Abstract
Background
The pathophysiology of so called 'cough variant asthma' has not received a great deal of research interest and opinion lies divided as to whether it is really asthma or not. The proponents of cough variant asthma suggest a therapeutic trial of medications usually used to treat asthma
Objectives
To determine the effectiveness of inhaled ß2 agonists in non‐specific chronic cough in children over the age of 2 years.
Search methods
CENTRAL, MEDLINE and EMBASE were searched. Reference lists were checked and trial authors were contacted. 'Grey' literature including theses, internal reports, and non‐peer reviewed journals were sought. Searches are current as of February 2006.
Selection criteria
All randomised (randomised and quasi‐randomised) controlled clinical trials in which inhaled ß2 agonists were given for chronic cough in children over 2 years of age were included. Two reviewers independently assessed articles for inclusion and methodological quality.
Data collection and analysis
Data for trials of salbutamol versus placebo were extracted by both reviewers and entered into the Cochrane Collaboration software program Review Manager, version 4.2
Main results
In children presenting with isolated chronic cough there was no significant difference between salbutamol treated group and placebo group.
Authors' conclusions
Salbutamol was no different from placebo in reducing the frequency of cough measured objectively or scored subjectively.
Plain language summary
Inhaled beta2‐agonists for non‐specific chronic cough in children
The existence of cough variant asthma (cough as the only respiratory symptom without any evidence of airway obstruction) is controversial. This review raises the appropriateness of the common practice of using inhaled ß2 agonists in the treatment of children with cough without any other evidence of airway obstruction. The review found that there is nothing at present to suggest that treatment with ß2 agonists will be beneficial in treating nonspecific isolated cough in children.
Background
Chronic cough is generally accepted as a cough that is persistent for more than 3 weeks. All children cough at some time, but for most this is just a normal response to common viral infections of the respiratory tract. Some, however, persist with a troublesome cough. In a child with isolated cough, a detailed history and examination, followed in a small number of cases by targeted investigations, should allow the child to be placed in one of five broad diagnostic categories. These are: a normal child who is chronically exposed to an irritant (e.g. smoke, fresh paints, new carpeting, dry air heating, old pillow, furry pet); a child with a serious illness such as cystic fibrosis, tuberculosis etc.; a child with non‐serious, but treatable causes of cough, for example gastro‐oesophageal reflux or postnasal drip; a child with an asthma syndrome; and a child where there is an overestimation of symptoms by either the child or family due to psychological or other reasons (Bush 2002). There are those who do not fit into these categories and are labelled as having nonspecific chronic cough, that is isolated cough without any other associated manifestations that could be related to a background aetiology.
Inhaled ß2 agonists have long been used for the treatment of acute asthma symptoms in both adults and children. To maximize the amount of drug that reaches the airways and minimise systemic absorption and side effects, small doses of the drug can be inhaled in aerosol and powder form (Skoner 2001).
The pathophysiology of so called 'cough variant asthma' has not received a great deal of research interest and opinion lies divided as to whether it is really asthma or not. Currently there are proponents for (Doan 1992; Cloutier 1981) and against (McKenzie 1995; Ninan 1995) the diagnosis of asthma in children with cough as the sole respiratory symptom, so called 'cough variant asthma'. The proponents of the entity of cough variant asthma found that in patients presenting solely with chronic cough:
The diagnosis of asthma was confirmed by an exercise provocation test in some children (Konig 1981);
Obvious clinical asthma developed in some patients after long‐term follow‐up (Hannaway 1982);
Personal and family histories of atopy and blood eosinophilia suggested an allergic diathesis. Chest radiographs showed hyperinflation in many and spirometry showed bronchospasm (Yahav 1982);
They responded to a therapeutic trial of asthma medication (e.g. inhaled corticosteroids), and relapsed on stopping medications with a second response to recommencing them (Bush 2002).
On this evidence the proponents of cough variant asthma suggest a therapeutic trial of medications usually used to treat asthma (Doan 1992). Wright 1996 on the other hand found that children with recurrent cough in isolation of other symptoms differ significantly from those with asthma, with or without cough, in airway hyperresponsiveness and atopy, among other factors. Another study found that children with persistent cough had less morbidity and less atopy compared with children with wheeze (Faniran 1998). Despite these differences and the unclear pathophysiology, for many general practitioners and paediatricians the most practicable approach to isolated chronic cough is a therapeutic trial of ß2‐agonists and or inhaled corticosteroids. As ß2 agonists are one of the main therapies used for this condition we aim to conduct a systematic review of the evidence that supports their use in chronic cough.
Objectives
To determine the effectiveness of inhaled ß2 agonists in non‐specific chronic cough in children over the age of 2 years.
Methods
Criteria for considering studies for this review
Types of studies
All randomised (randomised and quasi‐randomised) controlled clinical trials in which inhaled ß2 agonists were given for chronic cough in children over 2 years of age were included.
Types of participants
Children aged 2 years or over presenting with non‐specific chronic cough for more than 3 weeks in all settings (inpatient or outpatient, general practice and the home). Studies involving children under the age of 2 years were excluded since different pathophysiologies may occur in babies and young infants. Children with associated wheezy chest were also excluded.
Types of interventions
Inhaled ß2 agonists, delivered by any means; aerosol, nebulised or by metered dose inhaler (MDI); with or without a holding chamber +/‐ a mask. All doses and dosing regimes were included. Studies including comparison of: ß2 agonist with placebo, ß2 agonist with any other drug, and comparison between different types of ß2 agonists were included.
Types of outcome measures
Primary outcomes
Improvement in cough frequency
Secondary outcomes
Cough severity score charts.
