Abstract
Humoral responses to COVID-19 vaccines in people living with HIV (PLWH) remain incompletely characterized. We measured circulating antibodies against the SARS-CoV-2 spike protein receptor-binding domain (RBD), ACE2 displacement and viral neutralization activities one month following the first and second COVID-19 vaccine doses, and again 3 months following the second dose, in 100 adult PLWH and 152 controls. All PLWH were receiving suppressive antiretroviral therapy, with median CD4+ T-cell counts of 710 (IQR 525–935) cells/mm3, though nadir CD4+ T-cell counts ranged as low as <10 cells/mm3. After adjustment for sociodemographic, health and vaccine-related variables, HIV infection was associated with lower anti-RBD antibody concentrations and ACE2 displacement activity after one vaccine dose. Following two doses however, HIV was not significantly associated with the magnitude of any humoral response after multivariable adjustment. Rather, older age, a higher burden of chronic health conditions, and dual ChAdOx1 vaccination were associated with lower responses after two vaccine doses. No significant correlation was observed between recent or nadir CD4+ T-cell counts and responses to two vaccine doses in PLWH. These results indicate that PLWH with well-controlled viral loads and CD4+ T-cell counts in a healthy range generally mount strong initial humoral responses to dual COVID-19 vaccination. Factors including age, co-morbidities, vaccine brand, response durability and the rise of new SARS-CoV-2 variants will influence when PLWH will benefit from additional doses. Further studies of PLWH who are not receiving antiretroviral treatment or who have low CD4+ T-cell counts are needed, as are longer-term assessments of response durability.
Subject terms: HIV infections, Outcomes research, Vaccines
Introduction
COVID-19 vaccination is expected to benefit people living with HIV (PLWH)1, since they may be at increased risk for severe COVID-19 as a result of immunosuppression, higher rates of multi-morbidity or social determinants of health2–5. Our understanding of immune responses to COVID-19 immunization in PLWH however remains incomplete, in part because relatively few PLWH were included in the clinical trials for the COVID-19 vaccines that have now been widely administered in Canada and Europe (~196 for the BNT162b2 mRNA vaccine6,7, 176 for the mRNA-1273 mRNA vaccine8 and 54 and 103 PLWH respectively in the UK and South Africa for the ChAdOx1 viral vector vaccine9). Furthermore, immune response data from PLWH in these trials are currently only available for ChAdOx110,11. Real-world COVID-19 vaccine immune response data from PLWH are also limited. While all three of these vaccines have shown effectiveness following their initial mass rollouts12–14, and while clinical trial and observational data have shown robust vaccine-induced humoral immune responses in the general population15–17, impaired responses have been reported in certain immunocompromised groups including solid organ transplant recipients18,19, cancer patients20–22, and individuals on immunosuppressive or immune-depleting therapies23–25.
While antiretroviral therapy durably suppresses HIV to undetectable levels in plasma, restores CD4+ T-cell numbers, and can reverse HIV-induced immune dysfunction to a substantial extent26–29, persistent immunopathology can nevertheless lead to blunting of immune responses to vaccination in PLWH30–32. COVID-19 vaccine immunogenicity data in PLWH are emerging33–38, but many of these studies have featured limited numbers of PLWH and/or controls, and few have adjusted for chronic health conditions that may also impair immune responses39 (Levy et al.33 is an exception). Here, we longitudinally characterize SARS-CoV-2-specific humoral immune responses after COVID-19 immunization in 100 PLWH and 152 control participants ranging from 22 to 88 years of age, with samples analyzed one month after the first and second vaccine doses, and again 3 months after the second dose.
Results
Cohort characteristics and COVID-19 vaccine rollout in British Columbia, Canada
Characteristics of the 100 PLWH and 152 controls are shown in Table 1. All PLWH were receiving antiretroviral therapy; the most recent plasma viral load, measured a median of 32 (Interquartile range [IQR] 7–54) days before enrolment, was <50 copies HIV RNA/mL for 95 PLWH, and between 71 and 162 copies/mL for the remaining five PLWH, though prior values were <50 copies/mL in all five of these cases. The most recent CD4+ T-cell count, measured a median of 44 (Interquartile range [IQR] 18–136) days before enrolment, was 710 (IQR 525–935; range 130–1800) cells/mm3. The estimated nadir CD4+ T-cell count, recorded a median of 8 (IQR 3.4–15) years before enrolment, was 280 (IQR 120–490; range <10–1010) cells/mm3.
Table 1.
Participant characteristics.
| Characteristic | PLWH (n = 100) | Controls (n = 152) |
|---|---|---|
| HIV-related variables | ||
| Receiving antiretroviral therapy, n (%) | 100 (100%) | – |
| Most recent plasma viral load, copies HIV RNA/mL, median [IQR] | <50 [<50–<50] | – |
| Most recent CD4+ T-cell count in cells/mm3, median [IQR] | 710 [525–935] | – |
| Nadir CD4+ T-cell count in cells/mm3, median [IQR] | 280 [120–490] | – |
| Sociodemographic and health variables | ||
| Age in years, median [IQR] | 54 [40–61] | 47 [35–70] |
| Male sex at birth, n (%) | 88 (88%) | 50 (33%) |
| Ethnicity, n (%) | ||
| White/Caucasian | 69 (69%) | 78 (51%) |
| Black | 5 (5%) | 1 (0.7%) |
| Asian | 10 (10%) | 59 (38%) |
| Latin American | 8 (8%) | 4 (2.6%) |
| Middle Eastern/Arab | 3 (3%) | 0 (0%) |
| Mixed ethnicity | 4 (4%) | 8 (5.3%) |
| Not disclosed | 1 (1%) | 2 (1.3%) |
| COVID-19 convalescent (anti-N Ab+) at entry, n (%) | 8 (8%) | 15 (10%) |
| Number of chronic health conditions, median [IQR] | 0 [0–1] | 0 [0–1] |
| Hypertension, n (%) | 15 (15%) | 22 (14.5%) |
| Diabetes, n (%) | 6 (6%) | 6 (3.9%) |
| Asthma, n (%) | 8 (8%) | 15 (9.9%) |
| Obesity, n (%) | 15 (15%) | 14 (9.2%) |
| Chronic lung disease, n (%) | 4 (4%) | 3 (2%) |
| Chronic liver disease, n (%) | 4 (4%) | 1 (0.7%) |
| Chronic kidney disease, n (%) | 1 (1%) | 1 (0.7%) |
| Chronic heart disease, n (%) | 1 (1%) | 4 (2.6%) |
| Chronic blood disease, n (%) | 1 (1%) | 2 (1.3%) |
| Cancer, n (%) | 5 (5%) | 4 (2.6%) |
| Immunosuppression, n (%) | 3 (3%) | 0 (0%) |
| At least one of the above, n (%) | 46 (46%) | 50 (33%) |
| Vaccine-related variables | ||
| mRNA vaccine for first dose, n (%) | 83 (83%) | 148 (97%) |
| First dose type | ||
| BNT162b2, n (%) | 60 (60%) | 133 (87.5%) |
| mRNA-1273, n (%) | 23 (23%) | 15 (10%) |
| ChAdOx1, n (%) | 17 (17%) | 4 (2.6%) |
| mRNA vaccine for second dose, n (%) | 91 (91%) | 151 (99.3%) |
| Complete vaccine regimen details | ||
| mRNA–mRNA | 83 (83%) | 148 (97%) |
| ChAdOx1–mRNA (heterologous) | 8 (8%) | 3 (2%) |
| ChAdOx1–ChAdOx1 | 8 (8%) | 1 (0.7%) |
| ChAdOx1–not disclosed | 1 (1%) | – |
| Time between doses in days, median [IQR] | 58 [53–68] | 89 [65–98] |
| Specimen-related variables | ||
| Pre-vaccine specimen, n (%) | 66 (66%) | 148 (97%) |
| Specimen one month after first dose, n (%) | 98 (98%) | 149 (98%) |
| Day of collection one month after first dose, median [IQR] | 30 [29–32] | 30 [28–32] |
| Specimen one month after second dose, n (%) | 96 (96%) | 151 (99%) |
| Day of collection one month after second dose, median [IQR] | 30 [29–30] | 30 [29–32] |
| Specimen three months after second dose, n (%) | 92 (92%) | 148 (97%) |
| Day of collection three months after second dose, median [IQR] | 90 [90–92] | 90 [89–91] |
PLWH and controls were similar in terms of age, but were different in terms of sex and ethnicity, with the PLWH group including more males and white ethnicity (Table 1). PLWH and controls had similar burdens of chronic health conditions (median 0; IQR 0–1; range 0–3 conditions in both groups), where 46% and 33% of PLWH and controls, respectively, had at least one condition. The most commonly reported conditions were hypertension, obesity, and asthma. There is some evidence that all three of these conditions can negatively affect immune responses to vaccination against other pathogens40–44 as well as COVID-1945–47, where high-dose corticosteroid treatments used to treat severe asthma could also blunt vaccine responses48. At study entry, 8% of PLWH and 10% of controls were identified as COVID-19 convalescent based on the presence of anti-N antibodies. An additional one (1%) PLWH and four (2.6%) controls developed anti-N antibodies during follow-up consistent with SARS-CoV-2 infection after vaccination. These participants were retained in the “COVID-19 naive at study entry” group, as excluding them did not affect overall results.
