Abstract
Neurodegenerative diseases, including Alzheimer's disease (AD), Parkinson's disease (PD), and Huntington's disease (HD), are characterized by the progressive degeneration of neurons. Although the etiology and pathogenesis of neurodegenerative diseases have been studied intensively, the mechanism is still in its infancy. In general, most neurodegenerative diseases share common molecular mechanisms, and multiple risks interact and promote the pathologic process of neurogenerative diseases. At present, most of the approved drugs only alleviate the clinical symptoms but fail to cure neurodegenerative diseases. Numerous studies indicate that dietary plant polyphenols are safe and exhibit potent neuroprotective effects in various neurodegenerative diseases. However, low bioavailability is the biggest obstacle for polyphenol that largely limits its adoption from evidence into clinical practice. In this review, we summarized the widely recognized mechanisms associated with neurodegenerative diseases, such as misfolded proteins, mitochondrial dysfunction, oxidative damage, and neuroinflammatory responses. In addition, we summarized the research advances about the neuroprotective effect of the most widely reported dietary plant polyphenols. Moreover, we discussed the current clinical study and application of polyphenols and the factors that result in low bioavailability, such as poor stability and low permeability across the blood-brain barrier (BBB). In the future, the improvement of absorption and stability, modification of structure and formulation, and the combination therapy will provide more opportunities from the laboratory into the clinic for polyphenols. Lastly, we hope that the present review will encourage further researches on natural dietary polyphenols in the treatment of neurodegenerative diseases.
1. Introduction
Neurodegenerative diseases, including Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD), amyotrophic lateral sclerosis (ALS), and multiple sclerosis (MS), are a group of incurable heterogeneous diseases. They are characterized by the gradual degeneration of the function and structure of neurons and overactivation of microglia in the central nervous system (CNS) [1]. To date, the accurate molecular mechanisms related to the pathogenesis and progression of neurodegenerative diseases are not well elucidated [2]. Although each neurodegenerative disease exhibits the respective pathological features, they also share some common molecular mechanisms, such as the aggregation of misfolded proteins, oxidative damage, mitochondrial dysfunction, DNA damage, neuroexcitotoxicity, biometal dyshomeostasis, neurotrophic impairment, and neuroinflammatory responses [3, 4]. Among them, the aggregated misfolded proteins have become the pathological hallmarks in many neurodegenerative diseases. For example, the extracellular deposition of amyloid-β (Aβ) fibrils and intracellular hyperphosphorylated Tau are found in the brain of AD. In addition, Lewy bodies containing α-synuclein, mutant huntingtin (mHtt), mutant superoxide dismutase 1 (SOD1), and TAR DNA-Binding Protein 43 (TDP-43) are closely associated with the pathogenesis of PD, HD, and ALS, respectively [5]. It is known to us that these misfolded proteins are increasingly accumulated with ageing and induce oxidative stress by generating excessive reactive oxygen species (ROS) and reactive nitrogen species (RNS), which is accompanied by mitochondrial dysfunction, DNA damage, neuroexcitotoxicity, and ultimately neuronal death [6]. In addition, neuroinflammation plays a critical role in the early onset and late-stage of neurodegenerative diseases [7]. Microglia known as the resident macrophage cells in the brain are chronically activated by the Pathogen-Associated Molecular Patterns or Danger-Associated Molecular Patterns (PAMPs/DAMPs), such as misfolded protein aggregates, bacteria, viruses, lipopolysaccharides (LPS), and many environmental toxins. Then, the sustained activated microglia subsequently release several cytokines and induce proinflammatory responses [8]. Therefore, neuronal death and microglial overactivation are two major indicators for the pathological development and process of neurodegenerative diseases. Emerging evidence indicates that the autophagy-lysosome pathway (ALP) and the ubiquitin-proteasome system (UPS) are two important processes that facilitate the clearance of misfolded proteins and damaged or unnecessary organelles, such as mitochondria [9]. At the early onset of neurodegenerative diseases, ALP and UPS acting as collaborators play protective roles in the degradation of toxin misfolded proteins, resistance to oxidative stress, and suppression of neuroinflammation [10, 11]. However, the normal function of ALP and UPS is impaired with ageing by the increasingly accumulated misfolded proteins and toxins [12, 13]. In this review, we summarized the current well-studied molecular mechanisms closely associated with the development of neurodegenerative diseases, including the aggregation of misfolded proteins, oxidative damage, mitochondrial dysfunction, DNA damage, excitotoxicity, biometal dyshomeostasis, and neuroinflammatory responses. However, the molecular mechanism of neurodegenerative diseases is still in its infancy and requires further in-depth investigations.
At present, there are currently many drugs developed and approved for the improvement of the symptoms of patients with neurodegenerative diseases in the clinical, but few of them can cure these diseases. More seriously, there might have side effects that appeared owing to the long-term use. In addition, many drugs, such as bapineuzumab, gantenerumab, and solanezumab, were recently declared failures during the clinical trial [14, 15]. Therefore, the accurate molecular mechanism and discovery of targeted drugs for the treatment of neurodegenerative diseases are still urgent and attract more and more attention [16]. In this review, we summarized the main current therapies and their mechanisms of action, neuroprotective effects, and limitations in various neurodegenerative diseases (Table 1). In view of the diversity of pathogenic mechanisms, the combinational therapies or the discovery and development of drugs with multitargets bring new hope for the treatment of neurodegenerative diseases. Therefore, more and more attentions are paid to natural medicine such as traditional Chinese medicines (TCMs) with multicompounds, multitargets, and multieffect properties. TCMs originating from natural products have a 2000-year history of treating diseases in China and have been proved to be safe and effective. To date, various kinds of bioactive compounds, including alkaloids, polyphenols, and saponins, are isolated and identified from natural plants. Among them, polyphenols, an important type of natural product, are mainly widely distributed in natural dietary plants. They are commonly divided into flavonoids and nonflavonoids which are subclassified into phenolic acids, stilbenes, lignans, curcuminoids, and coumarins. The modern pharmacological studies demonstrate that these polyphenols exhibit potential neuroprotective effects including the inhibition of neuronal death and the attenuation of neuroinflammatory responses in vitro and in vivo [17]. In this review, we summarized the research advances about the neuroprotective effect of the most widely reported dietary plant polyphenols in various cellular and animal models of neurodegenerative diseases. In addition, we discussed the current clinical study and application of polyphenols and the factors that result in low bioavailability. In the future, we hope that the improvement of absorption and stability, the modification of structure and formulation, and the combination therapy will provide more opportunities from the laboratory into the clinic for polyphenols. The present review will aid the researchers to know the research advances of polyphenols in neurodegenerative diseases. Lastly, we hope further researches will be encouraged for natural dietary polyphenols in the treatment of neurodegenerative diseases.
Table 1.
The main current therapies and their mechanisms, effects, and limitations for neurodegenerative diseases.
| Drugs | Mechanisms | Main effects | Main limitations | Diseases |
|---|---|---|---|---|
| Donepezil, Ralantamine, Rivastigmine | Inhibiting acetylcholinesterase | Increasing levels of synaptic acetylcholine | Increasing cognitive impairment; low CNS selectivity; gastrointestinal toxicity (nausea, vomiting, and diarrhea) | AD [18–24] |
| Memantine | Antagonizing N-methyl-D-aspartate-receptor (NMDAR) | Blocking glutamate from accessing NMDA receptors | Inability to slow down the progression of the disease | |
| Aducanumab | Human, immunoglobulin gamma 1 (IgG1) monoclonal antibody | Reducing aggregated soluble and insoluble forms of Aβ | High cost and failure to show definite effect in clinical trials | |
| Levodopa+Carbidopa | Inhibiting DA precursor and DOPA decarboxylase | Increasing DA levels in SNc | Wearing and movement disorders; dizziness and gastrointestinal upset | PD [25–28] |
| Pramipexole and Apomorphine | Agitating DA | Activating DA receptors | Less effective than levodopa; worsen dyskinesia | |
| Selegiline, Rasagiline, and Safinamide | Inhibiting monoamine oxidase B (MAO-B) | Preventing DA metabolism | Mild efficacy in monotherapy | |
| Gocovri (Amantadine) | Antivirus | Reducing levodopa-induced dyskinesia | Several side effects including psychosis, edema, constipation, and livedo reticularis | |
| Trihexyphenidyl | Antagonizing muscarinic acetylcholine receptor | Reducing tremor | Serious side effects including memory impairment, confusion, and hallucinations | |
| Levodopa+Carbidopa+Istradefylline | Inhibiting DA precursor, DOPA decarboxylase, and antagonizing A2A receptor | Reducing the “off” episodes | Higher incidence of treatment-emergent adverse events (TEAEs) and dyskinesia | |
| Levodopa+Carbidopa+Opicapone | Inhibiting DA precursor, DOPA decarboxylase, and catechol-o-methyl transferase (COMT) | Reducing the “off” episodes | Higher incidence of TEAEs and worsen dyskinesia than istradefylline | |
| Tetrabenazine (TBZ; Xenazine™) and deutetrabenazine (AUSTEDO™) | Inhibiting vesicular monoamine transporter type 2 (VMAT2) | Treating chorea associated with HD and tardive dyskinesia | Inability to slow down the progression of the disease | HD [29] |
| Riluzole | Blocking the presynaptic release of glutamate | Inhibiting the excitotoxicity | High cost and modest efficacy | ALS [30–33] |
| Edaravone (RADICAVATM) | Antioxidant | Protecting neuronal cells from oxidative stress, ameliorating motor dysfunction | Limited patient population |
2. The Common Molecular Mechanisms of Neurodegenerative Diseases
2.1. Aggregation of Misfolded Proteins
The aggregation of misfolded proteins is recognized to be the common pathological feature of neurodegenerative diseases, such as Aβ and hyperphosphorylated Tau in AD, mutant α-synuclein in PD, and mHtt in HD, as well as SOD1 and TDP-43 in ALS [5, 34, 35] (Figure 1). It is known to us that ALP and UPS are two major intracellular elimination pathways for the clearance of these neurotoxic proteins in neurons and other cells in the brain [9, 36–38]. In the early onset of neurodegenerative disease, these toxic misfolded proteins are degraded via ALP and UPS pathways or effectively engulfed by microglia and astrocytes under normal physiological conditions. However, there is a growing body of studies showing that these misfolded protein aggregates are increasingly accumulated with ageing, accompanied by dysregulated or impaired ALP and UPS, which is implicated in the late stage of various neurodegenerative diseases [39]. Lastly, the normal function of neurons is becoming lost, and the microglia are overactivated, which ultimately results in neuronal death and proinflammatory responses [40] (Figure 1). For example, many accumulated autophagosomes and autophagic vesicles in the brain of AD patients are observed at the late stage of autophagy flux under immunoelectron microscopy [41]. In addition, autophagy is activated in the brain cells of AD patients and APP/PS1 mice. However, autophagy is impaired with ageing as revealed by the accumulation of Aβ-containing autophagic vesicles [42]. Therefore, autophagy plays a protective mechanism that fights against toxic protein-induced neuronal death and neuroinflammation at the early stage of AD, while the normal function of autophagy is impaired by the overgenerated toxic misfolded proteins (e.g., Aβ and Tau). In PD, emerging evidence indicates that the accumulation of mutant genes, including α-synuclein, Parkin, and ubiquitin carboxy-terminal hydrolase L1 (UCHL-1), is closely associated with the dysfunction of ALP and UPS [43]. At the early stage of PD, autophagy participates in the clearance of misfolded proteins, damaged mitochondria, and generated ROS. However, autophagy is impaired in the brain of PD toxin-induced animals or transgenic mice with PD. For instance, the mRNA level of ubiquitinated α-synuclein is significantly increased in the brain of 1-methy-4-phenyl-1,2,3,6-tetrahydropyridine- (MPTP-) induced mice [44]. In addition, the impaired lysosome is accompanied by the accumulation of α-synuclein in mice which are chronically injected with probenecid and MPTP [45]. There is a growing body of evidence showing that UPS plays an important role in the degradation of soluble mHtt, but almost 90% of long-lived or large aggregated proteins such as mHtt can only be degraded via ALP [46]. For example, rapamycin, a potent autophagy inducer, significantly accelerates the autophagic degradation of mHtt, while autophagy inhibitors including 3-methyladenine (3-MA) and bafilomycin A1 attenuate the effect of rapamycin [47, 48]. Taken together, the aggregation of misfolded proteins is the pathological hallmarks of neurodegenerative diseases, while ALP and UPS act as a protective mechanism that timely clears the misfolded protein aggregates to maintain cellular homeostasis at the early stage of neurodegenerative diseases. However, misfolded proteins are increasingly accumulated with ageing, which dysregulates the normal functions of ALP and UPS [49]. Therefore, the discovery of ALP or UPS enhancers that target the clearance of misfolded proteins and damaged organelles is recognized to be a promising therapeutic strategy for neurodegenerative diseases.
Figure 1.
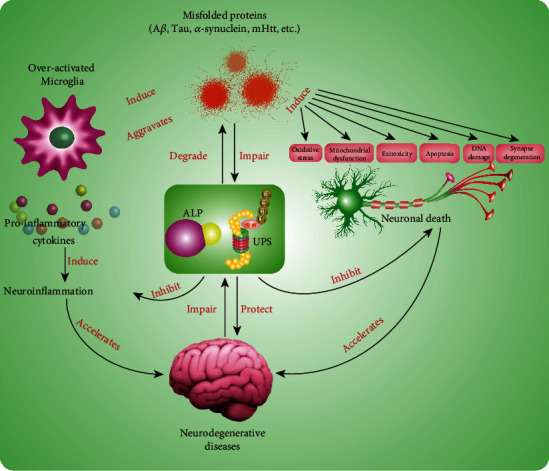
The role of misfolded proteins in neurodegenerative diseases. The misfolded proteins, including Aβ, Tau, α-synuclein, and mHtt, induce the overactivation of microglia and neuronal death. The overactivated microglia release the amount of proinflammatory cytokines, including IL-6, IL-1β, IL-18, and TNF-α, and then induce neuroinflammation. Meanwhile, the overactivation of microglia aggravates the aggregation of misfolded proteins. Neuronal death was induced by misfolded proteins through multiple mechanisms, including oxidative stress, mitochondrial dysfunction, excitotoxicity, apoptosis, DNA damage, and synapse degeneration. Both neuroinflammation and neuronal death accelerate the progress of neurodegenerative diseases. However, both ALP and UPS acting as two major degradation pathways not only clear the misfolded proteins but also inhibit neuroinflammation and neuronal death in the early stage of neurodegenerative diseases. However, the overaccumulation of misfolded proteins and degenerated brain impair the normal function of ALP and UPS.
2.2. Oxidative Stress
In general, oxidative stress is caused by the imbalance between oxidation and antioxidation when the free radicals including superoxide anion radical and hydroxyl radical are overgenerated and cannot be cleared timely and effectively [50, 51]. Oxidative stress is currently implicated in various diseases, such as neurodegenerative diseases, ageing, atherosclerosis, and cancers. It is characterized by mitochondrial dysfunction and abnormal accumulation of transition metals, which causes mitochondrial DNA (mtDNA) mutations, changes in membrane permeability, calcium dyshomeostasis, lipid oxidation generation, and protein carbonylation [52]. Emerging evidence indicates that the brain cells are more susceptible to oxidative damage owing to the high oxygen consumption and the weak antioxidant defence ability [53]. The mechanistic studies demonstrate that oxidative stress is a critical inducer of neuronal death and neuroinflammation in neurodegenerative diseases [54]. It is reported that the misfolded protein aggregates (Aβ, Tau, a-synuclein, mHtt, etc.) damage the normal function of mitochondria, which then induces the generation of amounts of ROS [6, 55]. In turn, excessive ROS levels promote the aggregation of the pathological proteins [56]. For example, oxidative stress is reported to promote Aβ deposition, Tau hyperphosphorylation, and the subsequent loss of synapses and neurons in AD [57] and also induce the degeneration of dopaminergic neurons in the substantia nigra of PD brain [58, 59]. In addition, oxidative stress also overactivated microglia and induces neuroinflammation [60], while neuroinflammation further aggravates the accumulation of misfolded proteins and induces oxidative stress [61]. Collectively, a vicious circle among oxidative stress, misfolded proteins, neuronal death, and neuroinflammation is formed, which collaboratively induces the onset of neurodegenerative diseases and accelerates the progress and development of pathology. At present, several studies indicate that the activation of Kelch-like ECH-associated protein 1/nuclear factor erythroid 2-related factor 2/antioxidant response element (Keap1/Nrf2/ARE) pathway has a certain neuroprotective effect in numerous cellular and animal models of neurodegenerative diseases. However, there is limited clinical evidence showing that Nrf2 activation is a clinical target in neurodegenerative disease except for MS [62, 63] (Figure 2). Thus, more clinical studies are needed to be carried out for the validation and confirmation of the neuroprotective effect of Nrf2 target and its activators. Collectively, the discovery of antioxidants targeting the inhibition of oxidative stress to suppress neuronal death and neuroinflammation is an effective therapeutic strategy for neurodegenerative diseases.
Figure 2.
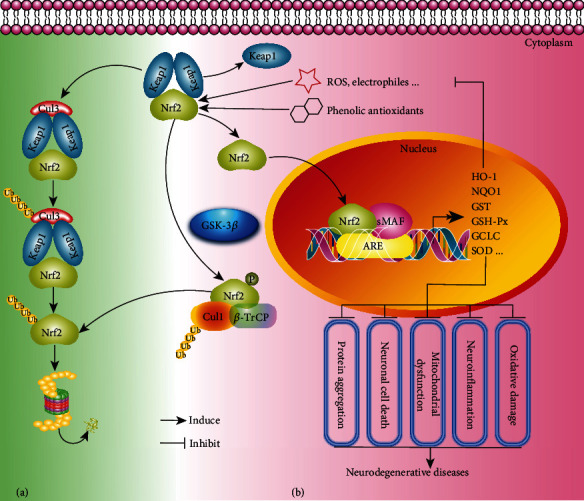
The regulation of the Keap1-Nrf2 pathway under the stimulation of ROS and electrophiles or the treatment of phenolic antioxidants in neurodegenerative diseases. Under basic conditions, Keap1, functioning as a substrate adaptor protein for Cullin3-based Cullin-RING E3 ubiquitin ligase complex around the Cullin3 (Cul3) scaffold protein, mediates the ubiquitination and proteasomal degradation of Nrf2. Under Nrf2 activation, the generated ROS or electrophiles alter the interaction between Nrf2 and its repressors under oxidative stress, resulting in the accumulation of Nrf2 in the cytoplasm and the translocation of Nrf2 into the nucleus, while the phenolic antioxidants (exogenous activator) enhance the effect of the endogenous activator on the Nrf2 pathway, thereby accelerating the dissociation of Nrf2 from Keap1 and leading to more Nrf2 translocation into the nucleus under the conditions of oxidative stress. Through the binding with Maf and ARE, Nrf2 regulates the expression of its downstream target genes, including heme oxygenase-1 (HO-1), NADPH Quinone Dehydrogenase 1 (NQO1), glutathione S-transferase (GST), glutathione peroxidase (GSH-Px), Glutamate-Cysteine Ligase Catalytic Subunit (GCLC), and superoxide dismutase (SOD). Alternatively, Nrf2 is phosphorylated by GSK-3β; then, β-transducin repeat-containing protein (β-TrCP) mediates its interaction with a Cul1 ubiquitin ligase complex to promote the proteasomal degradation of Nrf2, thereby inhibiting the expression of cytoprotective genes. The upregulation of cytoprotective genes prevents the generation of ROS levels, as well as oxidative damage, neuroinflammation, mitochondrial dysfunction, neuronal cell death, and protein aggregation.
2.3. Mitochondrial Dysfunction
Mitochondria, membrane-bound organelles located in the cytoplasm of almost all eukaryotic cells, are a cellular powerhouse, which generate energy for cells in the form of adenosine triphosphate (ATP) [64, 65]. Emerging evidence indicates that mitochondria play a crucial role in cellular development and function, including growth, differentiation, proliferation, and metabolism [66]. In neurodegenerative diseases, the accumulated toxic misfolded proteins and many neurotoxins damage the mitochondria in neurons and microglia [67]. There is a growing body of evidence showing that mitochondrial dysfunction is closely associated with the development of neurodegenerative diseases [68–72]. Mechanistic studies demonstrate that mitochondrial dysfunction leads to the excessive generation of free radicals, decreased ATP levels and mitochondrial membrane potential (MMP), calcium dyshomeostasis, mitochondrial permeability transition, mtDNA mutations, and perturbed mitochondrial dynamics [67] (Figure 3). In 12-month-old APPsw and APP/PS1 mouse models of AD, mitochondrial Aβ levels are closely associated with mitochondrial dysfunction and cognitive impairment [73]. In addition, mutant APP and Aβ enter mitochondria and interact with mitochondrial-related proteins, then disrupt the electron transport chain (ETC) and induce the generation of ROS, and decrease the cellular ATP levels [74, 75]. In PD, neurotoxins, such as MPTP, rotenone, and paraquat, induce dopaminergic neuronal death through the direct inhibition of the activity of mitochondrial complex I [76, 77]. In the brain of HD patients, the activity of the respiratory chain complexes is decreased, which was accompanied by the abnormal mitochondrial morphology [78]. In addition, the postmortem brain samples of HD patients exhibit impaired mitochondrial complexes II, III, and IV of the ETC [79]. Moreover, mtDNA oxidative damage-mediated impaired complex I is reported to contribute to the pathogenicity of MS [72]. The mitochondrial antioxidant defence system including SOD and catalase plays important role in clearing the endogenous free radicals effectively. In AD and familial and sporadic ALS patients, the expression level of mitochondrial SOD is decreased [80]. Therefore, maintenance of the normal function of mitochondria and the discovery of targeted drugs can effectively mitigate the progress of neurodegenerative diseases.
Figure 3.
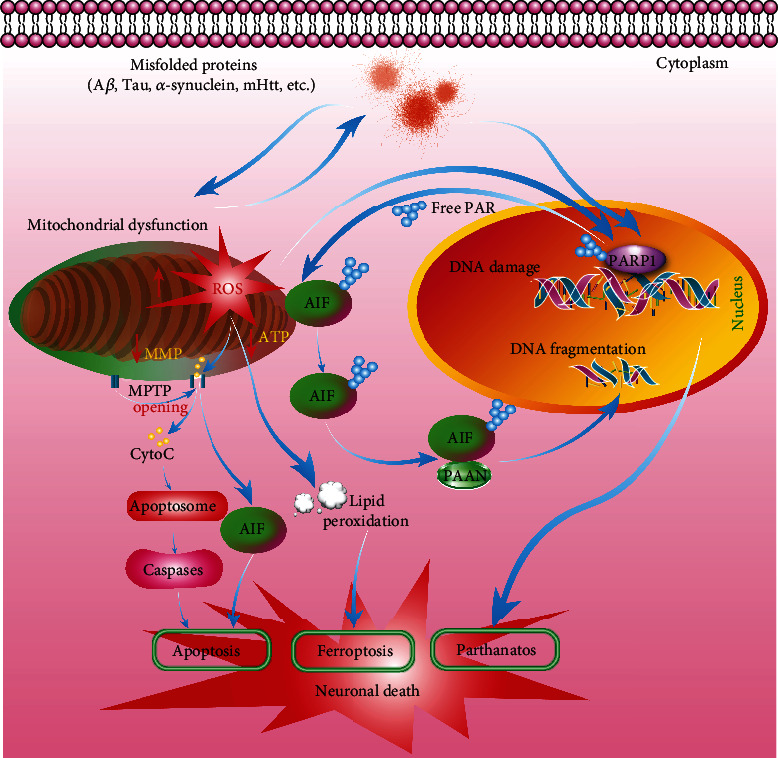
The mitochondrial dysfunction and DNA damage in neurodegenerative diseases. The increasingly accumulated misfolded proteins (Aβ, Tau, α-synuclein, mHtt, etc.) damage the normal function of mitochondria, thus resulting in the opening of the mitochondrial permeability transition pore (MPTP). The damaged mitochondria exhibit reduced ATP levels, increased ROS generation, decreased MMP, and increased release of cytochrome c (CytoC) into the cytosol, which promotes the formation of the apoptosome and subsequent proteolytical cleavage of procaspase-3 and procaspase-7, into the activated forms. Meanwhile, the loss of MMP results in the release of apoptosis-inducing factor (AIF) that is on the cytosolic side of the outer membrane of the mitochondria into the cytosol. The activation of caspases and accumulation of AIF ultimately induce neuronal cell apoptosis. In addition, the generation of large amounts of ROS induces the production and accumulation of lipid peroxidation, which indicates that neurons undergo ferroptosis. It is worth noting that the damaged mitochondria in turn further exacerbate the aggregation of misfolded proteins. In addition, the increasingly accumulated misfolded proteins induce DNA damage in the nucleus. The damaged DNA then activates PARP-1, which catalyzes PAR formation. The free PAR translocates from the nucleus to the cytosol and mitochondria where it binds AIF, inducing AIF release from the mitochondria. Then, AIF binds the parthanatos AIF-associated nuclease (PAAN) and translocates to the nucleus and causes the generation of DNA fragmentation, which induces neuronal cell death via parthanatos.
2.4. DNA Damage
Deoxyribonucleic acid (DNA), an important genetic material in cells, functions as the passer of genetic information with high fidelity. Otherwise, the cells undergo senescence and death when the DNA is damaged and cannot be repaired effectively. Therefore, DNA damage is implicated in various diseases, such as cancer, ageing, and neurodegenerative diseases [81–83]. There is a growing body of studies showing that DNA damage or defective DNA repair system is recognized to be a shared pathogenic mechanism, which is closely associated with the development of neurodegenerative diseases [84] (Figure 3). Oxidative DNA, DNA strand breaks, and DNA damage response (DDR) are the main lesions in neurodegenerative diseases [85, 86]. Among them, oxidative stress is especially sensitive to DNA damage and has attracted increasing attention. The high metabolic rate and high ROS levels decrease the ratio of antioxidant to prooxidant enzymes and induce oxidative stress [87]. It is reported that the base excision repair (BER) pathway consisting of DNA glycosylase changes with ageing in neurodegenerative diseases, which is primarily involved in the repair of oxidative lesions. In the brain of AD, the expressions of mitochondrial uracil DNA glycosylase and betaOGG1 glycosylase are found to be decreased [87]. At the same time, elevated DNA strand breaks, the reduced expression of DNA double-strand breaks (DSBs), repair proteins including the DNA-dependent protein kinase catalytic subunit (DNA-PKcs) and Mre11-Rad50-Nbs1 (MRN) complex proteins, and the activity of BER are identified in AD patients [88, 89]. In addition, the increased levels of oxidative lesions and single-strand breaks (SSBs) lead to serious damage of mtDNA in the neurons of ALS and PD [90, 91]. Furthermore, HD patient fibroblasts exhibit DNA oxidative lesions because the DNA repair system is impaired by mHtt [92]. Taken together, inhibition of DNA damage and the discovery of drugs that can repair DNA damage are important therapeutic strategies for neurodegenerative diseases.
2.5. Excitotoxicity
Excitotoxicity is a process that is triggered by the activation of the glutamate receptors owing to the pathologically high neurotransmitters such as glutamate, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), or N-methyl-D-aspartic acid (NMDA). Under excitotoxicity, the dendrites become degenerated and nerve cells undergo damage or even death (Figure 4). Therefore, excitotoxicity acting as a common pathogenic mechanism plays a key role in the development of various neurodegenerative diseases. Glutamate and aspartate are two major neurotransmitters that are widely distributed in neurons located in the cerebral cortex and hippocampus. They play important roles in regulating memory and learning functions. Emerging evidence indicates that the glutamate receptor is overactivated by excitatory amino acids, which damages neurons via multiple ways, including the impairment of calcium buffering, generation of free radicals, activation of the mitochondrial permeability transition (MPT), and its resultant secondary excitotoxicity [93]. The overexpression of NMDA or AMPA-type glutamate receptors is reported to induce neuronal apoptosis in vivo and in vitro [94]. In addition, the expression of NMDA receptors (NMDARs) is closely associated with mitochondrial activity, and NMDAR agonists lead to mitochondrial toxin-induced striatal damage [95]. For example, kynurenic acid (KA) and quinolinic acid (QA) induce neuronal apoptosis via activating the nuclear factor kappa B (NF-κB) signaling pathway and upregulating the expressions of p53 and c-Myc [96, 97]. At present, the excitotoxicity hypothesis has been widely studied in the molecular mechanism of HD. In addition to neuronal death and neuroinflammation, mHtt is also reported to enhance the activity of NMDAR and disturb the calcium signaling pathway, ultimately leading to neuronal death [98]. Further study revealed that mHtt activates NMDAR via the postsynaptic density protein- (PSD-) 95 [99] and NR1A/NR2B known as the main NMDAR subtype in neostriatal medium-size spiny neurons [100]. In addition, the early cognitive deficit is paralleled with the activation of glutamatergic signaling in AD [101]. Emerging evidence shows that glutamate- or Aβ-induced oxidative stress and the generation of lipid peroxidation are closely associated with the activation of NMDAR in hippocampal neurons [102]. In PD, Parkin is reported to regulate the function and stability of excitatory glutamatergic synapses, while the knockdown of Parkin or overexpression of mutant Parkin results in the proliferation of glutamatergic synapses and excitotoxicity [103]. MK-801, a noncompetitive antagonist of NMDAR, is demonstrated to inhibit MPTP-induced excitotoxicity in dopaminergic neurons [104]. Therefore, neuronal excitotoxicity plays an important role in the progression of neurodegenerative diseases, while inhibitors of excitotoxicity have become promising candidates for the treatment of neurodegenerative diseases.
Figure 4.
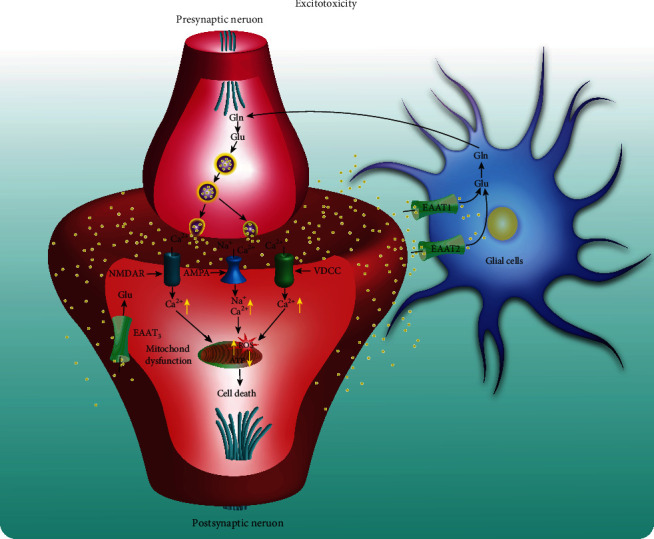
The role of excitotoxicity in neurodegenerative diseases. In presynaptic neurons, glutamate (Glu) is generated through the conversion of Glutamine (Gln) with the action of glutaminase. Glu is stored in the vesicles by vesicular glutamate transporters (vGLUTs). Then, Glu is released from the vesicles and out of presynaptic neurons owing to the depolarization of the presynaptic membrane. Then, Glu binds with the ionotropic glutamate receptors (iGluRs), such as N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors in the postsynaptic membrane, and generates an action potential. The binding of Glu with AMPA results in Na+ influx and consequent membrane depolarization and opening of voltage-dependent Ca2+ channels (VDCC). Meanwhile, the binding of Glu with NMDA receptors (NMDARs) leads to the opening of the NMDA receptor channel under depolarizing conditions, resulting in large amounts of Ca2+ influx. Finally, the increased levels of cytoplasmic Ca2+ induce the uptake of Ca2+ uptake into the mitochondria, which then induces the production of reactive oxygen species (ROS) and decreases ATP levels, ultimately resulting in neuronal cell death. The excitatory amino acid transporter 3 (EAAT3) is a transporter of Glu present at the postsynaptic neuronal element. In addition, the excessively released Glu in the synaptic cleft is transported into astrocytes through the EAAT1 and EAAT2 transporters. In astrocytes, Glu is recycled and converted to Gln which is transported to neurons and converted into Glu again.
