Abstract
Objective
We evaluated the relevance of using the smudge cell percentage in the blood smear as a prognostic marker in CLL.
Methods
In this prospective study, 42 untreated Senegalese patients with CLL were enrolled. The diagnosis was established, based on the peripheral blood count and flow cytometry using the Matutes score. Cytogenetic aberrations, assessed by fluorescence in situ hybridization (FISH), were available for 30 patients, while the immunoglobulin heavy chain genes (IGVH) mutation status was performed by next-generation sequencing (NGS) in 24 patients. The SC percentage was determined in the blood smear, as previously described. Statistical analyses were executed using the GraphPad Prism 8.
Results
The mean age was 63 years (48 - 85) and the male: female sex ratio was 4.66. A low SC (< 30%) percentage was correlated with Binet stage B/C (p = 0.0009), CD38 expression (p = 0.039), unmutated IGVH status (p = 0.0009) and presence of cytogenetic abnormalities (for del 13q, p = 0.0012, while for other cytogenetic aberrations, p = 0.016). An inverse correlation was found between the SC percentage and the absolute lymphocyte count (r = -0.51) and patients with higher percentage of SCs had a prolonged survival. However, there was no correlation between the SC percentage and age (p = 0.41) or gender (median, 19% for males vs. 20% for females; p = 0.76).
Conclusion
When less than 30%, the SC was associated with a poor prognosis in CLL. Easy and affordable, the percentage of SCs in a blood smear could be a reliable prognostic marker, accessible to all CLL patients, mainly those in developing countries.
Keywords: Smudge cells, Blood smear, Prognostic marker, CLL, Senegal
Introduction
Chronic lymphocytic leukemia (CLL) is the most common type of leukemia in Caucasians. It is a heterogeneous disease which can present in an indolent form that will not necessitate treatment for several years, or as an aggressive and life-threatening leukemia.1,2 Despite the Binet et al. and Rai et al. staging systems3,4 which are the reference clinical staging systems to evaluate patient outcomes, this heterogeneity in the course persists, even in patients within a unique staging category. It has important impacts on survival times from diagnosis and clinical approaches, as well as treatment strategies.
Currently, different useful prognostic biomarkers are utilized in CLL, such as cytogenetic aberrations (del 17p, del 11q, del 13q and trisomy 12) evaluated by FISH, expression of specific proteins on, or in, CLL cells (70-kD ζ-associated protein, CD38, CD49d…), or molecular aberrations (TP 53, IGVH mutation, NOTCH1, MYD88, BIRC3 …). Thus, shorter survival times can be established for patients with early-stage, biologically aggressive disease.1,2,5 However, these tests are high-priced and demand equipment and substantial technical expertise and hence, are not always available to many CLL patients in poor countries. Consequently, more affordable prognostic tests which are easier, but above all reliable, are badly needed.
Smear or smudge cells (SCs) are broken CLL B cells present in blood smears of CLL patients. For almost a century, SCs were thought to be simply an artifact during the slide preparation. It has been noticed that smudge generation is linked up with the content of the cytoskeletal protein vimentin existing in leukemic cells.6, 7, 8 Nowakowski et al.9 showed that the percentage of SCs in a routine blood smear is an independent prognostic factor in patients with CLL and patients with a high percentage of SCs experience prolonged survival.
The present study, evaluated the correlation between the SC percentage in a routine blood smear with other prognostic factors in Senegalese CLL patients. We also investigated the influence of the percentage of SCs on the overall survival (OS).
Methods
Patients
In this 4-year prospective study, the blood smears of 42 untreated Senegalese patients with B-CLL were analyzed. There were initially 45 patients, but 3 of them were lost earlier. CLL is the most common type of leukemia in Western populations, being rarer in Asian and African people. This could explain the limited number of cases
The diagnosis of CLL was based on:
-
-
Peripherical blood count with persistent lymphocytosis of more than 5 × 109/L for 3 months, and;
-
-
flow cytometry using the Matutes scoring:10 CD5+, CD23+, weak monotypic light chain Ig (λ or κ light chain), weak expression, or absence, of FMC7 and 79b or CD22. The diagnosis of B-CLL was made when the Matutes score was 4 or 5.
Patient characteristics, clinical data, disease course and overall survival were available for all patients and the median follow-up time from diagnosis was 36 months.
