Abstract
In temperate regions the annual pattern of wood development is characterized by the formation of radially narrow and thick walled latewood cells. This takes place at the later part of the growing season when cambial cell division declines. To gain new insight into the regulation of this process, micro-analytical techniques were used to visualize the distribution of indole-3-acetic acid (IAA), soluble carbohydrates, and activities of sucrose (Suc)-metabolizing enzymes across the cambial region tissues in Scots pine (Pinus sylvestris). The total amount of IAA in the cambial region did not change with latewood initiation. But its radial distribution pattern was altered, resulting in an increased concentration in the cambial meristem and its recent derivatives. Thus, initiation of latewood formation and cessation of cambial cell division is not a consequence of decreased IAA concentrations in dividing and expanding cells. Rather, IAA most likely has a role in defining the altered developmental pattern associated with latewood formation. Carbohydrates and enzyme activities showed distinctive radial distribution patterns. Suc peaked in the phloem and decreased sharply to low levels across the cambial zone, whereas fructose and glucose reached their highest levels in the maturing tracheids. Suc synthase was the dominating Suc cleaving enzyme with a peak in the secondary wall-forming tracheids and in the phloem. Soluble acid invertase peaked in dividing and expanding cells. Suc-phosphate synthase had its highest activities in the phloem. Activities of cell wall bound invertase were low. The absence of major seasonal variations indicates that carbohydrate availability is not a trigger for latewood initiation. However, steep concentration gradients of the sugars suggest a role for sugar signaling in vascular development.
The annual transition from earlywood to latewood formation is a conspicuous developmental switch in temperate region trees. Latewood is induced during the later part of the growing season, when cell division activity in the cambial meristem declines. It involves a reduction in radial expansion and an increase in wall thickening of the cambial derivatives. Thus, earlywood is characterized by large-diameter and thin-walled tracheids, whereas latewood is composed of narrow diameter tracheids with thick cell walls. The induction of latewood cells and cambial dormancy offers a natural system by which we can gain new insight into the regulation of the basic growth processes of cell division and cell morphology.
Early investigators concluded that the initiation of latewood formation was induced by shortening of the photoperiod, and was associated with cessation of apical and needle growth at a time when current year needles had become net exporters of photosynthetic assimilate (Richardson and Dinwoodie, 1960; Larson, 1964; Gordon and Larson, 1968). It was also observed that exogenous IAA could cause cambia that were forming narrow-diameter latewood trach-eids to revert to forming large-diameter earlywood tracheids (Larson, 1960). These observations have led to a long-lasting dogma that propounds that the induction of both narrow-diameter latewood tracheids and growth cessation are induced by a reduction in IAA concentration in cambial tissues, whereas the induction of thick cell walls associated with the latewood tracheids is a result of an increased carbohydrate availability (Larson, 1969b).
This idea has inspired several investigations on the seasonal variation of auxin and carbohydrates in wood-forming tissues. Investigations into the concentration of IAA in samples containing the combined tissues from the cambial region (i.e. mature phloem plus dividing, expanding, and maturing phloem and xylem cells) collected during the earlywood/latewood transition have yielded conflicting results (Little and Wareing, 1981; Savidge et al., 1982; Savidge and Wareing, 1984; Sandberg and Ericsson, 1987; Sundberg, et al., 1987, 1990, 1993; Little and Pharis, 1995; Eklund et al., 1998) This may partly be explained by the fact that endogenous indole-3-acetic acid (IAA) is present as a steep concentration gradient across developing secondary vascular tissues, as recently demonstrated in Scots pine and hybrid aspen (Uggla et al., 1996, 1998; Tuominen et al., 1997; Sundberg et al., 2000). From this observation it is evident that the IAA concentration in the cells of specific developmental stages, such as division and expansion, cannot be judged from estimates based on samples comprising the combined cambial region tissues. Moreover, seasonal variation of the IAA concentration as calculated in earlier studies will be perturbed by the unavoidable variation in the amount and proportion of different cambial region tissues in the samples and will not necessarily reflect the amount of IAA supplied to cambial tissues by polar transport (Uggla et al., 1998; Sundberg et al., 2000). Therefore, these data are difficult to interpret. However, the observation of a steep concentration gradient across developing cambial tissues inspired the idea that IAA influences developmental patterns by providing positional information (Uggla et al., 1996) with support being obtained from studies with both Scots pine and hybrid aspen (Tuominen et al., 1997; Uggla et al., 1998; Sundberg et al., 2000).
There have only been a few studies on carbohydrates in relation to seasonal variation in cambial growth (Gordon and Larson, 1968; Parkerson and Whitmore, 1972; Savidge, 1991; Sundberg et al., 1993). The distribution patterns of carbohydrates across the different tissues of the cambial region have not been determined and, in analogy to the case of IAA, available information is therefore difficult to evaluate. Moreover, it is not just the presence of carbohydrates that is important, the capacity of tissues to use them in metabolic processes is just as significant or perhaps even more so. Thus, information concerning the activities of sugar-metabolizing enzymes would be very helpful. Such information has not been available for the separate tissues involved in wood formation. An understanding of the sugar dynamics in cambial tissues is also of interest in the light of recent evidence for sugar signaling in developmental control. Soluble carbohydrates such as Suc and Glc induce gene expression and seem to interact in, for example, light and hormonal responses (Sheen et al., 1999).
To gain new insight about the regulation of the earlywood/latewood transition, a detailed analysis of IAA and carbohydrates was performed in Scots pine (Pinus sylvestris) to evaluate their role in the transition process. The use of tangential cryosectioning of the cambial region, in combination with microscale analytical techniques, enabled us to report herein on the radial distribution patterns of IAA, Suc, Glc, Fru, and activities of Suc synthase (SuSy), acid invertase (AI), and Suc-phosphate synthase (SPS), across the tissues of the cambial region during latewood initiation.