2.6. Biometal Dyshomeostasis
In general, metals are divided into essential and nonessential metals according to the human body needs. The essential metals include chromium, iron (Fe), copper (Cu), manganese (Mn), calcium (Ca), and zinc (Zn). They act as cofactors of enzymes to regulate cellular bioactivity. Although essential metals are important for the function of the human body, they are usually present in trace amounts. Emerging evidence indicates that essential metals exert important physiological functions in different regions of the brain, while the deficiency of essential metals in the brain commonly results in the abnormal biological process and promotes the progression of neurodegenerative diseases [105–107]. At the same time, the overaccumulation of metals in the brain also induces various detrimental events, such as oxidative damage, mitochondrial dysfunction, protein misfolding, autophagy dysfunction, neuronal death, and neuroinflammation. Therefore, intracellular metal dyshomeostasis is implicated in various neurodegenerative diseases [108, 109]. In AD, abnormal or excessive Ca released from the endoplasmic reticulum (ER) results in the disruption of intracellular Ca dyshomeostasis and ultimately leads to memory loss and cognitive dysfunction [110]. In addition, metals, including Zn, Cu, and Fe, are reported to promote Aβ aggregation and induce oxidative stress. Meanwhile, Cu accumulated in neurofibrillary tangles (NFTs) binds to Tau protein and accelerates the aggregation of Tau in vitro [111, 112]. In 6-OHDA- or MPTP-induced animal models of PD, the content of iron in the brain is found to be increased [113], and the accumulated iron leads to the degeneration and ferroptosis of nigrostriatal dopaminergic neurons [114]. In addition, Mn inhibits glycolysis and energy metabolism, which ultimately results in excitotoxicity and dysregulation of cytoskeletal integrity in YAC128Q mice, an animal model of HD [115]. Furthermore, the aberrant copper-protein interaction also promotes the progression of HD by modulating the huntingtin structure and interfering with brain lactate-energy metabolism [116]. In ALS, lead (Pb) and selenium (Se) are demonstrated to be the common risks [117, 118]. In addition, Zn and Cu acting as cofactors for SOD1 contribute to the progression of ALS [119]. Therefore, biometal homeostasis plays an important role in CNS, while the imbalance of biometals will accelerate the development of neurodegenerative diseases.
2.7. Neurotrophic Impairment
Neurotrophins are important regulators for the survival, development, function, and plasticity of neurons [120]. In general, neurotrophic factors are grouped into three major families, including neurotrophins, glia cell-line-derived neurotrophic factor (GDNF), and neurokinins. The neurotrophins are further subdivided into nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), GDNF, neurotrophin-3 (NT-3), and neurotrophin-4. There is a growing body of evidence indicating that these neurotrophic factors inhibit cell death and improve neuronal proliferation and maturation, as well as enhance the growth and function of cholinergic and dopaminergic neurons [121, 122], while neurotrophic impairment contributes to the pathogenesis of neurodegenerative diseases [123]. Among them, BDNF, a key neurotrophic factor, regulates cell death and survival of neurons via multiple signaling pathways including c-Jun N-terminal kinase (JNK), Ras homolog gene family member (RhoA), NF-κB, mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt), and phospholipase C-γ (PLC-γ) (Figure 5). In AD, changes in the level of neurotrophic factors including BDNF, NGF, and GDNF are closely associated with the development of disease [124]. Among them, NGF is recognized as a key neurotrophic factor for the development of the cholinergic system [125]. In addition, neurotrophic factor alterations are observed in many preclinical and clinical cases of PD [126]. For example, decreased levels of BDNF in the dopaminergic area were demonstrated to be associated with the progression of PD [127]. Furthermore, GDNF, another important neurotrophic factor, is reported to play an important role in the regulation of the survival, differentiation, and maintenance of motor and dopaminergic neurons [128]. In HD, intracerebral transplantation of BDNF-overexpressing human neural stem cells promotes the migration, differentiation, and functional recovery of neurons in the unilateral QA-lesioned striatum of HD rat [129]. In addition, ciliary neurotrophic factor (CNTF) improves motor function and survival, decreases neuronal degeneration and muscle atrophy in the wobbler mouse model of ALS. In the SOD1G93A mice, tumor necrosis factor α- (TNF-α-) triggered GDNF is found to limit the degeneration of motor neurons and slow down the progression of disease [130]. Taken together, neurotrophic impairment is a key mechanism in neurodegenerative diseases, and the maintenance of normal levels of neurotrophic factors in neurons is a promising strategy for the treatment of neurodegenerative diseases.
Figure 5.
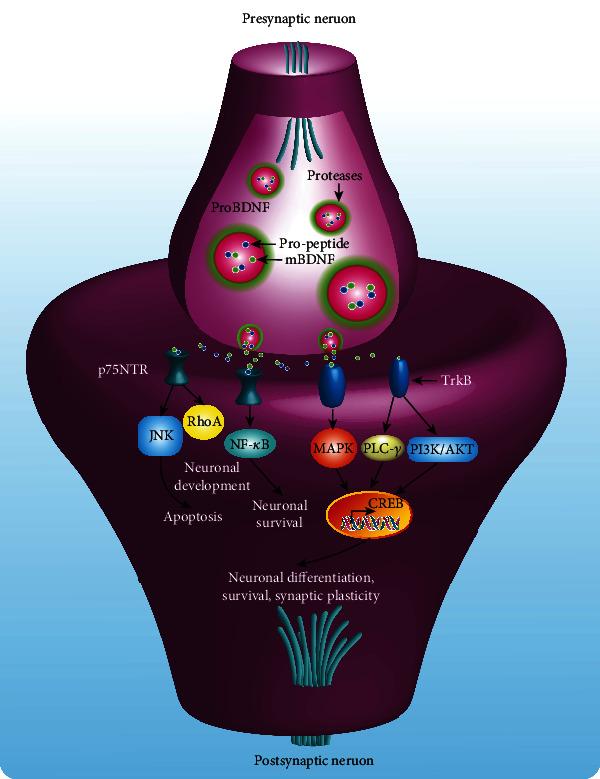
The key role of BDNF in the regulation of neuronal cell death and survival. Pre-proBDNF undergoes processing and cleavage to generate proBDNF, which is further processed to mature BDNF (mBDNF). Both proBDNF and mBDNF are stored in the proteases. ProBDNF undergoes low levels of constitutive release, while mBDNF associated with synaptic plasticity is released in an activity-dependent manner. Then, the signaling cascades are activated by the interaction of BDNF isoforms with the cell receptors located on the membrane of postsynaptic neurons, including the p75 neurotrophin receptor (p75NTR) and tropomyosin receptor kinase B (TrkB) receptor. Among them, proBDNF has a greater affinity with the p75NTR and forms the proBDNF/p75/sortilin complex, which leads to the activation of c-Jun N-terminal kinase (JNK), Ras homolog gene family member (RhoA), and nuclear factor kappa B (NF-κB) signaling pathways, subsequently induces apoptosis, neuronal growth and development, and neuronal survival, respectively. In addition, mBDNF binds with TrbB and forms the mBDNF/TrkB receptor complex, which activates the following signaling pathways, including mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt), and phospholipase C-γ (PLC-γ). Then, the transcription factor cAMP response element-binding protein (CREB) and transcription of genes are activated. Gene modulation induces neuronal differentiation, survival, and synaptic plasticity.
2.8. Neuroinflammatory Responses
Microglia, the resident immune cells in the brain, play a key role in maintaining brain homeostasis and constitute the first line of defence against brain intrusion and lesions. The chronic activation of microglia under the stimulation of DAMPs/PAMPs induces the proinflammatory response and releases multiple proinflammatory mediators, including cytokines, prostaglandins, and chemokines, which are found to be elevated in the cerebrospinal fluid (CSF) and postmortem brain tissue [131] (Figure 1). Recently, inflammasome-mediated neuroinflammation has been implicated in various neurodegenerative diseases [132]. Among them, NLRP3 is the most common and well-studied inflammasome, which is implicated in the pathological development of neurodegenerative diseases [133]. In Aβ-induced BV-2 cells and APP/PS1 mice, the NLRP3 inflammasome is activated and amounts of proinflammatory cytokines including IL-1β, IL-6, IL-18, and TNF-α are subsequently secreted, which are accompanied by the cognitive decline and memory loss of APP/PS1 mice [134]. In addition, microglia are also overactivated, and the proinflammatory responses are induced in MPTP-induced PD mice [135]. Moreover, mHtt-induced abnormal activation of microglia is found to be correlated with the severity of disease in midstate HD patients [136, 137]. The mechanistic study finds that the NF-κB signaling pathway is activated by mHtt, and the proinflammatory cytokines such as IL-6 and IL-8 are released [138]. In the TDP-43-overexpressed brain of LPS-treated mice, the microglia and astrocytes are overactivated. Meanwhile, the permeability of BBB is vulnerable under the stimulation of proinflammatory responses [139]. Therefore, neuroinflammation has been an important indicator of pathological development, which is implicated in various neurodegenerative diseases, and the discovery of drugs targeting the inhibition of neuroinflammation is useful for the treatment of neurodegenerative diseases.
3. The Potential Treatment of Dietary Plant Polyphenols for Neurodegenerative Diseases
Polyphenols are mainly from rich natural resources and are characterized by the presence of large multiples of phenol structural units. In general, most of the polyphenols are commonly found in dietary plants, such as the seed or skin of fruits (e.g., grape, litchi, rambutan, mangosteen, and pitahaya), vegetables (e.g., legumes, cereals, and cauliflower), various kinds of tea leaves, and also many medical herbs (e.g., Scutellaria baicalensis, ginkgo leaves, and Lycium barbarum) [140]. Emerging evidence indicates that polyphenols exhibit multiple bioactivities, including antioxidation, clearance of free radicals, anticancer, anti-inflammation, cardiovascular protection, brain protection, and prevention of obesity and diabetes. It is worth noting that most of the polyphenols manifest potential therapeutic effects in the in vitro and in vivo models of neurodegenerative diseases. However, the poor stability and low bioavailability largely limit their neuroprotective effects [141]. In this review, we summarized the neuroprotective effect and molecular mechanism of the most reported and representative polyphenols (Table 2) and the natural dietary plants enriching polyphenols in various neurodegenerative diseases (Table 3). Meanwhile, we also discussed the barricades and possibilities for polyphenols from bench to bedside.
Table 2.
The potential effect and molecular mechanism of the representative polyphenols in various neurodegenerative diseases.
| Polyphenols | Sources | Mechanisms | Models (dosage) | Diseases |
|---|---|---|---|---|
| Quercetin | Apples, berries, onions, and capers | Inhibition of misfolded proteins, antioxidative stress, antineuroinflammation | APP695-transfected SH-SY5Y cells (100 nM), Aβ25-35-induced PC-12 cells (80 μM), 6-OHDA-induced MN9D cells (30 μM), rotenone- and iron supplement-induced rats (50 mg/kg), MPTP-induced rats (50 mg/kg), neruo-2a cells transfected with 16Q Htt and 150 Htt (100 μM), aluminum-induced rats (10 mg/kg) | AD [145, 148–150], PD [151–154, 318], HD [155], ALS [156, 157] |
| Hesperidin | Orange and lemon | Antineuroinflammation, antioxidative stress, antiapoptosis | Aβ1-42-injected mice (50 mg/kg), Aβ1-42/LPS-induced BV-2 or HT22 cells (50 μM), H2O2-induced PC-12 cells (50 μM), 3-NP-induced rats (100 mg/kg) | AD [167, 168], PD [170], HD [171], MS [172] |
| Anthocyanins | Blueberries, cherries, raspberries, purple grapes, and blackcurrants | Inhibition of misfolded proteins, anti-neuroinflammation, and antioxidative stress | Aβ-induced HT22 cells and rats (0.2 mg/mL and 4 mg/kg), LPS-induced BV-2 cells (100 μg/mL), Aβ and α-synuclein-induced PC-12 cells (50 μM) | AD [176, 179–181, 184, 185], PD [185], ALS [186] |
| Epigallocatechin-3-gallate | Green tea | Antineuroinflammation, antioxidative stress, antiapoptosis, metal-chelating ability | LPS-induced PBMCs (40 μM), D-galactose-induced mice (2 mg/kg), Aβ-induced EOC 13.31 microglia (20 μM), APP/PS1 mice (2 mg/kg), H2O2- or Aβ-induced PC-12 cells (200 μM) | AD [191, 194, 196–198], HD [319], ALS [320] |
| Apigenin | Parsley, celery, oranges, and grape fruit | Inhibition of misfolded proteins, antineuroinflammation, antioxidative stress | APP/PS1 mouse (40 mg/kg), Aβ25-35-induced amnesic mice (20 mg/kg), rotenone-induced rats (20 mg/kg), MPTP-induced mice (20 mg/kg) | AD [204, 205], PD [200, 207] |
| Genistein | Soybeans | Inhibition of misfolded proteins, antineuroinflammation, antioxidative stress | EAE mice (300 mg/kg) | AD [210, 212, 214], PD [211], MS [215] |
| Gallic acid | Grape seed, rose flowers, sumac, oak, and witch hazel | Inhibition of misfolded proteins, antineuroinflammation, antioxidative stress | κ-CN fibril-induced PC-12 cells (100 μM), APP/PS1 mice (20 mg/kg), LPS- and Aβ-induced BV-2 and primary microglia cells (50 μM), APP/PS1 mice (20 mg/kg), 6-OHDA-induced SH-SY5Y cells (1 μg/mL), 6-OHDA-induced Wistar rats (200 mg/kg), AlCl3-induced Wistar rats (200 mg/kg), EAE mice (2 mg/mouse) | AD [219, 220], PD [223–225], ALS [226], MS [227] |
| Chlorogenic acid | Apple, cherry, tea | Inhibition of misfolded proteins, antineuroinflammation, antioxidative stress, antiapoptosis | Aβ-induced SH-SY5Y cells (50 μM), APP/PS1 mice (20 mg/kg), α-synuclein-induced PC-12 cells (100 μM), 6-OHDA-induced SH-SY5Y cells (100 μM), 6-OHDA-induced SD male rats (60 mg/kg), MPTP-induced mice (100 mg/kg) | AD [231, 232], PD [230, 233–236] |
| Hydroxytyrosol | Olive oil | Antineuroinflammation, antioxidative stress, antiapoptosis, and antimitochondrial dysfunction | 7PA2 cells (5 μM), APP/PS1 mice (5 mg/kg), Aβ25-35-treated astrocytes (5 μM), MPP(+)-induced rat PD model (1.5 mg/kg), PC-12 cells (10 μM), SHSY-5Y cells (1 μM) | AD [243, 247–249], PD [250–252] |
| Resveratrol | Grapes, raspberries, mulberries, and peanuts | Inhibition of misfolded proteins, antineuroinflammation, antioxidative stress | 3xTg-AD mice (100 mg/kg), Aβ-induced activation of microglial cells (50 nM), Aβ-induced human neural stem cells (10 μM), MPTP-induced mice (50 mg/kg), A53T α-synuclein transgenic mouse (50 mg/kg), rotenone-induced SH-SY5Y cells (50 μM), 6-OHDA-induced rats (40 mg/kg), MPTP-induced mice (10 mg/kg), YAC128 mice (1 mg/kg) and N171-82Q transgenic mice (25 mg/mouse), thimerosal-induced SH-SY5Y cells (1 μM) and VSC4.1 cells (20 μM), cuprizone-intoxicated C57Bl/6 mice (250 mg/kg), EAE and TMEV-IDD mice (250 mg/kg) | AD [259, 260, 263, 268–270], PD [271–276], HD [256, 277, 278], ALS [281], MS [282–284] |
| Schisandrin B | Schisandra chinensis | Inhibition of misfolded proteins, antineuroinflammation, antioxidative stress | Aβ1-42-induced SH-SY5Y cells (10 μg/mL), N2A/SWE cells (10 μM), Aβ-induced PC-12 cells (25 μM), APP/PS1 mice (30 mg/kg), 6-OHDA-induced rats (80 mg/kg), paraquat- or tBHP-induced PC-12 cells (15 μM), 3-NP-induced PC-12 cells (15 μM), LPS-treated primary microglia (20 μM), and ICR mice (20 mg/mL) | AD [288, 290, 292], PD [293–295], HD [296] |
| Curcumin | Curcuma longa | Inhibition of misfolded proteins, antineuroinflammation, antioxidative stress, chelating metal ions | Tg2576 mouse model of AD (500 mg/kg), APP/PS1 mice (150 mg/kg), LPS-stimulated BV-2 cells (20 μM), MPP(+)-induced SH-SY5Y cells (40 μM), ALS patients (600 mg/day) | AD [298, 299, 301, 303], PD [304, 305], HD [306, 307], ALS [308], |
| Imperatorin | Angelica dahurica, Glehnia littoralis, and Niphogeton | Antineuroinflammation, antioxidative stress | Scopolamine-induced mice (10 mg/kg), LPS-induced mice (10 mg/kg), PFHxS-induced cerebellar granule cells (0.5 μM) | AD [314] |
Table 3.
The potential effect and molecular mechanism of the representative natural dietary plants in various neurodegenerative diseases.
| Natural dietary plants | Components | Mechanisms | Models (dosage) | Diseases |
|---|---|---|---|---|
| Tea leaves | CG, ECG, and EGCG | Inhibition of misfolded proteins, antineuroinflammation, antioxidative stress, antiapoptosis | Aβ-induced PC-12 cells (150 μg/mL), glutamate-induced neuro-2a and HT22 cells (25 μg/mL), Aβ and α-synuclein aggregation in C. elegans (200 μg/mL), MPTP-induced monkeys (40 mg/kg) | AD [335, 336], PD [337] |
| Grape seed | Procyanidin, catechin, epicatechin, gallic acid, and epicatechin gallate | Antineuroinflammation, antioxidative stress, antiapoptosis | Tg2576 mice (200 mg/kg), transgenic Drosophila expressing human α-synuclein (0.64 mg/100 g of culture medium), 6-OHDA-induced rats (250 mg/kg), Q93httexon1 Drosophila R6/2 mice (100 mg/kg) | AD [327, 328], PD [329, 325], HD [326] |
| Litchi chinensis seed | Rutin, gallic acid, procyanidin B2, gallocatechin, epicatechin, epicatechin-3-gallate, catechin, procyanidin A1, and procyanidin A2 | Antineuroinflammation, antioxidative stress, antiapoptosis | Aβ25–35-induced PC-12 cells (7.60 mg/L), Aβ25–35-injected rats (480 mg/kg), STZ-induced rats (2.8 g/kg), DXM-induced HepG2 and HT22 cells (3.5 μg/mL), Aβ1-42-induced BV-2 cells (10 mg/L), SAMP8 mice (100 mg/kg) | AD [341–346] |
| Scutellaria baicalensis | Scutellarin, wogonin, baicalin, baicalein | Inhibition of misfolded proteins, antineuroinflammation, antioxidative stress, antiapoptosis | H2O2-induced PC-12 cells (baicalein: 40 μM), Aβ-induced SH-SY5Y cells (baicalein: 10 μM), rotenone-induced rats (baicalein: 100 mg/kg), MPTP-induced mice (baicalein: 560 mg/kg) | AD [356, 357, 360, 364], PD [354, 361–363] |
| Ginkgo leaves | Ginkgolic acid | Inhibition of misfolded proteins, antineuroinflammation, antioxidative stress, antiapoptosis | Aluminum-induced rats (100 mg/kg), APPswe-expressing neuro-2a cells (400 μg/mL), H2O2-induced SK-N-BE cells (25 μg/mL), chloride-induced SH-SY5Y cells (ginkgolic acid: 80 μM), Aβ1-42-induced BV-2 cells (90 μg/mL) | AD [365, 368, 369, 371], PD [366], HD [367] |
| Lycium barbarum | Tea polyphenols, caffeic acid, chlorogenic acid, ferulic acid, and anthocyanin | Inhibition of misfolded proteins, antineuroinflammation, antioxidative stress, antiapoptosis | Fibrillar Aβ1-42 and Aβ25-35 fragments induced primary rat cortical neurons (100 μg/mL), Aβ-induced neuronal cells (500 μg/mL), APP/PS1 mice (10 mg/kg), glutamate-induced PC-12 cells (200 μg/mL), H2O2-induced PC-12 cells (1000 μg/mL) | AD [376–378] |
3.1. Polyphenols
To date, there are thousands of polyphenols identified from natural dietary plants. In general, polyphenols are mainly classified into flavonoids and nonflavonoids. According to the hydroxylation mode and oxidation state, the flavonoids are subdivided into flavanols, flavanones, anthocyanins, flavonols, flavones, and isoflavones, while the nonflavonoids mainly include phenolic acids, phenolic alcohols, stilbenes, lignans, curcuminoids, and coumarins (Figure 6) [142].
Figure 6.
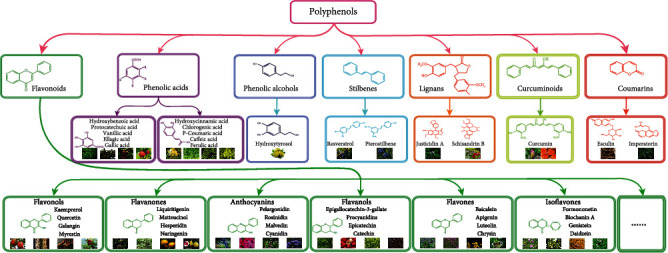
The classification of polyphenols. Polyphenols mainly include flavonoids, phenolic acids, phenolic alcohols, stilbenes, lignans, curcuminoids, and coumarins. Flavonoids are subclassified into flavanols, flavanones, anthocyanins, flavonols, flavones, isoflavones, etc. Phenolic acids are divided into hydroxybenzoic acids and hydroxycinnamic acids. The representative image of plants enriching the corresponding polyphenols.
3.1.1. Flavonoids
Flavonoids are a large group of plant polyphenolic metabolites. They are commonly found in a variety of diets, including fruits and vegetables. Structurally, most flavonoids share a 1,2-diphenylpropane or 1,3-diphenylpropane (C6-C3-C6) skeleton [143]. In general, flavonoids are classified into 12 major types according to their chemical structures. Among them, the representative compounds of flavonols, flavanones, anthocyanins, flavonols, flavones, and isoflavones are the most common and widely reported polyphenols. The representative compounds include kaempferol, quercetin, galangin, myricetin, liquiritigenin, matteucinol, hesperidin, and naringenin; pelargonidin, rosinidin, malvidin, cyanidin, procyanidins, epicatechin, and catechin; and baicalein, apigenin, luteolin, chrysin formononetin, biochanin A, genistein, and daidzein. Several studies show that these flavonoids exert a potent neuroprotective effect in various neurodegenerative diseases via antioxidant, antiapoptosis, and anti-inflammatory responses.
(1) Quercetin. Quercetin, also known as 3,3′,4′,5,7-pentahydroxyflavone, belongs to flavonols. It is widely found in fruits and vegetables, such as apples, berries, onions, and capers [144]. Therefore, quercetin is recognized to be safe and displays various biological and health-promoting effects. To date, several studies indicate that quercetin protects against neurodegenerative diseases through multiple mechanisms, such as inhibition of the aggregation of misfolded proteins [145], antioxidative stress [146], and anti-inflammatory responses [147]. In APP695-transfected SH-SY5Y cells, quercetin not only exhibits antiamyloidogenic and fibril-disaggregating effects but also reduces the cytotoxicity and oxidative stress [145]. Meanwhile, quercetin decreases the levels of lactate dehydrogenase (LDH), acetylcholinesterase (AChE), and malondialdehyde (MDA), while increasing the protein levels of SOD, GSH-Px, plasma levels of catalase (CAT), and total antioxidant capacity (T-AOC) in Aβ25-35-induced PC-12 cells via the sirtuin1/Nrf2/HO-1 pathway [148]. It is reported that beta-secretase-1 (BACE-1) plays an important role in the generation of Aβ fragments, while quercetin can inhibit the activity of the BACE-1 enzyme through the formation of hydrogen bonds with BACE-1 [149]. In triple transgenic AD (3xTg-AD) mice, quercetin significantly decreases the protein expressions of extracellular Aβ and Tau and inhibits the proinflammatory responses in the hippocampus and amygdala, which is manifested by improvements in cognitive and behavioural function. In addition, quercetin inhibits the hyperphosphorylation of Tau, oxidative stress, and apoptosis in okadaic acid- (OA-) induced PC-12 cells via the PI3K/Akt/GSK3β, MAPKs, and NF-κB signaling pathways [150]. In multiple PD toxin (e.g., 6-OHDA, MPTP, and rotenone)-induced nerve cells and animals, quercetin exerts potent neuroprotective effect [135]. For example, quercetin protects MN9D cells against 6-OHDA-induced neurotoxicity and reverses behavioural deficits, striatal dopamine depletion, and the loss of tyrosine hydroxylase (TH) neuronal cells in MitoPark transgenic mice. The mechanistic study found that the protein kinase D1- (PKD1-) Akt pathway is activated by quercetin [151]. In addition, quercetin attenuates rotenone-induced behavioural impairment and oxidative stress [152]. Most importantly, the combination of quercetin with piperine shows superior neuroprotective effects in antioxidative and anti-inflammatory in rotenone- and iron supplement-induced rats [153] and also in MPTP-induced rats [154]. In neuro-2a cells transiently transfected with 16Q huntingtin (Htt) and 150 Htt, quercetin increases cell viability and clears the mHtt aggregates via the upregulation of UPS activity [155]. In addition, quercetin binds to the SOD1 dimer, then blocks its fibrillization, and reduces the cytotoxicity of SOD1 fibrils in ALS [156, 157]. Emerging evidence indicates that the excessive accumulation of metal ions generates amounts of ROS levels and induces neurotoxicity, which favours the pathological process in various neurodegenerative diseases [158–160], while the treatment of quercetin improves the viability and inhibits the proinflammatory responses via inhibiting the production of ROS levels and its resultant apoptosis [161, 162]. Taken together, quercetin shows a potent neuroprotective effect in neurodegenerative diseases. However, its narrow therapeutic window, low bioavailability, and poor solubility limit its clinical application [163–165]. Thus, the structure and formulation modifications are required for quercetin to further increase its neuroprotective effect.
(2) Hesperidin. Hesperidin is a flavanone glycoside that exists in fruits including orange and lemon [166]. Emerging studies indicate that hesperidin possesses multiple neuroprotective activities, including the inhibition of oxidative damage [167], the suppression of neuroinflammation [168], and antiapoptosis [168]. For instance, in Aβ1-42-injected mice and Aβ1-42/LPS-induced BV-2 or HT22 cells, hesperidin exhibits potent neuroprotective effects mainly involving the inhibition of oxidative stress, antineuroinflammation, and antiapoptosis. Meanwhile, it also improves cognitive function via the Nrf2/HO-1 and TLR4/NF-κB signaling pathways [167, 168]. In addition, hesperidin inhibits H2O2-induced oxidative stress via regulating the ER and TrkA signaling pathways [169] and inhibits LPS-induced apoptosis via increasing Bcl-2 protein levels and reducing the expression of phosphorylated-c-Jun N-terminal kinases (p-JNK), Bax, and caspase-3 [168]. In the 6-OHDA-induced mouse model of PD, hesperidin reduces the degeneration of DA neurons in the substantia nigra pars compacta (SNpc) via preventing mitochondrial dysfunction and inhibiting the activity of caspase-3 and caspase-9 [170]. In addition, hesperidin attenuates iron-induced mortality, oxidative stress, and mitochondrial dysfunction and restores DA levels in the Drosophila melanogaster model of PD [170]. 3-Nitropropionic acid (3-NP), an inhibitor of succinate dehydrogenase, is commonly used to induce an animal model of HD. The treatment of hesperidin can inhibit 3-NP-induced neurotoxicity and attenuate oxidative stress, dysfunction of mitochondrial complex enzymes, and locomotor activity [171]. Furthermore, hesperidin also inhibits neuroinflammation as revealed by the increased production of IL-10 and transforming growth factor- (TGF-) β in the mouse model of MS [172]. Regarding the permeability of hesperidin through the BBB [173], hesperidin is believed to be a promising compound for the treatment of neurodegenerative diseases.
(3) Anthocyanins. Anthocyanins, a type of water-soluble flavonoid, are widely found in many coloured fruits and vegetables, including blueberries, cherries, raspberries, purple grapes, and blackcurrants [174]. Thus, anthocyanins as our daily diet are safe for the human body [175]. To date, there are many bioactive anthocyanins identified, mainly including cyanidin, malvidin, delphinidin, and pelargonidin. Anthocyanins are reported to exert a neuroprotective effect in vitro and in vivo, including the inhibition of Aβ [176], the attenuation of oxidative damage [177], and the suppression of inflammatory responses [178]. In Aβ-induced HT22 cell and rat models of AD, anthocyanins restore cell viability, increasing the MMP and the level of intracellular free Ca2+. Meanwhile, anthocyanins decrease the protein expressions of Bax, caspase-3, caspase-9, Aβ, APP, P-Tau, and BACE-1 [179]. Anthocyanins including anthocyanoside, malvidin, and malvidin-3-O-glucoside isolated from Vaccinium myrtillus are demonstrated to inhibit the formation of Aβ1-42 and Aβ1-40 fibrils in neuro-2a cells [180, 181]. Besides, anthocyanins attenuate glutamate-induced oxidative stress via increasing the levels of GSH and GSSG and stimulating the expression of endogenous Nrf2 and HO-1 [182]. At the same time, anthocyanins inhibit glutamate-induced mitochondrial depolarization and ROS generation via reducing the intracellular Ca2+ levels [183]. In amyloid-beta oligomer- (AβO-) induced HT22 cells, anthocyanins reduce neurotoxicity via regulating PI3K/Akt/Nrf2 signaling pathways [184]. In addition, anthocyanins inhibit LPS-induced expression of NO and PGE2 and suppress the production of proinflammatory cytokines including TNF-α and IL-1β in BV-2 cells via the NF-κB and Akt/MAPK signaling pathways [178]. Protocatechuic acid, a major metabolite of anthocyanin, is reported to inhibit the aggregations of Aβ and α-synuclein and ultimately recovers the cell viability of PC-12 cells [185]. In addition, protocatechuic acid also lessens the severity of pathological symptoms and slows down the progression of disease in the mouse model of ALS [186]. Moreover, the ability of anthocyanins to cross the BBB suggests that anthocyanins may be a promising drug for the treatment of neurodegenerative diseases [187]. Although studies indicate that anthocyanins possess potential therapeutic effects on certain neurodegenerative diseases, the effect of anthocyanins on more models of neurodegenerative diseases needs to be further confirmed and explored.