The study was authorized by the Cheikh Anta Diop University (Dakar, Senegal) “Research Ethics Committee”. Each CLL patient signed a free and informed consent form before participating in this study.
Lymphocyte count
The peripheral blood count was performed on the Sysmex XT2000i ™ (Sysmex Diagnostics, Japan).
Peripheral blood smear examination
Blood smears of our patients were prepared from EDTA-anticoagulated blood. A manual method was used and always performed by the same technician. The May-Grunwald-Giemsa staining was used for all slides. Each patient had 2 slides and each slide was examined by the same biologist. This professional was not aware either of the clinical features or the progress of the patients. In sequence, the results of 2 readouts were compared and if there were a sizable difference between 2 readouts (more than 3%), a third one was obtained and the mean of the 3 readouts was calculated.
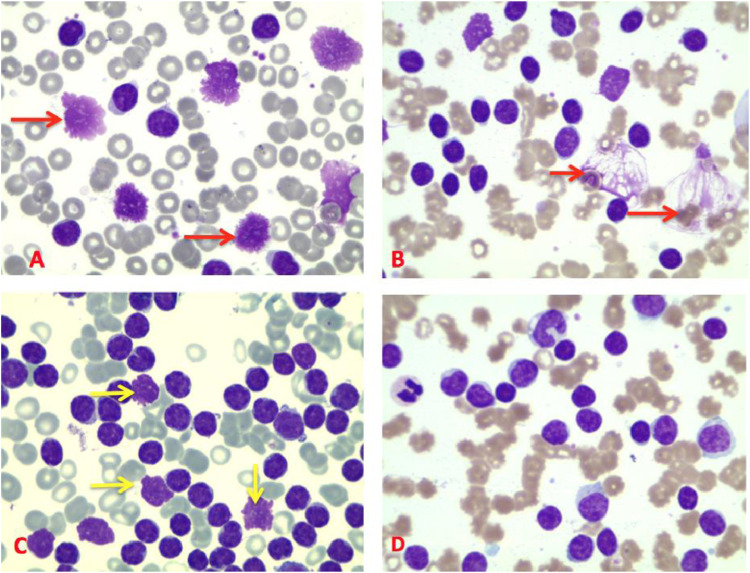
Smudge cells were defined as ruptured cells with no intact cytoplasm and nuclear membrane (Figure 1). The percentage of SCs was calculated, as previously reported.8,9 The SC percentage was calculated by counting 200 lymphocytes and/or smudge cells, the SC number then being divided by the total number of cells counted (smudge cells + intact lymphocytes) and multiplied by 100%: SC% = SC number /200 (lymphocytes + SC) x 100.
Figure 1.
Blood smears of different CLL patients (x 100). A and B: different forms of SC (red arrows). C: SC with disrupted nuclear membrane (yellow arrows). D: Blood smear without SC.
* represent the degree of significance. *: p = 0.01, **: p = 0.001, ***: p = 0.0001.Ns = not significant.
Immunophenotype
The B-cell phenotype was studied at the time of diagnosis using the FacsCalibur™ flow cytometer (Becton Dickinson, CA, USA). The antibodies employed were: CD45-APC/ CD19-PerCP/ CD5-PE/ CD23-PE/ FMC7- FITC/ lambda-PE/ kappa-FITC and CD22-FITC. The CD38 expression was defined at the same time as the Matutes scoring. The data were acquired and analyzed with the BD CellQuest Pro software (Becton Dickinson).
Cytogenetic analysis
Chromosomal aberrations were evaluated in the peripheral blood by FISH using Vysis probes (Abbott) according to the manufacturer's procedure. Abnormalities tested for were: del17p13 (TP53 probe), del13q14 (D13S319 probe), trisomy 12 (CEP 12 DNA Probe) and del 11q22 (ATM probe).
IGVH mutation status
The extraction of total RNA from CLL samples was performed with Trizol according to the manufacturer's recommendations (Invitrogen, Life Technologies, Carlsbad, CA).
