Summary
Background
Ischemic heart disease (IHD) is a leading cause of mortality, particularly for men. Few interventions have focused on protecting specifically men. Emerging evidence may implicate testosterone. Neurokinin 3 receptor (NK3R) antagonists, an existing class of drugs being considered as treatments for reproductive conditions in women, affect testosterone; this study addresses genetic validation of their use to prevent IHD in men.
Methods
A one-sample Mendelian randomization (MR) study using the UK Biobank cohort study, based on independent (r2 < 0.005) genetic variants predicting testosterone in men (n = 157738) at genome wide significance in the target gene for NK3R antagonists (TACR3), was used to assess associations with IHD (cases=15056, non-cases=151964) and positive control outcomes (relative age voice broke, children fathered, hypertension) in men and a negative control outcome (IHD) in women using summary statistics. A two-sample MR study using the PRACTICAL consortium was used for the positive control outcome of prostate cancer.
Findings
Two relevant TACR3 genetic variants (rs116646027 and rs1351623) were identified in men. Genetically mimicked NK3R antagonists were inversely associated with IHD (odds ratio 0.54 per standard deviation lower testosterone, 95% confidence interval 0.31, 0.94) and with control outcomes (older relative age voice broke, fewer children and lower risk of hypertension and prostate cancer) as expected in men and in women (unrelated to IHD).
Interpretation
Genetic validation of a role of NK3R antagonists in IHD suggests their consideration as a new means of preventing IHD in men. Whether they protect against prostate cancer might bear further consideration.
Keywords: NK3R, IHD, Sex, Evolutionary biology, Men
Research in context.
Evidence before this study
Cardiovascular disease rates are higher in men than women and contribute to shorter lives in men than women. Few interventions designed to address this disparity exist, although recent research suggests testosterone may be a contributing factor. A widely developed class of drugs, neurokinin 3 receptor (NK3R) antagonists, originally developed as anti-psychotics, are now being tested as a treatment for reproductive conditions in women. NK3R antagonists are known to reduce testosterone and have been proposed as a treatment for cardiovascular disease, which might be particularly relevant in men. No study has assessed cardiovascular effects of NK3R antagonists, according to this search in PubMed (“neurokinin 3 receptor cardiovascular human”)
Added value of this study
This quasi-experimental study provides genetic validation suggesting that NK3R antagonists may protect men against ischemic heart disease, as well as having expected associations for several control outcomes (higher relative age voice broke, fewer children fathered, and lower risk of hypertension and prostate cancer).
Implications of all the available evidence
As well as NK3R antagonists being considered as treatment for reproductive conditions in women, they should also be considered as a means of addressing sexual disparities in health and lifespan.
Alt-text: Unlabelled box
Ischemic heart disease (IHD) is the leading cause of death globally with higher mortality rates in men than women.1 Development of new means of preventing and treating cardiovascular disease has stalled recently.2 To address this gap chronic diseases, similar to infectious diseases, are increasingly seen within an evolutionary biology framework,3 where drivers of growth and reproduction may trade-off against long-term health. Correspondingly, interventions, such as calorie restriction, targeting the drivers of growth, have long been investigated as potential interventions.4 One of the newer drugs for type 2 diabetes, particularly recommended for those at higher risk of cardiovascular disease,5 is thought to be a calorie restriction mimetic.6 Interventions targeting sex hormones are widely used to treat hormone-related cancers.7,8 Emerging quasi-experimental evidence suggests that androgens may be involved in cardiovascular disease,9, 10, 11 and one of its key risk factors, hypertension.12 Correspondingly, one of the most effective drugs for preventing and treating cardiovascular disease, statins, reduces testosterone13,14 and operates partially by that pathway in men.14 Many recommendations concerning a healthy lifestyle likely reduce testosterone in men,15 few interventions for IHD have explicitly addressed this oppurtunity.15
An existing drug class, originally developed as antipsychotics, currently being considered as a treatment for reproductive conditions in women,16 i.e., neurokinin 3 receptor (NK3R) antagonists, also modulates testosterone.16 As such, NK3R antagonists represent an existing drug class that might provide a new means of combating cardiovascular disease,17 particularly in men. However, no relevant experimental evidence exists. Mendelian randomization provides an alternative, by leveraging the random allocation of genetic material at conception to obtain estimates largely free of the confounding that can bias observational studies.18 Mendelian randomization studies can clarify mechanistic pathways19 and likely drug effects, and have foreshadowed results of clinical trials.20 This study assessed genetic validation, or otherwise, of NK3R antagonists as preventing IHD in men. Androgens or their precursors would be expected to affect fertility and have been associated with hypertension12 and prostate cancer12,21 so these were all considered as positive control outcomes. Finally, testosterone is much lower in women than men,22 so associations with IHD in women were considered as a negative control outcome.