RESULTS
Developmental Patterns and Anatomy
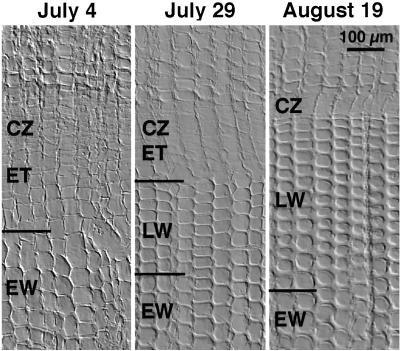
The sampling dates were selected to represent typical phases during the earlywood to latewood transition (Fig. 1). On July 4, an average of 21 ± 1.2 sd tracheary elements had been formed, still without any signs of latewood characteristics. The cambial zone, which consisted of 7.6 ± 0.4 cells per radial file, was still actively dividing. Samples from July 29 represent the initial stage of latewood formation as judged from the narrow radial diameter of the 4 to 7 most recent secondary wall-forming tracheids, in comparison with previously formed cells. At this date, an average of 29 ± 6.7 tracheids had been formed, and the cambial zone consisted of 5.9 ± 0.2 cells per radial file, still actively dividing. On August 19, an average of 37 ± 5.1 tracheids had been produced. The expansion zone was almost absent, and the number of cells in the cambial zone had decreased to 4.9 ± 1.0 and cell production was essentially complete. Typical latewood tracheids had been produced and cell wall thickening was still ongoing in the most recent tracheids. During the sampling period, the number of cells per radial cell file in the zone of tracheid expansion decreased from 8.1 ± 0.2 cells on July 4, to 3.1 ± 0.6 cells on July 29, and finally to 1.3 ± 0.6 cells on August 19. The radial diameter of the tracheids produced decreased as well. Fully expanded earlywood tracheids had radial diameters of approximately 52 μm, compared with approximately 32 μm for typical latewood tracheids. The two most recently formed fully expanded latewood tracheids in the August 19 sample had radial diameters of approximately 13 μm. The number of cells per cell file in the zone of secondary wall formation increased slightly during the sampling period, e.g. 13 ± 1.3 cells on July 4, 14 ± 2.8 on July 29, and 16 ± 5.5 on August 19.
Figure 1.
Representative transverse sections from the cambial region of Scots pine trees during the earlywood to latewood transition. On July 4, typical earlywood tracheids had been formed. On July 29, the radial width of the expansion zone had decreased, and tracheids undergoing secondary wall formation were radially narrow. On August 19, the expansion zone was almost absent, the most recent tracheids were very narrow and the thick wall of the latewood tracheids was obvious. CZ, Cambial zone; ET, expanding tracheids; EW, tracheids with earlywood characteristics; LW, tracheids with latewood characteristics.
Distribution Patterns of IAA, Carbohydrates, Enzyme Activities, and Total Protein across the Cambial Region
IAA
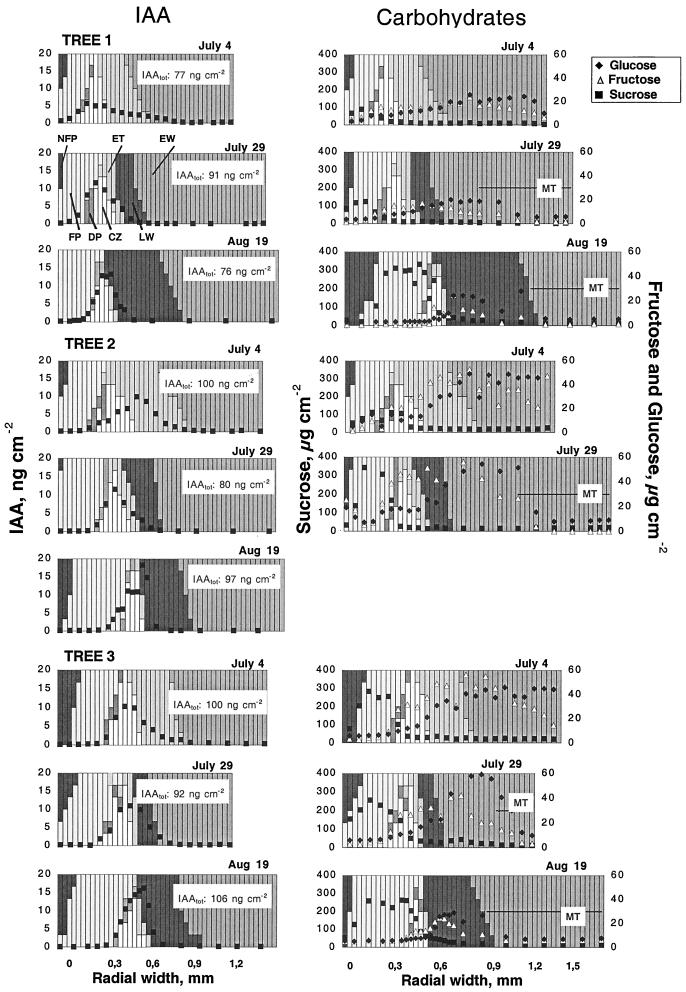
From a peak level associated with the cambial zone, the IAA concentration steeply decreased toward phloem and xylem, reaching low and relatively stable levels close to the transition between expanding and secondary wall-forming cells (Fig. 2). This pattern of a steep concentration gradient across the developing cambial derivatives has been described and discussed earlier for Scots pine (Uggla et al., 1996, 1998) and hybrid aspen (Tuominen et al., 1997). An exceptional distribution pattern was evident in tree 2 sampled on July 4, where the peak IAA level was associated with expanding xylem derivatives. This anomaly is ascribed to a longitudinal wavyness of the cambial zone. During the sampling period a consistent increase in peak concentration of IAA was evident, but the amount of IAA (IAAtot) in all cambial region tissues was fairly constant. It is of consequence that the radial width of the IAA distribution decreased in close association with the decrease in the combined radial width of the cambial and expansion zones.
Figure 2.
Radial distribution patterns of IAA and carbohydrates across the cambial meristem and its differentiating and mature derivatives at different dates in three different trees. Each column represents a 30-μm-thick tangential section and its relative composition of different tissues. The different shadings of the columns are explained in the figure. Endogenous content of the substance per cm2 section is indicated by the symbol in the column. For IAA, the total amount per cm2 of all cambial region tissues (IAAtot; i.e. the integrated area below the gradient) is indicated at the upper right corner for each position. NFP, Nonfunctional phloem; FP, functional phloem; DP, developing phloem; CZ, cambial zone; ET, expanding tracheids; EW, maturing earlywood tracheids forming secondary wall; LW, maturing latewood tracheids forming secondary wall; MT, mature (dead) tracheids (only indicated in the right column).
Carbohydrates and Enzyme Activities
Suc is supplied to the tissues of the cambial region via phloem sieve cells and indeed the phloem contained the highest levels of Suc (Fig. 2). Peak values from 100 μg cm−2 section up to 400 μg cm−2 section were recorded. Based on estimates of the total protein content, this would correspond to approximately 8.3 and 33 μg μg−1 protein, respectively. From dry weight measurements of samples and previous determinations of a water content of 70% in the phloem (Uggla et al., 1996), this would result in a maximum concentration of Suc in the phloem ranging from 6% to 23% of the water content (97 to 390 mm), or 130 to 530 mg per g dry weight. From the phloem, the level of Suc steeply decreased toward the cambial zone, reaching lowest levels in the developing xylem cells.
