Abstract
Neoadjuvant immunotherapy and chemotherapy have improved the major pathological response (MPR) in patients with early-stage operable non-small cell lung cancer (NSCLC). This study aimed to assess whether the presence of targetable driver mutations affects the efficacy of the combination of immunotherapy and chemotherapy. We enrolled patients with early-stage operable NSCLC who received preoperative neoadjuvant therapy between January 1, 2017, and December 30, 2020. Neoadjuvant therapy was delivered with platinum-doublet chemotherapy; moreover, pembrolizumab was added at the attending physician’s discretion based on patient’s request. Pathological responses were assessed; moreover, disease-free survival was estimated. Next-generation sequencing was performed in case sufficient preoperative biopsy specimens were obtained. We included 23 patients; among them, 11 received a combination of neoadjuvant immunotherapy and chemotherapy while 12 received neoadjuvant chemotherapy alone. The MPR and pathological complete response rates were 54.5% and 27.3%, respectively, in patients who received a combination of neoadjuvant immunotherapy and chemotherapy. These rates were significantly higher than those in patients who only received neoadjuvant chemotherapy. Three patients in the combination group experienced disease recurrence during the follow-up period even though two of them showed an MPR. These three patients had targetable driver mutations, including an EGFR exon 20 insertion, EGFR exon 21 L858R substitution, and MET exon 14 skipping. Only one patient who remained disease-free had a targetable driver mutation. Among patients with early-stage operable NSCLC requiring neoadjuvant therapy, comprehensive genomic profiling is crucial before the administration of the combination of neoadjuvant immunotherapy and chemotherapy.
Subject terms: Non-small-cell lung cancer, Cancer immunotherapy
Introduction
Tumor relapse in patients with stage II and III non-small cell lung cancer (NSCLC) remains a great challenge. The 5-year survival rates in patients with N1- and N2-positive NSCLC are 49% and 36%, respectively1. Although neoadjuvant chemotherapy significantly improves the overall survival in patients with operable NSCLC, the absolute benefit was reported only to be 6% in the 5-year overall survival rate2. There is a need for novel innovative drug combination strategies to improve the postoperative overall survival. Immunotherapy has improved the overall survival in patients with advanced NSCLC. Phase 3 studies have demonstrated that pembrolizumab or atezolizumab significantly improve overall survival among patients with high programmed death-ligand 1 (PD-L1) expression3,4. Combining immunotherapy and chemotherapy has further improved the overall survival of patients with stage IV NSCLC5,6. Given the success of immune checkpoint inhibitors in advanced NSCLC, there is growing evidence regarding their use in patients with early-stage operable NSCLC.
Forde et al. reported that neoadjuvant nivolumab allowed a major pathological response (MPR) rate of 45% in patients with stage II to III operable NSCLC7. However, a subsequent larger phase II LCMC3 study reported that neoadjuvant atezolizumab for untreated stage IB–IIIB operable NSCLC without EGFR or ALK mutations allowed an MPR rate of only 20% (30/147; 95% CI: 14–28%) and a pathological complete response (pCR) rate of only 7% (10/147; 95% CI: 3–12%)8. A single arm cohort study also revealed that neoadjuvant pembrolizumab provides MPR of only 27% and pCR of only 13.3%9. To improve the pathological response, there is increasing evidence regarding the surgical outcomes through the combination of neoadjuvant immunotherapy and chemotherapy. A phase 2 single-arm multicenter NADIM study on the treatment efficacy of neoadjuvant nivolumab and chemotherapy reported that 83% and 63% of the patients had MPR and pCR, respectively10. Moreover, a multicenter single-arm phase 2 study on neoadjuvant atezolizumab and chemotherapy found that 57% and 33% of the patients had MPR and pCR, respectively11. The recent phase 3 checkmate 816 study observed MPR and pCR in 46.8% and 30.5%, respectively, in patients who received neoadjuvant nivolumab and chemotherapy followed by surgery12. Current ongoing phase 3 trials also evaluated the role of different immunotherapy and chemotherapy combination in neoadjuvant therapy (ClinicalTrials.gov identifier: NCT03425643, NCT03456063, and NCT03800134). However, the wide variation of MPR rates indicates the need for studies to determine a predictive biomarker.
There remains controversial evidence regarding predictive biomarkers. Studies on the PD-L1 expression level as a predictive biomarker have reported inconsistent results10–12. Additionally, the studies using tumor mutation burden7 or circulating tumor DNA12 as predictive biomarkers were also limited. This study aimed to analyze the treatment outcomes of the combination of neoadjuvant pembrolizumab and chemotherapy, as well as the association of treatment efficacy with predictive biomarkers, including PD-L1 expression level and genomic alterations.
Material and method
Patient population
We retrospectively reviewed patients who diagnosed with stage I to III NSCLC at a tertiary hospital from January 1, 2017, to December 30, 2020. All patients had undergone Chest computed tomography (CT) scans, whole-body bone scans, and brain imaging (CT or magnetic resonance imaging) before treatment. Then, the patients were staged based on the tumor, node, metastasis (TNM) system proposed by the American Joint Committee on Cancer, 7th edition. Baseline characteristics, including age, sex, tumor histology, and TNM stage, were recorded. Transbronchial lymph node aspiration or positron emission tomography was performed to define the lymph node stage. After multidisciplinary team discussion, neoadjuvant chemotherapy was recommended for a proportion of patients, except for patients with unresectable disease, poor performance status, or relatively limited disease which did not require extensive surgical resection. The multidisciplinary team consisted of various specialists, including chest physicians, thoracic surgeons, medical oncologists, radiation oncologists, radiologists, nuclear medicine physicians, and pathologists. The treatment strategy of patients with complicated condition or stage III tumor were discussed at the weekly meeting. Neoadjuvant chemotherapy was administered using a 4-cycle platinum-doublet regimen (cisplatin, carboplatin, and docetaxel). Moreover, two doses of pembrolizumab 100 mg were administered at the attending physician’s discretion based on patient’s request, instead of a pre-selected patient group. We then perform retrospective analysis of treatment efficacy among patients who received neoadjuvant chemotherapy. The informed consent was obtained from all participants. This study was reviewed and approved by the Review Board and Ethics Committee of the National Cheng Kung University Hospital (NCKUH B-ER-109-182). All data were anonymized following the approved guidelines and the Declaration of Helsinki.
