Abstract
Background
: Colon cancer is potentially curable by surgery. Although adjuvant chemotherapy benefits patients with stage III disease, there is uncertainty of such benefit in stage II colon cancer. A systematic review of the literature was performed to better define the potential benefits of adjuvant therapy for patients with stage II colon cancer.
Objectives
: To determine the effects of adjuvant therapy on overall survival and disease‐free survival in patients with stage II colon cancer.
Search methods
: Ovid MEDLINE(R) (1986‐2007), EMBASE (1980‐2007), and EBM Reviews ‐ Cochrane Central Register of Controlled Trials ( to 2007) were searched using the medical headings "colonic neoplasms", "colorectal neoplasms", "adjuvant chemotherapy", "adjuvant radiotherapy" and "immunotherapy", and the text words "colon cancer" and "colonic neoplasms". In addition, proceedings from the annual meetings of the American Society of Clinical Oncology and the European Society of Medical Oncology (1996 to 2004) as well as personal files were searched for additional information.
Selection criteria
: Randomized trials or meta‐analyses containing data on stage II colon cancer patients undergoing adjuvant therapy versus surgery alone.
Data collection and analysis
: Three reviewers summarized the results of selected studies. The main outcomes of interest were overall and disease‐free survival, however, data on toxicity and treatment delivery were also recorded.
Main results
: With regards to the effect of adjuvant therapy on stage II colon cancer, the pooled relative risk ratio for overall survival was 0.96 (95% confidence interval 0.88, 1.05). With regards to disease‐free survival, the pooled relative risk ratio was 0.83 (95% confidence interval 0.75, 0.92).
Authors' conclusions
: Although there was no improvement in overall survival in the pooled analysis, we did find that disease‐free survival in patients with stage II colon cancer was signficantly better with the use of adjuvant therapy. It seems reasonable to discuss the benefits of adjuvant systemic chemotherapy with those stage II patients who have high risk features, including obstruction, perforation, inadequate lymph node sampling or T4 disease. The co‐morbidities and likelihood of tolerating adjuvant systemic chemotherapy should be considered as well. There exists a need to further define which high‐risk features in stage II colon cancer patients should be used to select patients for adjuvant therapy. Also, researchers must continue to search for other therapies which might be more effective, shorter in duration and less toxic than those available today.
Keywords: Humans; Antineoplastic Combined Chemotherapy Protocols; Antineoplastic Combined Chemotherapy Protocols/therapeutic use; Chemotherapy, Adjuvant; Colonic Neoplasms; Colonic Neoplasms/pathology; Colonic Neoplasms/therapy; Immunotherapy; Neoplasm Staging; Randomized Controlled Trials as Topic
Plain language summary
Adjuvant therapy for completely resected stage II colon cancer
Colon cancer is the second most common cause of cancer deaths in the Western world. A large proportion of colon cancer patients can be cured by surgical resection alone. For those patients with lymph node positive (stage III) disease, the recurrence rate can exceed 50% and adjuvant chemotherapy has been shown to significantly reduce the risk of recurrence. In patients without lymph node involvement (stage I and II), the prognosis is quite good with surgery alone, with survival rates of 75% to 95% at 5 years. However, some patients with high risk stage II disease have a relapse rate approaching that of stage III colon cancer patients. Due to the effectiveness of systemic chemotherapy in stage III disease, a similar approach has been considered for patients with stage II disease. We performed a systematic review looking at all randomized clinical trials evaluating stage II colon cancer patients and adjuvant therapy versus surgery alone. Our review found that adjuvant therapy ‐either systemic or regional chemotherapy or immunotherapy‐ can improve the outcomes of stage II patients. In counselling individual patients, the advice given should be conditioned by the patient's age and comorbidities. In addition, the high risk features of the tumour should also be considered when contemplating the benefits of systemic therapy in patients with stage II colon cancer. Further investigation is needed to elucidate which patient and tumour factors can be used to select stage II colon cancer patients for adjuvant therapy. There also exists a need to continue to search for other adjuvant therapies which might be more effective, shorter in duration and less toxic than those available today.
Background
Colon cancer affects 50 to 60 people per 100,000 population and is the second most common cause of cancer death after lung cancer in North America. Approximately half of patients diagnosed with colon cancer ultimately die of the disease. The prognosis of newly diagnosed colon cancer patients is determined by the clinico‐pathologic stage of the disease. In stage II disease there is tumour penetration through the bowel wall beyond the submucosa, but there is no involvement of the regional lymph nodes or other distant sites (Table 1). Overall survival for this group of patients is on average 75% five years after surgery alone. However, this is a heterogeneous group of patients and some have poorer outcomes, similar to that of patients with stage III disease with a 5‐year overall survival of 50% (O'Connell 2004). High risk features include tumours which penetrate the bowel wall, adhere or invade surrounding structures, perforation, obstruction, poorly differentiated tumours, extramural vascular invasion and aneuploidy (O'Connell 2004; Galandiuk 1992). The number of lymph nodes examined can also affect prognosis and fewer than 12 examined lymph nodes may result in a worse outcome (Baxter 2005; Berger 2005). Extensive genomic alterations in colon cancer (Grady 2004) may have prognostic consequences, such as chromosome 18q mutation and microsatellite stability (Halling 1999; Graziano 2003; Popat 2005). Adjuvant therapy has been recommended for stage III colon cancer patients because it decreases relapse and mortality rates by 30% to 40% when compared to observation alone after surgery (NIH 2000). Although there is no biological reason why adjuvant therapy will not have a similar effect on stage II patients, ongoing controversy exists as to whether adjuvant therapy should also be advised for patients with stage II colon cancer. For this reason, numerous clinical trials have been performed in this setting. Results of these trials have been inconclusive to date. The explanation for this in the past has been that insufficient numbers of patients have been enrolled in a single trial to demonstrate any overall survival benefit (Buyse 2001; Gill S, 2004). In view of the low power of the individual studies, a systematic review of the literature was undertaken to collect data on stage II colon cancer patients investigated in randomized clinical trials comparing adjuvant therapy to observation alone after surgery.
1. Staging of Colorectal Cancer.
| TMN | Stage | Extension To | Dukes |
| T1N0M0 | I | Mucosa | A |
| T2N0M0 | I | Muscularis | B1 |
| T3N0M0 | II | Subserosa/Perciloic Tissue | B2 |
| T4N0M0 | II | Visceral Per/Other Organs | B3 |
| T1‐2N1M0 | IIIA | N1 1‐3 lymph nodes | C |
| T3‐4N1M0 | IIIB | as above | C |
| Tany N2M0 | IIIC | N2 >3lymph nodes | C |
| TanyNanyM1 | IV | Metastatic Disease | D |
Objectives
1.‐ To determine if adjuvant therapy improves the survival of stage II colon cancer patients compared with surgery alone.
2.‐ To determine if the group of patients with poor prognostic factors (high‐risk stage II) benefit more in regards to overall survival than those without poor prognostic features.
3.‐ To determine if improvements in disease‐free survival parallel those of overall survival.
4.‐ To determine compliance with treatment and associated toxicity.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled clinical trials (RCTs) and meta‐analyses of RCTs comparing adjuvant therapy to surgery alone.
Types of participants
Patients with completely resected stage II colon carcinoma of any age or sex.
Types of interventions
Adjuvant therapy may include any form of chemotherapy, radiotherapy and/or immunotherapy compared to observation alone immediately before or after surgery.
Inclusion criteria
Articles were selected for inclusion in this systematic review if the following criteria were met: 1. Randomized clinical trials or meta‐analyses of RCTs; 2. Include patients with completely resected stage II colon cancer. This includes studies that explore adjuvant therapy in patients with other stages of colon cancer. In addition it includes studies in which a subset of patients have rectal cancer. 3. Adjuvant therapy is compared with observation alone after surgery; 4. Data on patient survival at 5 years is available, with a median follow‐up of at least 48 months.
Exclusion criteria
1. Trials published before 1987. Buyse et al (Buyse 1988) summarized the results of randomized trials of adjuvant therapy for colorectal cancer up to that year. Major methodological concerns about the design of the trials, lack of standardization of surgery and staging and therapy not presently used would make further delving into those data uninformative. 2. Trials without a surgery only control arm. 3. Trials that do not allow for the separate calculation of survival data for stage II colon cancer patients. 4. Studies with less than 48 months of median follow‐up.
Types of outcome measures
1.‐ Number of deaths at 5 years. (Mortality rate at 5 years) 2.‐ Number of tumour recurrences at 5 years. (Recurrence rate at 5 years) 3.‐ Number of patients experiencing adverse events related to treatment: deaths, hospitalizations, neutropenic sepsis, grade 3 or higher gastrointestinal toxicity and other grade 3 or higher toxicities. 4.‐ Number of patients receiving >80% of the prescribed protocol treatment. 5.‐ Overall planned dose intensity of the adjuvant treatment. 6.‐ Number of patients who received cointervention or control patients who received adjuvant treatment. 7.‐ Number of patients with high‐risk factors such as T4 disease, perforation, obstruction, poorly differentiated tumours.
Search methods for identification of studies
Ovid MEDLINE(R) (1950 through March Week 1 2007), EMBASE (1980 through 2007 Week 11) and EBM Reviews ‐ Cochrane Central Register of Controlled Trials ( through 1st Quarter 2007) were searched using the medical headings "colonic neoplasms", "colorectal neoplasms", "adjuvant chemotherapy", "adjuvant radiotherapy" and "immunotherapy", and the text words "colon cancer" and "colonic neoplasms". In addition, proceedings from the annual meetings of the American Society of Clinical Oncology and the European Society of Medical Oncology (1996 to 2004) were searched for reports of recently completed or ongoing trials on stage II colon cancer patients. Personal reprint files, consultation with colleagues and reference lists of relevant studies were also searched.
CCTR, EMBASE, Ovid MEDLINE(R) search strategy
1. Colonic Neoplasms/ [MeSH] 2. Colorectal Neoplasms/ [MeSH] 3. (colon OR colorectal OR colonic).ti.[title] 4. (malignan$ OR Neoplas$ OR cancer OR carcinoma OR adenocarcinoma).ti. 5. 3 AND 4 6. 1 OR 2 OR 5 7. Chemotherapy, Adjuvant/ [MeSH] 8. radiotherapy, adjuvant/ [MeSH] 9. Immunotherapy/ [MeSH] 10. adjuv$.mp. 11. 7 OR 8 OR 9 OR 10 12. 6 AND 11 13. (clinical AND trial).ab.ti. [abstract or title] 14. Clinical Trials/ [MeSH] 15. clinical trial.pt [publication type] 16.random$.mp. 17.Random Allocation/ [MeSH] 18.therapeutic use$.mp. 19. 13 OR 14 OR 15 OR 16 OR 17 OR 18 20. 12 and 19
Data collection and analysis
Development of the systematic review
This review is an update of a previous guideline on the use of adjuvant therapy in stage II colon cancer produced by the Gastrointestinal Cancer Disease Site Group of the Cancer Care Ontario Program on Evidence‐based Care (Figueredo 1997) and a more recent overview published as the background for the American Society of Clinical Oncology guideline on the same topic (Figueredo 2004). While the previous reviews followed the methodology of the practice guideline cycle described elsewhere (Browman 1995), the present systematic review follows the Cochrane Collaboration methodology.
Data Collection The three reviewers summarized articles of selected trials using a paper data collection form. Although mortality at 5 years was the main outcome of interest, data relating to disease‐free survival, patient toxicities and treatment delivery were also recorded. Significant toxicity was categorized for the present review as that causing death, hospitalization, major infection, major gastrointestinal disorder or other major events. Toxicity criteria were assessed using the World Health Organization toxicity criteria. Treatment delivery was measured by compliance with the treatment and the projected dose intensity of the regimen.
Assessment of Study Quality Study quality was assessed according to the four types of biases described by Feinstein (Feinstein 1985). Studies comparing adjuvant therapy to observation do not have blinding for treatment assignment or outcome measurement. Given this significant deficit of adjuvant trials, we concentrated on considering other areas of biases as follows:
1.Selection bias. In considering only randomized controlled trials, a major source of selection bias is eliminated. We also recorded whether or not allocation concealment took place. Finally, we also compared patient characteristics with known or potential prognostic significance (age, sex, disease stage, comorbidity). 2.Performance bias: Both contamination (control group patients receiving the intervention) and cointervention (different care for the two randomized groups such as variation in follow‐up) were evaluated for each selected study. 3.Attrition bias: This is an important source of bias in adjuvant trials. Although some loss of trial participants is expected, we looked for a clear description of these losses. We considered losses in the range of <10% to be reasonable. 4.Detection bias: As outcome assessments in adjuvant trials are not blinded, we have chosen the most objective of the outcomes: whether the patient is alive or not. Other outcomes are more subjective. Disease recurrence diagnosis depends on patient contact with physician and the use of screening tests for recurrences. Although follow‐up schedule and tests are the same for treated and control patients after treatment completion, there is more intensive testing and follow‐up during adjuvant treatment and this may introduce some bias in early recurrence diagnosis.
To classify the studies according to the biases present, the following considerations will be taken into account:
Major biases: ‐ No concealment of randomization ‐ More than 10% of randomized patients lost for analysis ‐ Control group gets treatment in >10% of patients ‐ For determination of disease‐free survival, detection of recurrences during period of adjuvant treatment.
Minor biases: ‐ Doubt in regard to concealment of randomization ‐ Differences in baseline characteristics (p <0.05) ‐ Control group gets treatment in <10% of patients ‐ Cointervention favours one group
Studies will be classified as follows:
Risk of Bias Interpretation Bias Assessment
Low unlikely to alter results No major or one minor bias Moderate may alter results One major or up to 3 minor biases High likely to alter results One or more major or minor biases Studies with moderate and high biases will be considered separately in a sensitivity analysis.
Analysis
Results were recorded with regards to an intention to treat analysis.
Pooled analysis was performed using the RevMan version 4.2.7software. Results were expressed as relative risks or risk ratios (RR) with 95% confidence intervals. RR less than 1.0 favours the experimental group, indicating that patients on the experimental group (adjuvant therapy) experienced fewer events than the control group (observation after surgery) (Sinclair 1994). Data were analyzed using the random effects model (DerSimonian 1986).
Meta‐analysis results were investigated for clinical and statistical heterogeneity. In the case of significant statistical heterogeneity, outlier results were looked for and cumulative meta‐analysis was used to detect trials that caused the heterogeneity.
To avoid treatment heterogeneity, the analysis of trials was performed according to the adjuvant treatments given. For example, patients receiving common chemotherapy such as 5‐fluorouracil (5‐FU) plus either levamisole or leucovorin, were considered together. The same analysis was carried out for patients receiving regional portal vein infusion chemotherapy or adjuvant immunotherapy.
Similar analyses were performed for disease recurrence, toxicity and treatment compliance.
The issue of Hazard Ratio (HR) versus Relative Risk (RR): Hazard ratios typically give a better description and measure of the difference in survival between two randomized groups. Hazard ratios also describe the total time experience of the groups rather than at a single time post. However, this single point measurement relates to the total number of patients over the total time of the trial and it can give a good estimate of the difference. Our group selected RR over HR for the purposes of this meta‐analysis given the significant difficulty in obtaining HR for many trials. We thought that by selecting out trials for review according to this criterium, this would result in substantial bias. We did obtain HR's for some of the larger adjuvant trials, including those using 5‐FU plus leucovorin and/or levamisole. We found that the HR's were in fact very similar to RR's.
Results
Description of studies
The original literature search identified 1732 abstracts. An initial screening by title reduced the number of relevant reports to 312. Of these 312 abstracts, 159 abstracts appeared to deal with curatively resected stage II colorectal cancer patients who had been randomized to adjuvant therapy or observation. Upon review of the full publications, 117 articles were considered potentially relevant according to the inclusion criteria. These included 84 publications reporting on clinical trials of which 32 were accessories to the main trials. Furthermore, there were 33 systematic reviews, with most of these including meta‐analyses within them. Of the meta‐analyses, 4 contained data to corroborate or update trials also identified using the search strategy. Figure 1 summarizes the overall results of the search strategy employed. Five additional publications were found in personal files (Gray 1988; Gray 1991; LIMAG 1997;Schlag 1990; Harris 2000). Figure 1 (see Additional Figures) depicts the trials classified according to the adjuvant therapy received as well as the total number of patients and the percentage of patients with stage II disease. Tables 2 to 6 provide more details about the trials reviewed, showing both the wide range of therapies tested and the forthcomings in reporting significant data.
1.

Table 1.
Included studies Twenty‐five publications reporting on 33 trials and 17 meta‐analyses are shown in the Table of Included Studies. We only included randomized phase III clinical trials (RCTs) and meta‐analyses of RCTs with a control arm consisting of surgery, and other arms consisting of adjuvant therapy. The validity of the trial results is measured by the risk of bias. This risk was determined to be low in 26 studies, moderate in 9 and high in none.
The participants in the trials were patients with completely resected colon (n=7731) or rectal (n=7600) cancers. There were 4 trials reported that included stage II colon cancer patients alone (n=3384). Patients were excluded if there was any evidence of previous malignancy, prior treatment with chemotherapy or radiation, abnormal blood counts or liver or renal function or severe co‐morbidities. Most trials were performed in one country (n=16), although some were international (n=16) or data from single institutions (n=3). Patient accrual in these trials occurred between the years 1976 and 2002. The number of participants in each study ranged from 78 to 1738 and had a median age of approximately 62 years and a male/female ratio of 1.11. The median follow‐up of patients was more than 60 months in 21 trials.
The interventions investigated included a variety of treatments: systemic chemotherapy (n=16), regional chemotherapy (n=6) and immunotherapy (n=7). A description of the treatment(s) is given for each trial. Six trials had 2 treatment arms.
All included studies had risk ratios and 95% confidence intervals calculated for all stage II colon cancer patients. 5‐year overall survival and disease free survival were recorded. In view of the variety of treatments tested, a pooling of trial results was deemed to be reasonable only within certain treatment groups. These groups were determined a priori and included 5‐fluorouracil (5‐FU) plus nitrosoureas, mitomycin C and fluoro‐pyrimidines, 5‐FU plus levamisole and/or folinic acid, portal vein infusion (PVI) of 5‐FU alone or with mitomycin C and specific versus non‐specific immunotherapy.
In addition to the randomised clinical trials, 19 meta‐analyses were also included. Two of these include primary results of 10 RCTs (IMPACT B2 1999; Glimelius 2005). Another meta‐analysis provides data on stage II patients not available in the original publication (Mamounas 1999). The other meta‐analyses utilize either published reports or directly obtained individual patient data. These latter trials investigate a variety of factors in regard to the benefits and harms of the adjuvant therapies and ways to predict prognosis.
Excluded Studies
Clinical trials were excluded if there was no observation arm or separate data on DFS or OS for stage II patients or if they were accessories to the main trial publication. For completeness of the review the available data of excluded RCTs with an observation arm are depicted in Tables 1 through 6 together with data from the included studies. Meta‐analyses were excluded if they were similar to previous publications by authors of the same group. All of the above information is listed in the Table of Excluded Studies.
Risk of bias in included studies
Twenty‐five publications reporting on 33 trials and 17 meta‐analyses are shown in the Table of Included Studies. All included studies were either randomized phase III clinical trials (RCTs) or meta‐analyses of RCTs, with a control arm consisting of surgery, and other arms consisting of adjuvant therapy. The validity of the trial results was measured by the risk of bias. This risk was determined to be low in 26 studies, moderate in 9 and high in none.
Effects of interventions
Adjuvant systemic chemotherapy (Figure 2, Figure 3, Figure 4 & Figure 5 in Additional Figures)
2.

Table 2.
Adjuvant Systemic Chemotherapy with Early Regimens
3.

Table 2b.
4.

Table 3.
Adjuvant Systemic Chemotherapy with commonly used regimens
5.