The radial distributions of Fru and Glc across cambial region tissues were different from that of Suc. Both compounds were present in low levels in the phloem and increased toward the xylem, reaching their highest levels at 20 to 60 μg cm−2 section (1.2–3.8 μg μg−1 protein) in the zone of secondary wall formation. The water content in this zone was estimated to be 60%. A Glc or Fru content of 50-μg cm−2 section would therefore correspond to a concentration of 92 mm or 3.3% of the water content. The centripetal increase in concentration was initiated earlier for Fru than for Glc, resulting in a Fru to Glc ratio of approximately 2 in the cambial zone. The ratio decreased toward 1 in the zone of secondary wall formation. From this position and inwards, the level of Fru consistently decreased, whereas the level of Glc remained stable across the entire zone of maturing xylem. Both sugars were present in low levels in the mature xylem. Due to failure in MS analysis, data from August 19 is missing for one of the trees.
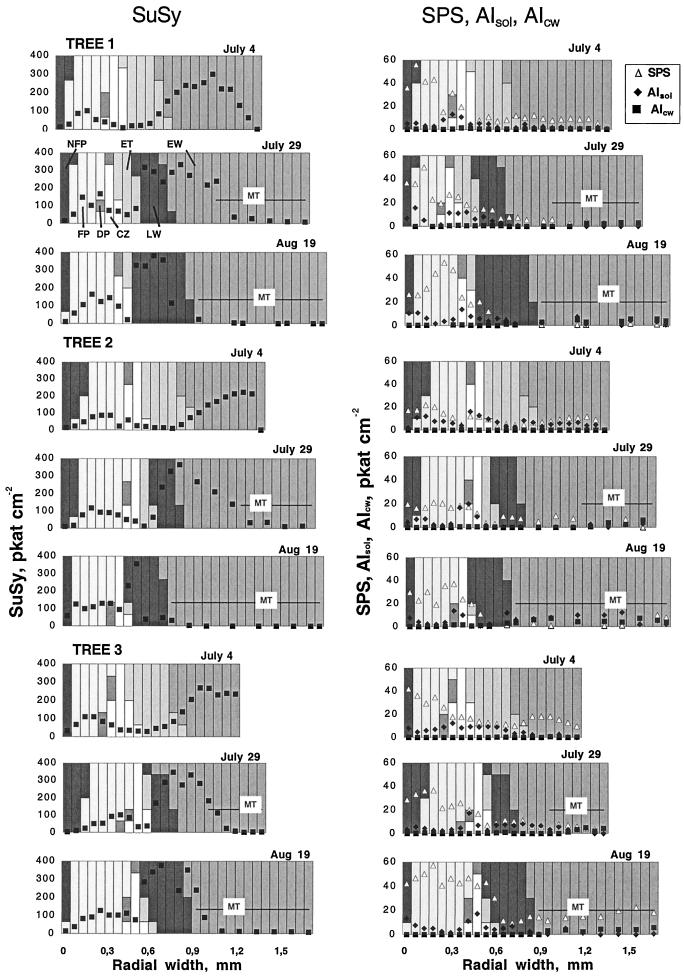
The extractable activity of the Suc metabolizing enzymes, SuSy, AI, and SPS, was also analyzed (Fig. 3). SuSy was found to be the dominating Suc cleaving enzyme with a consistent peak activity in the zone of secondary wall formation, reaching values of 200 to 400 pkat cm−2 section (12–25 pkat μg−1 protein). A tendency of increasing values during the earlywood to latewood transition was evident. Another peak of approximately 100 pkat cm−2 section (8.3 pkat μg−1 protein) was found in the phloem. The other Suc cleaving enzyme, AI, was present in two forms, denoted AIsol and AIcw. The AIsol activity was highest in the zones of cambial cell division and cell expansion, and, to a lesser degree, toward the point of transition between functional and non-functional phloem with activities approximately 10 to 20 pkat cm−2 section (0.7–1.3 pkat μg−1 protein) The AIcw activity was low (approximately 0.2 pkat cm−2 section) in all cambial region tissues. The activity increased to rates between 3 and 7 pkat cm−2 section only in mature xylem (lack of expression on a protein basis is due to non-detectable levels of total protein in this tissue).
Figure 3.
Radial distribution patterns of Suc-metabolizing enzyme activities across the cambial meristem and its differentiating and mature derivatives at different dates in three different trees. Each column represents two 30-μm-thick tangential sections and their relative composition of different tissues. For explanations, see Figure 2.
The strong dominance of SuSy in Suc cleaving activity in the phloem and the secondary wall forming xylem is apparent from the SuSy to AIsol activity ratios, which was between 20 and 400, and between 50 and 3,700, respectively. In the cambial and expansion zones, however, the SuSy to AIsol ratio was lower, indicating a more pronounced role for AIsol in these tissues. SPS, which is the key enzyme for Suc synthesis, showed considerable activity in all sampled tissues, although the highest values, between 20 and 50 pkat cm−2 section (1.7–4.2 pkat μg−1 protein), were restricted to the phloem.
The general appearance of the radial distribution pattern of carbohydrates and Suc-metabolizing enzymes across the cambial region, as described above, did not change in any typical trend during the sampling period except for an increase in the SuSy activity associated with latewood tracheids forming secondary walls. However, the amounts and peak values varied considerably between different trees and dates.
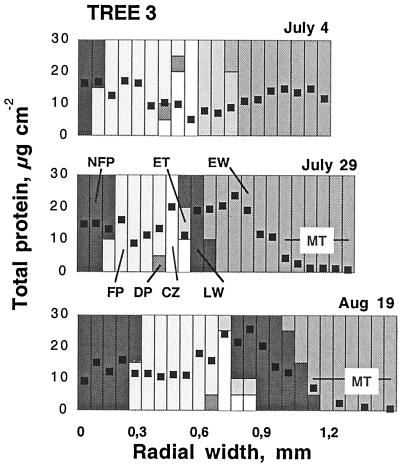
Protein
The radial distribution pattern of total protein across the cambial region was determined for one tree (Fig. 4). Protein content varied from 5 to 25 μg cm−2 section, except within the zone of mature tracheids, where it decreased to non-detectable levels. The protein data are used as an alternative basis for the expression of carbohydrate and enzyme activity. However, as the variation in protein content was relatively small between different tissues, this did not change the radial profiles of the enzyme activities very much. The one exception is for the estimates in samples from the late phase of secondary wall formation and mature tracheids where expression on a protein basis would increase the estimates.
