Abstract
Sprouts of chicory (Cichorium intybus), a vegetable grown in the dark, have a slightly bitter taste associated with the presence of guaianolides, eudesmanolides, and germacranolides. The committed step in the biosynthesis of these compounds is catalyzed by a (+)-germacrene A synthase. Formation of the lactone ring is the postulated next step in biosynthesis of the germacrene-derived sesquiterpene lactones. The present study confirms this hypothesis by isolation of enzyme activities from chicory roots that introduce a carboxylic acid function in the germacrene A isopropenyl side chain, which is necessary for lactone ring formation. (+)-Germacrene A is hydroxylated to germacra-1(10),4,11(13)-trien-12-ol by a cytochrome P450 enzyme, and is subsequently oxidized to germacra-1(10),4,11(13)-trien-12-oic acid by NADP+-dependent dehydrogenase(s). Both oxidized germacrenes were detected as their Cope-rearrangement products elema-1,3,11(13)-trien-12-ol and elema-1,3,11(13)-trien-12-oic acid, respectively. The cyclization products of germacra-1(10),4,11(13)-trien-12-ol, i.e. costol, were also observed. The (+)-germacrene A hydroxylase is inhibited by carbon monoxide (blue-light reversible), has an optimum pH at 8.0, and hydroxylates β-elemene with a modest degree of enantioselectivity.
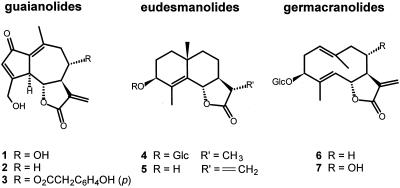
Wild chicory (Cichorium intybus) is a blue-flowered composite plant that has spread all over the world from the Mediterranean and east Asia. Since the seventeenth century it has been cultivated (var sativum) for its bitter roots that were roasted and used in hot coffee-like beverages. Although the use of its roots was displaced by genuine coffee from Coffea arabica, sprouts of chicory var foliosum that are grown in the dark became popular as a vegetable (Belgian endive) halfway through the nineteenth century. Nowadays it is a common crop in Belgium, northern France, and The Netherlands (Weeda et al., 1991; Vogel et al., 1996). The bitter taste of chicory is associated with the presence of sesquiterpene lactones, in particular the guaianolides lactucin (1) (Fig. 1), 8-deoxylactucin (2), and lactupicrin (3). Smaller amounts of eudesmanolides and germacranolides are also present in the plant (Pyrek, 1985; Seto et al., 1988; Price et al., 1990; van Beek et al., 1990).
Figure 1.
The major sesquiterpene lactones of chicory: the guaianolides lactucin (1), 8-deoxylactucin (2), and lactupicrin (3); the eudesmanolides sonchuside C (4) and cichoriolide A (5); and the germacranolides sonchuside A (6) and cichorioside C (7).
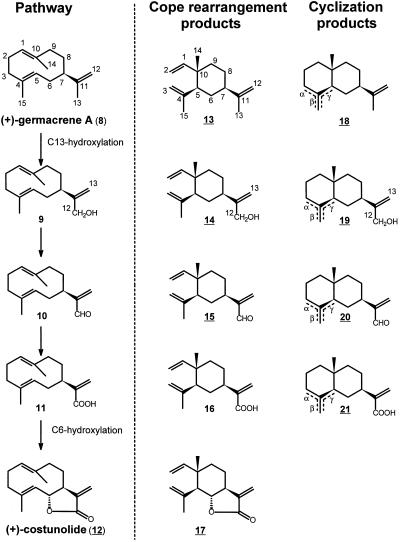
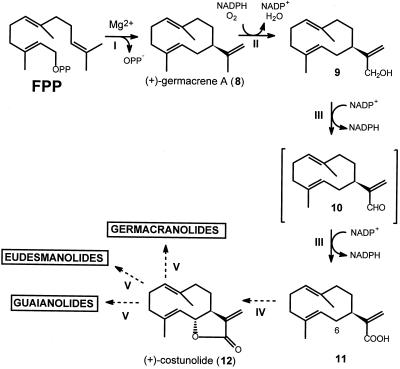
Sesquiterpene lactones are a major class of plant secondary metabolites and over 4,000 different structures have been elucidated. A wealth of information is available about the structural aspects and biological activities of these type of compounds (e.g. Picman, 1986), but little is known about their biosynthesis. By far the largest group of naturally occurring sesquiterpene lactones is the germacranolides, and the majority of sesquiterpene lactones are thought to evolve from this class. The simplest member of the germacranolides (+)-costunolide (12) (Fig. 2) is generally accepted as the common intermediate of all germacranolide-derived lactones (Geissman, 1973; Herz, 1977; Fischer et al., 1979; Seaman, 1982; Song et al., 1995).
Figure 2.
Proposed biosynthetic route from (+)-germacrene A (8) to (+)-costunolide (12) via germacra-1(10),4,11(13)-trien-12-ol (9), germacra-1(10),4,11(13)-trien-12-al (10), and germacra-1(10),4,11(13)-trien-12-oic acid (11). At the right side of the dotted line, compounds are shown that can be formed from these unstable germacrenes: the heat-induced Cope-rearrangement products (-)-β-elemene (13), (-)-elema-1,3,11(13)-trien-12-ol (14), (-)-elema-1,3,11(13)-trien-12-al (15), elema-1,3,11(13)-trien-12-oic acid (16), and dehydrosaussurea lactone (17); and the acid-induced cyclization products selinene (18) (γ-selinene is usually named selina-4, 11-diene), costol (19), costal (20), and costic acid (21). Compounds with underlined numbers have all been identified in costus roots; (+)-germacrene A (8) and germacra-1(10),4,11(13)-trien-12-al (10) were isolated from other plant species. Note that after hydroxylation the numbering of carbon atoms 12 and 13 is inverted.
(+)-Costunolide was first isolated from costus (Saussurea lappa Clarke) roots by Paul et al. (1960) and Somasekar Rao et al. (1960), and has since been found with other sesquiterpene lactones in various plants (Fischer et al., 1979). Among them is lettuce, a species that is closely related to chicory and also contains the bitter tasting compounds lactucin (1) and lactupicrin (3) (Takasugi et al., 1985; Price et al., 1990).
We have recently demonstrated that the sesquiterpenoid backbone of the sesquiterpene lactones in chicory is formed by a (+)-germacrene A synthase, which cyclizes farnesyl diphosphate (FPP) to (+)-germacrene A (8) (Fig. 2; de Kraker et al., 1998). This (+)-germacrene A is not further transformed into a guaiane or an eudesmane, indicating that functionalization of the molecule precedes its cyclization. Studies on the biosynthesis of santonin (Barton et al., 1968) suggested that lactone formation precedes any other oxidation of the sesquiterpenoid ring system (Cordell, 1976), and various authors have proposed a biosynthetic route (Fig. 2) from (+)-germacrene A (8) toward (+)-costunolide (12) (Geissman, 1973; Herz, 1977; Seaman, 1982; Fischer, 1990; Song et al., 1995). In this hypothetical route (+)-germacrene A (8) is hydroxylated to germacra-1(10),4,11(13)-trien-12-ol (9), which is further oxidized via germacra-1(10),4,11(13)-trien-12-al (10) to germacra-1(10),4,11(13)-trien-12-oic acid (11). The germacrene acid is thought to be hydroxylated at the C6-position and subsequent loss of water leads to the formation of a lactone ring such as present in (+)-costunolide (12).
