Abstract
Introduction
Patients with cancer (PC) are at high risk of acquiring COVID-19 and can develop more serious complications. Deeper understanding of vaccines immunogenicity in this population is crucial for adequately planning vaccines programs. The ONCOVac study aimed to comprehensively assess the immunogenicity of mRNA-1273 vaccine in terms of humoral and cellular response.
Methods
We conducted a prospective, single-center study including patients with solid tumours treated with cyclin-dependent kinases 4 and 6 inhibitors (CDK4/6i), immunotherapy (IT) or chemotherapy (CT). Patients were enrolled previously to vaccination with mRNA-1273. We also involved health care workers (HCW) to serve as a control group. We took blood samples before first dose administration (BL), after first dose (1D), and after second dose (2D). The primary objective was to compare the rate and magnitude of T cell response after second dose whereas safety and humoral response were defined as secondary objectives. We also collected patient reported outcomes after both the first and second vaccine dose and a six-month follow-up period to diagnose incident COVID-19 cases was planned.
Results
The rate of specific anti-S serologic positivity (anti-S IgG cut-off point at 7,14 BAU/mL) was significantly higher in HCW compared to PC after 1D (100% versus 83.8%; p = 0.04), but similar after 2D (100% versus 95.8%; p = 0.5). This difference after 1D was driven by PC treated with CT (100% versus 64.5%; p = 0.001). Cellular response after 2D was significantly lower in PC than in HCW for both CD4+ (91.7% versus 59.7%; p = 0.001) and CD8+ (94.4% versus 55.6%; p < 0.001) T cells. We found a difference on pre-existing CD4+ T cell response in HCW comparing to PC (36% and 17%, p = 0.03); without difference in pre-existing CD8+ T cell response (31% and 23%, p = 0.5). After excluding patients with pre-existing T cell response, PC achieved even lower CD4+ (50.9% versus 95.5%, p < 0.001) and CD8+ (45.5% versus 95.5%, p < 0.001) T cell response compared with HCW. Regarding safety, PC reported notably more adverse events than HCW (96.6% versus 69.2%, p < 0.001).
Conclusion
We demonstrated that PC showed a similar humoral response but a lower T cell response following two doses of mRNA-1273 vaccination. Further studies are needed to complement our results and determine the implication of low T cell response on clinical protection of PC against COVID-19.
Keywords: SARS-CoV-2, Solid tumours, Cellular response, Humoral response, Vaccination
1. Introduction
Coronavirus disease 2019 (COVID-19) pandemic has led to more than 245 million cases and 5 million of deaths globally since the end of 2019 [1]. There has been an unprecedented global effort to develop vaccines against COVID-19, which has been a crucial step to control the pandemic. In general population, vaccines efficacy ranges between 60% and 94% and presents a good safety profile [[2], [3], [4], [5], [6], [7]].
Patients with cancer (PC) are at high risk of acquiring COVID-19 due to their immunosuppression and higher exposition rate with frequent hospital visits. In addition, they can develop more serious complications with COVID-19 infection [[8], [9], [10]]. For these reasons, COVID-19 vaccination is highly recommended in these patients [11,12].
Data about the efficacy and safety of vaccines in PC comes from heterogeneous prospective studies [[13], [14], [15], [16], [17], [18], [19], [20], [21], [22]]. Many of them include patients with solid and hematological tumors, with different types of cancer treatments and/or using different vaccines. Due to its heterogeneity, it is difficult to elucidate the specific influence of different cancer treatments on the vaccine's humoral and cellular responses [13,17,22].
It is widely agreed that humoral response to vaccine is poor after the first administration but reaches similar rates of positive seroconversion after the second dose, when compared with a healthy population. However, antibody median titers are usually lower than in health care workers (HCW). It is still not clear how important could be this for a long-term protection [23].
Besides seroconversion, T cell response in this immunosuppressed population has emerged as an important tool to address the immunogenicity against COVID-19, and several studies even described a lower T cell response after COVID-19 infection, including CD8+ T cells and CD4+ T cells [24,25], and this could be correlated with COVID-19 severity [24].
Furthermore, T cell response could be crucial to protect against the disease with new viral variants, as recently demonstrated [26]. However, there is a paucity of data about vaccine T cell response in PC, especially by considering the possible influence of different anticancer therapies in the immune mechanisms of T cell response. Moreover, this cellular response to vaccine could be altered in the presence of cross-reactive immunity, an important issue since pre-existing T cell response to SARS-CoV-2 has been observed in 30%–60% of unexposed individuals [27,28].
To accordingly elucidate the rate of both humoral and cellular response according to pre-existing T cell response and type of anticancer therapy, we evaluated the efficacy and safety of mRNA-1273 SARS-CoV-2 vaccination in 3 prospectively selected cohorts of patients with solid tumors treated with broadly used cancer treatments such as cytotoxic chemotherapy (CT), immunotherapy (IT), and cyclin-dependent kinases 4 and 6 inhibitors (CDK4/6i), using as control a cohort of HCW.
2. Methods
We conducted a prospective study at the Ramon y Cajal University Hospital in Madrid. Consecutive PC of three different types of therapy, aged 18 years or older visiting our Oncology Department previously to be vaccinated (from April 21 to May 13) were offered to participate in the study. As mentioned, three types of participants were enrolled: individuals with solid tumours treated with CDK4/6i (cohort A), those receiving IT (cohort B), and patients who had received or were receiving CT (cohort C). In addition, HCWs were enrolled to serve as control group. In all cohorts, patients with a history of clinical COVID-19 or anti-N baseline positive serology against SARS-CoV-2 were excluded from the study.
