Abstract
We investigated the kinetics of the neutralizing antibody responses to the severe acute respiratory syndrome-coronavirus-2 delta variant over the course of 1 year in 16 patients infected at the beginning of the pandemic. In patients with severe disease, neutralizing responses to the delta variant were detectable, albeit at lower levels than responses to the wild type. Neutralizing responses to the delta variant were undetectable, however, in asymptomatic persons. This finding implies that the vaccination strategy for persons with past natural infection should depend on the severity of the previous infection.
Keywords: COVID-19, SARS-CoV-2, Delta Variant, FRNT
Graphical Abstract
The severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) delta variant (B.1.617.2), first detected in India in October 2020, was classified as a Variant of Concern on May 11, 2021, due to its high transmissibility and potential immune escape.1 Recent reports have indicated that the delta variant reduces sensitivity to neutralization by serum from vaccinated individuals and previously infected individuals.2,3,4 Most of these studies, however, were cross-sectional surveys and mainly evaluated patients with mild infection. Neutralizing antibody titers in patients with severe coronavirus disease 2019 (COVID-19) were found to be maintained at high levels for up to 1 year,5 suggesting that they might be less susceptible to reinfection. To determine the timing of their vulnerability to reinfection with the delta variant, neutralizing antibody titers were assessed in blood samples collected at regular intervals.
Participants deemed eligible for this prospective longitudinal study consisted of patients with real-time reverse transcription polymerase chain reaction-confirmed COVID-19 admitted to the Biocontainment Unit of Seoul National University Hospital (SNUH) between February 1, 2020 and June 30, 2020. This study also enrolled asymptomatic persons infected with SARS-CoV-2 who were isolated in a community treatment center (CTC) operated by SNUH between March 5, 2020 and April 9, 2020.
During their stay in the CTC, patients were comprehensively evaluated twice daily by physicians using a video consultation system.6 Asymptomatic persons were defined as those with body temperature < 37.5°C and no symptoms (e.g., subjective fever, myalgia, rhinorrhea, sore throat, cough, sputum, chest discomfort) during the entire CTC stay. According to whether or not oxygen therapy was required, we classified pneumonia cases as mild or severe. Plasma samples were collected at 3 to 4 month intervals.
SARS-CoV-2 spike (S1 domain)-specific IgG was semi-quantitively measured using an anti-SARS-CoV-2 enzyme-linked immunosorbent assay (ELISA) kit (Euroimmun, Luebeck, Germany). Optical density ratios were interpreted as negative (< 0.8), borderline (≥ 0.8 to < 1.1), or positive (≥ 1.1), according to the manufacturer’s instructions. Neutralizing antibodies were detected using an immunocolourimetric-based focus reduction neutralization test (FRNT) against two strains of SARS-CoV-2, SARS-CoV-2/wt (NCCP No. 43326), obtained from the National Culture Collection for Pathogen, and SARS-CoV-2/B.1.617.2 (GenBank accession No. MZ853946) isolated from a nasopharyngeal swab taken from a patient with COVID-19. Heat inactivated plasma samples were serially diluted from 1:20 to 1:12,500. Foci were visualized by sequentially incubating the serum of a rabbit immunized with SARS-CoV-2 nucleocapsid protein, goat anti-rabbit IgG antibody conjugated to alkaline phosphatase (Invitrogen, Waltham, MA, USA), and nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (Roche, Basel, Switzerland). The FRNT50 antibody titers were calculated by non-linear regression analysis using a mode of inhibitor vs normalized response in GraphPad Prism version 9.1.2 (GraphPad Software, San Diego, CA, USA).
Data were analyzed from 12 patients with COVID-19 pneumonia and four persons with asymptomatic SARS-CoV-2 infection. None of these patients was vaccinated against COVID-19 during the study period. The clinical characteristics of the patients are summarized in Table 1.