However, germacrenes are notoriously unstable compounds, susceptible to proton-induced cyclizations and heat-induced (e.g. steam distillation and gas chromatography [GC] analysis) Cope rearrangement (Takeda, 1974; Bohlman et al., 1983; Reichardt et al., 1988; Teisseire, 1994; de Kraker et al., 1998). None of the intermediates between (+)-germacrene A and (+)-costunolide has ever been isolated, apart from germacra-1(10),4,11(13)-trien-12-al (10), which was isolated with greatest difficulty from Vernonia glabra and could not be separated from its cyclization product costal (20) (Bohlman et al., 1983). Most likely as a result of this instability, the hypothetical biosynthetic route for (+)-costunolide has merely been based on the isolation from costus roots of the Cope-rearrangement products (-)-elema-1,3,11(13)-trien-12-ol (14) and (-)-elema-1,3,11(13)-trien-12-al (15), and the proton-induced cyclization products costol (19), costal (20), and costic acid (21) (Bawdekar and Kelkar, 1965; Bawdekar et al., 1967; Maurer and Grieder, 1977). Besides the reported (+)-germacrene A synthase from chicory roots (de Kraker et al., 1998), thus far no other enzyme has been isolated that might be involved in this proposed pathway from FPP to (+)-costunolide.
The aim of the present research was to find enzymes in chicory that are involved in oxidation of the germacrene A isopropenyl side chain, and thus to investigate the initial steps in the formation of the lactone ring, as present in (+)-costunolide and other germacrene-derived sesquiterpene lactones.
RESULTS
Demonstration of (+)-Germacrene A Hydroxylase Activity
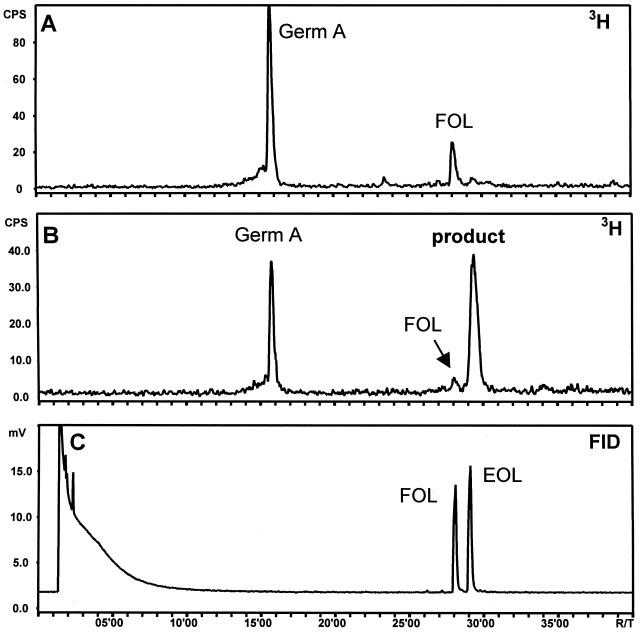
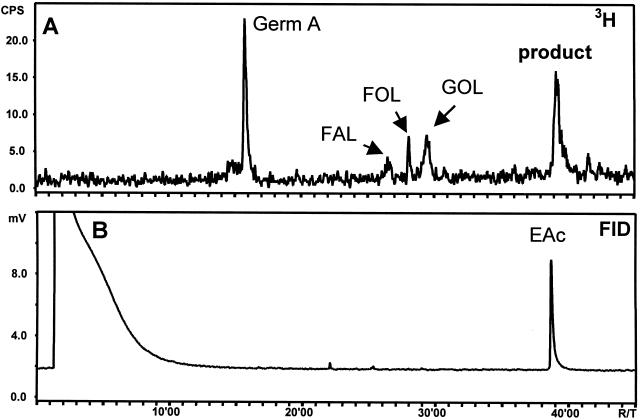
Radio-GC analysis of the pentane-ether extract from the incubation of a 20,000g chicory root supernatant with [3H]FPP revealed a peak of germacrene A as a result of the (+)-germacrene A synthase present in the supernatant (Fig. 3A). In the presence of NADPH, 60% of the (+)-germacrene A was converted into a more polar compound that eluted from the GC column at a higher temperature (Fig. 3B). In both incubations a small amount of farnesol was also formed due to non-specific phosphohydrolases, but it was efficiently reduced by the addition of 6 mm sodium orthovanadate to the assay buffer (Croteau and Karp, 1974). Farnesol formation decreased even further in the presence of the NADPH-regenerating system, likely because Glc-6-P (1 mm) acts as a competitive inhibitor on the phosphohydrolases.
Figure 3.
Radio-GC analyses of the products formed in incubations of a 20,000g supernatant from chicory roots with [3H]FPP in the absence (A) or presence (B) of an NADPH-regenerating system. In the presence of NADPH the produced [3H]germacrene A (8; germ A) converted into a more polar product. C shows the response of the flame ionization detector (FID) to a standard solution of trans,trans-farnesol (FOL) and (-)-elema-1,3,11(13)-trien-12-ol (14) (EOL), injected under the same GC conditions.
In a GC-mass spectrometry (MS) measurement at an injection port temperature of 250°C, germacrene A was detected as its Cope-rearrangement product β-elemene (13). The unknown product was displayed as a sharp peak with the same retention time and mass spectrum as (-)-elema-1,3,11(13)-trien-12-ol (14), the Cope-rearrangement product of germacra-1(10),4,11(13)-trien-12-ol (9). Lowering the injection port temperature to 150°C yielded the slightly fronting peak of germacrene A (de Kraker et al., 1998), but the peak of elema-1,3,11(13)-trien-12-ol was replaced by a broad “hump” in the baseline that started at the position of elema-1,3,11(13)-trien-12-ol and stretched over a 2-min period. If the germacrene alcohol is not rearranged in the injection port, it apparently rearranges during migration through the GC column (HP5-MS) to the faster migrating elema-1,3,11(13)-trien-12-ol and is observed as a broad peak similar to the one described for 7-hydroxygermacrene (Stahl, 1984). Germacra-1(10),4,11(13)-trien-12-ol is also expected to rearrange on the column of the radio GC (cold on column injection), but was nevertheless detected as a somewhat broadened peak that co-elutes with a standard of elema-1,3,11(13)-trien-12-ol (Fig. 3, B and C). It seems that on the Carbowax column of the radio GC there is only little difference in retention time between the elemene and germacrene alcohol.
Considerable amounts of costol (19) were detected when, during the extraction-filtration procedure, aluminum oxide was replaced by silica gel and larger amounts of MgSO4 were used (data not shown). Costol is the acid-induced cyclization product of germacra-1(10),4,11(13)-trien-12-ol, just as costal (20) is derived from germacra-1(10),4,11(13)-trien-12-al (10) (Bohlman et al., 1983). Its observation confirms the conclusion that (+)-germacrene A is enzymatically converted into the somewhat unstable germacra-1(10),4,11(13)-trien-12-ol. Further oxidation of the germacrene alcohol was not observed in any of the (+)-germacrene A hydroxylase assays.
