Abstract
Purpose of Review
Glioblastoma is the commonest primary brain cancer in adults whose outcomes are amongst the worst of any cancer. The current treatment pathway comprises surgery and postoperative chemoradiotherapy though unresectable diffusely infiltrative tumour cells remain untreated for several weeks post-diagnosis. Intratumoural heterogeneity combined with increased hypoxia in the postoperative tumour microenvironment potentially decreases the efficacy of adjuvant interventions and fails to prevent early postoperative regrowth, called rapid early progression (REP). In this review, we discuss the clinical implications and biological foundations of post-surgery REP. Subsequently, clinical interventions potentially targeting this phenomenon are reviewed systematically.
Recent Findings
Early interventions include early systemic chemotherapy, neoadjuvant immunotherapy, local therapies delivered during surgery (including Gliadel wafers, nanoparticles and stem cell therapy) and several radiotherapy techniques. We critically appraise and compare these strategies in terms of their efficacy, toxicity, challenges and potential to prolong survival. Finally, we discuss the most promising strategies that could benefit future glioblastoma patients.
Summary
There is biological rationale to suggest that early interventions could improve the outcome of glioblastoma patients and they should be investigated in future trials.
Keywords: Glioblastoma; Radiotherapy; Intraoperative radiotherapy; Radiation; Brachytherapy; Neoadjuvant, Neurosurgery; Preoperative; Progression; Stem cells; Gliadel; Immunotherapy; Radiosurgery
Introduction
Glioblastoma is the commonest primary malignant brain tumour in adults [1]. The median overall survival with standard treatment, comprising surgery and postoperative chemoradiotherapy, is just 15 months [2]. Despite decades of research, the 5-year survival remains < 5% and modern treatment fails to halt local recurrence, which occurs in > 80% of patients within 2 cm of the original surgical cavity [3, 4].
In the time between surgery and radiotherapy, remnant tumour cells remain untreated causing rapid early progression (REP), which is associated with a shorter survival [5–13]. This highlights the limitations of the current glioblastoma treatment pathway and the desperate need for new strategies. One approach involves intensified upfront therapy, which could provide timely treatment to a favourable tumour microenvironment, to counter mechanisms leading to REP and improve patient outcome. Importantly, this approach is different to simply earlier commencement of standard postoperative chemoradiotherapy, which could have a negative effect on outcome [14–16].
This review will explore the biological rationale and clinical landscape of early interventions in newly diagnosed glioblastoma, based mostly on preclinical and early phase clinical trial data. Our aim is to stimulate novel treatment approaches to improve the outcome of this deadly disease.
Biological Justifications
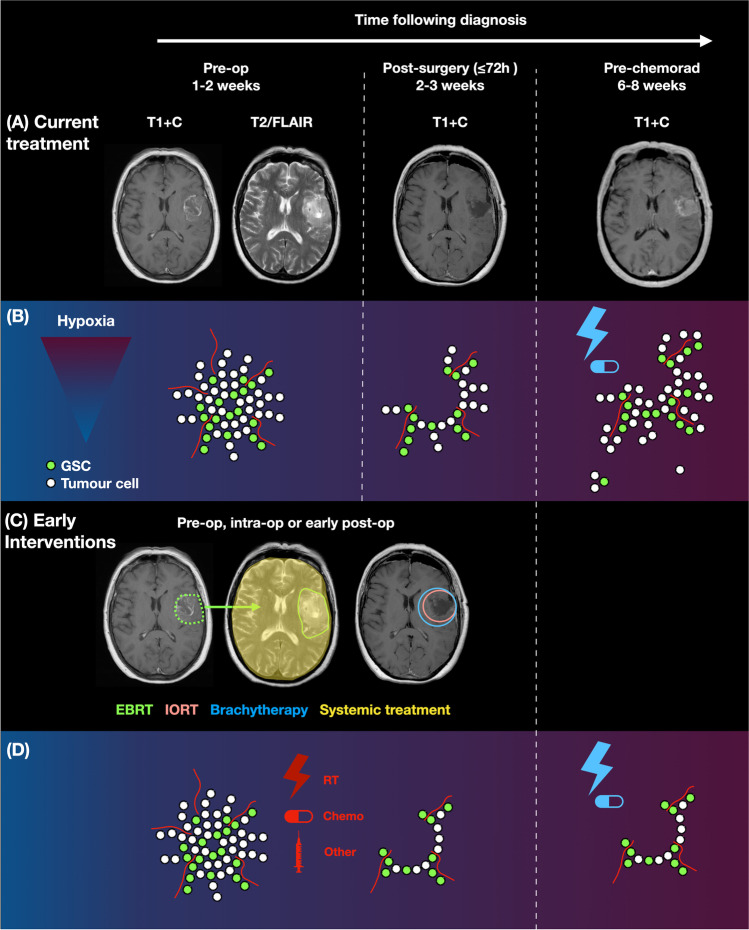
There are several biological advantages to earlier interventions (Fig. 1).
Fig. 1.
Biological rationale for early therapeutic interventions in newly diagnosed glioblastoma. A Limitations of the current treatment pathway for glioblastoma: serial MRI scans of a 63-year-old male who presented with seizures and dysphasia and was diagnosed with a glioblastoma. Scans are displayed preoperatively, postoperatively and pre-chemoradiotherapy demonstrating the development of rapid early progression (REP) in the time interval between surgery and postoperative chemoradiotherapy. B Cartoon representation of tumour cells through the current treatment pathway. Cells in the invasive margin of glioblastoma remain untreated for several weeks and may contribute to REP, potentiated by the negative biological effects of surgery. The current treatment pathway fails to prevent REP and adjuvant treatment is also delivered to a relatively more hypoxic postoperative tumour bed. C Sites of action of early therapeutic interventions including radiotherapy and systemic therapies. D Cartoon representation of the beneficial effects of early interventions on tumour cells, leading to fewer tumour cells with time, through earlier treatment of the invasive margin and subsequent prevention of REP. Early interventions also act on a relatively less hypoxic microenvironment and could increase the effectiveness of chemoradiotherapy. Abbreviations: T1 + C, T1 with contrast; Pre-op, preoperative; GSC, glioma stem cell; chemorad, chemoradiotherapy; EBRT, external beam radiation therapy; IORT, intraoperative radiotherapy; RT, radiotherapy; chemo, chemotherapy
Rapid Early Progression
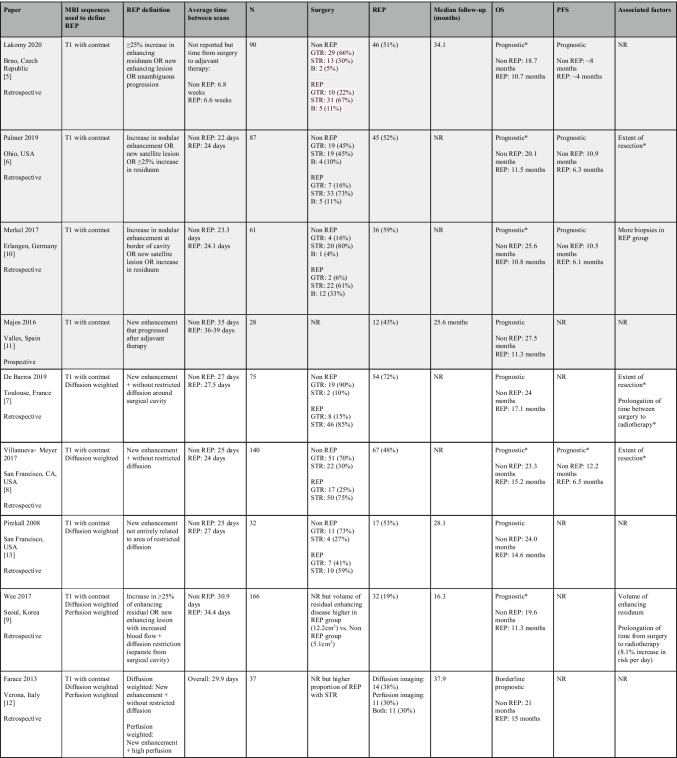
Approximately half of all glioblastoma patients develop macroscopically observed REP between surgery and postoperative radiotherapy, which is associated with a shorter survival (Table 1 and Fig. 1A) [5–13]. From a biological perspective, macroscopically observed REP is associated with extent of resection and volume of residual disease and more frequently observed in patients with subtotal resection [6, 8, 10]. In addition, preclinical studies suggest that surgery potentiates the proliferative and migratory state of glioblastoma cells on a microscopic level [17, 18]. In one clinical study of patients with multifocal glioblastoma where just one tumour was biopsied, the biopsied tumour grew faster than the non-biopsied tumour, with an increase in tumour cell motility, migration and proliferation [17]. The poorer outcomes of patients after subtotal resection could be in part explained by REP [19].
Table 1.