After inclusion, participants received two mRNA-1273 vaccinations intramuscularly, 28 days apart. The vaccination program for PC was designed by the public healthcare system authorities, and we could not control the timing of the vaccine administration within the anticancer therapy schedule. The primary objective was to analyse and compare the rate and magnitude of T cell response at least 25 days after the second dose of vaccine, whereas humoral response defined as specific serologic response was used as secondary objective.
Three blood samples were obtained from each patient: before the administration of the first vaccine dose (baseline, BL), at least 21 days after the administration of the first vaccine dose (1D), and three to four weeks after the second vaccine dose (2D). For the different analysis, 30 mL of venous blood were obtained in EDTA tubes and processed within 2 h after the collection. After the centrifugation, the plasma fraction was stored to −80 °C, while the cellular fraction was diluted in phosphate-buffered saline (PBS) followed by a Ficoll density gradient centrifugation for the isolation of PBMCs, which were subsequently washed and frozen with foetal bovine serum (FBS) and dimethyl sulfoxide (DMSO) 8%. Initially, antibodies to the SARS-CoV-2 nucleocapsid (COVID-19-SARS-CoV-2 IgG ELISA, Demeditech, Germany; positivity threshold 11 relative units (RU)/mL) was performed to identify those with previous infection. In the three consecutive samples, specific antibodies to SARS-COV-2 Spike protein (SARS-CoV-2 IgG II Quant Alinity; Abbott, Maidenhead, UK; positivity threshold 50 arbitrary units (AU)/mL) was measured. Following WHO recommendation for standardization of anti-SARS-CoV-2 immunoglobulin determination, we converted our antibody levels units to BAU/mL (binding antibody units, conversion factor 0.142) [29]. Consequently, for specific antibodies to SARS-COV-2 Spike protein positivity threshold is equivalent to 7,14 BAU/mL.
Cellular immune response was assessed at baseline and after complete vaccination (2D time point). Briefly, SARS-CoV-2-specific CD4+ and CD8+ T cells were measured using in vitro stimulation with SARS-CoV-2 peptide pools of viral proteins encompassing the spike (S), membrane (M), and nucleocapsid (N), followed by quantitation of CD4+ and CD8+ T cell specific interferon (IFN)-γ in live cell flow cytometry, using peripheral blood mononuclear cell (PBMC) samples from all subjects. It was considered reactive if the proportion of positive cells in stimulated wells was at least 2-fold higher in comparison with the negative control wells (unstimulated).
Patient reported outcomes were collected after both the first and the second vaccine doses. Adverse events were recorded by the participants using self-completed questionnaires after each vaccine dose.
The study was approved by our institutional ethics committee (Ramon y Cajal University Hospital Ethic Committee, EC 412/21). All the participants provided written informed consent before their inclusion in the study. The study was conducted following the Good Clinical Practice guidelines and the World Medical Association Declaration of Helsinki.
2.1. Statistical analysis
Continuous variables were expressed as the median and interquartile range (IQ25-75) and categorical variables by frequencies and proportions. Comparisons between groups were performed using two-tailed statistical tests, chi-square or Fisher's exact tests for categorical variables, and Mann–Whitney test or 1-way analysis of variance (Kruskal–Wallis test) with Dunn's correction for multiple comparisons, as appropriate. Paired samples were compared using Wilcoxon-signed rank test. Statistical significance was defined as two-sided p values <0.05. Statistical analysis was performed with STATA version 15 software, and GraphPadPrism version 8 for figures.
3. Results
3.1. Participants
Overall, 131 patients were enrolled in the study, but 17 patients were excluded at BL for serological evidence of previous SARS-CoV-2 infection. Therefore, 114 participants were the final sample of the study, 36 HCW and 78 PC. PC were subdivided in three groups: 26 patients treated with CDK4/6i, 20 patients treated with IT and 32 patients treated with CT.
Characteristics of enrolled individuals are shown in Table 1 . Specifically, cancer subtypes and the different anticancer treatments administered are available in Supplementary Table S1. As it can be observed, PC were older and had more comorbidities compared to HCWs. It should be noted that 9% and 6.4% of PC were receiving treatment with steroids (more than 10 mg daily of prednisone or equivalent) and anticoagulation therapy (with new oral anticoagulants or low-molecular-weight heparin), respectively.
Table 1.
Patient characteristics.
| HCW (n = 36) | PC (n = 78) | |
|---|---|---|
| Ageb | 46.7 (39.9–58.8) | 58.5 (51.2–68.7)a |
| Female | 69.4% | 73.1% |
| Hypertension | 11.1% | 33.3%a |
| Diabetes | 5.6% | 11.5% |
| Dyslipidaemia | 25% | 24.4% |
| Cardiopathy | 5.6% | 11.5% |
| Pneumopathy | 5.6% | 6.4% |
| Thrombotic event | – | 7.8% |
| Smoking | 50% | 44.9% |
| Steroids | – | 9% |
| Anticoagulation | – | 6.4% |
Results with p < 0.05 compared to controls.
Expressed as median (IQ25–75).
3.2. Humoral response (Table 2, Fig. 1)
Table 2.
Results of immunogenicity of SARS-CoV-2 vaccine.
| HCW | PC | iCDK4/6 | IT | CT | ||
|---|---|---|---|---|---|---|
| Positive serology (%) | After 1D | 100% | 83.8%a | 100% | 94.4% | 64.5%a |
| After 2D | 100% | 95.8% | 100% | 100% | 89.7% | |
| Anti-S IgG (BAU/mL) b | After 1D | 142 | 91 | 367a | 78 | 38a |
| After 2D | 1766 | 1540 | 3427 | 2034 | 613 | |
| Anti-S CD4 T cell response (%) | BL | 36.1% | 16.7%a | 23.1% | 20% | 9.4%a |
| After 2D | 91.7% | 59.7%a | 69.2%a | 58.8%a | 51.7%a | |
| Anti-S CD8 T cell response | BL | 30.6% | 23.1% | 34.6% | 25% | 12.5% |
| After 2D | 94.4% | 55.6%a | 69.2%a | 64.7%a | 37.9%a |
Results with p < 0.05 compared with HCW.
