Abstract
Background
Household air pollution from biomass cookstoves is a major contributor to global morbidity and mortality, yet little is known about exposures to nitrogen dioxide (NO2).
Objective
To characterize NO2 kitchen area concentrations and personal exposures among women with biomass cookstoves in the Peruvian Andes.
Methods
We measured kitchen area NO2 concentrations at high-temporal resolution in 100 homes in the Peruvian Andes. We assessed personal exposure to NO2 in a subsample of 22 women using passive samplers.
Results
Among 97 participants, the geometric mean (GM) highest hourly average NO2 concentration was 723 ppb (geometric standard deviation (GSD) 2.6) and the GM 24-hour average concentration was 96 ppb (GSD 2.6), 4.4 and 2.9 times greater than WHO indoor hourly (163 ppb) and annual (33 ppb) guidelines, respectively. Compared to the direct-reading instruments, we found similar kitchen area concentrations with 48-hour passive sampler measurements (GM 108 ppb, GSD 3.8). Twenty-seven percent of women had 48-hour mean personal exposures above WHO annual guidelines (GM 18 ppb, GSD 2.3). In univariate analyses, we found that roof, wall, and floor type, as well as higher SES, was associated with lower 24-hour kitchen area NO2 concentrations.
Practical Implications
Kitchen area concentrations and personal exposures to NO2 from biomass cookstoves in the Peruvian Andes far exceed WHO guidelines. More research is warranted to understand the role of this understudied household air pollutant on morbidity and mortality and to inform cleaner-cooking interventions for public health.
Keywords: biomass cookstove, environmental justice, household air pollution, indoor air pollution, nitrogen dioxide, women’s health
1. | INTRODUCTION
Nearly 40% of households worldwide use biomass fuels, such as charcoal, wood, and dung, as their primary source of cooking fuel.1 These biomass cookstoves produce high levels of household air pollution (HAP) which can exceed WHO indoor guidelines2 by orders of magnitude.3 HAP from cookstoves is typically characterized by short-term, high-concentration spikes during cooking which gradually decay to background levels once cooking is complete. Women and children specifically face higher exposure to smoke from biomass cookstoves due to traditional labor roles and closer proximity to and involvement with cooking activities.4,5 Emissions from biomass cookstoves are complex mixtures of a range of health-threatening pollutants,6 yet the existing cookstove literature has focused on particulate matter and carbon monoxide (CO) as the components of HAP with the greatest impact on morbidity and mortality.7 Exposure to HAP has been associated with acute lower respiratory infection in children,8 COPD,8–11 lung cancer,12,13 and increased blood pressure14,15 and was responsible for an estimated 1.6 million premature deaths worldwide in 2017.16
Little is known about nitrogen dioxide (NO2) emissions and exposures from biomass cookstoves, in spite of NO2 being a known by-product of biomass combustion6,17–19 and a regulated hazard to human health.18 According to the United States Environmental Protection Agency (US EPA), exposure to NO2 is causally related to respiratory effects.18 A growing body of literature suggests associations between NO2 exposure and the development20 and exacerbations21–24 of pediatric asthma, COPD,25,26 worse lung function in adults and children,27–33 and all-cause mortality.34,35 High-concentration spikes of NO2 exposure are known to have different effects on health than chronic exposures,18 and the WHO has developed separate annual and hourly guidelines for indoor NO2 concentrations.2 A few studies in Asia and Africa have measured 24- or 48-hour time-weighted average (TWA) concentrations of NO2 in homes with biomass cookstoves. With one exception,36 all studies reported mean kitchen area TWA concentrations between 1.5 and 6.5 times greater 37–43 than the WHO indoor annual guideline.2 However, to our knowledge, no study has characterized NO2 concentrations from biomass cookstoves at high-temporal resolution to quantify the potentially health-relevant concentration spikes from cooking or to assess whether kitchen concentrations exceed hourly guidelines. Additionally, we know of no assessments of personal exposure to NO2 from cooking with biomass fuel. The dearth of knowledge on NO2 exposures from biomass cookstoves leaves a critical gap in our understanding of the full impacts of biomass cookstoves on global morbidity and mortality and of the pollutant reductions needed to improve public health through cleaner-cooking interventions. To address this gap, this study aims to characterize daily and hourly kitchen area concentrations and personal exposures to NO2 among women who use biomass cookstoves in rural Peru.
2. | METHODS
2.1. | Study setting and design
This study was nested within a randomized controlled, field trial of a liquefied-petroleum gas (LPG) cleaner-cooking intervention in the Puno province of Peru. Puno is a rural, agricultural region in the southern Peruvian Andes at 3,825 meters above sea level. Openfire biomass-burning stoves are prevalent, and cow dung is the most common cooking fuel.44 We enrolled study participants from predominantly Indigenous Aymara communities where daily life generally revolves around subsistence farming and small-scale quinoa and potato production. In these low-density communities, homes are located a median distance of 101 meters from the closest neighboring home45 and local sources of ambient air pollution are minimal. Most households have separate kitchen and sleeping structures, which together surround a central courtyard.
In the Cardiopulmonary Outcomes and Household Air Pollution Trial (CHAP),45 180 women aged 25–64 years who used biomass fuel daily were enrolled and randomized 1:1 into an LPG intervention group or a control group. Participants in the intervention group received an LPG stove and free fuel delivered to their homes and installed by trained research staff for one year. Participants in the control group continued to use biomass and will receive an LPG stove and fuel after the first year of the study. The current study is based on results from the CHAP trial’s baseline assessments of women who used biomass cookstoves daily. Participants’ demographic data were collected via questionnaire and kitchen characteristics were directly observed by trained field research staff. Wealth status was calculated using the Demographic and Health Survey (DHS) wealth quintiles, which were previously determined based on a nationally representative survey conducted in Peru.46 We assigned each participant in our study a score based on their ownership of assets considered by the DHS (computer, bookshelf, windows with curtains, sofa, toilet connected to the sewer, reinforced concrete roof, and brick or cement walls).47 We then classified participants into one of the five wealth quintiles based on their total score. Additional information about enrollment and assessments in the CHAP trial has been previously published.45
We randomly selected a subsample of 100 participants from the overall trial (n = 180) for NO2 kitchen area assessment and inclusion in this study. Of those 100 participants, 22 participants were randomly selected to also receive personal exposure assessment. All participants provided informed consent verbally, and the trial received approval from the Johns Hopkins School of Public Health Institutional Review Board, AB PRISMA Ethical Institutional Committee, and the Universidad Peruana Cayetano Heredia Institutional Review Board.
