Abstract
Thrombosis in the circulation system can lead to major myocardial infarction and cardiovascular deaths. Understanding thrombosis formation is necessary for developing safe and effective treatments. In this work, using digital light processing (DLP)-based 3D printing, we fabricated sophisticated in vitro models of blood vessels with internal microchannels that can be used for thrombosis studies. In this regard, photoacoustic microscopy (PAM) offers a unique advantage for label-free visualization of the 3D-printed vessel models, with large penetration depth and functional sensitivity. We compared the imaging performances of two PAM implementations: optical-resolution PAM and acoustic-resolution PAM, and investigated 3D-printed vessel structures with different patterns of microchannels. Our results show that PAM can provide clear microchannel structures at depths up to 3.6 mm. We further quantified the blood oxygenation in the 3D-printed vascular models, showing that thrombi had lower oxygenation than the normal blood. We expect that PAM can find broad applications in 3D printing and bioprinting for in vitro studies of various vascular and other diseases.
Keywords: Photoacoustic imaging, photoacoustic microscopy, bioprinting, digital light processing, blood oxygenation
Introduction
Thrombosis is the principal cause of cardiovascular-related diseases1,2, which may also play a role in the development of cancers3. For example, ischemic stroke, which affects more than 0.7 million Americans each year4, occurs when a thrombus shuts off an artery supplying blood to the brain. About 87% of stroke incidences are ischemic stroke in the brain,5 and most heart attacks are caused by acute arterial thrombosis6. In order to study thrombosis-induced diseases and develop effective treatment regimes, it is highly important to develop biological models that can be used for precisely monitoring the blood clot formation.
Three-dimensional (3D) printing as an emerging technology for biomedical applications, provides the possibility of building vascular constructs with complex geometrical, molecular, and cellular features based on digital files7–10. 3D-printed microchannels allow the emulation of blood vessels, enabling in vitro thrombosis studies. Digital light processing (DLP)-based 3D printing utilizes patterns projected from the digital micromirror device (DMD) or liquid crystal display (LCD) to photopolymerize the liquid ink into volumetric constructs in a layer-by-layer manner7,11–13. Compared with other nozzle-based printing approaches such as those using the extrusion mechanism, DLP-based 3D printing shows superiority in printing resolution at the micrometer scale10,11,13–17. Additionally, DLP-based 3D printing enables a higher printing speed when projecting an entire two-dimensional (2D) pattern each time, instead of the slower point-by-point laser scanning in stereolithography apparatus (SLA)-based printing18. Furthermore, using clinical image data as printing files, DLP-based 3D printing can produce patient-specific scaffolds for tissue modeling, tissue regeneration, and medical devices19–21. Thus, with high resolution, high speed, and rich design flexibility, DLP-based 3D printing has great potential in the rapid fabrication of sophisticated structures such as vascular and thrombosis models.
On the other hand, it is oftentimes necessary to image the internal structures of 3D-printed samples for accurate analyses of structural and functional information. However, the bulky volumes of most printed constructs and the optical properties of the tissue-mimicking hydrogel materials usually result in strong optical scattering and/or absorption, which makes it challenging to use high-resolution optical microscopy that is plagued by the limited imaging depth22,23. For example, the imaging depth of confocal microscopy is limited to 100–200 μm24. Two-photon microscopy improves the imaging depth by using longer excitation wavelengths, which reduces optical attenuation and broadens optical focusing25, but the imaging depth remains at around 1 mm. Different from other optical imaging technologies, photoacoustic microscopy (PAM), a hybrid imaging modality that acoustically detects optical absorption contrast via the photoacoustic (PA) effect, achieves relatively deep penetration with scalable spatial resolutions, because biological tissue is orders of magnitude more transparent to sound than to light26,27. PAM has been widely used for imaging single cells28–30, microvasculature31,32, organs33, brain activities34,35, blood flow36–38, oxygen saturation of hemoglobin (sO2)39, molecular labels40–43 and tumors44. There are two major PAM implementations: i) In optical-resolution PAM (OR-PAM), the excitation is tightly focused, and the penetration depth can reach 1 mm; ii) In acoustic-resolution PAM (AR-PAM), the excitation light is only loosely focused to fulfill the entire acoustic detection volume and the lateral resolution is determined by ultrasonic focusing at depths of a few transports mean free paths45. The penetration depth of AR-PAM can reach 5 mm.
In this work, we have demonstrated that both OR-PAM and AR-PAM are capable of imaging various 3D-printed vascular and thrombosis models. Compared with OR-PAM, AR-PAM can achieve deeper penetration and facilitate better visualization of volumetric structures. PAM can differentiate oxy-hemoglobin (HbO2) and deoxy-hemoglobin (HbR), and thus can quantitatively measure the blood oxygenation of the thrombosis46. Overall, PAM may become a useful tool for studying the morphologies and functions of 3D-printed constructs for thrombosis studies and beyond.
Methods
Synthesizing gelatin methacryloyl (GelMA) and hyaluronic acid methacrylate (HAMA)
GelMA was synthesized according to previously reported method47–50. In brief, gelatin from cold-water fish skin (10 g, Sigma-Aldrich, USA) was dissolved in phosphate-buffered saline (PBS, ThermoFisher, USA; 100 mL) at the concentration of 10 wt.%. Methacrylic anhydride (MA, Sigma-Aldrich; 12 mL) was then added dropwise and was reacted with gelatin solution at 50 °C for 2 h. After diluting the solution 1:1 with warm PBS (40 °C) to stop the reaction, dialysis was conducted for 7 days against deionized (DI) water using dialysis membrane (molecular weight cut off = 12–14 kDa, Spectrum Chemical, USA) at 40 °C to remove low-molecular weight impurities. Subsequently, the solution was lyophilized and stored at −20 °C in dark until use.
The synthesis of HAMA followed the procedure as previously described47. Briefly, 4.0 g of HA (molecular weight = 500 kDa) was fully dissolved in 200 mL of DI water at 4 °C. 133.3 mL of dimethylformamide (DMF, Sigma-Aldrich) and 7.88 mL of MA were added into HA solution under vigorous stirring. The pH of the solution was regulated to pH 8–9 with 1-M sodium hydroxide (Sigma-Aldrich) solution. The reaction was kept at 4 °C under continuous stirring for another 18 h. Subsequently, 0.5-M NaCl (Sigma-Aldrich) was dissolved in the mixture, and the mixture was precipitated in a doubled volume of ethanol (Sigma-Aldrich). HAMA was then collected as white pellets after precipitation. The precipitate was washed with ethanol for 3 times before being dissolved in DI water and the solution was dialyzed against DI water for 5 days. The purified product was obtained by lyophilization and stored at −20 °C until use.”
DLP-based 3D printing system
The in-house DLP-based 3D printer is consisted of a projector (ViewSonic PA503W), a custom-built ink vat, and a programmable, movable building platform14,51. As shown in Figure 1(a), the DMD chip (resolution: 1280 × 800 pixels) inside the projector projects printing patterns with visible light (25 mW cm−2 of light intensity) onto the ink vat. The building platform allows us to obtain the 3D-printed construct via layer-by-layer photopolymerization.
Figure 1. Principles of DLP-based 3D printing and PAM imaging.
