Abstract
Root border cells are living cells that surround root apices of most plant species and are involved in production of root exudates. We tested predictions of the hypothesis that they participate in detection and avoidance of aluminum (Al) toxicity by comparing responses of two snapbean (Phaseolus vulgaris) cultivars (cv Dade and cv Romano) known to differ in Al resistance at the whole-root level. Root border cells of these cultivars were killed by excess Al in agarose gels or in simple salt solutions. Percent viability of Al-sensitive cv Romano border cells exposed in situ for 96 h to 200 μm total Al in an agarose gel was significantly less than that of cv Dade border cells; similarly, relative viability of harvested cv Romano border cells was significantly less than that of cv Dade cells after 24 h in 25 μm total Al in a simple salt solution. These results indicate that Al-resistance mechanisms that operate at the level of whole roots also operate at the cellular level in border cells. Al induced a thicker mucilage layer around detached border cells of both cultivars. Cultivar Dade border cells produced a thicker mucilage layer in response to 25 μM Al compared with that of cv Romano cells after 8 h of treatment and this phenomenon preceded that of observed cultivar differences in relative cell viability. Release of an Al-binding mucilage by border cells could play a role in protecting root tips from Al-induced cellular damage.
Acid soils occur in nearly one-half of all nonirrigated, arable lands in the world (Fageria et al., 1988), particularly in the tropics. In addition, soil acidification due to fertilizers or acid rain caused by industrial pollution are an increasing threat to agricultural and natural ecosystems in temperate regions (van Breemen, 1985). Acidic conditions in soil solubilize aluminum (Al), resulting in phytotoxicity.
The physiological mechanism by which Al damages cells is not known (Kochian, 1995). Inhibition of root growth is the first easily observable symptom of Al toxicity (Foy, 1988), which occurs within 1 to 2 h (Clarkson, 1965). The root apex appears to be the primary site of Al toxicity because exposure to Al of the terminal 2 to 3 mm of a maize root is required for inhibition of growth (Ryan et al., 1993). The initial sites of Al uptake in maize are the root cap and root mucilage, as shown by staining with hematoxylin, a compound that binds Al in vitro to form a colored complex (Bennet et al., 1985).
Comparative studies of Al tolerance in 22 species in seven families have established that plants can resist the toxic effects of Al. There are many proposed mechanisms of Al tolerance in plants that involve external avoidance or internal tolerance (Foy, 1988; Taylor, 1991). Substantial evidence exists that one mechanism of Al avoidance is chelation of Al by organic acids released by roots in response to excess Al (Miyasaka et al., 1991; Delhaize et al., 1993; Pellet et al., 1995).
A second possible mechanism of Al avoidance is adsorption of Al by negatively charged root mucilages, which prevents migration of Al into the root meristem. In one study mucilage of an Al-tolerant cultivar of cowpea bound more than one-half of the total Al content of the apical 1 cm of root tips, and its removal depressed root elongation in the presence of Al (Horst et al., 1982). Li et al. (2000) also have demonstrated that Al binds strongly to maize mucilage.
In most studies the material termed “mucilage” has included all extracellular materials that can be removed from the tip by immersion in water or by manual wiping with a tissue. This material actually is a complex biological mixture of high-Mr mucilage secreted by the root cap, a population of several thousand metabolically active root “border” cells, and an array of cell wall fragments that are solubilized as cells separate from the cap (Hawes et al., 1998). Border cells per se express a unique profile of mRNAs and proteins (Brigham et al., 1995) and many of these proteins are secreted into the external environment. Together, the mixture of root cap mucilage, border cells, and their associated exudates comprises up to 98% of the total exudate released from healthy young roots and its production is tightly regulated by the root in response to environmental and endogenous signals (Hawes et al., 2000).
In the absence of free water, border cells and their encasing mucilage form a tight sheath around the periphery of the cap, and root cap turnover remains in a quiescent state (Hawes et al., 1998). Upon removal of cells by abrasion or by immersion of the root tip in water, border cell and mucilage production are reinitiated within 1 to 2 min, concomitantly with a global switch in gene expression throughout the root cap (Brigham et al., 1998) and production of a new set of tip exudates within a 25-h period (Hawes and Lin, 1990). In solution culture, border cell populations, root cap mucilage, and all other materials secreted by border cells and the root cap dissociate from the roots and are removed from the root tip periphery as they are produced. After detachment from root tips of plants grown in hydroponic culture, border cells settle to the bottom of the vessel where they can survive for months (Knudson, 1919). In soil, little is known about the fate of border cells, but these detached cells can reduce the mechanical friction of the growing root under some conditions (Bengough and McKenzie, 1997).
Border cells provide a means of packaging exudates in living cells, which act as a chemical, physical, and biological interface between roots and soil. To our knowledge, the possible role of root border cells in Al-tolerance mechanisms has been largely ignored. Fiskesjo (1990) found that exposure to Al resulted in cytoplasmic structures in cells within the root cap of onion and she proposed a novel hypothesis that toxic Al could be removed by separation of root border cells from the cap.
In this paper we confirm the differential Al resistance of whole root systems of two snapbean (Phaseolus vulgaris) cultivars (cv Dade and cv Romano) in simple salt solution and in agarose gels. Further experiments were carried out to examine the possibility that differences in border cells and/or associated root exudates are involved in Al resistance by comparing border cell number, viability, and appearance in situ around root tips or in suspension. We present evidence consistent with the hypothesis that root border cells have the capacity to protect root apices from Al toxicity, possibly through exudation of an Al-binding mucilage.