2.2. | Exposure assessment of nitrogen dioxide
2.2.1. | Kitchen area assessment
We monitored kitchen area NO2 concentrations for 48 hours in each household. We used direct-reading monitors to sample at high-temporal resolution in 100 households and collected additional, time-integrated measurements via passive samplers in a subsample of 20 households. All kitchen area samplers were placed in a wire birdcage suspended from the ceiling, located at 1.0 meter horizontal distance from the edge of the stove combustion zone and 1.5 meters above the floor. This standard location was chosen to approximate the breathing zone of a woman cooking at the stove. Field staff used a measuring tape to ensure consistent placement of the samplers in relation to the cookstove.
We sampled kitchen area NO2 concentrations at high-temporal resolution using Aeroqual Series 500 portable direct-reading monitors with NO2 sensor heads (Aeroqual Limited, Auckland, New Zealand). Measurements were recorded at one-minute temporal resolution throughout the 48-hour sample, with support from two auxiliary batteries to extend the sampling duration due to the lack of electricity in many households. To mitigate imprecision between sensors, we co-located Aeroqual monitors in the field office every four months and subjected all monitors to NO2 concentrations ranging from ambient concentration to approximately 1000 ppb, using an LPG stove as the emissions source. We calculated intercept and slope adjustments for each sensor with robust linear regression using Siegel repeated medians (mblm R package v0.12.1; Komsta, 2019), comparing individual sensor readings with the median measurement from all collocated sensors. We found no evidence of a linear pattern in the drift of individual sensors across multiple collocations over time, and all collocations were pooled to create a single slope and intercept adjustment for each device. To determine the limit of detection (LOD), two devices were brought to the Johns Hopkins University for comparison with a gold standard reference instrument (model 42c, Thermo Environmental Instruments Inc, Franklin). The LOD was calculated as three times the standard deviation (SD) of the difference between these two devices and reference instrument-confirmed zero-air from a dynamic gas calibrator (model 146i, Thermo Environmental Instruments Inc, Franklin). We estimated an LOD of 20 ppb, and 36% of all collected 1-minute measurements fell beneath the LOD. All concentrations <20 ppb were replaced with LOD/sqrt(2) ≈ 14.1 ppb, which is similar to a recent modeled estimate48 of ambient NO2 conditions in the Puno region (12 ppb). After twelve months of use, sensor heads were decommissioned and replaced with new, factory-calibrated sensors, as recommended by the manufacturer for high-concentration settings.
In a subset of 20 households, we sampled time-integrated kitchen area NO2 concentrations with Ogawa Passive Samplers (Ogawa USA, Pompano Beach). Samples were analyzed at the Universidad Peruana Cayetano Heredia in Lima, Peru, using standard colorimetric methods.49 Temperature and relative humidity were measured during each sample by a collocated Enhanced Children’s Monitor (RTI Inc, Research Triangle Park) for calculation of the final TWA NO2 concentration. Temperature data for one sample were missing due to instrument failure and were imputed using the median temperature from all kitchen samples. Field blanks were taken every 10th sample, and we calculated the LOD as the mean plus SD*3 concentration among blanks. We estimated the LOD to be 2.6 ppb, similar to the manufacturer recommended lower range of accuracy (2 ppb), and none of the passive sampler kitchen area concentrations fell beneath the LOD.
2.2.2. | Personal exposure assessment
We assessed personal exposure for 48 hours using Ogawa Passive Samplers as described above. These passive badge samplers are small and lightweight and, unlike the heavier Aeroqual direct-reading monitors, can be easily worn by participants. The passive samplers were attached to aprons which had been altered to hold personal exposure monitors for PM2.5 and CO and were provided to the participants.45 Participants were asked to wear the apron at all times during waking hours and to place the apron nearby when sleeping or bathing. The NO2 sampler and temperature and relative humidity loggers were placed over each participant’s central chest region, to approximate conditions in each woman’s breathing zone. One personal sample had missing temperature data, which was replaced with the median temperature among all personal samples. We used the same LOD of 2.6 ppb for personal exposure samples, and one personal exposure sample (4%) which was below the LOD was replaced with LOD/sqrt(2) ≈ 1.8 ppb.
2.3. | Statistical methods
All samples from the direct-reading kitchen monitors that did not meet a minimum duration of 20 hours were excluded from the analysis. We hypothesized that mean kitchen area concentrations would largely be driven by a small number of biomass cooking events throughout the day and that samples that lasted less than the targeted 48 hours would be biased by which cooking events were or were not captured in the final sampling day. As 15% of sample durations were >24 hours but ≤44 hours, we used only data from sampling hours 0–24 to calculate a 24-hour mean, for consistency. Because of the high-altitude setting, we assumed conditions of 10°C and an altitude of 3825 MASL to estimate an atmospheric pressure of 625 hPa and convert mass concentration guidelines to setting-specific ppb (annual, 40 μg/m3 = 33 ppb; hourly 200 μg/m3 = 163 ppb).2 We calculated the highest hourly average concentration as the highest 60-minute centered rolling mean throughout the entire duration of each kitchen area sample. To understand the duration of high exposures, we estimated the number of hours per day where kitchen area concentration exceeded 163 ppb, the WHO indoor NO2 hourly guideline.2 We calculated summary statistics for the direct-reading measurements and the time-integrated passive samplers. We performed a Shapiro-Wilk test of the log-transformed 24-hour mean kitchen area concentrations, which failed to reject the null of a lognormal distribution (P-value = .052). Therefore, we calculated and reported geometric mean (GM) and geometric standard deviation (GSD) for descriptive results of NO2 concentrations, as a compliment to the arithmetic mean and standard deviation. We also estimated the Spearman’s correlation coefficient of 48-hour passive samples comparing concurrent measurements of kitchen area concentration and personal exposure.