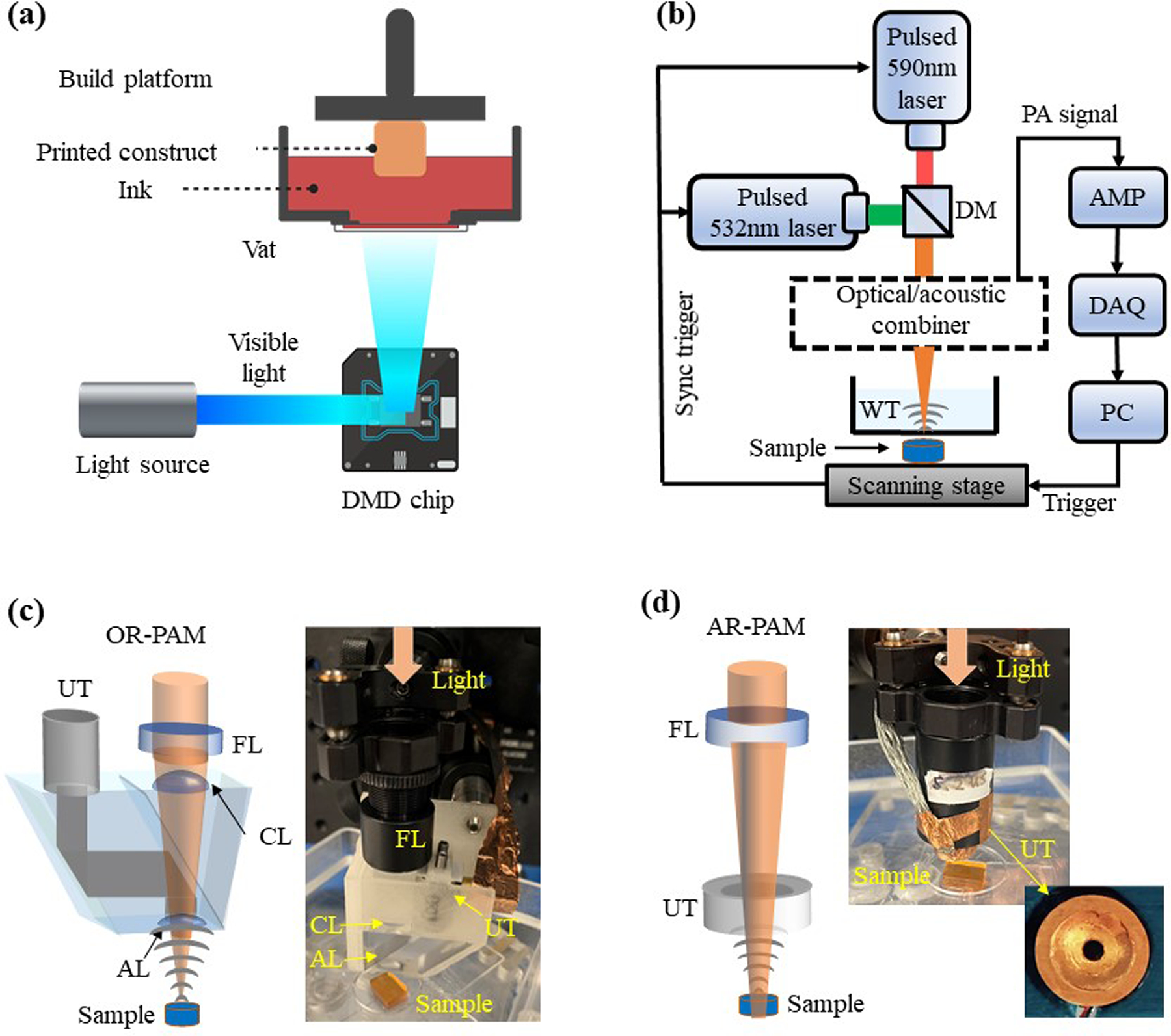
(a) Schematic of the DLP-based 3D-printing system. (b) Schematic of the PAM system with different optical-acoustic beam combining for OR-PAM or AR-PAM. AMP, amplifier; DAQ, data-acquisition; WT, water tank. (c) The optical-acoustic beam combining for OR-PAM, with a silicone oil layer that transmits light and reflects sound. AL, acoustic lens; CL, correction lens; FL, focusing lens; UT, ultrasonic transducer. (d) The optical-acoustic beam combining for AR-PAM, with a ring-shaped ultrasonic transducer.
3D printing methodology
Printing inks were prepared by mixing 40 v/v% poly(ethylene glycol)-diacrylate (PEGDA, molecular weight (Mw) = 575 Da, Sigma-Aldrich), 1-mM/10-mM tris(2,2-bipyridyl) dichlororuthenium (II) hexahydrate (Ru)/sodium persulfate (SPS) (Advanced Biomatrix), and photoabsorber (Ponceau 4R, Sigma-Aldrich). Liquid inks were held in the vat with the Teflon AF 2400 film (50-μm thickness, Random Technologies) at the bottom, which is hydrophobic and oxygen-permeable for decreasing material adhesion52. During printing, the build-platform was lowered to the first printing layer, and the ink was cured by a sequence of patterns at a speed of 10 to 70 μm s−1 and with a layer height of 350 μm. Once the printing procedure was completed, the 3D constructs were carefully removed from the glass slide on the build-platform with a razor blade and washed with phosphate-buffered saline (PBS, Thermo Fisher). We used SolidWorks to design the printing models, sliced them with the open-source DLP slicer (https://formlabs.com/blog/open-source-dlp-slicer/, Formlabs), and finally loaded the sliced PNG images into our DLP-printing software.
PAM
In this study, we adapted our previously developed OR-PAM and AR-PAM systems, shown in Figure 1(b). In OR-PAM, the laser beam was tightly focused into a diffraction-limited spot, and the lateral resolution was primarily determined by the optical focal spot size53. OR-PAM has a limited penetration depth of ~1 mm with a lateral resolution of ~3.7 μm, as shown in Figure S1(a). Figure 1(c) is the schematic of the reflection-mode OR-PAM system. The excitation light was provided by a fiber laser (VPFL-G-20, Spectral Physics) at 532 nm and a dye laser (Credo, Sirah) at 590 nm. The laser beams were combined by a dichroic mirror (DMLP605, Thorlabs), focused by an objective lens, and delivered to the sample surface with a pulse energy of 800nJ at 532 nm and 1.8 μJ at 590 nm. The resultant PA signals were then detected by an ultrasonic transducer with a central frequency of 30 MHz (V214-BB-RM, Olympus-NDT). Figure 1(d) is the schematic of the AR-PAM system, which can achieve a penetration depth beyond the ballistic regime. The light sources were the same as OR-PAM. The laser beams were weakly focused onto the sample surface with a pulse energy of 1.7 μJ at 532 nm and 5.8 μJ at 590 nm. The resultant PA signals were then detected by a customized ring-shaped focused ultrasonic transducer with a central frequency of 20 MHz. The ring-shaped ultrasonic transducer has a central aperture to pass the excitation light. With acoustically defined lateral resolution, both ballistic and scattered photons that reach the target contribute to the final PA signals. The lateral resolution of the AR-PAM system is ~50 μm, as shown in Figure S1(b).
Blood clot preparation
Whole bovine blood was purchased from Lampire Biological Laboratories. 10 v/v% of 0.1-M CaCl2 aqueous solution (Sigma-Aldrich) was added to the blood. The blood was mixed, drained into a centrifuge tube, and sealed for 20 minutes to induce clotting54.
Blood-perfused microchannel with different oxygen concentration
The samples with different oxygen level were made by perfusing the 3D-printed microchannel using the blood with different oxygen concentrations. The whole bovine blood (7200801, Lampire Biological Laboratories) has the highest oxygen concentration, while lower concentration can be obtained by pumping the blood with medical grade nitrogen (NF200, Airgas). In this study, four different blood oxygen levels were made by pumping nitrogen for 10s, 1min, 3 min and 5 min, respectively
Measurement of absorption spectrum
The absorption spectrum of the photoabsorber (Ponceau 4R) was measured in the range of 380 to 740 nm using a microplate reader (SpectraMax M3, Molecular devices).
Results
Fabrication of vascular constructs using DLP-based 3D printing
Using the visible light-based DLP printer, 3D constructs were fabricated with the ink composed of 40 v/v% PEGDA (Mw = 575 Da) and photoinitiator (1-mM/10-mM Ru/SPS), in the presence of photoabsorber (Ponceau 4R). Figure 2(a) illustrates the photo-polymerization process, in which the photons absorbed by the Ru/SPS promote their cleavage, resulting in the generation of free radicals. Ru/SPS, a visible-light photoinitiating system, has been used as an effective and biocompatible photoinitiator in numerous biomedical applications55,56. The free radicals then react with the vinyl bonds in PEGDA to form the hydrogel. PEGDA is arguably the most commonly used synthetic material in DLP-based 3D printing, taking advantage of its good biocompatibility and proper mechanical properties that are needed for shape fidelity in 3D construction11,12,57.