Expressed as median.
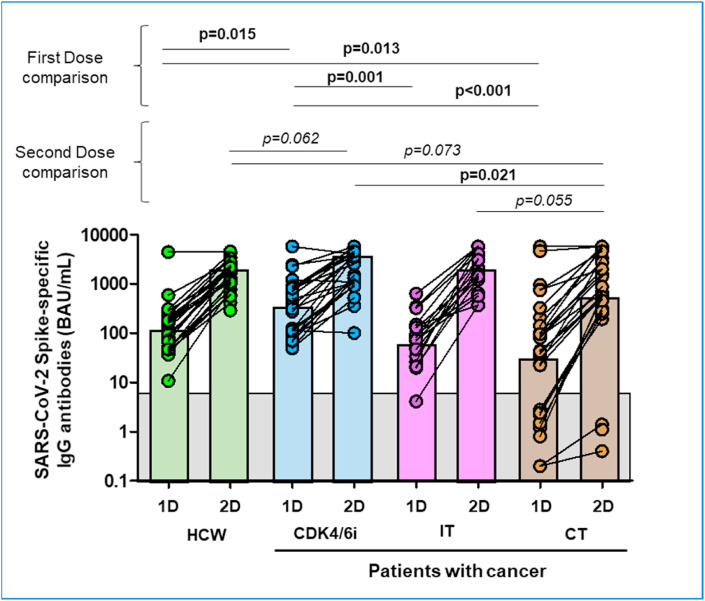
Fig. 1.
SARS-CoV-2 Spike-specific IgG antibody response after first and second dose of the vaccine.
Following vaccination, the rate specific anti-S serologic positivity (anti-S IgG cut-off point at 7,14 BAU/mL) was significantly higher in HCW compared to PC after 1D (100% versus 83.8%; p = 0.04), but similar after 2D (100% versus 95.8%; p = 0.5), but this difference was driven by PC treated with CT after 1D (100% versus 64.5%; p = 0.001) with no differences after 2D. However, the rate of humoral response of CDK4/6i and IT treated cohorts were similar compared to HCW at any timepoint. Regarding the magnitude of response, anti-S IgG levels were 1.15-fold higher in HCW compared to PC; and 2.88-fold higher when compared to PC treated with CT. No statistically significant differences were observed between PC treated with CDK4/6i and IT compared to HCW .
Considering a cut-off point at 300 BAU/mL, serologic positivity after 2D was similar between PC and HCW (97% versus 89%; p = 0.27). Only PC treated with CT showed significant lower rate of humoral response compared to HCW (97% versus 76%; p = 0.018).
3.3. Cellular response (Table 2, Fig. 2)
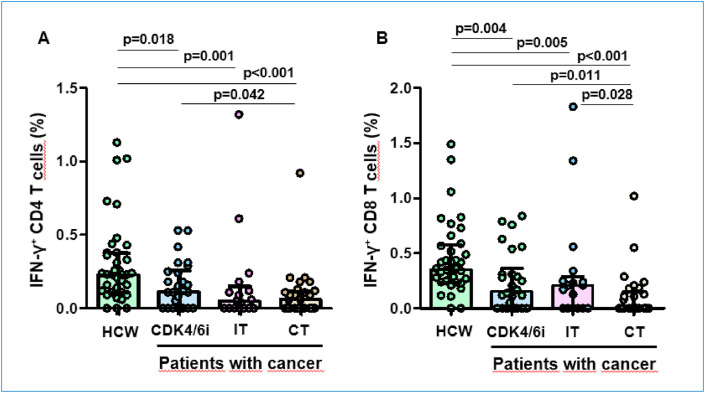
Fig. 2.
SARS-CoV-2 spike-specific T cell response after vaccine.
At baseline, pre-existing CD4+ T cell response was found in 36% of HCW and 17% of PC (p = 0.03), and pre-existing CD8+ T cell response in 31% and 23% (p = 0.5), and again the lower proportion of cross-reactivity was found in those treated with CT (p = 0.01 for CD4+ T cells).
Globally, the rate of cellular response after 2D was significantly lower in PC than in HCW for both anti-S CD4 (91.7% versus 59.7%; p = 0.001) and CD8 (94.4% versus 55.6%; p < 0.001) T cells. This effect was consistent among all PC subgroups (CDK4/6i, IT and CT) for both anti-S CD4 and CD8 T cells (p < 0.05). Furthermore, the magnitude of response measured by the percentage of IFN-g producing CD4+ and CD8+ T cells was also significantly lower in PC in comparison with HCW, showing a decreasing quantity from CDK4/6i treated patients, to those with immunotherapy and to chemotherapy cohort.
We did not find any predictive factor for this poor cellular response among the baseline patient characteristics.
