Abstract
In the present paper we investigated the effect of the sucrose (Suc) analog palatinose on potato (Solanum tuberosum) tuber metabolism. In freshly cut discs of growing potato tubers, addition of 5 mm palatinose altered the metabolism of exogenously supplied [U-14C]Suc. There was slight inhibition of the rate of 14C-Suc uptake, a 1.5-fold increase in the rate at which 14C-Suc was subsequently metabolized, and a shift in the allocation of the metabolized label in favor of starch synthesis. The sum result of these changes was a 2-fold increase in the absolute rate of starch synthesis. The increased rate of starch synthesis was accompanied by a 3-fold increase in inorganic pyrophosphate, a 2-fold increase in UDP, decreased UTP/UDP, ATP/ADP, and ATP/AMP ratios, and decreased adenylate energy charge, whereas glycolytic and Krebs cycle intermediates were unchanged. In addition, feeding palatinose to potato discs also stimulated the metabolism of exogenous 14C-glucose in favor of starch synthesis. In vitro studies revealed that palatinose is not metabolized by Suc synthases or invertases within potato tuber extracts. Enzyme kinetics revealed different effects of palatinose on Suc synthase and invertase activities, implicating palatinose as an allosteric effector leading to an inhibition of Suc synthase and (surprisingly) to an activation of invertase in vitro. However, measurement of tissue palatinose levels revealed that these were too low to have significant effects on Suc degrading activities in vivo. These results suggest that supplying palatinose to potato tubers represents a novel way to increase starch synthesis.
In growing potato (Solanum tuberosum) tubers, Suc arriving via the phloem is degraded via Suc synthase (SuSy) to Fru and UDP-Glc, which are converted to hexose phosphates by fructokinase and UDP-Glc pyrophosphorylase, respectively. Hexose phosphates are then imported into the plastid and used for starch synthesis via plastidial phosphoglucomutase (Tauberger et al., 2000), ADP-Glc pyrophosphorylase (AGPase), and the various isoforms of starch synthase (for review, see ap Rees and Morell, 1990; Kruger, 1997).
There has been considerable interest to increase the efficiency of this pathway and thus increase starch accumulation within potato tubers by conventional plant breeding or genetic manipulation strategies (Stark et al., 1991; Schafer-Pregl et al., 1998; Trethewey et al., 1998). Transgenic approaches have focussed primarily on the modulation of Suc catabolism (Sonnewald et al., 1997; Trethewey et al., 1998) or of the plastidial starch synthetic pathway (Stark et al., 1991; Tauberger et al., 2000). To date, the only successful transgenic approaches have resulted from the overexpression of a bacterial AGPase (Stark et al., 1991) or of the Arabidopsis amyloplastial ATP/ADP translocator (Tjaden et al., 1998). Attempts were previously made to improve the starch yield of potato tubers by the expression of a more efficient pathway of Suc degradation, consisting of a yeast invertase and a bacterial glucokinase (Trethewey et al., 1998). However, despite the fact that the transgenic tubers exhibited reduced levels of Suc and elevated levels of hexose-phosphates, ATP and glycerate-3-phosphate (3-PGA) with respect to wild-type tubers, this attempt failed. This is intriguing because hexose phosphates, ATP, and 3-PGA represent the immediate precursors for and the activator of the AGPase reaction, respectively (for review, see Preiss, 1988). Moreover, these hexose-phosphates were found to partition to glycolysis at the cost of starch synthesis, resulting in a decreased starch accumulation within these lines (Trethewey et al., 1998). When taken together, all these studies reveal that the regulation of starch synthesis in potato tubers is more complex than initially expected and a simple increase in the concentration of the precursors may not be sufficient to drive starch synthesis.
There is now compelling evidence that Suc is not only the major transport form of assimilates in higher plants, but also serves as a source of signals regulating a variety of different genes and processes in various plant organs (Koch, 1996; Chiou and Bush, 1998; Smeekens, 1998). In source tissues Suc has been shown to repress photosynthetic genes (Krapp et al., 1993; Krapp and Stitt, 1995), whereas in sink tissues, genes involved in Suc degradation (Salanoubat and Beliard, 1989; Fu and Park, 1995) and storage function (Rocha-Sosa et al., 1989; Müller-Röber et al., 1990) are induced. Furthermore, studies on bean embryos demonstrate a strong spatial correlation between high invertase activities, high Glc concentrations, and zones of cell division, as well as between high SuSy activities, high Suc concentrations, and starch storing zones (Weber et al., 1998).
The above studies on the role of Suc as a signal provide a plausible explanation for the inhibition of starch accumulation within tubers of potato engineered to express a yeast invertase and a bacterial glucokinase in their cytosol, since these tubers revealed a massive drop in the levels of Suc (Trethewey et al., 1998). Independent evidence supporting this hypothesis comes from studies with wild-type potato tubers in which the rate of starch synthesis was modulated by alterations in the carbohydrate supply. Starch synthesis was found to be inhibited when Suc supply was interrupted by detaching growing wild-type tubers for 24 h from their mother plant. Further studies revealed that this inhibition could be prevented by feeding Suc (but not Glc) into the tuber via the detached stolon (Geiger et al., 1998). In addition, when Suc was supplied externally to wild-type tuber slices, there was a rapid stimulation of starch synthesis that was accompanied by a decrease in the levels of hexose-phosphates and 3-PGA (Geiger et al., 1998). However, in both instances the mechanisms for these changes remain unclear and it is as yet unknown whether Suc itself, its metabolism, or a downstream product of Suc catabolism triggers this response.
Sugar analogs are useful tools by which information relating to signaling mechanism can be derived, since when used carefully they allow the discrimination of the effects of the analogous sugar per se and the effect of a metabolic product of this sugar (Roitsch et al., 1995; Martin et al., 1997; Pego et al., 1999). In the present study we used the Suc analog palatinose (6-O-α-d-glucopyranosyl-d-Fru; isomaltulose), which is functionally very similar to Suc in that it is a disaccharide composed of glucosyl and fructosyl moieties; however, it differs in the nature of the bond between these moieties consisting of an α rather than a β linkage. The primary aim of this work was to address whether palatinose, like Suc, was able to stimulate starch synthesis within developing potato tubers. To investigate the effect of palatinose on metabolic fluxes, we fed [U-14C]Suc and [U-14C]Glc independently to potato tuber slices in the presence and absence of palatinose, and then analyzed the metabolism of the labeled sugars. To elucidate possible reasons for the palatinose-induced changes in metabolism we analyzed the levels of various metabolites and nucleotides involved in the pathway of Suc to starch, as well as the palatinose levels within the tissue. Finally, we analyzed the effects of palatinose on the kinetics of Suc-degrading enzymes using potato tuber extracts in vitro. The data will be discussed in the context of current models for sugar sensing in plant tissues.