Figure 2. Fabrication of vascular constructs using DLP-based 3D printing.
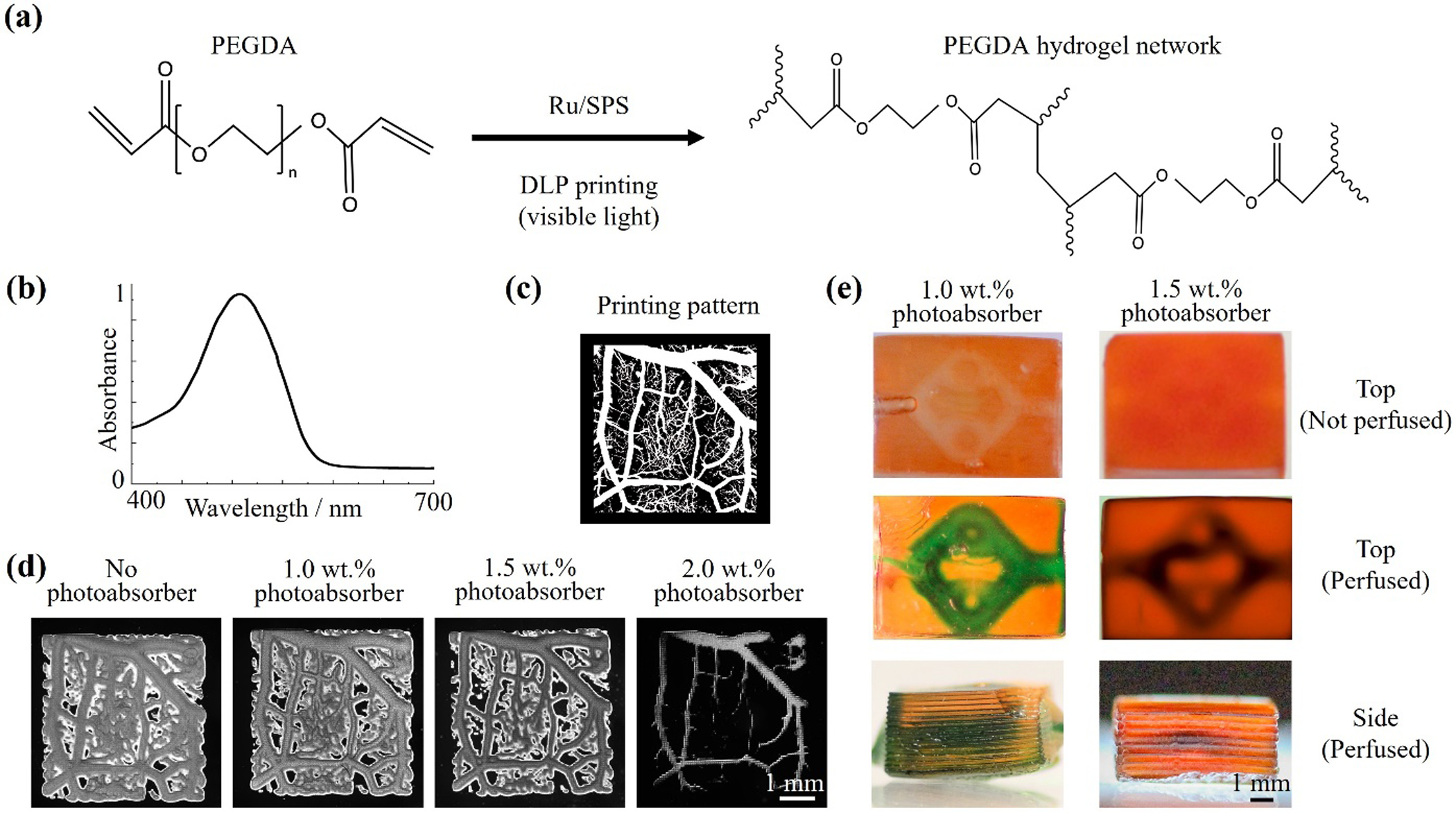
(a) The formation of PEGDA hydrogel through visible-light irradiation in the presence of Ru/SPS as photoinitiator. (b) The absorption spectrum of the photoabsorber (Ponceau 4R). (c) The designed digital printing pattern of a vascular network with branched structures. (d) Micrographs of printed 2D PEGDA samples containing different concentrations of photoabsorber. (e) Photographs of 3D constructs printed with 40 v/v% PEGDA (Mw = 575 Da), 1-mM/10-mM Ru/SPS, and 1.0 wt.% or 1.5 wt.% photoabsorber. The internal microchannels were perfused with a green dye to improve visibility.
In DLP-based printing, except for the pixel size of the projector, the printing resolution also relies on the concentration of the photoabsorber, which limits the light penetration to achieve the desired printing layer thickness12,58. In our printing platform, each pixel could be observed in the printed construct with the size of 60 μm (Figure S2). Additionally, we chose the red food coloring, Ponceau 4R, as the photoabsorber in our visible light-based system because of its high cytocompatibility and strong absorbance in the visible-light range (380–600 nm, Figure 2(b)). The 2D vascular-like structures with 5-μm- to 500-μm-diameter branches were printed using inks containing 40 v/v% PEGDA (Mw = 575 Da), 1-mM/10-mM Ru/SPS, and 0.0 wt.% to 2.0 wt.% Ponceau 4R. As shown in Figure 2(c)–2(d), in the absence of the photoabsorber, the branches smaller than 125 μm could not be identified in the printed pattern, and the main vascular parts presented larger crosslinking areas likely because of the fast diffusion of the chemical species. After adding 1.0 wt.% or 1.5 wt.% photoabsorber into the ink, the printing fidelity was improved. Details of the branches could be observed in the construct printed with the ink containing 1.5 wt.% photoabsorber. However, some vascular branches disappeared when the photoabsorber was further increased to 2.0 wt.% due to insufficient crosslinking. Therefore, the inks with 1.0 wt.% and 1.5 wt.% Ponceau 4R were chosen to fabricate the 3D vascular constructs for subsequent PAM imaging and thrombosis studies.
We printed 3D vascular constructs (8 mm, 6 mm, and 4 mm in length, width, and height, respectively) with multi-vascular architectures with the total printing time of roughly 1 minute each. After perfusing the microchannels with a water-soluble green dye (brilliant green, Sigma-Aldrich), the internal microchannels could be visualized from the photographs captured with a camera (Figures 2(e) and S3). However, the water-soluble dye quickly diffused into the printed hydrogels, leading to the blurred edges of the microchannels observed in the images. To this end, we anticipated that the PAM systems could provide better imaging the 3D-printed constructs and detect the internal geometries.
OR-PAM imaging of the 3D-printed samples with the low photoabsorber concentration
To obtain at high-resolution the structures of the 3D-printed vascular constructs, we first applied OR-PAM to image the samples with 1.0 wt.% photoabsorber, which were relatively transparent and allowed the focused light penetrating deeper. Figure 3(a) shows the designs and photographs of three different printed structures that mimic various blood vessels, including the “Y” structure, the “single-loop” structure, and the “double-loop” structure. Figure 3(b) shows the maximum amplitude projection (MAP) OR-PAM images of the three different patterns with the microchannels perfused by bovine whole blood, acquired at 532 nm. The microchannels inside the construct could be clearly imaged, shown in Figure 3(c). The printed structures with the flat microchannels were clearly resolved. The depths of the printed microchannels, which were 1.6 mm for the “Y” structure, 1.3 mm for the “single-loop” structure, and 1.7 mm for the “double-loop” structure, respectively, could be accurately measured by the side-view MAP images. As shown in Figure 3(d), the depth-coded images matched well with their corresponding side-view MAP images, which further demonstrated the 3D nature of these structures. As shown in the volumetric rendering Videos S1–S3, OR-PAM was able to image not only the 3D-printed vascular microchannel perfused with blood, but also the printed hydrogel constructs due to the presence of the photoabsorber, which could be useful in evaluating the 3D-printing quality.
Figure 3. OR-PAM images of vascular constructs with 1.0 wt.% photoabsorber.
