Abstract
Introduction
The usefulness of smartphone-based application software as a way to manage adverse events (AEs) after vaccination is well known. The purpose of this study is to clarify the usefulness and precautions of employing a smartphone application for collecting AEs after the administration of Comirnaty®️.
Methods
Healthcare workers (HCWs) who were vaccinated with Comirnaty®️ were asked to register for the application software and to report AEs for 14 days after vaccination. AEs were self-reported according to severity. The software was set to output an alert in case of fever.
Results
The number of HCWs who received the first dose was 2,551, and 2,406 (94.3%) reported their vaccinations. 2,547 received the second dose, and 2,347 (92.1%) reported their vaccinations. With the first dose, the reporting rate stayed above 83.3% until the final day. On the other hand, that of the second dose decreased rapidly after 6 days. The most frequent symptom was “pain at injection site” (more than 70%). Severe AEs were 6.6% after the second dose, with 0.6% visiting a clinic. Many AEs peaked on the day after administration and disappeared within 1 week. There were few reports of fever.
Conclusion
Smartphone applications can be used to collect information on AEs after vaccination. Application settings and dissemination are necessary to maintain the reporting rate of HCWs.
Keywords: Adverse event, Application software, SARS-CoV2, Vaccine
1. Introduction
Increasing vaccination coverage is considered essential for the reduction of SARS-CoV-2 cases and the severity of the disease [1,2]. In February 2021, Japan granted special approval for Pfizer's Comirnaty®️, a vaccine against SARS-CoV-2 infections, for the prevention of health hazards [3]. Comirnaty®️ was a newly synthesized messenger RNA vaccine with an unprecedented development scheme [3]. In Japan, both the public and the government were hopeful for infection-preventing effects of the new vaccine, while being concerned about potential adverse effects (AEs). The Japan government decided to give priority to healthcare workers (HCWs) to curb the spread of infectious diseases and save critically ill patients.
Chiba University Hospital (CUH), the regional center of excellence for infectious disease care, has 850 beds and about 3,000 health care workers (HCWs). Vaccination was initiated at the time of increasing coronavirus infection cases. A medical infrastructure development was needed to prevent coronavirus infection. Also, strategies for monitoring health conditions are crucial. Therefore, health condition management by the use of application software after vaccination had been promoted among HCWs.
There have been several reports on post-vaccination AE management systems. V-safe and SmartVax are the representative smartphone applications that were used in the United States and Australia, respectively [[4], [5], [6], [7]]. It is considered that AEs information obtained from the smartphone applications differs depending on the application usage and setting. However, the characteristics of smartphone-obtaining information from HCWs are still largely unknown. The purpose of this study is to clarify its usefulness and necessary precautions when employing the smartphone application for collecting AEs after the administration of Comirnaty®️.
2. Materials and methods
2.1. Study design
Since the vaccination schedule must be spread over at least a 3-week period, it was conducted on weekdays from March 15th to March 19th and April 5th to April 9th, 2021. Vaccination was performed at the COVID-19 Vaccine Center of CUH. We provided various information to HCWs to encourage them to get vaccinated. Also, we were concerned about the possibility that post-vaccination fever might cause many HCWs to be absent from work. In case of fever, HCWs were recommended to take acetaminophen, as it is an antipyretic analgesic with no or few anti-inflammatory effects, unlike non-steroidal anti-inflammatory drugs [8,9]. We also provided a list of over-the-counter products available at nearby pharmacies. As the basic flow of the vaccination process, HCWs were pre-screened after registration. When a physician judged them to be ready for vaccination, they were injected with the vaccine. They were then directed to a waiting area and observed for 15 min for their general condition. If the HCWs were then judged by a physician to require attention for immediate AEs during a preliminary examination, the HCWs were placed on standby for 30 min. In addition, if anaphylaxis was suspected, the Medical Emergency Team (MET) consisting of staff from the emergency department was asked to handle the situation. All vaccinations and aseptic preparations were performed by professional pharmacists and professional nurses. They properly controlled the temperature of the vaccine during storage, transportation, and preparation. Vaccination receivers were over 20 years of age, and all HCWs worked at our hospital as hospital employees such as physicians, nurses, pharmacists, nutritionists, physical therapists, laboratory technicians, clinical radiologists, clerks, researchers, etc. Vaccination was voluntary and registration for use of the application was also voluntary, with exclusion criteria consisting of those declining and those hoping to be vaccinated at other institutions. Questionnaire surveillance via application was conducted at a single site at CUH in Chiba, Japan.