In total, 1 μg of whole extracted RNA per patient was transcribed into complementary DNA using the QuantiTect Reverse Transcription Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Resulting cDNA underwent polymerase chain reaction amplification of the IGVH locus using modified BIOMED-2 framework region 1 consensus primers in combination with a consensus J segment primer, as previously described.11 The analysis of the data was performed using the Roche (Basel, Switzerland) proprietary software package for the 454 GS Junior system (Roche). Image acquisition and treatment and signal processing were performed during the run. The bioinformatical analysis was executed using the 454 GS Junior system, as previously described.11
Statistical analysis
The data were compiled using the Microsoft Excel, statistical analyses were performed and figures were created using the GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA).
The continuous variables were described as the mean with the standard deviation for normally distributed data, while we assessed the proportion to characterize qualitative variables. The Pearson's correlation analysis was employed to evaluate the association between the SC percentage and other continuous variables. The significance of differences between groups was estimated using the non-parametric Mann–Whitney test or an unpaired t-test. The overall survival (OS) was calculated from the date of diagnosis until death. The Kaplan–Meier survival curve and the log-rank test were used to compare the survival between different groups.
The confidence interval of 95% was used for the estimations and statistical tests were considered significant when the p-value was below 0.05.
Results
In this prospective study, 42 Senegalese patients with untreated CLL were enrolled. Epidemiological characteristics (age and gender), clinical features (tumor syndrome and Binet staging) and biological prognostic factors (absolute lymphocyte count, cytogenetic abnormalities, CD38 expression and IGVH mutation status) are detailed in Table 1.
Table 1.
Patient characteristics.
| Patient | Gender | Age (years) | Tumor syndrome | Lymphocytes 109/L | Smudge cells (%) | Binet Stage | CD38 Expression | Del13 q (n = 30) | Del11q (n = 30) | Trisomy 12 (n = 30) | Del17p (n = 30) | IGVH mutational Status (n = 24) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 63 | Absence | 33.62 | 48 | A | Negative | - | - | - | - | M |
| 2 | F | 50 | P | 298.80 | 11 | C | + | - | - | Presence | - | UM |
| 3 | M | 68 | P | 52.25 | 26 | C | + | Presence | - | - | - | UM |
| 4 | M | 53 | P | 831.14 | 12 | B | + | Presence | Presence | - | - | UM |
| 5 | M | 54 | P | 225.50 | 8 | C | Negative | Presence | - | - | - | UM |
| 6 | M | 69 | P | 199.50 | 8 | B | + | Presence | - | - | - | UM |
| 7 | M | 80 | P | 49.64 | 55 | A | Negative | - | - | - | - | M |
| 8 | M | 63 | P | 230.00 | 21 | C | + | - | - | Presence | Presence | UM |
| 9 | M | 50 | P | 58.00 | 32 | C | + | - | Presence | - | - | UM |
| 10 | M | 51 | P | 88.55 | 14.5 | C | + | Presence | - | - | - | UM |
| 11 | M | 63 | P | 145.78 | 14.5 | B | Negative | - | - | - | Presence | UM |
| 12 | M | 59 | P | 42.60 | 31 | C | + | Presence | - | Presence | - | UM |
| 13 | M | 67 | Absence | 6.48 | 80 | A | Negative | - | - | - | - | M |
| 14 | F | 67 | Absence | 6.40 | 42 | A | + | Presence | - | - | - | UM |
| 15 | M | 70 | P | 491.40 | 11 | B | + | Presence | - | Presence | Presence | UM |
| 16 | F | 57 | P | 12.80 | 39 | C | + | - | - | - | - | M |
