Abstract
Purpose
The purpose of the study is to assess the global risk of extracolonic secondary primary cancers (SPCs) in patients with colorectal cancer (CRC).
Methods
Studies of SPC in patients with CRC were included if they reported the standardised incidence ratio (SIR) for extracolonic SPCs in patients with CRC compared with the general population. Pooled summary estimates were calculated using a random-effects model.
Results
A total of 7,716,750 patients with CRC from 13 retrospective cohort studies that reported extracolonic SPC incidence were included. The overall risk of several SPCs was significantly higher in patients with CRC compared with the general population, including cancers of the urinary bladder (pooled SIR 1.19, 95% confidence interval (CI) 1.06–1.33; p = 0.003), female genital tract (1.88, 1.07–3.31; p = 0.03), kidney (1.50, 1.19–1.89; p = 0.0007), thorax (lung, bronchus and mediastinum) (1.16, 1.01–1.32; p = 0.03), small intestine (4.26, 2.58–7.01; p < 0.0001), stomach (1.22, 1.07–1.39; p = 0.003), and thyroid (1.40, 1.28–1.53; p < 0.0001), as well as melanoma (1.28, 1.01–1.62; p = 0.04). There was also a decreased risk of developing cancer of the gall bladder (0.75, 0.60–0.94; p = 0.01).
Conclusion
Patients with CRC had a significantly increased risk of extracolonic SPCs compared with the general population. These findings highlight the need to develop research strategies for the management of second primary cancer in patients with CRC.
Supplementary information
The online version contains supplementary material available at 10.1007/s00384-022-04105-x.
Keywords: Colorectal cancer, Second primary cancer, Multiple malignancies, Risk factors, Population-based study, Meta-analysis
Introduction
Colorectal cancer (CRC) is the fourth most common cancer type in the world and the third most deadly, accounting for about 10% of all incident cancers and cancer-related deaths each year [1, 2]. Although there have been improvements in the prognosis of patients with CRC due to recent advances in the screening, early detection, and treatment of CRC [3], the disease remains an important health issue worldwide. In addition, there has been an unexplained increase among young people [3–7]. This expanding population of CRC survivors faces long-term health concerns [8], such as the increased risk of developing second primary cancers (SPCs) [1, 9–21]. The reasons for this elevated risk remain unelucidated; however, various hypotheses have been posited in recent years, particularly familial genetic predispositions such as Lynch syndrome [22, 23], similar tumorigenic epigenetic changes in response to environmental exposures, or carcinogens related to tissues originating from the same germ layer [17], as well as specific mutations common to CRC and certain second cancers [24]. While the risk of synchronous and metachronous multiple malignancies of the colorectum have been well documented [20], evidence for the risk of extracolonic SPCs among CRC survivors has been less consistent [9–14, 17, 25, 26]. Around the world, CRC has been associated with extracolonic SPCs, including but not limited to malignancies of the urinary bladder [10, 12, 13, 25, 26], breast [11, 12, 27], kidney [10, 12], ovary [11, 12], pancreas [11–13, 25, 26], prostate [11, 12], stomach [11, 13, 25, 26], small intestine [11–13, 25, 26], and endometrium [12]. These mixed findings are indicative of the vast heterogeneity among countries and demonstrate the need to determine these risks to inform strategies for subsequent cancer surveillance following the management of primary CRC. Therefore, we carried out a systematic review and meta-analysis to investigate the risk of extracolonic SPCs in patients with CRC compared with the general population.
Methods
This systematic review and meta-analysis were done according to pre-specified criteria and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for the reporting of meta-analyses.
Data sources and searches
We searched PubMed, Embase, Scopus, and the Cochrane electronic database for studies published from each database’s inception to 27 Dec 2021, assessing the risk of SPCs in patients with CRC, using the following search terms: “colorectal cancer”, “bowel cancer”, “second cancer”, “second primary cancer”, “second malignancies”, “multiple primary cancer”, “multiple primary malignancies”, and “multiple primaries”.
Inclusion and exclusion criteria
Articles were eligible for inclusion if they reported the risk of extracolonic SPCs in patients with CRC, in terms of standardised incidence ratio (SIR). We included only studies that reported SIR estimates in our analyses since they provided an indirect method of adjustment for age and gender. No restrictions were applied to age, gender, comorbidities, duration, or location of the study, nor method of reporting cancer diagnoses. Articles without sufficient data, without reported individual extracolonic SPC risk, on second or multiple metachronous CRC, synchronous second or multiple cancers, centred on treatment modalities, and with overlapping populations and time periods were excluded. Only articles published in English were considered. The titles and abstracts of potentially eligible articles according to these eligibility criteria, and any duplicates, were excluded. Full-text articles were retrieved for studies that met the eligibility criteria. At this point, we excluded studies that did not include patients with CRC or did not report the SIR with respective 95% confidence intervals (CIs).
Data extraction and quality assessment
Data were extracted from all eligible studies using predefined data extraction form: study characteristics (study design, year of publication, and corresponding author), study setting (location and period), study population characteristics (sample size, age, and gender of the patients), and outcomes (duration of follow-up and cancer incidence per cancer type). Diagnosis and confirmation of CRC and SPCs were done according to the criteria of each study. The corresponding authors of the studies, or the national registry databases used as a data source in the original studies, were consulted for additional information if required. The methodological quality evaluation of each cohort study was based on the Newcastle–Ottawa Scale.