3.4. Patients without cross-reactivity at baseline (Fig. 3)
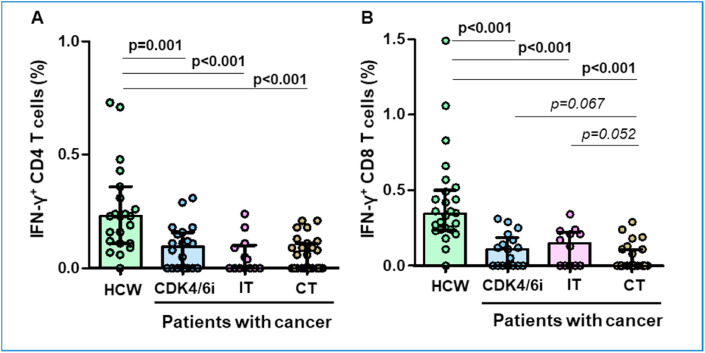
Fig. 3.
SARS-CoV-2 spike-specific T cell response after vaccine in individuals with no cellular response before vaccine.
To avoid the possible interference of pre-exisiting T cell response in the rate and magnitude of response, especially among HCWs, we repeated the humoral and cellular response analysis after withdrawing those participants with T cell cross-reactivity. In these 82 patients (22 healthy volunteers, 17 patients treated with CDK4/6i, 15 with IT and 28 with CT), humoral response was similar between controls and PC after full vaccination. It should be noted that CDK4/6i patients reached significantly higher IgG levels after 1D compared with controls (p = 0.02); and CT patients had notably lower IgG titres after 1D (p = 0.02) and 2D (p = 0.03) compared to controls. In addition, the rate of positive serology in CT patients was particularly lower than controls both after 1D (64.3%; p = 0.007) and 2D (88.5%; p = 0.1).
At a cut-off point of 300 BAU/mL, we found that PC treated with CDK4/6i reached higher rate of positive serology after 1D compared with controls (56%; p = 0.02), without significant differences after 2D. Additionally, CT patients showed lower humoral response after 2D compared to controls (76%; p = 0.02).
Regarding cellular response, PC achieved lower CD4 T cell response (50.9% versus 95.5%, p < 0.001) compared with HCW. These results were consistent within the subgroups of patients treated with CDK4/6i (64.7%; p = 0.02), IT (41.7%; p = 0.001) and CT (46.2%; p < 0.001). Comparable results were found for CD8 T cell response compared with controls (45.5% versus 95.5%, p < 0.001) and among the predefined subgroups of CDK4/6i (58.8%; p = 0.007), IT (58.3%; p = 0.14) and CT (30.8%; p < 0.001). Again, the magnitude of T cell response was also significantly blunted in the different groups of PC, especially for CD4+ T cell response after vaccine (Fig. 3).
3.5. Safety (Table 3, Fig. 4)
Table 3.
Adverse events after vaccine administration.
| Adverse event | HCW (n = 26) | PC (n = 88) | p | CDK4/6 inhibitors (n = 34) | Immunotherapy (n = 23) | Chemotherapy (n = 31) |
|---|---|---|---|---|---|---|
| All | 69.2% | 96.6% | <0.01 | 100% | 91.3% | 96.8% |
| Local pain | 65.4% | 93.2% | <0.01 | 97.1% | 91.3% | 90.3% |
| Local erythema | 8% | 27.3% | 0.06 | 38.2% | 21.7% | 19.4% |
| Local oedema | 28% | 43.2% | 0.25 | 47.1% | 39.1% | 41.9% |
| Fatigue | 42.3% | 70.1% | 0.02 | 75.8% | 56.5% | 74.2% |
| Headache | 20% | 49.4% | 0.01 | 69.7% | 30.4% | 41.9% |
| Myalgias | 32% | 48.3% | 0.18 | 60.6% | 39.1% | 41.9% |
| Arthralgias | 32% | 36.8% | 0.81 | 45.5% | 39.1% | 25.8% |
| Vomiting | 4% | 13.8% | 0.29 | 15.2% | 13% | 12.9% |
| Diarrhoea | – | 19.5% | – | 27.3% | 13% | 16.1% |
| Chills | 20% | 44.8% | 0.04 | 63.6% | 34.8% | 32.3% |
| Fever | 12% | 31% | 0.07 | 33.3% | 34.8% | 25.8% |
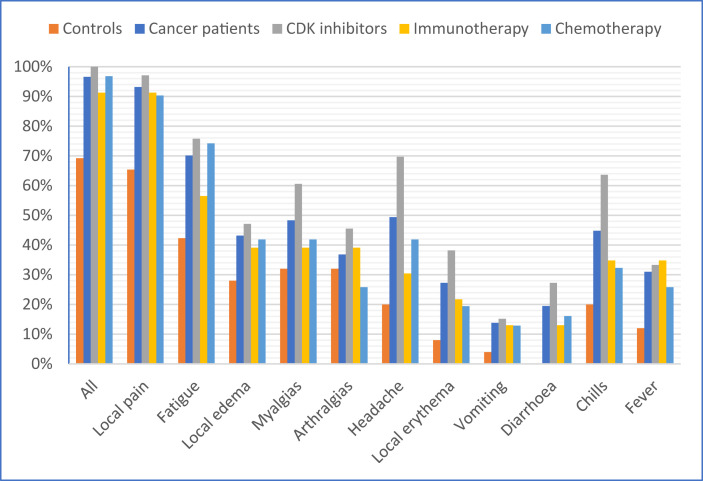
Fig. 4.
Frequency of adverse events after vaccination.
Overall, PC reported notably more adverse events than HCW (96.6% versus 69.2%, p < 0.001). The proportion of patients experiencing local pain, fatigue, headache, and chills was significantly higher in PC compared with HCW (p < 0.05). Symptomatic treatment for adverse events was equally administered in PC (44.2%) and controls (42.3%). None of the patients or controls experienced serious adverse events or needed hospitalization secondary to vaccination.