| 17 | F | 80 | P | 91.40 | 20 | C | + | Presence | - | Presence | - | UM |
| 18 | M | 60 | P | 82.30 | 27 | C | + | - | - | Presence | - | UM |
| 19 | M | 85 | P | 219.00 | 19 | C | + | Presence | - | - | Presence | UM |
| 20 | M | 54 | P | 87.80 | 43 | A | Negative | - | - | - | - | UM |
| 21 | M | 60 | P | 16.38 | 17 | C | + | Presence | Presence | - | - | UM |
| 22 | M | 63 | P | 789.20 | 12 | C | Negative | Presence | - | - | - | UM |
| 23 | M | 54 | Absence | 51.76 | 31 | C | + | Presence | - | - | - | M |
| 24 | M | 67 | P | 201.52 | 19 | B | + | Presence | - | Presence | - | UM |
| 25 | M | 60 | P | 171.00 | 10 | C | + | Presence | Presence | - | - | |
| 26 | M | 72 | P | 15.00 | 41 | C | Negative | - | - | - | - | |
| 27 | M | 58 | P | 7.10 | 37 | A | Negative | - | - | - | - | |
| 28 | M | 73 | P | 241.00 | 13 | B | + | Presence | - | Presence | - | |
| 29 | M | 74 | P | 12.70 | 39 | C | Negative | - | - | - | - | |
| 30 | M | 63 | P | 734.00 | 21 | C | Negative | - | Presence | - | - | |
| 31 | M | 68 | Absence | 5.20 | 31 | B | Negative | |||||
| 32 | M | 48 | P | 165.93 | 19 | C | + | |||||
| 33 | M | 60 | P | 85.50 | 16.5 | C | + | |||||
| 34 | M | 52 | P | 156.30 | 14 | C | + | |||||
| 35 | F | 48 | P | 365.80 | 11 | C | + | |||||
| 36 | F | 78 | P | 311.00 | 15 | B | + | |||||
| 37 | F | 54 | Absence | 17.20 | 35 | B | + | |||||
| 38 | M | 82 | P | 150.90 | 12 | A | Negative | |||||
| 39 | F | 64 | P | 869.00 | 10 | C | + | |||||
| 40 | M | 55 | P | 114.42 | 15 | C | + | |||||
| 41 | F | 67 | P | 191.00 | 18 | C | + | |||||
| 42 | F | 70 | P | 79.20 | 29 | C | + |
F: female; M: male; P: presence; M: mutated; UM: unmutated; IGVH: immunoglobulin heavy chain.
The average age of our population was 63 years (ranging from 48 to 85), while the sex ratio was 4.66 (33 males and 9 females). The median total lymphocyte count was 102.9 × 109/L (ranging from 5.2 to 869) (95% CI 58–191). Most of the patients had progressive disease upon entering the study (Binet stage B and C, respectively, in 21.4% (9 patients) and 62% (26 patients) and CD38 was expressed in 69% of the cases. Chromosomal abnormalities, performed by FISH, were detected in 30 patients. Among them, 59% had del 13q and 26.7% trisomy 12, while del 11q and del17p were present in 5 and 4 patients, respectively. A total of 19/24 patients had unmutated IGVH status. Most of the patients were treated with reduced doses of CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) to avoid severe cytopenia. Five of them were treated with the association rituximab-cyclophosphamide.
The median follow-up time was 36 months and during this period, 26 deaths were observed.
The deaths were mainly secondary to infections (19 cases), most of them being pulmonary infections. Two patients developed a Richter syndrome and 3 patients had severe cytopenia, leading to death. The cause of death was unknown in 2 cases.
Smudge cell percentage in CLL patients
The mean of SC percentage was 24.46 (ranging from 8 to 80) (95% CI: 19.78 to 29.15), while the median was 19%.
Using 30% as the threshold value, as recommended by Nowakowski et al.,9,10 only 14 patients had an SC percentage > 30% (positive value), while 28 patients had a negative value (SC percentage<30%).
Association between smudge cell percentage and other prognostic factors
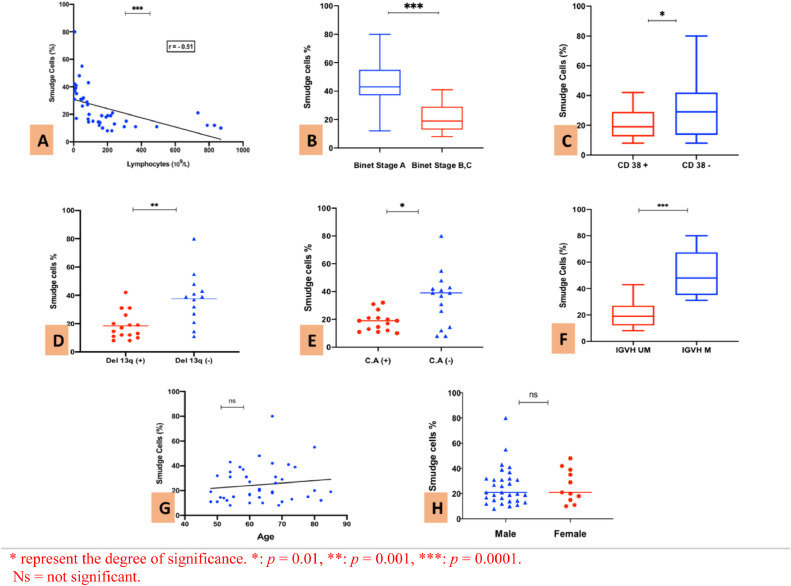
The percentage of SC was inversely associated with lymphocytosis: a decreasing trend in the lymphocyte count was observed when the SC percentage was high (>30%) (r = −0.51; p = 0.0006) (Figure 2A).