Rapid early progression (REP) in glioblastoma. Studies have been grouped into two groups: those using just T1 contrast enhancement as an indicator of REP (shaded light grey) and those using T1 contrast enhancement in combination with diffusion and/or perfusion weighted imaging (shaded white)
*Indicates significance in multivariate analysis. Abbreviations: GTR, gross total resection; STR, subtotal resection; B, biopsy; REP, rapid early progression; OS, overall survival; PFS, progression-free survival; NR, not reported
Hypoxia
Neoadjuvant treatment may better target tumours due to the higher preoperative tumoural blood flow and less hypoxia compared to postoperative residual tumours [20]. Magnetic resonance imaging (MRI) studies have demonstrated the ischaemic side effects of surgery, which are correlated with more aggressive recurrence patterns. Indeed, postoperative diffusion-weighted MRI demonstrates areas of restricted diffusion indicative of ischaemia, in up to 90% of patients and more frequently after gross total resection (GTR) [21]. Although GTR improves outcome, ischaemic lesions are independently associated with a shorter survival and multifocal tumour recurrence [22]. In addition, hypoxia is associated with treatment resistance and is independently related to worse outcomes for glioblastoma patients [23, 24]. Hypoxia alters the glioblastoma microenvironment towards a more aggressive phenotype largely through increased hypoxia-inducible-factor (HIF) signalling. These transcription factors have several effects including: maintenance of cancer stem cell stemness and promotion of tumour cell dedifferentiation [25, 26]; upregulation of vascular endothelial growth factor (VEGF) mediated angiogenesis [26]; increased chemokine signalling to promote vasculogenesis [26]; upregulation of microRNAs involved in resistance to temozolomide [27]; promotion of a proneural-to-mesenchymal transition [28]; and a shift towards a growth promoting metabolome with increased rates of glycolysis [28]. Treatment of a biological system containing fewer aggressive hypoxic regions may therefore improve the overall outcome.
Untreated Tumour Cells
Glioblastoma is a diffusely infiltrative disease whose microscopic margins extend beyond those visible on modern imaging or intraoperative appearance [29]. Unresected remnant cells are exposed to surgery induced tumour potentiating factors. In addition, these cells are not treated for at least 4–6 weeks, the average time period between surgery and postoperative chemoradiotherapy [14]. This time period is longer than the predicted doubling time of the disease [30]. Furthermore, temporal heterogeneity of glioblastoma increases with time, with an increase in overall mutation burden and subclones after completion of treatment [31]. Targeting an earlier biological tumour system with less heterogeneity may therefore translate into clinical benefit.
In summary, these observations suggest a role for earlier interventions that could include traditional modalities like surgery, radiotherapy and chemotherapy, and novel approaches including targeted agents such as immunotherapy. The following sections will provide an overview of these strategies.
Clinical Interventions
Surgery
Early repeat surgery could be used as a treatment for REP. It is currently performed for unintended residual disease, though done very rarely given the widespread use of tools to aid maximal safe resection. When employed, outcomes appear to be favourable, though length of stay is increased [32, 33]. However, early repeat surgery may not be technically possible and/or considered too high risk in most cases because of the location of the remnant/progressive tumour [5, 10]. This surgical risk assessment is crucial, as demonstrated in a large UK national study of residual enhancing disease in glioblastoma. Although 44/80 patients had residual enhancing disease, none underwent further surgery [34]. Additional arguments against surgery include the fact it may delay postoperative therapy further for untreated cells and does not exploit the favourable early tumour microenvironment.
Systemic Treatment
Temozolomide
Systemic treatments could exploit and target the biological justifications for early interventions. Careful consideration is required as to the exact time point of use, which could be preoperatively or in the early postoperative period. In reference to the latter, chemotherapy could in theory result in immunosuppression and wound healing difficulties. However, this was not demonstrated in a recent case–control study of temozolomide use within 7 days after surgery, followed by the Stupp protocol (6 weeks radiotherapy and concurrent temozolomide). Indeed, a significantly longer survival was noted in the early temozolomide group (23.0 months vs. 17.0 months), with no significant increase in complications [35]. These findings require validation, though early temozolomide may be of specific benefit to patients with O6-methylguanine-DNA methyltransferase (MGMT) promotor methylation. Future research should also explore the increased survival noted in REP patients with MGMT promotor methylation versus those with unmethylated promotors, to test whether early temozolomide use could counter REP cellular processes [6].
Immunotherapy
Neoadjuvant immunotherapy is of great interest for newly diagnosed glioblastoma (Table 2) after encouraging preliminary results in recurrent glioblastoma [36]. Cloughesy et al. found neoadjuvant immunotherapy to be superior to postoperative immunotherapy alone in recurrent glioblastoma. Thirty-five patients were randomised to neoadjuvant/postoperative anti-programmed death ligand 1 (anti PD-1) or postoperative anti-PD1 alone, prior to re-resection. The median survival was almost doubled with neoadjuvant treatment (13.7 vs. 7.5 months) [36]. Neoadjuvant treatment upregulated the expression of genes related to key immune pathways such as interferon gamma responsiveness to a greater extent compared to standard postoperative therapy. This effect may translate to patients with newly diagnosed glioblastoma and data is awaited from an ongoing trial (NCT04583020) [37]. Preliminary experience of 3 patients treated with neoadjuvant anti PD-1 is encouraging (2 out of 3 patients with newly diagnosed glioblastoma survived ≥ 28 months) [38].
Table 2.
Clinical trials of early time point interventions for glioblastoma that are currently recruiting or soon to begin. *POBIG stands for PreOperative Brain Irradiation in Glioblastoma—an upcoming phase I dose escalation trial of neoadjuvant radiotherapy for newly diagnosed glioblastoma at the senior author’s institution. Abbreviations: BET, bromodomain and extra-terminal domain; PD-1, programmed death ligand 1; CDK, cyclin dependent kinase; CTLA-4, cytotoxic
| Reference | Phase | Patients | Trial intervention (see legend) | Time period | Other treatment |
|---|---|---|---|---|---|
|
(POBIG*) Manchester, UK |
I | Newly diagnosed | Radiotherapy | Neoadjuvant | None |
|
London, UK |
II | Newly diagnosed | Ipilimumab | Neoadjuvant | Not specified |
|
Multi-centre USA |
I | Newly diagnosed | Aglatimagene besadenovec (AdV-tk, gene therapy) injected into wall of surgical cavity | Intraoperative | Early postoperative valacyclovir and nivolumab |
|
INTRAGO-II International multi-centre |
III | Newly diagnosed | Intraoperative radiotherapy (Intrabeam device) | Intraoperative | Not specified |
|
Mannheim, Germany |
II | Newly diagnosed | Gamma knife radiosurgery | Early postoperative (24–72 h) | Not specified |
|
Beijing, China |
II | Newly diagnosed suitable for surgical resection | Camrelizumab (anti PD-1) | Neoadjuvant + adjuvant |
Surgical resection 60 Gy radiotherapy Temozolomide |
|
PA, USA |
II | Newly diagnosed suitable for surgical resection |
Radiotherapy Temozolomide |
Neoadjuvant | Not specified |
|
Multi-centre USA |
I/II | Recurrent gliomas suitable for salvage surgical resection |
CC-90010 (BET protein inhibitor) |
Neoadjuvant | Not specified |
|
Shanghai, China |
II | Recurrent suitable for resection | Camrelizumab (anti PD-1) and dendritic cell vaccine (or placebo) | Neoadjuvant + adjuvant | None |
|
Multi-centre USA |
I | Recurrent suitable for resection | Sapanisertib (mTOR inhibitor) | Neoadjuvant + adjuvant | Not specified |
|
Boston, USA |
I | Recurrent suitable for resection | Nivolumab (anti PD-1) ± ipilimumab (anti CTLA-4) | Neoadjuvant | Not specified |
|
San Francisco, USA |
I | Recurrent suitable for resection | Nivolumab (anti PD-1) ± ipilimumab (anti CTLA-4) | Neoadjuvant | Not specified |
|
Multi-centre USA |
0/II | Recurrent suitable for resection with suitable mutation (e.g. Rb, CDKN2A, mTOR +) | Ribociclib (CDK4/6 inhibitor) and everolimus (mTOR inhibitor) | Neoadjuvant | Not specified |
|
Barrow, USA |
I | Recurrent suitable for resection, with retinoblastoma positivity | Ribociclib (CDK4/6 inhibitor) | Neoadjuvant | Not specified |
Use of neoadjuvant immunotherapy requires careful consideration of other treatments employed in this time period that can have immunomodulatory effects. Steroids are one example that can negate the potentiating effects of surgery [17]. However, steroids are not by themselves cytotoxic and may have offset the survival benefit of postoperative immunotherapy in the CheckMate 143 trial [39]. Such concerns have led to reluctance to use high-dose steroids alongside immunotherapy.
Local Therapies at the Time of Surgery
Gliadel Wafers
Local intraoperative therapies can overcome the drug constraints of the blood–brain-barrier. Gliadel wafers (carmustine in a biodegradable polymer—providing the alternative name of carmustine wafers) deliver local chemotherapy over a period of 5–7 days, with degradation of the polymer occurring over 5–6 weeks [40]. The only completed randomised trial to evaluate their efficacy is from the pre-temozolomide era and although a marginal improvement in overall survival was noted in the Gliadel group, this was not statistically significant during long-term follow-up [41, 42]. There are also concerns about the toxicity of this treatment with respect to high seizure rates and wound complications, though these are not universally reported, and were comparable to non-Gliadel patients in the aforementioned trial and large centre experiences [42–44]. This controversy has limited the eligibility of patients with Gliadel to participate in future trials [45]. The combined efficacy of Gliadel and temozolomide is currently being evaluating in an ongoing randomised trial [46]. Other intraoperative strategies such as direct intratumoural injection of carmustine and local immunotherapy have achieved disappointing clinical results [47, 48].