RESULTS
Differential Resistance of Snapbean Cultivars
In Simple Salt Solution
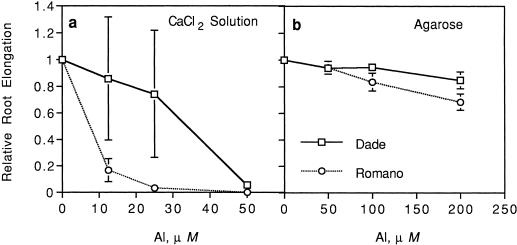
Compared with controls, relative root elongation of both cultivars ranged from 0.18 to 0.50 within 24 h at 12.5 to 50 μm Al. However, roots of cv Dade exposed to 12.5 to 25 μm Al continued to elongate over time, whereas cv Romano tips tended to turn brown and ceased to grow in Al. Thus, significant cultivar differences in relative root elongation rates in response to Al were evident at 72 h (data not shown) and 96 h after the start of treatments (Fig. 1a). Cultivar Dade had significantly greater relative root elongation compared with cv Romano (P = 0.049 and 0.04, respectively) at 12.5 and 25 μm Al. These results confirmed previous observations that these two cultivars vary in Al sensitivity at the whole-root level when grown in solution (Miyasaka et al., 1991).
Figure 1.
Relative root elongation of two snapbean cultivars (cv Dade and cv Romano) with increasing total Al levels after 96 h in CaCl2 solution (a; 0, 12.5, 25, and 50 μm) agarose gel (b; 0, 50, 100, and 200 μm). Significant Al (P = 0.0003 and 0.0001), cultivar (P = 0.02 and 0.007), and Al × cultivar (P = 0.047 and 0.008) effects were found in CaCl2 solution and agarose gel, respectively. Error bars represent ses of mean.
In Agarose
Since all root exudates at the tip, including border cells, are dissociated away from roots grown in solution culture, this experiment was designed to compare cultivar sensitivity under conditions that allowed retention of the normal configuration of root tip components. Both cultivars exhibited reduced relative root elongation of 0.63 to 0.84 within 24 h after exposure to 100 or 200 μm Al. Primary roots of both cultivars tended to curve away from the Al-containing agarose, but the filter paper placed over the roots prevented loss of contact with it. In contrast to results in solution culture, root tips of Al-sensitive cv Romano did not die at the higher Al levels. Similar to solution culture, a significant cultivar difference in root growth was apparent only after 96 h when cv Dade had significantly greater relative root elongation at 200 μm Al compared with that of cv Romano (Fig. 1b; P = 0.02). These results confirmed that cultivar differences in Al sensitivity seen in solution culture could be reproduced using agarose gels.
Snapbean Border Cells
The number of viable border cells in both snapbean cultivars increased significantly (P = 0.0001) with increasing root length until a maximum of 4,400 (± 280) was reached after 1.75 cm. No significant cultivar differences in the number of viable border cells were detected. A maximal number of viable border cells were found previously in other legume species (Hawes and Pueppke, 1986).
Aluminum Effect on Border Cells
In Situ Cultivar Response in Agarose
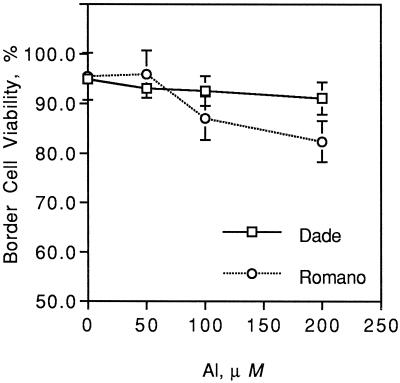
Increasing Al levels significantly decreased percent viability of border cells of both snapbean cultivars in situ (Fig. 2). Aluminum-sensitive cv Romano had significantly lower border cell viability at 200 μm total Al compared with Al-resistant cv Dade (P = 0.01) at 96 h when cultivar differences in relative root elongation were observed (Fig. 1b). No significant difference in total number of border cells was observed due to treatments (data not shown).
Figure 2.
Border cell viability of two snapbean cultivars (cv Dade and cv Romano) exposed in situ to increasing levels of total Al (0, 50, 100, and 200 μm) in agarose gels after 96 h. Significant Al (P = 0.0001) and Al × cultivar (P = 0.009) effects were found. Error bars represent ses of mean.
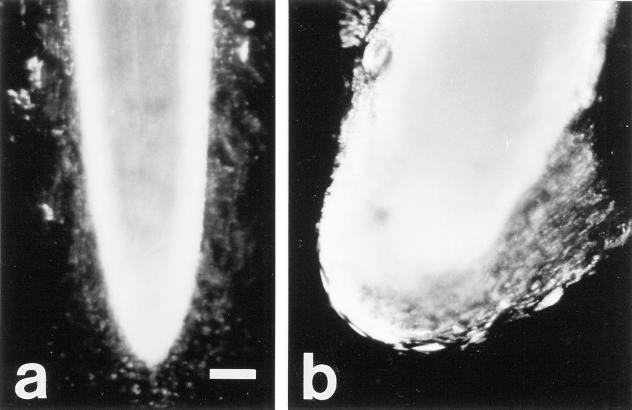
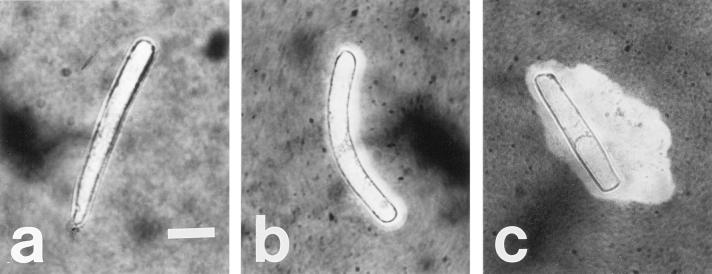
A conspicuous difference in the appearance of border cell populations between the two cultivars was observed at 96 h of exposure to Al treatments. When seedlings were removed from agarose and root tips were placed into water for approximately 60 s, control border cells of both cultivars dispersed readily into suspension (Fig. 3a), as did those of cv Dade roots exposed to Al. However, border cells of cv Romano roots grown at 200 μm Al clumped together and did not disperse into solution (Fig. 3b). These results suggested that responses to Al of border cells and/or their associated exudates vary between the two cultivars.
