Abstract
Background
Multisystem inflammatory syndrome (MIS-C) is a clinical presentation reported in children related to Coronavirus-19 infection who present with a toxic shock like syndrome. Vitamin D deficiency has been postulated to play a role with severity of coronavirus infection in adult patients and other viral respiratory infections.
Objective
This study aims to investigate if severe vitamin D deficiency was associated with increased disease severity and cardiac involvement in MIS-C.
Methods
This is a retrospective and single center study. We included hospitalized patients less than 18 years of age with diagnosis of MIS-C between March and July 2020. Severe vitamin D deficiency was defined as 25-OH vitamin D level < 10 ng/ml within 48 h of admission. The composite outcome severe disease included patients requiring inotropes, mechanical ventilation, and extracorporeal membrane oxygenation.
Results
Of the 31 patients with MIS-C, 45% were male and 58% were African American. The median age was 8 (1–13) years. Ten patients had severe vitamin D deficiency with a mean level of 7.2 ng/ml. Ninety percent of patients with severe vitamin D deficiency had severe disease (P < 0.001). Patients with severe vitamin D deficiency had an increased risk of cardiac involvement (P < 0.001).
Conclusions
We describe a potential association between severe vitamin D deficiency and severe disease in children presenting with MIS-C. Severe vitamin D deficiency predisposes patients for cardiovascular involvement and may play a critical role in the host immune response to COVID-19 infection. Future prospective studies at the basic science and clinical level should be pursued to better delineate this association.
Keywords: Vitamin D, Covid-19 infection, Severe disease, Cardiac involvement
1. Introduction
The outbreak of the novel coronavirus disease (Covid-19) has caused a dramatic impact worldwide with more than 160 million cases diagnosed and more than 3 million deaths since the onset of this global pandemic [1]. Initial reports described the pediatric population as low risk for severe Covid-19 disease [2], [3]. However, in the last several months, a life-threatening presentation in the pediatric population, known as multisystem inflammatory syndrome in children (MIS-C), has emerged related to a late presentation of Covid-19 infection [4], [5], [6], [7], [8]. The clinical presentation of MIS-C has varied in severity; some patients have mild disease, some require critical care, and unfortunately, some have died [9], [10], [11], [12]. Recently, adult studies have postulated a link between severity of Covid-19 infection and severe vitamin D deficiency, based on observational studies in the Northern Hemisphere. These countries seemed to share lower levels of vitamin D due to reduced sunlight exposure [13], [14], [15]. Previous reports have been published regarding the properties of vitamin D and their implications in the acute viral respiratory syndrome caused by Covid-19 [16], [17], [18]. Our standalone tertiary care Children's Hospital is located in the heart of downtown Detroit, which has seen one of the severe initial outbreaks of Covid-19 in the United States. As a result, we were part of the group reporting one of the first pediatric case series of MIS-C [12]. The objective of the study was to investigate if severe vitamin D deficiency was associated with increased disease severity and cardiac involvement in children with MIS-C.
2. Methods
This is a single center retrospective study including patients ≤ 18 years of age who met criteria for MIS-C upon admission to our hospital. We defined MIS-C by the Centers for Disease Control and Prevention (CDC) definition: Patients <21 years of age with fever, laboratory evidence of inflammation, and evidence of clinically severe illness requiring hospitalization, with multisystem ≥2 organ involvement (cardiac, renal, respiratory, hematologic, gastrointestinal, dermatologic or neurological) and no alternative plausible diagnoses and positive for current or recent SARS-CoV-2 infection by RT-PCR, serology, or antigen test; or exposure to a suspected or confirmed Covid-19 case within the 4 weeks prior to the onset of symptoms [19]. We excluded patients who were found to have other infectious etiology for their initial clinical presentation and those with incomplete medical records. The study period corresponded from March to July 2020. The main variable was severe vitamin D deficiency, and the primary outcome was the composite variable severe disease, which was defined as positive if the patients required inotropic support, mechanical ventilation, or venoarterial extracorporeal membrane oxygenation (VA ECMO), or if the disease resulted in death. The secondary outcome was cardiac involvement, consisting of abnormal electrocardiogram, ventricular dysfunction, coronary abnormalities, or pericardial effusion on echocardiogram. Ventricular dysfunction was defined as a shortening fraction less than 28% or ejection fraction less than 50%. The Institutional Review Board at Wayne State University and the Detroit Medical Center research institute approved this study. This was a retrospective chart review, the Institutional Review Boards at Wayne State University waived parental informed consent.