Methods
This Mendelian randomization study, using two-sample methods, takes advantage of the largest relevant publicly available sex-specific genetic summary statistics, i.e., from the UK Biobank (http://www.nealelab.is/uk-biobank), for the exposure, i.e., genetic mimics of NK3R antagonists (in standardized units of testosterone), and for the main outcome, i.e., IHD. UK Biobank genetic summary statistics were also used for the positive control outcomes (fertility (relative age voice broke and children fathered) and hypertension), the negative control outcome (IHD in women) and any multivariable Mendelian randomization analysis. The PRACTICAL consortium23 was used for the positive control outcome of prostate cancer. Finally, Biobank Japan (BBJ)24, 25, 26 was used for replication because it has sex-specific genetic associations with IHD. As such, this study largely used a one-sample Mendelian randomization study design, with a two-sample design used for prostate cancer and replication for IHD in BBJ.
The UK Biobank is a large cohort study of half a million people, intended to be aged 40 to 69 years at recruitment in Great Britain from 2006 to 2010, and thereafter followed-up via record linkage to hospital records and death (https://www.ukbiobank.ac.uk).27 Summary sex-specific UK Biobank quality controlled genetic associations for 13.7 million variants, used here, pertain to men (n < = 167020) or women (n < = 194,174) of white British descent adjusted for age, age2 and 20 principal components. PRACTICAL is a consortium of case series, case-control and cohort studies of prostate cancer mainly in men (cases mean age 65.7 years, controls mean age 61.6 years) of European descent23; quality-controlled summary genetic associations, used here, were adjusted for principal components and study relevant covariates stratified by country and study.23 BBJ is a hospital-based registry study of about 200,000 people (mean age 62 years, 53.1% male), followed-up via medical records and national death registration,24,25 and supplemented by 34,793 controls from existing Japanese population based cohort studies.26 Summary sex-specific BBJ quality controlled genetic associations with IHD for 8.9 million variants, pertain to 109,347 men of East Asian descent adjusted for age and the top 5 principal components.
Genetically mimicked NK3R antagonists
Genetic mimics of NK3R antagonists were obtained from UK Biobank summary statistics (http://www.nealelab.is/uk-biobank) for testosterone in 157,738 men, as all genome wide significant (p < 5 × 10−8) independent (r2 < 0.005) variants with minor allele frequency (MAF) >0.01 from the relevant target gene (+-50 kbp), TACR3,28 predicting testosterone with F-statistic>10. To assess possible pleiotropic effects of the selected genetic variants via other biasing pathways, their associations with other phenotypes were checked using two curated genotype to phenotype cross-references (http://www.phenoscanner.medschl.cam.ac.uk and https://gwas.mrcieu.ac.uk/phewas/).
Ischemic heart disease in men and women
Summary genetic associations with IHD from the UK Biobank, defined using a wide definition, international classification of diseases 10 codes I20–5 of events, comprised 15,056 cases and 151,964 non-cases in men, and 5801 cases and 188,373 non-cases in women.
Control outcomes in men
Relative age voice broke, on a comparative scale, i.e., younger than average, about average and older than average, (n = 144,645), number of children fathered (n = 154,888) and hypertension (cases = 49,744, non-cases = 117,244) were self-reported at UK Biobank recruitment. Prostate cancer (cases = 79,148, controls = 61,106) was defined as diagnosis.23
Consideration of potential confounders
Statins were considered as potential confounders because of their well-known effects on IHD, including specifically in men,14 and their potential relevance to NK3R antagonism. The effect of statins in men was mimicked by rs12916 as previously.14
Replication in Biobank Japan
IHD in BBJ was defined as myocardial infarction, stable angina, and unstable angina. In men there were 21,611 cases and 87,736, controls.
Co-localization analysis
Co-localization was used to assess the posterior probability of the TACR3 gene sharing the same one causal variant for testosterone and IHD or for testosterone and each of the four positive control outcomes.29 The default prior probabilities were used, i.e., 10−4 for an association with testosterone only, 10−4 for an association with the outcome only and 10−5 for an association with both.29 A posterior probability of more than 0.8 was taken as evidence of colocalization.29 The prior probabilities for an association with one trait only are well-established, but the choice of prior for an association with both traits needs careful thought,30 and with a prior of 10−6 possibly more suitable for small samples, as here, and a larger prior possibly more suitable for theoretically based associations, as here.30 So sensitivity analysis was conducted using a prior of 10−4 and 10−3 for an association with both traits.
