Abstract
SMAD3 plays a central role in cancer metastasis, and its hyperactivation is linked to poor cancer outcomes. Thus, it is critical to understand the upstream signaling pathways that govern SMAD3 activation. Here, we report that SMAD3 underwent methylation at K53 and K333 (K53/K333) by EZH2, a process crucial for cell membrane recruitment, phosphorylation, and activation of SMAD3 upon TGFB1 stimulation. Mechanistically, EZH2-triggered SMAD3 methylation facilitated SMAD3 interaction with its cellular membrane localization molecule (SARA), which in turn sustained SMAD3 phosphorylation by the TGFB receptor. Pathologically, increased expression of EZH2 expression resulted in the accumulation of SMAD3 methylation to facilitate SMAD3 activation. EZH2-mediated SMAD3 K53/K333 methylation was upregulated and correlated with SMAD3 hyperactivation in breast cancer, promoted tumor metastasis, and was predictive of poor survival outcomes. We used 2 TAT peptides to abrogate SMAD3 methylation and therapeutically inhibit cancer metastasis. Collectively, these findings reveal the complicated layers involved in the regulation of SMAD3 activation coordinated by EZH2-mediated SMAD3 K53/K333 methylation to drive cancer metastasis.
Keywords: Oncology
Keywords: Cancer, Molecular biology
Introduction
Tumor metastasis is highly responsible for tumor-related death (1–4). During metastasis, epithelial cells gradually discard their differentiated characteristics, including losing their cellular polarity and cell-cell adhesion capacity, thereby gaining mesenchymal characteristics such as invasion, migration, and motility, which is also referred to as epithelial-mesenchymal transition (EMT) (5, 6). TGFB plays a key role in development, inflammation, homeostasis, and multiple diseases, including tumor metastasis and EMT (4, 7–9). In premalignant cells, TGFB acts as a potent tumor suppressor by inhibiting cell proliferation, promoting apoptosis, and maintaining genome stability (10, 11). However, TGFB signaling orchestrates the EMT, and cancer cells use TGFB to create an immune-suppressive tumor microenvironment (TME), which suppresses the antitumor immune reactions that foster tumor progression and metastasis (1, 2, 5, 9, 12–15). The cellular effect induced by TGFB occurs via specific type I and II serine/threonine kinase receptors, followed by SMAD2 and SMAD3 (R-SMADs) C-terminal phosphorylation and then formation of a complex with SMAD4 (16). SMAD3, one of the receptor-regulated R-SMADs, is directly phosphorylated at the C-terminal SSXS motif by the TGFB type I receptor (TBRI) and plays an essential role in the canonical TGFB signaling pathway (17). Following the binding of R-SMADs to common SMAD, the complex is translocated into the nucleus, where it binds to transcriptional coactivators or corepressors and regulates transcription (15). Interestingly, Recent studies show that the biological function of SMAD3 opposes that of SMAD2 in tumor metastasis, and silencing SMAD2 promotes tumor metastasis, whereas SMAD3 KO inhibits tumor metastasis (18, 19). Given these confusing phenomena, the mechanism underlying the regulation of TGFB signaling merits in-depth investigation.
Posttranslational modifications (PTMs) of nonhistone proteins, such as phosphorylation, acetylation, methylation, and ubiquitination, are involved in protein stability, catalytic activity, and protein-protein interaction (20, 21). Dysregulation of these modifications induced severe diseases including cancer (22). Recently, lysine methylation of nonhistone proteins has been identified as a prevalent PTM (23–26). SMAD3 is subject to extensive PTMs, including phosphorylation, ubiquitination, and acetylation, which are important for SMAD3 activation, translocation, and stability (27–33). However, whether lysine methylation of the key protein SMAD3 in the TGFB1 signaling pathway plays a crucial role in TGFB/SMADs signaling activation has, to our knowledge, yet to be investigated.
Here, we report a PTM for SMAD3, methylation. We show that K53 and K333 (K53/K333)methylation of SMAD3 was crucial for SMAD3 cell membrane recruitment, phosphorylation, and biological function, stimulated by TGFB1. Deletion of SMAD3 K53/K333 methylation can dramatically inhibit tumor metastasis. Therefore, this study reveals that EZH2-mediated SMAD3 K53/K333 methylation was necessary for SMAD3 transcriptional activity and that targeting SMAD3 K53/K333 methylation might offer a potential therapeutic strategy for the treatment of TGFB/SMAD tumors.
Results
Methylation of SMAD3 is essential for TGFB1-mediated SMAD3 activity.
To identify potentially novel SMAD3 PTMs, we first performed a co-IP assay to isolate SMAD3 protein from HEK293T cells and then conducted mass spectrometric analysis. Interestingly, we found that methylation was one of the potential SMAD3 PTMs (Supplemental Table 1; supplemental material available online with this article; https://doi.org/10.1172/JCI152394DS1). Of note, our mass spectrometric analysis led us to other studies that also reported SMAD3 PTMs, such as phosphorylation (Supplemental Table 1). Protein lysine methylation has been identified as a prevalent PTM and is an important regulator of cellular signal transduction. To prove the mass spectrometry analysis and further investigate whether the lysine of SMAD3 can be methylated, we carried out a co-IP assay to isolate the SMAD3 protein from HEK293T cells and then used the pan–lysine trimethylation (pan–K-me3) antibody for detection. Notably, we observed that the lysine of SMAD3 could be trimethylated (Supplemental Figure 1A). Moreover, the trimethylation levels, but not the di- or monomethylation levels, of SMAD3 were increased by TGFB1 stimulation (Figure 1A and Supplemental Figure 1, B and C). SMAD3 phosphorylation at S423/S425 (SMAD3S423/S425 phosphorylation) was necessary for its transcriptional activity. Next, to determine whether there is a relationship between SMAD3 lysine trimethylation and SMAD3S423/S425 phosphorylation, we shortened the duration of TGFB1 stimulation of HEK293T cells. Importantly, upon TGFB1 stimulation, we found that lysine trimethylation of SMAD3 occurred before SMAD3S423/S425 phosphorylation (Supplemental Figure 1D and Figure 1B). Furthermore, the global histone methylation inhibitor 3-deazaneplanocin A (DZneP) (34) inhibited SMAD3 lysine trimethylation and SMAD3S423/S425 phosphorylation (Supplemental Figure 1E). On the basis of our previous mass spectrometric results, we hypothesized that lysines 53, 81, 117, and 333 of SMAD3 might be potential methylation sites (Supplemental Figure 1F).
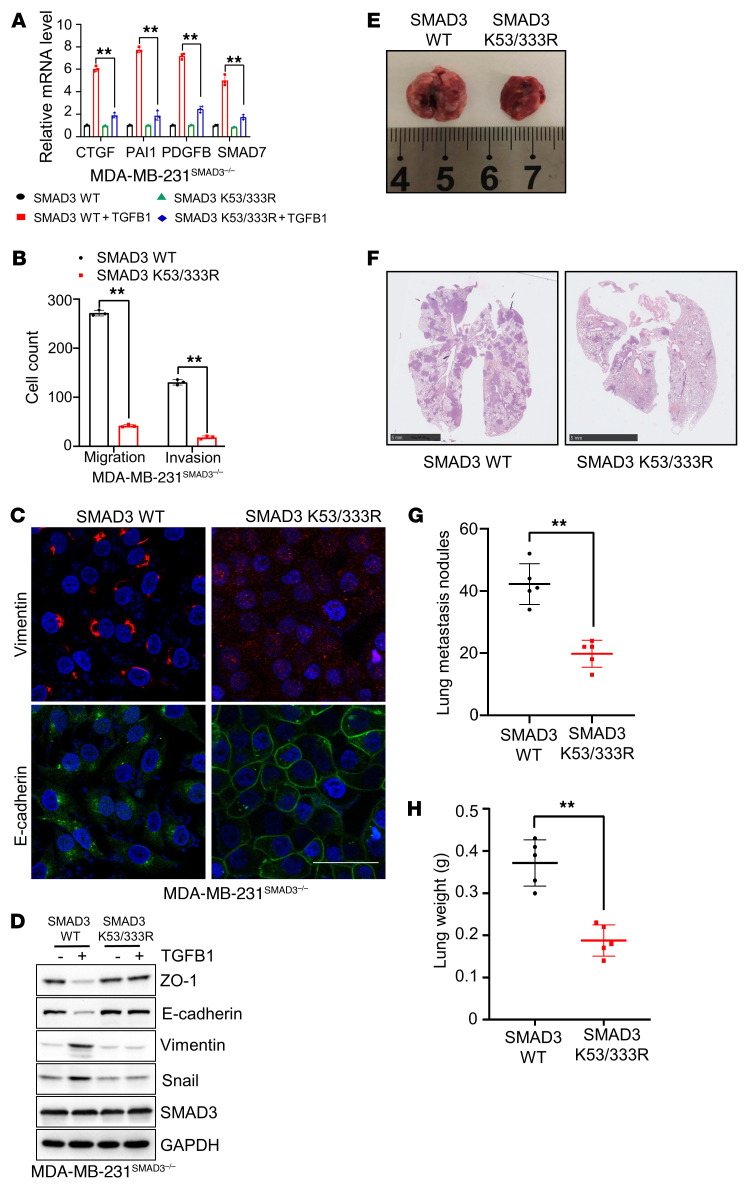
Figure 1. SMAD3 K53/K333 methylation is critical for SMAD3 activation.