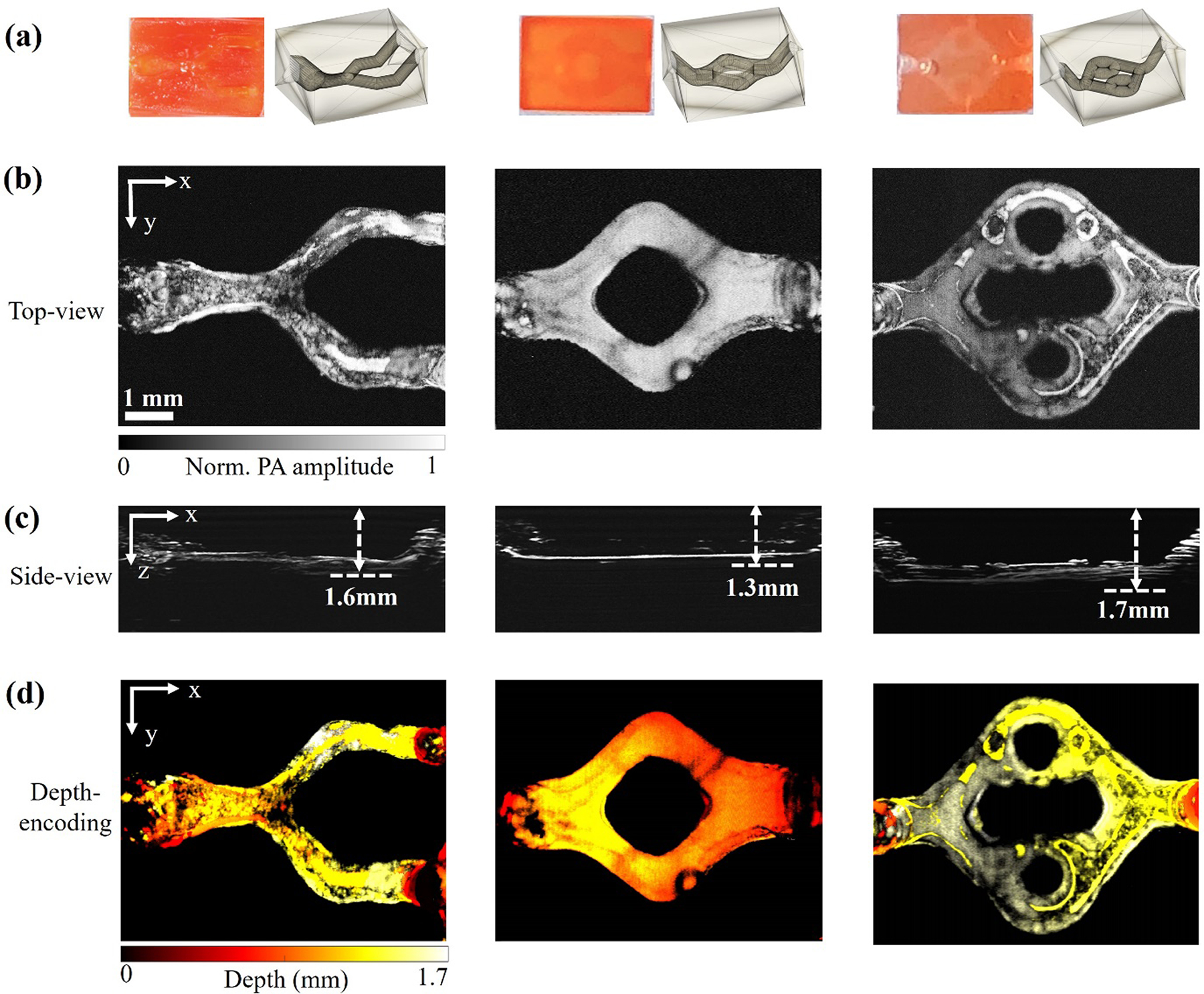
(a) Photographs of three different types of printed 3D structures: Left: “Y-shaped” structure; Middle: “single-loop” structure; Right: “double-loop” structure. (b) Corresponding top-view MAP images of the three constructs. (c) Corresponding side-view MAP images of the three constructs. (d) Corresponding depth-coded images of the three constructs.
AR-PAM imaging of the 3D-printed samples with the high photoabsorber concentration
Compared with 3D-printed samples containing the low-concentration photoabsorber (1.0 wt.%), the higher photoabsorber concentration (1.5 wt.%) could increase the sample’s shape fidelity and is preferred for printing more sophisticated 3D structures. Two 3D-printed constructs both with 1.5 wt.% photoabsorber were initially fabricated for further PAM studies. As shown in Figure 4(a), the samples printed with 1.5 wt.% photoabsorber were darker in color than those containing 1.0 wt.% photoabsorber (Figure 3(a)), and were accordingly more difficult to identify their internal structures perfused by the blood. Because the higher photoabsorber concentration increases both the sample’s absorption and optical scattering, the samples could be effectively imaged by neither traditional optical microscopy, nor OR-PAM, as shown in Figures 4(b) and S4(a). OR-PAM was not able to detect the sample’s depth due to the low signal-to-noise ratio (SNR), as shown in Figure S4(b).
Figure 4. AR-PAM images of vascular constructs printed with 1.5 wt.% photoabsorber.
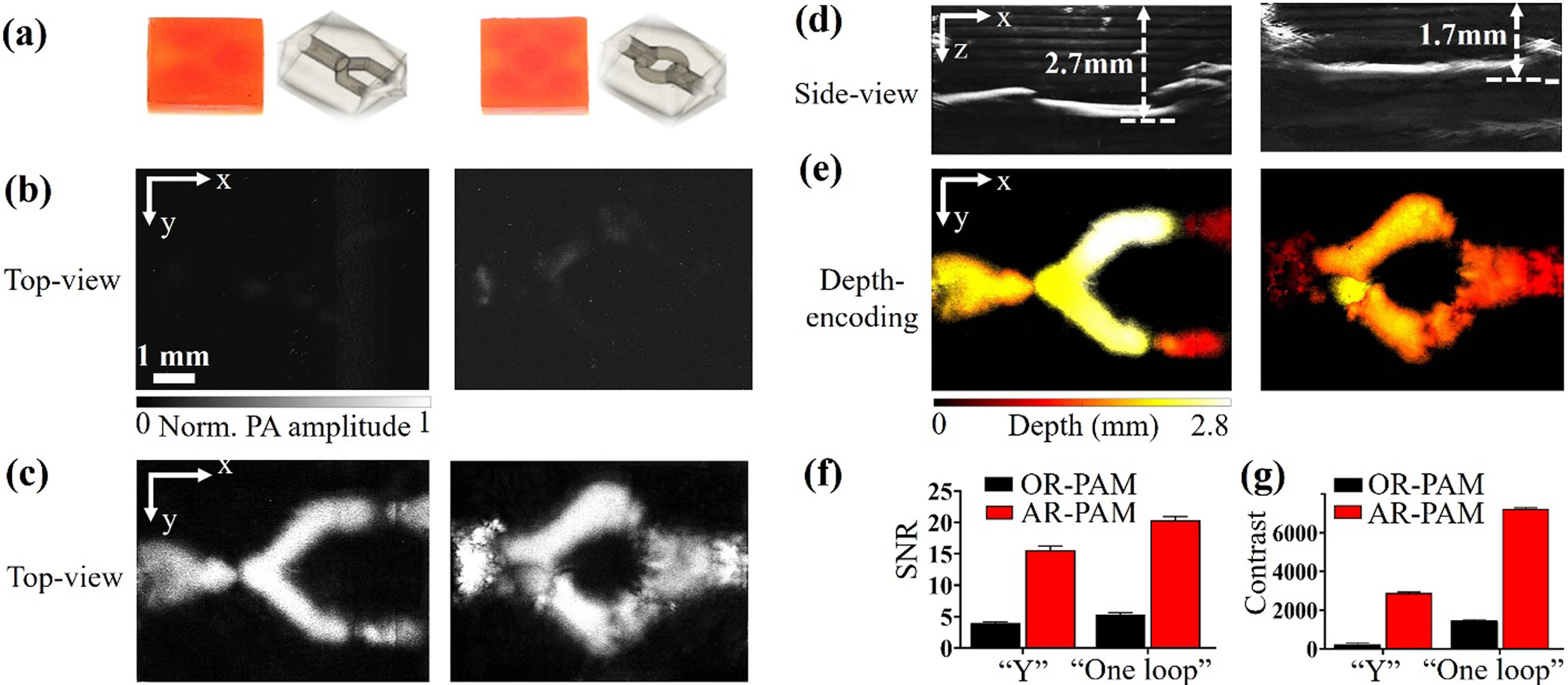
(a) Photographs of two printed structures: Left, “Y-shaped” structure; Right, “single-loop” structure. (b) Corresponding top-view MAP images of the two structures acquired by OR-PAM. (c) Corresponding top-view MAP images of the two structures acquired by AR-PAM. (d) Corresponding side-view MAP images acquired by AR-PAM. (e) Corresponding depth-coded images acquired by AR-PAM. (f) Comparisons of SNRs and (g) contrasts of the structures imaged by OR-PAM and AR-PAM.