Table 1. Clinical characteristics of the patients.
| Patients | Age | Sex | Symptom | Pneumonic infiltration | Severity | Max.O2 demand | DM | HTN | Lowest lymphocyte count, /μL | Peak hs-CRP, mg/dL | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 20 | Male | Absent | None | Asymptomatic | - | - | - | NA | NA | - |
| B | 21 | Female | Absent | None | Asymptomatic | - | - | - | NA | NA | - |
| C | 25 | Male | Absent | None | Asymptomatic | - | - | - | NA | NA | - |
| D | 26 | Male | Absent | None | Asymptomatic | - | - | - | NA | NA | - |
| E | 41 | Female | Present | Subtle | Mild | - | - | - | NA | NA | - |
| F | 72 | Female | Present | Subtle | Mild | - | - | - | 889 | 1.26 | Remdesivir, baricitinib |
| G | 43 | Male | Present | Apparent | Mild | - | + | + | 2,032 | 3.98 | Lopinavir/ritonavir |
| H | 69 | Female | Present | Apparent | Mild | - | - | + | 1,393 | 3.89 | Lopinavir/ritonavir |
| I | 39 | Male | Present | Apparent | Severe | NP 3 L/min | - | - | 1,017 | 18.27 | - |
| J | 67 | Male | Present | Apparent | Severe | NP 3 L/min | + | + | 774 | 13.32 | Remdesivir, baricitinib |
| K | 48 | Male | Present | Apparent | Severe | NP 4 L/min | - | - | 483 | 25.73 | Remdesivir, dexamethasone |
| L | 51 | Male | Present | Apparent | Severe | HFNC | - | + | 540 | 20.32 | Remdesivir, lopinavir/ritonavir |
| M | 65 | Male | Present | Apparent | Severe | HFNC | + | - | 1,078 | 7.09 | Remdesivir, baricitinib |
| N | 69 | Female | Present | Apparent | Severe | HFNC | - | + | 570 | 20.89 | Remdesivir, lopinavir/ritonavir |
| O | 61 | Male | Present | Apparent | Severe | MV | + | + | 548 | 30.45 | Remdesivir, baricitinib, lopinavir/ritonavir |
| P | 76 | Female | Present | Apparent | Severe | MV | - | + | 838 | 18.66 | Remdesivir |
DM = diabetes mellitus, HTN = hypertension, hsCRP = high-sensitivity C-reactive protein, NA = not applicable, NP = nasal prong, HFNC = high flow nasal cannula, MV = mechanical ventilation.
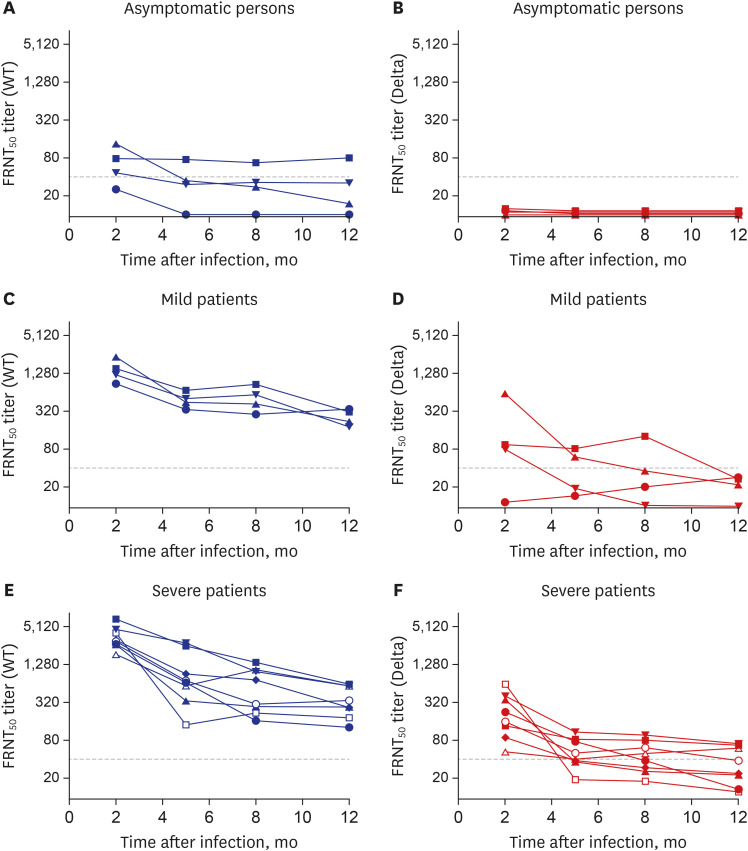
All eight patients with severe disease and all four with mild disease, but only one (25%) of the four individuals with asymptomatic infection, had FRNT50 titers ≥ 40 against SARS-CoV-2/wt at 1 year (Fig. 1). The FRNT50 titers against the delta variant in all convalescent samples were reduced (median, 12.6-fold; range, 2.2-through 219.4-fold). None of the asymptomatic individuals had FRNT50 titers ≥ 40 against the delta variant. All eight patients with severe disease had FRNT50 titers ≥ 40 against the delta variant 2 months after infection, whereas five (62.5%) and three (37.5%) had FRNT50 titers ≥ 40 at 5 months and 1 year, respectively.