Statistical analysis
F-statistics were approximated by squared estimate for genetic variant on testosterone divided by its variance. Inverse variance weighted Mendelian randomization estimates were obtained from a meta-analysis of genetic variant specific Wald estimates (genetic variant on outcome divided by genetic variant on exposure) using fixed or multiplicative random effects as appropriate. For multivariable Mendelian randomization instruments for exposure and potential confounder were combined, correlated instruments (r2 < 0.005) dropped and the conditional F-statistics calculated.31
Statistical analysis was conducted using R 4.0.2.32 The MR-Base33 R package ld_clump was used to obtain independent variants, the Mendelianrandomization package to obtain estimates, metafor to obtain forest plots, MVMR to obtain the conditional F-statistics and coloc for the co-localization analysis. An established approximation was used to convert genetic associations reported as probabilities of events to logodds.34 This study only uses genetic summary statistics previously created from information and materials previously collected with informed consent. The data extracted to conduct the MR analyses is given in Supplementary Tables 1, 3 and 5.
Role of the funding source
This study had no funding
Results
Genetic instruments mimicking NK3R antagonists
Of the 936 genetic variants from TACR3 +/- 50 kbp with MAF > 0.01 two independent genetic variants (rs116646027 and rs1351623) (TACR3) predicted testosterone at genome wide significance in men, F-statistics 88.2 and 58.2, respectively (Supplementary Table 1). Other associations at genome wide significance for these variants were almost entirely with testosterone related attributes, i.e., body composition, height, pubertal characteristics and bone density (Supplementary Table 2).
Associations of genetically mimicking of NK3R antagonists with IHD and the control outcomes
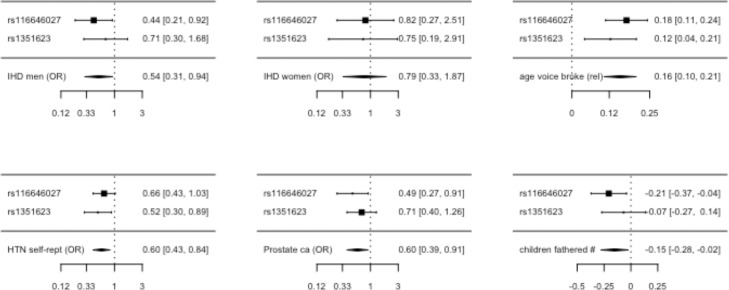
Genetically mimicking NK3R antagonists was associated with lower IHD risk in men (Table 1). Genetically mimicking NK3R antagonists was also associated with all the control outcomes as expected, i.e., had no association with IHD in women, but was associated with older relative age voice broke, fewer children and lower risk of hypertension and prostate cancer in men (Table 1). Associations tended to be driven by rs116646027, which was associated in men with IHD and all the positive control outcomes except hypertension (Figure 1).
Table 1.
Mendelian randomization estimates for associations of genetically mimicking NK3R antagonists (based on rs116646027 and rs1351623, TACR3) with ischemic heart disease in men and the control outcomes (ischemic heart disease in women, relative age voice broke, number of children fathered, self-reported hypertension and prostate cancer in men) per standard deviation lower testosterone in men from the UK Biobank (http://www.nealelab.is/uk-biobank) and the PRACTICAL consortium23 (prostate cancer).
| Outcome | sex | type | estimate | 95% confidence interval | p-value | Q statistic (p-value) |
|---|---|---|---|---|---|---|
| Odds ratio | ||||||
| Ischemic heart disease | men | main | 0.54 | 0.31 to 0.94 | 0.030 | 0.67 (0.41) |
| women | control | 0.79 | 0.33 to 1.87 | 0.591 | 0.01 (0.92) | |
| beta | ||||||
| Relative age voice broke | men | control | 0.16 | 0.10 to 0.21 | 6.2e-9 | 0.89 (0.34) |
| Number of children | men | control | -0.15 | -0.28 to -0.02 | 0.021 | 1.07 (0.30) |
| Odds ratio | ||||||
| Hypertension | men | control | 0.60 | 0.43 to 0.84 | 0.003 | 0.47 (0.49) |
| Prostate cancer | men | control | 0.60 | 0.39 to 0.91 | 0.016 | 0.70 (0.40) |
The data underlying this table is given in Supplementary Table 3.
