Abstract
Preterm delivery of low-birth weight infants is considered a leading cause of morbidity and mortality among neonates. Various studies have reported a positive correlation between periodontal disease (PD) and premature birth (PB) and yet no population-based study has assessed the impact of PD severity and treatments on premature birth. This cohort study used Taiwan’s national medical records (1999–2012, included 1,757,774 pregnant women) to investigate the association between PD severity and PB. Women with PD during the 2-year period prior for giving birth were more likely to have PB (11.38%) than those without PD (10.56%; p < 0.001). After variables adjustment, the advanced PD group had OR of 1.09 (95% CI 1.07–1.11) for PB, the mild PD group had OR of 1.05 (95% CI 1.04–1.06), while no-PD group had OR of 1. Increased PD severity was related to higher risk of PB. When stratified by age, the highest ORs for PB were those aged from 31 to 35 years in both mild PD group (OR = 1.09, 95% CI 1.07–1.11) and advanced PD group (OR = 1.13, 95% CI 1.09–1.17). Improving periodontal health before or during pregnancy may prevent or reduce the occurrence of adverse pregnancy outcomes and therefore maternal and perinatal morbidity and mortality.
Subject terms: Periodontics, Preterm birth
Introduction
A preterm delivery or premature birth (PB) entails an infant with a gestational age (GA) of less than 37 weeks at delivery1. According to a systematic analysis corporate with the World Health Organization (WHO) an estimation of 14.9 billion of infants were born preterm, which consist of 11.1% of newborn babies worldwide2–5 and approximately 10% of births in Taiwan were premature in 20196. Despite advances in reproductive and neonatal medicine, the preterm birth rate has not declined, and no treatments are effective in prolonging pregnancy to prevent preterm delivery. Preterm delivery of low-birthweight (LBW) infants is considered a leading cause of morbidity and mortality among neonates, and it is a major global public health concern.
The short-term health outcomes for PB babies including respiratory distress, chronic lung diseases, apnea, feeding difficulties, necrotizing enterocolitis, gastroesophageal reflux, immature immune system and cardiovascular disorder, as a consequence, PB babies often need neonatal intensive care unit (NICU) for close monitor of health signs7. An observational study in Denmark suggested that premature infants under 32 weeks receiving standard care in the NICU cost average of 14,300 euros8. As in Canada, a cost average of $30,572 for infants with GA of 29–32 weeks; and $100,440 for those with GA of < 29 weeks. In the United stated, hospital costs averaged $202,700 for babies at 25 weeks GA, substantially decreased to $1100 for 38 weeks GA infants9. Studies also showed that mothers caring for preterm infants may experience heightened levels of stress, anxiety, and depression in both during and beyond NICU stay10. Mother who give birth to PB babies need an average of 3 years to withdraw from work compared to mother who give birth to full GA infants in order to take a close eye on their infants11. As for long term complication, PB babies who survived, there was a higher risk of cerebral paralysis, neurodevelopmental disorders, mental retardation, epilepsy, respiratory system diseases, pathologic heart conditions, also disorders of psychological development, behavior, and emotion, as well as disabilities that affecting work and social capacities while adulthood12,13. Expenses for PB babies are high in regards of neonatal intensive care and continuous medical care and education fees. The cost of medical care for premature infants impose an economic burden on families and health care systems, additionally to the emotional impact on parents14.
Multiple factors have been reported to be associated with PB, such as high or low maternal age, a relatively low family socioeconomic stratum, poor nutritional status, smoking, drug use, hypertension, diabetes, genitourinary tract infection, cervical incompetence, multiple pregnancies, and stress15. Infection and inflammation have also been proposed as causes of preterm rupture of membranes and preterm delivery16. Both generalized and localized infections of the genitourinary system might affect gestational length and lead to premature delivery17,18.
Periodontal disease (PD), caused by Gram-negative anaerobic bacteria, is a highly prevalent disease characterized by chronic inflammation. PD affects 10% to 60% of the global population and destroys periodontal supporting tissue, resulting in tooth mobility and even tooth loss2,3,19. Causal relationships between PD and a variety of systemic diseases have been studied. PD increases the risk of ischemic stroke, acute myocardial infarction, esophageal cancer, rheumatoid arthritis20, and dementia21,22. The relationship between PD and adverse pregnancy outcomes has also been discussed23–25.
Higher levels of progesterone and estrogen are produced during pregnancy, which increases periodontal vascular permeability and the amount of crevicular fluid and causes gingival edema, swelling, and inflammation26. A tendency to develop gingivitis during pregnancy increases the prevalence of PD. Proinflammatory mediators such as prostaglandins, cytokines, and interleukin I, induced by oral flora, also cause advanced PD27. Although studies have reported a positive correlation between PD and adverse pregnancy outcomes—for example, preterm delivery28–30, others have reported otherwise23,31.
To our knowledge, no population-based study has assessed the impact of PD severity and PD treatments on premature delivery. This cohort study used national medical records retrieved from Taiwan’s National Health Insurance Research Database (NHIRD) to investigate the association between PD severity and preterm delivery.
Methods
Data source
The investigators conducted a retrospective nationwide cohort study by analyzing data in Taiwan’s NHIRD from 1999 to 2012. The NHIRD contains more than 99% of the medical claims data of 23 million residents of Taiwan. The research data were obtained from the NHIRD program, managed by the Health and Welfare Data Science Center, Taiwan to provide useful epidemiological information for basic and clinical research in Taiwan. This database contains demographic information and claims data, including diagnoses and procedure codes. Diagnoses recorded in the database are coded according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). All information allowing a specific patient to be identified was encrypted32.