3.6. Follow up
Our study also included a follow-up period of 6 months to detect symptomatic COVID-19 cases. After a scheduled phone contacts during this period, only two cases were observed. None of them required hospitalization. Seven patients died during this period without evidence of COVID-19 infection.
4. Discussion
The ONCOVac study was designed to prospectively evaluate the serologic status, immunogenicity, and safety of mRNA-1273 vaccine in three cohorts of advanced PC under different oncologic treatments, ruling out the possibility of previous undiagnosed SARS-CoV-2 infection and the existence of pre-existing T cell immunity. Our data demonstrated that T cell response is significantly blunted in percentage and magnitude in all the PC, a striking finding that could confirm the special risk situation for these patients.
The different cohorts were carefully selected based on the widespread use of these cancer therapies in oncology, and their different mechanisms of action, which could potentially influence on the immune system and thus, on vaccination efficacy. Both CDK4/6i and CT constitute immunosuppressive therapies causing mainly neutropenia through reversible bone marrow suppression by cell cycle arrest or depletion of precursor myeloid cells by apoptotic death. On the contrary, immune checkpoint inhibitors (ICI) restore an exhausted immunity and enhance cytotoxic immune response which could, hypothetically, increase the rate of response to COVID-19 vaccines.
In the entire cohort, our results showed lower seroconversion rates compared with HCW after the first dose, without differences after the second dose. These results agree with previous published studies addressing this question and confirm that the humoral response pattern in PC is gradual and slower than in a noncancer population, especially for those with haematologic malignancies. After two doses of COVID-19 vaccines, the majority of patients become seropositive [23,30,31].
This difference after the first dose was mainly driven by a lower humoral response in chemotherapy patients, while CDK4/6i and IT responded similarly to HCW at any time point. In fact, patients receiving CDK4/6i or IT had antibody titers 6- and 3-fold higher than those patients who received CT, respectively. This is also in line with available data showing that among patients receiving CT, there is a higher proportion of patients with a weak or lack of response after the first vaccine dose [32]. Also, consistent with our results, published data with CDK4/6i treated patients confirmed that SARS-CoV-2 neutralizing antibody titers were similar to HCW after the first dose of vaccine in patients with breast cancer [33].
Notably, we observed a good humoral response in those patients receiving immunotherapy, although contradictory data have been published on the influence of IT on vaccine efficacy in terms of humoral response. A recent study documented only 25% of appropriate neutralizing antibodies titers in PC under IT compared with 65.7% of HCW after the first dose [19]. Conversely, the VOICE trial described seroconversion rates of 99.3% and 100% among patients receiving IT and IT-based treatment, respectively [34].
Our study was able to evaluate the anti-S T cell response after two doses of vaccine, important information since few data about cellular response are currently available. We observed a very limited response in the rate and magnitude of CD4+ and CD8+ T cell response after two doses of the vaccine in PC comparing to noncancer population. Notably, we found that the overall rate of CD4+ T cell response was lower than expected in all the cohorts of PC compared with HCW (59.7% versus 91.7%), oscillating from 51.7% of cellular response for those patients receiving CT to 69.2% for those treated with CDK4/6i.
The lack of T cell response could be of vital importance in cancer population, since antigen-specific CD4+ T cell response plays an important role in antigen-specific B cell development, maturation and survival [35,36], and antigen-specific memory CD4+ and CD8+ T cells are likely to be less impacted by antibody escape mutations in variant viral strains [26,37].
This concerning data could be controversial for those patients receiving IT, considering the mechanism of action of these drugs, as well as data from a previous report showing an increased T cell immunity after SARS-CoV-2 infection [38]. Nevertheless, our results are in line with an exploratory finding of the CAPTURE trial, that highlighted a negative impact of IT on cellular immune responses [39]. The reason for this unexpected low T cell response to mRNA-1273 COVID-19 vaccine in this population is unknown and investigation in this issue should be encouraged.
In our study, for the first time, we also present data on the importance of pre-existing, cross-reactive, T cell immunity in PC. We showed that this cross-reactive immunity is also lower in patients with different types of cancer in comparison with HCW, and even lower in those receiving chemotherapy. We have previously demonstrated that cross-reactivity is associated with a better cellular response to the vaccine in noncancer patients [40]. Therefore, we were able to avoid the bias associated with the presence of this cross-reactivity immunity, showing that the differences between PC and patients without cancer in the rate and magnitude of T cell response were even higher after excluding these cases.
In terms of safety, PC presented significantly more adverse events than HCW (96.6% versus 69.2%, p < 0.001), mainly driven by local pain, fatigue, headache, and chills (p < 0.05). This contrasts with available literature showing lower incidence of local (36% versus 52%) and systemic (25 versus 32%) symptoms for PC compared to healthy control after BNT162b2 vaccine [20]. On the other hand, in a study that specifically addressed the safety of IT, PC experienced similar systemic symptoms with the exception of myalgia [41]. In our opinion, this information should be interpreted with caution given the coexistence of cancer or treatment derived symptoms and heterogeneity between groups.
Our study has some limitations in addition to the small sample size. First, we did not include the analysis of neutralizing antibodies; although a good correlation between neutralizing antibodies and anti-spike antibodies has been observed [42]. Second, the cohorts were different in other factors such as age or sex, that could contribute to differences in the rate of immune response to vaccine. The median age of our control group is statistically younger, and age inversely correlates with response to vaccines. The fact that 9% of PC were receiving concurrent steroids, which may affect the results is usual in the cancer patient population.