Nanoparticles and Stem Cell Therapy
There is significant preclinical interest in the use of nanotechnology and human stem cells, to enhance delivery of anti-tumour therapies and target specific tumour cells. Nanoparticles can be conjugated to anti-tumour molecules and enhance drug delivery [49]. For example, the topoisomerase inhibitor camptothecin was conjugated to a nanoparticle hydrogel self-assembly drug system and injected into the surgical cavity of a mouse glioblastoma model after resection. The median survival of mice treated with nanoparticles was almost doubled [50]. In addition to mere vehicles, nanotechnology can encompass biocargoes including small interfering ribonucleic acids (siRNAs) and DNA altering technology (e.g. CRISPRs/Cas9), to directly target specific molecular alterations [51]. In this way, intratumoural injection of liposomes containing interferon-beta in five high-grade gliomas achieved an encouraging overall survival of 17 months [52].
Human stem cells show tropism to brain pathology such as tumours and can cross the blood–brain-barrier, offering another vehicle system. They can be delivered systemically or locally. Human adipose derived mesenchymal stem cells (hAMSCs) are of particular interest as, compared to embryonic stem cells, they are derived from readily available adipose tissue, are more genetically stable and have a lower senescence ratio [53]. Preclinical experience is promising, particularly when combined with nanotechnology, to avoid potential immunogenicity that can accompany viral transfection [54].
Viral and Vector Mediated Therapy
Naturally occurring viruses such as adenoviruses and herpes simplex viruses can be genetically engineered to target glioblastoma cellular proteins and exert anti-tumourigenic effects when administered systemically or locally. The resulting cytotoxicity and cell lysis (‘oncolysis’) can induce an immune response, further strengthening the overall effect [55]. Early-phase studies evaluating oncolytic viral therapy delivered to the surgical bed for recurrent glioblastoma have reported a high rate of adverse events (39–67%), so the technique requires further evaluation [55]. An alternative approach is called gene-mediated cytotoxic immunotherapy—using locally delivered viral vectors in combination with anti-viral agents and immune-modulating agents, to stimulate a systemic vaccine effect [56]. Aglatimagene besadenovec (AdV-tk) is an adenoviral vector expressing the herpes simplex virus thymidine kinase, which is currently being evaluated in this way for newly diagnosed glioblastoma (NCT03576612) [57].
Radiotherapy
Dose and Dose Escalation
Postoperative radiotherapy utilises modern external beam radiation therapy (EBRT) techniques such as intensity modulated radiation therapy (IMRT) and volumetric modulated arc therapy (VMAT), which increase dose conformity. Nonelderly patients receive 60 Gy, though doses up to 75 Gy are well tolerated [58]. However, most contemporary trials of dose escalation have found no improvement in outcome when doses above 60 Gy are given at the conventional postoperative time point [59–64]. Alternative strategies are therefore required and the following sections will review those with clinical results. Notably, FLASH radiotherapy, involving instantaneous high-dose radiation therapy, has not yet made it to clinical testing although preclinical results are promising [65].
Intraoperative Radiotherapy
Intraoperative radiotherapy (IORT) using photons or electrons is administered after maximal resection. Modern IORT for gliomas uses Intrabeam (Carl Zeiss®), a mobile device that delivers low-energy photons (30–50 kV), thus alleviating the need for operating room radiation shielding [66]. Treatment is delivered through spherical applicators depending on the size of the surgical cavity [66].
In a recent phase II trial—INTRAGO (INTRAoperative radiotherapy for GliOblastoma), 15 patients with newly diagnosed glioblastoma were treated with a median IORT dose of 25 Gy. Encouraging results were obtained with 2/15 cases of local progression and a median overall survival of 17.8 months. The two cases of local progression included one patient that received the lowest dose of radiation at 20 Gy and one who could not receive postoperative concurrent chemoradiotherapy. Radiation necrosis (n = 5) occurred at each dose of radiation in roughly equal numbers [67]. These results were affirmed by other centres reporting a median OS of around 18 months with IORT for glioblastoma [68, 69]. A future phase III trial (INTRAGO II) will test the efficacy of intrabean based IORT (NCT02685605) [70].
Brachytherapy
Brachytherapy involves administration of radioactive isotopes into the tumour or surgical cavity that decay with time, releasing radiation to surrounding tissue. In glioblastoma, brachytherapy has been most commonly used with iodine-125 (I-125), though two phase III randomised trials did not find that it improved survival [71–73].
Recent studies have reported favourable outcomes with brachytherapy in the temozolomide era [73]. Furthermore, advances in brachytheraphy technology have improved its safety, leading to more support for its use [74]. New brachytherapy systems such as the GammaTile® include absorbable radioisotope carrier systems embedding caesium-131 (Cs-131) seeds. These do not require surgical removal and they prevent direct contact between radioactive seeds and the brain surface. From an efficacy perspective, Cs-131 has a shorter half-life than radioisotopes such as I-125, providing a cumulative radiation boost earlier than I-125 [74]. Early experience in brain metastases has demonstrated excellent local control of 100% [75, 76]. The rates of radiation necrosis with Cs-131 also appear lower than I-125, reported at 0–11% [76, 77]. Studies evaluating GammaTile® for glioblastoma are ongoing (NCT04427384) [78].
External Beam Radiation Therapy
Use of EBRT has two potential time points of use—(1) in the early postoperative stage or (2) preoperatively.
Early (≤ 3 Weeks) Postoperative External Beam Radiation Therapy
Earlier commencement of standard postoperative chemoradiotherapy does not show survival benefit [14, 15]. Some studies have evaluated early postoperative SRS (≤ 3 weeks) that is additional to standard chemoradiotherapy. Smith et al. reported a phase I/II trial Gamma-Knife SRS delivered ≤ 2 weeks postoperatively (including Gliadel wafers). Thirty patients were included and the median overall survival was < 12 months overall [79]. In contrast to these disappointing results, Duma et al. described a favourable overall survival of 23 months in 174 patients using postoperative Gamma-Knife SRS to the FLAIR abnormality. Treatment was given a median of 18 days postoperatively. Thus, early postoperative EBRT in the form of SRS has not demonstrated conclusive efficacy. A future trial will test SRS ≤ 48 h postoperatively for residual tumour [80].
Preoperative External Beam Radiation Therapy
Preoperative radiotherapy for brain metastases is safe [81] but has only been historically tested in glioblastoma patients with whole brain radiotherapy (WBRT) as described by Seiler et al. in 1979 [82]. In 10 patients with new suspected glioblastomas, 30–40 Gy WBRT over 3–4 weeks was given preoperatively, followed by a 2–3-week delay to surgery with subsequent postoperative WBRT of 25–40 Gy in 3–4 weeks. Although this is an outdated and abandoned technique, a relatively favourable median survival of 12 months was achieved in this pre-temozolomide era experience [82]. Preoperative radiotherapy is an interesting strategy to explore in the modern era using contemporary radiotherapy techniques of IMRT/VMAT.
Preoperative chemoradiation is currently being trialled prospectively (NCT04209790) in biopsy confirmed glioblastoma patients [83]. Prior tissue confirmation of histological or molecular diagnosis has the potential of delaying the treatment, morbidity and mortality [84]. The possibility of misdiagnosis is rare using modern MRI techniques, that have a high sensitivity, but the effects of EBRT on prognostic molecular mutations in glioblastoma is unclear and will require further evaluation [85, 86].
Discussion
Early interventions have advantages and disadvantages (Table 3 and Fig. 1C). Their common advantage is the potential to target tumour cells that are otherwise only treated 6–8 weeks after diagnosis, whilst stimulated to become more biologically aggressive from surgery. These cells are potentiated by the post-surgical inflammatory response that stimulates wound repair. Patient factors are also an important consideration as not all patients may be suited to each technique. For example, early postoperative therapies suit patients presenting acutely unwell requiring urgent surgical intervention.
Table 3.
