Highlights
-
•
CXB appears to be an efficacious technique for rectal cancer treatment and may allow rectal preservation in selected patients.
-
•
These GEC ESTRO ACROP recommendations recommend dose schemes in for rectal CXB.
-
•
These recommendations advise reporting of tumour depth to enable future refinement of dose prescription and target definition.
-
•
The routine collection and publication of outcome data including patient reported outcomes (PROs) is recommended.
Keywords: GEC ESTRO, Groupe Européen de CuriethérapieEuropean Society for Radiotherapy and Oncology; CXB, Contact X-ray brachytherapy; EBRT, External beam radiotherapy; TME, Total mesorectal excision; cCR, complete clinical response; kV, Kilovoltage; TEMS, Transanal endoscopic microsurgery; GTN, Glyceryl-trinitrate; GTV, Gross tumour volume; CTV, Clinical target volume; PTV, Planning target volume; MRI, Magnetic resonance imaging; US, Ultrasound; NTCP, Normal tissue complication probability; ACROP, Advisory Committee for Radiation Oncology Practice
Keywords: Consensus recommendations, Contact X-Ray brachytherapy, Papillon treatment, Rectal cancer
Abstract
Purpose
To issue consensus recommendations for contact X-Ray brachytherapy (CXB) for rectal cancer covering pre-treatment evaluation, treatment, dosimetric issues and follow-up. These recommendations cover CXB in the definitive and palliative setting.
Methods
Members of GEC ESTRO with expertise in rectal CXB issued consensus-based recommendations for CXB based on literature review and clinical experience. Levels of evidence according to the Oxford Centre for Evidence based medicine guidance are presented where possible.
Results
The GEC ESTRO ACROP consensus recommendations support the use of CXB to increase the chances of clinical complete remission and cure for patients who are elderly with high surgical risk, surgically unfit or refusing surgery. For palliative treatment, the use of CXB is recommended for symptomatic relief and disease control. The use of CXB in an organ-preservation setting in surgically fit patients is recommended within the setting of a clinical trial or registry.
Conclusions
The GEC ESTRO ACROP recommendations for CXB are provided. Recommendations towards standardisation of reporting and prescription are given. Practitioners are encouraged to follow these recommendations and to develop further clinical trials to examine this treatment modality and increase the evidence base for its use. The routine collection of outcomes both clinical and patient-reported is also encouraged.
Aim
A steering committee was convened to develop these recommendations. Members of the Groupe Européen de Curiethérapie European Society for Radiotherapy and Oncology (GEC ESTRO) with expertise in rectal brachytherapy were invited to join a panel to review recommendations and provide feedback. Recommendations were based on literature review and clinical experience. Feedback was provided by consensus review of recommendations and group discussion. Majority consensus was met if 90% or more of the group agreed with the recommendation. Where applicable, level of evidence as per the Oxford Centre for Evidence Based Medicine is given [1], see Appendix 1. Voting took place in one meeting hosted by GEC ESTRO and any areas not achieving majority consensus were further discussed by electronic means. The guidelines were reviewed and approved by the ESTRO ACROP (Advisory Committee for Radiation Oncology Practice) .
The aim of these recommendations is to develop guidance for referring and treating practitioners for the assessment, treatment and follow-up of patients receiving contact X-Ray brachytherapy (CXB) for rectal cancer and to start to standardise dose reporting for future outcome comparison.
Introduction
Rectal cancer is a leading cause of cancer morbidity and mortality worldwide. In 2020 colorectal cancer was the fourth most common malignancy worldwide [2]. It’s likely that approximately a third of cases were rectal cancer since in 2010–2012 in the UK rectal cancer comprised 32% of colorectal cancer cases in men and 23% in women [3]. In 2016–2018 43% of colorectal cancer cases occurred in patients aged 75 or over [4]. Rectal cancer can be treated with a variety of modalities. Surgery is the mainstay of treatment with chemotherapy and neo-adjuvant external beam radiotherapy (EBRT) traditionally used for more advanced disease. However, surgery can result in formation of a permanent stoma and the risks of postoperative morbidity and mortality rise with advancing age [5]. Therefore, there is increasing interest in developing alternative methods of disease control that decrease the risks of morbidity and mortality associated with surgery, especially in an elderly population. There are few randomised studies comparing organ preservation to rectal excision. Five year results from the GRECCAR 2 study which 148 randomised patients with a good clinical response from neo-adjuvant chemo-radiotherapy (residual tumour ≤ 2 cm) to local excision or total mesorectal excision (TME) have shown that there was no difference in oncologic outcomes between the techniques [6]. There were 5 local recurrences in each group, all of which were endoluminal with no pelvic lymph node recurrence, importantly one local recurrence occurred in the 5th year of follow up emphasising the important of long-term surveillance. Rectal preservation strategies utilising radiotherapy without surgery have also been described and non-randomised studies appear to show similar results to surgical series for disease-free and overall survival, with slightly higher local recurrence (often termed regrowth in the non-operative setting) [7], [8]. However, the optimal dose of radiotherapy required to achieve complete clinical response (cCR) is unknown. Consequently, different techniques have been used to escalate the dose of radiation delivered to the tumour, CXB is one of these.
Brachytherapy is the delivery of radiotherapy in very close proximity to a tumour. Due to its very steep dose gradient, brachytherapy enables the delivery of a very high dose to the tumour with a simultaneous decrease in the volume of normal tissue irradiated compared to conventional EBRT. Therefore brachytherapy can increase treatment efficacy [9] while keeping toxicity in check [10]. As such, brachytherapy has been used successfully for curative organ sparing treatment of rectal cancer either alone for limited T1 tumours, or in combination with EBRT +/- concurrent chemotherapy for more advanced disease. Rectal cancer patients can be divided into three groups: group 1; Resectable tumour and surgically fit, group 2; resectable tumour and not surgically fit, group 3; unresectable tumour or palliative. The justification and choice of brachytherapy modality depends on which of these groups the patient falls into and the individual characteristics of the tumour. As well as the potential efficacy of the technique, the potential toxicity must also be considered [11]. The choice of technique may also vary according to local equipment availability.