Changes in capsaicin cough receptor sensitivity.
Indicators of exacerbations (for example, increased use of bronchodilators, use of oral steroids, hospital admission or physician attendance).
Patient and / or parent assessment.
Physician's assessment.
Search methods for identification of studies
Electronic searches
The Cochrane Central Register of Controlled Trials (CENTRAL) Issue 1 2006; MEDLINE (1966‐February 2006) and EMBASE (1980‐February 2006) were searched using the search strategies detailed in Table 1.
1. Search strategies used for Medline, Embase and Central.
| Database searched | Terms used |
| CENTRAL | #1. COUGH explode all trees (MeSH); #2. cough*; #3. (#1 or #2); #4. ADRENERGIC BETA‐AGONISTS explode tree 2 (MeSH); #5. agonist*; #6. (receptor next agonist*); #7. (adrenergic next agonist*); #8. beta‐2; #9. b2; #10. (#8 or #9); #11. (#5 or #6 or #7); #12. (#10 and #11); #13. (bronchodilator* near agent*); #14. (albuterol or aminophylline* or atropine* or bricanyl or budesonide or clenbuterol or cromakalim or dyphylline or ephedrine or epinephrine or fenoterol or hexoprenaline or ipratropium or isoetharine or isoproterenol or khellin or (nitric next oxide) or orciprenaline or procaterol or s‐nitrosoglutathione or nitrosothiols or salbutamol or terbutaline or theobromine or theophylline or tretoquinol or ventolin); #15. (#4 or #12 or #13 or #14); #16. NEBULIZERS AND VAPORIZERS explode tree 1 (MeSH); #17. ADMINISTRATION INHALATION explode tree 1 (MeSH); #18. (aerosol* or inhal* or atomi* or nebuli* or volatili*); #19. (#16 or #17 or #18); #20. (#3 and #15 and #19) #21. (child* or paediat* or pediat* or adolesc* or infan* or toddler* or bab* or young* or preschool* or pre‐school or (pre next school) or newborn* or new‐born* or (new next born*) or neonat* or neo‐nat*); #22. (#20 and #21) |
| MEDLINE (combined with RCT filter) | 1. exp COUGH/ 2. cough$.mp. 3. 1 or 2 4. exp Child/ or exp infant/ or exp adolescent/ 5. exp Pediatrics/ 6. (paediatric$ or pediatric$ or child$ or adolescen$ or infant$ or young$ or preschool$ or pre‐school$ or newborn$ or new‐born$ or neonat$ or neo‐nat$).mp. 7. 4 or 5 or 6 8. exp Adrenergic beta‐Agonists/ 9. agonist$.mp. 10. (receptor adj5 agonist$).mp. 11. (adrenergic adj5 agonist$).mp. 12. (beta‐2 or beta2 or b2).mp. 13. 9 or 10 or 11 14. 12 and 13 15. (bronchodilat$ adj5 agent$).mp. 16. (albuterol or aminophylline$ or atropine$ or bricanyl or budesonide or clenbuterol or cromakalim or dyphylline or ephedrine or epinephrine or fenoterol or hexoprenaline or ipratropium or isoetharine or isoproterenol or khellin or (nitric adj3 oxide) or orciprenaline or procaterol or s‐nitrosoglutathione or nitrosothiols or salbutamol or terbutaline or theobromine or theophylline or tretoquinol or ventolin).mp. 17. 8 or 14 or 15 or 16 18. exp "Nebulizers and Vaporizers"/ 19. exp administration inhalation/ 20. (aerosol$ or inhal$ or atomi$ or nebuli$ or volatili$).mp. 21. 18 or 19 or 20 22. 3 and 17 and 21 23. 22 and 7 |
| EMBASE (combined with RCT filter) | 1. exp COUGHING/ 2. cough$.mp. 3. 1 or 2 4. exp Child/ or exp infant/ or exp adolescent/ 5. exp PEDIATRICS/ 6. (paediatric$ or pediatric$ or child$ or adolescen$ or infant$ or young$ or preschool$ or pre‐school$ or newborn$ or new‐born$ or neonat$ or neo‐nat$).mp. 7. 4 or 5 or 6 8. exp Beta Adrenergic Receptor Stimulating Agent/ 9. agonist$.mp. 10. (receptor adj5 agonist$).mp. 11. (adrenergic adj5 agonist$).mp. 12. (beta‐2 or beta2 or b2).mp. 13. 9 or 10 or 11 14. 12 and 13 15. (bronchodilat$ adj5 agent).mp. 16. (albuterol or aminophylline$ or atropine$ or bricanyl or budesonide or clenbuterol or cromakalim or dyphylline or ephedrine or epinephrine or fenoterol or hexoprenaline or ipratropium or isoetharine or isoproterenol or khellin or (nitric adj3 oxide) or orciprenaline or procaterol or s‐nitrosoglutathione or nitrosothiols or salbutamol or terbutaline or theobromine or theophylline or tretoquinol or ventolin).mp. 17. 8 or 14 or 15 or 16 18. exp NEBULIZER/ 19. exp VAPORIZER/ 20. exp Inhalational Drug Administration/ 21. (aerosol$ or inhal$ or atomi$ or nebuli$ or volatili$).mp. 22. 18 or 19 or 20 or 21 23. 3 or 17 or 22 24. 7 and 23 |
Searching other resources
Reference lists in primary sources were searched. Personal communication with authors and experts in the field were made in order to trace other unpublished sources. "Grey literature" including theses, internal reports, non‐peer reviewed journals were sought.