Study cohort
The investigation included 1,757,774 pregnant women who first gave birth (primiparas) during the 13-year study period. The targeted subjects were separated into no-PD and PD groups depended on being diagnosed with PD or not. Furthermore, the researchers used ICD-9-CM and PD treatment codes to define PD severity and assembled into mild PD and advanced PD groups. The ages of the women ranged from 20 to 45 years.
Definition of PD
PD is caused by specific bacterial biofilm which accumulates and forms non-calcified dental plaque and calcified dental calculus around the teeth. The micro-bacterial biofilm can induce gingival inflammation, alveolar bone destruction and even tooth loss if without optimal oral hygiene maintenance or PD treatments. The PD subjects diagnosed with gingivitis, acute or chronic periodontitis were coded as 523.0–523.5 in the ICD-9-CM. These codes represent various PD stages and symptoms, which can appear simultaneously in multiple areas around oral cavity. All subjects who received any PD treatment were identified with the ICD-9-CM code for PD in the NHI program. The subjects who suffered from advanced PD with teeth removal required, the diagnosis would be PD rather than other dental diseases, for example, tooth caries or trauma. However, ICD-9-CM codes do not imply any particular level of inflammation of PD. In order to assign subjects to PD groups, the researchers used the most intensive PD treatment received by the patients as the indicator for grading the severity of PD. Dentists would chart a subject’s periodontal probing depth and check full-mouth dental X-rays as baseline records before treatment. If the subjects with gingivitis or mild PD, non-invasive treatments were preformed such as local periodontal emergency treatment or dental prophylaxis. On the other hand, if the subject meets the criteria (periodontal probing depth > 5 mm or evidence of alveolar bony destruction) for moderate to advanced PD, more intensive treatments such as subgingival curettage, root planning, periodontal flap surgery or tooth extraction will be necessary33,34. Thus, the different periodontal treatment codes could imply the severity of PD (dental prophylaxis < PD intensive treatment and tooth extraction due to PD).
The subjects were separated into no-PD (n = 922,846) and PD (n = 834,928) groups according to ICD-9-CM code from 523.0 to 523.5. Those with a PD diagnosis who did not receive PD treatment (n = 12,696) were excluded from the PD group due to unable to grading their PD severity from any PD treatment that they received. Those who received periodontal treatments (n = 822,232) were separated into mild and advanced PD subgroups according to the most advanced PD treatment they received within 2 years prior to the day of their first birth delivery. The mild PD group (n = 654,180) received local emergency periodontal treatment or dental prophylaxis (tooth scaling). The advanced PD group (n = 168,052) received more intensive periodontal treatment (eg, subgingival curettage, root planning, periodontal flap operation, or tooth extraction due to severe PD; Fig. 1).
Figure 1.
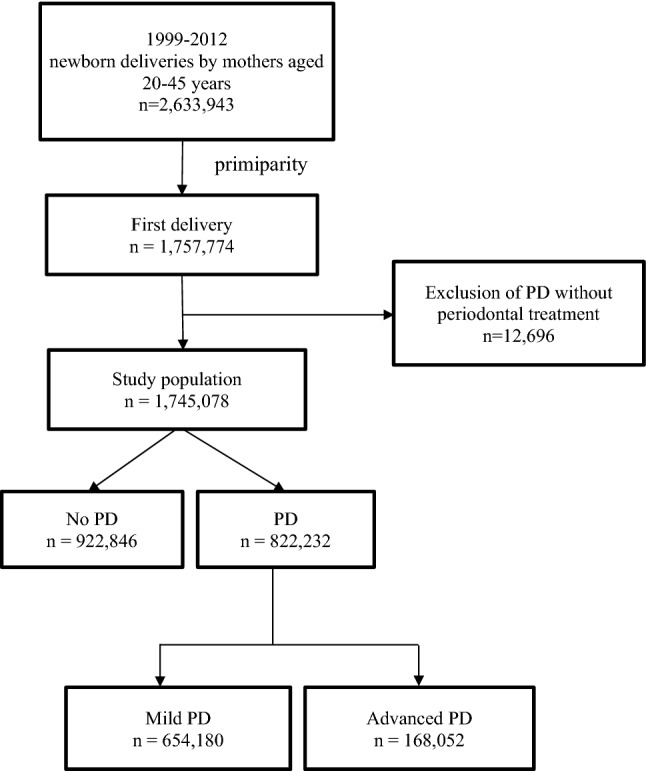
Flowchart of study population selection.
Measures of dependent variables
The subjects diagnosed as having PB were coded as ICD-9-CM 644, 765.0, 765.1, or 765.20 to 765.28.
Other confounding variables
Data on outpatient visits for multifetal pregnancy (ICD-9-CM 651), placental abnormalities (ICD-9-CM 641), diabetes mellitus complicating pregnancy (ICD-9-CM 648.0–648.8), vaginal infection (ICD-9-CM 616), autoimmune diseases (ICD-9-CM 710 and 714), hypertension complicating pregnancy (ICD-9-CM 642), or psychological factors (ICD-9-CM 290–319) within pregnancy period before delivery were included as individual obstetric histories. Other variables included were age and monthly income. The women were separated into 4 age groups (20–25, 26–30, 31–35, and > 35 years). Monthly income comprised 4 categories according to documented monthly income: dependent, < NT$20,000, NT$20,000–$39,999, and ≥ NT$40,000 (NT$32 = approximately US$1). Homemakers and unemployed workers were classified as dependent. Additionally, people without a documented monthly income (self-employed, retired, small shop proprietor, or those with a low income) can enroll in the health insurance scheme through labor unions or associations (e.g., farmer associations) or through local government offices, and they were assigned to the low monthly income group with an insurable wage of less than NT$20,00035.