All participants received two COVID-19 vaccine doses between December 2020 and August 2021, with 97% of controls receiving an mRNA vaccine for their first dose compared to 83% of PLWH (Table 1). This is because health care workers, who represent 59% of controls, were eligible for vaccination before ChAdOx1 was approved in Canada, while members of the public, including PLWH, received the vaccine(s) recommended for their age group during the mass rollout. More PLWH received heterologous (ChAdOx1/mRNA) regimens compared to controls (8% and 2%, respectively). Heterologous regimens were administered in Canada after mRNA vaccines were universally recommended as second doses49 due to reports of rare thrombotic events associated with the ChAdOx1 vaccine50. The between-dose interval was also longer for controls (median 89 days, versus 58 for PLWH). This is because the province of British Columbia extended the dose interval to 112 days beginning on March 1, 2021 due to limited vaccine supply51. As a result, health care workers who were vaccinated around that time waited the longest for their second doses, while those vaccinated at a later date waited a shorter time between doses, as supply increased. Samples were collected prior to vaccination where possible (66% of PLWH and 97% of controls), one month after the first vaccine dose (98% of both PLWH and controls), one month after the second dose (96% of PLWH and 99% of controls) and again 3 months after the second dose (92% of PLWH and 97% of controls).
Anti-RBD binding antibody responses after first and second vaccine doses
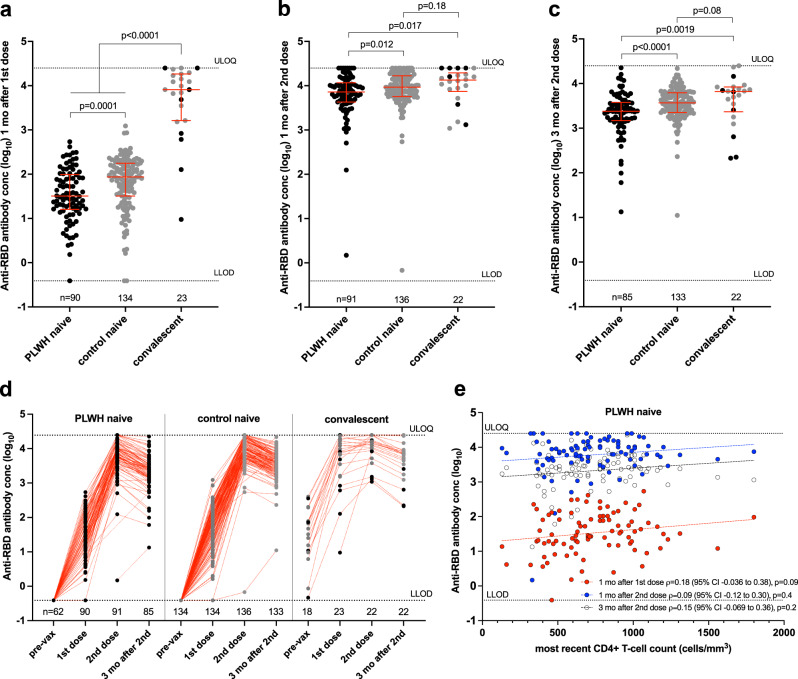
Among participants naive to COVID-19 at study entry, all but three (one PLWH and two controls) developed anti-RBD antibodies after one vaccine dose, though overall concentrations in PLWH (median 1.51 [IQR 1.20–1.99] log10 U/mL) were on average ~0.4 log10 lower than controls (median 1.94 [IQR 1.51–2.25] log10 U/mL; Mann–Whitney p = 0.0001) (Fig. 1a). In contrast, and consistent with studies demonstrating robust immune responses after one vaccine dose in individuals with prior COVID-1952,53, anti-RBD antibody concentrations in COVID-19 convalescent participants (median 3.91 [IQR 3.21–4.26] log10 U/mL) were >2 log10 higher than in the COVID-19 naive PLWH or control participants (both p < 0.0001). In the univariable analyses presented here, convalescent participants were grouped together as there is insufficient power to further stratify by PLWH versus control subgroups, though responses were slightly lower among the former; Mann–Whitney p = 0.17). In multivariable analyses controlling for sociodemographic, health and vaccine-related variables, the strongest independent predictors of lower antibody responses after one dose were older age (every decade of age was associated with an adjusted ~0.1 log10 lower response; p = 0.0002), and a higher number of chronic health conditions (every additional condition associated with an adjusted 0.14 log10 lower response; p = 0.0053) (Table 2). HIV infection was also associated with an adjusted 0.2 log10 lower antibody response after one vaccine dose (p = 0.034). Prior COVID-19 was associated with an adjusted 1.88 log10 higher response after one dose (p < 0.0001).
Fig. 1. Binding antibody responses to spike RBD following one and two COVID-19 vaccine doses.
a Binding antibody responses to the SARS-CoV-2 spike RBD in serum one month following the first COVID-19 vaccine dose in PLWH (black circles) and controls (grey circles) who were COVID-19 naive at study entry. Convalescent participants, denoting those with anti-N antibodies at study entry, are presented as a single group but colored by subgroup as above. The numbers of participants analyzed are indicated at the bottom of the plot. Red bars and whiskers represent the median and IQR. p-values were computed using the Mann–Whitney U-test and are uncorrected for multiple comparisons. U-statistics were 4238 (PLWH naive vs. control naive), 93 (PLWH naive vs. convalescent), and 173 (control naive vs. convalescent). LLOD lower limit of detection, ULOQ upper limit of quantification. b Binding antibody responses one month after the second dose, presented as in (a). U-statistics were 4976 (PLWH naive vs. control naive), 675 (PLWH naive vs. convalescent) and 1226 (control naive vs. convalescent). c Binding antibody responses three months after the second dose, presented as in (a). U-statistics were 3841 (PLWH naive vs. control naive), 539 (PLWH naive vs. convalescent) and 1123 (control naive vs. convalescent). d Binding antibody responses plotted longitudinally for each group, beginning with the pre-vaccine time point. Red dotted lines connect participants’ longitudinal measurements. The numbers of participants analyzed are indicated at the bottom of the plot. e Correlation between most recent CD4+ T-cell count and binding antibody responses one month after the first dose (red circles; n = 90), one month after the second dose (blue circles; n = 91) and three months following the second dose (clear circles; n = 85). Matching-coloured dotted lines help visualize the trend.
Table 2.
Multivariable analyses of the relationship between sociodemographic, health and vaccine-related variables on immunogenicity measures after first and second COVID-19 vaccine doses.
| Outcome | Variable | 1 mo after 1st: Est (95% CI), pa | 1 mo after 2nd: Est (95% CI), pa | 3 mo after 2nd: Est (95% CI), pa |
|---|---|---|---|---|
| Anti-RBD Abs (log10) | HIV | −0.20 (−0.38 to −0.015); p = 0.034 | −0.023 (−0.18 to 0.14); p = 0.78 | −0.13 (−0.28 to 0.011); p = 0.07 |
| Age (per decade increment) | −0.094 (−0.14 to −0.046); p = 0.0002 | −0.057 (−0.097 to −0.017); p = 0.0055 | −0.035 (−0.072 to 0.00097); p = 0.056 | |
| Male sex | −0.13 (−0.31 to 0.045); p = 0.14 | −0.018 (−0.16 to 0.12); p = 0.80 | 0.051 (−0.079 to 0.18); p = 0.44 | |
| White ethnicity | −0.12 (−0.28 to 0.037); p = 0.13 | 0.059 (−0.070 to 0.19; p = 0.37 | 0.062 (−0.055 to 0.18); p = 0.30 | |
| Chronic cond. (per # incr) | −0.14 (−0.24 to −0.043); p = 0.0053 | −0.11 (−0.19 to −0.034); p = 0.0053 | −0.098 (−0.17 to −0.024); p = 0.01 | |
| ChAdOx1 as first vaccine | −0.24 (−0.51 to 0.031); p = 0.083 | – | – | |
| Dual ChAdOx1 regimen | – | −0.63 (−0.97 to −0.29); p = 0.0003 | −0.70 (−1.0 to −0.40); p < 0.0001 | |
| Dose interval (per week incr) | – | 0.025 (0.0022 to 0.047); p = 0.031 | 0.028 (0.0074 to 0.048); p = 0.008 | |
| Days since vaccine | 0.023 (−0.0011 to 0.047); p = 0.061 | −0.0022 (−0.024 to 0.020); p = 0.84 | 0.0026 (−0.014 to 0.019); p = 0.75 | |
| COVID-19 convalescent | 1.88 (1.63 to 2.13); p < 0.0001 | 0.071 (−0.14 to 0.28); p = 0.50 | 0.10 (−0.082 to 0.29); p = 0.27 | |
| ACE2 displ. (%)b | HIV | −10.95 (−20.35 to −1.56); p = 0.023 | 0.64 (−5.274 to 6.547); p = 0.83 | −6.05 (−16.08 to 3.98); p = 0.24 |
| Age (per decade increment) | −1.47 (−3.14 to 0.41); p = 0.13 | −1.62 (−2.72 to −0.52); p = 0.0042 | −2.32 (−4.24 to −0.41); p = 0.018 | |
| Male sex | −6.94 (−13.25 to −0.62); p = 0.031 | −2.17 (−6.09 to 1.77); p = 0.28 | −0.81 (−7.71 to 6.09); p = 0.82 | |
| White Ethnicity | −5.46 (−10.95 to 0.031); p = 0.051 | 1.181 (−2.28 to 4.65); p = 0.50 | 1.50 (−4.51 to 7.51); p = 0.62 | |
| Chronic cond. (per # incr) | −0.85 (−4.29 to 2.58); p = 0.63 | −2.71 (−4.85 to −0.58); p = 0.013 | −2.51 (−6.27 to 1.24); p = 0.19 | |
| ChAdOx1 as first vaccine | −18.77 (−28.34 to −9.21); p = 0.0001 | – | – | |