CXB was first used in Germany then France and was popularized by Professor Papillon in Lyon [12], thus is often called the Papillon technique. Since the inception of the technique, technology has improved and the majority of modern series use a dedicated 50 kV X-ray tube with high voltage generator (Ariane, Derby, UK). A further rectal CXB machine delivering radiation via a miniature 50 kV X-Ray source is under investigation (Axxent, Xoft iCAD, San Jose, California, USA). Since both machines utilise 50 kV, dosimetry and dose fall-off for the miniature source is similar to the X-ray tube though treatment times are longer (dose delivery is 2.1 Gy/minute compared to 10–12 Gy/minute) [13]. A new applicator has been developed for use with the Axxent device with lateral windows and the ability to visualise the tumour and allow quality control monitoring during treatment. The applicator modifications aim to allow all patients to be treated in the supine position and shorten the treatment time to 2 min. The Whistle study NCT 04336202 will examine clinical outcomes using the miniature X-Ray source technique with the newer applicator in combination with EBRT in 45 non-operable elderly patients [14]. A HDR after-loaded applicator (Varian, Palo Alto, USA) with a dose profile similar to 50 kV X-rays is in development but has not yet been used clinically [15], [16]. This applicator has a slanted applicator edge for easier placement and Monte Carlo modelling shows similar dosimetry and dose fall off to 50 kV X-Rays with slightly longer treatment times (e.g. under 9 min for a 20 mm applicator).
Since there is no published clinical evidence on the Xoft or the HDR afterloader CXB techniques yet, these recommendations will focus on the use of 50 kV X-Rays delivered directly onto the rectal mucosa using a 50 kV X-Ray tube.
Use of CXB has been described for potentially operable patients as part of an active surveillance organ preservation technique or for patients who are not medically fit for surgery or refuse surgery. An international group, ICONE (International COntact radiotherapy NEtwork), collaborates over techniques, outcomes, data collection and development of randomised trials to examine the technique. In France, Haute Autorité de Santé (HAS) certified CXB as an efficient treatment for rectal cancer stages T1-3, N0 in October 2008 [17]. In the UK, the National Institute for Health and Care Excellence (NICE) assessed the use of CXB for early rectal cancer in September 2015 and issued recommendations for its use in medically operable and inoperable populations [18]. NICE only recommends CXB within clinical trials for patients with locally advanced rectal cancer [19].
It is important to consider an organ-preserving approach from the outset when choosing a patient’s initial treatment strategy so that appropriate EBRT treatment target volumes can be selected. For patients who are surgically fit, it should be documented that CXB is being undertaken as an organ sparing approach and the patient is aware that local regrowth rates may be higher than TME and that surgery will still be recommended in cases of incomplete response or local regrowth. It is recommended that these patients who are surgically fit should be treated either within a clinical trial or in a registry with a well-defined follow-up protocol in line with international standards [20]. The patient should also be aware that since the rate of local regrowth in the rectum and mesorectum may be higher following organ preservation with radiotherapy alone than with TME more intensive surveillance is required following CXB than after TME surgery so that early surgical intervention can be instituted if there is regrowth. It is also important to explain that surgery may be more complex several years after pelvic EBRT due to the development of fibrosis, risking greater morbidity.
Patient selection
Age and co-morbidity are important considerations when selecting an organ-preservation approach. In young patients undergoing any pelvic radiotherapy the risks of infertility or second cancer [21] may be higher and must therefore be taken into consideration when considering an organ-preservation approach. In very old patients, age may be a factor, Faiz et al. demonstrated that patients over 90 have a 10% higher one-year mortality following surgery for distal bowel cancers than proximal tumours [5]. Smith et al. performed decision modelling which demonstrated the organ preserving techniques may offer a 10% survival benefit in patients over 80 [22]. Therefore strategies avoiding major pelvic surgery may be preferable in very old patients. Allowing the patient to be fully involved in a shared decision making process is paramount. Further research into patient reported outcomes is essential to aiding this process.
Table 1 demonstrates the selection criteria for surgically fit patients with low rectal cancer who wish to have CXB as part of an organ-preserving treatment. These criteria have been developed using clinical experience and expert opinion. For surgically unfit or very old patients with early T1-T2 rectal cancers the criteria can be slightly wider, particularly for histopathologic parameters. Patients with larger cancers must have a good response to EBRT to be considered for CXB boost, as the residual tumour must fit within the maximal size of the CXB treatment applicator, which is 3 cm. Patients who refuse surgery can also be considered for CXB even if they do not fulfil all of the selection criteria in Table 1 since it is likely to offer improved sphincter preservation than EBRT alone, level 1b [23].
Table 1.
Inclusion and Exclusion criteria for definitive local CXB treatment in surgically fit patients.
| Inclusion | |
| 1. | Mobile non-ulcerative exophytic tumour < 10 cm from anal verge (due to applicator length) |
| 2. | Tumour < 3 cm at the time of CXB (due to applicator size) |
| 3. | Clinically and radiologically staged T1 or 2 or 3a/N0/M0 (unanimous consensus). T3b or N1 (limited) with good downstaging following EBRT (majority consensus) |
| 4. | Well/moderately differentiated tumour (unanimous consensus), poorly differentiated (minority consensus to include these patients) |
| 5. | No lymphovascular or venous invasion (majority consensus) |
| Exclusion | |
| 1. | Mucinous tumours |
| 2. | Tumour within the anal canal |
| 3. | Patients not wanting follow-up |
| 4. | Anterior tumour following TEMS surgery in women (potentially higher fistula risk)* |
| 5. | Patients who cannot undergo MRI surveillance** |
*relative contraindication.
**relative contraindication for older patients who could undergo CT and endorectal ultrasound imaging surveillance instead.
Patients that have undergone excision of malignant polyps can also be offered CXB +/- EBRT to decrease the risk of local regrowth, level 2b [24]. Patients with low-risk (see Table 2) incompletely excised or close margins pT1 tumours following local excision (R1 resection) can have CXB to the tumour site if they are not fit for further surgery. EBRT to the mesorectum only can be considered depending on the calculated lymph node risk [25], tumour location in the rectum and patient fitness. Patients with pT1 SM2 or above or pT2 tumours following local excision can have CXB to the excision site combined with EBRT +/- chemotherapy, depending on patient fitness, if they are either surgically unfit or if they wish to pursue an organ-preserving approach when completion surgery would otherwise require permanent stoma formation [24].
Table 2.