Figure 1.
Forest plots showing the SNP-specific and overall Mendelian randomization estimates for associations of genetically mimicking NK3R antagonists (based on rs116646027 and rs1351623, TACR3) with ischemic heart disease in men and the negative control outcome (ischemic heart disease in women) and the positive control outcomes in men (relative age voice broke, hypertension, prostate cancer and number of children fathered in men) per standard deviation lower testosterone in men from the UK Biobank and the PRACTICAL consortium23 (prostate cancer).
Multivariable associations of genetically mimicking NK3R antagonists with IHD in men
The association of genetically mimicking NK3R antagonists with IHD was similar after adjusting for genetically mimicked statins (Supplementary Table 5). Genetically mimicked statins were also inversely associated with IHD.
Replication in Biobank Japan
Only one instrument for NK3R antagonists was available in BBJ, because rs116646027 is monomorphic in East Asians. Using rs1351623 and the association with testosterone from UK Biobank, the magnitude of association with IHD was similar to that in UK Biobank (odds ratio (OR) 0.66, 95% confidence interval (CI) 0.36 to 1.23).
Co-localization analysis
Colocalization analysis of 936 genetic variants from TACR3 +/- 50 kbp with MAF>0.01 identified rs116646027 as the variant with the largest posterior probability for both testosterone and IHD. The overall posterior probability was 14.3% using a prior of 10−5 for both traits, but 94.3% using a prior of 10−3. Colocalization for testosterone and the positive control outcomes, similarly identified rs116646027 (or a complete correlate rs17289915) as the variant with the largest posterior probability. The overall posterior probability using a prior of 10−5 for both traits was 98.8% for relative age of voice break, 10.1% for number of children, 9.4% for hypertension and 17.4% for prostate cancer; all were over 90% using a prior of 10−3 (Supplementary Table 6).
Discussion
Consistent with expectations from evolutionary biology and previous evidence concerning the role of testosterone in IHD,9, 10, 11 genetically mimicked NK3R antagonism in men appeared to beneficial for IHD, as well as for hypertension and prostate cancer but at the expense of reducing fertility. Genetically mimicked NK3R antagonism in men was not associated with the control outcome of IHD in women.
Physiologically, NK3R centrally regulates steroid precursors, particularly gonadotropin releasing hormone. Correspondingly rare variants in TACR3 are associated with isolated hypogonadotropic hypogonadism.28 Pharmacological targeting of TACR3 has been suggested as a means of treating sex-steroid related diseases, such as breast and prostate cancer.28 NK3R antagonists are thought likely to be safe in clinical practise.35 Current investigation of clinical applications of NK3R antagonists is focused on treatment of menopausal symptoms and possibly other benefits of suppression of sex-hormone levels in women with less consideration of corresponding benefits for men.36 Experiments in spontaneously hypertensive rats have shown that NK3R antagonism normalizes mean arterial pressure.37,38 Similarly, the means by which testosterone might cause IHD have not been extensively explored but might encompass thrombotic or related processes, such as thromboxane A2,39 nitric oxide,40 the haemostatic system41 or coronary plaque volume.42 Neurokinins affect both the brain and the gut, so effects mediated by the gut brain axis43 are possible but untested.
Despite providing new evidence about a novel way of addressing the specific need for IHD prevention in men with minimal confounding, this study has limitations inherent in the design. Mendelian randomization relies on the instrumental variable analysis assumptions of relevance, independence and exclusion-restriction. To address relevance, the instruments for genetically mimicking NK3R antagonists were only extracted from the target gene (TACR3) based on the strength of their associations with testosterone in men because NK3R antagonists target TACR3 and reduce testosterone.16 Bio-available testosterone may be more physiologically relevant; the same instruments and a similar interpretation were obtained using bioavailable testosterone from the UK Biobank (data not shown). The F-statistics were also high indicating adequate strength of the instruments. Colocalization did not unequivocally indicate that rs116646027 was the causal variant in TACR3 underlying the observed Mendelian randomization associations using the default priors, apart from for relative age voice broke. However, a larger prior probability for both traits might be more suitable for a hypothesis driven study,30 and did suggest colocalization.