| Dual ChAdOx1 regimen | – | −29.48 (−38.50 to −20.47); p < 0.0001 | −33.5 (−48.59 to −18.41); p < 0.0001 | |
| Dose interval (per week incr) | – | −0.24 (−0.92 to 0.43); p = 0.48 | −0.89 (−2.03 to 0.25); p = 0.12 | |
| Days since vaccine | 0.52 (−0.32 to 1.37); p = 0.22 | −0.12 (−0.70 to 0.47); p = 0.70 | −0.41 (−1.28 to 0.45); p = 0.35 | |
| EDTA as anticoagulant | 6.25 (−3.74 to 16.23); p = 0.22 | 1.17 (−5.57 to 7.90); p = 0.73 | 11.88 (0.50 to 23.25); p = 0.041 | |
| COVID-19 convalescent | 36.37 (27.68 to 45.05); p < 0.0001 | 2.84 (−2.75 to 8.44); p = 0.32 | 9.35 (−0.048 to 18.76); p = 0.051 | |
| Viral neut (log2)b,c | HIV | −0.28 (−0.62 to 0.056); p = 0.10 | 0.17 (−0.51 to 0.84); p = 0.63 | −0.2 (−0.88 to 0.49); p = 0.58 |
| Age (per decade increment) | −0.047 (−0.11 to 0.017); p = 0.15 | −0.18 (−0.31 to −0.054); p = 0.0055 | −0.13 (−0.26 to 0.0043); p = 0.058 | |
| Male sex | −0.1 (−0.33 to 0.12); p = 0.38 | −0.37 (−0.82 to 0.077); p = 0.10 | 0.062 (−0.41 to 0.54); p = 0.80 | |
| White ethnicity | 0.057 (−0.14 to 0.25); p = 0.57 | −0.16 (−0.56 to 0.24); p = 0.42 | −0.032 (−0.45 to 0.38); p = 0.88 | |
| Chronic cond. (per # incr) | 0.046 (−0.078 to 0.17); p = 0.47 | −0.29 (−0.54 to −0.047); p = 0.02 | −0.16 (−0.42 to 0.099); p = 0.23 | |
| ChAdOx1 as first vaccine | −0.14 (−0.48 to 0.21); p = 0.44 | – | – | |
| Dual ChAdOx1 regimen | – | −1.37 (−2.40 to −0.35); p = 0.0088 | −1.54 (−2.58 to −0.51); p = 0.0037 | |
| Dose interval (per week incr) | – | 0.049 (−0.028 to 0.13); p = 0.21 | −0.0018 (−0.080 to 0.077); p = 0.96 | |
| Days since vaccine | 0.024 (−0.061 to 0.55); p = 0.12 | −0.0092 (−0.076 to 0.058); p = 0.79 | −0.044 (−0.10 to 0.015); p = 0.14 | |
| EDTA as anticoagulant | 0.3 (−0.061 to 0.66); p = 0.1 | 0.83 (0.061 to 1.60); p = 0.035 | 0.43 (−0.36 to 1.21); p = 0.28 | |
| COVID-19 convalescent | 3.9 (3.60 to 4.22); p < 0.0001 | 1.07 (0.43 to 1.70); p = 0.0011 | 1.612 (0.97 to 2.26); p < 0.0001 |
aFor each study visit (one month after the 1st vaccine dose; 1 month after the 2nd dose, and 3 months after the 2nd dose), we report the Estimate (with the 95% Confidence Interval in parentheses); followed by the p-value. Statistically significant p-values are in bold.
bAnalyses performed on plasma (i.e. ACE2 displacement and viral neutralization) additionally correct for the anticoagulant used, with ACD as the reference category. Analyses of anti-RBD concentration do not correct for this variable because this assay was performed on serum collected in the same tube type.
cFor viral neutralization, reciprocal plasma dilutions were log2 transformed prior to multivariable analysis.
An extended version of this table, which also lists the F-statistics and model degrees of freedom, can be found in the Supplementary information.
The second vaccine dose substantially boosted anti-RBD binding antibody concentrations in all but two participants: one PLWH with immunodeficiency due to a chronic blood disorder, and one >80-year-old control participant with three chronic health conditions (Fig. 1b). One month after the second dose, antibody concentrations in COVID-19 naive PLWH (median 3.86 [IQR 3.63–4.07] log10 U/mL) were only ~0.1 log10 lower than those in naive controls (median 3.97 [IQR 3.76–4.22] log10 U/mL; Mann–Whitney p = 0.012), and only ~0.2 log10 lower than in convalescent participants (median 4.13 [IQR 3.87–4.29] log10 U/mL; Mann–Whitney p = 0.017. In multivariable analyses controlling for sociodemographic, health and vaccine-related variables however, HIV infection was no longer associated with antibody concentrations one month after the second vaccine dose (p = 0.78, Table 2). Rather, older age, a greater number of chronic conditions and having received two ChAdOx1 doses were independently predictive of weaker responses at this time point, with every 10 years of older age, each additional chronic condition and having received dual ChAdOx1 doses associated with 0.057 log10, 0.11 log10 and 0.63 log10 lower antibody concentrations, respectively (all p < 0.01). A longer dose interval was also associated with marginally higher antibody concentrations at this time point (0.025 log10 per additional week, p = 0.031). One month after the second dose, there was no longer a significant association between prior COVID-19 and antibody response (p = 0.5).
By 3 months following the second vaccine dose, antibody concentrations had declined in all participants by an average of ~0.4–0.5 log10 (Fig. 1c, d). Among PLWH and controls who were naive to COVID-19 at study entry, antibody levels had declined to median concentrations of 3.38 [IQR 3.17–3.58] log10 U/mL in PLWH and 3.57 [IQR 3.35–3.79] log10 U/mL in controls (Mann–Whitney p < 0.0001), while those in convalescent participants had declined to a median of 3.82 [IQR 3.37–3.92] log10 U/mL (Fig. 1c). The association between HIV infection and lower antibody concentrations at this time point however did not remain statistically significant in multivariable analyses, though the p-value was marginal (p = 0.07, Table 2; Supplementary Table 2). Rather, a greater number of chronic conditions and having received two ChAdOx1 doses remained statistically significantly associated with weaker responses at this time point (p = 0.01 and p < 0.0001, respectively), while a longer dose interval remained associated with higher antibody concentrations (p = 0.008).
Among PLWH who were naive to COVID-19 at study entry, we observed a weak positive correlation between the most recent CD4+ T-cell count and antibody concentration after one dose that was not statistically significant (Spearman’s ρ = 0.18, p = 0.09), but no significant relationship at either time point after the second dose (Fig. 1e). Similarly, we observed a weak positive relationship between nadir CD4+ T-cell count and antibody concentration after one dose that was not statistically significant (Spearman’s ρ = 0.19, p = 0.07), but no significant relationship at either time point after the second dose (Supplementary Fig. 1).
ACE2 receptor displacement activities after first and second vaccine doses
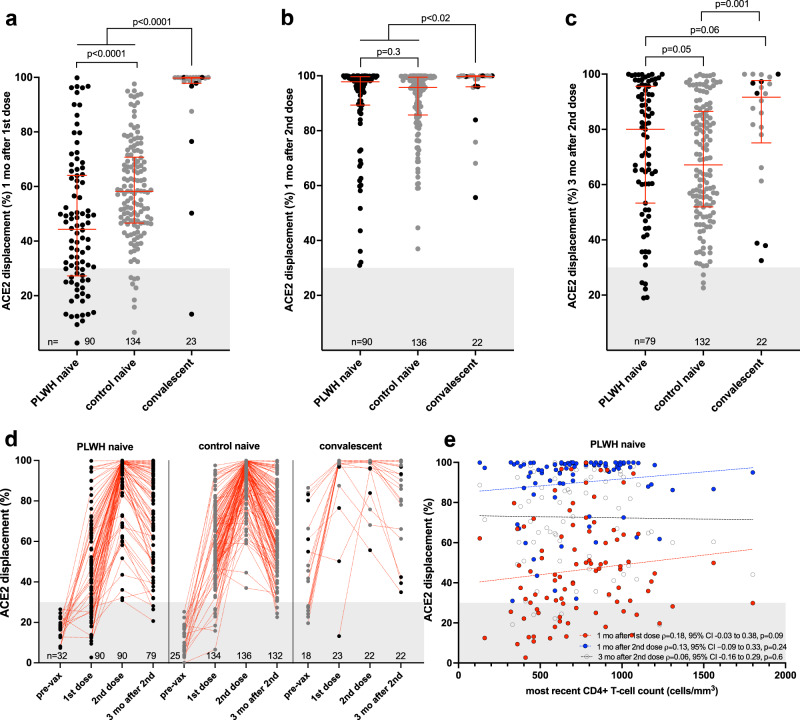
We next assessed the ability of plasma to block the RBD-ACE2 interaction, which represents a higher throughput approach to estimate potential viral neutralization activity (also referred to as a surrogate viral neutralization test54). After one vaccine dose, PLWH and controls who were COVID-19 naive at study entry exhibited median 44% (IQR 27–64%) and 58% (IQR 47–71%) ACE2 displacement activities, respectively, indicating lower function among PLWH (Mann–Whitney p < 0.0001) (Fig. 2a). In contrast, convalescent participants exhibited a median 99.7% (IQR 97.8–99.9%) ACE2 displacement activity after one dose (Mann–Whitney p < 0.0001 compared to both naive groups). In multivariable analyses, HIV infection remained significantly associated with an adjusted 11% lower ACE2 displacement activity after one vaccine dose (p = 0.023), with male sex (adjusted ~7% lower activity compared to female sex, p = 0.031) and having received ChAdOx1 as the first dose (adjusted 18.8% lower activity compared to an mRNA vaccine as first dose, p = 0.0001) remaining additional independent predictors of lower ACE2 displacement activity. Prior COVID-19 remained associated with an adjusted 36% higher ACE2 displacement activity following one vaccine dose (p < 0.0001).