Table 3b.
This form of adjuvant treatment had the largest number of trials. The trials were grouped into categories of early chemotherapy regimens, more contemporary chemotherapy regimens, and those using miscellaneous treatments no longer in use (intraluminal 5‐FU, radiation to the liver and razoxane). Early chemotherapy trials tested adjuvant therapy with a fluoro‐pyrimidine plus either a nitrosourea or mitomycin C (Figure 2(see Additional Figures)). Four trials tested a combination of 5‐FU and nitrosoureas, and one of these (Wolmark 1988) was the first to demonstrate significant benefits in DFS and OS for combined stage II and III colon cancer patients. There was no significant interaction between treatment effect and stage. However, two other similar trials did not confirm these findings (Panettiere 1988; Abdi 1989) and 10‐year results of the positive trial found that the apparent benefits disappeared over time (Smith 2004). All of these trials reported separate data for stage II patients. A pooled analysis revealed a RR for death of 0.93; (95% CI 0.71, 1.10). Compliance with treatment (>80% of planned dose) was noted in 41% of patients in the only trial describing it (Wolmark 1988). The same trial also described 3 toxic deaths due to acute leukemia and severe GI toxicity in 15% of patients. Renal failure occurred in one patient each of 2 trials (Abdi 1989; Wolmark 1988).
The other group of 9 trials, using a variety of fluoro‐pyrimidines ‐mostly administered orally and often combined with mitomycin C‐, showed benefits in both DFS and OS when considering all patients, and one trial (Watanabe 2006) demonstrated significant benefit in DFS for stage II patients. The published results did not allow for pooling of results in stage II patients (Figure 2 and Figure 3(see Additional Figures)). However, the use of oral fluoro‐pyrimidines as adjuvant therapy in Japan was the topic of 4 pooled analyses (Sakamoto 1999; Sakamoto 2001; Sakamoto 2004; and Sakamoto 2005). The 2001 report by Sakamoto et al. was excluded since only one of these trials compared adjuvant therapy with observation after surgery (Ito 1996). This trial and two others (Yasutomi 1997; the other not reviewed here), which tested carmofur (HCFU) as adjuvant therapy, either alone or with mitomycin C, were pooled in an individual patient data meta‐analysis (Sakamoto 2005). When all patients were considered, there was a significant benefit for those receiving treatment: DFS of 77% vs. 71% (p=0.003); OS 80% vs. 76% (p=0.043). For patients with stage II colon cancer, the absolute difference favouring treatment for DFS was 6.6% (p=0.032) and for OS was 6.8% (p=0.086). The two other pooled analyses, using similar methodology, investigated the value of adjuvant oral HCFU or 5‐FU for one year on the outcome of patients with both colon and rectal cancer. One of the analyses (Sakamoto 2004) considered trials where the randomization was performed centrally. In patients with stage II disease receiving adjuvant therapy there was a hazard ratio reduction (21%±SE 9%) in both colon and rectal cancer patients. In a previous analysis (Sakamoto 1999), where patient randomization was by the closed envelop method, there was no benefit for stage II patients receiving adjuvant oral fluoro‐pyrimidines. Compliance with planned drug doses was 71% and 76%, and toxicity was reported as mild in one trial (CCCSG Japan 1995). In another trial (Watanabe 2006) compliance with drug doses was 59% for mitomycin C, 93% for 5‐FU and 59% for HCFU.
The contemporary chemotherapy consisted of 5‐FU plus either levamisole and/or folinic acid (Figure 4 and Figure 5(see Additional Figures)).
Six individual trials have tested levamisole alone or combined with 5‐FU, and all except one (Dahl 2003) have reported separate data for stage II patients (Figure 4). None of the individual trials have demonstrated a benefit for adjuvant therapy in stage II patients. Pooled results from 4 trials reporting on stage II patients (Windle 1987; Laurie 1989; Moertel 1995 and Taal 2001) showed a RR for mortality of 0.88; (95% CI 0.71, 1.10). Compliance with 5‐FU + levamisole was 80% at 6 months and 69% at 12 months in one trial (Taal 2001) and in another trial (Moertel 1995) 30% of patients discontinued treatment, mostly due to severe nausea, at a median of 5 months. No deaths were reported in either trial but later reports of a severe leukoencephalophaty (Hook 1992) which affects up to 5% of treated patients (Figueredo 1995), led to the abandonment of levamisole as adjuvant treatment.
Thirteen trials testing the combination of 5‐FU plus folinic acid, with or without levamisole, have been reported either individually or in pooled analyses involving 1016 (IMPACT B2 1999) and 1520 (Glimelius 2005) colon cancer patients (Figure 4 and Figure 5(see Additional Figures)). Separate data for stage II patients was available in two individual trials (Francini 1994; Zaniboni 1997) which are also included in the pooled analysis of 5 trials (IMPACT B2 1999). Neither the individual trials nor the meta‐analyses demonstrated significant benefits for adjuvant therapy. This adjuvant therapy was well tolerated: 85% to 98% of patients received the planned doses of the drugs (IMPACT B2 1999). Toxicity did not include deaths but severe nausea/vomiting (4%), stomatitis (11%) and diarrhea (8%) did occur. Glimelius et al. (Glimelius 2005) also noted that 67% of patients received the planned number of treatment courses of 5‐FU ± levamisole ± folinic acid. In a pooled analysis of 7 trials using 5‐FU + levamisole or 5‐FU + folinic acid (Sargent 2001) it was noted that grade 3 or higher gastrointestinal toxicity was more common with 5‐FU + levamisole than with 5‐FU + folinic acid. With regards to leucopenia, the reverse was true. However, these comparisons were carried out between rather than within trials. Of interest, it should be noted that patients >70 years of age appeared to tolerate systemic adjuvant treatment similar to their younger counterparts.
A later pooled analysis of 3302 patients with stage II and III colon cancer investigated in 7 clinical trials comparing observation to adjuvant therapy with 5‐FU combined with levamisole or folinic acid has been performed using individual patient data (Gill 2004). Applying a Cox proportional hazard regression model, the authors estimated 5‐year DFS and OS according to T stage, nodal status and histological tumour grade. As expected, the larger benefits were observed in patients with node positive disease. In patients without nodal involvement, regardless of T stage and tumour grade, the absolute percent benefit in DFS for treated versus observed patients was 4% to 6%, with wide confidence intervals. The OS benefit for these stage II patients was smaller than the DFS, and the results were consistent across the various age and tumour grade groups.
Regional Chemotherapy (Figure 6 and Figure 7(see Additional Figures))
6.

Table 4.
Adjuvant regional chemotherapy
7.

Table 4b.
Given that most recurrences of colon and rectal cancer occur within the area of the portal vein system, particularly the liver, regional chemotherapy delivers a higher concentration of drug to the portal/hepatic circulation. Several trials evaluated this delivery route. Enthusiasm for this approach followed the significant survival benefits, especially in stage II patients, reported by Taylor et al. (Taylor 1985). This regional chemotherapy treatment was well tolerated and delivered one week after surgery.
At least 13 other trials have attempted to reproduce these early results, but only one has demonstrated a benefit in DFS (Wolmark 1990). All these trials used 5‐FU except one using floxiridine (Schlag 1990). Some of these trials also tested the hypothesis that either heparin (Gray 1987; Fielding 1992; Nitti 1997) or urokinase (Wereldsma 1990), which was used to maintain patent the portal vein access, may have some anti‐tumour effect. However, no benefit was detected in these studies. Four trials also intensified the chemotherapy by adding mitomycin C to 5‐FU, and, again, no benefit was demonstrated. Four published trials (Wolmark 1990; Beart 1990; Fielding 1992 and Laffer 1995) reported separate data for stage II patients.
Considering that the effect of chemotherapy by portal vein infusion is small and that the reported trial samples have a small to moderate number of patients, two meta‐analyses have been published. The initial one (Gray 1991) pooled results of 6 trials of chemotherapy by portal vein infusion and found significant benefits in overall survival, but did not give separate data for stage II patients. The Liver Infusion Meta‐analysis Group (LIMAG 1997) used individual patient data obtained from 10 trials (9 excluding the original Taylor's study) and had a total of 3088 patients, most of whom had colon cancer. The odds of death were reduced for the treated patients by 11%±5% (p=0.04) for the entire sample and by 5%±2% (p=0.01) for Dukes' A and B stages combined.
Three trials investigated peritoneal chemotherapy (Figure 7 ‐ additional figures). One of them only reported preliminary findings (Graf 1994). Another trial combined intraperitoneal chemotherapy combined with intravenous chemotherapy for a prolonged period of time and was therefore included in the 5‐FU plus folinic acid trials (Scheithauer 1995). The only trial that truly tested the intraperitoneal route with mature results was the trial reported by Vaillant et al. (Vaillant 2000). There was a trend towards improved outcomes, including overall survival in stage II patients.
A single study has investigated chemotherapy via hepatic artery infusion (Sadahiro 2004) (Figure 7). The reported results for the entire sample, including colon cancer patients with stages I, II and III, were significant for both DFS and OS; and there was a trend noted for improved overall survival in stage II colon cancer patients.
Immunotherapy (Figure 8 and Figure 9(see Additional Figures))
8.

Table 5.
Adjuvant immunotherapy
9.

Table 5b.
The interest in non‐toxic, immune‐stimulating treatments to facilitate rejection of residual cancer cells after apparent complete surgical excision has been widely investigated in colorectal adjuvant clinical trials. The immune therapy tested was categorized as being either specific, using altered tumour cells or antibodies, or non‐specific, using known immune stimulants such as BCG, interleukin‐2, interferon and others.
Our search identified 10 trials of non‐specific immunotherapy (Figure 8(see Additional Figures)): three trials tested BCG (Prohm 1988; Wolmark 1988; Abdi 1989), 3 tested interleukin‐2 (Nichols 1992; Nichols 1993, Brivio 2006), and one each tested PAPUA ‐polyadenyl‐polyuridylic acic‐ (Lacour 1992), PSK ‐a protein‐bound polysaccharide‐ (Nio 1992) and PBG KP‐45 ‐propionibacterium granulosum ‐ (Isenberg 1994).
Two trials reported conflicting results with the use of BCG. One trial (Wolmark 1988) showed benefit in overall survival (51% vs. 56%; p=0.02) whereas the other (Abdi 1989) reported poorer outcomes for treated patients. Of the 3 trials testing interleukin‐2, only one (Monga 2006) reported overall survival results favouring treatment. This benefit was not observed in patients with stage II disease. The only other trial reporting survival data (Isenberg 1994) tested PAPUA and demonstrated a significant trend favouring treatment and especially in stage II patients.
Among the 7 trials of adjuvant specific immunotherapy (Figure 8 and Figure 9(see Additional Figures)), four have tested the combination of altered tumour cell plus BCG administered by intradermal inoculations over 1 to 18 months (Vermorken 1999; Harris 2000; Hoover 1993; Gray 1989). Only one individual trial (Vermorken 1999) found an improvement for treated patients compared to untreated controls. This benefit was particularly prominent and significant in stage II patients. Pooling the results of 3 of these trials (excluding Hoover 1993) showed a RR for death of 0.91 (95% CI 0.70 ‐ 1.18). A critical review of 3 trials excluding Gray, 1989 (Hanna 2001) performed one meta‐analysis for all randomized patients and another for a selected group of "analyzable patients". The latter group required definite protocol eligibility and vaccines with >70% of viable cells and a dose of 107 tumour cells per immunization. The meta‐analysis of all 723 patients demonstrated a significant improvement in DFS (Odds Ratio 0.75; CI 0.56, 1.00). The pooled analysis of analyzable patients also demonstrated a significant delay in recurrences (p=0.028) which also occurred in stage II patients (80% vs. 63%; p=0.032). Most of the benefit was seen in patients who developed an induration of >5 mm at the site of the inoculum. Based on these findings, the investigators concluded that specific immunotherapy is an important adjuvant modality, particularly valuable for stage II patients. The different results of the 3 trials was attributed to variability in the immunogenicity of the vaccine, status of the host immune system and whether patients developed systemic cell‐mediated immunity. These 3 trials prepared the vaccine by initial enzymatic dissociation of tumour fragments, freezing the cells in liquid nitrogen for 30 days, thawing, washing and then irradiating the cells which were then added to BCG prior to administration. The fourth trial of specific immunotherapy (Gray 1989) prepared the tumour cells similarly, but used Vibrio‐cholera neuraminidase prior to freezing and irradiation. The tumour cells were not mixed with BCG, but instead they were applied to the site where BCG was previously inoculated. This trial did not report benefit for the treatment in spite of 18 months of immunotherapy.
Two trials reported initial results on the use of edrecolomab adjuvant therapy (Hartung 2005; Colacchio 2004). Edrecolomab is an antibody against glycoprotein 17‐1A found on the surface of colorectal tumour cells. It was successfully tested in patients with stage III colorectal cancer (Riethmuller 1994). The 2 trials reported results after median follow‐up times of 34 and 44 months, respectively. No significant difference in survival between treated and observed patients was noted. A meta‐analysis of the results at this early stage showed a RR for death of 0.88; 0.65, 1.20). Longer follow‐up is required before a final judgement can be made about this form of adjuvant therapy.
Another specific antibody 105AD7, mimicking the glycoprotein CD55 on the tumour cell surface, was tested together with BCG administered intradermally or with alum administered intramuscularly (Ullenhag 2006). These vaccinations were continued for 2 years. The available publications reported only immune responses, and did not report survival outcomes.
Other treatments (Figure 10(see Additional Figures))
10.

Table 6.
Miscellaneous therapies
Four placebo‐controlled trials tested histamine‐2 receptor antagonists (H‐2RA) in the adjuvant treatment of resected colorectal cancer (Svendsen 1995; Kelly 1999; Nielsen 2002; Kapoor 2005). Although the exact mechanism of action is unknown, cimetidine demonstrated cytotoxicity to colorectal cancers and stimulation of lymphocyte function in vitro. Other investigators noted improved survival for patients with rectal cancer containing lymphocytic infiltrates. Two of the trials administered cimetidine or ranitidine for 24 to 60 months whereas the other two used a higher dose of cimetidine or famotidine for 5 to 7 days. These trials showed a trend toward better survival for treated patients but none of them reached statistical significance. It was noted that the treatment effect was found only in patients who did not receive blood transfusions in the peri‐operative period (Nielsen 2002).
One report described a proposal for an adjuvant trial involving the COX‐2 inhibitor, Rofecoxib (Pendlebury 2003). It has been suggested that COX‐2 inhibition leads to a reduction in cancer cell invasion and metastasis. This VICTOR trial was stopped when serious cardiovascular side‐effects of the COX‐2 inhibitors were described. A recent report indicates that the incidence of cardiovascular events was significant in patients on rofecoxib compared to those receiving a placebo (estimated relative risk 2.66; p=0.04) (Kerr 2007).
Hazard Ratios versus Relative Risk Ratios We calculated hazard ratios for several of the trials included in the meta‐analysis to illustrate that there was very little difference between calculated hazard ratios and relative risk ratios. See Figure 11 and Figure 12 in Additional Figures.
11.