Tumor response and survival analysis
The tumor response to neoadjuvant therapy was evaluated using radiologic and pathological criteria. The chest CT scan was arranged after completing neoadjuvant therapy to determine the radiological response and at subsequent 12-week intervals after surgery until disease recurrence. Disease-free survival was calculated from the date of surgery until the date of radiological progression based on the Response Evaluation Criteria in Solid Tumors v1.113 or death, with censoring at the date of the last follow-up if the patient did not show recurrence. The percentage of residual viable tumors, necrosis, and stroma in post-operative specimens was quantified by a pathologist as per the IASLC multidisciplinary recommendations14. We used the average value of the percentage of residual tumor to determine the pathological response as described by Junker et al.15 and IASLC recommendation14. As proposed by Junker et al., the regression status was classified into four categories as follows: score I (no tumor regression), score IIa (tumor regression with ≥ 10% viable tumors), score IIb (tumor regression with < 10% viable tumors), and score III (no viable tumors in both primary tumors and regional lymph nodes). The classification proposed by the IASLC includes two categories, with MPR being defined as < 10% residual tumor and pCR being defined as no viable tumor discovered16.
To determine the predictive factors for pathological response, a lung pathologist assessed the preoperative and postoperative tumor specimens following established recommendations of the World Health Organization17. The PD-L1 expression level, which was presented as a tumor proportion score, was assessed using the Dako 22C3 pharmDx system (Agilent Technologies Inc., Santa Clara, CA, USA) assay18. Additionally, in case sufficient biopsy specimens were obtained, the targetable driver mutation was assessed through next-generation sequencing using the QIAGEN GeneReader NGS system and QIAact Lung All-in-One assay.
Statistical analysis
Categorical variables were compared using the chi-square test or Fisher’s exact test. Continuous variables were compared using Student’s t-test or the Wilcoxon rank-sum test. Statistical analysis was conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). All reported P values were two‐sided.
Results
Patients
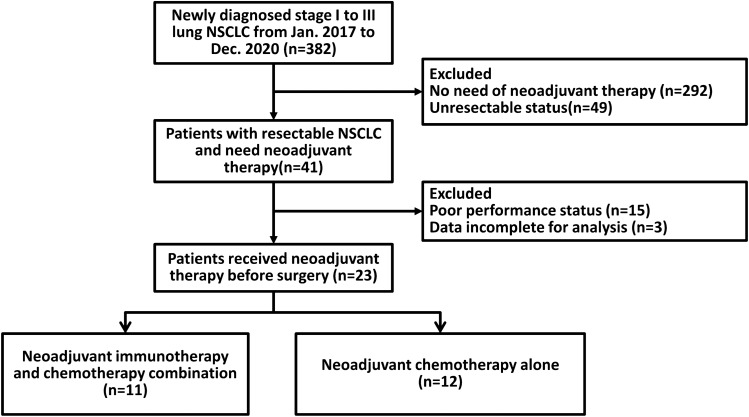
A total of 23 patients were enrolled, including 11 and 12 patients received neoadjuvant pembrolizumab and chemotherapy (combination group) and neoadjuvant chemotherapy only (chemotherapy group), respectively. Figure 1 presents a detailed flowchart for enrolling participants. Table 1 summarizes the baseline characteristics of the patients. The median age was 63.8 years; moreover, 18 of the participants were male. The histological subtypes included adenocarcinoma (n = 11) and squamous cell carcinoma (n = 12). Further, 21 patients had stage III disease; further, two patients had stage II disease but required neoadjuvant therapy. There was no difference in baseline characteristics between the combination and chemotherapy group, including age, sex, histological subtype, tumor size, nodal status, and stage distribution (Table 1).
Figure 1.
Flow chart describing the enrollment of patients.
Table 1.
Baseline characteristics.
| Total population (N = 23) | Combined chemotherapy and immunotherapy (N = 11) | Chemotherapy alone (N = 12) | P value | |
|---|---|---|---|---|
| Age, median (years) | 63.8 | 61.0 | 64.0 | 0.554 |
| ≥ 60 | 16 | 7 | 9 | |
| < 60 | 7 | 4 | 3 | |
| Sex, n (%) | 0.692 | |||
| Male | 18 | 9 | 9 | |
| Female | 5 | 2 | 3 | |
| Histology | 0.827 | |||
| Adenocarcinoma | 11 | 5 | 6 | |
| Squamous cell carcinoma | 12 | 6 | 6 | |
| Tumor size | 0.286 | |||
| ≥ 3 cm | 22 | 10 | 12 | |
| < 3 cm | 1 | 1 | 0 | |
| Nodal involvement, n (%) | 0.538 | |||
| N1 | 5 | 3 | 2 | |
| N2 | 18 | 8 | 10 | |
| Stage, n (%) | 0.799 | |||
| II | 2 | 1 | 1 | |
| IIIA | 10 | 4 | 6 | |
| IIIB | 11 | 6 | 5 | |
Tumor response and survival analysis
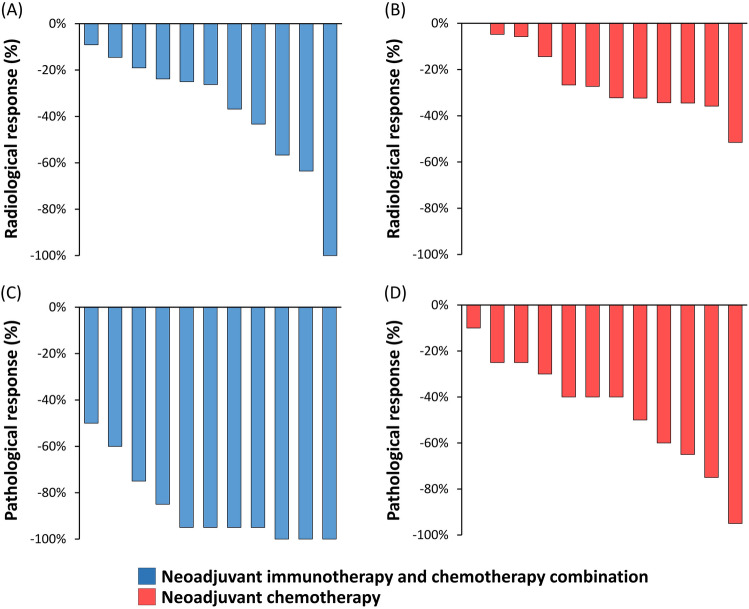
The median follow-up period was 18.3 months. The radiological objective response rate (ORR) in the combination and chemotherapy groups were 45.5% (Fig. 2A) and 58.3%, (Fig. 2B), respectively (P = 0.537). Similarly, the disease control rates were 100% in both groups. For pathological assessment, the combination group had a significantly higher MPR rate (63.6%, Fig. 2C) than the chemotherapy group (8.3%, Fig. 2D) (P = 0.005). Furthermore, the combination group had a significantly higher pCR (27.3%, Fig. 2C) than the chemotherapy group (0%, Fig. 2D) (P = 0.052). Table 2 details the tumor response and pathological assessment of the combination group. All patients with an MPR had pathological nodal clearing (postoperative N0 status, Table 2). There was no significant correlation between the radiological response and pathological response. The immunohistochemical staining of PD-L1 and the H&E stain of pathological response were illustrated in supplementary material Figs. S1 and S2.