2.1. Data collection
Demographic and clinical data included age, gender, race, weight, height, and comorbidities. Laboratory measurements collected included 25-OH vitamin D level, serum calcium level, serum phosphorus level, nasal swab PCR for SARS-CoV-2 (Covid-19 PCR), immunoglobulin G antibody assay for SARS-CoV-2 (Covid-19 IgG), troponin levels, brain natriuretic peptide (BNP), and creatinine. As part of the initial assessment of all suspected MIS-C cases, a thorough laboratory evaluation, which included 25-OH vitamin D levels, was routinely obtained in order to define this new infectious entity. Initial echocardiography and electrocardiography data were collected. The method to quantify ejection fraction was the area-length or bullet method using the formula V = 5 / 6 × short-axis basal area × LV length, where the short-axis basal area was measured from parasternal short-axis views, and LV length was measured from apical 4-chamber view. The other method used was the M-mode measurement of shortening fraction (SF); this calculated the percentage change in LV volume from end diastole to end systole using parasternal short-axis view of the left ventricle [20]. Severe vitamin D deficiency was defined as Vit D < 10 ng/ml and normal vitamin D levels are considered to be >20 ng/ml [21]. Length of stay in the hospital and the pediatric intensive care unit (PICU) were also obtained. In addition, we calculated the vasoactive-inotropic score (VIS), widely used scoring system for cardiovascular support in pediatric patients that predicts morbidity and mortality in PICU [22], [23].
2.2. Statistical analysis
Statistical analysis was performed using SPSS 25 software for PC (IBM Inc.). Demographic continuous variables were expressed as median with interquartile range (IQR) and the categorical variables were expressed in absolute counts with percentage. The entire cohort was divided into two groups based on our main variable vitamin D deficiency. The Student t-test and the Chi square test were used as appropriate for statistical comparisons between the groups with and without severe vitamin D deficiency. Logistic regression analysis was used to evaluate the association between vitamin D and all other statistically significant variables in relationship to our primary outcome. Secondary analysis was performed to evaluate the association of cardiac involvement to severe vitamin D deficiency. Statistical significance was taken as a P value of <0.05.
3. Results
The study cohort included 31 patients, with median (IQR) age of 8 (1–13) years, median weight of 32.3 (16–67.7) kg, median height of 130 (93–158) cm, and 14 (45.2%) were males. The cohort included 18 African American patients (58.1%), 4 Caucasian patients (12.9%), and 9 patients of other races (29%). There were 12 (38.7%) patients who had one or more comorbidities. The most common comorbidities were asthma (n = 6), obesity (n = 6), and type 1 diabetes (n = 2). Other comorbidities included systemic hypertension, obstructive sleep apnea, seizure disorder, and propionic acidemia. The baseline demographic and clinic characteristics of the entire cohort are depicted in Table 1 .
Table 1.
Baseline demographic characteristics.
| Characteristics (median (IQR) OR n %) | Patients (N = 31) |
|---|---|
| Age, years | 8 (1–13) |
| Weight, kg | 32.3 (16–67.7) |
| Height, cm | 130 (93–158) |
| Male | 14 (45.1%) |
| Caucasian | 4 (12.9%) |
| African American | 18 (58.1%) |
| Other races | 9 (29%) |
| Severe vitamin D deficiency (≤10 ng/ml) | 10 (32.2%) |
| Covid-19 PCR positive | 10 (32.2%) |
| Covid-19 IgG positive | 15 (67.7%) |
| Need for inotrope support | 14 (4.5%) |
| Need for mechanical ventilation | 6 (19.3%) |
| Need for VA ECMO | 2 (6.5%) |
| Mortality | 0 (0%) |
Data is presented as n (%) for categorical variables and mean (25th–75th percentiles) for continuous variables. Covid-19: novel coronavirus disease; VA ECMO: venoarterial extracorporeal membrane oxygenation.