Table 7.
12.
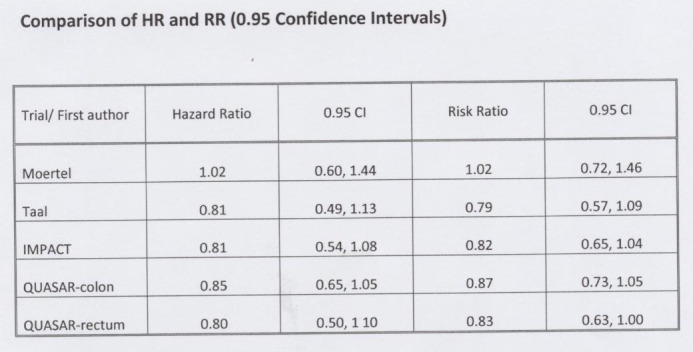
Table 8.
Other published meta‐analyses
Following the ground‐breaking pooled analysis by Buyse and collaborators (Buyse 1988a) the next two meta‐analyses continued to investigate results of colorectal cancer adjuvant therapy for all stages together. In one study (Zalcberg 1996) it was found that the planned total dose of 5‐FU during the first 3 months of treatment predicted for the size of survival benefit. The odd ratios for death were 0.71 for >10 gm, 0.79 for 8 to 10 gm, 0.93 for <8 gm and 1.04 for oral 5‐FU. Further, the value of adding levamisole was questioned because its benefit disappeared when the 5‐FU dose was considered. Dube (Dube 1997) performed a meta‐analysis of 39 clinical trials published between 1959 and 1993 and found the odds ratio for death predicted an absolute 5‐year overall survival benefit of 5% for colon cancer and 9% for rectal cancer.
A guideline developed by the Cancer Care Ontario, Provincial Guideline Initiative, Gastrointestinal Disease Site Group was the first to focus on stage II colon cancer (Figueredo 1997). It pooled results of 11 trials published after 1987 comparing adjuvant treatment versus observation after surgery. It found that neither systemic nor portal vein infusion chemotherapy provided significant survival benefits. Therefore, it recommended no standard adjuvant therapy for stage II colon cancer but discussion of potential benefits in patients at high risk of recurrence and participation in trials with an observation arm.
In 1999, two meta‐analyses investigated the value of adjuvant therapy in stage II colon cancer. The individual patient data meta‐analysis of 5 RCTs using 5‐FU plus folinic acid demonstrated the 5‐year absolute OS benefit of treated patients was 2% (p=0.398) (IMPACT B2 1999). The second study (Mamounas 1999) pooled results of 4 consecutive adjuvant trials in colon cancer by the National Surgical Adjuvant Breast & Bowel Project (NSABP). Two of the trials tested adjuvant therapy versus observation (Wolmark 1988 and Wolmark 1990) but the two other trials compared two adjuvant therapies. The results of the analysis suggested a 30% reduction in mortality for stage II patients. Further, this finding was supported by the lack of interaction between treatment and stage described in each of the individual trials.
The Cancer Care Ontario Guideline for the adjuvant treatment of stage II colon cancer was updated (Figueredo 2004). Eighteen trials and several meta‐analyses were considered and the pooled results for the different forms of adjuvant therapy remained inconclusive. The recommendations given, together with those of the accompanying article (Benson 2004), discussed the uncertainty of the benefits and suggested these be discussed with patients, particularly those at high risk of recurrence. Another meta‐analysis (Wan 2005) included the 4 trials considered by Mamounas, the IMPACT‐B2 data, that of Taal (Taal 2001) and also included results of a non‐randomized trial. The results showed a significant benefit of adjuvant therapy for stage II colon cancer.
In a statistical analysis of the question of adjuvant therapy in stage II colon cancer Buyse (Buyse 2001) noted that meta‐analysis may not be the most efficient tool searching for true benefits of treatment as it considers only a portion of the trial population. Either the lack of interaction between treatment and stage or an individual patient data with subgroup analysis may give the best answer short of a massive clinical trial. A group of investigators (Sargent, 1999) had already begun to collect individual patient information in a large data‐base of contemporary adjuvant systemic chemotherapy trials using 5‐FU plus either levamisole or folinic acid. The data was used then to determine patients who may benefit from the adjuvant treatment (Gill 2004). A Cox proportional hazard regression model was used to predict DFS and OS considering as variables treatment, age, sex, tumour location, T stage, nodal status and histological grade of malignancy. Treatment benefits were observed across age, sex, tumour location, T stage and grade. The benefit in 5‐year DFS for treated patients, compared to observation after surgery, was 4% to 6% regardless of T stage and grade. The 5‐year OS benefit for adjuvant therapy ranged from 2% to 4% regardless of age and grade, with a 1% larger benefit for T4 compared to T3 stage. Neither of the values were individually significant. Moertel 1995 had already performed a multivariate analysis with data from the reported trial as well that from Laurie 1989, where the end‐points were DFS or OS and the variables were age ±61 years, sex, delay of treatment after surgery (±21 days), adhesion or invasion to other organ, obstruction, perforation and location of the primary tumour within the colon. Neither of the investigated variables had an interaction with the 5‐FU plus levamisole adjuvant treatment on disease recurrence or survival.
Another study (Sargent 2005) tested whether DFS at 3 years could be used as a surrogate end‐point for 5‐year OS. The correlation obtained for the two was 0.89. This correlation was explained because 80% of recurrences occur within 3 years and 91% of these recurrences lead to death within 5 years. This correlation was found for both the treatment and control arms of the trials. The author cautioned, however, that marginally significant benefits in DFS may not translate into significant advantages in 5‐year OS.
One of the initial studies of the above group (Sargent 2001) explored whether adjuvant chemotherapy benefits extended to elderly patients. For this pooled analysis 7 clinical trials involving 3351 patients were tested for death and toxicity according to 4 age groups (<50, 51‐50, 61‐70 and >70 years). No significant interaction was detected for overall survival (p=0.61) or for recurrence (p=0.33). Deaths without evidence of recurrence, however, were more common with increasing age (2% in patients <50 years old; 13% for those >70 years old). Toxicity was not related to age except for severe leucopenia. There was no separate data for treatment effect on stage II patients.
Two other publications have examined the outcomes of African‐Americans compared to Caucasians in adjuvant trials in attempting to explain the poorer prognosis for African‐American patients in the population at large. While the first study (Dignam 1999) including patients in 5 trials lead by NSABP found an 8% higher recurrence rate (p=0.27) and a 21% higher risk of death (p=0.004) for African‐Americans, the other study (McCollum 2002) investigating a large single trial of several 5‐FU adjuvant regimens did not find differences in either disease‐free or overall survival. In the second study, however, the toxicity for African‐Americans was significantly lower than for Caucasians, including diarrhea, nausea, vomiting, stomatitis and overall (p<0.005).
Discussion
To better understand the extensive literature on the heterogeneous adjuvant treatments for stage II colon cancer, the discussion will be divided into three sections: systemic chemotherapy, regional chemotherapy and immunotherapy.
Systemic chemotherapy The systemic administration of chemotherapy has been the most intensely investigated and 5‐FU and its analogs the main components of the regimens. The nitrosoureas are no longer used due to toxicities which include acute leukemias (Wolmark 1988). Mitomycin C has remained active in Japanese adjuvant trials, mainly as an induction agent followed by 5‐FU or analogs (HCFU or carmofur, UFT or uracil plus ftorafur). These regimens have demonstrated in meta‐analyses (Sakamoto 2005 and Sakamoto 2004) significant benefits for stage II colon cancer in DFS (6%; p=0.003) and OS (4%; p=0.043). Most trials in the Western world have used 5‐FU by bolus intravenous injection, only recently substituted by continuous intravenous infusion or by the oral analog capecitabine (5‐deoxy‐5‐fluorouridine or 5'DFUR). Initially 5‐FU was combined with levamisole and later with folinic acid. The benefit of levamisole was questioned early in a meta‐analysis (Zalcberg 1996) when the dose of 5‐FU was found the determinant factor in improving survival. Further, its delayed toxicity which included a leukoencephalopathy and the demonstration of the value of folinic acid led to its abandonment. In the reviewed trials only 5‐FU by IV bolus injection, combined with levamisole and/or folinic acid has been used. These regimens of adjuvant treatment are considered contemporaneous because have been standard therapy for stage III colon cancer until recently and have been advocated for stage II high‐risk patients. The definite beneficial advantage of adjuvant therapy with these regimens in stage III have not been conclusive for stage II disease patients. A meta‐analysis of 5‐FU plus folinic acid adjuvant trials (IMPACT B2 1999) revealed minimal increase in OS (absolute risk difference 2%; p=0.08). A more ambitious pooled analysis (Gill 2004) attempted to determine benefits for subgroup of patients with different stage of disease, histological malignancy grade and age. In stage II patients the benefits in DFS and OS were small (4% to 6%) and not statistically significant. A more recent massive adjuvant trial with 5‐FU±folinic acid±levamisole including over 3000 patients (Gray 2004) confirmed this non‐significant benefit. It is probable that pooling the results of this trial with the data‐base used by Gill will provide statistically significant results albeit the benefit will still be in the range of 1% to 6%. This information as well as early results from a trial 5‐FU+ folinic acid alone or combined with oxaliplatin opened again the debate of advising adjuvant therapy for stage II colon cancer (Midgley 2005; Sobrero 2006; Andre 2006; Monga 2006). However, an update of the oxaliplatin trial (De Gramont 2007) showed again significant benefits for stage III patients but not for stage II patients. For this latter group of patients the DFS risk difference was 3.8% (p=0.258) and for OS 0.1% (p=0.996).
Regional chemotherapy The attempt to increase drug concentration in the organs of the portal vein system, the most affected by first site of recurrence, chemotherapy by portal vein, peritoneal and hepatic artery infusion have been tested. Ten published adjuvant trials have administered chemotherapy by portal vein infusion for one week immediately after surgery. Only 4 trials provide separate data for stage II patients. A meta‐analysis of the trials revealed no benefit for this treatment. A previous meta‐analysis (Gray, 1991) pooled results of 6 trials but did not report separate data for stage II patients. A more inclusive study (LIMAG, 1997) used individual patient data and included 4000 patients participating in 10 trials. It demonstrated a small but significant decrease in the risk of death for patients with stages I and II colon cancer combined (5%±2%; p=0.01). Chemotherapy by peritoneal infusion (Vaillant 2000) as well as by hepatic artery infusion (Sadahiro 2004), as single modalities, have also reported to induce a trend toward improved survival in stage II patients. Regional chemotherapy as an adjuvant for colon cancer has been mostly abandoned because the technical difficulties of the procedures and similar results from systemic chemotherapy.
Immunotherapy Attempts to boost the immune response against residual cancer cells after apparent complete surgery have been widely investigated. The non‐specific immune stimulation has utilized a variety of agents. BCG was initially investigated alone (Prohm 1988; Wolmark 1988; Abdi 1989) or combined iwith chemotherapy (Panettiere 1988). The only study demonstrating a persistent benefit in survival, even after 12‐year follow‐up did not show an improvement in DFS (Wolmark 1988; Smith 2004). Other non‐specific immune stimulants (Interferon, lnterleukin‐2, PAPUA, etc.) have been tested but separate data for stage II colon cancer patients are not available except for one study (Isenberg 1994). This small study, using propiobacterium granulosum preparation KP‐ 45 intravenously in the perioperative period observed a significant decrease in mortality and recurrence for treated patients with stage II disease. These results should be considered preliminary and would require confirmation in a larger trial. Specific immunotherapy has been included in 4 trials using altered tumour cells plus BCG and 2 trials using the antibody edrecolomab. Among RCTs testing active specific immunotherapy (Hoover 1993; Gray 1988; Vermorken 1999; Harris 2000) only one has demonstrated a survival benefit for stage II colon cancer (Vermorken 1999). A critical review and pooling the results of three of those trials (Hanna 2001) has demonstrated overall survival benefits, particularly when including only patients who were eligible and received and responded to active vaccination. The recurrence‐free survival in stage II patients favoured treatment (80% vs 63%; p=0.0032). No data is given for overall survival. Although this active specific tumour cell vaccination appears to have a larger benefit than other forms of treatment and requires only a short duration of treatment witn only minor side‐effects, it is a complicated procedure requiring a dedicated laboratory to obtain an active vaccine and a responsive host. An easier form of specific immunotherapy utilizes an antibody to a glycoprotein of the cancer cell surface. Edrecolomab, produced commercially, was found effective in reducing risk in stage III colorectal cancer (Riethmuller 1994) and was then tested in stage II colon cancer (Hartung 2005; Colacchio 2004). Neither of these latter trials have shown improved outcomes for treated patients but follow‐up has been shorter than the usual 5‐year mark. One of the trials has included the measure of various prognostic factors in tumour tissues which may be relevant for future selection of patients and therapeutic approaches.
Authors' conclusions
Implications for practice.
In a statistical perspective about whether patients with stage II colon cancer should receive adjuvant therapy (Buyse 2001) it is stated that to investigate benefits, four statistical approaches can be used short of a massive RCT: 1) consider overall treatment effects regardless of the stage; 2) estimate benefits for stage II patients only in a meta‐analysis; 3) compare the relative benefits among patients with stage II and stage III disease; and 4) perform tests of interaction between treatment and stage. All these tests have been done as well as a massive clinical trial. A benefit has been demonstrated by most procedures but the benefit is small even when statistically significant. The lack of major treatment effect in stage II colon cancer can be attributed to the good survival of patients treated with only surgery (75% to 80% at 5 years) and the competing mortality from causes other than colon cancer (Buyse 2001). This phenomenon had already been reported (Nauta 1989). These investigators compared the survival of patients with stage II colon cancer participating in 2 Gastro Intestinal Tumour Study Group (GITSG) trials to age, sex and race adjusted cohorts of healthy people. Further, they subdivided both cohorts into four age groups (<50, 50‐60, 60‐70 and > 70 years). The survival of patients with stage II colon cancer was similar for all 4 age groups. This has also been observed in the pooled analyses of Sargent and Gill (Sargent 2001; Gill 2004). The survival of the healthy cohorts, however, decreased steadily from the <50 to the >70 year groups. The difference between expected survival for the four age groups was 23% for those <50 years and declined to almost nil for those >70 years. As most patients with stage II colon cancer are over 60 years, it is clear that the potential benefit in survival must be small as has been found in all other trials and pooled analyses.
It is also important that the definition of stage II colon cancer be restricted to those with at least 10 to 13 lymph nodes examined (Baxter 2005; Berger 2005) and to those with T4 disease which have been resected in block and who did not have a perforated tumour. Otherwise, it is possible that lax criteria for stage II disease may include patients at higher risk of undetected lymph node metastases or spillage of cancer cells in the peritoneal cavity at surgery. These and other potential prognostic factors have bee described (Nathanson 1994; Hase 1998).
Patients with resected colon cancer should be staged appropriately, restricting the label stage II for those who had no tumour involvement in at least 10 to 13 regional lymph nodes, had real en block resection for T4 tumours and had no evidence of tumour perforation into the peritoneal cavity. These patients have an excellent prognosis from the cancer and, if over age 60 years, unlikely to benefit from adjuvant therapy. Patients less than 60 years old with co‐morbidities should be assessed in regard to the potential to survive the next 5 and possibly 10 years. If their probability of survival is lesser than a corresponding age cohort, then adjuvant therapy might not be beneficial.
All these factors and uncertainty of the benefit of adjuvant therapy must be clearly explained to patients stressing that to save one life treatment must be given to 17 to 100 patients. Or inversely that 16 to 99 patients will receive treatment for no gain. If systemic chemotherapy is planned it may include 5‐FU + leucovorin or oral capecitabine for 6 months. There is not enough evidence yet to support the use of additional drugs, such as oxaliplatin in patients with stage II colon cancer. Toxicity occurrence must also enter this discussion as potential non‐toxic therapies may become available and this small gain may then be more acceptable.
Implications for research.
Clinical adjuvant trials involving patients with stage II colon cancer must be restrictive in their definition of the stage. Further investigation of healthy cohorts are needed as the ones cited by Nauta are from the 1980's. Other prognostic factors may have to be included in the selection of patients considered at high risk such as genetic changes. Randomization of patients to an observation arm is still ethical if patients are well informed.
What's new
| Date | Event | Description |
|---|---|---|
| 14 May 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 3, 2005 Review first published: Issue 3, 2008
| Date | Event | Description |
|---|---|---|
| 1 May 2008 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We must acknowledge the original support of the Cancer Care Ontario Provincial Guideline Initiative , Gastrointestinal Disease Site Group chaired by Dr. J. Maroun. Their support and comments were invaluable during the development of the guidelines on stage II colon cancer published in 1997 and 2004. A special thanks is extended to the Research Coordinators of the group B. Rumble, M. Charette and L. Zuraw for their help in literature search, editing of text and tabulation of results and meta‐analyses. We must also aknowledge Dr. K. Zbuk for help in the development and coordination of the original protocol for the present review.
Data and analyses
Comparison 1. Deaths.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Deaths for Stage II Colon Cancer ‐ By subcategory | 28 | 18975 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.91, 1.02] |
| 1.1 Adjuvant Systemic Chemotherapy | 16 | 7097 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.87, 1.05] |
| 1.2 Adjuvant Regional Chemotherapy | 25 | 8775 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.89, 1.08] |
| 1.3 Adjuvant Immunotherapy | 8 | 3103 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.77, 1.06] |
1.1. Analysis.

Comparison 1 Deaths, Outcome 1 Deaths for Stage II Colon Cancer ‐ By subcategory.
Comparison 2. Recurrences.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recurrences for Stage II Colon Cancer ‐ by subcategory | 18 | 11772 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.77, 0.92] |
| 1.1 Adjuvant Systemic Chemotherapy | 18 | 8642 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.75, 0.91] |
| 1.2 Adjuvant Regional Chemotherapy | 2 | 456 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.46, 1.30] |
| 1.3 Adjuvant Immunotherapy | 6 | 2674 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.70, 1.11] |
2.1. Analysis.

Comparison 2 Recurrences, Outcome 1 Recurrences for Stage II Colon Cancer ‐ by subcategory.
Comparison 3. Systemic Chemotherapy Deaths.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Systemic Chemotherapy Deaths | 15 | 4457 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.89, 1.11] |
| 1.1 5‐FU and Nitrosourea Chemotherapy Regimens | 3 | 470 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.71, 1.33] |
| 1.2 Miscellaneous Treatments | 1 | 374 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.93, 1.53] |
| 1.3 Mitomycin C and Mostly Oral Fluoropyrimidines | 1 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.59, 2.09] |
| 1.4 5‐FU and Levamisole | 5 | 749 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.74, 1.19] |
| 1.5 5‐FU and Folinic Acid Regimens | 5 | 2562 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.80, 1.10] |
3.1. Analysis.

Comparison 3 Systemic Chemotherapy Deaths, Outcome 1 Systemic Chemotherapy Deaths.
Comparison 4. Regional Chemotherapy Deaths.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Regional Chemotherapy Deaths | 6 | 1393 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.74, 1.68] |
| 1.1 Portal Vein Infusion | 4 | 1101 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.76, 2.18] |
| 1.2 Intraperitoneal Infusion | 1 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.56, 1.92] |
| 1.3 Hepatic Artery Infusion | 1 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.20, 1.23] |
4.1. Analysis.

Comparison 4 Regional Chemotherapy Deaths, Outcome 1 Regional Chemotherapy Deaths.
Comparison 5. Immunotherapy Deaths.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Immunotherapy Deaths | 8 | 3091 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.78, 1.14] |
| 1.1 Non‐specific Immunotherapy | 3 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.34, 2.95] |
| 1.2 Specific Immunotherapy | 5 | 2652 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.78, 1.14] |
5.1. Analysis.