Figure 2.
A: Simple linear regression between SC percentage and lymphocytes count. B: Percentage of SC in Binet stage A and Binet stages B/C. C: SC percentage depending on CD38 expression. D: SC percentage regarding the presence or absence of 13q deletion. E: SC percentage in patients with at least one C.A (Trisomy 12 and/or del 11q and/or del 17p). F: SC percentage in patients with unmutated or mutated IGVH status. G: Correlation between SC percentage and age. H: SC percentage depending on gender.
The SC percentage was lower in patients with progressive disease (Binet stage B or C) and the median was 43% in Binet stage A and only 19% in Binet stage B/C, respectively (p = 0.0009) (Figure 2B).
The SC percentage was higher in CD38- patients, compared to CD38+ patients, the median being at 30.2% (ranging from 8 to 80) vs. 19% (ranging from 8 to 42), respectively (p = 0.039) (Figure 2C).
Patients with the 13q deletion as the unique chromosomal aberration had a lower percentage of SCs than patients without the 13q deletion, the median being at 15.75% vs. 39%, respectively (p = 0.0012) (Figure 2D).
Regarding the other cytogenetic abnormalities (CA) (trisomy 12, del11q and del17p), 15 patients had at least one abnormality and their presence was associated with a lower percentage of SCs, the median being at 19% with CA+ vs. 39% with CA- (p = 0.016) (Figure 2E).
Similarly, a significant link was also found between unmutated IGVH and a lower percentage of SCs and patients with mutated status had a higher percentage of SCs (p = 0.0009) (Figure 2F).
The SC percentage was markedly higher in patients with mutated IGVH status (median = 48%), compared to patients with unmutated IGVH status (median = 19%) (p = 0.0009).
However, there was no association between the SC percentage and age (p = 0.41) (Figure 2G) or gender, the median being at 19% for males vs. 20% for females (p = 0.76) (Figure 2H).
Percentage of smudge cells and survival
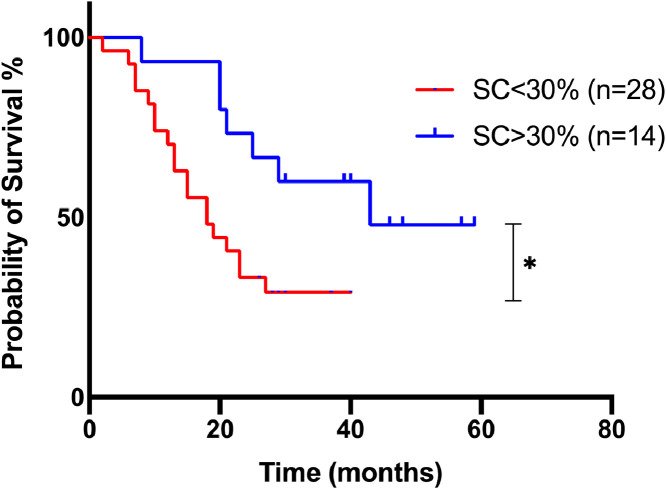
A significant association was found between the SC percentage and the overall survival. The 3-year survival estimate was 29.6% vs. 53.3% for patients with an SC percentage < 30% vs. more than 30%, respectively (p = 0.024) (Figure 3). Sixty-one percent of the patients died during the follow-up, 19 patients in the group with < 30% of SCs and 7 in the group with > 30% of SCs.
Figure 3.
Kaplan-Meier survival curves of 42 CLL patients, regarding the SC percentage.