Figure 2.
The radiological response in patients who received the combination of neoadjuvant immunotherapy and chemotherapy (A) and in those who received neoadjuvant chemotherapy alone (B). The pathological response in patients who received the combination of neoadjuvant immunotherapy and chemotherapy (C) and in those who received neoadjuvant chemotherapy alone (D).
Table 2.
Pathological response of patients who received neoadjuvant chemotherapy and immunotherapy.
| ID | Histology | PD-L1 (%) | Tumor size (cm) | Nodal status | Pathological response | Junker Regression | Pathological assessment according to IASLC (%) | DFS (months) | Recurrence | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biopsy | Surgery | Biopsy | Surgery | Biopsy | Surgery | Tumor | Necrosis | Stroma | ||||||
| 1 | Squamous | 95 | 90 | 9.6 | 3.5 | 2 | 0 | PR | IIa | 50 | 0 | 50 | 22.30 | No |
| 2 | Adenocarcinoma | 0 | 100 | 6.1 | 4.5 | 1 | 0 | PR | IIa | 40 | 10 | 50 | 9.31 | No |
| 3 | Adenocarcinoma | 90 | 90 | 3.8 | 2.4 | 2 | 2 | PR | IIa | 25 | 0 | 75 | 12.13 | Yes |
| 4 | Squamous | 75 | 95 | 6.3 | 4.8 | 2 | 0 | PR | IIa | 15 | 50 | 35 | 17.48 | No |
| 5 | Squamous | 0 | 100 | 5 | 5.3 | 1 | 0 | MPR | IIb | 5 | 65 | 30 | 18.16 | No |
| 6 | Adenocarcinoma | 90 | 100 | 3.2 | 2.4 | 2 | 0 | MPR | IIb | 5 | 0 | 95 | 15.54 | Yes |
| 7 | Adenocarcinoma | 20 | 70 | 6 | 3.4 | 2 | 0 | MPR | IIb | 5 | 35 | 60 | 9.31 | No |
| 8 | Adenocarcinoma | 90 | 70 | 6.6 | 6 | 2 | 0 | MPR | IIb | 5 | 20 | 75 | 9.25 | Yes |
| 9 | Squamous | 60 | NAa | 3 | 1.3 | 1 | 0 | pCR | III | 0 | 0 | 100 | 1.67 | No |
| 10 | Squamous | NAa | NAa | 4.3 | 0 | 2 | 0 | pCR | III | 0 | 0 | 100 | 30.20 | No |
| 11 | Squamous | 20 | NAa | 2.1 | 1.7 | 2 | 0 | pCR | III | 0 | 20 | 80 | 14.66 | No |
MPR major pathological response, pCR pathological complete response, PR partial response.
aNot available because of insufficient tissue.
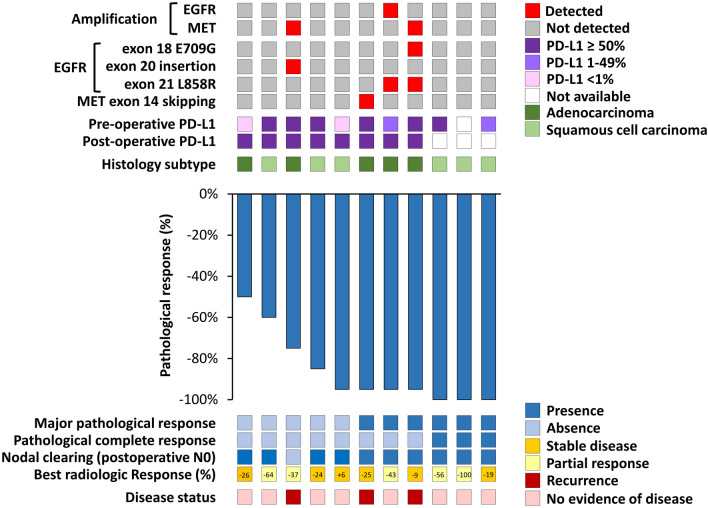
Figure 3 summarizes the relationship between pathological response and individual patient characteristics, including genomic alterations, histological subtypes, PD-L1 expression level, and disease status. Neither the PD-L1 expression level nor the histological subtype could predict the MPR. During the follow-up period, three patients in the combination group experienced disease recurrence. All three patients had high PD-L1 expression in preoperative biopsy specimens; among them, two patients had an MPR in postoperative assessment. After next-generation sequencing, all three patients had targetable driver mutations, including an EGFR exon 20 insertion, complex EGFR mutation (exon 21 L858R substitution and exon 18 E709G substitution), and MET exon 14 skipping (splice donor site mutation). Further, two of these patients harbored MET amplification, with copy number gains of 2.89 and 3.94, respectively. Contrastingly, there was a significantly lower frequency of targetable driver mutations among the remaining eight patients with a disease-free status during the follow-up period, with only one patient having a targetable driver mutation (EGFR exon 21 L858R substitution) and EGFR amplification (copy number gain 10.23) (P = 0.007). The detailed genomic sequencing data of individual patients were listed in supplementary material Fig. S3.
