Abstract
Background
Hypokalemic periodic paralysis (HPP) is a rare muscle disorder characterized by episodic muscle weakness that can lead to respiratory failure. This disorder is a common manifestation of renal tubular acidosis. Renal tubular acidosis can occur associated with various systemic disorders such as Sjogren's syndrome and thyroid disorders.
Case presentation
A 58-year-old woman complained of weakness in all extremities. In the last 3 years, the patient was diagnosed with hypothyroidism and got recurrent hypokalemia without vomiting or diarrhea. The examination showed blood pressure of 110/70 mmHg, pulse rate of 98 ×/m, temperature of 36.8 °C, and respiratory rate of 20 ×/m. Motor strength 2 in all four extremities. The ECG examination showed 1st degree AV block. Laboratory examination found hypokalemia, metabolic acidosis with a normal anion gap of 13.8 meq/L, urine pH 8.0, urine anion gap 41 mmol/h. FT4 1.89 ng/dL, TSH 1.21 IU/mL. Anti TPO 20.6 IU/mL, ANA profile: strong positive SS-A (Ro), Ro-52, SS-B (La) which indicates Sjogren's syndrome.
Discussion
HPP is a rare case so the accuracy of diagnosis increases the success of treatment.
Conclusion
The patient was diagnosed with HPP and renal tubular acidosis based on hypokalemia, metabolic acidosis, alkaline urine, and positive urine anion gap.
Keywords: Autoimmune, HPP, Hypothyroid, Renal tubular acidosis, Sjogren's syndrome
Highlights
-
•
Hypokalemic periodic paralysis (HPP) is a rare case that was recently reported in Indonesia.
-
•
Early diagnosis of HPP and renal tubular acidosis play important role in disease management.
-
•
The diagnosis of HPP and renal tubular acidosis, in this case, was reviewed from the laboratory data and the emerging clinical aspects.
1. Introduction
Hypokalemic periodic paralysis (HPP) is a neuromuscular disorder characterized by periodic skeletal muscle weakness that can cause respiratory muscle failure and even death [1]. The prevalence of HPP is 1 in 100,000 cases [2]. Muscle weakness depends on changes in serum potassium level (<3.5 mmol/dL) which can be due to primary or secondary causes. Primary causes are generally autosomal dominant, while secondary causes include diuretic use, loss from the gastrointestinal tract, renal tubular acidosis (RTA), primary hyperaldosteronism, Barter's syndrome, hyperthyroidism, and hypothyroidism [3].
Renal tubular acidosis refers to a transport defect characterized by the kidney's inability to excrete acid (H+) and reabsorb bicarbonate (HCO3-) with the clinical syndrome of metabolic acidosis with a normal anion gap, hyperchloremia, and impaired urine acidification [4]. Renal tubular acidosis can be caused by primary causes due to genetic mutations or secondary causes [5]. We report based on Surgical Case Report (SCARE) 2020 guidelines [6].
2. Case Presentation
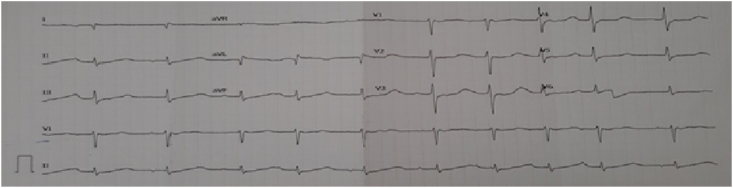
A 58-year-old woman with hypokalemia and hypothyroidism. She had a complaint of weakness in all four limbs. The patient has had a history of hypothyroidism since 3 years ago and is still on Eutirox 1 × 100 mg treatment. For the last 3 years, she has been hospitalized 7 times because of repeated hypokalemia. The patient previously had no complaints of vomiting or diarrhea. Physical examination: blood pressure of 130/70 mmHg, pulse of 98 ×/minute, temperature of 36.8 °C, breathing of 20 ×/minute. The following are the results of laboratory tests which can be seen in Table 1. The results of the electrocardiographic examination can be seen in Fig. 1.
Table 1.
Laboratory examination.