Outcome measures
The primary outcome measure was the incidence of extracolonic SPCs in patients with CRC, reported as SIRs. The SIR was defined in each study as the number of observed cancers in patients with CRC compared with the number of expected cancers in the general population. Specific details of how the expected number of neoplasms were calculated in each study have been summarised in Supplementary Table 1.
Statistical analyses
We used random-effects meta-analysis to assess the risk of extracolonic SPCs in patients with CRC. To calculate the pooled SIR of SPCs, we combined the extracted study-specific estimates and corresponding 95% CIs using the DerSimonian and Laird random-effects model [28]. The Newcastle–Ottawa Scale was used to assess the risk of bias of the included studies [29]. Studies with a rating of 6 or higher were considered high quality. The heterogeneity across studies was assessed using the I2 statistic (I2 0–25%, mild heterogeneity; I2 25–50% moderate heterogeneity; I2 > 50%, large heterogeneity) [19]. We used funnel plots to assess the potential for small-study effects (publication bias). All statistical analyses used RevMan (version 5.4.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration). All statistical tests used a two-sided α value of 0.05 for statistical significance.
Results
Literature search
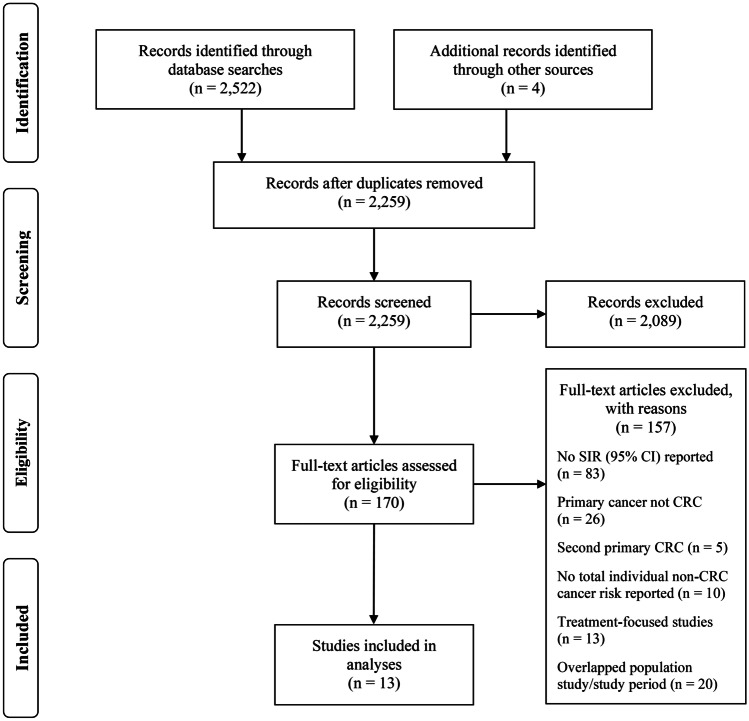
Searches returned 2522 records, with an additional 4 records identified through reference lists, of which 2259 were excluded after an initial screening of duplicates, titles, and abstracts. Full texts were retrieved for 170 studies and assessed for eligibility (Fig. 1). Thirteen studies published between 1999 and 2021, including 7,716,750 patients (2.01 × 109 person-years) with CRC that reported extracolonic SPCs cancer incidence, were included in the meta-analysis according to our inclusion criteria [16, 18, 21, 27, 30–38]. The median Newcastle–Ottawa rating for the studies included was 8 (interquartile range (IQR) (7–8)). The population characteristics and outcomes of the included studies are summarised in Table 1. The median age of the study populations ranges from 56 to 73.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart
Table 1.
Characteristics and quality of included studies
| Study ID | Study type | Country and data source | Study population, definition, and inclusion criteria | Primary cancer diagnosis timeframe and follow-up duration | Sample size (N) | Men | Women | Age (years) | Second primary cancer type(s); number of cases | Second primary cancers SIR (95% CI) | Newcastle–Ottawa Scale rating* |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bright et al. [16] | Retrospective cohort study | England and Wales; Office for National Statistics and Welsh Cancer registry | Men and women with > 5-year diagnosis of cancer; aged 15–39 years | 1971–2006; 16.8 years (median) | 200,945 | 76,666 (38.0%) | 124,279 (62.0%) | 15–39 |
Breast: 74 Lung and bronchus: 48 Urinary bladder: 32 Prostate: 33 Melanoma: 20 Ovary: 19 Oral: 16 Corpus uteri: 68 Kidney: 23 Non-Hodgkin lymphoma: 19 Brain: 21 Oesophagus: 12 Pancreas: 9 Other female genital: 27 Stomach: 14 Leukaemia: 9 |
1.80 (1.06–3.07) 1.30 (0.90–1.70) 2.10 (1.40–2.90) 1.20 (0.80–1.70) 1.50 (0.90–1.10) 1.90 (1.10–2.9) 1.80 (1.00–2.90) 7.20 (5.60–9.10) 3.00 (1.90–4.50) 1.50 (0.90–2.30) 3.00 (1.80–4.60) 1.60 (0.80–2.90) 1.30 (0.06–2.50) 3.30 (2.20–4.80) 2.00 (1.10–3.40) 1.30 (0.60–1.30) |
7 |
| Caini et al. [30] | Retrospective cohort study | Italy; European Institute of Oncology database | Men and women with diagnosis of non-cutaneous malignancy | 2000–2010; 4 years (median) | 52,354 | 15,706 (30.0%) | 36,648 (70.0%) | 56 (median) | Melanoma: 9 | 1.37 (0.71–2.63) | 4 |