Figure 3.
The correlation between pathological response and individual patient characteristics, including genomic data, histological subtype, PD-L1 expression, and disease status, in patients who received the combination of neoadjuvant immunotherapy and chemotherapy.
Discussion
Our findings demonstrated that neoadjuvant pembrolizumab and chemotherapy provided better MPR and pCR compared with neoadjuvant chemotherapy, which was consistent with previous findings10–12. The pCR rate in the combination group was compatible with findings of the recent phase 3 study Checkmate 816, which demonstrated that the combination of neoadjuvant nivolumab and chemotherapy allowed a pCR rate of 24.0%. Moreover, all our patients with pCR presented with nodal clearing (downstaging from N2 to N0)12. Consistent with the aforementioned studies, we found that the combination of neoadjuvant immunotherapy and chemotherapy could be the mainstay treatment modality in patients with operable early-stage NSCLC requiring neoadjuvant therapy.
Numerous preclinical studies have provided evidence that neoadjuvant immunotherapy can provide better survival for patients with resectable tumors compared with administration of the same therapy in adjuvant settings. Immune checkpoint inhibitors can activate suppressed intratumoral cytotoxic T cells, which mediate antitumor immune responses, as well as promote interactions between cytotoxic T cells and antigen‐presenting cells19. A preclinical study regarding the efficacy of various immunotherapies (anti-CD25, anti-PD-1, or combined anti-PD-1 and anti-CD137) reported that neoadjuvant therapy had improved long-term survival over adjuvant therapy in murine models of triple‐negative breast cancer20. Pathological assessment of the resected tumor revealed an increased density of intratumoral cytotoxic T cells, which may be associated with improved treatment efficacy20. However, a clinical study demonstrated suboptimal MPR and pCR with neoadjuvant immunotherapy8. There is a need for further research regarding new combinations for neoadjuvant therapy.
Preclinical findings have demonstrated that combining chemotherapy and immunotherapy may provide better survival benefits. This combination could inhibit tumor growth and stimulate immune reactions to tumor cells. In addition to increasing the release of tumor neoantigens to augment immunogenicity, chemotherapy inhibits regulatory T cells and enhances the intratumoral activity of cytotoxic T cells21,22. Chemotherapy could block the signaling of signal transducer and activator of transcription 6, enhance the effector immune response by modulating the expression of PD-L1 and mannose-6-phosphate receptor, and stimulate immunogenic cell death through the production of adenosine triphosphate and high-mobility group protein box-123,24. Additionally, the phase 3 Checkmate816 study observed better pCR in patients receiving the combination of neoadjuvant nivolumab and chemotherapy12. Taken together, the combination of neoadjuvant immunotherapy and chemotherapy should be the mainstay strategy for patients with early-stage NSCLC.
However, biomarkers for predicting patients who could benefit from combination therapy remain unclear. A previous study on the efficacy of immunotherapy and chemotherapy in advanced NSCLC reported similar hazard ratios of overall survival were similar among patients with different PD-L1 expression levels5,6. However, patients with PD-L1 expression levels > 50% had the longest overall survival when they received the combination of immunotherapy and chemotherapy5,6. The phase 2 NADIM study, which used nivolumab and chemotherapy as neoadjuvant therapy, reported that PD-L1 expression levels > 25% could predict MPR with 65% sensitivity and 100% specificity10. However, in the Checkmate 816 study, both patients with positive and negative PD-L1 expression benefited from neoadjuvant nivolumab combined with chemotherapy12. Further, the phase 2 study using neoadjuvant atezolizumab and chemotherapy reported that the MPR was not dependent on the PD-L1 expression level11. In our study, the PD-L1 expression level varied widely across both patients with and without MPR. Future prospective studies should assess the association of the PD-L1 expression level with pathological response.
Another unfavorable prognostic factor of immunotherapies in patients with advanced NSCLC is the presence of EGFR or ALK mutations. A meta-analysis demonstrated that using immune checkpoint inhibitors did not benefit the overall survival of patients with EGFR mutations25. However, it remains unclear whether targetable driver mutations decrease the efficacy of the combination of neoadjuvant immunotherapy and chemotherapy. Previous studies on the combination of neoadjuvant immunotherapy and chemotherapy have employed different patient enrollment strategies. The NADIM study10 excluded patients with EGFR or ALK mutations; contrastingly, the phase 2 study11 using atezolizumab enrolled all patients with early-stage lung cancer. Although the MPR rate was similar across these studies, they did not perform disease-free survival analysis in the subgroup of patients with targetable driver mutations9,11. In our study, three of the four patients with targetable driver mutations had MPR after receiving the combination of neoadjuvant immunotherapy and chemotherapy. However, all three patients who experienced disease recurrence had targetable driver mutations, including an EGFR exon 20 insertion, EGFR exon 21 L858R substitution, and MET exon 14 skipping. To our knowledge, this is the first study to demonstrate that an EGFR exon 20 insertion or MET exon 14 skipping can shorten the disease-free survival after administration of the combination of neoadjuvant immunotherapy and chemotherapy. Future studies should investigate better neoadjuvant therapy for patients with targetable driver mutations.