Fig. 1. Neutralizing antibody responses against SARS-CoV-2/wt and the SARS-CoV-2 B.1.671.2 (Delta) variant in serially collected samples from 12 symptomatic patients and four asymptomatic individuals. (A) FRNT50 titers against SARS-CoV-2/wt in asymptomatic persons. (B) FRNT50 titers against the delta variant in asymptomatic persons. (C) FRNT50 titers against SARS-CoV-2/wt in individuals who had recovered from mild COVID-19. (D) FRNT50 titers against the delta variant in individuals who had recovered from mild COVID-19. (E) FRNT50 titers against SARS-CoV-2/wt in individuals who had recovered from severe COVID-19. (F) FRNT50 titers against the delta variant in individuals who had recovered from severe COVID-19. Titers ≤ 10 were regarded as 10 and dotted lines indicate 1:40 titer.
WT = wild type, FRNT = focus reduction neutralization test, SARS-CoV-2 = severe acute respiratory syndrome-coronavirus-2, COVID-19 = coronavirus disease 2019.
The S1 ELISA optical density correlated well with FRNT50 antibody titers against SARS-CoV-2/wt (correlation coefficient, 0.72) and the delta variant (correlation coefficient, 0.60). Of the 57 S1 ELISA-positive plasma samples, 54 (94.7%) had FRNT50 titers ≥ 40 against wild-type virus, but only 26 (48.1%) had FRNT50 titers ≥ 40 against the delta variant (Supplementary Fig. 1).
Neutralizing antibodies play an essential role in virus clearance and have been considered to be a critical immune factor for protection against viral disease. The presence of neutralizing antibodies was found to correlate with protection from reinfection with SARS-CoV-2.7,8 One modeling study suggested that an in vitro neutralization titer between 1:10 and 1:30 was required for 50% protection against detectable SARS-CoV-2 infection.9
The widespread variant strains raise concerns about reinfection due to reduced neutralization by antibodies induced in patients previously infected with COVID-19.4 This study found that the plasma samples from asymptomatic persons barely neutralized the delta variant. In mildly infected patients, the neutralizing antibody titers against the delta variant were low at 2 months after infection, suggesting that individuals recovering from asymptomatic or mild SARS-CoV-2/wt infection may be at risk of reinfection with the delta variant. Because immune responses were much greater in patients with severe than mild COVID-19,10 severely infected patients have been regarded as less susceptible to reinfection. The present study, however, found that neutralizing antibody titers against the delta variant in patients with severe COVID-19 decreased to ≤ 1:40 beginning 5 months after infection, suggesting that these patients were susceptible to reinfection by the delta variant.
Although the delta variant has been reported to be less susceptible to neutralization by serum from vaccinated individuals,4 a two-dose vaccination regimen generated high sero-neutralization levels against the delta variant after 8 to 16 weeks.2,3 The two-dose effectiveness of the AstraZeneca and Pfizer vaccines against the delta variant was estimated to be 60% and 88%, respectively.11 In persons who had recovered from COVID-19, the administration of a single dose of vaccine boosted the humoral immune response to a higher level than the response in previously uninfected persons who had received a second dose.12 These results strongly suggest that vaccination of previously infected individuals is likely to protect against variant strains, including the delta variant. The results of the present study indicated that the vaccination strategy for persons with past natural infection should depend on the severity of infection and that the vaccination of individuals who had recovered from COVID-19 should not be postponed, especially in asymptomatic persons.
Ethics statement
The Institutional Review Boards (IRBs) of Seoul National University Hospital approved the study (IRB No. H-2004-158-1118). Written informed consent was obtained from all patients.
ACKNOWLEDGMENTS
The authors thank Kyung Sook Ahn, MD, Director of Gyeongsan City Health Center, for administrative support, and Areum Jo and Su Jin Choi of the Seoul National University Hospital Biomedical Research Institute for technical support.