(4) Epigallocatechin-3-Gallate. Epigallocatechin-3-gallate (EGCG), the major component in green tea, belongs to tea polyphenols and exhibits various biological activities in the CNS [188], including antioxidative stress [189], metal-chelating ability [190], the inhibition of neuroinflammatory responses [191], and antiapoptosis [192]. In LPS-induced peripheral mononuclear blood cells (PBMCs), EGCG decreases the production of inflammatory cytokines, including TNF-α, IL-1β, and IL-6 [193]. Meanwhile, EGCG attenuates the expressions of Aβ and APP in the hippocampal neurons of D-galactose-induced AD mice [194]. Additionally, EGCG effectively remodels the structure of fibrillated amyloid proteins including α-synuclein and Aβ into nontoxic aggregations [195]. Through chelation with iron, EGCG reduces the expressions of iron-regulated APP and Aβ in Chinese hamster ovary cells, which are overexpressed with the APP “Swedish” mutation [196]. In Aβ-induced EOC 13.31 microglia, EGCG inhibits the neuroinflammatory responses by decreasing the expressions of TNFα, IL-1β, IL-6, and iNOS via negatively regulating the ROS-mediated NF-κB pathway and activating the Nrf2/HO-1 pathway [197]. Moreover, the anti-inflammatory effect of EGCG is validated in APP/PS1 mice as evidenced by the inactivation of NLRP3 and caspase-11-dependent inflammasome via the TLR4/NF-κB pathway [191]. In addition, EGCG protects PC-12 cells against H2O2- or Aβ-induced apoptosis through activating the PI3K/Akt pathway and inhibiting the GSK-3 pathway [198]. Therefore, this evidence suggests that EGCG has the potential to be developed into a new drug in the prevention and treatment of neurodegenerative diseases.
(5) Apigenin. Apigenin, known as 4′,5,7-trihydroxyflavone, belongs to the flavones and is widely found in common fruits and vegetables, such as parsley, celery, oranges, and grapefruit, particularly abundant in the chamomile plant [199]. Emerging evidence indicates that apigenin exerts a neuroprotective effect, including the inhibition of misfolded proteins [200], antineuroinflammation [201, 202], and antioxidant effects [203]. In the APP/PS1 mouse model of AD, apigenin reduces the Aβ plaque burden, inhibits oxidative stress, and improves memory impairment via the ERK/CREB/BDNF pathway [204]. In addition, apigenin is proven to improve learning and memory abilities in Aβ25-35-induced amnesic mice. Meanwhile, apigenin also reduces oxidative damage, suppresses the activity of AChE, and increases the levels of BDNF, TrkB, and phospho-CREB [205]. In chronic unpredictable mild stress- (CUMS-) induced rats, apigenin inhibits oxidative stress, upregulates PPARγ expression, and suppresses the activation of NLRP3 inflammasome and the subsequent production of IL-1β and IL-18 [206]. In addition, apigenin inhibits the aggregation of α-synuclein and increases the expression of TH and dopamine D2 receptors in the rotenone-induced rat model of PD [200]. Meanwhile, apigenin protects dopaminergic neurons against oxidative injury, inhibits microglial activation, and enhances the levels of TH and BDNF in the MPTP-induced mouse model of PD [207]. Although the present studies suggest the neuroprotective effect of apigenin in AD and PD, the bioavailability, absorption, and metabolism of apigenin in vivo remain unclear [208]. Therefore, further studies associated with its pharmacokinetic parameters are still needed to be explored, which help the development of apigenin as a new drug for the treatment of neurodegenerative diseases.
(6) Genistein. In soybeans, isoflavones are the major component, which is reported to alleviate Aβ1-42-induced impairment of learning and memory ability via regulating the RAGE/LRP-1 pathway in Wistar rats [209]. Genistein, a polyphonic compound of soy isoflavones, has been reported to exert a neuroprotective effect in various neurodegenerative diseases, such as AD and PD [210, 211]. For instance, genistein improves Aβ-triggered cognitive impairment and scavenges the free radicals in vivo [210]. Meanwhile, genistein blocks the hyperphosphorylation of Tau by reducing the intracellular Ca2+ levels and promoting its autophagic clearance [210]. The mechanistic study indicates that genistein decreases the intracellular Ca2+ levels through activating the calcium/calmodulin-dependent protein kinase IV (CAMK4) [212]. In addition, genistein inhibits ischemic oxidative damage and improves behavioural deficits via the eNOS/Nrf2/HO-1 signaling pathway [213] and also protects cerebrovascular endothelial cells against Aβ25-35-induced oxidative damage via activating the Nrf2 and PI3K pathways [214]. In 6-OHDA-induced rat models of Parkinsonism (P) and Parkinsonism+ovariectomized (OP), genistein effectively improves spatial learning and memory impairment [211]. Furthermore, the oral genistein administration also reduces the neuronal demyelination and inhibits the secretion of IFN-γ, IL-12, and TNF-α in the splenocyte and brain of the early phase of experimental allergic encephalomyelitis (EAE) mouse, a relevant model of MS [215]. Collectively, genistein as the major component in soybeans is safe and exhibits the potential component beneficial effect in neurodegenerative diseases.
3.1.2. Phenolic Acids
Phenolic acids usually refer to the phenolic compounds with a carboxylic acid group on the benzene ring. They are mainly divided into hydroxybenzoic acid and hydroxycinnamic acid. Phenolic acids usually exist in the binding form of amides, esters, or glycosides in a variety of dietary plants, such as plant seeds, fruit peels, and vegetable leaves. Numerous studies show that this type of polyphenols is potential therapeutic value in neurodegenerative diseases [216].
(1) Gallic Acid. Gallic acid, also known as 3,4,5-trihydroxy benzoic acid, belongs to hydroxybenzoic acid and is found in a variety of plants including grape seed, rose flowers, sumac, oak, and witch hazel [217]. In general, gallic acid exists in the free state of ester derivatives and polymers via the hydrolysis of terpenoids and polyphenol tannins [218]. A mounting body of researches shows that gallic acid exhibits the inhibition of misfolded proteins [219], antioxidant [219], and anti-inflammatory [220] effect in various models of neurodegenerative diseases [221]. For example, gallic acid is identified to be the most active component in grape seed extract that inhibits the formation of κ-CN fibrils and reduced the toxicity of κ-CN in PC-12 cells [222]. Meanwhile, gallic acid also inhibits the expression of Aβ protein, reduces the activity of BACE-1, inhibits neuroinflammation, and stabilizes the oxidative stress in the brain, ultimately attenuating the impaired learning and memory of APP/PS1 mice [219]. In addition, gallic acid acting as a histone acetyltransferase inhibitor decreases LPS- or Aβ-induced NF-κB acetylation and cytokine production in BV-2 and primary microglia cells and Institute of Cancer Research (ICR) mice, thereby effectively inhibiting the neuroinflammation and neuronal cell death [220]. At the same time, gallic acid severing as a free radical scavenger prevents lipid peroxidation, reduces ROS levels, and increases the expression of SOD1 and GPx1 in APP/PS1 mice and AlCl3-induced Wistar rats [219]. In 6-OHDA-induced SH-SY5Y cells, gallic acid ameliorates the disruption of MMP, reduces the level of ROS, and inhibits apoptosis or cell death through activating the TrkB/CREB/BDNF and AKT/Keap1/Nrf2 signaling pathways [223]. In vivo, gallic acid is demonstrated to counteract oxidative stress by increasing the contents of total thiol and GPx and decreasing the levels of MDA in the hippocampus and striatum tissues of 6-OHDA-induced Wistar rats [224, 225]. In the AlCl3-induced Wistar rat model of ALS, gallic acid effectively improves learning ability and motor coordination via improving the antioxidant status, preventing glutamate excitotoxicity, inhibiting caspase-3 activation, and decreasing the production of proinflammatory cytokines [226]. The molecular docking analysis and in silico analysis predicted that gallic acid is a novel agonist of aryl hydrocarbon receptor (Ahr). It can inhibit the proinflammatory responses and increase the level of transforming growth factor-β (TGF-β) in EAE mice [227]. Although a large number of studies show that gallic acid has therapeutic effects on a variety of neurodegenerative diseases through multiple pathways, further researches are required to investigate its safety and effectiveness in clinical.
(2) Chlorogenic Acid. Chlorogenic acid (CGA), known as 3,4′,5-trihydroxy-stilbene, is the most abundant isomer of caffeoylquinic acid, which belongs to hydroxycinnamic acid and is rich in the dietary fruits and vegetable [228]. Numerous studies indicate that CGA exerts a neuroprotective effect including anti-inflammatory responses [229], antioxidative stress [230], antiapoptosis [231] and the inhibition of misfolded proteins [232, 233]. In Aβ-induced SH-SY5Y cells and APP/PS1 mice, CGA can promote the activity of lysosomes and restore the autophagic flux in the brain cells, thereby improving cognitive impairments via the mTOR/TFEB signaling pathway [232]. Besides, CGA inhibits apoptosis, improves the antioxidant capacity, and inhibits mitochondrial injury in Aβ-induced hippocampal neurons [231]. In the Tet-Off system, which controls the cytotoxicity of α-synuclein, CGA significantly inhibits the oxidation of dopamine and the interaction of oxidized dopamine with α-synuclein and degrades the oligomerization of α-synuclein in PC-12 cells [233]. In addition, CGA inhibits oxidative stress and ERS by reducing the expression levels of C/EBP homologous protein (CHOP) and GRP94. Meanwhile, CGA also inhibits the apoptosis in 6-OHDA-induced SH-SY5Y cells [230]. In vivo, CGA is validated to reverse motor dysfunction via enhancing the activity of antioxidant enzymes including SOD and GSH-Px in the striatum of 6-OHDA-induced Sprague-Dawley male rats [230]. Furthermore, CGA alleviates the MPTP-induced PD symptoms of mice through the anti-inflammatory and antioxidant mechanisms, which mainly involves the increased activity of SOD and CAT, decreased release of TNF-α, IL-1β, and NO, and the increased secretion of IL-10 via the NF-κB signaling pathway [234]. It is reported that mitochondrial-mediated apoptotic senescence of DA neurons is implicated in MPTP-intoxicated PD mouse, while the treatment of CGA can inhibit the ratio of Bax/Bcl-2 and caspase-3 activation, which is associated with the downregulation of GSK3β via activating the Akt/ERK signaling pathway [235, 236]. Taken together, numerous studies are suggesting that CGA exhibits considerable protective effects in various neurodegenerative diseases. However, further efforts such as the modification of the formulation and the improvement of stability are required to push forward its clinical use.
3.1.3. Hydroxytyrosol
At present, 30 different phenolic compounds, including oleacein, tyrosol and hydroxytyrosol, were identified from olive oil. Olive oil is the most important resource in the Mediterranean region, which has been associated with many health benefits [237–239]. The pharmacological studies show that olive oil phenols exhibit neuroprotective effects in various neurodegenerative diseases such as AD [240], PD [241], and HD [242].
Among these polyphenols, hydroxytyrosol belonging to phenolic alcohol is also found in diverse vegetable species and exerts powerful antioxidant and anti-inflammatory effect [243, 244]. Most importantly, hydroxytyrosol is able to pass through the BBB [245]. As far as we know, mitochondrial dysfunction is one of the key cellular hallmarks of neurodegenerative diseases [246]. In the 7PA2 cell cellular model simulating Aβ toxicity of AD, hydroxytyrosol can restore the energy deficiency to maintain mitochondrial function [247]. Meanwhile, hydroxytyrosol ameliorates the neuronal impairment in APP/PS1 mice via modulating mitochondrial oxidative stress, neuroinflammation, and apoptosis [248]. In addition, the treatment of hydroxytyrosol increases the cell viability in Aβ25-35-treated astrocytes via improving insulin sensitivity and restoring insulin signal transduction [249]. In 1-methyl-4-phenylpyridinium (MPP(+))-induced rat model of PD, hydroxytyrosol and its derivatives decrease lipid fluorescence products (LFP) and increase striatal dopamine levels and brain GSH/GSSG ratio, as well as inhibit the monoamine oxidase (MAO) isoforms and prevent neurotoxicity [250, 251]. In addition, hydroxytyrosol is demonstrated to inhibit the enzymatic and spontaneous oxidation of endogenous dopamine in PC-12 cells with MAO inhibition [252]. Moreover, it has been shown that the combination of hydroxytyrosol with donepezil which forms a novel hydroxytyrosol-donepezil hybrid has potential neuroprotective effect compared to drug alone [243]. In summary, the neuroprotective effects of olive oil phenols such as hydroxytyrosol have been determined, but the mechanisms behind these effects need to be further elucidated.
3.1.4. Resveratrol
Stilbenes belong to natural polyphenols in which two phenyl parts are connected by the methylene of two carbon. Structurally, stilbenes are characterized by the replacement of two benzene rings with hydroxyl and methoxy groups. In general, stilbenes are not as common as other polyphenols, which exist in some plants in the form of glycosylation [142].
Resveratrol, known as 3,4,5-trihydroxystilbene, belongs to stilbenes, which is widely investigated and found to be abundant in dietary plants, including grapes, raspberries, mulberries, and peanuts [253]. Increasing studies suggest that resveratrol exerts antiageing and neuroprotective effects [254–257]. At present, the phase II clinical trials of resveratrol for AD patients are ongoing [258]. In 3xTg-AD mice, resveratrol improves memory loss and brain pathology as evidenced by the reduced protein expressions of Aβ and P-Tau in the hippocampus. The mechanism mainly involves the enhancement of proteostasis, the increased level of amyloid-degrading enzyme neprilysin, the reduced activity of BACE-1, and the increased activity of proteasome [259]. In addition to the degradation via the proteasome, the production and aggregation of Aβ are also reduced by resveratrol via direct binding to Aβ peptide [260] and autophagy induction [261]. Moreover, resveratrol promotes the insulin-degrading enzyme- (IDE-) dependent degradation of Aβ42 monomer and its fragments [262]. In addition, the upregulation of SIRT1 and downregulation of CD147 by resveratrol are closely associated with the abrogation of hypoxia-induced upregulation of exosomal Aβ [263, 264]. In intracerebroventricularly injected Aβ mice and Aβ-induced activation of microglia, resveratrol significantly inhibits the activation of NLRP3 inflammasome and reduces the release of proinflammatory cytokines, including IL-6, TNF-α, and IL-1β, which ultimately alleviates the learning and cognitive decline of mice [265–267]. In addition, resveratrol exerts antioxidative effects via decreasing the intracellular levels of MDA and ROS and correspondingly increasing the levels of SOD and GSH in Aβ1-42-induced PC-12 cells, which is correlated with the upregulation of HO-1 expression through activating the PI3K/AKT/Nrf2 signaling pathway [268]. Furthermore, the upregulation of adenosine monophosphate-activated protein kinase (AMPK) and SIRT1 is associated with the antineuroinflammation and antioxidative stress effect of resveratrol in Aβ-induced human neural stem cells [269, 270]. In MPTP-induced PD mouse and A53T α-synuclein transgenic mouse, resveratrol inhibits the expression of α-synuclein via upregulating the level of MicroRNA-214, thereby improving the motor dysfunction [271–273]. In addition, resveratrol inhibits rotenone-induced apoptosis in SH-SY5Y cells and promotes the degradation of α-synuclein via the AMPK/SIRT1-mediated autophagy induction in PC-12 cells overexpressing α-synuclein [274]. In vivo, resveratrol attenuates oxidative damage and dopamine depletion in 6-OHDA-induced PD rats [275]. Most importantly, the combinational use of resveratrol with L-Dopa alleviates the loss of dopaminergic neurons, attenuates the activation of astrocytes, and inhibits the protein levels of Bax and caspase-3 in MPTP-induced PD mice, which is more superior than resveratrol or L-Dopa alone [276]. In multiple models of HD, including the PC-12/HttQ103 cell line, Drosophila expressing mutant Httex1, and the R6/2 mice, resveratrol increases the survival of PC-12 cells and prolongs the lifespan of flies and R6/2 mice. Meanwhile, resveratrol alleviates the pathology of Drosophila and R6/2 mice via activating the ERK signaling pathway [277]. In addition, resveratrol protects the normal function of mitochondrial and improves the motor coordination and learning ability in YAC128 mice and N171-82Q transgenic mice through the AMPK, SIRT1, and peroxlsome proliferator-activated receptor-γ coactlvator-1α (PGC-1α) pathways [256, 278]. In thimerosal-induced SH-SY5Y and VSC4.1 cells overexpressing mutant SOD1-G93A, resveratrol increases the cell viability via the upregulation of SIRT1 [279, 280]. At the same time, resveratrol can prolong the lifespan of SOD1-G93A ALS mice [281]. In addition, resveratrol attenuates neuronal damage and promotes myelin regeneration via enhancing Olig1 and SIRT1 expression in cuprizone-intoxicated C57Bl/6 mice and EAE mice [282, 283]. However, another study reports that resveratrol significantly exacerbates demyelination and inflammation without neuroprotection in the EAE and Theiler's murine encephalomyelitis virus-induced demyelinating disease (TMEV-IDD) models of MS [284]. Therefore, resveratrol exhibits a potent neuroprotective effect in various neurodegenerative diseases. However, further studies about how to increase its safety and bioavailability are also required before it implements clinical trials.
3.1.5. Schisandrin B
The lignans are formed by oxidative dimerization of two or more phenylpropane units [285]. Lignans are usually found in a wide variety of plant-based food, including grains, vegetables, and fruits in the form of aglycone, ester, or glycoside [286]. A large number of studies show that lignans such as Schisandrin B, justicidin A, and matairesinol have anti-inflammatory, antioxidant, and neuroprotective effects [36, 287]. Among them, Schisandrin B is one of the most abundant lignans presenting in the traditional Chinese medical herb Schisandra chinensis (SC) belonging to the medicine food homology (MFH) species. The modern pharmacological studies demonstrate that Schisandrin B exerts protective effects on neurodegenerative diseases through multiple pathways, including the inhibition of misfolded proteins [288], antioxidative stress [289], and the inhibition of inflammatory responses [287]. For instance, Schisandrin B protects SH-SY5Y cells against Aβ1-42-induced injury via increasing the mRNA and protein expressions of DNA methylation (DNMT), including DNMT3A and DNMT3B [290]. Besides, Schisandrin B significantly reduces the secretion of Aβ levels in N2A/SWE cells by inhibiting the transcription and translation of BACE-1 [288]. Meanwhile, Schisandrin B also antagonizes Aβ-mediated cell damage by decreasing the expression of vacuolar sorting 35 and APP in PC-12 cells [291]. GSK-3β is a key enzyme that is responsible for the hyperphosphorylation of Tau protein. It is reported that Schisandrin B increases the expression of p-GSK-3β (Ser9) but decreases the expressions of p-GSK-3β (Tyr216) and p-GSK-3β (Tyr279) and ultimately inhibits the activity of GSK-3β and the protein expression of Tau in APP/PS1 mice [292]. In the 6-OHDA-induced rat model of PD, Schisandrin B downregulates miR-34a expression and activates the Nrf2 pathway to reduce neuronal damage [293]. In addition, Schisandrin B protects differentiated PC-12 cells against paraquat- or tert-butylhydroperoxide- (tBHP-) induced oxidant injury via enhancing GSH redox cycling and cellular GSH levels [294, 295]. In 3-NP-induced PC-12 cells, Schisandrin B inhibits the ratio of necrotic and apoptotic cells through enhancing cellular glutathione redox status and ameliorating the cellular energy crisis, which is regulated by suppressing the JNK-mediated activation of pyruvate dehydrogenase (PDH) [296]. In addition, Schisandrin B alleviates neuroinflammatory responses as demonstrated by the decreased levels of proinflammatory cytokines, including NO, TNF-α, PGE2, IL-1β, and IL-6, in LPS-treated primary microglia and ICR mice via the TLR4-dependent MyD88/IKK/NF-κB signaling pathway [287]. Taken together, Schisandrin B offers a promising therapeutic prospect in neurodegenerative diseases.
3.1.6. Curcumin
Curcumin (1,7-bis[4-hydroxy-3-methoxyphenyl]-1,6-heptadiene-3,5-dione), also known as diferuloylmethane, is the major active ingredient of turmeric derived from the rhizome of Curcuma longa [297]. Accumulating lines of literatures show that curcumin possesses various neuroprotective effects, such as inhibiting the aggregation of misfolded proteins [298], chelating metal ions [299], antioxidation [300], and attenuating neuroinflammation [301]. In an early study, curcumin is demonstrated to effectively inhibit the formation of Aβ oligomers and fibrils. Meanwhile, curcumin binds plaques and reduces Aβ in the Tg2576 mouse model of AD [298]. In addition, curcumin inhibits the oligomerization of Tau and disintegrated preformed Tau filaments in vitro [302]. In the APP/PS1 transgenic mouse model of AD and LPS-stimulated BV-2 microglia, curcumin attenuates Aβ-induced neuroinflammation as evidenced by the inactivation of microglia and astrocytes and reduced production of proinflammatory cytokines via activating the activity of PPARγ and inhibiting the NF-κB signaling pathway [301, 303]. In addition, curcumin functionated as a metal chelator interacts with copper or iron to inhibit metal-induced Aβ aggregation and toxicity, as well as inhibits the inflammatory responses [299]. In PD, curcumin binds to α-synuclein oligomers and fibrils to decrease the cytotoxicity in SH-SY5Y cells [304]. In the MPP(+)-induced SH-SY5Y cell model of PD, curcumin increases cell viability as evidenced by the improvement of cell morphology. Meanwhile, curcumin also promotes cell proliferation and inhibits apoptosis via the upregulation of HSP90 protein [305]. In HD, curcumin prevents the formation of htt72Q-GFP (a Q-rich) and Het-s-GFP (a non Q-rich) aggregates in yeast, which is closely associated with the downregulation of Vps36, a component of the endosomal sorting complex required for transport (ESCRT-II) [306]. In addition, curcumin encapsulated solid lipid nanoparticles (C-SLNs) rescue mitochondrial swelling and reduce the levels of lipid peroxidation and ROS in 3-NP-treated rats [307]. In a clinical trial, ALS patients who were treated with curcumin showed a slight slowdown in disease progression and an improvement of aerobic metabolism and oxidative damage [308]. Furthermore, curcumin shows potent neuroprotective effects in antioxidants and inhibition of NLRP3 inflammasome activation [309, 310]. The formulation modification of curcumin using nanotechnology effectively is a promising strategy for the penetration of curcumin through BBB [311]. Taken together, the current evidence suggests that curcumin is a promising polyphenol for the treatment of neurodegenerative diseases.
3.1.7. Imperatorin
Imperatorin, known as [9-(3-methyl but-2-acyloxy)-7H-furo[3,2-g]chromen-7-one] belongs to natural coumarins, which is widely found in the plants, including Angelica dahurica, Glehnia littoralis, and Niphogeton [312]. At present, only a few studies show that imperatorin and other coumarins possess neuroprotective effects, including antineuroinflammation and antioxidative damage [313]. In the scopolamine-induced mouse model of AD, imperatorin significantly reverses the memory impairment and reduces oxidative damage as evidenced by an increase in the activity of antioxidant enzymes (e.g., SOD, GPx, and glutathione reductase (GR)) and a decrease in the level of MDA [314]. In addition, imperatorin improves memory impairment via decreasing the levels of AChE, TNF-α, and IL-6 and upregulating the level of BDNF in the brain of LPS-induced mice [313]. Meanwhile, the imperatorin analogues show selective inhibition toward butyrylcholinesterase (BuChE) rather than AChE [315]. The in silico analysis using Autodock 4.2, Pre-ADMET, and molinspiration tools predicts that imperatorin is a potent inhibitor of COX-1, HO-1, and LOX-1. It exhibits low toxicity and a better ability to cross BBB [316]. Perfluorohexanesulfonate (PFHxS), one of the major perfluoroalkyl compounds, is widely distributed in environmental contaminants and reported to induce neuronal apoptosis, while the treatment of imperatorin effectively inhibits PFHxS-induced apoptosis of cerebellar granule cells (CGC) via the inhibition of NMDA receptor/intracellular calcium-mediated ERK pathway, suggesting that imperatorin may be a promising therapeutic candidate for the treatment of neurological disorders associated with neuroexcitotoxic damage [317]. However, the current studies on the neuroprotective effect of imperatorin are limited; thus, more in vivo and in vitro data and evidence are needed to confirm its neuroprotective effect and elucidate the molecular mechanism of imperatorin in neurodegenerative diseases.
3.2. Natural Materials Enriching Polyphenols
3.2.1. Grape Seed
Grape (Putao in Chinese) is a kind of fruit that can be used for making wine, jam, grape juice, and jelly, while the grape seed is extracted and developed into various health products. Several studies suggest that the grape seed is rich in polyphenols mainly including procyanidin, catechin, epicatechin, gallic acid, and epicatechin gallate [321–323]. Therefore, the grape seed extract is widely reported to exhibit antioxidative, antiapoptosis, and anti-inflammatory effects in various neurodegenerative diseases [324–326]. For instance, grape seed-derived polyphenolics inhibit the production and accumulation of Aβ and reduce the level of Aβ in vitro and in vivo [327, 328]. In a transgenic Drosophila expressing human α-synuclein, grape seed extract scavenges oxygen free radicals and reduces the level of ROS and the production of toxic secondary products, ultimately slowing down the damage of mitochondria [329]. In addition, grape seed-derived polyphenols inhibit neuroinflammation via the NF-κB signaling pathway in 6-OHDA-induced rats and also reduce the apoptosis of midbrain dopaminergic neurons by inhibiting the activity of caspase-3 [325]. In the Q93httexon1 Drosophila model of HD, the grape seed polyphenolic extract significantly improves the lifespan of drosophila, which is further confirmed in the R6/2 mice [326]. Therefore, the grape is not only a delicious fruit but also exhibits a potent neuroprotective effect.
3.2.2. Tea Leaves
In most countries, especially in China and Japan, tea drinking is very popular and has become a local culture. In general, most of the tea is obtained from the leaves of different spices of Camellia sinensis. To date, the detailed ingredients in tea leaves have been well-elucidated and most of them are tea polyphenols, known as catechins, which account for almost 30-42% of dry tea leaves [330]. The pharmacological studies show that the total extract of tea leaves is reported to have a potent neuroprotective effect in various neurodegenerative diseases [331, 332]. For example, green tea catechin inhibits the activity of β- and γ-secretase to reduce the generation of Aβ [333]. In addition, thirty-three phenolic compounds are identified from the extract of fermented tea, and the anti-Aβ aggregation and antiapoptotic effects of three tea polyphenols, including (-)-catechin gallate (CG), (-)-epicatechin gallate (ECG), and EGCG, are confirmed [334]. In addition to green tea, other types of tea including white tea, oolong tea, and black tea also significantly inhibit the formation of Aβ aggregates and protect PC-12 cells against Aβ-induced neurotoxicity [335]. In addition, the oolong tea extract can reduce the intracellular ROS levels and increase the gene expressions of GPx, GSTs, SODs, and GAP-43, as well as improve the average neurite length in neuro-2a cells. Meanwhile, the oolong tea extract is validated to inhibit Aβ-induced paralysis, chemotaxis deficiency, and α-synuclein aggregation in C. elegans [336]. In MPTP-induced PD monkeys, green tea polyphenols alleviate motor impairment and dopaminergic neuronal injury [337]. Taken together, tea leaves as the raw materials of daily drinking are beneficial for our health and exhibit a potent neuroprotective effect in various neurodegenerative diseases; thus, tea drinking is recognized as a good habit for people to prevent neurodegenerative diseases.
3.2.3. Litchi chinensis Seed
Litchi chinensis (Lizhi in Chinese), commonly known as lychee, belongs to a subtropical fruit. It is popular for its nutritional value and taste. In China, the seed of Litchi chinensis as TCMs is prescribed in many formulas for a long time owing to its great medicinal value [338]. The chemical studies show that the components in the seed of Litchi chinensis mainly are polyphenols, such as rutin, gallic acid, procyanidin B2, gallocatechin, epicatechin, and epicatechin-3-gallate [339]. In our research group, a purified active fraction named lychee seed fraction enriching polyphenol (LSP) is obtained from lychee seed and its neuroprotective effect is investigated in multiple models of AD [340]. For example, LSP inhibits neuronal apoptosis and improves cognitive function in PC-12 cells [341] and Aβ25–35-injected rats [342, 343]. In addition, LSP effectively reduces the levels of glucose, insulin, Aβ, advanced glycation end products (AGEs), and Tau in a streptozotocin- (STZ-) induced rat model of type II diabetes mellitus (T2DM) [344]. In dexamethasone- (DXM-) induced HepG2 and HT22 cells, LSP significantly improves insulin resistance (IR) and inhibits Tau proteins via the IRS-1/PI3K/Akt/GSK-3β pathway. Meanwhile, polyphenols including catechin, procyanidin A1, and procyanidin A2 are identified to be the bioactive components [345]. Furthermore, LSP inhibits neuroinflammation via the NF-κB pathway in Aβ1-42-induced BV-2 cells [346], and catechin and proanthocyanidins A2 are identified to be the active components [338]. As is known to us, NLRP3 inflammasome-mediated inflammation plays an important role in neurodegenerative diseases, while autophagy negatively regulates the activation of NLRP3 inflammasome. Our recent studies demonstrate that LSP inhibits Aβ1-42-induced activation of NLRP3 inflammasome via autophagy induction in vitro and in vivo [340, 347]. Endoplasmic reticulum stress (ERS) is related to protein misfolding and contributes to the development of neurodegenerative diseases [348]. Oligomerized lychee fruit-derived polyphenol (OLFP) is reported to reduce the ERS in nerve cells via upregulating the mRNA expression of Wolfram syndrome-1 (Wfs1) in SAMP8 mice [349]. Although Litchi chinensis seed exhibits a potential therapeutic effect in AD, its effects on other neurodegenerative diseases are still unknown and need to be further investigated in the future.