Additionally, there is still controversy about the antibody's titer cutoff considered protective due to a lack of technique and assays harmonization across published studies, which make it difficult to establish a uniform threshold. In order to overcome this issue, we present the results using both, the SARS-CoV-2 IgG II Quant Alinity threshold (50 AU/mL, or 7,14 BAU/mL), and the cutoff more recently recommended as protective of 300 BAU/mL [43].
Finally, in the follow-up period we did not find high incidence of COVID-19 infections in this cohort and, therefore, we cannot link the absence or a weaker T cell response with a higher risk of COVID-19 infection.
In conclusion, we demonstrate that PC showed a blunted T cell response following mRNA vaccination, slightly modified by the type of cancer therapy and the pre-existing immunity. Although it seems that antigen-specific memory CD4+ and CD8+ T cells are an important weapon against variant viral strains, it remains to be determined if the specific T cell response can protect individuals against COVID-19. This new data together with previously published literature [43], reinforce the idea of administering a booster dose in this population.
CRediT author statement
Alfonso Cortés: Conceptualization, Project administration, Writing- Original draft preparation, Writing- Reviewing and Editing, Supervision. Jose Luis Casado: Conceptualization, Methodology, Writing- Reviewing and Editing, Supervision. Federico Longo: Conceptualization, Formal Analysis, Writing- Reviewing and Editing. Juan José Serrano: Conceptualization, Data curation, Writing- Reviewing and Editing, Cristina Saavedra: Writing- Reviewing and Editing, Resources. Hector Velasco: Investigation. Adrián Martin: Investigation. Jesús Chamorro: Data curation. Diana Rosero: Data curation. María Fernández: Resources. María Gion: Resources. Noelia Martínez Jañez: Resources. Ainara Soria: Resources. Teresa Alonso: Resources. Iñigo Martínez: Resources. Yolanda Lage: Resources. Elena Lopez Miranda: Resources. María Eugenia Olmedo: Resources. Pablo Reguera: Resources. Pablo Gajate: Resources. Javier Molina: Resources. Eva Guerra: Resources. Raquel Fuentes: Resources. Beatriz Romero: Investigation. Mario J Rodriguez-Dominguez: Investigation. Alejandro Vallejo: Formal Analysis, Reviewing and Editing. Alfredo Carrato: Reviewing and Editing.
Funding
Medical Oncology Department unrestricted research funds.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Alfonso Cortes declares the payment for presentations/speaker bureaus/manuscript-writing/educational events from GSK, AstraZeneca, Roche, MSD and Eisai. Alfonso Cortes declares consulting fees from Clovis, Lilly, Pfizer, GSK, Ferrer and Roche. Alfonso Cortes has received research grants from Pfizer. Alfonso Cortes declares support for attending meetings from Roche, Daiichi and Pfzer. Alfonso Cortes is co-founder of ONCARE. Jose Luis Casado and Federico Longo have not conflict of interest to declare. Juan José Serrano declares speaker bureau from Pierre-Fabre and travel/accomodation/expenses from Novartis. Cristina Saavedra declares travel/accomodation/expenses from Lilly and Pfizer. Hector Velasco, Alejandro Vallejo and Adrian San Miguel have not conflict of interest to declare. Jesus Chamorro and Diana Rosero have not conflict of interest to declare. María Fernandez, María Gion and Noelia Martinez have not conflict of interest to declare. Ainara Soria has not conflict of interest to declare. Teresa Alonso declares: Scientific Consultancy Role (speaker and advisory role) and travel grant from IPSEN, Pfizer, Bayer, Sanofi, Janssen, Astellas, Adacap, Eisai, Lilly, Novartis, BMS, Roche. Teresa Alonso declares the participation in Clinical trials from Roche, BMS, MSD, Pfizer, Novartis, IPSEN, Exelixis, Astrazeneca-Medimmune, Janssen, Lilly, Eisai, Astellas. Teresa Alonso declares research support from Roche, Pfizer, IPSEN. Iñigo Martinez and Yolanda Lage have not conflict of interest to declare. Elena López declares to receive advisory/consultancy honorarium from AstraZeneca, Pfizer, Roche, Novartis. Elena López has received speaker bureau/expert testimony honorarium from Roche, Novartis , Eisai, Astra Zeneca; and has received travel/accommodation/expenses from Roche, Novartis. María Eugenia Olmedo has not conflict of interest to declare. Pablo Reguera has not conflict of interest to declare. Pablo Gajate declares travel and educational support from BMS, MSD, Pfizer, Ipsen, Sanofi-Genzyne, Roche and Jansen. Pablo Gajate declares advisor and delivered lectures for BMS, MSD, Merck Serono, Pfizer, Ipsen, Roche, Adacap, Eisai, Sanofi-Genzyme, Novartis and Jansen. Javier Molina declares consultant, advisory or speaker roles for IPSEN, Roche, Pfizer, Sanofi, Janssen, and BMS. Javier Molina has received research grants from Pfizer, IPSEN and Roche. Eva Guerra declares: advisory/consultancy honorarium from AstraZeneca-MSD, Clovis Oncology, GSK-Tesaro, PharmaMar, Roche. Eva Guerra has received speaker bureau/expert testimony honorarium from AstraZeneca, PharmaMar, Roche, GSK; and has received travel/accommodation/expenses from Roche, TESARO, and Baxter. Raquel Fuentes, Beatriz Romero and Mario J Rodriguez-Dominguez have not conflict of interest. Alfredo Carrato has not conflict of interest to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejca.2022.02.017.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.WHO COVID-19 dashboard. World Health Organization; Geneva: 2020. https://covid19.who.int/ Available online: [Google Scholar]