Ethical approval
The study received full approval from the Taipei City Hospital Institutional Review Board (IRB no. TCHIRB-10603107-E). The institutional review board waived the requirement for written informed consent, as all patient-identifying information was encrypted. All methods in this study were performed in accordance with relevant guidelines and regulations. This study is also in accordance with the Declaration of Helsinki.
Data analysis
Baseline characteristics of participants were compared non-premature birth and premature birth using the chi-square test for categorical variables. The predictors for PB were analyzed using multivariate logistic regression, and adjusted for all other confounding factors, including mother’s age, monthly income, individual obstetric history, and PD condition. Stratified logistic regression analysis by age group was used to estimate the effect of PD on PB. All statistical analyses were performed using the SAS (version 9.4; SAS Institute, Inc., Cary, NC) statistical software package.
Results
The maternal age impact to PB
Among the 1,745,078 puerperae, 191,036 had PB delivery, resulting in an overall PB rate of 10.95%. The group aged over 35 years had the highest PB rate (12.46%); that with the lowest rate (10.34%) was the 26 to 30-year-old group (p < 0.001) The PB rate (11.39%) in the 20 to 25-year-old group was higher than in the group comprising those aged 25 to 35 inclusive (Table 1).
Table 1.
Characteristics of study population.
| Variable | Nonpremature birth | Premature birth | p valuea | ||
|---|---|---|---|---|---|
| n = 1,554,042 | Rate (%) | n = 191,036 | Rate (%) | ||
| Mother age (years) | |||||
| 20 ≤ Age ≤ 25 | 368,046 | 88.61 | 47,315 | 11.39 | < 0.001 |
| 25 < Age ≤ 30 | 629,297 | 89.66 | 72,566 | 10.34 | |
| 30 < Age ≤ 35 | 426,998 | 89.01 | 52,698 | 10.99 | |
| Age > 35 | 129,701 | 87.54 | 18,457 | 12.46 | |
| Monthly income | |||||
| Dependent | 354,617 | 89.03 | 43,685 | 10.97 | < 0.001 |
| < NT$20,000 | 465,966 | 89.43 | 55,076 | 10.57 | |
| NT$20,000–$40,000 | 522,330 | 89.23 | 63,016 | 10.77 | |
| > NT$40,000 | 211,129 | 87.83 | 29,259 | 12.17 | |
| Individual obstetric history | |||||
| Multifetal pregnancy | 6,906 | 47.21 | 7,721 | 52.79 | < 0.001 |
| Placental abnormality | 153,632 | 83.18 | 31,057 | 16.82 | < 0.001 |
| Diabetes mellitus complicating pregnancy | 190,966 | 85.36 | 32,745 | 14.64 | < 0.001 |
| Vaginal infection | 1,391,265 | 88.99 | 172,105 | 11.01 | < 0.001 |
| Autoimmune disease | 482,45 | 85.87 | 7,942 | 14.13 | < 0.001 |
| Hypertension complicating pregnancy | 30,975 | 71.70 | 12,224 | 28.30 | < 0.001 |
| Psychological factor(s) | 306,618 | 87.03 | 45,683 | 12.97 | < 0.001 |
| Periodontal disease | |||||
| No | 825,399 | 89.44 | 97,447 | 10.56 | < 0.001 |
| Yes | 728,643 | 88.62 | 93,589 | 11.38 | |
| Periodontal disease | |||||
| No PD | 825,399 | 89.44 | 97,447 | 10.56 | < 0.001 |
| Mild PD | 580,190 | 88.69 | 73,990 | 11.31 | |
| Advanced PD | 148,453 | 88.34 | 19,599 | 11.66 | |
ap values were obtained from Chi-squared test for categorical variables.
The association between incomes and PB
The risk of PB in those with a monthly income of ≥ NT$40 000 was higher than in the groups with lower monthly incomes (PB rate = 12.17% vs 10.97%, 10.57%, and 10.77% in the dependent, < NT$20 000, and NT$20 000–$39 999 groups, respectively; p < 0.001).
The impact of comorbidity to PB
Women with multifetal pregnancy had the highest PB rate (52.79%). Those with a placental abnormality (PB rate = 16.82%), gestational diabetes (PB rate = 14.64%), vaginal infection (PB rate = 11.01%), autoimmune disease (PB rate = 14.13%), hypertension complicating pregnancy (PB rate = 28.30%), or psychological disease (PB rate = 12.97%) had higher rates of PB (p < 0.001) than those without comorbidities.
Role of periodontal disease in preterm birth
Women with PD during the 2-year period prior to giving birth were more likely to have PB (11.38%) than those without PD (10.56%; p < 0.001). PB for those with advanced PD was more likely than for those with mild PD (11.66% vs 11.31%; p < 0 0.001; Table 1).
After adjusting for age, monthly income, and individual obstetric history, the pregnant women with PD had a higher risk of PB (odds ratio [OR] = 1.06, 95% confidence interval [CI 1.05–1.07; Appendix Table A1). The ORs of PB were further compared among the no-PD, mild PD, and advanced PD groups; after adjustment for other variables, the advanced PD group had an OR of 1.09 (95% CI 1.07–1.11), the mild PD group an OR of 1.05 (95% CI 1.04–1.06), and the no-PD group an OR of 1 (Table 2).
Table 2.