There is growing evidence regarding the use of neoadjuvant EGFR-tyrosine kinase inhibitors (TKIs) in patients with early-stage operable EGFR-mutant NSCLC26. Earlier phase 2 single-arm studies using EGFR-TKI as neoadjuvant therapy revealed ideal ORR, MPR, and disease-free survival27,28. Another phase 2 study reported that patients receiving neoadjuvant erlotinib had a higher MPR rate and significantly longer disease-free survival than those receiving neoadjuvant chemotherapy29, which suggests that neoadjuvant targeted therapy may be a better strategy. Given the improved survival allowed by combined chemotherapy and gefitinib in patients with advanced-stage EGFR-mutant NSCLC30,31, the ongoing phase 3 NEOADAURA study is evaluating the treatment efficacy of the combination of neoadjuvant osimertinib and chemotherapy32. Additionally, the LCMC4 trial (ClinicalTrials.gov identifier: NCT04712877) will further validate the role of comprehensive genomic profiling and neoadjuvant targeted therapy in patients with early-stage operable NSCLC. Neoadjuvant targeted therapy might be an alternative for patients with targetable driver mutations.
There are several possible explanations regarding the decreased efficacy of immune checkpoint inhibitors in patients with targetable driver mutations. Previous studies reported that patients with EGFR, ALK, ROS1, and RET mutations carry relatively low tumor mutation burden (TMB) (3–6 mutations/Mb, 2–4 mutations/Mb, 4 mutations/Mb, and 4.8 mutations/Mb, respectively)33,34. Another study reported that > 50% and ≈ 10% of patients with EGFR mutations had a PD-L1 expression level < 1% and > 50%, respectively35. The low TMB and PD-L1 expression levels might be associated with a low response rate and short progression-free survival with the use of PD-1 inhibitors36. Additionally, high PD-L1 expression levels may be secondary to the signaling activity of the EGFR pathway, including the IL-6/JAK/STAT3 and p-ERK1/2/p-c-Jun pathways, rather than being the interacting molecule with intratumoral immune cells37. Moreover, the presence of targetable driver mutations is associated with an immunosuppressive tumor environment. For example, an EGFR mutation is associated with a decreased density of intratumoral cytotoxic T cells38; moreover, it results in immune escape by overactivation of regulatory T cells via the EGFR/GSK-3β/Foxp3 pathway39. Additionally, the EGFR-mutant cancer cells secrete colony stimulating factor 1, which converts tumor-associated macrophages into the M2 phenotype40. The low TMB/PD-L1 levels and immunosuppressive tumor microenvironment result in poor response to immune checkpoint inhibitors. Taken together, the presence of targetable driver mutations might be associated with early recurrence in patients receiving the combination of neoadjuvant immunotherapy and chemotherapy.
This study has some limitations. First, this was a single-center small-sample sized study. However, the baseline characteristics were similar between the combination and chemotherapy groups. In addition, because patients with poor performance status were not recommended to received neoadjuvant therapy by multidisciplinary team (Fig. 1), all patients in both groups had good performance status. Moreover, the MPR and pCR rates were similar to those reported in the phase 3 Checkmate 816 study for patients who received the combination of neoadjuvant nivolumab and chemotherapy. Our participants may represent the general patient population. Second, although patients with MPR showed varying PD-L1 expression levels, which implies that PD-L1 could not predict the MPR rate, the sample size was limited. Nonetheless, previous findings regarding the role of PD-L1 in predicting the MPR rate remain inconsistent9–12. Moreover, the MPR rate may not be associated with disease-free survival in patients with targetable driver genes. There is a need for future prospective studies to validate the predictive biomarkers for the combination of neoadjuvant immunotherapy and chemotherapy. Third, the genomic tests were retrospectively assessed and only four patients in present study had targetable driver mutations, which precludes definitive conclusions regarding the role of targetable driver mutations in patients who received the combination of neoadjuvant immunotherapy and chemotherapy. Despite the limitation in patient number, our study highlighted the potential deteriorated effect of the targetable driver mutations in combined neoadjuvant immunotherapy and chemotherapy. Moreover, data regarding the role of targetable driver genes in disease-free survival remain unclear. Furthermore, one patient who suffered from disease progression after the combination of neoadjuvant immunotherapy and chemotherapy followed by surgery had MET exon 14 skipping. This genetic alteration has not been reported as a poor prognostic factor for the combination of immunotherapy and chemotherapy before. Forth, one of four patients with targetable driver mutations remained in disease-free status, which implied that the immunotherapy and chemotherapy combination might provide benefit in certain group of patients with targetable driver mutations. In a phase 2 study regarding the efficacy of neoadjuvant atezolizumab and chemotherapy, two of four patients with EGFR mutation had pCR11. However, in the study, disease-free survival in that patient subgroup was not analyzed. Given the nature of decreased immune cell infiltration in NSCLC with targetable driver mutation, whether the combination of immunotherapy could provide survival benefit warrant more clinical studies41. Moreover, the ongoing LCMC4 study (ClinicalTrials.gov identifier: NCT03515837) and phase 3 NEOADAURA will provide the data regarding neoadjuvant targeted therapy, which could provide more information about the choice of neoadjuvant therapy in patients with early stage NSCLC and targetable driver mutations.
In summary, among patients with operable stage II to IIIA NSCLC preoperatively receiving the combination of neoadjuvant immunotherapy and chemotherapy, the presence of targetable driver mutations may be associated with early recurrence. Therefore, comprehensive genomic profiling might have important role in patients with early-stage NSCLC. Future prospective studies are warranted to validate our findings.
Supplementary Information
Acknowledgements
This study was based in part on data sourced from the Cancer Data Bank of the National Cheng Kung University Hospital. We also thank the Molecular Medicine Core Laboratory, Clinical Medicine Research Center, National Cheng Kung University Hospital for technical support, experimental design, and data analysis with the GeneReader NGS system.