3.2.4. Scutellaria baicalensis
Scutellaria baicalensis (Huangqin in Chinese) belonging to the Lamiaceae is widely used in China medicine hospitals. The bioactive products of Scutellaria baicalensis are mainly flavonoids, including scutellarin, wogonin, baicalin, and baicalein. The pharmacological effects of Scutellaria baicalensis including anti-inflammation [350], antioxidation [351], and neuroprotection [352] are widely reported. Meanwhile, numerous studies show that Scutellaria baicalensis extract or its derived components exhibit potent neuroprotective effects in various neurodegenerative diseases [353–355]. For instance, baicalein inhibits the aggregation of Aβ and a-synuclein, as well as slows down aggregated fibre-induced neurotoxicity [356]. The thioflavin T (ThT) assay indicates that baicalein promotes the degradation of Aβ. Meanwhile, simulation and docking studies reveal that baicalein inhibits Tau aggregation through covalent modification [357]. In addition, baicalein slows down H2O2-induced apoptosis and maintains normal mitochondrial function via regulating the expression of Bcl-2 and Bax in PC-12 cells [358]. Baicalein also attenuates ERS-induced neuronal injury via reducing the expression of CHOP, glucose-regulated protein 78 (GRP78), the cleavage of X-box binding protein-1 (XBP1) and activating transcription factor 6α (ATF6) and phosphorylation of eukaryotic initiation factor-2α (eIF2) and MAPK pathways [359]. Baicalin, another flavonoid in Scutellaria baicalensis, reacts with copper directly and inhibits the Aβ aggregation and oxidative stress in SH-SY5Y cells [360]. In addition, baicalin inhibits neurotoxicity via HO-1-mediated autophagy induction in the rotenone-induced rat model of PD [361]. In addition to the inhibition of neuronal death, the component in Scutellaria baicalensis also inhibits the overactivation of microglia and the production of proinflammatory cytokines [362]. For example, baicalein inhibits neuroinflammation by negatively regulating the NLRP3/caspase-1/GSDMD pathway in MPTP-induced mice and inhibiting the NF-κB and MAPK signaling pathways in rotenone-induced rats [354, 363]. Based on the above evidence, we employed UHPLC-DAD-TOF/MS analysis after a preincubation of Scutellaria baicalensis extract with Aβ1-42 to identify the potential inhibitors of Aβ fibrillization. Finally, baicalein and baicalin are found to have the highest binding affinity with Aβ, suggesting that baicalein and baicalin are the strongest inhibitors of Aβ fibrillization in Scutellaria baicalensis [364]. Taken together, the above evidence suggests that Scutellaria baicalensis and its derived flavonoids exert a potent neuroprotective effect.
3.2.5. Ginkgo Leaves
Ginkgo biloba, commonly known as the maidenhair tree, is one of the oldest living tree species. The dried green leaf of Ginkgo biloba is a popular supplement and is commonly used in the treatment of early-stage AD [365], PD [366], and HD [367]. In the aluminum-induced rat model of AD, ginkgo leaves derived polyphenols reduce the accumulation of Aβ and improve the symptoms of AD rats via the upregulation of heat shock proteins (HSPs) [368]. In addition, ginkgo leaf extract exerts antioxidative effects via reducing the levels of ROS and RNS and increasing the contents of total superoxide dismutase (T-SOD), CAT, and GSH-Px in APPswe-expressing neuro-2a cells [369]. At the same time, the ginkgo leaf extract also inhibits H2O2-induced apoptosis via blocking the p53 pathway and reducing Bax/Bcl-2 ratio in SK-N-BE neuroblastoma cells [370]. Ginkgolic acid, a polyphenic compound, is reported to activate autophagy and clear α-synuclein aggregates in potassium chloride-induced SH-SY5Y cells [366]. In addition, ginkgo leaf extract can degrade poly-Q protein by increasing the activity of the proteasome via the Keap1/Nrf2 pathway [367]. Furthermore, emerging studies show that ginkgo leaf extract inhibits Aβ1-42-induced neuroinflammatory responses via the P38 MAPK pathway in BV-2 microglial cells [371]. Therefore, ginkgo leaf polyphenols are demonstrated to be safe and have medical value in the prevention and treatment of neurodegenerative diseases. At present, ginkgo leaf extract has been developed into a top-selling herbal supplement.
3.2.6. Lycium Fruits
Lycium fruits from the plant Lycium barbarum (Gouqi in Chinese) are commonly been used as traditional medicine and food supplement in China for a long history. It is a traditional homology of medicine and food in Chinese medicine. The chemical studies show that Lycium fruits are rich in polysaccharides, tea polyphenols, caffeic acid, chlorogenic acid, ferulic acid, and anthocyanin [372, 373]. Although numerous studies show that polysaccharides exert potent neuroprotective effects, there are still many reports about the polyphenols and extract of Lycium fruits in various neurodegenerative diseases [374, 375]. In fibrillar Aβ1-42 or Aβ25-35 fragment induced primary rat cortical neurons, pretreatment with Lycium barbarum extract inhibits the release of LDH and the activity of caspase-3 via the JNK pathway [376]. In addition, the pretreatment of the alkaline extract of Lycium barbarum attenuates Aβ-induced apoptosis and neuronal cell death via activating the AKT pathway [377]. In vivo, Lycium barbarum extract significantly reduces the level of Aβ1–42 in hippocampal tissue and improves the learning and memory ability of APP/PS1 mice [378]. In glutamate-induced PC-12 cells, Lycium barbarum extract markedly increases cell viability and decreases the release of LDH, Ca2+ overload, ROS generation, and cell apoptosis [379]. In addition, Lycium fruit polyphenols also inhibit the level of intracellular ROS and decrease the expression of caspase-3/-8/-9 in H2O2-induced PC-12 cells [380]. Furthermore, Lycium barbarum extract significantly attenuates the intracellular ROS accumulation and MMP loss and increases the total levels of GSH in MPP(+)-induced PC-12 cells [381]. Therefore, both polysaccharides and polyphenols are two kinds of components in Lycium barbarum contributing to neuroprotection in various neurodegenerative diseases.
4. Indirect Beneficial Effect of Plant Polyphenols on Neurodegenerative Diseases
BBB is the barrier between plasma and brain cells formed by the walls of brain capillaries and glial cells and the barrier between plasma and CSF formed by the choroid plexus [382]. Extensive tight junctions are essential to maintain the integrity of the BBB, making it difficult for macromolecules and nonlipid soluble molecules to pass through [383]. Small molecules and fat-soluble substances can cross the BBB by passive diffusion and selective active transport, such as various nutrients, water, ions, organic anions, amino acids, and macromolecules (glucose) [384]. At the same time, BBB prevents the invasion of microorganisms and toxins in circulating blood to damage brain tissues. Therefore, the BBB has important biological significance for maintaining the basic stability of the internal environment of brain tissues and the normal physiological state of the CNS [385]. However, the presence of the BBB severely prevents most drugs such as many polyphenols from entering the brain to exert their effects [386].
Although a large number of studies have shown that some plant polyphenols can cross the BBB and reach the brain to exert a neuroprotective effect, there are still many polyphenols reported having indirect beneficial effects in neurodegenerative diseases [387–389]. There is growing evidence of stronger two-way communication between the gut and the brain through the neural, endocrine, and immune systems, called the brain-gut axis [390]. The gut microbiota refers to the multiple microorganisms that have coevolved in the human gut, such as symbiotic bacteria, viruses, fungi, and protozoa, which maintain homeostasis in the host by regulating digestion, immunity, metabolism, and various neurological functions. Recent studies have shown a tight association between dysbiosis of the intestinal flora and several neurodegenerative diseases, such as AD and PD [391]. Therefore, targeting regulation of the intestinal microbiota is an important strategy for the treatment of neurodegenerative diseases [389]. For example, it has been found that curcumin plays a neuroprotective role by affecting intestinal microorganisms [392]. Specifically, curcumin improves the cognition of APP/PS1 mice via altering the abundance of key bacterial species associated with AD, including Prevotella and Bacteroides [392–395]. Meanwhile, the intestinal microorganisms produce active metabolites such as demethylcurcumin and bisdemethoxycurcumin via transforming curcumin, which indirectly enhances the neuroprotective effect of curcumin [396, 397]. In addition, reduced cerebral blood flow is one of the common early features of AD, and the strict control of cardiovascular risk factors can reduce the risk of developing dementia. Therefore, the regulation of cerebral perfusion is recognized as another indirect pathway that is crucial to regulating brain function [389]. Clinical trials have shown that polyphenols are associated with enhanced cerebral blood flow and cerebral oxygenation, thus exerting neuroprotective effects [389, 398]. For example, cocoa flavonoids and curcumin are reported to increase blood flow to the cerebral cortex, thus improving cognition [399–402]. Furthermore, metabolic disorders are associated with neurodegenerative diseases, and the improvement of metabolism is also an indirect way for the treatment of neurodegenerative diseases [389]. It has been demonstrated that lychee seed polyphenols and cocoa flavonoids have therapeutic potential for AD by improving insulin resistance [345, 399]. Taken together, these indirect protective effects of plant polyphenols on neurodegenerative diseases should not be ignored.
5. Clinical Study and Application of Plant Polyphenols
To date, some clinical studies have been conducted and confirmed the neuroprotective actions of plant polyphenols, such as the ability to suppress misfolded protein accumulation and neuroinflammation, the ability to protect neurons from neurotoxin damage, and the potential to promote memory, cognition, and other brain functions. For instance, resveratrol intake (200 mg/d) for 26 weeks significantly improved memory, glucose metabolism, and functional connectivity of the hippocampus in older adults compared with the placebo treatment [403]. Turner et al. conducted a randomized, double-blind, placebo-controlled trial of resveratrol and found that resveratrol with high dose is safe and well tolerated in individuals with mild-to-moderate AD [404]. In addition, the cosupplementation of piperine with resveratrol can improve the bioavailability and efficacy of resveratrol in cognition and cerebral blood flow (CBF). Meanwhile, resveratrol also can decrease CSF MMP9, modulate neuroinflammation, and induce adaptive immunity [405]. Moreover, resveratrol attenuated the decline in minimental status examination (MMSE) scores and progressive decline in CSF Aβ40 levels, as well as the activity of daily living (ADL) scores, but did not alter the Tau level [406]. Curcumin, another potent neuroprotective agent, can cross the BBB due to its lipophilicity. Baum et al. imposed a double-blind, placebo-controlled clinical trial on Chinese patients (n = 34) who presented a decline in memory and cognitive function. After the treatment of curcumin (1 g or 4 g daily) for six months, the patients exhibited almost no significant improvement in cognitive function as compared to the placebo treatment [407]. This study suggested that the low bioavailability of curcumin is the biggest issue for its use in the treatment of neurodegenerative diseases. In addition, curcumin may be helpful for the early diagnosis of AD. Cheng et al. found that magnetic nanoparticles (MNPs) made of superparamagnetic iron oxide (SPIO) conjugated with curcumin had the potential for noninvasive diagnosis of AD using magnetic resonance imaging (MRI) [408]. Moreover, a recent study examined the effect of curcumin on cognition and mood in 60 healthy adults aged 60-85. The results showed that working memory and mood were significantly improved after 4 weeks of treatment with curcumin, confirming the potential psychological and cognitive benefits of curcumin in older populations [409]. Emerging evidence indicates that EGCG can cross the BBB to increase the memory and learning ability of the ageing brain, as well as inhibit cognitive dysfunction and reduce oxidative damage [410, 411]. De la Torre et al. demonstrated that EGCG significantly reversed the cognitive deficits of patients with Down syndrome (DS) and improved memory recognition, working memory, and quality of life [412]. A double-blind, placebo-controlled, crossover investigation demonstrated that a single dose of orally administered EGCG could modulate localized CBF parameters which are not associated with changes in cognitive performance or mood in healthy humans [413]. Similarly, another double-blind, placebo-controlled crossover study found that EGCG significantly increased the overall electroencephalography (EEG) activity in different brain regions and self-rated calmness but reduced self-rated stress compared to the placebo group, suggesting that patients who supplemented with EGCG exhibited a more relaxed and attentive state [414].
Researches indicate that the chronic consumption of flavonoids is associated with cognitive improvement. For instance, Kean et al. imposed a randomized, double-blind, placebo-controlled trial in healthy older adults and found that the chronic daily consumption of flavanone-rich 100% orange juice over 8 weeks is beneficial for cognitive function in healthy older adults [415]. In addition, the consumption of hesperidin-rich orange juice could enhance objective and subjective cognition throughout 6 h in healthy middle-aged adults [416]. Moreover, Lamport et al. demonstrated that consumption of flavanone-rich citrus juice in quantities enhanced CBF in 44 healthy young adults (18–30) in an acute, randomized, single-blind, placebo-controlled, clinical study [417].
To sum up, the current clinical trials confirm the efficacy of plant dietary polyphenols in neurodegenerative diseases. However, the current evidence is still very scarce. Several reasons such as low bioavailability and poor study design may be responsible for the discrepancy between the preclinical experiment and clinical trial. Therefore, increasing attention was paid to improving the bioavailability of plant polyphenols before the clinical trials.
6. Bioavailability of Plant Polyphenols
A large number of studies have found that the bioavailability of plant polyphenols is relatively poor, which greatly limits their efficacy [418, 419]. For example, the bioavailability of curcumin in the brain after oral administration is very low, and the systemic available dose is less than 1% of the administered dose [419]. EGCG absorption from decaffeinated green tea administered orally is only about 0.1%–0.15% in rats and about 12–26% in mice [420]. In addition, the BBB, a physical barrier that regulates the entry of substances into the brain and ensures homeostasis in the body, is the biggest barrier for drugs entering the brain [421]. Thus, plant polyphenols are administered at doses much higher than the effective circulating concentrations in the body [420, 422]. Emerging evidence indicates that polyphenols are metabolized in the body, and the metabolites take effect actually in most cases [423]. Therefore, bioavailability emerged as an unavoidable challenge for the application and development of plant polyphenols. The absorption of plant polyphenols is mainly related to the physicochemical properties of drugs, the route of administration, the absorption environment, and so on [424, 425]. For example, the insufficient water solubility of curcumin leads to low absorption and ultimately low bioavailability [426]. Thus, nanotechnology is used to improve the solubility of curcumin, and the formation of polylactic-coglycolic acid copolymer with curcumin improved the bioavailability by 40 times compared to the administration of curcumin alone in rats [427]. In addition, nanoparticulation also significantly prolonged the retention of curcumin in the cerebral cortex and hippocampus [427]. Meanwhile, other formulation modifications, including microemulsion carriers containing surfactants, oils, and cosurfactants, are also used. The optimal formulation consisting of Capryol 90 (oil), Cremophor RH40 (surfactant), and Transcutol P aqueous solution (cosurfactant) could increase the solubility of curcumin up to 32.5 mg/mL with a 22-fold increase in bioavailability [428]. In addition, curcumin formed an amorphous solid dispersion with a matrix consisting of hydroxypropyl methylcellulose, lecithin, and isomaltose, and its bioavailability increased almost 13-fold [429]. Furthermore, the formation of complexes of polyphenols with phosphatidylcholine (PC) [430], hyaluronic acid [431], polyethylene glycol [432], and dendrimer [433] can improve the solubility and bioavailability of curcumin. For example, curcumin-PC complexes reach more than triple plasma concentrations and higher area under the curve (AUC) at the same concentrations in rats as compared to curcumin alone [430]. Meanwhile, the combination of drugs also can promote the bioavailability of plant polyphenols. When EGCG was coadministered with ascorbic acid, piperine, and sucrose, respectively, the bioavailability of EGCG was significantly improved, which may be related to the inhibition of oxidative degradation of EGCG in the gastrointestinal tract, inhibition of intestinal glucuronidation, slowing down of gastrointestinal transport, and increasing retention time of EGCG [434, 435]. Distribution is the process by which drugs are absorbed and circulated in the blood to various tissues and intracellular fluids [436]. First, plant polyphenols with better fat solubility are more likely to cross the BBB and enter the brain [437]. Due to its lipophilic nature, curcumin can easily cross the BBB [438]. Metabolism refers to the chemical structural transformation of drugs [439]. After entering the body, plant polyphenols are metabolized by various enzymes, such as cytochrome P450 [440] and catechol-O-methyltransferase (COMT) [441]. After oral administration of curcumin, its glucuronide conjugates or sulfate conjugates could be detected in the blood [442]. Therefore, curcumin undergoes extensive metabolism upon arrival in the large intestine [441], which significantly affects the bioavailability and efficacy of curcumin [443].
Although a growing number of studies have shown that many plant polyphenols have promising effects in the treatment of neurodegenerative diseases, the low bioavailability still largely limits their neuroprotective effects. Nanoformulation is a technological system for loading and industrialization of drug molecules at the nanoscale using special carriers. Nanomodified drugs have the advantages of improving the solubility of insoluble drugs, extending the half-life of drugs, avoiding drug cytotoxicity, and increasing drug BBB permeability [444]. In this review, we made an in-depth analysis of the effect of some nanoformulations on the improvement of the bioavailability and stability of curcumin, one of the most common polyphenols, in the last decade. For instance, in curcumin-loaded lipid-PLGA nanobubbles (Cur-NBs), the solubility of curcumin was greatly increased 5-fold compared to curcumin alone [445]. In addition, curcumin-loaded PLGA nanoparticles (C-NPs) extend the half-life of curcumin and the retention time of curcumin in the cerebral cortex by 96% and in the hippocampus by 83% [427]. Curcumin delivered to nanostructured lipid carriers (NLC-Cur) has a relative lower IC50 of 20 μg/mL and can enhance the targeting of curcumin to the brain, leading to the concentration of curcumin in vivo is 6.4-fold compared to curcumin alone [446]. In HCMEC/D3 cell monolayer permeation model, transferrin-functionalized lipid nanoparticles enhanced the BBB permeability of curcumin by 1.5-fold [447]. In addition, a lipoprotein resembling protein-free nanostructured lipid carrier (PS80-NLC) significantly enhances the affinity of curcumin with bEnd.3 cells and effectively promotes the BBB penetration and brain accumulation of curcumin [448]. Moreover, gamma scintillation studies showed that curcumin-loaded solid lipid nanoparticles (C-SLNSs) improve the brain bioavailability by 16.4 for oral administration and by 30-fold for intravenous administration compared to curcumin alone. Therefore, nanoformulations are useful tools that can improve the BBB penetration and bioavailability of plant polyphenols to exert better neuroprotective effects. However, the clinical effects of nanomodified plant polyphenols in the treatment of neurodegenerative diseases still have a long way to go.
7. Concluding Remarks and Future Perspectives
Neurodegenerative diseases are characterized by the progressive loss of the structure and function of neurons and the overactivated inflammatory responses. Emerging evidence indicates that the pathological mechanisms of neurodegenerative diseases are complicated and remain unelucidated. Commonly, the aggregation of misfolded proteins, DNA damage, mitochondrial dysfunction, oxidative stress, excitotoxicity, biometal dyshomeostasis, neurotrophic impairment, and neuroinflammatory responses are implicated in most of the neurodegenerative diseases (Figure 7). In addition, although many drugs are in clinical trials, only a small part of these drugs are successfully developed and approved for the treatment of neurodegenerative diseases. Therefore, the in-depth investigation of the mechanism and drug discovery is still essential in the future.
Figure 7.
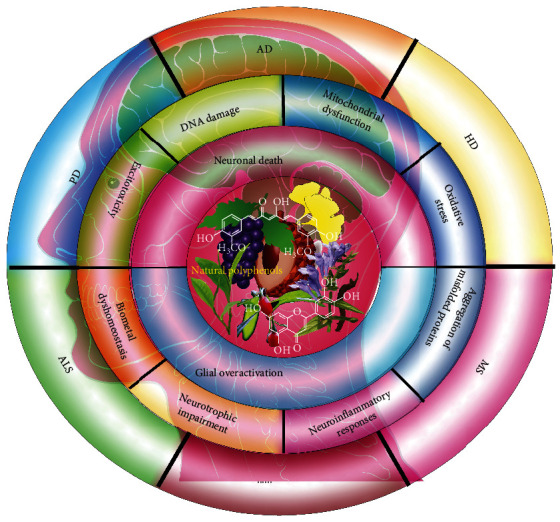
The potential treatment of natural polyphenols in neurodegenerative diseases. The natural plants especially the dietary food, such as grape, green tee, and litchi, enrich polyphenols, together with the widely reported polyphenols, such as resveratrol, curcumin, and quercetin, exhibit potent neuroprotective effects mainly involving inhibition of neuronal death and glial overactivation. The molecular mechanisms associated with the progress of neurogenerative diseases (AD, PD, HD, ALS, MS, etc.) include DNA damage, mitochondrial dysfunction, oxidative stress, excitotoxicity, biometal dyshomeostasis, neurotrophic impairment, neuroinflammatory responses, and the aggregation of misfolded proteins.
Polyphenols are complex plant secondary metabolites, which are mainly from dietary plants and exhibit a variety of pharmacological activities, such as antioxidant, anti-inflammatory, anticancer, liver protection, and neuroprotection [140, 449, 450]. Although most of the polyphenols are demonstrated to exhibit neuroprotective effects in various cellular and animal models, there are still very limited polyphenols or plant extracts that are developed into new drugs for the treatment of neurodegenerative diseases. In this respect, only 18 polyphenols are reported to have clinical studies by the US National Institute of Health (NIH). In addition to the poor stability, the literatures indicate that poor absorption, rapid metabolism and systemic elimination, inefficient delivery systems, and selective permeability across the BBB are also serious issues, which largely limit the bioavailability and neuroprotective effects of polyphenols in neurodegenerative diseases [451]. With the development of pharmaceutics, nanoencapsulation of polymeric nanoparticles or liposomes was employed to increase the permeability across BBB and improve the bioavailability of polyphenols. For example, an in silico validation along with the synthesis of CGA-loaded polymeric nanoparticles (CGA-NPs) by ionic gelation method is developed to overcome its pharmacological limitations and improve its stability in targeting neurodegenerative diseases [452]. In addition, liposomal resveratrol exhibits a more pronounced antioxidative effect as evidenced by the radical scavenging ability and reduction in ROS production when compared to resveratrol alone [453]. In LPS-stimulated HMC3 cells and murine acute brain slices, the liposomal curcumin shows a better effect in attenuated neuroinflammatory and reactive astrogliosis reactions than free curcumin [454]. Furthermore, the combinational use of polyphenols with other known compounds with neuroprotective effects is a promising strategy for improving their neuroprotective effects. It is reported that quercetin can function as an effective adjuvant to levodopa therapy might through its COMT/MAO inhibition property in the treatment of PD [455]. With the development of medical chemistry, increasing derivates are synthesized based on the polyphenols with the best neuroprotective effect. According to the structure of resveratrol, a series of compounds are designed and synthesized for the treatment of AD. Among them, compound 5d can be across the BBB and exhibit low toxicity in mice at doses of up to 2000 mg/kg [456]. Therefore, with the evidence suggesting the potential neuroprotective effect of polyphenols and dietary plants in various neurodegenerative diseases (Figure 7), more technologies and strategies on how to improve the absorption and stability, the modification of structure and formulation, and combination therapy are developing, which provide more opportunities from the laboratory into the clinic for polyphenols in the treatment of neurodegenerative diseases.
Acknowledgments
This work was supported by Grants from the National Natural Science Foundation of China (Grant Nos. 81903829 and 81801398), the Science and Technology Planning Project of Sichuan Province, China (Grant Nos. 22GJHZ0008, 2021YJ0180, and 2020YJ0494), the Macao Science and Technology Development Fund of Macao SAR (Project Nos.: SKL-QRCM(MUST)-2020-2022 and MUST-SKL-2021-005), the Southwest Medical University (Grant Nos. 2021ZKZD015, 2021ZKZD018, and 2021ZKMS046), and the Joint project of Luzhou Municipal People's Government and Southwest Medical University, China (Grant No. 2020LZXNYDJ37).
Contributor Information
Xiao-Gang Zhou, Email: zxg@swmu.edu.cn.
Da-Lian Qin, Email: dalianqin@swmu.edu.cn.
An-Guo Wu, Email: wuanguo@swmu.edu.cn.
Data Availability
All data generated or analyzed in this study are available from the corresponding author on reasonable request.
Conflicts of Interest
All authors have disclosed that they do not have any conflicts of interest.
Authors' Contributions
Da-Lian Qin, Xiao-Gang Zhou, and An-Guo Wu conceived the paper. Lu Yan, Min-Song Guo, and Wei Ai wrote the original manuscript. Yue Zhang, Feng-Dan Zhu, Yong Tang, Hua Li, and Mao Li collected the data in the tables. An-Guo Wu and Chong-Lin Yu drew the figures. Lu Yu, Qi Chen, and Jian-Ming Wu checked all the references and manuscript. All authors approved the final version of the manuscript. Lu Yan, Min-Song Guo, and Yue Zhang contributed equally to this work.