| Parameters | Results |
|---|---|
| Clinical chemistry | |
| Potassium (mmol/L) | 2.3 |
| Sodium (mmol/L) | 140 |
| Chloride (mmol/L) | 110 |
| BUN (mg/dL) | 17 |
| Serum Creatinine (mg/dL) | 1.06 |
| Albumin (g/dL) | 3.36 |
| AST (U/L) | 16 |
| ALT (U/L) | 34 |
| Arterial Blood Gas | |
| pH | 7.26 |
| pCO2 (mmHg) | 25 |
| HCO3 (mmol/L) | 11.2 |
| Base Excess (mmol/L) | −15.9 |
| Anion Gap (mEq/L) | 13.8 |
| Immunological test | |
| FT4 | 1.89 |
| TSH | 0.121 |
| Anti-TPO | 20 |
| Ro-52 | 86 |
| SSB | 89 |
| SSA | 50 |
| Anti HCV | Non-Reactive |
| HbsAg | Non-Reactive |
| Urine analysis | |
| pH | 8.0 |
| Leucocyte | Negative |
| Protein | 2+ |
| Nitrite | Negative |
| Ketone | Negative |
| Glucose | Negative |
| Urobilinogen | Negative |
| Bilirubin | Negative |
| Erythrocyte | 2+ |
| Potassium (mmol/24 h) | 82 |
| Sodium (mmol/24 h) | 229 |
| Chloride (mmol/24 h) | 270 |
| Phosphate (mg/24 h) | 504 |
| Calcium (mg/24 h) | 182 |
| Creatinine (mg/24 h) | 394 |
| Coagulation function | |
| PPT (s) | 10.3 |
| APTT (s) | 24.9 |
| Hematology | |
| WBC (× 103/μL) | 15.35 |
| % Neu | 89.3 |
| % Lym | 5.4 |
| % Mono | 3.7 |
| % Eos | 0.7 |
| % Baso | 0.1 |
| RBC (× 106/μL) | 4.41 |
| Hb (g/dL) | 11.6 |
| Hematocrit (%) | 37.3 |
| Mean corpuscular volume (fL) | 84.3 |
| Mean corpuscular haemoglobin (pg) | 26.2 |
| Mean corpuscular haemoglobin concentration (g/dL) | 31.1 |
| Platelet | 427 |
Fig. 1.
ECG results.
The patient was also examined for neurological status such as Glasgow Coma Scale (GCS) of compos mentis [7], neck rigidity (−), all cranial nerve of normal [8], decrease motoric skills of the upper and lower extremity, the sensory skill of normal, physiology reflex of normal, and nothing pathology reflex. Detailed motoric skills assessment can be seen in Table 2. Based on the motor examination performed, the patient experienced weakness in all four extremities (quadriparesis). History, physical examination, and laboratory investigations on the patient concluded as hypokalemia periodic paralysis. This patient was treated to manage his emergency with IVFD therapy WIDA KN-2 1500 cc/day, KSR 3 × 600 mg, Ranitidine 2 × 50 mg, Metoclopramide 3 × 10 mg, Euthyrox 1 × 100 mg.
Table 2.
Motoric assessment of upper and lower extremity.
| Assessment | Upper extremity |
Lower extremity |
||
|---|---|---|---|---|
| Dextra | Sinistra | Dextra | Sinistra | |
| Strength | 2 | 2 | 2 | 2 |
| Movement | Limited | Limited | Limited | Limited |
| Tonus | Normal | Normal | Normal | Normal |
| Muscle | Eutrophic | Eutrophic | Eutrophic | Eutrophic |
| Muscle clonus | – | – | – | – |
3. Discussion
Hypokalemic periodic paralysis is a common manifestation of renal tubular acidosis. Renal tubular acidosis is defined as the inability of the renal tubules to acidify urine, under conditions of normal or near-normal glomerular filtration rate [1,9]. Under conditions of acidosis, the renal tubules are unable to excrete acid (H+) or reabsorb bicarbonate (HCO3-) which results in a clinical syndrome such as metabolic acidosis with a normal anion gap. Renal tubular acidosis is mainly divided into 4 types, namely distal renal tubular acidosis (type 1), proximal renal tubular acidosis (type 2), hyperkalemic renal tubular acidosis, and type 3 renal tubular acidosis which is a mixture of proximal and distal renal tubular acidosis. This type 3 RTA is very rare. Distal renal tubular acidosis is characterized by reduced acid secretion in the urine, while proximal renal tubular acidosis is caused by impaired reabsorption of bicarbonate (HCO3-). Type 4 RTA shows acid-base disturbances that occur due to deficiency or aldosterone resistance. Distal renal tubular acidosis is the most common type of renal tubular acidosis [4,10,11].
Distal renal tubular acidosis can be caused by primary or secondary causes. The most common “acquired” secondary causes of distal renal tubular acidosis are autoimmune diseases such as Sjogren's syndrome, systemic lupus erythematosus, rheumatoid arthritis, hypothyroidism, hyperthyroidism, Hashimoto thyroiditis [1]. Renal tubular acidosis is very rarely reported in patients with thyroid disorders such as hypothyroidism [9]. Hypothyroidism can cause renal tubular acidosis through a defect in the Na–K ATPase pump in the cortical and/or tubular medulla so that the function of the Na–K ATPase pump is reduced, causing decreased H+ elimination [12,13].