Sesquiterpenoid Dehydrogenase Activities
Incubations of (-)-Elema-1,3,11(13)-trien-12-ol (14) and (-)-Elema-1,3,11(13)-trien-12-al (15)
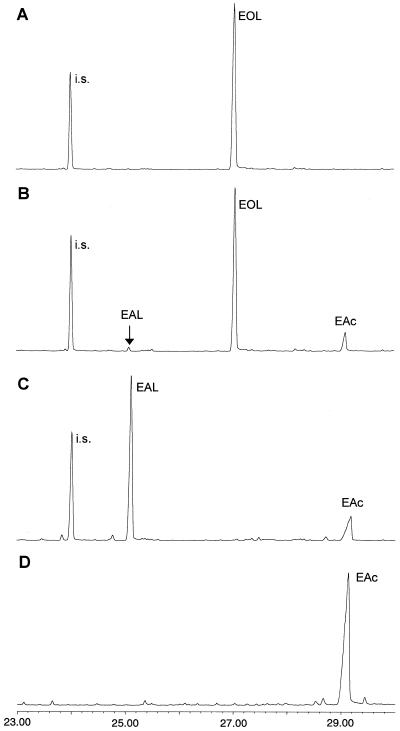
(-)-Elema-1,3,11(13)-trien-12-ol (14) (100 μm) was converted into elema-1,3,11(13)-trien-12-oic acid (16) by a 20,000g chicory root supernatant in the presence of NADP+ at pH 10 (Fig. 4, B and D). This reaction did not take place in the presence of NAD+ or without cofactor (Fig. 4A). After centrifugation at 200,000g, dehydrogenase activity was retained in the supernatant, showing that it originates from soluble enzyme(s). Elema-1,3,11(13)-trien-12-al (15) was only detected in minute quantities, which might be explained by the chemical reactivity of the isopropenal side chain. Elema-1,3,11(13)-trien-12-al added in 10 μm concentration to a solution of 0.5 mg/mL bovine serum albumin (a protein concentration similar to the supernatant) or 2 mm dithiothreitol (DTT) was only extractable for 20% to 50% in comparison with equal amounts of elematrien-12-al that had been added to demineralized water. DTT could, however, not be omitted from the assay buffer, since no dehydrogenase activity was detected in its absence consistent with the literature about dehydrogenases (Kjonaas et al., 1985; Ikeda et al., 1991).
Figure 4.
Identification by GC-MS of the products formed by a 20,000g supernatant from chicory roots incubated with (-)-elema-1,3,11(13)-trien-12-ol (14) (EOL) in the absence (A) or presence of NADP+ (B); or with (-)-elema-1,3,11(13)-trien-12-al (15) (EAL) and NAD+ (C). The produced elema-1,3,11(13)-trien-12-oic acid (16) (EAc) has the same retention time as the synthesized standard (D).
(-)-Elema-1,3,11(13)-trien-12-al (15) (200 μm) was oxidized to elema-1,3,11(13)-trien-12-oic acid most effectively in the presence of NAD+ (Fig. 4C), whereas a smaller aldehyde dehydrogenase activity (≤15% of maximum activity) was detected in the presence of NADP+ or absence of any cofactor. Boiled controls showed no conversion at all. Enzyme activity without cofactor has also been observed for the abietadienal dehydrogenase of grand fir (Funk and Croteau, 1994).
Incubation of [3H]Germacra-1(10),4,11(13)-trien-12-ol (9)
Incubation of [3H]germacra-1(10),4,11(13)-trien-12-ol (9) with the 20,000g supernatant and NADP+ showed a new broadened peak in the [3H]trace of the radio GC, whose front co-elutes with a standard of elema-1,3,11(13)-trien-12-oic acid (16) (Fig. 5). During GC-MS analysis a small peak was detected with the mass spectrum and retention time of elema-1,3,11(13)-trien-12-oic acid. This peak should result from Cope rearrangement of germacra-1(10),4,11(13)-trien-12-oic acid (11) in the GC-MS injection port. Formation of the germacrene/elemene aldehyde was not detected.
Figure 5.
A, Radio-GC analyses of the products formed in an incubation of 20,000g supernatant with NADP+ and a pentane-ether extract from the (+)-germacrene A hydroxylase assay of Figure 3B, containing [3H]germacra-1(10),4,11(13)-trien-12-ol (9; GOL) and smaller amounts of [3H]germacrene A (8) (germ A) and [3H]farnesol (FOL). A more polar product is formed together with a minute amount of [3H]farnesal (FAL). Trace B shows the response of the FID to a standard solution of elema-1,3,11(13)-trien-12-oic acid (16) (EAc).
Characterization of the (+)-Germacrene A Hydroxylase
Hydroxylation of (+)-germacrene A was optimal at a pH of 8.0 with 60% of maximal enzyme activity at pH 7.5 and 9.0 (no difference in activity between Bis-Tris and Tris). The reaction required NADPH. NADH was less effective as a reductant (Table I). The combination of both cofactors showed an additive effect, which is not uncommon for plant cytochrome P450 systems and is believed to result from the participation of NADH:cytochrome b reductase and NADPH:cytochrome P450 reductase in electron transfer to cytochrome P450 (West, 1980; Funk and Croteau, 1994). Flushing the reaction mixture for 1.5 min with Argon prior to incubation caused a 69% decrease of enzyme activity because of O2 depletion. An assay buffer without flavins (flavin adenine dinucleotide [FAD] and flavin mononucleotide [FMN]) gave 18% loss of hydroxylase activity; however, omitting these flavins from the extraction buffer resulted in a loss of more than 70% in enzyme activity (data not shown).
Table I.
Requirements for (+)-germacrene A hydroxylase activity
| Additiona | Percentage Enzyme Activityb ± sd |
|---|---|
| None | 3 ± 0.5 |
| 1 mm NADPH | 100 ± 9.8 |
| 1 mm NADH | 30 ± 4.7 |
| 1 mm NADPH + 1 mm NADH | 120 ± 11 |
| 1 mm NADPH − flavins | 82 ± 4.8 |
| 1 mm NADPH + argon atmosphere | 31 ± 5.1 |
Flavins were present during incubation, unless otherwise mentioned; NAD(P)H was regenerated during incubation.
100% enzyme activity corresponds to an elematriene-12-ol peak size of 0.96 × internal standard (5 nmol cis-neridol in each assay).
The results described above and the observation that the enzyme activity resided in the 150,000g pellet support the involvement of a cytochrome P450 enzyme in the hydroxylation of (+)-germacrene A. This was confirmed by the effect of cytochrome P450 inhibitors on (+)-germacrene A hydroxylase activity: cytochrome C (100 μm) caused 97% inhibition; miconazole (100 μm) caused 30% inhibition; aminobenzotriazole (100 μm) 26% inhibition; metyrapone (1 mm) caused 23% inhibition; and clotrimazole (100 μm) caused 16% inhibition. Somewhat unexpectedly, all of them except cytochrome C could inhibit (+)-germacrene A synthase activity as well.
The strongest proof for the involvement of a cytochrome P450 enzyme is blue-light reversible inhibition of enzyme activity by CO (West, 1980; Mihaliak et al., 1993). An atmosphere of 80% CO + 20% O2 inhibits (+)-germacrene A hydroxylase by 69% (Table II). The corresponding decrease of the elema-1,3,11(13)-trien-12-ol peak was accompanied by an increase in the GC-MS peak size of β-elemene, because the production of (+)-germacrene A from FPP was not affected by CO. The inhibitory effect of CO on (+)-germacrene A hydroxylase activity could be convincingly reversed by blue light (450 nm).
Table II.