Low risk features following T1 tumour local excision.
| 1. | Well/moderately differentiated adenocarcinoma |
| 2. | No Lympho -vascular invasion |
| 3. | Tumour size < 3 cm |
| 4. | Resection margin ≥ 1 mm |
| 5. | Depth of penetration Kikuchi stage sm1 |
Clinical evaluation and practical aspects
All patients should undergo an MRI scan of the pelvis for full staging unless it is contra-indicated. Endorectal ultrasound scans are very helpful, particularly for differentiating between T1 or T2 disease. A CT scan of the chest and abdomen should be performed to stage the cancer outside the pelvis. Ideally prior to offering treatment to the patient, the imaging and histopathology of each patient should be discussed in a multi-disciplinary setting with a specialist radiologist, clinical/radiation oncologist and colorectal surgeon present. Other members may include a histopathologist and clinical nurse specialist or similar patient advocate. A geriatric medical assessment may be beneficial in the elderly. Determination of surgical fitness should follow recognised local and national recommendations with high-risk anaesthetic assessment if required. The choice of treatment should depend on the therapeutic goal and should be carefully evaluated against possible alternatives regarding safety, toxicity and efficacy, as well as the potentially invasive nature of the procedure. If a patient is surgically fit and brachytherapy is being considered as part of an organ preservation approach, the patient should have the opportunity to discuss treatment options and possible toxicities with both the surgeon and the clinical/radiation oncologist to ensure that they are aware of all available options.
At the time of CXB, it is helpful to review the original diagnostic endoscopy report and imaging. The endoscopist may tattoo to aid applicator positioning e.g. small dots in four corners around the lesion, especially if the tumour is > 3 cm at presentation and EBRT will be used prior to CXB. If the patient is on aspirin or anti-coagulation treatment, consideration can be given to stopping it before the procedure. Guidance regarding endoscopy in patients on antiplatelet or anticoagulant therapy may aid decision making in this area [26]. Patients requiring long-term anti-coagulation should be counselled that there is a higher risk of the late complication of bleeding following brachytherapy treatment. It is acceptable to treat the patient without any previous dietary preparation.
Timing of CXB treatment
CXB is a superficial radiotherapy treatment. The dose is prescribed at the applicator surface. The dose falls off rapidly and is approximately 60% at 0.5 cm and 40% at 1 cm (with slight variation according to applicator size) [27], [28]. Exophytic tumour which extends inside the applicator will receive a proportionally higher dose, this is difficult to quantify due to the steep dose gradient close to the X-Ray target. Therefore patients whose tumours extend deeper into the rectal wall (T2 and above) will also need EBRT to treat the deep aspects of the tumour. The EBRT should cover the mesorectum to cover the risk of microscopic nodal metastases and other nodal volumes at risk of metastases dependant on tumour staging.
If EBRT has been used as initial treatment, a break of two to six weeks between end of EBRT treatment and CXB has sometimes been used, especially in historical series. The OPERA trial used a 2–3 week interval between CXB and EBRT. A shorter break (or no break at all) may theoretically be preferable to minimise the risks of tumour repopulation. Patient comfort or tumour size may in some cases necessitate a slightly longer interval allowing resolution of EBRT toxicity or tumour shrinkage. There is no data available on what the optimal overall treatment time should be or the magnitude of the detrimental effect if this interval is exceeded [9]. MRI should be performed following EBRT and prior to brachytherapy to assess response in tumours which were bulky at presentation (T3B or above) to ensure that the post EBRT tumour thickness is suitable for the depth dose profile of CXB. Most practitioners perform this MRI response assessment between 3 and 7 weeks after the EBRT, with brachytherapy performed soon after the MRI. Ideally the residual tumour should extend <5 mm deep to the mucosal surface at the time of CXB due to the relatively superficial depth penetration of the 50 kV X-Rays.
Practical aspects of the CXB procedure
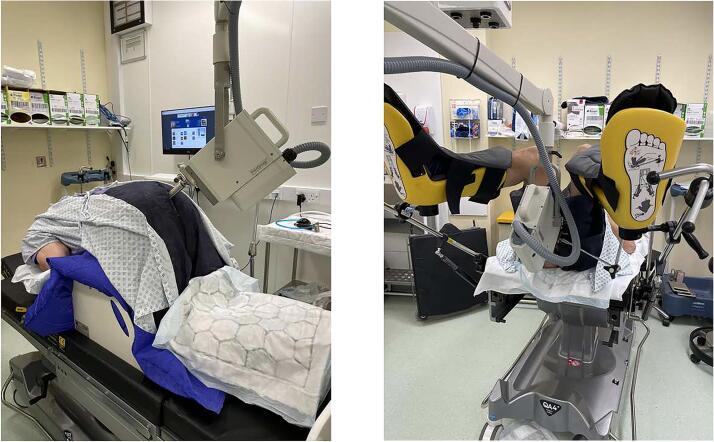
On arrival, the patient will receive an enema and empty their bowels. Glyceryl-trinitrate (GTN) ointment can be applied around the anal sphincter at the time of enema insertion if it is not medically contra-indicated. Topical lidocaine 2% gel can be inserted into the ano-rectum prior to applicator insertion for additional pain relief. A variety of treatment positions can be used for rectal brachytherapy. Use of the ‘knee-chest’ prone position for CXB, see Fig. 1, causes the sphincter to relax and the rectum to open giving optimal views possibly with less patient discomfort. However, this may not be ideal for posterior tumours or may not be possible for elderly, frail patients. In this instance, the lithotomy position can be used. Occasionally a lateral lying position can be used. Most institutions do not routinely use sedation for rectal brachytherapy, though use of inhaled nitrous oxide, intravenous sedation or general anaesthesia can be considered in patients who experience discomfort and are medically fit. Following the procedure, the patient may have postural hypotension, especially if they have been prone for treatment, so they must rise slowly and with support.
Fig. 1.
1A-Photograph to demonstrate a patient in the ‘knee-chest’ position for CXB. 1B-Photograph to demonstrate a patient in the lithotomy position receiving CXB.
Immediately prior to treatment, an assessment is made of the tumour or scar using digital palpation of the rectum and rigid rectoscopy. An applicator of the appropriate size is selected. Clinical experience has shown that the applicator should encompass the tumour with an approximately 2 mm margin, level 5. This often results in applicator size decreasing with subsequent fractions. Verrijssen et al. demonstrated on excised tumour specimens that 95% of the tumour cells lie within a 5.5 mm margin of the macroscopic visible tumour [29]. Overall, 80% of tumours had no surrounding microscopic tumour cells. This information should be taken into account when selecting an applicator to cover visible disease residual. If no tumour is visible, the applicator should be selected to cover the scar or site of original tumour. Local anaesthetic gel is placed around the rectum and on the applicator. The treatment applicator is gently introduced into the rectum. The central obturator is removed from the tube. The applicator position is checked by direct visualisation and the applicator is positioned over the tumour/scar. If there is bleeding which impedes views, topical application of tranexamic acid may be helpful. The applicator is clamped into place. A pre-treatment photograph is taken using the external camera. The treatment tube is gently inserted. An independent check is made of the time required for treatment delivery and the treatment is administered. It is important to watch the patient carefully during the treatment and interrupt treatment if applicator movement is detected. If the applicator or tumour moves during the treatment it may be appropriate to administer a 4th fraction at lower dose, dependant on tumour response by fraction 3, evidence level 5. A maximal CXB dose of 110 Gy over 4 fractions is considered safe [30]. Using a 50 kV X-Ray tube, it may be safe to occasionally manually hold the treatment applicator in place during treatment if the physician uses appropriate radiation protection measures and monitors cumulative radiation dose to ensure that annual limits are not exceeded and that local radiation protection procedures are followed.