Early therapeutic interventions for newly diagnosed glioblastoma: advantages and disadvantages of different interventions
| Early therapeutic interventions for newly diagnosed glioblastoma: an evaluation of different interventions | ||||||
|---|---|---|---|---|---|---|
| Second surgery | Systemic agents | IORT | Brachytherapy | Pre-op EBRT | Early post-op EBRT | |
| Advantages | • Can allow definitive removal of unintended residual disease that itself is associated with REP |
• Neoadjuvant immunotherapy may be more effective than adjuvant • Temozolomide may be of benefit to patients with MGMT promotor methylation • Combination with local radiotherapy techniques is possible |
• Promising results in early phase trials • Logistically easy to implement • Potentially less dose to organs at risk • High surface dose |
• Potentially less dose to organs at risk • High surface dose • Modulation of spatial dose is possible to some extent |
• Uses available technology that can deliver radiation with precision • Spatial dose modulation with potential to cover all disease • Accurate estimation of dose to organs at risk • Opportunity to study short-term irradiation response |
• Uses existing technology that can deliver radiation with precision • More precise target and margin delineation on MRI • Accurate estimation of dose to organs at risk |
| Disadvantages |
• Increased risk of complications related to surgery and anaesthesia • Could delay postoperative chemoradiotherapy • Negative biological effects of surgery |
• Systemic side effects may increase risk of postoperative complications (e.g. poor wound healing, infection) |
• Cost of technology • Need for training • On-site availability of radiotherapy delivery experts • Shape of surgical cavity must be appropriate • Need for intraoperative imaging • Only targets 5–10-mm margin from surgical cavity • No spatial dose modulation |
• Has not demonstrated efficacy in previous randomised trials • Cost of technology • Need for training • On-site availability of radiotherapy delivery experts • Difficult to precisely calculate dose to residuum/organs at risk |
• Treatment on basis of imaging diagnosis alone • Requires logistical alignments • Patients requiring urgent surgery may not be suitable • The effect of radiotherapy on clinically relevant markers is unclear |
• May have ‘missed window’ to counter negative biological effects of surgery • Patients with complications of surgery may not be able to have this therapy • First attempts did not provide promising results |
From a biological point of view, postoperative treatment strategies have the common disadvantage that tumour potentiating effects of surgery may have already occurred. In addition, surgery can induce stem cell like changes in peritumoural astrocytes that may not be reversible [87]. Furthermore, surgery leads to immune cell infiltration that promotes tumour cell proliferation and potentially decreases radiosensitivity [88]. In this regard, preoperative treatment given in advance of the operation theoretically renders tumour cells less aggressive at the time of surgery. Indeed, radiation induced cellular senescence in glioblastoma appears to be time dependent. Zhang et al. exposed the LN229 glioblastoma cell line to 2–8 Gy radiation and found increased cell senescence and decreased cell cycle checkpoint regulation at 7 days versus 12 hours post-irradiation [89]. From an oxygenation point of view, preoperative tissue is also more oxygenated compared to postoperative residuum, making it plausibly more sensitive to preoperative EBRT and neoadjuvant immunotherapy [23, 90].
Other biological considerations for early interventions relate to the potential of mutual synergism. Targeted agents could be used with early time point radiotherapy strategies, though clinical results using radiosensitisers and immunotherapy in the normal postoperative setting are disappointing to date [91]. Indeed, the CheckMate 498 and 548 randomised trials that evaluated nivolumab (anti-programmed death ligand-1) have failed to show an improvement in overall survival with combined immunotherapy and radiotherapy for newly diagnosed glioblastoma patients [92, 93].
Advances in nanotechnology may facilitate delivery of drugs in a more efficient way, or even as radiosensitisers in their own right, as with gold nanoparticles that can absorb ionising radiation [49]. This may be particularly effective with longer courses of radiation. Neoadjuvant immunotherapy and early time point radiotherapy strategies may be of interest as preclinical data corroborates their combined efficacy [89, 94]. However, more data is needed to understand how immunotherapy can best be combined with radiotherapy using more advanced model systems replicating the immune microenvironment of glioblastoma.
Several considerations are important for early time point radiotherapy. Current EBRT techniques allow individualised treatment plans enabling dose boosts and custom margins (i.e. dose modulation). The use of protons in particular allows dose modulation whilst increasing normal tissue sparing [95]. However, more data is required regarding the early time point irradiation response to advanced preoperative EBRT strategies. In contrast to EBRT, IORT delivers a highly spherical dose of radiation to a 5–10-mm margin, though not all surgical cavities may be suitably shaped for this technique. Brachytherapy delivers a radiation dose that is dependent on surgical implantation technique and 5–8 mm around the surgical cavity with newer GammaTile® technology [74]. Notably, IORT/brachytherapy dose distributions potentially do not reach more distally invasive glioblastoma cells. This phenomenon could explain the discrepancy seen between the local and overall progression free survival seen in the INTRAGO trial (17.8 months versus 11.3 months) [67]. Preoperative EBRT and neoadjuvant immunotherapy have challenges in that they require patient treatment based on imaging diagnosis alone to prevent additional surgical interventions. They also require consideration of the timing of surgery in particular for severely symptomatic patients.
Although there is strong biological rationale to support early interventions for newly diagnosed glioblastoma, clinical evidence is currently at an early stage and/or retrospective in design, with inherent selection bias that precludes extended analysis of outcomes. Of interest, early interventions have demonstrated benefit in other solid tumours. For example, neoadjuvant chemotherapy/radiotherapy can downstage locally advanced breast cancer, sarcoma and several gastrointestinal cancers, improving the likelihood of organ preserving gross total resection [96–99]. This does not always translate into a long-term survival benefit however, as demonstrated for retroperitoneal sarcoma [100]. As glioblastoma is a diffusely infiltrative solid tumour, total tumour removal is not possible or even the aim of therapy, but rather, extending survival, which itself has proved challenging with the current treatment pathway. Future outcome data from larger trials is awaited to evaluate the efficacy and role of early interventions for glioblastoma.
Conclusion
There is biological rationale to suggest that early interventions could benefit the outcome of glioblastoma patients. Additional therapy at an earlier time point treats a better oxygenated tumour in a treatment naïve biological system, with less molecular heterogeneity. Neoadjuvant immunotherapy and early time point radiotherapy strategies are of specific interest as they can target invasive tumour cells that cannot be resected. Early interventions could finally lead to the long-awaited improvement in survival for glioblastoma and require further investigation.
Author Contribution
• Manuscript concept: GB, AQ, DT.
• Draft manuscript preparation: MW, DT, CM, JO, DC, LA, GB.
• Figure generation: MW, GB, DT, AQ.
• Manuscript editing: DT, CM, DC, JO, DC, LA, AQ, GB.
• Finalisation of manuscript: MW, GB, DT, AQ.
Data Availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Declarations
Ethics Approval
This article type did not require ethical approval or participant consent.
Consent
This article type did not require patient consent.
Conflict of Interest
The authors declare no competing interests.
Footnotes
This article is part of the Topical collection on Neuro-oncology