Figure 4.
Radial distribution patterns of total protein content across the cambial meristem and its differentiating and mature derivatives at different dates. For explanations, see Figure 2.
DISCUSSION
The morphology of tracheary elements is determined by the rate and duration at which the developing cambial derivatives expand and form their secondary walls (Whitmore and Zahner, 1966; Wilson and Howard, 1968; Skene, 1969, 1972; Wodzicki, 1971; Denne, 1974; Dodd and Fox, 1990). The developmental phases of cell expansion and secondary wall formation are separated in space, giving rise to discrete developmental zones in a radial direction. The duration each derivative will remain in a particular developmental zone is governed by the radial width of the zone and the rate of cell production from the cambial meristem. Thus, the morphology of tracheary elements is a result of the combined effects of factors that: (a) determine developmental patterns, and (b) affect rates of cell division, cell expansion, and secondary wall formation. The formation of latewood, in particular, is a result of slower rates of cell division, decreased rates and duration of cell expansion, and a longer duration of secondary wall formation (Whitmore and Zahner, 1966; Wilson, 1966; Gregory, 1971; Wodzicki, 1971; Dodd and Fox, 1990).
The earlywood to latewood transition was not associated with a decreased supply of IAA to the cambial tissues (as reflected in total amount of IAA in the cambial region) (Figs. 2 and 5). This agrees with earlier studies of IAA contents in slow-growing cambia at the base of Scots pine trees during the same transitional phase (Sundberg et al., 1990, 1993). Although a decrease in the amount of IAA has been observed in the more rapidly-growing cambia in balsam fir during the earlywood to latewood transition (Sundberg et al., 1987), it must be concluded that latewood formation is not induced by a reduction in IAA supply. The detailed analysis in this study revealed that the earlywood to latewood transition was correlated with a reduction in the width of the radial distribution of IAA, and consequently, an increased peak concentration of IAA in the cambial meristem and its most recent derivatives. This demonstrates unequivocally that cessation of cambial cell division activity at the end of the growing season is not induced by a decreased IAA concentration in meri-stematic tissue. This is in accordance with the finding that IAA concentrations measured in dormant cambial zone cells at mid-winter were similar to concentrations in the dividing meristem at mid-summer (Uggla et al., 1996). It also agrees with the repeated finding of high IAA levels in cambial region tissues during dormancy (Little and Wareing, 1981; Savidge and Wareing, 1984; Sandberg and Ericsson, 1987; Sundberg et al., 1990; Savidge, 1991; Eklund et al., 1998). Furthermore, it is consistent with the observation that cessation of cambial activity cannot be inhibited by exogenous supply of IAA (Little and Bonga, 1974; Sundberg et al., 1987). The factor(s) inducing cessation of cell division activity in the cambial meristem as a consequence of shorter photoperiods therefore remains to be identified.
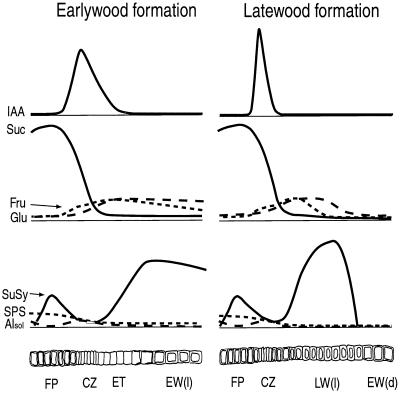
Figure 5.
Schematic drawing describing the generalized distribution patterns of IAA, carbohydrates, and Suc-metabolizing enzyme activities across the cambial meristem and its differentiating and mature derivatives at earlywood and latewood formation. FP, Functional phloem; CZ, cambial zone; ET, expanding tracheids; EW(l), maturing (living) earlywood tracheids; EW(d), mature (dead) earlywood tracheids; LW(l), maturing (living) latewood tracheids.
It can also be concluded that the decreased radial diameter of latewood tracheids is not due to a decreased IAA concentration in the expanding tracheids, ruling out a role for IAA in regulating the reduced rate of cell expansion observed to be involved in latewood formation (Dodd and Fox, 1990). However, our results show that the point along the IAA radial gradient where the concentration dropped steeply to low levels was closely related to the transition from the zone of expansion to the zone of secondary wall formation. This is consistent with previous findings in old stems of Scots pine (Uggla et al., 1996, 1998). Thus, we suggest that the developmental regulation of decreased width of the expansion zone is mediated by the decreased width of the IAA gradient. It is clear, however, that the altered appearance of IAA distribution is not a consequence of a decreased IAA supply but is caused by the yet unidentified mechanism(s) that determines the distance between the IAA peak in the cambial meristem and the margin of the concentration gradient.
The radial patterns of carbohydrates and of Suc metabolizing enzymes found in this study were related to tissue-specific processes (Figs. 2, 3, and 5). The Suc synthetic capacity (SPS activity) is predominantly confined to the functioning and non-functioning phloem. With Suc contents of up to 53% of dry matter, these tissues constitute an important source of carbon. Similar Suc contents (up to 66%) have been reported for source needles of gymnosperms (Blechschmidt-Schneider et al., 1997). As the compartmentation of Suc between axial translocation paths (sieve cells) and storage tissue (parenchyma) is not known, one can only speculate about the association between SPS-activities and Suc-pools and thus the possible occurrence of starch-Suc interconversions. The starch content in the living bark of Norway spruce and Scots pine (Sundberg et al., 1993; Egger et al., 1996) and outer sapwood of Scots pine (Höll, 1997) steadily decreases from the onset of cambial activity until late summer. This indicates that Suc in the radial translocation path (the rays) is partly derived from stored starch. However, unloading from the phloem is also likely and the dominant activities of SuSy may supply energy for unloading and loading in the phloem (Fu and Park, 1995).
Across the dividing and expanding tissues of the cambial region, the levels of Suc steeply decreased toward the xylem side, whereas the monomeric sugars gradually increased. This is associated with the peak activity of AIsol in accordance with observations of high activities of AIsol in a wide range of expanding tissues, such as young sink leaves (Morris and Arthur, 1984; Pate et al., 1985), elongating pods (Sung et al., 1994), expanding internodes, gravistimulated pulvini, fibrous roots, flower petals, and early stages of fruit expansion (Quick and Schaffer, 1996). Soluble AI has been demonstrated to be induced by exogenous IAA in a variety of tissues (Morris, 1996). Here, we demonstrate a close correlation between the presence of endogenous IAA and AIsol activity in the primary walled tissues, confirming recent observations reported from eggplant and melon (Lee et al., 1997a, 1997b, 1997c). However, the increased AIsol activity toward the non-functional phloem was not correlated with increased IAA content. Although our data support the view that IAA induces soluble AI activity, this induction seems to be differently regulated in different tissues.