Carbon monoxide inhibition and blue-light reversal of (+)-germacrene A hydroxylase activity
| Assay Conditionsa | Percentage Enzyme Activityb ± sd |
|---|---|
| Air (≈ 80% N2+ 20% O2) | |
| Dark | 100 ± 2.9 |
| 450 nm light | 92 ± 6.7 |
| 80% CO + 20% O2 | |
| Dark | 31 ± 1.5 |
| 450 nm light | 50 ± 6.7 |
1 mm NADPH-regenerating system was present in all incubations, but no flavins were added.
100% enzyme activity corresponds to an elematriene-12-ol peak size of 1.03 × internal standard (5 nmol cis-neridol in each assay).
Enantioselectivity of the (+)-Germacrene A Hydroxylase
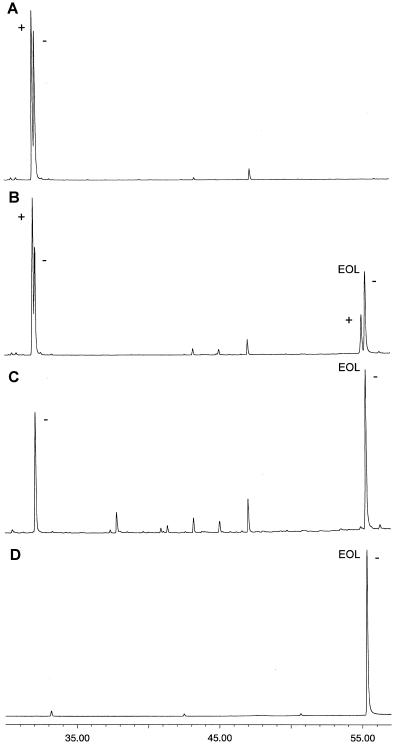
A microsomal preparation from chicory roots was able to convert β-elemene (13) into elema-1,3,11(13)-trien-12-ol (14). Addition of 100 μm β-elemene to the (+)-germacrene A hydroxylase assay reduced the conversion of [3H]germacrene A into [3H]germa-cratrien-12-ol with 37%. This substrate competition shows that both hydroxylations are most likely catalyzed by the same enzyme, so β-elemene can be used to investigate the enantioselectivity of the (+)-germacrene A hydroxylase. The amount of (+)-β-elemene hydroxylated was only two times less than that of (-)-β-elemene (Fig. 6), the structural analog of (+)-germacrene A. This indicates a modest enantioselectivity of the (+)-germacrene A hydroxylase.
Figure 6.
Determination of the stereochemical preference of the (+)-germacrene A hydroxylase using GC-MS equipped with an enantioselective column in selected ion monitoring-mode (m/z 119, 121, 145, 147, and 189). A, Incubation of (±)-β-elemene in the absence of NADPH (blank). B, Incubation of (±)-β-elemene in the presence of NADPH, resulting in a mixture of elema-1,3,11(13)-trien-12-ol (14) enantiomers (EOL). C, Incubation of (-)-β-elemene giving (-)-elema-1,3,11(13)-trien-12-ol. D, Standard of (-)-elema-1,3,11(13)-trien-12-ol (EOL).
DISCUSSION
The present results have established the pathway for biosynthesis in chicory roots of germacra-1(10),4,11(13)-trien-12-oic acid (11), a compound en route to the germacrene-derived sesquiterpene lactones. The reported oxidation of the (+)-germacrene A isopropenyl side chain supports the hypothesis that formation of the lactone ring precedes any cyclization or other oxidation of the germacrene framework (Cordell 1976; de Kraker et al., 1998). Formation of (+)-costunolide (12) itself has not yet been demonstrated, but the results presented are in line with the proposed pathway for this germacranolide, which is structurally most closely related to (+)-germacrene A (Geissman, 1973; Herz, 1977; Fischer et al., 1979; Seaman, 1982; Song et al., 1995).
The biosynthesis of germacrene-derived sesquiterpene lactones (Fig. 7) starts with the cyclization of FPP into (+)-germacrene A (8) by a sesquiterpene cyclase (reaction I; de Kraker et al., 1998). In the next step (reaction II), the isopropenyl side chain of this (+)-germacrene A (8) is subjected to a hydroxylation reaction, resulting in the formation of germacra-1(10),4,11(13)-trien-12-ol (9). This sesquiterpene alcohol is visible as its Cope-rearrangement product (-)-elema-1,3,11(13)-trien-12-ol (14) in GC-MS measurements at high injection port temperatures, and it is detected as costol (19) when extracted under acidic conditions. The involvement of a cytochrome P450 enzyme in this membrane-bound hydroxylation reaction is clearly demonstrated by its NADPH and O2 dependence, by the blue-light reversible inhibition by CO, and by the inhibitory effect of various established cytochrome P450 inhibitors (West, 1980; Mihaliak et al., 1993).
Figure 7.
Proposed biosynthetical route for the germacrene-derived sesquiterpene lactones present in chicory. I, Cyclization of FPP to (+)-germacrene A (8) by a sesquiterpene synthase. II, Hydroxylation of the isopropenyl side chain by (+)-germacrene A hydroxylase, a cytochrome P450 enzyme. III, Oxidation of germacratrien-12-ol (9) to germacratrien-12-oic acid (11) by NADP+-dependent dehydrogenase(s) via the intermediate germacratrien-12-al (10). IV, The not-yet-demonstrated hydroxylation at the C6-position of germacratrien-12-oic acid and subsequent lactonization to (+)-costunolide (12). V, The postulated formation of guaiane, eudesmane, and germacrane lactones.
The conversion of germacra-1(10),4,11(13)-trien-12-ol (9) into germacra-1(10),4,11(13)-trien-12-oic acid (11) (Fig. 7; reaction III) is catalyzed by water-soluble pyridine nucleotide dependent dehydrogenase(s). This enzyme activity oxidizes germacrene alcohol (9) as well as (-)-elema-1,3,11(13)-trien-12-ol (14) to their corresponding acids (11 and 16) solely in the presence of NADP+. Because two hydrides have to be abstracted to obtain the acid, we assume that the stoichiometry is two NADP+ molecules for each molecule of germacrene/elemene acid formed. Since we worked with a root crude extract it is uncertain whether formation of germacra-1(10),4,11(13)-trien-12-oic acid (11) from the germacrene alcohol is catalyzed by one or more dehydrogenase(s), and whether oxidation occurs via germacra-1(10),4,11(13)-trien-12-al (10) or within one enzymatic step. The aldehyde was not detected in incubations with the germacrene alcohol and only in trace amounts in incubations with the elemene alcohol. A study of the oxidation of perilla alcohol to perillic acid by bacterial extracts (Dhavalikar et al., 1966) could not demonstrate the presence of the expected aldehyde intermediate either. Isopropenal side chains are sensitive toward nucleophilic attack, and both proteins and DTT were demonstrated to “bind” elema-1,3,11(13)-trien-12-al (15). It could be a reason why these aldehydes are not or hardly detectable in enzyme assays. Despite all of that, enzymatic oxidation of elema-1,3,11(13)-trien-12-al (15) to the elemene acid (16) could be established with the chicory root supernatant, but strikingly, NAD+ was preferred over NADP+ and some conversion occurred also in the absence of any cofactor.
