocks Myofibroblast Differentiation and Extracellular Matrix Protein Production
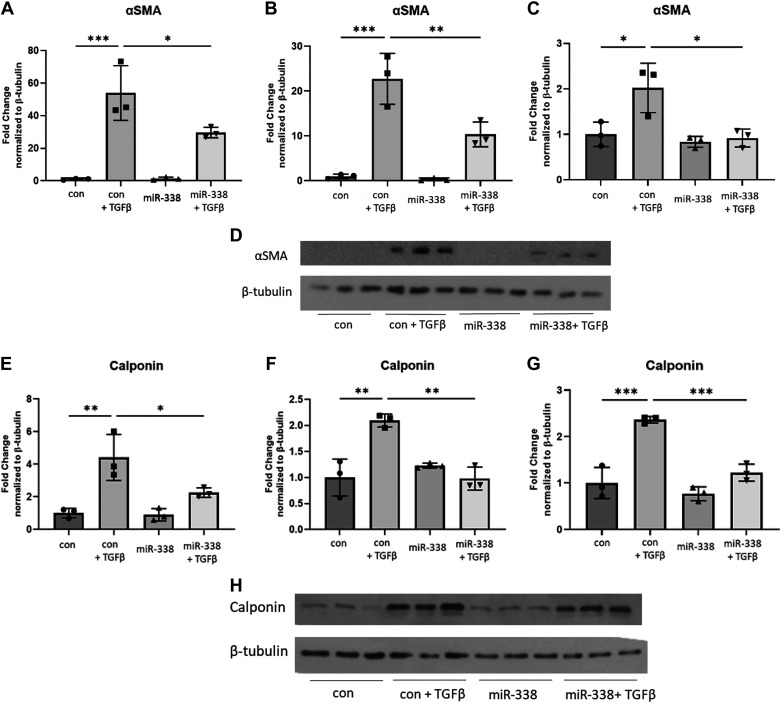
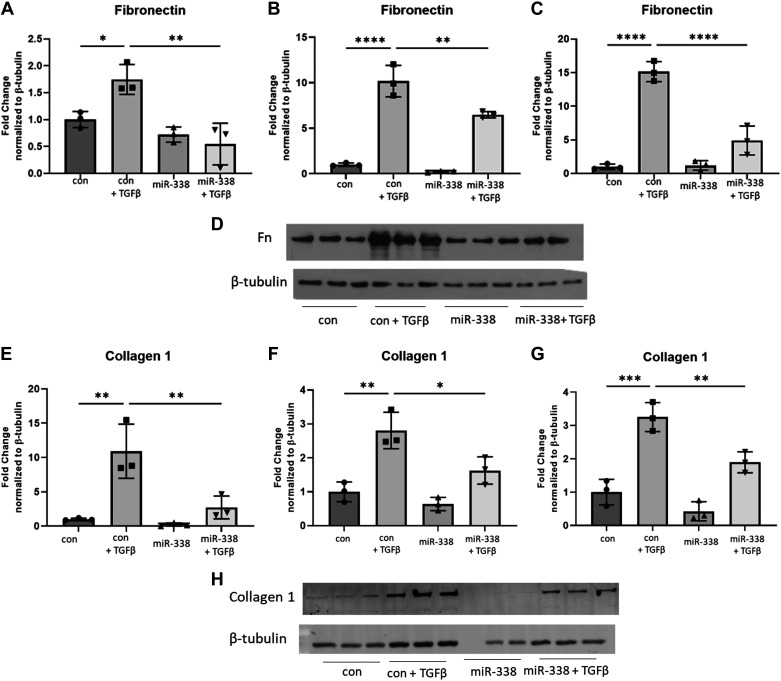
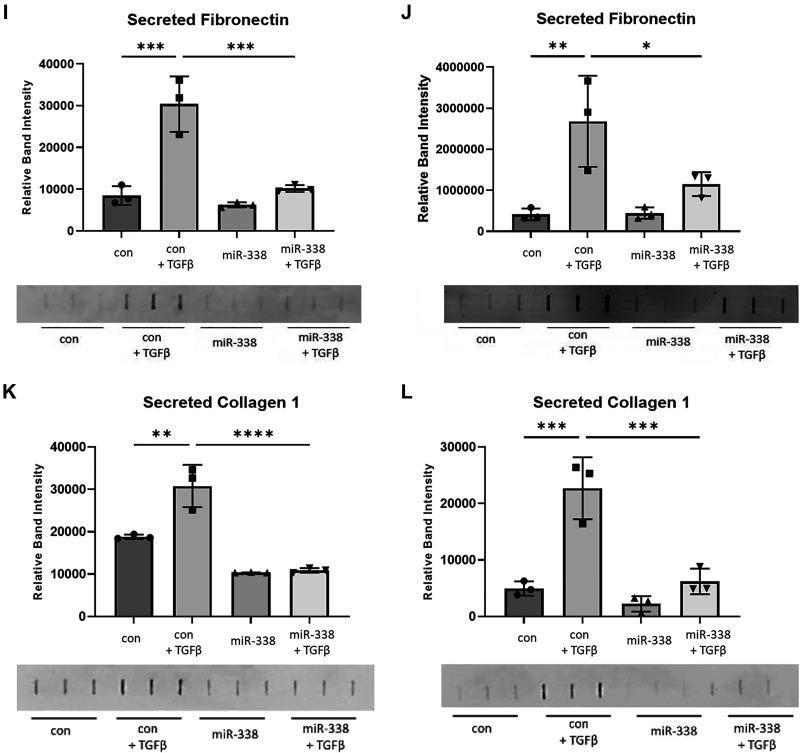
Given the significant downregulation of miR-338-3p in fibrotic lung tissue, we hypothesized that exogenous supplementation with miR-338-3p would block profibrotic phenotypes in vitro. To test this hypothesis, primary human lung fibroblasts were treated with TGFβ 6 h before transfection with miR-338-3p. Protein was isolated after 72 h to assess expression of the myofibroblast markers α smooth muscle actin (αSMA) and calponin (25, 30). Calponin is a calcium binding protein, which regulates actin and myosin dynamics in smooth muscle and is identified to regulate α smooth muscle actin (31–33). Although less commonly used as a myofibroblast marker compared with αSMA, our laboratory group has demonstrated calponin is a reliable and reproducible marker of myofibroblast differentiation (23, 25, 34–37). We found miR-338-3p effectively blocked TGFβ-induced protein expression of αSMA (Fig. 2, A–C) and as calponin (Fig. 2, D–F) in fibroblasts isolated from three different donors. miR-338-3p also blocked TGFβ-induced fibronectin (Fig. 3, A–C) and collagen 1 production (Fig. 3, D–F) and as well as secreted fibronectin (Fig. 3, G–H) and secreted collagen 1 (Fig. 3, I–J). This indicates miR-338-3p inhibits myofibroblast differentiation and matrix production.
Figure 2.
miR-338-3p blocks myofibroblast differentiation. Primary human lung fibroblasts from three different donors were treated with 200 nM nontargeting control miRNA or miR-338-3p and/or 1 ng/mL of TGFβ. Each donor is graphed on a separate axis with one representative Western blot shown. Raw blots are provided in the supplement. A–C: protein was harvested after 72 h to examine αSMA protein expression. D: representative western blot for αSMA protein expression. E–G: protein was harvested after 72 h to assess calponin expression. H: representative Western blot for calponin protein expression. Data analysis was performed with three technical replicates (*P < 0.05, **P < 0.01, ***P < 0.005, by ANOVA).