SuSy activity was at its lowest level in the primary walled tissues and peaked in the zone of maturing tracheids where the secondary wall is formed. Here SuSy became the predominant enzyme and may be a measure of the sink strength of this tissue (Sung et al., 1993). The high SuSy activity in tracheids that are forming secondary walls may reflect its association with the synthesis of cell wall polysaccharides; SuSy has been suggested to be a part of the cellulose synthase complex (Amor et al., 1995). Moreover, it is very likely that SuSy is needed to sustain lignin biosynthesis (Hauch and Magel, 1998). This idea is supported by the high activities of SuSy, as expressed on a total protein basis, during the lignification process (data not shown) in the late phase of cell maturation when cellulose deposition is decreasing (Larson, 1969a). In the mature xylem, levels of soluble carbohydrates and enzyme activities were low. SuSy and AIsol activities were partly replaced by AIcw. This could be taken as evidence for apoplastic transport of Suc in rays of the mature xylem.
The only consistent variation in carbohydrates and activities of Suc metabolizing enzymes found during the earlywood/latewood transition was an increase in SuSy activity in tracheids undergoing secondary wall formation. But the impact of this increase on Suc turnover is probably minor. The lack of obvious seasonal changes in carbohydrate availability in our data is consistent with previous observations that latewood formation is under developmental, rather than metabolic, control. That is, it is the duration and not the rate of wall material deposition that causes the thicker cell walls of the latewood tracheids (Whitmore and Zahner, 1966; Skene, 1969, 1972; Wodzicki, 1971; Denne, 1974).
Although no variation in soluble sugars during earlywood/latewood transition was observed it is of interest to note that, similar to IAA, Suc, Glc, and Fru all exhibited steep concentration gradient across the developing vascular tissues. Much molecular and genetic evidence have recently emerged showing that soluble sugars are not only resources for energy and components for structures and storage but are also developmental regulators in plants, and cross talk between sugar and plant hormone signaling have been revealed (Sheen et al., 1999). A concentration gradient of Glc across developing cotyledons of Vicia faba was recently documented, and Glc was suggested to act as a morphogen in embryo development (Borisjuk et al., 1998). In callus and pith tissue a role for Suc as a developmental signal in the induction of xylem and phloem elements is well established (Wetmore and Rier, 1963; Jeffs and Northcote, 1966, 1967; Warren Wilson et al., 1994). When investigating the specificity of different sugars, Jeffs and Northcote (1967) found that Suc together with maltose and trehalose where the only sugars that induced both xylem and phloem elements. Glc and Fru induced xylem elements only, whereas other monosaccharides investigated had no effect. Moreover, ratios of Suc and auxin was observed to affect the direction of differentiation into phloem and xylem (Wetmore and Rier, 1963; Jeffs and Northcote, 1966), and opposing concentration gradients of Suc and auxin has been hypothesized to provide positional information for xylem and phloem development (Warren Wilson and Warren Wilson, 1984). Our data provide the first evidence for concentration gradients of soluble sugars across developing vascular tissues in plants, and with the accumulating evidence for sugars as developmental signals it seems likely that these gradients provides positional information for pattern formation in cambial growth.
MATERIALS AND METHODS
Plant Material
The plant material consisted of 44-year-old Scots pine (Pinus sylvestris) trees growing in northern Sweden (64° 21′ N, 19° 46′ E). Three trees were sampled at eight dates from early July to early September. Blocks (2 by 10 cm), consisting of extraxylary tissues and a few annual rings, were collected at breast height around the stem. The blocks were immediately frozen in liquid nitrogen, transported to the laboratory on dry ice, and stored at −80°C. Small samples (2 by 2 cm) were collected in parallel, and, after transverse sectioning with a razor-blade, were subjected to microscopic inspection to monitor the progression of latewood formation. On the basis of this assessment, samples from three dates, representative of different phases during the earlywood to latewood transition, were selected for further analysis. The dates were: July 4, during formation of earlywood tracheids; July 29, during the initial stage of latewood formation; and August 19, during the late stage of latewood formation (Fig. 1).
Sample Preparation and Anatomical Characterization
Analysis was performed on 30-μm-thick, longitudinal tangential sections from tissues of the cambial region. The sections were obtained by centripetal tangential cryosectioning of an approximate 3- by 15-mm specimen obtained from a 2- by 10-cm block. The sampling and anatomical characterization of the tissues in each section was performed in accordance to Uggla et al. (1996, 1998).
IAA and carbohydrates were measured in alternate sections from the same series of sections for the two first collection dates and in separate section series for the last date. Suc metabolic enzymes and total protein were measured in separate series of sections, and each sample consisted of two pooled sections. For total protein, only one tree per sampling date was analyzed.
The number of cells per radial cell file in the cambial zone, the zones of expansion and secondary wall formation, and the mature earlywood and latewood, were determined for each series of sections. The outer radial diameters of earlywood, mid-latewood, and the most recently formed latewood tracheids were measured for three tracheids in each of nine radial files on a transverse section from the latest sampling date. As the border between earlywood to latewood was determined before secondary wall thickening had been completed, the often-used Mork definition of latewood (Denne, 1988) could not be used. Therefore, latewood tracheids were distinguished by their relatively narrow radial diameters (Fig. 1).
Quantification of IAA
Endogenous IAA was quantified using an isotope dilution mass spectrometry technique, according to the method of Edlund et al. (1995). One to 6 ng [13C6]IAA (Cambridge Isotope Laboratories, Woburn, MA) was added to each sample as an internal standard. Analysis was performed by gas chromatography (GC)-selected reaction monitoring-mass spectrometry (MS), using a JMS-SX/SX102A instrument (JEOL, Tokyo).