Neither germacra-1(10),4,11(13)-trien-12-oic acid (11) nor elema-1,3,11(13)-trien-12-oic acid (16) has ever been isolated from a natural source (Buckingham, 1999), but we managed to detect a small amount of elema-1,3,11(13)-trien-12-oic acid in costus resinoid with the help of the chemically produced standard (J.-W. de Kraker, unpublished data). In general, cyclic mono- and sesquiterpenoid acids are rather uncommon in plants (Bauer et al., 1990) and little is known about their biosynthesis.
The established pathway for germacrene A carboxylic acid resembles the biodegradation of limonene via perillic acid in bacteria (Dhavalikar et al., 1966) and the biosynthesis of monoterpenoid aldehydes/ketones in plants (e.g. McConkey et al., 2000), but with regard to the dehydrogenases it differs from the diterpenoid pathways of kaurenoic and abietic acid (Funk and Croteau, 1994). We assume that in the biosynthesis of the sesquiterpene endoperoxide artemisinin by Artemisia annua oxidation of the isopropenyl side chain of amorphadiene is catalyzed in the same way as the oxidation of (+)-germacrene A to germacrene acid (Bouwmeester et al., 1999).
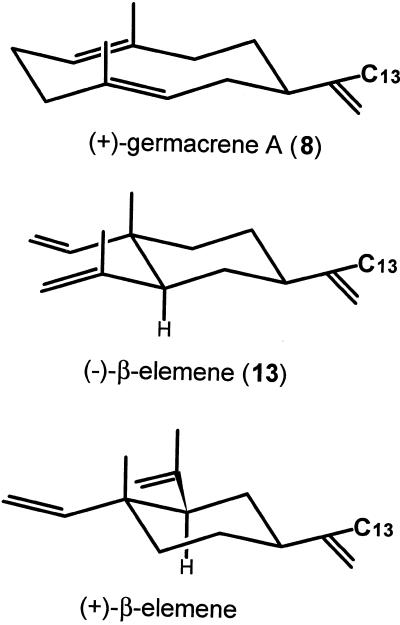
Cytochrome P450 enzymes involved in plant terpenoid secondary metabolism are known to be rather substrate specific (West, 1980; Mihaliak et al., 1993). Nevertheless, β-elemene (13) is hydroxylated by the (+)-germacrene A hydroxylase of chicory and competitively inhibits the hydroxylation of (+)-germacrene A, probably because of the strong similarity in three-dimensional structure between (+)-germacrene A and β-elemene (Fig. 8). β-Elemene contains two isopropenyl side chains, but its hydroxylation is regioselective and only elema-1,3,11(13)-trien-12-ol (14) is formed. When the C13-containing isopropenyl tails of the β-elemene enantiomers are depicted in the same way (Fig. 8), there are no evident differences at the side of hydroxylation and the enzyme only shows a modest degree of enantioselectivity toward these enantiomers. A similar degree of enantioselectivity is also observed for the hydroxylation of limonene in peppermint (C3), spearmint (C6), and perilla (C7; Karp et al., 1990), though the limonene C6-hydroxylase in caraway has a 10-fold preference for the (+)-enantiomer (Bouwmeester et al., 1998). The modest enantioselectivity of the (+)-germacrene A hydroxylase has no influence on the stereochemistry of the sesquiterpene lactones present in chicory, as in vivo only (+)-germacrene A is offered to the enzyme (de Kraker et al., 1998).
Figure 8.
Three-dimensional structures of (+)-germacrene A, (-)-β-elemene, and (+)-β-elemene demonstrating the resemblance of these compounds at the site of hydroxylation (C13-position).
In general, all guaianolides, eudesmanolides, and germacranolides are thought to origin from (+)-costunolide (12) (Fischer, 1990; Fig. 7; reaction V) and the same holds true for the bitter sesquiterpene lactones of chicory. Formation of (+)-costunolide from germacrene acid (11) involves a C6-hydroxy-lation, after which formation of the lactone ring can be completed (reaction IV). This probably cytochrome P450 catalyzed step is currently under investigation.
MATERIALS AND METHODS
Materials
Fresh roots of cultivated chicory (Cichorium intybus L. cv Focus) harvested during late summer were obtained from a grower in Veenendaal, The Netherlands. The chicory roots were cut into small pieces, frozen in liquid nitrogen, and were stored at −80°C. An FPP solution was obtained from Sigma (Zwijndrecht, The Netherlands) and was concentrated to 10 μm (de Kraker et al., 1998); [1-3H]FPP (16.0 Ci mmol−1, 200 μCi mL−1) was obtained from Amersham (Buckinghamshire, UK). trans,trans-Farnesol, cis-nerolidol, and trans-caryophyllene were purchased from Fluka (Buchs, Switzerland). (±)-β-Elemene and (-)-β-elemene (13) were a gift from Prof. Dr. W.A. König (Hamburg University, Germany). (-)-Elema-1,3,11(13)-trien-12-ol (14) was a gift from Dr. B. Maurer (Firmenich SA, Geneva). Spectra and retention times of costol (19) were recorded from costus root oil (Pierre Chauvet SA, Seillans, France; Maurer and Grieder, 1977). Ether (diethyl ether) and pentane were redistilled before use.
Preparation of Elema-1,3,11(13)-trien-12-oic Acid (16)
(-)-Elema-1,3,11(13)-trien-12-al (15) necessary for the synthesis of elema-1,3,11(13)-trien-12-oic acid was isolated from 5 g of costus root oil that had been stirred twice for 6 h with 10 g of MnO2 (Merck, Darmstadt, Germany) in 100 mL of pentane. The aldehydes and ketones were extracted from the oil with 3 g of Girard P and were separated by column chromatography as described by Maurer and Grieder (1977). It yielded 13 mg of (-)-elema-1,3,11(13)-trien-12-al (15), 15 mg of β-costal (20), 22 mg of a α/γ-costal mixture (20), and smaller amounts of trans-bergamota-2,12-dienal and ionone. To obtain elema-1,3,11(13)-trien-12-oic acid (16), 2 mg of the corresponding aldehyde was dissolved in 1.2 mL of tert-butyl alcohol and 0.3 mL of 2-methyl-2-butene to which 0.5 mL of a solution of sodium dihydrogen phosphate and sodium chlorite (0.1 g mL−1 each) was carefully added (Balkrishna et al., 1981). The mixture was vigorously shaken overnight at room temperature. Volatile components were afterward removed under vacuum and the residue was dissolved in 1.5 mL of demineralized water and 20 μL of 2-methyl-2-butene. On ice, the aqueous layer was acidified with 20 μL of 5 m HCl to pH 3 and was quickly extracted with four portions of 1.5 mL of ether. The combined ether layers were washed with 1 mL of cold demineralized water and were dried with MgSO4. After removal of ether, elema-1,3,11(13)-trien-12-oic acid (16) was obtained as a white powder (1.5 mg). 1H-Nuclear magnetic resonance (NMR; 400 MHz, CDCl3): δ 1.04 (s, 3H, Me14); δ 1.2 to 1.7 (m, 6H); δ 1.74 (br s, 3H, Me15); δ 2.11 (dd, 1H, H5, J5, 6 = 3.4 Hz, J5, 6′ = 13 Hz); δ 2.54 (dddd, 1H, H7, J6, 7 = J7, 8 = 3 Hz, J6′, 7 = J7, 8′ = 12 Hz); δ 4.60 (br s, 1H, H3); δ 4.80 (m, 1H, H3'); δ 4.92 (dd, 1H, H2, J1, 2 = 10 Hz, J2, 2′ = 1.4 Hz); δ 4.96 (dd, 1H, H2′, J1, 2′ = 18 Hz, J2, 2′ = 1.4 Hz); δ 5.71 (s, 1H, H13); δ 5.85 (dd, H1, J1, 2 = 10 Hz, J1, 2′ = 18 Hz); δ 6.33 (s, 1H, H13′). 13C-NMR (400 MHz, DEPT, CDCl3) δ 17.0 (q), δ 25.3 (q), δ 27.6 (t), δ 30.1 (s), δ 33.7 (t), δ 39.8 (d), δ 40.1 (t), δ 52.9 (d), δ 110.5 (t), δ 112.7 (t), δ 125.2 (t), δ 145.0 (s), δ 147.8 (s), δ 150.4 (d), δ 171.5 (s). EIMS (70 eV) m/z: 234 [M]+ (1), 219 (11), 201 (5), 189 (18), 81 (100), 205 (4), 193 (7), 177 (20), 173 (13), 147 (18), 133 (22), 121 (31), 119 (29), 117 (15), 107 (38), 105 (47), 93 (48), 91 (76), 79 (84), 77 (58), 68 (59), 67 (94), 65 (35), 55 (45), 53 (83), 41 (97), 39 (67).
