Abstract
With the aim to specifically study the molecular mechanisms behind photoinhibition of photosystem I, stacked spinach (Spinacia oleracea) thylakoids were irradiated at 4°C with far-red light (>715 nm) exciting photosystem I, but not photosystem II. Selective excitation of photosystem I by far-red light for 130 min resulted in a 40% inactivation of photosystem I. It is surprising that this treatment also caused up to 90% damage to photosystem II. This suggests that active oxygen produced at the reducing side of photosystem I is highly damaging to photosystem II. Only a small pool of the D1-protein was degraded. However, most of the D1-protein was modified to a slightly higher molecular mass, indicative of a damage-induced conformational change. The far-red illumination was also performed using destacked and randomized thylakoids in which the distance between the photosystems is shorter. Upon 130 min of illumination, photosystem I showed an approximate 40% inactivation as in stacked thylakoids. In contrast, photosystem II only showed 40% inactivation in destacked and randomized thylakoids, less than one-half of the inactivation observed using stacked thylakoids. In accordance with this, photosystem II, but not photosystem I is more protected from photoinhibition in destacked thylakoids. Addition of active oxygen scavengers during the far-red photosystem I illumination demonstrated superoxide to be a major cause of damage to photosystem I, whereas photosystem II was damaged mainly by superoxide and hydrogen peroxide.
Photosynthesis requires light. Excessive irradiation may, however, lead to over-saturation of the photosynthetic light reactions, which eventually can cause photoinhibitory damage to the photosynthetic apparatus (Powles, 1984; Aro et al., 1993). Under many photoinhibitory conditions, photosystem II is preferentially damaged (Prasil et al., 1992; Aro et al., 1993; Andersson and Barber, 1996). However, illumination of cold-sensitive plants at chilling temperatures leads to a predominant photoinhibition of photosystem I (Havaux and Davaud, 1994; Sonoike and Terashima, 1994; Terashima et al., 1994; Sonoike 1995, 1996b; Sonoike et al., 1995; Tjus et al., 1998a, 1999). Recent findings demonstrated significant inactivation of photosystem I by low light treatment at low temperatures also in cold-tolerant barley (Tjus et al., 1998a, 1998b, 1999; Teicher et al., 2000) and rye (Ivanov et al., 1998). This demonstrates that photoinhibition of photosystem I at chilling temperatures is a general phenomenon in higher plants.
In isolated thylakoid membranes, photosystems I and II are equally vulnerable to photoinhibition at non-chilling temperatures (Satoh, 1970a, 1970b; Inoue et al., 1986; 1989; Sonoike, 1995; Tjus, 1995). This suggests that photosystem I is protected in vivo by systems that are lost or inactivated during thylakoid isolation. The protective systems include CuZn-superoxide dismutase and ascorbate peroxidase, which are located close to photosystem I in the chloroplast (Asada, 1996). These enzymes scavenge superoxide and hydrogen peroxide, which are produced near the reducing side of photosystem I (Asada, 1996). Decreased efficiency in the cold of CuZn-superoxide dismutase and ascorbate peroxidase combined with acceptor side accumulation of reducing power may together explain the photosystem I photoinhibition at chilling temperatures (Havaux and Davaud, 1994; Asada, 1996; Sonoike, 1996b; Tjus et al., 1998a).
Because photosystem I is a potentially dangerous creator of active oxygen, it may exert damage also on its surroundings. Photosystem II photodamage normally occurs through endogenous formation of singlet oxygen (Vass et al., 1992) or through long-lived P680+/TyrZ+ at the donor side (Jägerschöld et al., 1990). Recent observations show that also the action of superoxide contribute to damages leading to the degradation of the photosystem II D1 reaction center protein during donor-side and acceptor-side photoinhibition of photosystem II (Henmi, 1997). The oxygen radicals generated around photosystem I may thus through diffusion be dangerous also for neighboring photosystem II complexes. In higher plants photosystem I is situated in the stroma-exposed lamellae and thereby largely separated from photosystem II, which is preferentially located in the appressed granal region of the thylakoid membranes (Anderson and Andersson, 1982). An important beneficial effect of the lateral heterogeneity of the thylakoid membrane could be to keep photosystem II well apart from the potentially “toxic” photosystem I.
To test these possibilities we have in the present study illuminated isolated spinach (Spinacia oleracea) thylakoids, well-stacked or destacked and randomized, with specific photosystem I light in the presence of externally added photosystem I electron donors. Furthermore, the mode of damage-action of the created dangerous oxygen species was investigated by the addition of several active oxygen scavengers during the light treatments. The results clearly demonstrate for the first time the sensitivity of photosystem II to active oxygen generated by photosystem I and they suggest that hydrogen peroxide and superoxide are the most damaging forms of active oxygen for this kind of spillover damage.