We used linear regression to examine associations between 24-hour mean kitchen area NO2 concentrations and characteristics of participants and their kitchens. We log-transformed NO2 concentrations to meet model assumptions of homoscedasticity. All regression estimates were transformed to represent the percent difference in NO2 concentration (the dependent variable) associated with a unit increase in the independent variables. We used simple linear regression to analyze the single variable associations between 24-hour mean NO2 and age (in one-year increments), education (primary school or less vs. secondary school), SES quintiles (1st [lowest] vs. 2nd or 3rd), chimney (none vs. roof opening vs. chimney), roof type (thatch/natural fiber/other vs. corrugated metal), wall type (adobe without plaster vs. adobe with plaster/other), floor type (earth vs. cement), and number of windows (0, 1, 2+). We did not include kitchen area entrances in this analysis as all observed kitchens had exactly one entrance.
We also examined the correlation between co-located direct-reading monitors and passive samplers in kitchens, however a substantial number of co-located direct-reading monitors did not achieve the entire 48-hour duration of the passive samplers due to insufficient battery power. All analyses were performed using R (www.r-project.org).
3. | RESULTS
3.1. | Participant characteristics
We successfully completed assessments for 97 participants (Table 1), as three kitchen area assessments did not meet a minimum duration of 20 hours due to battery failure. The average age among participants was 48 years, and 95% of participants were in the two lowest national wealth quintiles. Within participant homes, kitchens generally had either a non-specific opening in the roof or no ventilating structures, with walls made of adobe and earthen floors. We observed a range of 0–6 windows in the kitchens with a median of one window.
TABLE 1.
Characteristics of study participants and their kitchens in Puno, Peru
| N (%) or Mean (SD) | |
|---|---|
| Number of participants | 97 |
| Age in years | 48 (10) |
| Education | |
| Primary or less | 57 (59%) |
| Secondary | 40 (41%) |
| National SES quintile | |
| 1 (lowest) | 55 (57%) |
| 2 | 37 (38%) |
| 3 | 5 (5%) |
| 4 | 0 (0%) |
| 5 (highest) | 0 (0%) |
| Cookstove ventilation | |
| Chimney | 9 (9%) |
| Roof opening | 48 (49%) |
| No cookstove ventilation | 40 (41%) |
| Roof type | |
| Corrugated metal | 43 (44%) |
| Natural fiber (thatch) | 53 (55%) |
| Other | 1 (1%) |
| Wall type | |
| Adobe/mud with plaster | 26 (27%) |
| Adobe/mud without plaster | 65 (67%) |
| Other | 6 (6%) |
| Floor type | |
| Dirt | 89 (92%) |
| Cement | 8 (8%) |
| Kitchen windows (#) | |
| 0 | 38 (39%) |
| 1 | 42 (43%) |
| 2 + | 17 (18%) |
| Kitchen doors/entryways (#) | |
| 1 | 97 (100%) |
Note: SD, standard deviation; SES, socio-economic status.
3.2. | Kitchen area concentrations of nitrogen dioxide
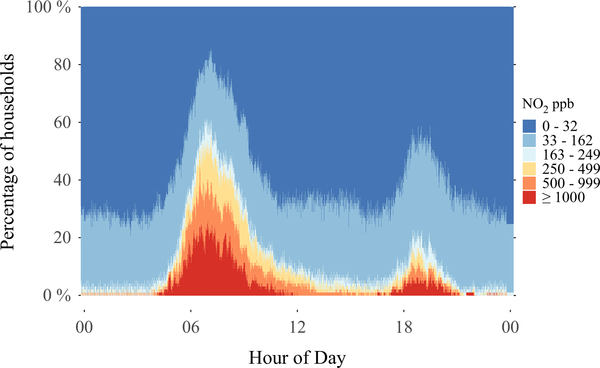
Results from the direct-reading instruments at one-minute temporal resolution indicate short-term concentration spikes in kitchen areas orders of magnitude above the WHO indoor annual and hourly guidelines. These data are presented as a bar plot of study-wide concentrations throughout each minute of the day (Figure 1). Dark blue indicates the proportion of households with kitchen NO2 concentrations <33 ppb at a given time of day, with other colors representing increasingly higher concentration bins as indicated in the legend. A substantial proportion of kitchens experience high concentrations during common cooking times (5:00–9:00 and 18:00–20:00 hours). For example, at approximately 8:00 hours, NO2 concentrations were ≥1000 ppb in 20% of households (red color), ≥500 ppb in 40% of households (red and orange colors), and ≥the WHO indoor hourly guideline of 163 ppb in 60% of households (red, orange, yellow, and light blue colors).
FIGURE 1.
Prevalence of indoor NO2 concentrations by calendar minute in 97 kitchens with biomass cookstoves in rural Peru. For example, at approximately 8:00 h, NO2 concentrations were ≥1000 ppb in 20% of households (red color), ≥500 ppb in 40% of households (red and orange colors), and ≥ the WHO indoor hourly guideline of 163 ppb in 60% of households (red, orange, yellow, and light blue colors)
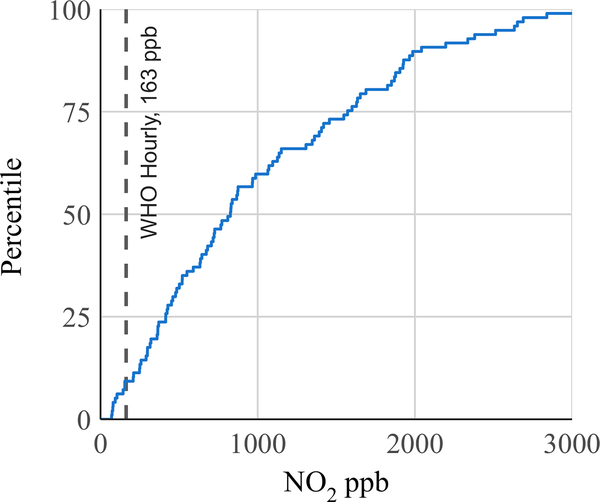
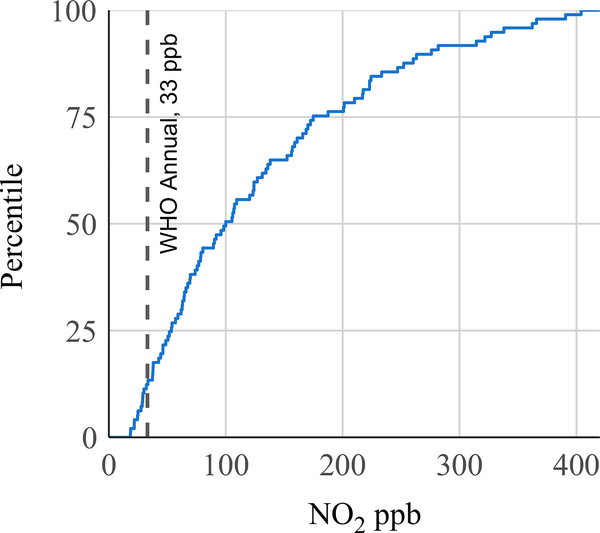