RESULTS
Feeding Palatinose Alters Metabolism of Suc in Potato Tuber Slices
To investigate whether the Suc analog palatinose affects Suc metabolism in potato tubers, freshly cut slices of growing potato tubers were incubated with 20 mm [U-14C]Suc in the presence of 0, 5, 20, or 100 mm palatinose for 2 h, and the fate of the label was analyzed (Fig. 1, A–K). Uptake of [U-14C]Suc was slightly (4%, 20%, or 24%) decreased after feeding 5, 20, or 100 mm palatinose, respectively (Fig. 1A). Palatinose feeding led to a 1.5-fold increase in the rate of 14C-Suc degradation (Fig. 1B), which rose from about 7% of the absorbed label in the absence of palatinose to 10%, 9%, and 9.5% of the absorbed label when 5, 20, and 100 mm palatinose were fed, respectively. The distribution of the metabolized label between starch, phosphate esters, organic acids, and amino acids is shown in Figure 1, C through F, in which the results are expressed as a percentage of the total metabolized label. In the presence of 5, 20, or 100 mm palatinose, an increased proportion of the metabolized label entered starch (Fig. 1C) and a decreased proportion was retained in the phosphate ester pool (Fig. 1D); the proportion of label entering organic acids and amino acids remained unaltered (Fig. 1, E and F). Furthermore, the total radiolabel present in starch at the end of the incubation increased 2-fold in response to palatinose (calculated from Fig. 1, A–C).
Figure 1.
Addition of palatinose affects the metabolism of 14C-Suc by potato tuber slices. Freshly cut slices of growing potato tubers were incubated for 2 h in the presence of 20 mm [U-14C]Suc (specific activity 1.4 kBq/μmol) with and without addition of 5, 20, or 100 mm palatinose, before they were washed and extracted to determine label distribution. A, [14C]Suc absorbed by the tissue; B, percentage of the absorbed label that is metabolized to other compounds. Incorporation of 14C into starch (C), phosphate ester (D), organic acids (E), and amino acids (F) is expressed as a percentage of the label metabolized. The specific activity of the hexose phosphate pool (G) was estimated by dividing the label retained in the phosphate ester pool by the summed carbon of the hexose phosphates (see Fig. 2N). The values were corrected by dividing by 2 to give the mean specific activity during the course of the 2 h. The absolute fluxes to starch (H), glycolysis (I), and the summed fluxes to starch and glycolysis (K) were calculated using the specific activity of the hexose-phosphate pool. The results are means ± se (n = 4).
The absorbed 14C-Suc will mix with internal unlabeled pools, so movement of label will not necessarily reflect fluxes into various pools (Geigenberger et al., 1997). Interpretation of labeling experiments is especially complicated when treatments are compared that modify label uptake or turnover. The specific activity of the hexose phosphate pool (Fig. 1G) was estimated from the label retention in the phosphate ester faction (Fig. 1D) and the summed carbon in the hexose phosphate pool (see below, Fig. 2N), and was used to estimate the absolute rates of starch synthesis (Fig. 1H) and glycolytic flux (Fig. 1I). The assumptions involved in calculating the specific activity of the hexose phosphate pool are discussed in Geigenberger et al. (1997). With increasing concentrations of palatinose, there was a progressive decrease in the specific activity of the hexose-phosphate pool (Fig. 1G), following the decrease in 14C-Suc uptake (Fig. 1A). Feeding 5, 20, or 100 mm palatinose led to a 2.6-, 2.0-, or 2.4-fold increase in the absolute flux to starch, respectively (Fig. 1H; the increase being significant, P < 0.05, when analyzed using the Student's t test). This large stimulation in starch synthesis most probably results from the combination of an increased rate of Suc mobilization (Fig. 1B), and an increase in the proportion of the metabolized Suc that is then allocated to starch (Fig. 1C). The increase in starch synthesis was already maximal at 5 mm palatinose and showed no further increase at higher concentrations of palatinose, indicating that this starch synthesis responds very sensitively to palatinose. Coupled to the stimulation of starch synthesis there was a slight, but significant increase in the glycolytic flux (estimated as the sum of the fluxes to organic acids and amino acids) following palatinose feeding (Fig. 1I). When these fluxes are summed to provide an estimate of the total fluxes deriving from Suc, there is a striking 2-fold increase following addition of palatinose (significant P < 0.05; see Fig. 1K), which confirms that the analog stimulates the rate of Suc degradation.
Figure 2.
Metabolite and nucleotide levels in tuber slices incubated with 0, 5, 20, or 100 mm palatinose for 2 h, in parallel to the slices used in Figure 1. A, Suc; B, Glc; C, Fru; D, UDP-Glc; E, UTP; F, UDP; G, UTP/UDP ratio; H, PPi; I, ATP; J, ADP; K, AMP; L, ATP/ADP ratio; M, energy charge; N, hexose phosphates; O, 3-PGA; and P, pyruvate. The results are means ± se (n = 4).
Effect of Palatinose Feeding on Metabolite Levels in Potato Tuber Discs
To investigate the reason(s) for the observed stimulation of the Suc-to-starch conversion, the levels of various metabolic intermediates of the pathway were analyzed from equivalent tuber disc samples. Levels of Suc (Fig. 2A), Glc (Fig. 2B), and Fru (Fig. 2C) remained unaltered following addition of 5 mm palatinose, but were decreased slightly when higher concentrations of palatinose (20 and 100 mm) were supplied to the discs. Palatinose feeding did not affect UDP-Glc levels (Fig. 2D), but resulted in a minor increase in UTP (Fig. 2E) and a marked increase in UDP levels (up to 1.7-fold; Fig. 2F), whereas the derived UTP/UDP ratio (Fig. 2G) decreased by approximately 40%. These changes were accompanied by a 3-fold increase in the levels of inorganic pyrophosphate (PPi; Fig. 2H).
The levels of ATP increased only slightly (Fig. 2I), whereas ADP (Fig. 2J) and AMP (Fig. 2K) increased up to 2- and 5-fold, respectively, resulting in a decreased ATP/ADP ratio (Fig. 2L) and a drop in the adenylate energy charge (Fig. 2M) in response to palatinose. Summation of the total uridinylates (Fig. 2, D–F) and the total adenylates (Fig. 2, I–K) revealed that these were also increased.
The levels of glycolytic and Krebs cycle intermediates were not significantly altered in response to palatinose. There were no substantial changes in hexose phosphate (Fig. 2N) and 3-PGA levels (Fig. 2O), pyruvate increased only slightly (Fig. 2P), and the levels of isocitrate and α-oxoglutarate remained unaltered (data not shown). The data indicate that palatinose feeding results in a marked stimulation of Suc-to-starch transition without major alterations in the levels of phosphorylated sugars and organic acids.