Enzyme Isolation and Assay for (+)-Germacrene A Hydroxylase Activity
We prepared a cell-free extract of chicory roots that contained the sesquiterpene synthase (germacrene A synthase) and the microsomal-bound cytochrome P450 enzymes (e.g. sesquiterpene hydroxylases), similar to the approach of Threlfall and Whitehead (1988) that had demonstrated hydroxylation of 5-epi-aristolochene in tobacco (Whitehead et al., 1989). Twenty-five grams of frozen root material was homogenized in a Sorvall Omni-mixer (Newtown, CT) with 2.5 g of insoluble polyvinylpolypyrrolidone and 40 mL of buffer containing 50 mm Tris (pH 7.5), 50 mm sodium meta-bisulfite, 50 mm ascorbic acid, 10 mm MgCl2, 5 mm DTT, 2.5 mm EDTA (disodium), 5 μm FAD, 5 μm FMN, and 20% (v/v) glycerol (buffer A). The slurry was filtered through pre-moistened cheesecloth with an additional 10 mL of buffer and was centrifuged for 20 min at 20,000g at 4°C. The supernatant was filtered through rough glass wool and was desalted with an Econo-Pac 10Dg column (Bio-Rad, Hercules, CA) to buffer B. This buffer contained 25 mm Tris (pH 7.5), 10 mm MgCl2, 2 mm DTT, 1 mm ascorbic acid, 5 μm FAD, 5 μm FMN, 10% (v/v) glycerol, and 6 mm sodium orthovanadate to suppress phosphohydrolase activity (Croteau and Karp, 1979). A 1-mL aliquot of the desalted supernatant was incubated with 20 μm [3H]FPP (50 Ci mol−1) for 1 h at 30°C in the absence or presence of an NADPH-regenerating system. The NADPH-regenerating system consisted of 1 mm NADPH, 5 mm Glc-6-P, and 1.2 IU Glc-6-P dehydrogenase (all from Sigma).
After incubation the assay was extracted twice with 1 mL of 20% (v/v) ether in pentane and the organic phase was filtered through a glasswool-plugged (dimethyl chlorosilane-treated glasswool; Chrompack, Bergen op Zoom, The Netherlands) Pasteur pipette that contained 0.90 g of aluminum oxide (grade III) and a little anhydrous MgSO4. The column was washed with 1.5 mL of ether and the extract was carefully concentrated to approximately 50 μL under a stream of nitrogen. Samples of 1 μL were analyzed by radio GC and GC-MS. Radio-GC analyses was achieved by cold on column injection according to Bouwmeester et al. (1999), using a final oven temperature of 240°C. Signals of the FID and radioactivity detector were synchronized with [3H]farnesol (a gift of dr. E. Wallaart, University of Groningen). GC-MS analysis was essentially carried out as before (de Kraker et al., 1998) at an injection port temperature of 250°C and an oven programmed for 4 min at 55°C followed by a ramp of 5°C to 280°C.
Assay for Sesquiterpenoid Dehydrogenase Activities
The involvement of dehydrogenases in the oxidation of germacra-1(10),4,11(13)-trien-12-ol (9) was initially investigated with (-)-elema-1,3,11(13)-trien-12-ol (14). A 20,000g supernatant was prepared as described above, but MgCl2, EDTA, and flavins were omitted from buffer A. The supernatant was desalted to buffer C containing 25 mm Gly (pH 10.0), 10% (v/v) glycerol and 2 mm DTT (Bouwmeester et al., 1998). Aliquots of 1 mL were incubated at 30°C with 100 μm of substrate and 1 mm NAD+, 1 mm NADP+, or no cofactor. Boiled controls were included. After 90 min, 20 μm cis-nerolidol was added as internal standard and each incubation was acidified with 20 μL of 5 m HCl. The incubation mixture was extracted three times with 1 mL of ether and the combined ether layers were filtered through a Pasteur pipette that contained 0.45 g of silica and a little anhydrous MgSO4. The pipette was rinsed with 1 mL of ether and the extract was concentrated to approximately 150 μL and analyzed by GC-MS. Experiments were repeated with 200 μm of (-)-elema-1,3,11(13)-trien-12-al (15).
[3H]Germacra-1(10),4,11(13)-trien-12-ol (9) was produced in (+)-germacrene A hydroxylase assays that consisted of 1.5 mL of supernatant and 50 μm [3H]FPP (40 Ci mol−1). The pentane-ether extracts of two of such assays were combined, concentrated to approximately 10 μL, and added to one dehydrogenase assay. After incubation, the reaction mixture was carefully acidified with 0.5 m HCl to pH 3 and extracted with ether. The extract was concentrated to approximately 50 μL and was analyzed by radio GC and GC-MS.
Characterization of the (+)-Germacrene A Hydroxylase
(+)-Germacrene A (8) necessary for characterization of the (+)-germacrene A hydroxylase was produced in 1.5-mL enzyme assays consisting of 150,000g supernatant, 50 μm FPP (de Kraker et al., 1998), and an additional 6 mm sodium orthovanadate. The pentane-ether extracts of several of these incubations were combined and the volume was carefully reduced under a flow of nitrogen. The concentration of (+)-germacrene A was estimated by comparison with a 10 μm trans-caryophyllene solution using GC-MS; in general, one incubation of 50 μm FPP with 150,000g supernatant would give sufficient substrate for one hydroxylase experiment (approximately 10 μm germacrene A).
The optimum pH of the (+)-germacrene A hydroxylase was determined by desalting the 20,000g supernatant to buffer B without MgCl2, in which 25 mm Tris was replaced by 50 mm Bis-Tris of pH 6.5 to 9.0 in 0.5-unit increments. After 30 min of incubation 5 μm of cis-nerolidol was added as internal standard and the incubation mixtures were extracted and analyzed by GC-MS. Areas of the product peak were compared with those of cis-nerolidol.