The GM highest hourly average NO2 concentration was 723 ppb (GSD 2.6) (Table 2), over four times greater than the hourly guideline of 163 ppb. The distribution of highest hourly averages per sample is presented as a modified empirical cumulative distribution function plot (Figure 2). The x-axis represents NO2 concentration and the y-axis represents the percent of observations with hourly average concentrations less than the corresponding concentration. Ninety-one percent of kitchens had highest hourly average concentrations above the WHO indoor hourly guideline (163 ppb), and 40% of households had hourly averages which exceeded 1000 ppb. Kitchen concentrations exceeded the hourly guideline for a mean duration of 3.0 hours or 12.5% of daily time (Table 2). The GM 24-hour TWA NO2 concentration from the direct-reading instruments was 96 ppb (GSD 2.2) (Table 2), 2.9 times greater than the WHO annual guideline of 33 ppb. In a modified empirical cumulative distribution plot of 24-hour TWA kitchen concentrations, only 12% of kitchens had 24-hour TWA NO2 concentrations below the annual guideline and 51% of households had 24-hour TWA concentrations more than three times (99 ppb) greater than the WHO annual guideline (Figure 3). In the subsample of 20 kitchens where NO2 was measured using passive samplers, one sample was lost during processing. In the successful 19 kitchen area passive samples, the GM 48-hour TWA NO2 concentration was 108 ppb (Table 2).
TABLE 2.
Nitrogen dioxide kitchen concentrations and personal exposures among women with biomass cookstoves in Puno, Peru
| N | Mean | SD | GM | GSD | Min | 25th %ile | Median | 75th %ile | Max | |
|---|---|---|---|---|---|---|---|---|---|---|
| Kitchen area: direct-reading | ||||||||||
| Highest hourly means (ppb) | 97 | 1027 | 781 | 723 | 2.6 | 70 | 415 | 826 | 1572 | 3901 |
| 24-h means (ppb) | 97 | 129 | 97 | 96 | 2.2 | 18 | 54 | 100 | 175 | 404 |
| Daily hours > 163 ppb | 97 | 3.0 | 2.1 | 2.0 | 3.4 | 0.0 | 1.3 | 2.8 | 4.5 | 9.8 |
| Kitchen area: passive badge | ||||||||||
| 48-h means (ppb) | 19 | 199 | 192 | 108 | 3.8 | 7 | 67 | 117 | 387 | 593 |
| Personal exposure: passive badge | ||||||||||
| 48-h means (ppb) | 22 | 23 | 16 | 18 | 2.3 | 2 | 11 | 19 | 34 | 64 |
Note: GM, geometric mean; GSD, geometric standard deviation; ppb, parts per billion; SD, standard deviation.
FIGURE 2.
Distribution of highest hourly mean NO2 concentrations in 97 kitchens with biomass cookstoves in rural Peru, with WHO indoor NO2 hourly guideline of 163 ppb. The x-axis represents NO2 concentration and the y-axis represents the percent of observations with hourly average concentrations less than the corresponding concentration
FIGURE 3.
Distribution of 24-h mean NO2 concentrations in 97 kitchens with biomass cookstoves in rural Peru, with WHO indoor NO2 annual guideline of 33 ppb
3.3. | Associations between participant and kitchen characteristics and nitrogen dioxide concentrations
In the single variable regression analysis, higher SES, corrugated metal roofs (vs. thatch/natural/other), walls of adobe with plaster/other (vs. adobe without plaster), and cement floors (vs. earth) were associated with lower 24-hour mean kitchen area NO2 concentrations (Table 3). Notably, neither chimney type nor number of kitchen windows were associated with kitchen area NO2 concentration.
TABLE 3.
Associations of 24-h mean NO2 concentrations in 97 kitchen areas of biomass cookstove users in Puno, Peru, presented as percent concentration difference associated with participant and kitchen characteristics
| Univariate | |
|---|---|
| Age (one-year increase) | 1.3 (−0.4, 3.0) |
| Education | |
| Primary or less | Ref |
| Secondary | −14.6 (−38.7, 18.9) |
| SES | |
| 1st quintile (lowest) | Ref |
| 2nd or 3rd quintile | −29.4 (−48.9, −2.5)* |
| Chimney | |
| None | Ref |
| Opening | 3.8 (−26.3, 46.1) |
| Chimney | −37.0 (−65.1, 13.5) |
| Roof type | |
| Thatch/natural/other | Ref |
| Corrugated metal | −49.1 (−62.3, −31.3)* |
| Wall type | |
| Adobe w/o plaster | Ref |
| Adobe w plaster/Other | −31.9 (−51.5, −4.3)* |
| Floor type | |
| Earth | Ref |
| Cement | −48.2 (−71.0, −7.5)* |
| Windows | |
| 0 | Ref |
| 1 | −4.9 (−33.9, 36.7) |
| 2 + | 4.5 (−34.9, 67.8) |
Note: SES, socio-economic status.
P-value < 0.05.
3.4. | Personal exposure to nitrogen dioxide
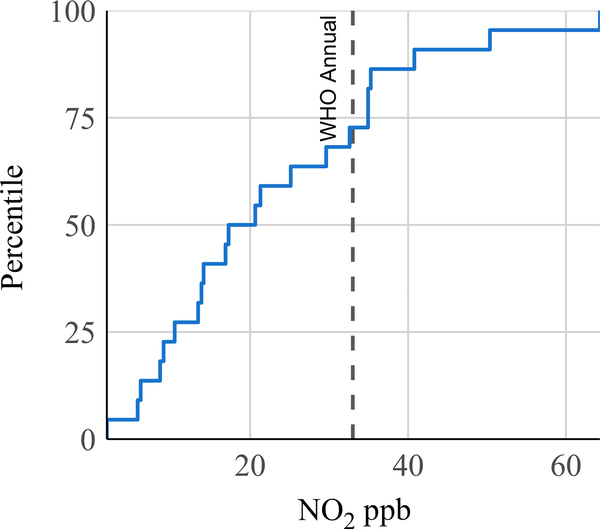
Personal exposure to NO2 was sampled in 22 women using passive samplers attached to aprons worn by participants. The GM time-integrated personal exposure to NO2 during a 48-hour sample was 18 ppb (GSD 2.3) (Table 2). Twenty-seven percent of women experienced mean personal exposures in excess of the WHO annual guideline (33 ppb) (Figure 4). Among 19 concurrent passive samples of NO2 kitchen area concentration and personal exposure, we observed a Pearson correlation coefficient of 0.43.