(A and B) HEK293T cells and MDA-MB-231 cells were serum starved and treated with TGFB1 (5 ng/mL) for the indicated durations, and whole-cell extracts (WCEs) were collected for IP with anti-SMAD3 antibody, followed by immunoblot (IB) analysis. (C) HEK293T cells were transfected with WT HA-SMAD3 or mutant plasmids as indicated and treated with TGFB1 (5 ng/mL). WCEs were then collected for IP with anti-HA antibody, followed by IB analysis. (D) SMAD3 K53/K333 site aa in different species. (E) HEK293T cells were transfected with WT HA-SMAD3 or K53/333R-mutant plasmids and then treated with TGFB1 (5 ng/mL). WCEs were collected for IP with anti-HA antibody, followed by IB analysis. (F) ddH2O (10 μL) containing different peptides (0.1–0.75 μg) was added onto the PVDF membranes, followed by IB analysis using a K53-specific trimethylation antibody (anti–SMAD3 K53me3) and a K333-specific trimethylation antibody (anti–SMAD3 K333me3). (G) MDA-MB-231 cells were serum starved and treated with TGFB1 (5 ng/mL), and WCEs were collected for IP with anti-SMAD3 antibody, followed by IB analysis with SMAD3 K53/K333 trimethylation–specific antibodies. (H) MDA-MB-231SMAD3–/– cells were stably transfected with WT SMAD3 or SMAD3 K53/333R plasmids and treated with TGFB1 (5 ng/mL). WCEs were collected for IP with anti-SMAD3 antibody, followed by IB analysis. All immunoblotting was performed 3 times, independently, with similar results.
To further investigate the methylation sites of SMAD3 upon TGFB1 stimulation, we constructed methylation-deficient variants of SMAD3 (K53R, K81R, K117R, and K333R), which were expressed in HEK293T cells with or without TGFB1 stimulation. The data showed that only the K53R and K333R variants partly abrogated the trimethylation upregulation of SMAD3 under TGFB1 stimulation (Figure 1C). Of note, the K53 and K333 sites were highly conserved among various species (Figure 1D). Moreover, the special methylation-deficient variant of SMAD3, K53/333R, completely abrogated the trimethylation upregulation of SMAD3 and dramatically inhibited SMAD3S423/S425 phosphorylation under TGFB1 stimulation (Figure 1E). Next, we generated a K53-specific trimethylation antibody (anti–SMAD3 K53me3) and a K333-specific trimethylation antibody (anti–SMAD3 K333me3), which specifically recognized SMAD3 K53/K333 trimethylation using a dot-blot assay (Figure 1F). In addition, IHC analysis of lung metastasis led by MDA-MB-231 SMAD3 WT and KO cell lines further confirmed that anti–phosphorylated SMAD3S423/S425 (anti–p-SMADS423/S425) , anti–SMAD3 K53me3, and anti–SMAD3 K333me3 antibodies specifically recognized SMAD3S423/S425 phosphorylation and K53/K333 trimethylation (Supplemental Figure 2I). Using the anti–SMAD3 K53me3 and anti–SMAD3 K333me3 antibodies, we further confirmed that SMAD3 K53/K333 trimethylation was induced by TGFB1 treatment (Figure 1G). Next, we generated a SMAD3-KO MDA-MB-231 cell line using the CRISPR/Cas9 system (Supplemental Figure 1H) and subsequently induced ectopically stable expression of a methylation-deficient variant of SMAD3, K53/333R, in SMAD3-KO MDA-MB-231 cells (Supplemental Figure 1I). Compared with SMAD3 WT, we found that SMAD3 K53/333R dramatically reduced SMAD3S423/S425 phosphorylation under TGFB1 stimulation (Figure 1H). Therefore, SMAD3 K53/K333 trimethylation was induced by TGFB1 stimulation and necessary for SMAD3S423/S425 phosphorylation.
Absence of SMAD3 methylation inhibits its oncogenic functions in vivo and in vitro.
TGFB is a potent inducer of EMT, whereby epithelial progenitor cells lose polarity, downregulate cell-cell adhesions, and migrate and invade to generate or regenerate tissues (14, 35). Beyond the contribution of TGFB-induced EMT to tumor invasion and metastatic dissemination, the TGFB pathway induces gene responses that support the ability of cancer cells to infiltrate and colonize specific organs (19). To reveal the potential biological functions of SMAD3 K53/K333 methylation and their role in TGFB pathway activation, we first measured the mRNA levels of CTGF, PAI1, PDGFB, and SMAD7, which are transcriptionally regulated by the SMAD2-SMAD3 complex and are predicted to activate the canonical TGFB pathway activation in cells with or without TGFB1 treatment (11, 36). The results showed that SMAD3 K53/333R cells had significantly lower mRNA levels of CTGF, PAI1, PDGFB, and SMAD7 compared with levels in SMAD3 WT cells upon TGFB1 treatment (Figure 2A and Supplemental Figure 2, A and B). We then showed that the migratory and invasive abilities of SMAD3 K53/333R cells dramatically decreased compared with what we observed with SMAD3 WT cells (Figure 2B and Supplemental Figure 2C). Furthermore, SMAD3 K53/333R cells had higher protein expression of ZO-1 and E-cadherin and lower protein expression of vimentin and snail compared with SMAD3 WT cells (Figure 2, C and D). Under the TGFB1 stimulation condition, the protein expression levels of ZO-1, E-cadherin, vimentin, and snail in SMAD3 K53/333R cells showed no visual change compared with expression levels in SMAD3 WT cells (Figure 2D). These observations indicate that TGFB1-induced SMAD3 K53/333 methylation is critical for SMAD3 function and TGFB pathway activation. To determine whether methylation-deficient mutants of SMAD3 abrogate tumor metastasis in vivo, we injected SMAD3 WT and SMAD3 K53/333R MDA-MB-231 cells into the tail veins of 4-week-old female BALB/c nude mice. The results showed that SMAD3 K53/333R cells generated fewer metastatic nodules and lower tumor weights than did SMAD3 WT cells (Figure 2, E–H). Furthermore, the biological function of SMAD3 K53/333R cells phenocopied that of SMAD3-KO cells (Supplemental Figure 2, D–H). Taken together, these results revealed that the methylation of SMAD3 on K53 and K333 was crucial for TGFB/SMAD signaling pathway activation and the oncogenic functions of SMAD3.
Figure 2. Deletion of SMAD3 K53/K333 methylation inhibits the SMAD3 oncogene in vitro and in vivo.
(A) MDA-MB-231SMAD3–/– cells were stably transfected with WT SMAD3 or SMAD3 K53/333R plasmids and treated with TGFB1 (5 ng/mL). Quantitative RT-PCR analysis of TGFB/SMAD3 signaling pathway downstream genes, including CTGF, PAI1, PDGFB, and SMAD7, in the indicated cells with or without TGFB1 (5 ng/mL) treatment. (B) Quantitative analysis of Transwell assay in the indicated cells. (C) IF and (D) IB analysis of EMT markers in the indicated cells. Scale bar: 50 um. (E and F) Representative lung image (E) and H&E-stained lung sections (F). Scale bars: 5 mm. (G and H) Scatter plots showing lung metastatic nodules (G) and lung weights (H). All immunoblots were performed 3 times, independently, with similar results. Data indicate the mean ± SD. **P < 0.05, by 2-tailed Student’s t test (A, B, G, and H).
EZH2 methylates SMAD3 and promotes its activation.
Based on the finding that K53/333 methylation of SMAD3 was essential for TGFB/SMAD signaling pathway activation and oncogenic functions, we then sought to determine which methyltransferase(s) mediated SMAD3 K53/333 methylation. Given our observation of K53 and K333 upregulation and TGFB1-induced SMAD3 trimethylation, we hypothesized that the physical interaction between potential methyltransferase(s) and SMAD3 should also be enhanced by TGFB1 stimulation. We transfected HA-SMAD3 or a plasmid vector control into HEK293T cells and then pulled down HA-SMAD3 using an anti-HA antibody under TGFB1 treatment and nontreatment conditions. The pulled-down proteins were then subjected to systematic mass spectrometric analysis to identify potential methyltransferase(s) that may have triggered SMAD3 K53/333 methylation.