Discussion
Also known as smear cells, basket cells, or shadows of Gumprecht, SCs were first described in 1896, in the blood smear of patients with “lymphocytic leukemia” by Gumprecht.12 Initially, their formation was considered an artefact produced during the slide preparation because of “cell fragility”. The interaction between the glass of the slide and CLL cells leads to disrupted cytoplasmic and nuclear membranes.8, 13
Nowakowski et al.7 discovered that SC formation is linked up with the content of the cytoskeletal protein, vimentin, present in leukemic cells. They noticed that a high vimentin content resulted in a low percentage of SCs and is associated with a shorter time to initial therapy in early-stage CLL. However, the high vimentin expression has been shown to be linked with a poor prognosis and metastatic potential in colorectal14 and breast cancer.15, 16 Some studies revealed that the SC percentage could be a reliable prognostic marker in patients with CLL.9, 13, 17
To the best of our knowledge, this is the first study in Africa comparing the percentage of SCs in a blood smear and the other prognostic factors in patients with CLL. It is obvious that the percentage of SCs has some potential benefits over the other prognostic biomarkers routinely used in CLL: it is accessible worldwide because blood smear examination is necessary for diagnosis and it can be easily available for patients in poor countries.
In this current study, we used a manual method to prepare the slides and calculated the SC percentage using the Nowakowski method, as previously described.9 In our cohort, the mean percentage of SCs was 24.46%, ranging from 8 to 80, while the median was 19%. This median was lower, compared to the previous studies with the medians of 28%, 24% and 21%, respectively.9,13,17 As in these previous studies, no correlation was found between the SC percentage and age or gender, however, the low SC percentage was related to the ZAP70 expression, CD38 positivity, unmutated IGVH, or shorter overall survival. In other words, the low SC percentage is associated with a poor prognosis in CLL.
It has been reported that the percentage of SC does not vary during the disease and represents not only a substitute marker of disease burden, but also reflects biologic characteristics of the leukemic cells.9
Using the cut-off value of 30% recommended by Nowakowski, 66.7% of the patients had a negative value (SC < 30%) and only one-third of the patients had a positive value (SC > 30%). It seemed that the majority of our CLL patients had a poor prognosis regarding the SC percentage. Hence, we did a bivariate analysis to verify if the low percentage of SC is correlated with other poor prognosis factors in CLL.
In this cohort, the overall survival was higher in the patients with SC > 30% (43 months) than in the patients with SC < 30% (19 months) (p = 0.024). Godja et al.18 found the 5-year survival rate was lower (51%) in patients with the SC < 30% than in patients with the SC > 30% (81%).
Moreover, previous studies have shown that the SC percentage was significantly lower in unmutated IGVH patients and CD38- and ZAP70-positive patients.9,13,17,18 The ZAP70 expression was not evaluated in our study; however, we found a significant correlation between a low SC percentage and a CD38 positivity (p = 0.039, as well as between a low percentage of SC and an unmutated IGVH status (p = 0.0009).
Regarding the clinical staging (Rai or Binet), results were different depending on the studies; Johansson et al.18 did not find any association between the SCs and Binet stage, while in other studies, the SC < 30% was associated with progressive disease (Rai III or IV or Binet stage C).9,18 When we treated the SC percentage as a continuous variable, patients with early disease (Binet stage A) had a higher SC percentage (mean = 45%) than patients with progressive disease (Binet B/C, mean = 20%) (p = 0.0009).
The prognostic relevance of hyperlymphocytosis in CLL remains uncertain. Shvidel et al., in 2015,20 showed that patients presenting with hyperleukocytosis (> 100 × 109/L) at the time of diagnosis generally had a more aggressive clinical course and standard prognostic biomarkers were not valid in this patient category.
In the previous studies,9,17,18 no correlation was found between the SC percentage and the absolute lymphocyte count. In the present study, the SC percentage was inversely correlated with lymphocytosis (r = −0.51; p = 0.0006). Multicenter studies would be necessary to better specify the place of the absolute lymphocyte count in the list of prognostic biomarkers of CLL.
The deletion of the 13q is the most frequent cytogenetic abnormality in CLL and indicates a good prognosis.2,5,19 It may seem surprising that in our study, patients with the del13q had a lower percentage of SCs than patients without the 13q deletion: (median 15.75% vs. 39%; p = 0.0012). While the del13q is considered to have a good prognosis, the percentage of nuclei carrying the deletion is relevant to the clinical course of patients. Indeed, when more than 80% of the nuclei carry the deletion, the 13q deletion then becomes a factor of poor prognosis.21 Out of 16 patients with the 13q deletion in our cohort, only 2 had a percentage of deleted nuclei less than 80%. This could explain why this deletion is associated with a lower SC percentage in our study and therefore, a poor prognosis.