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013–2017. Neuro-oncology. 2020;22:iv1–iv96. doi: 10.1093/neuonc/noaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for R, Treatment of Cancer Brain T, Radiotherapy G, National Cancer Institute of Canada Clinical Trials G Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Choucair AK, Levin VA, Gutin PH, Davis RL, Silver P, Edwards MS, Wilson CB. Development of multiple lesions during radiation therapy and chemotherapy in patients with gliomas. J Neurosurg. 1986;65:654–658. doi: 10.3171/jns.1986.65.5.0654. [DOI] [PubMed] [Google Scholar]
- 4.Poon MTC, Sudlow CLM, Figueroa JD, Brennan PM. Longer-term (>/= 2 years) survival in patients with glioblastoma in population-based studies pre- and post-2005: a systematic review and meta-analysis. Sci Rep. 2020;10:11622. doi: 10.1038/s41598-020-68011-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lakomy R, Kazda T, Selingerova I, Poprach A, Pospisil P, Belanova R, Fadrus P, Smrcka M, Vybihal V, Jancalek R, Kiss I, Muckova K, Hendrych M, Knight A, Sana J, Slampa P, Slaby O (2020) Pre-radiotherapy progression after surgery of newly diagnosed glioblastoma: corroboration of new prognostic variable. Diagnostics (Basel) 10. 10.3390/diagnostics10090676 [DOI] [PMC free article] [PubMed]
- 6.Palmer JD, Bhamidipati D, Shukla G, Sharma D, Glass J, Kim L, Evans JJ, Judy K, Farrell C, Andrews DW, Wang ZW, Peiper SC, Werner-Wasik M, Shi W. Rapid early tumor progression is prognostic in glioblastoma patients. Am J Clin Oncol. 2019;42:481–486. doi: 10.1097/COC.0000000000000537. [DOI] [PubMed] [Google Scholar]
- 7.De Barros A, Attal J, Roques M, Nicolau J, Sol JC, Cohen-Jonathan-Moyal E, Roux FE. Impact on survival of early tumor growth between surgery and radiotherapy in patients with de novo glioblastoma. J Neurooncol. 2019;142:489–497. doi: 10.1007/s11060-019-03120-3. [DOI] [PubMed] [Google Scholar]
- 8.Villanueva-Meyer JE, Han SJ, Cha S, Butowski NA. Early tumor growth between initial resection and radiotherapy of glioblastoma: incidence and impact on clinical outcomes. J Neurooncol. 2017;134:213–219. doi: 10.1007/s11060-017-2511-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wee CW, Kim E, Kim TM, Park CK, Kim JW, Choi SH, Yoo RE, Lee ST, Kim IH. Impact of interim progression during the surgery-to-radiotherapy interval and its predictors in glioblastoma treated with temozolomide-based radiochemotherapy. J Neurooncol. 2017;134:169–175. doi: 10.1007/s11060-017-2505-x. [DOI] [PubMed] [Google Scholar]
- 10.Merkel A, Soeldner D, Wendl C, Urkan D, Kuramatsu JB, Seliger C, Proescholdt M, Eyupoglu IY, Hau P, Uhl M. Early postoperative tumor progression predicts clinical outcome in glioblastoma-implication for clinical trials. J Neurooncol. 2017;132:249–254. doi: 10.1007/s11060-016-2362-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Majos C, Cos M, Castaner S, Pons A, Gil M, Fernandez-Coello A, Macia M, Bruna J, Aguilera C. Preradiotherapy MR imaging: a prospective pilot study of the usefulness of performing an MR examination shortly before radiation therapy in patients with glioblastoma. AJNR Am J Neuroradiol. 2016;37:2224–2230. doi: 10.3174/ajnr.A4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farace P, Amelio D, Ricciardi GK, Zoccatelli G, Magon S, Pizzini F, Alessandrini F, Sbarbati A, Amichetti M, Beltramello A. Early MRI changes in glioblastoma in the period between surgery and adjuvant therapy. J Neurooncol. 2013;111:177–185. doi: 10.1007/s11060-012-0997-y. [DOI] [PubMed] [Google Scholar]
- 13.Pirzkall A, McGue C, Saraswathy S, Cha S, Liu R, Vandenberg S, Lamborn KR, Berger MS, Chang SM, Nelson SJ. Tumor regrowth between surgery and initiation of adjuvant therapy in patients with newly diagnosed glioblastoma. Neuro Oncol. 2009;11:842–852. doi: 10.1215/15228517-2009-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buszek SM, Al Feghali KA, Elhalawani H, Chevli N, Allen PK, Chung C. Optimal timing of radiotherapy following gross total or subtotal resection of glioblastoma: a real-world assessment using the National Cancer Database. Sci Rep. 2020;10:4926. doi: 10.1038/s41598-020-61701-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blumenthal DT, Won M, Mehta MP, Gilbert MR, Brown PD, Bokstein F, Brachman DG, Werner-Wasik M, Hunter GK, Valeinis E, Hopkins K, Souhami L, Howard SP, Lieberman FS, Shrieve DC, Wendland MM, Robinson CG, Zhang P, Corn BW. Short delay in initiation of radiotherapy for patients with glioblastoma-effect of concurrent chemotherapy: a secondary analysis from the NRG Oncology/Radiation Therapy Oncology Group database. Neuro Oncol. 2018;20:966–974. doi: 10.1093/neuonc/noy017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osborn VW, Lee A, Garay E, Safdieh J, Schreiber D. Impact of timing of adjuvant chemoradiation for glioblastoma in a large hospital database. Neurosurgery. 2018;83:915–921. doi: 10.1093/neuros/nyx497. [DOI] [PubMed] [Google Scholar]
- 17.Alieva M, Margarido AS, Wieles T, Abels ER, Colak B, Boquetale C, Jan Noordmans H, Snijders TJ, Broekman ML, van Rheenen J. Preventing inflammation inhibits biopsy-mediated changes in tumor cell behavior. Sci Rep. 2017;7:7529. doi: 10.1038/s41598-017-07660-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okolie O, Bago JR, Schmid RS, Irvin DM, Bash RE, Miller CR, Hingtgen SD. Reactive astrocytes potentiate tumor aggressiveness in a murine glioma resection and recurrence model. Neuro Oncol. 2016;18:1622–1633. doi: 10.1093/neuonc/now117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown TJ, Brennan MC, Li M, Church EW, Brandmeir NJ, Rakszawski KL, Patel AS, Rizk EB, Suki D, Sawaya R, Glantz M. Association of the extent of resection with survival in glioblastoma: a systematic review and meta-analysis. JAMA Oncol. 2016;2:1460–1469. doi: 10.1001/jamaoncol.2016.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomsen H, Steffensen E, Larsson EM. Perfusion MRI (dynamic susceptibility contrast imaging) with different measurement approaches for the evaluation of blood flow and blood volume in human gliomas. Acta Radiol. 2012;53:95–101. doi: 10.1258/ar.2011.110242. [DOI] [PubMed] [Google Scholar]
- 21.Bette S, Wiestler B, Kaesmacher J, Huber T, Gerhardt J, Barz M, Delbridge C, Ryang YM, Ringel F, Zimmer C, Meyer B, Boeckh-Behrens T, Kirschke JS, Gempt J. Infarct volume after glioblastoma surgery as an independent prognostic factor. Oncotarget. 2016;7:61945–61954. doi: 10.18632/oncotarget.11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bette S, Barz M, Huber T, Straube C, Schmidt-Graf F, Combs SE, Delbridge C, Gerhardt J, Zimmer C, Meyer B, Kirschke JS, Boeckh-Behrens T, Wiestler B, Gempt J. Retrospective analysis of radiological recurrence patterns in glioblastoma, their prognostic value and association to postoperative infarct volume. Sci Rep. 2018;8:4561. doi: 10.1038/s41598-018-22697-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gray LH, Conger AD, Ebert M, Hornsey S, Scott OC. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol. 1953;26:638–648. doi: 10.1259/0007-1285-26-312-638. [DOI] [PubMed] [Google Scholar]
- 24.Spence AM, Muzi M, Swanson KR, O’Sullivan F, Rockhill JK, Rajendran JG, Adamsen TC, Link JM, Swanson PE, Yagle KJ, Rostomily RC, Silbergeld DL, Krohn KA. Regional hypoxia in glioblastoma multiforme quantified with [18F]fluoromisonidazole positron emission tomography before radiotherapy: correlation with time to progression and survival. Clin Cancer Res. 2008;14:2623–30. 10.1158/1078-0432.CCR-07-4995. [DOI] [PMC free article] [PubMed]
- 25.Seidel S, Garvalov BK, Wirta V, von Stechow L, Schanzer A, Meletis K, Wolter M, Sommerlad D, Henze AT, Nister M, Reifenberger G, Lundeberg J, Frisen J, Acker T. A hypoxic niche regulates glioblastoma stem cells through hypoxia inducible factor 2 alpha. Brain. 2010;133:983–995. doi: 10.1093/brain/awq042. [DOI] [PubMed] [Google Scholar]