3.1. Severe vitamin D deficiency and disease severity
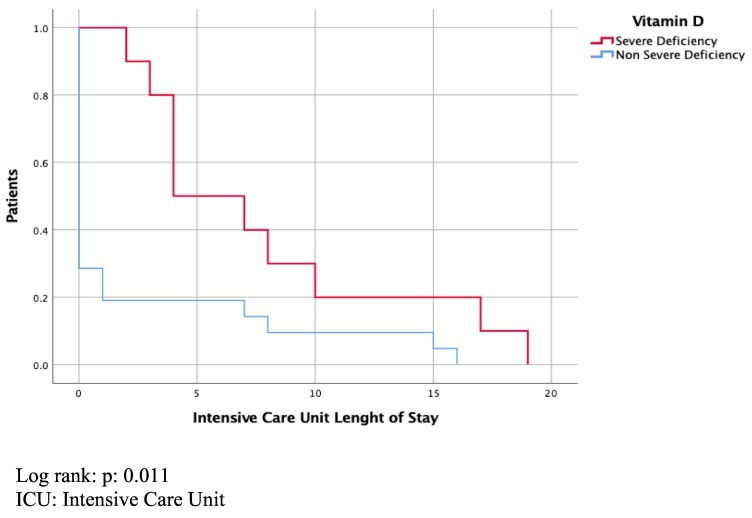
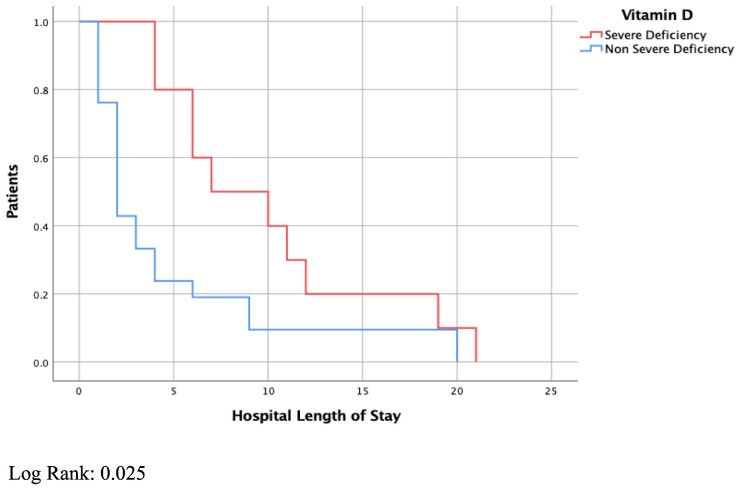
There were 10 (32.3%) patients with severe vitamin D deficiency with a mean (±standard deviation) 25-OH vitamin D level of 7.2 (±0.42) ng/ml. This group had lower levels of serum calcium (8.3 mg/dl vs. 9.2 mg/dl, P < 0.001), and phosphorus (3.4 mg/dl vs. 4.3 mg/dl, P = 0.051) compared with the rest of the cohort. The rest of the cohort had a mean 25-OH vitamin D level of 22.8 (±9.9) ng/ml. Patients with severe vitamin D deficiency had longer ICU (P = 0.02) and hospital length of stay (P = 0.05) than the rest of the cohort (Kaplan-Meier analysis, Fig. 1, Fig. 2 ). Patients with severe vitamin D deficiency were more likely to have severe disease (P = 0.001). In the group with severe vitamin D deficiency, 9 (90%) patients were positive for our composite outcome of severe disease. All patients with severe vitamin D deficiency and severe disease were positive for Covid-19 IgG.
Fig. 1.
ICU length of stay
Log rank: P: 0.011.
ICU: intensive care unit.
Fig. 2.
Hospital length of stay
Log Rank: 0.025.
In the group of patients with severe disease (n = 14); 14 (100%) patients required inotropic support, 6 (42.8%) patients needed invasive mechanical ventilation, and 2 (14%) patients required VA ECMO support. There were no deaths during the study period. Table 2 compares other variables between the group with severe disease to the rest of the cohort.
Table 2.