Figure 3.
miR-338-3p blocks TGFβ-induced matrix protein production. Primary human lung fibroblasts from three different donors were treated with 200 nM nontargeting control microRNA or miR-338-3p ±1 ng/mL TGFβ. A–C: protein was harvested at 72 h to assess fibronectin expression. D: representative Western blot. E–G: protein was harvested at 72 h to assess collagen 1 expression. H: representative Western blot. Secreted matrix proteins were evaluated from cells isolated from two donors. I and J: supernatants were collected at 72 h posttreatment to assess secreted fibronectin. F–H and K and L: supernatants were collected at 72 h posttreatment to assess secreted collagen. All experiments performed in technical triplicate (P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.0001 by ANOVA).
miR-338-3p Inhibits TGFβ-Induced Proliferation
To assess the functional consequences of miR-338-3p in primary human lung fibroblasts, cellular proliferation and ATP production were assessed. There are several reports in the literature that miR-338-3p inhibits proliferation in the context of cancer; however, there are no data on primary human cell types (38–41). We used trypan blue to assess the number of live cells after fibroblasts were transfected with a nontargeting microRNA or miR-338-3p and TGFβ where indicated. We observed miR-338-3p does not block proliferation at baseline but is effective at preventing TGFβ-induced proliferation (Fig. 4A). We also examined ATP production, as we hypothesized miR-338-3p may target glycolysis and thus, alter the metabolic productivity of the cell; however, there was no difference in ATP production with miR-338-3p (Fig. 4B). To confirm these results were not the consequence of cell death, cytotoxicity assays were performed via detection of LDH in the supernatants. We assessed cytotoxicity before and following transfection with miR-338-3p to ensure there were no differences before transfection that would affect the posttransfection results. There were no differences in cytotoxicity across treatment groups, indicating these results are not due to cell death (Fig. 4, C and D).
miR-338-3p Does Not Inhibit Glycolytic Enzyme Expression
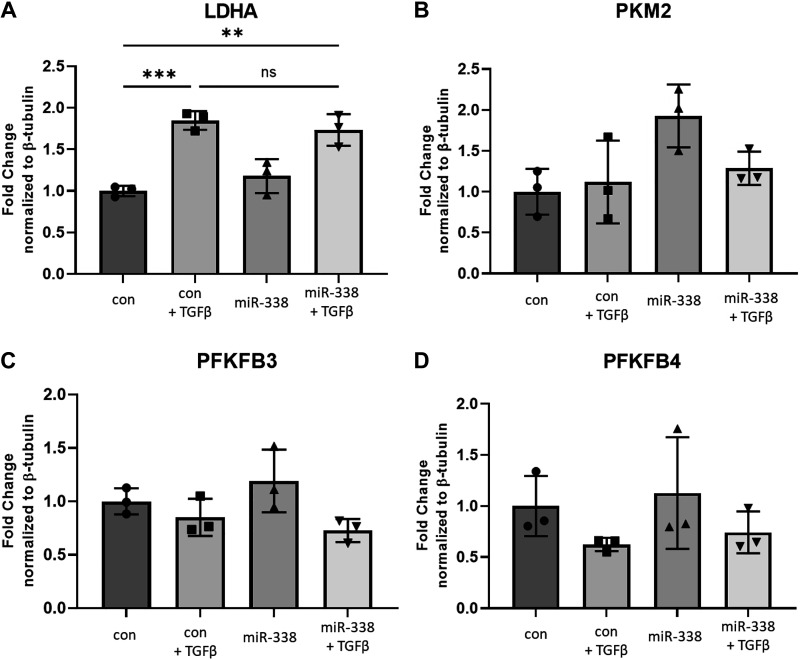
miR-338-3p is reported to target several glycolytic enzymes and oppose the Warburg effect in malignancy (42–44). It is well established that fibroblasts isolated from patients with pulmonary fibrosis exhibit a glycolytic shift similar to what is observed in many cancers (13, 45–47). Our group has shown LDHA is increased in pulmonary fibrosis (13) and that inhibition of LDHA blocks myofibroblast differentiation (14) and prevents the development of pulmonary fibrosis in two different animal models (15, 16). Inhibition of other glycolytic enzymes such as 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3) and pyruvate dehydrogenase kinase 1 (PDK1) also inhibits fibrosis (45, 48). Our initial interest in miR-338-3p was based on a predicted binding domain for miR-338-3p in the 3'-UTR of LDHA (Supplemental Fig. S1). The potential for pleiotropic inhibition of glycolytic enzymes by miR-338-3p was novel and led us to hypothesize miR-338-3p would block myofibroblast differentiation, at least in part, via direct regulation of LDHA.
Primary human lung fibroblasts were treated with 1 ng/mL TGFβ to induce myofibroblast differentiation and were subsequently transfected with miR-338-3p or a nontargeting control miRNA. As previously reported, TGFβ induced LDHA expression, but this was not attenuated by introduction of miR-338-3p (Fig. 5A), indicating miR-338-3p does not block myofibroblast differentiation via inhibition of LDHA expression. We next assessed if other glycolytic enzymes were inhibited by miR-338-3p. In several different cancer cell lines, miR-338-3p blocks the expression of PKM2 (44, 49, 50) and has a predicted binding domain for miR-338-3p (Supplemental Table S1). However miR-338-3p does not decrease PKM2 protein expression in primary human lung fibroblasts (Fig. 5B). We examined the expression of PFKFB3 and PFKFB4 as they are rate controlling enzymes of glycolysis (51). miR-338-3p did not alter expression of PFKFB3 (Fig. 5C) or PFKFB4 (Fig. 5D). This led us to conclude that miR-338-3p does not block myofibroblast differentiation by targeting glycolytic enzyme expression in primary human lung fibroblasts.
Figure 5.
miR-338-3p does not alter glycolytic enzyme expression. Primary human lung fibroblasts were treated with 200 nM nontargeting control miRNA or miR-338-3p ±1 ng/mL TGFβ. Protein was harvested at 72 h to assess expression of LDHA (A), PKM2 (B), PFKFB3 (C), and PFKFB4 (D). n = 3/group (***P < 0.005 by ANOVA). miRNA, microRNA.
miR-338-3p Does Not Interfere with TGFβ Signaling
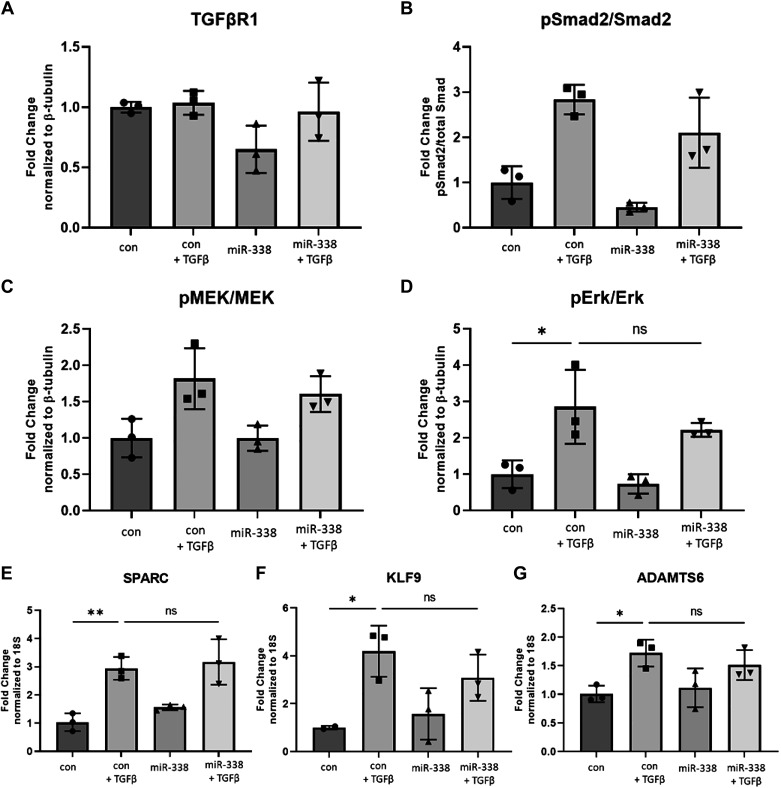
Having established that miR-338-3p opposes TGFβ-induced myofibroblast differentiation and matrix protein production, we sought to determine if miR-338-3p suppresses TGFβ signaling. TGFβ binds and cross-activates TGFBR1 and TGFBR2 resulting in the induction of numerous canonical and noncanonical pathways downstream (52). miR-338-3p has a predicted binding domain in the 3'-UTR of TGFβR1 (Supplemental Table S1); however, miR-338-3p did not significantly alter TGFβR1 expression (Fig. 6A). Phosphorylation of Smad2/3 is the hallmark of canonical TGFβ signaling (53) and is required for myofibroblast differentiation (54). miR-338-3p did not inhibit TGFβ-induced Smad2 phosphorylation (Fig. 6B). The induction of MEK/ERK axis is one example of noncanonical TGFβ signaling that is relevant to the development of pulmonary fibrosis in vivo (55). In ovarian cancer cell lines, miR-338-3p inhibits Met-induced ERK phosphorylation (41). In human lung fibroblasts, miR-338-3p does not inhibit TGFβ-induced MEK1/2 (Fig. 6C) or ERK1/2 phosphorylation (Fig. 6D).