Figure 3.
Effect of Al exposure in situ on border cells of two snapbean cultivars a, cv Dade roots exposed to 0 Al in agarose with the blurring of border cells was due to dispersal of cells into solution; b, cv Romano roots exposed to 200 μm total Al in agarose with border cells clumping together and not separating readily from each other or the root apex. Scale indicates 0.5 mm.
Responses of Detached cv Dade Border Cells
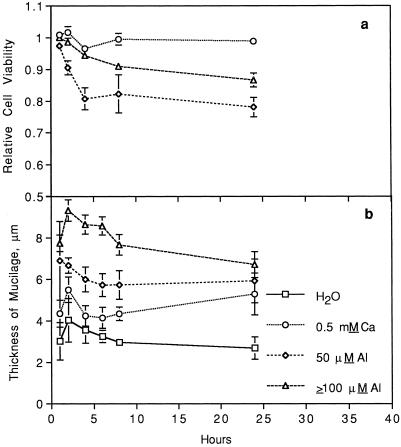
In the previous experiment, border cells of cv Dade exposed in situ to Al appeared resistant to Al damage. To test whether this Al resistance was an intrinsic characteristic of border cells, populations from cv Dade roots were harvested and their responses to Al in solution culture examined. The presence of Al significantly decreased relative viability of harvested cv Dade border cells (Fig. 4a). This decrease in viability of cv Dade border cells, which was initiated within 1 h, appeared nearly linear to 4 h after exposure to Al and then it leveled off between 8 to 24 h. Surprisingly, the relative viability of harvested border cells exposed to 50 μm Al was significantly lower than those of cells exposed to higher Al treatments (P = 0.0001). The percentage mortality of border cells in the water treatment was not significantly different from that of cells in the 0.5 mm CaCl2 treatment.
Figure 4.
a, Cell viability of detached cv Dade border cells in response to increasing hours of exposure to increasing levels of total Al (0, 50, and ≥100 μm) in 0.5 mm CaCl2 relative to that of water controls. Relative viability of cells was calculated as (percent viability at × Al level)/(Percent viability in water). b, Thickness of the mucilage layer of detached cv Dade border cells in response to increasing hours of exposure to treatment solutions containing water only or increasing levels of total Al (0, 50, and ≥100 μm) in 0.5 mm CaCl2. For relative cell viability, significant treatment (P = 0.0001), hours of exposure (linear effect: P = 0.0001; quadratic effect: P = 0.0001), and treatment × hours of exposure (P = 0.001) effects were found. For thickness of border cell mucilage, significant treatment (P = 0.0001) and hours of exposure (linear effect: P = 0.002) effects were found. Error bars represent ses of mean.
Microscopic examination of cv Dade border cells stained with India ink revealed that individual cells exposed to Al were surrounded by a conspicuous layer of mucilage (Fig. 5, b and c) compared with the thin layer found in water controls (Fig. 5a). A time-course examination of this phenomenon showed that cv Dade border cells developed a thicker mucilage layer within 1 h of exposure to Al compared with controls in water (Fig. 4b). The width of the mucilage layer that developed at ≥100 μm Al was significantly greater than that which developed in response to 50 μm (Fig. 4b; P = 0.0001). Thickness of this cell mucilage layer was correlated positively with cell viability in response to Al dosage. For example, relative cell viability at Al levels ≥100 μm was 0.91 at 4 h, whereas that at 50 μm Al was much less at 0.79 (Fig. 4a).
Figure 5.
Exudation of mucilage around detached cv Dade border cells exposed to water (a), 100 μm total Al (b), and 200 μm total Al (c). India ink was used to visualize the mucilage around border cells because it does not penetrate this layer. Scale indicates 20 μm.
A significantly thicker mucilage layer also developed around cells exposed to 0.5 mm CaCl2 compared with those of cells in water (Fig. 4b; P = 0.01), although this layer was much thinner than those found after exposure to Al. Over 24 h of exposure to treatments, thickness of the mucilage layer around border cells decreased in a small, but significant, linear pattern.
Cultivar Responses of Detached Border Cells
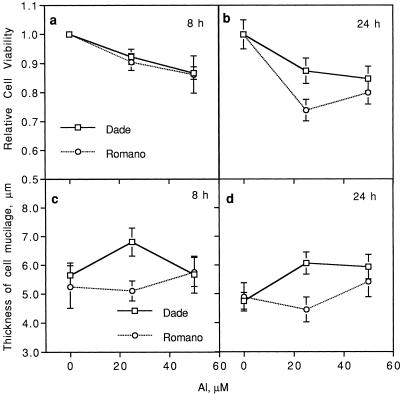
Exposure of harvested border cells from both cultivars to excess Al significantly decreased percent viability relative to control cells in water (P = 0.0001; Fig. 6, a and b). Increasing hours of exposure to Al treatments significantly decreased relative viability of border cells. Border cells of Al-sensitive cv Romano exhibited significantly greater relative mortality compared with those of cv Dade, particularly at 24 h in 25 μm Al (P = 0.002).