Fig. 2. Ability of vaccine-induced antibodies to block ACE2-receptor binding following one and two COVID-19 vaccine doses.
a ACE2 displacement activities of plasma antibodies one month following the first COVID-19 vaccine dose in PLWH (black circles) and controls (grey circles) who were COVID-19 naive at study entry. Convalescent participants are colored by subgroup. The numbers of participants analyzed are indicated at the bottom of the plot. Red bars and whiskers represent median and IQR. Grey shaded area denotes the range of values observed in pre-vaccine plasma from COVID-19 naive participants (see d). p-values were computed using the Mann–Whitney U-test and are uncorrected for multiple comparisons. U-statistics were 4002 (PLWH naive vs. control naive), 144 (PLWH naive vs. convalescent) and 255 (control naive vs. convalescent). b ACE2 displacement activities one month after the second dose, presented as in (a). U-statistics were 5618 (PLWH naive vs. control naive), 660 (PLWH naive vs. convalescent) and 917 (control naive vs. convalescent). c ACE2 displacement activities 3 months after the second dose, presented as in (a). U-statistics were 4353 (PLWH naive vs. control naive), 644 (PLWH naive vs. convalescent) and 839 (control naive vs. convalescent). d ACE2 displacement activities plotted longitudinally for each group. Pre-vaccine measurements were performed only on a subset of COVID-19 naive participants to estimate assay background, shown as grey shading. The numbers of participants analyzed are indicated at the bottom of the plot. Red dotted lines connect participants’ longitudinal measurements. e Correlation between most recent CD4+ T-cell count and ACE2 displacement activities one month after the first dose (red circles; n = 90), one month after the second dose (blue circles; n = 90) and three months following the second dose (clear circles; n = 79). Matching-coloured dotted lines help visualize the trend.
One month following the second vaccine dose, the median ACE2 displacement activity in COVID-19 naive PLWH and controls rose to >95% in both groups and there was no longer a statistically significant difference between them (median 97.8% [IQR 89.3–99.6%] in PLWH versus 95.7% [85.7–99.5%] in controls, Mann–Whitney p = 0.3) (Fig. 2b). Furthermore, while the median ACE2 displacement activity in convalescent individuals (median 99.7% [IQR 96.0–100%]) remained statistically significantly higher than both naïve groups (both p < 0.02), the magnitude of this difference was marginal. In multivariable analyses, older age, a larger number of chronic conditions, and dual ChAdOx1 vaccination—but not HIV—were independently associated with lower ACE2 displacement function at this time point (adjusted 1.6% lower ACE2 displacement function for every decade of older age, 2.7% lower function for every additional health condition, and 29% lower function for dual ChAdOx1 vaccination; all p < 0.02) (Table 2).
By 3 months following the second vaccine dose, ACE2 displacement activity in COVID-19 naive PLWH and controls had declined to medians of 80% [IQR 53–96%] versus 67% [IQR 52–87%] respectively (Mann–Whitney p = 0.05), while the activity in convalescent participants had declined to a median of 92% [IQR 75–98%] (Fig. 2c, d). In multivariable analyses correcting for sociodemographic, health and vaccine-related variables, older age and having received two ChAdOx1 doses—but not HIV—were statistically significantly associated with weaker responses at this time point (p = 0.018 and p < 0.0001, respectively; Table 2).
Among PLWH who were naive to COVID-19 at study entry, we observed a weak positive correlation between recent CD4+ T-cell count and ACE2 displacement activity after one dose that was not statistically significant (Spearman’s ρ = 0.18, p = 0.09), but no association at either time point after two doses (Fig. 2e). Similarly, we observed a weak positive relationship between nadir CD4+ T-cell count and ACE2 displacement activity after one dose that was not statistically significant (Spearman’s ρ = 0.2, p = 0.06), but no association at either time point following two doses (Supplementary Fig. 1).
Viral neutralization activity after first and second vaccine doses
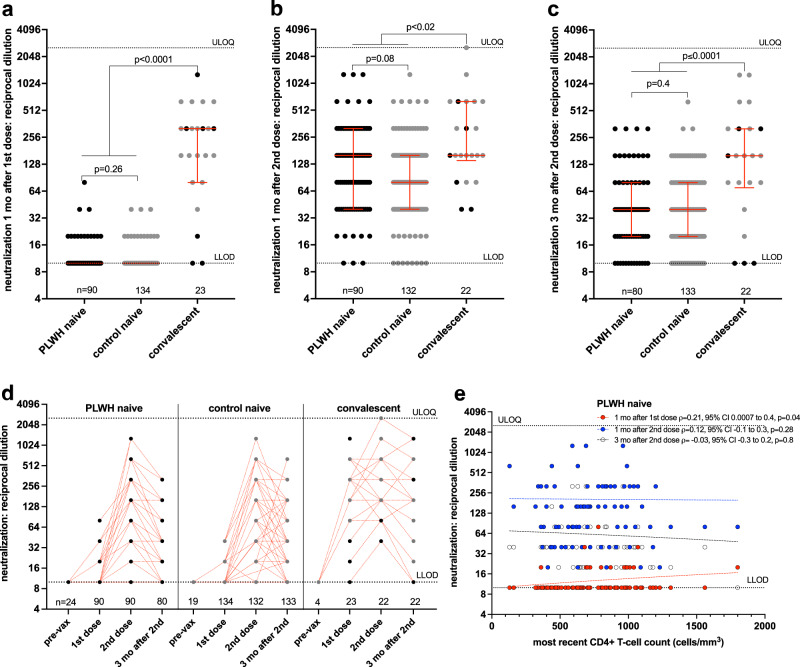
After one vaccine dose, plasma from most COVID-19 naive participants displayed weak or no ability to neutralize live SARS-CoV-2, with no significant differences between PLWH and controls (median and IQR undetectable in both groups; Mann–Whitney p = 0.26) (Fig. 3a). In contrast, neutralization activities in COVID-19 convalescent individuals were significantly higher, where the median reciprocal plasma dilution needed to achieve neutralization was 320 (IQR 80–320; p < 0.0001 compared to both naive groups) (Fig. 3a). Consistent with this, only COVID-19 convalescent status was significantly associated with higher neutralization activity in multivariable analyses after one dose (Table 2).
Fig. 3. Ability of vaccine-induced antibodies to neutralize live SARS-CoV-2 following one and two COVID-19 vaccine doses.
a Viral neutralization activities, defined as the highest reciprocal plasma dilution at which neutralization was observed in all triplicate assay wells, one month following the first COVID-19 vaccine dose in PLWH (black circles) and controls (grey circles) who were COVID-19 naive at study entry. Convalescent participants are colored by subgroup. The numbers of participants analyzed are indicated at the bottom of the plot. Red bars and whiskers represent median and IQR. p-values were computed using the Mann–Whitney U-test and are uncorrected for multiple comparisons. U-statistics were 5721 (PLWH naive vs. control naive), 116 (PLWH naive vs. convalescent) and 158 (control naive vs. convalescent). LLOD: assay lower limit of detection. ULOQ: assay upper limit of quantification. b Viral neutralization activities one month after the second vaccine dose, presented as in (a). U-statistics were 5131 (PLWH naive vs. control naive), 664 (PLWH naive vs. convalescent) and 777 (control naive vs. convalescent). c Viral neutralization activities 3 months after the second dose, presented as in (a). U-statistics were 4984 (PLWH naive vs. control naive), 431 (PLWH naive vs. convalescent) and 686 (control naive vs. convalescent). d Viral neutralization activities plotted longitudinally for each group. Pre-vaccine measurements were performed only on a subset of COVID-19 naive and convalescent participants, none of whom had detectable neutralization activity at this time. The numbers of participants analyzed are indicated at the bottom of the plot. Note that many values are superimposed. Red dotted lines connect participants’ longitudinal measurements. e Correlation between most recent CD4+ T-cell count and viral neutralization activities one month after the first dose (red circles, n = 90), one month after the second dose (blue circles, n = 90) and three months following the second dose (clear circles, n = 80). Matching-coloured dotted lines help visualize the trend.