Inhibition of the (+)-germacrene A hydroxylase by established cytochrome P450 inhibitors was demonstrated with microsomal pellets. They were prepared by centrifugation of 8 mL of 20,000g supernatant (without MgCl2) at 150,000g and stored at −80°C under argon. Before use the pellets were resuspended in 2 mL of buffer B without MgCl2, combined, and divided into 1-mL aliquots. Inhibitors were dissolved in ethanol: clotrimazole (10 mm), miconazole (10 mm), aminobenzotriazole (10 mm), and metyrapone (100 mm); cytochrome C (10 mm) was dissolved in buffer. Ten microliters of one of these solutions was added to 1 mL of enzyme preparation 15 min prior to the addition of (+)-germacrene A and NADPH-regenerating system, after which the incubations were continued for 1 h. Inhibitory effects were expressed relative to a control for the solvent, and each inhibitor was tested in duplicate.
For demonstration of blue-light reversible CO inhibition of (+)-germacrene A hydroxylase activity, a mixture of 80% CO and 20% O2 was prepared from pure CO and O2. In 4.5-mL septum-capped vials, 50 μm FPP and an NADPH-regenerating system were added to 1 mL of 20,000g supernatant that had been desalted to buffer B devoid of flavins. Reaction mixtures were slowly bubbled with 50 mL of the gas mixture via a needle inserted through the (vented) septum. CO-treated vials and non-CO-treated control vials were incubated on a rotary shaker in a climate chamber at 30°C in blue light or protected from the light by aluminum foil. Blue light was obtained by passing a beam of a 100 W H44GS-100 mercury lamp (Sylvania, Winchester, KY; ballast no. 1A024, Grainger, Morton Grove, IL) through a thin-layer chromatography chamber (width of 7 cm) filled with a 10% (w/v) CuSO4 solution (Karp et al., 1987). After 1 h of incubation, 5 μm cis-nerolidol was added as internal standard and the assays were extracted and analyzed by GC-MS. Each treatment was done in triplicate. In similar experiments the effects of an Argon atmosphere, NADH, and the absence of flavins were tested on (+)-germacrene A hydroxylase activity.
Enantioselectivity of the (+)-Germacrene A Hydroxylase
The enantioselectivity of the (+)-germacrene A hydroxylase was investigated with (-)-β-elemene (13) and (±)-β-elemene, as we had no source of (-)-germacrene A at our disposal. Aliquots of 1 mL of resuspended microsomal pellet were incubated with 20 μm β-elemene in the presence or absence of an NADPH-regenerating system. Extracts of the enzyme assay were analyzed on an enantioselective column (de Kraker et al., 1998) programmed at 45°for 4 min, a ramp of 2°C min−1 to 170°C, and a final time of 10 min; spectra were recorded in Scan mode and Selected Ion Monitoring Mode (m/z 119, 121, 145, 147, and 189). In a separate experiment, 100 μm of (-)-β-elemene was added to the standard germacrene A hydroxylase assay to demonstrate whether it competed as a substrate with [3H]germacrene A. Production of [3H]germacratrien-12-ol was measured by radio CG and compared with that of incubations where only 10 μL of ethanol (the solvent of β-elemene) had been added.
ACKNOWLEDGMENTS
The authors are most grateful to Dr. B. Maurer (Firmenich SA, Switzerland) for the gift of (-)-elema-1,3,11(13)-trien-12-ol, and to Prof. W.A. König (Hamburg University, Germany) for the gift of the β-elemene enantiomers. We would also like to thank J. de Mik for the gift of the chicory roots, Dr. M.A. Posthumus for GC-MS analyses of the costus root oil, A. van Veldhuizen for collecting NMR data, W.P.A. Boschman for fixing the HGL-file converter, and F.W.A. Verstappen, Dr. L.H. Stevens, and Dr. C.O. Schmidt for their useful suggestions.
LITERATURE CITED
- Balkrishna BL, Childers WE, Pinnick JR, Pinnick HW. Oxidation of α, β-unsaturated aldehydes. Tetrahedron. 1981;37:2091–2096. [Google Scholar]
- Barton DHR, Moss GP, Whittle JA. Investigations on the biosynthesis of steroids and terpenoids part I: a preliminary study of the biosynthesis of santonin. J Chem Soc (C) 1968;XX:1813–1818. [Google Scholar]
- Bauer K, Garbe D, Surburg H. Common Fragrance and Flavor Materials: Preparation, Properties and Uses. Ed 2. Weinheim, Germany: VCH Verlagsgesellschaft; 1990. [Google Scholar]
- Bawdekar AS, Kelkar GR. Terpenoids: LXVIII. Structure and absolute configuration of costic acid: a new sesquiterpene acid from costus root oil. Tetrahedron. 1965;21:1521–1528. [Google Scholar]
- Bawdekar AS, Kelkar GR, Bhattacharyya SC. Terpenoids: CIV. Costol fraction of costus root oil. Tetrahedron. 1967;23:1993–1996. [Google Scholar]
- Bohlman F, Ates N, Jakupovic J. Hirsutinolides from South African Vernonia species. Phytochemistry. 1983;22:1159–1162. [Google Scholar]
- Bouwmeester HJ, Gershenzon J, Konings MCJM, Croteau R. Biosynthesis of the monoterpene limonene and carvone in the fruit of caraway: I. Demonstration of enzyme activities and their changes with development. Plant Physiol. 1998;117:901–912. doi: 10.1104/pp.117.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwmeester HJ, Wallaart TE, Janssen MHA, van Loo B, Jansen BJM, Posthumus MA, Schmidt CO, de Kraker J-W, König WA, Franssen MCR. Amorpha-4,11-diene synthase catalyzes the first probable step in artemisinin biosynthesis. Phytochemistry. 1999;52:843–854. doi: 10.1016/s0031-9422(99)00206-x. [DOI] [PubMed] [Google Scholar]
- Buckingham J. Dictionary of Natural Products on CD-ROM, version 8.1. London: Chapman & Hall; 1999. [Google Scholar]
- Cordell GA. Biosynthesis of sesquiterpenes. Chem Rev. 1976;76:425–460. [Google Scholar]
- Croteau R, Karp F. Biosynthesis of monoterpenes: hydrolysis of bornyl pyrophosphate, an essential step in camphor biosynthesis, and hydrolysis of geranyl pyrophosphate, the acyclic precursor of camphor by enzymes from sage (Salvia officinalis) Arch Biochem Biophys. 1979;198:523–532. doi: 10.1016/0003-9861(79)90527-7. [DOI] [PubMed] [Google Scholar]
- de Kraker J-W, Franssen MCR, de Groot A, König WA, Bouwmeester HJ. (+)-Germacrene A biosynthesis: the committed step in the biosynthesis of sesquiterpene lactones in chicory. Plant Physiol. 1998;117:1381–1392. doi: 10.1104/pp.117.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhavalikar RS, Rangachari PN, Bhattacharyya PK. Microbiological transformations of terpenes: Part IX. Pathways of degradation of limonene in a soil pseudomonad. Indian J Biochem. 1966;3:158–164. [PubMed] [Google Scholar]