Every day for two weeks after vaccination, application registrants received a notification to report their health condition, and they recorded only solicited events. On this questionnaire, the following items were not listed: type of work, history of medical conditions, and allergies. The severity of AEs was graded by a four-point scale (none, mild, moderate, severe). All other AEs were recorded as unsolicited ones. The AEs grading was a simple modification of a standard scale, as was used by Greenberg et al. [10]. The grade of AEs was subjectively based. To clarify the appearance of AEs, the vaccinated HCWs were stratified in 10-year age groups and classified by gender and frequency of administration. In addition, the frequency and severity of each AE were reported over time.
2.2. Data collection
We used the application software respon:sum (Smart119 Inc., Chiba, JP) as a system to quickly manage the health condition of HCWs. Respon:sum is useful for keeping track of the daily attendance and emergency call-up of staff in case of disasters [11]. Parts of the AE data used in this study are analyzed differently than in our previous report [12]. Every day for 2 weeks after vaccination, application enrollees receive a reminder to report their health condition. Information security and the protection of personal information were taken into consideration when listing the survey items. The investigated AEs were fever, joint pain, fatigue, chillness, headache, myalgia, nausea, erythema at injection site, induration at injection site, pain at injection site, and clinic visits. Fever after SARS-CoV2 infection is an early monitoring indicator of disease onset. However, a high frequency of fever is known to occur post vaccination. A message was pre-set on the application to “consult your supervisor about whether you can go to work” if you have a fever of 37.5 °C or higher during the 14-day observation period after vaccination. These AE items did not include occupation, underlying illness, medications taken, or history of allergies. The vaccinated HCWs were led to a waiting space and their general conditions were observed for 15–30 min. Immediate-type AEs such as urticaria, respiratory distress, and hypotension were not included in the items. AEs on the day of vaccination were managed, they were not included in the survey, and data were collected from the day after vaccination. This notification could be sent to a specified email address or to their mobile phone. It was not mandatory to fill in all the items related to AEs. To register with respon:sum, the target HCWs were contacted prior to vaccination, and they were also instructed on its usage at the reception post vaccination.
2.3. Statistical analysis
The data were entered into Excel 2016 (Microsoft, Redmond, WA) and transferred to JMP® Pro 13.0.0 (SAS Institute Inc., Cary, NC, USA) for statistical analysis. Chi-square test or Fisher's exact test was applied for comparison between two or more sample proportions, with a P-value below 0.05 considered statistically significant.
Ethical approval
This study was approved by the Research Ethics Committee of the Graduate School of Medicine and School of Medicine, Chiba University, and was conducted in accordance with the principles of the Declaration of Helsinki and Japanese regulatory requirements (No. HS202106-08).