Severe disease.
| Clinical characteristic Mean (SD) or n (%) |
Severe disease (n = 14) | No severe disease (n = 17) | P value |
|---|---|---|---|
| Age, years | 10.4 (4.4) | 6.1 (5.6) | 0.03 |
| Comorbidities | 9 (64.3%) | 3 (17.6%) | <0.01 |
| PICU length of stay, days | 8.79 (5.7) | 0.18 (0.5) | <0.01 |
| Hospital length of stay, days | 11.4 (6) | 2.2 (1) | <0.01 |
| Mechanical ventilation | 6 (42.8%) | 0 (0%) | <0.01 |
| Inotropic support | 14 (100%) | 0 (0%) | <0.01 |
| VA ECMO | 2 (14%) | 0 (0%) | <0.01 |
SD: standard deviation, Covid-19: novel coronavirus disease; ICU: pediatric intensive care unit; VA ECMO: veno-arterial extracorporeal membrane oxygenation.
The group with severe vitamin D deficiency had an increased risk of severe disease by MIS-C (OR: 28.8; 95% CI; 2.9–286.4; P < 0.01). Table 3 compares several other variables like comorbidities, peak troponin, peak BNP, peak creatinine, and peak GFR between the groups with severe vitamin D deficiency and the rest of the cohort. In the group with severe vitamin D deficiency, the mean peak VIS score during the first 72 h was 6.6 (±6.5) with a mean duration of inotropes of 67.5 (±56.3) hours. There was no independent association by multivariate logistic regression analysis between severe vitamin D deficiency and any of the variables relevant by univariate analysis in a model that included age, ethnicity, comorbidities, peak BNP, peak creatinine, peak GFR, VIS score, ICU and hospital length of stay. Similarly, there was no independent association between severe disease and any of the variables relevant by univariate analysis in a model that included age, comorbidities, PICU length of stay, hospital length of stay, mechanical ventilation, inotropic support, and VA ECMO requirement.
Table 3.
Severe vitamin D deficiency.
| Clinical characteristic Mean (SD) or n (%) |
Severe vit D deficiency (n = 10) | Normal vit D (n = 21) | P value |
|---|---|---|---|
| Age, years | 11.3 (3.3) | 7.2 (5.8) | 0.052 |
| Weight, kg | 55.6 (30.6) | 38.2 (36) | 0.199 |
| Height, cm | 148 (19) | 117 (36) | 0.02 |
| Male gender | 4 (40%) | 10 (47%) | 0.69 |
| African American race | 8 (80%) | 10 (47%) | 0.17 |
| Comorbidities | 5 (50%) | 7 (33%) | 0.37 |
| Severe disease | 9 (90%) | 5 (24%) | 0.001 |
| Covid-19 PCR, positive | 4 (40%) | 6 (29%) | 0.5 |
| Covid-19 IgG, positive | 9 (90%) | 6 (28%) | 0.005 |
| Ca, mg/dL | 8.3 (0.5) | 9.2 (0.5) | 0.001 |
| Peak troponin, ng/L | 968 (1765) | 303 (755) | 0.148 |
| Peak BNP, pg/mL | 2152 (1758) | 516 (926) | 0.003 |
| Peak creatinine, mg/dL | 1.4 (1.2) | 0.7 (0.6) | 0.034 |
| Peak GFR, ml/min | 63 (30) | 94 (44) | 0.05 |
| PICU length of stay, days | 7.8 (5.9) | 2.2 (4.9) | 0.02 |
| Hospital length of stay, days | 10 (5.9) | 4.6 (5.6) | 0.05 |
| VIS score | 6.6 (6.5) | 1.9 (4.8) | 0.03 |
| Left ventricular ejection fraction, % | 44.9 (15.5) | 64 (9.4) | <0.001 |
| Left ventricular shortening fraction, % | 22.8 (9.2) | 34.5 (6.3) | <0.001 |
SD: standard deviation, Covid-19: novel coronavirus disease; BNP: brain natriuretic peptide; GFR: glomerular filtration rate; PICU: pediatric intensive care unit; VIS: vasoactive-inotropic score.