Palatinose Feeding Stimulates Partitioning of Glc to Starch
We further investigated the effect of palatinose on carbohydrate metabolism by following the metabolism of radiolabeled Glc. This experiment allowed us to evaluate whether palatinose is effecting starch synthesis by stimulating Suc degradation or whether it is acting further downstream in the starch synthetic pathway. In addition, since Suc degradation in growing tubers is regulated by a cycle of Suc synthesis and degradation (Geigenberger and Stitt, 1993), we wanted to ensure that the observed stimulation of Suc breakdown (Fig. 1A) was not negated by a complementary increase in the rate of Suc resynthesis.
Tuber slices were incubated with 2 mm [U-14C]Glc in the presence and absence of 50 mm palatinose (separate incubations were carried out in the presence of 50 mm mannitol to provide an osmotic control). Palatinose feeding led to 20% increase in the rate of Glc uptake (Table I), but did not change the proportion of the absorbed label that was metabolized (more than 95% of the absorbed Glc was metabolized in each case). In the presence of palatinose the proportion of the metabolized label entering starch was significantly increased, whereas the proportion entering organic acids and amino acids was decreased (Table I). The proportion of label entering phosphate ester and Suc remained unchanged (Table I). Partitioning of label was not significantly altered after feeding 50 mm mannitol, indicating that the changes observed following palatinose feeding are not due to osmotic effects. These data are in close agreement with previous studies (Geigenberger et al., 1997, 1999) in demonstrating that feeding 50 mm mannitol to potato tuber slices does not influence the partitioning of carbon toward starch.
Table I.
Addition of palatinose affects metabolism of 14C-Glc by potato tuber slices
| Parameter | Metabolism of 14C-Glc by
Tuber Slices Incubated for 2 h in 2 mm
[U-14C]Glc in the Presence of:
|
||
|---|---|---|---|
| No additions | 50 mm Mannitol | 50 mm palatinose | |
| 14C-Glc absorbed (dpm disc−1) | 18,285 ± 1,865 | 17,110 ± 1,098 | 21,392 ± 1,383 |
| 14C-Glc metabolized (% of absorbed) | 97.0 ± 0.2 | 95.5 ± 0.3 | 95.9 ± 0.1 |
| % of Metabolized label converted to: | |||
| Starch | 46.9 ± 2.1 | 50.0 ± 2.7 | 57.1 ± 3.6 |
| Suc | 12.4 ± 0.7 | 12.4 ± 1.2 | 12.5 ± 1.7 |
| Phosphate ester | 16.9 ± 2.2 | 13.3 ± 2.1 | 16.6 ± 2.0 |
| Organic acids | 10.0 ± 2.0 | 12.6 ± 2.0 | 5.2 ± 2.7 |
| Amino acids | 12.2 ± 0.7 | 11.5 ± 1.1 | 8.9 ± 0.5 |
| Specific activity hexose-P (dpm/nmol) | 20.4 ± 0.9 | 20.5 ± 1.1 | 22.0 ± 1.6 |
| Starch flux (nmol g fresh wt−1 min−1) | 772 ± 20 | 816 ± 94 | 1112 ± 90 |
| Flux to Suc (nmol g fresh wt−1 min−1) | 206 ± 22 | 203 ± 34 | 243 ± 33 |
| Glycolytic flux (nmol g fresh wt−1 min−1) | 367 ± 21 | 391 ± 40 | 275 ± 67 |
| Starch/glycolysis ratio | 2.1 ± 0.1 | 2.1 ± 0.3 | 4.4 ± 1.2 |
Freshly cut slices of growing potato tubers were incubated for 2 h in the presence of 2 mm [U-14C]Glc (specific activity 1.4 kBq μmol−1) with and without addition of 50 mm palatinose (or 50 mm mannitol as an osmotic control) before they were washed and extracted to determine label distribution. The specific activity of the hexose phosphate pool was estimated by dividing the label retained in the phosphate ester pool by the summed carbon of the hexose phosphates, and was used to calculate absolute fluxes to starch, Suc, and glycolysis. The results are means ± se (n = 4).
The specific activity of the phosphate-ester pool was estimated from label retention in the phosphate-ester fraction (Table I) and the summed carbon in the hexose-phosphate pool (data not shown). This was then multiplied by the label entering starch, Suc, or the summed label entering organic acids and amino acids to provide estimates of the absolute rates of starch synthesis, Suc resynthesis, or glycolysis, respectively. Palatinose led to a 1.4-fold increase in the absolute flux to starch (significant, P < 0.05), whereas glycolytic and Suc synthetic fluxes were not significantly changed (Table I). The results document that the stimulation of the unidirectional rate of Suc degradation is not accompanied by a similar stimulation of Suc resynthesis, implying that feeding palatinose leads to decreased rates of Suc cycling, and a stimulation of net Suc degradation.
Palatinose Is Not Metabolized by SuSy or Invertase
To establish whether the palatinose can be metabolized by Suc-degrading enzymes, the activities of SuSy, acid invertase, and alkaline invertase were assayed in the presence of 100 mm Suc or 100 mm palatinose using desalted potato tuber extracts. SuSy was the major Suc cleaving activity in the potato extracts. SuSy activity, when palatinose was supplied as substrate, represented only 0.5% of the activity measured when Suc was supplied as substrate: the activities were 1,620 ± 120 and 9 ± 1 nmol g fresh weight−1 (means ± se, n = 4). The extracts used for kinetic analysis contained only marginal invertase activities, representing less than 1% of the Suc catabolic activity, as is typical of potato tuber extracts (e.g. Merlo et al., 1993; Trethewey et al., 1998). When palatinose was supplied as substrate, activities of alkaline invertase and acid invertase were not detectable (data not shown). In addition to this, gas chromatography mass spectrometry (GC-MS) analysis revealed that no possible cleavage products of the analog appeared when potato tuber extracts were incubated with 50 mm palatinose for 12 h (data not shown). The results document that palatinose is not metabolized by any of the sucrolytic activities of potato tuber extracts.
In a separate experiment 100 mm palatinose or 100 mm Suc was incubated with 0.2 units of commercially available yeast invertase (Sigma, Munich) for up to 12 h. Hexoses formed following this incubation were then detected using conventional assay techniques. No hydrolytic products were detected in the palatinose incubation, whereas Suc was readily cleaved into its constitutive hexosyl moieties (data not shown).