Figure 6.
Relative viability of detached border cells from two snapbean cultivars after 8 (a) and 24 h (b) of exposure to increasing levels of Al (0, 25, and 50 μm) in 0.5 mm CaCl2. Thickness of the mucilage layer around border cells from two snapbean cultivars (cv Dade and cv Romano) in response to increasing levels of Al (0, 25, and 50 μm) in 0.5 mm CaCl2 at 8 (c) and 24 h (d) after initiation of treatments. For relative cell viability, significant Al (P = 0.0001), cultivar (P = 0.04), and hours of exposure (P = 0.002) effects were found. For thickness of mucilage layer, significant treatment (P = 0.0001), cultivar (P = 0.01), and treatment by hours of exposure interaction (P = 0.008) effects were found. Error bars represent ses of mean.
Exposure of border cells from both cultivars to Al increased thickness of the mucilage layer around cells of both cultivars (Fig. 6, c and d). Al-resistant cv Dade border cells had thicker mucilage layers compared with those of cv Romano cells, at 8 (P = 0.03) and 24 h (P = 0.02) after exposure to 25 μm Al. Similar effects of CaCl2 and time on thickness of the cell mucilage layer were found for both cultivars as described earlier for cv Dade alone.
DISCUSSION
The root tip is the primary site of Al toxicity in higher plants (Ryan et al., 1993) and its encasing mucilaginous capsule has been implicated as a protective source of materials that prevent the uptake of Al into root meristems (Horst et al., 1982). To our knowledge, the response to Al and the possible impact on Al resistance of the thousands of metabolically active border cells that are a major constituent of the root tip capsule have never been examined. In the current paper two snapbean cultivars that vary in their susceptibility to Al were used to test the hypothesis that border cells are involved in the detection and avoidance of Al toxicity.
Two snapbean cultivars previously found to vary in susceptibility to Al damage in soil (Foy et al., 1972) or nutrient solution (Miyasaka et al., 1991) were shown to vary in sensitivity of their root tips to Al-induced damage when grown in a simple salt solution. Both cultivars exhibited reduced root growth within 24 h, but root tips of the Al-sensitive cv Romano tended to became necrotic and ceased growth altogether in the presence of Al, whereas root tips of cv Dade remained functional and continued to elongate.
Immersion of roots in solution results in continuous dispersal of border cells from their usual location surrounding root apices, making it difficult to study the possible function of root border cells in protecting root apices from Al injury. Therefore, experimental conditions were established using agarose gels. Although one disadvantage of agarose is that speciation of Al is not possible because of uncertainties about complexation reactions between agarose and Al, such gels proved to be an excellent medium for growth of snapbean roots. In addition, these Al-containing gels allowed evaluation of Al responses of roots with an intact border cell-mucilage capsule present at the root tip. Cultivar differences in Al resistance that were observed earlier in a simple salt solution were confirmed to occur when roots were grown on Al-containing agarose after 96 h.
Snapbean cultivars were characterized for their formation of root border cells. Viable border cells increased in number with increasing root length until a maximum number that did not differ between snapbean cultivars was reached. Thereafter, the number of live border cells did not increase, indicating that the root cap tightly regulated the number of live border cells, as has been found for other plant species (Hawes and Pueppke, 1986; Hawes and Brigham, 1992; Hawes et al., 1998).
High levels of Al killed border cells of both snapbean cultivars in situ on roots in agarose gels or in vitro in simple salt solutions. Border cells are living cells that are able to remain viable for as long as 90 d in hydroponic culture (Knudson, 1919), and they are not pre-programmed for cell death as implied in the commonly used term “sloughed root cap cells.” Since the number of live border cells is regulated, increased cell death by Al could provide a signal to the roots that a toxin has been encountered in the rhizosphere.
A conspicuous cultivar difference in viability of border cells and in their appearance was detected 96 h after exposure to 200 μm Al in situ, when cultivar differences in root elongation were observed. Cultivar Romano border cell viability was significantly reduced by Al, but cv Dade viability decreased very little. In addition, whereas border cells of cv Dade exposed to Al behaved like controls and dispersed into suspension immediately upon immersion in water, cv Romano border cells formed a cohesive aggregate that did not detach from the root tip. Such a reaction could involve Al damage to the root cap's normal ability to produce border cells. For example, inhibition of cell wall solubilizing enzyme activity in the root cap results in a similar phenotype (Wen et al., 1999). It is possible that Al-induced exudation of citrate by cv Dade roots (Miyasaka et al., 1991) could prevent Al from binding to the root cap, border cells, and/or their surrounding mucilage. Li et al. (2000) showed that citrate could be used to desorb Al bound to maize mucilage.