Data collection and analysis
Selection of studies
The full texts of potential studies for inclusion were obtained to decide if they should be included or not by AT. A list of excluded studies was made, with reasons for their exclusion documented. In order to make sure these decisions were made properly, a second reviewer (MM) independently selected the studies to be included as well. No disagreement occurred so a third reviewer was not needed.
Data extraction and management
Data for trials was extracted by both reviewers and entered into the Cochrane Collaboration software program Review Manager. Since only one study was identified no meta‐analysis is possible at present.
Assessment of risk of bias in included studies
Studies included underwent quality assessment performed independently by both reviewers, using two methods. The first involves using the Cochrane approach to assessment of allocation of concealment. Trials were scored using the following principles: Grade A: Adequate concealment Grade B: Unclear concealment Grade C: Clearly inadequate concealment
Secondly, each study was assessed using a 1 to 5 scale described by Jadad 1996 and summarised as follows: Was the study described as randomised? (1=yes; 0=no) Was the study described as double blind? (1=yes; 0=no) Was there a description of withdrawals and dropouts? (1=yes; 0=no) Was the method of randomisation clearly described and appropriate? (1=yes; 0=no) Was the method of double blinding well described and appropriate? (1=yes; 0=no). (Deducting one point when the methods for randomisation or blinding were inappropriate). Inter‐reviewer reliability was measured by using Kappa and weighted Kappa statistics.
Assessment of heterogeneity
The trial characteristics that may have influenced the observed treatment effect was to be examined. Statistical heterogeneity was to be investigated by sensitivity analysis.
Sensitivity analysis provides an approach for testing how robust the results of a review are relative to key decisions and assumptions that have been made in the process of conducting the review. Two factors were to be investigated:
1. Publication bias ‐ the existence of publication bias was to be examined using a funnel plot trial quality; 2. The effects of overall trial quality on the pooled result was to be examined using both the Cochrane approach and that of Jadad 1996.
Data synthesis
All future included trials will be combined using the RevMan Analyses 1.0.2. For dichotomous variables, individual and pooled statistics will be calculated as relative risks with 95% confidence intervals. For continuous outcomes, individual and pooled statistics will be calculated as weighted mean differences or standard mean differences, as indicated, with 95% confidence intervals. We will pool data with a Fixed effect model. Since there does not appear to be consensus as to which method is superior, we will report both models where statistical heterogeneity as measured by the I square statistic is greater than 20%, or where the Chi‐squared test for heterogeneity is significant at the P=0.1 level.
Subgroup analysis and investigation of heterogeneity
Where future heterogeneity is observed, we will explore variation in treatment effects by conducting the following a priori subgroup analyses of clinical characteristics:
Females versus males
Age (2‐5 years versus 6‐18 years)
Co‐interventions versus none
Different doses of ß2‐agonists
Duration of ß2‐agonists administration (< 6 weeks vs >/= 6 weeks administration)
Results
Description of studies
Results of the search
Electronic search: 516 citations retrieved; 506 excluded on basis of abstract: mainly being related to chronic cough of asthmatic subjects and/or adults. Ten papers were obtained in full text form. Nine papers did not meet the inclusion criteria (see Characteristics of excluded studies).
Included studies
One publication met the criteria for inclusion in the review (Chang 1998).
Study design
The study was a randomised, double‐blind parallel group trial. The method of allocation was by sequential random number with treatments assigned based upon the number.
Population
43 participants from an outpatient's clinic were recruited who were not experiencing current chest infections, and had a recent history of persistent cough.
Interventions
Participants were randomised to receive inhaled salbutamol or placebo two puffs twice a day (100 mcg salbutamol per puff) for 5‐7 days. Therapies administered via a metered dose inhaler (MDI) with a spacer.
Outcomes assessed
The principal outcome assessed was the cough frequency. Chang 1998 studied cough frequency over 24 hrs at day 5‐7 of the study .Other outcomes reported in the study included symptom scores using cough score charts (both parent and child completed), as well as measuring the lowest capsaicin concentration required to stimulate 2 coughs or more (Cth) and 5 coughs or more (C5). Airway responsiveness was assessed by inhalations of hypertonic saline (4.5%) using an ultrasonic nebuliser (the dose of hypertonic saline was increased successively by doubling the inhalation time). FEV1 was measured 60 seconds after each inhalation time and the test ceased when the FEV1 had fallen by more 15% or more or when a cumulative inhalation time of 15.5 minutes had been achieved. The presence of airway hyper‐responsiveness (AHR positive) was defined as a fall in FEV1 of 15% or more from the baseline at any during the hypertonic saline challenge.
Risk of bias in included studies
The overall quality of reporting of methods in the study included was fair. It was described as randomised and double blind. As assessed by the Jadad method, a score of 3 was given. The number of withdrawals and the reason for withdrawals were clearly stated. The concealment of allocation was not adequate as it was undertaken by the trialists directly.
Effects of interventions
Inhaled ß2‐agonists versus placebo
Symptoms
Chang 1998 reported that cough frequency and other outcome variables did not change significantly in either treatment group and there was no significant difference between the groups. There was also no significant difference in the change in cough frequency between the AHR positive and the AHR negative groups. The confidence intervals are wide and this study can neither confirm or rule out clinical benefit from inhaled β2‐agonists in chronic cough in children.
Other outcomes
There was no significant difference in the change in Cth and C5 (reflecting cough receptor sensitivity) in either group.
Based upon canister weights 28 of the 38 children who completed the study (13 in ß2‐agonist group, and 15 in the placebo group) were compliant with the treatment protocol.