| Chung et al. [31] | Retrospective cohort study | South Korea; Cancer Registry database at Severance Hospital, Seoul, Korea | Men and women with a diagnosis of CRC; aged 45–74 years | 2001–2009; 40.1 months (median) | 4822 | 2981 (61.8) | 1841 (38.2) | 61 (median) | Pancreas: 13 | 14.44 (12.71–16.16) | 5 |
| Cluze et al. [32] | Retrospective cohort study | France; Cancer Registry of Isère | Men and women with a diagnosis of breast, prostate, or colorectal cancer; aged > 15 years | 1989–1997; 3.5 years (mean) | 14,353 | 6314 (44.0%) | 8039 (56.0%) | 66.4 (mean) |
Upper aerodigestive tract: 10 Oesophagus: 3 Stomach: 3 Small intestine: 5 Liver and hepatic ducts: 3 Pancreas: 0 Lung: 16 Skin: 9 Breast: 18 Female genitals: 5 Prostate: 25 Kidney: 5 Urinary bladder: 9 |
1.33 (0.69–2.33) 1.10 (0.23–3.22) 0.70 (0.23–1.64) 10.70 (3.47–24.97) 0.70 (0.23–1.63) 0.23 (0.01–1.29) 1.21 (0.77–1.79) 1.52 (0.92–2.38) 1.22 (0.77–1.85) 1.00 (0.40–2.06) 1.11 (0.79–1.53) 1.90 (0.76–3.92) 1.40 (0.72–2.44) |
8 |
| Crocetti et al. [36] | Retrospective cohort study | Italy; Italian cancer registries | Men and women with a diagnosis of thyroid cancer; aged < 85 years | 1998–2012; < 7 years (median) | 6,984,420 | 3,340,798 (47.8%) | 3,643,622 (52.2%) | < 85 | Thyroid: 230 | 1.40 (1.30–1.60) | 8 |
| Dasgupta et al. [18] | Retrospective cohort study | Australia; Queensland Cancer Registry | Men and women with a diagnosis of invasive CRC; aged 20–79 years | 1996–2005; 4.2 years (median) | 15,755 | 9091 (57.7%) | 6664 (42.3%) | 64 (mean) |
Stomach: 38 Small intestine: 20 Pancreas: 33 Lung: 202 Melanoma: 168 Breast (female): 115 Uterus: 25 Prostate: 265 Kidney: 57 Urinary bladder: 73 Non-Hodgkin lymphoma: 42 Myeloma: 21 |
1.43 (1.01–1.97) 4.84 (2.96–7.48) 1.19 (0.82–1.67) 1.40 (1.22–1.61) 1.37 (1.17–1.59) 1.22 (1.01–1.47) 1.57 (1.01–2.31) 1.14 (1.01–1.29) 1.61 (1.21–2.07) 1.25 (0.98–1.57) 1.02 (0.73–1.38) 1.32 (0.82–2.02) |
8 |
| He et al. [33] | Retrospective cohort study | USA; National Cancer Institute SEER database | Men and women with a diagnosis of CRC; Aged > 18 years | 1973–2013; 7.3 years (mean) | 44,106 | 25,514 (58.0%) | 18,592 (42.0%) | > 18 |
Oropharynx: 999 Oesophagus: 576 Stomach: 1225 Small intestine: 585 Liver: 371 Gallbladder: 106 Pancreas: 1478 Lung and bronchus: 7400 Melanoma: 1254 Breast: 4949 Cervix uteri: 166 Corpus uteri: 1273 Ovary: 525 Prostate: 8101 Urinary bladder: 2947 Kidney: 68 Brain: 360 Thyroid: 410 Myeloma: 611 Leukaemia: 1306 |
0.95 (0.90–1.02) 1.08 (1.00–1.17) 1.16 (1.09–1.22) 3.13 (2.89–3.40) 0.76 (0.69–0.85) 0.65 (0.54–0.79) 0.99 (0.94–1.04) 1.00 (0.98–1.03) 0.89 (0.85–0.95) 0.99 (0.96–1.02) 0.96 (0.82–1.12) 1.22 (1.15–1.29) 0.88 (0.81–0.96) 0.91 (0.89–0.93) 1.00 (0.97–1.04) 1.07 (1.01–1.13) 0.85 (0.77–0.95) 1.30 (1.18–1.43) 0.86 (0.79–0.93) 0.90 (0.85–0.95) |
9 |
| Lee et al. [34] | Retrospective cohort study | Taiwan; Taiwan’s National Health Insurance Database | Men and women with a diagnosis of CRC | 1996–2011; 4.03 years (median) | 98,876 | 55,729 (56.4%) | 43,147 (43.6%) | 67 (median) |
Oesophagus: 77 Stomach: 299 Liver/biliary tract: 636 Pancreas: 100 Lung and mediastinum: 843 Skin: 127 Breast: 275 Women genital: 257 Cervix uteri: 92 Corpus uteri: 106 Ovary: 59 Prostate: 455 Urinary bladder: 259 Kidney: 191 Thyroid: 73 |
0.87 (0.68–1.08) 1.02 (0.91–1.14) 0.90 (0.83–0.97) 1.01 (0.82–1.23) 1.18 (1.10–1.26) 1.12 (0.93–1.33) 1.20 (1.06–1.25) 1.64 (1.45–1.85) 0.98 (0.79–1.20) 0.32 (2.62–3.87) 2.00 (1.52–2.57) 1.19 (1.09–1.31) 1.31 (1.16–1.48) 1.45 (1.25–1.67) 1.71 (1.34–2.15) |
8 |
| Levi et al. [21] | Retrospective cohort study | Switzerland; Vaud Cancer Registry | Men and women with a diagnosis of CRC or adenomatous polyps | 1974–1994; average follow-up unknown | 5261 | . | . | . |