We underlined that the association between low SC percentage and poor prognostic factors seems to be specific to CLL. Indeed, in a recent study, Chang et al.22 showed that in all cancers, patients with an SC percentage higher than 50 had poor outcomes, compared to those with an SC percentage less than 50. For that reason, the clinical meaning of the SC in other neoplasias remains undefined and further investigations are needed.
In this current study, we demonstrated that a low percentage of SCs (< 30%) was associated with many poor prognostic factors in Senegalese patients with CLL: Binet stage B/C, CD38 positivity, unmutated IGVH status and cytogenetic abnormalities. In addition, patients with a high SC percentage (> 30%) had a prolonged survival.
However, some limitations of our study should to be raised: small sample size and short median follow-up. As CLL seems to be rare, but more aggressive, in the African population,23 multicentric studies with large series and long follow-ups are advised to state that the SC percentage is an independent and solid prognostic factor in CLL.
Conclusion
With a readily attainable, easy to perform and minimum-requirement technique, the SC percentage should be considered as an interesting and reliable tool in the prognostication of CLL, mostly in developing countries.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
We thank Prof. Lorenzo Leoncini (University of Siena, Italy) for his help in studying the mutational status of IGVH in Senegalese patients.
References
- 1.Rai K.R., Jain J. Chronic lymphocytic leukemia: Then and now. Am J Hematol. 2016;91:330–340. doi: 10.1002/ajh.24282. [DOI] [PubMed] [Google Scholar]
- 2.Hallek M. Chronic lymphocytic leukemia: 2020 update on diagnosis, risk stratification and treatment. Am J Hematol. 2019;94:1266–1287. doi: 10.1002/ajh.25595. [DOI] [PubMed] [Google Scholar]
- 3.Binet J.L., Auquier A., Dighiero G., Chastang C., Piguet H., Goasguen J., et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer. 1981;48:198–204. doi: 10.1002/1097-0142(19810701)48:1<198::aid-cncr2820480131>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 4.Rai K.R., Sawitsky A., Cronkite E.P., Chanana A.D., Levy R.N., Pasternack B.S. Clinical staging of chronic lymphocytic leukemia. Blood. 1975;46:219–234. [PubMed] [Google Scholar]
- 5.Baliakas P., Mattsson M., Stamatopoulos K., Rosenquist R. Prognostic indices in chronic lymphocytic leukaemia: where do we stand how do we proceed? J Intern Med. 2016;279:347–357. doi: 10.1111/joim.12455. [DOI] [PubMed] [Google Scholar]
- 6.Brown M.J., Hallam J.A., Colucci-Guyon E., Shaw S. Rigidity of circulating lymphocytes is primarily conferred by vimentin intermediate filaments. J Immunol. 2001;166:6640–6646. doi: 10.4049/jimmunol.166.11.6640. [DOI] [PubMed] [Google Scholar]
- 7.Nowakowski G.S., Lee Y.K., Bone N.D., Morice W.G., Barnidge D., Jelinek D.F., et al. Proteomic analysis of chronic lymphocytic leukemia cells identifies vimentin as a novel prognostic factor for aggressive disease. Blood. 2005;106(11):707. [Google Scholar]
- 8.Nowakowski G.S., James D., Shanafelt T.D., Geyer S.M., Laplant B.R., Call T.G., et al. Using smudge cells on routine blood smears to predict clinical outcome in chronic lymphocytic leukemia: A universally available prognostic test. Mayo Clin Proc. 2007;82(4):449–453. doi: 10.4065/82.4.449. [DOI] [PubMed] [Google Scholar]
- 9.Nowakowski G.S., James D., Shanafelt T.D., Zent C.S., Call T.G., Bone N.D., et al. Percentage of smudge cells on routine blood smear predicts survival in chronic lymphocytic leukemia. J Clin Oncol. 2009;27:1844–1849. doi: 10.1200/JCO.2008.17.0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matutes E., Owusu-Ankomah K., Morilla R., Garcia Marco J., Houlihan A., Que T.H., et al. The immunological profile of B-cell disorders and proposal of a scoring system for the diagnosis of CLL. Leukemia. 1994;8:1640–1645. [PubMed] [Google Scholar]