Withdrawals: Five children (four from the active treatment group and one from the placebo group did not complete the study due to personal reasons (three), use of salbutamol in addition to trial medications (one in the placebo group), and a refusal to take the trial medications (one from the active group). Thirty‐eight children completed the study. It is not clear whether the trialists conducted an intention‐to‐treat analysis.
Discussion
This review raises the appropriateness of the common practice of using inhaled ß2 agonists in the treatment of children with cough without any other evidence of airway obstruction. Identifying children who are unlikely to benefit from medications used for asthma could not only potentially prevent the diagnostic labelling of asthma in a large group of children but also save these children from the use of unnecessary medications and their associated side effects, as well as save their parents from any unnecessary expense.
The promotion of the diagnosis of asthma based on the symptom of cough alone has led to the overuse of ß2 agonists in children with recurrent cough (McKenzie 1995). The findings of a lack of response to inhaled medications used routinely for asthma, irrespective of the presence of AHR, are supported by epidemiological data (Clifford 1989; Lombard 1997). Clifford 1989 found no association between cough and either AHR or atopy after controlling for wheeze, and they suggested that, in the absence of wheeze, the significance of cough and dyspnoea for the diagnosis of asthma in epidemiological studies should be questioned.
This review supports growing epidemiological evidence that cough alone is a poor marker for asthma (Ninan 1995; Wright 1996). McFadden 1975 were the first to describe cough as a presenting symptom of asthma in adults. All subjects in this study had exertional dyspnoea and abnormal baseline expiratory flow volume loops consistent with moderate airways obstruction. Subsequent studies of cough variant asthma included adults and children without any abnormality of their baseline pulmonary function (Cloutier 1981; Cheriyan 1994; Hannaway 1982). Many of these studies used asthma medications for adults and children with cough variant asthma but had no placebo arm, and results were based on subjective reporting of cough, which is known to be unreliable (Hsu 1994, Archer 1985). Cheriyan 1994 was a retrospective study that reported eight of 10 adult patients free from cough while on either oral or inhaled corticosteroids after three months. In the earlier adult (Armitage 1994) and paediatric (Cloutier 1981) studies, the subjects' cough responded to oral theophylline within 2‐5 days, in contrast to the prolonged course of corticosteroids used by other studies (Cheriyan 1994). The study included in this review is not comparable to the study of Hannaway 1982 that included children with abnormal baseline lung function or chest physical examination. The subjects in the included study had normal physical examination and spirometry. In the Hannaway 1982 study, a third of the 32 children involved had abnormal physical examination of the chest and 12 of the children who were able to perform pulmonary function tests had abnormalities in the expiratory flow loop. Similarly, in another study (Konig 1981) six of the eight children who were able to perform pulmonary function tests had FEV1 predicted values of approximately 85%.
Some children with persistent cough no doubt have unrecognised wheeze, either because it is poorly perceived by their parents or it is not present at the time they present to a physician. It has also been suggested that this group has a higher wheeze threshold (Koh 1993). The fact that inhaled corticosteroid has helped the coughers in the study by Davies 1999 does not mean that they necessarily have asthma. It may be that children with recurrent, persistent, and isolated cough occupy an intermediate clinical position between children with no cough and those with wheeze with respect to atopy (Lewis 1989), prognosis for asthma (Wright 1996, Powell 1996), family history of asthma (Ninan 1995), response to bronchodilator (Konig 1981), and episodes of respiratory morbidity (Levy 1984). However, coughers appear to have normal lung function measured by spirometry and airway responsiveness (Chang 1998; Wright 1996).
A positive relation between isolated cough and atopic family history as markers for the later development of wheeze has been noted (Powell 1996), and others have also found that children with cough and no wheeze had more atopy than controls but less than asthmatics (Wright 1996; Lewis 1989). Eczema and hay fever, however, occurred no more in coughers than controls (Ninan 1995).
Although cough is often used as a marker of asthma instability (Isles 1993) the severity of cough has been shown to be unrelated to the airway calibre as measured by FEV1, peak expiratory flow or its variability (Hsu 1994). In the laboratory, medications (e.g. cromolyn, atropine) that inhibit the bronchoconstriction that occurs with broncho‐ provocative challenges do not alter the cough response (Hansson 1988, Sheppard 1983). Conversely, other medications (lignocaine, codeine) that inhibit the induced cough have no effect on broncho‐constriction (Hansson 1988; Fuller 1988). In human airways, the pathways for cough and bronchoconstriction are distinctly different (Sheppard 1983; Fuller 1988; Shimuzu 1996). While the trigger for cough and airway narrowing may be the same, the symptom of cough may persist despite adequate treatment of bronchoconstriction. It is, therefore, not surprising that the AHR positive children in Chang 1998 study responded to salbutamol and placebo in a similar manner to the AHR negative children.
The number of subjects included in the study included in our review was small, but did achieve a power of 80% based on an expected difference of 70% in the frequency of cough as measured by cough meter. Whether this is generalisable depends on two questions. Firstly, is it safe to assume in a small study that any differences between treatment and placebo would not have been missed? Secondly, is a 70% reduction in cough frequency a reasonable representation of treatment success? The primary outcome of improvement of cough and how it is measured is crucial to this issue. Clearly there are difficulties in measuring cough, especially at night. Any future studies should aim to design a validated tool for assessing cough in order to reduce uncertainty in the measurement of cough. Hence the major problem with the included study is the limited ability to generalise the results based on 80% power. In addition the dose was lower than standard asthma care as outlined in BTS guidelines on asthma (given that the authors has taken a pure asthma slant rather than other possible effects of salbutamol such as possible effects on cough receptors, cough pathway, airway clearance etc).