RESULTS
Illumination of Spinach Thylakoids with Photosystem I-Specific Red Light
Earlier studies have shown that active oxygen created around photosystem I during low-intensity white light illumination at chilling temperatures damages photosystem I (Tjus et al., 1998a). We have now investigated the molecular mechanisms leading to the photoinhibition of photosystem I using isolated spinach thylakoid membranes. During thylakoid isolation a large part of the superoxide dismutase was supposedly lost due to its loose association with the thylakoid surface (Asada, 1996). Ascorbate peroxidase is more tightly bound to the membranes, but any remaining ascorbate peroxidase would have been inactivated by the lack of ascorbate during isolation (Asada, 1996). The isolated thylakoids were illuminated with red light specific for photosystem I excitation. Normal well-stacked thylakoids with the photosystems situated well apart except for border regions, and destacked and randomized thylakoids with photosystems I and II intermixed were subjected to the far-red light treatment. At first, the thylakoid membranes were checked for their degree of stacking by subjecting them to French press treatment followed by differential centrifugation. The stacked thylakoid membranes demonstrated a good separation between low-speed (10,000g) pellets of high yield with a low chlorophyll a/b ratio of 2.6, representative of grana stacks with high photosystem II content, and high-speed (40,000 and 100,000g) pellets of low yield with a high chlorophyll a/b ratio of 5.3 and 7.4, respectively, typical of stroma lamellae dominated by photosystem I. The destacked membranes showed an intermediate chlorophyll a/b ratio between 2.7 and 3.3 in all fractions and a higher yield of the high-speed fractions. This evidences random rupture of a homogeneous thylakoid structure without stacking, which results in a similar composition of the differently sized thylakoid fragments derived from the press treatment, in agreement with previous studies (Åkerlund et al., 1976).
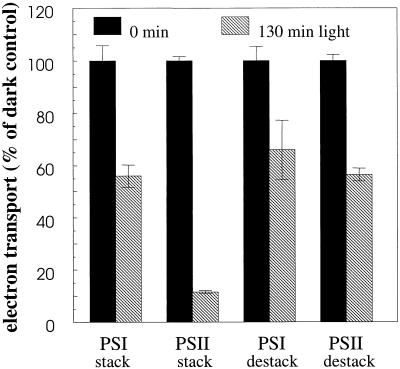
After illumination of stacked thylakoid membranes for 130 min with photosystem I light only, photosystem II was surprisingly inactivated by as much as 88%, whereas photosystem I showed only a 44% decrease as compared with the dark controls (Fig. 1). Illumination of the destacked thylakoids also displayed a clear inactivation of photosystem II, but to a much smaller degree than demonstrated in the stacked membranes, with 44% inactivation after 130 min of light treatment (Fig. 1). Photosystem I was only slightly more stable in the destacked membranes, with 34% inactivation after 130 min illumination (Fig. 1).
Figure 1.
Electron transport of well-stacked and destacked spinach thylakoid membranes illuminated with photosystem I-specific far-red light (>715 nm at 20°C for 130 min) in the presence of externally added photosystem I electron donors. Electron transport rates were assayed using an oxygen electrode. Error bars indicate sd with n = 2 to 3. For stacked thylakoids 100% activity was −111 μmol O2 and 140 μmol O2 (mg chlorophyll) −1 h−1 for photosystem I and photosystem II, respectively, and for destacked thylakoids 100% activity was −103 μmol O2 and 200 μmol O2 (mg chlorophyll) −1 h−1 for photosystem I and photosystem II, respectively.
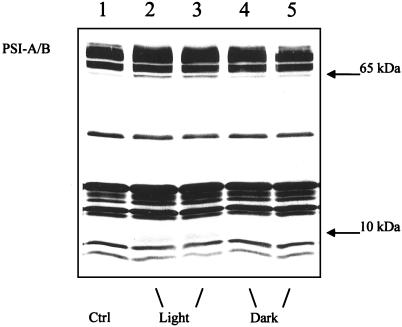
The illuminated samples were further examined by SDS-PAGE and immunoblotting to detect changes in the polypeptide pattern. When immunoblots were prepared using holo-photosystem I antibodies, no actual decrease in the amount of photosystem I polypeptides was observed. Therefore, saturated immunoblots were used with the specific aim to identify if photosystem I was partially damaged as revealed by the appearance of degradation products (Fig. 2). In the stacked thylakoid membranes the illumination induced polypeptides around 65 kD just below the photosystem I-A/B reaction center proteins and newly appearing polypeptides around 10 kD (Fig. 2). Polypeptides around 65 kD were previously shown to result from photoinhibition in vivo of barley and cucumber leaves and to represent breakdown products of PSI-A and PSI-B (Tjus et al., 1999). The dark control samples contained much smaller amounts of the 65-kD bands, presumably caused by active oxygen species generated by other sources than light. Minor degradation in the dark was also seen in previous in vivo studies when active oxygen scavenging was inhibited (Tjus et al., 1998a). With destacked thylakoids, no degradation fragments were identified in light or dark samples (data not shown)
Figure 2.
Immunoblot analysis of stacked spinach thylakoid membranes isolated after illumination with photosystem I-specific far-red light (>715 nm at 20°C for 130 min) in the presence of externally added photosystem I electron donors. The blot was incubated with antibodies directed against the holo-photosystem I complex isolated from barley. Each sample applied contained 1.0 μg of chlorophyll. 1, 0 control. 2 + 3, 130 min of illumination. 4 + 5, 130 min of darkness.