3.2. Cardiac involvement
There were 13 (41.9%) patients who had cardiac involvement. It consisted of 12 patients with ventricular dysfunction, 4 patients with coronary artery involvement, 5 patients had pericardial effusion and 9 patients had an abnormal electrocardiogram. The group with cardiac involvement had a mean (SD) 25-OH vitamin D level of 9.6 (4.3) ng/ml vs. 23.7 (10.6) ng/ml for the rest of the cohort (P < 0.01). The group with severe vitamin D deficiency had an increased risk of cardiac involvement (OR: 38.3; 95% CI; 3.7–395.3; P < 0.01). Additionally, those with severe vitamin D deficiency had reduced left ventricular ejection and shortening fraction compared to the group with non-severe vitamin D deficiency (P < 0.001). There was no association by multivariate logistic regression analysis between cardiac involvement and any of the variables included in the model (age, ethnicity, comorbidities, severe vitamin D deficiency, peak BNP, peak creatinine, peak GFR, VIS score, ICU and hospital length of stay).
4. Discussion
We report an association between severe vitamin D deficiency and severe disease in children with MIS-C. In addition, severe vitamin D deficiency was associated with cardiac involvement, prolonged ICU, and hospital length of stay. To our knowledge, this is the first study to report an association between vitamin D deficiency and illness severity in pediatric patients with MIS-C. Although no variable remained associated by multivariate analysis, we postulate that the reason for this was the small sample size and that several variables were individually associated with severe disease, hence, making regression analysis difficult to interpret in a small sample cohort.
4.1. Severe vitamin D deficiency and severe disease
Previous studies in adults have described an association between low vitamin D levels and Covid-19 infection [24], [25]. Merzon et al. included 7807 individuals who had vitamin D levels and were tested for Covid-19. They reported that low vitamin D; defined in their study as <30 ng/mL; was a risk factor for infection and hospitalization, independent of demographic characteristics and previous medical conditions [24]. D'Avolio et al. reported the association between lower vitamin D levels and positive PCR test for Covid-19. Their cohort included 107 patients, of which 27 patients were positive for Covid-19 PCR. Vitamin D levels were lower in the group with Covid-19 infection, median of 11.1 ng/ml vs. 24.6 ng/ml (P = 0.004) [25]. In a different manner, our cohort included only pediatric patients with evidence of Covid-19 infection and reported that severe vitamin D deficiency was associated with increased morbidity.
Previous studies in critically ill children have shown an association between severe disease and vitamin D deficiency [26], [27]. Moreover, a recent study reported that a single dose of vitamin D given to pediatric patients with vitamin D deficiency could reduce the incidence of septic shock in specific patient populations [28]. Furthermore, a meta-analysis in 2017 reported that vitamin D deficiency in patients admitted to the pediatric ICU could lead to increased mortality, higher incidence of multi organ dysfunction, and overall worse clinical course for the respective illness [29]. Similarly, we describe association between severe MIS-C and severe vitamin D deficiency in our cohort. Although vitamin D deficiency is a common occurrence in our community, it is notable that almost all children with severe MIS-C had concurrent severe vitamin D deficiency. The etiology of this association can be related to the known immune modulatory role of vitamin D, since the current accepted pathophysiology of MIS-C is related to an altered and exaggerated immune response [30].
4.2. Cardiac involvement
The role of vitamin D in cardiovascular pathophysiology has been studied extensively, with vitamin D deficiency being a common finding in patients with cardiovascular disease [31]. It has been postulated that vitamin D plays an essential role in endothelial function, mediated by vitamin D receptors (VDRs). These VDRs are expressed in many cells and tissues of the cardiovascular system, including vascular smooth muscles, endothelium, and myocardium [32]. Furthermore, vitamin D regulates numerous genes involved in the pathogenesis of cardiovascular disease, controlling cell proliferation and differentiation, apoptosis, oxidative stress, membrane transport, matrix homeostasis, and cell adhesion. Endothelial stress leads to proliferation and migration of vascular smooth muscle cells which is inhibited by 25(OH)D3 and 1,25(OH)2D3 [33].