Figure 6.
miR-338-3p does not inhibit TGFβ-induced signaling pathways. Primary human lung fibroblasts were treated with 200 nM nontargeting control microRNA or miR-338-3p ±1 ng/mL TGFβ. Protein was harvested 24 h after treatment to assess expression of TGFβR1 (A) and phosphorylation of Smad2 (B), MEK (C), and ERK (D). RNA was harvested 48 h posttreatment and evaluated for expression of SPARC (E), KLF9 (F), and ADAMTS6 (G). All experiments were performed in triplicate (*P < 0.05, **P < 0.01 by ANOVA).
Owing to the significant inhibition of matrix protein production, we examined if miR-338-3p inhibited matricellular proteins induced by TGFβ including SPARC, KLF9, and ADAMTS6. Secreted protein acidic rich in cysteine (SPARC) is a matrix binding protein and chaperone that assists in collagen processing and fibril formation and fibronectin (56, 57). Recapitulating what is reported in the literature, TGFβ induces SPARC gene expression. However, miR-338-3p does not block SPARC (Fig. 6E). Krüppel-like factor 9 (KLF9) is a zinc finger transcription factor known to regulate fibroblast proliferation and differentiation (58). Although not well studied in fibrosis, KLF9 serves as a transcriptional activator of collagen 1 gene expression (59, 60). Here we show TGFβ induces KLF9 gene expression in human lung fibroblasts, but miR-338-3p does not attenuate this induction (Fig. 6F). Similarly, ADAM metallopeptidase with thrombospondin type 1 motif 6 (ADAMTS6) is a transcription factor that positively regulates collagen gene expression (59) and contains a predicted binding domain for miR-338-3p (Supplemental Fig. S1); however, miR-338-3p did not inhibit ADAMTS6 gene expression (Fig. 6G).
miR-338-3p Does Not Block Cell Cycle Proteins
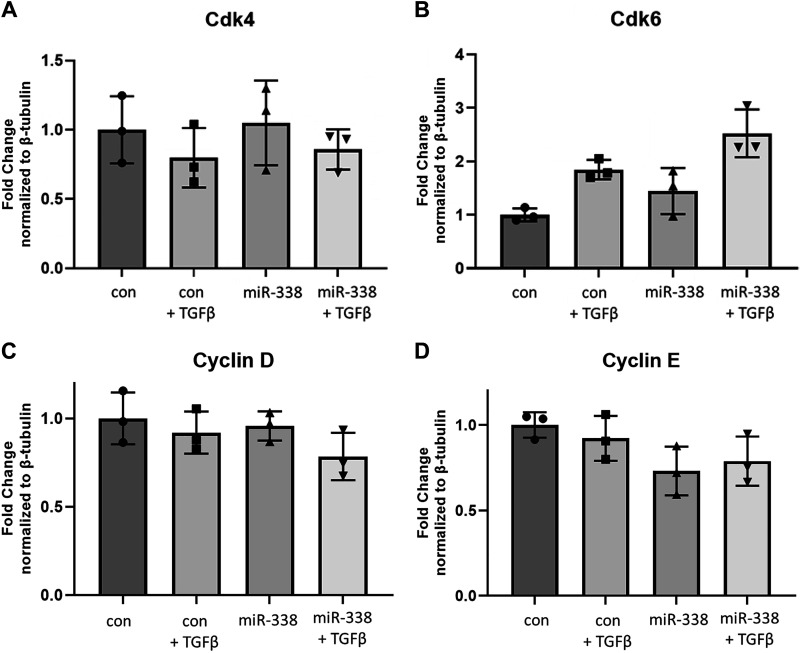
In hepatic stellate cells, miR-338-3p inhibits collagen 1 protein expression. This was contingent on cell cycle regulation, specifically through inhibition of cyclin-dependent kinase 4 (Cdk4; Supplemental Table S1) (39). miR-338-3p has also been shown to inhibit cyclin dependent kinase 2 (Cdk2) (61) and cyclin D (40, 62). In addition, in our primary human lung fibroblasts, miR-338-3p inhibited TGFβ-induced proliferation (Fig. 4A). To examine whether miR-338-3p blocked myofibroblast differentiation through regulation of the cell cycle, we examined protein expression of various cell cycle proteins 72 h after transfection with miR-338-3p. miR338-3p did not alter the protein expression of Cdk4 (Fig. 7A), Cdk6 (Fig. 7B), Cyclin D (Fig. 7C), or Cyclin E (Fig. 7D).
Figure 7.
miR-338-3p does not inhibit target cell cycle proteins. Primary human lung fibroblasts were treated with 200 nM nontargeting control microRNA or miR-338-3p ±1 ng/mL TGFβ. Protein was harvested 72 h after treatment to assess expression of Cdk4 (A) Cdk6 (B), Cyclin D (C), and Cyclin E (D). All experiments were performed in triplicate, statistical analysis by ANOVA (*P < 0.05, **P < 0.01 by ANOVA).
miR-338-3p Blocks Myofibroblast Differentiation via Induction of PTEN
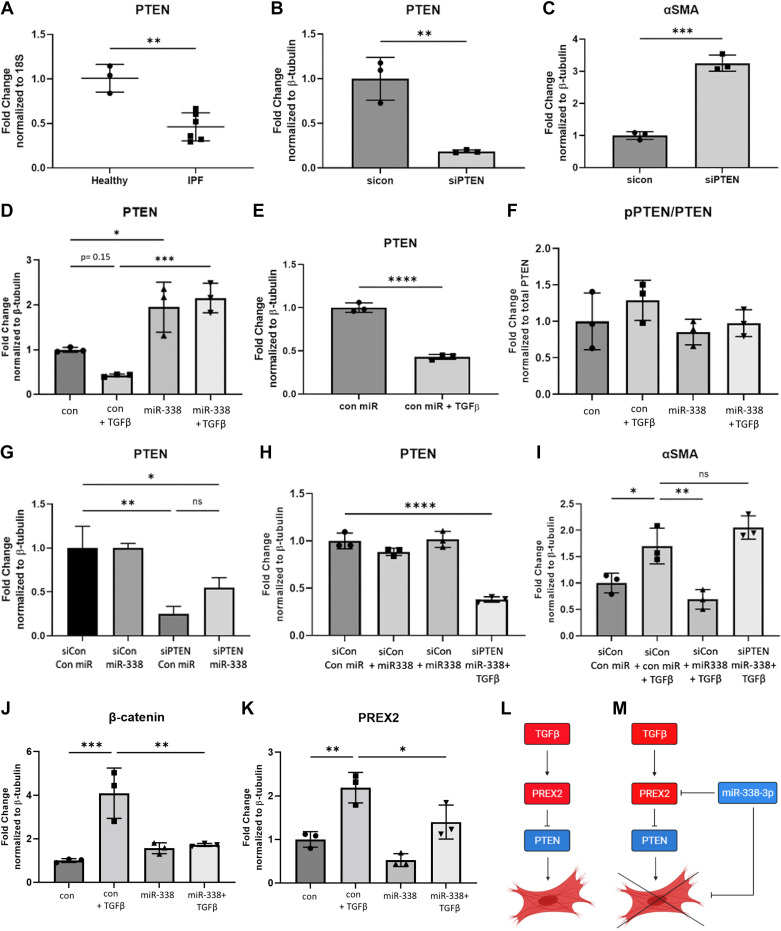
Tumor suppressor phosphatase and tensin homolog (PTEN) is a protein and lipid phosphatase recognized to have decreased activity in cancer (63–65), autoimmune disease (66, 67) and various types of fibrosis (68–70). In our lung tissue samples, we observed PTEN is downregulated in whole lung tissue of patients with IPF (Fig. 8A). In fibroblasts isolated from patients with pulmonary fibrosis, there is a loss of PTEN gene and protein expression as well as a decrease in PTEN phosphatase activity (71). Fibroblasts that have low PTEN expression also exhibit increased αSMA expression, indicating the loss of PTEN drives myofibroblast differentiation in vivo (72). In vitro inhibition of PTEN activity resulted in myofibroblast differentiation whereas overexpression of PTEN was protective (72). Furthermore, numerous publications have demonstrated PTEN induction blocks fibroblast proliferation in response to various stimuli, including TGFβ (72–75).