To improve the penetration depth, we applied AR-PAM to image these samples printed with 1.5 wt.% photoabsorber, taking advantage of the deep penetration of the acoustic focusing. As expected, the samples with 1.5 wt.% photoabsorber could be clearly imaged by AR-PAM with blood perfusion, shown in Figure 4(c). The internal microchannels were detected at various depths beyond 1 mm, shown in Figure 4(d). The depth-coded images of the two constructs are shown in Figure 4(e), demonstrating a 2.7-mm depth for the “Y-shaped” structure and 1.7-mm depth for the “single-loop” structure. The volumetric renderings of the AR-PAM images are shown in Videos S4–S5.
Comparing the results obtained by OR-PAM and AR-PAM, we found that both the SNR and contrast of the AR-PAM images were substantially higher than those of the OR-PAM images. As shown in Figure 4(f), the SNR of the AR-PAM image was 3.9 times higher than that of the OR-PAM image for the “Y-shaped” structure and 3.8 times higher for the “single-loop” structure. The contrast of the AR-PAM image was 12.8 times higher than that of the OR-PAM image for the “Y-shaped” structure and 4.9 times higher for the “single-loop” structure, as shown in Figure 4(g). These results demonstrated that AR-PAM performed better than OR-PAM in imaging the 3D-printed samples containing higher photoabsorber concentrations.
We further demonstrated the versatility of our PAM system in imaging other 3D objects printed with additional types of biomaterials. GelMA has been widely applied for light‐assisted bioprinting due to the abundant intrinsic bioactive moieties for cell adhesion59. We prepared the ink consisted of 15 wt.% GelMA, 1-mM/10-mM Ru/SPS, and 1.5 wt.% photoabsorber and printed 3D structures at the speed of 20 μm s-1. We also formulated the mixture of GelMA and HAMA as another ink type. As can be seen from Figure S6, the “single-loop” structures were also successfully fabricated using GelMA and GelMA/HAMA-based inks and imaged through AR-PAM, beside the construct printed with PEGDA.
Functional imaging of the 3D-printed thrombosis models
Blood oxygenation is an important indicator of tissue hypoxia and can be used for characterizing the thrombi that typically feature low oxygenation60,61. The oxygen saturation of hemoglobin (sO2) can be computed by using multi-wavelength measurement62. In this work, we used two wavelengths at 532 nm and 590 nm for sO2 measurement on 3D-printed vascular constructs with 1.5 wt.% photoabsorber. We have demonstrated that the AR-PAM is capable of measuring the sO2 change by imaging a printed vascular sample perfused using the blood with different oxygen concentrations. As shown in Figure S5, the change of the oxygen levels can be clearly observed when the average sO2 of the blood samples decreased from 0.93 to 0.55. Figure 5(a) shows the sO2 images of the ‘Y-shaped’ and ‘single-loop’ constructs filled with oxygenated bovine whole blood, acquired by OR-PAM. Again, it was challenging for OR-PAM to provide accurate sO2 measurement if possible at all, due to the limited penetration depth. By contrast, the sO2 images acquired by AR-PAM clearly showed the uniformly high oxygenation level of the internal microchannels, as shown in Figure 5(b).
Figure 5. Comparison of the sO2 images obtained by OR-PAM and AR-PAM on two 3D-printed structures.
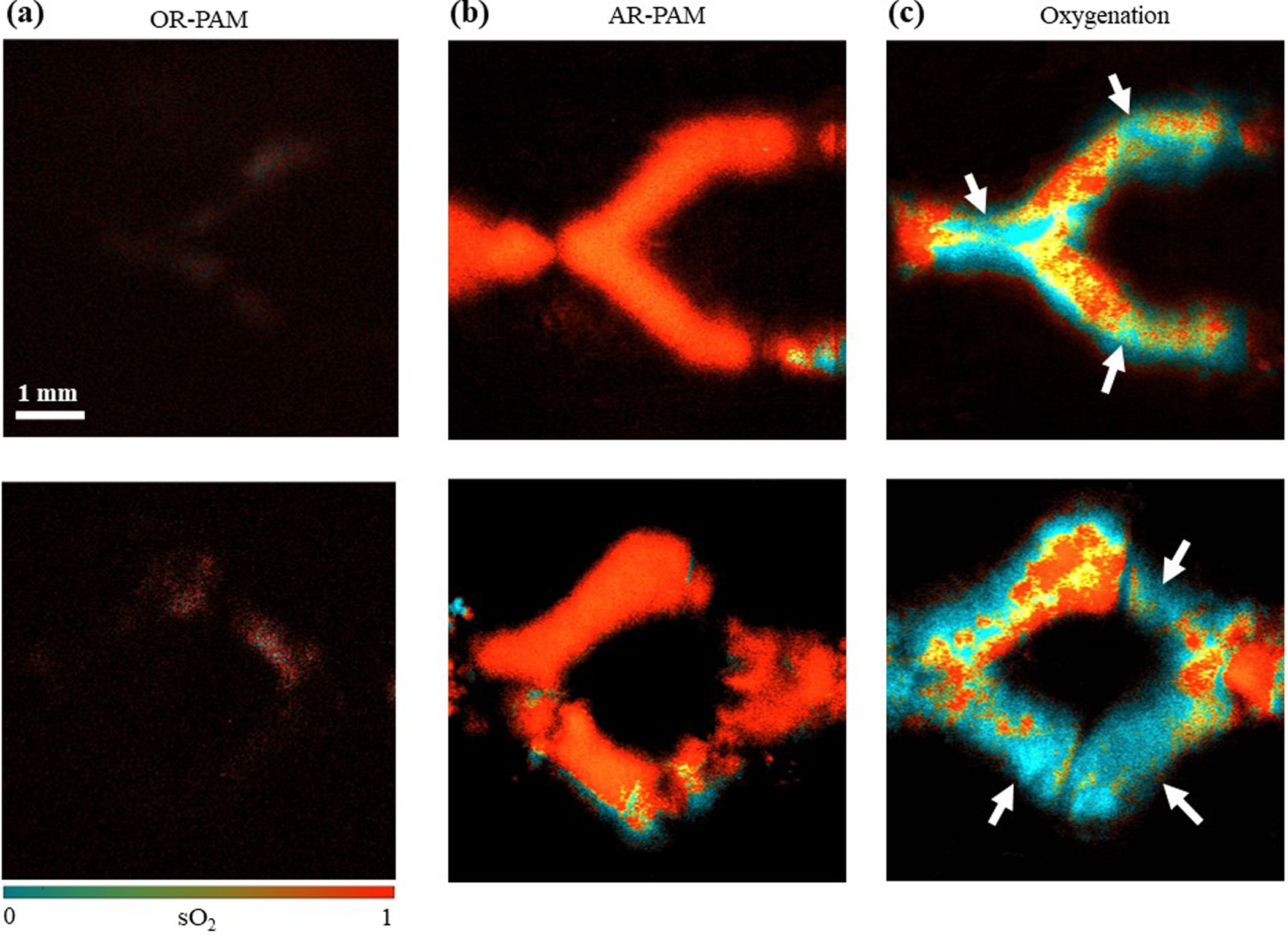
Top row, “Y-shaped” structure; Bottom row, “single-loop” structure. (a) OR-PAM sO2 images of samples perfused with oxygenated blood. (b) AR-PAM sO2 images of the samples perfused with oxygenated blood. (c) AR-PAM sO2 images of the samples filled with thrombosis. The arrows point out the thrombi locations.