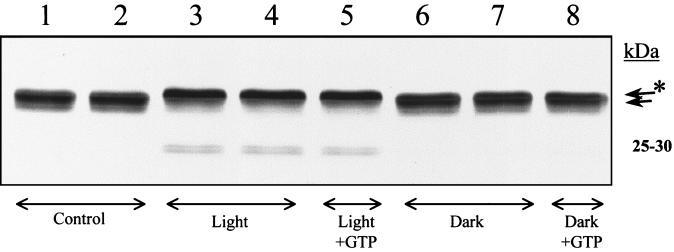
The fate of the photosystem II D1 protein during the far-red illumination was investigated using a specific D1 antibody. Despite the large decrease in photosystem II activity during illumination, no clearly detectable decrease in the amount of the D1 protein could be detected (Fig. 3). However, in the stacked thylakoids the illumination resulted in two new polypeptide components represented as a faint doublet at 25 to 30 kD (Fig. 3, lanes 3 and 4). This indicates the induction of a partial degradation of the D1 protein. More conspicuously, the illumination resulted in the conversion of the D1 polypeptide into a form that migrated slightly slower on the gel (Fig. 3, lanes 3 and 4). In the stacked samples where 88% of photosystem II was inactivated, nearly quantitative conversion of the D1 protein to the “upper” form was observed. In the destacked membranes in which most of photosystem II was still active, a similar change in migration of D1 was hardly detectable (data not shown). Dark incubation of membranes with GTP and Mg2+-ions after the illumination, aiming to facilitate primary proteolysis of damage-tagged D1 (Spetea et al., 1999), did not change the ratio between the differently migrating D1 protein forms (Fig. 3, lanes 5 and 8).
Figure 3.
Immunoblot analysis of stacked spinach thylakoid membranes isolated after illumination with photosystem I-specific far-red light (>715 nm at 20°C for 130 min) in the presence of externally added photosystem I electron donors. The blot was incubated with antibodies directed against the photosystem II D1 reaction center protein. Each sample applied contained 1.0 μg of chlorophyll. 1 + 2, 0 control. 3 + 4, 130 min of illumination. 5, 130 min of illumination followed by a 90-min incubation with 0.35 mm GTP and 5 mm Mg2+-ions. 6 + 7, 130 min of darkness. 8, 130 min of darkness followed by a 90-min incubation with 0.35 mm GTP and 5 mm Mg2+-ions.
Effect of Active Oxygen Scavengers during Red Light Illumination of Thylakoid Membranes
The molecular mechanisms behind the photoinhibitory damage induced by the photosystem I-specific illumination was further investigated by illumination of stacked thylakoid membranes using far-red light (>715 nm) in the presence of scavengers of different active oxygen species and measurement of their ability to prevent photoinhibitory damage.
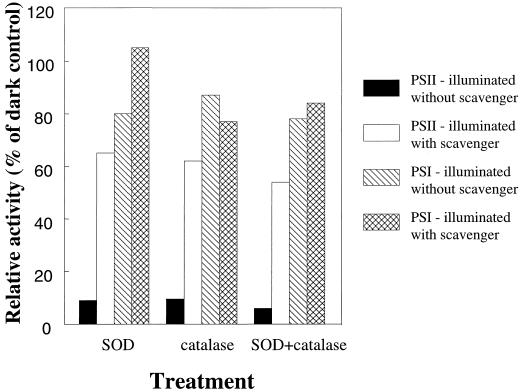
The most efficient scavengers were superoxide dismutase and catalase, which lowered the photo-inactivation of photosystem II from about 90% to about 35% (Fig. 4). Photo-inactivation of photosystem I was decreased from 51% to 24% in the presence of superoxide dismutase, whereas catalase did not protect photosystem I. Propyl gallate, which scavenges hydroxyl radicals, lowered the photoinactivation of photosystem I from 20% to 16%. This small difference may not be significant, but indicates a small protective effect of propyl gallate for photosystem I. Propyl gallate partially inactivated the photosystem II electron transport in dark control samples, lowering the photosystem II activity by approximately 50%. Therefore, the ability of propyl gallate to protect photosystem II is difficult to assess. Propyl gallate did not affect photosystem I activity in the dark controls.
Figure 4.
Electron transport of stacked spinach thylakoid membranes illuminated with photosystem I-specific far-red light (>715 nm at 20°C for 100 min) in the presence of externally added photosystem I electron donors and in the absence or the presence of the active oxygen scavengers superoxide dismutase (SOD), catalase, or SOD and catalase combined. Electron transport rates were assayed using an oxygen electrode. All rates are relative to activities of dark control samples from parallel dark experiments with otherwise identical set-up. Control treatment values are derived from illumination without scavengers present. 100% activity was −356 μmol O2 and 220 μmol O2 (mg chlorophyll) −1 h−1 for photosystem I and photosystem II, respectively.
DISCUSSION
In earlier studies we have shown that photoinhibition of barley photosystem I at chilling temperatures results in a primary damage to the electron transport cofactors and subsequent partial degradation of the reaction center proteins PSI-A and PSI-B (Tjus et al., 1999). Inhibition of superoxide dismutase and ascorbate peroxidase by KCN treatment of the leaves resulted in accelerated photoinhibition of photosystem I (Tjus et al., 1998a, 1998b, 1999), which suggested the involvement of active oxygen in the photosystem I damage. We also showed that illumination of KCN pre-treated barley leaves caused serious photoinhibitory inactivation of photosystem II (Tjus et al., 1998a). This suggested that toxic oxygen species created around photosystem I severely affect photosystem II if not effectively scavenged.
Under the typical experimental conditions used in studies of photoinhibition, both photosystems are excited and it is therefore difficult to distinguish primary damage in photosystem II from a possible indirect damage caused by active oxygen generated by photosystem I. To circumvent this ambiguity we developed a protocol for the specific excitation of photosystem I using far-red light >715 nm and included electron donors for photosystem I. The striking result from the photosystem I-specific excitation was a much higher degree of damage to photosystem II than to photosystem I. It is clear that the active oxygen generated by photosystem I damages photosystem II in the absence of any photochemistry in the photosystem II complex. This result emphasizes the high sensitivity of photosystem II to oxidative damage. In contrast, oxidative damage to photosystem I is only substantial when photosystem I is active in photochemistry, i.e. when the photosystem I acceptors are reduced (Sonoike, 1996b).