To address independence, potential confounders of genetically mimicked NK3R antagonism on IHD were considered, such as statins, but the estimate for IHD was unchanged. In addition, all the genetic associations used were adjusted for principal components and genetic confounding is uncommon.44 To address exclusion-restriction, other consequences of the genetic instruments were identified. However most other associations at genome wide significance were plausible consequences of testosterone (Supplementary Table 2). In addition, genetic studies are open to selection bias, particularly for outcomes which cannot be observed because they, or a fatal competing risk, occurred before recruitment.45 However, the UK Biobank and BBJ participants were not that old, when IHD tends to occur before other cardiovascular conditions, such as stroke,46 making survival to recruitment less of an issue. UK Biobank participants are healthier than the general population,47 so the number of cases may be under-estimated. Such under-representation of cases is likely to be independent of genetic make-up and so does not necessarily cause a bias48 although it may reduce precision. The UK Biobank was used for exposure and most outcomes. Lack of independence between genetic variants on exposure and genetic variants on outcome can bias towards the conventional observational estimates in finite samples. Such, bias is particularly likely when the expected F-statistic is small,49 the sample is relatively small (<100,000),50 or the MR-Egger estimate is used.50 Here, the F-statistics were quite large, the sample size was ∼150,000 and only the inverse variance weighted estimate was used, so substantial bias from conducting the study mainly in one-sample would not be expected. However, the possibility of spurious findings due to type 1 error cannot be excluded, although the consistency across outcomes is somewhat re-assuring. Canalization could also create bias, however expected associations were evident for the control outcomes across the lifespan (Table 1). The samples pertain to people of European descent, however, causes usually act consistently but are not always relevant.51 Replication was limited by the lack of sex-specific genetic summary statistics for IHD in Europeans, and the lack of comprehensive genetic associations with testosterone for other populations. Meta-analys of the available estimate for IHD in East Asians with the association for both SNPs in UK Biobank gave OR 0.59, 95% CI 0.39 to 0.89. Routine provision of sex-specific GWAS would facilitate the development of sex informed disease prevention, so as to address disparities.
Proposed use of NK3R antagonists in women suggests they have an acceptable safety profile.52 Given the extensive availability of NK3R antagonists35 and the lack of new drugs targeting IHD in men further investigation of their use in men to protect against IHD and prostate cancer is warranted. Notably the association of genetically mimicking NK3R antagonists with lower risk of IHD in men appeared to be independent of the effects of genetically mimicked statins, consistent with the possibility that NK3R antagonists could be acting on IHD in men via an underexploited target. Drugs acting via testosterone may raise the risk of diabetes.53 However, statins increasing diabetes risk does not outweigh their benefits for cardiovascular disease prevention.54 Finally, the colocalization analysis suggested, albeit as a post hoc hypothesis with a high posterior probability, that one shared genetic variant could be relevant to NK3R antagonism and pubertal timing in boys. As such, NK3R antagonists could also be relevant to modulating pubertal timing in boys. More generally, this study also raises the question as to whether any related neurokinins might relate in men to the same diseases. Notably drugs targeting NK1R (substance p) antagonism are a commonly used anti-emetic.55
Conclusion
An existing drug class (NK3R antagonists) could be used specifically in men to address IHD risk, possibly with the added benefit of reducing risk of prostate cancer. Further investigation of the role of NK3R antagonists in men is warranted complementary to investigation of their potential use in women to treat reproductive conditions.
Contributors
CMS conducted the study. The data used is publicly available and provided in the Supplementary tables. S Li and G Yang very kindly re-checked the analysis.
Declaration of interests
There are no conflicts of interest.
Acknowledgments
The PRACTICAL consortium, CRUK, BPC3, CAPS, PEGASUS. The Prostate cancer genome-wide association analyses are supported by the Canadian Institutes of Health Research, European Commission's Seventh Framework Programme grant agreement n° 223175 (HEALTH-F2-2009-223175), Cancer Research UK Grants C5047/A7357, C1287/A10118, C1287/A16563, C5047/A3354, C5047/A10692, C16913/A6135, and The National Institute of Health (NIH) Cancer Post-Cancer GWAS initiative grant: No. 1 U19 CA 148537-01 (the GAME-ON initiative).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.103901.