After treatment tube removal, a post-treatment photograph is taken using the external camera.
Target volume definitions and CXB dose schemes
At the time of CXB, the gross tumour volume (GTV) comprises the visible rectal tumour. Any residual ulcer will also be considered GTV as it is likely to contain tumour cells. The clinical target volume (CTV) is defined as the GTV plus a margin of up to 5.5 mm (historically a minimum of 2 mm margin has been used in most series) or the post-operative scar in the setting of previous tumour excision. If the scar is very large the histologic margins can be subtracted from the scar to ensure coverage. A margin does not need to be added for planning target volume (PTV) since the tumour is directly visualised throughout. CXB dose is prescribed at the exit applicator surface (all doses described in these guidelines are at the exit applicator surface). Dose prescription is not adapted to parameters such as tumour thickness and therefore a minimal tumour dose cannot currently be described. Due to the inability to image at the time of applicator placement, the doses to the deeper aspects of the tumour or structures at risk of toxicity, such as vascular plexus, cannot currently be determined. This is an area that would benefit from future research. It is recommended to report the tumour thickness at the start of treatment as determined by MRI and/or endorectal US, if possible noting what component is exophytic from the mucosal surface.
Quality control for contact brachytherapy should be as per published guidance [31] and supported by appropriate external audit [32]. If the applicator is not fully applied to the mucosa but is at an oblique angle, it must be recognised that some of the tumour will receive a lower total dose than the prescription dose, the prescription dose is not changed in this instance. As definitive treatment, a variety of dose schemes have been employed over time. When 60 Gy in 2 fractions was used in combination with EBRT as non-operative treatment of rectal cancer, higher rates of local regrowth were seen than in published studies which used higher CXB doses [33], thus higher doses are recommended in these guidelines, level 2a. A variety of doses have been employed as a boost to pelvic EBRT. Table 4 presents results of modern retrospective series using a median dose of at least 80 Gy in 3 fractions as a boost dose before or after pelvic EBRT. In general, fractions are administered at two weekly intervals to allow time for response assessment and resolution of normal tissue toxicity between fractions.
Table 4.
Table to present recent results of Contact X-Ray Brachytherapy (CXB) combined with external beam radiotherapy (EBRT) for rectal adenocarcinoma.
| Name, year | Recruitment period | Total no. of patients reported (no. receiving CXB alone) | EBRT dose | CXB dose | Response rates | Toxicity |
|---|---|---|---|---|---|---|
| Gérard, 2002 [49] | 1986–1998 | 63 | 39 Gy/13F | 35–140 Gy/1-4F (mean 80 Gy/3F) | cCR 58/63 (92%) LR-18/58 (31%) OS-64% 5 year, 45% 10 year |
0% G3/4 toxicity |
| Sun Myint, 2017 [47] | 2003–2012 | 200 (17) | 45/25F + chemo (127), 39 Gy/13F or 25 Gy/5F if unfit (56) | Mean 90/3F before or after | cCR 144/200 (72%)-plus 8 /38 had pathCR at surgery LR 16/144 (11%) DFS 53% 5 years |
0% G3/4 toxicity 39% G1/2 bleeding 30% G1 rectal ulcer |
| Dhadda, 2017 [41] | 2011–2015 | 45 (3) |
45 Gy/25F + capecitabine or 25 Gy/5F if unfit | 90/3F before or after | cCR 36/42 (86%) LR 5/36 (14%) 2 year |
1/42 (2%) G3 toxicity |
| Frin, 2017 [39] | 2002–2014 | 112 (20) |
50 Gy/25F ± chemo |
60–90/2-3F before or after | T2-early T3, N0 group 43/45 (96%) cCR LR 3/43 (11%) 5 yr |
9% (8/92) late G3 toxicity (including 3 fistulae post APER) 4/45 in T2-early T3,N0 group |
| Gérard, 2019 [50] | 2002–2016 | 74 (2) |
50 Gy/25F (69) + chemo (49/69) 25 Gy/5F (3) |
90–110 Gy/3-4F before or after | cCR 69/74 (93%) at 1 year (cCR 31/74 at 14 weeks) LR 7/71 (10%) 5 yr DFS 88% 3 yr OS 74% 3 yr |
11% (8/74) late G3 toxicity Rectal bleeding 34% late G1/2, 10% G3 |
ERUS Endo rectal ultrasound.
F fractions.
cCR clinical complete response.
LR local relapse.
OS overall survival.
Patients with T1N0 low risk tumours (see Table 1) who have a low predicted lymph node risk can receive CXB alone (no EBRT or surgery) after appropriate counselling. The dose used in this instance is 110 Gy/4 fractions (30 Gy/30 Gy/30 Gy/20 Gy). If there is insufficient tumour shrinkage by the time of the third fraction (defined as little or no change in tumour size) then surgery should be considered instead in operable patients as this implies that the tumour may not be very radiosensitive and may have been under-staged with MRI. For inoperable patients the third fraction of CXB should be administered and EBRT added to cover any tumour penetration not covered by CXB and to deliver dose to the mesorectal lymph nodes as the tumour is likely to be more aggressive than initial staging suggested (level 5 evidence). In a study of 194 patients who received local excision with CXB afterwards due to high-risk features 55% of patients also received EBRT [34]. The local recurrence rate was 7.7% at 77 months median follow up. Organ preservation was achieved in 95% of patients. This compares with other studies of similar patients showing local recurrence rates of 7% after completion surgery [35] or 14% after adjuvant EBRT [36].