Palatinose Acts as an Inhibitor of SuSy, But Stimulates Invertase Activity
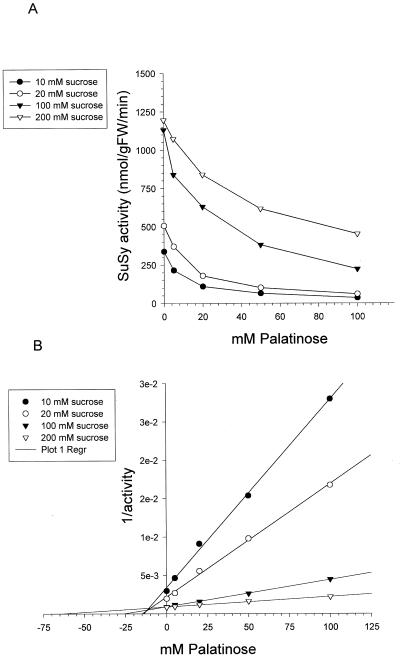
To investigate whether palatinose could have stimulated Suc degradation by acting as an activator of Suc-degrading enzymes, the activities of SuSy, acid invertase, or alkaline invertase were assayed in the presence of Suc together with various concentrations of palatinose (0, 5, 10, 20, 50, and 100 mm) using desalted potato tuber extracts. It is surprising that addition of palatinose led to a strong inhibition of SuSy when 10 mm Suc was used in the assay (Fig. 3, A and B). The inhibition of SuSy was increasingly diminished when higher Suc concentrations (up to 200 mm) were added, indicating that the inhibition is competitive to Suc. The Ki values were 13, 15, 25, and 60 mm in the presence of 10, 20, 100, and 200 mm Suc, respectively (calculated from Fig. 3, A and B). Glc and Fru are known inhibitors of SuSy in various plant tissues (Doehlert, 1987; Dancer et al., 1990a). However, palatinose did not affect the Fru or the Glc inhibition observed for potato tuber SuSy (data not shown).
Figure 3.
Inhibition of potato tuber SuSy by palatinose in the presence of 10, 20, 100, and 200 mm Suc. A, SuSy activity plotted versus the palatinose concentration in the assay. B, Dixon plot. The results are means of two replicate assay incubations.
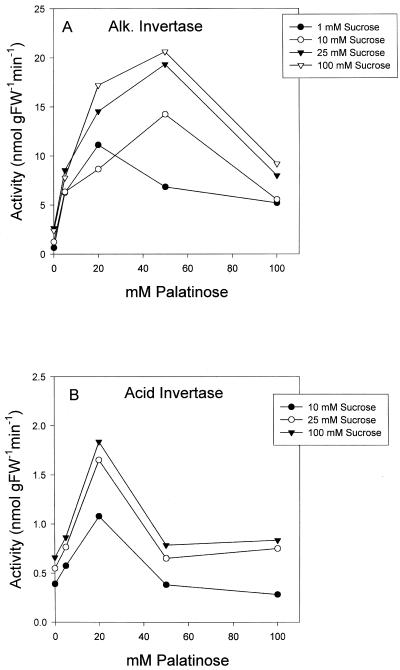
In contrast, addition of palatinose led to an activation of alkaline and acid invertase activity using Suc as a substrate (Fig. 4, A and B). The stimulation was already evident with 5 mm palatinose, was maximal between 20 and 50 mm palatinose, and decreased again with higher palatinose concentrations. The reasons for the decreased extent or absence of stimulation at higher palatinose concentrations are not known. Addition of 0.5 mm palatinose had no significant effect on invertase activities (data not shown). The extent by which the invertases were activated was not affected by the Suc concentration used in the assay. There are several reports in the literature documenting that invertase from stored potato tubers and other tissues is regulated by an endogenous inhibitor (Pressey, 1967; Krausgrill et al., 1996). To test whether palatinose prevents invertase to bind to its inhibitor we repeated the assays (see above) using a potato extract that had been foamed by shaking for 90 min, or by including 20 mm dithiothreitol or 10 mm N-ethyl-maleimide in the assay. These treatments have been used previously to inactivate the invertase inhibitor in potato tuber extracts (Pressey, 1967; Ovalle et al., 1995). None of these treatments affected the palatinose-induced activation of alkaline or acid invertase significantly (data not shown), indicating that palatinose is not interacting with the inhibitor.
Figure 4.
Activation of potato tuber invertase by palatinose in the presence of 1, 20, 50, and 100 mm Suc. A, Alkaline invertase; B, acid invertase. The results are means of two replicate assay incubations.
Analysis of Palatinose Levels in the Discs
GC-MS analysis (Roessner et al., 2000) was used to investigate the uptake of palatinose by the tuber slices. After a 2-h incubation in 5, 20, or 100 mm palatinose, slices were rinsed three times with buffer, blotted with tissue paper, extracted in methanol, and palatinose levels were analyzed. Palatinose levels were 15, 150, and 1,300 nmol g fresh weight−1 in discs that were incubated in 5, 20, and 100 mm palatinose, respectively (mean values of two separate incubations). Slices incubated in 100 mm palatinose were also analyzed after 10 sequential washes with buffer, but the level of palatinose in the tissue only went down minimally to 940 nmol g fresh weight−1, compared with 1,300 nmol g fresh weight−1 after three washes. The estimated concentrations of palatinose in the tissue (0.01–1.3 mm) are well below the Ki of palatinose for SuSy (see above), and in a range where no activation of invertases were observed (see above). GC-MS analysis of the incubation medium during the 2-h incubation indicated that no cleavage products of palatinose, such as Glc, Fru, or UDP-Glc appeared during the time course of the experiment (data not shown).
DISCUSSION
Palatinose Acts as a Non-Metabolizable Suc Analog That Is Only Poorly Absorbed by Potato Tuber Slices
Our results demonstrate that palatinose is not metabolized by SuSy or invertase or any other Suc cleaving activities present in potato tuber extracts. This contrasts with 1-fluoro-Suc, another Suc analog, which is rapidly cleaved by SuSy, but acts a poor substrate of invertase (Schmalstig and Hitz, 1987a, 1987b), indicating that different analogs of Suc are differently discriminated by Suc metabolizing enzymes.