At one month after the second vaccine dose, viral neutralization activities in COVID-19 naive PLWH and controls increased substantially in both groups, with naive PLWH achieving neutralization at a median reciprocal plasma dilution of 160 (IQR 40–320) compared to a median of 80 (IQR 40–160) in controls (p = 0.08) (Fig. 3b). The viral neutralization activities of COVID-19 convalescent individuals (median reciprocal dilution of 160; IQR 140–640) remained marginally higher than COVID-19 naive individuals at this time point (p < 0.02 for both comparisons). Consistent with the other humoral functions evaluated, multivariable analyses identified older age, a higher number of chronic conditions, and dual ChAdOx1 vaccination—but not HIV—as being independently associated with lower viral neutralization activity after two COVID-19 vaccine doses (p ≤ 0.02; Table 2). COVID-19 convalescent status also remained significantly associated with higher neutralization activity at this time point (p = 0.0011).
By 3 months following the second vaccine dose, neutralization activity had declined an average of two- to four-fold in all participants, to median reciprocal dilutions of 40 [IQR 20–80] in both COVID-19 naive PLWH and controls (Mann–Whitney p = 0.4), versus 160 [IQR 70–320] in convalescent participants (p < 0.0001 for both comparisons) (Fig. 3c, d). In multivariable analyses, the only two variables that were statistically significantly associated with neutralization activity at this time point were having received two ChAdOx1 doses (associated with lower neutralization activity, p = 0.0037) and COVID-19 convalescent status (associated with higher neutralization activity, p < 0.0001; Table 2).
Among PLWH who were naive to COVID-19 at study entry, we observed a weak positive correlation between recent CD4+ T-cell count and viral neutralization activity after one dose (Spearman’s ρ = 0.21, p = 0.04), but this association no longer remained at either time point after two doses (Fig. 3e). We observed no significant correlations between nadir CD4+ T-cell count and viral neutralization activity at any time point evaluated (Supplementary Fig. 1).
Humoral response against the SARS-CoV-2 delta variant
Given recent concerns that certain SARS-CoV-2 variants may be more transmissible or evade aspects of host immunity55,56, we examined the ACE2 displacement activity in plasma against the widespread B.1.617.2 (Delta) variant. After one vaccine dose, plasma from all groups was impaired in its ability to block ACE2 receptor engagement by the Delta RBD compared to the original wild-type (Wuhan) RBD, where the average (median) magnitude of this impairment was ~5%, ~15% and ~1% for COVID-19 naive PLWH, naive controls and convalescents, respectively (Wilcoxon-matched pairs signed rank test, all p ≤ 0.0001) (Fig. 4a). One month after the second vaccine dose, these impairments remained, albeit at a lower magnitude (a median of ~2%, ~7% and ~1% for naive PLWH, naive controls and convalescents, respectively, all p < 0.0001; Fig. 4b). By 3 months after the second vaccine dose, the impairments again became more pronounced (a median of ~6%, ~8% and ~6% for naive PLWH, naive controls and convalescents, respectively, all p < 0.0001; Fig. 4c). Given the strong correlations between ACE2 displacement and viral neutralization activities observed in our study against the wild-type strain (overall Spearman’s ρ = 0.84, p < 0.0001; Supplementary Fig. 2), these results suggest that vaccine-elicited humoral responses may be less able to prevent infection by the Delta variant, which is consistent with a recent report showing reduced ability of plasma from convalescent and vaccinated individuals to neutralize this strain57.
Fig. 4. ACE2 displacement activities against the original and Delta SARS-CoV-2 variants after one and two doses of COVID-19 vaccine.
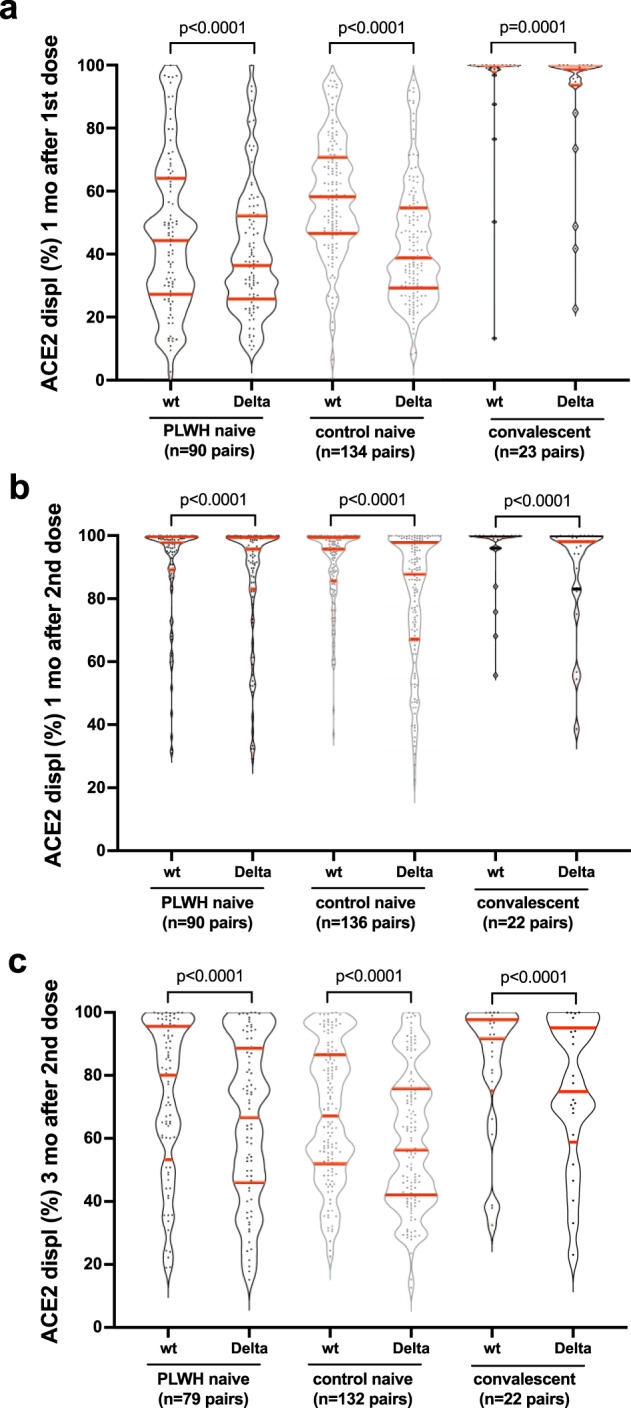
a ACE2 displacement activities of plasma antibodies against the original wild-type (wt) and Delta variant Spike-RBD in naive PLWH, naive controls, and convalescent individuals one month after the first vaccine dose. Horizontal red lines depict the median, 1st and 3rd quartiles. p-values were computed using the Wilcoxon matched-pairs signed rank test and are uncorrected for multiple comparisons. b Responses one month after the second vaccine dose, displayed as in (a). c Responses 3 months after the second vaccine dose, displayed as in (a).
Discussion
Our results add to a growing body of evidence that adult PLWH receiving stable antiretroviral therapy, who have suppressed plasma HIV loads and who have CD4+ T-cell counts in a healthy range, generally mount robust initial humoral immune responses to two COVID-19 vaccine doses10,33–35,37,38. Though HIV infection was associated with marginally (0.2 log10) lower overall anti-RBD antibody concentrations and ~11% lower ACE2 displacement activities following a single vaccine dose after adjustment for sociodemographic, health, and vaccine-related variables, we observed no statistically significant effect of HIV infection on any humoral response at either 1 or 3 months after the second vaccine dose after multivariable adjustment (though at the 3-month time point there was a trend towards lower anti-RBD antibody concentrations in PLWH compared to controls; Table 2). Rather, older age and a higher burden of chronic health conditions were independently associated with weaker humoral responses after two vaccine doses, consistent with previous reports39,58–61. Also consistent with previous reports62,63, having received two ChAdOx1 doses, as opposed to a heterologous or autologous mRNA vaccine regimen, was associated with significantly lower “peak” humoral responses (measured one month following the second dose). A recent study has reported that, while humoral responses to the mRNA vaccines initially reach high levels but wane considerably thereafter, immune responses induced by a COVID-19 viral vector vaccine induced lower median titers that remained more steady over time64, however in our study humoral responses remained significantly lower at 3 months after the second dose in individuals who received two ChAdOx1 vaccines. A longer interval between first and second COVID-19 vaccine doses (where the maximum interval among study participants was 122 days) was also associated with marginally higher binding antibody concentrations, though not ACE2 displacement or viral neutralization activities, which is partially consistent with reports of improved antibody and T-cell responses using extended dosing intervals of the BNT162b2 mRNA vaccine65. Indeed, Canada’s unique adoption of a longer (up to 112 days) dose interval yields insight into the magnitude of peak humoral responses following such an extended regimen. It is interesting that the anti-RBD antibody concentrations following two doses measured in the present study are generally higher than in studies of individuals who had shorter dose intervals that also employed the Roche Elecsys Anti-SARS-CoV-2 S assay, even though responses following the first dose were similar66–69. Comparing values across studies should be done with caution however, as the assay quantitative range varies based on the maximum sample dilution performed. It is also notable that at 3 months following the second vaccine dose, having COVID-19 prior to vaccination remained strongly associated with higher virus neutralization activity and marginally higher ACE2 displacement activity (Table 2). This is consistent with reports of superior “hybrid immunity” in previously infected individuals after vaccination70,71, which is characterized by the presence of higher affinity antibodies that display enhanced neutralization potency and variant cross-recognition activity.