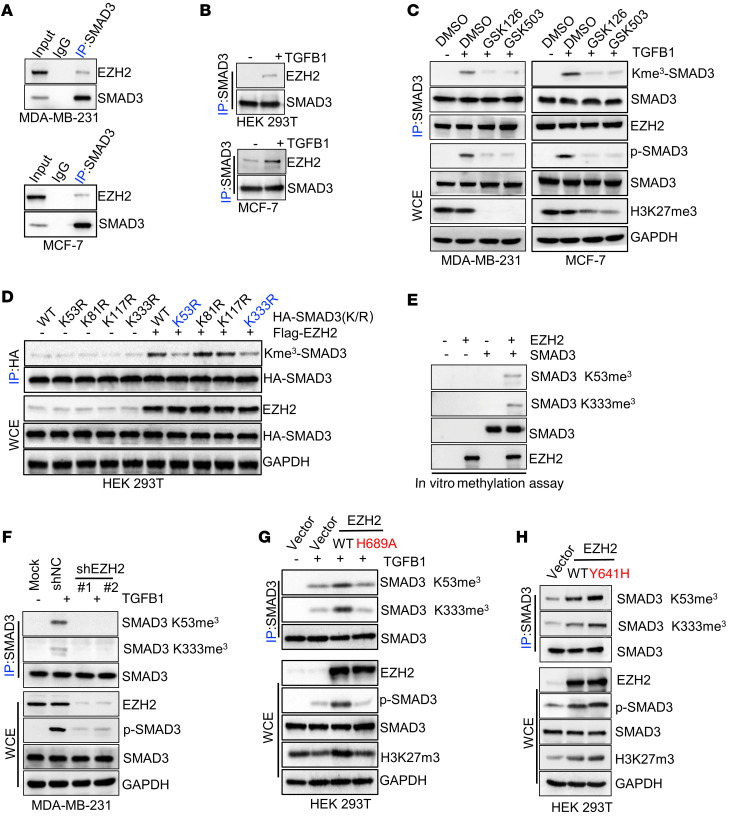
The mass spectrometric results showed that the physical interaction between EZH2 and SMAD3 was dramatically enhanced by TGFB1 stimulation (Supplemental Table 2). EZH2 is a histone H3 methyltransferase capable of catalyzing the trimethylation of lysine 27 of histone H3 (H3K27me3) (37) and further confirmed the physical interaction between the SMAD2-SMAD3 complex and EZH2, which could be significantly enhanced by TGFB1 treatment (Figure 3, A and B, and Supplemental Figure 3, A–D). An in vitro GST pull-down assay further demonstrated the direct binding between SMAD3 and EZH2 (Supplemental Figure 3E). Next, we sought to determine whether EZH2 could methylate SMAD3. Because the aa sequence of SMAD3 is highly conserved with that of SMAD2, we transfected Flag-EZH2 and HA-SMAD2/3 into HEK293T cells and subsequently pulled down HA-SMAD2/3, using an anti–pan–K-me3 antibody for detection. Notably, we found that EZH2 mediated the trimethylation of SMAD3, but not SMAD2 (Supplemental Figure 3F). Remarkably, upon TGFB1 treatment, the interaction between EZH2 and SMAD3 was enhanced, and SMAD3 trimethylation was induced (Supplemental Figure 3G). SMAD3 trimethylation and phosphorylation were enhanced in EZH2-overexpressing cells (Supplemental Figure 3H), while SMAD3 trimethylation and phosphorylation were reduced in cells with EZH2 knockdown or treated with EZH2 inhibitors (GSK126 and GSK503; Figure 3C and Supplemental Figure 3I). Next, we tried to confirm whether EZH2 triggered SMAD3 trimethylation at K53/K333 and found that the double-methylation–deficient variant of SMAD3 K53/333R abrogated the methylation of SMAD3 upregulation induced by EZH2, while overexpression of EZH2 did not affect other potential methylation sites, such as K81 and K117 (Figure 3D and Supplemental Figure 4A). Importantly, the in vitro methylation assay validated that EZH2 could trimethylate SMAD3 at the K53 and K333 sites (Figure 3E and Supplemental Figure 4B). In addition, we found that silencing of EZH2 markedly inhibited SMAD3 K53/K333 trimethylation (Figure 3F and Supplemental Figure 4C), whereas EZH2 overexpression enhanced SMAD3 K53/K333 trimethylation and SMAD3S423/S425 phosphorylation under TGFB1 stimulation. Meanwhile, overexpression of WT EZH2, but not the EZH2 H689A mutant deficient in methyltransferase activity (38, 39), enhanced basal and TGFB1-induced SMAD3 at K53/K333 trimethylation and SMAD3S423/S425 phosphorylation (Figure 3G and Supplemental Figure 4D). Furthermore, the gain-of-methyltransferase function EZH2 mutant EZH2 Y641H (40) showed higher levels of SMAD3 K53/K333 trimethylation compared with EZH2 WT (Figure 3H). We found that P38 could be phosphorylated under TGFB1 stimulation. Recent studies revealed that P38-mediated EZH2 T372 phosphorylation induced its cytoplasmic localization to promote breast cancer metastasis (41, 42). Hence, we further investigated whether EZH2 T372 phosphorylation was necessary for TGFB1-mediated, EZH2-catalyzed SMAD3 K53/K333 methylation. Notably, we found that gain of WT EZH2, but not the EZH2 T372A mutant, enhanced basal TGFB1-induced SMAD3 at K53/K333 trimethylation and SMAD3S423/S425 phosphorylation, although cells ectopically expressing EZH2 T372A had higher histone 3 at K27 trimethylation than did cells ectopically expressing WT EZH2 (Supplemental Figure 4E). Collectively, our data suggest that EZH2 triggered SMAD3 trimethylation at the K53 and K333 sites. Therefore, EZH2 is a SMAD3 methyltransferase responsible for SMAD3 K53/K333 trimethylation and SMAD3S423/S425 phosphorylation in response to TGFB1 stimulation.
Figure 3. SMAD3 methylation is triggered by EZH2.
(A) WCEs of MDA-MB-231 and MCF-7 cells were collected and subjected to co-IP and IB assays. (B) HEK293T and MCF-7 cells were serum starved and treated with TGFB1 (5 ng/mL), and WCEs were collected for IP with anti-SMAD3 antibody, followed by IB analysis. (C) MDA-MB-231 and MCF-7 cells were treated with TGFB1 (5 ng/mL) and the EZH2 inhibitors GSK126 or GSK503, and WCEs were collected for IP with anti-SMAD3 antibody, followed by IB analysis. (D) HEK293T cells were transfected with WT HA-SMAD3 or mutant plasmids and a Flag-EZH2 plasmid as indicated/WCEs were then collected for IP with anti-HA antibody, followed by IB analysis. (E) Immunoprecipitated EZH2 from HEK293 cells was incubated with SAM along with SMAD3 protein for in vitro methylation of SMAD3. The methylated proteins were separated by SDS-PAGE, and SMAD3 methylation was analyzed by IB using anti–SMAD3 K53/K333 trimethylation–specific antibodies. (F) MDA-MB-231 cells silenced with control (shNC) or EZH2 shRNA (nos. 1 and 2) were treated with TGFB1 (5 ng/mL), and WCEs were collected for IP with anti-SMAD3 antibody, followed by IB analysis. (G) HEK293T cells were transfected with vector, EZH2WT, or EZH2H689A and then treated with TGFB1 (5 ng/mL). WCEs were collected for IP with anti-SMAD3 antibody, followed by IB analysis. (H) HEK293T cells were transfected with vector, EZH2WT, or EZH2Y641H, and WCEs were collected for IP with anti-SMAD3 antibody, followed by IB analysis. All immunoblotting was performed 3 times, independently, with similar results.
EZH2-mediated tumor metastasis depends on SMAD3 methylation.
EZH2, a histone H3 methyltransferase capable of catalyzing trimethylation on lysine 27 of histone H3 (H3K27me3), mediates the metastasis of various types of tumors (39, 43–45). In addition, the TGFB signaling pathway plays a crucial role in tumor metastasis (11, 36, 46). Signaling of both EZH2 and TGFB was found to be crucial for EMT of tumor cells and tumor metastasis. On the basis of these findings, we assumed that there is a relationship between EZH2 and the TGFB1 signaling pathway. We show in our study that the migratory and invasive abilities of cells induced by TGFB1 could be inhibited by silencing EZH2 (Figure 4A and Supplemental Figure 5, A–C). Moreover, knockdown of EZH2 in SMAD3 WT and SMAD3 K53/333R cells resulted in decreased SMAD3S423/S425 phosphorylation in SMAD3 WT cells, but not in SMAD3 K53/333R–expressing cells, whose oncogenic capacity was lower than that of WT cells (Figure 4B), a finding that was consistent with the results seen with overexpression of EZH2 in SMAD3 WT and SMAD3 K53/333R cells (Supplemental Figure 5D). Moreover, overexpression of EZH2, but not EZH2 H689A, induced EMT in SMAD3 WT cells, but not in SMAD3 K53/333R or SMAD3-KO cells (Figure 4C and Supplemental Figure 5E). We also showed that EZH2 promoted cell metastasis in SMAD3 WT cells, but not in SMAD3 K53/333R or SMAD3-KO cells, whereas the loss-of-function EZH2 H689A mutant showed no such effect (Figure 4, D and E, and Supplemental Figure 5, F and G). A further in vivo xenograft study also showed that EZH2 could significantly increase mouse lung metastatic nodule formation with WT SMAD3–expressing cells, but not with SMAD3 K53/333R–expressing cells (Figure 4, F–H). Taken together, our results indicate that EZH2 methylated SMAD3 at K53 and K333, triggering tumor metastasis.
Figure 4. EZH2 promotes cell migration and invasion dependent on methylation of SMAD3 at K53 and K333.
(A) Quantitative analysis of Transwell assay in the indicated MDA-MB-231 cells treated with TGFB1 (5 ng/mL). (B) MDA-MB-231SMAD3–/– cells were stably transfected with WT SMAD3 or SMAD3 K53/333R plasmids and silenced with control or EZH2 shRNA (nos. 1 and 2). WCEs were collected for IB analysis. (C) WT Flag-EZH2 or a Flag-EZH2 H689A plasmid was transfected into MDA-MB-231SMAD3–/– cells ectopically expressing WT SMAD3 or SMAD3 K53/333R, and WCEs were collected for IB analysis. (D and E) A Transwell cell invasion assay was performed using MDA-MB-231SMAD3–/– cells stably transfected with WT SMAD3 or SMAD3 K53/333R plasmids and transfected with a vector, EZH2WT, or EZH2H689A. Representative images (D) and quantitative analysis (E). Original magnification, ×200. (F–H). Representative lung image (F),H&E-stained lung sections (G), and scatter plot showing lung weights (H). Scale bars: 5 mm. All immunoblotting was performed 3 times, independently, with similar results. Data indicate the mean ± SD. **P < 0.05, by 2-tailed Student’s t test (A, E, and H).
SMAD3 K53/K333 methylation is necessary for SMAD3 membrane localization and phosphorylation.