References
- 1.Golpich M., Amini E., Mohamed Z., Azman Ali R., Mohamed Ibrahim N., Ahmadiani A. Mitochondrial dysfunction and biogenesis in neurodegenerative diseases: pathogenesis and treatment. CNS Neuroscience & Therapeutics . 2017;23(1):5–22. doi: 10.1111/cns.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kausar S., Wang F., Cui H. The role of mitochondria in reactive oxygen species generation and its implications for neurodegenerative diseases. Cell . 2018;7(12):p. 274. doi: 10.3390/cells7120274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jellinger K. A. Basic mechanisms of neurodegeneration: a critical update. Journal of Cellular and Molecular Medicine . 2010;14(3):457–487. doi: 10.1111/j.1582-4934.2010.01010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bossy-Wetzel E., Schwarzenbacher R., Lipton S. A. Molecular pathways to neurodegeneration. Nature Medicine . 2004;10(S7):S2–S9. doi: 10.1038/nm1067. [DOI] [PubMed] [Google Scholar]
- 5.Wells C., Brennan S. E., Keon M., Saksena N. K. Prionoid proteins in the pathogenesis of neurodegenerative diseases. Frontiers in Molecular Neuroscience . 2019;12 doi: 10.3389/fnmol.2019.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abramov A. Y., Berezhnov A. V., Fedotova E. I., Zinchenko V. P., Dolgacheva L. P. Interaction of misfolded proteins and mitochondria in neurodegenerative disorders. Biochemical Society Transactions . 2017;45(4):1025–1033. doi: 10.1042/BST20170024. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X., Dong H., Wang F., Zhang J. Mast cell deficiency protects mice from surgery-induced neuroinflammation. Mediators of Inflammation . 2020;2020:7. doi: 10.1155/2020/1921826.1921826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou H., Qu Z., Mossine V. V., et al. Proteomic analysis of the effects of aged garlic extract and its FruArg component on lipopolysaccharide-induced neuroinflammatory response in microglial cells. PLoS One . 2014;9(11, article e113531) doi: 10.1371/journal.pone.0113531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nedelsky N. B., Todd P. K., Taylor J. P. Autophagy and the ubiquitin-proteasome system: collaborators in neuroprotection. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease . 2008;1782(12):691–699. doi: 10.1016/j.bbadis.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frake R. A., Ricketts T., Menzies F. M., Rubinsztein D. C. Autophagy and neurodegeneration. Journal of Clinical Investigation . 2015;125(1):65–74. doi: 10.1172/JCI73944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKinnon C., Tabrizi S. J. The ubiquitin-proteasome system in neurodegeneration. Antioxidants & Redox Signaling . 2014;21(17):2302–2321. doi: 10.1089/ars.2013.5802. [DOI] [PubMed] [Google Scholar]
- 12.Zheng Q., Huang T., Zhang L., et al. Dysregulation of ubiquitin-proteasome system in neurodegenerative diseases. Frontiers in Aging Neuroscience . 2016;8 doi: 10.3389/fnagi.2016.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menzies F. M., Fleming A., Caricasole A., et al. Autophagy and neurodegeneration: pathogenic mechanisms and therapeutic opportunities. Neuron . 2017;93(5):1015–1034. doi: 10.1016/j.neuron.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 14.Yagi T., Kosakai A., Ito D., et al. Establishment of induced pluripotent stem cells from centenarians for neurodegenerative disease research. PLoS One . 2012;7(7, article e41572) doi: 10.1371/journal.pone.0041572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanzione P., Tropepi D. Drugs and clinical trials in neurodegenerative diseases. Annali dell'Istituto Superiore di Sanità . 2011;47(1):49–54. doi: 10.4415/ANN_11_01_11. [DOI] [PubMed] [Google Scholar]
- 16.Feng L., Wang X., Peng F., et al. Walnut protein hydrolysates play a protective role on neurotoxicity induced by d-galactose and aluminum chloride in mice. Molecules . 2018;23(9):p. 2308. doi: 10.3390/molecules23092308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milke L., Aschenbrenner J., Marienhagen J., Kallscheuer N. Production of plant-derived polyphenols in microorganisms: current state and perspectives. Applied Microbiology and Biotechnology . 2018;102(4):1575–1585. doi: 10.1007/s00253-018-8747-5. [DOI] [PubMed] [Google Scholar]
- 18.Ellis J. M. Cholinesterase inhibitors in the treatment of dementia. The Journal of the American Osteopathic Association . 2005;105(3):145–158. [PubMed] [Google Scholar]
- 19.Atri A. Current and future treatments in Alzheimer's disease. Seminars in Neurology . 2019;39(2):227–240. doi: 10.1055/s-0039-1678581. [DOI] [PubMed] [Google Scholar]
- 20.Moss D. E., Perez R. G., Kobayashi H. Cholinesterase inhibitor therapy in Alzheimer's disease: the limits and tolerability of irreversible CNS-selective acetylcholinesterase inhibition in primates. Journal of Alzheimer's Disease . 2017;55(3):1285–1294. doi: 10.3233/JAD-160733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imbimbo B. P. Pharmacodynamic-tolerability relationships of cholinesterase inhibitors for Alzheimers disease. CNS Drugs . 2001;15(5):375–390. doi: 10.2165/00023210-200115050-00004. [DOI] [PubMed] [Google Scholar]
- 22.Folch J., Busquets O., Ettcheto M., et al. Memantine for the treatment of dementia: a review on its current and future applications. Journal of Alzheimer's Disease . 2018;62(3):1223–1240. doi: 10.3233/JAD-170672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mukhopadhyay S., Banerjee D. A primer on the evolution of aducanumab: the first antibody approved for treatment of Alzheimer's disease. Journal of Alzheimer's Disease . 2021;83(4):1537–1552. doi: 10.3233/JAD-215065. [DOI] [PubMed] [Google Scholar]
- 24.Dhillon S. Aducanumab: first approval. Drugs . 2021;81(12):1437–1443. doi: 10.1007/s40265-021-01569-z. [DOI] [PubMed] [Google Scholar]
- 25.Reich S. G., Savitt J. M. Parkinson's disease. Medical Clinics of North America . 2019;103(2):337–350. doi: 10.1016/j.mcna.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Stanga S., Caretto A., Boido M., Vercelli A. Mitochondrial dysfunctions: a red thread across neurodegenerative diseases. International Journal of Molecular Sciences . 2020;21(10):p. 3719. doi: 10.3390/ijms21103719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rascol O., Payoux P., Ory F., Ferreira J. J., Brefel-Courbon C., Montastruc J. L. Limitations of current Parkinson's disease therapy. Annals of Neurology . 2003;53(S3):S3–S15. doi: 10.1002/ana.10513. [DOI] [PubMed] [Google Scholar]
- 28.Singh A., Gupta D., Dhaneria S., Sheth P. G. Istradefylline versus opicapone for "off" episodes in Parkinson's disease: a systematic review and meta-analysis. Annals of Neurosciences . 2021;28(1-2):65–73. doi: 10.1177/09727531211046362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Potkin K. T., Potkin S. G. New directions in therapeutics for Huntington disease. Future Neurology . 2018;13(2):101–121. doi: 10.2217/fnl-2017-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gittings L. M., Sattler R. Recent advances in understanding amyotrophic lateral sclerosis and emerging therapies. Faculty Reviews . 2020;9 doi: 10.12703/b/9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller R. G., Mitchell J. D., Moore D. H., Cochrane Neuromuscular Group Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND) Cochrane Database of Systematic Reviews . 2012;2012 doi: 10.1002/14651858.CD001447.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mejzini R., Flynn L. L., Pitout I. L., Fletcher S., Wilton S. D., Akkari P. A. ALS genetics, mechanisms, and therapeutics: where are we now? Frontiers in Neuroscience . 2019;13 doi: 10.3389/fnins.2019.01310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abe K., Itoyama Y., Sobue G., et al. Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients. Amyotroph Lateral Scler Frontotemporal Degener . 2014;15(7-8):610–617. doi: 10.3109/21678421.2014.959024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo M., Zhu F., Qiu W., et al. High-throughput screening for amyloid- β binding natural small-molecules based on the combinational use of biolayer interferometry and UHPLC −DAD-Q/TOF-MS/MS. Acta Pharmaceutica Sinica B . 2021 doi: 10.1016/j.apsb.2021.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun Y., Jiang X., Pan R., et al. Escins isolated from Aesculus chinensis Bge. promote the autophagic degradation of mutant huntingtin and inhibit its induced apoptosis in HT22 cells. Frontiers in Pharmacology . 2020;11 doi: 10.3389/fphar.2020.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu A. G., Pan R., Law B. Y., et al. Targeting autophagy as a therapeutic strategy for identification of liganans from _Peristrophe japonica_ in Parkinson 's disease. Signal Transduction and Targeted Therapy . 2021;6(1) doi: 10.1038/s41392-020-00442-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu A. G., Wong V. K., Xu S. W., et al. Onjisaponin B derived from Radix Polygalae enhances autophagy and accelerates the degradation of mutant α-Synuclein and huntingtin in PC-12 cells. International Journal of Molecular Sciences . 2013;14(11):22618–22641. doi: 10.3390/ijms141122618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yi-Ling W., Jing Y. O. U., Jing-Jie C. A. O., et al. Screening of the ubiquitin-proteasome system activators for anti-Alzheimer's disease by the high-content fluorescence imaging system. Chinese Journal of Natural Medicines . 2022;20(1):33–42. doi: 10.1016/S1875-5364(22)60152-3. [DOI] [PubMed] [Google Scholar]
- 39.Lilienbaum A. Relationship between the proteasomal system and autophagy. International Journal of Biochemistry and Molecular Biology . 2013;4(1):1–26. [PMC free article] [PubMed] [Google Scholar]
- 40.Kosloski L. M., Ha D. M., Hutter J. A., et al. Adaptive immune regulation of glial homeostasis as an immunization strategy for neurodegenerative diseases. Journal of Neurochemistry . 2010;114(5):1261–1276. doi: 10.1111/j.1471-4159.2010.06834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nilsson P., Saido T. C. Dual roles for autophagy: degradation and secretion of Alzheimer's disease Aβ peptide. BioEssays . 2014;36(6):570–578. doi: 10.1002/bies.201400002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu W. H., Cuervo A. M., Kumar A., et al. Macroautophagy—a novel β-amyloid peptide-generating pathway activated in Alzheimer's disease. Journal of Cell Biology . 2005;171(1):87–98. doi: 10.1083/jcb.200505082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lim K. L., Tan J. M. Role of the ubiquitin proteasome system in Parkinson's disease. BMC Biochemistry . 2007;8(Supplement 1):p. S13. doi: 10.1186/1471-2091-8-S1-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vila M., Vukosavic S., Jackson-Lewis V., Neystat M., Jakowec M., Przedborski S. α-Synuclein up-regulation in substantia nigra dopaminergic neurons following administration of the parkinsonian toxin MPTP. Journal of Neurochemistry . 2000;74(2):721–729. doi: 10.1046/j.1471-4159.2000.740721.x. [DOI] [PubMed] [Google Scholar]
- 45.Magalhaes J., Gegg M. E., Migdalska-Richards A., Schapira A. H. Effects of ambroxol on the autophagy-lysosome pathway and mitochondria in primary cortical neurons. Scientific Reports . 2018;8(1) doi: 10.1038/s41598-018-19479-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarkar S., Rubinsztein D. C. Huntington's disease: degradation of mutant huntingtin by autophagy. FEBS Journal . 2008;275(17):4263–4270. doi: 10.1111/j.1742-4658.2008.06562.x. [DOI] [PubMed] [Google Scholar]
- 47.Sap K. A., Guler A. T., Bezstarosti K., et al. Global Proteome and Ubiquitinome Changes in the Soluble and Insoluble Fractions of Q175 Huntington Mice Brains. Molecular & Cellular Proteomics . 2019;18(9):1705–1720. doi: 10.1074/mcp.RA119.001486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aravindan S., Chen S., Choudhry H., Molfetta C., Chen K. Y., Liu A. Y. C. Osmolytes dynamically regulate mutant Huntingtin aggregation and CREB function in Huntington's disease cell models. Scientific Reports . 2020;10(1):p. 15511. doi: 10.1038/s41598-020-72613-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin D. S., Ho C. S., Huang Y. W., et al. Impairment of proteasome and autophagy underlying the pathogenesis of leukodystrophy. Cell . 2020;9(5):p. 1124. doi: 10.3390/cells9051124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Angelova P. R., Abramov A. Y. Role of mitochondrial ROS in the brain: from physiology to neurodegeneration. FEBS Letters . 2018;592(5):692–702. doi: 10.1002/1873-3468.12964. [DOI] [PubMed] [Google Scholar]
- 51.Wu A. G., Teng J. F., Wong V. K., et al. Novel steroidal saponin isolated from _Trillium tschonoskii_ maxim. exhibits anti-oxidative effect via autophagy induction in cellular and _Caenorhabditis elegans_ models. Phytomedicine . 2019;65, article 153088 doi: 10.1016/j.phymed.2019.153088. [DOI] [PubMed] [Google Scholar]
- 52.Singh A., Kukreti R., Saso L., Kukreti S. Oxidative stress: a key modulator in neurodegenerative diseases. Molecules . 2019;24(8):p. 1583. doi: 10.3390/molecules24081583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nunomura A., Honda K., Takeda A., et al. Oxidative damage to RNA in neurodegenerative diseases. Journal of Biomedicine and Biotechnology . 2006;2006:6. doi: 10.1155/JBB/2006/82323.82323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Battaglini M., Marino A., Carmignani A., et al. Polydopamine nanoparticles as an organic and biodegradable multitasking tool for neuroprotection and remote neuronal stimulation. ACS Applied Materials & Interfaces . 2020;12(32):35782–35798. doi: 10.1021/acsami.0c05497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Angelova P. R., Abramov A. Y. Alpha-synuclein and beta-amyloid - different targets, same players: calcium, free radicals and mitochondria in the mechanism of neurodegeneration. Biochemical and Biophysical Research Communications . 2017;483(4):1110–1115. doi: 10.1016/j.bbrc.2016.07.103. [DOI] [PubMed] [Google Scholar]
- 56.Höhn A., Weber D., Jung T., et al. Happily (n)ever after: aging in the context of oxidative stress, proteostasis loss and cellular senescence. Redox Biology . 2017;11:482–501. doi: 10.1016/j.redox.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Z., Zhong C. Oxidative stress in Alzheimer's disease. Neuroscience Bulletin . 2014;30(2):271–281. doi: 10.1007/s12264-013-1423-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jenner P., Dexter D. T., Sian J., Schapira A. H., Marsden C. D., The Royal Kings And Queens Parkinson's Disease Research Group Oxidative stress as a cause of nigral cell death in Parkinson's disease and incidental Lewy body Disease. Annals of Neurology . 1992;32(S1):S82–S87. doi: 10.1002/ana.410320714. [DOI] [PubMed] [Google Scholar]
- 59.Serra J. A., Domínguez R. O., de Lustig E. S., et al. Parkinson's disease is associated with oxidative stress: comparison of peripheral antioxidant profiles in living Parkinson's, Alzheimer's and vascular dementia patients. Journal of Neural Transmission . 2001;108(10):1135–1148. doi: 10.1007/s007020170003. [DOI] [PubMed] [Google Scholar]
- 60.Liao D., Chen Y., Guo Y., et al. Salvianolic acid B improves chronic mild stress-induced depressive behaviors in rats: involvement of AMPK/SIRT1 signaling Pathway. Journal of Inflammation Research . 2020;13:195–206. doi: 10.2147/JIR.S249363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Choi J., Zheng Q., Katz H. E., Guilarte T. R. Silica-based nanoparticle uptake and cellular response by primary microglia. Environmental Health Perspectives . 2010;118(5):589–595. doi: 10.1289/ehp.0901534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brandes M. S., Gray N. E. NRF2 as a therapeutic target in neurodegenerative diseases. ASN Neuro . 2020;12 doi: 10.1177/1759091419899782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gold R., Kappos L., Arnold D. L., et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. New England Journal of Medicine . 2012;367(12):1098–1107. doi: 10.1056/NEJMoa1114287. [DOI] [PubMed] [Google Scholar]
- 64.Sturm A., Mollard V., Cozijnsen A., Goodman C. D., McFadden G. I. Mitochondrial ATP synthase is dispensable in blood-stage Plasmodium berghei rodent malaria but essential in the mosquito phase. Proceedings of the National Academy of Sciences of the United States of America . 2015;112(33):10216–10223. doi: 10.1073/pnas.1423959112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun X., Wu A., Kwan Law B. Y., et al. The active components derived from Penthorum chinense Pursh protect against oxidative-stress-induced vascular injury via autophagy induction. Free Radical Biology and Medicine . 2020;146:160–180. doi: 10.1016/j.freeradbiomed.2019.10.417. [DOI] [PubMed] [Google Scholar]
- 66.Reja R., Venkatakrishnan A. J., Lee J., et al. MitoInteractome: mitochondrial protein interactome database, and its application in 'aging network' analysis. BMC Genomics . 2009;10(Supplement 3) doi: 10.1186/1471-2164-10-S3-S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Du H., Yan S. S. Mitochondrial medicine for neurodegenerative diseases. The International Journal of Biochemistry & Cell Biology . 2010;42(5):560–572. doi: 10.1016/j.biocel.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Swerdlow R. H. The neurodegenerative mitochondriopathies. Journal of Alzheimer's Disease . 2009;17(4):737–751. doi: 10.3233/JAD-2009-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Banerjee R., Starkov A. A., Beal M. F., Thomas B. Mitochondrial dysfunction in the limelight of Parkinson's disease pathogenesis. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease . 2009;1792(7):651–663. doi: 10.1016/j.bbadis.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Almeida S., Sarmento-Ribeiro A. B., Januário C., Rego A. C., Oliveira C. R. Evidence of apoptosis and mitochondrial abnormalities in peripheral blood cells of Huntington's disease patients. Biochemical and Biophysical Research Communications . 2008;374(4):599–603. doi: 10.1016/j.bbrc.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 71.Hervias I., Beal M. F., Manfredi G. Mitochondrial dysfunction and amyotrophic lateral sclerosis. Muscle & Nerve . 2006;33(5):598–608. doi: 10.1002/mus.20489. [DOI] [PubMed] [Google Scholar]
- 72.Alharbi M. A., Al-Kafaji G., Khalaf N. B., et al. Four novel mutations in the mitochondrial ND4 gene of complex I in patients with multiple sclerosis. Biomedical Reports . 2019;11(6):257–268. doi: 10.3892/br.2019.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dragicevic N., Mamcarz M., Zhu Y., et al. Mitochondrial amyloid-beta levels are associated with the extent of mitochondrial dysfunction in different brain regions and the degree of cognitive impairment in Alzheimer's transgenic mice. Journal of Alzheimer's Disease . 2010;20(s2):S535–S550. doi: 10.3233/JAD-2010-100342. [DOI] [PubMed] [Google Scholar]
- 74.Manczak M., Anekonda T. S., Henson E., Park B. S., Quinn J., Reddy P. H. Mitochondria are a direct site of A beta accumulation in Alzheimer's disease neurons: implications for free radical generation and oxidative damage in disease progression. Human Molecular Genetics . 2006;15(9):1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- 75.Liu Y., Yu B. MicroRNA-186-5p is expressed highly in ethanol-induced cardiomyocytes and regulates apoptosis via the target gene XIAP. Molecular Medicine Reports . 2019;19(4):3179–3189. doi: 10.3892/mmr.2019.9953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Luo Y., Hoffer A., Hoffer B., Qi X. Mitochondria: a therapeutic target for Parkinson's disease? International Journal of Molecular Sciences . 2015;16(9):20704–20730. doi: 10.3390/ijms160920704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu A. G., Zeng W., Wong V. K., et al. Hederagenin and α-hederin promote degradation of proteins in neurodegenerative diseases and improve motor deficits in MPTP-mice. Pharmacological Research . 2017;115:p. 25. doi: 10.1016/j.phrs.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 78.Seppet E., Gruno M., Peetsalu A., et al. Mitochondria and energetic depression in cell pathophysiology. International Journal of Molecular Sciences . 2009;10(5):2252–2303. doi: 10.3390/ijms10052252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gu M., Gash M. T., Mann V. M., Javoy-Agid F., Cooper J. M., Schapira A. H. Mitochondrial defect in Huntington's disease caudate nucleus. Annals of Neurology . 1996;39(3):385–389. doi: 10.1002/ana.410390317. [DOI] [PubMed] [Google Scholar]
- 80.Chen S., Sayana P., Zhang X., Le W. Genetics of amyotrophic lateral sclerosis: an update. Molecular Neurodegeneration . 2013;8(1) doi: 10.1186/1750-1326-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hoeijmakers J. H. DNA damage, aging, and cancer. New England Journal of Medicine . 2009;361(15):1475–1485. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- 82.Chen D., Lan J., Pei W., Chen J. Detection of DNA base-excision repair activity for oxidative lesions in adult rat brain mitochondria. Journal of Neuroscience Research . 2000;61(2):225–236. doi: 10.1002/1097-4547(20000715)61:2<225::AID-JNR13>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 83.Maynard S., Fang E. F., Scheibye-Knudsen M., Croteau D. L., Bohr V. A. DNA damage, DNA repair, aging, and neurodegeneration. Cold Spring Harbor Perspectives in Medicine . 2015;5(10) doi: 10.1101/cshperspect.a025130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ross C. A., Truant R. DNA repair: a unifying mechanism in neurodegeneration. Nature . 2017;541(7635):34–35. doi: 10.1038/nature21107. [DOI] [PubMed] [Google Scholar]
- 85.Madabhushi R., Pan L., Tsai L. H. DNA damage and its links to neurodegeneration. Neuron . 2014;83(2):266–282. doi: 10.1016/j.neuron.2014.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Katyal S., McKinnon P. J. DNA strand breaks, neurodegeneration and aging in the brain. Mechanisms of Ageing and Development . 2008;129(7-8):483–491. doi: 10.1016/j.mad.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Canugovi C., Misiak M., Ferrarelli L. K., Croteau D. L., Bohr V. A. The role of DNA repair in brain related disease pathology. DNA Repair . 2013;12(8):578–587. doi: 10.1016/j.dnarep.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Adamec E., Vonsattel J. P., Nixon R. A. DNA strand breaks in Alzheimer's disease. Brain Research . 1999;849(1-2):67–77. doi: 10.1016/S0006-8993(99)02004-1. [DOI] [PubMed] [Google Scholar]
- 89.Shackelford D. A. DNA end joining activity is reduced in Alzheimer's disease. Neurobiology of Aging . 2006;27(4):596–605. doi: 10.1016/j.neurobiolaging.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 90.Lopez-Gonzalez R., Lu Y., Gendron T. F., et al. Poly(GR) in C9ORF72-related ALS/FTD compromises mitochondrial function and increases oxidative stress and DNA damage in iPSC-derived motor neurons. Neuron . 2016;92(2):383–391. doi: 10.1016/j.neuron.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bender A., Krishnan K. J., Morris C. M., et al. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nature Genetics . 2006;38(5):515–517. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- 92.Maiuri T., Mocle A. J., Hung C. L., Xia J., van Roon-Mom W. M., Truant R. Huntingtin is a scaffolding protein in the ATM oxidative DNA damage response complex. Human Molecular Genetics . 2017;26(2):395–406. doi: 10.1093/hmg/ddw395. [DOI] [PubMed] [Google Scholar]
- 93.Javitt D. C., Zukin S. R. The role of excitatory amino acids in neuropsychiatric illness. The Journal of Neuropsychiatry and Clinical Neurosciences . 1990;2(1):44–52. doi: 10.1176/jnp.2.1.44. [DOI] [PubMed] [Google Scholar]
- 94.Shah S. A., Lee H. Y., Bressan R. A., Yun D. J., Kim M. O. Novel osmotin attenuates glutamate-induced synaptic dysfunction and neurodegeneration via the JNK/PI3K/Akt pathway in postnatal rat brain. Cell Death & Disease . 2014;5(1, article e1026) doi: 10.1038/cddis.2013.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fan M. M., Raymond L. A. N-methyl-D-aspartate (NMDA) receptor function and excitotoxicity in Huntington's disease. Progress in Neurobiology . 2007;81(5-6):272–293. doi: 10.1016/j.pneurobio.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 96.Coyle J. T., Schwarcz R. Lesion of striatal neurones with kainic acid provides a model for Huntington's chorea. Nature . 1976;263(5574):244–246. doi: 10.1038/263244a0. [DOI] [PubMed] [Google Scholar]
- 97.Liang Z. Q., Wang X. X., Wang Y., et al. Susceptibility of striatal neurons to excitotoxic injury correlates with basal levels of Bcl-2 and the induction of P53 and c-Myc immunoreactivity. Neurobiology of Disease . 2005;20(2):562–573. doi: 10.1016/j.nbd.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 98.Zhang H., Li Q., Graham R. K., Slow E., Hayden M. R., Bezprozvanny I. Full length mutant huntingtin is required for altered Ca2+ signaling and apoptosis of striatal neurons in the YAC mouse model of Huntington's disease. Neurobiology of Disease . 2008;31(1):80–88. doi: 10.1016/j.nbd.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Song C., Zhang Y., Parsons C. G., Liu Y. F. Expression of polyglutamine-expanded huntingtin induces tyrosine phosphorylation of N-methyl-D-aspartate receptors. Journal of Biological Chemistry . 2003;278(35):33364–33369. doi: 10.1074/jbc.M304240200. [DOI] [PubMed] [Google Scholar]
- 100.Zeron M. M., Chen N., Moshaver A., et al. Mutant huntingtin enhances excitotoxic cell death. Molecular and Cellular Neuroscience . 2001;17(1):41–53. doi: 10.1006/mcne.2000.0909. [DOI] [PubMed] [Google Scholar]
- 101.Parameshwaran K., Dhanasekaran M., Suppiramaniam V. Amyloid beta peptides and glutamatergic synaptic dysregulation. Experimental Neurology . 2008;210(1):7–13. doi: 10.1016/j.expneurol.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 102.De Felice F. G., Velasco P. T., Lambert M. P., et al. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. Journal of Biological Chemistry . 2007;282(15):11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- 103.Helton T. D., Otsuka T., Lee M. C., Mu Y., Ehlers M. D. Pruning and loss of excitatory synapses by the parkin ubiquitin ligase. Proceedings of the National Academy of Sciences of the United States of America . 2008;105(49):19492–19497. doi: 10.1073/pnas.0802280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moring J., Niego L. A., Ganley L. M., Trumbore M. W., Herbette L. G. Interaction of the NMDA receptor noncompetitive antagonist MK-801 with model and native membranes. Biophysical Journal . 1994;67(6):2376–2386. doi: 10.1016/S0006-3495(94)80724-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li K., Reichmann H. Role of iron in neurodegenerative diseases. Journal of Neural Transmission . 2016;123(4):389–399. doi: 10.1007/s00702-016-1508-7. [DOI] [PubMed] [Google Scholar]
- 106.Desai V., Kaler S. G. Role of copper in human neurological disorders. The American Journal of Clinical Nutrition . 2008;88(3):855S–858S. doi: 10.1093/ajcn/88.3.855S. [DOI] [PubMed] [Google Scholar]
- 107.Prasad A. S. Impact of the discovery of human zinc deficiency on health. Journal of the American College of Nutrition . 2009;28(3):257–265. doi: 10.1080/07315724.2009.10719780. [DOI] [PubMed] [Google Scholar]
- 108.Chen P., Miah M. R., Aschner M. Metals and neurodegeneration. F1000Research . 2016;5:p. 366. doi: 10.12688/f1000research.7431.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mezzaroba L., Alfieri D. F., Colado Simão A. N., Vissoci Reiche E. M. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology . 2019;74:230–241. doi: 10.1016/j.neuro.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 110.Wang Y., Shi Y., Wei H. Calcium dysregulation in Alzheimer's disease: a target for new drug development. Journal of Alzheimer's disease & Parkinsonism . 2017;7(5) doi: 10.4172/2161-0460.1000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ma Q., Li Y., Du J., et al. Copper binding properties of a tau peptide associated with Alzheimer's disease studied by CD, NMR, and MALDI-TOF MS. Peptides . 2006;27(4):841–849. doi: 10.1016/j.peptides.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 112.Ma Q. F., Li Y. M., Du J. T., et al. Binding of copper (II) ion to an Alzheimer's tau peptide as revealed by MALDI-TOF MS, CD, and NMR. Biopolymers . 2005;79(2):74–85. doi: 10.1002/bip.20335. [DOI] [PubMed] [Google Scholar]
- 113.Salazar J., Mena N., Hunot S., et al. Divalent metal transporter 1 (DMT1) contributes to neurodegeneration in animal models of Parkinson's disease. Proceedings of the National Academy of Sciences of the United States of America . 2008;105(47):18578–18583. doi: 10.1073/pnas.0804373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Oestreicher E., Sengstock G. J., Riederer P., Olanow C. W., Dunn A. J., Arendash G. W. Degeneration of nigrostriatal dopaminergic neurons increases iron within the substantia nigra: a histochemical and neurochemical study. Brain Research . 1994;660(1):8–18. doi: 10.1016/0006-8993(94)90833-8. [DOI] [PubMed] [Google Scholar]
- 115.Wegrzynowicz M., Holt H. K., Friedman D. B., Bowman A. B. Changes in the striatal proteome of YAC128Q mice exhibit gene-environment interactions between mutant huntingtin and manganese. Journal of Proteome Research . 2012;11(2):1118–1132. doi: 10.1021/pr200839d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fox J. H., Kama J. A., Lieberman G., et al. Mechanisms of copper ion mediated Huntington's disease progression. PLoS One . 2007;2(3):p. e334. doi: 10.1371/journal.pone.0000334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fang F., Kwee L. C., Allen K. D., et al. Association between blood lead and the risk of amyotrophic lateral sclerosis. American Journal of Epidemiology . 2010;171(10):1126–1133. doi: 10.1093/aje/kwq063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vinceti M., Bonvicini F., Rothman K. J., Vescovi L., Wang F. The relation between amyotrophic lateral sclerosis and inorganic selenium in drinking water: a population-based case-control study. Environmental Health . 2010;9(1) doi: 10.1186/1476-069X-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Trumbull K. A., Beckman J. S. A role for copper in the toxicity of zinc-deficient superoxide dismutase to motor neurons in amyotrophic lateral sclerosis. Antioxidants & Redox Signaling . 2009;11(7):1627–1639. doi: 10.1089/ars.2009.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sofroniew M. V., Howe C. L., Mobley W. C. Nerve growth factor signaling, neuroprotection, and neural repair. Annual Review of Neuroscience . 2001;24(1):1217–1281. doi: 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- 121.Connor B., Dragunow M. The role of neuronal growth factors in neurodegenerative disorders of the human brain. Brain Research Reviews . 1998;27(1):1–39. doi: 10.1016/S0165-0173(98)00004-6. [DOI] [PubMed] [Google Scholar]