FIGURE 4.
Distribution of 48-h mean personal exposure to NO2 among 22 women who use biomass cookstoves in rural Peru, with WHO indoor NO2 annual guideline of 33 ppb
4. | DISCUSSION
To our knowledge, this is the first study to measure kitchen area NO2 concentrations at high-temporal resolution and personal exposure to NO2 from biomass cookstoves. Using direct-reading instruments allows for the characterization of concentration spikes from cooking events, which are known to have different health impacts than chronic NO2 exposures and are poorly quantified by integrated sampling methods. Among women with biomass cookstoves in Peru, 91% of households sampled had highest hourly NO2 concentrations above the WHO indoor hourly guideline, with a GM 4.4 times above the same guideline. Chronic exposures were also excessive, and two different sampling methods recorded GM daily kitchen area NO2 concentrations approximately three times greater than the WHO indoor annual guideline. We observed that 27% of women in this study experienced 48-hour TWA personal exposures to NO2 that exceeded annual guidelines.
The mean NO2 kitchen area concentrations we observed in this study (129 ppb arithmetic mean, direct-reading), where dung is the predominant biomass fuel, are similar to those observed by Colbeck et al in biomass-burning kitchens in rural Pakistan (136 ppb).39 An assessment of NO2 in 36 homes in rural Kenya who used a mix of crop residues and wood as fuel observed 24-hour mean concentrations of 90 ppb.43 Padhi et al reported mean kitchen NO2 concentrations of 72 ppb among 378 households in rural India who used a mix of wood, cow dung, agricultural residue, and dried leaves.41 The largest assessment to date, by Kumie et al, recorded a mean of 52 ppb NO2 among 17,215 samples of kitchen areas in Ethiopia where burning of dung is common.37 Cookstove emissions of NO2 are partially a function of biomass fuel type and physical combustion processes.50 However, the relative importance of differences in cooking behaviors associated with different fuel types (eg cooking duration, number of cooking events, household sociodemographic differences, or confounding by regional social and cultural traditions) and kitchen characteristics (eg household ventilation) is poorly understood. In studies which specifically report wood as the primary biomass fuel,36,38,40,42 mean NO2 concentrations are lower (≈30 ppb) than observed in this and other studies in which dung, agricultural residue, or a mix of biomass fuels were used. Lower NO2 emissions from wood compared to dung fuel were also observed in brief assessments of kitchen area NO2 concentrations while cooking in rural India.51 It is worth noting that in spite of possibly lower concentrations in wood-burning kitchens, all but one study36 of wood-fueled cookstoves report mean concentrations that exceed WHO annual guidelines.
While 27% of participants had 48-hour personal exposures above the WHO annual guideline, GM personal exposures were 5 and 6 times lower than direct-reading and passive sampler kitchen area concentrations, respectively. Though we are aware of no prior HAP studies which compare kitchen area concentrations and personal exposures to NO2, a systematic review by the WHO of biomass-related PM2.5 estimated PM2.5 personal exposures 3.6 times lower than PM2.5 kitchen area concentrations.52 In our rural setting, sources of ambient air pollution are minimal and personal exposures are likely dominated by cooking-related exposures while inside the kitchen area. Qualitative research in our study has shown that women frequently do not stay inside the kitchen throughout the entire cooking event. Compared to populations in settings with higher ambient air pollution, participants in our study likely have lower personal exposure to NO2 and other air pollutants when outside the kitchen area, and a larger difference between kitchen area concentrations and personal exposures is plausible.
In our single variable analysis of participant and kitchen characteristics and 24-hour kitchen area NO2 concentration, higher SES as well as type of roof (metal), wall (plastered adobe), and floor (cement) were associated with lower kitchen area NO2. A 2014 study by Pollard et al examining kitchen characteristics and PM2.5 concentrations in rural Puno53 did not find an association between kitchen area PM2.5 and SES, though they did observe a lack of association between kitchen area PM2.5 and windows similar to our study. The apparent lack of importance of number of kitchen windows may be related to the cold climate in Puno, as windows are often kept closed for comfort and may have a relatively minor impact on ventilation. Pollard also reported an association between metal roofs and lower kitchen area PM2.5, which we found for lower kitchen area NO2. It is plausible that these associations are related to ceiling ventilation. In Puno, corrugated metal roofing sheets are commonly attached to the rafters in a manner which creates a gap between the roof and the kitchen wall, potentially facilitating ventilation. In contrast, thatch roofs a few decimeters thick often sit directly on top of the kitchen wall, providing limited airflow. If roof material or design is related to kitchen area NO2 concentration, it provides a potential point of intervention to reduce NO2 exposures from biomass cookstoves.
We observed similar GM kitchen area daily concentrations using the passive samplers and the direct-reading monitors. While the number of passive samples was relatively small, these passive samplers are considered a gold standard method, and the similar magnitude of daily concentrations observed corroborates the high daily concentrations reported by the direct-reading monitors. We intended to compare measurements from collocated direct-reading monitors and passive samplers, yet many of the direct-reading samples did not achieve the full 48 hours of duration of the passive samplers, due to battery failure. To determine if the first and second days of direct-reading sampling were interchangeable, we compared mean concentrations between hours 0–24 and hour 24 through the end of each direct-reading sample. We estimated an adjusted R2 of 0.33 using the mean concentration during the first day of sampling to predict mean concentrations during the second day. Because of the substantial within-household variability between the first and second sampling days with the direct-reading monitors, we concluded that the measurements of physically collocated direct-reading monitors (ranging from 20 to 48 hours duration) and passive sampler (≈48 hours duration) measurements were not directly comparable given the differences in sampling duration.