Footnotes
Funding: This work was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) and was funded by the Korean government (MSIT) (grant No. 2021M3A9H5020761 and 2021M3A9I2080498) and by the SNUH Research Fund (grant No. 04-2021-0210).
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Park WB, Cho NH.
- Data curation: Choe PG, Kim Y, Chang E, Kang CK.
- Formal analysis: Choe PG, Kim NJ, Cho NH, Park WB, Oh MD.
- Funding acquisition: Cho NH, Park WB.
- Investigation: Choe PG, Kang CK.
- Methodology: Kim Y, Cho NH, Park WB.
- Supervision: Cho NH, Park WB.
- Validation: Choe PG, Kim Y, Chang E, Kang CK, Kim NJ, Cho NH, Park WB, Oh MD.
- Writing - original draft: Choe PG, Park WB.
- Writing - review & editing: Choe PG, Kim Y, Chang E, Kang CK, Kim NJ, Cho NH, Park WB, Oh MD.
SUPPLEMENTARY MATERIAL
Correlation between anti-S1 IgG ELISA optic density ratios and FRNT50 titers against severe acute respiratory syndrome-coronavirus-2/wt (A) and the delta variant (B) in plasma serially sampled from asymptomatic and symptomatic coronavirus disease 2019 patients. Vertical and horizontal dotted lines indicate the cut-off values (1.1) of the optical density ratio between borderline and positive results and 1:40 titers, respectively.
References
- 1.Singh J, Rahman SA, Ehtesham NZ, Hira S, Hasnain SE. SARS-CoV-2 variants of concern are emerging in India. Nat Med. 2021;27(7):1131–1133. doi: 10.1038/s41591-021-01397-4. [DOI] [PubMed] [Google Scholar]
- 2.Edara VV, Pinsky BA, Suthar MS, Lai L, Davis-Gardner ME, Floyd K, et al. Infection and vaccine-induced neutralizing-antibody responses to the SARS-CoV-2 B.1.617 variants. N Engl J Med. 2021;385(7):664–666. doi: 10.1056/NEJMc2107799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM, et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596(7871):276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 4.Liu C, Ginn HM, Dejnirattisai W, Supasa P, Wang B, Tuekprakhon A, et al. Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum. Cell. 2021;184(16):4220–4236.e13. doi: 10.1016/j.cell.2021.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choe PG, Kang CK, Kim KH, Yi J, Kim ES, Park SW, et al. Persistence of neutralizing antibody response up to 1 year after asymptomatic or symptomatic SARS-CoV-2 infection. J Infect Dis. 2021;224(6):1097–1099. doi: 10.1093/infdis/jiab339. [DOI] [PubMed] [Google Scholar]
- 6.Choe PG, Kang EK, Lee SY, Oh B, Im D, Lee HY, et al. Selecting coronavirus disease 2019 patients with negligible risk of progression: early experience from non-hospital isolation facility in Korea. Korean J Intern Med. 2020;35(4):765–770. doi: 10.3904/kjim.2020.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C, et al. COVID-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385(16):1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lumley SF, O’Donnell D, Stoesser NE, Matthews PC, Howarth A, Hatch SB, et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. 2021;384(6):533–540. doi: 10.1056/NEJMoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 10.Choe PG, Kang CK, Suh HJ, Jung J, Kang E, Lee SY, et al. Antibody responses to SARS-CoV-2 at 8 weeks postinfection in asymptomatic patients. Emerg Infect Dis. 2020;26(10):2484–2487. doi: 10.3201/eid2610.202211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385(7):585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anichini G, Terrosi C, Gandolfo C, Gori Savellini G, Fabrizi S, Miceli GB, et al. SARS-CoV-2 antibody response in persons with past natural infection. N Engl J Med. 2021;385(1):90–92. doi: 10.1056/NEJMc2103825. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Correlation between anti-S1 IgG ELISA optic density ratios and FRNT50 titers against severe acute respiratory syndrome-coronavirus-2/wt (A) and the delta variant (B) in plasma serially sampled from asymptomatic and symptomatic coronavirus disease 2019 patients. Vertical and horizontal dotted lines indicate the cut-off values (1.1) of the optical density ratio between borderline and positive results and 1:40 titers, respectively.