- 122.Sullivan A. M., O'Keeffe G. W. Neurotrophic factor therapy for Parkinson's disease: past, present and future. Neural Regeneration Research . 2016;11(2):205–207. doi: 10.4103/1673-5374.177710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bossers K., Meerhoff G., Balesar R., et al. Analysis of gene expression in Parkinson's disease: possible involvement of neurotrophic support and axon guidance in dopaminergic cell death. Brain Pathology . 2009;19(1):91–107. doi: 10.1111/j.1750-3639.2008.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Budni J., Bellettini-Santos T., Mina F., Garcez M. L., Zugno A. I. The involvement of BDNF, NGF and GDNF in aging and Alzheimer's disease. Aging and Disease . 2015;6(5):331–341. doi: 10.14336/AD.2015.0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nilbratt M., Porras O., Marutle A., Hovatta O., Nordberg A. Neurotrophic factors promote cholinergic differentiation in human embryonic stem cell-derived neurons. Journal of Cellular and Molecular Medicine . 2010;14(6b):1476–1484. doi: 10.1111/j.1582-4934.2009.00916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nagatsu T., Sawada M. Biochemistry of postmortem brains in Parkinson's disease: historical overview and future prospects. Neuropsychiatric Disorders An Integrative Approach . 2007 doi: 10.1007/978-3-211-73574-9_14. [DOI] [PubMed] [Google Scholar]
- 127.Ziebell M., Khalid U., Klein A. B., et al. Striatal dopamine transporter binding correlates with serum BDNF levels in patients with striatal dopaminergic neurodegeneration. Neurobiology of Aging . 2012;33(2):428.e1–428.e5. doi: 10.1016/j.neurobiolaging.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 128.Chermenina M., Schouten P., Nevalainen N., Johansson F., Orädd G., Strömberg I. GDNF is important for striatal organization and maintenance of dopamine neurons grown in the presence of the striatum. Neuroscience . 2014;270:1–11. doi: 10.1016/j.neuroscience.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 129.Kim H. S., Jeon I., Noh J. E., et al. Intracerebral transplantation of BDNF-overexpressing human neural stem cells (HB1.F3.BDNF) promotes migration, differentiation and functional recovery in a rodent model of Huntington's disease. Experimental Neurobiology . 2020;29(2):130–137. doi: 10.5607/en20011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gouel F., Rolland A. S., Devedjian J. C., Burnouf T., Devos D. Past and future of neurotrophic growth factors therapies in ALS: from single neurotrophic growth factor to stem cells and human platelet lysates. Frontiers in Neurology . 2019;10 doi: 10.3389/fneur.2019.00835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Garwood C. J., Cooper J. D., Hanger D. P., Noble W. Anti-inflammatory impact of minocycline in a mouse model of tauopathy. Frontiers in Psychiatry . 2010;1 doi: 10.3389/fpsyt.2010.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu P., Li H., Wang Y., et al. Harmine ameliorates cognitive impairment by inhibiting NLRP3 inflammasome activation and enhancing the BDNF/TrkB signaling pathway in STZ-induced diabetic rats. Frontiers in Pharmacology . 2020;11 doi: 10.3389/fphar.2020.00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wu A. G., Zhou X. G., Qiao G., et al. Targeting microglial autophagic degradation in NLRP3 inflammasome-mediated neurodegenerative diseases. Ageing Research Reviews . 2021;65:p. 101202. doi: 10.1016/j.arr.2020.101202. [DOI] [PubMed] [Google Scholar]
- 134.Qiu W. Q., Ai W., Zhu F. D., et al. Polygala saponins inhibit NLRP3 inflammasome-mediated neuroinflammation via SHP-2-Mediated mitophagy. Free Radical Biology & Medicine . 2022;179:76–94. doi: 10.1016/j.freeradbiomed.2021.12.263. [DOI] [PubMed] [Google Scholar]
- 135.Bournival J., Plouffe M., Renaud J., Provencher C., Martinoli M. G. Quercetin and sesamin protect dopaminergic cells from MPP+-induced neuroinflammation in a microglial (N9)-neuronal (PC12) coculture system. Oxidative Medicine and Cellular Longevity . 2012;2012:11. doi: 10.1155/2012/921941.921941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Moscovitch-Lopatin M., Weiss A., Rosas H. D., et al. Optimization of an HTRF assay for the detection of soluble mutant huntingtin in human buffy coats: a potential biomarker in blood for Huntington disease. PLoS Currents . 2010;2, article Rrn1205 doi: 10.1371/currents.RRN1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Simmons D. A., Casale M., Alcon B., Pham N., Narayan N., Lynch G. Ferritin accumulation in dystrophic microglia is an early event in the development of Huntington's disease. Glia . 2007;55(10):1074–1084. doi: 10.1002/glia.20526. [DOI] [PubMed] [Google Scholar]
- 138.Khoshnan A., Ko J., Watkin E. E., Paige L. A., Reinhart P. H., Patterson P. H. Activation of the I B kinase complex and nuclear Factor-B contributes to mutant huntingtin neurotoxicity. The Journal of Neuroscience . 2004;24(37):7999–8008. doi: 10.1523/JNEUROSCI.2675-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zamudio F., Loon A. R., Smeltzer S., et al. TDP-43 mediated blood-brain barrier permeability and leukocyte infiltration promote neurodegeneration in a low-grade systemic inflammation mouse model. Journal of Neuroinflammation . 2020;17(1):p. 283. doi: 10.1186/s12974-020-01952-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pandey K. B., Rizvi S. I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Medicine and Cellular Longevity . 2009;2(5):5370–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Perrone L., Sampaolo S., Melone M. A. B. Bioactive phenolic compounds in the modulation of central and peripheral nervous system cancers: facts and misdeeds. Cancers . 2020;12(2):p. 454. doi: 10.3390/cancers12020454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Singla R. K., Dubey A. K., Garg A., et al. Natural polyphenols: chemical classification, definition of classes, subcategories, and structures. Journal of AOAC International . 2019;102(5):1397–1400. doi: 10.5740/jaoacint.19-0133. [DOI] [PubMed] [Google Scholar]
- 143.Wen W., Alseekh S., Fernie A. R. Conservation and diversification of flavonoid metabolism in the plant kingdom. Current Opinion in Plant Biology . 2020;55:100–108. doi: 10.1016/j.pbi.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 144.Nie J., Liu X. Quercetin alleviates generalized hyperalgesia in mice with induced adenomyosis. Molecular Medicine Reports . 2017;16(4):5370–5376. doi: 10.3892/mmr.2017.7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jiménez-Aliaga K., Bermejo-Bescós P., Benedí J., Martín-Aragón S. Quercetin and rutin exhibit antiamyloidogenic and fibril-disaggregating effects in vitro and potent antioxidant activity in APPswe cells. Life Sciences . 2011;89(25-26):939–945. doi: 10.1016/j.lfs.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 146.Bao D., Wang J., Pang X., Liu H. Protective effect of quercetin against oxidative stress-induced cytotoxicity in rat pheochromocytoma (PC-12) cells. Molecules . 2017;22(7):p. 1122. doi: 10.3390/molecules22071122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lee M., McGeer E. G., McGeer P. L. Quercetin, not caffeine, is a major neuroprotective component in coffee. Neurobiology of Aging . 2016;46:113–123. doi: 10.1016/j.neurobiolaging.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 148.Yu X., Li Y., Mu X. Effect of Quercetin on PC12 Alzheimer’s Disease Cell Model Induced by Aβ25-35 and Its Mechanism Based on Sirtuin1/Nrf2/HO-1 Pathway. BioMed Research International . 2020;2020:10. doi: 10.1155/2020/8210578.8210578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shimmyo Y., Kihara T., Akaike A., Niidome T., Sugimoto H. Flavonols and flavones as BACE-1 inhibitors: structure-activity relationship in cell-free, cell-based and in silico studies reveal novel pharmacophore features. Biochimica et Biophysica Acta (BBA) - General Subjects . 2008;1780(5):819–825. doi: 10.1016/j.bbagen.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 150.Jiang W., Luo T., Li S., et al. Quercetin protects against okadaic acid-induced injury via MAPK and PI3K/Akt/GSK3β signaling pathways in HT22 hippocampal neurons. PLoS One . 2016;11(4, article e0152371) doi: 10.1371/journal.pone.0152371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ay M., Luo J., Langley M., et al. Molecular mechanisms underlying protective effects of quercetin against mitochondrial dysfunction and progressive dopaminergic neurodegeneration in cell culture and MitoPark transgenic mouse models of Parkinson's Disease. Journal of Neurochemistry . 2017;141(5):766–782. doi: 10.1111/jnc.14033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.El-Horany H. E., El-Latif R. N., ElBatsh M. M., Emam M. N. Ameliorative effect of quercetin on neurochemical and behavioral deficits in rotenone rat model of Parkinson's disease: modulating autophagy (quercetin on experimental Parkinson's disease) Journal of Biochemical and Molecular Toxicology . 2016;30(7):360–369. doi: 10.1002/jbt.21821. [DOI] [PubMed] [Google Scholar]
- 153.Sharma S., Raj K., Singh S. Neuroprotective effect of quercetin in combination with piperine against rotenone- and iron supplement-induced Parkinson's disease in experimental rats. Neurotoxicity Research . 2020;37(1):198–209. doi: 10.1007/s12640-019-00120-z. [DOI] [PubMed] [Google Scholar]
- 154.Singh S., Jamwal S., Kumar P. Neuroprotective potential of quercetin in combination with piperine against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. Neural Regeneration Research . 2017;12(7):1137–1144. doi: 10.4103/1673-5374.211194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chakraborty J., Rajamma U., Jana N., Mohanakumar K. P. Quercetin improves the activity of the ubiquitin-proteasomal system in 150Q mutated huntingtin-expressing cells but exerts detrimental effects on neuronal survivability. Journal of Neuroscience Research . 2015;93(10):1581–1591. doi: 10.1002/jnr.23618. [DOI] [PubMed] [Google Scholar]
- 156.Bhatia N. K., Modi P., Sharma S., Deep S. Quercetin and baicalein act as potent antiamyloidogenic and fibril destabilizing agents for SOD1 fibrils. ACS Chemical Neuroscience . 2020;11(8):1129–1138. doi: 10.1021/acschemneuro.9b00677. [DOI] [PubMed] [Google Scholar]
- 157.Ip P., Sharda P. R., Cunningham A., Chakrabartty S., Pande V., Chakrabartty A. Quercitrin and quercetin 3-β-d-glucoside as chemical chaperones for the A4V SOD1 ALS-causing mutant. Protein Engineering, Design and Selection . 2017;30(6):431–440. doi: 10.1093/protein/gzx025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Garza-Lombó C., Posadas Y., Quintanar L., Gonsebatt M. E., Franco R. Neurotoxicity linked to dysfunctional metal ion homeostasis and xenobiotic metal exposure: redox signaling and oxidative stress. Antioxidants & Redox Signaling . 2018;28(18):1669–1703. doi: 10.1089/ars.2017.7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Faller P. Copper and zinc binding to amyloid-beta: coordination, dynamics, aggregation, reactivity and metal-ion transfer. Chembiochem . 2009;10(18):2837–2845. doi: 10.1002/cbic.200900321. [DOI] [PubMed] [Google Scholar]
- 160.Sharma D. R., Wani W. Y., Sunkaria A., et al. Quercetin attenuates neuronal death against aluminum-induced neurodegeneration in the rat hippocampus. Neuroscience . 2016;324:163–176. doi: 10.1016/j.neuroscience.2016.02.055. [DOI] [PubMed] [Google Scholar]
- 161.Zubčić K., Radovanović V., Vlainić J., et al. PI3K/Akt and ERK1/2 signalling are involved in quercetin-mediated neuroprotection against copper-induced injury. Oxidative Medicine and Cellular Longevity . 2020;2020:14. doi: 10.1155/2020/9834742.9834742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bahar E., Kim J. Y., Yoon H. Quercetin attenuates manganese-induced neuroinflammation by alleviating oxidative stress through regulation of apoptosis, iNOS/NF-κB and HO-1/Nrf2 pathways. International Journal of Molecular Sciences . 2017;18(9):p. 1989. doi: 10.3390/ijms18091989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liu Y., Gong Y., Xie W., et al. Microbubbles in combination with focused ultrasound for the delivery of quercetin-modified sulfur nanoparticles through the blood brain barrier into the brain parenchyma and relief of endoplasmic reticulum stress to treat Alzheimer's disease. Nanoscale . 2020;12(11):6498–6511. doi: 10.1039/C9NR09713A. [DOI] [PubMed] [Google Scholar]
- 164.Pinheiro R. G. R., Granja A., Loureiro J. A., et al. RVG29-functionalized lipid nanoparticles for quercetin brain delivery and Alzheimer's disease. Pharmaceutical Research . 2020;37(7):p. 139. doi: 10.1007/s11095-020-02865-1. [DOI] [PubMed] [Google Scholar]
- 165.Qi Y., Guo L., Jiang Y., Shi Y., Sui H., Zhao L. Brain delivery of quercetin-loaded exosomes improved cognitive function in AD mice by inhibiting phosphorylated tau-mediated neurofibrillary tangles. Drug Delivery . 2020;27(1):745–755. doi: 10.1080/10717544.2020.1762262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li C., Schluesener H. Health-promoting effects of the citrus flavanone hesperidin. Critical Reviews in Food Science and Nutrition . 2017;57(3):613–631. doi: 10.1080/10408398.2014.906382. [DOI] [PubMed] [Google Scholar]
- 167.Ikram M., Muhammad T., Rehman S. U., et al. Hesperetin confers neuroprotection by regulating Nrf2/TLR4/NF-κB signaling in an Aβ mouse model. Molecular Neurobiology . 2019;56(9):6293–6309. doi: 10.1007/s12035-019-1512-7. [DOI] [PubMed] [Google Scholar]
- 168.Muhammad T., Ikram M., Ullah R., Rehman S. U., Kim M. O. Hesperetin, a citrus flavonoid, attenuates LPS-induced neuroinflammation, apoptosis and memory impairments by modulating TLR4/NF-κB signaling. Nutrients . 2019;11(3):p. 648. doi: 10.3390/nu11030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hwang S. L., Yen G. C. Effect of hesperetin against oxidative stress via ER- and TrkA-mediated actions in PC12 cells. Journal of Agricultural and Food Chemistry . 2011;59(10):5779–5785. doi: 10.1021/jf104632a. [DOI] [PubMed] [Google Scholar]
- 170.Poetini M. R., Araujo S. M., Trindade de Paula M., et al. Hesperidin attenuates iron-induced oxidative damage and dopamine depletion in Drosophila melanogaster model of Parkinson's disease. Chemico-Biological Interactions . 2018;279:177–186. doi: 10.1016/j.cbi.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 171.Kumar P., Kumar A. Protective effect of hesperidin and naringin against 3-nitropropionic acid induced Huntington's like symptoms in rats: possible role of nitric oxide. Behavioural Brain Research . 2010;206(1):38–46. doi: 10.1016/j.bbr.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 172.Haghmorad D., Mahmoudi M. B., Salehipour Z., et al. Hesperidin ameliorates immunological outcome and reduces neuroinflammation in the mouse model of multiple sclerosis. Journal of Neuroimmunology . 2017;302:23–33. doi: 10.1016/j.jneuroim.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 173.Roohbakhsh A., Parhiz H., Soltani F., Rezaee R., Iranshahi M. Neuropharmacological properties and pharmacokinetics of the citrus flavonoids hesperidin and hesperetin -- A mini-review. Life Sciences . 2014;113(1-2):1–6. doi: 10.1016/j.lfs.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 174.Pérez-Cano F. J., Massot-Cladera M., Rodríguez-Lagunas M. J., Castell M. Flavonoids affect host-microbiota crosstalk through TLR modulation. Antioxidants . 2014;3(4):649–670. doi: 10.3390/antiox3040649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wu X., Beecher G. R., Holden J. M., Haytowitz D. B., Gebhardt S. E., Prior R. L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. Journal of Agricultural and Food Chemistry . 2006;54(11):4069–4075. doi: 10.1021/jf060300l. [DOI] [PubMed] [Google Scholar]
- 176.Ma H., Johnson S. L., Liu W., et al. Evaluation of polyphenol anthocyanin-enriched extracts of blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry for free radical scavenging, reactive carbonyl species trapping, anti-glycation, anti-β-amyloid aggregation, and microglial neuroprotective effects. International Journal of Molecular Sciences . 2018;19(2):p. 461. doi: 10.3390/ijms19020461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rahman M. M., Ichiyanagi T., Komiyama T., Sato S., Konishi T. Effects of anthocyanins on psychological stress-induced oxidative stress and neurotransmitter status. Journal of Agricultural and Food Chemistry . 2008;56(16):7545–7550. doi: 10.1021/jf800930s. [DOI] [PubMed] [Google Scholar]
- 178.Jeong J. W., Lee W. S., Shin S. C., Kim G. Y., Choi B. T., Choi Y. H. Anthocyanins downregulate lipopolysaccharide-induced inflammatory responses in BV2 microglial cells by suppressing the NF-κB and Akt/MAPKs signaling pathways. International Journal of Molecular Sciences . 2013;14(1):1502–1515. doi: 10.3390/ijms14011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Badshah H., Kim T. H., Kim M. O. Protective effects of anthocyanins against amyloid beta-induced neurotoxicity in vivo and in vitro. Neurochemistry International . 2015;80:51–59. doi: 10.1016/j.neuint.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 180.Yamakawa M. Y., Uchino K., Watanabe Y., et al. Anthocyanin suppresses the toxicity of Aβ deposits through diversion of molecular forms in in vitro and in vivo models of Alzheimer's disease. Nutritional Neuroscience . 2016;19(1):32–42. doi: 10.1179/1476830515Y.0000000042. [DOI] [PubMed] [Google Scholar]
- 181.Shih P. H., Wu C. H., Yeh C. T., Yen G. C. Protective effects of anthocyanins against amyloid β-peptide-induced damage in neuro-2A cells. Journal of Agricultural and Food Chemistry . 2011;59(5):1683–1689. doi: 10.1021/jf103822h. [DOI] [PubMed] [Google Scholar]
- 182.Shah S. A., Amin F. U., Khan M., et al. Anthocyanins abrogate glutamate-induced AMPK activation, oxidative stress, neuroinflammation, and neurodegeneration in postnatal rat brain. Journal of Neuroinflammation . 2016;13(1):p. 286. doi: 10.1186/s12974-016-0752-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yang J. S., Perveen S., Ha T. J., Kim S. Y., Yoon S. H. Cyanidin-3-glucoside inhibits glutamate-induced Zn2+ signaling and neuronal cell death in cultured rat hippocampal neurons by inhibiting Ca2+-induced mitochondrial depolarization and formation of reactive oxygen species. Brain Research . 2015;1606:9–20. doi: 10.1016/j.brainres.2015.02.028. [DOI] [PubMed] [Google Scholar]
- 184.Ali T., Kim T., Rehman S. U., et al. Natural dietary supplementation of anthocyanins via PI3K/Akt/Nrf2/HO-1 pathways mitigate oxidative stress, neurodegeneration, and memory impairment in a mouse model of Alzheimer's disease. Molecular Neurobiology . 2018;55(7):6076–6093. doi: 10.1007/s12035-017-0798-6. [DOI] [PubMed] [Google Scholar]
- 185.Hornedo-Ortega R., Álvarez-Fernández M. A., Cerezo A. B., Richard T., Troncoso A. M. A., Garcia-Parrilla M. A. C. Protocatechuic acid: inhibition of fibril formation, destabilization of preformed fibrils of amyloid-β and α-synuclein, and neuroprotection. Journal of Agricultural and Food Chemistry . 2016;64(41):7722–7732. doi: 10.1021/acs.jafc.6b03217. [DOI] [PubMed] [Google Scholar]
- 186.Koza L. A., Winter A. N., Holsopple J., et al. Protocatechuic acid extends survival, improves motor function, diminishes gliosis, and sustains neuromuscular junctions in the hSOD1G93A mouse model of amyotrophic lateral sclerosis. Nutrients . 2020;12(6):p. 1824. doi: 10.3390/nu12061824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Winter A. N., Bickford P. C. Anthocyanins and their metabolites as therapeutic agents for neurodegenerative disease. Antioxidants . 2019;8(9):p. 333. doi: 10.3390/antiox8090333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yu J., Jia Y., Guo Y., et al. Epigallocatechin-3-gallate protects motor neurons and regulates glutamate level. FEBS Letters . 2010;584(13):2921–2925. doi: 10.1016/j.febslet.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 189.Srividhya R., Kalaiselvi P. Neuroprotective potential of epigallo catechin-3-gallate in PC-12 cells. Neurochemical Research . 2013;38(3):486–493. doi: 10.1007/s11064-012-0940-9. [DOI] [PubMed] [Google Scholar]
- 190.Avramovich-Tirosh Y., Reznichenko L., Mit T., et al. Neurorescue activity, APP regulation and amyloid-beta peptide reduction by novel multi-functional brain permeable iron- chelating- antioxidants, M-30 and green tea polyphenol, EGCG. Current Alzheimer Research . 2007;4(4):403–411. doi: 10.2174/156720507781788927. [DOI] [PubMed] [Google Scholar]
- 191.Zhong X., Liu M., Yao W., et al. Epigallocatechin-3-gallate attenuates microglial inflammation and neurotoxicity by suppressing the activation of canonical and noncanonical inflammasome via TLR4/NF-κB pathway. Molecular Nutrition & Food Research . 2019;63(21, article e1801230) doi: 10.1002/mnfr.201801230. [DOI] [PubMed] [Google Scholar]
- 192.Mandel S., Reznichenko L., Amit T., Youdim M. B. Green tea polyphenol (-)-epigallocatechin-3-gallate protects rat PC12 cells from apoptosis induced by serum withdrawal independent of P13-Akt pathway. Neurotoxicity Research . 2003;5(6):419–424. doi: 10.1007/BF03033171. [DOI] [PubMed] [Google Scholar]
- 193.Liu J. B., Zhou L., Wang Y. Z., et al. Neuroprotective activity of (-)-epigallocatechin gallate against lipopolysaccharide-mediated cytotoxicity. Journal of Immunology Research . 2016;2016:10. doi: 10.1155/2016/4962351.4962351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.He M., Liu M. Y., Wang S., et al. Research on EGCG improving the degenerative changes of the brain in AD model mice induced with chemical drugs. Zhong Yao Cai . 2012;35(10):1641–1644. [PubMed] [Google Scholar]
- 195.Ehrnhoefer D. E., Bieschke J., Boeddrich A., et al. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nature Structural & Molecular Biology . 2008;15(6):558–566. doi: 10.1038/nsmb.1437. [DOI] [PubMed] [Google Scholar]
- 196.Reznichenko L., Amit T., Zheng H., et al. Reduction of iron-regulated amyloid precursor protein and beta-amyloid peptide by (-)-epigallocatechin-3-gallate in cell cultures: implications for iron chelation in Alzheimer's disease. Journal of Neurochemistry . 2006;97(2):527–536. doi: 10.1111/j.1471-4159.2006.03770.x. [DOI] [PubMed] [Google Scholar]
- 197.Wei J. C.-C., Huang H. C., Chen W. J., Huang C. N., Peng C. H., Lin C. L. Epigallocatechin gallate attenuates amyloid β-induced inflammation and neurotoxicity in EOC 13.31 microglia. European Journal of Pharmacology . 2016;770:16–24. doi: 10.1016/j.ejphar.2015.11.048. [DOI] [PubMed] [Google Scholar]
- 198.Koh S. H., Kim S. H., Kwon H., et al. Epigallocatechin gallate protects nerve growth factor differentiated PC12 cells from oxidative-radical-stress-induced apoptosis through its effect on phosphoinositide 3-kinase/Akt and glycogen synthase kinase-3. Molecular Brain Research . 2003;118(1-2):72–81. doi: 10.1016/j.molbrainres.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 199.McKay D. L., Blumberg J. B. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.) Phytotherapy Research . 2006;20(8):619–633. doi: 10.1002/ptr.1936. [DOI] [PubMed] [Google Scholar]
- 200.Anusha C., Sumathi T., Joseph L. D. Protective role of apigenin on rotenone induced rat model of Parkinson's disease: suppression of neuroinflammation and oxidative stress mediated apoptosis. Chemico-Biological Interactions . 2017;269:67–79. doi: 10.1016/j.cbi.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 201.Chen L., Xie W., Xie W., Zhuang W., Jiang C., Liu N. Apigenin attenuates isoflurane-induced cognitive dysfunction via epigenetic regulation and neuroinflammation in aged rats. Archives of Gerontology and Geriatrics . 2017;73:29–36. doi: 10.1016/j.archger.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 202.Choi J. S., Islam M. N., Ali M. Y., Kim E. J., Kim Y. M., Jung H. A. Effects of C-glycosylation on anti-diabetic, anti-Alzheimer's disease and anti-inflammatory potential of apigenin. Food and Chemical Toxicology . 2014;64:27–33. doi: 10.1016/j.fct.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 203.Zhang F., Li F., Chen G. Neuroprotective effect of apigenin in rats after contusive spinal cord injury. Neurological Sciences . 2014;35(4):583–588. doi: 10.1007/s10072-013-1566-7. [DOI] [PubMed] [Google Scholar]
- 204.Zhao L., Wang J. L., Liu R., Li X. X., Li J. F., Zhang L. Neuroprotective, anti-amyloidogenic and neurotrophic effects of apigenin in an Alzheimer's disease mouse model. Molecules . 2013;18(8):9949–9965. doi: 10.3390/molecules18089949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Liu R., Zhang T., Yang H., Lan X., Ying J., Du G. The flavonoid apigenin protects brain neurovascular coupling against amyloid-β₂₅-₃₅-induced toxicity in mice. Journal of Alzheimer's Disease . 2011;24(1):85–100. doi: 10.3233/JAD-2010-101593. [DOI] [PubMed] [Google Scholar]
- 206.Li R., Wang X., Qin T., Qu R., Ma S. Apigenin ameliorates chronic mild stress-induced depressive behavior by inhibiting interleukin-1β production and NLRP3 inflammasome activation in the rat brain. Behavioural Brain Research . 2016;296:p. 318. doi: 10.1016/j.bbr.2015.09.031. [DOI] [PubMed] [Google Scholar]
- 207.Patil S. P., Jain P. D., Sancheti J. S., Ghumatkar P. J., Tambe R., Sathaye S. RETRACTED: Neuroprotective and neurotrophic effects of Apigenin and Luteolin in MPTP induced parkinsonism in mice. Neuropharmacology . 2014;86:192–202. doi: 10.1016/j.neuropharm.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 208.Siddique Y. H., Jyoti S. Alteration in biochemical parameters in the brain of transgenic _Drosophila melanogaster_ model of Parkinson 's disease exposed to apigenin. Integrative Medicine Research . 2017;6(3):245–253. doi: 10.1016/j.imr.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Xi Y. D., Li X. Y., Ding J., et al. Soy isoflavone alleviates Aβ1-42-induced impairment of learning and memory ability through the regulation of RAGE/LRP-1 in neuronal and vascular tissue. Current Neurovascular Research . 2013;10(2):144–156. doi: 10.2174/1567202611310020007. [DOI] [PubMed] [Google Scholar]
- 210.Uddin M. S., Kabir M. T. Emerging signal regulating potential of genistein against Alzheimer's disease: a promising molecule of Interest. Frontiers in Cell and Development Biology . 2019;7 doi: 10.3389/fcell.2019.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Arbabi E., Hamidi G., Talaei S. A., Salami M. Estrogen agonist genistein differentially influences the cognitive and motor disorders in an ovariectomized animal model of Parkinsonism. Iranian Journal of Basic Medical Sciences . 2016;19(12):1285–1290. doi: 10.22038/ijbms.2016.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ye S., Wang T. T., Cai B., et al. Genistein protects hippocampal neurons against injury by regulating calcium/calmodulin dependent protein kinase IV protein levels in Alzheimer's disease model rats. Neural Regeneration Research . 2017;12(9):1479–1484. doi: 10.4103/1673-5374.215260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wang R., Tu J., Zhang Q., et al. Genistein attenuates ischemic oxidative damage and behavioral deficits via eNOS/Nrf2/HO-1 signaling. Hippocampus . 2013;23(7):634–647. doi: 10.1002/hipo.22126. [DOI] [PubMed] [Google Scholar]
- 214.Xi Y. D., Yu H. L., Ding J., et al. Flavonoids protect cerebrovascular endothelial cells through Nrf2 and PI3K from β-amyloid peptide-induced oxidative damage. Current Neurovascular Research . 2012;9(1):32–41. doi: 10.2174/156720212799297092. [DOI] [PubMed] [Google Scholar]
- 215.Jahromi S. R., Arrefhosseini S. R., Ghaemi A., Alizadeh A., Sabetghadam F., Togha M. Effect of oral genistein administration in early and late phases of allergic encephalomyelitis. Iranian Journal of Basic Medical Sciences . 2014;17(7):509–515. [PMC free article] [PubMed] [Google Scholar]
- 216.Kumar N., Goel N. Phenolic acids: natural versatile molecules with promising therapeutic applications. Biotechnology Reports . 2019;24, article e00370 doi: 10.1016/j.btre.2019.e00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Khan B. A., Mahmood T., Menaa F., et al. New perspectives on the efficacy of gallic acid in cosmetics & nanocosmeceuticals. Current Pharmaceutical Design . 2018;24(43):5181–5187. doi: 10.2174/1381612825666190118150614. [DOI] [PubMed] [Google Scholar]
- 218.Daglia M., Di Lorenzo A., Nabavi S. F., Talas Z. S., Nabavi S. M. Polyphenols: well beyond the antioxidant capacity: gallic acid and related compounds as neuroprotective agents: you are what you eat! Current Pharmaceutical Biotechnology . 2014;15(4):362–372. doi: 10.2174/138920101504140825120737. [DOI] [PubMed] [Google Scholar]
- 219.Mori T., Koyama N., Yokoo T., et al. Gallic acid is a dual α/β-secretase modulator that reverses cognitive impairment and remediates pathology in Alzheimer mice. Journal of Biological Chemistry . 2020;295(48):16251–16266. doi: 10.1074/jbc.RA119.012330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kim M. J., Seong A. R., Yoo J. Y., et al. Gallic acid, a histone acetyltransferase inhibitor, suppresses β-amyloid neurotoxicity by inhibiting microglial-mediated neuroinflammation. Molecular Nutrition & Food Research . 2011;55(12):1798–1808. doi: 10.1002/mnfr.201100262. [DOI] [PubMed] [Google Scholar]
- 221.Hajipour S., Sarkaki A., Farbood Y., Eidi A., Mortazavi P., Valizadeh Z. Effect of gallic acid on dementia type of Alzheimer disease in rats: electrophysiological and histological studies. Basic and Clinical Neuroscience . 2016;7(2):97–106. doi: 10.15412/J.BCN.03070203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Liu Y., Pukala T. L., Musgrave I. F., Williams D. M., Dehle F. C., Carver J. A. Gallic acid is the major component of grape seed extract that inhibits amyloid fibril formation. Bioorganic & Medicinal Chemistry Letters . 2013;23(23):6336–6340. doi: 10.1016/j.bmcl.2013.09.071. [DOI] [PubMed] [Google Scholar]
- 223.Chandrasekhar Y., Phani Kumar G., Ramya E. M., Anilakumar K. R. Gallic acid protects 6-OHDA induced neurotoxicity by attenuating oxidative stress in human dopaminergic cell line. Neurochemical Research . 2018;43(6):1150–1160. doi: 10.1007/s11064-018-2530-y. [DOI] [PubMed] [Google Scholar]
- 224.Mansouri M. T., Farbood Y., Sameri M. J., Sarkaki A., Naghizadeh B., Rafeirad M. Neuroprotective effects of oral gallic acid against oxidative stress induced by 6-hydroxydopamine in rats. Food Chemistry . 2013;138(2-3):1028–1033. doi: 10.1016/j.foodchem.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 225.Sameri M. J., Sarkaki A., Farbood Y., Mansouri S. M. Motor disorders and impaired electrical power of pallidal EEG improved by gallic acid in animal model of Parkinson's disease. Pakistan Journal of Biological Sciences . 2011;14(24):1109–1116. doi: 10.3923/pjbs.2011.1109.1116. [DOI] [PubMed] [Google Scholar]
- 226.Maya S., Prakash T., Goli D. Evaluation of neuroprotective effects of wedelolactone and gallic acid on aluminium-induced neurodegeneration: Relevance to sporadic amyotrophic lateral sclerosis. European Journal of Pharmacology . 2018;835:41–51. doi: 10.1016/j.ejphar.2018.07.058. [DOI] [PubMed] [Google Scholar]
- 227.Abdullah A., Maged M., Hairul-Islam M. I., et al. Activation of aryl hydrocarbon receptor signaling by a novel agonist ameliorates autoimmune encephalomyelitis. PLoS One . 2019;14(4, article e0215981) doi: 10.1371/journal.pone.0215981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Naveed M., Hejazi V., Abbas M., et al. Chlorogenic acid (CGA): a pharmacological review and call for further research. Biomedicine & Pharmacotherapy . 2018;97:67–74. doi: 10.1016/j.biopha.2017.10.064. [DOI] [PubMed] [Google Scholar]
- 229.Gao W., Wang C., Yu L., et al. Chlorogenic acid attenuates dextran sodium sulfate-induced ulcerative colitis in mice through MAPK/ERK/JNK pathway. BioMed Research International . 2019;2019:13. doi: 10.1155/2019/6769789.6769789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Shan S., Tian L., Fang R. Chlorogenic acid exerts beneficial effects in 6-hydroxydopamine-induced neurotoxicity by inhibition of endoplasmic reticulum stress. Medical Science Monitor . 2019;25:453–459. doi: 10.12659/MSM.911166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Shi M., Sun F., Wang Y., Kang J., Zhang S., Li H. CGA restrains the apoptosis of Aβ25-35-induced hippocampal neurons. International Journal of Neuroscience . 2020;130(7):700–707. doi: 10.1080/00207454.2019.1702547. [DOI] [PubMed] [Google Scholar]