Univariate and multivariate logistic regression of factors associated with preterm birth.
| Variable | Crude | Adjusted | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Mother age | ||||
| 20 ≤ Age ≤ 25 | 1.00 | – | 1.00 | – |
| 25 < Age ≤ 30 | 0.90 | 0.89–0.91 | 0.87 | 0.86–0.88 |
| 30 < Age ≤ 35 | 0.96 | 0.95–0.97 | 0.90 | 0.88–0.91 |
| Age > 35 | 1.11 | 1.09–1.13 | 1.01 | 0.99–1.02 |
| Monthly income | ||||
| Dependent | 1.00 | – | 1.00 | – |
| < NT$20,000 | 0.96 | 0.95–0.97 | 0.96 | 0.95–0.98 |
| NT$20,000–$40,000 | 0.98 | 0.97–0.99 | 1.00 | 0.98–1.01 |
| > NT$40,000 | 1.13 | 1.11–1.14 | 1.13 | 1.11–1.15 |
| Individual obstetric history | ||||
| Multifetal pregnancy | 9.46 | 9.15–9.77 | 9.03 | 8.73–9.33 |
| Placental abnormality | 1.77 | 1.75–1.79 | 1.72 | 1.70–1.74 |
| Diabetes mellitus complicating pregnancy | 1.48 | 1.46–1.50 | 1.41 | 1.39–1.42 |
| Vaginal infection | 1.06 | 1.05–1.08 | 1.00 | 0.98–1.01 |
| Autoimmune disease | 1.35 | 1.32–1.39 | 1.29 | 1.25–1.32 |
| Hypertension complicating pregnancy | 3.36 | 3.29–3.43 | 3.16 | 3.09–3.23 |
| Psychological factor(s) | 1.28 | 1.26–1.29 | 1.25 | 1.24–1.27 |
| Periodontal disease | ||||
| No PD | 1.00 | – | 1.00 | – |
| Mild PD | 1.08 | 1.07–1.09 | 1.05 | 1.04–1.06 |
| Advanced PD | 1.12 | 1.10–1.14 | 1.09 | 1.07–1.11 |
Regarding the ORs of the 4 age groups for PB and comparing the without, mild, and advanced PD groups after variable adjustment, ORs were significantly higher in the advanced PD group compared with the mild PD group for all age groups and for the no-PD group (OR = 1). The highest ORs for PB in the 2 PD groups were for those aged 31–35 years (OR = 1.09, 95% CI 1.07–1.11 in mild PD group; OR = 1.13, 95% CI 1.09–1.17 in the advanced PD group; Table 3).
Table 3.
Multivariate analysis of the association between periodontal status and preterm birth according to mother’s age (years).
| Variable | 20 ≤ Age ≤ 25 | 25 < Age ≤ 30 | 30 < Age ≤ 35 | Age > 35 | ||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Monthly income | ||||||||
| Dependent | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – |
| < NT$20,000 | 0.97 | 0.95–1.00 | 0.96 | 0.94–0.98 | 0.95 | 0.92–0.97 | 0.98 | 0.93–1.03 |
| NT$20,000–$40,000 | 0.94 | 0.92–0.96 | 1.00 | 0.98–1.03 | 1.02 | 0.99–1.05 | 1.07 | 1.01–1.12 |
| > NT$40,000 | 1.05 | 0.97–1.13 | 1.14 | 1.11–1.18 | 1.13 | 1.10–1.16 | 1.15 | 1.10–1.21 |
| Individual obstetric history | ||||||||
| Multifetal pregnancy | 7.37 | 6.77–8.02 | 9.10 | 8.61–9.63 | 9.79 | 9.26–10.35 | 8.92 | 8.10–9.81 |
| Placental abnormality | 1.65 | 1.61–1.69 | 1.70 | 1.66–1.73 | 1.79 | 1.74–1.83 | 1.85 | 1.76–1.94 |
| Diabetes mellitus complicating pregnancy | 1.50 | 1.46–1.55 | 1.45 | 1.42–1.48 | 1.35 | 1.32–1.38 | 1.23 | 1.18–1.28 |
| Vaginal infection | 1.00 | 0.96–1.04 | 1.00 | 0.98–1.03 | 1.01 | 0.98–1.04 | 0.96 | 0.92–1.00 |
| Autoimmune disease | 1.20 | 1.13–1.28 | 1.26 | 1.21–1.31 | 1.33 | 1.27–1.39 | 1.38 | 1.29–1.48 |
| Hypertension complicating pregnancy | 2.67 | 2.55–2.80 | 3.21 | 3.09–3.32 | 3.40 | 3.26–3.54 | 3.49 | 3.29–3.70 |
| Psychological factor(s) | 1.33 | 1.30–1.36 | 1.25 | 1.23–1.28 | 1.21 | 1.19–1.24 | 1.19 | 1.14–1.23 |
| Periodontal disease | ||||||||
| No PD | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – |
| Mild PD | 1.00 | 0.98–1.03 | 1.06 | 1.04–1.08 | 1.09 | 1.07–1.11 | 1.03 | 0.99–1.06 |
| Advanced PD | 1.06 | 1.03–1.10 | 1.09 | 1.06–1.12 | 1.13 | 1.09–1.17 | 1.09 | 1.02–1.15 |
Discussion
A population-based cohort study was conducted to investigate associations among age, monthly income, individual obstetric history, PD severity, and the risk of premature delivery. With regard to maternal age, a meta-analysis performed by Pinheiro et al. concluded that women aged over 35 years were generally at a higher risk of adverse obstetrical and perinatal outcomes36. That advanced maternal age increases the risk of extreme preterm birth has also been discussed36–40. The findings accord with those of the present study, in which the group with a maternal age older than 35 years had the highest preterm birth rate. The maternal age groups of 25–30 years and 30–35 years (OR = 0.87, 95% CI 0.86–0.88; OR = 0.90, 95% CI 0.88–0.91, respectively) were associated with significantly lower risk of PB than the under-25 age group after confounding adjustment (Table 2). Fuchs et al. reported a similar finding for Quebec, Canada. The rate of spontaneous preterm delivery was elevated in women aged 20 to 24 years, and lower prematurity was observed for women aged 30–34 years. The adjusted OR for PB followed a U-shaped distribution from 20 to 24, 25 to 29, 30 to 34, 35 to 39, and 40 or more years37.