To separate the Al effects on border cells from those on root tips, experiments were carried out using detached border cells. Cultivar Dade border cells in solution produced a surprising result: cell death in response to Al began immediately, but then virtually ceased after 4 to 8 h. This rapid killing of border cells in response to Al was unlike the 6-h lag period before death occurred in tobacco cells exposed to Al and Fe (Ikegawa et al., 1998). It was similar to the response to pathotoxins such as victorin or Helminthosporium maydis race T toxin, which caused linear decreases in percent viability of border cells of oat and maize over a 10- to 24-h period, respectively (Hawes and Wheeler, 1982). However, in contrast to this linear decrease in cell viability due to pathotoxins, cv Dade border cell death leveled off after 4 to 8 h of exposure to Al. This unique effect was found in concert with a surprising discovery that viability was lower in response to 50 μm Al compared with levels ≥100 μm Al. Such border cell death kinetics and dosage response are distinct from patterns seen in response to any other toxins and stresses tested previously (Hawes and Wheeler, 1982; Hawes and Brigham, 1992; Hawes et al., 1998).
The abrupt halt in a linear rate of cell death suggested the reduction of Al toxicity and/or a loss of cellular uptake or sensitivity. One possibility that could account for such results is a change in pH; however, in the current experiments, solution pH of Al-containing treatments did not increase by more than 0.3 pH units over a 24-h period. Kinraide and Parker (1990) reported that Al phytotoxicity to another legume, soybean, was greater at pH 4.8 than 4.2. It was apparent that the reduced mortality in cv Dade border cells occurred by a physiological mechanism other than an increase in extracellular pH.
An alternative possibility is that the observed production of a conspicuous mucilage layer surrounding individual border cells prevented further uptake of soluble Al. Production of this polysaccharide layer around detached border cells was temporally and spatially correlated with reduced cell death in cv Dade border cells: induction of this layer preceded the cessation of linear cell death rates, and thicker layers were correlated with lower mortality. Detached border cells have been shown previously to produce extracellular polysaccharides after detachment from roots (W.D. Bauer, unpublished data, cited in Hawes and Brigham, 1992). Induction of mucilage production by border cells is not a general response to toxins or pathogens, but rather a specific response to certain stress factors. For example, border cells of pea produced a mucilage layer in response to Agrobacterium tumefaciens, but not in response to Escherichia coli (Hawes and Brigham, 1992).
Cultivar differences in response to Al of whole roots were found also in detached root border cells exposed to Al. Harvested border cells of Al-resistant cv Dade had significantly greater relative viability compared with those of cv Romano when exposed to 25 μm total Al for 24 h. Thus, cultivar differences in whole root response to Al were expressed also at the cellular level in detached border cells in the absence of roots. This differential response to Al in border cells could not be due to Al-induced exudation of citrate by cv Dade roots (Miyasaka et al., 1991), because border cells were harvested from roots not exposed to Al.
Mucilage production by detached border cells of Al-sensitive cv Romano was measurably less than that of cv Dade border cells after 8 h of exposure to 25 μm Al and this difference preceded cultivar differences in relative viability observed after 24 h in 25 μm Al. Such results suggest that Al-induced mucilage exudation in border cells is a cellular self-protection response. In an alternate manner, it is possible that cv Dade border cells have another Al-resistance mechanism that allows them to survive and continue to produce mucilage that is not involved in protection from Al. Preliminary results showed that exudation of organic acids by border cells is not involved, because no significant cultivar difference in exudation of citrate by border cells was observed in the presence of Al (S.C. Miyasaka, unpublished data). Root border cells could serve as a good model system to study cellular responses to Al and genotypic variation in Al resistance.
How border cells and their Al-inducible mucilage layer might contribute to the observed cultivar differences in root growth are not known. Al bound to mucilage was found to be non-phytotoxic to maize root cells and yet Al-induced root elongation was not affected by the presence or absence of mucilage (Li et al., 2000). In a similar manner, Ryan et al. (1993) removed the root cap of maize roots without altering Al-induced inhibition of root elongation over 24 h. It is important to note here that both treatments were applied in solution culture in which mucilage and border cells are continually dispersed away from the root tip. Since control and treated root tips would be devoid of much mucilaginous materials, the possible impact of its Al-chelating abilities cannot be evaluated in these two studies. In addition, use of root elongation as the sole measure of Al toxicity is not sufficient to characterize root responses to excess Al. Longer term Al-avoidance mechanisms that protect the apical root meristem may be more important for continued root growth.
It will be of interest in future studies to test the hypothesis that this border cell mucilage layer is one mechanism of Al avoidance in some species. An Al-induced mucilage layer surrounding each of several thousand cells encapsulating the root tip could provide a significant barrier to Al uptake into the root. Such a protective effect of root border cells in avoidance of Al toxicity could help to explain the differences in Al resistance found between plants grown in solution culture where border cells are continually dispersed away from the root versus those grown in soil or sand culture (Horst et al., 1990) or in agarose. Previous studies have suggested that mucilaginous materials at the root tip do have the potential to prevent uptake of Al into the root (Horst et al., 1982). Also, Ryan et al. (1993) found a greater penetration of the hematoxylin stain for Al into the meristematic region of decapped roots compared with intact roots. In addition, maize mucilage separated away from root tips was shown to tightly bind Al and maize roots without mucilage had a slightly higher Al content than control roots (Li et al., 2000). Based on those reported experimental protocols, border cells must have been a component of the tested mixture, though it is impossible to assess their relative contribution to the Al-binding activity.
This paper is the first to report that Al toxicity kills root border cells and that cultivar differences in response to excess Al of whole-root systems are exhibited also by detached border cells. Al induces increased exudation of mucilage around detached border cells, and the presence of this mucilage layer in cv Dade cells is associated with an inhibition of cell death. In addition, compared with cv Dade cells, harvested cv Romano border cells had a significantly thinner mucilage layer and decreased cell viability due to Al exposure under certain conditions. This research opens up interesting avenues to explore further the role of root border cells in detection and avoidance of Al toxicity. Transgenic roots with altered production of border cells (Wen et al., 1999) could be used to test the possibility that border cells and their associated mucilages help to protect plant health by inhibiting Al uptake into roots.