3. Results
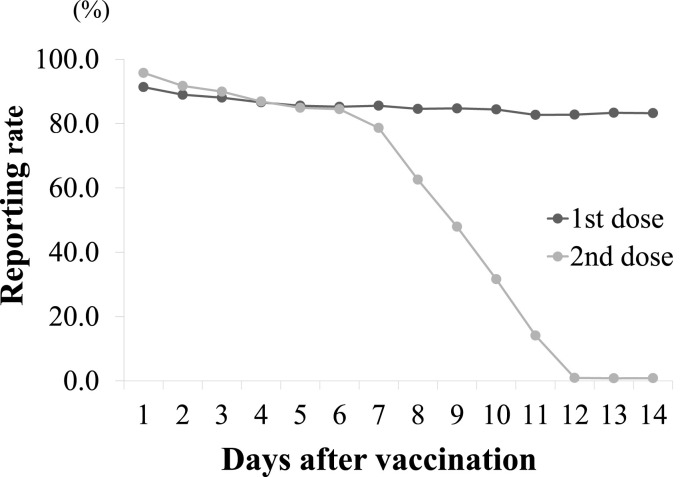
2,551 HCWs were vaccinated with the first dose, and 2,406 (94.3%) reported their vaccinations. 2,547 HCWs were vaccinated with the second dose, and 2,347 (92.1%) reported their vaccinations. The number of HCWs who waited for 30 min after the first dose was 117, and MET was dispatched to 2 patients. The number of HCWs who waited for 30 min after the second dose was 115, and MET intervention was not required. In both groups, there was no diagnosis of anaphylaxis, and the recovery course was mild. Table 1 shows the distribution of HCWs who received the vaccine and reported it on respon:sum. The number of HCWs reporting after the first dose was 921 for males and 1,485 for females, and that after the second dose was 898 for males and 1,449 for females. By age groups, 30-39-year-olds accounted for 32% or more, and those under 40 years old totaled about 59%. The number of reporters in the 50–59 age group was 335 after the first dose, but that after the second dose increased by 4–339. Looking at the changes in daily reported rates, it was over 83.3% until the final day of the first dose. After the second dose, the reported rate (86.9%) until day 4 was similar to that after the first dose (86.6%). On the other hand, the reported rate of the second dose decreased rapidly after 6 days (Fig. 1 ).
Table 1.
Demographics of study participants who submitted survey reports.
| 1st dose |
2nd dose |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (year) | n | (%) | male | (%) | female | (%) | n | (%) | male | (%) | female | (%) |
| 20–29 | 657 | (27.3) | 156 | (16.9) | 501 | (33.7) | 627 | (26.7) | 152 | (16.9) | 475 | (32.8) |
| 30–39 | 772 | (32.1) | 397 | (43.1) | 375 | (25.3) | 750 | (32.0) | 382 | (42.5) | 368 | (25.4) |
| 40–49 | 551 | (22.9) | 223 | (24.2) | 328 | (22.1) | 541 | (23.1) | 218 | (24.3) | 323 | (22.3) |
| 50–59 | 335 | (13.9) | 99 | (10.7) | 236 | (15.9) | 339 | (14.4) | 101 | (11.2) | 238 | (16.4) |
| ≧60 | 91 | (3.8) | 46 | (5.0) | 45 | (3.0) | 90 | (3.8) | 45 | (5.0) | 45 | (3.1) |
| Total | 2,406 | 921 | 1,485 | 2,347 | 898 | 1,449 | ||||||
Fig. 1.
Changes in health status reporting rate
The black and gray lines indicate the reporting rates after the first and second doses, respectively.
Table 2 shows the frequency of AEs during 14 days post vaccination. The proportion of HCWs reporting any AEs was significantly higher after the second dose (92.4%) than after the first dose (88.7%). When AEs were classified, the majority were mild or moderate. The frequency of AEs was categorized by severity, revealing that it increased significantly after the second dose compared to the first dose. The most frequent symptom was “pain at injection site” (more than 70%). Severe AEs were 6.6% after the second dose, with 0.6% visiting a clinic. The number of HCWs who reported severe AEs was 27 after the first dose and 154 after the second dose. The number of HCWs who were absent from work within 2 weeks after vaccination was 31 after the first dose and 158 after the second dose.
Table 2.