GC-MS analysis revealed palatinose levels of 15, 150, or 1,300 nmol g fresh weight−1 in potato discs incubated in 5, 20, or 100 mm palatinose, respectively. Given that palatinose cannot be metabolized, the low level of palatinose in the tissue indicates that absorption of the analog by the discs is very poor. The low levels of palatinose within potato tuber discs suggests that it cannot be effectively transported and as such is in agreement with the results of Delrot and coworkers who demonstrated that palatinose is not recognized (M'Batchi et al., 1985) or transported (M'Batchi and Delrot, 1988) by the Suc transporter of protoplasts derived from soybean cotyledons of broad bean leaves. It is interesting that the rate of [U-14C]Suc uptake into potato tuber discs decreases slightly, but significantly, following incubation of palatinose and unlike the effects of palatinose on metabolism, this decrease appears to be concentration dependent (Fig. 1A). This finding supports that of Schmitt et al. (1984) who showed that 100 mm palatinose caused a slight (14%) reduction in the rate of [U-14C]Suc uptake by soybean cotyledon protoplasts. That the Suc transporter can transport Suc, but not palatinose suggests that the β-fructofuranoside bond or a component of the fructosyl moiety of Suc must participate in binding to the carrier in the potato tuber as has previously been found in protoplasts derived from soybean cotyledons (Schmitt et al., 1984).
Palatinose Stimulates Suc to Starch Conversion in Potato Tuber Slices
The results of this paper show that palatinose leads to a marked stimulation of Suc degradation and starch synthesis when fed to potato tuber slices in vivo (see Fig. 1), and to a differential allosteric regulation of SuSy and invertase in vitro (Figs. 3 and 4). This demonstrates that palatinose has direct effects on tuber metabolism, despite being a non-metabolizable sugar.
Three lines of evidence indicate that palatinose is acting downstream of Suc degradation under in vivo conditions. First, palatinose also stimulated the metabolism of exogenously supplied 14C-Glc to starch (Table I). Second, palatinose levels within the tissue were below the levels required to exert significant effects on Suc-degrading enzymes (0.01–1 mm). Third, palatinose feeding did not result in increased levels of hexoses or hexose phosphates and measurements indicated that the level of UDP-Glc was also unchanged within the tuber tissue despite these metabolites being the direct cleavage products of Suc. We, therefore, propose that starch synthesis was stimulated directly via a signaling mechanism triggered by palatinose and acted independently of the levels of glycolytic intermediates (see below for a more detailed discussion of the putative mechanisms). At present we do not know at which enzymatic step this regulation occurs; stimulation of starch synthesis could involve regulation of transport processes at the amyloplast envelope, plastidial phosphoglucomutase, AGPase, or the polymerizing reactions of starch biosynthesis. Further studies including subcellular analyses of metabolite concentrations are in progress to clarify the mechanism of this regulation.
The stimulation of starch synthesis in turn leads to an increase in Suc mobilization. We propose that the increase in Suc degradation is due to an increase in the levels of UDP and PPi, both acting as cosubstrates in the reversible reactions of SuSy and UGPase (Fig. 2). It has been shown for potato tubers and other plant tissues that SuSy and UGPase catalyze reactions that are close to equilibrium (Geigenberger and Stitt, 1993; Geigenberger et al., 1993, 1997) and strongly substrate limited in vivo (Jelitto et al., 1992; Loef et al., 1999).
The increase of UDP was due to an increased proportion of the total uridine-nucleotide pool being present as UDP, and was accompanied by a 40% decrease in the UTP/UDP ratio (see Fig. 2). There was also a decrease in the ATP/ADP and ATP/AMP ratios, as well as a drop in the adenylate energy charge consistent with increased consumption in biosynthetic processes. Previous studies have shown that the UTP/UDP and ATP/ADP ratios are equilibrated by the action of a nucleotide-5-diphosphate kinase in plant tissues (Dancer et al., 1990b). The results indicate that palatinose triggers ATP consumption in the tissue by activating starch biosynthesis. The consequential increase in UDP will then stimulate Suc degradation via SuSy (see above). A similar scenario occurs in growing potato tubers in response to an increased assimilate supply (Geigenberger and Stitt, 2000).
The increase in PPi levels could be due to a stimulation of various PPi-producing processes (including starch biosynthesis) or to an inhibition of various PPi-consuming reactions (Stitt, 1998). It has been shown recently that a direct inhibition of starch biosynthesis via antisense inhibition of AGPase expression leads to a decreases in the level of PPi (Farré et al., 2000). This would indicate that PPi, which is produced in the plastid during starch synthesis, is, at least in part, recycled back into the cytosol to fuel Suc degradation via SuSy. We, therefore propose that the stimulation of starch synthesis by palatinose leads to a concomitant increase in PPi, which in turn stimulates Suc degradation.
Palatinose Acts as an Allosteric Effector Leading to an Activation of Invertase and an Inhibition of SuSy in Vitro
Palatinose is not metabolized by invertase or SuSy, but can act as an allosteric effector for both enzymes. It is interesting that palatinose affects SuSy and invertase in different ways. It leads to an inhibition of SuSy, but an activation of invertase. Although the inhibition of SuSy occurred competitively with respect to Suc, the activation of invertase was unaffected by Suc concentration in the assay. The activation of invertase activity also occurred under conditions where the endogenous invertase inhibitor has been irreversibly destroyed, indicating that allosteric activation occurred. Invertases have been characterized in the literature as exhibiting a remarkable lack of regulatory properties (Avigard, 1982; Kruger, 1997) and there is no evidence to date for a strong regulation of invertase activity in response to metabolic factors. In fact, to our knowledge this is therefore the first time that allosteric regulation of invertase activity has been demonstrated. It is possible that Suc has an analogous stimulating effect on invertase activity acting not only as a substrate, but also as a modulator of invertase activity. Such regulation would be especially important in tissues where Suc is degraded mainly via invertase (i.e. growing seeds), providing a key link between Suc supply and its mobilization; however, further experimentation is required to test this hypothesis.
Implications for Sugar Sensing in Plant Storage Organs
Our results show that the non-metabolizable Suc analog palatinose leads to a direct stimulation of starch synthesis in potato tuber slices without requiring an intervening increase in metabolite levels. A similar stimulation of starch synthesis was also observed when external Suc was fed to potato tuber slices or to intact tubers (Geiger et al., 1998; Loef et al., 2001), or in potato tubers attached to the plant when they were analyzed at the end of the photoperiod when assimilate import rates and internal Suc concentrations were high (Geigenberger and Stitt, 2000). On the basis of these data we propose that palatinose promotes an endogenous signal cascade that is involved in the stimulation of starch synthesis in response to Suc.