Photosystem I was damaged to the same extent irrespective of stacking or destacking of the membranes. Photosystem II was inactivated to the same degree as photosystem I upon illumination of de-stacked thylakoids. In contrast, illumination of stacked thylakoid membranes resulted surprisingly in a much higher degree of damage to photosystem II. Thus, photosystem II was more protected from photoinhibition in the destacked and randomized thylakoids, compared with its normal location “hidden” inside the grana stacks. This suggests that some damaging factor created during photoinhibition is located inside the grana stacks, or that a protection mechanism of the thylakoid membrane is confined to nonappressed regions. In vivo, with intact scavenging systems, the location of the sensitive photosystem II in the grana stacks may in fact be advantageous, since the damaging oxygen species can be partly quenched before they can reach photosystem II.
The large inactivation of photosystem II activity induced by the far-red photosystem I light was not accompanied by a similar degree of D1 degradation and only some minor polypeptide fragmentation occurred. However, part of the D1 protein was converted to a more slowly migrating form during the illumination (Fig. 3). Direct photodamage of the D1 protein is known to result in a phosphorylated form of D1, which is protected from immediate degradation (Rintamäki et al., 1995; Salonen et al., 1998). The phosphorylated form of the D1 protein migrates more slowly than the unmodified form in SDS-PAGE. In the experiments reported here no ATP was added to the thylakoids during the illumination, and we consider it unlikely that an endogenously derived ATP pool in the thylakoids would be sufficient to cause substantial D1 phosphorylation. This interpretation was further substantiated experimentally. Treatment with alkaline phosphatase did not convert the slowly migrating form of D1 into the faster migrating protein form (data not shown). In addition, immunostaining using phospho-Thr antibodies, which are known to recognize the phosphorylated D1 protein (Rintamäki et al., 1997), gave no response (data not shown). Induction of D1-phosphorylation by photosystem I light is also opposed by the findings from studies of Spirodela oligorrhiza plants, which demonstrated that the D1 protein was phosphorylated during white light illumination, but was de-phosphorylated by subsequent far-red illumination (Elich et al., 1993). These results further support the conclusion that the D1 protein was not phosphorylated by the far-red light treatments used in the current study. GTP and Mg2+-ions facilitate primary proteolysis of damage-tagged D1 protein (Spetea et al., 1999).
In the present study, inclusion of these factors did not result in increased D1 degradation (Fig. 3). It is possible that the process of primary proteolysis of the D1 protein could have been more significant with addition also of ATP and zinc-ions to accelerate the secondary proteolysis of primary D1 fragments (Hundal et al., 1998) and to avoid product inhibition of the primary proteolysis. The observed increase in the apparent molecular mass of the D1 protein is more likely explained by a direct oxidative damage to the D1 protein imposed by superoxide and/or hydrogen peroxide formed by photosystem I during the light treatment. This damage may have changed the molecular structure of D1 and thereby its migration in SDS-PAGE. The change in structure could be different from the one occurring during “normal” direct photosystem II photoinactivation where singlet oxygen generated within the reaction center is known to be the dominating active oxygen species (Andersson and Barber, 1996). A different structure of a damaged D1 protein compared with that normally encountered during direct photosystem II damage might be less easily recognized by the proteolytic enzyme. In an alternate manner, the D1 proteolytic system may require activation by light of lower wavelengths.
The photoinhibition induced during photosystem I-specific illumination of thylakoids could be largely prevented by addition of active oxygen scavenging enzymes. With respect to photosystem I, superoxide dismutase protected against photoinhibition, whereas catalase had no protective effect (Fig. 4). This suggests superoxide, but not hydrogen peroxide to be damaging to photosystem I. In opposition to this, Sonoike (1996a) showed upon illumination of spinach chloroplasts no protection of photosystem I with addition of superoxide dismutase, but in agreement with our results, showed indication of protection by the superoxide scavenger butylcatechol. With superoxide dismutase present, the burst of hydrogen peroxide close to target molecules in photosystem I may lead to production of highly reactive hydroxyl radicals (Asada, 1996). In our present experiments direct scavenging of hydroxyl radicals by ascorbate may, however, have taken place, as the illumination was performed in the presence of 7.5 mm ascorbate to keep the photosystem I electron donor dichlorophenol indophenol (DCIP) reduced throughout the illumination. The normal chloroplastic content of ascorbate is 10 to 20 mm (Halliwell and Gutteridge, 1989; Asada, 1996). Our control illuminations also contained ascorbate and resulted in clear photo-inactivation of both photosystems, but with highest effect on photosystem II (Fig. 4). The reaction rate of ascorbate with superoxide is not high (Asada, 1996). However, the limited photo-inactivation of photosystem I in the control illumination could in part be due to direct scavenging by ascorbate of highly reactive hydroxyl radicals or singlet oxygen created from non-scavenged superoxide (Halliwell and Gutteridge, 1989; Asada, 1996). In agreement with this, Sonoike (1996a) found a small protection from photoinhibition of photosystem I by including 10 mm ascorbate during illumination of spinach chloroplasts. In the present study propyl gallate, which is an efficient scavenger of hydroxyl radicals, showed only little protection of photosystem I, whereas Sonoike (1996a) previously showed more pronounced protection of photosystem I by propyl gallate. The smaller protection detected by us may further reflect ascorbate as being a potential quencher of hydroxyl radicals. Preliminary studies using dimethyl sulfoxide as a trap for singlet oxygen (Jakob and Heber, 1996) decreased the photodamage to photosystem II by approximately 20%, but did not protect photosystem I. As singlet oxygen is highly reactive with a diffusion controlled reaction rate (Asada, 1996), this damage possibly originated from in situ created singlet oxygen in photosystem II derived from superoxide and/or hydrogen peroxide migrating from photosystem I (Halliwell and Gutteridge, 1989).