Regarding PD in relation to PB after age stratification, ORs were found significantly higher in the advanced PD subjects among all age groups (Table 3). We concluded that pregnant women with advanced PD have a higher risk of PB compared to women without PD among all age groups. In the mild PD group, PB risks were also found higher in the groups of 25 < Age ≤ 30 and 30 < Age ≤ 35 but did not show a significant increase in women younger than 25 and older than 35 age groups. In this study, women aged between 25 and 35 years consist of 68% of puerperae, therefore, PD preventions and treatments toward fertile females should be emphasized especially to women with 25–35 years old to reduce the PB rate in Taiwan.
In the present study, women with a higher monthly income had significantly higher risk of PB. However, in studies by Lohana et al. and Morgen et al., SES and preterm delivery did not exhibit a significant association41,42. Additionally, controversial results were reported for an investigation conducted by Kramer and Dickute, who concluded that low SES, low educational attainment, and occupation were risk factors for LBW16,43. The variables in our database of Taiwan’s NHIRD do not include other SES-related variables such as education, smoking, domicile and occupation. However, studies have found that income inequality might lead to unfavorable health outcomes and increase the risk of preterm birth during pregnancy44,45. Some studies have pointed out the relationship between income and health condition correlated with one another in two hypotheses: 1. Income directly affects basic living conditions. 2. Income indirectly affects human health status by influencing their social and living environment46. In other words, the less public health services provided by society, the more important personal income is to health.
Taiwan is a country with well-developed medical and health care systems. Multiple policies aid and encourage the public to access personal medical needs. The coverage rate of national health insurance provided by the government reached as high as 99.9%47. Additionally, medical accessibility in Taiwan is extremely high compared to other well-developed and developing countries. As for low-income households, the government offers extra medical and premium subsidies. Therefore, the influence of people with low income on medical usage may have less impact in contrast with other countries. The results of this study found that the higher the income, the greater the risk of preterm birth. This might be a result of women with higher salaries and job positions being more engaged to work that develops a higher physical and mental stress of working. We recommend more research and discussion on the relationship between personal income and PB, especially in regions with mature medical insurance systems.
Pregnant women with diabetes or autoimmune or psychological diseases have a higher risk of preterm delivery48. Those who have a multifetal pregnancy have the highest risk of preterm delivery, followed by those with hypertension complicating pregnancy after cofounding adjustment49–51.
Having a hypertensive disorder during the perinatal period can lead to fetal morbidity, resulting in intrauterine growth restriction and preterm birth. This condition further affects preterm offspring; whether through inherited genetic factors or continued pathophysiology, they have an increased risk of type 2 diabetes, hypertension, and cardiometabolic diseases in adulthood52,53. In research by da Silva et al., an increased level of core blood adipokines in preterm infants exposed to maternal hypertension during pregnancy was reported. In addition, a decreased level of ghrelin has been associated with preeclampsia54,55.
Periodontitis is a highly prevalent chronic inflammatory disease. Studies have suggested a correlation between multiple systemic diseases such as diabetes, cardiovascular disease, cancer, and dementia and premature delivery56; systemic inflammatory responses have been determined to be related to elevated concentrations of circulating inflammatory markers, to form pathogen depositions, neural plaques, and atherosclerosis thrombogenesis, and to induce vascular changes and multiple systemic diseases22,57,58. Mediators of systemic inflammation such as cytokines, chemokines, prostaglandins, and C-reactive protein (CRP) have been proved in many studies to be associated with PD59,60. Pregnant women with PD and elevated CRP levels being at increased risk for adverse pregnancy outcomes was reported by Ruma et al61.
PD affects a large proportion of pregnant woman and occurs in up to 15% of fertile women41. The potential causal association between the presence of maternal PD and several adverse pregnancy outcomes is systemic abnormal immunological changes in the fetal/placental unit, which are elevated by PD pathogens and lead to pregnancy complications28,30,62. Another hypothesis regarding the mechanism of PD and adverse pregnancy outcomes is that the PD pathogen can colonize the placenta, causing local inflammation and resulting in prematurity and other adverse pregnancy outcomes63.
In the present study, primiparas with PD had a higher risk of premature delivery than those without PD. In addition, those with advanced PD had a much higher PB risk compared with those with mild PD. Thus, increased PD severity was related to a higher risk of premature delivery. In the conclusions of Lohana, M. H. et al., PD was a potential risk factor for preterm LBW and, as maternal PD severity increased, the proportion of preterm and LBW deliveries also increased41.
According to a study by Albert et al., pregnant women who receive prophylactic dental treatment have a better PD condition and a lower risk of preterm birth than those who do not64. In Taiwan, National Health Insurance offers pregnant women an additional free periodontal prophylaxis (in addition to the twice yearly treatment available to all the insured older than 13 years) to prevent PD. The mechanisms underlying the association between PD and premature delivery have not been fully elucidated. PD is a preventable and treatable disease, and pregnant women should improve their oral hygiene to alleviate periodontal tissue inflammation and reduce the risk of PB.