Appendix. Supplementary materials
References
- 1.Bugiardini R., Cenko E. Sex differences in myocardial infarction deaths. Lancet. 2020;396(10244):72–73. doi: 10.1016/S0140-6736(20)31049-7. [DOI] [PubMed] [Google Scholar]
- 2.Jackson N., Atar D., Borentain M., et al. Improving clinical trials for cardiovascular diseases: a position paper from the Cardiovascular Round Table of the European Society of Cardiology. Eur Heart J. 2016;37(9):747–754. doi: 10.1093/eurheartj/ehv213. [DOI] [PubMed] [Google Scholar]
- 3.Wells J.C.K., Nesse R.M., Sear R., Johnstone R.A., Stearns S.C. Evolutionary public health: introducing the concept. Lancet. 2017;390(10093):500–509. doi: 10.1016/S0140-6736(17)30572-X. [DOI] [PubMed] [Google Scholar]
- 4.Kirkham A.A., Beka V., Prado C.M. The effect of caloric restriction on blood pressure and cardiovascular function: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr. 2021;40(3):728–739. doi: 10.1016/j.clnu.2020.06.029. [DOI] [PubMed] [Google Scholar]
- 5.Li S., Vandvik P.O., Lytvyn L., et al. SGLT-2 inhibitors or GLP-1 receptor agonists for adults with type 2 diabetes: a clinical practice guideline. BMJ. 2021;373:n1091. doi: 10.1136/bmj.n1091. [DOI] [PubMed] [Google Scholar]
- 6.Hoong C.W.S., Chua M.W.J. SGLT2 inhibitors as calorie restriction mimetics: insights on longevity pathways and age-related diseases. Endocrinology. 2021;162(8) doi: 10.1210/endocr/bqab079. [DOI] [PubMed] [Google Scholar]
- 7.Cao Q., Bai P., Shi D., et al. CYP17 inhibitors improve the prognosis of metastatic castration-resistant prostate cancer patients: a meta-analysis of published trials. J Cancer Res Ther. 2020;16(5):990–1001. doi: 10.4103/jcrt.JCRT_295_18. [DOI] [PubMed] [Google Scholar]
- 8.Abufaraj M., Iwata T., Kimura S., et al. Differential impact of gonadotropin-releasing hormone antagonist versus agonist on clinical safety and oncologic outcomes on patients with metastatic prostate cancer: a meta-analysis of randomized controlled trials. Eur Urol. 2021;79(1):44–53. doi: 10.1016/j.eururo.2020.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Luo S., Au Yeung S.L., Zhao J.V., Burgess S., Schooling C.M. Association of genetically predicted testosterone with thromboembolism, heart failure, and myocardial infarction: mendelian randomisation study in UK Biobank. BMJ. 2019;364:l476. doi: 10.1136/bmj.l476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pott J., Bae Y.J., Horn K., et al. Genetic association study of eight steroid hormones and implications for sexual dimorphism of coronary artery disease. J Clin Endocrinol Metab. 2019;104(11):5008–5023. doi: 10.1210/jc.2019-00757. [DOI] [PubMed] [Google Scholar]
- 11.Schooling C.M., Luo S., Au Yeung S.L., et al. Genetic predictors of testosterone and their associations with cardiovascular disease and risk factors: a Mendelian randomization investigation. Int J Cardiol. 2018;267:171–176. doi: 10.1016/j.ijcard.2018.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohammadi-Shemirani P., Chong M., Pigeyre M., Morton R.W., Gerstein H.C., Paré G. Effects of lifelong testosterone exposure on health and disease using Mendelian randomization. Elife. 2020;9 doi: 10.7554/eLife.58914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schooling C.M., Au Yeung S.L., Freeman G., Cowling B.J. The effect of statins on testosterone in men and women, a systematic review and meta-analysis of randomized controlled trials. BMC Med. 2013;11:57. doi: 10.1186/1741-7015-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schooling C.M., Zhao J.V., Au Yeung S.L., Leung G.M. Investigating pleiotropic effects of statins on ischemic heart disease in the UK Biobank using Mendelian randomisation. Elife. 2020;9 doi: 10.7554/eLife.58567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schooling C.M., Zhao J.V. Investigating the association of testosterone with survival in men and women using a Mendelian randomization study in the UK Biobank. Sci Rep. 2021;11(1) doi: 10.1038/s41598-021-93360-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skorupskaite K., Anderson R.A. Hypothalamic neurokinin signalling and its application in reproductive medicine. Pharmacol Ther. 2022;230 doi: 10.1016/j.pharmthera.2021.107960. [DOI] [PubMed] [Google Scholar]
- 17.Schooling CM. Tachykinin neurokinin 3 receptor antagonists: a new treatment for cardiovascular disease? Lancet. 2017;390(10095):709–711. doi: 10.1016/S0140-6736(16)31648-8. [DOI] [PubMed] [Google Scholar]