Comparison 5 Immunotherapy Deaths, Outcome 1 Immunotherapy Deaths.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdi 1989.
| Methods | 1. RCT 2. Risk of bias: Low | |
| Participants | 1. Colon & rectal cancer 2. Stages II & III 3. Alberta, Canada 4. Years: 1976‐83 5. N = 253 6. Age: median 57.1 years 7. Sex M/F: 143/ 110 8. Follow‐up: ‐schedule & tests described ‐ duration: mean 83.4 months | |
| Interventions | 1. Observation 2a. Semustine 130mg/m2 po day 1 & 5‐FU 325 mg/m2 days 1‐5 and 375 mg/m2 days 36‐40; every 10 weeks; for 20 months. 2b. BCG 120 mg po every 28 days for 5 years. 3. Duration: 20 months 4. Delay after surgery: up to 6 weeks | |
| Outcomes | 1. Dose given: N/A 2. Toxicity: ‐ no deaths ‐ grade 3 GI: N/A ‐ grade 3 other: 1 renal failure | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Beart 1990.
| Methods | 1. RCT 2. Risk of bias: Low | |
| Participants | 1. Colon & rectal cancer 2. Stages II & III 3. US; NCCTG 4. Years: N/A 5. N = 224 6. Age: 66 median 7. Sex M/F: 103/116 8. Follow‐up: ‐ schedule: yes ‐ tests: yes 9: Duration: 66 months median | |
| Interventions | 1. Observation 2. 5‐FU 500 mg/m2/day x 7 | |
| Outcomes | 1. Dose given: >80% in 100 pts. <50% in 10 pts. 2. Toxicity: none significant 3. % 5‐year OS: ‐all pts: 68 /68 ‐stage II: 82/82 4. % 5‐year DFS: N/A | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Benson 2004.
| Methods | 1. Recommendations based on meta‐analyses | |
| Participants | 1. Colon cancer 2. Stage II 3. International 4. Years: 1987‐2004 5. N=N/R 6. Age: N/R 7. Sex: N/R | |
| Interventions | 1. Observation 2. Various chemotherapies including 5‐FU | |
| Outcomes | 1. Dose given: N/R 2. Toxicity: N/R 3. % 5‐yr OS: N/R 4. % 5‐yr DFS: N/R | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
CCCSG Japan 1995.
| Methods | 1. RCT 2. Risk of bias: Low | |
| Participants | 1. Colon & rectal cancer (here we consider only colon). 2. Stages II & III 3. Japan, CCCSG 4. Years: 1984‐85 5. N = 899 6. Age: mean 59 7. Sex M/F: 458/441 8. Follow‐up: ‐schedule & tests described ‐median duration: >60 months | |
| Interventions | 1. Observation 2a. Mitomycin C 12 mg intraportally day 0 and 6 mg days 7 & 14 & at 2, 4 & 6 months; plus 5‐FU 200 mg po daily from day 14. 2b. As above except no intraportal chemotherapy. 3. Duration: 6 months 4. Delay after surgery: 0 | |
| Outcomes | 1. Dose prescribed: overall 71% to 76% of drug doses given. 2. Toxicity: reported as mild. 3. % 5‐yr OS: ‐ all pts: 80/80/82 ‐ Stg II: 90/84/88 4. % 5‐yr DFS: ‐ all pts: 76/77/81 ‐ Stg II: 88/88/89 | |
| Notes | Patients with rectal cancer not considered. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Chlebowski 1988.
| Methods | 1. RCT 2. Risk of bias: Low | |
| Participants | 1. Colon & rectal cancer 2. Stages II & III 3. US, WCSG 4. Years 1976‐78 5. N = 78 6. Age: median 57 years 7. Sex M/F: 42/36 8. Follow‐up: ‐ schedule & tests described ‐ duration: median 90 months | |
| Interventions | 1. Placebo 2. Levamisole 2.5 mg/K/day on days 1 & of each week. 3. Duration: 18 months 4. Delay after surgery: N/A 3. | |
| Outcomes | 1. Dose given: all 52 had >80% of planned dose 2. Toxicity: 1 death due to agranulocytosis. 3. % 5‐year OS: ‐ all colon pts: 67/60 ‐ Stg II colon: 84/79 4. % 5‐year DFS: ‐ all pts: N/A ‐ Stg II: N/A | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Colacchio 2004.
| Methods | 1. RCT 2. Risk of bias: Low | |
| Participants | 1. Colon 2. Stage II 3. US, Canada; CALGB 4. Years: 1997‐2002 5. N = 1738 6. Age: N/A 7. Sex M/F: N/A 8. Follow‐up: ‐schedule & tests: N/A ‐ duration: 32 to 34 months, median | |
| Interventions | 1. Observation 2. Edrecolomab 500 mg and then 100 mg IV every month, times 4. 3. Duration: 4 months 4. Delay after surgery: N/A | |
| Outcomes | 1. Dose given: N/A 2. Toxicity: ‐ 0 deaths ‐ Grade 3‐4 GI toxcity: 13% 3. % 5‐year OS: ‐ all pts: 93/94 ‐ Stg II: 93/94 4. % 5‐year DFS: ‐ all pts: 88/88 ‐ Stg II: 88/88 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Dignam 1999.
| Methods | 1. Meta‐analysis of 5 RCTs 2. Risk of bias: Low | |
| Participants | 1. Colon cancer 2. Stages I to III 3. US, NSABP 4. Years: 1977‐1994 5. N=5969 whites & 663 blacks 6. Age: 52.6% > 60 yrs 7. Sex M/F: 3606/3026 8. Follow‐up: N/R | |
| Interventions | 1. Observation or systemic chemotherapy 2. Systemic or PVI chemotherapy 3. Duration: variable 4. Delay after surgery: variable | |
| Outcomes | 1. Dose given: N/R 2. Toxicity: N/R 3. % 5‐yr OS: 21% excess risk of death for blacks compared with whites (p=0.004) 4. % 5‐yr DFS: 8% excess risk of recurrence for blacks compared with whites (p=0.27) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Dube 1997.
| Methods | 1. Meta‐analysis of 29 RCTs 2. Risk of bias: Low | |
| Participants | 1. Colon & rectal cancer 2. Stages II & III 3. International 4. Years: 1950‐90 5. N=N/R 6. Age: N/R 7. Sex M/F: N/R 8. Follow‐up: N/R | |
| Interventions | 1. Observation 2. Various chemotherapies including 5‐FU 3. Duration: N/R 4. Delay after surgery: N/R | |
| Outcomes | 1. Dose given: N/R 2. Toxicity: N/R 3. % 5‐yr OS: OR for death for colon cancer 0.81 (95% CI: 0.69, 0.94) or an absolute 5% increase in OS 4. % 5‐yr DFS: N/R | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Fielding 1992.
| Methods | 1. RCT 2. Risk of bias: moderate (>10% drop‐outs; difference in well differentiated tumours ‐p= 0.04‐) | |
| Participants | 1. Colon & rectal cancer 2. Stages I, II, III 3. UK, LBCP 4. Years: 1981‐84 5. N=398 6. Age: N/A 7. Sex M/F: N/A 8. Follow‐up: ‐schedule described, but not the tests. ‐duration: >84 months | |
| Interventions | 1. Observation 2a. 5‐FU 1000mg plus heparin 10,000 u by PVI daily. 2b. Heparin 10,000 u by PVI daily. 3. Duration: 7 days 4. Delay after surgery: 0 | |
| Outcomes | 1. Dose given: treatment was incomplete in 11% on heparin & 18% on 5‐FU/heparin 2. Toxicity: ‐ 1 death due to neutropenic sepsis ‐other: N/A 3. % 5‐year OS: ‐ all pts: 77/73/82 ‐ Stg II: 93/81/90 4. % 5‐year DFS: N/A | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Figueredo 1997.
| Methods | 1. Meta‐analysis of 11 RCTs 2. Risk of bias: Low | |
| Participants | 1. Colon & rectal cancer 2. Stage II 3. International 4. Years: 1987‐96 5. N=N/R 6. Age: N/R 7. Sex M/F: N/R 8. Follow‐up: N/R | |
| Interventions | 1. Observation 2. Various treatments 3. Duration: variable 4. Delay after surgery: N/R | |
| Outcomes | 1. Dose given: N/R 2. Toxicity: described 3. % 5‐yr OS: OR for death for systemic chemotherapy 1.02 (95% CI: 0.80, 1.30) and for PVI chemotherapy 0.62 (95% CI: 0.35, 1.11) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Figueredo 2004.
| Methods | 1. Meta‐analysis of 13 RCTs and 1 M‐A 2. Low | |
| Participants | 1. Colon & rectal cancer 2. Stages II & III 3. International 4. Years: 1987‐2004 5. N=4187 6. Age: N/R 7. Sex M/F: N/R 8. Follow‐up: N/R | |
| Interventions | 1. Observation 2. Various treatments 3. Duration: N/R 4. Delay after surgery: variable | |
| Outcomes | 1. Dose given: N/R 2. Toxicity: N/R 3. % 5‐yr OS: RR for death 0.87 (95% CI: 0.75, 1.01); also reported for groups receiving 5‐FU + levamisole, 5‐FU + folinic acid and PVI chemotherapy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Francini 1994.
| Methods | 1. RCT 2. Risk of bias: Low | |
| Participants | 1. Colon cancer 2. Stages II & III 3. Siena, Italy 4. Years: 1985‐90 5. N = 398 6. Age: N/A 7. Sex M/F: N/A 8. Follow‐up: ‐schedule & tests described ‐Duration: 65 months | |
| Interventions | 1. Observation 2. 5‐FU 400mg/m2 and folinic acid 200mg/m2 IV days 1‐5, every 4 weeks 3. Duration: 1 year 4. Delay after surgery: up to 3 weeks | |
| Outcomes | 1. Dose given: N/A 2. Toxicity: ‐ 0 deaths ‐ Grade 3 GI: 21 diarrhea, 20 stomatitis ‐ Grade 3 other: 3 dermatitis, 13 conjunctivitis 3. % 5‐yr OS: ‐ all pts: 65/79 (p=0.0044) ‐ Stg II pts: 86/89 (p=0.005) 4. % 5‐yr DFS: ‐ all pts: 59/74 (p=0.005) ‐ Stg II pts: 77/83 | |
| Notes | Included in IMPACT B2, 1999 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Gill 2004.
| Methods | 1. Meta‐analysis of 7 RCTs 2. Risk of bias: Low | |
| Participants | 1. Colon cancer 2. Stages II & III 3. International 4. Years: 1989‐98 5. N=3302 6. Age: 56%>60 years 7. Sex M/F: 1753/1649 8. Follow‐up: N/R | |
| Interventions | 1. Observation 2. 5‐FU plus either levamisole or folinic acid 3. Duration: 6 to 12 months 4. Delay after surgery: N/R | |
| Outcomes | 1. Dose given: N/R 2. Toxicity: N/R 3. % 5‐yr DFS and OS: calculated according to prognostic subsets | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Glimelius 2005.
| Methods | 1. Meta‐analysis of 5 RCTs 2. Risk of bias: Low | |
| Participants | 1. Colon & rectal cancer 2. Stages II & III 3. Scandinavia 4. Years: 1991‐97 5. N = 2170 (colon 1520) 6. Age: 62‐66 7. Sex M/F: 1212/999 8. Follow‐up: ‐schedule & tests not described, variable for each institution ‐duration: >60 months | |
| Interventions | 1. Observation 2. Either of 5 regimens: ‐ 5‐FU & levamisole as in Moertel, 1995 ‐ 5‐FU & folinic acid as in O'Connell, 1997 ‐ 5‐FU 500mg/m2 plus folinic acid 60mg/m2 IV days 1 & 2, every 2 weeks, 10 times (Nordic regimen) ‐ 5‐FU & folinic acid as in O'Connell, 1997 plus levamisole as in Moertel, 1995 ‐ 5‐FU & folinic acid as in Nordic plus levamisole as in Moertel, 1995 3. Duration: 5‐12 months 4. Delay after surgery: 30‐42 days | |
| Outcomes | 1. Dose given: 67% of pts received the planned number of treatments 2. Toxicity: N/A 3. % 5‐year OS: ‐ all colon pts: N/A ‐ Stg II colon: 79/79 4. % 5‐year DFS: ‐ all pts: N/A ‐ Stg II: N/A | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Gray 1989.
| Methods | 1. RCT 2. Risk of bias: Low | |
| Participants | 1. Colon & rectal cancer 2. Stages II & III 3. Australia & New Zealand 4. Years 1978‐81 5. N = 293 6. Age: mean 63‐64 years 7. Sex M/F: 160/133 8. Follow‐up: ‐schedule & tests described ‐duration: >60 months | |
| Interventions | 1. Observation 2. Autologous tumour cells plus BCG by scarification every 2 weeks for 2 months; monthly 10 times; then every 3 monts. ‐Duration: 2 years ‐Delay after surgery: 2 weeks | |
| Outcomes | 1. Dose given: N/A 2. Toxicity: 1 death due to renal failure (immune complexes in the mesangium of the kidneys) 3. % 5‐year OS: ‐ all pts: 54/55 ‐ Stg II: 64/63 4. % 5‐year DFS: ‐ all pts: 56/55 ‐ Stg II: 61/65 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Gray 1991.
| Methods | 1. Meta‐analysis of 6 RCTs of PVI chemotherapy 2. Risk of bias: Moderate | |
| Participants | 1. Colon & rectal cancer 2. Stages I to III 3. International 4. Years: pre 1990 5. N = 2201 6. Age: N/A 7. Sex: N/A 8. Follow‐up: ‐ schedule: N/A ‐ tests: N/A ‐ duration: N/A | |
| Interventions | 1. Observation 2. 5‐FU +/‐ mitomycin C by PVI 3. Duration: 7 days 4. Delay after surgery: none | |
| Outcomes | 1. Dose given: N/A 2. Toxicity: N/A 3. % 5‐yr OS: ‐ all pts: OR for death 31% +/‐ 8% ‐ stg II: N/A 4. % 5‐yr DFS: N/A | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Gray 2007.
| Methods | 1. RCT 2. Risk of bias: low | |
| Participants | 1. Colon and rectal cancer 2. Stages II and III | |
| Interventions | 1. Observation 2. 5‐FU or levamisole | |
| Outcomes | 1. Dose given: N/A 2. Toxicity: N/A | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Hanna 2001.
| Methods | 1. Meta‐analysis of 3 RCTs 2. Risk of bias: moderate | |
| Participants | 1. Colon & rectal cancer 2. Stages II & III 3. US & Holland 4. Years: 1980‐99 5. N = 728 6. Age: N/A 7. Sex: N/A 8. Follow‐up: ‐schedule N/A ‐tests: N/A ‐duration: >60 months | |
| Interventions | 1. Observation 2. Autologour tumour cells + BCG intradermally 3. Duration: 2 to 6 months. 4. Delay postop.: N/A | |
| Outcomes | 1. Dose given: N/A
2. Toxicity: mild, local
3. % 5‐year OS:
N/A except for subgroups
4. % 5‐year DFS:
‐all pts: 66 /77
HR=0.75, p=0.05
‐stage II: 63 /80 ‐stage II: |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Harris 2000.
| Methods | 1. RCT 2. Risk of bias: Low | |
| Participants | 1. Colon cancer 2. Stages II & III 3. US, Canada; ECOG 4. Years: 1986‐93 5. N = 412 6. Age: 66 years, median 7. Sex M/F: 216/194 8. Follow‐up: ‐schedules & tests described ‐ Duration: median 80 months | |
| Interventions | 1. Observation 2. Intradermal irradiated tumour cells plus BCG at weeks 0, 1 & 3, the latter without BCG 3. Duration: 1 month 4. Delay after surgery: 4‐5 weeks | |
| Outcomes | 1. Dose given: N/A 2. Toxicity: ‐ 0 deaths ‐ ulceration, draining and crusting in 79 patients 3. % 5‐yr OS: ‐ all pts: 64/62 ‐ Stg II pts: 70/69 4. % 5‐yr DFS: ‐ all pts: 59/58 ‐ Stg II pts: 66/64 | |
| Notes | see also Hanna review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Hartung 2005.
| Methods | 1. RCT 2. Risk of bias: Moderate (17% drop‐out) | |
| Participants | 1. Colon cancer 2. Stage II 3. Manheim, Germany 4. Years: 1997‐2000 5. N = 312 6. Age: median 64 years 7. Sex M/F: 118/186 8. Follow‐up: ‐schedule & tests described ‐duration: median 42 months | |
| Interventions | 1. Observation 2. Edrecolomab 500mg IV and then 100mg IV every month, 4 times. 3. Duration: 4 months 4. Delay after surgery: up to 6 weeks. | |
| Outcomes | 1. Dose given: N/A 2. Toxicity: ‐0 deaths ‐no Grade 3 toxicity 3. % 5‐year OS: ‐ all pts: 87/90 ‐ Stg II: 87/90 4. % 5‐year DFS: ‐ all pts: 76/76 ‐ Stg II: 76/76 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
IMPACT B2 1999.
| Methods | 1. Meta‐analysis of 5 RCTs 2. Risk of bias: Low | |
| Participants | 1. Colon cancer 2. Stage II only 3. International 4. Years: 1982‐91 5. N = 1016 6. Age: 62 years 7. Sex M/F: 559/457 8. Follow‐up: ‐schedule & tests not described ‐ Duration: median 62 to 102 months | |
| Interventions | 1. Observation 2. 5‐FU 370‐425 mg/m2 plus folinic acid 20‐200 mg/m2 days 1‐5, every month 3. Duration: 6 months except one 12 months. 4. Delay after surgery: 3‐6 weeks. | |
| Outcomes | 1. Dose given: median relative dose‐intensity (given/planned) 85%‐98% 2. Toxicity: ‐ 0 deaths ‐ Grade 3 GI: nausea/vomiting 4%; stomatitis 11% and diarrhea 8%. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Isenberg 1994.
| Methods | 1. RCT 2. Risk of bias: Moderate ( uncertain allocation concealment) | |
| Participants | 1. Colon & rectal cancer 2. Stages I, II & III 3. Koln, Germany 4. Years: 1983‐86 5. N = 97 6. Age: mean 61 & 64 years 7. Sex M/F: 45/56 8. Follow‐up: ‐schedule & tests: standard in the clinic. ‐duration: 96 months | |
| Interventions | 1. Observation 2. Propiobacterium granulosum KP‐45 10mg IV on days ‐7 to +3 3. Duration 10 days 4. Pre‐ & post‐surgery | |
| Outcomes | 1. Dose given: all >80% 2. Toxicity: fever/chills in 43, headaches in 28 vomiting in 16 3. % 6‐year OS: ‐ all pts: 34/54 ‐ Stg II: 45/90 4. % 5‐year DFS: ‐ all pts: 34/54 ‐ Stg II: 45/90 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kimura 1995.
| Methods | 1. RCT 2. Risk of bias: Low | |
| Participants | 1. Colon & rectal cancer 2. Stages II & III 3. Tottori Univ., Japan 4. Years: 1985‐88 5. N = 110 6. Age: mean 63 years 7. Sex M/F: 52/58 8. Follow‐up: ‐schedule & tests: N/A ‐ duration: >60 months | |
| Interventions | 1. Observation 2. 5‐FU 3mg/K/day by PVI for 14 days plus Mitomycin C 10mg by PVI or IV on days 0, 7, 14, plus 5‐FU 150mg/day po for 6 months after infusion 2. Duration: 6 months 3. Delay after surgery: 0 | |
| Outcomes | 1. Dose given: N/A 2. Toxicity: N/A 3. % 5‐year OS: N/A 4. % 5‐year DFS: ‐all pts: 74/81 ‐ Stg II: 82/92 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Kingston 1993.
| Methods | 1. RCT 2. Risk of bias: Low | |
| Participants | 1. Colon & rectal cancer 2. Stages II & III 3. England, UK 4. Years 1981‐83 5. N = 603 6. Age: median 66‐67 years 7. Sex M/F: 341/262 8. Follow‐up: ‐schedule & tests described ‐duration: >60 months | |
| Interventions | 1. Observation 2. Razoxane 125mg twice daily, 5 days per week 3. Duration: 2 years 4. Delay after surgery: up to 8 weeks | |
| Outcomes | 1. Dose given: >80% in 17%, 50‐80% in 18% | |
| Notes | Trial cut short due to worse outcome for treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Laffer 1995.
| Methods | 1. RCT 2. Risk of bias: Low | |
| Participants | 1. Colon & rectal cancer 2. Stages I, II & III 3. Switzerland, SAKK 4. Years: 1981‐87 5. N = 505 6. Age: 62 years 7. Sex M/F: 277/228 8. Follow‐up: ‐schedule & tests described ‐ Duration: 96 months | |
| Interventions | 1. Observation 2. 5‐FU 500mg/m2 plus heparin 5000 u, days 1‐7 and mitomycin C 10 mg/m2 on day 1 only, all by PVI. 3. Duration: 7 days 4. Delay after surgery: 0 | |
| Outcomes | 1. Dose given: 69% of patients received >80% planned doses. 2. Toxicity: N/A 3. %5‐yr OS: ‐ all pts: 55/66 (p=0.026) ‐ all colon: 61/72 ‐ Stg II pts: 69/77 4. % 5‐yr DFS: ‐ all pts: 48/57 (p=0.051) ‐ all colon: 55/66 ‐ Stg II: 63/68 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Laurie 1989.
| Methods | 1. RCT 2. Risk of bias: Low | |
| Participants | 1. Colon & rectal cancer 2. Stages II & III 3. US, NCCTG 4. Years 1980s 5. N = 408 6. Age: median 61 years 7. Sex M/F: 204/204 8. Follow‐up: ‐schedule & tests described ‐duration: 93 months | |
| Interventions | 1. Observation 2a. Levamisole 150mg days 1, 2 & 3 every 2 weeks. 2b. Levamisole as above plus 5‐FU 450mg/m2 days 1‐5 and beginning day 28, weekly. 3. Duration: 1 year 4. Delay after surgery: up to 5 weeks. | |
| Outcomes | 1. Dose given: >80% of planned dose for levamisole in 95% and for 5‐FU+levamisole 83% of patients. 2. Toxicity: 1 case of severe dermatitis due to levamisole. 3. % 5‐year OS: ‐all pts: 57/59/62 ‐ Stg II: 58/76/74 4. % 5‐year DFS: ‐all pts: 45/58/59 ‐Stg II: 58/76/74 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
LIMAG 1997.
| Methods | 1. Meta‐analysis of 10 RCTs 2. Risk of bias: Low | |
| Participants | 1. Colon & rectal cancer 2. Stages I to III 3. International 4. Years: 1979‐96 5. N = 3499 6. Age: 65 years median 7. Sex M/F: 1696/1390 8. Follow‐up: ‐ schedule: N/A ‐ tests: N/A ‐ duration: > 60 months | |
| Interventions | 1. Observation 2. 5‐FU +/‐ mitomycin C by PVI x 7 days 3. Delay after surgery: nil | |
| Outcomes | 1. Dose given: N/A 2. Toxicity: N/A 3. % 5‐yr OS: ‐ all pts: 57/61 ‐ stg II: 71/76 (p=0.01) 4. % 5‐yr DFS: N/A ‐ all pts: | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Mamounas 1999.
| Methods | 1. Meta‐analysis of 4 RCTs 2. Risk of bias: moderate | |
| Participants | 1. Colon cancer 2. Stages II & III 3. US 4. Years: 1977‐90 5. N = 4006 6. Age: mid60's 7. Sex M/F: 2081/1739 8. Follow‐up: ‐schedule: described ‐tests: described ‐duration: .60 months | |
| Interventions | 1a. Observation vs. MOF or PVI 5‐FU in 2 trials 1b. MOF or FU/Lev vs. FUFA in 2 trials | |
| Outcomes | 1. Dose given: N/A 2. Toxicity: N/A 3. % 5‐year OS: ‐all pts: Obs. vs MOF: 60/67 Obs vs. PVI: 67/74 MOF vs. FUFA: 66/76 (p<0.001) FU/Lev vs. FUFA: 70/75 ‐stage II pts: Obs. vs. MOF: 72/75 Obs. vs PVI: 76/88 (p=0.01) MOF vs FUFA: 84/92 (p=0.05) FU/Lev vs. FUFA: 81/85 4. % 5‐year DFS: all pts: N/A ‐stage II: N/A | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
McCollum 2002.
| Methods | 1. Meta‐analysis of arms in an RCT 2. Risk of bias: Low | |