Author contributions
P.-L.S., J.-Y.C., C.-Y.C., and C.-C.L. contributed to the design, data analysis; P.-L.S. and C.-C.L.contributed to the writing of the manuscript; Y.-L.C., W.-L.C., K.-Y.L., and C.-L.H. contributed experiment support; J.-S.T., S.-C.Y., C.-W.C., Y.-L.W., Y.-L.T., C.-C.C., Y.-T.Y., C.-Y.L., and W.-C.S. contributed to the data analysis; all authors reviewed and contributed to the preparation of the final manuscript.
Funding
The present study was funded by the Center of Applied Nanomedicine at National Cheng Kung University and the Featured Areas Research Center Program within the framework of the Higher Education Sprout Project of the Ministry of Education, Taiwan (grant no. MOST 109-2314-B-006-083 and MOST 108-2314-B-006-092-MY2 from the Ministry of Science and Technology, Taiwan, and grant no. NCKUH-11002003 from National Cheng Kung University Hospital, Tainan, Taiwan.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Po-Lan Su and Jung-Yueh Chen.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-07423-w.
References
- 1.Asamura H, et al. The International Association for the Study of Lung Cancer lung cancer staging project: Proposals for the revision of the n descriptors in the forthcoming of the TNM classification for lung cancer. J. Thorac. Oncol. 2015;10:1675–1684. doi: 10.1097/jto.0000000000000678. [DOI] [PubMed] [Google Scholar]
- 2.Burdett S, Stewart LA, Rydzewska L. A systematic review and meta-analysis of the literature: Chemotherapy and surgery versus surgery alone in non-small cell lung cancer. J. Thorac. Oncol. 2006;1:611–621. [PubMed] [Google Scholar]
- 3.Reck M. Updated analysis of KEYNOTE-024: Pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J. Clin. Oncol. 2019;37:537–546. doi: 10.1200/jco.18.00149. [DOI] [PubMed] [Google Scholar]
- 4.Herbst RS, Giaccone G, De Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, Morise M, Felip E, Andric Z, Geater S, Ozguroglu M, Zou W, Sandler A, Enquist I, Komatsubara K, Deng Y, Kuriki H, Wen X, McCleland M, Mocci S, Jassem J, Spigel DR. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N. Engl. J. Med. 2020;383:1328–1339. doi: 10.1056/NEJMoa1917346. [DOI] [PubMed] [Google Scholar]
- 5.Rodríguez-Abreu D, Powell SF, Hochmair MJ, Gadgeel S, Esteban E, Felip E, Speranza G, De Angelis F, Domine M, Cheng SY, Bischoff HG, Peled N, Reck M, Hui R, Garon EB, Boyer M, Kurata T, Yang J, Pietanza MC, Souza F, Garassino MC. Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: Protocol-specified final analysis from KEYNOTE-189. Ann. Oncol. 2021 doi: 10.1016/j.annonc.2021.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Paz-Ares L, Vicente D, Tafreshi A, Robinson A, Parra HS, Mazieres J, Hermes B, Cicin I, Medgyasszay B, Rodriguez-Cid J, Okamoto I, Lee S, Ramlau R, Vladimirov V, Cheng Y, Deng X, Zhang Y, Bas T, Piperdi B, Halmos B. A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol-specified final analysis of KEYNOTE-407. J. Thorac. Oncol. 2020;15:1657–1669. doi: 10.1016/j.jtho.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, Zahurak M, Yang S, Jones DR, Broderick S, Battafarano RJ, Velez MJ, Rekhtman N, Olah Z, Naidoo J, Marrone K, Verde F, Guo H, Zhang J, Caushi JX, Chan HY, Sidhom JW, Scharpf RB, White JR, Gabrielson E, Wang H, Rosner G, Rusch V, Wolchok JD, Merghoub T, Taube JM, Velculescu V, Topalian SL, Brahmer JR, Pardoll DM. Neoadjuvant PD-1 blockade in resectable lung cancer. N. Engl. J. Med. 2018;378:1976–1986. doi: 10.1056/NEJMoa1716078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee J, Chaft J, Nicholas A, Patterson A, Waqar S, Toloza E, Haura E, Raz D, Reckamp K, Merritt R, Owen D, Finley D, Mcnamee C, Blasberg J, Garon E, Mitchell J, Doebele R, Baciewicz F, Nagasaka M, Pass H, Schulze K, Phan S, Johnson A, Bunn P, Johnson B, Kris M. PS01.05 surgical and clinical outcomes with neoadjuvant atezolizumab in resectable stage IB–IIIB NSCLC: LCMC3 trial primary analysis. J. Thorac. Oncol. 2021;16:S59–S61. doi: 10.1016/j.jtho.2021.01.320. [DOI] [Google Scholar]
- 9.Eichhorn F, Klotz LV, Kriegsmann M, Bischoff H, Schneider MA, Muley T, Kriegsmann K, Haberkorn U, Heussel CP, Savai R. Neoadjuvant anti-programmed death-1 immunotherapy by pembrolizumab in resectable non-small cell lung cancer: First clinical experience. Lung Cancer (Amsterdam, Netherlands). 2021;153:150–157. doi: 10.1016/j.lungcan.2021.01.018. [DOI] [PubMed] [Google Scholar]
- 10.Provencio M, Nadal E, Insa A, García-Campelo MR, Casal-Rubio J, Dómine M, Majem M, Rodríguez-Abreu D, Martínez-Martí A, De Castro Carpeño J, Cobo M, Vivanco GL, Del Barco E, Caro RB, Viñolas N, ArandaI B, Viteri S, Pereira E, Royuela A, Casarrubios M, Antón CS, Parra ER, Wistuba I, Calvo V, Laza-Briviesca R, Romero A, Massuti B, Cruz-Bermúdez A. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): An open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21:1413–1422. doi: 10.1016/s1470-2045(20)30453-8. [DOI] [PubMed] [Google Scholar]
- 11.Shu CA, Gainor JF, Awad MM, Chiuzan C. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: An open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21:786–795. doi: 10.1016/s1470-2045(20)30140-6. [DOI] [PubMed] [Google Scholar]
- 12.Forde, P.M. et al. Nivolumab + platinum-doublet chemotherapy vs chemotherapy as neoadjuvant treatment for resectable (IB-IIIA) non-small cell lung cancer in the phase 3 CheckMate 816 trial. in AACR Annual Meeting2021: Abstract CT003 (2021).