Importantly, among PLWH in our study, all of whom were receiving suppressive antiretroviral treatment, we observed only a very weak positive correlation between the most recent CD4+ T-cell count and humoral responses, and only after the first vaccine dose. This association disappeared following the second vaccine dose. While CD4+ T-cell counts <250 cells/mm3 have been associated with lower antibody levels following one COVID-19 vaccine dose36,72, and in some studies also after two doses38, we were unable to confirm this finding as only two PLWH in the present study had CD4+ T-cell counts in this range, and both mounted strong vaccine responses. Moreover, although we found weak positive correlations between nadir CD4+ T-cell counts (which were as low as <10 cells/mm3 in our cohort) and both anti-RBD antibody concentrations and ACE2 displacement activities following one dose, these associations no longer remained following the second dose. Furthermore, we observed no association between viral neutralization activity and nadir CD4 T+ cell count after either vaccine dose. Taken together with a recent study that reported a lack of association between nadir CD4+ T-cell count and antibody response after two doses of BNT162b238, this indicates that, for PLWH currently receiving suppressive antiretroviral therapy and whose CD4+ T-cell counts are currently in a healthy range, having had low CD4 T+ cell counts in the past will not necessarily compromise immune responses to COVID-19 vaccines presently.
We also observed that the ability of vaccine-induced plasma antibodies to disrupt the ACE2/RBD interaction was modestly yet significantly reduced against the now widespread SARS-CoV-2 Delta variant compared to the original strain for all participant groups. Given the ability of SARS-CoV-2 variants to evade at least some aspects of vaccine-elicited immunity55, this suggests that all individuals, regardless of HIV status, will remain more susceptible to infection by this variant, even after vaccination.
Our study has several limitations. Our results may not be generalizable to PLWH who are not receiving antiretroviral therapy, who have CD4+ T-cell counts <200 cells/mm3, or who have complex co-morbidities (54% of PLWH in our study reported no chronic health conditions). Our study did not include children or adolescents living with HIV. As the precise immune correlates of protection for SARS-CoV-2 transmission and disease severity remain incompletely characterized73, the implications of our results on individual-level protection from SARS-CoV-2 infection and COVID-19 remain uncertain. The relationship between vaccine-induced antibody concentrations in blood and at mucosal sites, which may be a better correlate of protection, is also incompletely understood, though a recent study identified anti-RBD IgG antibodies in saliva in 100% of participants following a two-dose COVID-19 vaccine series74. We did not investigate vaccine-induced T-cell responses, though two recent studies have demonstrated comparable anti-Spike T-cell responses in PLWH compared to controls10,34. Our study was not designed nor powered to investigate potential differences in immune responses between the two mRNA vaccines66,75,76. Though our study was longitudinal, the latest time point analyzed was 3 months after the second vaccine dose, so additional durability data are needed.
Taken together with existing data10,33–35, our results indicate that adults with HIV receiving suppressive antiretroviral therapy and who have CD4+ T-cell counts in the healthy range, mount broadly comparable “peak” humoral immune responses to two COVID-19 vaccine doses compared to individuals without HIV. Furthermore, although immune responses naturally decline over time in all persons, we observed no statistically significant differences between PLWH and controls in the magnitude of any humoral measure at 3 months after the second vaccine dose after multivariable adjustment, though the trend towards marginally lower anti-RBD antibody concentrations (but not other measures) in PLWH at this timepoint underscore the need for ongoing assessments of response durability. Moreover, we found no evidence that a low nadir CD4+ T-cell count negatively influenced the response to COVID-19 vaccination in this group. Rather, our results identified older age, a higher burden of chronic health conditions, and having received a dual ChAdOx1 regimen (as opposed to a heterologous or dual mRNA vaccine regimen)—but not HIV—as significant negative modulators of humoral responses following two-dose COVID-19 vaccination.
These results indicate that PLWH with well-controlled viral loads and CD4+ T-cell counts in a healthy range generally mount strong initial humoral responses to dual COVID-19 vaccination. Factors such as older age, co-morbidities, initial vaccine regimen type, response durability and the rise of new SARS-CoV-2 variants will influence when PLWH (as well as individuals without HIV) will benefit from additional vaccine doses. Further studies of PLWH who are not receiving antiretroviral treatment and/or who have low CD4+ T-cell counts are needed, as are longer-term assessments of vaccine response durability.
Methods
Participants and sampling
A total of 100 adult PLWH were recruited through three HIV care clinics in Vancouver, British Columbia (BC), Canada and through community outreach. Control samples were obtained from 152 individuals without HIV, of which 128 were also included in a parallel study of COVID-19 vaccine responses across the adult age spectrum, where the majority (N = 89; 59%) were health care workers77. HIV-negative status of control participants was determined by self-report. Serum and plasma were collected prior to COVID-19 vaccination (where possible), 1 month after the first COVID-19 vaccine dose, and at 1 and 3 months after the second dose. Plasma was collected in either ethylenediaminetetraacetic acid (EDTA) or anticoagulant citrate dextrose (ACD). Specimens were processed on the day of collection and frozen until analysis. COVID-19 convalescent individuals were identified at study entry by the presence of serum antibodies against the SARS-CoV-2 nucleoprotein (N).
Ethics approval
This study complied with all relevant ethical regulations for work with human participants, and all participants provided written informed consent. This study was approved by the University of British Columbia/Providence Health Care and Simon Fraser University Research Ethics Boards.
Data sources
Sociodemographic data, chronic health conditions and COVID-19 vaccination information were collected by self-report and confirmed through medical records where available. We assigned a score of 1 for each of the following 11 chronic health conditions: hypertension, diabetes, asthma, obesity (defined as having a body mass index ≥30), chronic diseases of lung, liver, kidney, heart or blood, cancer, and immunosuppression due to chronic conditions or medication, to generate a total score ranging from 0 to 11. Clinical information for PLWH was recovered from the BC Centre for Excellence in HIV/AIDS Drug Treatment Program Database, which houses clinical records for all PLWH receiving care in BC. For PLWH, having a recent CD4+ T-cell count <200 cells/mm3 was classified as “immunosuppression” in the chronic health conditions score.
Binding antibody assays
We measured total binding antibodies against SARS-CoV-2 N and spike receptor binding domain (RBD) in serum using the Roche Elecsys Anti-SARS-CoV-2 and Elecsys Anti-SARS-CoV-2 S assays, respectively. Both are electro-chemiluminescence sandwich immunoassays. Post-infection, both assays should be positive, whereas post-mRNA vaccination only the S assay should be positive, enabling identification of convalescent samples. The S assay reports results in arbitrary units/mL (U/mL), calibrated against an external standard. For the S assay, the manufacturer indicates that arbitrary unit values can be considered equivalent to international binding antibody units (BAU) as defined by the World Health Organization and the measurement range is from 0.4 to 25,000 U/mL78.
ACE2 displacement assay
We assessed the ability of plasma antibodies to block the interaction between RBD and the ACE2 receptor using the V-plex SARS-CoV-2 Panel 11 (ACE2) kit on a MESO QuickPlex SQ120 instrument (Meso Scale Discovery) at the manufacturer’s recommended 1:20 dilution. ACE2 displacement was calculated as 100−[Arbitrary Units (AU) of ACE2 binding in the presence of plasma/AU of ACE2 binding in the absence of plasma] and reported as a percentage. All samples were assessed in replicate experiments; results from one representative experiment are shown.
Live virus neutralization assay
Neutralizing activity in plasma was examined using a live SARS-CoV-2 infectivity assay in a Containment Level 3 facility. Assays were performed using isolate USA-WA1/2020 (BEI Resources) and VeroE6-TMPRSS2 (JCRB-1819) target cells. Viral stock was adjusted to 50 TCID50/200 µl in DMEM in the presence of serial 2-fold dilutions of plasma (from 1/20 to 1/2560), incubated at 4 °C for 1 h and then added to target cells in 96-well plates in triplicate. Cultures were maintained at 37 °C with 5% CO2 and the appearance of viral cytopathic effect (CPE) was recorded 3 days post-infection. Neutralizing activity is reported as the highest reciprocal plasma dilution necessary to prevent CPE in all three replicate wells. Samples exhibiting only partial or no neutralization at the lowest dilution of 1/20 were coded as having a reciprocal dilution of “10”, defined as below the limit of detection in this assay.