Chang 1998 recorded cough over 24 hours using a cough diary. By using video recordings in Davies 1999, as with other studies (Picciotto 1998), were able to make observations of children with nocturnal cough in their own homes. This method eliminates difficulties with diary card reporting and the problems associated with recording cough of co‐sleepers.
Chang 1998 found that inhaled salbutamol had no significant effect on cough frequency or score, irrespective of the presence of AHR. It could be argued that the infrequent use of salbutamol trial affected the outcome measures. Salbutamol was used twice a day rather than four times a day, with the aim of improving compliance. Moreover, regular use of ß2 agonists (three to four times a day) may worsen asthma symptoms, increase the risk of severe asthma or cause enhanced AHR (Busse 1994). As compliance and treatment success were similar in both treatment arms, improved compliance and a larger daily dose of salbutamol are unlikely to alter the final results. In addition, there is no gold standard on acceptable compliance and devices that record the use of the medication cannot confirm if the medication was actually consumed (Rand 1994).
A potential criticism for the study included in this review is that silent gastro‐oesophageal reflux and sinusitis were not thoroughly investigated. In children, these diagnoses as a cause of cough, rather than an association, are controversial. In adults with gastro‐oesophageal reflux (but no respiratory symptoms), reflux was found to be a cofactor and not the cause of cough (Ferrari 1995). The exclusion of sinusitis is extremely difficult. In a prospective study, 50% of the 137 children younger than 13 years (Diament 1987) had computed tomography sinus scans consistent with sinusitis but all were asymptomatic.
One of the most important observations made in the studies included in this review is that children improved on placebo. Of those who were followed up, most continued with improved symptoms. Since follow up was six months to one year after the study, this could simply reflect the natural history of the tendency of cough to improve with time (Brooke 1995).
Authors' conclusions
Implications for practice.
While cough is associated with asthma (McFadden 1975), when no other evidence of airway obstruction is present one small study has shown that irrespective of the presence of AHR, inhaled salbutamol provided no advantage over placebo in reducing the frequency of cough measured objectively or scored subjectively. However, on the basis of one small trial we cannot exclude the possibility that in children with recurrent cough without other evidence of airway obstruction inhaled ß2‐agonist might be beneficial. Until there is a greater body of evidence on the treatment of non‐specific cough, no recommendation can be made.
Implications for research.
The common problem of cough in childhood and its relation with airway obstruction deserves further investigation.
More randomised controlled studies are needed to study the efficacy of asthma medications in isolated chronic cough. Since no benefit has been shown from 2 puffs twice a day (100 mcg salbutamol per puff) of inhaled salbutamol it might be worth undertaking a larger study using higher doses of inhaled salbutamol or other types of ß2 agonists to investigate whether a subgroup of children with non‐specific chronic cough who have never wheezed, are likely to benefit. However the adverse events of using higher doses ( e.g. tachycardia, tremor, anxiety, etc) could be problematic. It might also be worth comparing the pros and cons of Inhaled corticosteroids versus inhaled ß2 agonists in these cases. Key to the successful study of non‐specific cough is exclusion of asthma and it is recommended that in children able to perform lung function, reversible airways obstruction and/or airway hyper responsiveness is studied as part of assessment for suitability in a study of non‐specific cough.
What's new
| Date | Event | Description |
|---|---|---|
| 30 July 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 2, 2003 Review first published: Issue 3, 2005
| Date | Event | Description |
|---|---|---|
| 19 May 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank the support staff of the Cochrane Airways Group (Toby Lasserson & Liz Arnold) for assistance in the electronic search and retrieval of papers. We would like to thank Dr Anne Chang who was kind enough to supply us with extra details concerning her study.
Data and analyses
Comparison 1. Inhaled salbutamol (400 mcg/day) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cough frequency in 24 hrs | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Symptom score (parent completed) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Symptom score (child completed) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 C5 (lowest concentration of capsaicin required to stimulate 5 coughs or more) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Cth (lowest concentration of capsaicin required to stimulate 2 coughs or more) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Compliance (N deemed compliant by trialists) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Withdrawals | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
1.1. Analysis.
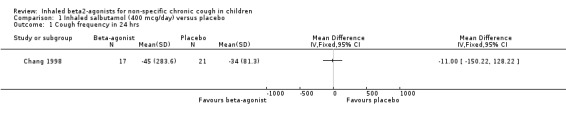
Comparison 1 Inhaled salbutamol (400 mcg/day) versus placebo, Outcome 1 Cough frequency in 24 hrs.
1.2. Analysis.
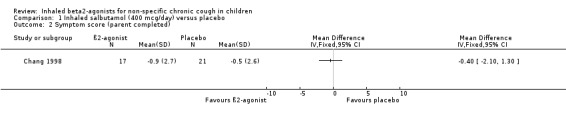
Comparison 1 Inhaled salbutamol (400 mcg/day) versus placebo, Outcome 2 Symptom score (parent completed).
1.3. Analysis.
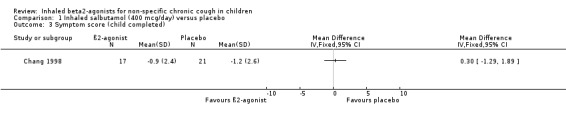
Comparison 1 Inhaled salbutamol (400 mcg/day) versus placebo, Outcome 3 Symptom score (child completed).
1.4. Analysis.
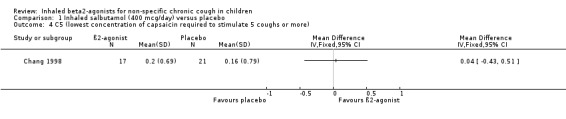
Comparison 1 Inhaled salbutamol (400 mcg/day) versus placebo, Outcome 4 C5 (lowest concentration of capsaicin required to stimulate 5 coughs or more).