Superoxide dismutase and catalase significantly protected the photosystem II complex (Fig. 4). When not scavenged, the rather long-lived superoxide anions created at photosystem I diffuse toward photosystem II. Part or most of the superoxide is converted into hydrogen peroxide during the diffusion, through auto-disproportonation or residual superoxide dismutase activity before reaching photosystem II. This suggests superoxide and hydrogen peroxide originating from photosystem I to be potentially damaging species to the photosystem II complex. The protective effect from catalase when present alone can be explained as scavenging of hydrogen peroxide created from superoxide through auto-disproportionation. With superoxide dismutase added, the superoxide is rapidly converted into hydrogen peroxide, which would be damaging to photosystem II in the absence of an efficient scavenging system. Therefore, it is somewhat surprising that superoxide dismutase alone actually protected photosystem II. However, as discussed above, the superoxide dismutase catalyzed burst of hydrogen peroxide close to reactive molecules within photosystem I is likely to result in generation of hydroxyl radicals. It is possible that the hydroxyl radicals and remaining hydrogen peroxide generated near photosystem I are largely scavenged by ascorbate before reaching the more distant photosystem II complexes. In the control illumination without added scavengers, the superoxide, which is not very reactive with ascorbate, is more gradually converted into hydrogen peroxide by the relatively slow auto-disproportionation, and therefore, the hydrogen peroxide might reach photosystem II before being scavenged by ascorbate. In this way the hydrogen peroxide concentration near photosystem II may actually be higher than when superoxide dismutase is added and all the hydrogen peroxide is generated more distantly from photosystem II.
Earlier studies have shown a selective photodamage to photosystem I in cold-sensitive plants during weak white-light illumination at chilling temperatures (Havaux and Davaud, 1994; Terashima et al., 1994). Later studies showed these conditions to induce photoinactivation of both photosystems in cold-tolerant plants and furthermore to highly increase the damage to both photosystems upon inhibition of active oxygen enzymes (Tjus et al., 1998). The differences between cold-sensitive and cold-tolerant species with respect to photoinhibition is suggested to originate in differing sensitivities of the active oxygen scavenging enzymes toward cold and in difference in how to cope with distribution of excess excitation light (Havaux and Davaud, 1994; Sonoike 1996b; Tjus et al., 1998a, 1999). Thus, under normal conditions superoxide is created and mainly scavenged around photosystem I, whereas conditions with diminished oxygen scavenging lead to damages. The reactive oxygen radicals are expected to hit first in photosystem I, where created, and then to diffuse toward photosystem II.
As shown in this investigation, photosystem II is much more sensitive to damaging oxygen species than photosystem I. Thus, prevention of damage to photosystem II may be one of the advantages of having lateral heterogeneity with photosystem I and photosystem II largely located in different domains of the thylakoid membrane.
In conclusion, photosystem I generates active oxygen that is damaging not only to itself, but clearly also to photosystem II. Superoxide and hydrogen peroxide, originating from the reducing side of photosystem I when photosystem II activity is silenced, diffuse to photosystem II and cause great damage if not scavenged, even greater than the damage to photosystem I itself.
MATERIALS AND METHODS
Isolation of Thylakoid Membranes
Spinach (Spinacia oleracea) was purchased from a local market and thylakoid membranes were isolated. For isolation of well-stacked thylakoids, leaves were homogenized in a blender equipped with exchangeable razor blades (Kannangara et al., 1977) in 50 mm Bis-Tris (2-[bis(hydroxyethyl)amino]-2-(hydroxymethyl)-1-propane-1,3-diol, pH 6.8), 300 mm Suc, 10 mm MgCl2, and 10 mm NaCl. Chloroplasts were pelleted by centrifugation at 1,000g for 5 min, resuspended in the same buffer, and re-pelleted at 2,000g for 5 min. Osmotic rupture of the chloroplasts was achieved by incubation in 50 mm Bis-Tris (pH 6.8), 50 mm Suc, 10 mm MgCl2, and 10 mm NaCl for 5 min after which the thylakoid membranes were obtained by centrifugation at 2,000g for 5 min. The thylakoid membranes were washed three times in 10 mm Bis-Tris (pH 6.8), 100 mm Suc, 10 mm MgCl2, and 10 mm NaCl, re-pelleted as above, and finally re-suspended and homogenized in the same buffer.
Destacked thylakoid membranes were prepared with the same procedure as above except that MgCl2 was omitted in all steps. The thylakoid membranes carry a net negative charge, but the presence of cations, especially divalent cations, keeps the thylakoid membranes stacked by screening the electrostatic repulsion (Barber, 1980, 1982; Sculley et al., 1980). NaCl was included at 10 mm to avoid osmotic swelling and rupture of the thylakoid membranes, but this concentration is still sufficiently low to allow destacking (Smillie et al., 1976). To facilitate migration and randomization of the photosynthetic complexes in the destacked thylakoid membranes (Sundby et al., 1986), the membranes were further incubated for 40 min at 20°C at approximately 2 mg of chlorophyll per milliliter after the last resuspension in 10 mm Bis-Tris (pH 6.8), 100 mm Suc, and 10 mm NaCl. Well-stacked thylakoids were incubated the same way, but with addition of 10 mm MgCl2 when stacked and destacked membranes were compared in the same experiment.