This study has many strengths, including the use of monitors with high-temporal resolution to detect time-weighted average concentrations and acute concentration spikes, the use of both passive and direct-reading samplers to take field measurements, and the collection of personal exposure samples. This study is limited by the relatively small number of personal samples, though this is the first assessment of personal exposure to NO2 from biomass cookstoves to the authors’ knowledge. While direct-reading instruments allowed us to observe NO2 concentrations at high-temporal resolution among a much larger sample of kitchen areas, we do not know how these concentrations relate to true personal exposures as individual behavior such as timing and duration of time spent in the kitchen or proximity to other sources of NO2 is unknown. Furthermore, while Aeroqual NO2 monitors were calibrated in high-altitude Puno, the estimation of the LOD was performed at approximately sea level in Baltimore, USA. A recent study of Aeroqual O3 monitors, with similar electrochemical sensors to the NO2 monitors in this study, found comparable correlations with reference-instruments in both high-altitude (approximately 1650 MASL) Denver, USA, and low-altitude (approximately 300 MASL) Atlanta, USA.54 While the effect of atmospheric pressure on electrochemical sensors is expected to be minimal by design, we cannot rule out the possibility of bias in our LOD estimates from varying altitude.
5. | CONCLUSIONS
Current efforts to reduce morbidity and mortality from emissions from biomass cookstoves have focused on PM2.5 and CO as the primary risks to health. However, this study provides evidence of acute and long-term NO2 area concentrations and personal exposures at levels known to harm health, using two different sampling methods. We also found evidence that NO2 concentrations are associated with kitchen roof, wall, and floor materials, which may be actionable points of intervention to reduce NO2 exposure among biomass fuel users. While this study is based in the Peruvian Andes, studies in Pakistan, Kenya, India, and Ethiopia have also indicated high mean concentrations of NO2 with a similar magnitude in homes with biomass cookstoves. If NO2 exposures from biomass cookstoves are present in other settings globally, the public health community may be underestimating true morbidity and mortality from biomass cookstove emissions by focusing only on the risks from PM2.5 and CO. Children in particular are known to be vulnerable to NO2 exposure, which can lead to the development and exacerbation of pediatric asthma and limit lung development with permanent effect. Further attention is warranted to characterize the global presence of acute and chronic NO2 exposures from biomass cookstoves, understand how this is impacting the health of women, children, and others who cook with biomass fuel, and ensure that cleaner-cooking public health interventions are effective at reducing not only PM2.5 and CO, but also NO2.
Practical Implications.
Epidemiologic studies and public health interventions of household air pollution (HAP) have focused on particulate matter and carbon monoxide as the byproducts of biomass combustion of greatest concern to public health. Little is known about HAP-related exposures to nitrogen dioxide (NO2), a known byproduct of biomass combustion and a threat to respiratory health.
We measured NO2 kitchen area concentrations at high-temporal resolution and personal exposure among women with biomass cookstoves in Peru. We found NO2 kitchen area concentrations and personal exposures which far exceed WHO annual and hourly guidelines.
Further attention is warranted to understand the impact of HAP-associated chronic and acute exposures to NO2 on morbidity and mortality, and to inform cleaner-cooking interventions that aim to reduce exposures to all health-threatening household air pollutants.
ACKNOWLEDGEMENTS
Financial support for the CHAP trial was received from the Global Environmental and Occupational Health, Fogarty International Center, United States National Institutes of Health (1U2RTW010114-01); the Clean Cooking Alliance of the United Nations Foundation (UNF 16–80), and the Johns Hopkins Center for Global Health. The Center for Global Non-Communicable Disease Research and Training field site in Puno, Peru, also received generous support from Mr William and Bonnie Clarke III. JLK and KNW were supported by the NIH Fogarty International Center, NINDS, NIMH, NHBLI, and NIEHS under NIH Research Training Grant # D43 TW009340 and the Johns Hopkins Center for Global Health. JLK was also supported by the Ruth L. Kirschstein Institutional National Research Service Award (5T32ES007141-33) funded by the NIH/NIEHS. KNW was also supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number T32HL007534. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding information
National Heart, Lung, and Blood Institute, Grant/Award Number: T32HL007534; National Institute of Environmental Health Sciences, Grant/Award Number: 5T32ES007141-33 and D43 TW009340; Global Alliance for Clean Cookstoves, Grant/Award Number: UNF 16–80; Johns Hopkins Center for Global Health; Mr. William and Bonnie Clarke III; Fogarty International Center, Grant/Award Number: 1U2RTW010114-01 and D43 TW009340; Johns Hopkins; Center for Global Health
APPENDIX
Cardiopulmonary outcomes and Household Air Pollution (CHAP) trial Investigators: Steering Committee: William Checkley MD PhD (Johns Hopkins University, Baltimore, MD, USA), Gustavo F Gonzales MD (Universidad Peruana Cayetano Heredia, Lima, Peru), Luke Naeher PhD (University of Georgia, Athens, GA, USA), Joshua Rosenthal PhD (National Institutes of Health, Bethesda, MD, USA), N Kyle Steenland PhD (Emory University, Atlanta, Georgia, USA). Johns Hopkins University Investigators: Theresa Aguilar, Vanessa Burrowes PhD, Magdalena Fandiño-Del-Rio MSc, Elizabeth C Fung MSPH, Dina Goodman MSPH, Steven A Harvey PhD, Phabiola Herrera MD, Josiah L Kephart PhD, Kirsten Koehler PhD, Alexander Lee, Kathryn A Lee MPH, Catherine H Miele MD MPH, Mitra Moazzami MSPH, Lawrence H. Moulton PhD, Saachi Nangia, Carolyn O’Brien MSPH, Suzanne Simkovich MD MS, Timothy Shade, Lena Stashko MSPH, Ariadne Villegas-Gomez MSPH, Kendra N Williams PhD, Abigail Winiker MSPH. Asociación Benéfica PRISMA Investigators: Marilú Chiang MD MPH, Gary Malpartida, Carla Tarazona-Meza MPH. Washington University Investigators: Victor Davila-Roman MD, Lisa de las Fuentes MD. Emory University Investigators: Dana Barr Boyd PhD, Maria Jolly MSPH, Angela Rozo.