Figure 8.
miR-338-3p prevents TGFβ-mediated downregulation of PTEN and targets the PREX2-PTEN axis to inhibit myofibroblast differentiation. A: human lung tissue (Table 1) was examined for PTEN gene expression. PTEN knockdown was performed, and protein expression of PTEN (B) and αSMA (C) was assessed 72 h after transfection. Fibroblasts were transfected with 200 nM nontargeting miRNA or miR-338-3p with or without 1 ng/mL TGFβ to assess PTEN protein expression (D) and observe the effect of TGFβ on PTEN expression (E). In addition, phosphorylation status of PTEN (F) examined. Primary human lung fibroblasts were cotransfected with 100 nM of a nontargeting control siRNA (siCon) or siRNA targeting PTEN (siPTEN) and 100 nM of a nontargeting control miRNA (con miR) or miR-338-3p and 1 ng/mL TGFβ where indicated. 72 h after transfection, PTEN expression was assessed to determine knockdown efficiency (G–H) and αSMA protein expression was examined (I). Cells transfected with a nontargeting miRNA or miR-338-3p were examined for the expression of β-catenin (J) and PREX2 (K). We propose miR-338-3p is an antifibrotic regulator, which is capable of inducing PTEN expression through the inhibition of PREX2, a negative regulator of PTEN, which then inhibits myofibroblast differentiation (L and M). All experiments were performed in triplicate. Data were analyzed by t test and ANOVA (*P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.0001). miRNA, microRNA.
As miR-338-3p inhibits proliferation in fibroblasts but does not directly affect cell cycle protein expression, we examined if PTEN induction may be related to the antifibrotic functions of miR-338-3p. Confirming reports from the literature, we observed knockdown of PTEN-induced myofibroblast differentiation, as assessed by αSMA expression (Fig. 8, B and C). To understand whether miR-338-3p inhibits myofibroblast differentiation via PTEN, we examined both total and phosphorylated protein levels of PTEN via Western blot. We found, as is reported in the literature (72), TGFβ downregulated total PTEN protein expression. Although not statistically significant by ANOVA (Fig. 8D), this downregulation was highly significant by t test (Fig. 8E) and is likely biologically relevant. miR-338-3p not only prevented TGFβ-mediated downregulation but also additionally induced PTEN protein expression (Fig. 8D). miR-338-3p did not affect the phosphorylation status of PTEN (Fig. 8E).
Given the published data demonstrating the antifibrotic potential of PTEN, we hypothesized miR-338-3p inhibits myofibroblast differentiation via induction of PTEN. To test if miR-338-3p requires the induction of PTEN to block myofibroblast differentiation, cells were transfected with either a nontargeting siRNA or PTEN siRNA and a nontargeting miRNA or miR-338-3p. From these experiments, we were able to maintain low levels of PTEN while also delivering miR-338-3p to assess myofibroblast differentiation. After 16 h, the medium was replaced either without a treatment or with 1 ng/mL TGFβ. Seventy-two hours after the transfection, protein lysates were harvested to assess myofibroblast differentiation. The total concentration of genetic material delivered never exceeded 200 nM, which does not induce any cytotoxicity (Fig. 4D). To confirm we achieved comparable levels of knockdown of PTEN across treatment conditions, we compared efficiency through the examination of PTEN protein expression (Fig. 8, G and H). The levels of PTEN were not statistically different across the PTEN knockdown groups, indicating the observed effects are a result of miR-338-3p rather than variable PTEN expression. When fibroblasts were transfected with a nontargeting siRNA and miR-338-3p in conjunction with TGFβ, we observed miR-338-3p inhibited myofibroblast differentiation (Fig. 8I). However, when fibroblasts were cotransfected with PTEN siRNA and miR-338-3p, miR-338-3p no longer inhibited myofibroblast differentiation (Fig. 8I). These data demonstrate that PTEN expression is required for miR-338-3p to inhibit myofibroblast differentiation.
β-Catenin is part of the Wnt pathway that is upregulated in IPF. Inhibition of this pathway prevents the development of pulmonary fibrosis in vivo and numerous publications have linked PTEN to β-catenin/Wnt signaling (70, 76–80). Therefore, we wanted to determine whether miR-338-3p-mediated induction of PTEN also inhibited β-catenin expression. We observed miR-338-3p effectively inhibited TGFβ-induced total β-catenin protein (Fig. 8J). This indicates Wnt signaling may be impacted within this signaling pathway as well.
Our data demonstrate miR-338-3p requires PTEN to inhibit myofibroblast differentiation (Fig. 8I). However, miRNAs function through negative regulation of proteins via binding to the 3'-UTR. So to more completely identify the mechanism of action of miR-338-3p, we examined negative regulators of PTEN. We hypothesized miR-338-3p treatment caused protein degradation of a negative regulator of PTEN, resulting in increased PTEN expression. Of the known PTEN inhibitors, PREX2 has proposed to have a binding domain for miR-338-3p (61), which would make direct miR-338-3p-PREX2 regulation feasible. There are few publications describing the role of PREX2, and it remains unstudied in the context of fibrosis and myofibroblast differentiation.
In fibroblasts, we observed TGFβ induced PREX2 protein expression and this induction was attenuated with miR-338-3p (Fig. 8K). This led us to theorize a potential mechanism of action for miR-338-3p in fibroblasts. Under conditions with insufficient miR-338-3p, TGFβ induces PREX2, which inhibits PTEN and drives myofibroblast differentiation (Fig. 8L). However, when there is sufficient miR-338-3p present, miR-338-3p is able to block PREX2, enabling the induction of PTEN, which inhibits myofibroblast differentiation (Fig. 8M). As such, miR-338-3p serves as an important negative regulator of myofibroblast differentiation, and the loss of miR-338-3p in the IPF lung may drive disease progression.
DISCUSSION
MicroRNAs are increasingly recognized as important regulators of human disease. They have great translational potential as biomarkers and novel therapeutics. Due to the many potential targets of any one individual miRNA, it is often difficult to identify the precise mechanism(s) of action. Adding to this difficulty, the same miRNA may have different targets and effects across cell types.
Here, we identify miR-338-3p is downregulated in IPF and models of fibrosis (Fig. 1). This novel finding highlights the potential impact of microRNA dysregulation in the context of pulmonary fibrosis. We hypothesized that miRNAs lost in fibrosis may have antifibrotic potential. We demonstrate miR-338-3p blocks myofibroblast differentiation, as assessed by protein expression of αSMA and calponin (Fig. 2). In addition, miR-338-3p blocked the production of extracellular matrix proteins fibronectin and collagen 1 (Fig. 3). Both of these extracellular matrix proteins are upregulated in the IPF lung and are prominent constituents of the scar tissue observed in IPF. In the context of liver fibrosis, one group proposed that downregulation of a network of miRNAs, including miR-338-3p, causes dysregulation of the microenvironment, which results in an exacerbated profibrotic response (81). Combined, this is an indication that miR-338-3p could be involved in the regulation of the wound healing response across tissue types.
We also examined functional outcomes associated with miR-338-3p delivery. As observed in other cell types, miR-338-3p inhibits proliferation, in this case in response to TGFβ (Fig. 4). Due to the reported ability of miR-338-3p to inhibit metabolic processes, we examined ATP production; however, miR-338-3p did not alter ATP production. In addition, we confirmed these results were not a consequence of cytotoxicity (Fig. 4).
Several pathways were investigated to uncover the mechanism of action of miR-338-3p. Initially, our interest in miR-338-3p was related to the reported and predicted targets of miR-338-3p, notably glycolytic enzyme expression. These reports are exclusively in cancer cell lines where glycolytic enzyme expression is much higher than what would be observed in a noncancerous environment. miR-338-3p had no effect on LDHA, PFKBFB3, PFBFB4, or PKM2 in primary human lung fibroblasts (Fig. 5). There are several reports of miR-338-3p inhibiting glycolysis. We next examined if TGFβ signaling was altered as a result of treatment with miR-338-3p. Smad2/3 phosphorylation is required for myofibroblast differentiation and is representative of canonical TGFβ signaling; miR-338-3p did not block Smad2 phosphorylation. MEK1/2 and ERK1/2 phosphorylation, a representative of a noncanonical TGFβ pathway, were also unchanged by miR-338-3p (Fig. 6). Given the ability of miR-338-3p to block matrix proteins, we examined TGFβ regulated transcription factors known to induce fibronectin and collagen 1. Here again miR-338-3p had no effect on gene expression of SPARC, KLF9, or ADAMTS6 (Fig. 6).