- Fischer NH. Sesquiterpene lactones: biogenesis and biomimetic transformations. In: Towers G, Towers H, editors. Biochemistry of the Mevalonic Acid Pathway to Terpenoids. New York: Plenum Press; 1990. pp. 161–201. [Google Scholar]
- Fischer NH, Olivier EJ, Fischer HD. The biogenesis and chemistry of sesquiterpene lactones. In: Herz W, Grisebach H, Kirby GW, editors. Progress in the Chemistry of Organic Natural Products. Vol. 38. New York: Springer-Verlag; 1979. [Google Scholar]
- Funk C, Croteau R. Diterpenoid resin acid biosynthesis in conifers: characterization of two cytochrome P450-dependent monooxygenases and an aldehyde dehydrogenase involved in abietic acid biosynthesis. Arch Biochem Biophys. 1994;308:258–266. doi: 10.1006/abbi.1994.1036. [DOI] [PubMed] [Google Scholar]
- Geissman TA. The biogenesis of sesquiterpene lactones of the compositae. In: Runeckles VC, Marby TJ, editors. Recent Advances in Phytochemistry. Vol. 6. New York: Academic Press; 1973. pp. 65–95. [Google Scholar]
- Herz W. Sesquiterpene lactones in the compositae. In: Heywood VH, Harborne JB, Turner BL, editors. The Biology and Chemistry of the Compositae. London: Academic Press; 1977. pp. 337–357. [Google Scholar]
- Ikeda H, Esaki N, Nakai S, Hashimoto K, Uesato S, Soda K, Fujita T. Acyclic monoterpene primary alcohol:NADP+ oxidoreductase of Rauwolfia serpentina cells: the key enzyme in biosynthesis of monoterpene alcohols. J Biochem. 1991;109:341–347. [PubMed] [Google Scholar]
- Karp F, Harris JL, Croteau R. Metabolism of monoterpenes: demonstration of the hydroxylation of (+)-sabinene to (+)-cis-sabinol by an enzyme preparation from sage (Salvia officinalis) leaves. Arch Biochem Biophys. 1987;256:179–193. doi: 10.1016/0003-9861(87)90436-x. [DOI] [PubMed] [Google Scholar]
- Karp F, Mihaliak CA, Harris JL, Croteau R. Monoterpene biosynthesis: specificity of the hydroxylations of (-)-limonene by enzyme preparations from peppermint (Mentha piperita), spearmint (Mentha spicata) and perilla (Perilla frutescens) leaves. Arch Biochem Biophys. 1990;276:219–226. doi: 10.1016/0003-9861(90)90029-x. [DOI] [PubMed] [Google Scholar]
- Kjonaas RB, Venkatachalam KV, Croteau R. Metabolism of monoterpenes: oxidation of isopiperitenol to isopiperitenone and subsequent isomerization to piperitenone by soluble enzyme preparations from peppermint (Mentha piperita) leaves. Arch Biochem Biophys. 1985;238:49–60. doi: 10.1016/0003-9861(85)90139-0. [DOI] [PubMed] [Google Scholar]
- Maurer B, Grieder A. Sesquiterpenoids from costus root oil (Saussurea lappa Clarke) Helv Chim Acta. 1977;60:2177–2190. doi: 10.1002/hlca.19770600710. [DOI] [PubMed] [Google Scholar]
- McConkey ME, Gershenzon J, Croteau RB. Developmental regulation of monoterpene biosynthesis in the glandular trichomes of peppermint. Plant Physiol. 2000;122:215–223. doi: 10.1104/pp.122.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaliak CA, Karp F, Croteau . Cytochrome P-450 terpene hydroxylases. In: Lea PJ, editor. Methods in Plant Biochemistry: Enzymes of Secondary Metabolism. Vol. 9. London: Academic Press; 1993. pp. 261–279. [Google Scholar]
- Paul A, Bawdekar AS, Joshi RS, Somesekar Roa A, Kelkar GR, Bhattacharyya SC. Terpenoids XX: examination of costus root oil. Perfume Essential Oil Rec. 1960;15:115–120. [Google Scholar]
- Picman AK. Review article number 7: biological activities of sesquiterpene lactones. Biochem Syst Ecol. 1986;14:255–281. [Google Scholar]
- Price KR, DuPont MS, Shepherd R, Chan HW-S, Fenwick GR. Relationship between the chemical and sensory properties of exotic salad crops: colored lettuce (Lactuca sativa) and chicory (Cichorium intybus) J Sci Food Agric. 1990;53:185–192. [Google Scholar]
- Pyrek JST. Sesquiterpene lactones of Cichorium intybus and Leontodon autumnalis. Phytochemistry. 1985;24:186–188. [Google Scholar]
- Reichardt PB, Anderson BJ, Clausen TP, Hoskins LC. Thermal instability of germacrone: implications for gas chromatographic analysis of thermally unstable analytes. Can J Chem. 1988;67:1174–1177. [Google Scholar]
- Seaman FC. Sesquiterpene lactones as taxonomic characters in the Asteraceae. Bot Rev. 1982;48:124–145. [Google Scholar]
- Seto M, Miyase T, Umehara K, Ueno A, Hirano Y, Otani N. Sesquiterpene lactones from Cichorium endivia L. and C. intybus L. and cytotoxic activity. Chem Pharm Bull. 1988;36:2423–2429. doi: 10.1248/cpb.36.2423. [DOI] [PubMed] [Google Scholar]
- Somasekar Roa A, Kelkar GR, Bhattacharyya SC. Terpenoids: XXI. The structure of costunolide, a new sesquiterpene lactone from costus root oil. Tetrahedron. 1960;9:275–283. [Google Scholar]
- Song Q, Gomez-Barrios ML, Hopper EL, Hjortso MA, Fischer NH. Biosynthetic studies of lactucin derivatives in hairy root cultures of Lactuca floridana. Phytochemistry. 1995;40:1659–1665. [Google Scholar]
- Stahl E. Das ätherische Öl aus Thymus praecox spp. articus isländischer Herkunft. Planta Med. 1984;50:157–160. doi: 10.1055/s-2007-969659. [DOI] [PubMed] [Google Scholar]
- Takasugi M, Okinaka S, Katsui N, Masamune T, Shirata A, Ohuchi M (1985) Isolation and structure of lettucinin A, a novel guaianolide phytoalexin from Lactuca sativa var. capitata (Compositae). J Chem Soc Chem Commun 621–622
- Takeda K. Stereospecific cope rearrangement of the germacrene-type sesquiterpenes. Tetrahedron. 1974;30:1525–1534. [Google Scholar]
- Teisseire PJ. Chemistry of Fragrant Substances. New York: VCH Publishers; 1994. pp. 193–289. [Google Scholar]
- Threlfall DR, Whitehead IM. Coordinated inhibition of squalene synthetase and induction of enzymes of sesquiterpenoid phytoalexin biosynthesis in cultures of Nicotiana tabacum. Phytochemistry. 1988;27:2567–2580. [Google Scholar]
- van Beek TA, Maas P, King BM, Leclercq E, Voragen AGJ, de Groot Ae. Bitter sesquiterpene lactones from chicory roots. J Agric Food Chem. 1990;38:1035–1038. [Google Scholar]
- Vogel G, Hartmann HD, Krahnstöver K. Handbuch des speziellen Gemüsebaues. Stuttgart, Germany: Ulmer; 1990. pp. 84–144. [Google Scholar]
- Weeda EJ, Westra R, Westra CH, Westra C. Nederlandse oecologische flora: wilde planten en hun relaties 4. Amsterdam: IVN; 1991. pp. 152–154. [Google Scholar]
- West CA. Hydroxylases, monooxygenases, and cytochrome P-450. In: Davis DD, editor. The Biochemistry of Plants. Vol. 2. London: Academic Press; 1980. pp. 317–342. [Google Scholar]
- Whitehead IM, Threlfall DR, Ewing DF. 5-Epi-aristolochene is a common precursor of the sesquiterpenoid phytoalexins capsidiol and debneyol. Phytochemistry. 1989;28:775–779. [Google Scholar]