- 26.Colwell N, Larion M, Giles AJ, Seldomridge AN, Sizdahkhani S, Gilbert MR, Park DM. Hypoxia in the glioblastoma microenvironment: shaping the phenotype of cancer stem-like cells. Neuro Oncol. 2017;19:887–896. doi: 10.1093/neuonc/now258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ge X, Pan MH, Wang L, Li W, Jiang C, He J, Abouzid K, Liu LZ, Shi Z, Jiang BH. Hypoxia-mediated mitochondria apoptosis inhibition induces temozolomide treatment resistance through miR-26a/Bad/Bax axis. Cell Death Dis. 2018;9:1128. doi: 10.1038/s41419-018-1176-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Talasila KM, Rosland GV, Hagland HR, Eskilsson E, Flones IH, Fritah S, Azuaje F, Atai N, Harter PN, Mittelbronn M, Andersen M, Joseph JV, Hossain JA, Vallar L, Noorden CJ, Niclou SP, Thorsen F, Tronstad KJ, Tzoulis C, Bjerkvig R, Miletic H. The angiogenic switch leads to a metabolic shift in human glioblastoma. Neuro Oncol. 2017;19:383–393. doi: 10.1093/neuonc/now175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burger PC, Heinz ER, Shibata T, Kleihues P. Topographic anatomy and CT correlations in the untreated glioblastoma multiforme. J Neurosurg. 1988;68:698–704. doi: 10.3171/jns.1988.68.5.0698. [DOI] [PubMed] [Google Scholar]
- 30.Burnet NG, Jena R, Jefferies SJ, Stenning SP, Kirkby NF. Mathematical modelling of survival of glioblastoma patients suggests a role for radiotherapy dose escalation and predicts poorer outcome after delay to start treatment. Clin Oncol. 2006;18:93–103. doi: 10.1016/j.clon.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 31.Barthel FP, Johnson KC, Varn FS, Moskalik AD, Tanner G, Kocakavuk E, Anderson KJ, Abiola O, Aldape K, Alfaro KD, Alpar D, Amin SB, Ashley DM, Bandopadhayay P, Barnholtz-Sloan JS, Beroukhim R, Bock C, Brastianos PK, Brat DJ, Brodbelt AR, Bruns AF, Bulsara KR, Chakrabarty A, Chakravarti A, Chuang JH, Claus EB, Cochran EJ, Connelly J, Costello JF, Finocchiaro G, Fletcher MN, French PJ, Gan HK, Gilbert MR, Gould PV, Grimmer MR, Iavarone A, Ismail A, Jenkinson MD, Khasraw M, Kim H, Kouwenhoven MCM, LaViolette PS, Li M, Lichter P, Ligon KL, Lowman AK, Malta TM, Mazor T, McDonald KL, Molinaro AM, Nam DH, Nayyar N, Ng HK, Ngan CY, Niclou SP, Niers JM, Noushmehr H, Noorbakhsh J, Ormond DR, Park CK, Poisson LM, Rabadan R, Radlwimmer B, Rao G, Reifenberger G, Sa JK, Schuster M, Shaw BL, Short SC, Smitt PAS, Sloan AE, Smits M, Suzuki H, Tabatabai G, Van Meir EG, Watts C, Weller M, Wesseling P, Westerman BA, Widhalm G, Woehrer A, Yung WKA, Zadeh G, Huse JT, De Groot JF, Stead LF, Verhaak RGW, Consortium G Longitudinal molecular trajectories of diffuse glioma in adults. Nature. 2019;576:112–120. doi: 10.1038/s41586-019-1775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schucht P, Murek M, Jilch A, Seidel K, Hewer E, Wiest R, Raabe A, Beck J. Early re-do surgery for glioblastoma is a feasible and safe strategy to achieve complete resection of enhancing tumor. PLoS ONE. 2013;8:e79846. doi: 10.1371/journal.pone.0079846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Troya-Castilla M, Kaen A, Marquez-Rivas FJ, Infante-Cossio P, Rius Diaz F, Narros Gimenez JL, Gonzalez-Pombo M, Cancela P, Segura Fernandez-Nogueras M, Arraez Sanchez MA. Impact of early reoperation on the prognosis of patients operated on for glioblastoma. World Neurosurg. 2020;139:e592–e600. doi: 10.1016/j.wneu.2020.04.072. [DOI] [PubMed] [Google Scholar]
- 34.Ma R, Chari A, Brennan PM, Alalade A, Anderson I, Solth A, Marcus HJ, Watts C, British Neurosurgical Trainee Research C Residual enhancing disease after surgery for glioblastoma: evaluation of practice in the United Kingdom. Neurooncol Pract. 2018;5:74–81. doi: 10.1093/nop/npx023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang H, Zeng W, Ren X, Cui Y, Li M, Yang K, Elbaroody M, Lin S. Super-early initiation of temozolomide prolongs the survival of glioblastoma patients without gross-total resection: a retrospective cohort study. J Neurooncol. 2019;144:127–135. doi: 10.1007/s11060-019-03211-1. [DOI] [PubMed] [Google Scholar]
- 36.Cloughesy TF, Mochizuki AY, Orpilla JR, Hugo W, Lee AH, Davidson TB, Wang AC, Ellingson BM, Rytlewski JA, Sanders CM, Kawaguchi ES, Du L, Li G, Yong WH, Gaffey SC, Cohen AL, Mellinghoff IK, Lee EQ, Reardon DA, O’Brien BJ, Butowski NA, Nghiemphu PL, Clarke JL, Arrillaga-Romany IC, Colman H, Kaley TJ, de Groot JF, Liau LM, Wen PY, Prins RM. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25:477–86. 10.1038/s41591-018-0337-7. [DOI] [PMC free article] [PubMed]
- 37.Neoadjuvant PD-1 in newly diagnosed glioblastoma (NCT04583020). https://clinicaltrials.gov/ct2/show/NCT04583020. Accessed 2nd November 2021
- 38.Schalper KA, Rodriguez-Ruiz ME, Diez-Valle R, Lopez-Janeiro A, Porciuncula A, Idoate MA, Inoges S, de Andrea C, Lopez-Diaz de Cerio A, Tejada S, Berraondo P, Villarroel-Espindola F, Choi J, Gurpide A, Giraldez M, Goicoechea I, Gallego Perez-Larraya J, Sanmamed MF, Perez-Gracia JL, Melero I. Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat Med. 2019;25:470–476. doi: 10.1038/s41591-018-0339-5. [DOI] [PubMed] [Google Scholar]
- 39.Reardon DA, Brandes AA, Omuro A, Mulholland P, Lim M, Wick A, Baehring J, Ahluwalia MS, Roth P, Bahr O, Phuphanich S, Sepulveda JM, De Souza P, Sahebjam S, Carleton M, Tatsuoka K, Taitt C, Zwirtes R, Sampson J, Weller M. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020;6:1003–1010. doi: 10.1001/jamaoncol.2020.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fleming AB, Saltzman WM. Pharmacokinetics of the carmustine implant. Clin Pharmacokinet. 2002;41:403–419. doi: 10.2165/00003088-200241060-00002. [DOI] [PubMed] [Google Scholar]
- 41.Westphal M, Ram Z, Riddle V, Hilt D, Bortey E, Executive Committee of the Gliadel Study G (2006) Gliadel wafer in initial surgery for malignant glioma: long-term follow-up of a multicenter controlled trial. Acta Neurochir (Wien) 148: 269–275; discussion 275 10.1007/s00701-005-0707-z [DOI] [PubMed]
- 42.Westphal M, Hilt DC, Bortey E, Delavault P, Olivares R, Warnke PC, Whittle IR, Jaaskelainen J, Ram Z. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5:79–88. doi: 10.1093/neuonc/5.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gutenberg A, Lumenta CB, Braunsdorf WE, Sabel M, Mehdorn HM, Westphal M, Giese A. The combination of carmustine wafers and temozolomide for the treatment of malignant gliomas. A comprehensive review of the rationale and clinical experience. J Neurooncol. 2013;113:163–174. doi: 10.1007/s11060-013-1110-x. [DOI] [PubMed] [Google Scholar]
- 44.Attenello FJ, Mukherjee D, Datoo G, McGirt MJ, Bohan E, Weingart JD, Olivi A, Quinones-Hinojosa A, Brem H. Use of Gliadel (BCNU) wafer in the surgical treatment of malignant glioma: a 10-year institutional experience. Ann Surg Oncol. 2008;15:2887–2893. doi: 10.1245/s10434-008-0048-2. [DOI] [PubMed] [Google Scholar]
- 45.Nabors LB, Portnow J, Ahluwalia M, Baehring J, Brem H, Brem S, Butowski N, Campian JL, Clark SW, Fabiano AJ, Forsyth P, Hattangadi-Gluth J, Holdhoff M, Horbinski C, Junck L, Kaley T, Kumthekar P, Loeffler JS, Mrugala MM, Nagpal S, Pandey M, Parney I, Peters K, Puduvalli VK, Robins I, Rockhill J, Rusthoven C, Shonka N, Shrieve DC, Swinnen LJ, Weiss S, Wen PY, Willmarth NE, Bergman MA, Darlow SD. Central nervous system cancers, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18:1537–1570. doi: 10.6004/jnccn.2020.0052. [DOI] [PubMed] [Google Scholar]
- 46.Kadota T, Saito R, Kumabe T, Mizusawa J, Katayama H, Sumi M, Igaki H, Kinoshita M, Komori T, Ichimura K, Narita Y, Nishikawa R. A multicenter randomized phase III study for newly diagnosed maximally resected glioblastoma comparing carmustine wafer implantation followed by chemoradiotherapy with temozolomide with chemoradiotherapy alone; Japan Clinical Oncology Group Study JCOG1703 (MACS study) Jpn J Clin Oncol. 2019;49:1172–1175. doi: 10.1093/jjco/hyz169. [DOI] [PubMed] [Google Scholar]
- 47.Ursu R, Carpentier A, Metellus P, Lubrano V, Laigle-Donadey F, Capelle L, Guyotat J, Langlois O, Bauchet L, Desseaux K, Tibi A, Chinot O, Lambert J, Carpentier AF. Intracerebral injection of CpG oligonucleotide for patients with de novo glioblastoma-a phase II multicentric, randomised study. Eur J Cancer. 2017;73:30–37. doi: 10.1016/j.ejca.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 48.Jenkinson MD, Smith TS, Haylock B, Husband D, Shenoy A, Vinjamuri S, Walker C, Pietronigro D, Warnke PC. Phase II trial of intratumoral BCNU injection and radiotherapy on untreated adult malignant glioma. J Neurooncol. 2010;99:103–113. doi: 10.1007/s11060-010-0113-0. [DOI] [PubMed] [Google Scholar]
- 49.Chakroun RW, Zhang P, Lin R, Schiapparelli P, Quinones-Hinojosa A, Cui H (2018) Nanotherapeutic systems for local treatment of brain tumors. Wiley Interdiscip Rev Nanomed Nanobiotechnol 10. 10.1002/wnan.1479 [DOI] [PMC free article] [PubMed]