Given their similar response with respect to starch synthesis, the crucial differences between Suc and palatinose allow two conclusions to be drawn concerning the regulation of starch synthesis by sugar in developing potato tubers. First, palatinose cannot be metabolized by potato tubers, indicating that the putative signaling cascade invoking the increased synthesis of starch will not involve palatinose metabolism or a metabolic product of the analog as an initial trigger. Second, palatinose affects starch synthesis at very low tissue concentrations (approximately 15 μm if we assume that palatinose is evenly distributed throughout the cell, and approximately 150 μm if we assume that palatinose is restricted to the cytosol). Our in vitro studies suggest that no significant inhibition of SuSy or activation of invertase will occur in this concentration range. This makes it very unlikely that a direct effect of palatinose on the kinetics of Suc-degrading enzymes is responsible for the alteration in carbon partitioning. Furthermore, these palatinose levels are three orders of magnitude lower than those of Suc in the tubers (approximately 20,000 nmol g fresh weight−1). We, therefore, speculate that palatinose binds to a factor located at the plasma membrane or has a very high affinity to an endogenous factor located inside the cell, triggering the metabolic response. The existence of such factors in plants have recently been postulated (Lalonde et al., 1999; Fernie et al., 2000), however, far more experimental evidence is required to ascertain the role and significance of these. Furthermore, the finding that palatinose inhibits the uptake of externally supplied 14C-Suc into the tissue (see Fig. 1A) suggests that binding of palatinose to a Suc carrier may occur; however, further studies are required to test this hypothesis. Moreover, the rapidity of the metabolic response to palatinose (within 2 h) indicates that it may consist of post-transcriptional regulation (i.e. protein phosphorylation) rather than changes in gene expression.
In conclusion, supplying palatinose to potato tuber discs provides a novel way to increase starch synthesis. This elevated rate of starch synthesis occurs despite the fact that palatinose is only poorly absorbed and not metabolized by potato tuber slices, and is independent of changes in the levels of glycolytic intermediates. Further studies are required, including expression of bacterial genes converting Suc to palatinose (see Huang et al., 1998) in different cellular compartments of potato tubers, to clarify the sensing mechanism and to elucidate the signal transduction pathway involved in this response.
MATERIALS AND METHODS
Plant Material
Potato (Solanum tuberosum L. cv Desirée, Saatzucht Fritz Lange, Bad Schwartau, Germany) plants were grown in soil (3-L pots) supplemented with Hakaphos grün (100 g per 230 L of soil; BASF, Ludwigshafen, Germany) in a growth chamber (350 μmol photons m−2 s−1 irradiance, 14-h/10-h day/night regime, 20°C, 50% relative humidity). Growing tubers from 9-week-old daily watered plants with high activities of SuSy, which is taken as an indicator for rapidly growing tubers (Merlo et al., 1993), were used for the experiments.
Enzyme Analysis
Tuber extracts were prepared and desalted by centrifugation through Sephadex-G25 columns as in Geigenberger et al. (1998). SuSy, acid invertase, and alkaline invertase were measured according to Dancer et al. (1990a). Substrate concentrations were varied as stated in the text.
Metabolite and Nucleotide Analysis
Tissue slices were frozen in liquid nitrogen, extracted with trichloroacetic acid, and metabolites and nucleotides were measured as given in Geigenberger et al. (1998). The recovery of small, representative amounts of each metabolite through the extraction, storage, and assay procedures has been documented previously (see Jelitto et al., 1992; Merlo et al., 1993; Geigenberger et al., 1994).
Measurement of Palatinose
Potato tuber tissue (200 mg) was extracted in 1,400 μL of methanol as described by Roessner et al. (2000) and 50 μL of 13.2 mm ribitol was added as an internal standard for quantification. The mixture was extracted for 15 min at 70°C, mixed vigorously with 1 volume of water, centrifuged at 2,200g, and 1 mL of the supernatant was reduced to dryness in vacuo. The residue was redissolved and derivatized for 90 min at 30°C (in 100 μL of 20 mg mL−1 methoxyamine hydrochloride in pyridine) followed by a 30 min treatment at 37°C (with 100 μL N-methyl-N-[trimethylsilyl]trifluroacetamide). One-microliter samples were then injected with a split ratio of 25:1 using a hot needle technique. The GC-MS system consisted of an AS 2000 autosampler, a GC 8000 gas chromatograph, and a Voyager quadrupole mass spectrometer (ThermoQuest, Manchester, UK). GC-MS parameters were performed as described by Roessner et al. (2000). The palatinose was quantified by comparison with the peak area of a calibration curve derived from the specific ion trace 361 of palatinose to the peak area derived from the specific ion trace 319 of the added internal standard ribitol.
Labeling Experiments
Tuber discs (diameter of 8 mm, thickness of 2 mm) were cut directly from growing tubers attached to the fully photosynthesizing mother plant, washed three times with 10 mm MES [2-(N-morpholino) ethane sulfonic acid]-KOH (pH 6.5), and were then incubated (eight discs in a volume of 4 mL in a 100-mL Erlenmeyer flask shaken at 90 rpm) for 2 h in 20 mm Suc including 1.4 KBq μmol−1 U-[14C]Suc or 2 mm Glc including 1.4 KBq μmol−1 U-[14C]Glc (Amersham-Buchler, Freiburg, Germany) and supplementary respiratory substrate as described in the text. Then discs were harvested, washed three times in buffer (eight discs per 100 mL), and frozen in liquid nitrogen.
Fractionation of 14C-Labeled Tissue Extracts
Discs were extracted with 80% (v/v) ethanol at 80°C (1 mL per two discs), re-extracted in two subsequent steps with 50% (v/v) ethanol (1 mL per two discs for each step), the combined supernatants were dried under an air stream at 40°C and were taken up in 1 mL of water (“soluble fraction”). They were then separated into neutral, anionic, and basic fractions by ion-exchange chromatography. The neutral fraction (3.5 mL) was freeze dried, taken up in 100 μL of water, and was further analyzed by thin-layer chromatography (Geigenberger et al., 1997). To measure phosphate esters, samples (150 μL) of the soluble fraction were incubated in 50 μL of buffer (10 mm MES-KOH, pH 6.0) with or without 1 unit of potato acid phosphatase (Grade II, Boehringer Mannheim) for 3 h at 37°C, boiled for 2 min, and then analyzed by ion-exchange chromatography (Geigenberger et al., 1997). The insoluble material left after ethanol extraction was homogenized, taken up in 1 mL of water, and was counted for starch. In discs from growing tubers, starch accounts to over 90% of the label in the insoluble fraction (Geigenberger et al., 1994).
ACKNOWLEDGMENTS
We are indebted to Mark Stitt and Lothar Willmitzer for critical readings of the manuscript.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft (P.G.) and the Max-Planck Gesellschaft (A.R.F.).