| Participants | 1. Colon cancer 2. Stages II & III 3. US, Intergroup 0089 4. Years: 198?‐92 5. N=3759 6. Age: median 64 years 7. Sex M/F: 1846/1534 8. Follow‐up: N/R | |
| Interventions | Systemic chemotherapies 1, 2, 3 & 4 3. Duration: 6 to 12 months 4. Delay after surgery: N/R | |
| Outcomes | 1. Dose given: N/R 2. Toxicity: ‐Grade 3+ diarrhea: 23% whites, 8% blacks ‐Grade 3+ stomatitis: 12% whites, 9% blacks ‐other Grade 3+ toxicities not different 3. %5‐yr OS: ‐blacks/whites: 65%/66% ‐blacks/whites: 57%/58% | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
McDermott 2003.
| Methods | 1. RCT 2. Risk of bias: Moderate (rectal cancer pts receive RT; other characteristics not described in abstract) | |
| Participants | 1. Colon & rectal cancer 2. Stages II & III 3. Belfast, UK 4. Years: 1994‐97 5. N = 254 6. Age: N/A 7. Sex M/F: N/A 8. Follow‐up: ‐schedule & tests: N/A ‐duration: median 80 months | |
| Interventions | 1. Observation 2. 5‐FU 400mg/m2 plus folinic acid 200mg/m2 by IV boluses on day 1 and 5‐FU 400mg/m2 by IV infusion over 22 hours for 2 days; every 2 weeks. 3. Duration: 4 months 4. Delay after surgery: N/A | |
| Outcomes | 1. Dose given: N/A 2. Toxicity: ‐1 death ‐Grade 3 diarrhea in 0.8% 3. % 5‐year OS: ‐ all pts: N/A ‐ stage II: 73/76 4. % 5‐year DFS: ‐ all pts: N/A ‐ stage II: N/A | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Moertel 1995.
| Methods | 1. RCT 2. Risk of bias: Low | |
| Participants | 1. Colon cancer 2. Stage II 3. US, Canada, Intergroup 4. Years 1984‐87 5. N = 318 6. Age: 61‐62 median 7. Sex M/F: 183/135 8. Follow‐up: ‐schedule & tests described ‐duration: median 74 months | |
| Interventions | 1. Observation 2. Levamisole 150mg days 1, 2 & 3 every 2 weeks and 5‐FU 450mg/m2 days 1‐5 and starting on day 28, weekly. 3. Duration: 1 year 4. Delay after surgery up to 5 weeks | |
| Outcomes | 1. Dose given: NR 2. Toxicity: NR 3. % 5‐year OS: ‐Stg II: 61/60 4. % 5‐year DFS: ‐ Stg II: 56/61 | |
| Notes | For Dose given & Toxicity see Moertel, 1990 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Panettiere 1988.
| Methods | 1. RCT 2. Risk of bias: Moderate (drop‐outs 12%) | |
| Participants | 1. Colon & rectal cancer 2. Stages II & III 3. USA ‐ SWOG 4. Years 1977‐80 5. 37 months 6. N = 279 7. Age: median 60 years 8. Sex M/F: 156/123 9. Follow‐up: ‐schedule & tests described ‐duration: median 84 months | |
| Interventions | 1. Observation 2a. Semustine 175mg/m2 po day 1 & 5‐FU 400mg/m2 days 1, 8 & 15; all every 8 weeks 2b. As above plus BCG 600 M org. orally q 2weeks 3. Duration: 1 year 4. Delay after surgery: 2‐6 weeks | |
| Outcomes | 1. Dose given: N/A 2. Toxicity: N/A 3. % 5‐year OS: ‐all pts: 61/62/50 ‐Stg II: 88/66/58 4. % 5‐year DFS: ‐all pts: 47/47/45 ‐ Stg II: N/A | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Sadahiro 2004.
| Methods | 1. RCT 2. Risk of bias: Low | |
| Participants | 1. Colon cancer 2. Stages I, II & III 3. Tokai, Japan 4. Years: 1994‐2001 5. N = 305 6. Age: mean 60 years 7. Sex M/F: 185/120 8. Follow‐up: ‐schedule & tests described ‐duration: 59 months | |
| Interventions | 1. Observation 2. 5‐FU 250mg/day plus dexamethasone 4mg by hepatic artery infusion. 3. Duration: 3 weeks 4. Delay after surgery: from week before to 2 weeks after surgery | |
| Outcomes | 1. Dose given: N/A 2. Toxicity: ‐0 deaths ‐0 significant Grade 3 3. % 5‐year OS: ‐all pts: 82/94 (p=0.0005) ‐Stg II: 84/92 4. % 5‐year DFS: ‐all pts: 72/86 (p=0.0022) ‐Stg II: 79/88 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Sakamoto 2004.
| Methods | 1. Meta‐analysis of 3 RCTs 2. Risk of bias: Low | |
| Participants | 1. Colon & rectal cancer 2. Stages I to III 3. Japan 4. Years: 1986‐90 5. N = 5233 6. Age: median 59 7. Sex M/F: 2944/2289 8. Follow‐up: ‐schedule: N/A ‐tests: N/A ‐duration: >60 months | |
| Interventions | 1. Observation 2. IV mitomycin C or 5‐FU followed by oral 5‐FU, UFT or HCFU 3. Duration: 1 year 4. Delay after surgery N/A | |
| Outcomes | 1. Dose given: N/A 2. Toxicity: N/A 3. % 5‐year OS: For all colon cancer, HR 0.86 (0.73, 1.00). For stage II: decrease by 21% SE 9%; absolute OS difference 4.3%. 4. % 5‐year DFS: HR 0.87 (075, 0.99); absolute DFS difference 4.7%. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Sakamoto 2005.
| Methods | 1. Meta‐analysis of 3 RCTs 2. Risk of bias: Low | |
| Participants | 1. Colon cancer 2. Stages Dukes B & C 3. Japan 4. Years: 1987‐90 5. N = 2152 6. Age: N/A 7. Sex: N/A 8. Follow‐up: ‐schedule: N/A ‐tests: N/A ‐duration: >60 months | |
| Interventions | 1. Observation 2. 5‐FU or mitomycin C IV followed by oral HCFU (Carmofur) 3. Duration: 6 weeks to 12 months 4. Delay after surgery: N/A | |
| Outcomes | 1. Dose given: N/A 2. Toxicity: N/A 3. % 5‐year OS: ‐all pts: 76/80 (HR = 0.82 (0.68, 0.99)) ‐stage II: 82/89 4. % 5‐ year DFS: ‐all pts: 71/77 (HR = 0.77 (0.65, 0.91) ‐stage II: 78 / 85 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Sargent 2001.
| Methods | 1. Meta‐analysis of 7 RCTs 2. Risk of bias: Low | |
| Participants | 1. Colon cancer 2. Stages II & III 3. International 4. Years: 1978‐2000 5. N=3351 6. Age: 53%>61 yrs 7. Sex M/F: N/R 8. Follow‐up: N/R | |
| Interventions | 1. Observation 2. Systemic chemotherapy with 5‐FU + levamisole or folinic acid 3. Duration: 6 to 12 months 4. Delay after surgery: 4 to 7 weeks | |
| Outcomes | 1. Dose given: N/R 2. Toxicity: ‐leucopenia and N/V more common with 5‐FU + levamisole ‐stomatitis and diarrhea more common with 5‐FU + leucovorin | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Sargent 2005.
| Methods | 1. Meta‐analysis of 18 RCTs 2. Risk of bias: Low | |
| Participants | 1. Colon cancer 2. Stages I, II & III 3. International 4. Years: 1977‐99 5. N=20,898 6. Age: 58%>60 yrs 7. Sex M/F: 55%/45% 8. Follow‐up: N/R | |
| Interventions | 1. Observation or chemotherapy 2. Systemic chemotherapy 3. Duration: variable 4. Delay after surgery: variable | |
| Outcomes | 1. Dose given: N/R 2. Toxicity: N/R 3. 3‐yr DFS predicts 5‐yr OS (correlation for hazard ratios 0.92) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Taal 2001.
| Methods | 1. RCT 2. Risk of bias: Moderate (RT in rectal & some colon patients) | |
| Participants | 1. Colon & rectal cancer 2. Stages II & III 3. Netherlands, NACCP 4. Years: 1990‐96 5. N = 1029 6. Age: 60 7. Sex M/F: 599/439 | |
| Interventions | 1. Observation 2. Levamisole 150 mg days 1, 2 & 3 every 2 weeks plus 5‐FU 450mg/m2 days1‐5 and starting on day 28, weekly. 3. Duration: 1 year 4. Delay after surgery: up to 8 weeks | |
| Outcomes | 1. Dose given: compliance with treatment 80% at 6 months and 69% at 1 year 2. Toxicity: N/A 3. % 5‐yr OS: ‐ all pts: 58/60 ‐ stageII: 73/79 4. % 5‐year DFS: ‐ all pts: 51/56 ‐ stage II: 65/71 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Vaillant 2000.
| Methods | 1. RCT 2. Risk of bias: Moderate (16% drop‐out, 2 co‐intervention) | |
| Participants | 1. Colon cancer 2. Stages II & III 3. France 4. Years: 1986‐91 5. N = 267 6. Age: mean 63 years 7. Sex M/F: 124/143 8. Follow‐up: ‐schedule not described but tests yes ‐duration: 58 months | |
| Interventions | 1. Observation 2. 5‐FU 600mg/m2 by intraperitoneal infusion over 3 hours, days 1 to 6. 3. Duration: 6 days 4. Delay after surgery: 0 | |
| Outcomes | 1. Dose given: >80% for 103; 50‐80% for 8 and <50% for 12 patients 2. Toxicity: ‐0 deaths ‐0 Grade 3 3. % 5‐year OS: ‐all pts: 69/74 ‐Stg II: 79/88 4. % 5‐year DFS: N/A | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Vermorken 1999.
| Methods | 1. RCT 2. Risk of bias: Low | |
| Participants | 1. Colon cancer 2. Stages II & III 3. Amsterdam, Netherlands 4. Years: 1987‐96 5. N = 254 6. Age: 65, 66 7. Sex M/F: 136/118 8. Follow‐up: ‐schedule & tests described ‐duration: 64 months | |
| Interventions | 1. Observation 2. Autologous tumour cell preparation plus BCG 1 M organisms intradermal on day 0, weeks 1 and 3, and at 6 months 3. Duration: 6 months 4. Delay after surgery: 4‐5 weeks | |
| Outcomes | 1. Dose given: all had >80% 2. Toxicity: mild 3. % 5‐year OS: ‐all pts: 71/75 ‐Stg II: 73/83 (p=0.01) 4. % 5‐year DFS: ‐all pts: 61/70 ‐Stg II: 63/77 (p=0.018) | |
| Notes | see also Hanna, 2001 review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Wan 2005.
| Methods | 1. Meta‐analysis of 8 studies (11 RCTs and 1 prospective group) 2. Risk of bias: High | |
| Participants | 1. Colon & rectal cancer 2. Stage II 3. International 4. Years: 1993‐2002 5. N=6518 6. Age: N/R 7. Sex M/F: N/R 8. Follow‐up: N/R | |
| Interventions | 1. Observation or systemic chemotherapy 2. Systemic or PVI chemothearpy with 5‐FU +/‐ levamisole +/‐ folinic acid 3. Duration: N/R 4. Delay after surgery: N/R | |
| Outcomes | 1. Dose given: N/R 2. Toxicity: N/R 3. % 5‐yr OS: Stg II: RR 0.79 (95% CI: 0.7, 0.9) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Watanabe 2006.
| Methods | 1. RCT 2. Risk of bias: Low | |
| Participants | 1. Colon cancer 2. Stages II & III 3. Japan, JFMTC 4. Years: 1989‐90 5. N = 984 6. Age: median 62 years 7. Sex M/F: 507/494 8. Follow‐up: ‐schedule & tests not described ‐duration: >60 months | |
| Interventions | 1. Observation 2. Mitomycin C 6mg/m2 days 0, 7 & 28 ans every month to 6 months plus 5‐FU 250mg IV days 1‐8 plus HCFU (Carmofur) 300 mg daily from day 14 to one year. 3. Duration: 1 year 4. Delay after surgery: 0 | |
| Outcomes | 1. Dose prescribed: ‐MitoC: 59.6% ‐5‐FU: 93.2% ‐HCFU: 59.5% 2. Toxicity: no deaths; not graded 3. % 5‐year OS: ‐all pts: 78/82 ‐Stg II: N/A 4. % 5‐year DFS: ‐all pts: 72/78 ‐Stg II: 85/93 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Windle 1987.
| Methods | 1. RCT 2. Risk of bias: Low | |
| Participants | 1. Colon & rectal cancer. 2. Stages I, II & III 3. Leicester, UK 4. Years: 1979‐82 5. N = 131 6. Age: median 60 years 7. Sex M/F: 67/64 8. Follow‐up: ‐schedule & tests described ‐Duration: >60 months | |
| Interventions | 1. Observation 2a. 5‐FU 1gm IV days 0, 1 & 2, then weekly. 2b. 5‐FU as above plus levamisole 150mg po days 0‐2 3. Duration: 6 months 4. Delay after surgery: 0 | |
| Outcomes | 1. Dose prescribed: all patients had > 80% 2. Toxicity: N/A 3. % 5‐yr OS: ‐all pts: 56/48/68 ‐Stg II pts: 60/64/64 4. % 5‐yr DFS: ‐all pts: 56/48/68 ‐ Stg II pts: N/A | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wolmark 1988.
| Methods | 1. RCT 2. Risk of bias: Moderate (difference in patient characteristics) | |
| Participants | 1. Colon cancer 2. Stages II & III 3. US & Canada, NSABP 4. Years 1977‐83 5. N = 1116 6. Age: median 65 years 7. Sex M/F: 692/424 8. Follow‐up: ‐schedule & tests described ‐duration: mean 77.3 months | |
| Interventions | 1. Observation 2a. Semustine 130 mg/m2 day 1 plus 5‐FU 325 mg/m2 days 1‐5 and 375mg/m2 days 36‐40; every 10 weeks. 2b. BCG 600 M org. by scarification, weekly 12 times and every other week 33 times. 3. Duration: 20 months 4. Delay after surgery: 3‐6 weeks | |
| Outcomes | 1. Dose prescribed: >80% in 159/383 2. Toxicity: ‐3 toxic deaths; acute leukemia ‐ Grade 3 GI: 15% ‐ Grade 3 other: 1 case nephrotoxicity 3. % 5‐year OS: ‐all pts: 59/67/67 ‐Stg II: 72/75/NR 4. % 5‐year DFS: ‐all pts: 51/58/56 ‐Stg II: N/A | |
| Notes | for data on Stg II pts see Mamounas, 1999 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Wolmark 1990.
| Methods | 1. RCT 2. Risk of bias: Moderate (22% drop‐out due to pre‐randomization) | |
| Participants | 1. Colon cancer 2. Stages I, II & III 3. US, Canada; NSABP 4. Years: 1984‐88 5. N = 898 6. Age: 65 7. Sex M/F: 527/374 8. Follow‐up: ‐schedule & tests described ‐duration: >60 months | |
| Interventions | 1. Observation 2. 5‐FU 600 mg/m2 plus heparin 5000 u by PVI daily 3. Duration: 7 days 4. Delay after surgery: 0 | |
| Outcomes | 1. Dose given: N/A 2. Toxicity: ‐ 0 deaths ‐ 0 Grade 3 3. % 5‐year OS: ‐all pts: 73/81 ‐Stg II: 88/76 4. % 5‐year DFS: ‐all pts: 64/72 (p=0.02) ‐Stg II: N/A | |
| Notes | for data on Stg II pts see Mamounas, 1999 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Zalcberg 1996.
| Methods | 1. Meta‐analysis of 17 RCTs 2. Risk of bias: Low | |
| Participants | 1. Colon & rectal cancer 2. Stages II & III 3. International 4. Years: 1976‐90 5. N=3221 6. Age: N/A 7. Sex M/F: N/A 8. Follow‐up: ‐schedule & tests: N/A ‐ duration: N/A | |
| Interventions | 1. Observation 2. Various chemotherapy regimens including 5‐FU 3. Duration: variable 4. Delay after surgery: variable | |
| Outcomes | 1. Dose given: N/R 2. Toxicity: N/R 3. % 5‐yr OS: OR for death 0.82 (95% CI: 0.74, 0.91) 4. % 5‐yr DFS: N/G 5. The larger the planned dose in the first 3 months, the larger the benefit in survival. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Zaniboni 1997.
| Methods | 1. RCT 2. Risk of bias: Low | |
| Participants | 1. Colon cancer 2. Stages II & III 3. Italy, GIVIO 4. Years: 1989‐91 5. N = 869 6. Age: median 62 years 7. Sex M/F: 451/437 8. Follow‐up: ‐schedule & tests not described ‐duration: median 64 months | |
| Interventions | 1. Observation 2. 5‐FU 370mg/m2 plus folinic acid 200 mg/m2 on days 1‐5, every 28 days. 3. Duration: 6 months 4. Delay after surgery: up to 5 weeks. | |
| Outcomes | 1. Dose given: 5% patients discontinued because side‐effects. | |
| Notes | Study included in IMPACT‐B2, 1999. QoL measured. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
OS = overall survival DFS = disease‐free survival
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahn 2001 | not an RCT |
| Alikhan 2004 | comment |
| Alliot 2004 | multiple letters about a trial with no observation arm |
| Arnaud 1988 | Stage III only |
| Arnaud 1989 | Stage III only |
| Boussen 1999 | no observation arm; median follow‐up 25.8 months |
| Brivio 2006 | Trial of Obs. vs. Interleukin‐2 preoperatively. No separate data for stage II patients. |
| Bukowski 1987 | Review |
| Buyse 1988 | Meta‐analysis of trials published before 1987 of observation vs. Radiotherapy +/‐ chemotherapy in colorectal cancer. No separate data for stage II patients. |
| Buyse 1989 | French version of Buyse, 1988 |
| Chlebowski 1994 | Accessory to Chlebowski, 1998 |
| Cremona 1997 | Accesory to AXIS, 2003 |
| Dahl 2003 | Trial of Obs. vs. 5‐FU + levamisole Abstract: no separate data for stage II patients |
| De Gramont 2003 | abstract only |
| Fata 2002 | not an RCT; analysis of registry data |
| Fietkau 1998 | Norwegian commentary about a Norwegian trial in rectal cancer |
| Focan 2000 | no observation arm |
| Franchi 1991 | Trial of Obs. vs. 5‐FU + folinic acid + Mitomycin C Preliminary report on toxicity; no survival data. |
| Gambill 2001 | Accesory to Hartung, 2005 |
| Garcia 1996 | Trial of Obs. vs. Mitomycin C + Ftorafur No separate data for Stage II patients |
| Gennatas 2002 | no observation arm |
| Graf 1994 | Trial of 5_FU infusion to peritoneal cavity. Early report on Tolerance and toxicity. No survival data. |
| Gray 1988 | Accesory to Gray, 1989 |
| Gray 2004 | QUASAR trial of Obs. vs. 5‐FU + folinic acid +/‐ levamisole. Abstract: no separate data for stage II patients. |
| Grem 2004 | commentary |
| Grundmann 1988 | Acesory to Isenberg, 1994 |
| GTSG 1991 | Trial of Obs. vs. radiotherapy to liver plus 5‐FU. No separate data for stage II patients. |
| Hafstrom 1988 | abstract about a review |
| Hafstrom 1990 | Stage III data only |
| Haller 2001 | Review; not an RCT |
| Harder 1990 | Accesory to SAKK, 1995 Data on transfusion effect |
| Hashimoto 1994 | Neocarzinostatin into bowel wall. No RCT |
| Hess 1997 | Trial comparing two treatments; no observation arm. |
| Hoover 1989 | Accesory to Hoover, 1993 |
| Hoover 1993 | Specific immunotherapy vs. observation. No separate data for stage II patients. |
| IMPACT 1995 | Pooling of results of 5 RCTs comparing Obs. vs. 5‐FU + folinic acid, and including patients with colon cancer, stages II and III. Later report, IMPACT‐B2, 1999 gives results for stage II only. |
| Ito 1996 | Trail of Obs. vs. carmofur (HCFU) No separate data for stage II patients |
| Kapoor 2005 | Trial of Obs. vs Famotidine. No survival data. |
| Kato 2002 | Trial of Obs. vs. UFT. No separate data for stage II patients |
| Kelly 1999 | Trial of Obs. vs. Cimetidine. No separate data for stage II patients. |
| Kerr 2000 | Review; not an RCT |
| Knuth 1988 | Commentary; not an RCT |
| Kodaira 1989 | Accessory to Kunii, 1995 (CCCSG of Japan) |
| Kodaira 1997 | Review and meta‐analysis |
| Kunii 1987 | Accesory to Matsuda, 1991 |
| Kunii 1995 | Trial of observation vs. mitomycin C + UFT. Noseparate data for stage II patients. |
| Lacour 1992 | Trial of Obs. vs. PAPUA (polyadenylic polyuridylic acid). No data for stage II patients. |
| Laffer 1987 | Accesory to SAKK, 1995 |
| Laffer 1992 | Accesory to SAKK, 1995 |
| Laffer 1992a | Accesory to SAKK, 1995 |
| Lise 1987 | Review and meta‐analysis; no separate Stage II data |
| Marangolo 1989 | Trial of Obs. vs. 5‐FU + lomustine No data for stage II patients |
| Marsoni 2001 | Accessory to IMPACT‐B, 1999 |
| Matsuda 1991 | Trial of Obs. vs. Ftorafur vs. ACNU + Ftorafur No separate data for stage II patients |
| Matsumoto 2002 | no observation arm |
| Metzger 1987 | Accesory to SAKK 1995 |
| Metzger 1989 | Accesory to SAKK 1995 Data on effect of transfusion |
| Metzger 1990 | Accesory to SAKK 1995 |
| Mitomi 1990 | no observation arm |
| Mitomi 1993 | no observation arm |
| Moertel 1995 | Stage III data only |
| Moertel 1990 | Accessory to Moertel CG, 1995 |
| Moertel 1993 | Accessory to GTSG, 1991 |
| Mori 2002 | not an RCT; review |
| Mukai 2003 | not an RCT |
| Nichols 1992 | Trial of Interleukin‐2 vs. observation Report on immune responses. No survival data. |
| Nichols 1993 | Trial of Interleukin‐2 + Interferon vs observation. Report on immune response. No survival data. |
| Nielsen 2002 | Trial of Obs. vs Ranitidine. No separate data for stage II patients. |
| Niimoto 1988 | Accesory to Matsuda, 1991 |
| Nio 1992 | Trial of Obs. vs. PSK orally. No survival data. |
| Nishida 1993 | Accesory to Kunii, 1995 |
| Nitti 1997 | Trial of Obs. vs. 5‐FU + heparin by PVI. No searate data for stage II patients. |
| O DH Jr. 2000 | Commentary; not an RCT |
| O'Connell 1997 | Obs. vs 5‐FU + folinic acid No separate data for stage II patients. Data included, however, in the IMPACT‐B2 meta‐analysis |
| Pater 1994 | Background for the IMPACT meta‐analysis |
| Pendlebury 2003 | Protocol for a trial of Obs. vs. Rofecoxib. Trial abandoned after demonstration of increased cardiovascular morbidity with COX‐2 inhibitors (Kerr, 2007). |
| Petrasch 1997 | Commentary; not an RCT |
| Piedbois 2000 | Accessory to Buyse, 1988 |
| Prohm 1988 | Trial of Obs. vs. BCG Abstract: no survival data. |
| Ragnhammar 2001 | Review of chemotherapy for colorectal cancer |
| Reddy 2004 | No observation arm; stage III data only; median follow‐up < 48 months |
| Reddy 2005 | summary; not an RCT |
| Riethmuller 1994 | Trial of Edrecolomab vs. observation. Only patients with stage III disease. |
| Rougier 1993 | not an RCT; review and trial proposal |
| Rougier 1998 | Trial of Obs. vs. 5‐FU + heparin by PVI. No separate data for stage II patients. |