To verify the thylakoid stacking/destacking, French press treatment combined with differential centrifugation (Sane et al., 1970) was applied to the isolated thylakoids. The randomized thylakoid membranes were diluted to 0.5 mg chlorophyll per milliliter with low salt buffer for the destacked membranes and with buffer complemented with 10 mm MgCl2 for the stacked membranes. The thylakoid membranes were passed through the French press cell three consecutive times at 4,000 psi, applying a drop flow of approximately 20 mL/min. The eluate was diluted 3-fold in low salt buffer and was subjected to differential centrifugation at 10,000g for 30 min, 40,000g for 30 min, and 100,000g for 60 min at 4°C in an ultracentrifuge using a Beckman 70 Ti rotor (Beckman Instruments, Fullerton, CA). Chlorophyll was determined according to Arnon (1949).
Photosystem I-Specific Illumination of Stacked and Destacked Spinach Thylakoid Membranes
After rapid isolation, stacked or destacked spinach thylakoids were diluted to 5-mL aliquots. The destacked samples contained 0.1 mg of chlorophyll per milliliter, 10 mm Bis-Tris (pH 6.8), 100 mm Suc, 10 mm NaCl, 7.5 mm sodium ascorbate, and 150 μm of DCIP. The amount of ascorbate was carefully titrated to allow reduction of DCIP throughout the illumination. The stacked thylakoids were further supplemented with 10 mm MgCl2. Illumination was performed using a KL1500 lamp (Schott, Glostrup, Denmark) with fiber optics directed from above into a beaker containing the sample. The sample was continuously stirred by a magnetic flea and kept at 20°C by a surrounding water bath. The illumination was performed in the absence of background light from other light sources. The white light from the KL 1500 lamp was filtered through KG1, RG650, and RG715 filters (Schott), which absorbed excessive heat and resulted in red light >715 nm of about 30 μmol photons m−2 s−1. This light was exciting the photosystem I reaction center on the far-red absorption tail of the P700 reaction center chlorophyll, but did not excite the P680 reaction center of photosystem II. This was verified by measuring oxygen evolution directly in the thylakoid sample, using illuminated mixtures as above with 1.5 mm PpBQ added as photosystem II electron acceptor. No oxygen evolving activity could be detected with red light above 715 nm, whereas normal photosystem II activity was attained when using white light. Two parallel illumination set-ups were used when comparing stacked and destacked thylakoids. An identical, but light-protected set-up was used for dark control samples, which showed high stability throughout the treatment period. All thylakoids were treated for 130 min after which samples were withdrawn. At the same time, illuminated and dark control thylakoids were incubated with 0.35 mm GTP (Sigma, St. Louis) and 5 mm MgCl2 in the dark for 90 min at 25°C to facilitate primary proteolysis of damage-tagged D1 protein (Spetea et al., 1999). All thylakoid aliquots were immediately frozen in liquid nitrogen and stored at −80°C until further analyzed.
Photosystem I-Specific Illumination of Thylakoids in the Presence of Active Oxygen Scavengers
Illumination with two parallel light set-ups as above was performed on stacked thylakoid membranes with and without addition of active oxygen scavengers. The scavengers used were 1 unit/μL superoxide dismutase (horseradish; Sigma) scavenging superoxide, 4.5 units μL−1 catalase (bovine liver, Sigma) scavenging hydrogen peroxide, and 2 mm propyl gallate scavenging hydroxyl radicals (Sonoike, 1996a).
Electron Transport Measurements
Photosystem I and II activities were measured in an oxygraph as in Tjus et al. (1998a). To optimize membrane stability, the assay conditions were modified as follows: Photosystem I electron transport was measured at saturating white light using a Clark-type oxygen electrode and a 2-mL reaction mixture composed of 50 mm Bis-Tris (pH 6.8), 200 mm Suc, 10 mm MgCl2, 10 mm NaCl, 50 μm methyl viologen, 230 μm DCIP, 1.2 mm sodium ascorbate, 14 mm NH4Cl, 14 μm 3-(3,4-dichlorophenyl)-1,1-dimethylurea, 7 mm sodium-azide, and thylakoids corresponding to 20 μg of chlorophyll. In a similar manner, photosystem II electron transport was assayed in a 2-mL reaction mixture composed of 50 mm Bis-Tris (pH 6.8), 200 mm Suc, 10 mm MgCl2, 10 mm NaCl, 1.5 mm PpBQ, and thylakoids corresponding to 20 μg of chlorophyll.
Electrophoresis and Immunoblot Analyses
SDS-PAGE was carried out in 8% to 25% linear gradient gels prepared according to Fling and Gregerson (1986) using Mini-Protean electrophoresis chambers (Bio-Rad, Hercules, CA). To identify and quantify the photosystem I polypeptides, immunoblotting was carried out in a semi-dry blotting device by transferring proteins to a nitro-cellulose membrane. The membrane was incubated first with polyclonal rabbit antibodies raised against the appropriate polypeptides and subsequently with swine-anti-rabbit IgG conjugated with alkaline phosphatase (Dakopatts, Denmark). The blot was developed by a standard color reaction using tetrazolium blue and bromo-chloro-indolyl-phosphate. The developed immunoblots were scanned with a JX330 scanner (1,200 × 600 dpi, Sharp, Ballerup, Denmark).