The randomised Lyon R96-02 [23], [37] trial employed 85 Gy in 3 fractions (30 Gy/30 Gy/25 Gy) with 39 Gy in 13 fractions EBRT for patients who were operable but a non-operative approach was preferred for medical co-morbidity, age or patient choice, level 1b. These CXB and EBRT dose/fractionation schemes were also used by Gerard et al. in later published prospective series, level 2b [38], [39]. Sun Myint et al. treated a proportion of surgically unfit patients with either 25 Gy in 5 fractions or 45 Gy in 25 fractions EBRT and a CXB boost of 110 Gy in 4 fractions (30 Gy/30 Gy/30 Gy/20 Gy) [30]. However the majority of patients in published cohorts have been treated with either 25 Gy in 5 fractions or 45 Gy in 25 fractions EBRT and CXB 90 Gy in 3 fractions (30 Gy per fraction), level 2b [40], [41]. Use of 25 Gy in 5 fractions EBRT as an alternative to ‘long-course’ EBRT +/- chemotherapy has not been widely studied but is considered acceptable practice, with studies in the pre-surgical setting showing similar local control outcomes to both regimens [42], [43].
The ICONE consensus view was that using the same dose per fraction was less likely to result in radiation administration errors, level 5 with majority consensus. It was also felt that using a higher dose (110 Gy in 4 fractions) as a boost to EBRT outside a trial setting had not significantly improved control and risked higher toxicity, but may be used safely in some circumstances where it is felt that clinical conditions during treatment may have resulted in less than the intended 90 Gy being delivered to the tumour over 3 fractions, such as oblique applicator placement or applicator movement during a fraction, level 5 with majority consensus. Entry of potentially operable patients into clinical trials is recommended [44]. The OPERA trial (NCT02505750) comparing a CXB boost to an EBRT boost in medically fit patients receiving chemo-radiotherapy to the pelvis has now closed to recruitment and results are awaited. Other patients receiving CXB should be entered into a registry such as the NICE recommended database (www.colorectaldatabase.co.uk) which enables reporting of outcomes by centre but also enables combining of outcomes for all participating centres.
Following local tumour excision there may be a risk of tumour recurrence. Bach et al. have defined risk factors for tumour regrowth following local excision [45]. Patients with low risk pT1 tumours (see Table 2) unfit for or unsuitable for transanal endoscopic mucosal surgery (TEMS) full-thickness excision can be treated with CXB alone. Following TEMS for patients with higher risk features and R0 tumours a lower CXB dose can be delivered (60 Gy in 2 fractions CXB) but following R1 tumours if there is visible residual at the time of CXB a higher dose may be required (90 Gy in 3 fractions CXB), level 2b. Patients with higher risk pT1 tumours or pT2/3 tumours require EBRT in addition to CXB to sterilise potentially positive mesorectal lymph nodes. Frin et al. help to define the recommendation for EBRT using a risk of positive lymph nodes > 10% [39]. There is no majority consensus on dose following local excision. 30–40 Gy in 2 fractions CXB has been described, level 2b [39], with EBRT using the parameters described above, with a local regrowth rate of 4% (1/20 patients). In an older series Gerard et al. treated 43 patients with a brachytherapy boost following local excision, 30/43 received 45–50 Gy in 3 fractions CXB alone, 1/43 received 15 Gy in 1 fraction CXB and EBRT and the remaining patients had interstitial implants +/- EBRT [46]. The local regrowth rate was 2.3% (1/43) with perirectal nodal relapse in 4.7% (2/43), level 2b. In the UK, 60 Gy in 2 fractions CXB alone is used for low risk cancers following local excision with the addition of EBRT for higher risk tumours 45 Gy in 25 fractions with capecitabine or for less fit patients EBRT alone 25 Gy in 5 fractions, level 2b.
The data on toxicity following CXB is relatively limited. The toxicity of CXB appears to be acceptable with an overall grade 3 toxicity of 3%, level 2a [11]. Sun Myint et al. showed a rate of bleeding in 28% of patients (grade 1–3), increased in patients on anti-coagulation [47]. 10% of patients in this series required argon plasma coagulation. Future registries and randomised studies are encouraged to prospectively collect toxicity data and patient reported outcomes.
In the setting of a rectal cancer developing in a pelvis previously irradiated for another cancer, CXB alone can be used if surgery is declined or contra-indicated, level 5. This is less likely to be curative in nature, depending on the depth of tumour invasion or other adverse tumour characteristics and the subsequent risk of nodal relapse or local recurrence. If local regrowth has occurred following EBRT alone for rectal cancer using an organ-preserving approach and surgery is contra-indicated due to metastatic disease or intercurrent illness, CXB can be used. In both instances, up to 110 Gy in 4 fractions (30 Gy/30 Gy/ 30 Gy/20 Gy) can be used dependent on previous EBRT dose and time-interval since treatment. This approach may provide better control than chemotherapy or no treatment but the numbers of patients treated are small so although the procedure appears to be safe, full data on efficacy are unknown. Retreatment using CXB for local regrowth following initial CXB treatment has been used but there is insufficient evidence for safety and efficacy. Therefore this should only be used for patients in whom surgery would be contra-indicated and the patient should be appropriately consented for a potential increase in post-treatment toxicity.
Currently there is no method available to reconstruct the cumulative dose to the tumour and bowel wall following EBRT and CXB, hampering the development of dose response relations to tumoural tissue or normal tissue complication probability (NTCP) relations. Dose reporting at the applicator surface is currently the only method that is practical and is therefore the recommended way to report dose. However, it is noted that this method holds no direct relation towards the dose received by critical structures in the bowel wall responsible for toxicity, or dose received by the deeper aspects of the tumour. There is rapid dose fall off for CXB delivered using the 50 kV X-Ray tube and this must be taken into account as other methods of CXB are developed and dose/fractionation schemes are chosen. If available, endorectal ultrasound can be used to determine tumour depth. Development of this technique is encouraged and it may be used in the future to improve dose reporting and prediction of response.
Conclusions
Contact X-Ray brachytherapy appears to be an efficacious technique for the treatment of rectal cancer and may allow rectal preservation in selected patients. It appears to be safe, with acceptable toxicity, and is particularly valuable for medically frail or very old patients in whom an organ-sparing approach is likely to result in lower mortality than surgery [22]. Economic analysis demonstrates that use of CXB in potentially operable patients with a low rectal cancer is a cost-effective approach [48]. These GEC ESTRO ACROP recommendations recommend the dose schemes in Table 3 for rectal CXB, though other schemes may be considered based on institutional experience and calculated biological equivalence. Entry into clinical trials is recommended for appropriate patients.
Table 3.