The used of the nationwide population-based database of the NHIRD make the study more credible as it provides sufficient sample size and statistical power to assess the association between PD and PB. However, there are several limitations in the study. The NHIRD is mainly conduct and processed by the Bureau of National Health Insurance (BNHI). In order to prevent miscoding or misclassification by the usage of administrative data, BNHI routinely samples the patient charts from different medical institutes to validate the quality of the database. However, the lack of information of other PB associated factors such as education, occupation, body mass index, and smoking status et al., which cannot be provided from NHIRD, that might reduce accuracy and feasibility of interpreting the analytic outcomes.
Conclusion
Improving periodontal health before or during pregnancy may prevent or reduce the occurrence of adverse pregnancy outcomes and therefore maternal and perinatal morbidity and mortality. Further studies are required to investigate the mechanism linking PD with adverse pregnancy outcomes. Understanding the impacts of PD on pregnancy can provide empirical evidence for the design of health policy and assist in the development of effective prevention and welfare programs to address adverse pregnancy outcomes.
Supplementary Information
Acknowledgements
The authors are grateful to the members of Department of Education and Research, Taipei City Hospital, Taiwan for their valuable contributions in data management and statistical analysis.
Author contributions
All authors contributed to the conception and design of the work. Y.L.L. drafted the manuscript. D.C. and C.L.L. were involved in conception and project coordination. H.Y.H., F.S.C. performed the data analysis and S.Y.C. performed the data collection. C.Y.Y. has provided appropriate information when revising the manuscript. All authors critically reviewed, edited and approved the final manuscript.
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-07425-8.
References
- 1.WHO. WHA Global Nutrition Targets 2025: Low Birth Weight Policy Brief. World Health Organisationhttps://www.who.int/nutrition/topics/globaltargets_lowbirthweight_policybrief.pdf (2014).
- 2.Blencowe H, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: A systematic analysis and implications. Lancet (London, England) 2012;379:2162–2172. doi: 10.1016/s0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 3.Walani SR. Global burden of preterm birth. Int. J. Gynaecol. Obstet. 2020;150:31–33. doi: 10.1002/ijgo.13195. [DOI] [PubMed] [Google Scholar]
- 4.Liu L, et al. Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet (London, England) 2012;379:2151–2161. doi: 10.1016/s0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 5.Blencowe H, et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod. Health. 2013;10:S2. doi: 10.1186/1742-4755-10-s1-s2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.HPA. Birth notification data. Taiwanhttps://www.hpa.gov.tw/Pages/Detail.aspx?nodeid=649&pid=13646 (2021).
- 7.Institute of Medicine Committee on Understanding Premature, B. & Assuring Healthy, O. The National Academies Collection: Reports funded by National Institutes of Health. Washington (DC). (2007).
- 8.Rasmussen MK, et al. Cost analysis of neonatal tele-homecare for preterm infants compared to hospital-based care. J. Telemed. Telecare. 2020;26:474–481. doi: 10.1177/1357633x19843753. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert WM, Nesbitt TS, Danielsen B. The cost of prematurity: Quantification by gestational age and birth weight. Obstet. Gynecol. 2003;102:488–492. doi: 10.1016/s0029-7844(03)00617-3. [DOI] [PubMed] [Google Scholar]
- 10.Miles MS, Holditch-Davis D, Schwartz TA, Scher M. Depressive symptoms in mothers of prematurely born infants. J. Dev. Behav. Pediatr. JDBP. 2007;28:36–44. doi: 10.1097/01.DBP.0000257517.52459.7a. [DOI] [PubMed] [Google Scholar]
- 11.Fox H, Callander E. Cost of preterm birth to Australian mothers: Assessing the financial impact of a birth outcome with an increasing prevalence. J. Paediatr. Child Health. 2021;57:618–625. doi: 10.1111/jpc.15278. [DOI] [PubMed] [Google Scholar]
- 12.Moster D, Lie RT, Markestad T. Long-term medical and social consequences of preterm birth. N. Engl. J. Med. 2008;359:262–273. doi: 10.1056/NEJMoa0706475. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro S, McCormick MC, Starfield BH, Krischer JP, Bross D. Relevance of correlates of infant deaths for significant morbidity at 1 year of age. Am. J. Obstet. Gynecol. 1980;136:363–373. doi: 10.1016/0002-9378(80)90863-7. [DOI] [PubMed] [Google Scholar]
- 14.Rios JD, et al. Costs of neonatal intensive care for canadian infants with preterm birth. J. Pediatr. 2021;229:161–167. doi: 10.1016/j.jpeds.2020.09.045. [DOI] [PubMed] [Google Scholar]
- 15.Schneider HG, Göbbels E, Apel EM. Simplification of plaque index method of Quigley and Hein. Stomatologie der DDR. 1989;39:91–94. [PubMed] [Google Scholar]
- 16.Kramer MS. Determinants of low birth weight: Methodological assessment and meta-analysis. Bull. World Health Organ. 1987;65:663–737. [PMC free article] [PubMed] [Google Scholar]
- 17.Andrews WW, Goldenberg RL, Hauth JC. Preterm labor: emerging role of genital tract infections. Infect. Agents Dis. 1995;4:196–211. [PubMed] [Google Scholar]
- 18.Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am. J. Obstet. Gynecol. 1992;166:1515–1528. doi: 10.1016/0002-9378(92)91628-n. [DOI] [PubMed] [Google Scholar]
- 19.Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol. 1994;2000(5):78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 20.Chen HH, et al. Association between a history of periodontitis and the risk of rheumatoid arthritis: A nationwide, population-based, case-control study. Ann. Rheum. Dis. 2013;72:1206–1211. doi: 10.1136/annrheumdis-2012-201593. [DOI] [PubMed] [Google Scholar]