Adverse events within 14 days after vaccination.
| 1st dose |
2nd dose |
||||
|---|---|---|---|---|---|
| n = 2,406 | (%) | n = 2,347 | (%) | P value | |
| Any adverse events | 2,134 | (88.7) | 2,168 | (92.4) | <0.01 |
| Mild | 1,505 | (62.6) | 1,008 | (42.9) | <0.01 |
| Moderate | 601 | (25.0) | 1,003 | (42.7) | |
| Severe | 27 | (1.1) | 154 | (6.6) | |
| Pain at injection site | 1,706 | (70.9) | 1,811 | (77.2) | <0.01 |
| Myalgia | 1,112 | (46.2) | 1,378 | (58.7) | <0.01 |
| Fatigue | 565 | (23.5) | 1,407 | (59.9) | <0.01 |
| Headache | 485 | (20.2) | 1,159 | (49.4) | <0.01 |
| Induration at injection site | 307 | (12.8) | 402 | (17.1) | <0.01 |
| Joint pain | 274 | (11.4) | 1,003 | (42.7) | <0.01 |
| Chillness | 140 | (5.8) | 983 | (41.9) | <0.01 |
| Erythema at injection site | 92 | (3.8) | 194 | (8.3) | <0.01 |
| Nausea | 85 | (3.5) | 229 | (9.8) | <0.01 |
| Fever ≥ 37.5 °C | 11 | (0.5) | 231 | (9.8) | <0.01 |
| Clinic visit | 9 | (0.4) | 13 | (0.6) | 0.40 |
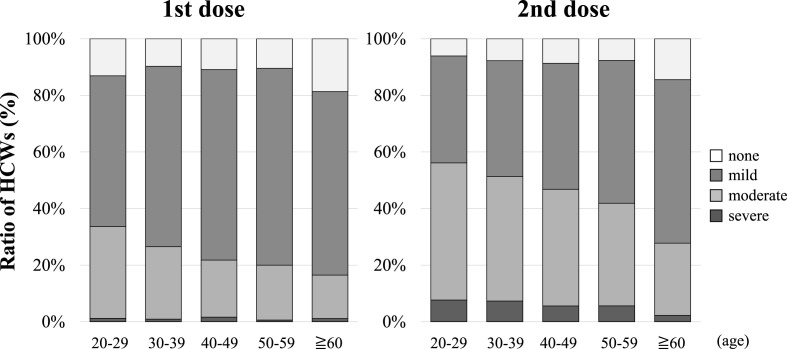
Fig. 2 shows the status of AEs according to age group. The proportions reporting “none,” “mild,” “moderate,” and “severe” (1st vs. 2nd dose) in the 20–29 age group were “13.1% vs. 6.1%," “53.3% vs. 37.8%," “32.4% vs. 48.5%," and “1.2% vs. 7.7%," respectively. For those aged 60 years and older, the percentages were “18.7% vs. 14.4%," “64.8% vs. 57.8%," “15.4% vs. 25.6%," and “1.1% vs. 2.2%," respectively. There was a non-significant increase in the frequency of moderate and severe AEs with the second dose compared with the first dose in any of the age groups. The proportion of serious AEs reported was higher in the younger age groups.
Fig. 2.
Classification of adverse events by age
The severity of adverse events during the study period after vaccination is shown by age. HCWs, health care workers.
AEs compared according to gender are shown in Table 3 . The detailed results of AEs showed that the frequency of females was higher in all items. Significant differences were observed between the two vaccinations at joint pain, fatigue, chillness, headache, myalgia, nausea, erythema at injection site, and induration at injection site. There was a significant difference between fever> = 37.5 °C and pain at injection site in females after the second dose. AEs reported in more than 50% of the patients are described by gender as follows. In males, the number of items increased from 1 after the first dose (pain at injection site) to 3 after the second dose (pain at injection site, muscle pain, fatigue). In females, the number of items increased from 2 after the first dose (pain at injection site, muscle pain) to 4 after the second dose (pain at injection site, muscle pain, fatigue, headache).
Table 3.