MATERIALS AND METHODS
Aseptic Conditions
Aseptic conditions were maintained to prevent mortality of border cells due to microorganisms and to prevent microbial breakdown of released exudates. Solutions or gels without Al were autoclaved, then filter-sterilized Al solutions were added. Sterility in solutions was checked by plating 100 μL onto agar plates containing Luria broth. Only minor microbial contamination was found at the completion of experiments.
Based on preliminary trials, seeds of snapbean (Phaseolus vulgaris) cv Dade were surface-sterilized by placing them in 95% (v/v) ethanol for 3 min, then in 8.8 m hydrogen peroxide for 30 min, followed by soaking them in sterile water for 1 h. Seeds of cv Romano were surface-sterilized by placing them in 95% (v/v) ethanol for 1 min, followed by 15 min in 8.8 m hydrogen peroxide, and 6 h of soaking in sterile water. Seedlings were germinated in the dark at 24°C for 3 to 4 d in Petri dishes containing filter paper (Whatman No. 4, Whatman, Clifton, NJ) placed over a gel containing 10 g agar L−1 and 1 mm CaCl2.
Statistical Analyses
Analysis of variance was conducted using Statistical Analysis Systems (1982) programs to determine treatment and interaction effects. Most experiments followed a randomized complete block design unless specified otherwise. Single degree-of-freedom contrasts determined whether significant differences existed between specific treatments. When a series of measurements were taken over time, analysis of variance was conducted as a repeated measures design with main plots of treatments and subplots of time unless stated otherwise. A probability level of 0.05 or less was considered to be statistically significant.
Differential Resistance of Snapbean Cultivars
In Simple Salt Solution
Cultivar Dade was selected as an Al-resistant cultivar and cv Romano was selected as an Al-sensitive cultivar based on previous work by Foy et al. (1972) in an Al-toxic soil and by Miyasaka et al. (1991) in complete nutrient solution. To confirm differential resistance of these cultivars, seedlings were grown at four Al levels (0, 12.5, 25, and 50 μm) added as AlCl3 into 0.5 mm CaCl2 adjusted initially to pH 4.5. There were eight treatments (four Al × two cultivars) and six blocks.
To avoid formation of polymeric Al, the methods of Tice et al. (1992) were followed, but modified by preparing Al-containing solutions from a stock solution of 4 mm AlCl3 in 0.1 mm HCl. An initial solution pH of 4.5 was selected because a preliminary study confirmed differential response of two snapbean cultivars in complete nutrient solution at this pH level.
Germinated snapbean seedlings were grown in the dark for an additional 2 to 4 d in sterilization trays containing 10 g agar L−1 and 1 mm CaCl2. One seedling was placed over 800 mL of aerated treatment solution in an environmentally controlled growth chamber maintained at a 16-h photoperiod, 14 μmol m−2 s−1 photon flux density, and a 24°C day and a 22°C night temperature. All subsequent experiments were conducted under the same environmental conditions in the growth chamber unless specified otherwise.
Root lengths were measured at 24, 48, 72, and 96 h after initiation of treatments. Root elongation rates were calculated as the increase in root length over a 24-h period. Relative root elongation rates were calculated as (root elongation at X μm Al)/(Root elongation at 0 Al). Analysis of variance and single degree-of-freedom contrasts were conducted at 96 h after treatment initiation. Solution pH was measured after 96 h, and it ranged from 4.6 to 4.9, but was not significantly affected by Al treatment or cultivar.
In Agarose
Seedlings of cv Dade and cv Romano were grown at four levels of Al (0, 50, 100, and 200 μm) added as AlCl3 to gels containing 10 g of agarose L−1 and 0.5 mm CaCl2. There were eight treatments (four Al levels × two cultivars) and seven blocks.
For Al treatments of 100 μm or lower, 5 mm HCl was used to adjust initially the solution pH to 4.5, whereas for the 200 μm Al treatment, 0.1 m KOH was added. Using the method of Calba et al. (1996), total soluble Al levels were determined to be 0, 52, and 114 μm for the 0, 100, and 200 μm Al treatments, respectively.
Seedlings were initially placed on top of filter paper (Whatman No. 4, Whatman) over agarose treatments. However, the most obvious effect of Al compared with controls under these conditions was that roots of both cultivars immediately changed their direction of growth at a 90 degree angle away from agarose gels containing >50 um Al. Similar results were found by Hasenstein et al. (1988) in maize roots exposed to Al. To force continued contact with Al, filter paper was placed over and under seedlings.
Three seedlings of each cultivar were placed in 4-L glass pans containing agarose gels. The top of the glass pans were covered with aluminum foil and placed at a 70 degree angle in a growth chamber. Root lengths were measured 24, 48, 72, and 96 h after initiation of treatments. Relative root elongation rate was calculated as described previously. Analysis of variance and single degree-of-freedom contrasts were conducted at 96 h after start of treatments.
Snapbean Border Cells
To characterize the number and viability of border cells from two snapbean cultivars, the primary root lengths of cv Dade and cv Romano seedlings were measured. Ten seedlings from six categories (0.51–1.0, 1.01–1.5, 1.51–2.0, 2.01–2.5, 2.51–3.0, and 3.01–3.5 cm) were selected and the border cells from each seedling were harvested into 500 μL of deionized water. The experimental design was a fully randomized one with 12 treatments (two cultivars × six root lengths) and 10 replicates.