- 21.Lee YL, Hu HY, Huang LY, Chou P, Chu D. Periodontal disease associated with higher risk of dementia: Population-based cohort study in Taiwan. J. Am. Geriatr. Soc. 2017;65:1975–1980. doi: 10.1111/jgs.14944. [DOI] [PubMed] [Google Scholar]
- 22.Lee YL, et al. Dental prophylaxis and periodontal treatment are protective factors to ischemic stroke. Stroke. 2013;44:1026–1030. doi: 10.1161/strokeaha.111.000076. [DOI] [PubMed] [Google Scholar]
- 23.Davenport ES, et al. Maternal periodontal disease and preterm low birthweight: Case–control study. J. Dent. Res. 2002;81:313–318. doi: 10.1177/154405910208100505. [DOI] [PubMed] [Google Scholar]
- 24.López NJ, Smith PC, Gutierrez J. Higher risk of preterm birth and low birth weight in women with periodontal disease. J. Dent. Res. 2002;81:58–63. doi: 10.1177/002203450208100113. [DOI] [PubMed] [Google Scholar]
- 25.Yeo BK, Lim L-P, Paquette DW, Williams RC. Periodontal disease—The emergence of a risk for systemic conditions: pre-term low birth weight. Ann. Acad. Med. Singapore. 2005;34:111–116. [PubMed] [Google Scholar]
- 26.Carrillo-de-Albornoz A, Figuero E, Herrera D, Bascones-Martínez A. Gingival changes during pregnancy: II. Influence of hormonal variations on the subgingival biofilm. J. Clin. Periodontol. 2010;37:230–240. doi: 10.1111/j.1600-051X.2009.01514.x. [DOI] [PubMed] [Google Scholar]
- 27.Usin MM, et al. Association between maternal periodontitis and preterm and/or low birth weight infants in normal pregnancies. J. Maternal Fetal Neonatal Med. 2016;29:115–119. doi: 10.3109/14767058.2014.987751. [DOI] [PubMed] [Google Scholar]
- 28.Daalderop LA, et al. Periodontal disease and pregnancy outcomes: Overview of systematic reviews. JDR Clin. Transl. Res. 2018;3:10–27. doi: 10.1177/2380084417731097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar A, et al. Association of maternal periodontal health with adverse pregnancy outcome. J. Obstet. Gynaecol. Res. 2013;39:40–45. doi: 10.1111/j.1447-0756.2012.01957.x. [DOI] [PubMed] [Google Scholar]
- 30.Offenbacher S, et al. Periodontal infection as a possible risk factor for preterm low birth weight. J. Periodontol. 1996;67:1103–1113. doi: 10.1902/jop.1996.67.10s.1103. [DOI] [PubMed] [Google Scholar]
- 31.Iheozor-Ejiofor, Z., Middleton, P., Esposito, M. & Glenny, A. M. Treating periodontal disease for preventing adverse birth outcomes in pregnant women. Cochrane Datab. Syst. Rev. 6, Cd005297. 10.1002/14651858.CD005297.pub3 (2017). [DOI] [PMC free article] [PubMed]
- 32.Lin LY, Warren-Gash C, Smeeth L, Chen PC. Data resource profile: The national health insurance research database (NHIRD) Epidemiol. Health. 2018;40:e2018062. doi: 10.4178/epih.e2018062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet (London, England) 2005;366:1809–1820. doi: 10.1016/s0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 34.Fogacci MF, Vettore MV, Thomé Leão AT. The effect of periodontal therapy on preterm low birth weight: A meta-analysis. Obstet. Gynecol. 2011;117:153–165. doi: 10.1097/AOG.0b013e3181fdebc0. [DOI] [PubMed] [Google Scholar]
- 35.MOL. Labor Statistics Survey in Taiwan. Ministry of labor, Taiwanhttps://statfy.mol.gov.tw/statistic_DB.aspx (2021).
- 36.Pinheiro, R. L., Areia, A. L., Mota Pinto, A. & Donato, H. Advanced maternal age: adverse outcomes of pregnancy, a meta-analysis. Acta Med. Portugues.32, 219–226. 10.20344/amp.11057 (2019). [DOI] [PubMed]
- 37.Fuchs F, Monet B, Ducruet T, Chaillet N, Audibert F. Effect of maternal age on the risk of preterm birth: A large cohort study. PLoS ONE. 2018;13:e0191002. doi: 10.1371/journal.pone.0191002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu B, et al. Association between maternal pre-pregnancy obesity and preterm birth according to maternal age and race or ethnicity: A population-based study. Lancet Diabetes Endocrinol. 2019;7:707–714. doi: 10.1016/s2213-8587(19)30193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Londero AP, Rossetti E, Pittini C, Cagnacci A, Driul L. Maternal age and the risk of adverse pregnancy outcomes: A retrospective cohort study. BMC Preg. Childbirth. 2019;19:261. doi: 10.1186/s12884-019-2400-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waldenström U, Cnattingius S, Vixner L, Norman M. Advanced maternal age increases the risk of very preterm birth, irrespective of parity: A population-based register study. BJOG Int. J. Obstet. Gynaecol. 2017;124:1235–1244. doi: 10.1111/1471-0528.14368. [DOI] [PubMed] [Google Scholar]
- 41.Lohana MH, Suragimath G, Patange RP, Varma S, Zope SA. A Prospective cohort study to assess and correlate the maternal periodontal status with their pregnancy outcome. J. Obstet. Gynaecol. India. 2017;67:27–32. doi: 10.1007/s13224-016-0920-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morgen, C. S., Bjørk, C., Andersen, P. K., Mortensen, L. H. & Nybo Andersen, A. M. Socioeconomic position and the risk of preterm birth—a study within the Danish National Birth Cohort. Int. J. Epidemiol.37, 1109–1120. 10.1093/ije/dyn112 (2008). [DOI] [PubMed]
- 43.Dickute J, et al. Maternal socio-economic factors and the risk of low birth weight in Lithuania. Medicina (Kaunas) 2004;40:475–482. [PubMed] [Google Scholar]
- 44.Vilda D, Wallace M, Dyer L, Harville E, Theall K. Income inequality and racial disparities in pregnancy-related mortality in the US. SSM Popul. Health. 2019;9:100477. doi: 10.1016/j.ssmph.2019.100477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wallace ME, Mendola P, Chen Z, Hwang BS, Grantz KL. Preterm Birth in the Context of Increasing Income Inequality. Matern. Child Health J. 2016;20:164–171. doi: 10.1007/s10995-015-1816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marmot M. The influence of income on health: Views of an epidemiologist. Health Aff. 2002;21:31–46. doi: 10.1377/hlthaff.21.2.31. [DOI] [PubMed] [Google Scholar]
- 47.NHIA. Unversal Health Coverage in Taiwan. National Health Insurance Administration, Ministry of Health and Welfarehttps://www.nhi.gov.tw/English/Content_List.aspx?n=4D7051840BF42F52&topn=ED4A30E51A609E49 (2021).