- 50.Schiapparelli P, Zhang P, Lara-Velazquez M, Guerrero-Cazares H, Lin R, Su H, Chakroun RW, Tusa M, Quinones-Hinojosa A, Cui H. Self-assembling and self-formulating prodrug hydrogelator extends survival in a glioblastoma resection and recurrence model. J Control Release. 2020;319:311–321. doi: 10.1016/j.jconrel.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 51.Glaser T, Han I, Wu L, Zeng X. Targeted nanotechnology in glioblastoma multiforme. Front Pharmacol. 2017;8:166. doi: 10.3389/fphar.2017.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wakabayashi T, Natsume A, Hashizume Y, Fujii M, Mizuno M, Yoshida J. A phase I clinical trial of interferon-beta gene therapy for high-grade glioma: novel findings from gene expression profiling and autopsy. J Gene Med. 2008;10:329–339. doi: 10.1002/jgm.1160. [DOI] [PubMed] [Google Scholar]
- 53.Li Q, Wijesekera O, Salas SJ, Wang JY, Zhu M, Aprhys C, Chaichana KL, Chesler DA, Zhang H, Smith CL, Guerrero-Cazares H, Levchenko A, Quinones-Hinojosa A. Mesenchymal stem cells from human fat engineered to secrete BMP4 are nononcogenic, suppress brain cancer, and prolong survival. Clin Cancer Res. 2014;20:2375–2387. doi: 10.1158/1078-0432.CCR-13-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mangraviti A, Tzeng SY, Gullotti D, Kozielski KL, Kim JE, Seng M, Abbadi S, Schiapparelli P, Sarabia-Estrada R, Vescovi A, Brem H, Olivi A, Tyler B, Green JJ, Quinones-Hinojosa A. Non-virally engineered human adipose mesenchymal stem cells produce BMP4, target brain tumors, and extend survival. Biomaterials. 2016;100:53–66. doi: 10.1016/j.biomaterials.2016.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu VM, Shah AH, Vallejo FA, Eichberg DG, Luther EM, Shah SS, Komotar RJ, Ivan ME. Clinical trials using oncolytic viral therapy to treat adult glioblastoma: a progress report. Neurosurg Focus. 2021;50:E3. doi: 10.3171/2020.11.FOCUS20860. [DOI] [PubMed] [Google Scholar]
- 56.Wheeler LA, Manzanera AG, Bell SD, Cavaliere R, McGregor JM, Grecula JC, Newton HB, Lo SS, Badie B, Portnow J, Teh BS, Trask TW, Baskin DS, New PZ, Aguilar LK, Aguilar-Cordova E, Chiocca EA. Phase II multicenter study of gene-mediated cytotoxic immunotherapy as adjuvant to surgical resection for newly diagnosed malignant glioma. Neuro Oncol. 2016;18:1137–1145. doi: 10.1093/neuonc/now002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.GMCI, nivolumab, and radiation therapy in treating patients with newly diagnosed high-grade gliomas (GMCI) (NCT03576612). https://clinicaltrials.gov/ct2/show/NCT03576612. Accessed November 2nd 2021
- 58.Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int J Radiat Oncol Biol Phys. 1979;5:1725–1731. doi: 10.1016/0360-3016(79)90553-4. [DOI] [PubMed] [Google Scholar]
- 59.Piroth MD, Pinkawa M, Holy R, Klotz J, Schaar S, Stoffels G, Galldiks N, Coenen HH, Kaiser HJ, Langen KJ, Eble MJ. Integrated boost IMRT with FET-PET-adapted local dose escalation in glioblastomas. Results of a prospective phase II study. Strahlentherapie und Onkologie : Organ der Deutschen Rontgengesellschaft [et al]. 2012;188:334–9. 10.1007/s00066-011-0060-5. [DOI] [PubMed]
- 60.Tsien CI, Brown D, Normolle D, Schipper M, Piert M, Junck L, Heth J, Gomez-Hassan D, Ten Haken RK, Chenevert T, Cao Y, Lawrence T. Concurrent temozolomide and dose-escalated intensity-modulated radiation therapy in newly diagnosed glioblastoma. Clin Cancer Res. 2012;18:273–279. doi: 10.1158/1078-0432.CCR-11-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Monjazeb AM, Ayala D, Jensen C, Case LD, Bourland JD, Ellis TL, McMullen KP, Chan MD, Tatter SB, Lesser GJ, Shaw EG. A phase I dose escalation study of hypofractionated IMRT field-in-field boost for newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2012;82:743–748. doi: 10.1016/j.ijrobp.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsien C, Moughan J, Michalski JM, Gilbert MR, Purdy J, Simpson J, Kresel JJ, Curran WJ, Diaz A, Mehta MP, Radiation Therapy Oncology Group T Phase I three-dimensional conformal radiation dose escalation study in newly diagnosed glioblastoma: Radiation Therapy Oncology Group Trial 98–03. International journal of radiation oncology, biology, physics. 2009;73:699–708. doi: 10.1016/j.ijrobp.2008.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Werner-Wasik M, Scott CB, Nelson DF, Gaspar LE, Murray KJ, Fischbach JA, Nelson JS, Weinstein AS, Curran WJ Jr. Final report of a phase I/II trial of hyperfractionated and accelerated hyperfractionated radiation therapy with carmustine for adults with supratentorial malignant gliomas. Radiation Therapy Oncology Group Study 83–02. Cancer. 1996;77:1535–43. 10.1002/(SICI)1097-0142(19960415)77:8%3c1535::AID-CNCR17%3e3.0.CO;2-0. [DOI] [PubMed]
- 64.Gondi V, Pugh S, Tsien C, Chenevert T, Gilbert M, Omuro A, McDonough J, Aldape K, Srinivasan A, Rogers CL, Shi W, Suh JH, Algan O, Nedzi LA, Chan MD, Bahary JP, Mehta MP. Radiotherapy (RT) dose-intensification (DI) using intensity-modulated RT (IMRT) versus standard-dose (SD) RT with temozolomide (TMZ) in newly diagnosed glioblastoma (GBM): preliminary results of NRG Oncology BN001. Int J Radiat Oncol Biol Phys. 2020;108:S22–S23. doi: 10.1016/j.ijrobp.2020.07.2109. [DOI] [Google Scholar]
- 65.Montay-Gruel P, Acharya MM, Goncalves Jorge P, Petit B, Petridis IG, Fuchs P, Leavitt R, Petersson K, Gondre M, Ollivier J, Moeckli R, Bochud F, Bailat C, Bourhis J, Germond JF, Limoli CL, Vozenin MC. Hypofractionated FLASH-RT as an effective treatment against glioblastoma that reduces neurocognitive side effects in mice. Clin Cancer Res. 2021;27:775–784. doi: 10.1158/1078-0432.CCR-20-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weil RJ, Mavinkurve GG, Chao ST, Vogelbaum MA, Suh JH, Kolar M, Toms SA. Intraoperative radiotherapy to treat newly diagnosed solitary brain metastasis: initial experience and long-term outcomes. J Neurosurg. 2015;122:825–832. doi: 10.3171/2014.11.JNS1449. [DOI] [PubMed] [Google Scholar]
- 67.Giordano FA, Brehmer S, Murle B, Welzel G, Sperk E, Keller A, Abo-Madyan Y, Scherzinger E, Clausen S, Schneider F, Herskind C, Glas M, Seiz-Rosenhagen M, Groden C, Hanggi D, Schmiedek P, Emami B, Souhami L, Petrecca K, Wenz F. Intraoperative radiotherapy in newly diagnosed glioblastoma (INTRAGO): an open-label, dose-escalation phase I/II trial. Neurosurgery. 2019;84:41–49. doi: 10.1093/neuros/nyy018. [DOI] [PubMed] [Google Scholar]
- 68.Sarria GR, Sperk E, Han X, Sarria GJ, Wenz F, Brehmer S, Fu B, Min S, Zhang H, Qin S, Qiu X, Hanggi D, Abo-Madyan Y, Martinez D, Cabrera C, Giordano FA. Intraoperative radiotherapy for glioblastoma: an international pooled analysis. Radiother Oncol. 2020;142:162–167. doi: 10.1016/j.radonc.2019.09.023. [DOI] [PubMed] [Google Scholar]
- 69.Lyons M, Phang I, Eljamel S. The effects of PDT in primary malignant brain tumours could be improved by intraoperative radiotherapy. Photodiagnosis Photodyn Ther. 2012;9:40–45. doi: 10.1016/j.pdpdt.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 70.Intraoperative radiotherapy in newly diagnosed glioblastoma multiforme (INTRAGO-II) (NCT02685605). https://clinicaltrials.gov/ct2/show/NCT02685605. Accessed November 2nd 2021
- 71.Laperriere NJ, Leung PM, McKenzie S, Milosevic M, Wong S, Glen J, Pintilie M, Bernstein M. Randomized study of brachytherapy in the initial management of patients with malignant astrocytoma. Int J Radiat Oncol Biol Phys. 1998;41:1005–1011. doi: 10.1016/s0360-3016(98)00159-x. [DOI] [PubMed] [Google Scholar]
- 72.Selker RG, Shapiro WR, Burger P, Blackwood MS, Arena VC, Gilder JC, Malkin MG, Mealey JJ, Jr., Neal JH, Olson J, Robertson JT, Barnett GH, Bloomfield S, Albright R, Hochberg FH, Hiesiger E, Green S, Brain Tumor Cooperative G (2002) The Brain Tumor Cooperative Group NIH Trial 87–01: a randomized comparison of surgery, external radiotherapy, and carmustine versus surgery, interstitial radiotherapy boost, external radiation therapy, and carmustine. Neurosurgery 51: 343–355; discussion 355–347 [PubMed]
- 73.Barbarite E, Sick JT, Berchmans E, Bregy A, Shah AH, Elsayyad N, Komotar RJ. The role of brachytherapy in the treatment of glioblastoma multiforme. Neurosurg Rev. 2017;40:195–211. doi: 10.1007/s10143-016-0727-6. [DOI] [PubMed] [Google Scholar]
- 74.Gessler DJ, Ferreira C, Dusenbery K, Chen CC. GammaTile((R)): surgically targeted radiation therapy for glioblastomas. Future Oncol. 2020 doi: 10.2217/fon-2020-0558. [DOI] [PubMed] [Google Scholar]