Quantification of Carbohydrates
In the sections destined for carbohydrate analysis, Suc, Fru, and Glc were measured. Before extraction, each section was freeze-dried, weighed, and then placed in boiling water for 10 min in a sealed tube (Eppendorf Scientific, Westbury, NY) to inactivate endogenous hydrolytic enzyme activities. The sample was then homogenized in liquid nitrogen with a conical metal pestle connected to an electrical drill. Five-hundred microliters of distilled water, containing 20 μg phenyl-β-d-glucoside (Sigma, St. Louis) as an internal standard, was added to the Eppendorf tube, and the sample was extracted with continuous shaking for 1 h. After centrifugation, the supernatant was transferred to a test tube and evaporated to dryness. Prior to GC-MS analysis the sample was derivatized by incubation for 60 min at 70°C in 100 μL 3% (w/v) hydroxylamine (Sigma) in freshly distilled pyridine. It was then silylated with 25 μL N-methyl-N-trimethylsilyltrifluoroacetamide with 1% (w/v) trimethylchlorosilane (Pierce Chemical, Rockford, IL) for 15 min at 70°C, before dilution with heptane to approximately 10 mL. The derivatization procedure resulted in oxime-trimethylsilyl derivatives of Fru and Glc and the trimethylsilyl derivative of Suc.
Quantitative analysis was performed by GC-high resolution-selected ion monitoring-MS. The samples were injected split-less by an autosampler (model 7,673, Hewlett-Packard, Palo Alto, CA) into a gas chromatograph (model 5,890, Hewlett-Packard) equipped with a 25 m × 0.25 mm i.d.-fused silica capillary column with a chemically bound 0.25 μm CP-SIL-5 CB/MS stationary phase (Chrompack, Amsterdam). The injector temperature was 260°C, and the column temperature was held at 70°C for 2 min, then increased by 30°C min−1 to 160°C, and finally by 5°C min−1 to 230°C. The column effluent was introduced into the ion source of a JEOL, JMS-SX/SX102A tandem mass spectrometer (JEOL). The mass spectrometer was used in the single MS-mode. The interface and the ion source temperatures were 270°C. Ions were generated with 70 eV at an ionization current of 150 μA. High resolution-selected ion monitoring measurements at a resolution of 5,000 were performed using accelerating voltage switching from 10 kV. The dwell time was 50 ms, and m/z 205.115, m/z 217.116, m/z 307.1581, m/z 319.1581, and m/z 361.1687 values were recorded. Perfluorokerosene was used as a reference compound.
Calibration curves were obtained using 1.25 ng to 40 ng Fru, Glc, and Suc with 7.5 ng phenyl-b-d-glucoside as the internal standard. As syn- and anti-conformation resulted in double peaks for the analysis of Fru and Glc, both peaks were integrated for standard curves and samples. The identity of the quantified compounds were verified by full scan mass spectrometry. The absence of interfering compounds in the quantitative analysis was verified by comparing ratios between different fragments. All data were processed by the MS-MP7010D data system (JEOL).
Measurement of Enzyme Activities
In the freeze-dried sections destined for analysis of Suc-metabolizing enzymes, the activities of SPS, SuSy, and the two forms of AI, denoted soluble acid invertase (AIsol), and cell wall bound acid invertase (AIcw) were measured. After weighing on an ultramicro balance (Sartorius Ultramicro, Göttingen, Germany), each pair of sections, ranging between 0.2 and 1.5 mg dry weight, were transferred to Pyrex tubes (2.3 mm i.d.), containing 7 mg of Polyclar AT. The material was homogenized under liquid nitrogen cooling, using a high-speed dental drill (Meisinger 21/023H; Guttenberger et al., 1998). Crude extracts were prepared by the subsequent addition of 255 μL of extraction medium (0.1 m/0.3 m Tris/borate, pH 7.5, containing 1% [w/v] bovine serum albumin [BSA] and 14 mm β-mercaptoethanol), gentle mixing on ice for 20 min and centrifugation (8 min, 8,000g). AIcw was extracted by re-extracting the pellet with extraction medium supplemented with NaCl (100 μL, 1 m NaCl final concentration). Ten microliters of each of the crude extracts was assayed for enzyme activities using a microplate reader system (340 ATTC, SLT, Salzburg, Austria) according to Egger and Hampp (1993), modified for the specific characteristics of the pine enzymes. SPS activity was measured under non-limiting conditions (21.5 mm Fru-6-P, 7.7 mm UDP-Glc, 55 mm Glc-6-P, all final concentrations; pH 7.5; 65 μL total volume) via the formation of UDP. UDP was assayed as the consumption of NADH in the presence of pyruvate kinase and lactate dehydrogenase (0.36 mm NADH, 1 mm phosphoenolpyruvate, 1.2 mm MgCl2, 15.5 units of lactate dehydrogenase/mL, 8.6 units of pyruvate kinase/mL). The determination of SuSy activity was based on a two-step assay. First, UDP-Glc was formed by the enzyme in the specific step (total volume of 40 μL, pH 7.1, 250 mm HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid], 250 mm Suc, 6.25 mm UDP). Second, the product, UDP-Glc, was quantified via UDP-Glc dehydrogenase in the indicator step (300 mm Gly buffer, 14 mm NAD, 0.043 unit of UDP-Glc dehydrogenase/mL; pH 8.3; total volume of 70 μL). Activities of AIsol and AIcw were assayed by measuring the amount of Glc and Fru (150 mm HEPES, 1.5 mm MgSO4, 1.55 mm NADP, 4.07 mm ATP, 4.3 units of phospho-Glc-isomerase/mL, 2.2 units of Glc-6-P dehydrogenase/mL, 9.2 units of hexokinase/mL; pH 7.0; total volume of 70 μL) formed from Suc in the specific step (68 mm citrate/86 mm phosphate buffer, 220 mm Suc; pH 4.0; total volume of 45 μL). For all enzyme assay, blanks were run for each individual sample and thus for each tissue. In the blanks the specific substrate was omitted to assay unspecific background, such as endogenous metabolite pools of e.g. UDP-glucose, pyridine nucleotides, or tissue endogenous enzyme activities.
Measurement of Protein
Protein was determined by a dot-blot assay combining high sensitivity with minimal interference by extract constituents (Guttenberger et al., 1991). The assay is based on the binding of protein (2 μL of aliquots of crude extracts prepared using extraction medium without BSA) to a cellulose acetate membrane. After staining (with the fluorescent dye, benzoxanthene yellow) and washing procedures the spots were eluted and the protein was fluorometrically quantified against BSA standards.
Footnotes
This work was supported by the Swedish Council for Forestry and Agricultural Sciences, by the Swedish Natural Sciences Research Council, and by Deutschen Akademischen Austausch Dienst.