Lipshultz et al. described how low PTH level and subsequently lower intracellular calcium concentrations in patients with chronic renal disease correlated strongly with ischemia, depressed contractibility, and adverse outcomes [34]. Similarly, Margosian et al. described that vitamin D deficiency and elevated PTH levels were associated with lower LV mass z-scores while FGF-23 was inversely related to end-diastolic septal thickness [35]. Similarly, we described that patients with MIS-C and severe vitamin D deficiency had reduced left ventricular ejection and shortening fraction compared to their counterparts with MIS-C and normal vitamin D levels, although our cohort was small.
Additionally, McNally et al. reported vitamin D levels in patients following pediatric cardiac surgery. They demonstrated that vitamin D deficiency was associated with prolonged mechanical ventilation and ICU stay after pediatric cardiac surgery [31]. Similarly, Dohain et al. found that postoperative vitamin D level was inversely associated with postoperative inotropic requirement in pediatric patients following cardiac surgery [36]. We postulate that patients with severe vitamin D deficiency have non-ideal baseline endothelial function, oxidative stress, membrane transport, and cell matrix homeostasis. These factors would put them at higher risk for significant cardiovascular involvement and predispose them to myocardial malfunction, endothelial dysfunction, peripheral edema, shock, pulmonary edema, and hypotension, which are all key clinical manifestations of severe cardiac involvement and illness due to MIS-C.
4.3. Limitations
The study is a single center retrospective study with a relatively small number of patients. In addition, data collection and statistical analysis were limited to data already recorded in the medical records. Despite our retrospective design, one of the strengths of our study compared with similar reports in adults is that vitamin D levels were obtained in patients meeting criteria for MIS-C within 2 days of hospital admission. Unfortunately, we were not able to obtain additional laboratory (parathyroid hormone level) or imaging data (wrist X ray) that would further support the diagnosis of vitamin D deficiency as these tests were not routinely obtained during their hospitalization due to the critical nature of their illness. However, the patients in our severe vitamin D deficiency group did have overall lower levels of calcium and phosphorous compared to the rest of the cohort, which suggests that these patients likely had severe vitamin D deficiency.
5. Conclusion
We describe a potential association between severe vitamin D deficiency and severe disease in children presenting with MIS-C due to Covid-19. Severe vitamin D deficiency predisposes patients for cardiovascular involvement due to altered cellular homeostasis mediated by widespread VDRs in the cardiovascular system. Furthermore, the immune modulatory activity of vitamin D may play a critical role in the host immune response to Covid-19 infection. Future prospective studies at the basic science and clinical level should be pursued to better delineate this association.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human medical regulations and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the ethical committee of Children's Hospital of Michigan.
Disclosure
This was a retrospective chart review by the IRB (Institutional Review Boards) at Wayne University waived parental informed consent.
CRediT authorship contribution statement
Diana Torpoco Rivera: conceptualization, methodology, formal analysis, writing - original draft. Amrit Misra: resources, formal analysis, writing - review & editing. Yamuna Sanil: supervision, writing - review & editing. Natalie Sabzghabaei: supervision, writing - review & editing. Raya Safa: supervision, writing - review & editing. Richard Garcia: formal analysis, supervision, writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.WHO Coronavirus disease 2019 (Covid-19) 2021. Weekly epidemiological update on COVID-19. 18 May. [Google Scholar]