LITERATURE CITED
- ap Rees T, Morrell S. Carbohydrate metabolism in developing potatoes. Am Potato J. 1990;67:835–847. [Google Scholar]
- Avigard G. Sucrose and other disaccharides. In: Lowus TA, Tanner W, editors. Encyclopedia of Plant Physiology. Heidelberg: Springer-Verlag; 1982. pp. 217–347. [Google Scholar]
- Chiou TJ, Bush DR. Sucrose as a signal molecular in assimilate partitioning. Proc Natl Acad Sci USA. 1998;95:4784–4788. doi: 10.1073/pnas.95.8.4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancer J, Hatzfield WD, Stitt M. Cytosolic cycles regulate the turnover of sucrose in heterotrophic cell-suspension cultures of Chernopodium rubrum L. Planta. 1990a;182:223–231. doi: 10.1007/BF00197115. [DOI] [PubMed] [Google Scholar]
- Dancer J, Neuhaus HE, Stitt M. Subcellular compartmentation of uridine nucleotides and nucleoside-5-diphodphate kinase in leaves. Plant Physiol. 1990b;92:637–641. doi: 10.1104/pp.92.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehlert DC. Substrate inhibition of maize endosperm sucrose synthase by fructose and its interaction with glucose inhibition. Plant Sci. 1987;52:153–157. [Google Scholar]
- Farré EM, Geigenberger P, Willmitzer L, Trethewey RN. A possible role for pyrophosphate in the coordination of cytosolic and plastidial carbon metabolism within the potato tuber. Plant Physiol. 2000;123:661–668. doi: 10.1104/pp.123.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie AR, Riesmeier JW, Martiny A, Ramalingam S, Willmitzer L, Trethewey RN. Consequences of the expression of a bacterial glucokinase, both in combination with and independently of a yeast-derived invertase, in potato tubers. Aust J Plant Physiol. 2000;27:827–833. [Google Scholar]
- Fu H, Park WD. Sink and vascular associated sucrose synthase functions are encoded by different gene classes in potato. Plant Cell. 1995;7:1395–1403. doi: 10.1105/tpc.7.9.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenberger P, Hajirezaei M, Geiger M, Deiting U, Sonnewald U, Stitt M. Overexpression of pyrophosphatase leads to increased sucrose degradation and starch synthesis, increased activities of enzymes involved in sucrose-starch interconversion, and increased levels of nucleotides in growing potato tubers. Planta. 1998;205:428–434. doi: 10.1007/s004250050340. [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Langenberger S, Wilke I, Heineke D, Heldt HW, Stitt M. Sucrose is metabolised by sucrose synthase and glycolysis within the phloem complex of Ricinus communis L. seedlings. Planta. 1993;190:446–453. [Google Scholar]
- Geigenberger P, Merlo L, Reimholz R, Stitt M. When growing potato tubers are detached from their mother plant there is a rapid inhibition of starch synthesis, involving inhibition of ADP glucose pyrophosphorylase. Planta. 1994;193:486–493. [Google Scholar]
- Geigenberger P, Reimholz R, Deiting U, Sonnewald U, Stitt M. Decreased expression of sucrose phosphate synthase strongly inhibits the water stress-induced synthesis of sucrose in growing potato tubers. Plant J. 1999;19:119–129. doi: 10.1046/j.1365-313x.1999.00506.x. [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Reimholz R, Geiger M, Merlo L, Canale V, Stitt M. Regulation of sucrose and starch metabolism in potato tubers in response to short-term water deficit. Planta. 1997;201:502–518. [Google Scholar]
- Geigenberger P, Stitt M. Sucrose synthase catalyzes a readily reversible reaction in vivo in developing potato tubers and other plant tissues. Planta. 1993;190:440–450. doi: 10.1007/BF00194429. [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Stitt M. Growing potato tubers exhibit diurnal changes in sucrose, metabolites, starch synthesis, and Agps transcript that are suppressed in transformants with decreased expression of sucrose phosphate synthase. Plant J. 2000;23:795–806. doi: 10.1046/j.1365-313x.2000.00848.x. [DOI] [PubMed] [Google Scholar]
- Geiger M, Stitt M, Geigenberger P. Metabolism in slices from growing potato tubers responds differently to addition of glucose and sucrose. Planta. 1998;206:234–244. [Google Scholar]
- Huang JH, Hsu LH, Su YC. Conversion of sucrose to isomaltulose by Klebsiella planticola. J Micro Biotechnol. 1998;21:22–27. [Google Scholar]
- Jelitto T, Sonnewald U, Willmitzer L, Hajirezeai M, Stitt M. Inorganic pyrophosphate content and metabolites in potato and tobacco plants expressing E. coli pyrophosphate in their cytosol. Planta. 1992;188:238–244. doi: 10.1007/BF00216819. [DOI] [PubMed] [Google Scholar]
- Koch KE. Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:509–540. doi: 10.1146/annurev.arplant.47.1.509. [DOI] [PubMed] [Google Scholar]
- Krapp A, Hofmann B, Schäfer C, Stitt M. Regulation of the expression of rbcS and other photosynthetic genes by carbohydrates: a mechanism for the sink regulation of photosynthesis. Plant J. 1993;3:817–828. [Google Scholar]
- Krapp A, Stitt M. An evaluation of direct and indirect mechanisms for the sink regulation of photosynthesis in spinach: changes in gas exchange, metabolites, enzyme activities and steady-state transcript levels after cold girdling spinach leaves. Planta. 1995;192:313–323. [Google Scholar]
- Krausgrill S, Sander A, Greiner S, Weil M, Rausch T. Regulation of cell-wall invertase by a proteinaceous inhibitor. J Exp Bot. 1996;47:1193–1198. doi: 10.1093/jxb/47.Special_Issue.1193. [DOI] [PubMed] [Google Scholar]
- Kruger NJ. Carbohydrate synthesis and degradation. In: Dennis DT, Turpin DH, Lefebvre DD, Layzell DB, editors. Plant Metabolism. Harlow, UK: Longman; 1997. pp. 83–104. [Google Scholar]
- Lalonde S, Boles E, Hellmann H, Barker L, Patrick JW, Frommer WB, Ward JM. The dual function of sugar carriers: transport and sugar sensing. Plant Cell. 1999;11:707–726. doi: 10.1105/tpc.11.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loef I, Stitt M, Geigenberger P. Feeding orotate leads to a specific increase in uridine nucleotide levels, resulting in a stimulation of sucrose degradation and starch synthesis in discs of growing potato tubers. Planta. 1999;209:314–323. doi: 10.1007/s004250050638. [DOI] [PubMed] [Google Scholar]