ACKNOWLEDGMENT
Dr. Torill Hundal is greatly acknowledged for generously donating D1 antibodies.
Footnotes
This work was supported by the Nordic Joint Committee for Agricultural Research.
LITERATURE CITED
- Åkerlund H-E, Andersson B, Albertsson P-Å. Isolation of Photosystem II-enriched membrane vesicles from spinach chloroplasts by phase partition. Biochim Biophys Acta. 1976;449:525–535. doi: 10.1016/0005-2728(76)90161-4. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Andersson B. The architecture of photosynthetic membranes: lateral and transverse organisation. Trends Biochem Sci. 1982;7:288–292. [Google Scholar]
- Andersson B, Barber J. Mechanisms of photodamage and protein degradation during photoinhibition of Photosystem II. In: Baker NR, editor. Photosynthesis and the Environment. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1996. pp. 101–121. [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplasts: polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1–14. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aro E-M, Virgin I, Andersson B. Photoinhibition of Photosystem II: inactivation, protein damage and turnover. Biochim Biophys Acta. 1993;1143:113–134. doi: 10.1016/0005-2728(93)90134-2. [DOI] [PubMed] [Google Scholar]
- Asada K. Radical production and scavenging in chloroplasts. In: Baker NR, editor. Photosynthesis and the Environment. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1996. pp. 123–150. [Google Scholar]
- Barber J. Membrane surface charges and potentials in relation to photosynthesis. Biochim Biophys Acta. 1980;594:253–308. doi: 10.1016/0304-4173(80)90003-8. [DOI] [PubMed] [Google Scholar]
- Barber J. Influence of surface charges on thylakoid structure and function. Annu Rev Plant Physiol. 1982;23:261–295. [Google Scholar]
- Elich TD, Edelman M, Mattoo AK. Dephosphorylation of photosystem II core proteins is light-regulated in vivo. EMBO J. 1993;12:4857–4862. doi: 10.1002/j.1460-2075.1993.tb06175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling SP, Gregerson DS. Peptide and protein molecular weight determination by electrophoresis using a high-molarity Tris buffer system without urea. Anal Biochem. 1986;155:83–88. doi: 10.1016/0003-2697(86)90228-9. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Ed 2. Oxford: Clarendon Press; 1989. [Google Scholar]
- Havaux M, Davaud A. Photoinhibition of photosynthesis in chilled potato leaves is not correlated with a loss of Photosystem II activity: preferential inactivation of Photosystem I. Photosynth Res. 1994;40:75–92. doi: 10.1007/BF00019047. [DOI] [PubMed] [Google Scholar]
- Henmi T. Oxygen is required for the degradation of the D1 protein in Photosystem II during the donor-side photoinhibition. Plant Physiol Suppl. 1997;114:1036. [Google Scholar]
- Hundal T, Spetea C, Lohmann F, Andersson B. ATP- and zinc-dependent proteolysis of the D1 protein primary fragments: possible involvement of the FtsH protease. In: Garab G, editor. Photosynthesis: Mechanisms and Effects. III. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 2023–2026. [Google Scholar]
- Inoue K, Fujii Y, Yokoyama E, Matsuura K, Hiyama K, Sakurai H. The photoinhibition site of photosystem I in isolated chloroplasts under extremely reducing conditions. Plant Cell Physiol. 1989;30:65–71. [Google Scholar]
- Inoue K, Sakurai H, Hiyama T. Photoinactivation of photosystem I in isolated chloroplasts. Plant Cell Physiol. 1986;27:961–968. [Google Scholar]
- Ivanov AG, Morgan RM, Gray GR, Velitchkova MY, Huner NPA. Temperature/light dependent development of selective resistance to photoinhibition of photosystem I. FEBS Lett. 1998;430:288–292. doi: 10.1016/s0014-5793(98)00681-4. [DOI] [PubMed] [Google Scholar]
- Jägerschöld C, Virgin I, Styring S. Light-dependent degradation of the D1 protein in photosystem II is accelerated after inhibition of the water splitting reaction. Biochemistry. 1990;29:6179–6186. doi: 10.1021/bi00478a010. [DOI] [PubMed] [Google Scholar]
- Jakob B, Heber U. Photoproduction and detoxification of hydroxyl radicals in chloroplasts and leaves and relation to photoinactivation of photosystems I and II. Plant Cell Physiol. 1996;37:629–635. [Google Scholar]
- Kannangara CG, Gough SP, Hansen B, Rasmussen JN, Simpson DJ. A homogenizer with replaceable razor blades for bulk isolation of active barley plastids. Carlsberg Res Commun. 1977;42:431–439. [Google Scholar]
- Powles SB. Photoinhibition of photosynthesis induced by visible light. Annu Rev Plant Physiol. 1984;35:15–44. [Google Scholar]
- Prasil O, Adir N, Ohad I. Dynamics of photosystem II: mechanism of photoinhibition and recovery process. In: Barber J, editor. Topics in Photosynthesis. Vol. 11. Amsterdam, The Netherlands: Elsevier; 1992. pp. 295–348. [Google Scholar]
- Rintamäki E, Kettunen R, Tyystjärvi E, Aro E-M. Light-dependent phosphorylation of D1 reaction center protein of photosystem II: hypothesis for the functional role in vivo. Physiol Plant. 1995;93:191–195. [Google Scholar]