Phosphorylation of SMAD3 by TGFB type 1 receptors primarily occurs at the plasma membrane (17). To understand how EZH2-mediated SMAD3 K53/K333 methylation regulates SMAD3S423/S425 phosphorylation and activation in response to TGFB1, we determined whether EZH2-mediated SMAD3 K53/K333 methylation regulated SMAD3 membrane recruitment upon TGFB1 stimulation. Our data showed that EZH2 deficiency or introduction of the SMAD3 K53/333R mutation impaired basal and TGFB1-mediated SMAD3 membrane recruitment (Figure 5, A–D, and Supplemental Figure 6, A–D). This indicates that EZH2-mediated SMAD3 K53/K333 methylation regulates SMAD3S423/S425 phosphorylation by regulating SMAD3 membrane recruitment.
Figure 5. SMAD3 K53/K333 methylation is essential for its membrane localization.
(A) Membrane and cytosolic fractions from MDA-MB-231SMAD3–/– cells stably transfected with WT SMAD3 or SMAD3 K53/333R plasmids were collected and subjected to IB analysis. (B) Membrane fractions from MDA-MB-231SMAD3–/– cells stably transfected with WT SMAD3 or SMAD3 K53/333R plasmids and treated with TGFB1 (5 ng/mL) were collected and subjected to IB analysis. (C) MDA-MB-231SMAD3–/– cells were stably transfected with WT SMAD3 or SMAD3 K53/333R plasmids and treated with TGFB1 (5 ng/mL). IF images show the cellular localization of SMAD3. Scale bar: 10 μm. (D) Membrane and cytosolic fractions from MDA-MB-231 cells silenced with control or EZH2 shRNA (no. 1) were collected and subjected to IB analysis. (E) HEK293T cells were transfected with WT HA-SMAD3 or HA-SMAD3 K53/333R plasmids and treated with TGFB1 (5 ng/mL), and WCEs were collected for IP with anti-HA antibody, followed by IB analysis. (F) HEK293T cells were silenced with control or EZH2 shRNA (nos. 1 and 2), and WCEs were collected for IP with anti-SMAD3 antibody, followed by IB analysis. (G) Co-IP of endogenous SMAD3 from MDA-MB-231 cells transfected with EZH2WT or EZH2H689A, followed by IB analysis. (H) Co-IP of endogenous SMAD3 from MDA-MB-231 cells transfected with EZH2WT or EHZ2Y641H, followed by IB analysis. All immunoblotting was performed 3 times, independently, with similar results.
SMAD anchor for receptor activation (SARA) belongs to a large family of proteins containing the Fab1, YOTB, Vac1, and EEA1 protein (FYVE) domain, which confers the ability to interact with phosphatidylinositol 3-phosphate (PI3P), a phospholipid of membranes highly enriched in endosomes and directly involved in the recruitment of proteins (47). SARA contains structural motifs that interact with SMAD2 and SMAD3 (SMAD2/3), as well as a C-terminal region, all of which are required for interaction with the TBRI, turning on TGFB signaling by triggering SMAD2/3 membrane recruitment, phosphorylation, and nuclear translocation (35, 48, 49). We found that EZH2-mediated SMAD3 K53/K333 methylation was essential for SMAD3 membrane recruitment and phosphorylation at S423/S425 and that the activation of TGFB signaling was similar to that of SARA. We then studied the crosstalk between SMAD3 K53/K333 methylation, as well as the interaction between SARA and SMAD3. Strikingly, the results showed that a methylation-deficient SMAD3 K53/333R had an attenuated interaction with SARA compared with SMAD3 WT, while silencing of EZH2 had a similar result (Figure 5, E and F, and Supplemental Figure 6, E and F). Furthermore, overexpression of WT EZH2, but not EZH2 H689A, increased the physical interaction between SMAD3 and SARA (Figure 5G and Supplemental Figure 6G), whereas the EZH2 Y641H mutant further enhanced the binding between SMAD3 and SARA compared with WT EZH2 (Figure 5H). Consistently, we found that silencing of SARA diminished SMAD3S423/S425 phosphorylation but not SMAD3 methylation (Supplemental Figure 6H). Thus, we found that EZH2-mediated SMAD3 K53/K333 methylation was essential for SARA-SMAD3 interaction, which in turn mediated SMAD3 membrane recruitment, phosphorylation at S423/S425, and activation of TGFB signaling.
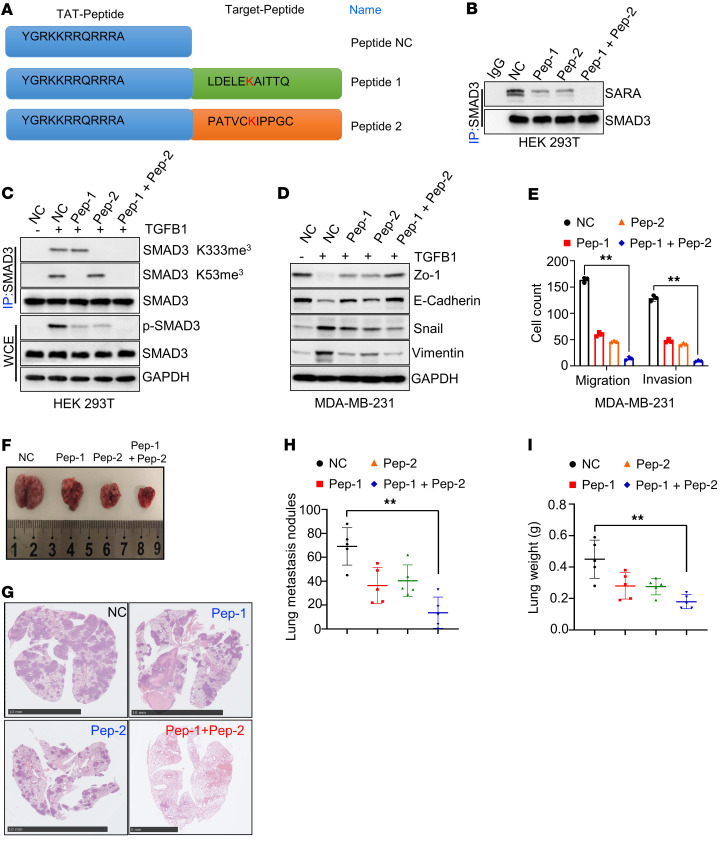
Inhibition of SMAD3 methylation with synthesized peptides blocks cancer cell metastasis.
Given that EZH2-mediated SMAD3 K53/K333 methylation is necessary for TGFB/SMAD signaling pathway activation and cancer metastasis, we investigated whether SMAD3 methylation could be therapeutically targeted. To evaluate whether nonmethylated peptides containing methylation sites could inhibit endogenous SMAD3 methylation and further restrain cancer metastasis, we first synthesized peptides that contained SMAD3 methylation sites (48–58 aa for peptide 1 and 328–338 aa for peptide 2) with a trans-activator of transcription (TAT) tag placed in its N-terminal region (with a TAT domain as a negative control) (Figure 6A). To determine whether TAT peptides could restrain SMAD3 methylation and phosphorylation, we first treated cells with TAT peptide 1 and/or TAT peptide 2. Interestingly, we found that TAT peptide 1 and TAT peptide 2 synergistically inhibited the interaction between SMAD3 and SARA (Figure 6B). Importantly, TAT peptide 1 markedly inhibited EZH2/TGFB1-mediated SMAD3 K53 methylation, but not SMAD3 K333 methylation, and TAT peptide 2 could silence EZH2/TGFB1-mediated SMAD3 K333 methylation, but not SMAD3 K53 methylation (Figure 6C). Combining TAT peptides 1 and 2 markedly inhibited EZH2-mediated SMAD3 K53/K333 methylation, as well as cancer cell EMT (Figure 6, C and D). In addition, immunofluorescence (IF) analysis showed that combining TAT peptides 1 and 2 markedly impaired TGFB1-mediated SMAD3 membrane recruitment and nuclear localization (Supplemental Figure 7A). Transwell assays showed that the TAT peptides could inhibit cancer cell migratory and invasive abilities (Figure 6E and Supplemental Figure 7B). To test whether the TAT peptides inhibited breast cancer lung metastasis in vivo, we injected MDA-MB-231 cells into the tail vein of mice. Three days after injection, we injected nonmethylation TAT peptides or TAT control peptides (100 mL, 1 mg/mL per mouse) into the tail vein every 3 days. All mice were sacrificed 2 months later. The results showed that the lung metastatic nodules and lung weights in the nonmethylation TAT peptide–treated group were significantly decreased compared with those in the TAT control peptide–treated group, whereas peptides 1 and 2 had synergistic effects on the inhibition of tumor metastasis, indicating that nonmethylated peptides inhibited MDA-MB-231 lung colonization (Figure 6, F–I). Thus, targeting SMAD3 methylation may be a potential therapeutic strategy to reverse the oncogenic processes.
Figure 6. Targeting SMAD3 K53/K333 methylation inhibits cancer metastasis.
(A) The aa sequence of different TAT peptides. (B and C) HEK293T cells were treated with different TAT peptides (Pep-1, Pep-2) and TGFB1 (5 ng/mL), and WCEs were collected for IP with anti-SMAD3 antibody, followed by IB analysis. (D) MDA-MB-231 were silenced with TAT peptides and treated with TGFB1 (5 ng/mL), and WCEs were collected for IB analysis. (E) Quantitative analysis of Transwell cell migration and invasion assays using MDA-MB-231 cells treated with different TAT peptides. (F and G) Representative lung image (F) and H&E-stained lung sections (G). Scale bars: 10 mm. (H and I) Scatter plots show the number of lung metastatic nodes (I) and lung weights (H). All immunoblotting was performed 3 times, independently, with similar results. Data indicate the mean ± SD. **P < 0.05, by 2-tailed Student’s t test.