Dose recommendations for Contact X-Ray Brachytherapy (see appendix 1 to describe the Grade of recommendation).
| Stage | Dose and number of fractions | Notes |
|---|---|---|
| Initial Treatment | ||
| Post excision (low-risk T1, see Table 2) | Preferred 40–60 Gy in 2F, Grade B Acceptable 50 Gy in 3F (20 Gy/15 Gy/15 Gy), Grade C |
|
| Post excision (T2 or high-risk T1, see Table 2) | Preferred 40–60 Gy in 2F (completely excised) 90 Gy in 3F (disease residual), Grade B |
With EBRT 45–50 Gy/25F + capecitabine if fit or 25 Gy/5F if less fit |
| T1N0 | 110 Gy in 4F (30 Gy/30 Gy/30 Gy/20 Gy), Grade C |
If inadequate tumour response at 3rd F fit patients should proceed to surgery and unfit patients should have 3rdF and then have EBRT 45–50 Gy/25F + capecitabine if fit or 25 Gy/5F if less fit |
| T2/3N0/1 (surgically fit) T4 or any N2 (surgically unfit or palliative) |
Preferred 90 Gy in 3F (30 Gy/30 Gy/30 Gy), Grade C Acceptable 85 Gy in 3F (30 Gy/30 Gy/25 Gy), Grade B 110 Gy in 4F (30 Gy/30 Gy/30 Gy/20 Gy), Grade D |
With EBRT 45–50 Gy/25F + capecitabine if fit or 25 Gy/5F if less fit |
| Retreatment | ||
| Any | 90 Gy in 3F (30 Gy/30 Gy/30 Gy) 110 Gy in 4F (30 Gy/30 Gy/30 Gy/20 Gy), Grade D |
Consideration must be given to previous radiotherapy doses |
Abbreviations.
EBRT external beam radiotherapy.
F fraction.
Gy Gray.
Use of the Guildford database to collect clinical data is recommended for UK CXB patients in the NICE guidance for CXB (www.colorectaldatabase.co.uk) and this database can also be used for CXB patients internationally as well as surgical patients for comparison. The routine collection and publication of outcome data including patient reported outcomes (PROs) is recommended. Further development of clinical trials is encouraged. These recommendations recommend definitive, boost and retreatment doses as described in Table 3. These recommendations advise reporting of tumour depth to enable future refinement of dose prescription and target definition.
Disclaimer
These recommendations represent the views of the authors, the GI GEC-ESTRO working party and the ICONE group regarding currently accepted treatment and have been reviewed by the ESTRO ACROP committee. The suggested doses result from published evidence and clinical experience. The clinician should use their judgment to select appropriate treatment approaches, including dose and fractionation for their patients. The recommendations will be updated as clinical experience increases.
Declaration of Competing Interest
JP Gerard is the medical advisor of Ariane Medical Systems Company, Derby, UK
F Verhaegen, M Berbee and EJ van Limbergen have submitted a patent on an endorectal probe device a patent for effecting radiation treatment of colorectal cancerous tissue in the rectum of a human or animal subject which is pending to Maastricht University.
All other authors state they have no conflicts of interest
Appendix 1.
Grades of recommendation
A Consistent level 1 studies
B Consistent level 2 or 3 studies or extrapolations from level 1 studies
C Level 4 studies or extrapolations from level 2 or 3 studies
D Level 5 evidence or troublingly inconsistent or inconclusive studies of any level
Levels of evidence
1a Systematic review of randomised controlled trials
1b Individual randomised control trial
1c All or none§
2a Systematic review (with homogeneity*) of cohort studies
2b Individual cohort study (including low quality randomised controlled trial; for example, <80% follow-up)
2c ‘Outcomes’ research; ecological studies
3a Systematic review (with homogeneity*) of case- control studies
3b Individual case-control study
4 Case series (and poor quality case series and cohort studies)
5 Expert opinion
*In this context, homogeneity means a systematic review that is free of worrisome variations (heterogeneity) in the directions and degrees of results between individual studies. Not all systematic reviews with statistically significant heterogeneity need be worrisome, and not all worrisome heterogeneity need be statistically significant. As noted above, studies displaying worrisome heterogeneity should be tagged with a ‘-’ at the end of their designated level.
§Met when all patients died before the treatment became available, but some now survive on it; or when some patients died before the Rx became available, but none now die on it.
§§In this context, poor-quality cohort study means one that failed to clearly define comparison groups and/or failed to measure exposures and outcomes in the same (preferably blinded), objective way in both exposed and non-exposed individuals and/or failed to identify or appropriately control known confounders and/or failed to carry out a sufficiently long and complete follow-up of patients. In this context, poor quality case-control study means one that failed to clearly define comparison groups and/or failed to measure exposures and outcomes in the same (preferably blinded), objective way in both cases and controls and/or failed to identify or appropriately control known confounders.
References
- 1.http://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009 [accessed on 22/11/202Secondary].
- 2.https://gco.iarc.fr/today/online-analysis-multi-bars [accessed on 22/11/2021]. Secondary.
- 3.http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer/incidence - heading-Three accessed on 22/11/2021. Secondary.
- 4.http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer/incidence - heading-One accessed on 22/11/2021. Secondary.