Oropharynx: 16 Oesophagus: 18 Stomach: 32 Small intestine: 4 Liver: 6 Gallbladder: 6 Pancreas: 6 Lung: 50 Melanoma: 12 Breast (female): 80 Cervix uteri: 10 Corpus uteri: 8 Ovary: 2 Prostate: 96 Urinary bladder: 22 Kidney: 16 Non-Hodgkin lymphoma: 12 Leukaemia: 8 |
0.84 (0.40–1.60) 1.36 (0.60–2.60) 1.16 (0.70–1.90) 1.89 (0.20–6.80) 0.63 (0.10–1.80) 0.71 (0.10–2.10) 0.29 (0.10–0.90) 0.70 (0.50–1.00) 0.96 (0.40–2.10) 1.29 (0.90–1.80) 1.73 (0.60–4.00) 0.63 (0.20–1.60) 0.21 (0.00–1.20) 1.19 (0.90–1.60) 0.88 (0.50–1.60) 1.25 (0.50–2.50) 0.84 (0.30–1.80) 0.59 (0.20–1.50) |
8 |
| Utada et al. [27] | Retrospective cohort study | Japan; Nagasaki Prefecture Cancer Registry | Men and women with a diagnosis of primary cancer | 1985–2007; 4.3 years (mean) | 174,477 | . | . | . |
Stomach: 751 Pancreas: 137 Ovary: 34 |
1.37 (1.28–1.47) 1.21 (1.02–1.43) 1.83 (1.27–2.56) |
7 |
| Ye et al. [35] | Retrospective cohort study | Australia; Tasmanian Cancer Registry | Men and women with a diagnosis of cancer > 2 months; aged > 15 years | 1980–2009; 6.9 years (mean) | 51,802 | 28,242 (54.5%) | 23,560 (45.5%) | 66.2 (median) |
Lung: 121 Skin: 80 Prostate: 182 |
1.13 (0.80–1.58) 1.88 (1.38–2.55) 1.15 (0.90–1.48) |
8 |
| Zheng et al. [37] | Retrospective cohort study | Sweden; Swedish Cancer Registry | Men and women with a diagnosis of bladder or upper urinary tract cancer | 1990–2015; average follow-up unknown | 49,584 | 36,614 (74%) | 12,970 (26.0%) | 73 (median) |
Urinary bladder: 521 Kidney: 22 |
1.11 (1.02 –1.21) 1.32 (0.83–1.54) |
8 |
| Zheng et al. [38] | Retrospective cohort study | Sweden; Swedish Cancer Registry | Men and women with a diagnosis of hepatobiliary cancer | 1990–2015; 36 months (median) | 19,995 | 10,102 (64.9%) | 9893 (49.5%) | 72 (median) |
Gallbladder: 44 Bile duct: 61 |
0.85 (0.61–1.14) 1.20 (0.92–1.54) |
8 |
*Newcastle–Ottawa Scale ratings ≥ 6 were considered high quality
Risk of extracolonic SPCs in CRC patients
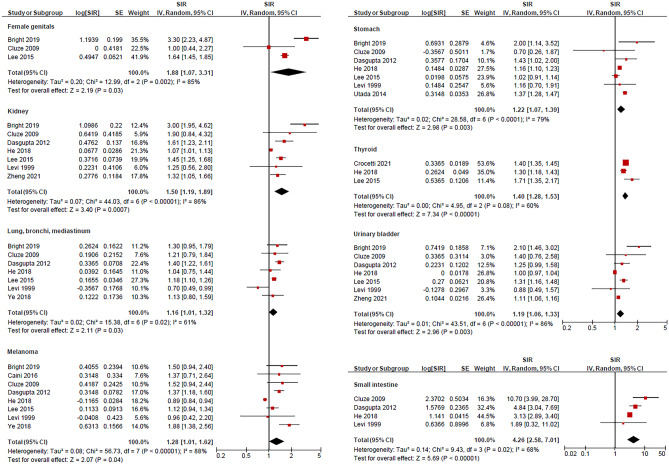
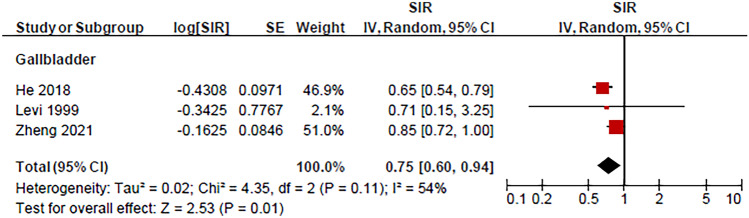
We analysed the risk of extracolonic SPCs in patients with CRC among 13 studies reporting SIR (Table 1). The risk of several second primary cancers was significantly higher in patients with CRC compared with the general population’s risk of developing respective primary cancers. The risk of subsequent malignancies was greatest in the small intestine (pooled SIR = 4.26 (95% CI = 2.58–7.01; p < 0.0001)) from four studies [18, 21, 32, 33]; followed by the female genitals (1.88 (1.07–3.31; p = 0.03)) from three studies [16, 32, 34]; kidney (1.50 (1.19–1.89; p = 0.0007)) from seven studies [16, 18, 21, 32–34, 37]; thyroid (1.40 (1.28–1.53; p < 0.0001)) from three studies [33, 34, 36]; skin (melanoma) (1.28 (1.01–1.62; p = 0.04)) from eight studies [16, 18, 21, 30, 32–35]; stomach 1.22 ((1.07–1.39; p = 0.003)) from seven studies [16, 18, 21, 27, 32–34]; urinary bladder (1.19 (1.06–1.33; p < 0.0001)) from seven studies [16, 18, 21, 32–34, 37]; and lung, bronchi, and mediastinum (1.16 (1.01–1.32; p = 0.03)) from seven studies [16, 18, 21, 32–35]; Fig. 2. In contrast, there was a decreased risk of second primary gall bladder cancer (pooled SIR = 0.75; 95% CI = 0.60–0.94; p = 0.01) from three studies [21, 33, 38]; Fig. 3). There was no significant difference in the risk of second primary cancers of the prostate, pancreatic, ovaries, oesophagus, upper aerodigestive tract, liver and biliary tract, breast, cervix, uterus, and brain, nor in non-Hodgkin lymphoma, leukaemia, and myeloma (p > 0.05). The median follow-up years for each SPC are outlined in Table 2. According to the studies included in our analysis, the median follow-up time for SPCs was 4.2 years.