LITERATURE CITED
- Amor Y, Haigler CH, Wainscott M, Delmer DP. A membrane-associated form of sucrose synthase and its potential role in synthesis of cellulose and callose in plants. Proc Natl Acad Sci USA. 1995;92:9353–9357. doi: 10.1073/pnas.92.20.9353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blechschmidt-Schneider S, Eschrich W, Jahnke S. Phloem loading, translocation and unloading processes. In: Rennenberg H, Eschrich W, Ziegler H, editors. Trees-Contributions to Modern Tree Physiology. Leiden, The Netherlands: Backhuys Publishers; 1997. pp. 139–163. [Google Scholar]
- Borisjuk L, Walenta S, Weber H, Mueller-Klieser W, Wobus U. High-resolution histographical mapping of glucose concentrations in developing cotelydons of Vicia faba in relation to mitotic activity and storage processes: glucose as a possible developmental trigger. Plant J. 1998;15:583–591. [Google Scholar]
- Denne MP. Effects of light intensity on tracheid dimensions in Picea sitchensis. Ann Bot. 1974;38:337–345. [Google Scholar]
- Denne MP. Definition of latewood according to Mork (1928) IAWA Bull New Ser. 1988;10:59–62. [Google Scholar]
- Dodd RS, Fox P. Kinetics of tracheid differentiation in Douglas-fir. Ann Bot. 1990;65:649–657. [Google Scholar]
- Edlund A, Eklöf S, Sundberg B, Moritz T, Sandberg G. A microscale technique for gas chromatography-mass spectrometry measurements of picogram amounts of indole-3-acetic acid in plant tissues. Plant Physiol. 1995;108:1043–1047. doi: 10.1104/pp.108.3.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger B, Einig W, Schlereth A, Wallenda T, Magel E, Loewe A, Hampp R. Carbohydrate metabolism in one- and two-year-old spruce needles, and stem carbohydrates from three months before until three months after bud break. Physiol Plant. 1996;96:91–100. [Google Scholar]
- Egger B, Hampp R. Invertase, sucrose synthase and sucrose phosphate synthase in lyophilized spruce needles: microplate reader assays. Trees. 1993;7:98–103. [Google Scholar]
- Eklund L, Little CHA, Riding RT. Concentrations of oxygen and indole-3-acetic acid in the cambial region during latewood formation and dormancy development in Picea abies stems. J Exp Bot. 1998;49:205–211. [Google Scholar]
- Fu H, Park WD. Sink- and vascular-associated sucrose synthase functions are encoded by different gene classes in potato. Plant Cell. 1995;7:1369–1385. doi: 10.1105/tpc.7.9.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JC, Larson PR. Seasonal course of photosynthesis, respiration, and distribution of 14C in young Pinus resinosa trees as related to wood formation. Plant Physiol. 1968;43:1617–1624. doi: 10.1104/pp.43.10.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory RA. Cambial activity in Alaskan white spruce. Am J Bot. 1971;58:160–171. [Google Scholar]
- Guttenberger M, Neuhoff V, Hampp R. A dot-blot assay for quantitation of nanogram amounts of protein in the presence of carrier ampholytes and other possibly interfering substances. Anal Biochem. 1991;196:99–103. doi: 10.1016/0003-2697(91)90124-c. [DOI] [PubMed] [Google Scholar]
- Guttenberger M, Zick H, Thelen H, Wallenda T, Hampp R. The effect of acid irrigation on enzyme activities of the single partners of ectomycorrhizas from a limed stand of Norway spruce (Picea abies [L.] Karst.) Plant Soil. 1998;199:71–81. [Google Scholar]
- Hauch S, Magel E. Extractable activities and protein content of sucrose phosphate synthase, sucrose synthase and neutral invertase in trunk tissues of Robinia pseudoacacia L. are related to cambial wood production and heartwood formation. Planta. 1998;207:266–274. [Google Scholar]
- Höll W. Storage and mobilization of carbohydrates and lipids. In: Rennenberg H, Eschrich W, Ziegler H, editors. Trees-Contributions to Modern Tree Physiology. Leiden, The Netherlands: Backhuys Publishers; 1997. pp. 197–211. [Google Scholar]
- Jeffs RA, Northcote DH. Experimental induction of vascular tissue in an undifferentiated plant callus. Biochem J. 1966;101:146–152. doi: 10.1042/bj1010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffs RA, Northcote DH. The influence of indol-3yl acetic acid and sugar on the pattern of induced differentiation in plant tissue culture. J Cell Sci. 1967;2:77–88. doi: 10.1242/jcs.2.1.77. [DOI] [PubMed] [Google Scholar]
- Larson PR. A physiological consideration of the springwood summerwood transition in red pine. For Sci. 1960;6:110–122. [Google Scholar]
- Larson PR. Contribution of different-aged needles to growth and wood formation of young red pines. For Sci. 1964;10:224–238. [Google Scholar]
- Larson PR. Incorporation of 14C in the developing walls of Pinus resinosa tracheids (earlywood and latewood) Holzforschung. 1969a;23:17–26. [Google Scholar]
- Larson PR. Wood formation and the concept of wood quality. Yale Univ Sch For Bull. 1969b;74:1–54. [Google Scholar]
- Lee T-H, Kato T, Kanayama Y, Ohno H, Takeno K, Yamaki S. The role of indole-3-acetic acid and acid invertase in the development of melon (Cucumis melon L. cv. Prince) fruit. J Jap Soc Hort Sci. 1997a;65:723–729. [Google Scholar]
- Lee T-H, Sugiyma A, Ofosu-Anim J, Takeno K, Ohno H, Yamaki S. Activation of sucrose-metabolizing enzymes and stimulation of sucrose uptake by auxin and sucrose in eggplant (Solanum melongena L.) J Plant Physiol. 1997b;150:297–301. [Google Scholar]
- Lee T-H, Sugiyma A, Takeno K, Ohno H, Yamaki S. Changes in content of indole-3-acetic acid and in activities of sucrose-metabolizing enzymes during fruit growth in eggplant (Solanum melongena L.) J Plant Physiol. 1997c;150:292–296. [Google Scholar]
- Little CHA, Bonga JM. Rest in the cambium of Abies balsamea. Can J Bot. 1974;52:1723–1730. [Google Scholar]
- Little CHA, Pharis RP. Hormonal control of radial and longitudinal growth in the tree stem. In: Gartner BL, editor. Plant Stems: Physiology and Functional Morphology. San Diego: Academic Press; 1995. pp. 281–319. [Google Scholar]