- 5.Faiz O., Haji A., Bottle A., Clark S.K., Darzi A.W., Aylin P. Elective colonic surgery for cancer in the elderly: an investigation into postoperative mortality in English NHS hospitals between 1996 and 2007. Colorectal Dis. 2011;13(7):779–785. doi: 10.1111/j.1463-1318.2010.02290.x. [DOI] [PubMed] [Google Scholar]
- 6.Rullier E., Vendrely V., Asselineau J., Rouanet P., Tuech J.-J., Valverde A., et al. Organ preservation with chemoradiotherapy plus local excision for rectal cancer: 5-year results of the GRECCAR 2 randomised trial. Lancet Gastroenterol Hepatol. 2020;5(5):465–474. doi: 10.1016/S2468-1253(19)30410-8. [DOI] [PubMed] [Google Scholar]
- 7.Chawla S., Katz A.W., Rauh S.M., Monson J.R.T. Can Surgery be Avoided After Preoperative Chemoradiation for Rectal Cancer in the Era of Organ Preservation? Current Review of Literature. Am J Clin Oncol. 2015;38(5):534–540. doi: 10.1097/COC.0000000000000122. [DOI] [PubMed] [Google Scholar]
- 8.Habr-Gama A., São Julião G.P., Vailati B.B., Fernandez L.M., Ortega C.D., Figueiredo N., et al. Organ Preservation Among Patients With Clinically Node-Positive Rectal Cancer: Is It Really More Dangerous? Dis Colon Rectum. 2019;62(6):675–683. doi: 10.1097/DCR.0000000000001337. [DOI] [PubMed] [Google Scholar]
- 9.Appelt A.L., Pløen J., Vogelius I.R., Bentzen S.M., Jakobsen A. Radiation Dose-Response Model for Locally Advanced Rectal Cancer After Preoperative Chemoradiation Therapy. Int J Radiat Oncol Biol Phys. 2013;85(1):74–80. doi: 10.1016/j.ijrobp.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fonteyne V., Ost P., Vanpachtenbeke F., Colman R., Sadeghi S., Villeirs G., et al. Rectal toxicity after intensity modulated radiotherapy for prostate cancer: which rectal dose volume constraints should we use? Radiother Oncol. 2014;113(3):398–403. doi: 10.1016/j.radonc.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 11.Verrijssen A.S., Opbroek T., Bellezzo M., et al. A systematic review comparing radiation toxicity after various endorectal techniques. Brachytherapy. 2019;18(1):71427–71485. doi: 10.1016/j.brachy.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Papillon J. Present status of radiation therapy in the conservative management of rectal cancer. Radiother Oncol. 1990;17(4):275–283. doi: 10.1016/0167-8140(90)90001-d. [DOI] [PubMed] [Google Scholar]
- 13.Richardson S., Garcia-Ramirez J., Lu W., Myerson R.J., Parikh P. Design and dosimetric characteristics of a new endocavitary contact radiotherapy system using an electronic brachytherapy source: A new endocavitary contact radiotherapy system. Med Phys. 2012;39(11):6838–6846. doi: 10.1118/1.4757915. [DOI] [PubMed] [Google Scholar]
- 14.https://clinicaltrials.gov/ct2/show/NCT04336202?term=04336202&draw=2&rank=1 accessed on 21/11/21.
- 15.Bellezzo M., Fonseca G.P., Verrijssen A.-S., Voncken R., Van den Bosch M.R., Yoriyaz H., et al. A novel rectal applicator for contact radiotherapy with HDR 192Ir sources. Brachytherapy. 2018;17(6):1037–1044. doi: 10.1016/j.brachy.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Bellezzo M., Fonseca G.P., Voncken R., Verrijssen A.-S., Van Beveren C., Roelofs E., et al. Advanced design, simulation, and dosimetry of a novel rectal applicator for contact brachytherapy with a conventional HDR 192Ir source. Brachytherapy. 2020;19(4):544–553. doi: 10.1016/j.brachy.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Haute Autorité de Santé. Radiothérapie de Contact. Rapport d’évaluation Technologique. Available at http://www.has-sante.fr/portail/upload/docs/application/pdf/2009-01/rapport_rtc.pdf, 2008.
- 18.National Institute for Clinical Excellence. Low energy contact X ray brachytherapy (the Papillon technique) for early stage rectal cancer NICE interventional procedure guidance [IPG532], 2015.
- 19.National Institute for Health and Care Excellence. Low-energy contacy X-Ray brachytherapy (the Papillon technique) for locally advanced rectal cancer IPG 659, 2019.
- 20.Rombouts A.J.M., Al-Najami I., Abbott N.L., Appelt A., Baatrup G., Bach S., et al. Can we Save the rectum by watchful waiting or TransAnal microsurgery following (chemo) Radiotherapy versus Total mesorectal excision for early REctal Cancer (STAR-TREC study)?: protocol for a multicentre, randomised feasibility study. BMJ Open. 2017;7(12):e019474. doi: 10.1136/bmjopen-2017-019474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warschkow R., Güller U., Cerny T., Schmied B.M., Plasswilm L., Putora P.M. Secondary malignancies after rectal cancer resection with and without radiation therapy: A propensity-adjusted, population-based SEER analysis. Radiother Oncol. 2017;123(1):139–146. doi: 10.1016/j.radonc.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Smith F.M., Rao C., Perez R.O., Bujko K., Athanasiou T., Habr-Gama A., et al. Avoiding radical surgery improves early survival in elderly patients with rectal cancer, demonstrating complete clinical response after neoadjuvant therapy: results of a decision-analytic model. Dis Colon Rectum. 2015;58(2):159–171. doi: 10.1097/DCR.0000000000000281. [DOI] [PubMed] [Google Scholar]
- 23.Ortholan C., Romestaing P., Chapet O., Gerard J.P. Correlation in Rectal Cancer Between Clinical Tumor Response After Neoadjuvant Radiotherapy and Sphincter or Organ Preservation: 10-Year Results of the Lyon R 96–02 Randomized Trial. Int J Radiat Oncol Biol Phys. 2012;83(2):e165–e171. doi: 10.1016/j.ijrobp.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Smith F.M., Pritchard D.M., Wong H., Whitmarsh K., Hershman M.J., Sun Myint A. A cohort study of local excision followed by adjuvant therapy incorporating a contact X-ray brachytherapy boost instead of radical resection in 180 patients with rectal cancer. Colorectal Dis. 2019;21(6):663–670. doi: 10.1111/codi.14584. [DOI] [PubMed] [Google Scholar]
- 25.Saraste D., Gunnarsson U., Janson M. Predicting lymph node metastases in early rectal cancer. Eur J Cancer. 2013;49(5):1104–1108. doi: 10.1016/j.ejca.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Veitch A.M., Vanbiervliet G., Gershlick A.H., Boustiere C., Baglin T.P., Smith L.-A., et al. Endoscopy in patients on antiplatelet or anticoagulant therapy, including direct oral anticoagulants: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines. Gut. 2016;65(3):374–389. doi: 10.1136/gutjnl-2015-311110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Croce O., Hachem S., Franchisseur E., Marcié S., Gérard J.-P., Bordy J.-M. Contact radiotherapy using a 50 kV X-ray system: Evaluation of relative dose distribution with the Monte Carlo code PENELOPE and comparison with measurements. Radiat Phys Chem. 2012;81(6):609–617. [Google Scholar]