Examination of the ANA profile (Anti-Nuclear Antibody) shows anti-SSA (Ro), Ro 52, SS-B (La) which leads to Sjogren's syndrome, systemic lupus erythematosus (SLE). SS-B (La) is said to be much more specific for Sjogren's syndrome, whereas SS-A/Ro is associated with many other autoimmune conditions but is frequently present in Sjogren's syndrome [14]. Sjogren's syndrome is a chronic autoimmune disease characterized by lymphocyte infiltration in the salivary and lacrimal glands [5,15]. Extra-glandular manifestations include neurological, renal, hepatic, cutaneous, respiratory, and vascular. The prevalence of Sjogren's syndrome is 1–3% of the total population, affecting women more than men with a ratio of 9:1. Sjogren's syndrome is found in two forms, namely primary Sjogren's syndrome with a single clinical manifestation, while secondary Sjogren's syndrome is accompanied by other autoimmune diseases such as rheumatoid arthritis, SLE, scleroderma [16].
The prevalence of renal tubular acidosis in patients with Sjogren's syndrome is 4.5–9%, generally occurring in middle age and only two-thirds are symptomatic [14]. Renal system involvement in Sjogren's syndrome is one of the extra-glandular manifestations and occurs in <10% of patients. Distal renal tubular acidosis, which is common in Sjogren's syndrome, is usually asymptomatic and often goes undetected in most cases. The most common electrolyte abnormality in distal renal tubular acidosis is hypokalemia, occurring in approximately 28–53% of patients. Hypokalemia may occur earlier than typical glandular symptoms and reveal previously undiagnosed Sjogren's syndrome [13]. Hypokalemia paralysis is an early symptom that appears in 7% of patients with Sjogren's syndrome [14,17].
The pathogenesis of distal renal tubular acidosis in patients with Sjogren's syndrome is not fully understood. Several studies indicate the absence or disruption of the H + -ATPase pump and the presence of autoantibodies against intercalated cells in the collecting duct and attack carbonic anhydrase (CA) in the distal nephron [11]. In addition, the presence of interstitial infiltration of lymphocytes and plasma cells that invade the tubular and epithelial membranes causes structural changes that lead to a secretory defect in the distal tubule [18]. Findings on kidney biopsies of patients with Sjogren's syndrome also show the absence of the H + -ATPase pump in the intercalated cells of the collecting duct [19].
Based on the diagnostic criteria of the American European Consensus Group Criteria of Sjogren's syndrome, this patient only had ocular symptoms (dry eyes), oral symptoms (dry mouth), and positive Anti-SSA (Ro) and Anti-SSB (La) autoantibodies. In this patient, several examinations to exclude the possibility of SLE, rheumatoid arthritis, and tests that support the diagnosis of Sjogren's syndrome could not be performed, including ANA test, rheumatoid factor, Schirmer test, salivary gland biopsy.
Quadriparesis is a neurological condition characterized by weakness in all four limbs. Management of quadriparesis in HPP Administration of potassium chloride 0.5–1.0 mEq/kg which can increase motor strength in all four extremities. The return of potassium concentration to normal levels maintains the balance of cation and anion ion channel gates in the process of excitation and inhibition of muscle cells [20,21].
4. Conclusion
Based on the symptoms of hypokalemia, weakness in all 4 extremities, metabolic acidosis with a normal anion gap, positive urine anion gap, and alkaline urine pH, the patient is diagnosed with periodic hypokalemia and renal tubular acidosis. The presence of objective complaints of dry eyes and dry mouth for more than 3 months, positive anti-SSA (Ro) anti-SSB (La) autoantibodies can be suspected to lead to a Sjogren's syndrome. This patient needs further examination to rule out the diagnosis of SLE, rheumatoid arthritis, and additional examinations that support the diagnosis of Sjogren's syndrome including salivary gland biopsy, Schirmer test.
Ethical approval
Not applicable.
Research registration
Not applicable.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Sources of funding
None.
Author contribution
All authors contributed toward data analysis, drafting, and revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgment
We would like to thank our editor “Fis Citra Ariyanto”.