- Rintamäki E, Salonen M, Suoranta U-M, Carlberg I, Andersson B, Aro E-M. Phosphorylation of light-harvesting complex II and photosystem II core proteins shows different irradiance dependent regulation in vivo. J Biol Chem. 1997;272:30476–30482. doi: 10.1074/jbc.272.48.30476. [DOI] [PubMed] [Google Scholar]
- Salonen M, Aro E-M, Rintamäki E. Reversible phosphorylation and turnover of the D1 protein under various redox states of photosystem II induced by low temperature photoinhibition. Photosynth Res. 1998;58:143–151. [Google Scholar]
- Sane PV, Goodchild DJ, Park RB. Characterization of chloroplast photosystems I and 2 separated by a non-detergent method. Biochim Biophys Acta. 1970;216:162–178. doi: 10.1016/0005-2728(70)90168-4. [DOI] [PubMed] [Google Scholar]
- Satoh K. Mechanism of photoinactivation in photosynthetic systems: I. The dark reaction in photoinactivation. Plant Cell Physiol. 1970a;11:15–27. [Google Scholar]
- Satoh K. Mechanism of photoinactivation in photosynthetic systems: II. The occurrence and properties of two different types of photoinactivation. Plant Cell Physiol. 1970b;11:29–38. [Google Scholar]
- Sculley MJ, Duniec JT, Thorne SW, Chow WS, Boardman NK. The stacking of chloroplast thylakoids: quantitative analysis of the balance of forces between thylakoid membranes of chloroplasts and the role of divalent cations. Arch Biochem Biophys. 1980;201:339–346. doi: 10.1016/0003-9861(80)90519-6. [DOI] [PubMed] [Google Scholar]
- Smillie RM, Henningsen KW, Nielsen NC, von Wettstein D. The influence of cations and methylamine on structure and function of thylakoid membranes from barley chloroplasts. Carlsberg Res Commun. 1976;41:27–56. [Google Scholar]
- Sonoike K. Selective photoinhibition of photosystem I in isolated thylakoid membranes from cucumber and spinach. Plant Cell Physiol. 1995;36:825–830. [Google Scholar]
- Sonoike K. Degradation of PsaB gene product, the reaction center subunit of Photosystem I, is caused during photoinhibition of photosystem I: possible involvement of active oxygen species. Plant Sci. 1996a;115:157–164. [Google Scholar]
- Sonoike K. Photoinhibition of photosystem I: its physiological significance in the chilling sensitivity of plants. Plant Cell Physiol. 1996b;37:239–247. [Google Scholar]
- Sonoike K, Terashima I. Mechanism of photosystem I photoinhibition in leaves of Cucumis sativus L. Planta. 1994;194:287–293. [Google Scholar]
- Sonoike K, Terashima I, Iwaki M, Itoh S. Destruction of photosystem I iron-sulphur centers in leaves of Cucumis sativus L. by weak illumination at chilling temperatures. FEBS Lett. 1995;362:235–238. doi: 10.1016/0014-5793(95)00254-7. [DOI] [PubMed] [Google Scholar]
- Spetea C, Hundal T, Lohmann F, Andersson B. GTP bound to chloroplast thylakoid membranes is required for light-induced, multienzyme degradation of the photosystem II D1 protein. Proc Natl Acad Sci USA. 1999;96:6547–6552. doi: 10.1073/pnas.96.11.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundby C, Larsson UK, Andersson B. Ionic effects on the lateral segregation of chlorophyll-proteins during restacking of thylakoid membranes. In: Barber J, Papa S, Papageorgiou G, editors. Ionic Interactions in Energy Transfer Biomembranes. New York: Plenum Publishers; 1986. pp. 237–249. [Google Scholar]
- Teicher HB, Møller BL, Scheller HV. Photoinhibition of photosystem I in field-grown barley (Hordeum vulgare L.): induction, recovery and acclimation. Photosynth Res. 2000;64:53–61. doi: 10.1023/A:1026524302191. [DOI] [PubMed] [Google Scholar]
- Terashima I, Funayama S, Sonoike K. The site of photoinhibition in leaves of Cucumis sativus L. at low temperatures is photosystem I, not photosystem II. Planta. 1994;193:300–306. [Google Scholar]
- Tjus SE. Photosystem I, Organisational and Functional Aspects. PhD thesis. Stockholm: Stockholm University; 1995. [Google Scholar]
- Tjus SE, Andersson B. Loss of the trans-thylakoid proton gradient is an early event during photoinhibitory illumination of chloroplast preparations. Biochim Biophys Acta. 1993;1183:315–322. [Google Scholar]
- Tjus SE, Møller BL, Scheller HV. Photosystem I is an early target of photoinhibition in barley illuminated at chilling temperatures. Plant Physiol. 1998a;116:755–764. doi: 10.1104/pp.116.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjus SE, Møller BL, Scheller HV. Photoinhibition of photosystem I damages both reaction center proteins PSI-A and PSI-B and acceptor-side located small photosystem I polypeptides. Photosynth Res. 1999;60:75–86. [Google Scholar]
- Tjus SE, Teicher HB, Møller BL, Scheller HV. Photoinhibitory damage of barley photosystem I at chilling temperatures induced under controlled illumination is also identified in the field. In: Garab G, editor. Photosynthesis: Mechanisms and Effects. III. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998b. pp. 2221–2224. [Google Scholar]
- Vass I, Styring S, Hundal T, Koivuniemi A, Aro E-M, Andersson B. Reversible and irreversible intermediates during photoinhibition of photosystem II: stable reduced QA species promotes chlorophyll triplet formation. Proc Natl Acad Sci USA. 1992;89:1408–1412. doi: 10.1073/pnas.89.4.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]