| Sakamoto 1999 | Earlier report on adjuvant carmofur ( HCFU) in colon cancer. See Sakamoto, 2005 |
| Sakamoto 2001 | Earlier report of adjuvant fluorpyrimidines in colorectal cancer. See Sakamoto, 2004 |
| SAKK 1997 | Accessory to SAKK, 1995 |
| Sargent 2004 | Abstract of data presented in Sargent, 2005 |
| Scheithauer 1995 | Trial of Obs. vs. 5‐FU + folinic acid both intraperitoneal and intravenous. No separate data for stage II patients; it is stated thewas no significant difference. |
| Seitz 1994 | Initial report on the IMPACT meta‐analysis |
| Smith 2004 | Accesory to Wolmark, 1988 10‐year results of that trial. |
| Suzuka 1999 | Preliminary report; Japanese |
| Svendsen 1995 | Trial of Obs. vs. Cimetidine. No separate data for stage II patients |
| The QCG 2004 | Trial of Obs. vs. 5‐FU + folinic acid +/‐ levamisole. Early report; abstract; no separate data for stage II patients. |
| Thomas 1987 | Accesory to GITSG, 1991 |
| Ullenhag 2006 | Trial of Obs. vs. antibody 105AD7. Preliminary report on immune responses. No survival data. |
| Ulsperger 1993 | not an RCT |
| Uyl‐de Groot 2005 | Accesory to Vermorken, 1999 |
| Vaillant 1998 | Accessory to Vaillant, 2000 |
| Van den Eertwegh 200 | Accesory to Vermorken, 1999 |
| Wan 1988 | Trial of Obs. vs. 5‐FU intraluminal + IV No survival data |
| Watanabe 2004 | Accesory to Watanabe, 2006 |
| Weber 1993 | Accesory to SAKK, 1995 |
| Weber 1995 | Accesory to SAKK, 1995 |
| Wein 2004 | Stage III data only; median follow‐up 45.3 months |
| Wereldsma 1990 | Trial of Obs. vs. 5‐FU + heparin vs. urokinase by PVI. No report of stage II data. |
| Wiesenfeld 1995 | Trial of Obs. vs. Interferon gamma. No separate data for stage II patients. |
| Wurschmidt 1995 | Stage III data only |
| Yamamoto 1998 | Tiral of Obs. vs. 5‐FU + folinic acid. No separate data for stage II patients. |
| Yanagi 2003 | Stage III data only; abstract |
| Yasutomi 1997 | Trial of Obs. vs. Mitomycin C + Carmofur (HCFU). No separate data for stage II patients. |
Contributions of authors
AF: Design of protocol, selection of publications, data collection, and writing of the text and design of tables
SM: selection of publications, data collection, performance of meta‐analysis, embedding tables, editing text & tables
MC: execution of search strategy, selection of publications, data collection, importation and management of references, editing text and tables
Declarations of interest
None
Edited (no change to conclusions)
References
References to studies included in this review
Abdi 1989 {published data only}
- Abdi EA, Hanson J, Harbora DE, Young DG, McPherson TA. Adjuvant chemoimmuno‐ and immunotherapy in Dukes' stage B2 and C colorectal carcinoma: a 7‐year follow‐up analysis. Journal of Surgical Oncology 1989;40(3):205. [DOI] [PubMed] [Google Scholar]
Beart 1990 {published data only}
- W BR, Jr, Moertel CG, Wieand HS, Leigh JE, Windschitl HE, Heerden JA, et al. Adjuvant therapy for resectable colorectal carcinoma with fluorouracil administered by portal vein infusion. A study of the Mayo Clinic and the North Central Cancer Treatment Group. Adjuvant therapy for resectable colorectal carcinoma with fluorouracil administered by portal vein infusion. A study of the Mayo Clinic and the North Central Cancer Treatment Group.. Archives of Surgery 1990;125(7):897. [DOI] [PubMed] [Google Scholar]
Benson 2004 {published data only}
- Benson AB, 3rd, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. Journal of Clinical Oncology 2004;22(16):3408. [DOI] [PubMed] [Google Scholar]
CCCSG Japan 1995 {published data only}
- Anonymous. Five‐year results of a randomized controlled trial of adjuvant chemotherapy for curatively resected colorectal carcinoma. The Colorectal Cancer Chemotherapy Study Group of Japan. Japanese Journal of Clinical Oncology 1995;25(3):91. [PubMed] [Google Scholar]
Chlebowski 1988 {published data only}
- Chlebowski RT, Nystrom S, Reynolds R, Weiner JM, Bateman JR. Long‐term survival following levamisole or placebo adjuvant treatment of colorectal cancer: a Western Cancer Study Group Trial. Oncology 1988;45(3):141. [DOI] [PubMed] [Google Scholar]
Colacchio 2004 {published data only}
- Colacchio T, Niedzwiecki D, Compton C, Warren R, Benson AIB, Goldberg R, Kerr D, Fields A, Hollis D, Mayer R. Phase III trial of adjuvant immunotherapy with MOAb 17‐1A following resection for stage II adenocarcinoma of the colon (CALGB 9581) [abstract]. Annual Meeting Proceedings of the American Society of Clinical Oncology. 2004:251.
Dignam 1999 {published data only}
- Dignam JJ, Colangelo L, Tian W, Jones J, Smith R, Wickerham DL, et al. Outcomes among African‐Americans and Caucasians in colon cancer adjuvant therapy trials: findings from the National Surgical Adjuvant Breast and Bowel Project.[see comment]. Journal of the National Cancer Institute 1999;91(22):1933. [DOI] [PubMed] [Google Scholar]
Dube 1997 {published data only}
- Dube S, Heyen F, Jenicek M. Adjuvant chemotherapy in colorectal carcinoma: results of a meta‐analysis. Diseases of the Colon & Rectum 1997;40(1):35. [DOI] [PubMed] [Google Scholar]
Fielding 1992 {published data only}
- Fielding LP, Hittinger R, Grace RH, Fry JS. Randomised controlled trial of adjuvant chemotherapy by portal‐vein perfusion after curative resection for colorectal adenocarcinoma.[see comment]. Lancet 1992;340(8818):502. [DOI] [PubMed] [Google Scholar]
Figueredo 1997 {published data only}
- Figueredo A, Germond C, Maroun J, Browman G, Walker‐Dilks C, Wong S. Adjuvant therapy for stage II colon cancer after complete resection. Provincial Gastrointestinal Disease Site Group. Cancer Prev Control 1997;1(5):379‐92. [PubMed] [Google Scholar]
Figueredo 2004 {published data only}
- Figueredo A, Charette ML, Maroun J, Brouwers MC, Zuraw L. Adjuvant therapy for stage II colon cancer: a systematic review from the Cancer Care Ontario Program in evidence‐based care's gastrointestinal cancer disease site group. Journal of Clinical Oncology 2004;22(16):3395. [DOI] [PubMed] [Google Scholar]
Francini 1994 {published data only}
- Francini G, Petrioli R, Lorenzini L, Mancini S, Armenio S, Tanzini G, et al. Folinic acid and 5‐fluorouracil as adjuvant chemotherapy in colon cancer. Gastroenterology 1994;106(4):899. [DOI] [PubMed] [Google Scholar]
Gill 2004 {published data only}
- Gill S, Loprinzi CL, Sargent DJ, Thome SD, Alberts SR, Haller DG, et al. Pooled analysis of fluorouracil‐based adjuvant therapy for stage II and III colon cancer: who benefits and by how much?[see comment]. Journal of Clinical Oncology 2004;22(10):1797. [DOI] [PubMed] [Google Scholar]
Glimelius 2005 {published data only}
- Glimelius B, Dahl O, Cedermark B, Jakobsen A, Bentzen SM, Starkhammar H, et al. Adjuvant chemotherapy in colorectal cancer: a joint analysis of randomised trials by the Nordic Gastrointestinal Tumour Adjuvant Therapy Group.[erratum appears in Acta Oncol. 2006;45(1):110]. Acta Oncologica 2005;44(8):904. [DOI] [PubMed] [Google Scholar]
Gray 1989 {published data only}
- Gray BN, Walker C, Andrewartha L, Freeman S, Bennett RC. Controlled clinical trial of adjuvant immunotherapy with BCG and neuraminidase‐treated autologous tumour cells in large bowel cancer. Journal of Surgical Oncology 1989;40(1):34. [DOI] [PubMed] [Google Scholar]
Gray 1991 {published data only}
- Gray R, James R, Mossman J, Stenning S. AXIS‐‐a suitable case for treatment. UK Coordinating Committee on Cancer Research (UKCCCR) Colorectal Cancer Subcommittee. Br J Cancer 1991;63(6):841‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gray 2007 {published data only}
- Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, Kerr DJ. QUASAR Collaborative Group: Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007. Dec. 15;370(9604):1980‐1. Dec. 15, 2007;370(9604):2020‐29. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hanna 2001 {published data only}
- G HM, Jr, C HH, Jr, Vermorken JB, Harris JE, Pinedo HM. Adjuvant active specific immunotherapy of stage II and stage III colon cancer with an autologous tumor cell vaccine: first randomized phase III trials show promise. Adjuvant active specific immunotherapy of stage II and stage III colon cancer with an autologous tumor cell vaccine: first randomized phase III trials show promise.. Vaccine 2001;19(17‐19):2576. [DOI] [PubMed] [Google Scholar]
Harris 2000 {published data only}
- Harris JE, Ryan L, Hoover HC, Jr, Stuart RK, Oken MM, Benson AB, 3rd, et al. Adjuvant active specific immunotherapy for stage II and III colon cancer with an autologous tumor cell vaccine: Eastern Cooperative Oncology Group Study E5283. J Clin Oncol 2000;18(1):148‐57. [DOI] [PubMed] [Google Scholar]
Hartung 2005 {published data only}
- Hartung G, Hofheinz RD, Dencausse Y, Sturm J, Kopp‐Schneider A, Dietrich G, et al. Adjuvant therapy with edrecolomab versus observation in stage II colon cancer: a multicenter randomized phase III study. Onkologie 2005;28(6‐7):347. [DOI] [PubMed] [Google Scholar]
IMPACT B2 1999 {published data only}
- Anonymous. Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer. International Multicentre Pooled Analysis of B2 Colon Cancer Trials (IMPACT B2) Investigators.[see comment]. Journal of Clinical Oncology 1999;17(5):1356. [PubMed] [Google Scholar]
Isenberg 1994 {published data only}
- Isenberg J, Ko H, Pulverer G, Grundmann R, Stutzer H, Pichlmaier H. Preoperative immunostimulation by Propionibacterium granulosum KP‐45 in colorectal cancer. Anticancer Research 1994;14(3B):1399. [PubMed] [Google Scholar]
Kimura 1995 {published data only}
- Kimura O, Kurayoshi K, Hoshino K, Sugezawa A, Makino M, Kaibara N. Prophylactic portal infusion chemotherapy as adjuvant therapy for the prevention of metachronous liver metastasis in colorectal cancer. Surgery Today 1995;25(3):211. [DOI] [PubMed] [Google Scholar]
Kingston 1993 {published data only}
- Kingston RD, Fielding JW, Palmer MK. An evaluation of the effectiveness and safety of razoxane when used as an adjunct to surgery in colo‐rectal cancer. Report of a controlled randomised study of 603 patients. International Journal of Colorectal Disease 1993;8(2):106. [DOI] [PubMed] [Google Scholar]
Laffer 1995 {published data only}
- Laffer U, Metzger U, Aeberhard P, Maibach R, Castiglione M, Goldhirsch A, et al. Long‐term results of single course of adjuvant intraportal chemotherapy for colorectal cancer. Lancet 1995;345(8946):349‐353. [PubMed] [Google Scholar]
Laurie 1989 {published data only}
- Laurie JA, Moertel CG, Fleming TR, Wieand HS, Leigh JE, Rubin J, et al. Surgical adjuvant therapy of large‐bowel carcinoma: an evaluation of levamisole and the combination of levamisole and fluorouracil. The North Central Cancer Treatment Group and the Mayo Clinic.[see comment]. Journal of Clinical Oncology 1989;7(10):1447. [DOI] [PubMed] [Google Scholar]
LIMAG 1997 {published data only}
- Liver Infusion Meta‐analysis Group. Portal vein chemotherapy for colorectal cancer: a meta‐analysis of 4000 patients in 10 studies. J Natl Cancer Inst 1997;89(7):497‐505. [PubMed] [Google Scholar]
Mamounas 1999 {published data only}
- Mamounas E, Wieand S, Wolmark N, Bear HD, Atkins JN, Song K, et al. Comparative efficacy of adjuvant chemotherapy in patients with Dukes' B versus Dukes' C colon cancer: results from four National Surgical Adjuvant Breast and Bowel Project adjuvant studies (C‐01, C‐02, C‐03, and C‐04)[see comment]. Journal of Clinical Oncology 1999;17(5):1349. [DOI] [PubMed] [Google Scholar]
McCollum 2002 {published data only}
- McCollum AD, Catalano PJ, Haller DG, Mayer RJ, Macdonald JS, Benson AB, 3rd, et al. Outcomes and toxicity in african‐american and caucasian patients in a randomized adjuvant chemotherapy trial for colon cancer. Journal of the National Cancer Institute 2002;94(15):1160. [DOI] [PubMed] [Google Scholar]
McDermott 2003 {published data only}
- McDermott U, Boyd RE, Houston RF, Kee F, McAleer JJ, Millar J, McAdams T, Sloan JM, Moorehead RJ, Wison RH. A phase III trial of short duration adjuvant chemotherapy with bolus/infusional 5 fluorouracil (FU) and folinic acid (FA) versus surgery alone in dukes' B and C colorectal cancer (CRC) [abstract]. Proceedings of the American Society of Clinical Oncology. 2003.
Moertel 1995 {published data only}
- Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Tangen CM, et al. Intergroup study of fluorouracil plus levamisole as adjuvant therapy for stage II/Dukes' B2 colon cancer. Journal of Clinical Oncology 1995;13(12):2936. [DOI] [PubMed] [Google Scholar]
Panettiere 1988 {published data only}
- Panettiere FJ, Goodman PJ, Costanzi JJ, B CA, Jr. , V V, McCracken JD, et al. Adjuvant therapy in large bowel adenocarcinoma: long‐term results of a Southwest Oncology Group Study. Journal of Clinical Oncology 1988;6(6):947. [DOI] [PubMed] [Google Scholar]
Sadahiro 2004 {published data only}
- Sadahiro S, Suzuki T, Ishikawa K, Yasuda S, Tajima T, Makuuchi H, et al. Prophylactic hepatic arterial infusion chemotherapy for the prevention of liver metastasis in patients with colon carcinoma: a randomized control trial.[see comment]. Cancer 2004;100(3):590. [DOI] [PubMed] [Google Scholar]
Sakamoto 2004 {published data only}
- Sakamoto J, Ohashi Y, Hamada C, Buyse M, Burzykowski T, Piedbois P, et al. Efficacy of oral adjuvant therapy after resection of colorectal cancer: 5‐year results from three randomized trials. Journal of Clinical Oncology 2004;22(3):484. [DOI] [PubMed] [Google Scholar]
Sakamoto 2005 {published data only}
- Sakamoto J, Hamada C, Rahman M, Kodaira S, Ito K, Nakazato H, et al. An individual patient data meta‐analysis of adjuvant therapy with carmofur in patients with curatively resected colon cancer. Japanese Journal of Clinical Oncology 2005;35(9):536. [DOI] [PubMed] [Google Scholar]
Sargent 2001 {published data only}
- Sargent DJ, Goldberg RM, Jacobson SD, Macdonald JS, Labianca R, Haller DG, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients.[see comment]. New England Journal of Medicine 2001;345(15):1091. [DOI] [PubMed] [Google Scholar]
Sargent 2005 {published data only}
- Sargent DJ, Wieand HS, Haller DG, Gray R, Benedetti JK, Buyse M, et al. Disease‐free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials.[see comment]. Journal of Clinical Oncology 2005;23(34):8664. [DOI] [PubMed] [Google Scholar]
Taal 2001 {published data only}
- Taal BG, Tinteren H, Zoetmulder FA. Adjuvant 5FU plus levamisole in colonic or rectal cancer: improved survival in stage II and III. Br J Cancer 2001;85(10):1437‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vaillant 2000 {published data only}
- Vaillant JC, Nordlinger B, Deuffic S, Arnaud JP, Pelissier E, Favre JP, et al. Adjuvant intraperitoneal 5‐fluorouracil in high‐risk colon cancer: A multicenter phase III trial.[see comment]. Annals of Surgery 2000;231(4):449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vermorken 1999 {published data only}
- Vermorken JB, Claessen AM, Tinteren H, Gall HE, Ezinga R, Meijer S, et al. Active specific immunotherapy for stage II and stage III human colon cancer: a randomised trial.[see comment]. Lancet 1999;353(9150):345. [DOI] [PubMed] [Google Scholar]
Wan 2005 {published data only}
- Wan XB, Pan ZZ, Chen G, Li LR, Wan DS. [Meta‐analysis of postoperative adjuvant chemotherapy for Dukes' B colorectal carcinoma]. Aizheng 2005;24(5):600. [PubMed] [Google Scholar]
Watanabe 2006 {published data only}
- Watanabe M, Kodaira S, Takahashi T, Tominaga T, Hojo K, Kato T, et al. Randomized trial of the efficacy of adjuvant chemotherapy for colon cancer with combination therapy incorporating the oral pyrimidine 1‐hexylcarbamoyl‐5‐fluorouracil. Langenbecks Archives of Surgery 2006;391(4):330. [DOI] [PubMed] [Google Scholar]
Windle 1987 {published data only}
- Windle R, Bell PR, Shaw D. Five year results of a randomized trial of adjuvant 5‐fluorouracil and levamisole in colorectal cancer. British Journal of Surgery 1987;74(7):569. [DOI] [PubMed] [Google Scholar]
Wolmark 1988 {published data only}
- Wolmark N, Fisher B, Rockette H, Redmond C, Wickerham DL, Fisher ER, et al. Postoperative adjuvant chemotherapy or BCG for colon cancer: results from NSABP protocol C‐01. Journal of the National Cancer Institute 1988;80(1):30. [DOI] [PubMed] [Google Scholar]
Wolmark 1990 {published data only}
- Wolmark N, Rockette H, Wickerham DL, Fisher B, Redmond C, Fisher ER, et al. Adjuvant therapy of Dukes' A, B, and C adenocarcinoma of the colon with portal‐vein fluorouracil hepatic infusion: preliminary results of National Surgical Adjuvant Breast and Bowel Project Protocol C‐02.[see comment]. Journal of Clinical Oncology 1990;8(9):1466. [DOI] [PubMed] [Google Scholar]
Zalcberg 1996 {published data only}
- Zalcberg JR, Siderov J, Simes J. The role of 5‐fluorouracil dose in the adjuvant therapy of colorectal cancer. Annals of Oncology 1996;7(1):42. [DOI] [PubMed] [Google Scholar]
Zaniboni 1997 {published data only}
- Zaniboni A, Labianca R, Marsoni S, Apolone G, Mosconi P, Tinazzi A, Torri V. GIVIO‐SITAC O1. A randomised trial of adjuvant 5‐fluorouracil and high‐dose folinic acid (HD‐FUFA) in patients with colon cancer: Long term results and evaluation of indicators of health related quality of life (QOL) and costs. Tumori 1997;83(3):2135‐2144. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ahn 2001 {published data only}
- Ahn JB, Shim KY, Jeung HC, Rha SY, Yoo NC, Kim NK, et al. Monthly 5‐days 5‐fluorouracil and low‐dose leucovorin for adjuvant chemotherapy in colon cancer. Cancer Letters 2001;167(2):215. [DOI] [PubMed] [Google Scholar]
Alikhan 2004 {published data only}
- Alikhan MA, V P, Kohli M. Re: Randomized trial of adjuvant therapy in colon carcinoma: 10‐year results of NSABP protocol C‐01.[comment]. Journal of the National Cancer Institute 2004;96(23):1794. [DOI] [PubMed] [Google Scholar]
Alliot 2004 {published data only}
- Alliot C, Ohwada S, Morishita Y. Adjuvant immunochemotherapy with oral Tegafur/Uracil plus PSK in patients with stage II or III colorectal cancer [1] (multiple letters). British Journal of Cancer 2004;91(6):1220‐1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arnaud 1988 {published data only}
- Arnaud JP, Buyse M, Adloff M, Nordlinger B, Pector JC, Duez N. Interim analysis of a double‐blind phase‐III clinical trial of adjuvant levamisole versus control in resectable Dukes‐C colon cancer: a study of the EORTC Gastrointestinal Tract Cancer Cooperative Group. Recent Results in Cancer Research 1988;110:101. [DOI] [PubMed] [Google Scholar]
Arnaud 1989 {published data only}
- Arnaud JP, Buyse M, Nordlinger B, Martin F, Pector JC, Zeitoun P, et al. Adjuvant therapy of poor prognosis colon cancer with levamisole: results of an EORTC double‐blind randomized clinical trial. British Journal of Surgery 1989;76(3):284. [DOI] [PubMed] [Google Scholar]
Boussen 1999 {published data only}
- Boussen H, Kallel N, Khomsi F, Jerbi G, Dhouib R, Ben Safta Z, et al. [Adjuvant chemotherapy after curative surgery for non‐metastatic colorectal carcinomas (2‐year results of 52 cases)]. Tunisie Medicale 1999;77(2):61. [PubMed] [Google Scholar]