There are several reports of the ability of miR-338-3p to block cell cycle proteins, including one report on hepatic stellate cells where miR-338-3p blocks collagen 1 production via inhibition of Cdk4 (39). However, in primary human lung fibroblasts, miR-338-3p did not block the expression of Cdk4, Cdk6, cyclin D, or cyclin E (Fig. 7). miRNAs have tissue- and cell-type-specific effects, so it is not surprising miR-338-3p utilizes other mechanisms in different organ systems. In addition, the hepatic stellate cells used in this manuscript were isolated from rats, so there may be significant interspecies differences as well.
Finally, we investigated the role of PTEN, a known antifibrotic mediator capable of inhibiting proliferation. Similar to miR-338-3p, PTEN is downregulated in pulmonary fibrosis and PTEN induction prevents myofibroblast differentiation. For the first time, we demonstrate that transfection of primary human lung fibroblasts with miR-338-3p induces PTEN expression and also prevents TGFβ-mediated downregulation of PTEN (Fig. 8). To prove miR-338-3p requires PTEN to effectively block myofibroblast differentiation, we transfected HLFs with a nontargeting siRNA or PTEN siRNA and cotransfected a nontargeting control miRNA or miR-338-3p. When cells were cotransfected with a nontargeting siRNA and miR-338-3p, we observed inhibition of myofibroblast differentiation, but when miR-338-3p was used in conjunction with PTEN siRNA, miR-338-3p no longer blocked myofibroblast differentiation (Fig. 8). Therefore, we conclude miR-338-3p inhibits myofibroblast differentiation via induction of PTEN.
MicroRNAs function to negatively regulate protein expression, so to more directly assess how miR-338-3p could induce PTEN expression, we examined PREX2, a known negative regulator of PTEN. We found miR-338-3p inhibits PREX2 expression. This led us to propose a mechanism of action for miR-338-3p in the context of TGFβ-induced myofibroblast differentiation. In a scenario with depleted miR-338-3p, TGFβ induces PREX2, which inhibits PTEN and results in myofibroblast differentiation. However, with sufficient miR-338-3p, miR-338-3p inhibits PREX2, which allows for restoration of PTEN expression and the inhibition of myofibroblast differentiation (Fig. 8). The downregulation of miR-338-3p in the IPF lung may represent a loss of homeostatic regulation that would normally inhibit myofibroblast differentiation and may oppose the progression of fibrosis, though animal studies would be required to determine the effects in vivo.
MiRNAs negatively regulate expression by binding to the 3′-UTR of targets that match a seed sequence. It is very novel to identify that a miRNA significantly upregulates a protein of interest. miR-338-3p targets PREX2, negative regulator of PTEN, in human lung fibroblasts, and it is the loss of a brake on PTEN expression that allows for this induction. In gastric cancer, miR-338-3p is reported to block PREX2. Suppression of PREX2 led to an induction of PTEN activity (61). P-Rex2 has not been studied in the context of fibrosis or TGFβ signaling. However, PREX2-dependent cell behavior represents a novel area of study in the context of fibrosis research. There are also other negative regulators of PTEN that may contribute to our proposed mechanism. MEK and ERK negatively regulate PTEN via c-Jun (82); however, this mechanism is unlikely given the inability of miR-338-3p to block MEK/ERK phosphorylation (Fig. 6). Other negative regulators of PTEN include nuclear factor-κB (NF-κB), which sequesters Creb binding protein (CBP)/P300 (83) or shank interacting protein-like 1 (SIPL1), which directly inhibits PTEN (84).
Our findings provide strong data that miR-338-3p inhibits TGFβ-induced myofibroblast differentiation and matrix protein production. miR-338-3p appears to have a distinct mechanism of action in human lung fibroblasts compared with cancer cells lines, highlighting the unique plasticity of miRNA function across cell types. For the first time, we report the ability of miR-338-3p to induce the antifibrotic mediator PTEN. In addition, miR-338-3p prevents TGFβ-mediated PTEN downregulation. This is an exciting and relevant finding as other researchers have demonstrated that overexpression of PTEN is antifibrotic and there are few mechanisms available to accomplish PTEN induction. Further work is needed to understand the interaction of miR-338-3p with the PTEN signaling axis. Ultimately, understanding the role of antifibrotic mediators like miR-338-3p and PTEN and the mechanisms that underlie their downregulation will provide insight into the pathogenesis of pulmonary fibrosis and widen the arena for further therapeutic development.
SUPPLEMENTAL DATA
Supplemental Tables S1 and S2 and Supplemental Fig. S1: https://doi.org/10.6084/m9.figshare.16867312.
GRANTS
This work was funded by R01HL127001 (to P.J.S.) and F31HL132453 (to J.L.J.); A.R. was supported by T32HL066988.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.R.R., J.L.J., C.F.W., P.J.S., and R.M.K. conceived and designed research; A.R.R. performed experiments; A.R.R. analyzed data; A.R.R. and R.M.K. interpreted results of experiments; A.R.R. prepared figures; A.R.R. drafted manuscript; A.R.R., J.L.J., C.F.W., P.J.S., and R.M.K. edited and revised manuscript; A.R.R., J.L.J., C.F.W., P.J.S., and R.M.K. approved final version of manuscript.
REFERENCES
- 1.Martinez FJ, Collard HR, Pardo A, Raghu G, Richeldi L, Selman M, Swigris JJ, Taniguchi H, Wells AU. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers 3: 17074, 2017. doi: 10.1038/nrdp.2017.74. [DOI] [PubMed] [Google Scholar]
- 2.Katzenstein AL, Myers JL. Idiopathic pulmonary fibrosis: clinical relevance of pathologic classification. Am J Respir Crit Care Med 157: 1301–1315, 1998. doi: 10.1164/ajrccm.157.4.9707039. [DOI] [PubMed] [Google Scholar]
- 3.O'Dwyer DN, Ashley SL, Moore BB. Influences of innate immunity, autophagy, and fibroblast activation in the pathogenesis of lung fibrosis. Am J Physiol Lung Cell Mol Physiol 311: L590–L601, 2016. doi: 10.1152/ajplung.00221.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabbiani G, Ryan GB, Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia 27: 549–550, 1971. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- 5.Bulvik R, Breuer R, Dvir-Ginzberg M, Reich E, Berkman N, Wallach-Dayan SB. SIRT1 deficiency, specifically in fibroblasts, decreases apoptosis resistance and is associated with resolution of lung-fibrosis. Biomolecules 10: 996, 2020. doi: 10.3390/biom10070996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romero Y, Bueno M, Ramirez R, Álvarez D, Sembrat JC, Goncharova EA, Rojas M, Selman M, Mora AL, Pardo A. mTORC1 activation decreases autophagy in aging and idiopathic pulmonary fibrosis and contributes to apoptosis resistance in IPF fibroblasts. Aging Cell 15: 1103–1112, 2016. doi: 10.1111/acel.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Álvarez D, Cárdenes N, Sellarés J, Bueno M, Corey C, Hanumanthu VS, Peng Y, D'Cunha H, Sembrat J, Nouraie M, Shanker S, Caufield C, Shiva S, Armanios M, Mora AL, Rojas M. IPF lung fibroblasts have a senescent phenotype. Am J Physiol Lung Cell Mol Physiol 313: L1164–L1173, 2017. doi: 10.1152/ajplung.00220.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aschner Y, Downey GP. Transforming growth factor-β: master regulator of the respiratory system in health and disease. Am J Respir Cell Mol Biol 54: 647–655, 2016. doi: 10.1165/rcmb.2015-0391TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eickelberg O, Köhler E, Reichenberger F, Bertschin S, Woodtli T, Erne P, Perruchoud AP, Roth M. Extracellular matrix deposition by primary human lung fibroblasts in response to TGF-β1 and TGF-β3. Am J Physiol Lung Cell Mol Physiol 276: L814–L824, 1999. doi: 10.1152/ajplung.1999.276.5.L814. [DOI] [PubMed] [Google Scholar]