To demonstrate the functional imaging capability of AR-PAM on thrombosis models, we perfused the 3D-printed vascular constructs and induced in situ blood clotting within the microchannels and quantified the sO2 in the microchannels. As shown in Figure 5(c), the thrombi had a much lower oxygenation level compared with the normal blood, which is consistent with the previously published results63,64. The arrows pointed out in Figure 5(c) are the thrombi locations. During the blood clot formation, the oxygen content inside the clot is reduced due to the lack of effective oxygen exchange with the surrounding medium13,39,63, which was efficiently reproduced in our 3D-printed vascular models. From Figure 5(c), we could also observe that a thrombus could form in both the relatively flat microchannels and the sloped inlet/outlet, demonstrating the accurate 3D localization provided by AR-PAM.
AR-PAM image of geometrically complex microchannel structures
As discussed above, the 3D constructs printed with 1.5 wt.% photoabsorber exhibit better shape fidelity that can support the production of more complex microchannel structures than those containing lower photoabsorber concentrations. Therefore, we printed a geometrically complex 3D structure featuring embedded dodecahedron-patterned microchannels with the dodecahedron diameter of 3.6 mm, as shown in Figure 6(a). The dodecahedron-patterned microchannels could not be imaged by traditional optical microscopy, but AR-PAM was able to access the entire volume, as shown in Video S6. Figures 6(b) and (c) show the top-view and 3D-view MAP AR-PAM images, respectively, of the dodecahedron-patterned microchannels, demonstrating the deep penetration of 3.6 mm. The sophisticated volumetric structure of the hollow ball was clearly depicted by the depth-coded image, as shown in Figure 6(d). A thrombosis study was also performed on the hollow ball construct. As shown in Figures 5(e)-(f), AR-PAM was able to quantity the sO2 levels over the entire hollow-ball construct, showing that the thrombi formed in the sample had a much lower oxygenation level than the normal blood. These results have strongly suggested that AR-PAM is capable of imaging complex 3D-printed structures otherwise not possible with most conventional imaging tools at sufficient resolution and sensitivity.
Figure 6. AR-PAM of a 3D-printed geometrically complex dodecahedron-patterned microchannel structure.
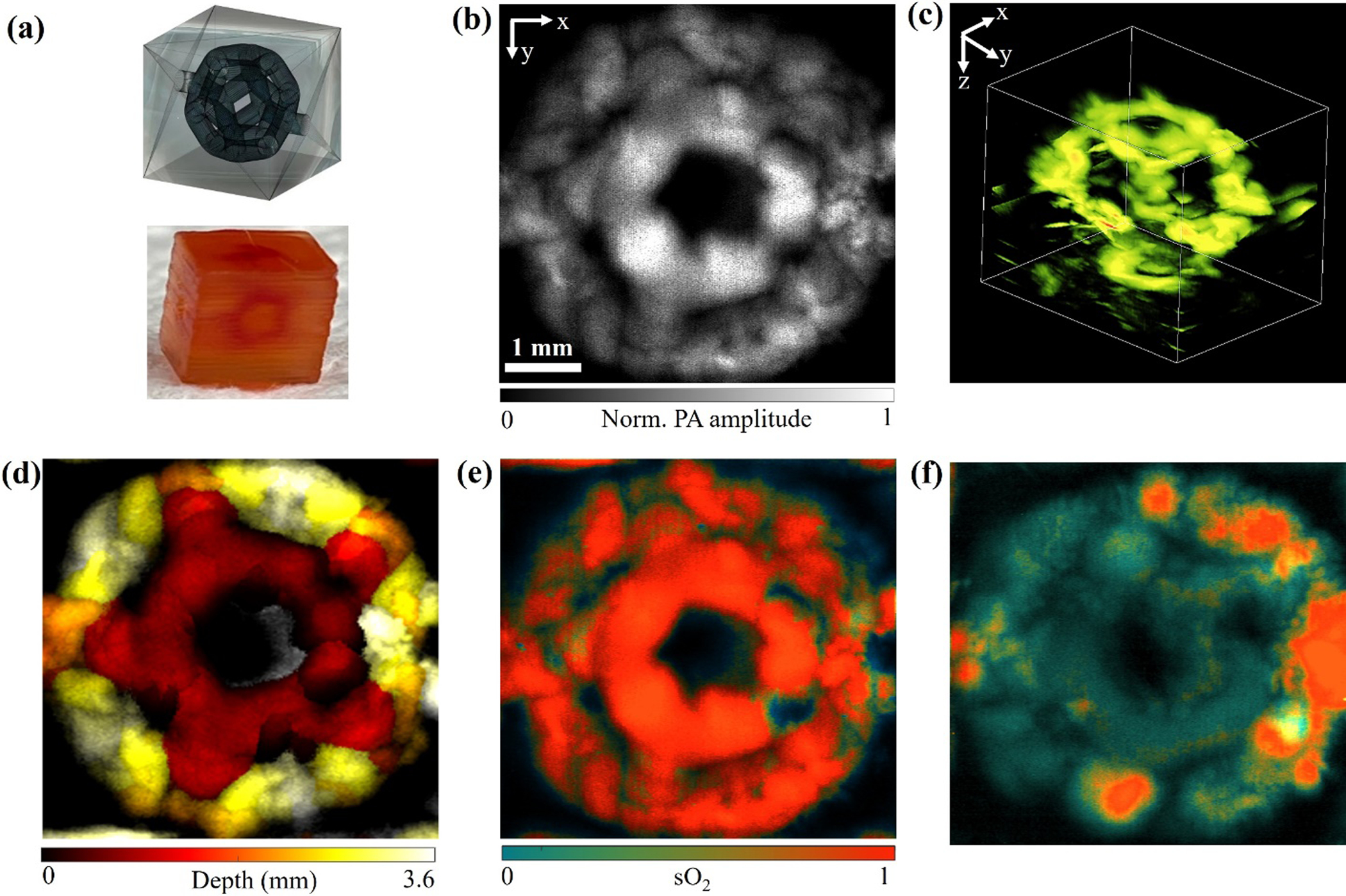
(a) Design and photograph of the geometrically complex dodecahedron-patterned microchannel structure. (b) top-view and (c) 3D-view AR-PAM MAP images of the structure perfused with bovine whole blood. (d) Depth-coded image. (e-f) sO2 images of the structure (e) perfused with oxygenated blood and (f) filled with thrombus.
Discussions and conclusion
Many cardiovascular diseases, such as myocardial infarction and ischemic stroke, are caused by thrombosis5. 3D-printed vascular constructs containing complex microchannel geometries can provide a powerful platform for in vitro thrombosis studies54,65. However, it is difficult for traditional optical imaging to observe the internal structures within bulky hydrogels, mainly because of three reasons: i) the photoabsorber (or other components) used in 3D printing, in particular DLP printing, is typically nonfluorescent and thus cannot be detected by fluorescence imaging; ii) the 3D-printed sample are usually thick, especially when printing more complex structures. The traditional optical imaging cannot image the thick samples because of the limit of the penetration depth even if there exist intrinsic fluorescence contrasts; and iii) more sophisticated structures can be printed by DLP printing with the addition of more photoabsorber, but the photoabsorber will further bring in stronger optical absorption that limit the penetration of ballistic photons. Combining rich optical contrast and deep acoustic penetration, PAM is a highly promising solution.