Brivio 2006 {published data only}
- Brivio F, Fumagalli L, Lissoni P, Nardone A, Nespoli L, Fattori L, et al. Pre‐operative immunoprophylaxis with interleukin‐2 may improve prognosis in radical surgery for colorectal cancer stage B‐C. Anticancer Research 2006;26(1B):599. [PubMed] [Google Scholar]
Bukowski 1987 {published data only}
- Bukowski RM. Clinical trials in colorectal cancer: experience of the Gastrointestinal Tumor Study Group and the Southwest Oncology Group. International Journal of Colorectal Disease 1987;2(2):100. [DOI] [PubMed] [Google Scholar]
Buyse 1988 {published data only}
- Buyse M, Zeleniuch‐Jacquotte A, Chalmers TC. Adjuvant therapy of colorectal cancer. Why we still don't know. JAMA 1988;259(24):3571‐8. [PubMed] [Google Scholar]
Buyse 1989 {published data only}
- Buyse M, Zeleniuch‐Jacquotte A, Chalmers TC. [Adjuvant chemotherapy of colorectal cancer: what can be learned from meta‐analysis?]. Bulletin du Cancer 1989;76(9):1021. [PubMed] [Google Scholar]
Chlebowski 1994 {published data only}
- Chlebowski RT, Lillington L, Nystrom JS, Sayre J. Late mortality and levamisole adjuvant therapy in colorectal cancer.[see comment]. British Journal of Cancer 1994;69(6):1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cremona 1997 {published data only}
- Cremona F, Izzo F, Ruffolo F, Palaia R, V P. Adjuvant therapy for resectable colorectal carcinoma with 5‐fluorouracil portal vein infusion. Journal of Chemotherapy 1997;9(2):140. [DOI] [PubMed] [Google Scholar]
Dahl 2003 {published data only}
- Dahl O TKMCEWJNMHEGTSAPNBOETJ. Only colon cancer patients with Dukes stage C benefit from adjuvant chemotherapy with 5‐fluorouracil and levamisole among 425 patients with operable colorectal cancer in a Norwegian randomised study [abstract]. European Journal of Cancer 2003;1(5):S324. [Google Scholar]
De Gramont 2003 {published data only}
- Gramont A, Banzi M, Navarro M, Tabernero J, Hickish T, Bridgewater J, Rivera F, Figer A, Fountzilas G, Andre T. Oxaliplatin/5 FU/LV in adjuvant colon cancer: results of the international randomized mosaic trial [abstract]. Proceedings of the American Society of Clinical Oncology. 2003.
Fata 2002 {published data only}
- Fata F, Mirza A, Wood GC, Nair S, Law A, Gallagher J, et al. Efficacy and toxicity of adjuvant chemotherapy in elderly patients with colon carcinoma: A 10‐year experience of the Geisinger Medical Center. Cancer 2002;94(7):1931‐1938. [DOI] [PubMed] [Google Scholar]
Fietkau 1998 {published data only}
- Fietkau R. [Adjuvant radio‐chemotherapy is effective in the treatment of Dukes B and C rectal cancer: results of a randomized controlled Norwegian trial]. Strahlentherapie und Onkologie 1998;174(9):490. [DOI] [PubMed] [Google Scholar]
Focan 2000 {published data only}
- Focan C, Bury J, Beauduin M, Herman ML, Vindevoghel A, Lecomte M, et al. Importance of 5‐fluorouracil dose‐intensity in a double randomised trial on adjuvant portal and systemic chemotherapy for Dukes B2 and C colorectal cancer. Anticancer Research 2000;20(6C):4665. [PubMed] [Google Scholar]
Franchi 1991 {published data only}
- Franchi F, Barone C, Ricevuto E, Cassano A, Astone A, Pozzo C, et al. 5‐Fluorouracil (FU) with folinic acid (FA) and mitomycin C (MMC) in the adjuvant treatment of colorectal carcinoma. Part I. Evaluation of toxicity. Medical Oncology & Tumor Pharmacotherapy 1991;8(2):69. [DOI] [PubMed] [Google Scholar]
Gambill 2001 {published data only}
- Gambill BD. Edrecolomab (Panorex) as adjuvant therapy for stage II colon cancer. Clinical Colorectal Cancer 2001;1(1):16. [DOI] [PubMed] [Google Scholar]
Garcia 1996 {published data only}
- Garcia Restoy E, Grau JJ, Daniels M. Mitomycin C and Ptorafur as adjuvant treatment in colorectal cancer. Neoplasia 1996;13(5):152‐157. [Google Scholar]
Gennatas 2002 {published data only}
- Gennatas C, Mouratidou D, Androulakis G, V G, Tsavaris N, Philippakis M, et al. Adjuvant systemic therapy protocol for Dukes' B2 and C resectable colon carcinoma. Tumori 2002;88(1):32. [PubMed] [Google Scholar]
Graf 1994 {published data only}
- Graf W, Westlin JE, Pahlman L, Glimelius B. Adjuvant intraperitoneal 5‐fluorouracil and intravenous leucovorin after colorectal cancer surgery: a randomized phase II placebo‐controlled study. International Journal of Colorectal Disease 1994;9(1):35. [DOI] [PubMed] [Google Scholar]
Gray 1988 {published data only}
- Gray BN, Walker C, Andrewartha L, Freeman S, Bennett RC. Melbourne trial of adjuvant immunotherapy in operable large bowel cancer. Australian & New Zealand Journal of Surgery 1988;58(1):43. [DOI] [PubMed] [Google Scholar]
Gray 2004 {published data only}
- Gray Rg, Barnwell J, Hills R, McConkey C, Williams N, Kerr D. QUASAR: A randomized study of adjuvant chemotherapy (CT) vs observation including 3238 colorectal cancer patients [abstract]. Annual Meeting Proceedings of the American Society of Clinical Oncology. 2004:246.
Grem 2004 {published data only}
- Grem JL. Mature results of adjuvant colon cancer trials from the fluorouracil‐only era.[comment]. Journal of the National Cancer Institute 2004;96(10):727. [DOI] [PubMed] [Google Scholar]
Grundmann 1988 {published data only}
- Grundmann R, Weber F, Kluger J, Wienand P, Pulverer G. [Preoperative nonspecific immunostimulation with Propionibacterium granulosum KP‐45 in patients with colorectal cancer. A prospective randomized study]. Chirurg 1988;59(4):272. [PubMed] [Google Scholar]
GTSG 1991 {published data only}
- The Gastrointestinal Tumor Study Group. Adjuvant therapy with hepatic irradiation plus fluorouracil in colon carcinoma. International journal of radiation oncology, biology, physics 1991;21(5):1151. [DOI] [PubMed] [Google Scholar]
Hafstrom 1988 {published data only}
- Hafstrom L. Postoperative adjuvant therapy. Scandinavian Journal of Gastroenterology ‐ Supplement 1988;149:179. [DOI] [PubMed] [Google Scholar]
Hafstrom 1990 {published data only}
- Hafstrom L, Domellof L, Rudenstam CM, Norryd C, Bergman L, Nilsson T, et al. Adjuvant chemotherapy with 5‐fluorouracil, vincristine and CCNU for patients with Dukes' C colorectal cancer. The Swedish Gastrointestinal Tumour Adjuvant Therapy Group. British Journal of Surgery 1990;77(12):1345. [DOI] [PubMed] [Google Scholar]
Haller 2001 {published data only}
- Haller DG. Update of clinical trials with edrecolomab: a monoclonal antibody therapy for colorectal cancer. Seminars in Oncology 2001;28(1 Suppl 1):25. [DOI] [PubMed] [Google Scholar]
Harder 1990 {published data only}
- Harder F, Laffer U, Berres M, Jaggi P, Metzger U. [Following curative resection of colorectal cancer, portal chemotherapy especially benefits non‐transfused patients]. Chirurg 1990;61(4):280. [PubMed] [Google Scholar]
Hashimoto 1994 {published data only}
- Hashimoto T, Saji S, Fukada D, Umemoto T, Kunieda K, Sugiyama Y, et al. [Studies on adjuvant chemotherapy using subserosal or submucosal administration of neocarzinostatin for gastric cancer and colorectal cancer]. Gan to Kagaku Ryoho [Japanese Journal of Cancer & Chemotherapy] 1994;21(13):2302. [PubMed] [Google Scholar]
Hess 1997 {published data only}
- Hess P, Heinicke JM, Laffer UT. [The importance of adjuvant chemotherapy in the treatment of colonic carcinoma: SAKK Study 40/93 (Swiss Work Group for Clinical Cancer Research)]. Revue Medicale de la Suisse Romande 1997;117(2):151. [PubMed] [Google Scholar]
Hoover 1989 {published data only}
- C HH, Jr. , G HM, Jr. Active immunotherapy in colorectal cancer. Seminars in Surgical Oncology 1989;5(6):436. [DOI] [PubMed] [Google Scholar]
Hoover 1993 {published data only}
- C HH, Jr, Brandhorst JS, Peters LC, Surdyke MG, Takeshita Y, Madariaga J, et al. Adjuvant active specific immunotherapy for human colorectal cancer: 6.5‐year median follow‐up of a phase III prospectively randomized trial.[see comment]. Adjuvant active specific immunotherapy for human colorectal cancer: 6.5‐year median follow‐up of a phase III prospectively randomized trial.[see comment].. Journal of Clinical Oncology 1993;11(3):390. [DOI] [PubMed] [Google Scholar]
IMPACT 1995 {published data only}
- International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators. Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. Lancet 1995;345(8955):939. [PubMed] [Google Scholar]
Ito 1996 {published data only}
- Ito K, Yamaguchi A, Miura K, Kato T, Baba S, Matsumoto S, et al. Oral adjuvant chemotherapy with carmofur (HCFU) for colorectal cancer: five‐year follow‐up. Tokai HCFU Study Group‐‐third study on colorectal cancer. Journal of Surgical Oncology 1996;63(2):107. [DOI] [PubMed] [Google Scholar]
Kapoor 2005 {published data only}
- Kapoor S, Pal S, Sahni P, Dattagupta S, Kanti Chattopadhyay T. Effect of pre‐operative short course famotidine on tumor infiltrating lymphocytes in colorectal cancer: a double blind, placebo controlled, prospective randomized study. Journal of Surgical Research 2005;129(2):172. [DOI] [PubMed] [Google Scholar]
Kato 2002 {published data only}
- Kato T, Ohashi Y, Nakazato H, Koike A, Saji S, Suzuki H, et al. Efficacy of oral UFT as adjuvant chemotherapy to curative resection of colorectal cancer: multicenter prospective randomized trial. Langenbecks Archives of Surgery 2002;386(8):575. [DOI] [PubMed] [Google Scholar]
Kelly 1999 {published data only}
- Kelly MD, King J, Cherian M, Dwerryhouse SJ, Finlay IG, Adams WJ, et al. Randomized trial of preoperative cimetidine in patients with colorectal carcinoma with quantitative assessment of tumor‐associated lymphocytes. Cancer 1999;85(8):1658. [DOI] [PubMed] [Google Scholar]
Kerr 2000 {published data only}
- Kerr DJ, Midgley RS. Immunotherapy for colorectal cancer: Potential application in an adjuvant setting. Seminars in Oncology 2000;27(5 SUPPL. 10):132‐137. [PubMed] [Google Scholar]
Knuth 1988 {published data only}
- Knuth A. [Adjuvant therapy in cancer of the large intestine]. Zeitschrift fur Gastroenterologie 1988;26(8):434. [PubMed] [Google Scholar]
Kodaira 1989 {published data only}
- Kodaira S, Kikuchi K, Inokuchi K, Komi N, Hattori T, Taguchi T, et al. [Cooperative study of surgical adjuvant chemotherapy of colorectal carcinoma (second study): a preliminary report. Cooperative Study Group of Surgical Adjuvant Chemotherapy of Colorectal Cancer in Japan]. Gan to Kagaku Ryoho [Japanese Journal of Cancer & Chemotherapy] 1989;16(10):3399. [PubMed] [Google Scholar]
Kodaira 1997 {published data only}
- Kodaira S. [The current status of postoperative adjuvant chemotherapy for colorectal cancer]. Gan to Kagaku Ryoho [Japanese Journal of Cancer & Chemotherapy] 1997;24(10):1230. [PubMed] [Google Scholar]
Kunii 1987 {published data only}
- Kunii Y, Kikuchi K, Kasai Y, Abe O, Kondo T, Taguchi T, et al. [Cooperative study of surgical adjuvant chemotherapy of colorectal cancer (first report): Investigation of background factors and adverse effects. Cooperative Study Group of Surgical Adjuvant Chemotherapy of Colorectal Cancer in Japan]. Gan to Kagaku Ryoho [Japanese Journal of Cancer & Chemotherapy] 1987;14(2):421. [PubMed] [Google Scholar]
Kunii 1995 {published data only}
- Kunii Y, Kikuchi K. [Cooperative study of surgical adjuvant chemotherapy for colorectal cancer. Cooperative Study Group of Surgical Adjuvant Chemotherapy for Colorectal Cancer in Japan]. Gan to Kagaku Ryoho [Japanese Journal of Cancer & Chemotherapy] 1995;22(10):1369. [PubMed] [Google Scholar]
Lacour 1992 {published data only}
- Lacour J, Laplanche A, Malafosse M, Gallot D, Julien M, Rotman N, et al. Polyadenylic‐polyuridylic acid as an adjuvant in resectable colorectal carcinoma: a 6 1/2 year follow‐up analysis of a multicentric double blind randomized trial. European Journal of Surgical Oncology 1992;18(6):599. [PubMed] [Google Scholar]
Laffer 1987 {published data only}
- Laffer U, Metzger U, Aeberhard P, Egeli R, Martinoli S, Muller W, Cavalli F, Durig M, Harder F. Adjuvant portal liver infusion after curative resection of colorectal cancer. A randomized multicenter study of the swiss cooperative group on clinical cancer research. Langenbecks Archiv fur Chirurgie 1987;372:889. [Google Scholar]
Laffer 1992 {published data only}
- Laffer U, Metzger U, Aeberhard P, Arigoni M, Arma S, Barras J, Egeli R, Martinoli S, Muller W, Schweizer W, Weber W, Pamplona S. Final evaluation of the randomized multicentric study SAKK 40/81: Adjuvant portal chemotherapy in resected colorectal carcinoma. Helvetica Chirurgica Acta 1992;58(5):755‐758. [PubMed] [Google Scholar]
Laffer 1992a {published data only}
- Laffer U, Metzger U, Pampalona S. Adjuvant portal liver infusion after radical resection of colorectal cancer: results of a randomized swiss multicenter trial. Journal of Cancer Research & Clinical Oncology 1992;118:R160. [Google Scholar]
Lise 1987 {published data only}
- Lise M, Gerard A, Nitti D, Zane D, Buyse M, Duez N, et al. Adjuvant therapy for colorectal cancer. The EORTC experience and a review of the literature. Diseases of the Colon & Rectum 1987;30(11):847. [DOI] [PubMed] [Google Scholar]
Marangolo 1989 {published data only}
- Marangolo M, Pezzuoli G, Marubini E, Amadori D, Boracchi P, Cocconi G, et al. Adjuvant chemotherapy with fluorouracil and CCNU in colon cancer. Results of a multicentric randomized study. Tumori 1989;75(3):269. [DOI] [PubMed] [Google Scholar]
Marsoni 2001 {published data only}
- Marsoni S, International Multicenter Pooled Analysis of Colon Cancer Trials I. Efficacy of adjuvant fluorouracil and leucovorin in stage B2 and C colon cancer. International Multicenter Pooled Analysis of Colon Cancer Trials Investigators. Seminars in Oncology 2001;28(1 Suppl 1):14. [DOI] [PubMed] [Google Scholar]
Matsuda 1991 {published data only}
- Matsuda T, Yasutomi M, Kikuchi K, Kasai Y, Abe O, Kondo T, et al. [Cooperative study of surgical adjuvant chemotherapy for colorectal cancer (third report): five‐year results. Cooperative Study Group of Surgical Adjuvant Chemotherapy for Colorectal Cancer in Japan]. Gan to Kagaku Ryoho [Japanese Journal of Cancer & Chemotherapy] 1991;18(3):461. [PubMed] [Google Scholar]
Matsumoto 2002 {published data only}
- Matsumoto S, Imaeda Y, Umemoto S, Kobayashi K, Suzuki H, Okamoto T. Cimetidine increases survival of colorectal cancer patients with high levels of sialyl Lewis‐X and sialyl Lewis‐A epitope expression on tumour cells.[see comment]. British Journal of Cancer 2002;86(2):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Metzger 1987 {published data only}
- Metzger U, Mermillod B, Aeberhard P, Gloor F, Bissat A, Egeli R, et al. Intraportal chemotherapy in colorectal carcinoma as an adjuvant modality. World Journal of Surgery 1987;11(4):452. [DOI] [PubMed] [Google Scholar]
Metzger 1989 {published data only}
- Metzger U, Laffer U, Egeli R, Barras J, Martinoli S, Muller W, et al. [Initial results of adjuvant portal liver infusion following radical surgery of colorectal cancer (Swiss Study Group for Epidemiologic and Clinical Cancer Research Study 40/81)]. Helvetica Chirurgica Acta 1989;56(4):455. [PubMed] [Google Scholar]
Metzger 1990 {published data only}
- Metzger U, Laffer U, Aeberhard P, Arigoni M, Arma S, Barras J, et al. Randomized multicenter trial of adjuvant intraportal chemotherapy for colorectal cancer (SAKK 40/81). An interim report. Acta Chirurgica Scandinavica 1990;156(6‐7):467. [PubMed] [Google Scholar]
Mitomi 1990 {published data only}
- Mitomi T, Tsuchiya S, Iijima N, Aso K, Suzuki K, Noto T, Ogawa N. A randomized controlled study on adjuvant immunochemotherapy with PSK in curatively resected colorectal cancer. Journal of Cancer Research & Clinical Oncology 1990;116:123‐130. [Google Scholar]
Mitomi 1993 {published data only}
- Mitomi T, Tsuchiya S, Iijima N, Aso K, Nishiyama K, Amano T, et al. Randomized controlled study on adjuvant immunochemotherapy with PSK in curatively resected colorectal cancer. 5 Years follow‐up after surgery (a final report). [Japanese]. Journal of Japan Society for Cancer Therapy 1993;28(1):71‐83. [Google Scholar]
Moertel 1995 {published data only}
- Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Tangen CM, et al. Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: a final report. Annals of Internal Medicine 1995;122(5):321. [DOI] [PubMed] [Google Scholar]
Moertel 1990 {published data only}
- Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Goodman PJ, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma.[see comment]. New England Journal of Medicine 1990;322(6):352. [DOI] [PubMed] [Google Scholar]
Moertel 1993 {published data only}
- Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA. Hepatic toxicity associated with fluorouracil plus levamisole adjuvant therapy.[see comment]. Journal of Clinical Oncology 1993;11(12):2386. [DOI] [PubMed] [Google Scholar]
Mori 2002 {published data only}
- Mori T. [Review of comparative studies of postoperative adjuvant chemotherapy after curatively resected colorectal cancer in Japan]. Gan to Kagaku Ryoho [Japanese Journal of Cancer & Chemotherapy] 2002;29(9):1532. [PubMed] [Google Scholar]
Mukai 2003 {published data only}
- Mukai M, Tajima T, Nakasaki H, Sato S, Ogoshi K, Makuuchi H. Efficacy of postoperative adjuvant oral immunochemotherapy in patients with Dukes' B colorectal cancer. Annals of Cancer Research & Therapy 2003;11(1‐2):201‐214. [Google Scholar]
Nichols 1992 {published data only}
- Nichols PH, Ramsden CW, Ward U, Sedman PC, Primrose JN. Perioperative immunotherapy with recombinant interleukin 2 in patients undergoing surgery for colorectal cancer. Cancer Research 1992;52(20):5765. [PubMed] [Google Scholar]
Nichols 1993 {published data only}
- Nichols PH, Ramsden CW, Ward U, Trejdosiewicz LK, Ambrose NS, Primrose JN. Peri‐operative modulation of cellular immunity in patients with colorectal cancer. Clinical & Experimental Immunology 1993;94(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nielsen 2002 {published data only}
- Nielsen HJ, Christensen IJ, Moesgaard F, Kehlet H, Danish RCCSG. Ranitidine as adjuvant treatment in colorectal cancer.[see comment]. British Journal of Surgery 2002;89(11):1416. [DOI] [PubMed] [Google Scholar]
Niimoto 1988 {published data only}
- Niimoto M, Kikuchi K, Kasai Y, Abe O, Kondo T, Taguchi T, et al. [A cooperative study of surgical adjuvant chemotherapy of colorectal cancer (second report): 3‐year survival rate. Cooperative Study Group of Surgical Adjuvant Chemotherapy of Colorectal Cancer in Japan]. Gan to Kagaku Ryoho [Japanese Journal of Cancer & Chemotherapy] 1988;15(8):2237. [PubMed] [Google Scholar]
Nio 1992 {published data only}
- Nio Y, Tsubono M, Tseng CC, Morimoto H, Kawabata K, Masai Y, et al. Immunomodulation by orally administered protein‐bound polysaccharide PSK in patients with gastrointestinal cancer. Biotherapy 1992;4(2):117. [DOI] [PubMed] [Google Scholar]
Nishida 1993 {published data only}
- Nishida O, Uchino J, Kikuchi K, Inokuchi K, Komi N, Hattori T, et al. [Cooperative Study of Surgical Adjuvant Chemotherapy for Colorectal Cancer (third report): five‐year results after surgery]. Gan to Kagaku Ryoho [Japanese Journal of Cancer & Chemotherapy] 1993;20(1):101. [PubMed] [Google Scholar]