- 75.Wernicke AG, Hirschfeld CB, Smith AW, Taube S, Yondorf MZ, Parashar B, Nedialkova L, Kulidzhanov F, Trichter S, Sabbas A, Ramakrishna R, Pannullo S, Schwartz TH. Clinical outcomes of large brain metastases treated with neurosurgical resection and intraoperative cesium-131 brachytherapy: results of a prospective trial. Int J Radiat Oncol Biol Phys. 2017;98:1059–1068. doi: 10.1016/j.ijrobp.2017.03.044. [DOI] [PubMed] [Google Scholar]
- 76.Wernicke AG, Yondorf MZ, Peng L, Trichter S, Nedialkova L, Sabbas A, Kulidzhanov F, Parashar B, Nori D, Clifford Chao KS, Christos P, Kovanlikaya I, Pannullo S, Boockvar JA, Stieg PE, Schwartz TH. Phase I/II study of resection and intraoperative cesium-131 radioisotope brachytherapy in patients with newly diagnosed brain metastases. J Neurosurg. 2014;121:338–348. doi: 10.3171/2014.3.JNS131140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brachman DG, Youssef E, Dardis CJ, Sanai N, Zabramski JM, Smith KA, Little AS, Shetter AG, Thomas T, McBride HL, Sorensen S, Spetzler RF, Nakaji P. Resection and permanent intracranial brachytherapy using modular, biocompatible cesium-131 implants: results in 20 recurrent, previously irradiated meningiomas. J Neurosurg. 2018;131:1819–1828. doi: 10.3171/2018.7.JNS18656. [DOI] [PubMed] [Google Scholar]
- 78.Registry of patients with brain tumors treated with STaRT (GammaTiles) (NCT0442738). https://clinicaltrials.gov/ct2/show/NCT04427384. Accessed November 2nd 2021
- 79.Smith KA, Ashby LS, Gonzalez LF, Brachman DG, Thomas T, Coons SW, Battaglia M, Scheck A. Prospective trial of gross-total resection with Gliadel wafers followed by early postoperative Gamma Knife radiosurgery and conformal fractionated radiotherapy as the initial treatment for patients with radiographically suspected, newly diagnosed glioblastoma multiforme. J Neurosurg. 2008;109(Suppl):106–117. doi: 10.3171/JNS/2008/109/12/S17. [DOI] [PubMed] [Google Scholar]
- 80.Brehmer S, Grimm MA, Forster A, Seiz-Rosenhagen M, Welzel G, Stieler F, Wenz F, Groden C, Mai S, Hanggi D, Giordano FA. Study protocol: early stereotactic Gamma Knife radiosurgery to residual tumor after surgery of newly diagnosed glioblastoma (Gamma-GBM) Neurosurgery. 2019;84:1133–1137. doi: 10.1093/neuros/nyy156. [DOI] [PubMed] [Google Scholar]
- 81.Asher AL, Burri SH, Wiggins WF, Kelly RP, Boltes MO, Mehrlich M, Norton HJ, Fraser RW. A new treatment paradigm: neoadjuvant radiosurgery before surgical resection of brain metastases with analysis of local tumor recurrence. Int J Radiat Oncol Biol Phys. 2014;88:899–906. doi: 10.1016/j.ijrobp.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 82.Seiler RW, Zimmermann A, Bleher EA, Markwalder H. Preoperative radiotherapy and chemotherapy in hypervascular, high-grade supratentorial astrocytomas. Surg Neurol. 1979;12:131–133. [PubMed] [Google Scholar]
- 83.Neoadjuvant chemoradiation for resectable glioblastoma (NeoGlio) (NCT04209790). https://clinicaltrials.gov/ct2/show/NCT04209790. Accessed 2nd November 2021
- 84.Riche M, Amelot A, Peyre M, Capelle L, Carpentier A, Mathon B. Complications after frame-based stereotactic brain biopsy: a systematic review. Neurosurg Rev. 2021;44:301–307. doi: 10.1007/s10143-019-01234-w. [DOI] [PubMed] [Google Scholar]
- 85.Park SM, Kim HS, Jahng GH, Ryu CW, Kim SY. Combination of high-resolution susceptibility-weighted imaging and the apparent diffusion coefficient: added value to brain tumour imaging and clinical feasibility of non-contrast MRI at 3 T. Br J Radiol. 2010;83:466–475. doi: 10.1259/bjr/34304111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brandes AA, Franceschi E, Paccapelo A, Tallini G, De Biase D, Ghimenton C, Danieli D, Zunarelli E, Lanza G, Silini EM, Sturiale C, Volpin L, Servadei F, Talacchi A, Fioravanti A, Pia Foschini M, Bartolini S, Pession A, Ermani M. Role of MGMT methylation status at time of diagnosis and recurrence for patients with glioblastoma: clinical implications. Oncologist. 2017;22:432–437. doi: 10.1634/theoncologist.2016-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hingtgen S, Figueiredo JL, Farrar C, Duebgen M, Martinez-Quintanilla J, Bhere D, Shah K. Real-time multi-modality imaging of glioblastoma tumor resection and recurrence. J Neurooncol. 2013;111:153–161. doi: 10.1007/s11060-012-1008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhu H, Leiss L, Yang N, Rygh CB, Mitra SS, Cheshier SH, Weissman IL, Huang B, Miletic H, Bjerkvig R, Enger PO, Li X, Wang J. Surgical debulking promotes recruitment of macrophages and triggers glioblastoma phagocytosis in combination with CD47 blocking immunotherapy. Oncotarget. 2017;8:12145–12157. doi: 10.18632/oncotarget.14553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang L, Cheng F, Wei Y, Zhang L, Guo D, Wang B, Li W. Inhibition of TAZ contributes radiation-induced senescence and growth arrest in glioma cells. Oncogene. 2019;38:2788–2799. doi: 10.1038/s41388-018-0626-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 91.Mathen P, Rowe L, Mackey M, Smart D, Tofilon P, Camphausen K. Radiosensitizers in the temozolomide era for newly diagnosed glioblastoma. Neurooncol Pract. 2020;7:268–276. doi: 10.1093/nop/npz057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bristol Myers Squibb (2019) Bristol-Myers Squibb announces phase 3 CheckMate -498 study did not meet primary endpoint of overall survival with Opdivo (nivolumab) plus radiation in patients with newly diagnosed MGMT-unmethylated glioblastoma multiforme. https://news.bms.com/news/corporate-financial/2019/Bristol-Myers-Squibb-Announces-Phase-3-CheckMate--498-Study-Did-Not-Meet-Primary-Endpoint-of-Overall-Survival-with-Opdivo-nivolumab-Plus-Radiation-in-Patients-with-Newly-Diagnosed-MGMT-Unmethylated-Glioblastoma-Multiforme/default.aspx. Accessed 23/11/20 2020
- 93.Bristol Myers Squibb (2019) Bristol-Myers Squibb provides update on phase 3 Opdivo (nivolumab) CheckMate -548 trial in patients with newly diagnosed MGMT-methylated glioblastoma multiforme. https://news.bms.com/news/corporate-financial/2019/Bristol-Myers-Squibb-Provides-Update-on-Phase-3-Opdivo-nivolumab-CheckMate--548-Trial-in-Patients-with-Newly-Diagnosed-MGMT-Methylated-Glioblastoma-Multiforme/default.aspx. Accessed 23/11/20 2020
- 94.Stessin AM, Clausi MG, Zhao Z, Lin H, Hou W, Jiang Z, Duong TQ, Tsirka SE, Ryu S. Repolarized macrophages, induced by intermediate stereotactic dose radiotherapy and immune checkpoint blockade, contribute to long-term survival in glioma-bearing mice. J Neurooncol. 2020;147:547–555. doi: 10.1007/s11060-020-03459-y. [DOI] [PubMed] [Google Scholar]
- 95.Grosshans DR, Mohan R, Gondi V, Shih HA, Mahajan A, Brown PD (2017) The role of image-guided intensity modulated proton therapy in glioma. Neuro-oncology 19: ii30-ii37 doi:10.1093/neuonc/nox002 [DOI] [PMC free article] [PubMed]
- 96.Collaborative EBCT, G,. Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19:27–39. 10.1016/S1470-2045(17)30777-5. [DOI] [PMC free article] [PubMed]
- 97.Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, Gebski V, Australasian Gastro-Intestinal Trials G. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681–692. doi: 10.1016/S1470-2045(11)70142-5. [DOI] [PubMed] [Google Scholar]
- 98.Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, Quirke P, Couture J, de Metz C, Myint AS, Bessell E, Griffiths G, Thompson LC, Parmar M. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373:811–820. doi: 10.1016/S0140-6736(09)60484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grobmyer SR, Maki RG, Demetri GD, Mazumdar M, Riedel E, Brennan MF, Singer S. Neo-adjuvant chemotherapy for primary high-grade extremity soft tissue sarcoma. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2004;15:1667–72. 10.1093/annonc/mdh431. [DOI] [PubMed]
- 100.Bonvalot S, Gronchi A, Le Pechoux C, Swallow CJ, Strauss D, Meeus P, van Coevorden F, Stoldt S, Stoeckle E, Rutkowski P, Rastrelli M, Raut CP, Hompes D, De Paoli A, Sangalli C, Honore C, Chung P, Miah A, Blay JY, Fiore M, Stelmes JJ, Dei Tos AP, Baldini EH, Litiere S, Marreaud S, Gelderblom H, Haas RL. Preoperative radiotherapy plus surgery versus surgery alone for patients with primary retroperitoneal sarcoma (EORTC-62092: STRASS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020;21:1366–1377. doi: 10.1016/S1470-2045(20)30446-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.