1.5. Analysis.
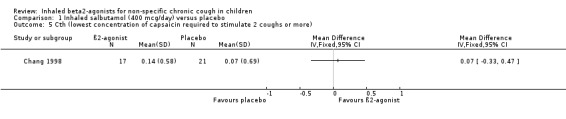
Comparison 1 Inhaled salbutamol (400 mcg/day) versus placebo, Outcome 5 Cth (lowest concentration of capsaicin required to stimulate 2 coughs or more).
1.6. Analysis.
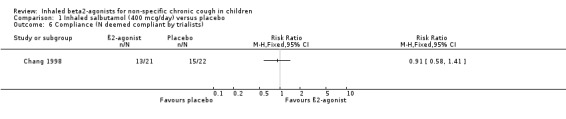
Comparison 1 Inhaled salbutamol (400 mcg/day) versus placebo, Outcome 6 Compliance (N deemed compliant by trialists).
1.7. Analysis.
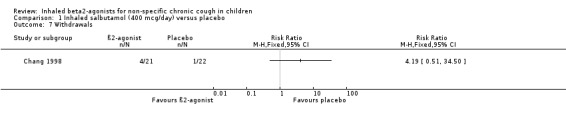
Comparison 1 Inhaled salbutamol (400 mcg/day) versus placebo, Outcome 7 Withdrawals.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chang 1998.
| Methods | Setting: outpatient department; Randomisation: RCT ;blocks of 6; Masking: DB Design: P.
Excluded: adequately stated (12); Withdrawal: adequately stated (5); Jadad score:3 Allocation was by sequential random number with treatments assigned based upon the number. The process of allocation was undertaken by the trialist. |
|
| Participants | Total eligible for inclusion:55; Number enrolled:43; Participants; males:19. Females:24; Number completed study: 38; Age:6‐17 years; Ethnicity: not given; Treatment groups: PHASE 1: SAL: 21; PLA: 22. PHASE 2: BDP: 22; PLA: 21. Baseline characteristics: well balanced. Inclusion criteria: Recurrent cough: two episodes of cough, each lasting two weeks in the past 12 months; experiencing an episode of cough. Exclusion Criteria: Moist, productive cough; bronchiectasis; whooping cough; immunodeficiency; clinical gastro‐oesophageal reflux; history of cardiac or neonatal pulmonary problems; abnormal cardiopulmonary physical examination and abnormal chest x ray. |
|
| Interventions | Phase 1: SAL versus PLA Phase 2: BDP versus PLA (participants re‐randomised in second phase to treatment groups. SAL/BDP: 2 puffs bd; 100 mcg per puff) PLA: 2 puffs bd Length of intervention: SAL: 5‐7 days; BDP 4‐5 weeks (8‐9 weeks in a subgroup) | |
| Outcomes | Number of coughs per 24 hrs measured objectively (24 hrs Holter monitor); subjective cough scores (charts;parent/child completed ); CRS; PD15; compliance if reduction more than 70% of canisters weight | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Allocation was by sequential random number with treatments assigned based upon the number. The process of allocation was undertaken by the trialist. |
DB = double‐blind; P = parallel; PLA = placebo; SAL = salbutamol; BDP = beclomethasone; bd = 2 x daily
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Belcher 1986 | Study conducted on adults and induced cough in normal subjects |
| Davies 1999 | RCT of steroids in paediatric cough |
| de Benedictis 1986 | Not RCT therapeutic trial |
| Ellul‐Micallef 1983 | Study conducted on adults |
| Irwin 1997 | Study conducted on adults |
| Lowry 1987 | Study conducted on adults and induced cough in normal subjects |
| Lui 1996 | Study conducted on adults and induced cough |
| Mulrennan 2002 | Study conducted in healthy adult smokers |
| Spelman 1991 | Not RCT |
Contributions of authors
AT: Protocol initiation, search result assessment, data extraction and analysis, write‐up and interpretation
MM: Protocol development, search result assessment, data extraction and analysis, interpretation
HV: interpretation
JM: interpretation
ML: interpretation
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Chang 1998 {published data only}
- Chang AB, Phelan PD, Carlin JB, Sawyer SM, Robertson CF. A randomised, placebo controlled trial of inhaled salbutamol and beclomethasone for recurrent cough. Archives of Disease in Childhood 1998;79:6‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Belcher 1986 {published data only}
- Belcher N, Rees PJ. Effects of pholcodine and salbutamol on citric acid induced cough in normal subjects. Thorax 1986;41:74‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davies 1999 {published data only}
- Davies M, Fuller P, Picciotto A, McKenzie SA. Persistent nocturnal cough:randomised controlled trial of high dose inhaled corticosteroid. Archives of Disease in Childhood 1999;81:38‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Benedictis 1986 {published data only}
- Benedictis FM, Canny GJ, Levison H. Methacholine Inhalational Challenge in the Evaluation of chronic cough in children. Journal of asthma 1986;23(6):303‐8. [DOI] [PubMed] [Google Scholar]
Ellul‐Micallef 1983 {published data only}