- 10.Conte E, Gili E, Fagone E, Fruciano M, Iemmolo M, Vancheri C. Effect of pirfenidone on proliferation, TGF-β-induced myofibroblast differentiation and fibrogenic activity of primary human lung fibroblasts. Eur J Pharm Sci 58: 13–19, 2014. doi: 10.1016/j.ejps.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Sánchez-Capelo A. Dual role for TGF-β1 in apoptosis. Cytokine Growth Factor Rev 16: 15–34, 2005. doi: 10.1016/j.cytogfr.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 12.O'Leary EM, Tian Y, Nigdelioglu R, Witt LJ, Cetin-Atalay R, Meliton AY, Woods PS, Kimmig LM, Sun KA, Gökalp GA, Mutlu GM, Hamanaka RB. TGF-β promotes metabolic reprogramming in lung fibroblasts via mTORC1-dependent ATF4 activation. Am J Respir Cell Mol Biol 63: 601–612, 2020. doi: 10.1165/rcmb.2020-0143OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kottmann RM, Kulkarni AA, Smolnycki KA, Lyda E, Dahanayake T, Salibi R, Honnons S, Jones C, Isern NG, Hu JZ, Nathan SD, Grant G, Phipps RP, Sime PJ. Lactic acid is elevated in idiopathic pulmonary fibrosis and induces myofibroblast differentiation via pH-dependent activation of transforming growth factor-β. Am J Respir Crit Care Med 186: 740–751, 2012. doi: 10.1164/rccm.201201-0084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kottmann RM, Trawick E, Judge JL, Wahl LA, Epa AP, Owens KM, Thatcher TH, Phipps RP, Sime PJ. Pharmacologic inhibition of lactate production prevents myofibroblast differentiation. Am J Physiol Lung Cell Mol Physiol 309: L1305–L1312, 2015. doi: 10.1152/ajplung.00058.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Judge JL, Owens KM, Pollock SJ, Woeller CF, Thatcher TH, Williams JP, Phipps RP, Sime PJ, Kottmann RM. Ionizing radiation induces myofibroblast differentiation via lactate dehydrogenase. Am J Physiol Lung Cell Mol Physiol 309: L879–L887, 2015. doi: 10.1152/ajplung.00153.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Judge JL, Nagel DJ, Owens KM, Rackow A, Phipps RP, Sime PJ, Kottmann RM. Prevention and treatment of bleomycin-induced pulmonary fibrosis with the lactate dehydrogenase inhibitor gossypol. PLoS One 13: e0197936, 2018. doi: 10.1371/journal.pone.0197936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol 15: 509–524, 2014. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 18.Bagnato G, Roberts WN, Roman J, Gangemi S. A systematic review of overlapping microRNA patterns in systemic sclerosis and idiopathic pulmonary fibrosis. Eur Respir Rev 26: 160125, 2017. doi: 10.1183/16000617.0125-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulz MH, Pandit KV, Lino Cardenas CL, Ambalavanan N, Kaminski N, Bar-Joseph Z. Reconstructing dynamic microRNA-regulated interaction networks. Proc Natl Acad Sci USA 110: 15686–15691, 2013. doi: 10.1073/pnas.1303236110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pandit KV, Corcoran D, Yousef H, Yarlagadda M, Tzouvelekis A, Gibson KF, Konishi K, Yousem SA, Singh M, Handley D, Richards T, Selman M, Watkins SC, Pardo A, Ben-Yehudah A, Bouros D, Eickelberg O, Ray P, Benos PV, Kaminski N. Inhibition and role of let-7d in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 182: 220–229, 2010. doi: 10.1164/rccm.200911-1698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120: 15–20, 2005. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 22.Backes C, Kehl T, Stöckel D, Fehlmann T, Schneider L, Meese E, Lenhof HP, Keller A. miRPathDB: a new dictionary on microRNAs and target pathways. Nucleic Acids Res 45: D90–D96, 2017. doi: 10.1093/nar/gkw926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baglole CJ, Reddy SY, Pollock SJ, Feldon SE, Sime PJ, Smith TJ, Phipps RP. Isolation and phenotypic characterization of lung fibroblasts. Methods Mol Med 117: 115–127, 2005. doi: 10.1385/1-59259-940-0:115. [DOI] [PubMed] [Google Scholar]
- 24.Judge JL, Lacy SH, Ku WY, Owens KM, Hernady E, Thatcher TH, Williams JP, Phipps RP, Sime PJ, Kottmann RM. The lactate dehydrogenase inhibitor gossypol inhibits radiation-induced pulmonary fibrosis. Radiat Res 188: 35–43, 2017. doi: 10.1667/RR14620.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferguson HE, Kulkarni A, Lehmann GM, Garcia-Bates TM, Thatcher TH, Huxlin KR, Phipps RP, Sime PJ. Electrophilic peroxisome proliferator–activated receptor-γ ligands have potent antifibrotic effects in human lung fibroblasts. Am J Respir Cell Mol Biol 41: 722–730, 2009. doi: 10.1165/rcmb.2009-0006OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burgy O, Fernandez Fernandez E, Rolandsson Enes S, Königshoff M, Greene CM, Bartel S. New players in chronic lung disease identified at the European Respiratory Society International Congress in Paris 2018: from microRNAs to extracellular vesicles. J Thorac Dis 10: S2983–S2987, 2018. doi: 10.21037/jtd.2018.08.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gangwar I, Kumar Sharma N, Panzade G, Awasthi S, Agrawal A, Shankar R. Detecting the molecular system signatures of idiopathic pulmonary fibrosis through integrated genomic analysis. Sci Rep 7: 1554, 2017. doi: 10.1038/s41598-017-01765-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oak SR, Murray L, Herath A, Sleeman M, Anderson I, Joshi AD, Coelho AL, Flaherty KR, Toews GB, Knight D, Martinez FJ, Hogaboam CM. A micro RNA processing defect in rapidly progressing idiopathic pulmonary fibrosis. PLoS One 6: e21253, 2011. doi: 10.1371/journal.pone.0021253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanna J, Hossain GS, Kocerha J. The potential for microRNA therapeutics and clinical research. Front Genet 10: 478, 2019. doi: 10.3389/fgene.2019.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shahar I, Fireman E, Topilsky M, Grief J, Schwarz Y, Kivity S, Ben-Efraim S, Spirer Z. Effect of endothelin-1 on α-smooth muscle actin expression and on alveolar fibroblasts proliferation in interstitial lung diseases. Int J Immunopharmacol 21: 759–775, 1999. doi: 10.1016/s0192-0561(99)00056-9. [DOI] [PubMed] [Google Scholar]
- 31.Winder SJ, Allen BG, Clément-Chomienne O, Walsh MP. Regulation of smooth muscle actin-myosin interaction and force by calponin. Acta Physiol Scand 164: 415–426, 1998. doi: 10.1111/j.1365-201x.1998.tb10697.x. [DOI] [PubMed] [Google Scholar]
- 32.Gimona M, Kaverina I, Resch GP, Vignal E, Burgstaller G. Calponin repeats regulate actin filament stability and formation of podosomes in smooth muscle cells. Mol Biol Cell 14: 2482–2491, 2003. doi: 10.1091/mbc.e02-11-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao H, Steffen MC, Ramos KS. Osteopontin regulates α-smooth muscle actin and calponin in vascular smooth muscle cells. Cell Biol Int 36: 155–161, 2012. doi: 10.1042/CBI20100240. [DOI] [PubMed] [Google Scholar]
- 34.Burgess HA, Daugherty LE, Thatcher TH, Lakatos HF, Ray DM, Redonnet M, Phipps RP, Sime PJ. PPARγ agonists inhibit TGF-β induced pulmonary myofibroblast differentiation and collagen production: implications for therapy of lung fibrosis. Am J Physiol Lung Cell Mol Physiol 288: L1146–L1153, 2005. doi: 10.1152/ajplung.00383.2004. [DOI] [PubMed] [Google Scholar]