Fig. 2.
Second primary cancers with an increased risk following primary colorectal cancer including cancers from female genitals, kidney, thorax (lung, bronchi, and mediastinum), stomach, thyroid, urinary bladder, and small intestine as well as melanoma. The red squares and their sizes represent the effect sizes and weights of the included studies, respectively. The black diamonds and their sizes represent the pooled effect size and their 95% confidence intervals, respectively. The centre line of no effect runs through the value 1. Points to the right of the centre line (> 1) indicate an increased risk, whereas points to the left of the centre line (< 1) indicate a decreased risk
Fig. 3.
Second primary cancers with a decreased risk of following primary colorectal cancer: gall bladder cancer. The red squares and their sizes represent the effect sizes and weights of the included studies, respectively. The black diamond and its size represent the pooled effect size and its 95% confidence intervals, respectively. The centre line of no effect runs through the value 1. Points to the right of the centre line (> 1) indicate an increased risk, whereas points to the left of the centre line (< 1) indicate a decreased risk
Table 2.
Median follow-up periods for the second primary cancers included in the meta-analysis
| Second cancer | Median follow-up years (IQR) |
|---|---|
| Urinary bladder | 4.2 (3.8–12.1) |
| Brain | 12.1 |
| Breast | 4.2 (3.8–12.1) |
| Cervix | 5.7 |
| Female genital | 4.0 (3.5–16.8) |
| Gallbladder | 7.3 |
| Kidney | 4.2 (3.8–12.1 |
| Leukaemia | 12.1 |
| Liver, hepatic duct, and biliary | 4.0 (3.5–7.3) |
| Lung, bronchus, and mediastinum | 4.2 (3.8–12.1) |
| Melanoma | 4.1 (3.9–9.7) |
| Myeloma | 5.8 |
| Non-Hodgkin lymphoma | 10.5 |
| Oesophagus | 5.7 (3.6–14.4) |
| Ovary | 7.3 (4.0–16.8) |
| Pancreas | 4.1 (3.5–9.7) |
| Prostate | 4.2 (3.8–12.1) |
| Small intestine | 4.2 (3.5–7.3) |
| Stomach | 4.2 (3.8–12.1 |
| Thyroid | 7.0 (4.0–7.3) |
| Upper aerodigestive tract | 7.3 (3.5–16.8) |
| Uterus | 4.2 (3.8–12.1) |
Publication bias
Heterogeneity was high in studies investigating the risk of urinary bladder, prostate, pancreatic, ovarian, stomach, kidney, lung, small intestine, upper aerodigestive tract, breast, uterine, thyroid, brain, female genital, and liver, hepatic duct, and biliary tract cancers, as well as melanoma, leukaemia, and myeloma. However, visual inspection of funnel plots showed no asymmetry which indicated no publication biases were present (Supplementary Fig. 1).
Discussion
The findings of this systematic review and meta-analysis suggest that patients with CRC have a significantly higher risk of extracolonic SPCs than the general population, including cancers of the urinary bladder, female genitals, kidney, lung, bronchus and mediastinum, small intestine, stomach, and thyroid, as well as melanoma. The greatest risk was observed for SPC of the small intestine, more than fourfold, compared with the general population, while the increased risk was relatively less for other sites (less than twofold).