- 232.Gao L., Li X., Meng S., Ma T., Wan L., Xu S. Chlorogenic acid alleviates Aβ25-35-Induced autophagy and cognitive impairment via the mTOR/TFEB signaling Pathway. Drug Design, Development and Therapy . 2020;Volume 14:1705–1716. doi: 10.2147/DDDT.S235969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Teraoka M., Nakaso K., Kusumoto C., et al. Cytoprotective effect of chlorogenic acid against α-synuclein-related toxicity in catecholaminergic PC12 cells. Journal of Clinical Biochemistry and Nutrition . 2012;51(2):122–127. doi: 10.3164/jcbn.D-11-00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Singh S. S., Rai S. N., Birla H., et al. Effect of chlorogenic acid supplementation in MPTP-intoxicated mouse. Frontiers in Pharmacology . 2018;9:p. 757. doi: 10.3389/fphar.2018.00757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Singh S. S., Rai S. N., Birla H., et al. Neuroprotective Effect of Chlorogenic Acid on Mitochondrial Dysfunction-Mediated Apoptotic Death of DA Neurons in a Parkinsonian Mouse Model. Oxidative Medicine and Cellular Longevity . 2020;2020:14. doi: 10.1155/2020/6571484.6571484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Miyazaki I., Isooka N., Wada K., Kikuoka R., Kitamura Y., Asanuma M. Effects of enteric environmental modification by coffee components on neurodegeneration in rotenone-treated mice. Cell . 2019;8(3):p. 221. doi: 10.3390/cells8030221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Visioli F., Franco M., Toledo E., et al. Olive oil and prevention of chronic diseases: summary of an international conference. Nutrition, Metabolism, and Cardiovascular Diseases . 2018;28(7):649–656. doi: 10.1016/j.numecd.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 238.Cicerale S., Lucas L., Keast R. Biological activities of phenolic compounds present in virgin olive oil. International Journal of Molecular Sciences . 2010;11(2):458–479. doi: 10.3390/ijms11020458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Visioli F., Poli A., Gall C. Antioxidant and other biological activities of phenols from olives and olive oil. Medicinal Research Reviews . 2002;22(1):65–75. doi: 10.1002/med.1028. [DOI] [PubMed] [Google Scholar]
- 240.Rigacci S. Olive oil phenols as promising multi-targeting agents against Alzheimer's disease. (Advances in Experimental Medicine and Biology). Natural Compounds as Therapeutic Agents for Amyloidogenic Diseases . 2015;863:1–20. doi: 10.1007/978-3-319-18365-7_1. [DOI] [PubMed] [Google Scholar]
- 241.Di Rosa G., Brunetti G., Scuto M., et al. Healthspan enhancement by olive polyphenols in C. elegans wild type and Parkinson's models. International Journal of Molecular Sciences . 2020;21(11) doi: 10.3390/ijms21113893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Tasset I., Pontes A. J., Hinojosa A. J., De la Torre R., Túnez I. Olive oil reduces oxidative damage in a 3-nitropropionic acid-induced Huntington's disease-like rat model. Nutritional Neuroscience . 2011;14(3):106–111. doi: 10.1179/1476830511Y.0000000005. [DOI] [PubMed] [Google Scholar]
- 243.Costanzo P., Oliverio M., Maiuolo J., et al. Novel hydroxytyrosol-donepezil hybrids as potential antioxidant and neuroprotective agents. Frontiers in Chemistry . 2021;9, article 741444 doi: 10.3389/fchem.2021.741444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Schaffer S., Podstawa M., Visioli F., Bogani P., Müller W. E., Eckert G. P. Hydroxytyrosol-rich olive mill wastewater extract protects brain cells in vitro and ex vivo. Journal of Agricultural and Food Chemistry . 2007;55(13):5043–5049. doi: 10.1021/jf0703710. [DOI] [PubMed] [Google Scholar]
- 245.Gallardo E., Palma-Valdés R., Espartero J. L., Santiago M. In vivo striatal measurement of hydroxytyrosol, and its metabolite (homovanillic alcohol), compared with its derivative nitrohydroxytyrosol. Neuroscience Letters . 2014;579:173–176. doi: 10.1016/j.neulet.2014.07.037. [DOI] [PubMed] [Google Scholar]
- 246.Lin M. Y., Sheng Z. H. Regulation of mitochondrial transport in neurons. Experimental Cell Research . 2015;334(1):35–44. doi: 10.1016/j.yexcr.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Visioli F., Rodríguez-Pérez M., Gómez-Torres Ó., Pintado-Losa C., Burgos-Ramos E. Hydroxytyrosol improves mitochondrial energetics of a cellular model of Alzheimer's disease. Nutritional Neuroscience . 2020:1–11. doi: 10.1080/1028415X.2020.1829344. [DOI] [PubMed] [Google Scholar]
- 248.Peng Y., Hou C., Yang Z., et al. Hydroxytyrosol mildly improve cognitive function independent of APP processing in APP/PS1 mice. Molecular Nutrition & Food Research . 2016;60(11):2331–2342. doi: 10.1002/mnfr.201600332. [DOI] [PubMed] [Google Scholar]
- 249.Crespo M. C., Tomé‐Carneiro J., Pintado C., Dávalos A., Visioli F., Burgos‐Ramos E. Hydroxytyrosol restores proper insulin signaling in an astrocytic model of Alzheimer's disease. BioFactors . 2017;43(4):540–548. doi: 10.1002/biof.1356. [DOI] [PubMed] [Google Scholar]
- 250.Pérez-Barrón G., Montes S., Aguirre-Vidal Y., et al. Antioxidant effect of hydroxytyrosol, hydroxytyrosol acetate and nitrohydroxytyrosol in a rat MPP(+) model of Parkinson's disease. Neurochemical Research . 2021;46(11):2923–2935. doi: 10.1007/s11064-021-03379-x. [DOI] [PubMed] [Google Scholar]
- 251.Perez-Barron G. A., Montes S., Rubio-Osornio M., et al. Hydroxytyrosol inhibits MAO isoforms and prevents neurotoxicity inducible by MPP+ invivo. Frontiers in Bioscience . 2020;12:25–37. doi: 10.2741/S538. [DOI] [PubMed] [Google Scholar]
- 252.Goldstein D. S., Jinsmaa Y., Sullivan P., Holmes C., Kopin I. J., Sharabi Y. 3,4-Dihydroxyphenylethanol (hydroxytyrosol) mitigates the increase in spontaneous oxidation of dopamine during monoamine oxidase inhibition in PC12 cells. Neurochemical Research . 2016;41(9):2173–2178. doi: 10.1007/s11064-016-1959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Ran G., Ying L., Li L., et al. Resveratrol ameliorates diet-induced dysregulation of lipid metabolism in zebrafish (Danio rerio) PLoS One . 2017;12(7, article e0180865) doi: 10.1371/journal.pone.0180865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Rao Y. L., Ganaraja B., Joy T., Pai M. M., Ullal S. D., Murlimanju B. V. Neuroprotective effects of resveratrol in Alzheimer's disease. Frontiers in Bioscience . 2020;12:139–149. doi: 10.2741/E863. [DOI] [PubMed] [Google Scholar]
- 255.Lee I. H. Mechanisms and disease implications of sirtuin-mediated autophagic regulation. Experimental & Molecular Medicine . 2019;51(9):1–11. doi: 10.1038/s12276-019-0302-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Pasinetti G. M., Wang J., Marambaud P., et al. Neuroprotective and metabolic effects of resveratrol: therapeutic implications for Huntington's disease and other neurodegenerative disorders. Experimental Neurology . 2011;232(1):1–6. doi: 10.1016/j.expneurol.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Bao J., Sack M. N. Protein deacetylation by sirtuins: delineating a post-translational regulatory program responsive to nutrient and redox stressors. Cellular and Molecular Life Sciences . 2010;67(18):3073–3087. doi: 10.1007/s00018-010-0402-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Smoliga J. M., Baur J. A., Hausenblas H. A. Resveratrol and health-a comprehensive review of human clinical trials. Molecular Nutrition & Food Research . 2011;55(8):1129–1141. doi: 10.1002/mnfr.201100143. [DOI] [PubMed] [Google Scholar]
- 259.Corpas R., Griñán-Ferré C., Rodríguez-Farré E., Pallàs M., Sanfeliu C. Resveratrol induces brain resilience against Alzheimer neurodegeneration through proteostasis enhancement. Molecular Neurobiology . 2019;56(2):1502–1516. doi: 10.1007/s12035-018-1157-y. [DOI] [PubMed] [Google Scholar]
- 260.Al-Edresi S., Alsalahat I., Freeman S., Aojula H., Penny J. Resveratrol-mediated cleavage of amyloid β1-42 peptide: potential relevance to Alzheimer's disease. Neurobiology of Aging . 2020;94:24–33. doi: 10.1016/j.neurobiolaging.2020.04.012. [DOI] [PubMed] [Google Scholar]
- 261.Jang J. H., Surh Y. J. Protective effect of resveratrol on beta-amyloid-induced oxidative PC12 cell death. Free Radical Biology and Medicine . 2003;34(8):1100–1110. doi: 10.1016/s0891-5849(03)00062-5. [DOI] [PubMed] [Google Scholar]
- 262.Krasinski C. A., Ivancic V. A., Zheng Q., Spratt D. E., Lazo N. D. Resveratrol sustains insulin-degrading enzyme activity toward Aβ42. ACS Omega . 2018;3(10):13275–13282. doi: 10.1021/acsomega.8b01913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Xie J., Li X., Zhou Y., et al. Resveratrol abrogates hypoxia-induced up-regulation of exosomal amyloid-β partially by inhibiting CD147. Neurochemical Research . 2019;44(5):1113–1126. doi: 10.1007/s11064-019-02742-3. [DOI] [PubMed] [Google Scholar]
- 264.Halle A., Hornung V., Petzold G. C., et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nature Immunology . 2008;9(8):857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Qi Y., Shang L., Liao Z., et al. Intracerebroventricular injection of resveratrol ameliorated Aβ-induced learning and cognitive decline in mice. Metabolic Brain Disease . 2019;34(1):257–266. doi: 10.1007/s11011-018-0348-6. [DOI] [PubMed] [Google Scholar]
- 266.Feng L., Zhang L. Resveratrol suppresses Aβ-induced microglial activation through the TXNIP/TRX/NLRP3 signaling pathway. DNA and Cell Biology . 2019;38(8):874–879. doi: 10.1089/dna.2018.4308. [DOI] [PubMed] [Google Scholar]
- 267.Huang T., Zhao J., Guo D., Pang H., Zhao Y., Song J. Curcumin mitigates axonal injury and neuronal cell apoptosis through the PERK/Nrf2 signaling pathway following diffuse axonal injury. Neuroreport . 2018;29(8):661–677. doi: 10.1097/WNR.0000000000001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Liang L., Wei H., Sun Y., Tian J. Anti-oxidative stress effects of curcumin on rat models of traumatic brain injury. Chinese Journal of Comparative Medicine . 2018;28(4):73–80. [Google Scholar]
- 269.Chiang M. C., Nicol C. J., Cheng Y. C. Resveratrol activation of AMPK-dependent pathways is neuroprotective in human neural stem cells against amyloid-beta-induced inflammation and oxidative stress. Neurochemistry International . 2018;115:1–10. doi: 10.1016/j.neuint.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 270.Dong Y. T., Cao K., Tan L. C., et al. Stimulation of SIRT1 attenuates the level of oxidative stress in the brains of APP/PS1 double transgenic mice and in primary neurons exposed to oligomers of the Amyloid-β peptide. Journal of Alzheimer's Disease . 2018;63(1):283–301. doi: 10.3233/JAD-171020. [DOI] [PubMed] [Google Scholar]
- 271.Wang Z. H., Zhang J. L., Duan Y. L., Zhang Q. S., Li G. F., Zheng D. L. MicroRNA-214 participates in the neuroprotective effect of resveratrol via inhibiting α-synuclein expression in MPTP-induced Parkinson's disease mouse. Biomedicine & Pharmacotherapy . 2015;74:252–256. doi: 10.1016/j.biopha.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 272.Zhang L. F., Yu X. L., Ji M., et al. Resveratrol alleviates motor and cognitive deficits and neuropathology in the A53T α-synuclein mouse model of Parkinson's disease. Food & Function . 2018;9(12):6414–6426. doi: 10.1039/c8fo00964c. [DOI] [PubMed] [Google Scholar]
- 273.Xia D., Sui R., Zhang Z. Administration of resveratrol improved Parkinson's disease-like phenotype by suppressing apoptosis of neurons via modulating the MALAT1/miR-129/SNCA signaling pathway. Journal of Cellular Biochemistry . 2019;120(4):4942–4951. doi: 10.1002/jcb.27769. [DOI] [PubMed] [Google Scholar]
- 274.Wu Y., Li X., Zhu J. X., et al. Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson's disease. Neurosignals . 2011;19(3):163–174. doi: 10.1159/000328516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Jin F., Wu Q., Lu Y. F., Gong Q. H., Shi J. S. Neuroprotective effect of resveratrol on 6-OHDA-induced Parkinson's disease in rats. European Journal of Pharmacology . 2008;600(1-3):78–82. doi: 10.1016/j.ejphar.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 276.Liu Q., Zhu D., Jiang P., et al. Resveratrol synergizes with low doses of L-DOPA to improve MPTP-induced Parkinson disease in mice. Behavioural Brain Research . 2019;367:10–18. doi: 10.1016/j.bbr.2019.03.043. [DOI] [PubMed] [Google Scholar]
- 277.Maher P., Dargusch R., Bodai L., Gerard P. E., Purcell J. M., Marsh J. L. ERK activation by the polyphenols fisetin and resveratrol provides neuroprotection in multiple models of Huntington's disease. Human Molecular Genetics . 2011;20(2):261–270. doi: 10.1093/hmg/ddq460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Naia L., Rosenstock T. R., Oliveira A. M., et al. Comparative mitochondrial-based protective effects of resveratrol and nicotinamide in Huntington's disease models. Molecular Neurobiology . 2017;54(7):5385–5399. doi: 10.1007/s12035-016-0048-3. [DOI] [PubMed] [Google Scholar]
- 279.Laudati G., Mascolo L., Guida N., et al. Resveratrol treatment reduces the vulnerability of SH-SY5Y cells and cortical neurons overexpressing SOD1-G93A to Thimerosal toxicity through SIRT1/DREAM/PDYN pathway. Neurotoxicology . 2019;71:6–15. doi: 10.1016/j.neuro.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 280.Wang J., Zhang Y., Tang L., Zhang N., Fan D. Protective effects of resveratrol through the up-regulation of SIRT1 expression in the mutant hSOD1-G93A-bearing motor neuron-like cell culture model of amyotrophic lateral sclerosis. Neuroscience Letters . 2011;503(3):250–255. doi: 10.1016/j.neulet.2011.08.047. [DOI] [PubMed] [Google Scholar]
- 281.Mancuso R., del Valle J., Modol L., et al. Resveratrol improves motoneuron function and extends survival in SOD1(G93A) ALS mice. Neurotherapeutics . 2014;11(2):419–432. doi: 10.1007/s13311-013-0253-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Ghaiad H. R., Nooh M. M., El-Sawalhi M. M., Shaheen A. A. Resveratrol promotes remyelination in cuprizone model of multiple sclerosis: biochemical and histological study. Molecular Neurobiology . 2017;54(5):3219–3229. doi: 10.1007/s12035-016-9891-5. [DOI] [PubMed] [Google Scholar]
- 283.Shindler K. S., Ventura E., Dutt M., Elliott P., Fitzgerald D. C., Rostami A. Oral resveratrol reduces neuronal damage in a model of multiple sclerosis. Journal of Neuro-Ophthalmology . 2010;30(4):328–339. doi: 10.1097/WNO.0b013e3181f7f833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Sato F., Martinez N. E., Shahid M., Rose J. W., Carlson N. G., Tsunoda I. Resveratrol exacerbates both autoimmune and viral models of multiple sclerosis. The American Journal of Pathology . 2013;183(5):1390–1396. doi: 10.1016/j.ajpath.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Barker D. Lignans. Molecules . 2019;24(7) doi: 10.3390/molecules24071424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Zanella I., Biasiotto G., Holm F., di Lorenzo D. Cereal lignans, natural compounds of interest for human health? Natural Product Communications . 2017;12(1):139–146. [PubMed] [Google Scholar]
- 287.Zeng K. W., Zhang T., Fu H., Liu G. X., Wang X. M. Schisandrin B exerts anti-neuroinflammatory activity by inhibiting the Toll-like receptor 4-dependent MyD88/IKK/NF-κB signaling pathway in lipopolysaccharide-induced microglia. European Journal of Pharmacology . 2012;692(1-3):29–37. doi: 10.1016/j.ejphar.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 288.Li Z., Liu X. C., Li R., Chang J. Reduction of Aβ generation by Schisandrin B through restraining beta-secretase 1 transcription and translation. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research . 2018;24:1219–1224. doi: 10.12659/MSM.905127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Jiang E. P., Li H., Yu C. R., et al. Schisandrin B protects PC12 cells against oxidative stress of neurodegenerative diseases. Neuroreport . 2015;26(6):360–366. doi: 10.1097/WNR.0000000000000354. [DOI] [PubMed] [Google Scholar]
- 290.Zhang M., Zheng H. X., Gao Y. Y., et al. The influence of Schisandrin B on a model of Alzheimer's disease using β-amyloid protein Aβ1-42-mediated damage in SH-SY5Y neuronal cell line and underlying mechanisms. Journal of Toxicology and Environmental Health, Part A . 2017;80(22):1199–1205. doi: 10.1080/15287394.2017.1367133. [DOI] [PubMed] [Google Scholar]
- 291.Yan M., Mao S., Dong H., et al. Schisandrin B protects PC12 cells by decreasing the expression of amyloid precursor protein and vacuolar protein sorting 35. Neural Regeneration Research . 2012;7(9):652–658. doi: 10.3969/j.issn.1673-5374.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Hu X. L., Guo C., Hou J. Q., et al. Stereoisomers of Schisandrin B are potent ATP competitive GSK-3β Inhibitors with neuroprotective effects against Alzheimer's disease: stereochemistry and biological activity. ACS Chemical Neuroscience . 2019;10(2):996–1007. doi: 10.1021/acschemneuro.8b00252. [DOI] [PubMed] [Google Scholar]
- 293.Ba Q., Cui C., Wen L., Feng S., Zhou J., Yang K. Schisandrin B shows neuroprotective effect in 6-OHDA-induced Parkinson's disease via inhibiting the negative modulation of miR-34a on Nrf2 pathway. Biomedicine & Pharmacotherapy . 2015;75:165–172. doi: 10.1016/j.biopha.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 294.Lam P. Y., Ko K. M. (-)Schisandrin B ameliorates paraquat-induced oxidative stress by suppressing glutathione depletion and enhancing glutathione recovery in differentiated PC12 cells. BioFactors . 2011;37(1):51–57. doi: 10.1002/biof.136. [DOI] [PubMed] [Google Scholar]
- 295.Lam P. Y., Leong P. K., Chen N., Ko K. M. Schisandrin B enhances the glutathione redox cycling and protects against oxidant injury in different types of cultured cells. BioFactors . 2011;37(6):439–446. doi: 10.1002/biof.179. [DOI] [PubMed] [Google Scholar]
- 296.Lam P. Y., Ko K. M. Beneficial effect of (-)Schisandrin B against 3-nitropropionic acid-induced cell death in PC12 cells. BioFactors . 2012;38(3):219–225. doi: 10.1002/biof.1009. [DOI] [PubMed] [Google Scholar]
- 297.Shen L. R., Parnell L. D., Ordovas J. M., Lai C. Q. Curcumin and aging. BioFactors . 2013;39(1):133–140. doi: 10.1002/biof.1086. [DOI] [PubMed] [Google Scholar]
- 298.Yang F., Lim G. P., Begum A. N., et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. Journal of Biological Chemistry . 2005;280(7):5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 299.Baum L., Ng A. Curcumin interaction with copper and iron suggests one possible mechanism of action in Alzheimer's disease animal models. Journal of Alzheimer's Disease . 2004;6(4):367–377. doi: 10.3233/jad-2004-6403. [DOI] [PubMed] [Google Scholar]
- 300.Altinay S., Cabalar M., Isler C., et al. Is chronic curcumin supplementation neuroprotective against ischemia for antioxidant activity, neurological deficit, or neuronal apoptosis in an experimental stroke model? Turkish Neurosurgery . 2017;27(4):537–545. doi: 10.5137/1019-5149.JTN.17405-16.0. [DOI] [PubMed] [Google Scholar]
- 301.Jin C. Y., Lee J. D., Park C., Choi Y. H., Kim G. Y. Curcumin attenuates the release of pro-inflammatory cytokines in lipopolysaccharide-stimulated BV2 microglia. Acta Pharmacologica Sinica . 2007;28(10):1645–1651. doi: 10.1111/j.1745-7254.2007.00651.x. [DOI] [PubMed] [Google Scholar]
- 302.Rane J. S., Bhaumik P., Panda D. Curcumin Inhibits tau aggregation and disintegrates preformed tau filaments in vitro. Journal of Alzheimer's Disease . 2017;60(3):999–1014. doi: 10.3233/JAD-170351. [DOI] [PubMed] [Google Scholar]
- 303.Liu Z. J., Li Z. H., Liu L., et al. Curcumin attenuates beta-amyloid-induced neuroinflammation via activation of peroxisome proliferator-activated receptor-gamma function in a rat model of Alzheimer's disease. Frontiers in Pharmacology . 2016;7 doi: 10.3389/fphar.2016.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Singh P. K., Kotia V., Ghosh D., Mohite G. M., Kumar A., Maji S. K. Curcumin modulates α-synuclein aggregation and toxicity. ACS Chemical Neuroscience . 2013;4(3):393–407. doi: 10.1021/cn3001203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Sang Q., Liu X., Wang L., et al. Curcumin protects an SH-SY5Y cell model of Parkinson's disease against toxic injury by regulating HSP90. Cellular Physiology and Biochemistry . 2018;51(2):681–691. doi: 10.1159/000495326. [DOI] [PubMed] [Google Scholar]
- 306.Verma M., Sharma A., Naidu S., Bhadra A. K., Kukreti R., Taneja V. Curcumin prevents formation of polyglutamine aggregates by inhibiting Vps36, a component of the ESCRT-II complex. PLoS One . 2012;7(8, article e42923) doi: 10.1371/journal.pone.0042923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Sandhir R., Yadav A., Mehrotra A., Sunkaria A., Singh A., Sharma S. Curcumin nanoparticles attenuate neurochemical and neurobehavioral deficits in experimental model of Huntington's disease. Neuromolecular Medicine . 2014;16(1):106–118. doi: 10.1007/s12017-013-8261-y. [DOI] [PubMed] [Google Scholar]
- 308.Chico L., Ienco E. C., Bisordi C., et al. Amyotrophic lateral sclerosis and oxidative stress: a double-blind therapeutic trial after curcumin supplementation. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders) . 2018;17(10):767–779. doi: 10.2174/1871527317666180720162029. [DOI] [PubMed] [Google Scholar]
- 309.Scapagnini G., Colombrita C., Amadio M., et al. Curcumin activates defensive genes and protects neurons against oxidative stress. Antioxidants & Redox Signaling . 2006;8(3-4):395–403. doi: 10.1089/ars.2006.8.395. [DOI] [PubMed] [Google Scholar]
- 310.Li Y., Li J., Li S., et al. Curcumin attenuates glutamate neurotoxicity in the hippocampus by suppression of ER stress-associated TXNIP/NLRP3 inflammasome activation in a manner dependent on AMPK. Toxicology and Applied Pharmacology . 2015;286(1):53–63. doi: 10.1016/j.taap.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 311.Cheng K. K., Yeung C. F., Ho S. W., Chow S. F., Chow A. H., Baum L. Highly stabilized curcumin nanoparticles tested in an in vitro blood-brain barrier model and in Alzheimer's disease Tg2576 mice. The AAPS Journal . 2013;15(2):324–336. doi: 10.1208/s12248-012-9444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Kozioł E., Skalicka-Woźniak K. Imperatorin-pharmacological meaning and analytical clues: profound investigation. Phytochemistry Reviews . 2016;15:627–649. doi: 10.1007/s11101-016-9456-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Chowdhury A. A., Gawali N. B., Shinde P., Munshi R., Juvekar A. R. Imperatorin ameliorates lipopolysaccharide induced memory deficit by mitigating proinflammatory cytokines, oxidative stress and modulating brain-derived neurotropic factor. Cytokine . 2018;110:78–86. doi: 10.1016/j.cyto.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 314.Budzynska B., Boguszewska-Czubara A., Kruk-Slomka M., et al. Effects of imperatorin on scopolamine-induced cognitive impairment and oxidative stress in mice. Psychopharmacology . 2015;232(5):931–942. doi: 10.1007/s00213-014-3728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Granica S., Kiss A. K., Jarończyk M., Maurin J. K., Mazurek A. P., Czarnocki Z. Synthesis of imperatorin analogs and their evaluation as acetylcholinesterase and butyrylcholinesterase inhibitors. Archiv der Pharmazie . 2013;346(11):775–782. doi: 10.1002/ardp.201300259. [DOI] [PubMed] [Google Scholar]
- 316.Varier K. M., Thangarajan S., Chinnasamy A. Effect of imperatorin in neuropathology of Parkinson's disease: an in silico study. Pharmaceutical and Clinical Research . 2017;9(8) [Google Scholar]
- 317.Lee E., Choi S. Y., Yang J. H., Lee Y. J. Preventive effects of imperatorin on perfluorohexanesulfonate-induced neuronal apoptosis via inhibition of intracellular calcium-mediated ERK pathway. The Korean Journal of Physiology & Pharmacology . 2016;20(4):399–406. doi: 10.4196/kjpp.2016.20.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Park E., Chun H. S. Protective effects of quercetin on dieldrin-induced endoplasmic reticulum stress and apoptosis in dopaminergic neuronal cells. Neuroreport . 2016;27(15):1140–1146. doi: 10.1097/WNR.0000000000000667. [DOI] [PubMed] [Google Scholar]
- 319.Ehrnhoefer D. E., Duennwald M., Markovic P., et al. Green tea (-)-epigallocatechin-gallate modulates early events in huntingtin misfolding and reduces toxicity in Huntington's disease models. Human Molecular Genetics . 2006;15(18):2743–2751. doi: 10.1093/hmg/ddl210. [DOI] [PubMed] [Google Scholar]
- 320.Koh S. H., Lee S. M., Kim H. Y., et al. The effect of epigallocatechin gallate on suppressing disease progression of ALS model mice. Neuroscience Letters . 2006;395(2):103–107. doi: 10.1016/j.neulet.2005.10.056. [DOI] [PubMed] [Google Scholar]
- 321.Herman F., Westfall S., Brathwaite J., Pasinetti G. M. Suppression of presymptomatic oxidative stress and inflammation in neurodegeneration by grape-derived polyphenols. Frontiers in Pharmacology . 2018;9 doi: 10.3389/fphar.2018.00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.González-Gallego J., García-Mediavilla M. V., Sánchez-Campos S., Tuñón M. J. Fruit polyphenols, immunity and inflammation. British Journal of Nutrition . 2010;104 doi: 10.1017/S0007114510003910. [DOI] [PubMed] [Google Scholar]
- 323.Dani C., Oliboni L. S., Agostini F., et al. Phenolic content of grapevine leaves (Vitis labrusca var. Bordo) and its neuroprotective effect against peroxide damage. Toxicology In Vitro . 2010;24(1):148–153. doi: 10.1016/j.tiv.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 324.Lian Q., Nie Y., Zhang X., et al. Effects of grape seed proanthocyanidin on Alzheimer's disease in vitro and in vivo. Experimental and Therapeutic Medicine . 2016;12(3):1681–1692. doi: 10.3892/etm.2016.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Ben Youssef S., Brisson G., Doucet-Beaupré H., et al. Neuroprotective benefits of grape seed and skin extract in a mouse model of Parkinson's disease. Nutritional Neuroscience . 2021;24(3):197–211. doi: 10.1080/1028415X.2019.1616435. [DOI] [PubMed] [Google Scholar]
- 326.Wang J., Pfleger C. M., Friedman L., et al. Potential application of grape derived polyphenols in Huntington's disease. Translational Neuroscience . 2010;1(2):95–100. doi: 10.2478/v10134-010-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Hayden E. Y., Yamin G., Beroukhim S., et al. Inhibiting amyloid β-protein assembly: Size-activity relationships among grape seed-derived polyphenols. Journal of Neurochemistry . 2015;135(2):416–430. doi: 10.1111/jnc.13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Wang J., Ho L., Zhao W., et al. Grape-derived polyphenolics prevent Abeta oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer's disease. Journal of Neuroscience . 2008;28(25):6388–6392. doi: 10.1523/JNEUROSCI.0364-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Long J., Gao H., Sun L., Liu J., Zhao-Wilson X. Grape extract protects mitochondria from oxidative damage and improves locomotor dysfunction and extends lifespan in a Drosophila Parkinson's disease model. Rejuvenation Research . 2009;12(5):321–331. doi: 10.1089/rej.2009.0877. [DOI] [PubMed] [Google Scholar]
- 330.Goenka P., Sarawgi A., Karun V., Nigam A. G., Dutta S., Marwah N. Camellia sinensis (tea): Implications and role in preventing dental decay. Pharmacognosy Reviews . 2013;7(14):152–156. doi: 10.4103/0973-7847.120515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Nouri Z., Fakhri S., El-Senduny F. F., et al. On the neuroprotective effects of naringenin: pharmacological targets, signaling pathways, molecular mechanisms, and clinical perspective. Biomolecules . 2019;9(11) doi: 10.3390/biom9110690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Zhou Z. D., Xie S. P., Saw W. T., et al. The therapeutic implications of tea polyphenols against dopamine (DA) neuron degeneration in Parkinson's disease (PD) Cell . 2019;8(8) doi: 10.3390/cells8080911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Lim H. J., Shim S. B., Jee S. W., et al. Green tea catechin leads to global improvement among Alzheimer's disease-related phenotypes in NSE/hAPP-C105 Tg mice. The Journal of Nutritional Biochemistry . 2013;24(7):1302–1313. doi: 10.1016/j.jnutbio.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 334.Rho T., Choi M. S., Jung M., Kil H. W., Hong Y. D., Yoon K. D. Identification of fermented tea (Camellia sinensis) polyphenols and their inhibitory activities against amyloid-beta aggregation. Phytochemistry . 2019;160:11–18. doi: 10.1016/j.phytochem.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 335.Li X., Smid S. D., Lin J., et al. Neuroprotective and anti-amyloid β effect and main chemical profiles of white tea: comparison against green, oolong and black tea. Molecules . 2019;24(10) doi: 10.3390/molecules24101926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Zhang S., Duangjan C., Tencomnao T., Liu J., Lin J., Wink M. Neuroprotective effects of oolong tea extracts against glutamate-induced toxicity in cultured neuronal cells and β-amyloid-induced toxicity in Caenorhabditis elegans. Food & Function . 2020;11(9):8179–8192. doi: 10.1039/d0fo01072c. [DOI] [PubMed] [Google Scholar]
- 337.Chen M., Wang T., Yue F., et al. Tea polyphenols alleviate motor impairments, dopaminergic neuronal injury, and cerebral α-synuclein aggregation in MPTP-intoxicated parkinsonian monkeys. Neuroscience . 2015;286:383–392. doi: 10.1016/j.neuroscience.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 338.Tang Y., Xiong R., Wu A. G., et al. Polyphenols derived from lychee seed suppress Aβ (1-42)-induced neuroinflammation. International Journal of Molecular Sciences . 2018;19(7) doi: 10.3390/ijms19072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Paliga M., Novello Z., Dallago R. M., et al. Extraction, chemical characterization and antioxidant activity of Litchi chinensis Sonn. and Avena sativa L. seeds extracts obtained from pressurized n-butane. Journal of Food Science and Technology . 2017;54(3):846–851. doi: 10.1007/s13197-016-2485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Qiu W. Q., Pan R., Tang Y., et al. Lychee seed polyphenol inhibits Aβ-induced activation of NLRP3 inflammasome via the LRP1/AMPK mediated autophagy induction. Biomedicine & Pharmacotherapy . 2020;130, article 110575 doi: 10.1016/j.biopha.2020.110575. [DOI] [PubMed] [Google Scholar]
- 341.Wang X., Zhang H., Liu J., et al. Inhibitory effect of lychee seed saponins on apoptosis induced by Aβ(25-35) through regulation of the apoptotic and NF-κB pathways in PC12 cells. Nutrients . 2017;9(4) doi: 10.3390/nu9040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Wang X., Wu J., Yu C., et al. Lychee seed saponins improve cognitive function and prevent neuronal injury via inhibiting neuronal apoptosis in a rat model of Alzheimer's disease. Nutrients . 2017;9(2) doi: 10.3390/nu9020105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Sun Y., Wu A., Li X., et al. The seed of Litchi chinensis fraction ameliorates hippocampal neuronal injury in an Aβ(25-35)-induced Alzheimer's disease rat model via the AKT/GSK-3β pathway. Pharmaceutical Biology . 2020;58(1):35–43. doi: 10.1080/13880209.2019.1697298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Tang Y., Yu C., Wu J., et al. Lychee seed extract protects against neuronal injury and improves cognitive function in rats with type II diabetes mellitus with cognitive impairment. International Journal of Molecular Medicine . 2018;41(1):251–263. doi: 10.3892/ijmm.2017.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Xiong R., Wang X. L., Wu J. M., et al. Polyphenols isolated from lychee seed inhibit Alzheimer's disease-associated Tau through improving insulin resistance via the IRS-1/PI3K/Akt/GSK-3β pathway. Journal of Ethnopharmacology . 2020;251, article 112548 doi: 10.1016/j.jep.2020.112548. [DOI] [PubMed] [Google Scholar]