SMAD3 methylation is upregulated in breast cancer and associated with worse overall survival.
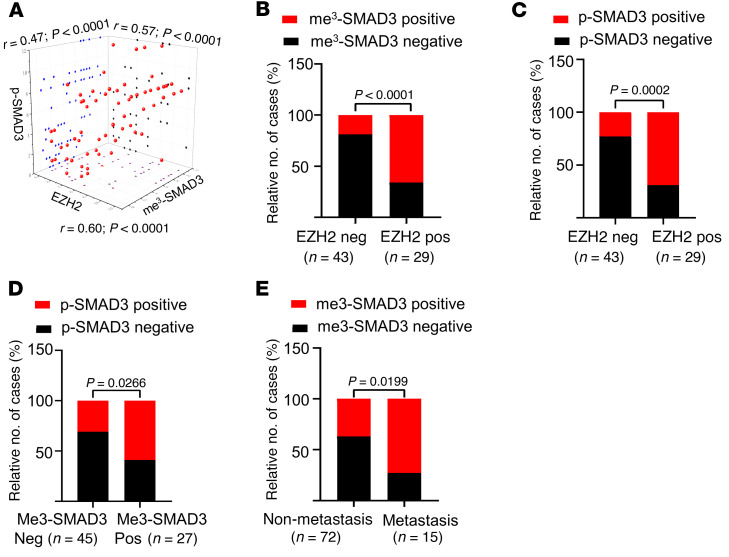
By examining the Gene Expression Profiling Interactive Analysis (GEPIA) databases, we found that EZH2 was not only upregulated in diverse human cancers but also overexpressed in many different types of breast cancer (Supplemental Figure 6C). Using the Kaplan-Meier Plotter (KM Plotter) database (50), we found that higher EZH2 expression was associated with poor survival outcomes for patients with breast cancer, as well as patients with breast cancer with lymph nodule metastasis (Supplemental Figure 8, A and B). To further investigate the relationship between EZH2 and SMAD3 methylation and phosphorylation in breast cancer, we performed IHC staining assays on breast carcinoma samples (Supplemental Figure 8C). SMAD3 methylation IHC signal scores were calculated using the following formula: (K333me3 IHC signal score + K53me3 IHC signal score)/2. The results consistently showed that EZH2 signals were positively correlated with SMAD3 methylation and phosphorylation signals (Figure 7, A–C). In addition, SMAD3 phosphorylation signals were also positively correlated with SMAD3 methylation signals (Figure 7, A and D). Moreover, SMAD3 methylation was highly expressed in the primary tumors of patients with metastatic breast cancer compared with expression in patients with nonmetastatic disease (Figure 7E and Supplemental Figure 8D). Next, we studied the relationship between SMAD3 methylation expression levels and the overall survival of patients with cancer. We evaluated SMAD3 methylation expression levels using microarray analysis of breast cancer specimens and found that SMAD3 methylation levels were negatively correlated with patients’ overall survival, and the multivariate analysis showed that SMAD3 methylation overexpression was an independent prognostic factor for patients with breast cancer (Figure 8, A and B, and Supplemental Figure 8E). Furthermore, we found that SMAD3 methylation IHC scores were positively correlated with lymph nodule–positive numbers and N (node) stage (Figure 8, C and D, and Supplemental Figure 8F). Collectively, our data underscore the importance of EZH2-mediated SMAD3 K53/K333 methylation in metastasis and indicate that SMAD3 methylation might function as an important factor in predicting overall survival of patients with breast cancer.
Figure 7. SMAD3 methylation levels are positively correlated with EZH2 and SMAD3 phosphorylation levels.
(A) Scatter plot of the IHC staining scores for EZH2, SMAD3 K53/K333 trimethylation, and SMAD3S423/S425 phosphorylation in breast cancer (n = 75). All P and r values were calculated with Spearman’s r test. (B–D) Quantitative IHC staining scores showing the correlation between EZH2, SMAD3 K53/K333 trimethylation, and SMAD3S423/S425 phosphorylation. neg, negat5ive; pos, positive. (E) Quantitative IHC staining score for staining of SMAD3 K53/K333 trimethylation in nonmetastatic primary breast tumors and lung-metastatic primary breast tumors. P = 0.0199. Statistical significance was calculated with the χ2 test.
Figure 8. SMAD3 K53/K333 methylation predicts poor survival of patients with cancer.
(A) Kaplan-Meier survival analysis comparing high and low levels of SMAD3 K53/K333 trimethylation using microarray results from a breast cancer specimen. P = 0.0004. (B) Cox regression analysis showing the significance of the relationship between SMAD3 K53/K333 trimethylation expression and prognosis for patients with breast cancer in the presence of other clinical variables. (C) Scatter plot of SMAD3 K53/K333 trimethylation IHC staining score comparing different N stages using microarray results from a breast cancer specimen (n = 80). P = 0.0051, by χ2 test. (D) Scatter plot of the number of cancer-positive lymph nodes comparing high and low levels of SMAD3 K53/K333 trimethylation using microarray results from a breast cancer specimen (n = 80). P = 0.0039, by χ2 test. (E) Working model of EZH2-mediated SMAD3 K53/K333 methylation crosstalk with TGFB-mediated SMAD3 phosphorylation, membrane localization, and activation.
Discussion
Our data reveal insight into the regulation of SMAD3 biological function. To determine whether SMAD3 could be methylated, we first isolated SMAD3 protein from cells and then incubated them with a pan–lysine trimethylation antibody or performed mass spectrometric analysis. We found that SMAD3 K53 and K333 could be trimethylated and that only SMAD3 trimethylation, but not dimethylation or monomethylation, was enhanced by TGFB1 stimulation. Deletion of SMAD3 methylation inhibited the interaction between SMAD3 and its localization to cellular membrane SARA, decreases its C-terminal phosphorylation, and significantly repressed cancer cell EMT, colony formation ability, and metastasis, which phenocopied SMAD3-KO cancer cells.
When treated with TGFB1, the SMAD2-SMAD3 complex could be respectively phosphorylated by the TGFB1 receptor at the C-terminals of SMAD2 and SMAD3 (10, 17). Based on our observation of SMAD3 methylation upregulated by TGFB1 stimulation, we found that the interaction between SMAD3 and EZH2 could be dramatically enhanced by TGFB1 treatment (Supplemental Table 2). Several studies showed that TGFB1 could activate P38 phosphorylation (10, 17, 41, 51). A recent study revealed that P38-mediated EZH2 phosphorylation induced its cytoplasmic localization to promote breast cancer metastasis (42). In our study, we identified SMAD3 as a substrate of EZH2 and showed that EZH2-mediated SMAD3 K53/K333 methylation crosstalks with TGFB1-mediated SMAD3 C-terminal phosphorylation. We believe this study expands our understanding of TGFB1/EZH2-mediated tumor metastasis.
EZH2 is amplified and overexpressed and is associated with poor survival in various cancers (52–54). Recent studies have identified EZH2 as a potential target for cancer treatment (55, 56). Consistently, we found that EZH2-mediated SMAD3 K53/K333 methylation was clearly upregulated in metastatic breast cancer and correlated with SMAD3 C-terminal phosphorylation. Furthermore, we found that SMAD3 K53/K333 methylation levels were highly consistent and associated with poor survival in breast cancer. Targeting SMAD3 methylation using TAT peptides 1 and 2 dramatically inhibited breast cancer metastasis (Figure 6). The mostly tumorigenesis function of EZH2 was previously thought to be dependent on EZH2-induced histone H3K27me3 modification. However, we found that EZH2-mediated tumor metastasis markedly abrogated the expression of SMAD3 K53/333R methylation–deficient mutant cells compared with that of WT SMAD3 cells. These results showed that EZH2-mediated biological function was partly dependent EZH2-mediated SMAD3 K53/K333 methylation.
Pharmacological targeting of SMAD3 activity, such as with SIS3 (a specific inhibitor of SMAD3 C-terminal phosphorylation), was proposed as a potential therapeutic strategy to treat various diseases, including metastatic cancer (57). Suppression of SMAD3 activity was shown to depress fibrosis, apoptosis, and inflammation in mouse kidneys with unilateral ureteral obstruction and inhibited the development of diabetic nephropathy in a mouse model of type 1 diabetes (58, 59). In an in vitro experiment, inhibition of SMAD3 C-terminal phosphorylation reversed ABCB1- and ABCG2-mediated multidrug resistance in cancer cell lines, E4BP4-mediated NK cell development, and tumor metastasis (57, 60, 61). In clinical experiments, EZH2 inhibitors were shown to impede the progression of many cancer types, including lymphoma and solid tumors (62, 63). We observed that when SMAD3 was in a hypermethylated state, the cancer cells were prone to EMT. We also showed that patients with breast cancer who had lung or lymphatic metastases had higher SMAD3 methylation levels. In our study, we introduced a therapeutic drug to target SMAD3 K53/K333 methylation. We found that TAT peptides inhibited not only TGFB/SMAD signaling pathway but the EZH2 signaling pathway as well. Importantly, although EZH2 inhibitors could be used to block EZH2/SMAD3 signaling, their side effects may limit their applications because of the inhibition of H3K27 trimethylation. Furthermore, other nonhistone substrates have also been identified, such as EZH2 catalysis of STAT3 methylation, which is essential for STAT3 transcriptional activation (38, 39), and SYMD2 stabilization, which was found to be regulated by EZH2-mediated SYMD2 methylation (43). In our study, the EZH2 inhibitors also had inhibitory roles in STAT3 and SYMD2 signaling. However, unlike EZH2 inhibitors that block H3K27 trimethylation, the targeting of TAT peptides was more specific, and the potential side effects might be more manageable.