- Ellul‐Micallef R. Effect of terbutaline sulphate in chronic "allergic" cough. British Medical journal 1983;287:940‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Irwin 1997 {published data only}
- Irwin RS, French CT, Smyrnios NA, Curley FJ. Interpretation of positive Results of a methacholine Inhalation Challenge and 1 week of Inhaled Bronchodilator Use in Diagnosing and Treating cough‐variant asthma. Archives of Internal Medicine 1997;157:1981‐7. [PubMed] [Google Scholar]
Lowry 1987 {published data only}
- Lowry R, Highenbottam T, Johnson T, Godden D. Inhibition of artificially induced cough in man by bronchodilators. British Journal of Clinical Pharmacology 1987;24:503‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lui 1996 {published data only}
- Lui PW, Hsing CH, Chu YC. Terbutaline inhalation suppresses fentanyl‐induced coughing. Canadian Journal of Anaesthesia 1996;43(12):1216‐9. [DOI] [PubMed] [Google Scholar]
Mulrennan 2002 {published data only}
- Mulrennan SA, Ojoo JC, Kastelik JA, Wright C, Thompson R, Redingotn AE, et al. Smoking, salbutamol and the cough reflex. Thorax 2002;57(Suppl 3):24. [Google Scholar]
Spelman 1991 {published data only}
- Spelman R. Two‐year follow up of the management of chronic or recurrent cough in children according. British Journal of General Practice 1991;41:406‐9. [PMC free article] [PubMed] [Google Scholar]
Additional references
Archer 1985
- Archer LN, Simpson H. Night cough counts and diary card scores in asthma. Archives of Disease in Childhood 1985;60:473‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Armitage 1994
- Armitage P, Berry G. Statistical methods in medical research. 3rd Edition. Oxford: Blackwell Scientific Publications, 1994:193‐4. [Google Scholar]
Brooke 1995
- Brooke AM, Lambert PC, Burton PR, Clarke C, Luyt DK, Simpson H. The natural history of respiratory symptoms in pre‐school children. American Journal of Respiratory and Critical Care Medicine 1995;152:1872‐8. [DOI] [PubMed] [Google Scholar]
BTS 1997
- British Thoracic Society, National Asthma Campaign, Royal College of Physicians of London, et al. British guidelines on asthma management: review and position statement. Thorax 1997;52:Suppl 1. [Google Scholar]
Bush 2002
- Bush A. Paediatric problems of cough. Pulmonary Pharmacology & Therapeutics 2002;15(3):309‐15. [DOI] [PubMed] [Google Scholar]
Busse 1994
- Busse WW, Maisiak R, Young KR Jr. Treatment regimen and side effects of treatment measures. American Journal of Respiratory & Critical Care Medicine 1994;149:44‐50. [DOI] [PubMed] [Google Scholar]
Census 1991
- Census Manual. East London and City Health Authority. 1991.
Chang 1996
- Chang AB, Phelan PD, Roberts RG, Robertson CF. Capsaicin cough receptor sensitivity test in children. European Respiratory Journal 1996;9:2220‐3. [DOI] [PubMed] [Google Scholar]
Chang 1997
- Chang AB, Phelan PD, Hoist D, Robertson CF. The effect of capsaicin on airway responsiveness to hypertonic saline in asthmatic and non asthmatic children. Pediatric Pulmonology 1997;23:412‐6. [DOI] [PubMed] [Google Scholar]
Chang 1997a
- Chang AB, Newman RG, Phelan PD, Robertson CF. A new use for an old Holter monitor: an ambulatory cough meter. European Respiratory Journal 1997;10:1637‐9. [DOI] [PubMed] [Google Scholar]
Cheriyan 1994
- Cheriyan S, Greenberger PA, Patterson R. Outcome of cough variant asthma treated with inhaled steroids. Annals of Allergy 1994;73:478‐80. [PubMed] [Google Scholar]
Clifford 1989
- Clifford RD, Howell JB, Radford M, Holgate ST. Associations between respiratory symptoms, bronchial response to methacholine, and atopy in two age groups of school children. Archives of Disease in Childhood 1989;64:1133‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cloutier 1981
- Cloutier MM, Loughlin GM. Chronic cough in children :a manifestation of airway hyperactivity. Pediatrics 1981;67:6‐12. [PubMed] [Google Scholar]
Diament 1987
- Diament MJ, Senac MO, Gilsanz V, Baker S, Gillespie T, Larsson S. Prevalence of incidental paranasal sinuses opacification in pediatric patients: a CT study. Journal of Computer Assisted Tomography 1987;11:426‐31. [DOI] [PubMed] [Google Scholar]
Doan 1992
- Doan T, Patterson R, Greenberger PA. Cough variant asthma: Usefulness of a diagnostic‐therapeutic trial with prednisone. Annals of Allergy 1992;69(6):505‐9. [PubMed] [Google Scholar]
East London 1996
- East London and the City: Health in the East. Annual Public Health Report 1995‐96.