- Loef I, Stitt M, Geigenberger P (2001) Increased adenine nucleotide levels modify the interaction between respiration and starch synthesis when adenine is fed to discs of growing potato tubers. Planta (in press) [DOI] [PubMed]
- Martin T, Hellmann H, Schmidt R, Willmitzer L, Frommer WB. Identification of mutants in metabolically regulated gene expression. Plant J. 1997;11:53–62. doi: 10.1046/j.1365-313x.1997.11010053.x. [DOI] [PubMed] [Google Scholar]
- M'Batchi B, Delrot S. Simulation of sugar exit from leaf tissues of Vicia faba L. Planta. 1998;174:340–348. doi: 10.1007/BF00959519. [DOI] [PubMed] [Google Scholar]
- M'Batchi B, Pichelin D, Delrot S. The effect of sugars on the binding of [203Hg]-p-chloromercuribenzenesulfonic acid to leaf tissues. Plant Physiol. 1985;79:537–542. doi: 10.1104/pp.79.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo L, Geigenberger P, Hajirezaei M, Stitt M. Changes of carbohydrates, metabolites and enzyme activities in potato tubers during development, and within a single tuber along a stolen-apex gradient. J Plant Physiol. 1993;142:392–402. [Google Scholar]
- Müller-Röber B, Kossmann J, Hannah LC, Willmitzer L, Sonnewald U. One of two different ADP-glucose pyrophosphorylase genes from potato responds strongly to elevated levels of sucrose. Mol Gen Genet. 1990;224:136–146. doi: 10.1007/BF00259460. [DOI] [PubMed] [Google Scholar]
- Ovalle R, Keyes AC, Ewing EE, Quimby FW. Purification and characterization of the acid-stable proteinaceous inhibitor of potato tuber invertase by non-ideal size exclusion chromatography. J Plant Physiol. 1995;147:334–340. [Google Scholar]
- Pego JV, Weisbeek PJ, Smeekens S. Mannose inhibits Arabidopsis germination via a hexokinase-mediated step. Plant Physiol. 1999;119:1017–1023. doi: 10.1104/pp.119.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss J. Biosynthesis of starch and its regulation. In: Preiss J, editor. The Biochemistry of Plants. Vol. 14. San Diego: Academic Press; 1988. pp. 181–254. [Google Scholar]
- Pressey R. Invertase inhibitor from potatoes: purification, characterization, and reactivity with plant invertases. Plant Physiol. 1967;44:1780–1786. doi: 10.1104/pp.42.12.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha-Sosa M, Sonnewald U, Frommer W, Stratmann M, Schell J, Willmitzer L. Both developmental and metabolic signals activate the promoter of the class I patatin gene. EMBO J. 1989;8:23–29. doi: 10.1002/j.1460-2075.1989.tb03344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessner U, Wagner C, Kopka J, Trethewey RN, Willmitzer L. Simultaneous analysis of metabolites in potato tuber by gas chromatography-mass spectrometry. Plant J. 2000;23:131–142. doi: 10.1046/j.1365-313x.2000.00774.x. [DOI] [PubMed] [Google Scholar]
- Roitsch T, Büttner M, Godt DE. Induction of apoplastic invertase of Chenopodium rubrum by D-glucose and a glucose analogue and tissue-specific expression suggest a role in source-sink regulation. Plant Physiol. 1995;108:285–294. doi: 10.1104/pp.108.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanoubat M, Beliard G. The steady-state level of potato sucrose synthase messenger RNA is dependent on wounding, anaerobiosis and sucrose concentration. Gene. 1989;84:181–185. doi: 10.1016/0378-1119(89)90153-4. [DOI] [PubMed] [Google Scholar]
- Schafer-Pregl R, Ritter E, Concililio L, Hesselbach J, Lovatti L, Walkemeier B, Thelen H, Salamini F, Gebhardt C. Analysis of quantitative trait loci (QTLs) and quantitative trait alleles (QTAs) for potato tuber yield and starch content. Theor Appl Genet. 1998;97:834–846. [Google Scholar]
- Schmalstig JD, Hitz WD. Transport and metabolism of a sucrose analog (1′-fluorosucrose) into Zea mays L. endosperm without invertase hydrolysis. Plant Physiol. 1987a;85:902–905. doi: 10.1104/pp.85.4.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalstig JG, Hitz WD. Contributions of sucrose synthase and invertase to the metabolism of sucrose in developing leaves: estimation by alternative substrate utilization. Plant Physiol. 1987b;85:407–412. doi: 10.1104/pp.85.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt MR, Hitz WD, Lin W, Giaquinita RT. Sugar transport into protoplasts isolated from developing soybean cotyledons. Plant Physiol. 1984;75:941–946. doi: 10.1104/pp.75.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S. Sugar regulation of gene expression in plants. Curr Opin Plant Biol. 1998;1:230–235. doi: 10.1016/s1369-5266(98)80109-x. [DOI] [PubMed] [Google Scholar]
- Sonnewald U, Hajiraezaei MR, Kossmann J, Heyer A, Trethewey RN, Willmitzer L. Expression of a yeast invertase in the apoplast of potato tubers increases tuber size. Nat Biotechnol. 1997;15:794–797. doi: 10.1038/nbt0897-794. [DOI] [PubMed] [Google Scholar]
- Stark DM, Timmermann KP, Barry GF, Preiss J, Kishore GM. Regulation of the amount of starch in plant tissues. Science. 1991;258:287–292. doi: 10.1126/science.258.5080.287. [DOI] [PubMed] [Google Scholar]
- Stitt M. Pyrophosphate as an energy donor in the cytosol of plant cells: an enigmatic alternative to ATP. Bot Acta. 1998;111:167–175. [Google Scholar]
- Tauberger E, Fernie AR, Emmermann M, Renz A, Kossmann J, Willmitzer L, Trethewey RN. Antisense inhibition of plastidial phosphoglucomutase provides compelling evidence that potato tuber amyloplasts import carbon from the cytosol in the form of glucose-6-phosphate. Plant J. 2000;23:43–53. doi: 10.1046/j.1365-313x.2000.00783.x. [DOI] [PubMed] [Google Scholar]
- Tjaden J, Möhlmann T, Kampfenkel K, Henrichs G, Neuhaus HE. Altered plastidic ATP/ADP-transporter activity influences potato (Solanum tuberosum L.) tuber morphology, yield and composition of tuber starch. Plant J. 1998;16:531–540. [Google Scholar]
- Trethewey RN, Geigenberger P, Riedel K, Hajirezaei MR, Sonnewald U, Stitt M, Riesmeier JW, Willmitzer L. Combined expression of glucokinase and invertase in potato tubers leads to a dramatic reduction in starch accumulation and a stimulation of glycolysis. Plant J. 1998;15:109–118. doi: 10.1046/j.1365-313x.1998.00190.x. [DOI] [PubMed] [Google Scholar]
- Weber H, Heim U, Golombek S, Borisjuk L, Wobus U. Assimilate uptake and the regulation of seed development. Seed Sci Res. 1998;8:331–345. [Google Scholar]