In summary, we identified a PTM of SMAD3, methylation. We showed that SMAD3 acted as a substrate for EZH2 and that EZH2-mediated SMAD3 K53/K333 methylation was essential for TGFB1-mediated SMAD3 recruitment to its cellular membrane location, SARA, and for C-terminal phosphorylation. Importantly, EZH2-mediated SMAD3 methylation not only rendered cancer cells more vulnerable to TGFB1 and promoted cancer cell EMT and metastasis, but also showed a positive correlation with poor patient survival (Figure 8E). Moreover, pharmacological inhibition of SMAD3 methylation dramatically inhibited cancer metastasis. We believe this study expands our understanding of TGFB1/EZH2-mediated tumor metastasis. Once EZH2 is amplified in cancer cells, TGFB1-mediated gene expression might be more vulnerable to activation and further facilitate tumor progression. Overall, TGFB1-mediated, EZH2-catalyzed SMAD3 K53/K333 methylation could function as a predictive marker of survival for patients with cancer and potentially serve as a therapeutic target for patients with metastatic cancer.
Methods
Cell lines and cell culturing.
Human MDA-MB-231, MCF-7, and HEK293T cells were purchased from the American Type Culture Collection (ATCC). MDA-MB-231 cells were maintained in L-15 medium (HyClone, GE Healthcare). MCF-7 and HEK293T cells were cultured in DMEM basal medium. FBS (10%, v/v; Gibco, Thermo Fisher Scientific) was added to the DMEM and L-15 basal culture medium. MDA-MB-231 cells were cultured at 37°C in a 100% air incubator, and other cells were cultured at 37°C in a 5% (v/v) CO2 incubator.
Antibodies and chemicals.
The following commercially available primary antibodies were used: anti-EZH2 (A11085, A13867, and A19577, ABclonal); anti–Flag tag (AE063, ABclonal and 14793S, Cell Signaling Technology); anti–HA tag (AE036, ABclonal and 3724T, Cell Signaling Technology); anti-SMAD3 (A19115, ABclonal and sc-101154, Santa Cruz Biotechnology); anti–p-SMAD3S423/S425 (AP0727, ABclonal); anti-SMAD2 (A19114, ABclonal); anti-GAPDH (sc-47724, Santa Cruz Biotechnology); anti-vimentin (5741, Cell Signaling Technology); anti–E-cadherin (610182, BD Transduction Laboratories); anti–ZO-1 (sc-33725, Santa Cruz Biotechnology); anti-Snail (3879, Cell Signaling Technology); anti–pan–monomethyl lysine (A18293, ABclonal); anti–pan–dimethyl lysine (14117, Cell Signaling Technology); anti–pan–trimethyl lysine (14680, Cell Signaling Technology); anti–trimethyl histone H3-K27 (A2363, ABclonal); anti–histone H3 (4499, Cell Signaling Technology); anti–caveolin-1 (A19006, ABclonal); anti-SARA (A16465, ABclonal); anti-HGS (A1970, ABclonal); and anti-DAB2 (A10349, ABclonal). The SMAD3 K53/K333 trimethylation rabbit polyclonal antibody was prepared by ABclonal (https://abclonal.com.cn). The following secondary antibodies were used in the immunofluorescence assays: anti–Dylight 549, goat anti–rabbit IgG (A23320, Abbkine); anti–Dylight 488, goat anti–mouse IgG (A23210, Abbkine); anti–Dylight 488, goat anti–rabbit IgG (A23220, Abbkine); and anti–Dylight 549, goat anti–mouse IgG (A23310, Abbkine). The following other small-molecule materials were used: GSK126 (T2079, Topscience); GSK503 (T1775,Topscience); TGFB1 (HY-P7118, MedChemExpress); and DiO (C1038, Beyotime).
Plasmid construction.
The expression vectors encoding pcDNA3.1-HA-SMAD3, pcDNA3.1-HA-SMAD2, and pcDNA3.1-Flag-EZH2 were purchased from AuGCT (http://www.augct.com). pLKO-AS3W-HA-SMAD3 and pLKO-AS3W-Flag-EZH2 plasmids were constructed by inserting the indicated DNAs into the pLKO-AS3W vector. The SMAD3 and EZH2 mutants were generated using a Mut Express II Fast Mutagenesis Kit V2 (C214-01, Vazyme). pLKO.1-lentiviral vectors expressing EZH2 shRNA were based on the following sequences: 5′-AAGAGGTTCAGACGAGCTGAT-3′ and 5′-GCTAGGTTAATTGGGACCAAA-3′.
Construction of cell lines.
We used pLKO.1-shEZH2, MD2-G, and the PPAX 3-pack system for the expression-silencing virus. We used the pLKO-AS3W–indicated genes, MD2-G, and the PPAX 3-pack system to generate high-expression viruses. SMAD3-KO virus was purchased from Genechem Shanghai. The sequences of the sgRNAs against the SMAD3-KO virus were as follows: 5′-GTGGTTCATCTGGTGGTCAC-3′; 5′-GCCGGCTCGCAGTAGGTAAC-3′ and 5′-ATTCGGAGCGCTTCTGCCTA-3′. All viruses were respectively transfected into the identified cells. After 12 hours, the medium was substituted with fresh complete medium. After 48 hours, 1 μg/mL puromycin was used to select the infected cells.
Mass spectrometric analyses.
Briefly, for protein elution, denaturation, reduction, and alkylation, beads samples obtained from the co-IP experiment were incubated with the reaction buffer containing sodium dehydrocholate (SDC), Tris (2 carboxyethyl) phosphine (TCEP), and chloroacetamide (CAA) at 95°C for 10 minutes. The supernatant was diluted 2 times with H2O. Trypsin (1 μg) was added for overnight digestion at 37°C. The next day, peptide purification was performed using SDB desalting columns. The peptide eluate was vacuum dried and stored at –20 °C for later use. Liquid chromatography tandem mass spectrometry (LC-MS/MS) data acquisition was carried out on a Q Exactive Plus LC-MS/MS mass spectrometer equipped with a nanospray source (Thermo Fisher Scientific). Peptides were dissolved in MS loading buffer (0.1% formic acid), loaded onto a C18 trap column through an autosampler, and then eluted into a C18 analytical column (50 μm × 150 mm, 2 μm particle size, 100 Å pore size). Mobile phase A (0.1% formic acid) and mobile phase B (90% ACN, 0.1% formic acid) were used to establish a 60-minute separation gradient. A constant flow rate was set at 300 nL/min. Data were acquired using a spray voltage of 2 kV, an ion funnel radio frequency (RF) of 40, and ion transfer tube temperature of 320°C. For data-dependent acquisition (DDA) mode analysis, each scan cycle consisted of 1 full-scan mass spectrum (resolution 70 K, scan range: 350–1800 m/z; automatic gain control [AGC]: 3 × 106; ion trap [IT]: 20 ms) followed by 15 MS/MS events (resolution 17.5 K; AGC: 2 × 105; IT: 50 ms). High-energy collision dissociation (HCD) collision energy was set at 28. The isolation window was set at 1.6 Da. The dynamic exclusion time was set at 35 seconds. MS raw data were analyzed with MaxQuant software (version 1.6.6) using the Andromeda database search algorithm and the MaxLFQ function. Spectra files were searched using the default parameters, except for label-free quantification mode, the minimum ratio count was set at 1, and the match-between-runs function was checked. The search results were filtered with a 1% FDR at both protein and peptide levels.
Cytosolic and membrane fractions.
Cytosolic and membrane fractions of the identified cells were isolated using a membrane protein extraction kit (C500049, Sangon Biotech) according to the manufacturer’s instructions.
Western blot and IP assays.
Cells were washed in cold PBS 3 times and lysed with NP-40 buffer for 30 minutes at 4°C. The protein concentration was measured with a Bicinchoninic Acid Assay (BCA) kit (Thermo Fisher Scientific). Membrane and cytosol fractions were isolated according to the manufacturer’s instructions (15F17B55, Boster Biological Technology). Next, 5× protein sample buffer (250 mM Tris–HCl [pH 6.8], 10% SDS, 30% glycerol, 5% β-mercaptoethanol, bromophenol blue) was added, followed by boiling of the samples at 95°C for 10 minutes. Proteins were separated by electrophoresis in an 8%~12% premade sodium dodecylsulfate-polyacrylamide minigel and then transferred onto a PVDF membrane. The membranes were incubated with the indicated antibodies overnight at 4°C, followed by incubation with HRP-conjugated secondary antibodies for 2 hours at room temperature. Immunoreactive bands were detected by chemiluminescence. For IP, the indicated primary antibodies were incubated with magnetic beads (HY-K0205, MedChemExpress [MCE]) overnight at 4°C and subsequently rotated with cell lysis, followed by Western blot analysis.
In vitro methylation assays and in vitro binding assays.