References
- 1.Sinha U., Sengupta N., Sinharay K., Sahana P.K. Recurrent hypokalemic paralysis: an atypical presentation of hypothyroidism. Indian journal of endocrinology and metabolism. 2013;17(1):174–176. doi: 10.4103/2230-8210.107880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yılmaz H., Kaya M., Özbek M., ÜUreten K., Safa Yıldırım İ. Hypokalemic periodic paralysis in Sjogren's syndrome secondary to distal renal tubular acidosis. Rheumatol. Int. 2013;33(7):1879–1882. doi: 10.1007/s00296-011-2322-z. [DOI] [PubMed] [Google Scholar]
- 3.Meregildo-Rodríguez E.D., Failoc-Rojas V.E. Case Report: recurrent hypokalemic periodic paralysis associated with distal renal tubular acidosis (type 1) and hypothyroidism secondary to Hashimoto's thyroiditis. F1000Research. 2018;7:1154. doi: 10.12688/f1000research.15662.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander R.T., Bitzan M. Renal tubular acidosis. Pediatr. Clin. 2019;66(1):135–157. doi: 10.1016/j.pcl.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Garza-Alpirez A., Arana-Guajardo A.C., Esquivel-Valerio J.A., Villarreal-Alarcón M.A., Galarza-Delgado D.A. Hypokalemic paralysis due to primary Sjögren syndrome: case report and review of the literature. Case reports in rheumatology. 2017:7509238. doi: 10.1155/2017/7509238. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A. The SCARE 2020 guideline: updating Consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 7.Bledsoe B.E., Casey M.J., Feldman J., Johnson L., Diel S., Forred W., et al. Glasgow coma Scale Scoring is often inaccurate. Prehospital Disaster Med. 2015;30(1):46–53. doi: 10.1017/s1049023x14001289. [DOI] [PubMed] [Google Scholar]
- 8.Damodaran O., Rizk E., Rodriguez J., Lee G. Cranial nerve assessment: a concise guide to clinical examination. Clin. Anat. 2014;27(1):25–30. doi: 10.1002/ca.22336. [DOI] [PubMed] [Google Scholar]
- 9.Yaxley J., Pirrone C. Review of the diagnostic evaluation of renal tubular acidosis. Ochsner J. 2016;16(4):525–530. [PMC free article] [PubMed] [Google Scholar]
- 10.Bagga A., Sinha A. Evaluation of renal tubular acidosis. Indian J. Pediatr. 2007;74(7):679–686. doi: 10.1007/s12098-007-0120-0. [DOI] [PubMed] [Google Scholar]
- 11.Soleimani M., Rastegar A. Pathophysiology of renal tubular acidosis: core curriculum 2016. Am. J. Kidney Dis. : the official journal of the National Kidney Foundation. 2016;68(3):488–498. doi: 10.1053/j.ajkd.2016.03.422. [DOI] [PubMed] [Google Scholar]
- 12.Virdee S., Greenan-Barrett J., Ciurtin C. A systematic review of primary Sjögren's syndrome in male and paediatric populations. Clin. Rheumatol. 2017;36(10):2225–2236. doi: 10.1007/s10067-017-3745-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romão V.C., Talarico R., Scirè C.A., Vieira A., Alexander T., Baldini C., et al. Sjögren's syndrome: state of the art on clinical practice guidelines. RMD open. 2018;4(Suppl 1) doi: 10.1136/rmdopen-2018-000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thorne I., Sutcliffe N. Sjögren's syndrome. Br. J. Hosp. Med. 2017;78(8):438–442. doi: 10.12968/hmed.2017.78.8.438. [DOI] [PubMed] [Google Scholar]
- 15.Stefanski A.L., Tomiak C., Pleyer U., Dietrich T., Burmester G.R., Dörner T. The diagnosis and treatment of Sjögren's syndrome. Deutsches Arzteblatt international. 2017;114(20):354–361. doi: 10.3238/arztebl.2017.0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rischmueller M., Tieu J., Lester S. Primary Sjögren's syndrome. Best Pract. Res. Clin. Rheumatol. 2016;30(1):189–220. doi: 10.1016/j.berh.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Cafaro G., Croia C., Argyropoulou O.D., Leone M.C., Orlandi M., Finamore F., et al. One year in review 2019: Sjögren's syndrome. Clin. Exp. Rheumatol. 2019;37(3):3–15. Suppl 118. [PubMed] [Google Scholar]
- 18.Mavragani C.P., Moutsopoulos H.M. Sjögren's syndrome: old and new therapeutic targets. J. Autoimmun. 2020;110:102364. doi: 10.1016/j.jaut.2019.102364. [DOI] [PubMed] [Google Scholar]
- 19.Fox R.I., Fox C.M., Gottenberg J.E., Dörner T. Treatment of Sjögren's syndrome: current therapy and future directions. Rheumatology. 2021;60(5):2066–2074. doi: 10.1093/rheumatology/kez142. [DOI] [PubMed] [Google Scholar]
- 20.Bansal N., Kumar P.A., Agarwal M.P., Aggarwal A. Recurrent hypokalemia leading to Flaccid quadriparesis: a renal or connective tissue disorder. Puert. Rico Health Sci. J. 2017;36(4):240–242. [PubMed] [Google Scholar]
- 21.Sardar Z., Waheed K.A.F., Javed M.A., Akhtar F., Bokhari S.R.A. Clinical and etiological Spectrum of hypokalemic periodic paralysis in a tertiary care hospital in Pakistan. Cureus. 2019;11(1) doi: 10.7759/cureus.3921. [DOI] [PMC free article] [PubMed] [Google Scholar]