To the best of our knowledge, this study has demonstrated the first application of PAM in imaging the 3D-printed vascular structures. PAM, especially AR-PAM, can overcome the penetration limit of traditional optical microscopy and image the complex 3D-printed vessel models. Our experimental results have shown that OR-PAM could clearly image the 3D-printed constructs with 1.0 wt.% photoabsorber but not those with 1.5 wt.% photoabsorber, suggesting its limited penetration depth when the absorption of the samples is increased. AR-PAM could image samples with 1.5 wt.% photoabsorber, benefiting from its large penetration depth. Complex structures such as the geometrically complex dodecahedron-patterned microchannels could be imaged by AR-PAM with a penetration depth of at least 3.6 mm. Furthermore, AR-PAM was able to quantify the sO2 levels of deep structures, which is useful for identifying the hypoxic thrombi from the surrounding blood.
There are two major technical limitations in this study. The first limitation is that the imaging speeds of both OR-PAM and AR-PAM systems are relatively slow due to the point-by-point raster scanning mechanism performed with the translational stages. The typical scanning speed is 5 mm/s and it usually takes more than 15 minutes to image one sample in this study. It is not fast enough to monitor the hemodynamics or the formation of the thrombosis in real time. The imaging speed can be improved by developing high-speed AR-PAM system using either the polygon scanner66 or the MEMS scanners67,68. Another limitation is that the lateral resolution decreases with an increased penetration depth. For OR-PAM, the system resolution is determined by the size of the optical focus, which enlarges due to the light diffusion in deep tissue. For AR-PAM, the lateral resolution can be calculated by . Herer NAa is the numerical aperture, which is determined by the geometric structure of the ultrasound transducer, while λa is the wavelength of the detected PA signal, which is inversely proportional to the central frequency of the PA signal. Due to the frequency dependent acoustic attenuation in tissue, the high frequency waves attenuate much faster than the low frequency waves. Therefore, the signal detected from the deeper region has a lower frequency and leads to worse imaging resolution.
It needs to be noted that the minimal blood vessel we could achieved was mainly determined by the resolution of 3D printing method, since the imaging resolutions were higher enough in our study (3.8 μm of OR-PAM and 28 μm for AR-PAM). The resolution of DLP-based 3D printing can be divided into resolutions in x-y and z. In the x-y plane, the resolution is primarily dependent on the pixel size of the DMD projecting the light patterns and the magnification of the projecting optics. The DMD chip (1280 × 800 pixels) inside our DLP printer projected patterns with visible light (25 mW cm−2 of light intensity), and delivered 38-μm resolution onto the ink vat after the magnification of our optical system. Additionally, the stepping motor resolution (5 μm in our printing system) and light penetration distance are the key factors affecting the z resolution. In this study, we chose Ponceau 4R as the photoabsorber due to its good absorption within the visible-light spectrum and the negligible toxicity. It has been demonstrated in our previous study that the reduced curing size and crosslinking thickness were observed in inks with higher concentrations of the photoabsorber (Ponceau 4R), which suggested that Ponceau 4R successfully controlled the optical penetration length for obtaining increased structure precision and higher circularity of channel printing11,50,69. The photoabsorber (Ponceau 4R) concentration applied in our 3D printing was 1.5 wt.%, resulting in the minimum diameter of printed channel to be 0.5 mm. The light penetration depth and channel diameter can be decreased by adding more photoabsorber, but the color from the photoabsorber may influence the imaging process.
In summary, we have demonstrated that PAM may provide a practically useful platform for imaging 3D-printed vascular structures. These vessel models allow us to develop a variety of disease models beyond thrombosis. The functional measurement by PAM in deep and complex structures present a unique opportunity for understanding disease development and even treatment efficacies. Future work includes longitudinal monitoring of the thrombosis formation in the microchannels over time, and multi-spectral analysis of the clot composition (e.g., lipid, fibrin, hemoglobin). We expect that the integration of PAM with 3D printing/bioprinting will likely enable numerous applications in tissue engineering and tissue model engineering, and can be readily expanded to other tissue types.
Supplementary Material
Acknowledgment
We thank Dr. Caroline Connor for editing the manuscript. This work was sponsored by American Heart Association Collaborative Sciences Award (18CSA34080277); Duke Institute of Brain Science Incubator Award; The United States National Institutes of Health (NIH) grants R21EB027981, R21EB027304, R21EB026175, R21EB030257, RF1NS115581 (BRAIN Initiative), R00CA201603, R01NS111039, R01EB028143, UG3TR003274; The United States National Science Foundation (NSF) CBET-EBMS-1936105; Chan Zuckerberg Initiative Grant (2020-226178); Brigham Research Institute.
References
- 1.DeCherney A & Berkowitz G Thrombosis and acute coronary-artery lesions in sudden cardiac ischemic death. N. Engl. J. Med 310, 1137–1140 (1982). [DOI] [PubMed] [Google Scholar]
- 2.Mai C & Hunt D Upper-extremity deep venous thrombosis: A review. Am. J. Med 124, 402–407 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Bick RL Cancer-Associated Thrombosis. N. Engl. J. Med 349, 109–111 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Lloyd-Jones D et al. Heart disease and stroke statistics - 2010 update: A report from the American heart association. Circulation 121, 948–954 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Engelter ST et al. Life-threatening orolingual angioedema during thrombolysis in acute ischemic stroke. J. Neurol 252, 1167–1170 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Mackman N Triggers, targets and treatments for thrombosis. Nature 451, 914–918 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang YS et al. 3D Bioprinting for Tissue and Organ Fabrication. Ann. Biomed. Eng 45, 148–163 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heinrich MA et al. 3D Bioprinting: from Benches to Translational Applications. Small 15, 1–47 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy SV, De Coppi P & Atala A Opportunities and challenges of translational 3D bioprinting. Nat. Biomed. Eng 4, 370–380 (2020). [DOI] [PubMed] [Google Scholar]
- 10.Wang M, Li W, Tang G, Garciamendez-Mijares CE & Zhang YS Engineering (Bio)Materials through Shrinkage and Expansion. Adv. Healthc. Mater 10, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li W et al. A Smartphone-Enabled Portable Digital Light Processing 3D Printer. Adv. Mater 33, 1–10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grigoryan B et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science (80-.). 364, 458–464 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das D & Pramanik M Combined ultrasound and photoacoustic imaging of blood clot during microbubble-assisted sonothrombolysis. J. Biomed. Opt 24, 1 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garciamendez-Mijares CE, Agrawal P, García Martínez G, Cervantes Juarez E & Zhang YS State-of-art affordable bioprinters: A guide for the DiY community. Appl. Phys. Rev 8, 031312 (2021). [Google Scholar]
- 15.Hwang HH, Zhu W, Victorine G, Lawrence N & Chen S 3D-Printing of Functional Biomedical Microdevices via Light- and Extrusion-Based Approaches. Small Methods 2, 1–18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shumer Daniel E, N. J. N. N. P. S. Recent Advances in Formulating and Processing Biomaterial Inks for Vat Polymerization-Based 3D Printing. Physiol. Behav 176, 139–148 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagla madhu. Aqueous Two-Phase Emulsion Bioink-Enabled 3D Bioprinting of Porous Hydrogels. Physiol. Behav 176, 100–106 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melchels FPW, Feijen J & Grijpma DW A review on stereolithography and its applications in biomedical engineering. Biomaterials 31, 6121–6130 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Goyanes A, Det-Amornrat U, Wang J, Basit AW & Gaisford S 3D scanning and 3D printing as innovative technologies for fabricating personalized topical drug delivery systems. J. Control. Release 234, 41–48 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Kinstlinger IS & Miller JS 3D-printed fluidic networks as vasculature for engineered tissue. Lab Chip 16, 2025–2043 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Ye W et al. 3D printing of gelatin methacrylate-based nerve guidance conduits with multiple channels. Mater. Des 192, 108757 (2020). [Google Scholar]