- 13.Tirkes T, Hollar MA, Tann M, Kohli MD, Akisik F, Sandrasegaran K. Response criteria in oncologic imaging: Review of traditional and new criteria. Radiographics. 2013;33:1323–1341. doi: 10.1148/rg.335125214. [DOI] [PubMed] [Google Scholar]
- 14.Travis WD, Dacic S, Wistuba I, Sholl L, Adusumilli P, Bubendorf L, Bunn P, Cascone T, Chaft J, Chen G, Chou TY, Cooper W, Erasmus JJ, Ferreira CG, Goo JM, Heymach J, Hirsch FR, Horinouchi H, Kerr K, Kris M, Jain D, Kim YT, Lopez-Rios F, Lu S, Mitsudomi T, Moreira A, Motoi N, Nicholson AG, Oliveira R, Papotti M, Pastorino U, Paz-Ares L, Pelosi G, Poleri C, Provencio M, Roden AC, Scagliotti G, Swisher SG, Thunnissen E, Tsao MS, Vansteenkiste J, Weder W, Yatabe Y. IASLC multidisciplinary recommendations for pathologic assessment of lung cancer resection specimens after neoadjuvant therapy. J. Thorac. Oncol. 2020;15:709–740. doi: 10.1016/j.jtho.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Junker K, Langner K, Klinke F, Bosse U, Thomas M. Grading of tumor regression in non-small cell lung cancer : Morphology and prognosis. Chest. 2001;120:1584–1591. doi: 10.1378/chest.120.5.1584. [DOI] [PubMed] [Google Scholar]
- 16.Hellmann MD, Chaft JE, William WN, Jr, Rusch V, Pisters KMW, Kalhor N, Pataer A, Travis WD, Swisher SG, Kris MG. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: Proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol. 2014;15:42–50. doi: 10.1016/s1470-2045(13)70334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E, Flieder DB, Geisinger K, Hirsch FR, Ishikawa Y, Kerr KM, Noguchi M, Pelosi G, Powell CA, Tsao MS, Wistuba I. WHO Panel. The 2015 World Health Organization classification of lung tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J. Thorac. Oncol. 2015;10:1243–1260. doi: 10.1097/jto.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 18.Roach C, Zhang N, Corigliano E, Jansson M, Toland G, Ponto G, Dolled-Filhart M, Emancipator K, Stanforth D, Kulangara K. Development of a companion diagnostic PD-L1 immunohistochemistry assay for pembrolizumab therapy in non-small-cell lung cancer. Appl. Immunohistochem. Mol. Morphol. 2016;24:392–397. doi: 10.1097/pai.0000000000000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li B, Chan HL, Chen P. Immune checkpoint inhibitors: Basics and challenges. Curr. Med. Chem. 2019;26:3009–3025. doi: 10.2174/0929867324666170804143706. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Blake SJ, Yong MC, Harjunpää H, Ngiow SF, Takeda K, Young A, O'Donnell JS, Allen S, Smyth MJ, Teng MW. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov. 2016;6:1382–1399. doi: 10.1158/2159-8290.cd-16-0577. [DOI] [PubMed] [Google Scholar]
- 21.Heinhuis KM, Ros W, Kok M, Steeghs N, Beijnen JH, Schellens JHM. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann. Oncol. 2019;30:219–235. doi: 10.1093/annonc/mdy551. [DOI] [PubMed] [Google Scholar]
- 22.Leonetti A, Wever B, Mazzaschi G, Assaraf YG, Rolfo C, Quaini F, Tiseo M, Giovannetti E. Molecular basis and rationale for combining immune checkpoint inhibitors with chemotherapy in non-small cell lung cancer. Drug Resist. Update. 2019;46:100644. doi: 10.1016/j.drup.2019.100644. [DOI] [PubMed] [Google Scholar]
- 23.Hato SV, Khong A, de Vries IJM, Lesterhuis W. Molecular pathways: The immunogenic effects of platinum-based chemotherapeutics. Clin. Cancer Res. 2014;20:2831–2837. doi: 10.1158/1078-0432.ccr-13-3141. [DOI] [PubMed] [Google Scholar]
- 24.Wanderley CW, Colón DF, Luiz JPM, Oliveira FF, Viacava PR, Leite CA, Pereira JA, Silva CM, Silva CR, Silva RL, Speck-Hernandez CA, Mota JM, Alves-Filho JC, Lima-Junior RC, Cunha TM, Cunha FN. Paclitaxel reduces tumor growth by reprogramming tumor-associated macrophages to an M1 profile in a TLR4-dependent manner. Cancer Res. 2018;78:5891–5900. doi: 10.1158/0008-5472.CAN-17-3480. [DOI] [PubMed] [Google Scholar]
- 25.Lee CK, Man J, Lord S, Links M, Gebski V, Mok T, Yang JCH. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer-A meta-analysis. J. Thorac. Oncol. 2017;12:403–407. doi: 10.1016/j.jtho.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Sun L, Guo YJ, Song J, Wang YR, Zhang SL, Huang LT, Zhao JZ, Jing W, Han CB, Ma JT. Neoadjuvant EGFR-TKI therapy for EGFR-mutant NSCLC: A systematic review and pooled analysis of five prospective clinical trials. Front Oncol. 2020;10:586596. doi: 10.3389/fonc.2020.586596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiong L, Li R, Sun J, Lou Y, Zhang W, Bai H, Wang H, Shen J, Jing B, Shi C, Zhong H, Gu A, Jiang L, Shi J, Fang W, Zhao H, Zhang J, Wang J, Ye J, Han B. Erlotinib as neoadjuvant therapy in stage IIIA (N2) EGFR mutation-positive non-small cell lung cancer: A prospective, single-arm, phase II study. Oncologist. 2019;24:157–164. doi: 10.1634/theoncologist.2018-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Fu F, Hu H, Wang S, Li Y, Hu H, Chen H. Gefitinib as neoadjuvant therapy for resectable stage II-IIIA non-small cell lung cancer: A phase II study. J. Thorac. Cardiovasc. Surg. 2021;161:434–442.e2. doi: 10.1016/j.jtcvs.2020.02.131. [DOI] [PubMed] [Google Scholar]