Border cells were harvested according to the method of Brigham et al. (1995). In a 10- to 20-μL aliquot, total number of border cells was counted and cell viability was determined using the vital stain, fluorescein diacetate (Hawes and Wheeler, 1982). Cell counts were made using a microscope (model D-7082, Zeiss, Jena, Germany) with an UV light source (DEBCO, Minneapolis). Percent viability was calculated as the number of live border cells divided by the total number of border cells.
Aluminum Effect on Border Cells
In Situ Cultivar Response in Agarose
As described earlier, seedlings of cv Dade and cv Romano were grown at four levels of Al (0, 50, 100, and 200 μm) in agarose gels. After 96 h, border cells were harvested from primary root tips of three seedlings. Total number of border cells and percent viability were calculated as described previously.
Responses of Detached cv Dade Border Cells to Al
To examine Al-induced effects on border cells alone, cells of cv Dade were harvested into water at approximately 24 border cells per 1 μL. An equivalent volume of treatment solutions was added to result in a water control and five final concentrations of Al (0, 50, 100, 150, and 200 μm) at 0.5 mm CaCl2 and pH 4.5. There were six Al treatments and six blocks. The cells were incubated in the dark at 24°C. Total border cell number and percentage viability were measured at 1, 2, 4, 8, and 24 h after initiation of treatments. Relative cell viability was calculated as (percent viability in × Al)/(percent viability in water controls). In a separate but identical trial, mucilage around border cells was visualized using India ink, which does not penetrate the polysaccharide layer. Thickness of this layer was measured in 15 randomly selected, live border cells per treatment replicate.
Solution pH was measured in another identical trial using a micro pH electrode (no. 13–644–6, Fisher Scientific, Pittsburgh) to assess the effect of solution pH on Al solubility. In Al-containing solutions, pH did not increase by more than 0.3 pH units during a 24-h period.
Cultivar Responses of Detached Border Cells
To determine the effect of excess Al on border cell viability of two snapbean cultivars, newly developed cells were harvested from seedlings of cv Dade and cv Romano into water. An equivalent volume of treatment solutions was added to result in a water control and three Al levels (0, 25, and 50 μm) at 0.5 mm CaCl2 and pH 4.5. There were eight treatments (four Al × two cultivars) and six blocks. For experiments on harvested border cells, cells were placed into incubation chambers maintained in the dark at 24°C. Total number of border cells and percent viability were measured at 4, 8, and 24 h after initiation of treatments. In a separate trial with identical treatments, thickness of the mucilage layer around border cells was measured on 15 randomly selected, live border cells in each treatment replicate at 2, 4, 8, and 24 h after initiation of treatments.
Lower percent viability of cv Romano border cells compared with those of cv Dade from roots less than 3.5 cm in length was observed in the absence of Al. To remove cells that might have been injured by the seed surface-sterilization technique, border cells were washed off root apices and then newly developed border cells formed over 24 h were utilized in this study. Hawes and Lin (1990) had demonstrated earlier that a complete set of border cells will form over a 25-h period. These newly formed border cells of cv Romano still had a lower viability in the absence of Al compared with those of cv Dade, so relative cell viability was calculated to allow a comparison of cultivar differences in response to Al.
ACKNOWLEDGMENTS
The authors would like to acknowledge Cisco Brothers of Idaho (Twin Falls) for their generous donation of snapbean seeds. In addition, they would like to acknowledge the late Dr. Merritt R. Nelson, former chairperson for Department of Plant Pathology at the University of Arizona, for his generous sharing of office space during the first author's sabbatical leave. Also, they would like to acknowledge the assistance of Ms. Fushi Wen of the Department of Plant Pathology at the University of Arizona in photographing roots and border cells. The authors also would like to thank Dr. Charles E. McCulloch of the Department of Epidemiology and Biostatistics, University of California, San Francisco, for his assistance in statistical analyses.
Footnotes
This work was supported by the National Research Initiative Competitive Grant Program/U.S. Department of Agriculture (grant nos. 97–35106–5060 and 98–35100–7002). This paper is journal series no. 4522 of the College of Tropical Agriculture and Human Resources, University of Hawaii, Honolulu, HI.