- Little CHA, Wareing PF. Control of cambial activity and dormancy in Picea sitchensis by indol-3-ylacetic and abscisic acids. Can J Bot. 1981;59:1480–1493. [Google Scholar]
- Morris DA. Hormonal regulation of sink-source relationships: an overview of potential mechanisms. In: Zamski E, Schaffer AA, editors. Photoassimilate Distribution in Plants and Crops. New York: Marcel Dekker Inc; 1996. pp. 441–465. [Google Scholar]
- Morris DA, Arthur ED. An association between acid invertase activity and cell growth during leaf epansion in Phaseolus vulgaris L. J Exp Bot. 1984;35:1369–1379. [Google Scholar]
- Parkerson RH, Whitmore FW. A correlation of stem sugars, starch, and lipid with wood formation in eastern white pine. For Sci. 1972;18:178–183. [Google Scholar]
- Pate JS, Peoples MB, van Bel AJE, Kuo J, Atkins CA. Diurnal water balance of the cowpea fruit. Plant Physiol. 1985;77:148–156. doi: 10.1104/pp.77.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick WP, Schaffer AA. Sucrose metabolism in sources and sinks. In: Zamski E, Schaffer AA, editors. Photoassimilate Distribution in Plants and Crops. New York: Marcel Dekker; 1996. pp. 115–156. [Google Scholar]
- Richardson SD, Dinwoodie JM. Studies on the physiology of xylem development. J Inst Wood Sci. 1960;6:3–13. [Google Scholar]
- Sandberg G, Ericsson A. Indole-3-acetic acid concentration in the leading shoot and living stem bark of Scots pine: seasonal variation and effects of pruning. Tree Physiol. 1987;3:173–183. doi: 10.1093/treephys/3.2.173. [DOI] [PubMed] [Google Scholar]
- Savidge RA. Seasonal cambial activity in Larix laricina saplings in relation to endogenous indol-3-ylacetic acid, sucrose, and coniferin. For Sci. 1991;37:953–958. [Google Scholar]
- Savidge RA, Heald JK, Wareing PF. Non-uniform distribution and seasonal variation of endogenous indol-3yl-acetic acid in the cambial region of Pinus contorta Dougl. Planta. 1982;155:89–92. doi: 10.1007/BF00402937. [DOI] [PubMed] [Google Scholar]
- Savidge RA, Wareing PF. Seasonal cambial activity and xylem development in Pinus contorta in relation to endogenous indol-3-yl-acetic and (S)-abscisic acid levels. Can J For Res. 1984;14:676–682. [Google Scholar]
- Sheen J, Zhou L, Jang J-C. Sugars as signaling molecules. Curr Opin Plant Biol. 1999;2:410–418. doi: 10.1016/s1369-5266(99)00014-x. [DOI] [PubMed] [Google Scholar]
- Skene DS. The period of time taken by cambial derivatives to grow and differentiate into tracheids in Pinus radiata D. Don. Ann Bot. 1969;33:253–262. [Google Scholar]
- Skene DS. The kinetics of tracheid development in Tsuga canadensis Carr. and its relation to tree vigour. Ann Bot. 1972;36:179–187. [Google Scholar]
- Sundberg B, Ericsson A, Little CHA, Näsholm T, Gref R. The relationship between crown size and ring width in Pinus sylvestris L. stems: dependence on indole-3-acetic acid, carbohydrates and nitrogen in the cambial region. Tree Physiol. 1993;12:347–362. doi: 10.1093/treephys/12.4.347. [DOI] [PubMed] [Google Scholar]
- Sundberg B, Little CHA, Cui K. Distribution of indole-3-acetic acid and the occurrence of its alkali-labile conjugates in the extraxylary region of Pinus sylvestris stems. Plant Physiol. 1990;93:1295–1302. doi: 10.1104/pp.93.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg B, Little CHA, Riding RT, Sandberg G. Levels of endogenous indole-3-acetic acid in the vascular cambium region of Abies balsamea trees during the activity-rest-quiescence transition. Physiol Plant. 1987;71:163–170. [Google Scholar]
- Sundberg B, Uggla C, Tuominen H. Cambial growth and auxin gradients. In: Savidge R, Barnett J, Napier R, editors. Cell and Molecular Biology of Wood Formation. Oxford: BIOS Scientific Publishers; 2000. pp. 169–188. [Google Scholar]
- Sung SS, Kormanik PP, Black CC. Vascular cambium sucrose metabolism and growth in loblolly pine (Pinus taeda L.) in relation to transplanting stress. Tree Physiol. 1993;12:243–258. doi: 10.1093/treephys/12.3.243. [DOI] [PubMed] [Google Scholar]
- Sung SS, Sheih WJ, Geiger DR, Black CC. Growth, sucrose synthase, and invertase activities of developing Phaseolus vulgaris L. fruits. Plant Cell Environ. 1994;17:419–426. [Google Scholar]
- Tuominen H, Puech L, Fink S, Sundberg B. A radial gradient of indole-3-acetic acid is related to secondary xylem development in hybrid aspen. Plant Physiol. 1997;115:577–585. doi: 10.1104/pp.115.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uggla C, Mellerowicz EJ, Sundberg B. Indole-3-acetic acid controls cambial growth in Pinus sylvestris (L.) by positional signaling. Plant Physiol. 1998;117:113–121. doi: 10.1104/pp.117.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uggla C, Moritz T, Sandberg G, Sundberg B. Auxin as a positional signal in pattern formation in plants. Proc Natl Acad Sci USA. 1996;93:9282–9286. doi: 10.1073/pnas.93.17.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren Wilson J, Roberts LW, Warren Wilson PM, Gresshoff PM. Stimulatory and inhibitory effects of sucrose concentration on xylogenesis in lettuce pith explants: possible mediation by ethylene biosynthesis. Ann Bot. 1994;73:65–73. [Google Scholar]
- Warren Wilson J, Warren Wilson PM. Control of tissue patterns in normal development and in regeneration. In: Barlow P, Carr D, editors. Positional Controls in Plant Development. Cambridge: Cambridge University Press; 1984. pp. 225–280. [Google Scholar]
- Wetmore RH, Rier JP. Experimental induction of vascular tissues in callus of angiosperms. Am J Bot. 1963;50:418–430. [Google Scholar]
- Whitmore FW, Zahner R. Development of the xylem ring in stems of young red pine trees. For Sci. 1966;12:198–210. [Google Scholar]
- Wilson BF. Mitotic activity in the cambial zone of Pinus strobus. Am J Bot. 1966;53:364–372. [Google Scholar]
- Wilson BF, Howard RA. A computer model for cambial activity. For Sci. 1968;14:77–90. [Google Scholar]
- Wodzicki TJ. Mechanisms of xylem differentiation in Pinus silvestris L. J Exp Bot. 1971;22:670–687. [Google Scholar]