In vitro methylation assays were performed as described previously (64). Briefly, EZH2 (ab132934, Abcam) and SMAD3 (ab151882, Abcam) proteins were purchased. Flag-tagged EZH2 protein was expressed and purified from HEK293T cells. Synthesized SMAD3 peptides from Abclone served as substrates. In each tube contained 1 μg Flag-tagged EZH2, 5 μL 5× protein lysine methyltransferase (PKMT) buffer (10 mM Tris–HCl [pH 8], 2% glycerol, 0.8 mM KCl, 1 mM MgCl2), 13 μM S-adenosyl-l-methionine (SAM), and GST-SMAD3–purified protein or SMAD3 peptides as substrates, with addition of H2O for a final volume of 25 μL. The reaction tubes were incubated at 37°C for 10 hours. The reaction was stopped by adding 5× protein sample buffer (250 mM Tris-HCl [pH 6.8], 10% SDS, 30% glycerol, 5% β-mercaptoethanol, bromophenol blue) followed by boiling of the samples at 95°C for 10 minutes. For the in vitro binding assays, purified EZH2 protein was incubated with GST or GST-SMAD3 fusion proteins bound to glutathione sepharose beads at 4°C overnight followed by Western blot analysis.
IHC analyses.
All clinical samples were obtained from patients with breast cancer who underwent surgical resection at Wuhan Tongji Hospital. None of the patients received chemotherapy or radiation therapy before surgery. For ICH analyses, tissue specimens were first cut into sections of 4 μm thickness and then fixed with 4% paraformaldehyde for 15 minutes at room temperature. The samples were stained with the indicated primary antibodies overnight at 4°C, followed by staining with secondary antibodies at room temperature for 1 hour. Two experienced pathologists received and scored the immunostaining results independently. The immunoreactive score (IRS) was used to quantify the IHC results. The percentage of positively stained tumor cells was scored as follows: 1 (<10%), 2 (10%–50%), 3 (50–75%), and 4 (>75%). Staining intensity was scored as follows: 0 = no staining; 1 = weak staining; 2 = moderate staining; 3 = strong staining. Finally, the IHC score was calculated by multiplying the score of the percentage of positively stained tumor cells and staining intensity, which ranged from 0 to 12.
Immunocytochemistry.
For immunocytochemistry, the indicated cells were fixed with 4% paraformaldehyde for 15 minutes at room temperature. Next, the cells were permeabilized in 5% Triton X-100 for 5 minutes and then cultured with primary antibodies for 1 hour at room temperature. Anti–mouse Alexa Fluor 488 or 594 dye–conjugated and/or anti–rabbit Alexa Fluor 488 or 594 dye–conjugated secondary antibodies were used. Cell nuclei were stained with DAPI. Then, the cells were imaged using a multiphoton confocal laser scanning or fluorescence microscope.
Transwell assays.
For Transwell migration and invasion assays, 4 × 104 cells (for invasion) or 10 × 104 cells (for migration) suspended in medium without FBS were seeded in the upper chamber membranes with or without Matrigel (BD Biosciences), and 500 μL complete medium was added to the lower chamber. After 6–12 hours, the cells were fixed with 4% paraformaldehyde for 15 minutes at room temperature and then stained with 0.1% Crystal Violet. Cells of the inner chamber were wiped out with a cotton swab. Cell numbers were calculated with a microscope.
Mouse xenograft assays.
Four-week-old female BALB/c nude mice were purchased from Beijing Huafukang Bioscience. The indicated cancer cells were collected and washed twice with PBS. Cells were resuspended with PBS and injected into the tail vein (4 × 105 cells per mouse). TAT control and TAT nonmethyl peptides (100 μL, 1 μg/mL) were injected into the tail vein at the indicated time intervals. After 2 months, the mice were sacrificed and their lungs isolated and imaged.
Statistics.
All statistical analysis was conducted using GraphPad Prism 8.0 (GraphPad Software) and SPSS 22.0 (IBM). All data represent the mean ± SD. Spearman’s r was calculated for ordinal data. Fisher’s exact test was used in the contingency tables. Pearson’s χ2 and Mann-Whitney U tests were used to assess the correction for between levels of protein expression. The difference was tested using a 2-tailed Student’s t test or 1-way ANOVA. A P value of less than 0.05 was considered statistically significant.
Study approval.
This study was approved by the Huazhong University of Science and Technology Ethics Committee. Written informed consent was obtained from all patients in this study. All mouse experiments were approved by the IACUC of Tongji Hospital.
Author contributions
CH and Fuqing Hu performed the majority of the experiments. CH and GW wrote the manuscript. CH, DS, AL, QW, Xiaowei She, GW, and JH designed the research studies and acquired data. YC, Fayong Hu, and FX helped analyze data. XL and YF provided reagents. LC and XS acquired data. XY provided advice for our study. All authors approved this study.
Supplementary Material
Acknowledgments
We thank members of the Medical Subcenter of the HUST Analytical and Testing Center for their help with data acquisition. We appreciate the assistance from members of GW’s laboratory and Junbo Hu’s laboratory. We are grateful to Xiangping Yang for helpful suggestions relating to our work. This study was supported by the National Natural Science Foundation of China (NSFC) (81874186, to JH; 81773113, 81974432, and 81922053, to GW) and by startup funding from Tongji Hospital (to GW).
Version 1. 01/27/2022
In-Press Preview
Version 2. 03/01/2022
Electronic publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2022, Huang et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: J Clin Invest. 2022;132(5):e152394.https://doi.org/10.1172/JCI152394.
Contributor Information
Changsheng Huang, Email: changshenghuang@yeah.net.
Fuqing Hu, Email: qinghufu@163.com.
Da Song, Email: 15802743491@163.com.
Xuling Sun, Email: tjhsxl@126.com.
Anyi Liu, Email: anyi_liu0316@163.com.
Qi Wu, Email: wqwd486356@163.com.
Xiaowei She, Email: xwshe97@163.com.
Yaqi Chen, Email: chenyqclaire@163.com.
Lisheng Chen, Email: clsmail1990@163.com.
Fayong Hu, Email: hufayong2013@163.com.
Feng Xu, Email: 13476111467@163.com.
Xuelai Luo, Email: luoxl@tjh.tjmu.edu.cn.
Yongdong Feng, Email: ydfeng@tjh.tjmu.edu.cn.
Xiangping Yang, Email: yangxp@hust.edu.cn.
Junbo Hu, Email: jbhu@tjh.tjmu.edu.cn.
Guihua Wang, Email: ghwang@tjh.tjmu.edu.cn.
References
- 1.Pei D, et al. Mesenchymal-epithelial transition in development and reprogramming. Nat Cell Biol. 2019;21(1):44–53. doi: 10.1038/s41556-018-0195-z. [DOI] [PubMed] [Google Scholar]
- 2.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147(2):275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen W, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 4.Colak S, Dijke Pt Targeting TGF-β signaling in cancer. Trends Cancer. 2017;3(1):56–71. doi: 10.1016/j.trecan.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brabletz T, et al. EMT in cancer. Nat Rev Cancer. 2018;18(2):128–134. doi: 10.1038/nrc.2017.118. [DOI] [PubMed] [Google Scholar]
- 7.Zhao X, et al. Targeting metabolic dysregulation for fibrosis therapy. Nat Rev Drug Discov. 2020;19(1):57–75. doi: 10.1038/s41573-019-0040-5. [DOI] [PubMed] [Google Scholar]
- 8.Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11(5):255–265. doi: 10.1038/nrcardio.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Batlle E, Massague J. Transforming growth factor-β signaling in immunity and cancer. Immunity. 2019;50(4):924–940. doi: 10.1016/j.immuni.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Derynck R, et al. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29(2):117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 11.Tang X, et al. SIRT7 antagonizes TGF-β signaling and inhibits breast cancer metastasis. Nat Commun. 2017;8(1):318. doi: 10.1038/s41467-017-00396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Itoh Y, et al. Smad3-STAT3 crosstalk in pathophysiological contexts. Acta Biochim Biophys Sin (Shanghai) 2018;50(1):82–90. doi: 10.1093/abbs/gmx118. [DOI] [PubMed] [Google Scholar]
- 13.Katz LH, et al. Targeting TGF-β signaling in cancer. Expert Opin Ther Targets. 2013;17(7):743–760. doi: 10.1517/14728222.2013.782287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mourskaia AA, et al. Targeting aberrant TGF-beta signaling in pre-clinical models of cancer. Anticancer Agents Med Chem. 2007;7(5):504–514. doi: 10.2174/187152007781668689. [DOI] [PubMed] [Google Scholar]
- 15.Mullen AC, et al. Master transcription factors determine cell-type-specific responses to TGF-β signaling. Cell. 2011;147(3):565–576. doi: 10.1016/j.cell.2011.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi YG, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113(6):685–700. doi: 10.1016/S0092-8674(03)00432-X. [DOI] [PubMed] [Google Scholar]
- 17.Massagué J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1(3):169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 18.Petersen M, et al. Smad2 and Smad3 have opposing roles in breast cancer bone metastasis by differentially affecting tumor angiogenesis. Oncogene. 2010;29(9):1351–1361. doi: 10.1038/onc.2009.426. [DOI] [PubMed] [Google Scholar]
- 19.Dzwonek J, et al. Smad3 is a key nonredundant mediator of transforming growth factor beta signaling in Nme mouse mammary epithelial cells. Mol Cancer Res. 2009;7(8):1342–1353. doi: 10.1158/1541-7786.MCR-08-0558. [DOI] [PubMed] [Google Scholar]