Classification of adverse reactions by gender.
| 1st dose |
2nd dose |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| male |
female |
male |
female |
|||||||
| n = 921 | (%) | n = 1,485 | (%) | P value | n = 898 | (%) | n = 1,449 | (%) | P value | |
| Any adverse events | 799 | (86.8) | 1,335 | (89.9) | 0.02 | 793 | (88.3) | 1,375 | (94.9) | <0.01 |
| Mild | 643 | (69.8) | 862 | (58.0) | <0.01 | 440 | (49.0) | 568 | (39.2) | <0.01 |
| Moderate | 149 | (16.2) | 452 | (30.4) | 318 | (35.4) | 685 | (47.3) | ||
| Severe | 7 | (0.8) | 20 | (1.3) | 34 | (3.8) | 120 | (8.3) | ||
| Pain at injection site | 635 | (68.9) | 1,071 | (72.1) | 0.10 | 649 | (72.3) | 1,162 | (80.2) | <0.01 |
| Myalgia | 367 | (39.8) | 745 | (50.2) | <0.01 | 473 | (52.7) | 905 | (62.5) | <0.01 |
| Fatigue | 159 | (17.3) | 406 | (27.3) | <0.01 | 459 | (51.1) | 948 | (65.4) | <0.01 |
| Headache | 91 | (9.9) | 394 | (26.5) | <0.01 | 325 | (36.2) | 834 | (57.6) | <0.01 |
| Induration at injection site | 87 | (9.4) | 220 | (14.8) | <0.01 | 107 | (11.9) | 295 | (20.4) | <0.01 |
| Joint pain | 65 | (7.1) | 209 | (14.1) | <0.01 | 299 | (33.3) | 704 | (48.6) | <0.01 |
| Chillness | 34 | (3.7) | 106 | (7.1) | <0.01 | 301 | (33.5) | 682 | (47.1) | <0.01 |
| Erythema at injection site | 18 | (2.0) | 74 | (5.0) | <0.01 | 32 | (3.6) | 162 | (11.2) | <0.01 |
| Nausea | 14 | (1.5) | 71 | (4.8) | <0.01 | 34 | (3.8) | 195 | (13.5) | <0.01 |
| Fever ≥ 37.5 °C | 2 | (0.2) | 9 | (0.6) | 0.22 | 51 | (5.7) | 180 | (12.4) | <0.01 |
| Clinic visit | 1 | (0.1) | 8 | (0.5) | 0.17 | 2 | (0.2) | 11 | (0.8) | 0.15 |
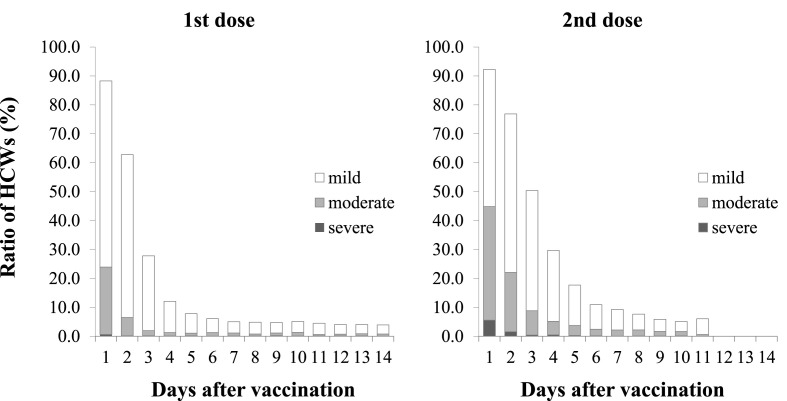
The duration of AEs by severity is shown in Fig. 3 . The percentages of any AEs reported by day 4 after the first dose were 88.3% (day 1), 62.8% (day 2), 27.8% (day 3), and 12.2% (day 4), respectively. The percentages of any AEs reported by day 4 after the second dose were 92.2% (day 1), 76.9% (day 2), 50.4% (day 3), and 29.7% (day 4), respectively. The difference in the percentage of AEs reported between the first and second doses was similar (4.0%) for day 1, but larger (22.6%) for day 3. For both doses, the highest percentage of AEs was reported on the day after administration (day 1), and it decreased thereafter. In addition, a higher percentage of AEs was reported up to day 11 after the second dose compared to the first dose. On the other hand, after day 12, some AEs were reported for the first dose, but not for the second dose. The rate of each AE reported over time is shown by severity (Supplemental figure). Most of the AEs improved over time. After the second dose, the reported rate of erythema at the injection site increased, peaking on day 3.