- 2.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadoff J., Gray G., Vandebosch A., Cárdenas V., Shukarev G., Grinsztejn B., et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384:2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., Tukhvatulin A.I., Zubkova O.V., Dzharullaeva A.S., et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet (London, England) 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia S., Zhang Y., Wang Y., Wang H., Yang Y., Gao G.F., et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21:39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giannakoulis V.G., Papoutsi E., Siempos I.I. Effect of cancer on clinical outcomes of patients with COVID-19: a meta-analysis of patient data. JCO Global Oncology. 2020:799–808. doi: 10.1200/GO.20.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saini K.S., Tagliamento M., Lambertini M., McNally R., Romano M., Leone M., et al. Mortality in patients with cancer and coronavirus disease 2019: a systematic review and pooled analysis of 52 studies. Eur J Cancer (Oxford, England: 1990) 2020;139:43–50. doi: 10.1016/j.ejca.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venkatesulu B.P., Chandrasekar V.T., Girdhar P., Advani P., Sharma A., Elumalai T., et al. A systematic review and meta-analysis of cancer patients affected by a novel coronavirus. JNCI Cancer Spectr. 2021;5:1–11. doi: 10.1093/jncics/pkaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribas A., Sengupta R., Locke T., Zaidi S.K., Campbell K.M., Carethers J.M., et al. Priority Covid-19 vaccination for patients with cancer while vaccine supply is limited. Cancer Discov. 2021;11:233–236. doi: 10.1158/2159-8290.CD-20-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garassino M.C., Vyas M., de Vries E.G.E., Kanesvaran R., Giuliani R., Peters S. The ESMO Call to Action on COVID-19 vaccinations and patients with cancer: Vaccinate. Monitor. Educate. Ann Oncol: Off J Eur Soc Med Oncol. 2021:579–581. doi: 10.1016/j.annonc.2021.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Addeo A., Shah P.K., Bordry N., Hudson R.D., Albracht B., Di Marco M., et al. Immunogenicity of SARS-CoV-2 messenger RNA vaccines in patients with cancer. Cancer Cell. 2021;39(8):1091–1098.e2. doi: 10.1016/j.ccell.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrière J., Chamorey E., Adjtoutah Z., Castelnau O., Mahamat A., Marco S., et al. Impaired immunogenicity of BNT162b2 anti-SARS-CoV-2 vaccine in patients treated for solid tumors. Ann Oncol: Off J Eur Soc Med Oncol. 2021:1053–1055. doi: 10.1016/j.annonc.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavanna L., Citterio C., Biasini C., Madaro S., Bacchetta N., Lis A., et al. COVID-19 vaccines in adult cancer patients with solid tumours undergoing active treatment: seropositivity and safety. A prospective observational study in Italy. Eur J Cancer. 2021;157:441–449. doi: 10.1016/j.ejca.2021.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Massarweh A., Eliakim-Raz N., Stemmer A., Levy-Barda A., Yust-Katz S., Zer A., et al. Evaluation of seropositivity following BNT162b2 messenger RNA vaccination for SARS-CoV-2 in patients undergoing treatment for cancer. JAMA Oncol. 2021;7:1133–1140. doi: 10.1001/jamaoncol.2021.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peeters M., Verbruggen L., Teuwen L., Vanhoutte G., Vande Kerckhove S., Peeters B., et al. Reduced humoral immune response after BNT162b2 coronavirus disease 2019 messenger RNA vaccination in cancer patients under antineoplastic treatment. ESMO Open. 2021;6:100274. doi: 10.1016/j.esmoop.2021.100274. Elsevier Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shmueli E.S., Itay A., Margalit O., Berger R., Halperin S., Jurkowicz M., et al. Efficacy and safety of BNT162b2 vaccination in patients with solid cancer receiving anticancer therapy - a single centre prospective study. Eur J Cancer (Oxford, England: 1990) 2021;157:124–131. doi: 10.1016/j.ejca.2021.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terpos E., Zagouri F., Liontos M., Sklirou A.D., Koutsoukos K., Markellos C., et al. Low titers of SARS-CoV-2 neutralizing antibodies after first vaccination dose in cancer patients receiving checkpoint inhibitors. J Hematol Oncol. 2021:86. doi: 10.1186/s13045-021-01099-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monin L., Laing A.G., Muñoz-Ruiz M., McKenzie D.R., Del Molino Del Barrio I., Alaguthurai T., et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22:765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shroff R.T., Chalasani P., Wei R., Pennington D., Quirk G., Schoenle M.V., et al. Immune responses to two and three doses of the BNT162b2 mRNA vaccine in adults with solid tumors. Nat Med. 2021;27:2002–2011. doi: 10.1038/s41591-021-01542-z. Springer US. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehmsen S., Asmussen A., Jeppesen S.S., Nilsson A.C., Østerlev S., Vestergaard H., et al. Antibody and T cell immune responses following mRNA COVID-19 vaccination in patients with cancer. Cancer Cell. 2021;39:1034–1036. doi: 10.1016/j.ccell.2021.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goshen-Lago T., Waldhorn I., Holland R., Szwarcwort-Cohen M., Reiner-Benaim A., Shachor-Meyouhas Y., et al. Serologic status and toxic effects of the SARS-CoV-2 BNT162b2 vaccine in patients undergoing treatment for cancer. JAMA Oncol. 2021;7:1507–1513. doi: 10.1001/jamaoncol.2021.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdul-Jawad S., Baù L., Alaguthurai T., Del Molino Del Barrio I., Laing A.G., Hayday T.S., et al. Acute immune signatures and their legacies in severe acute respiratory syndrome coronavirus-2 infected cancer patients. Cancer Cell. 2021;39:257–275.e6. doi: 10.1016/j.ccell.