- 2.Dong Y., Mo X., Hu Y., Qi X., Jiang F., Jiang Z., et al. Epidemiology of Covid-19 among children in China. Pediatrics. 2020 Jun;145(6) doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 3.Ludvigsson J.F. Systematic review of Covid-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088–1095. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebina-Shibuya R., Namkoong H., Shibuya Y., Horita N. Multisystem inflammatory syndrome in children (MIS-C) with Covid-19: insights from simultaneous familial Kawasaki disease cases. Int J Infect Dis. 2020;97:371–373. doi: 10.1016/j.ijid.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramcharan T., Nolan O., Lai C.Y., Prabhu N., Krishnamurthy R., Richter A.G., et al. Paediatric inflammatory multisystem syndrome: temporally associated with SARS-CoV-2 (PIMS-TS): cardiac features, management and short-term outcomes at a UK tertiary paediatric hospital. Pediatr Cardiol. 2020;41(7):1391–1401. doi: 10.1007/s00246-020-02391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaushik S., Aydin S.I., Derespina K.R., Bansal P.B., Kowalsky S., Trachtman R., et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2 infection: a multi-institutional study from New York City. J Pediatr. 2020;224:24–29. doi: 10.1016/j.jpeds.2020.06.045. 10.1016/j.jpeds.2020.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shekerdemian L.S., Mahmood N.R., Wolfe K.K., Riggs B.J., Ross C.E., McKiernan C.A., et al. International Covid-19 PICU collaborative. Characteristics and outcomes of children with coronavirus disease 2019 (Covid-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020;174(9):868–873. doi: 10.1001/jamapediatrics.2020.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chao J.Y., Derespina K.R., Herold B.C., Goldman D.L., Aldrich M., Weingarten J., Ushay H.M., Cabana M.D., Medar S.S. Clinical characteristics and outcomes of hospitalized and critically ill children and adolescents with coronavirus disease 2019 at a tertiary care medical Center in New York City. J Pediatr. 2020;223:14–19. doi: 10.1016/j.jpeds.2020.05.006. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capone C.A., Subramony A., Sweberg T., Schneider J., Shah S., Rubin L., et al. Characteristics, cardiac involvement, and outcomes of multisystem inflammatory disease of childhood (MIS-C) associated with SARS-CoV-2 infection. J Pediatr. 2020;224:141–145. doi: 10.1016/j.jpeds.2020.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakra N.A., Blumberg D.A., Herrera-Guerra A., Lakshminrusimha S. Multi-system inflammatory syndrome in children (MIS-C) following SARS-CoV-2 infection: review of clinical presentation, hypothetical pathogenesis, and proposed management. Children (Basel) 2020;7(7):69. doi: 10.3390/children7070069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies P., Evans C., Kanthimathinathan H.K., Lillie J., Brierley J., Waters G., et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study. Lancet Child Adolesc Health. 2020;(20):30215–30217. doi: 10.1016/S2352-4642(20)30215-7. S2352–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldstein L.R., Rose E.B., Horwitz S.M., Collins J.P., Newhams M.M., Son M.B.F., et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383(4):334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ilie P.C., Stefanescu S., Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res. 2020 Jul;32(7):1195–1198. doi: 10.1007/s40520-020-01570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jakovac H. Covid-19 and vitamin D-is there a link and an opportunity for intervention? Am J Physiol Endocrinol Metab. 2020;318(5) doi: 10.1152/ajpendo.00138.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panarese A., Shahini E. Letter: Covid-19, and vitamin D. Aliment Pharmacol Ther. 2020;51(10):993–995. doi: 10.1111/apt.15752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grant W.B., Lahore H., McDonnell S.L., Baggerly C.A., French C.B., Aliano J.L., et al. Evidence that vitamin D supplementation could reduce risk of influenza and Covid-19 infections and deaths. Nutrients. 2020;12(4):988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell F. Vitamin-D and Covid-19: do deficient risk a poorer outcome? Lancet Diabetes Endocrinol. 2020;8(7):570. doi: 10.1016/S2213-8587(20)30183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silberstein M. Vitamin D: A simpler alternative to tocilizumab for trial in Covid-19? Med Hypotheses. 2020;140:109767. doi: 10.1016/j.mehy.2020.109767. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CDC Covid-19 Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease. 2019. https://www.cdc.gov/mis-c/hcp/index.html