Nitti 1997 {published data only}
- Nitti D, Wils J, Sahmoud T, Curran D, Couvreur ML, Lise M, et al. Final results of a phase III clinical trial on adjuvant intraportal infusion with heparin and 5‐fluorouracil (5‐FU) in resectable colon cancer (EORTC GITCCG 1983‐1987). European Organization for Research and Treatment of Cancer. Gastrointestinal Tract Cancer Cooperative Group. European Journal of Cancer 1997;33(8):1209. [DOI] [PubMed] [Google Scholar]
O DH Jr. 2000 {published data only}
- O DH, Jr. Results from National Surgical Adjuvant Breast and Bowel project colon cancer trials.[comment]. Journal of Clinical Oncology 2000;18(1):235. [PubMed] [Google Scholar]
O'Connell 1997 {published data only}
- O'Connell MJ, Mailliard JA, Kahn MJ, Macdonald JS, Haller DG, Mayer RJ, et al. Controlled trial of fluorouracil and low‐dose leucovorin given for 6 months as postoperative adjuvant therapy for colon cancer. Journal of Clinical Oncology 1997;15(1):246. [DOI] [PubMed] [Google Scholar]
Pater 1994 {published data only}
- Pater JL. Timing the collaborative analysis of three trials comparing 5‐FU plus folinic acid (FUFA) to surgery alone in the management of resected colorectal cancer: a National Cancer Institute of Canada Clinical Trials Group (NCIC‐CTG) perspective. Statistics in Medicine 1994;13(13‐14):1337. [DOI] [PubMed] [Google Scholar]
Pendlebury 2003 {published data only}
- Pendlebury S, Duchesne F, Reed KA, Smith JL, Kerr DJ. A trial of adjuvant therapy in colorectal cancer: the VICTOR trial. Clinical Colorectal Cancer 2003;3(1):58. [DOI] [PubMed] [Google Scholar]
Petrasch 1997 {published data only}
- Petrasch S, Schmiegel W. [Adjuvant therapy of colon carcinoma: results of two Intergroup Studies (INT‐0035 and INT‐0089), a study by the German Cancer Aid 17‐1A Study Group and the IMPACT Study]. Zeitschrift fur Gastroenterologie 1997;35(1):49. [PubMed] [Google Scholar]
Piedbois 2000 {published data only}
- Piedbois P, Buyse M. Recent meta‐analyses in colorectal cancer. Current Opinion in Oncology 2000;12(4):362. [DOI] [PubMed] [Google Scholar]
Prohm 1988 {published data only}
- Prohm P, Seewald V, Hoffmann E. Adjuvant immunetherapy with BCG vaccine in patients with colorectal carcinoma A controlled randomized trial Preliminary report. Journal of Cancer Research & Clinical Oncology 1988;114:S66. [Google Scholar]
Ragnhammar 2001 {published data only}
- Ragnhammar P, Hafstrom L, Nygren P, Glimelius B, Care SBUgSCoTAiH. A systematic overview of chemotherapy effects in colorectal cancer. Acta Oncologica 2001;40(2‐3):282. [DOI] [PubMed] [Google Scholar]
Reddy 2004 {published data only}
- Reddy GK. Efficacy of adjuvant capecitabine compared with bolus 5‐fluorouracil/leucovorin regimen in dukes C colon cancer: results from the X‐ACT trial. Clinical Colorectal Cancer 2004;4(2):87. [DOI] [PubMed] [Google Scholar]
Reddy 2005 {published data only}
- Reddy GK. Recent data with oxaliplatin‐containing regimens in the adjuvant treatment of colorectal cancer. Clinical Colorectal Cancer 2005;5(2):83. [DOI] [PubMed] [Google Scholar]
Riethmuller 1994 {published data only}
- Riethmuller G, Schneider‐Gadicke E, Schlimok G, Schmiegel W, Raab R, Hoffken K, et al. Randomised trial of monoclonal antibody for adjuvant therapy of resected Dukes' C colorectal carcinoma. German Cancer Aid 17‐1A Study Group.[see comment]. Lancet 1994;343(8907):1177. [DOI] [PubMed] [Google Scholar]
Rougier 1993 {published data only}
- Rougier P, Nordlinger B. Large scale trial for adjuvant treatment in high risk resected colorectal cancers. Rationale to test the combination of loco‐regional and systemic chemotherapy and to compare l‐leucovorin + 5‐FU to levamisole + 5‐FU. Annals of Oncology 1993;4 Suppl 2:21. [DOI] [PubMed] [Google Scholar]
Rougier 1998 {published data only}
- Rougier P, Sahmoud T, Nitti D, Curran D, Doci R, Waele B, et al. Adjuvant portal‐vein infusion of fluorouracil and heparin in colorectal cancer: a randomised trial. European Organisation for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group, the Gruppo Interdisciplinare Valutazione Interventi in Oncologia, and the Japanese Foundation for Cancer Research.[see comment]. Lancet 1998;351(9117):1677. [DOI] [PubMed] [Google Scholar]
Sakamoto 1999 {published data only}
- Sakamoto J, Hamada C, Kodaira S, Nakazato H, Ohashi Y. Adjuvant therapy with oral fluoropyrimidines as main chemotherapeutic agents after curative resection for colorectal cancer: individual patient data meta‐analysis of randomized trials. Japanese Journal of Clinical Oncology 1999;29(2):78. [DOI] [PubMed] [Google Scholar]
Sakamoto 2001 {published data only}
- Sakamoto J, Kodaira S, Hamada C, Ito K, Maehara Y, Takagi H, et al. An individual patient data meta‐analysis of long supported adjuvant chemotherapy with oral carmofur in patients with curatively resected colorectal cancer. Oncology Reports 2001;8(3):697. [PubMed] [Google Scholar]
SAKK 1997 {published data only}
- Anonymous. Association between blood transfusion and survival in a randomised multicentre trial of perioperative adjuvant portal chemotherapy in patients with colorectal cancer. The Swiss Group for Clinical Cancer Research (SAKK). European Journal of Surgery 1997;163(9):693. [PubMed] [Google Scholar]
Sargent 2004 {published data only}
- Sargent DJ, Wieand S, Benedetti J, Labianca R, Haller DG, Shepherd LE, Seitz JF, Francini G, Gramont A, Goldberg RM. Disease‐free survival (DFS) vs. overall survival (OS) as a primary endpoint for adjuvant colon cancer studies: Individual patient data from 12,915 on patients on 15 randomized trials [abstract]. Annual Meeting Proceedings of the American Society of Clinical Oncology. 2004:246.
Scheithauer 1995 {published data only}
- Scheithauer W, Kornek G, Rosen H, Sebesta C, Marcell A, Kwasny W, et al. Combined intraperitoneal plus intravenous chemotherapy after curative resection for colonic adenocarcinoma. European Journal of Cancer 1995;31A(12):1981. [DOI] [PubMed] [Google Scholar]
Seitz 1994 {published data only}
- Seitz Jf ECMSSJSLZBMCRPBLGMTVTBSF. Adjuvant fluorouracil with high dose folinic acid (HD‐FUFA) increases survival in colon cancer: a prospective pooled analysis 9f 3 randomized tirals (RTs). Annals of Oncology 1994;5(Suppl 8):47. [Google Scholar]
Smith 2004 {published data only}
- Smith RE, Colangelo L, Wieand HS, Begovic M, Wolmark N. Randomized trial of adjuvant therapy in colon carcinoma: 10‐year results of NSABP protocol C‐01.[see comment]. Journal of the National Cancer Institute 2004;96(15):1128. [DOI] [PubMed] [Google Scholar]
Suzuka 1999 {published data only}
- Suzuka I, Izumi S, Onoda Y, Shiota K, Nishihara M, Nakagawa J, et al. [Usefulness of pharmacokinetic modulating chemotherapy (PMC) for the postoperative adjuvant therapy of colorectal carcinoma: a preliminary report]. Gan to Kagaku Ryoho [Japanese Journal of Cancer & Chemotherapy] 1999;26(14):2183. [PubMed] [Google Scholar]
Svendsen 1995 {published data only}
- Svendsen LB, Ross C, Knigge U, Frederiksen HJ, Graversen P, Kjaergard J, et al. Cimetidine as an adjuvant treatment in colorectal cancer. A double‐blind, randomized pilot study.[see comment]. Diseases of the Colon & Rectum 1995;38(5):514. [DOI] [PubMed] [Google Scholar]
The QCG 2004 {published data only}
- The QCG. QUASAR: a randomised study of adjuvant chemotherapy (CT) vs observation including 3239 colorectal cancer patients [abstract].. British Journal of Cancer 2004;Supplement:91. [Google Scholar]
Thomas 1987 {published data only}
- Thomas PR, Stablein DM, Steinberg SM, Barkin JS, Nauta RJ. The effects of liver directed radiotherapy and chemotherapy on liver function tests and hematological parameters in patients with surgically resected colon cancer: findings from the Gastrointestinal Tumor Study Group. American Journal of Gastroenterology 1987;82(8):738. [PubMed] [Google Scholar]
Ullenhag 2006 {published data only}
- Ullenhag GJ, Spendlove I, Watson NFS, Indar AA, Dube M, Robins RA, et al. A neoadjuvant/adjuvant randomized trial of colorectal cancer patients vaccinated with an anti‐idiotypic antibody, 105AD7, mimicking CD55. Clinical Cancer Research 2006;12(24):7389‐7396. [DOI] [PubMed] [Google Scholar]
Ulsperger 1993 {published data only}
- Ulsperger E, Rainer H, Locker G, Vetterlein M, Schiessel R, Hofbauer F, et al. Adjuvant vaccination in colorectal carcinoma. Annals of the New York Academy of Sciences 1993;690:360. [DOI] [PubMed] [Google Scholar]
Uyl‐de Groot 2005 {published data only}
- Uyl‐de Groot CA, Vermorken JB, G HM, Jr. , Verboom P, Groot MT, Bonsel GJ, et al. Immunotherapy with autologous tumor cell‐BCG vaccine in patients with colon cancer: a prospective study of medical and economic benefits. Vaccine 2005;23(17‐18):2379. [DOI] [PubMed] [Google Scholar]
Vaillant 1998 {published data only}
- Vaillant JC, Nordlinger B, Deuffic S, Arnaud JP, Pelissier E, Jaeck D, Favre JP, Grjean JP, Fourtanier G, Marre P, Letoublon C, Groupe CIPE. Adjuvant intraperitoneal chemotherapy can prevent the recurrence of stage B2 colon cancer 5 year results of a prospective randomized trial. Gastroenterologie Clinique et Biologique 1998;22:286. [Google Scholar]
Van den Eertwegh 200 {published data only}
- Eertwegh AJM, Claessen AME, Tinteren H, Gall HE, Meijer S, Scheper RJ, et al. Adjuvant active specific immunotherapy in stage II or stage III colon carcinoma patients: A prospective randomized trial. [Dutch]. Nederlands Tijdschrift voor Geneeskunde. Vol. 144(6)(pp 274‐279), 2000.Date of Publication: 05 FEB 2000. Nederlands Tijdschrift voor Geneeskunde 2000;144(6):274‐279. [Google Scholar]
Wan 1988 {published data only}
- Wan DS. [Reevaluation of intraluminal fluorouracil chemotherapy as an adjuvant to radical resection of colorectal cancer‐‐results of a randomized trial]. Chung‐Hua Chung Liu Tsa Chih [Chinese Journal of Oncology] 1988;10(5):388. [PubMed] [Google Scholar]
Watanabe 2004 {published data only}
- Watanabe M, Nishida O, Kunii Y, Kodaira S, Takahashi T, Tominaga T, et al. Randomized controlled trial of the efficacy of adjuvant immunochemotherapy and adjuvant chemotherapy for colorectal cancer, using different combinations of the intracutaneous streptococcal preparation OK‐432 and the oral pyrimidines 1‐hexylcarbamoyl‐5‐fluorouracil and uracil/tegafur. International Journal of Clinical Oncology 2004;9(2):98. [DOI] [PubMed] [Google Scholar]
Weber 1993 {published data only}
- Weber W, Laffer U, Metzger U. Adjuvant portal liver infusion with 5‐fluorouracil and mitomycin in colorectal cancer. SAKK. Anticancer Research 1993;13(5C):1839. [PubMed] [Google Scholar]
Weber 1995 {published data only}
- Weber W, Maibach R, Laffer U, Metzger U, Egeli R, Lorenz M, et al. Early hematological toxicity of adjuvant perioperative intraportal and intravenous chemotherapy with fluorouracil, mitomycin and heparin in colorectal cancer. Swiss Group for Clinical Cancer Research. Anticancer Research 1995;15(5B):2197. [PubMed] [Google Scholar]
Wein 2004 {published data only}
- Wein A, Lehnert T, Liersch T, Braumann D, Schaible A, Eckart M, Thiemann R, Teichmann W, Hohenberger W, Hahn EG. Toxicity and safety of weekly high‐dose 5‐FU as 24‐h infusion and folinic acid (AI0 regimen) in adjuvant therapy of UICC stage III colon cancer. InTACT: A multicenter phase III trial [abstract]. Annual Meeting Proceedings of the American Society of Clinical Oncology. 2004:267.
Wereldsma 1990 {published data only}
- Wereldsma JC, Bruggink ED, Meijer WS, Roukema JA, Putten WL. Adjuvant portal liver infusion in colorectal cancer with 5‐fluorouracil/heparin versus urokinase versus control. Results of a prospective randomized clinical trial (colorectal adenocarcinoma trial I). Cancer 1990;65(3):425. [DOI] [PubMed] [Google Scholar]
Wiesenfeld 1995 {published data only}
- Wiesenfeld M, O'Connell MJ, Wieand HS, Gonchoroff NJ, Donohue JH, J FR, Jr. , et al. Controlled clinical trial of interferon‐gamma as postoperative surgical adjuvant therapy for colon cancer. Journal of Clinical Oncology 1995;13(9):2324. [DOI] [PubMed] [Google Scholar]
Wurschmidt 1995 {published data only}
- Wurschmidt F, Baumann M. [Successful use of adjuvant immunotherapy in resected colorectal tumors]. Strahlentherapie und Onkologie 1995;171(3):175. [PubMed] [Google Scholar]
Yamamoto 1998 {published data only}
- Yamamoto T, Matsumoto K, Iriyama K. Potent effects of adjuvant chemotherapy using 5‐fluorouracil + leucovorin on DNA aneuploid colorectal cancer. International Journal of Clinical Oncology 1998;3(3):165‐170. [Google Scholar]
Yanagi 2003 {published data only}
- Yanagi H, Noda M, Yoshikawa R, Ikeuchi H, Gega M, Yamamura T, Kusunoki M. Randomized clinical trial of pharmacokinetic modulating chemotherapy (PMC) in combination with continuous 5 FU infusion plus oral UFT for adjuvant chemotherapy after curative resection for Dukes' C colorectal cancer patients [abstract]. Proceedings of the American Society of Clinical Oncology. 2003.
Yasutomi 1997 {published data only}
- Yasutomi M, Takahashi T, Kodaira S, Hojo K, Kato T, Ogawa M, et al. [Prospective controlled study on the usefulness of Carmofur as a postoperative adjuvant chemotherapy for colorectal cancer]. Gan to Kagaku Ryoho [Japanese Journal of Cancer & Chemotherapy] 1997;24(13):1953. [PubMed] [Google Scholar]
Additional references
Andre 2006
- Andre T, Sargent D, Tabernero J, O'Connell M, Buyse M, Sobrero A, et al. Current issues in adjuvant treatment of stage II colon cancer. Ann Surg Oncol 2006;13(6):887‐98. [DOI] [PubMed] [Google Scholar]
Baxter 2005
- Baxter NN, Virnig DJ, Rothenberger DA, Morris AM, Jessurun J, Virnig BA. Lymph node evaluation in colorectal cancer patients: a population‐based study. J Natl Cancer Inst 2005;97(3):219‐25. [DOI] [PubMed] [Google Scholar]
Berger 2005
- Berger AC, Sigurdson ER, LeVoyer T, Hanlon A, Mayer RJ, Macdonald JS, et al. Colon cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. J Clin Oncol 2005;23(34):8706‐12. [DOI] [PubMed] [Google Scholar]
Browman 1995
- Browman GP, Levine MN, Mohide EA, Hayward RS, Pritchard KI, Gafni A, et al. The practice guidelines development cycle: a conceptual tool for practice guidelines development and implementation. J Clin Oncol 1995;13(2):502‐12. [DOI] [PubMed] [Google Scholar]
Buyse 2001
- Buyse M, Piedbois P. Should Dukes' B patients receive adjuvant therapy? A statistical perspective. Semin Oncol 2001;28(1 Suppl 1):20‐4. [DOI] [PubMed] [Google Scholar]
De Gramont 2007
- Gramont A, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, et al. Oxaliplatin/5FU/LV in adjuvant colon cancer: Updated efficacy results of the MOSAIC trial, including survival, with a median follow‐up of six years. In: Journal of Clinical Oncology. Chicago, 2007 ASCO Annual Meeting Proceedings Part I.
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Feinstein 1985
- Feinstein AR. Clinical epidemiology. Philadelphia: Saunders, 1985. [Google Scholar]
Figueredo 1995
- Figueredo AT, Fawcet SE, Molloy DW, Dobranowski J, Paulseth JE. Disabling encephalopathy during 5‐fluorouracil and levamisole adjuvant therapy for resected colorectal cancer: a report of two cases. Cancer Invest 1995;13(6):608‐11. [DOI] [PubMed] [Google Scholar]
Galandiuk 1992
- Galandiuk S, Wieand HS, Moertel CG, Cha SS, Fitzgibbons RJ, Jr. , Pemberton JH, et al. Patterns of recurrence after curative resection of carcinoma of the colon and rectum. Surg Gynecol Obstet 1992;174(1):27‐32. [PubMed] [Google Scholar]
Grady 2004
- Grady WM. Genomic instability and colon cancer. Cancer Metastasis Rev 2004;23(1‐2):11‐27. [DOI] [PubMed] [Google Scholar]
Gray 1987
- Gray BN, deZwart J, Fisher R, Burns I, Hurley R, Ibister W, et al. In: Adjuvant Therapy of Cancer.. V. Grune & Stratton, 1987:p. 537‐46. [Google Scholar]
Graziano 2003
- Graziano F, Cascinu S. Prognostic molecular markers for planning adjuvant chemotherapy trials in Dukes' B colorectal cancer patients: how much evidence is enough?. Ann Oncol 2003;14(7):1026‐38. [DOI] [PubMed] [Google Scholar]
Halling 1999
- Halling KC, French AJ, McDonnell SK, Burgart LJ, Schaid DJ, Peterson BJ, et al. Microsatellite instability and 8p allelic imbalance in stage B2 and C colorectal cancers. J Natl Cancer Inst 1999;91(15):1295‐303. [DOI] [PubMed] [Google Scholar]
Hase 1998
- Hase K, Ueno H, Kuranaga N, Utsunomiya K, Kanabe S, Mochizuki H. Intraperitoneal exfoliated cancer cells in patients with colorectal cancer. Dis Colon Rectum 1998;41(9):1134‐40. [DOI] [PubMed] [Google Scholar]
Hook 1992
- Hook CC, Kimmel DW, Kvols LK, Scheithauer BW, Forsyth PA, Rubin J, et al. Multifocal inflammatory leukoencephalopathy with 5‐fluorouracil and levamisole. Ann Neurol 1992;31(3):262‐7. [DOI] [PubMed] [Google Scholar]
Kerr 2007
- Kerr DJ, Dunn JA, Langman MJ, Smith JL, Midgley RS, Stanley A, et al. Rofecoxib and cardiovascular adverse events in adjuvant treatment of colorectal cancer. N Engl J Med 2007;357(4):360‐9. [DOI] [PubMed] [Google Scholar]
Midgley 2005
- Midgley R, Kerr DJ. Adjuvant chemotherapy for stage II colorectal cancer: the time is right!. Nat Clin Pract Oncol 2005;2(7):364‐9. [DOI] [PubMed] [Google Scholar]
Monga 2006
- Monga DK, O'Connell MJ. Surgical adjuvant therapy for colorectal cancer: current approaches and future directions. Ann Surg Oncol 2006;13(8):1021‐34. [DOI] [PubMed] [Google Scholar]
Nathanson 1994
- Nathanson SD. Is there a role for clinical prognostic factors in staging patients with colorectal cancer?. Semin Surg Oncol 1994;10(3):176‐82. [DOI] [PubMed] [Google Scholar]
Nauta 1989
- Nauta R, Stablein DM, Holyoke ED. Survival of patients with stage B2 colon carcinoma. The Gastrointestinal Tumor Study Group experience. Arch Surg 1989;124(2):180‐2. [DOI] [PubMed] [Google Scholar]
NIH 2000
- NIH consensus conference. Adjuvant therapy for patients with colon and rectal cancer. Jama 1990;264(11):1444‐50. [PubMed] [Google Scholar]
O'Connell 2004
- O'Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst 2004;96(19):1420‐5. [DOI] [PubMed] [Google Scholar]
Popat 2005
- Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 2005;23(3):609‐18. [DOI] [PubMed] [Google Scholar]
Schlag 1990
- Schlag P, Saeger HD, Friedl P, Kettelhack C, SeBler MJ, Germoth T, et al. Adjuvant Intraportal FUDR‐chemotherapy in colon cancer patients.. In: Adjuvant Therapy of Cancer.. VI. Grune & Stratton, 1990:p. 439‐45. [Google Scholar]
Sinclair 1994
- Sinclair JC, Bracken MB. Clinically useful measures of effect in binary analyses of randomized trials. J Clin Epidemiol 1994;47(8):881‐9. [DOI] [PubMed] [Google Scholar]
Sobrero 2006
- Sobrero A. Should adjuvant chemotherapy become standard treatment for patients with stage II colon cancer? For the proposal. Lancet Oncol 2006;7(6):515‐6. [DOI] [PubMed] [Google Scholar]
Taylor 1985
- Taylor I, Machin D, Mullee M, Trotter G, Cooke T, West C. A randomized controlled trial of adjuvant portal vein cytotoxic perfusion in colorectal cancer. Br J Surg 1985;72(5):359‐63. [DOI] [PubMed] [Google Scholar]