- 346.Zhao Y., Zeng Y., Wu A., et al. Lychee seed fraction inhibits Aβ(1-42)-induced neuroinflammation in BV-2 cells via NF-κB signaling pathway. Frontiers in Pharmacology . 2018;9 doi: 10.3389/fphar.2018.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Xiong R., Zhou X. G., Tang Y., et al. Lychee seed polyphenol protects the blood-brain barrier through inhibiting Aβ(25-35)-induced NLRP3 inflammasome activation via the AMPK/mTOR/ULK1-mediated autophagy in bEnd.3 cells and APP/PS1 mice. Phytotherapy Research . 2021;35(2):954–973. doi: 10.1002/ptr.6849. [DOI] [PubMed] [Google Scholar]
- 348.Zhong H., Yu H., Chen J., et al. Hydrogen sulfide and endoplasmic reticulum stress: a potential therapeutic target for central nervous system degeneration diseases. Frontiers in Pharmacology . 2020;11:p. 702. doi: 10.3389/fphar.2020.00702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Sakurai T., Kitadate K., Nishioka H., et al. Oligomerised lychee fruit-derived polyphenol attenuates cognitive impairment in senescence-accelerated mice and endoplasmic reticulum stress in neuronal cells. The British Journal of Nutrition . 2013;110(9):1549–1558. doi: 10.1017/S000711451300086X. [DOI] [PubMed] [Google Scholar]
- 350.Qian Y., Chen Y., Wang L., Tou J. Effects of baicalin on inflammatory reaction, oxidative stress and PKDl and NF-kB protein expressions in rats with severe acute pancreatitis1. Acta Cirúrgica Brasileira . 2018;33(7):556–564. doi: 10.1590/s0102-865020180070000001. [DOI] [PubMed] [Google Scholar]
- 351.Chen D. S., Cao J. G., Zhu B., Wang Z. L., Wang T. F., Tang J. J. Baicalin attenuates joint pain and muscle dysfunction by inhibiting muscular oxidative stress in an experimental osteoarthritis rat model. Archivum Immunologiae et Therapiae Experimentalis (Warsz) . 2018;66(6):453–461. doi: 10.1007/s00005-018-0518-6. [DOI] [PubMed] [Google Scholar]
- 352.Fang J., Wang H., Zhou J., et al. Baicalin provides neuroprotection in traumatic brain injury mice model through Akt/Nrf2 pathway. Drug Design, Development and Therapy . 2018;12:2497–2508. doi: 10.2147/DDDT.S163951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Yimam M., Burnett B. P., Brownell L., Jia Q. Clinical and preclinical cognitive function improvement after oral treatment of a botanical composition composed of extracts fromScutellaria baicalensisandAcacia catechu. Behavioural Neurology . 2016;2016:9. doi: 10.1155/2016/7240802.7240802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Zhang X., Yang Y., Du L., Zhang W., Du G. Baicalein exerts anti-neuroinflammatory effects to protect against rotenone-induced brain injury in rats. International Immunopharmacology . 2017;50:38–47. doi: 10.1016/j.intimp.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 355.Yune T. Y., Lee J. Y., Cui C. M., Kim H. C., Oh T. H. Neuroprotective effect of Scutellaria baicalensis on spinal cord injury in rats. Journal of Neurochemistry . 2009;110(4):1276–1287. doi: 10.1111/j.1471-4159.2009.06214.x. [DOI] [PubMed] [Google Scholar]
- 356.Lu J. H., Ardah M. T., Durairajan S. S., et al. Baicalein inhibits formation of α-synuclein oligomers within living cells and prevents Aβ peptide fibrillation and oligomerisation. Chembiochem . 2011;12(4):615–624. doi: 10.1002/cbic.201000604. [DOI] [PubMed] [Google Scholar]
- 357.Sonawane S. K., Balmik A. A., Boral D., Ramasamy S., Chinnathambi S. Baicalein suppresses Repeat Tau fibrillization by sequestering oligomers. Archives of Biochemistry and Biophysics . 2019;675:p. 108119. doi: 10.1016/j.abb.2019.108119. [DOI] [PubMed] [Google Scholar]
- 358.Zhang S., Ye J., Dong G. Neuroprotective effect of baicalein on hydrogen peroxide-mediated oxidative stress and mitochondrial dysfunction in PC12 cells. Journal of Molecular Neuroscience . 2010;40(3):311–320. doi: 10.1007/s12031-009-9285-5. [DOI] [PubMed] [Google Scholar]
- 359.Choi J. H., Choi A. Y., Yoon H., et al. Baicalein protects HT22 murine hippocampal neuronal cells against endoplasmic reticulum stress-induced apoptosis through inhibition of reactive oxygen species production and CHOP induction. Experimental & Molecular Medicine . 2010;42(12):811–822. doi: 10.3858/emm.2010.42.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Yin F., Liu J., Ji X., Wang Y., Zidichouski J., Zhang J. Baicalin prevents the production of hydrogen peroxide and oxidative stress induced by Aβ aggregation in SH-SY5Y cells. Neuroscience Letters . 2011;492(2):76–79. doi: 10.1016/j.neulet.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 361.Kuang L., Cao X., Lu Z. Baicalein protects against rotenone-induced neurotoxicity through induction of autophagy. Biological & Pharmaceutical Bulletin . 2017;40(9):1537–1543. doi: 10.1248/bpb.b17-00392. [DOI] [PubMed] [Google Scholar]
- 362.Li F. Q., Wang T., Pei Z., Liu B., Hong J. S. Inhibition of microglial activation by the herbal flavonoid baicalein attenuates inflammation-mediated degeneration of dopaminergic neurons. Journal of Neural Transmission (Vienna) . 2005;112(3):331–347. doi: 10.1007/s00702-004-0213-0. [DOI] [PubMed] [Google Scholar]
- 363.Rui W., Li S., Xiao H., Xiao M., Shi J. Baicalein attenuates neuroinflammation by inhibiting NLRP3/caspase-1/GSDMD pathway in MPTP induced mice model of Parkinson's disease. International Journal of Neuropsychopharmacology . 2020;23(11):762–773. doi: 10.1093/ijnp/pyaa060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Yu L., Wu A. G., Wong V. K., et al. The new application of UHPLC-DAD-TOF/MS in identification of inhibitors on β-amyloid fibrillation from Scutellaria baicalensis. Frontiers in Pharmacology . 2019;10:p. 194. doi: 10.3389/fphar.2019.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Augustin S., Rimbach G., Augustin K., Schliebs R., Wolffram S., Cermak R. Effect of a short- and long-term treatment with Ginkgo biloba extract on amyloid precursor protein levels in a transgenic mouse model relevant to Alzheimer's disease. Archives of Biochemistry and Biophysics . 2009;481(2):177–182. doi: 10.1016/j.abb.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 366.Vijayakumaran S., Nakamura Y., Henley J. M., Pountney D. L. Ginkgolic acid promotes autophagy-dependent clearance of intracellular alpha-synuclein aggregates. Molecular and Cellular Neurosciences . 2019;101:p. 103416. doi: 10.1016/j.mcn.2019.103416. [DOI] [PubMed] [Google Scholar]
- 367.Stark M., Behl C. The Ginkgo biloba extract EGb 761 modulates proteasome activity and polyglutamine protein aggregation. Evidence-Based Complementary and Alternative Medicine . 2014;2014:14. doi: 10.1155/2014/940186.940186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Verma S., Sharma S., Ranawat P., Nehru B. Modulatory effects of Ginkgo biloba against amyloid aggregation through induction of heat shock proteins in aluminium induced neurotoxicity. Neurochemical Research . 2020;45(2):465–490. doi: 10.1007/s11064-019-02940-z. [DOI] [PubMed] [Google Scholar]
- 369.Chen L., Zhang C., Han Y., et al. <i>Gingko biloba</i> Extract (EGb) inhibits oxidative stress in neuro 2A cells overexpressing APPsw. BioMed Research International . 2019;2019:9. doi: 10.1155/2019/7034983.7034983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Di Meo F., Cuciniello R., Margarucci S., et al. Ginkgo biloba prevents oxidative stress-induced apoptosis blocking p53 activation in neuroblastoma cells, Antioxidants (Basel) . 2020;9(4) doi: 10.3390/antiox9040279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Meng M., Ai D., Sun L., Xu X., Cao X. EGb 761 inhibits Aβ1-42-induced neuroinflammatory response by suppressing P38 MAPK signaling pathway in BV-2 microglial cells. Neuroreport . 2019;30(6):434–440. doi: 10.1097/WNR.0000000000001223. [DOI] [PubMed] [Google Scholar]
- 372.Mocan A., Vlase L., Vodnar D. C., et al. Polyphenolic content, antioxidant and antimicrobial activities of Lycium barbarum L. and Lycium chinense Mill. leaves. Molecules . 2014;19(7):10056–10073. doi: 10.3390/molecules190710056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Zhou Z. Q., Xiao J., Fan H. X., et al. Polyphenols from wolfberry and their bioactivities. Food Chemistry . 2017;214:644–654. doi: 10.1016/j.foodchem.2016.07.105. [DOI] [PubMed] [Google Scholar]
- 374.Gao Y., Wei Y., Wang Y., Gao F., Chen Z. Lycium barbarum: a traditional Chinese herb and a promising anti-aging agent. Aging and Disease . 2017;8(6):778–791. doi: 10.14336/AD.2017.0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Xing X., Liu F., Xiao J., So K. F. Neuro-protective mechanisms of Lycium barbarum. Neuromolecular Medicine . 2016;18(3):253–263. doi: 10.1007/s12017-016-8393-y. [DOI] [PubMed] [Google Scholar]
- 376.Yu M. S., Leung S. K., Lai S. W., et al. Neuroprotective effects of anti-aging oriental medicine Lycium barbarum against beta-amyloid peptide neurotoxicity. Experimental Gerontology . 2005;40(8-9):716–727. doi: 10.1016/j.exger.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 377.Ho Y. S., Yu M. S., Lai C. S., So K. F., Yuen W. H., Chang R. C. Characterizing the neuroprotective effects of alkaline extract of Lycium barbarum on beta-amyloid peptide neurotoxicity. Brain research . 2007;1158:123–134. doi: 10.1016/j.brainres.2007.04.075. [DOI] [PubMed] [Google Scholar]
- 378.Zhang Q., Du X., Xu Y., Dang L., Xiang L., Zhang J. The effects of Gouqi extracts on Morris maze learning in the APP/PS1 double transgenic mouse model of Alzheimer's disease. Experimental and Therapeutic Medicine . 2013;5(5):1528–1530. doi: 10.3892/etm.2013.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Olatunji O. J., Chen H., Zhou Y. Lycium chinensis Mill attenuates glutamate induced oxidative toxicity in PC12 cells by increasing antioxidant defense enzymes and down regulating ROS and Ca(2+) generation. Neuroscience Letters . 2016;616:111–118. doi: 10.1016/j.neulet.2015.10.070. [DOI] [PubMed] [Google Scholar]
- 380.Gao H., Yuan X., Wang Z., Gao Q., Yang J. Profiles and neuroprotective effects of Lycium ruthenicum polyphenols against oxidative stress-induced cytotoxicity in PC12 cells. Journal of Food Biochemistry . 2020;44(1, article e13112) doi: 10.1111/jfbc.13112. [DOI] [PubMed] [Google Scholar]
- 381.Yao X. L., Wu W. L., Zheng M. Y., Li W., Ye C. H., Lu X. L. Protective effects of Lycium barbarum extract against MPP(+) -induced neurotoxicity in Caenorhabditis elegans and PC12 cells. Zhong Yao Cai . 2011;34(8):1241–1246. [PubMed] [Google Scholar]
- 382.Tang W., Zhu H., Feng Y., Guo R., Wan D. The impact of gut microbiota disorders on the blood-brain barrier. Infection and Drug Resistance . 2020;13:3351–3363. doi: 10.2147/IDR.S254403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Jiang L., Li S., Zheng J., Li Y., Huang H. Recent progress in microfluidic models of the blood-brain barrier. Micromachines (Basel) . 2019;10(6):p. 375. doi: 10.3390/mi10060375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Scioli Montoto S., Muraca G., Ruiz M. E. Solid lipid nanoparticles for drug delivery: pharmacological and biopharmaceutical aspects. Frontiers in Molecular Biosciences . 2020;7:p. 587997. doi: 10.3389/fmolb.2020.587997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Zhao Q., Qu R., Teng L., Yin C., Yuan Y. HO-1 protects the nerves of rats with cerebral hemorrhage by regulating the PI3K/AKT signaling pathway. Neuropsychiatric Disease and Treatment . 2019;15:1459–1468. doi: 10.2147/NDT.S197030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Ogawa K., Kato N., Kawakami S. Recent strategies for targeted brain drug delivery. Chemical & Pharmaceutical Bulletin (Tokyo) . 2020;68(7):567–582. doi: 10.1248/cpb.c20-00041. [DOI] [PubMed] [Google Scholar]
- 387.Silveira A. C., Dias J. P., Santos V. M., et al. The action of polyphenols in diabetes mellitus and Alzheimer's disease: a common agent for overlapping pathologies. Current Neuropharmacology . 2019;17(7):590–613. doi: 10.2174/1570159X16666180803162059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Schaffer S., Halliwell B. Do polyphenols enter the brain and does it matter? Some theoretical and practical considerations. Genes & Nutrition . 2012;7(2):99–109. doi: 10.1007/s12263-011-0255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Leclerc M., Dudonné S., Calon F. Can natural products exert neuroprotection without crossing the blood-brain barrier? International Journal of Molecular Sciences . 2021;22(7) doi: 10.3390/ijms22073356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Askarova S., Umbayev B., Masoud A. R., et al. The links between the gut microbiome, aging, modern lifestyle and Alzheimer's disease. Frontiers in Cellular and Infection Microbiology . 2020;10:p. 104. doi: 10.3389/fcimb.2020.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Fan X., Liu B., Zhou J., et al. High-fat diet alleviates neuroinflammation and metabolic disorders of APP/PS1 mice and the intervention with Chinese medicine. Frontiers in Aging Neuroscience . 2021;13:p. 658376. doi: 10.3389/fnagi.2021.658376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Sun Z. Z., Li X. Y., Wang S., Shen L., Ji H. F. Bidirectional interactions between curcumin and gut microbiota in transgenic mice with Alzheimer's disease. Applied Microbiology and Biotechnology . 2020;104(8):3507–3515. doi: 10.1007/s00253-020-10461-x. [DOI] [PubMed] [Google Scholar]
- 393.Shen L., Liu L., Ji H. F. Alzheimer's disease histological and behavioral manifestations in transgenic mice correlate with specific gut microbiome state. Journal of Alzheimer's Disease . 2017;56(1):385–390. doi: 10.3233/JAD-160884. [DOI] [PubMed] [Google Scholar]
- 394.Vogt N. M., Kerby R. L., Dill-McFarland K. A., et al. Gut microbiome alterations in Alzheimer's disease. Scientific Reports . 2017;7(1):p. 13537. doi: 10.1038/s41598-017-13601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Cattaneo A., Cattane N., Galluzzi S., et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiology of Aging . 2017;49:60–68. doi: 10.1016/j.neurobiolaging.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 396.Burapan S., Kim M., Han J. Curcuminoid demethylation as an alternative metabolism by human intestinal microbiota. Journal of Agricultural and Food Chemistry . 2017;65(16):3305–3310. doi: 10.1021/acs.jafc.7b00943. [DOI] [PubMed] [Google Scholar]
- 397.Chen P. T., Chen Z. T., Hou W. C., Yu L. C., Chen R. P. Polyhydroxycurcuminoids but not curcumin upregulate neprilysin and can be applied to the prevention of Alzheimer's disease. Scientific Reports . 2016;6:p. 29760. doi: 10.1038/srep29760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Roberts S. B., Franceschini M. A., Silver R. E., et al. Effects of food supplementation on cognitive function, cerebral blood flow, and nutritional status in young children at risk of undernutrition: randomized controlled trial. BMJ . 2020;370:p. m2397. doi: 10.1136/bmj.m2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Mastroiacovo D., Kwik-Uribe C., Grassi D., et al. Cocoa flavanol consumption improves cognitive function, blood pressure control, and metabolic profile in elderly subjects: the Cocoa, Cognition, and Aging (CoCoA) Study--a randomized controlled trial. The American Journal of Clinical Nutrition . 2015;101(3):538–548. doi: 10.3945/ajcn.114.092189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Spencer J. P., Vauzour D., Rendeiro C. Flavonoids and cognition: the molecular mechanisms underlying their behavioural effects. Archives of Biochemistry and Biophysics . 2009;492(1-2):1–9. doi: 10.1016/j.abb.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 401.Mo Y., Yue E., Shi N., Liu K. The protective effects of curcumin in cerebral ischemia and reperfusion injury through PKC-θ signaling. Cell Cycle . 2021;20(5-6):550–560. doi: 10.1080/15384101.2021.1889188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Bavarsad K., Barreto G. E., Hadjzadeh M. A., Sahebkar A. Protective effects of curcumin against ischemia-reperfusion injury in the nervous system. Molecular Neurobiology . 2019;56(2):1391–1404. doi: 10.1007/s12035-018-1169-7. [DOI] [PubMed] [Google Scholar]
- 403.Witte A. V., Kerti L., Margulies D. S., Flöel A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. The Journal of Neuroscience . 2014;34(23):7862–7870. doi: 10.1523/JNEUROSCI.0385-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Turner R. S., Thomas R. G., Craft S., et al. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology . 2015;85(16):1383–1391. doi: 10.1212/WNL.0000000000002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Wightman E. L., Reay J. L., Haskell C. F., Williamson G., Dew T. P., Kennedy D. O. Effects of resveratrol alone or in combination with piperine on cerebral blood flow parameters and cognitive performance in human subjects: a randomised, double-blind, placebo-controlled, cross-over investigation. The British Journal of Nutrition . 2014;112(2):203–213. doi: 10.1017/S0007114514000737. [DOI] [PubMed] [Google Scholar]
- 406.Moussa C., Hebron M., Huang X., et al. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer's disease. Journal of Neuroinflammation . 2017;14(1):p. 1. doi: 10.1186/s12974-016-0779-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Baum L., Lam C. W., Cheung S. K., et al. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. Journal of Clinical Psychopharmacology . 2008;28(1):110–113. doi: 10.1097/jcp.0b013e318160862c. [DOI] [PubMed] [Google Scholar]
- 408.Cheng K. K., Chan P. S., Fan S., et al. Curcumin-conjugated magnetic nanoparticles for detecting amyloid plaques in Alzheimer's disease mice using magnetic resonance imaging (MRI) Biomaterials . 2015;44:155–172. doi: 10.1016/j.biomaterials.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 409.Cox K. H., Pipingas A., Scholey A. B. Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. Journal of Psychopharmacology . 2015;29(5):642–651. doi: 10.1177/0269881114552744. [DOI] [PubMed] [Google Scholar]
- 410.Unno K., Takabayashi F., Yoshida H., et al. Daily consumption of green tea catechin delays memory regression in aged mice. Biogerontology . 2007;8(2):89–95. doi: 10.1007/s10522-006-9036-8. [DOI] [PubMed] [Google Scholar]
- 411.Schaffer S., Asseburg H., Kuntz S., Muller W. E., Eckert G. P. Effects of polyphenols on brain ageing and Alzheimer's disease: focus on mitochondria. Molecular Neurobiology . 2012;46(1):161–178. doi: 10.1007/s12035-012-8282-9. [DOI] [PubMed] [Google Scholar]
- 412.De la Torre R., De Sola S., Pons M., et al. Epigallocatechin-3-gallate, a DYRK1A inhibitor, rescues cognitive deficits in Down syndrome mouse models and in humans. Molecular Nutrition & Food Research . 2014;58(2):278–288. doi: 10.1002/mnfr.201300325. [DOI] [PubMed] [Google Scholar]
- 413.Wightman E. L., Haskell C. F., Forster J. S., Veasey R. C., Kennedy D. O. Epigallocatechin gallate, cerebral blood flow parameters, cognitive performance and mood in healthy humans: a double-blind, placebo-controlled, crossover investigation. Human Psychopharmacology . 2012;27(2):177–186. doi: 10.1002/hup.1263. [DOI] [PubMed] [Google Scholar]
- 414.Scholey A., Downey L. A., Ciorciari J., et al. Acute neurocognitive effects of epigallocatechin gallate (EGCG) Appetite . 2012;58(2):767–770. doi: 10.1016/j.appet.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 415.Kean R. J., Lamport D. J., Dodd G. F., et al. Chronic consumption of flavanone-rich orange juice is associated with cognitive benefits: an 8-wk, randomized, double-blind, placebo-controlled trial in healthy older adults. The American Journal of Clinical Nutrition . 2015;101(3):506–514. doi: 10.3945/ajcn.114.088518. [DOI] [PubMed] [Google Scholar]
- 416.Alharbi M. H., Lamport D. J., Dodd G. F., et al. Flavonoid-rich orange juice is associated with acute improvements in cognitive function in healthy middle-aged males. European Journal of Nutrition . 2016;55(6):2021–2029. doi: 10.1007/s00394-015-1016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Lamport D. J., Pal D., Macready A. L., et al. The effects of flavanone-rich citrus juice on cognitive function and cerebral blood flow: an acute, randomised, placebo-controlled cross-over trial in healthy, young adults. The British Journal of Nutrition . 2016;116(12):2160–2168. doi: 10.1017/S000711451600430X. [DOI] [PubMed] [Google Scholar]
- 418.Li H., Wang Z. Comparison in antioxidant and antitumor activities of pine polyphenols and its seven biotransformation extracts by fungi. PeerJ . 2017;5:p. e3264. doi: 10.7717/peerj.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Li G., Ruan L., Chen R., et al. Synergistic antidepressant-like effect of ferulic acid in combination with piperine: involvement of monoaminergic system. Metabolic Brain Disease . 2015;30(6):1505–1514. doi: 10.1007/s11011-015-9704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Filippi A., Ciolac O. A., Ganea C., Mocanu M. M. ErbB proteins as molecular target of dietary phytochemicals in malignant diseases. Journal of Oncology . 2017;2017:20. doi: 10.1155/2017/1532534.1532534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Agarwal R., Domowicz M. S., Schwartz N. B., et al. Delivery and tracking of quantum dot peptide bioconjugates in an intact developing avian brain. ACS Chemical Neuroscience . 2015;6(3):494–504. doi: 10.1021/acschemneuro.5b00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Gutierres V. O., Campos M. L., Arcaro C. A., et al. Curcumin pharmacokinetic and pharmacodynamic evidences in streptozotocin-diabetic rats support the antidiabetic activity to be via metabolite(s) Evidence-Based Complementary and Alternative Medicine . 2015;2015:13. doi: 10.1155/2015/678218.678218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Figueira I., Garcia G., Pimpão R. C., et al. Polyphenols journey through blood-brain barrier towards neuronal protection. Scientific Reports . 2017;7(1):p. 11456. doi: 10.1038/s41598-017-11512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Coelho M. M., Fernandes C., Remião F., Tiritan M. E. Enantioselectivity in drug pharmacokinetics and toxicity: pharmacological relevance and analytical methods. Molecules . 2021;26(11) doi: 10.3390/molecules26113113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Shin D., Lee S. J., Ha Y. M., et al. Pharmacokinetic and pharmacodynamic evaluation according to absorption differences in three formulations of ibuprofen. Drug Design, Development and Therapy . 2017;11:135–141. doi: 10.2147/DDDT.S121633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Zhao Z., Xie M., Li Y., et al. Formation of curcumin nanoparticles via solution-enhanced dispersion by supercritical CO2. International Journal of Nanomedicine . 2015;10:3171–3181. doi: 10.2147/IJN.S80434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Tsai Y. M., Chien C. F., Lin L. C., Tsai T. H. Curcumin and its nano-formulation: the kinetics of tissue distribution and blood-brain barrier penetration. International Journal of Pharmaceutics . 2011;416(1):331–338. doi: 10.1016/j.ijpharm.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 428.Jäger R., Lowery R. P., Calvanese A. V., Joy J. M., Purpura M., Wilson J. M. Comparative absorption of curcumin formulations. Nutrition Journal . 2014;13:p. 11. doi: 10.1186/1475-2891-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Chuah A. M., Jacob B., Jie Z., et al. Enhanced bioavailability and bioefficacy of an amorphous solid dispersion of curcumin. Food Chemistry . 2014;156:227–233. doi: 10.1016/j.foodchem.2014.01.108. [DOI] [PubMed] [Google Scholar]
- 430.Gupta N. K., Dixit V. K. Bioavailability enhancement of curcumin by complexation with phosphatidyl choline. Journal of Pharmaceutical Sciences . 2011;100(5):1987–1995. doi: 10.1002/jps.22393. [DOI] [PubMed] [Google Scholar]
- 431.Manju S., Sreenivasan K. Conjugation of curcumin onto hyaluronic acid enhances its aqueous solubility and stability. Journal of Colloid and Interface Science . 2011;359(1):318–325. doi: 10.1016/j.jcis.2011.03.071. [DOI] [PubMed] [Google Scholar]
- 432.Li J., Wang Y., Yang C., et al. Polyethylene glycosylated curcumin conjugate inhibits pancreatic cancer cell growth through inactivation of Jab1. Molecular Pharmacology . 2009;76(1):81–90. doi: 10.1124/mol.109.054551. [DOI] [PubMed] [Google Scholar]
- 433.Debnath S., Saloum D., Dolai S., et al. Dendrimer-curcumin conjugate: a water soluble and effective cytotoxic agent against breast cancer cell lines. Anti-Cancer Agents in Medicinal Chemistry . 2013;13(10):1531–1539. doi: 10.2174/18715206113139990139. [DOI] [PubMed] [Google Scholar]
- 434.Lambert J. D., Hong J., Kim D. H., Mishin V. M., Yang C. S. Piperine enhances the bioavailability of the tea polyphenol (-)-epigallocatechin-3-gallate in mice. The Journal of Nutrition . 2004;134(8):1948–1952. doi: 10.1093/jn/134.8.1948. [DOI] [PubMed] [Google Scholar]
- 435.Peters C. M., Green R. J., Janle E. M., Ferruzzi M. G. Formulation with ascorbic acid and sucrose modulates catechin bioavailability from green tea. Food Research International . 2010;43(1):95–102. doi: 10.1016/j.foodres.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Salt A. N., Plontke S. K. Pharmacokinetic principles in the inner ear: Influence of drug properties on intratympanic applications. Hearing Research . 2018;368:28–40. doi: 10.1016/j.heares.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Gottlieb K., Wacher V., Sliman J., Pimentel M. Review article: inhibition of methanogenic archaea by statins as a targeted management strategy for constipation and related disorders. Alimentary Pharmacology & Therapeutics . 2016;43(2):197–212. doi: 10.1111/apt.13469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Ono K., Hasegawa K., Naiki H., Yamada M. Curcumin has potent anti-amyloidogenic effects for Alzheimer's beta-amyloid fibrils in vitro. Journal of Neuroscience Research . 2004;75(6):742–750. doi: 10.1002/jnr.20025. [DOI] [PubMed] [Google Scholar]
- 439.Hua L., Chiang C. W., Cong W., et al. The cancer drug fraction of metabolism database. CPT: Pharmacometrics & Systems Pharmacology . 2019;8(7):511–519. doi: 10.1002/psp4.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Bártíková H., Boušová I., Jedličková P., Lněničková K., Skálová L., Szotáková B. Effect of standardized cranberry extract on the activity and expression of selected biotransformation enzymes in rat liver and intestine. Molecules . 2014;19(9):14948–14960. doi: 10.3390/molecules190914948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Scazzocchio B., Minghetti L., D'Archivio M. Interaction between gut microbiota and curcumin: a new key of understanding for the health effects of curcumin. Nutrients . 2020;12(9):p. 2499. doi: 10.3390/nu12092499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Stohs S. J., Chen O., Ray S. D., Ji J., Bucci L. R., Preuss H. G. Highly bioavailable forms of curcumin and promising avenues for curcumin-based research and application: a review. Molecules . 2020;25(6) doi: 10.3390/molecules25061397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Metzler M., Pfeiffer E., Schulz S. I., Dempe J. S. Curcumin uptake and metabolism. BioFactors . 2013;39(1):14–20. doi: 10.1002/biof.1042. [DOI] [PubMed] [Google Scholar]
- 444.Oliveira Pinho J., Matias M., Gaspar M. M. Emergent nanotechnological strategies for systemic chemotherapy against melanoma. Nanomaterials (Basel) . 2019;9(10) doi: 10.3390/nano9101455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Yan Y., Chen Y., Liu Z., et al. Brain delivery of curcumin through low-intensity ultrasound-induced blood-brain barrier opening via lipid-PLGA nanobubbles. International Journal of Nanomedicine . 2021;16:7433–7447. doi: 10.2147/IJN.S327737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Kuo Y. C., Chen C. L., Rajesh R. Optimized liposomes with transactivator of transcription peptide and anti-apoptotic drugs to target hippocampal neurons and prevent tau-hyperphosphorylated neurodegeneration. Acta Biomaterialia . 2019;87:207–222. doi: 10.1016/j.actbio.2019.01.065. [DOI] [PubMed] [Google Scholar]
- 447.Neves A. R., van der Putten L., Queiroz J. F., Pinheiro M., Reis S. Transferrin-functionalized lipid nanoparticles for curcumin brain delivery. Journal of Biotechnology . 2021;331:108–117. doi: 10.1016/j.jbiotec.2021.03.010. [DOI] [PubMed] [Google Scholar]
- 448.Meng F., Asghar S., Xu Y., et al. Design and evaluation of lipoprotein resembling curcumin-encapsulated protein-free nanostructured lipid carrier for brain targeting. International Journal of Pharmaceutics . 2016;506(1-2):46–56. doi: 10.1016/j.ijpharm.2016.04.033. [DOI] [PubMed] [Google Scholar]
- 449.Tringali C. Special issue: from natural polyphenols to synthetic bioactive analogues. Molecules . 2020;25(12) doi: 10.3390/molecules25122772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Li F., Zhao H., Xu R., et al. Simultaneous optimization of the acidified water extraction for total anthocyanin content, total phenolic content, and antioxidant activity of blue honeysuckle berries (Lonicera caerulea L.) using response surface methodology. Food Science & Nutrition . 2019;7(9):2968–2976. doi: 10.1002/fsn3.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Azam S., Jakaria M., Kim I. S., Kim J., Haque M. E., Choi D. K. Regulation of toll-like receptor (TLR) signaling pathway by polyphenols in the treatment of age-linked neurodegenerative diseases: focus on TLR4 signaling. Frontiers in Immunology . 2019;10:p. 1000. doi: 10.3389/fimmu.2019.01000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Agarwal V., Agarwal S., Kaur R., et al. In-silico validation and development of chlorogenic acid (CGA) loaded polymeric nanoparticle for targeting neurodegenerative disorders. Journal of Biomaterials and Nanobiotechnology . 2020;11(4):p. 279. [Google Scholar]
- 453.Vanaja K., Wahl M. A., Bukarica L., Heinle H. Liposomes as carriers of the lipid soluble antioxidant resveratrol: evaluation of amelioration of oxidative stress by additional antioxidant vitamin. Life Sciences . 2013;93(24):917–923. doi: 10.1016/j.lfs.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 454.Schmitt C., Lechanteur A., Cossais F., et al. Liposomal encapsulated curcumin effectively attenuates neuroinflammatory and reactive astrogliosis reactions in glia cells and organotypic brain slices. International Journal of Nanomedicine . 2020;15:3649–3667. doi: 10.2147/IJN.S245300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Singh A., Naidu P. S., Kulkarni S. K. Quercetin potentiates L-Dopa reversal of drug-induced catalepsy in rats: possible COMT/MAO inhibition. Pharmacology . 2003;68(2):81–88. doi: 10.1159/000069533. [DOI] [PubMed] [Google Scholar]
- 456.Lu C., Guo Y., Yan J., et al. Design, synthesis, and evaluation of multitarget-directed resveratrol derivatives for the treatment of Alzheimer's disease. Journal of Medicinal Chemistry . 2013;56(14):5843–5859. doi: 10.1021/jm400567s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed in this study are available from the corresponding author on reasonable request.