Previous studies have reported an increased risk of SPCs following CRC, particularly cancers of the urinary bladder [10, 12], kidney [10, 12], stomach [9, 10, 12], and the small intestine [9–12, 17], which are consistent with the results of our meta-analysis. Because of these findings, several possible mechanisms have been discussed. For example, some of the risks can be attributed to genetic predisposition, such as in cases of Lynch syndrome (hereditary non-polyposis colorectal cancer familial cancer syndrome), albeit rare [22, 23]. Another hypothesis pertains to the expectation that embryologically related tissues might respond in similar ways to environmental exposures or carcinogens and undergo comparable epigenetic changes conducive to tumourigenesis [17]. Indeed, the small intestine, stomach, urinary bladder, and lung share endoderm-derived epithelia and, therefore, may be linked in this manner. However, this was not supported by our observed decrease in the risk of second primary gall bladder cancer. Alternatively, specific mutations common to CRC and certain second primary malignancies may be responsible for the elevated risk. For instance, v-raf murine sarcoma viral oncogene homologue B1 (BRAF), one of the most frequently mutated protein kinase genes in human cancers, mutations are seen in melanoma, papillary thyroid carcinoma, and CRC [24]. In addition, in the follow-up of CRC, many of these SPCs of high prevalence (including cancers of the urinary bladder, female genitals, kidney, lung, bronchus and mediastinum, small intestine, and stomach) could be detected on the follow-up abdominal and chest computed tomography (CT) scan which may also contribute to higher pick-up rate of these SPCs.
Our study had several limitations. Misclassification of cancers in registry-based investigations may introduce over- or underestimation of SPC incidence rates. As we did not include metachronous CRC in our analysis, differentiating between SPCs and local recurrences was not an issue. Additionally, most studies reported attempts to prevent the inclusion of synchronous cancers by excluding subsequent cancers diagnosed within 2 months of the index CRC. There may have been some level of misclassification with respect to tumours arising in discrete sites, namely the lungs, bronchi, and mediastinum; upper aerodigestive tract; female genitals; and the liver, hepatic ducts, and biliary system. As such, we only pooled second cancers of discrete sites where explicitly consistent between individual studies for the robustness of our interpretations.
Although we anticipated and attempted to address heterogeneity in our planned analysis, it remained substantial for most pooled second cancers. This is likely due to epidemiological differences between studies, such as follow-up, the periods of time covered, changes in specific cancer demographics across time, varying selection criteria, and temporospatial differences in treatment modalities. Comparably moderate-to-high levels of heterogeneity have been previously observed and discussed in other meta-analyses on SPC [39, 40]. The heterogeneity in these meta-analyses can be largely attributed to differences in the magnitude of risk observed between studies. Ultimately, while we cannot be certain of the true magnitude of in the risk reported in the present study, our results are indicative of an increase in risk of specific second primary malignancies leading to further foci of research in the field.
There is lack of data to clearly document the effect of occurrence of SPC in the overall survival of patients with SPC. The survival of these patients is likely to be depending on the nature of the primary CRC and the SPC. If the CRC is of advanced stages and with residual cancer after resection as well as with mutation not amendable to target therapy, it is likely the survival is dismal and the impact of SPC on the survival is not apparent. On the other hand, if the CPC is of early stages and after curative resection, the survival of the patients with CRC is obviously affected and likely depend on the SPCs with high patients’ mortality and morbidity such as cancers of the thorax (lung, bronchus, and mediastinum) and melanoma [41, 42]. There are also SPCs such as from the urinary bladder, kidney, female genitals, small intestine, and stomach of similar diverse biological aggressiveness as CRC which will have impact of the survival on the patients. The only exception is in patients with SPC of thyroid cancer with is of increasing incidence worldwide. Thyroid cancer is mostly clinically indolent but could contribute to long-term morbidity of the patients with possibility of local recurrence, de-differentiation to clinical aggressive histological type, and thyroxine replacement therapy [43, 44].
In most clinical centres, the management of patients with CRC will be discussed in multidisciplinary team meeting and follow-up with standard protocols (such as radiology and endoscopic examinations) according to the prognostic parameters as well as personalised medical needs (such as comorbidity). Majority of the SPCs of relative higher prevalence could be detected by this means. Thus, awareness of the possibility of SPCs and adherence to protocols of follow-up of patients with CRC is important for best clinical practice. Nevertheless, we need more investigations to look at the length of intervals between CRC and SPCs. With the acknowledgement that the median follow-up time for SPCs from the studies included in our analysis was 4.2 years, it may be in some cases that SPCs may occur after the standard follow-up time for patients with CRC. Education on the patient and general practitioner of the issue should be of value to this group of patients. It is also important to have prospective clinical studies to address to the comorbidity issues and survival impacts in these patients.
Conclusion
The findings of this systematic review and meta-analysis suggest that patients with CRC have an increased risk of extracolonic SPCs compared with the general population, including cancers of the urinary bladder, female genitals, kidney, lung, bronchus and mediastinum, small intestine, stomach, and thyroid, as well as melanoma. Future studies monitoring SPC risk in patients with CRC are warranted as there is a need to develop surveillance and management strategies to decrease the burden of subsequent malignancies within this expanding population.
Supplementary information
Below is the link to the electronic supplementary material.