- 28.Fletcher C.L., Mills J.A., Baugh G.M., Roughton J. Comparison of 50 kV Facilities for Contact Radiotherapy. Clin Oncol. 2007;19(9):655–660. doi: 10.1016/j.clon.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Verrijssen A.S., Guillem J., Perez R., et al. Microscopic intramural extension of rectal cancer after neoadjuvant chemoradiation: A meta-analysis based on individual patient data. Radiother Oncol. 2019;144(1):37–45. doi: 10.1016/j.radonc.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myint A.S. Contact radiotherapy for elderly patients with early low rectal cancers. Br J Hosp Med (Lond). 2013;74(7):391–396. doi: 10.12968/hmed.2013.74.7.391. [DOI] [PubMed] [Google Scholar]
- 31.Furstoss C. COMP report: CPQR technical quality control guidelines for kilovoltage X ray radiotherapy machines. J Appl Clin Med Phys. 2018;19(2):18–21. doi: 10.1002/acm2.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Humbert-Vidan L., Sander T., Eaton D.J., Clark C.H. National audit of a system for rectal contact brachytherapy. Phys Imaging Radiat Oncol. 2017;1:1–5. [Google Scholar]
- 33.Birnbaum E.H., Ogunbiyi O.A., Gagliardi G., Fry R.D., Myerson R.J., Kodner I.J., et al. Selection criteria for treatment of rectal cancer with combined external and endocavitary radiation. Dis Colon Rectum. 1999;42(6):727–733. doi: 10.1007/BF02236926. [DOI] [PubMed] [Google Scholar]
- 34.Dhadda A.S., Sun Myint A., Thamphya B., et al. A multi-centre Nlysis of adjuvant contact X-Ray brachytherapy (CXB) in rectal cancer patients treated with local excsion-preliminary results of the CONTEM! study. Radiother and Oncol. 2021;162:195–201. doi: 10.1016/j.radonc.2021.07.021. [DOI] [PubMed] [Google Scholar]
- 35.Borstlap W.A.A., Coeymans T.J., Tanis P.J., Marijnen C.A.M., Cunningham C., Bemelman W.A., et al. Meta-analysis of oncological outcomes after local excision of pT1-2 rectal cancer requiring adjuvant (chemo) radiotherapy or completion surgery. BJS. 2016;103(9):1105–1116. doi: 10.1002/bjs.10163. [DOI] [PubMed] [Google Scholar]
- 36.Rackley T.P., Ma R.M.K., Brown C.J., Hay J.H. Transanal local excision for patients with rectal cancer: can radiation compensate for what is perceived as a non-definitive surgical approach? Dis Colon Rectum. 2016;59(3):173–178. doi: 10.1097/DCR.0000000000000544. [DOI] [PubMed] [Google Scholar]
- 37.Gerard J.-P., Chapet O., Nemoz C., Hartweig J., Romestaing P., Coquard R., et al. Improved Sphincter Preservation in Low Rectal Cancer With High-Dose Preoperative Radiotherapy: The Lyon R96-02 Randomized Trial. JCO. 2004;22(12):2404–2409. doi: 10.1200/JCO.2004.08.170. [DOI] [PubMed] [Google Scholar]
- 38.Gerard J.-P., Frin A.-C., Doyen J., Zhou F.X., Gal J., Romestaing P., et al. Organ preservation in rectal adenocarcinoma (T1) T2–T3 Nx M0. Historical overview of the Lyon Sud - nice experience using contact x-ray brachytherapy and external beam radiotherapy for 120 patients. Acta Oncol. 2015;54(4):550–556. doi: 10.3109/0284186X.2014.975840. [DOI] [PubMed] [Google Scholar]
- 39.Frin A.C., Evesque L., Gal J., Benezery K., François E., Gugenheim J., et al. Organ or sphincter preservation for rectal cancer. The role of contact X-ray brachytherapy in a monocentric series of 112 patients. Eur J Cancer. 2017;72:124–136. doi: 10.1016/j.ejca.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Sun Myint A., Grieve R.J., McDonald A.C., Levine E.L., Ramani S., Perkins K., et al. Combined modality treatment of early rectal cancer-the UK experience. Clin Oncol. 2007;19(9):674–681. doi: 10.1016/j.clon.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 41.Dhadda A.S., Martin A., Killeen S., Hunter I.A. Organ Preservation Using Contact Radiotherapy for Early Rectal Cancer: Outcomes of Patients Treated at a Single Centre in the UK. Clin Oncol. 2017;29(3):198–204. doi: 10.1016/j.clon.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 42.Ngan S.Y., Burmeister B., Fisher R.J., et al. Randomized tiral of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology group trial 01.04. J Clin Oncol. 2012;30(31):3827–3833. doi: 10.1200/JCO.2012.42.9597. [DOI] [PubMed] [Google Scholar]
- 43.Bujko K., Nowacki M.P., Nasierowska-Guttmejer A., Michalski W., Bebenek M., Kryj M. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006;93(10):1215–1223. doi: 10.1002/bjs.5506. [DOI] [PubMed] [Google Scholar]
- 44.https://clinicaltrials.gov/ct2/show/NCT02505750 [accessed on 22/11/2021].
- 45.Bach S.P., Hill J., Monson J.R.T., Simson J.N.L., Lane L., Merrie A., et al. A predictive model for local recurrence after transanal endoscopic microsurgery for rectal cancer. Br J Surg. 2009;96(3):280–290. doi: 10.1002/bjs.6456. [DOI] [PubMed] [Google Scholar]
- 46.Gérard J.P., Chapet O., Romestaing P., et al. Local excision and adjuvant radiotherapy for rectal adenocarcinoma T1–2 N0. Gastroenterol Clin Biol. 2000;24(4):430–435. [PubMed] [Google Scholar]
- 47.Sun Myint A., Smith F.M., Gollins S.W., Wong H., Rao C., Whitmarsh K., et al. Dose escalation using contact X-ray brachytherapy (Papillon) for rectal cancer: does it improve the chance of organ preservation? BJR. 2017;90(1080):20170175. doi: 10.1259/bjr.20170175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rao C., Smith F.M., Martin A.P., Dhadda A.S., Stewart A., Gollins S., et al. A Cost-Effectiveness Analysis of Contact X-ray Brachytherapy for the Treatment of Patients with Rectal Cancer Following a Partial Response to Chemoradiotherapy. Clin Oncol (R Coll Radiol) 2018;30(3):166–177. doi: 10.1016/j.clon.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 49.Gerard J.-P., Chapet O., Ramaioli A., Romestaing P. Long-term control of T2–T3 rectal adenocarcinoma with radiotherapy alone. Int J Radiat Oncol Biol Phys. 2002;54(1):142–149. doi: 10.1016/s0360-3016(02)02879-1. [DOI] [PubMed] [Google Scholar]
- 50.Gérard J.-P., Barbet N., Gal J., Dejean C., Evesque L., Doyen J., et al. Planned organ preservation for early T2–3 rectal adenocarcinoma: A French, multicentre study. Eur J Cancer. 2019;108:1–16. doi: 10.1016/j.ejca.2018.11.022. [DOI] [PubMed] [Google Scholar]