Statistical analysis
Comparisons of binary variables between groups were performed using Fisher’s exact test. Comparisons of continuous variables between groups were performed using the Mann–Whitney U-test (for unpaired data) or Wilcoxon test (for paired data). Correlations between continuous variables were performed using Spearman’s correlation. Multiple linear regression was employed to investigate the relationship between sociodemographic, health and vaccine-related variables and humoral outcomes. In addition to HIV infection, variables assessed included age (per decade increment), sex at birth (female as reference group), ethnicity (non-white as reference group), number of chronic health conditions (per number increment), type of vaccine received (mRNA vaccine as reference group), sampling date following vaccine dose (per day increment), and COVID-19 convalescent status at study entry (COVID-19 naive as reference group). Analyses performed following two doses additionally included the interval between doses (per week increment) and having received two ChAdOx1 doses (heterologous or dual mRNA vaccine regimen as the combined reference group). For the assays that tested plasma, which were the ACE2 displacement and viral neutralization assays, the models also corrected for the anticoagulant used (ACD as the reference group). All tests were two-tailed, with p < 0.05 considered statistically significant. Analyses were conducted using Prism v9.2.0 (GraphPad).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We thank the leadership and staff of Providence Health Care for their support of this study. We thank the phlebotomists and laboratory staff at St. Paul’s Hospital, the BC Centre for Excellence in HIV/AIDS and Simon Fraser University for assistance. Above all, we thank the participants, without whom this study would not have been possible. This work was supported by funding from Genome BC, the Michael Smith Foundation for Health Research, and the BCCDC Foundation for Public Health through a rapid SARS-CoV-2 vaccine research initiative in BC award (VAC-009 to Z.L.B., M.A.B.). It was also supported by the Public Health Agency of Canada (PHAC) through two COVID-19 Immunology Task Force (CITF) COVID-19 Awards (to Z.L.B., M.G.R., M.A.B. and to C.T.C., C.C., A.H.A.), the Canada Foundation for Innovation through Exceptional Opportunities Fund—COVID-19 awards (to C.J.B., M.A.B., M.N., M.L.D., R.P., Z.L.B.), a British Columbia Ministry of Health—Providence Health Care Research Institute COVID-19 Research Priorities Grant (to C.J.B.), the CIHR Canadian HIV Trials Network (CTN) (to A.H.A.) and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (R01AI134229 to R.P.). M.L.D. and Z.L.B. hold Scholar Awards from the Michael Smith Foundation for Health Research. L.Y.L. was supported by an SFU Undergraduate Research Award. G.U. and F.H.O. are supported by Ph.D. fellowships from the Sub-Saharan African Network for TB/HIV Research Excellence (SANTHE), a DELTAS Africa Initiative [grant # DEL-15-006]. The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)’s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust [grant # 107752/Z/15/Z] and the UK government. H.S. is supported by a CGS-M award from the Canadian Institutes of Health Research (CIHR). The views expressed in this publication are those of the authors and not necessarily those of PHAC, CITF, AAS, NEPAD Agency, Wellcome Trust, the Canadian or UK governments or other funders.
Author contributions
Z.L.B. and M.A.B. conceived and oversaw the study, drafted the manuscript and acquired funding. H.R.L. coordinated study operations, and collected, managed, curated and analyzed data. F.M., P.K.C., Y.S., M.C.D., F.Y., O.A., S.E., K.N., S.B., L.Y.L., R.K., S.S., N.M.-G., L.Y. B.G., G.U., F.H.O., K.A., H.S., and L.B. collected and/or analyzed data. J.T. and P.S. curated data. D.H., M.L.D., J.S., and M.G.R. oversaw the collection of the Roche Elecsys data and provided technical guidance and expertise. H.A., V.L., D.H., M.L.D., J.S., M.H., M.G.R., R.B., S.G., J.S.G., M.H. and M.H. provided clinical expertise, guidance and input. C.J.B. provided input on statistical analysis and analyzed data. M.N., and R.P. designed assays, conducted/oversaw data collection and provided guidance and input. C.T.C., C.C. and A.H.A. provided guidance and input and acquired additional funding. All authors discussed the results and implications and commented on the manuscript at all stages.
Data availability
The data supporting the findings of this paper comprise sociodemographic, health, clinical, vaccine and longitudinal laboratory measurement data from participants, including sensitive information such as HIV infection status. To protect participant privacy and confidentiality, data cannot be deposited into the public domain or shared openly. Data can however be shared with interested investigators through existing REB and institutionally approved processes. To request data, please contact the corresponding author with the data request. Data can be shared following completion of REB and institutional requirements (e.g. data transfer agreement). Making datasets of this type available to other researchers through this mechanism is in compliance with our REB and institutional requirements.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
6/9/2022
In the original version of this article, the given and family names of F. Harrison OMONDI were incorrectly structured. The name was displayed correctly in all versions at the time of publication. The original article has been corrected.
Supplementary information
The online version contains supplementary material available at 10.1038/s41541-022-00452-6.
References
- 1.BC Centre for Excellence in HIV/AIDS Committee on Drug Evaluation and Therapy. Committee Statement Update on the use of COVID-19 Vaccines in Persons Living with HIVhttp://bccfe.ca/therapeutic-guidelines/bc-cfe-cdet-statement-use-of-covid19-vaccines-persons-living-hiv (2021).
- 2.Geretti AM, et al. Outcomes of COVID-19 related hospitalization among people with HIV in the ISARIC WHO Clinical Characterization Protocol (UK): a prospective observational study. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciaa1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boulle A, et al. Risk factors for COVID-19 death in a population cohort study from the Western Cape Province, South Africa. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciaa1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tesoriero JM, et al. COVID-19 outcomes among persons living with or without diagnosed HIV infection in New York state. JAMA Netw. Open. 2021;4:e2037069. doi: 10.1001/jamanetworkopen.2020.37069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhaskaran K, et al. HIV infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV. 2021;8:e24–e32. doi: 10.1016/S2352-3018(20)30305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polack FP, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas SJ, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baden LR, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voysey M, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frater J, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: a single-arm substudy of a phase 2/3 clinical trial. Lancet HIV. 2021;8:e474–e485. doi: 10.1016/S2352-3018(21)00103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madhi SA, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in people living with and without HIV in South Africa: an interim analysis of a randomised, double-blind, placebo-controlled, phase 1B/2A trial. Lancet HIV. 2021;8:e568–e580. doi: 10.1016/S2352-3018(21)00157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dagan N, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N. Engl. J. Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tenforde MW, et al. Sustained effectiveness of Pfizer-BioNTech and moderna vaccines against COVID-19 associated hospitalizations among adults—United States, March–July 2021. Morb. Mortal. Wkly Rep. 2021;70:1156–1162. doi: 10.15585/mmwr.mm7034e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haas EJ, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh EE, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N. Engl. J. Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Widge AT, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N. Engl. J. Med. 2021;384:80–82. doi: 10.1056/NEJMc2032195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeewandara C, et al. Immune responses to a single dose of the AZD1222/Covishield vaccine in health care workers. Nat. Commun. 2021;12:4617. doi: 10.1038/s41467-021-24579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabinowich L, et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J. Hepatol. 2021;75:435–438. doi: 10.1016/j.jhep.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grupper A, et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am. J. Transpl. 2021;21:2719–2726. doi: 10.1111/ajt.16615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Massarweh A, et al. Evaluation of seropositivity following BNT162b2 messenger RNA vaccination for SARS-CoV-2 in patients undergoing treatment for cancer. JAMA Oncol. 2021;7:1133–1140. doi: 10.1001/jamaoncol.2021.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monin L, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22:765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palich R, et al. High seroconversion rate but low antibody titers after two injections of BNT162b2 (Pfizer-BioNTech) vaccine in patients treated with chemotherapy for solid cancers. Ann. Oncol. 2021;32:1294–1295. doi: 10.1016/j.annonc.2021.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moor MB, et al. Humoral and cellular responses to mRNA vaccines against SARS-CoV-2 in patients with a history of CD20 B-cell-depleting therapy (RituxiVac): an investigator-initiated, single-centre, open-label study. Lancet Rheumatol. 2021 doi: 10.1016/s2665-9913(21)00251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deepak P, et al. Effect of immunosuppression on the immunogenicity of mRNA vaccines to SARS-CoV-2: a Prospective Cohort Study. Ann. Intern. Med. 2021 doi: 10.7326/m21-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Apostolidis SA, et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021 doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plana M, et al. Immunological benefits of antiretroviral therapy in very early stages of asymptomatic chronic HIV-1 infection. AIDS. 2000;14:1921–1933. doi: 10.1097/00002030-200009080-00007. [DOI] [PubMed] [Google Scholar]
- 27.Kaufmann GR, et al. Rapid restoration of CD4 T cell subsets in subjects receiving antiretroviral therapy during primary HIV-1 infection. AIDS. 2000;14:2643–2651. doi: 10.1097/00002030-200012010-00003. [DOI] [PubMed] [Google Scholar]
- 28.Bart PA, et al. Immunological and virological responses in HIV-1-infected adults at early stage of established infection treated with highly active antiretroviral therapy. AIDS. 2000;14:1887–1897. doi: 10.1097/00002030-200009080-00002. [DOI] [PubMed] [Google Scholar]
- 29.Lundgren JD, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N. Engl. J. Med. 2015;373:795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El Chaer F, El Sahly HM. Vaccination in the adult patient infected with HIV: a review of vaccine efficacy and immunogenicity. Am. J. Med. 2019;132:437–446. doi: 10.1016/j.amjmed.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Kernéis S, et al. Long-term immune responses to vaccination in HIV-infected patients: a systematic review and meta-analysis. Clin. Infect. Dis. 2014;58:1130–1139. doi: 10.1093/cid/cit937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geretti AM, Doyle T. Immunization for HIV-positive individuals. Curr. Opin. Infect. Dis. 2010;23:32–38. doi: 10.1097/QCO.0b013e328334fec4. [DOI] [PubMed] [Google Scholar]
- 33.Levy I, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in people living with HIV-1. Clin. Microbiol. Infect. 2021 doi: 10.1016/j.cmi.2021.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woldemeskel BA, et al. The BNT162b2 mRNA vaccine elicits robust humoral and cellular immune responses in people living with HIV. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruddy JA, et al. Safety and antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in persons with HIV. AIDS. 2021 doi: 10.1097/qad.0000000000003017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nault, L. et al. Covid-19 vaccine immunogenicity in people living with HIV-1, Preprint at bioRxiv10.1101/2021.08.13.456258 (2021).