Author contribution
Dylan Robertson: writing—original draft, formal analysis, conceptualisation, methodology, investigations, resources, writing—review and editing; Alfred K Lam, Shu K Ng, and Peter D Baade contributed equally to this work: conceptualisation, writing—review and editing. All authors approved the final manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467–1480. doi: 10.1016/S0140-6736(19)32319-0. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Araghi M, Soerjomataram I, Bardot A, Ferlay J, Cabasag CJ, Morrison DS, et al. Changes in colorectal cancer incidence in seven high-income countries: a population-based study. Lancet Gastroenterol Hepatol. 2019;4:511–518. doi: 10.1016/s2468-1253(19)30147-5. [DOI] [PubMed] [Google Scholar]
- 4.Loomans-Kropp HA, Umar A (2019) Increasing incidence of colorectal cancer in young adults. J Cancer Epidemiol 2019:9841295. 10.1155/2F2019/2F9841295 [DOI] [PMC free article] [PubMed]
- 5.Vuik FE, Nieuwenburg SA, Bardou M, Lansdorp-Vogelaar I, Dinis-Ribeiro M, Bento MJ, et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut. 2019;68:1820–1826. doi: 10.1136/gutjnl-2018-317592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasi PM, Shahjehan F, Cochuyt JJ, Li Z, Colibaseanu DT, Merchea A. Rising proportion of young individuals with rectal and colon cancer. Clin Colorectal Cancer. 2019;18:e87–e95. doi: 10.1016/j.clcc.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Mauri G, Sartore-Bianchi A, Russo AG, Marsoni S, Bardelli A, Siena S. Early-onset colorectal cancer in young individuals. Mol Oncol. 2019;13:109–131. doi: 10.1002/1878-0261.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69:363–385. doi: 10.3322/caac.21565. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed F, Goodman MT, Kosary C, Ruiz B, Wu XC, Chen VW, et al. Excess risk of subsequent primary cancers among colorectal carcinoma survivors, 1975–2001. Cancer. 2006;107:1162–1171. doi: 10.1002/cncr.22013. [DOI] [PubMed] [Google Scholar]
- 10.Enblad P, Adami HO, Glimelius B, Krusemo U, Påhlman L. The risk of subsequent primary malignant diseases after cancers of the colon and rectum. A nationwide cohort study Cancer. 1990;65:2091–2100. doi: 10.1002/1097-0142(19900501)65:9/3C2091::aid-cncr2820650934/3E3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 11.McCredie M, Macfarlane GJ, Bell J, Coates M. Second primary cancers after cancers of the colon and rectum in New South Wales, Australia, 1972–1991. Cancer Epidemiol Biomarkers Prev. 1997;6:155–160. [PubMed] [Google Scholar]
- 12.Hemminki K, Li X, Dong C. Second primary cancers after sporadic and familial colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2001;10:793–798. [PubMed] [Google Scholar]
- 13.Evans HS, Møller H, Robinson D, Lewis CM, Bell CM, Hodgson SV (2002) The risk of subsequent primary cancers after colorectal cancer in southeast England. Gut 50:647–652. 10.1136/2Fgut.50.5.647 [DOI] [PMC free article] [PubMed]
- 14.Ringland CL, Arkenau HT, O’Connell DL, Ward RL. Second primary colorectal cancers (SPCRCs): experiences from a large Australian Cancer Registry. Ann Oncol. 2010;21:92–97. doi: 10.1093/annonc/mdp288. [DOI] [PubMed] [Google Scholar]
- 15.Guan X, Jin Y, Chen Y, Jiang Z, Liu Z, Zhao Z, et al. The incidence characteristics of second primary malignancy after diagnosis of primary colon and rectal cancer: a population based study. PLoS ONE. 2015;10:e0143067. doi: 10.1371/journal.pone.0143067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bright CJ, Reulen RC, Winter DL, Stark DP, McCabe MG, Edgar AB, et al. Risk of subsequent primary neoplasms in survivors of adolescent and young adult cancer (Teenage and Young Adult Cancer Survivor Study): a population-based, cohort study. Lancet Oncol. 2019;20:531–545. doi: 10.1016/S1470-2045(18)30903-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phipps AI, Chan AT, Ogino S. Anatomic subsite of primary colorectal cancer and subsequent risk and distribution of second cancers. Cancer. 2013;119:3140–3147. doi: 10.1002/cncr.28076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dasgupta P, Youlden DR, Baade PD. Multiple primary cancers among colorectal cancer survivors in Queensland, Australia, 1996–2007. Cancer Causes Control. 2012;23:1387–1398. doi: 10.1007/s10552-012-9990-1. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lam AK-Y, Gopalan V, Carmichael R, Buettner PG, Leung M, Smith R et al (2012) Metachronous carcinomas in colorectum and its clinicopathological significance. Internatl J Colorectal Dis 27:1303–1310. 10.3748/2Fwjg.v20.i22.6815 [DOI] [PubMed]
- 21.Levi F, Randimbison L, La Vecchia C, Te V-C, Franceschi S. Cancer risk following polyps or cancer of the large bowel in Vaud. Switzerland Int J Cancer. 1999;80:634–635. doi: 10.1002/(sici)1097-0215(19990209)80:4/3C634::aid-ijc26/3E3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 22.Lynch HT, Lynch PM, Lanspa SJ, Snyder CL, Lynch JF, Boland CR (2009) Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin Genet 76:1–18. 10.1111/2Fj.1399-0004.2009.01230.x [DOI] [PMC free article] [PubMed]