LITERATURE CITED
- Bengough AG, McKenzie BM. Sloughing of root cap cells decreases the frictional resistance to maize root growth. J Exp Bot. 1997;48:885–893. [Google Scholar]
- Bennet RJ, Breen CM, Fey MV. Aluminum uptake sites in the primary root of Zea mays L. S Afr J Plant Soil. 1985;2:1–7. [Google Scholar]
- Brigham LA, Woo H-H, Nicoll SM, Hawes MC. Differential expression of proteins and mRNAs from border cells and root tips of pea. Plant Physiol. 1995;109:457–463. doi: 10.1104/pp.109.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigham LA, Woo HH, Wen F, Hawes MC. Meristem-specific suppression of mitosis and a global switch in gene expression in the root cap of pea by endogenous signals. Plant Physiol. 1998;118:1223–1231. doi: 10.1104/pp.118.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calba H, Jaillard B, Fallavier P, Arvieu J-C. Agarose as a suitable substrate for use in the study of Al dynamics in the rhizosphere. Plant Soil. 1996;178:67–74. [Google Scholar]
- Clarkson DT. The effect of aluminum and some other trivalent metal cations on cell division in the root apices of Allium cepa. Ann Bot. 1965;29:309–315. [Google Scholar]
- Delhaize E, Ryan PR, Randall PJ. Aluminum tolerance in wheat (Triticum aestivum L.): II. Aluminum-stimulated excretion of malic acid from root apices. Plant Physiol. 1993;103:695–702. doi: 10.1104/pp.103.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fageria NK, Baligar VC, Wright RJ. Aluminum toxicity in crop plants. J Plant Nutr. 1988;11:303–319. [Google Scholar]
- Fiskesjo G. Occurrence and degeneration of “Al-structures” in root cap cells of Allium cepa L. after Al treatment. Hereditas. 1990;112:193–202. [Google Scholar]
- Foy CD. Plant adaptation to acid, aluminum-toxic soils. Commun Soil Sci Plant Ann. 1988;19:959–987. [Google Scholar]
- Foy CD, Fleming AL, Gerloff GC. Differential aluminum tolerance in two snapbean varieties. Agron J. 1972;64:815–818. [Google Scholar]
- Hasenstein KH, Evans ML, Stinemetz CJ, Moore R, Fondren WM, Koon EC, Higby MA, Smucker AJM. Comparative effectiveness of metal ions in inducing curvature of primary roots of Zea mays. Plant Physiol. 1988;86:885–889. doi: 10.1104/pp.86.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes MC, Brigham LA. Impact of root border cells on microbial populations in the rhizosphere. Adv Plant Pathol. 1992;8:119–148. [Google Scholar]
- Hawes MC, Brigham LA, Wen F, Woo HH, Zhu Y. Function of root border cells in plant health: pioneers in the rhizosphere. Annu Rev Phytopathol. 1998;36:311–327. doi: 10.1146/annurev.phyto.36.1.311. [DOI] [PubMed] [Google Scholar]
- Hawes MC, Gunawardena U, Miyasaka SC, Zhao X. The role of root border cells in plant defense. Trends Plant Sci. 2000;5:128–133. doi: 10.1016/s1360-1385(00)01556-9. [DOI] [PubMed] [Google Scholar]
- Hawes MC, Lin HJ. Correlation of pectolytic enzyme activity with the programmed release of cells from root caps of pea (Pisum sativum) Plant Physiol. 1990;94:1855–1859. doi: 10.1104/pp.94.4.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes MC, Pueppke SG. Sloughed peripheral root cap cells: yield from different species and callus formation from single cells. Am J Bot. 1986;73:1466–1473. [Google Scholar]
- Hawes MC, Wheeler HE. Factors affecting victorin-induced cell death: temperature and plasmolysis. Physiol Plant Pathol. 1982;20:137–144. [Google Scholar]
- Horst WJ, Klotz F, Szulkiewicz P. Mechanical impedance increases aluminum tolerance of soybean (Glycine max) roots. In: van Beusichem ML, editor. Plant Nutrition: Physiology and Applications. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1990. pp. 351–355. [Google Scholar]
- Horst WJ, Wagner A, Marschner H. Mucilage protects root meristems from aluminum injury. Z Pflanzenphysiol Bd. 1982;105:435–444. [Google Scholar]
- Ikegawa H, Yamamoto Y, Matsumoto H. Cell death caused by a combination of aluminum and iron in cultured tobacco cells. Physiol Plant. 1998;104:474–478. [Google Scholar]
- Kinraide TB, Parker DR. Apparent phytotoxicity of mononuclear hydroxy-aluminum to four dicotyledonous species. Physiol Plant. 1990;79:283–388. [Google Scholar]
- Knudson L. Viability of detached root cap cells. Am J Bot. 1919;6:309–310. [Google Scholar]
- Kochian LV. Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:237–260. [Google Scholar]
- Li XF, Ma JF, Hiradate S, Matsumoto H. Mucilage strongly binds aluminum but does not prevent roots from aluminum injury in Zea mays. Physiol Plant. 2000;108:152–160. [Google Scholar]
- Miyasaka SC, Buta JG, Howell RK, Foy CD. Mechanism of aluminum tolerance in snapbeans: root exudation of citric acid. Plant Physiol. 1991;96:737–43. doi: 10.1104/pp.96.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellet DM, Grunes DL, Kochian LV. Organic acid exudation as an aluminum-tolerance mechanism in maize (Zea mays L.) Planta. 1995;196:788–795. [Google Scholar]
- Ryan PR, Ditomaso JM, Kochian LV. Aluminum toxicity in roots: an investigation of spatial sensitivity and the role of the root cap. J Exp Bot. 1993;44:437–446. [Google Scholar]
- Statistical Analysis Systems Institute. SAS User's Guide: Statistics. Cary, NC: Statistical Analysis Systems Institute; 1982. [Google Scholar]
- Taylor GJ. Current views of the aluminum stress response: the physiological basis of tolerance. Curr Top Plant Biochem Physiol. 1991;10:57–93. [Google Scholar]
- Tice KR, Parker DR, DeMason DA. Operationally defined apoplastic and symplastic aluminum fractions in root tips of aluminum-intoxicated wheat. Plant Physiol. 1992;100:309–318. doi: 10.1104/pp.100.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Breemen N. Acidification and decline of Central European forests. Nature. 1985;315:16. [Google Scholar]
- Wen F, Zhu Y, Hawes MC. Effect of pectin methylesterase gene expression on pea root development. Plant Cell. 1999;11:1129–1140. doi: 10.1105/tpc.11.6.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]