Fig. 3.
Ratio of HCWs reporting adverse events and the day after vaccination
HCWs, health care workers.
4. Discussion
We managed the health of HCWs by introducing an application that allows us to quickly track the AEs of new vaccines. Comparing the reporting rates at the start of vaccination, the second dose was initially similar to the first. However, after the 6th day of the second dose the reporting rate dropped sharply. We wanted the information to maintain organization, but individuals have rights and it cannot be made mandatory. Chapin-Bardales et al. published information on AEs obtained using v-safe. In their report, there was a decrease in the number of v-safe participants at 14 days post-dose compared to 0–7 days post vaccination [5]. There was also a decrease in the number of participants with the second dose compared to the first dose. These results support our results and indicate that the reporting rate decreases with time since vaccination.
There are several other possible reasons for the decrease in reporting rate in this study. First, HCWs may have been keenly interested in post-vaccination AEs with the first dose. Therefore, the reporting rate of 80% or more may have been maintained. On the other hand, there were few HCWs in whom AEs appeared one week after the second dose. Many HCWs experienced AEs, but the frequency of persistent and serious AEs was low, and thus the familiarity of many HCWs with AEs may have reduced the reporting rates. Second, we recommended but did not force HCWs to report their health condition by respon:sum. We were using this application to manage the health of HCWs. Many HCWs may have believed that if they had no AEs they did not need to report. In order to maintain a high reporting rate using the application, high quality prior guidance and sharing of objectives are essential. Also, it may be effective to modify the application so that reporting is required. Third, most AEs are known to improve within a week. We were concerned about prolonged AEs because this was a new vaccine, so we set an observation period of two weeks. However, that period may have been too long. Based on the results of our study, the best observation period for obtaining a high reporting rate may be about 7 days. V-safe is a method with a varied reporting density: daily reporting for one week after vaccination, then weekly reporting, followed by monthly reporting [5]. Smartvax has users report adverse reactions in a single message on days 3–5 [7]. Modifying the timing and frequency of data collection based on these methods may improve the reporting rate.
The high-frequency AEs found in this study were injection site pain, fatigue, and headache, which were similar to the data reported in Japan and the package insert [13]. In the Japanese population, including our results, AEs with a frequency of more than 20% after the first dose were reported to be myalgia and headache. In addition, after the second dose, more than 40% of AEs were fatigue and headache [13,14]. Ossato et al. reported that the most frequent AEs were pain at the injection site, fatigue, and headache, and the most frequent AE after the second dose was malaise (52%), while other frequent AEs (chills, headache, muscular pain, joint pain, etc.) were less than 32% [15]. One of the influential factors is the difference in distribution according to age and gender of the target population. In addition, racial differences may also be involved in the frequency of AEs [16].
Since it is easy to report AEs using the smartphone application, even minor symptoms may be detected. However, there were few reports of fever, which was considered to be the most common type of AE. Prior to vaccination, as preliminary information we had explained to the staff that acetaminophen was effective in treating fever. Furthermore, since the system was designed to output alerts at 37.5 °C or higher, the possibility also exists that some HCWs made false declarations. The number of absent HCWs after the first dose was 31, and this grew to 158 after the second dose. The number of HCWs reporting severe AEs was 27 after the first dose and 154 after the second dose. We did not include whether they were absent or not in this survey. Therefore, the causal relationship between absenteeism and the severity of AEs is unknown. However, the number of daily absenteeism corresponds to the number of severe cases reported.
When the details of our findings are compared with those reported by the Ministry of Health, Labour and Welfare (MHLW), except for fever, fatigue and redness occurred less frequently, and headache, induration and local pain were comparable. In this study, we were able to obtain information on AEs such as chills and myalgia, which had not been disclosed by MHLW. These survey items were not included in the data released by MHLW and were set independently by modifying the application. At Chiba University Hospital, health management of HCWs was conducted at the time of influenza vaccination [[17], [18], [19]]. The data obtained in this study can be easily and quickly compiled and fed back to all staff, unlike previous paper-based data collections. The use of application software is considered to be very useful, as it allows for the rapid collection and compilation of AEs.