- 23.Win AK, Lindor NM, Young JP, Macrae FA, Young GP, Williamson E, et al. Risks of primary extracolonic cancers following colorectal cancer in Lynch syndrome. J Natl Cancer Inst. 2012;104:1363–1372. doi: 10.1093/jnci/djs351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pakneshan S, Salajegheh A, Smith RA, Lam AK-Y (2013) Clinicopathological relevance of BRAF mutations in human cancer. Pathology 45:346–356. 10.1097/pat.0b013e328360b61d [DOI] [PubMed]
- 25.Heard A, Roder D, Luke C. Multiple primary cancers of separate organ sites: implications for research and cancer control (Australia) Cancer Causes Control. 2005;16:475–481. doi: 10.1007/s10552-004-8023-0. [DOI] [PubMed] [Google Scholar]
- 26.Liang YH, Shao YY, Chen HM, Lai CL, Lin ZZ, Kuo RN, et al. Young patients with colorectal cancer have increased risk of second primary cancers. Jpn J Clin Oncol. 2015;45:1029–1035. doi: 10.1093/jjco/hyv137. [DOI] [PubMed] [Google Scholar]
- 27.Utada M, Ohno Y, Hori M, Soda M. Incidence of multiple primary cancers and interval between first and second primary cancers. Cancer Sci. 2014;105:890–896. doi: 10.1111/cas.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 29.Wells GA, Shea B, O’Connell Da, Peterson J, Welch V, Losos M et al (2011) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Available from: URL: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 30.Caini S, Radice D, Tosti G, Spadola G, Cocorocchio E, Ferrucci PF, et al. Risk of second primary malignancies among 1537 melanoma patients and risk of second primary melanoma among 52 354 cancer patients in Northern Italy. J Eur Acad Dermatol Venereol. 2016;30:1491–1496. doi: 10.1111/jdv.13645. [DOI] [PubMed] [Google Scholar]
- 31.Chung JW, Chung MJ, Bang S, Park SW, Song SY, Chung JB et al (2017) Assessment of the risk of colorectal cancer survivors developing a second primary pancreatic cancer. Gut Liver 11:728–732. 10.5009/2Fgnl16526 [DOI] [PMC free article] [PubMed]
- 32.Cluze C, Delafosse P, Seigneurin A, Colonna M. Incidence of second cancer within 5 years of diagnosis of a breast, prostate or colorectal cancer: a population-based study. Eur J Cancer Prev. 2009;18:343–348. doi: 10.1097/cej.0b013e32832abd76. [DOI] [PubMed] [Google Scholar]
- 33.He X, Wu W, Ding Y, Li Y, Si J, Sun L. Excessive risk of second primary cancers in young-onset colorectal cancer survivors. Cancer Med. 2018;7:1201–1210. doi: 10.1002/cam4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee YT, Liu CJ, Hu YW, Teng CJ, Tzeng CH, Yeh CM et al (2015) Incidence of second primary malignancies following colorectal cancer: a distinct pattern of occurrence between colon and rectal cancers and association of co-morbidity with second primary malignancies in a population-based cohort of 98,876 patients in Taiwan. Medicine 94:e1079. 10.1097/2FMD.0000000000001079 [DOI] [PMC free article] [PubMed]
- 35.Ye Y, Otahal P, Wills KE, Neil AL, Venn AJ. Temporal trends in the risk of second primary cancers among survivors of adult-onset cancers, 1980 through 2013: An Australian population-based study. Cancer. 2018;124:1808–1818. doi: 10.1002/cncr.31247. [DOI] [PubMed] [Google Scholar]
- 36.Crocetti E, Mattioli V, Buzzoni C, Franceschi S, Serraino D, Vaccarella S et al (2021) Risk of thyroid as a first or second primary cancer. A population-based study in Italy, 1998–2012. Cancer Med 10(19):6855–67. 10.1002/cam4.4193 [DOI] [PMC free article] [PubMed]
- 37.Zheng G, Sundquist K, Sundquist J, Försti A, Hemminki O, Hemminki K. Bladder and upper urinary tract cancers as first and second primary cancers. Cancer Rep. 2021 doi: 10.1002/cnr2.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng G, Sundquist K, Sundquist J, Chen T, Försti A, Hemminki A, et al. Second primary cancers after liver, gallbladder and bile duct cancers, and these cancers as second primary cancers. Clin Epidemiol. 2021;13:683–691. doi: 10.2147/CLEP.S318737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grantzau T, Overgaard J. Risk of second non-breast cancer among patients treated with and without postoperative radiotherapy for primary breast cancer: a systematic review and meta-analysis of population-based studies including 522,739 patients. Radiother Oncol. 2016;121:402–413. doi: 10.1016/j.radonc.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 40.Gilbert DC, Wakeham K, Langley RE, Vale CL. Increased risk of second cancers at sites associated with HPV after a prior HPV-associated malignancy, a systematic review and meta-analysis. Br J Cancer. 2019;120:256–268. doi: 10.1038/s41416-018-0273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ortega-Ortega M, Hanly P, Pearce A, Soerjomataram I, Sharp L. Paid and unpaid productivity losses due to premature mortality from cancer in Europe in 2018. Int J Cancer. 2022;150:580–593. doi: 10.1002/ijc.33826. [DOI] [PubMed] [Google Scholar]
- 42.Abe I, Lam AK (2021) Anaplastic thyroid carcinoma: Updates on WHO classification, clinicopathological features and staging. Histol Histopathol 36:239–248. 10.14670/HH-18-277 [DOI] [PubMed]
- 43.Lam AK. Squamous cell carcinoma of thyroid: a unique type of cancer in World Health Organization Classification. Endocr Relat Cancer. 2020;27:R177–R192. doi: 10.1530/ERC-20-0045. [DOI] [PubMed] [Google Scholar]
- 44.Lam AK, Lo CY, Lam KS. Papillary carcinoma of thyroid: A 30-yr clinicopathological review of the histological variants. Endocr Pathol. 2005;16:323–330. doi: 10.1385/ep:16:4:323. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.