Enarson 1987
- Enarson DA, Vedal S, Schulzer M, Dybuncio A, Chan‐Yeung M. Asthma, asthma‐like symptoms, chronic bronchitis, and the degree of bronchial hypereresponsiveness in epidemiological surveys. American Review of Respiratory Disease 1987;136:613‐67. [DOI] [PubMed] [Google Scholar]
Evald 1989
- Evald T, Munch EP, Kok‐Jensen A. Chronic non‐asthmatic cough is not affected by inhaled beclomethasone dipropionate. Allergy 1989;44:510‐14. [DOI] [PubMed] [Google Scholar]
Falconer 1993
- Falconer A, Oldman C, Helms P. Poor agreement between reported and recorded nocturnal cough in asthma. Pediatric Pulmonology 1993;15:209‐11. [DOI] [PubMed] [Google Scholar]
Faniran 1998
- Faniran AO, Peat JK, Woolcock AJ. Persistent cough: is it asthma. Archives of Disease in Childhood 1998;79(5):411‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferrari 1995
- Ferrari M, Oliveri M, Sembenini C. Tussive effect of capsaicin in patients with gastroesophageal reflux without cough. American Journal of Respiratory & Critical Care Medicine 1995;151:557‐61. [DOI] [PubMed] [Google Scholar]
Fuller 1988
- Fuller RW, Karisson JA, Choudry NB, Pride NB. Effect of inhaled and systemic opiates on responses to inhaled capsaicin in humans. Journal of Appled Physiology 1988;65:1125‐30. [DOI] [PubMed] [Google Scholar]
Fuller 1998
- Fuller P, Picciotto A, Davies M, McKenzie SA. Cough and sleep in inner city children. European Respiratory Journal 1998;12:426‐31. [DOI] [PubMed] [Google Scholar]
Glauster 1972
- Glauster FL. Variant asthma. Annals of Allergy 1972;30:457‐9. [PubMed] [Google Scholar]
Hannaway 1982
- Hannaway PJ, Hopper GDK. Cough variant asthma in children. Journal of the American Medical Association 1982;247(2):206‐8. [PubMed] [Google Scholar]
Hansson 1988
- Hansson L, Choudry NB, Fuller RW, Pride NB. Effect of nedocromil sodium on the airway response to inhaled capsaicin in normal subjects. Thorax 1988;43:935‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hsu 1994
- Hsu JY, Stone RA, Logan‐Sinclair RB, Worsdell M, Busst CM, Chung KF. Coughing frequency in patients with persistent cough: assessment using a 24 hour ambulatory recorder. European Respiratory Journal 1994;7:1246‐53. [DOI] [PubMed] [Google Scholar]
Isles 1993
- Isles A, Robertson CF. Treatment of asthma in children and adolescents: the need for a different approach. Medical Journal of Australia 1993;158:761‐3. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Koh 1993
- Koh YY, Chae SA, Min KU. Cough variant asthma is associated with a higher wheezing threshold than classic asthma. Clinical and Experimental Allergy 1993;23:696‐701. [DOI] [PubMed] [Google Scholar]
Konig 1981
- Konig P. Hidden asthma in childhood. American Journal of Diseases of Children 1981;135(11):1053‐5. [DOI] [PubMed] [Google Scholar]
Levy 1984
- Levy M, Bell L. General practice audit of asthma in childhood. British Medical journal 1984;289:1115‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 1989
- Lewis HM, Haeney M, Jeacock J, Thomas H. Chronic cough in a hospital population; its relationship to atopy and defects in host defence. Archives of Disease in Childhood 1989;64:1593‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lombard 1997
- Lombard E, Morgan WJ, Wright AL, Stein RT, Holberg CJ, Martinez FD. Cold air challenge at age 6 and subsequent incidence of asthma. American Journal of Respiratory & Critical Care Medicine 1997;156:1863‐9. [DOI] [PubMed] [Google Scholar]
McFadden 1975
- McFadden ER. Exertional dyspnea and cough as precludes to attacks of bronchial asthma.. New England Journal of Medicine 1975;292:555‐9. [DOI] [PubMed] [Google Scholar]
McKenzie 1994
- McKenzie SA. Cough‐but is it asthma?. Archives of Disease in Childhood 1994;70:1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
McKenzie 1995
- McKenzie S. Clinical features and their assessment. In: Silverman M editor(s). Childhood asthma and other wheezing disorders. London: Chapman & Hall, 1995:175‐200. [Google Scholar]
Ninan 1995
- Ninan TK, Macdonald L, Russel G. Persistent nocturnal cough in childhood. A population based study. Archives of Disease in Childhood 1995;73:403‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Picciotto 1998
- Picciotto A, Sturdy P, Hubbard M, Naish J, McKenzie SA. Prescribing for persistent cough in children. Respiratory Medicine 1998;92:638‐41. [DOI] [PubMed] [Google Scholar]
Powell 1996
- Powell CV, Primhak RA. Stability of respiratory symptoms in unlabelled wheezy illness and nocturnal cough. Archives of Disease in Childhood 1996;75:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rand 1994
- Rand CS, Wise RA. Measuring adherence to asthma medication regimens. American Journal of Respiratory & Critical Care Medicine 1994;149:S69‐76. [DOI] [PubMed] [Google Scholar]
Sheppard 1983
- Sheppard D, Rizk NW, Boushey HA, Bethel RA. Mechanism of cough and bronchoconstriction induced by distilled water aerosol. American Review of Respiratory Disease 1983;127:691‐4. [DOI] [PubMed] [Google Scholar]
Shimuzu 1996
- Shimuzu T, Mochizuki H, Tokuyama K, Morikawa A. Relationship between acid‐induced cough response and airway responsiveness and obstruction in children with asthma. Thorax 1996;51:284‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skoner 2001
- Skoner DP. Issues and challenges in pediatric asthma. Allergy 2001;56(66):18‐21. [DOI] [PubMed] [Google Scholar]
Tomerak 2003
- Tomerak A, McGlashen J, McKean M, Vyas H. Inhaled beta‐2 agonists for treating non‐specific chronic cough in children (Cochrane review). The Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wright 1996
- Wright AL, Holbergh CJ, Morgan WJ, Taussig LM, Halonenm, Martinez FD. Recurrent cough in childhood and its relation to asthma. American Journal Respiratory & Critical Care Medicine 1996;153:1259‐65. [DOI] [PubMed] [Google Scholar]
Yahav 1982
- Yahav Y, Katznelson D, Benzaray S. Persistent cough. A forme‐fruste of asthma. European Journal of Respiratory Diseases 1982;63(1):43‐6. [PubMed] [Google Scholar]