- 37.Jedicke N, et al. Humoral immune response following prime and boost BNT162b2 vaccination in people living with HIV on antiretroviral therapy. HIV Med. 2021 doi: 10.1111/hiv.13202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noe, S. et al. Humoral response to SARS-CoV-2 vaccines in people living with HIV. Infection 1–7 10.1007/s15010-021-01721-7 (2021). [DOI] [PMC free article] [PubMed]
- 39.Zimmermann, P. & Curtis, N. Factors that influence the immune response to vaccination. Clin. Microbiol. Rev. 32, 10.1128/cmr.00084-18 (2019). [DOI] [PMC free article] [PubMed]
- 40.Laratta CR, Williams K, Vethanayagam D, Ulanova M, Vliagoftis H. A case series evaluating the serological response of adult asthma patients to the 23-valent pneumococcal polysaccharide vaccine. Allergy Asthma Clin. Immunol. 2017;13:27. doi: 10.1186/s13223-017-0200-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoo KH, et al. Assessment of humoral and cell-mediated immune response to measles-mumps-rubella vaccine viruses among patients with asthma. Allergy Asthma Proc. 2010;31:499–506. doi: 10.2500/aap.2010.31.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheen YH, et al. Relationship between asthma status and antibody response pattern to 23-valent pneumococcal vaccination. J. Asthma. 2020;57:381–390. doi: 10.1080/02770903.2019.1575394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weber DJ, Rutala WA, Samsa GP, Santimaw JE, Lemon SM. Obesity as a predictor of poor antibody response to hepatitis B plasma vaccine. JAMA. 1985;254:3187–3189. doi: 10.1001/jama.1985.03360220053027. [DOI] [PubMed] [Google Scholar]
- 44.Sheridan PA, et al. Obesity is associated with impaired immune response to influenza vaccination in humans. Int. J. Obes. 2012;36:1072–1077. doi: 10.1038/ijo.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitsunaga T, et al. The evaluation of factors affecting antibody response after administration of the BNT162b2 vaccine: a prospective study in Japan. PeerJ. 2021;9:e12316. doi: 10.7717/peerj.12316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh AK, et al. Antibody response after first and second-dose of ChAdOx1-nCOV (Covishield(TM)®) and BBV-152 (Covaxin(TM)®) among health care workers in India: The final results of cross-sectional coronavirus vaccine-induced antibody titre (COVAT) study. Vaccine. 2021;39:6492–6509. doi: 10.1016/j.vaccine.2021.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uysal EB, Gümüş S, Bektöre B, Bozkurt H, Gözalan A. Evaluation of antibody response after COVID-19 vaccination of healthcare workers. J. Med. Virol. 2021 doi: 10.1002/jmv.27420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanania NA, et al. Immune response to influenza vaccination in children and adults with asthma: effect of corticosteroid therapy. J. Allergy Clin. Immunol. 2004;113:717–724. doi: 10.1016/j.jaci.2003.12.584. [DOI] [PubMed] [Google Scholar]
- 49.National Advisory Committee on Immunization (NACI). An Advisory Committee Statement (ACS)National Advisory Committee on Immunization (NACI): Recommendations on the Use of COVID-19 Vaccines (Public Health Agency of Canada, 2021).
- 50.Wise J. Covid-19: European countries suspend use of Oxford-AstraZeneca vaccine after reports of blood clots. BMJ. 2021;372:n699. doi: 10.1136/bmj.n699. [DOI] [PubMed] [Google Scholar]
- 51.National Advisory Committee on Immunization (NACI). An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI); Extended Dose Intervals for COVID-19 Vaccines To Optimize Early Vaccine Rollout and Population Protection in Canada in the Context of Limited Vaccine Supply (Public Health Agency of Canada, 2021).
- 52.Goel, R. R. et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals following mRNA vaccination. Sci. immunol.6, 10.1126/sciimmunol.abi6950 (2021). [DOI] [PMC free article] [PubMed]
- 53.Hirotsu Y, et al. Robust antibody responses to the BNT162b2 mRNA vaccine occur within a week after the first dose in previously infected individuals and after the second dose in uninfected individuals. Front. Immunol. 2021;12:722766. doi: 10.3389/fimmu.2021.722766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan CW, et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2–spike protein–protein interaction. Nat. Biotechnol. 2020;38:1073–1078. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- 55.Gupta RK. Will SARS-CoV-2 variants of concern affect the promise of vaccines? Nat. Rev. Immunol. 2021;21:340–341. doi: 10.1038/s41577-021-00556-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Plante JA, et al. The variant gambit: COVID-19’s next move. Cell Host Microbe. 2021;29:508–515. doi: 10.1016/j.chom.2021.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Planas D, et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596:276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 58.Pereira B, Xu XN, Akbar AN. Targeting inflammation and immunosenescence to improve vaccine responses in the elderly. Front. Immunol. 2020;11:583019. doi: 10.3389/fimmu.2020.583019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Merani S, Pawelec G, Kuchel GA, McElhaney JE. Impact of aging and cytomegalovirus on immunological response to influenza vaccination and infection. Front. Immunol. 2017;8:784. doi: 10.3389/fimmu.2017.00784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Collier DA, et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. 2021;596:417–422. doi: 10.1038/s41586-021-03739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Müller L, et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Normark J, et al. Heterologous ChAdOx1 nCoV-19 and mRNA-1273 vaccination. N. Engl. J. Med. 2021;385:1049–1051. doi: 10.1056/NEJMc2110716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schmidt T, et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat. Med. 2021;27:1530–1535. doi: 10.1038/s41591-021-01464-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Collier AY, et al. Differential kinetics of immune responses elicited by Covid-19 vaccines. N. Engl. J. Med. 2021 doi: 10.1056/NEJMc2115596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Payne RP, et al. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell. 2021;184:5699–5714.e5611. doi: 10.1016/j.cell.2021.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Steensels D, Pierlet N, Penders J, Mesotten D, Heylen L. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA. 2021 doi: 10.1001/jama.2021.15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Perkmann T, et al. Initial SARS-CoV-2 vaccination response can predict booster response for BNT162b2 but not for AZD1222. Int. J. Infect. Dis. 2021;110:309–313. doi: 10.1016/j.ijid.2021.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mueller T. Antibodies against severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) in individuals with and without COVID-19 vaccination: a method comparison of two different commercially available serological assays from the same manufacturer. Clin. Chim. Acta. 2021;518:9–16. doi: 10.1016/j.cca.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Di Meo A, et al. Evaluation of three anti-SARS-CoV-2 serologic Immunoassays for post-vaccine response. J. Appl. Lab. Med. 2021 doi: 10.1093/jalm/jfab087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Z, et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature. 2021;595:426–431. doi: 10.1038/s41586-021-03696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cho A, et al. Anti-SARS-CoV-2 receptor-binding domain antibody evolution after mRNA vaccination. Nature. 2021 doi: 10.1038/s41586-021-04060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ruddy JA, et al. Safety and antibody response to the first dose of severe acute respiratory syndrome coronavirus 2 messenger RNA vaccine in persons with HIV. AIDS. 2021;35:1872–1874. doi: 10.1097/QAD.0000000000002945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Feng S, et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 Infection. Nat. Med. 2021;27:2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ketas TJ, et al. Antibody responses to SARS-CoV-2 mRNA vaccines are detectable in saliva. Pathog. Immun. 2021;6:116–134. doi: 10.20411/pai.v6i1.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nanduri S, et al. Effectiveness of Pfizer-BioNTech and moderna vaccines in preventing SARS-CoV-2 infection among nursing home residents before and during widespread circulation of the SARS-CoV-2 B.1.617.2 (Delta) Variant—National Healthcare Safety Network, March 1–August 1, 2021. Morb. Mortal. Wkly Rep. 2021;70:1163–1166. doi: 10.15585/mmwr.mm7034e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abe, K. T. et al. Neutralizing antibody responses to SARS-CoV-2 variants in vaccinated Ontario long-term care home residents and workers. Preprint at bioRxiv10.1101/2021.08.06.21261721 (2021).
- 77.Brockman MA, et al. Reduced magnitude and durability of humoral immune responses to COVID-19 mRNA vaccines among older adults. J. Infect. Dis. 2021 doi: 10.1093/infdis/jiab592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mattiuzzo, G. et al. in WHO/BS/2020.2402 Establishment of the WHO International Standard and Reference Panel for Anti-SARS-CoV-2 Antibody (ed WHO Expert Committee on Biological Standardization) (World Health Organization, Geneva, 2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this paper comprise sociodemographic, health, clinical, vaccine and longitudinal laboratory measurement data from participants, including sensitive information such as HIV infection status. To protect participant privacy and confidentiality, data cannot be deposited into the public domain or shared openly. Data can however be shared with interested investigators through existing REB and institutionally approved processes. To request data, please contact the corresponding author with the data request. Data can be shared following completion of REB and institutional requirements (e.g. data transfer agreement). Making datasets of this type available to other researchers through this mechanism is in compliance with our REB and institutional requirements.