Footnotes
CONFLICT OF INTEREST
The authors report no conflict of interest, financial or otherwise.
The peer review history for this article is available at https://publons.com/publon/10.1111/ina.12653
Cardiopulmonary outcomes and Household Air Pollution (CHAP) trial Investigators present in Appendix.
REFERENCES
- 1.Bonjour S, Adair-Rohani H, Wolf J, et al. Solid fuel use for household cooking: Country and regional estimates for 1980–2010. Environ Health Perspect. 2013;121(7):784–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO (World Health Organization). WHO Guidelines for Indoor Air Quality: Selected Pollutants. Copenhagen, Denmark: WHO (World Health Organization) 2011. [PubMed] [Google Scholar]
- 3.Rehfuess E, Pope D, Bruce N, et al. WHO Indoor Air Quality Guidelines: Household Fuel Combustion - Review 6: Impacts of Interventions on Household Air Pollution Concentrations and Personal Exposure. Geneva; 2014. http://www.who.int/indoorair/guidelines/hhfc. Accessed May 21, 2019. [Google Scholar]
- 4.Desai MA, Mehta S, Smith KR. Indoor Smoke from Solid Fuels. Assessing the Environmental Burden of Disease at National and Local Levels. Geneva: Protection of the Human Environment, World Health Organization; 2004. http://apps.who.int/iris/bitstream/10665/42885/1/9241591358.pdf. Accessed October 4, 2018. [Google Scholar]
- 5.WHO. Burning Opportunity: Clean Household Energy for Health, Sustainable Development, and Wellbeing of Women and Children. 2016. http://apps.who.int/iris/bitstream/10665/204717/1/9789241565233_eng.pdf
- 6.Naeher LP, Brauer M, Lipsett M, et al. Woodsmoke health effects: A review. Inhal Toxicol. 2007;19(1):67–106. [DOI] [PubMed] [Google Scholar]
- 7.Bruce N, Smith KR. WHO Guidelines for Indoor Air Quality: Household Fuel- Review 4: Health Effects of Household Air Pollution Exposure. Geneva, Switzerland: WHO (World Health Organization); 2014. [Google Scholar]
- 8.Po JYT, FitzGerald JM, Carlsten C. Respiratory disease associated with solid biomass fuel exposure in rural women and children: Systematic review and meta-analysis. Thorax. 2011;66(3): 232–239. [DOI] [PubMed] [Google Scholar]
- 9.Siddharthan T, Grigsby MR, Goodman D, et al. Association between household air pollution exposure and chronic obstructive pulmonary disease outcomes in 13 low- and middle-income country settings. Am J Respir Crit Care Med. 2018;197:611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurmi OP, Semple S, Simkhada P, et al. COPD and chronic bronchitis risk of indoor air pollution from solid fuel: a systematic review and meta-analysis. Thorax. 2010;65(3):221–228. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Qin C, Lv J, et al. Solid fuel use and incident COPD in Chinese adults: findings from the China Kadoorie Biobank. Environ Health Perspect. 2019;127(5):057008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosgood HD, Wei H, Sapkota A, et al. Household coal use and lung cancer: Systematic review and meta-analysis of case-control studies, with an emphasis on geographic variation. Int J Epidemiol. 2011;40(3):719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruce N, Dherani M, Liu R, et al. Does household use of biomass fuel cause lung cancer? A systematic review and evaluation of the evidence for the GBD 2010 study. Thorax. 2015;70(5):433–441. [DOI] [PubMed] [Google Scholar]
- 14.Baumgartner J, Schauer JJ, Ezzati M, et al. Indoor air pollution and blood pressure in adult women living in rural China. Environ Health Perspect. 2011;119(10):1390–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young BN, Clark ML, Rajkumar S, et al. Exposure to household air pollution from biomass cookstoves and blood pressure among women in rural honduras: a cross-sectional study. Indoor Air. 2018;29(1):130–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanaway JD, Afshin A, Gakidou E, et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Stu. Lancet. 2018;392(10159):1923–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zelikoff JT, Lung CC, Cohen MD, Schlesinger RB. The toxicology of inhaled woodsmoke. J Toxicol Environ Heal - Part B Crit Rev. 2002;5(3):269–282. [DOI] [PubMed] [Google Scholar]
- 18.U.S. EPA. Integrated Science Assessment (ISA) for Oxides of Nitrogen - Health Criteria (Final Report, 2016). Washington, DC: U.S. EPA; 2016. [Google Scholar]
- 19.Kandpal JB, Maheshwari RC, Kandpal TC. Comparison of CO, NO2 and HCHO emissions from biomass combustion in traditional and improved cookstoves. Energy. 1994;19(11):1151–1155. [Google Scholar]
- 20.Achakulwisut P, Brauer M, Hystad P, Anenberg SC. Global, national, and urban burdens of paediatric asthma incidence attributable to ambient NO2 pollution: estimates from global datasets. Lancet Planet Heal. 2019;3:e166–e178. [DOI] [PubMed] [Google Scholar]
- 21.Hasselblad V, Eddy DM, Kotchmar DJ. Synthesis of environmental evidence: nitrogen dioxide epidemiology studies. J Air Waste Manage Assoc. 1992;42(5):662–671. [DOI] [PubMed] [Google Scholar]
- 22.Weinmayr G, Romeo E, De Sario M, Weiland SK, Forastiere F. Short-term effects of PM10 and NO2 on respiratory health among children with asthma or asthma-like symptoms: a systematic review and meta-analysis. Environ Health Perspect. 2009;118(4):449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belanger K, Holford TR, Gent JF, Hill ME, Kezik JM, Leaderer BP. Household levels of nitrogen dioxide and pediatric asthma severity. Epidemiology. 2013;24(2):320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paulin LM, Williams DAL, Peng R, et al. 24-h Nitrogen dioxide concentration is associated with cooking behaviors and an increase in rescue medication use in children with asthma. Environ Res. 2017;159:118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peel JL, Tolbert PE, Klein M, et al. Ambient air pollution and respiratory emergency department visits. Epidemiology. 2005;16(2):164–174. [DOI] [PubMed] [Google Scholar]