- 35.Epa AP, Thatcher TH, Pollock SJ, Wahl LA, Lyda E, Kottmann RM, Phipps RP, Sime PJ. Normal human lung epithelial cells inhibit transforming growth factor-β induced myofibroblast differentiation via prostaglandin E2. PLoS One 10: e0135266, 2015. doi: 10.1371/journal.pone.0135266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kulkarni AA, Thatcher TH, Olsen KC, Maggirwar SB, Phipps RP, Sime PJ. PPAR-γligands repress TGFβ-induced myofibroblast differentiation by targeting the PI3K/Akt pathway: implications for therapy of fibrosis. PLoS One 6: e15909, 2011. doi: 10.1371/journal.pone.0015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woeller CF, O'Loughlin CW, Roztocil E, Feldon SE, Phipps RP. Salinomycin and other polyether ionophores are a new class of antiscarring agent. J Biol Chem 290: 3563–3575, 2015. doi: 10.1074/jbc.M114.601872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen X, Wei L, Zhao S. miR-338 inhibits the metastasis of lung cancer by targeting integrin β3. Oncol Rep 36: 1467–1474, 2016. doi: 10.3892/or.2016.4928. [DOI] [PubMed] [Google Scholar]
- 39.Duan B, Hu J, Zhang T, Luo X, Zhou Y, Liu S, Zhu L, Wu C, Liu W, Chen C, Gao H. miRNA-338-3p/CDK4 signaling pathway suppressed hepatic stellate cell activation and proliferation. BMC Gastroenterol 17: 12, 2017. doi: 10.1186/s12876-017-0571-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fu X, Tan D, Hou Z, Hu Z, Liu G. miR-338-3p is down-regulated by hepatitis B virus X and inhibits cell proliferation by targeting the 3'-UTR region of CyclinD1. Int J Mol Sci 13: 8514–8539, 2012. doi: 10.3390/ijms13078514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang R, Shi H, Ren F, Feng W, Cao Y, Li G, Liu Z, Ji P, Zhang M. MicroRNA-338-3p suppresses ovarian cancer cells growth and metastasis: implication of Wnt/catenin β and MEK/ERK signaling pathways. J Exp Clin Cancer Res 38: 494, 2019. doi: 10.1186/s13046-019-1494-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Zheng J, Luo J, Zeng H, Guo L, Shao G. 125I suppressed the Warburg effect viaregulating miR-338/PFKL axis in hepatocellular carcinoma. Biomed Pharmacother 119: 109402, 2019. doi: 10.1016/j.biopha.2019.109402. [DOI] [PubMed] [Google Scholar]
- 43.Nie H, Li J, Yang XM, Cao QZ, Feng MX, Xue F, Wei L, Qin W, Gu J, Xia Q, Zhang ZG. Mineralocorticoid receptor suppresses cancer progression and the Warburg effect by modulating the miR-338-3p-PKLR axis in hepatocellular carcinoma. Hepatology 62: 1145–1159, 2015. doi: 10.1002/hep.27940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Q, Pan X, Zhu D, Deng Z, Jiang R, Wang X. Circular RNA MAT2B promotes glycolysis and malignancy of hepatocellular carcinoma through the miR-338-3p/PKM2 axis under hypoxic stress. Hepatology 70: 1298–1316, 2019. doi: 10.1002/hep.30671. [DOI] [PubMed] [Google Scholar]
- 45.Xie N, Tan Z, Banerjee S, Cui H, Ge J, Liu RM, Bernard K, Thannickal VJ, Liu G. Glycolytic reprogramming in myofibroblast differentiation and lung fibrosis. Am J Respir Crit Care Med 192: 1462–1474, 2015. doi: 10.1164/rccm.201504-0780OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bernard K, Logsdon NJ, Ravi S, Xie N, Persons BP, Rangarajan S, Zmijewski JW, Mitra K, Liu G, Darley-Usmar VM, Thannickal VJ. Metabolic reprogramming is required for myofibroblast contractility and differentiation. J Biol Chem 290: 25427–25438, 2015. doi: 10.1074/jbc.M115.646984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vallée A, Lecarpentier Y, Vallée JN. Thermodynamic aspects and reprogramming cellular energy metabolism during the fibrosis process. Int J Mol Sci 18: 2537, 2017. doi: 10.3390/ijms18122537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goodwin J, Choi H, Hsieh MH, Neugent ML, Ahn JM, Hayenga HN, Singh PK, Shackelford DB, Lee IK, Shulaev V, Dhar S, Takeda N, Kim JW. Targeting hypoxia-inducible factor-1α/pyruvate dehydrogenase kinase 1 axis by dichloroacetate suppresses bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol 58: 216–231, 2018. doi: 10.1165/rcmb.2016-0186OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu X, Zhu Q, Guo Y, Xiao Z, Hu L, Xu Q. LncRNA LINC00689 promotes the growth, metastasis and glycolysis of glioma cells by targeting miR-338-3p/PKM2 axis. Biomed Pharmacother 117: 109069, 2019. doi: 10.1016/j.biopha.2019.109069. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, Shi B, Chen J, Hu L, Zhao C. MiR-338-3p targets pyruvate kinase M2 and affects cell proliferation and metabolism of ovarian cancer. Am J Transl Res 8: 3266–3273, 2016. [PMC free article] [PubMed] [Google Scholar]
- 51.Yalcin A, Telang S, Clem B, Chesney J. Regulation of glucose metabolism by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatases in cancer. Exp Mol Pathol 86: 174–179, 2009. doi: 10.1016/j.yexmp.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 52.Grafe I, Alexander S, Peterson JR, Snider TN, Levi B, Lee B, Mishina Y. TGF-β family signaling in mesenchymal differentiation. Cold Spring Harb Perspect Biol 10: a022202, 2018. doi: 10.1101/cshperspect.a022202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vander Ark A, Cao J, Li X. TGF-β receptors: In and beyond TGF-β signaling. Cell Signal 52: 112–120, 2018. doi: 10.1016/j.cellsig.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 54.Ard S, Reed EB, Smolyaninova LV, Orlov SN, Mutlu GM, Guzy RD, Dulin NO. Sustained Smad2 phosphorylation is required for myofibroblast transformation in response to TGF-β. Am J Respir Cell Mol Biol 60: 367–369, 2019. doi: 10.1165/rcmb.2018-0252LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Madala SK, Schmidt S, Davidson C, Ikegami M, Wert S, Hardie WD. MEK-ERK pathway modulation ameliorates pulmonary fibrosis associated with epidermal growth factor receptor activation. Am J Respir Cell Mol Biol 46: 380–388, 2012. doi: 10.1165/rcmb.2011-0237OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bradshaw AD. The role of SPARC in extracellular matrix assembly. J Cell Commun Signal 3: 239–246, 2009. doi: 10.1007/s12079-009-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu Z, Mo L, Feng X, Huang M, Li L. Using bioinformatics approach identifies key genes and pathways in idiopathic pulmonary fibrosis. Medicine (Baltimore) 99: e22099, 2020. doi: 10.1097/MD.0000000000022099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang XL, Zhang D, Michel FJ, Blum JL, Simmen FA, Simmen RC. Selective interactions of Kruppel-like factor 9/basic transcription element-binding protein with progesterone receptor isoforms A and B determine transcriptional activity of progesterone-responsive genes in endometrial epithelial cells. J Biol Chem 278: 21474–21482, 2003. doi: 10.1074/jbc.M212098200. [DOI] [PubMed] [Google Scholar]
- 59.Ghosh AK. Factors involved in the regulation of type I collagen gene expression: implication in fibrosis. Exp Biol Med (Maywood) 227: 301–314, 2002. doi: 10.1177/153537020222700502. [DOI] [PubMed] [Google Scholar]
- 60.Chen A, Davis BH. The DNA binding protein BTEB mediates acetaldehyde-induced, jun N-terminal kinase-dependent αI(I) collagen gene expression in rat hepatic stellate cells. Mol Cell Biol 20: 2818–2826, 2000. doi: 10.1128/MCB.20.8.2818-2826.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guo B, Liu L, Yao J, Ma R, Chang D, Li Z, Song T, Huang C. miR-338-3p suppresses gastric cancer progression through a PTEN-AKT axis by targeting P-REX2a. Mol Cancer Res 12: 313–321, 2014. doi: 10.1158/1541-7786.MCR-13-0507. [DOI] [PubMed] [Google Scholar]