- 29.Zhong WZ, Chen KN, Chen C, Gu CD, Wang J, Yang XN. Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of stage IIIA-N2 EGFR-mutant non-small-cell lung cancer (EMERGING-CTONG 1103): A randomized phase II study. J. Clin. Oncol. 2019;37:2235–2245. doi: 10.1200/jco.19.00075. [DOI] [PubMed] [Google Scholar]
- 30.Hosomi Y, Morita S, Sugawara S, Kato T, Fukuhara T, Gemma A, Takahashi K, Fujita Y, Harada T, Minato K, Takamura K, Hagiwara K, Kobayashi K, Nukiwa T, Inoue A. Gefitinib alone versus gefitinib plus chemotherapy for non-small-cell lung cancer with mutated epidermal growth factor receptor: NEJ009 study. J Clin Oncol. 2020;38:115–123. doi: 10.1200/jco.19.01488. [DOI] [PubMed] [Google Scholar]
- 31.Noronha V, Patil VM, Joshi A, Menon N, Chougule A, Mahajan A, Janu A, Purandare N, Kumar R, More S, Goud S, Kadam N, Daware N, Bhattacharjee A, Shah S, Yadav A, Trivedi V, Behel V, Dutt A, Banavali SD, Prabhash K. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J. Clin. Oncol. 2020;38:124–136. doi: 10.1200/jco.19.01154. [DOI] [PubMed] [Google Scholar]
- 32.Tsuboi, M. et al. P03.02 neoadjuvant osimertinib with/without chemotherapy vs chemotherapy for EGFR mutated resectable NSCLC: NeoADAURA. in WCLC Annual Meeting. (2020).
- 33.Offin M, Rizvi H, Tenet M, Ni A, Sanchez-Vega F, Li BT, Drilon A, Kris MG, Rudin CM, Schultz N, Arcila ME, Ladanyi M, Riely GJ, Yu H, Hellmann MD. Tumor mutation burden and efficacy of EGFR-tyrosine kinase inhibitors in patients with EGFR-mutant lung cancers. Clin. Cancer Res. 2019;25:1063–1069. doi: 10.1158/1078-0432.ccr-18-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singal G, Miller PG, Agarwala V, Li G, Kaushik G, Backenroth D, Gossai A, Frampton GM, Torres AZ, Lehnert EM, Bourque D, O'Connell C, Bowser B, Caron T, Baydur E, Seidl-Rathkopf K, Ivanov I, Alpha-Cobb G, Guria A, He J, Frank S, Nunnally AC, Bailey M, Jaskiw A, Feuchtbaum D, Nussbaum N, Abernethy AP, Miller VA. Association of patient characteristics and tumor genomics with clinical outcomes among patients with non-small cell lung cancer using a clinicogenomic database. JAMA. 2019;321:1391–1399. doi: 10.1001/jama.2019.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoneshima Y, Ijichi K, Anai S, Ota K, Otsubo K, Iwama E, Tanaka K, Oda Y, Nakanishi Y, Okamoto I. PD-L1 expression in lung adenocarcinoma harboring EGFR mutations or ALK rearrangements. Lung Cancer (Amsterdam, Netherlands). 2018;118:36–40. doi: 10.1016/j.lungcan.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 36.Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, Chung HC, Kindler HL, Lopez-Martin JA, Miller WH, Jr, Italiano A, Kao S, Piha-Paul SA, Delord JP, McWilliams RR, Fabrizio DA, Aurora-Garg D, Xu L, Jinn F, Norwood K, Bang YJ. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21:1353–1365. doi: 10.1016/s1470-2045(20)30445-9. [DOI] [PubMed] [Google Scholar]
- 37.Chen N, Fang W, Zhan J, Hong S, Tang Y, Kang S, Zhang Y, He X, Zhou T, Qin T, Huang Y, Yi X, Zhang L. Upregulation of PD-L1 by EGFR activation mediates the immune escape in EGFR-Driven NSCLC: Implication for optional immune targeted therapy for NSCLC patients with EGFR mutation. J. Thorac. Oncol. 2015;10:910–923. doi: 10.1097/jto.0000000000000500. [DOI] [PubMed] [Google Scholar]
- 38.Dong ZY, Zhang JT, Liu SY, Su J, Zhang C, Xie Z, Zhou Q, Tu HY, Xu CR, Li-Xu Yan LX, Li YF, Zhong WZ, Wu YL. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD-1 blockade in non-small cell lung cancer. OncoImmunology. 2017;6:e1356145. doi: 10.1080/2162402x.2017.1356145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang S, Zhang Y, Wang Y, Ye P, Li J, Li H, Ding Q, Xia J. Amphiregulin confers regulatory T cell suppressive function and tumor invasion via the EGFR/GSK-3β/Foxp3 Axis. J. Biol. Chem. 2016;291:21085–21095. doi: 10.1074/jbc.M116.717892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santaniello A, Napolitano F, Servetto A, De Placido P, Silvestris N, Bianco C, Formisano L, Bianco R. Tumour microenvironment and immune evasion in EGFR addicted NSCLC: hurdles and possibilities. Cancers. 2019 doi: 10.3390/cancers11101419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang J, Zhang C, Zhong WZ. Neoadjuvant immunotherapy for non–small cell lung cancer: State of the art. Cancer Commun. 2021;41:287–302. doi: 10.1002/cac2.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.