- 48.Teresa C, Marian K, Bo J. Risk factors for spontaneous preterm delivery. Int. J. Gynaecol. Obstet. 2020;150:17–23. doi: 10.1002/ijgo.13184. [DOI] [PubMed] [Google Scholar]
- 49.Bramham, K. et al. Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. BMJ (Clinical research ed.)348, g2301. 10.1136/bmj.g2301 (2014). [DOI] [PMC free article] [PubMed]
- 50.Corrigan L, O'Farrell A, Moran P, Daly D. Hypertension in pregnancy: Prevalence, risk factors and outcomes for women birthing in Ireland. Preg. Hypertension. 2021;24:1–6. doi: 10.1016/j.preghy.2021.02.005. [DOI] [PubMed] [Google Scholar]
- 51.Magalhães E, et al. Pregnancy-induced hypertension, preterm birth, and cord blood adipokine levels. Eur. J. Pediatr. 2020;179:1239–1246. doi: 10.1007/s00431-020-03586-8. [DOI] [PubMed] [Google Scholar]
- 52.Bertagnolli M, Luu TM, Lewandowski AJ, Leeson P, Nuyt AM. Preterm birth and hypertension: is there a link? Curr. Hypertens. Rep. 2016;18:28. doi: 10.1007/s11906-016-0637-6. [DOI] [PubMed] [Google Scholar]
- 53.Mierzynski R, et al. Intra-uterine growth retardation as a risk factor of postnatal metabolic disorders. Curr. Pharm. Biotechnol. 2016;17:587–596. doi: 10.2174/1389201017666160301104323. [DOI] [PubMed] [Google Scholar]
- 54.Hamilcikan S, et al. The relationship of active ghrelin levels and intrauterine growth in preterm infants. Eur. J. Endocrinol. 2011;166:399–405. doi: 10.1530/EJE-11-0607. [DOI] [PubMed] [Google Scholar]
- 55.Warchoł M, et al. Association of cord blood ghrelin, leptin and insulin concentrations in term newborns with anthropometric parameters at birth. J Pediatr. Endocrinol. Metab. JPEM. 2018;31:151–157. doi: 10.1515/jpem-2017-0285. [DOI] [PubMed] [Google Scholar]
- 56.Nabet C, et al. Maternal periodontitis and the causes of preterm birth: The case-control Epipap study. J. Clin. Periodontol. 2010;37:37–45. doi: 10.1111/j.1600-051X.2009.01503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee Y-L, Hu H-Y, Chou P, Chu D. Dental prophylaxis decreases the risk of acute myocardial infarction: A nationwide population-based study in Taiwan. Clin. Interv. Aging. 2015;10:175–182. doi: 10.2147/CIA.S67854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Michaud DS, Liu Y, Meyer M, Giovannucci E, Joshipura K. Periodontal disease, tooth loss, and cancer risk in male health professionals: a prospective cohort study. Lancet Oncol. 2008;9:550–558. doi: 10.1016/s1470-2045(08)70106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Joshipura KJ, Wand HC, Merchant AT, Rimm EB. Periodontal disease and biomarkers related to cardiovascular disease. J. Dent. Res. 2004;83:151–155. doi: 10.1177/154405910408300213. [DOI] [PubMed] [Google Scholar]
- 60.Loos BG. Systemic markers of inflammation in periodontitis. J. Periodontol. 2005;76:2106–2115. doi: 10.1902/jop.2005.76.11-S.2106. [DOI] [PubMed] [Google Scholar]
- 61.Ruma M, et al. Maternal periodontal disease, systemic inflammation, and risk for preeclampsia. Am. J. Obstet. Gynecol. 2008;198(389):e381–385. doi: 10.1016/j.ajog.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 62.Varshney S, Gautam A. Poor periodontal health as a risk factor for development of pre-eclampsia in pregnant women. J. Indian Soc. Periodontol. 2014;18:321–325. doi: 10.4103/0972-124X.134569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Han YW, et al. Fusobacterium nucleatum induces premature and term stillbirths in pregnant mice: Implication of oral bacteria in preterm birth. Infect. Immun. 2004;72:2272–2279. doi: 10.1128/iai.72.4.2272-2279.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Albert DA, et al. An examination of periodontal treatment, dental care, and pregnancy outcomes in an insured population in the United States. Am. J. Public Health. 2011;101:151–156. doi: 10.2105/ajph.2009.185884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