- 62.Vivacqua A, Sebastiani A, Miglietta AM, Rigiracciolo DC, Cirillo F, Galli GR, Talia M, Santolla MF, Lappano R, Giordano F, Panno ML, Maggiolini M. miR-338-3p is regulated by estrogens through GPER in breast cancer cells and cancer-associated fibroblasts (CAFs). Cells 7: 203, 2018. doi: 10.3390/cells7110203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferrara MG, Martini M, D'Argento E, Forcella C, Vita E, Di Noia V, Sperduti I, Bilotta M, Ribelli M, Damiano P, Cannella A, Stefani A, Pilotto S, Carbone C, Piro G, Milella M, Tortora G, Bria E. PTEN Loss as a predictor of tumor heterogeneity and poor prognosis in patients with EGFR-mutant advanced non-small-cell lung cancer receiving tyrosine kinase inhibitors. Clinical Lung Cancer 22: 351–360, 2021. doi: 10.1016/j.cllc.2020.12.008. [DOI] [PubMed] [Google Scholar]
- 64.Smyth LM, Batist G, Meric-Bernstam F, Kabos P, Spanggaard I, Lluch A, Jhaveri K, Varga A, Wong A, Schram AM, Ambrose H, Carr TH, de Bruin EC, Salinas-Souza C, Foxley A, Hauser J, Lindemann JPO, Maudsley R, McEwen R, Moschetta M, Nikolaou M, Schiavon G, Razavi P, Banerji U, Baselga J, Hyman DM, Chandarlapaty S. Selective AKT kinase inhibitor capivasertib in combination with fulvestrant in PTEN-mutant ER-positive metastatic breast cancer. NPJ Breast Cancer 7: 44, 2021. doi: 10.1038/s41523-021-00251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vidotto T, Saggioro FP, Jamaspishvili T, Chesca DL, Picanço de Albuquerque CG, Reis RB, Graham CH, Berman DM, Siemens DR, Squire JA, Koti M. PTEN-deficient prostate cancer is associated with an immunosuppressive tumor microenvironment mediated by increased expression of IDO1 and infiltrating FoxP3+ T regulatory cells. Prostate 79: 969–979, 2019. doi: 10.1002/pros.23808. [DOI] [PubMed] [Google Scholar]
- 66.Heinicke F, Zhong X, Flåm ST, Breidenbach J, Leithaug M, Mæhlen MT, Lillegraven S, Aga AB, Norli ES, Mjaavatten MD, Haavardsholm EA, Zucknick M, Rayner S, Lie BA. MicroRNA expression differences in blood-derived CD19+ B cells of methotrexate treated rheumatoid arthritis patients. Front Immunol 12: 663736, 2021. doi: 10.3389/fimmu.2021.663736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bacalao MA, Satterthwaite AB. Recent advances in lupus B cell biology: PI3K, IFNγ, and chromatin. Front Immunol 11: 615673, 2020. doi: 10.3389/fimmu.2020.615673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Higgins DF, Ewart LM, Masterson E, Tennant S, Grebnev G, Prunotto M, Pomposiello S, Conde-Knape K, Martin FM, Godson C. BMP7-induced-Pten inhibits Akt and prevents renal fibrosis. Biochim Biophys Acta Mol Basis Dis 1863: 3095–3104, 2017. doi: 10.1016/j.bbadis.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 69.Lu S, Strand KA, Mutryn MF, Tucker RM, Jolly AJ, Furgeson SB, Moulton KS, Nemenoff RA, Weiser-Evans MCM. PTEN (phosphatase and tensin homolog) protects against Ang II (Angiotensin II)-induced pathological vascular fibrosis and remodeling—brief report. Arterioscler Thromb Vasc Biol 40: 394–403, 2020. doi: 10.1161/ATVBAHA.119.313757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vallée A, Lecarpentier Y, Guillevin R, Vallée JN. Interactions between TGF-β1, canonical WNT/β-catenin pathway and PPAR γ in radiation-induced fibrosis. Oncotarget 8: 90579–90604, 2017. doi: 10.18632/oncotarget.21234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.White ES, Thannickal VJ, Carskadon SL, Dickie EG, Livant DL, Markwart S, Toews GB, Arenberg DA. Integrin α4β1 regulates migration across basement membranes by lung fibroblasts: a role for phosphatase and tensin homologue deleted on chromosome 10. Am J Respir Crit Care Med 168: 436–442, 2003. doi: 10.1164/rccm.200301-041OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.White ES, Atrasz RG, Hu B, Phan SH, Stambolic V, Mak TW, Hogaboam CM, Flaherty KR, Martinez FJ, Kontos CD, Toews GB. Negative regulation of myofibroblast differentiation by PTEN (phosphatase and tensin homolog deleted on chromosome 10). Am J Respir Crit Care Med 173: 112–121, 2006. doi: 10.1164/rccm.200507-1058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.He Z, Deng Y, Li W, Chen Y, Xing S, Zhao X, Ding J, Gao Y, Wang X. Overexpression of PTEN suppresses lipopolysaccharide-induced lung fibroblast proliferation, differentiation and collagen secretion through inhibition of the PI3-K-Akt-GSK3β pathway. Cell Biosci 4: 2, 2014. doi: 10.1186/2045-3701-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fagone E, Conte E, Gili E, Fruciano M, Pistorio MP, Lo Furno D, Giuffrida R, Crimi N, Vancheri C. Resveratrol inhibits transforming growth factor-β-induced proliferation and differentiation of ex vivo human lung fibroblasts into myofibroblasts through ERK/Akt inhibition and PTEN restoration. Exp Lung Res 37: 162–174, 2011. doi: 10.3109/01902148.2010.524722. [DOI] [PubMed] [Google Scholar]
- 75.Geng J, Huang X, Li Y, Xu X, Li S, Jiang D, Liang J, Jiang D, Wang C, Dai H. Down-regulation of USP13 mediates phenotype transformation of fibroblasts in idiopathic pulmonary fibrosis. Respir Res 16: 124, 2015. doi: 10.1186/s12931-015-0286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cao H, Wang C, Chen X, Hou J, Xiang Z, Shen Y, Han X. Inhibition of Wnt/β-catenin signaling suppresses myofibroblast differentiation of lung resident mesenchymal stem cells and pulmonary fibrosis. Sci Rep 8: 13644, 2018. doi: 10.1038/s41598-018-28968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Königshoff M, Kramer M, Balsara N, Wilhelm J, Amarie OV, Jahn A, Rose F, Fink L, Seeger W, Schaefer L, Günther A, Eickelberg O. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest 119: 772–787, 2009. doi: 10.1172/JCI33950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou L, Yang K, Dunaway S, Abdel-Malek Z, Andl T, Kadekaro AL, Zhang Y. Suppression of MAPK signaling in BRAF-activated PTEN-deficient melanoma by blocking β-catenin signaling in cancer-associated fibroblasts. Pigment cell Melanoma Res 31: 297–307, 2018. doi: 10.1111/pcmr.12657. [DOI] [PubMed] [Google Scholar]
- 79.Guo F, Zhang H, Jia Z, Cui M, Tian J. Chemoresistance and targeting of growth factors/cytokines signalling pathways: towards the development of effective therapeutic strategy for endometrial cancer. Am J Cancer Res 8: 1317–1331, 2018. [PMC free article] [PubMed] [Google Scholar]
- 80.Kimura M, Hashimoto N, Kusunose M, Aoyama D, Sakamoto K, Miyazaki S, Ando A, Omote N, Imaizumi K, Kawabe T, Hasegawa Y. Exogenous induction of unphosphorylated PTEN reduces TGFβ-induced extracellular matrix expressions in lung fibroblasts. Wound Repair Regen 25: 86–97, 2017. [Erratum in Wound Repair Regen 27: 196, 2019] doi: 10.1111/wrr.12506. [DOI] [PubMed] [Google Scholar]
- 81.Winkler I, Bitter C, Winkler S, Weichenhan D, Thavamani A, Hengstler JG, Borkham-Kamphorst E, Kohlbacher O, Plass C, Geffers R, Weiskirchen R, Nordheim A. Identification of Pparγ-modulated miRNA hubs that target the fibrotic tumor microenvironment. Proc Natl Acad Sci USA 117: 454–463, 2020. doi: 10.1073/pnas.1909145117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hettinger K, Vikhanskaya F, Poh MK, Lee MK, de Belle I, Zhang JT, Reddy SA, Sabapathy K. c-Jun promotes cellular survival by suppression of PTEN. Cell Death Differ 14: 218–229, 2007. doi: 10.1038/sj.cdd.4401946. [DOI] [PubMed] [Google Scholar]
- 83.Vasudevan KM, Gurumurthy S, Rangnekar VM. Suppression of PTEN expression by NF-κB prevents apoptosis. Mol Cell Biol 24: 1007–1021, 2004. doi: 10.1128/MCB.24.3.1007-1021.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.He L, Ingram A, Rybak AP, Tang D. Shank-interacting protein-like 1 promotes tumorigenesis via PTEN inhibition in human tumor cells. J Clin Invest 120: 2094–2108, 2010. doi: 10.1172/JCI40778. [DOI] [PMC free article] [PubMed] [Google Scholar]