- 26.Schikowski T, Sugiri D, Ranft U, et al. Long-term air pollution exposure and living close to busy roads are associated with COPD in women. Respir Res. 2005;6(1):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schindler C, Ackermann-Liebrich U, Leuenberger P, et al. Associations between lung function and estimated average exposure to NO2 in eight areas of Switzerland. Epidemiology. 1998;9:405–411. [PubMed] [Google Scholar]
- 28.Gauderman WJ, Avol E, Gilliland F, et al. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med. 2004;351(11):1057–1067. [DOI] [PubMed] [Google Scholar]
- 29.Urman R, McConnell R, Islam T, et al. Associations of children’s lung function with ambient air pollution: Joint effects of regional and near-roadway pollutants. Thorax. 2014;69(6):540–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molter A, Agius RM, de Vocht F, et al. Long-term exposure to PM10 and NO2 in association with lung volume and airway resistance in the MAAS birth cohort. Environ Health Perspect. 2013;121(10):1232–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rojas-Martinez R, Perez-Padilla R, Olaiz-Fernandez G, et al. Lung function growth in children with long-term exposure to air pollutants in Mexico City. Am J Respir Crit Care Med. 2007;176(4):377–384. [DOI] [PubMed] [Google Scholar]
- 32.Oftedal B, Brunekreef B, Nystad W, Madsen C, Walker S-E, Nafstad P. Residential outdoor air pollution and lung function in schoolchildren. Epidemiology. 2008;19(1):129–137. [DOI] [PubMed] [Google Scholar]
- 33.Sekine K, Shima M, Nitta Y, Adachi M. Long term effects of exposure to automobile exhaust on the pulmonary function of female adults in Tokyo, Japan. Occup Environ Med. 2004;61(4):350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atkinson RW, Butland BK, Anderson HR, Maynard RL. Long-term concentrations of nitrogen dioxide and mortality. Epidemiology. 2018;29(4):460–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eum KD, Kazemiparkouhi F, Wang B, et al. Long-term NO 2 exposures and cause-specific mortality in American older adults. Environ Int. 2019;124:10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ni K, Carter E, Schauer JJ, et al. Seasonal variation in outdoor, indoor, and personal air pollution exposures of women using wood stoves in the Tibetan Plateau: Baseline assessment for an energy intervention study. Environ Int. 2016;94:449–457. [DOI] [PubMed] [Google Scholar]
- 37.Kumie A, Emmelin A, Wahlberg S, et al. Magnitude of indoor NO2 from biomass fuels in rural settings of Ethiopia. Indoor Air. 2009;19(1):14–21. [DOI] [PubMed] [Google Scholar]
- 38.Khalequzzaman M, Kamijima M, Sakai K, Chowdhury NA, Hamajima N, Nakajima T. Indoor air pollution and its impact on children under five years old in Bangladesh. Indoor Air. 2007;17(4):297–304. [DOI] [PubMed] [Google Scholar]
- 39.Colbeck I, Nasir ZA, Ali Z, Ahmad S. Nitrogen dioxide and household fuel use in the Pakistan. Sci Total Environ. 2010;409(2):357–363. [DOI] [PubMed] [Google Scholar]
- 40.Kilabuko JH, Matsuki H, Nakai S. Air quality and acute respiratory illness in biomass fuel using homes in Bagamoyo, Tanzania. Int J Environ Res Public Health. 2007;4(1):39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Padhi BK, Padhy PK. Domestic fuels, indoor air pollution, and children’s health: The case of rural India. Ann N Y Acad Sci. 2008;1140(1):209–217. [DOI] [PubMed] [Google Scholar]
- 42.Khalequzzaman M, Kamijima M, Sakai K, Hoque BA, Nakajima T. Indoor air pollution and the health of children in biomass-and fossil-fuel users of Bangladesh: Situation in two different seasons. Environ Health Prev Med. 2010;15(4):236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wafula EM. Indoor air pollution in a Kenyan village. East Afr Med J. 1990;67(1):24–32. [PubMed] [Google Scholar]
- 44.Hollada J, Williams KN, Miele CH, Danz D, Harvey SA, Checkley W. Perceptions of improved biomass and liquefied petroleum gas stoves in Puno, Peru: Implications for promoting sustained and exclusive adoption of clean cooking technologies. Int J Environ Res Public Health. 2017;14(2):182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fandiño-Del-Rio M, Goodman D, Kephart JL, et al. Effects of a liquefied petroleum gas stove intervention on pollutant exposure and adult cardiopulmonary outcomes (CHAP): Study protocol for a randomized controlled trial. Trials. 2017;18:518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Instituto Nacional de Estadística e Informática. Perú Encuesta Demográfica y de Salud Familiar - ENDES 2014; 2015. [Google Scholar]
- 47.Pullum TW. Abbreviating the wealth index to measure equity in health programs more easily. Glob Heal Sci Pract. 2016;4(1):4–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larkin A, Geddes JA, Martin RV, et al. Global land use regression model for nitrogen dioxide air pollution. Environ Sci Technol. 2017;51(12):6957–6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ogawa USA. NO, NO2, NOx and SO2 Sampling Protocol Using The Ogawa Sampler; 2006. http://ogawausa.com/wp-content/uploads/2017/11/prono-noxno2so206_206_1117.pdf
- 50.St HG, Aguilar-Villalobos M, Adetona O, et al. Exposure of pregnant women to cookstove-related household air pollution in urban and Periurban Trujillo, Peru. Arch Environ Occup Health 2015;70(1):10–18. [DOI] [PubMed] [Google Scholar]
- 51.Gautam SK, Suresh R, Sharma VP, Sehgal M. Indoor air quality in the rural India. Manag Environ Qual An Int J. 2013;24(2):244–255. [Google Scholar]
- 52.Balakrishnan K, Mehta S, Authors L, et al. WHO Indoor Air Quality Guidelines: Household Fuel Combustion - Review 5: Population Levels of Household Air Pollution and Exposures; 2014. http://www.who.int/indoorair/guidelines/hhfc. Accessed June 26, 2019.
- 53.Pollard SL, Williams DLDL, Breysse PN, et al. A cross-sectional study of determinants of indoor environmental exposures in households with and without chronic exposure to biomass fuel smoke Environ Heal. 2014;13(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feinberg S, Williams R, Hagler GSW, et al. Long-term evaluation of air sensor technology under ambient conditions in Denver Colorado. Atmos Meas Tech. 2018;11(8):4605–4615. [DOI] [PMC free article] [PubMed] [Google Scholar]