- 18.Smith G.D., Ebrahim S. Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32(1):1–22. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]
- 19.Schooling C.M., Freeman G., Cowling B.J. Mendelian randomization and estimation of treatment efficacy for chronic diseases. Am J Epidemiol. 2013;177(10):1128–1133. doi: 10.1093/aje/kws344. [DOI] [PubMed] [Google Scholar]
- 20.Holmes M.V., Ala-Korpela M., Smith G.D. Mendelian randomization in cardiometabolic disease: challenges in evaluating causality. Nat Rev Cardiol. 2017;14(10):577–590. doi: 10.1038/nrcardio.2017.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marrone M.T., Tsilidis K.K., Ehrhardt S., et al. When is enough, enough? When are more observational epidemiologic studies needed to resolve a research question: illustrations using biomarker-cancer associations. Cancer Epidemiol Biomark Prev. 2019;28(2):239–247. doi: 10.1158/1055-9965.EPI-18-0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clark R.V., Wald J.A., Swerdloff R.S., et al. Large divergence in testosterone concentrations between men and women: frame of reference for elite athletes in sex-specific competition in sports, a narrative review. Clin Endocrinol. 2019;90(1):15–22. doi: 10.1111/cen.13840. (Oxf) [DOI] [PubMed] [Google Scholar]
- 23.Schumacher F.R., Al Olama A.A., Berndt S.I., et al. Association analyses of more than 140,000 men identify 63 new prostate cancer susceptibility loci. Nat Genet. 2018;50(7):928–936. doi: 10.1038/s41588-018-0142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanai M., Akiyama M., Takahashi A., et al. Genetic analysis of quantitative traits in the Japanese population links cell types to complex human diseases. Nat Genet. 2018;50(3):390–400. doi: 10.1038/s41588-018-0047-6. [DOI] [PubMed] [Google Scholar]
- 25.Nagai A., Hirata M., Kamatani Y., et al. Overview of the BioBank Japan Project: study design and profile. J Epidemiol. 2017;27(3s):S2–s8. doi: 10.1016/j.je.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishigaki K., Akiyama M., Kanai M., et al. Large-scale genome-wide association study in a Japanese population identifies novel susceptibility loci across different diseases. Nat Genet. 2020;52(7):669–679. doi: 10.1038/s41588-020-0640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collins R. What makes UK Biobank special? Lancet. 2012;379(9822):1173–1174. doi: 10.1016/S0140-6736(12)60404-8. [DOI] [PubMed] [Google Scholar]
- 28.Topaloglu A.K., Reimann F., Guclu M., et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet. 2009;41(3):354–358. doi: 10.1038/ng.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giambartolomei C., Vukcevic D., Schadt E.E., et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 2014;10(5) doi: 10.1371/journal.pgen.1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallace C. Eliciting priors and relaxing the single causal variant assumption in colocalisation analyses. PLoS Genet. 2020;16(4) doi: 10.1371/journal.pgen.1008720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanderson E., Davey Smith G., Windmeijer F., Bowden J. An examination of multivariable Mendelian randomization in the single-sample and two-sample summary data settings. Int J Epidemiol. 2019;48(3):713–727. doi: 10.1093/ije/dyy262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.R Core Team. A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria 2020.
- 33.Hemani G., Zheng J., Elsworth B., et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7 doi: 10.7554/eLife.34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lloyd-Jones L.R., Robinson M.R., Yang J., Visscher P.M. Transformation of summary statistics from linear mixed model association on all-or-none traits to odds ratio. Genetics. 2018;208(4):1397–1408. doi: 10.1534/genetics.117.300360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muñoz M., Coveñas R. Neurokinin receptor antagonism: a patent review (2014-present) Expert Opin Ther Pat. 2020;30(7):527–539. doi: 10.1080/13543776.2020.1769599. [DOI] [PubMed] [Google Scholar]
- 36.Abbara A., Phylactou M., Dhillo W.S. Commentary on "pharmacodynamic activity of the novel neurokinin-3 receptor antagonist SJX-653 in healthy men. J Clin Endocrinol Metab. 2021;106(2):e1028–e1e30. doi: 10.1210/clinem/dgaa783. [DOI] [PubMed] [Google Scholar]