There are some limitations to this study. First, this was a single-center observational study, and selection bias could not be excluded. Second, the study population consisted predominantly of young women, and included only a small number of staff over 60 years of age. The proportion of women was higher than that of men, so gender differences may have played a role in increasing the frequency of reported AEs. Third, the AEs in this study were declared on a self-reported basis, without any medical diagnosis. Although the majority of HCWs were medical professionals, it is possible that a certain percentage of AEs did not originate from the vaccine. Fourth, information on immediate AEs was not included in the collection for this study. One of our objectives in collecting AEs was to manage the health of HCWs. Comirnaty®️ was considered to be important for identifying AEs such as anaphylaxis [13]. HCWs after vaccination waited for a certain time to be monitored by us. If any immediate AEs appeared, they were treated by MET. Since we were aware of AEs immediately after vaccination by this procedure, we considered it important to report AEs after the day following vaccination. In order to collect immediate AE data in the future, it will be necessary to set up a system to allow retrospective entry.
Based on our review, we believe that it is necessary to pay attention to the following points when using an application software. These consist of managing the system of the organization and the rights of individuals, providing non-misleading information, devising ways to improve the reporting rate, applying input items, managing alerts, and setting appropriate reporting periods. In conclusion, we clarified the usefulness and precautions of employing a smartphone application for collecting AEs after the administration of Comirnaty®️. We hope that this information will be used to provide information on AEs among HCWs in Japan.
Authorship statement
All authors meet the ICMJE authorship criteria. SY was the chief investigator. MU, YO, HC and HK contributed to data collection. KW, MY and TT contributed to data analysis. TN contributed to the manuscript revision. SY and HI performed data analysis and manuscript revision. TN and HI contributed to designing the study and manuscript revision. II and HI contributed to the conceptualization and design of the study and manuscript revision, and also supervised the study. All authors approve the manuscript for publication, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Declaration of competing interest
Taka-aki Nakada (TN) is an inventor and has submitted patents related to respon:sum system. TN serves as CEO, receives executive compensation and holds shares in Smart119 Inc., which develops the respon:sum system.
Acknowledgements
The authors would like to thank the Chiba University Hospital staff. We are grateful to Atsushi Sakamoto, Yumi Sato, and the other office staff who managed the entire vaccination schedule. The authors are also thankful to the COVID-19 practice support team members for their valuable contributions.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jiac.2022.02.020.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Alencar C.H., Cavalcanti L.P.G., Almeida M.M., Barbosa P.P.L., Cavalcante K.K.S., Melo D.N., et al. High effectiveness of SARS-CoV-2 vaccines in reducing COVID-19-related deaths in over 75-year-olds, ceara state, Brazil. Trav Med Infect Dis. 2021;6:129. doi: 10.3390/tropicalmed6030129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fabiani M., Ramigni M., Gobbetto V., Mateo-Urdiales A., Pezzotti P., Piovesan C. Vol. 26. 2021. p. 2100420. (Euro Surveill. Effectiveness of the Comirnaty (BNT162b2, BioNTech/Pfizer) vaccine in preventing SARS-CoV-2 infection among healthcare workers, Treviso province, Veneto region). Italy, 27 December 2020 to 24 March 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamb Y.N. BNT162b2 mRNA COVID-19 vaccine: first approval. Drugs. 