- 20.Carlson SM, Gozani O. Emerging technologies to map the protein methylome. J Mol Biol. 2014;426(20):3350–3362. doi: 10.1016/j.jmb.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu F, et al. PKM2 methylation by CARM1 activates aerobic glycolysis to promote tumorigenesis. Nat Cell Biol. 2017;19(11):1358–1370. doi: 10.1038/ncb3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albert M, Helin K. Histone methyltransferases in cancer. Semin Cell Dev Biol. 2010;21(2):209–220. doi: 10.1016/j.semcdb.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Ambler RP, Rees MW. ε-N‑methyl-lysine in bacterial flagellar protein. Nature. 1959;184:56–57. doi: 10.1038/184056b0. [DOI] [PubMed] [Google Scholar]
- 24.Webb KJ, et al. Identifcation of protein N-terminal methyltransferases in yeast and humans. Biochemistry. 2010;49(25):5225–5235. doi: 10.1021/bi100428x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy D. Lysine methylation signaling of non-histone proteins in the nucleus. Cell Mol Life Sci. 2019;76(15):2873–2883. doi: 10.1007/s00018-019-03142-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sergei Chuikov, et al. Regulation of p53 activity through lysine__methylation. Nature. 2004;432(7015):353–360. doi: 10.1038/nature03117. [DOI] [PubMed] [Google Scholar]
- 27.Xu Z, et al. SMURF2 regulates bone homeostasis by disrupting SMAD3 interaction with vitamin D receptor in osteoblasts. Nat Commun. 2017;8:14570. doi: 10.1038/ncomms14570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao S, et al. Ubiquitin ligase Nedd4L targets activated Smad2/3 to limit TGF-beta signaling. Mol Cell. 2009;36(3):457–468. doi: 10.1016/j.molcel.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoue Y, Imamura T. Regulation of TGF-beta family signaling by E3 ubiquitin ligases. Cancer Sci. 2008;99(11):2107–2112. doi: 10.1111/j.1349-7006.2008.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herhaus L, et al. OTUB1 enhances TGFβ signalling by inhibiting the ubiquitylation and degradation of active SMAD2/3. Nat Commun. 2013;4:2519. doi: 10.1038/ncomms3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li K, et al. Metformin suppresses melanoma progression by inhibiting KAT5-mediated SMAD3 acetylation, transcriptional activity and TRIB3 expression. Oncogene. 2018;37(22):2967–2981. doi: 10.1038/s41388-018-0172-9. [DOI] [PubMed] [Google Scholar]
- 32.Simonsson M, et al. The DNA binding activities of Smad2 and Smad3 are regulated by coactivator-mediated acetylation. J Biol Chem. 2006;281(52):39870–39880. doi: 10.1074/jbc.M607868200. [DOI] [PubMed] [Google Scholar]
- 33.Inoue Y, et al. Smad3 is acetylated by p300/CBP to regulate its transactivation activity. Oncogene. 2007;26(4):500–508. doi: 10.1038/sj.onc.1209826. [DOI] [PubMed] [Google Scholar]
- 34.Guo J, et al. AKT methylation by SETDB1 promotes AKT kinase activity and oncogenic functions. Nat Cell Biol. 2019;21(2):226–237. doi: 10.1038/s41556-018-0261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin HK, et al. Cytoplasmic PML function in TGF-beta signaling. Blood. 2004;104(11):481. doi: 10.1182/blood.V104.11.481.481. [DOI] [PubMed] [Google Scholar]
- 36.Tuncer E, et al. SMAD signaling promotes melanoma metastasis independently of phenotype switching. J Clin Invest. 2019;129(7):2702–2716. doi: 10.1172/JCI94295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asangani IA, et al. Characterization of the EZH2-MMSET histone methyltransferase regulatory axis in cancer. Mol Cell. 2013;49(1):80–93. doi: 10.1016/j.molcel.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dasgupta M, et al. STAT3-driven transcription depends upon the dimethylation of K49 by EZH2. Proc Natl Acad Sci U S A. 2015;112(13):3985–3990. doi: 10.1073/pnas.1503152112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim E, et al. Phosphorylation of EZH2 activates STAT3 signaling via STAT3 methylation and promotes tumorigenicity of glioblastoma stem-like cells. Cancer Cell. 2013;23(6):839–852. doi: 10.1016/j.ccr.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei FZ, et al. Epigenetic regulation of autophagy by the methyltransferase EZH2 through an MTOR-dependent pathway. Autophagy. 2015;11(12):2309–2322. doi: 10.1080/15548627.2015.1117734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Massague J. TGFbeta in cancer. Cell. 2008;134(2):215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anwar T, et al. p38-mediated phosphorylation at T367 induces EZH2 cytoplasmic localization to promote breast cancer metastasis. Nat Commun. 2018;9(1):2801. doi: 10.1038/s41467-018-05078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeng Y, et al. Regulation of EZH2 by SMYD2-mediated lysine methylation is implicated in tumorigenesis. Cell Rep. 2019;29(6):1482–1498. doi: 10.1016/j.celrep.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 44.Hirukawa A, et al. Targeting EZH2 reactivates a breast cancer subtype-specific anti-metastatic transcriptional program. Nat Commun. 2018;9(1):2547. doi: 10.1038/s41467-018-04864-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wan L, et al. Phosphorylation of EZH2 by AMPK suppresses PRC2 methyltransferase activity and oncogenic function. Mol Cell. 2018;69(2):279–291. doi: 10.1016/j.molcel.2017.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-b family signalling. Nature. 2003;425(6958):577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 47.Tsukazaki T, et al. SARA, a FYVE domain protein that recruits Smad2 to the TGFbeta receptor. Cell. 1998;95(6):779–791. doi: 10.1016/S0092-8674(00)81701-8. [DOI] [PubMed] [Google Scholar]
- 48.Chaikuad A, Bullock AN. Structural basis of intracellular TGF-β signaling: receptors and smads. Cold Spring Harb Perspect Biol. 2016;8(11):a022111. doi: 10.1101/cshperspect.a022111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu G, et al. Structural basis of Smad2 recognition by the Smad anchor for receptor activation. Science. 2000;289(5450):93–97. doi: 10.1126/science.287.5450.92. [DOI] [PubMed] [Google Scholar]
- 50.Gyorffy B, et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat. 2010;123(3):725–731. doi: 10.1007/s10549-009-0674-9. [DOI] [PubMed] [Google Scholar]
- 51.Edlund S, et al. Transforming growth factor-beta-induced mobilization of actin cytoskeleton requires signaling by small GTPases Cdc42 and RhoA. Mol Biol Cell. 2002;13(3):902–914. doi: 10.1091/mbc.01-08-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rao ZY, et al. EZH2 supports ovarian carcinoma cell invasion and/or metastasis via regulation of TGF-beta1 and is a predictor of outcome in ovarian carcinoma patients. Carcinogenesis. 2010;31(9):1576–1583. doi: 10.1093/carcin/bgq150. [DOI] [PubMed] [Google Scholar]
- 53.Karakashev S, et al. CARM1-expressing ovarian cancer depends on the histone methyltransferase EZH2 activity. Nat Commun. 2018;9(1):631. doi: 10.1038/s41467-018-03031-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fujii S, et al. MEK-ERK pathway regulates EZH2 overexpression in association with aggressive breast cancer subtypes. Oncogene. 2011;30(39):4118–4128. doi: 10.1038/onc.2011.118. [DOI] [PubMed] [Google Scholar]
- 55.Zhou L, et al. Targeting EZH2 enhances antigen presentation, antitumor immunity, and circumvents anti-PD-1 resistance in head and neck cancer. Clin Cancer Res. 2020;26(1):290–300. doi: 10.1158/1078-0432.CCR-19-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bugide S, et al. Inhibition of enhancer of zeste homolog 2 (EZH2) induces natural killer cell-mediated eradication of hepatocellular carcinoma cells. Proc Natl Acad Sci U S A. 2018;115(15):E3509–E3518. doi: 10.1073/pnas.1802691115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu CP, et al. SIS3, a specific inhibitor of Smad3 reverses ABCB1-and ABCG2-mediated multidrug resistance in cancer cell lines. Cancer Lett. 2018;433:259–272. doi: 10.1016/j.canlet.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ji X, et al. Specific inhibitor of Smad3 (SIS3) attenuates fibrosis, apoptosis, and inflammation in unilateral ureteral obstruction kidneys by inhibition of transforming growth factor β (TGF-β)/Smad3 signaling. Med Sci Monit. 2018;24:1633–1641. doi: 10.12659/MSM.909236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jinnin M, et al. Characterization of SIS3, a novel specific inhibitor of Smad3, and its effect on transforming growth factor-beta1-induced extracellular matrix expression. Mol Pharmacol. 2006;69(2):597–607. doi: 10.1124/mol.105.017483. [DOI] [PubMed] [Google Scholar]
- 60.Tang PM, et al. Smad3 promotes cancer progression by inhibiting E4BP4-mediated NK cell development. Nat Commun. 2017;8:14677. doi: 10.1038/ncomms14677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu RY, et al. JAK/STAT3 signaling is required for TGF-β-induced epithelial-mesenchymal transition in lung cancer cells. Int J Oncol. 2014;44(5):1643–1651. doi: 10.3892/ijo.2014.2310. [DOI] [PubMed] [Google Scholar]
- 62.Italiano A, et al. Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: a first-in-human, open-label, phase 1 study. Lancet Oncol. 2018;19(5):649–659. doi: 10.1016/S1470-2045(18)30145-1. [DOI] [PubMed] [Google Scholar]
- 63.Yap TA, et al. Phase I study of the novel enhancer of zeste homolog 2 (EZH2) inhibitor GSK2816126 in patients with advanced hematologic and solid tumors. Clin Cancer Res. 2019;25(24):7331–7339. doi: 10.1158/1078-0432.CCR-18-4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang G, et al. SETDB1-mediated methylation of Akt promotes its K63-linked ubiquitination and activation leading to tumorigenesis. Nat Cell Biol. 2019;21(2):214–225. doi: 10.1038/s41556-018-0266-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.