- 37.De Brito Gariepy H., Couture R. Blockade of tachykinin NK3 receptor reverses hypertension through a dopaminergic mechanism in the ventral tegmental area of spontaneously hypertensive rats. Br J Pharmacol. 2010;161(8):1868–1884. doi: 10.1111/j.1476-5381.2010.01008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lessard A., Laurin M., Yamaguchi N., Couture R. Central anti-hypertensive effect of tachykinin NK3 receptor antagonists in rat. Eur J Pharmacol. 2004;486(1):75–83. doi: 10.1016/j.ejphar.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 39.Ajayi A.A., Halushka P.V. Castration reduces platelet thromboxane A2 receptor density and aggregability. QJM. 2005;98(5):349–356. doi: 10.1093/qjmed/hci054. [DOI] [PubMed] [Google Scholar]
- 40.Rosselli M., Keller P.J., Dubey R.K. Role of nitric oxide in the biology, physiology and pathophysiology of reproduction. Hum Reprod Update. 1998;4(1):3–24. doi: 10.1093/humupd/4.1.3. [DOI] [PubMed] [Google Scholar]
- 41.Ferenchick G.S., Hirokawa S., Mammen E.F., Schwartz K.A. Anabolic-androgenic steroid abuse in weight lifters: evidence for activation of the hemostatic system. Am J Hematol. 1995;49(4):282–288. doi: 10.1002/ajh.2830490405. [DOI] [PubMed] [Google Scholar]
- 42.Budoff M.J., Ellenberg S.S., Lewis C.E., et al. Testosterone treatment and coronary artery plaque volume in older men with low testosterone. JAMA. 2017;317(7):708–716. doi: 10.1001/jama.2016.21043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steinhoff M.S., von Mentzer B., Geppetti P., Pothoulakis C., Bunnett N.W. Tachykinins and their receptors: contributions to physiological control and the mechanisms of disease. Physiol Rev. 2014;94(1):265–301. doi: 10.1152/physrev.00031.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith G.D., Lawlor D.A., Harbord R., Timpson N., Day I., Ebrahim S. Clustered environments and randomized genes: a fundamental distinction between conventional and genetic epidemiology. PLoS Med. 2007;4(12):e352. doi: 10.1371/journal.pmed.0040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schooling C.M., Zhao J.V., Au Yeung S.L., Kwok M.K. Letter in response to 'Bias in two-sample Mendelian randomization when using heritable covariable-adjusted summary associations'-'Interpreting Mendelian randomization studies pre-adjusted for the heritable covariable survival to recruitment. Int J Epidemiol. 2021;50(5):1744–1745. doi: 10.1093/ije/dyab126. [DOI] [PubMed] [Google Scholar]
- 46.Kesteloot H., Decramer M. Age at death from different diseases: the Flemish experience during the period 2000-2004. Acta Clin Belg. 2008;63(4):256–261. doi: 10.1179/acb.2008.047. [DOI] [PubMed] [Google Scholar]
- 47.Fry A., Littlejohns T.J., Sudlow C., et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol. 2017;186(9):1026–1034. doi: 10.1093/aje/kwx246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Westreich D. Berkson's bias, selection bias, and missing data. Epidemiology. 2012;23(1):159–164. doi: 10.1097/EDE.0b013e31823b6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burgess S., Davies N.M., Thompson S.G. Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol. 2016;40(7):597–608. doi: 10.1002/gepi.21998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Minelli C., Del Greco M.F., van der Plaat D.A., Bowden J., Sheehan N.A., Thompson J. The use of two-sample methods for Mendelian randomization analyses on single large datasets. Int J Epidemiol. 2021;50(5):1651–1659. doi: 10.1093/ije/dyab084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lopez P.M., Subramanian S.V., Schooling C.M. Effect measure modification conceptualized using selection diagrams as mediation by mechanisms of varying population-level relevance. J Clin Epidemiol. 2019;113:123–128. doi: 10.1016/j.jclinepi.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 52.Depypere H., Lademacher C., Siddiqui E., Fraser G.L. Fezolinetant in the treatment of vasomotor symptoms associated with menopause. Expert Opin Investig Drugs. 2021;30(7):681–694. doi: 10.1080/13543784.2021.1893305. [DOI] [PubMed] [Google Scholar]
- 53.Li S.Y., Zhao Y.L., Yang Y.F., et al. Metabolic effects of testosterone replacement therapy in patients with type 2 diabetes mellitus or metabolic syndrome: a meta-analysis. Int J Endocrinol. 2020;2020 doi: 10.1155/2020/4732021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ridker P.M., Pradhan A., MacFadyen J.G., Libby P., Glynn R.J. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet. 2012;380(9841):565–571. doi: 10.1016/S0140-6736(12)61190-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jin Z., Daksla N., Gan T.J. Neurokinin-1 antagonists for postoperative nausea and vomiting. Drugs. 2021;81(10):1171–1179. doi: 10.1007/s40265-021-01532-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.