- 20.Lopez L., Colan S.D., Frommelt P.C., et al. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the pediatric measurements writing Group of the American Society of echocardiography pediatric and congenital heart disease council. J Am Soc Echocardiogr. 2010;23(5):465–577. doi: 10.1016/j.echo.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 21.Sankar J., Ismail J., Das R., Dev N., Chitkara A., Sankar M.J. Effect of severe vitamin D deficiency at admission on shock reversal in children with septic shock: a prospective observational study. J Intensive Care Med. 2019;34(5):397–403. doi: 10.1177/0885066617699802. [DOI] [PubMed] [Google Scholar]
- 22.McIntosh A.M., Tong S., Deakyne S.J., Davidson J.A., Scott H.F. Validation of the vasoactive-inotropic score in pediatric sepsis. Pediatr Crit Care Med. 2017;18(8):750–757. doi: 10.1097/PCC.0000000000001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia R.U., Walters H.L., 3rd, Delius R.E., Aggarwal S. Vasoactive inotropic score (VIS) as biomarker of short-term outcomes in adolescents after cardiothoracic surgery. Pediatr Cardiol. 2016;37(2):271–277. doi: 10.1007/s00246-015-1273-7. [DOI] [PubMed] [Google Scholar]
- 24.Merzon E., Tworowski D., Gorohovski A., Vinker S., Golan Cohen A., Green I., et al. Low plasma 25(OH) Vitamin D level is associated with increased risk of Covid-19 infection: an Israeli population-based study. FEBS J. 2020 doi: 10.1111/febs.15495. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D'Avolio A., Avataneo V., Manca A., Cusato J., De Nicolò A., Lucchini R., et al. 25-HydroxyVitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2. Nutrients. 2020;12(5):1359. doi: 10.3390/nu12051359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madden K., Feldman H.A., Smith E.M., Gordon C.M., Keisling S.M., Sullivan R.M., et al. Vitamin D deficiency in critically ill children. Pediatrics. 2012;130(3):421–428. doi: 10.1542/peds.2011-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martineau A.R., Jolliffe D.A., Hooper R.L., Greenberg L., Aloia J.F., Bergman P., et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;15(356) doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y., Yang Z., Gao L., Cao Z., Wang Q. Effects of a single dose of vitamin D in septic children: a randomized, double-blinded, controlled trial. J Int Med Res. 2020;48(6) doi: 10.1177/0300060520926890. 300060520926890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNally J.D., Nama N., O'Hearn K., Sampson M., Amrein K., Iliriani K., McIntyre L., Fergusson D., Menon K. Vitamin D deficiency in critically ill children: a systematic review and meta-analysis. Crit Care. 2017;21(1):287. doi: 10.1186/s13054-017-1875-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biesalski H.K. Vitamin D deficiency and co-morbidities in Covid-19 patients – a fatal relationship? Nfs J. 2020;20:10–21. [Google Scholar]
- 31.McNally J.D., Menon K., Chakraborty P., Fisher L., Williams K.A., Al-Dirbashi O.Y., et al. Impact of anesthesia and surgery for congenital heart disease on the vitamin d status of infants and children: a prospective longitudinal study. Anesthesiology. 2013 Jul;119(1):71–80. doi: 10.1097/ALN.0b013e31828ce817. [DOI] [PubMed] [Google Scholar]
- 32.Apostolakis M., Armeni E., Bakas P., Lambrinoudaki I. Vitamin D and cardiovascular disease. Maturitas. 2018;115:1–22. doi: 10.1016/j.maturitas.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 33.Gunta S.S., Thadhani R.I., Mak R.H. The effect of Vitamin D status on risk factors for cardiovascular disease [published correction appears in Nat Rev Nephrol. 2013 Nov;9(11):626] Nat Rev Nephrol. 2013;9(6):337–347. doi: 10.1038/nrneph.2013.74. [DOI] [PubMed] [Google Scholar]
- 34.Lipshultz S.E., Somers M.J., Lipsitz S.R., Colan S.D., Jabs K., Rifai N. Serum cardiac troponin and subclinical cardiac status in pediatric chronic renal failure. Pediatrics. 2003;112(1 Pt 1):79–86. doi: 10.1542/peds.112.1.79. [DOI] [PubMed] [Google Scholar]
- 35.Margossian R., Williams P.L., Yu W., et al. Markers of bone mineral metabolism and cardiac structure and function in perinatally HIV-infected and HIV-exposed but uninfected children and adolescents. J Acquir Immune Defic Syndr. 2019;81(2):238–246. doi: 10.1097/QAI.0000000000002007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dohain A.M., Almogati J., Al-Radi O.O., Elassal A.A., Zaher Z.F., Fatani T.H., et al. Serum vitamin D status following pediatric cardiac surgery and association with clinical outcome. Eur J Pediatr. 2020;179(4):635–643. doi: 10.1007/s00431-019-03538-x. [DOI] [PubMed] [Google Scholar]