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mansi L., Spehner L., Daguindau E., Bouiller K., Almotlak H., Stein U., et al. Study of the SARS-CoV-2-specific immune T-cell responses in COVID-19-positive cancer patients. Eur J Cancer (Oxford, England: 1990) 2021;150:1–9. doi: 10.1016/j.ejca.2021.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tarke A., Sidney J., Methot N., Yu E.D., Zhang Y., Dan J.M., et al. Impact of SARS-CoV-2 variants on the total CD4+ and CD8+ T cell reactivity in infected or vaccinated individuals. Cell Rep Med. 2021;2:100355. doi: 10.1016/j.xcrm.2021.100355. ElsevierCompany. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., et al. Targets of T Cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501.e15. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le Bert N., Tan A.T., Kunasegaran K., Tham C.Y.L., Hafezi M., Chia A., et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584:457–462. doi: 10.1038/s41586-020-2550-z. England. [DOI] [PubMed] [Google Scholar]
- 29.Saker K., Escuret V., Pitiot V., Massardier-Pilonchéry A., Paul S., Mokdad B., et al. Evaluation of commercial anti-SARS-CoV-2 antibody assays and comparison of standardized titers in vaccinated health care workers. J Clin Microbiol. 2022;60 doi: 10.1128/JCM.01746-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corti C., Antonarelli G., Scotté F., Spano J.P., Barrière J., Michot J.M., et al. Seroconversion rate after vaccination against COVID-19 in patients with cancer-a systematic review. Ann Oncol: Off J Eur Soc Med Oncol. 2022;33:158–168. doi: 10.1016/j.annonc.2021.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becerril-Gaitan A., Vaca-Cartagena B.F., Ferrigno A.S., Mesa-Chavez F., Barrientos-Gutiérrez T., Tagliamento M., et al. Immunogenicity and risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection after coronavirus disease 2019 (COVID-19) vaccination in patients with cancer: a systematic review and meta-analysis. Eur J Cancer (Oxford, England: 1990) 2022;160:243–260. doi: 10.1016/j.ejca.2021.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shroff R.T., Chalasani P., Wei R., Pennington D., Quirk G., Schoenle M.V., et al. Immune responses to COVID-19 mRNA vaccines in patients with solid tumors on active, immunosuppressive cancer therapy. medRxiv: Preprint Server for Health Sciences. 2021 doi: 10.1101/2021.05.13.21257129. [DOI] [Google Scholar]
- 33.Zagouri F., Terpos E., Fiste O., Liontos M., Briasoulis A., Katsiana I., et al. SARS-CoV-2 neutralizing antibodies after first vaccination dose in breast cancer patients receiving CDK4/6 inhibitors. Breast (Edinburgh, Scotland) 2021;60:58–61. doi: 10.1016/j.breast.2021.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Veldt A.A.M., Oosting S.F., Dingemans A.M.C., Fehrmann R.S.N., GeurtsvanKessel C., Jalving M., et al. COVID-19 vaccination: the VOICE for patients with cancer. Nat Med. 2021;27:568–569. doi: 10.1038/s41591-021-01240-w. Springer US. [DOI] [PubMed] [Google Scholar]
- 35.Painter M.M., Mathew D., Goel R.R., Apostolidis S.A., Pattekar A., Kuthuru O., et al. Rapid induction of antigen-specific CD4+ T cells guides coordinated humoral and cellular immune responses to SARS-CoV-2 mRNA vaccination. bioRxiv. 2021 doi: 10.1016/j.immuni.2021.08.001. 2021.04.21.440862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng Y., Mentzer A.J., Liu G., Yao X., Yin Z., Dong D., et al. Broad and strong memory CD4(+) and CD8(+) T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol. 2020;21:1336–1345. doi: 10.1038/s41590-020-0782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geers D., Shamier M.C., Bogers S., den Hartog G., Gommers L., Nieuwkoop N.N., et al. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abj1750. United States. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yatim N., Boussier J., Tetu P., Smith N., Bruel T., Charbit B., et al. Immune checkpoint inhibitors increase T cell immunity during SARS-CoV-2 infection. Sci Adv. 2021;7:1–13. doi: 10.1126/sciadv.abg4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shepherd S.T.C., Fendler A., Au L., Byrne F., Wilkinson K., Wu M., et al. 1557O Adaptive immunity to SARS-CoV-2 infection and vaccination in cancer patients: the CAPTURE study. Ann Oncol. 2021;32:S1129. [Google Scholar]
- 40.Casado J.L., Haemmerle J., Vizcarra P., Rodriguez-Dominguez M., Velasco T., Velasco H., et al. T-cell response after first dose of BNT162b2 SARS-CoV-2 vaccine among healthcare workers with previous infection or cross-reactive immunity. Clin Transl Immunol. 2021;10:1–8. doi: 10.1002/cti2.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waissengrin B., Agbarya A., Safadi E., Padova H., Wolf I. Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol. 2021;22:581–583. doi: 10.1016/S1470-2045(21)00155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salazar E., Kuchipudi S.V., Christensen P.A., Eagar T., Yi X., Zhao P., et al. Convalescent plasma anti-SARS-CoV-2 spike protein ectodomain and receptor-binding domain IgG correlate with virus neutralization. J Clin Invest. 2020;130:6728–6738. doi: 10.1172/JCI141206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barrière J., Carles M., Audigier-Valette C., Re D., Adjtoutah Z., Seitz-Polski B., et al. Third dose of anti-SARS-CoV-2 vaccine for patients with cancer: should humoral responses be monitored? A position article. Eur J Cancer (Oxford, England: 1990) 2022;162:182–193. doi: 10.1016/j.ejca.2021.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.