- 22.Zhang YS, Wang LV & Xia Y Seeing Through the Surface: Non-invasive Characterization of Biomaterial–Tissue Interactions Using Photoacoustic Microscopy. Ann. Biomed. Eng 44, 649–666 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang YS & Yao J Imaging Biomaterial–Tissue Interactions. Trends Biotechnol. 36, 403–414 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang LV & Yao J A practical guide to photoacoustic tomography in the life sciences. Nat. Methods 13, 627–638 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mondal PP, Diaspro A, Mondal PP & Diaspro A Multiphoton Fluorescence Microscopy. Fundam. Fluoresc. Microsc 149–159 (2014). [Google Scholar]
- 26.Yao J & Wang LV Photoacoustic microscopy. Laser Photonics Rev. 7, 758–778 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cox B, Laufer JG, Arridge SR & Beard PC Quantitative spectroscopic photoacoustic imaging: a review. J. Biomed. Opt 17, 061202 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y et al. Noninvasive Photoacoustic Microscopy of Living Cells in Two and Three Dimensions through Enhancement by a Metabolite Dye. Angew. Chemie 123, 7497–7501 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang YS et al. Optical-Resolution Photoacoustic Microscopy for Volumetric and Spectral Analysis of Histological and Immunochemical Samples. Angew. Chemie 126, 8237–8241 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strohm EM, Moore MJ & Kolios MC Single Cell Photoacoustic Microscopy: A Review. IEEE J. Sel. Top. Quantum Electron 22, 137–151 (2016). [Google Scholar]
- 31.Zhang HF, Maslov K & Wang LV In vivo imaging of subcutaneous structures using functional photoacoustic microscopy. Nat. Protoc 2, 797–804 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Jeon S et al. In Vivo Photoacoustic Imaging of Anterior Ocular Vasculature: A Random Sample Consensus Approach. Sci. Rep 7, 1–9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong TTW et al. Label-free automated three-dimensional imaging of whole organs by microtomy-assisted photoacoustic microscopy. Nat. Commun 8, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Liao L et al. Imaging brain hemodynamic changes during rat forepaw electrical stimulation using functional photoacoustic microscopy. Neuroimage 52, 562–570 (2010). [DOI] [PubMed] [Google Scholar]
- 35.Stein EW, Maslov K & Wang LV Noninvasive, in vivo imaging of blood-oxygenation dynamics within the mouse brain using photoacoustic microscopy. J. Biomed. Opt 14, 020502 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen S-L, Xie Z, Carson PL, Wang X & Guo LJ In vivo flow speed measurement of capillaries by photoacoustic correlation spectroscopy. Opt. Lett 36, 4017 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheinfeld A & Eyal A Photoacoustic thermal diffusion flowmetry. Biomed. Opt. Express 3, 800 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brunker J & Beard P Velocity measurements in whole blood using acoustic resolution photoacoustic Doppler. Biomed. Opt. Express 7, 2789 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Das D, Sivasubramanian K, Rajendran P & Pramanik M Label-free high frame rate imaging of circulating blood clots using a dual modal ultrasound and photoacoustic system. J. Biophotonics 1–10 (2020). [DOI] [PubMed] [Google Scholar]
- 40.Weber J, Beard PC & Bohndiek SE Contrast agents for molecular photoacoustic imaging. Nat. Methods 13, 639–650 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Nie L et al. Palladium nanosheets as highly stable and effective contrast agents for in vivo photoacoustic molecular imaging. Nanoscale 6, 1271–1276 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Pu K et al. Semiconducting polymer nanoparticles as photoacoustic molecular imaging probes in living mice. Nat. Nanotechnol 9, 233–239 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Homan KA et al. Silver nanoplate contrast agents for in vivo molecular photoacoustic imaging. ACS Nano 6, 641–650 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cai X et al. Multi-scale molecular photoacoustic tomography of gene expression. PLoS One 7, 1–7 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jeon M, Kim J & Kim C Multiplane spectroscopic whole-body photoacoustic imaging of small animals in vivo. Med. Biol. Eng. Comput 54, 283–294 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Maharjan Sushila et al. Symbiotic Photosynthetic Oxygenation within 3D-Bioprinted Vascularized Tissues. Matter 4, 217–240 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gong J et al. Complexation-induced resolution enhancement of 3D-printed hydrogel constructs. Nat. Commun 11, 1–14 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maharjan S et al. Symbiotic photosynthetic oxygenation within 3D-bioprinted vascularized tissues. Matter 4, 217–240 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharifi F et al. A hepatocellular carcinoma–bone metastasis-on-a-chip model for studying thymoquinone-loaded anticancer nanoparticles. Bio-Design Manuf. 3, 189–202 (2020). [Google Scholar]
- 50.Levato R et al. High-resolution lithographic biofabrication of hydrogels with complex microchannels from low-temperature-soluble gelatin bioresins. Mater. Today Bio 12, 100162 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miri AK et al. Microfluidics-Enabled Multimaterial Maskless Stereolithographic Bioprinting. Adv. Mater 30, 1–9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tumbleston JR et al. Continuous liquid interface production of 3D objects. Science (80-.). 347, 1349–1352 (2015). [DOI] [PubMed] [Google Scholar]
- 53.Maslov K, Zhang HF, Hu S & Wang LV Optical-resolution photoacoustic microscopy for in vivo imaging of single capillaries. Opt. Lett 33, 929 (2008). [DOI] [PubMed] [Google Scholar]
- 54.Zhang YS et al. Bioprinted thrombosis-on-a-chip. Lab Chip 16, 4097–4105 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bertlein S et al. Thiol–Ene Clickable Gelatin: A Platform Bioink for Multiple 3D Biofabrication Technologies. Adv. Mater 29, 1–6 (2017). [DOI] [PubMed] [Google Scholar]
- 56.Lim KS et al. Visible Light Cross-Linking of Gelatin Hydrogels Offers an Enhanced Cell Microenvironment with Improved Light Penetration Depth. Macromol. Biosci 19, 1–14 (2019). [DOI] [PubMed] [Google Scholar]
- 57.Mau R, Nazir J, John S & Seitz H Preliminary Study on 3D printing of PEGDA Hydrogels for Frontal Sinus Implants using Digital Light Processing (DLP). Curr. Dir. Biomed. Eng 5, 249–252 (2019). [Google Scholar]
- 58.Ge L, Dong L, Wang D, Ge Q & Gu G A digital light processing 3D printer for fast and high-precision fabrication of soft pneumatic actuators. Sensors Actuators, A Phys. 273, 285–292 (2018). [Google Scholar]
- 59.Ying G, Jiang N, Yu C & Zhang YS Three-dimensional bioprinting of gelatin methacryloyl (GelMA). Bio-Design Manuf. 1, 215–224 (2018). [Google Scholar]
- 60.Hennen SN et al. Photoacoustic tomography imaging and estimation of oxygen saturation of hemoglobin in ocular tissue of rabbits. Exp. Eye Res 138, 153–158 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.R M Leach DFT Oxygen transport — 2. Tissue hypoxia. Clin. Rev 317, 1370–1373 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yao J et al. High-speed label-free functional photoacoustic microscopy of mouse brain in action. Nat. Methods 12, 407–410 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karpiouk AB et al. Combined ultrasound and photoacoustic imaging to detect and stage deep vein thrombosis: phantom and ex vivo studies. J. Biomed. Opt 13, 054061 (2008). [DOI] [PubMed] [Google Scholar]
- 64.Nakase H, Heimann A & Kempski O Alterations of regional cerebral blood flow and oxygen saturation in a rat sinus-vein thrombosis model. Stroke 27, 720–728 (1996). [DOI] [PubMed] [Google Scholar]
- 65.Costa PF et al. Mimicking arterial thrombosis in a 3D-printed microfluidic: In vitro vascular model based on computed tomography angiography data. Lab Chip 17, 2785–2792 (2017). [DOI] [PubMed] [Google Scholar]
- 66.Lan B et al. High-speed widefield photoacoustic microscopy of small-animal hemodynamics. Biomed. Opt. Express 9, 4689 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen M et al. High-speed functional photoacoustic microscopy using a water-immersible two-axis torsion-bending scanner. Photoacoustics 100309 (2021) doi: 10.1016/j.pacs.2021.100309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yao J et al. Wide-field fast-scanning photoacoustic microscopy based on a water-immersible MEMS scanning mirror. J. Biomed. Opt 17, 1 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang M et al. Digital Light Processing Based Bioprinting with Composable Gradients. Adv. Mater 2107038, 2107038 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