2021;81:495–501. doi: 10.1007/s40265-021-01480-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gee J., Marquez P., Su J., Calvert G.M., Liu R., Myers T., et al. First month of COVID-19 vaccine safety monitoring - United States, december 14, 2020-january 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:283–288. doi: 10.15585/mmwr.mm7008e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapin-Bardales J., Myers T., Gee J., Shay D.K., Marquez P., Baggs J., et al. Reactogenicity within 2 weeks after mRNA COVID-19 vaccines: findings from the CDC v-safe surveillance system. Vaccine. 2021;39:7066–7073. doi: 10.1016/j.vaccine.2021.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guedel D.S., Peters I.J., Banderet F., Epple V., Egli S., Mehling M., et al. Smartphone-based active vaccine safety surveillance (SmartVax) at a Swiss adult vaccination clinic - a pilot study. Swiss Med Wkly. 2021;151:w30090. doi: 10.4414/smw.2021.w30090. [DOI] [PubMed] [Google Scholar]
- 7.Salter S., Singh G., Nissen L., Trentino K., Murray K., Lee K., et al. Active vaccine safety surveillance of seasonal influenza vaccination via a scalable, integrated system in Western Australian pharmacies: a prospective cohort study. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-048109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertolini A., Ferrari A., Ottani A., Guerzoni S., Tacchi R., Leone S. Paracetamol: new vistas of an old drug. CNS Drug Rev. 2006;12:250–275. doi: 10.1111/j.1527-3458.2006.00250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beck D.H., Schenk M.R., Hagemann K., Doepfmer U.R., Kox W.J. The pharmacokinetics and analgesic efficacy of larger dose rectal acetaminophen (40 mg/kg) in adults: a double-blinded, randomized study. Anesth Analg. 2000;90:431–436. doi: 10.1097/00000539-200002000-00035. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg M.E., Lai M.H., Hartel G.F., Wichems C.H., Gittleson C., Bennet J., et al. Response to a monovalent 2009 influenza A (H1N1) vaccine. N Engl J Med. 2009;361:2405–2413. doi: 10.1056/NEJMoa0907413. [DOI] [PubMed] [Google Scholar]
- 11.https://smart119.biz/responsum/
- 12.Ikeda K., Nakada T.A., Kageyama T., Tanaka S., Yoshida N., Ishikawa T., et al. Spatiotemporal components of adverse reactions against BNT162b2 mRNA SARS-CoV-2 vaccine reflect different immunological processes. https://ssrn.com/abstract=3903937 Available at SSRN. [preprint)]
- 13.https://www.pmda.go.jp/drugs/2021/P20210212001/672212000_30300AMX00231_B101_1.pdf
- 14.https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/vaccine_hukuhannou-utagai-houkoku.html
- 15.Ossato A., Tessari R., Trabucchi C., Zuppini T., Realdon N., Marchesini F. Comparison of medium-term adverse reactions induced by the first and second dose of mRNA BNT162b2 (Comirnaty, Pfizer-BioNTech) vaccine: a post-marketing Italian study conducted between 1 January and 28 February 2021. Eur J Hosp Pharm. 2021 Jul 27 doi: 10.1136/ejhpharm-2021-002933. ejhpharm-2021-002933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang J., Du J., Duan R., Zhang X., Tao C., Chen Y. Characterization of the differential adverse event rates by race/ethnicity groups for HPV vaccine by integrating data from different sources. Front Pharmacol. 2018;9:539. doi: 10.3389/fphar.2018.00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Igari H., Segawa S., Watanabe A., Suzuki A., Watanabe M., Sakurai T., et al. Immunogenicity of a monovalent pandemic influenza A H1N1 vaccine in health-care workers of a university hospital in Japan. Microbiol Immunol. 2010;54:618–624. doi: 10.1111/j.1348-0421.2010.00254.x. [DOI] [PubMed] [Google Scholar]
- 18.Igari H., Watanabe A., Chiba H., Shoji K., Segawa S., Nakamura Y., et al. Effectiveness and safety of pandemic influenza A (H1N1) 2009 vaccine in healthcare workers at a university hospital in Japan. Jpn J Infect Dis. 2011;64:177–182. [PubMed] [Google Scholar]
- 19.Yamazaki S., Fujiwara M., Inoue C., Watanabe M., Takayanagi S., Taniguchi T., et al. Adverse events after the introduction of quadrivalent influenza vaccine in comparison with AH1pdm vaccine (2009) in Japan. Yakugaku Zasshi. 2019;139:469–474. doi: 10.1248/yakushi.18-00160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.