- 11.Amato T., Abate F., Piccaluga P., Iacono M., Fallerini C., Renieri A., et al. Clonality analysis of immunoglobulin gene rearrangement by next-generation sequencing in endemic Burkitt lymphoma suggests antigen drive activation of BCR as opposed to sporadic Burkitt lymphoma. Am J Clin Pathol. 2016;145:116–127. doi: 10.1093/ajcp/aqv011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gumprecht F. Leukozytenzerfall im Blute bei Leukamie und bei schweren Anamien. Dtsch Arch Klin Med. 1896;57:523–548. [Google Scholar]
- 13.Al-Kahiry W., Tawfik H.S., Sharshira H., Ghanem A., El-Gammal M., Mikhael I.L. Smudge cell percentage as a surrogate marker for ZAP-70 expression in patients with chronic lymphocytic leukemia. Blood Res. 2018;53:218–222. doi: 10.5045/br.2018.53.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ngan C.Y., Yamamoto H., Seshimo I., Tsujino T., Man-i M., Ikeda J.I., et al. Quantitative evaluation of vimentin expression in tumour stroma of colorectal cancer. Br J Cancer. 2007;96:986–992. doi: 10.1038/sj.bjc.6603651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raymond W.A., Leong A.S. Vimentin: A new prognostic parameter in breast carcinoma? J Pathol. 1989;158:107–114. doi: 10.1002/path.1711580205. [DOI] [PubMed] [Google Scholar]
- 16.Domagala W., Lasota J., Dukowicz A., Markiewski M., Striker G., Weber K., et al. Vimentin expression appears to be associated with poor prognosis in node-negative ductal NOS breast carcinomas. Am J Pathol. 1990;137:1299–1304. [PMC free article] [PubMed] [Google Scholar]
- 17.Johansson P., Eisele L., Klein-Hitpass L., Sellmann L., Dührsen U., Dürig, et al. Percentage of smudge cells determined on routine blood smears is a novel prognostic factor in chronic lymphocytic leukemia. Leuk Res. 2010;34:892–898. doi: 10.1016/j.leukres.2010.02.038. [DOI] [PubMed] [Google Scholar]
- 18.Gogia A., Raina V., Gupta R., Gajendra S., Kumar L., Sharma A., et al. Prognostic and Predictive significance of smudge cell percentage on routine blood smear in chronic lymphocytic leukemia. Clin Lymphoma Myeloma Leuk. 2014;14:514–517. doi: 10.1016/j.clml.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Rosenquist R., Cortese D., Bhoi S., Mansouri L., Gunnarsson R. Prognostic markers and their clinical applicability in chronic lymphocytic leukemia: Where do we stand? Leuk Lymph. 2013;54(11):2351–2364. doi: 10.3109/10428194.2013.783913. [DOI] [PubMed] [Google Scholar]
- 20.Shvidel L., Bairey O., Tadmor T., Braester A., Ruchlemer R., Finemann R., et al. Absolute lymphocyte count with extreme hyperleukocytosis does not have a prognostic impact in chronic lymphocytic leukemia. Anticancer Res. 2015;35(5):2861–2866. [PubMed] [Google Scholar]
- 21.Van Dyke D.L., Shanafelt T.D., Call T.G., Zent C.S., Smoley S.A., Rabe K.G., et al. A comprehensive evaluation of the prognostic significance of 13q deletions in patients with B-chronic lymphocytic leukemia. Br J Haematol. 2010;148:544–550. doi: 10.1111/j.1365-2141.2009.07982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang C.C., Sun J.T., Liou T.H., Kuo C.F., Bei C.H., Lin S.J., et al. Clinical significance of smudge cells in peripheral blood smears in hematological malignancies and other diseases. Asian Pac J Cancer Prev. 2016;17(4):1847–1850. doi: 10.7314/apjcp.2016.17.4.1847. [DOI] [PubMed] [Google Scholar]
- 23.Amato T., Sall A., Dièye T.N., Gozzetti A., Iacono M., Ambrosio M.R., et al. Preferential usage of specific immunoglobulin heavy chain variable region genes with unmutated profile and advanced stage at presentation are common features in patients with chronic lymphocytic leukemia from Senegal. Am J Clin Pathol. 2017;148:545–554. doi: 10.1093/ajcp/aqx105. [DOI] [PubMed] [Google Scholar]